Published online Jan 28, 2021. doi: 10.4329/wjr.v13.i1.29
Peer-review started: July 24, 2020
First decision: November 14, 2020
Revised: November 26, 2020
Accepted: December 4, 2020
Article in press: December 4, 2020
Published online: January 28, 2021
Processing time: 186 Days and 22.7 Hours
Redundant nerve roots (RNRs) of the cauda equina are often a natural evolutionary part of lumbar spinal canal stenosis secondary to degenerative processes characterized by elongated, enlarged, and tortuous nerve roots in the superior and/or inferior of the stenotic segment. Although magnetic resonance imaging (MRI) findings have been defined more frequently in recent years, this condition has been relatively under-recognized in radiological practice. In this study, lumbar MRI findings of RNRs of the cauda equina were evaluated in spinal stenosis patients.
To evaluate RNRs of the cauda equina in spinal stenosis patients.
One-hundred and thirty-one patients who underwent lumbar MRI and were found to have spinal stenosis between March 2010 and February 2019 were included in the study. On axial T2-weighted images (T2WI), the cross-sectional area (CSA) of the dural sac was measured at L2-3, L3-4, L4-5, and L5-S1 levels in the axial plane. CSA levels below 100 mm2 were considered stenosis. Elongation, expansion, and tortuosity in cauda equina fibers in the superior and/or inferior of the stenotic segment were evaluated as RNRs. The patients were divided into two groups: Those with RNRs and those without RNRs. The CSA cut-off value resulting in RNRs of cauda equina was calculated. Relative length (RL) of RNRs was calculated by dividing the length of RNRs at mid-sagittal T2WI by the height of the vertebral body superior to the stenosis level. The associations of CSA leading to RNRs with RL, disc herniation type, and spondylolisthesis were evaluated.
Fifty-five patients (42%) with spinal stenosis had RNRs of the cauda equina. The average CSA was 40.99 ± 12.76 mm2 in patients with RNRs of the cauda equina and 66.83 ± 19.32 mm2 in patients without RNRs. A significant difference was found between the two groups for CSA values (P < 0.001). Using a cut-off value of 55.22 mm2 for RNRs of the cauda equina, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) values of 96.4%, 96.1%, 89.4%, and 98.7% were obtained, respectively. RL was 3.39 ± 1.31 (range: 0.93-6.01). When the extension of RNRs into the superior and/or inferior of the spinal canal stenosis level was evaluated, it was superior in 54.5%, both superior and inferior in 32.8%, and inferior in 12.7%. At stenosis levels leading to RNRs of the cauda equina, 29 disc herniations with soft margins and 26 with sharp margins were detected. Disc herniation type and spondylolisthesis had no significant relationship with RL or CSA of the dural sac with stenotic levels (P > 0.05). As the CSA of the dural sac decreased, the incidence of RNRs observed at the superior of the stenosis level increased (P < 0.001).
RNRs of the cauda equina are frequently observed in patients with spinal stenosis. When the CSA of the dural sac is < 55 mm2, lumbar MRIs should be carefully examined for this condition.
Core Tip: In this study, magnetic resonance imaging findings of redundant nerve roots (RNRs) of the cauda equina were evaluated in patients with lumbar stenosis. The stenotic segment cross-sectional area (CSA) cut-off value that could lead to RNRs of the cauda equina was detected as 55.22 mm2. In patients with RNRs of the cauda equina, the average CSA was significantly lower than in patients who did not have RNRs. Disc herniation type and spondylolisthesis were not significantly associated with the relative length or CSA of the dural sac. It was found that the incidence of RNRs observed at the superior of the stenosis level increased as the CSA decreased.
- Citation: Gökçe E, Beyhan M. Magnetic resonance imaging findings of redundant nerve roots of the cauda equina. World J Radiol 2021; 13(1): 29-39
- URL: https://www.wjgnet.com/1949-8470/full/v13/i1/29.htm
- DOI: https://dx.doi.org/10.4329/wjr.v13.i1.29
The term redundant nerve roots (RNRs) of the cauda equina was first used by Cresmann and Pawl[1-3]. It is a condition in which nerve roots of the cauda equina have accompanying tortuosity and elongation and it develops secondary to spinal stenosis. It is not a new or separate disease but often a natural evolutionary part of lumbar spinal canal stenosis secondary to degenerative processes[4]. The developmental mechanism of this non-congenital elongated nerve root is probably the trapping of the nerve root at the level of stenosis. The most common symptoms in RNRs of the cauda equina are pain in the lower back and leg[3]. It has been reported that in patients with RNRs of the cauda equina, leg pain, paresthesia, and difficulty in walking are more pronounced than in patients with lumbar stenosis without RNRs and that they derive limited benefit from decompression surgery[4-6]. Radiologically, RNRs of the cauda equina were initially defined as serpiginous filling defects due to partial or total stenosis that prevents the passage of contrast material on myelography. Along with the increasing use of magnetic resonance imaging (MRI) for imaging the spinal canal, it is now predominantly considered as an MRI finding[2,4,7-14]. However, this condition has been relatively underrecognized in radiological practice[2,4]. The aim of the present study was to evaluate the imaging findings of RNRs of the cauda equina detected on the lumbar MRI of spinal stenosis patients.
The reports of 7424 patients in the picture archive and communication system (PACS) (SECTRA IDS7 PACS, Sweden) who underwent lumbar MRI in our hospital for various reasons between March 2010 and February 2019 were retrospectively examined for the expression “spinal stenosis”. One hundred and sixty-seven patients who were found to have the term "spinal stenosis" in lumbar MRI reports in PACS were examined for the presence of RNRs. One hundred and thirteen (67.7%) of these patients were female and 54 (32.3%) were male. The mean age was 60.7 ± 11.3 years (range 28-90). Sixty (35.9%) patients had low back pain, 54 (32.3%) had back and leg pain, 21 (12.6%) had leg pain, 13 (7.8%) had both low back and leg pain and claudication, nine (5.4%) had low back pain and claudication, eight (4.8%) had claudication and two (1.2%) had leg pain and claudication. Until 2017, MRI examinations were carried out using an 8-channel 1.5 T MRI machine (GE Signa Excite HD; GE Healthcare, Milwaukee, United States). A 16-channel 1.5 T MRI machine (GE Signa Explorer SV 25; GE Healthcare, Milwaukee, United States,) was used after 2017. A phased array spine coil was used on the lumbar MRI. Sequences and parameters obtained on lumbar MRI examinations were, respectively: sagittal plane T2-weighted (T2W) fast spin echo (FSE) sequences (TR: 3008 ms, TE: 91.9 ms, NEX: 2, slice thickness: 4 mm, gap distance: 1 mm, FOV: 29 cm, matrix: 320 x 224); sagittal plane T1W FSE sequences (TR: 602 ms, TE: 8.7 ms, NEX: 1.5, slice thickness: 4 mm, gap distance: 1 mm, FOV: 29 cm, matrix: 320 × 224); axial plane T2W (TR: 4647 ms, TE: 91.8 ms, NEX: 2, slice thickness: 4 mm, gap distance: 1 mm, FOV: 18 cm, matrix: 320 × 192). In those patients with spinal stenosis on lumbar MRI, the presence of RNRs was evaluated with consensus by two radiologists with 14 (E.G.) and eight (M.B.) years of work experience. Thirty-six patients with a history of craniospinal operations or spondylodiscitis and whose lumbar MRI examination was not of optimal image quality were excluded from the study. The number of patients not included in this study and the reasons for exclusion are shown in Table 1.
| The reason for exclusion | n |
| Spinal or cranial surgery history | 29 |
| Poor image quality | 3 |
| Spondylodiscitis | 2 |
| Spinal metastasis | 1 |
| Stenosis due to synovial cyst | 1 |
| Total | 36 |
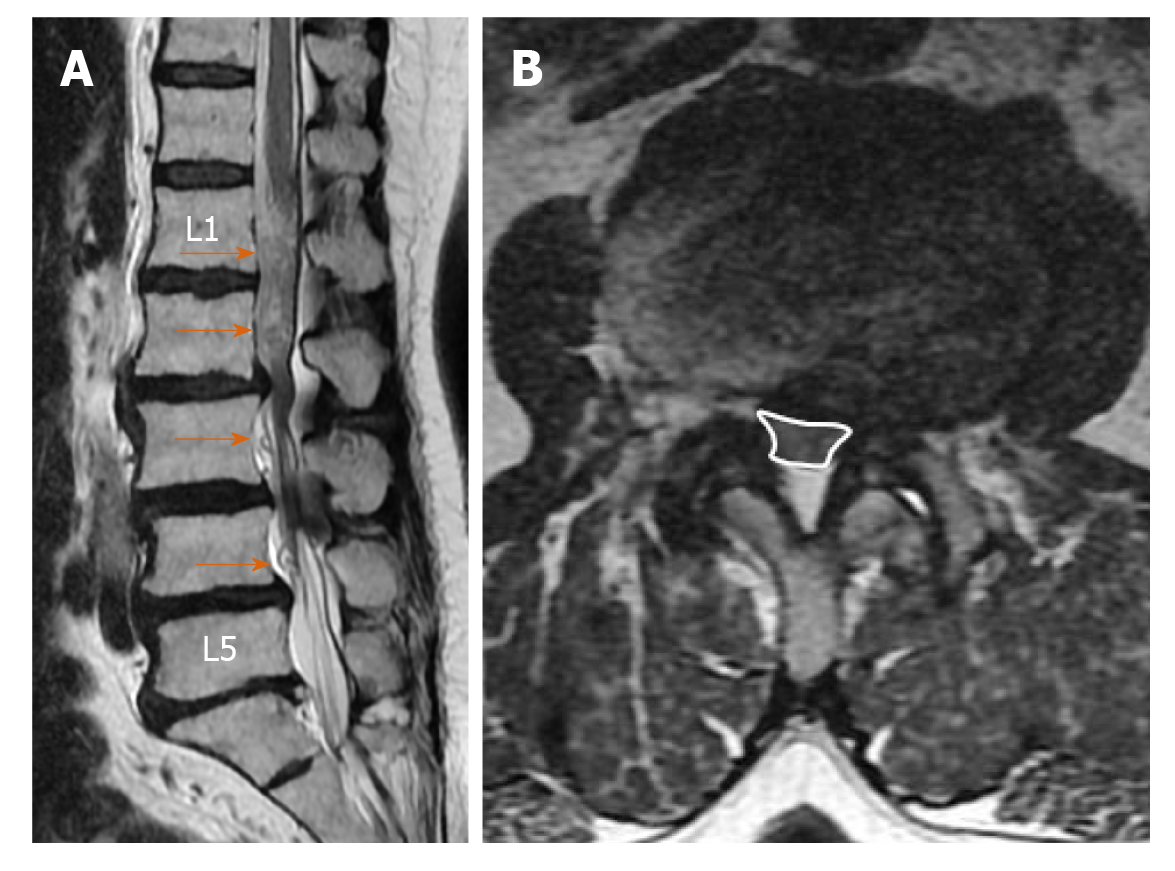
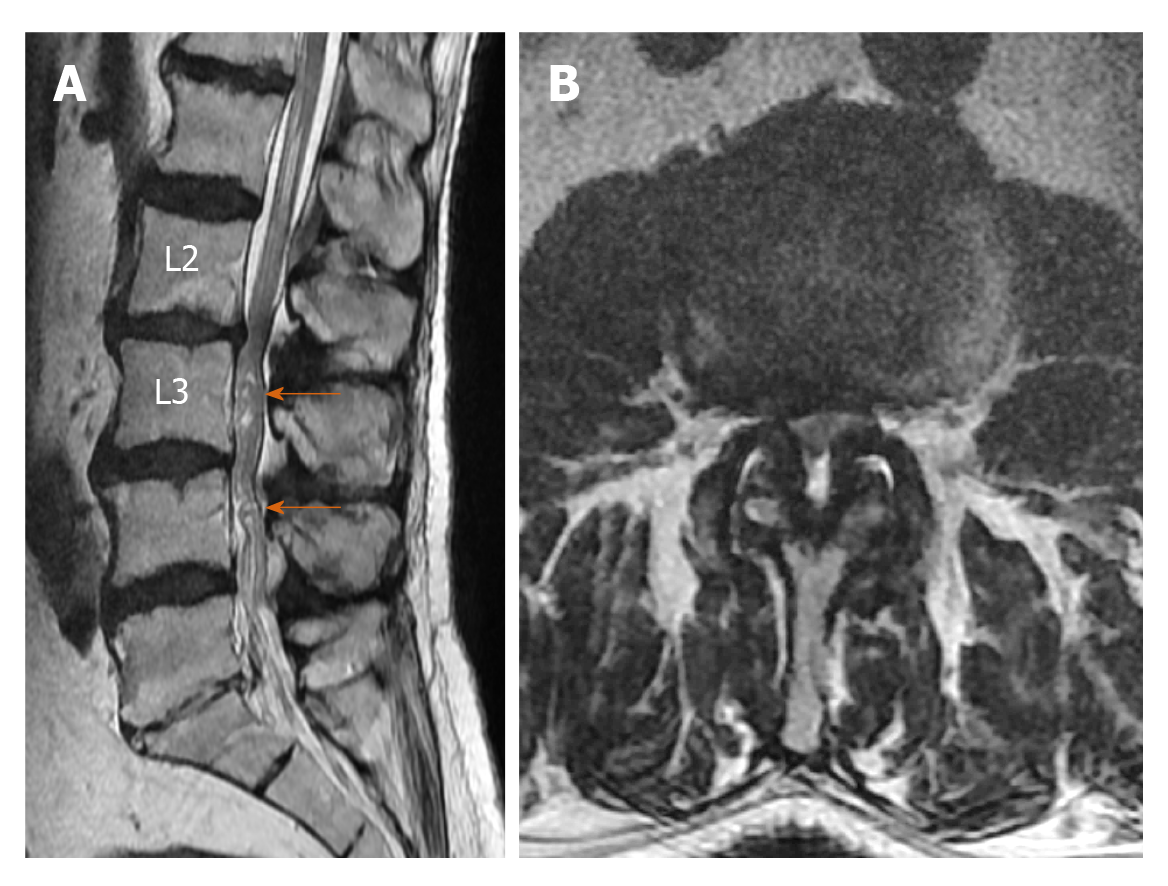
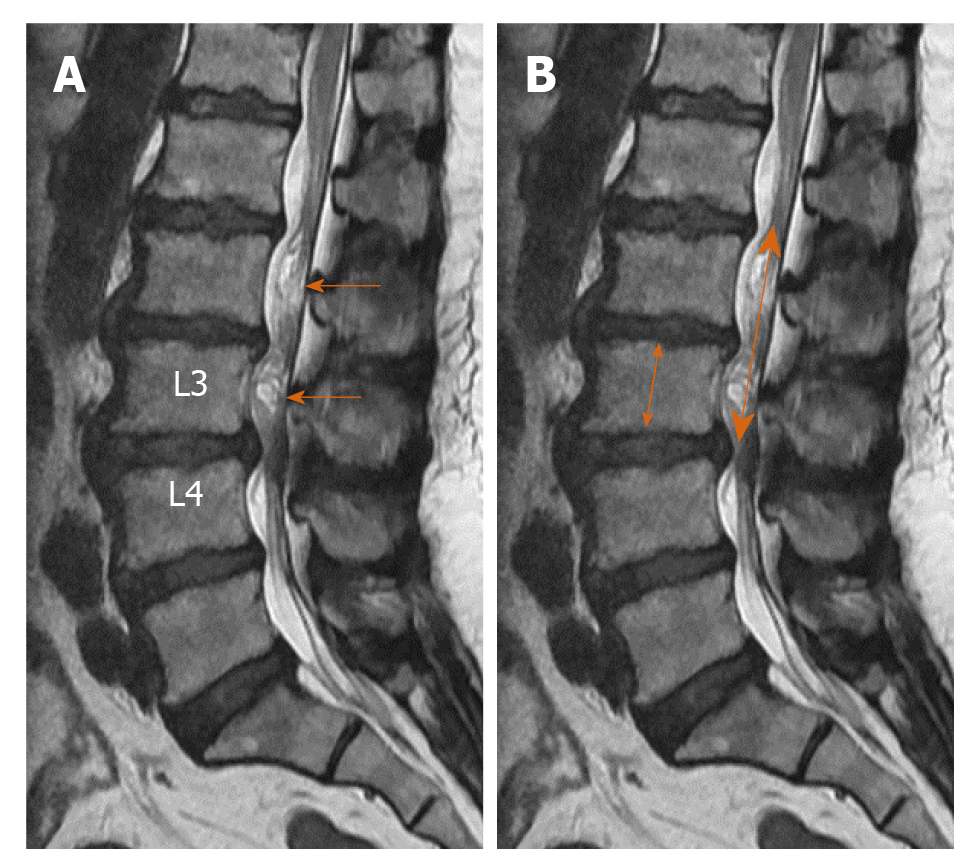
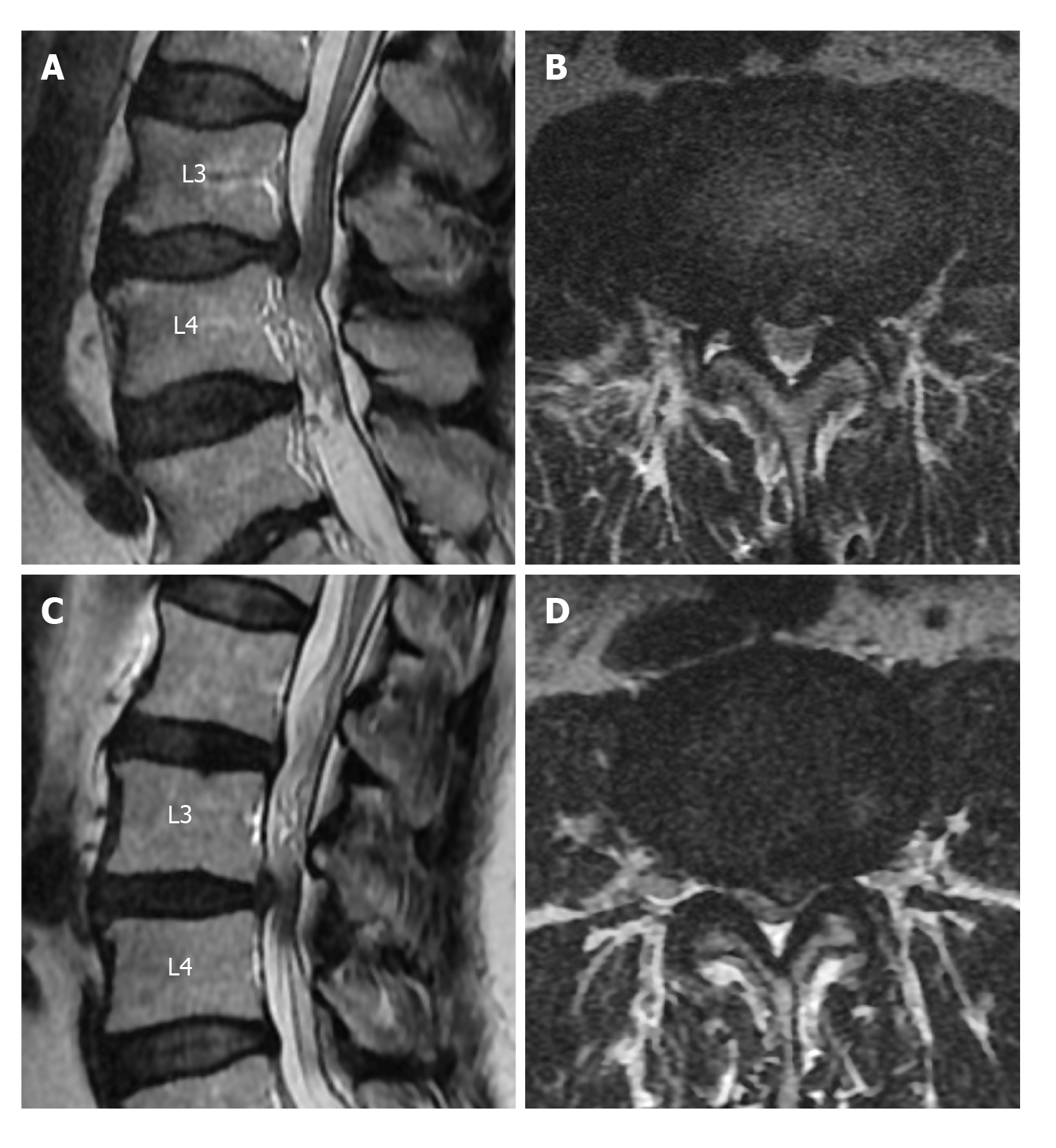
Elongation, expansion, and tortuosity in the stenotic segment superior and/or inferior of the cauda equina fibers on lumbar MRI were evaluated as RNRs of the cauda equina (Figure 1A). On T2W axial images in the PACS system, cross-sectional area (CSA) of the dural sac was manually drawn and measured at the narrowest section at L2-3, L3-4, L4-5, and L5-S1 intervertebral disc space levels in each patient (Figure 1B). Patients with CSAs under 100 mm2 at any of these spinal levels were considered to have spinal stenosis. Patients were divided into two groups: Those with stenosis and RNRs of the cauda equina and those with stenosis but without RNRs. In patients with spinal stenosis and RNRs at multiple levels, the narrowest CSA of the dural sac level was considered to be the level leading to RNRs of the cauda equina. Stenosis levels resulting in RNRs of the cauda equina and whether the RNRs were inferior or superior to the stenosis level were evaluated (Figures 1-3). On the T2W mid-sagittal MR image, relative length (RL) of RNRs was calculated by dividing the distance from the maximum stenosis level to the farthest level where redundant roots could be observed by the height of the vertebrae body superior to the stenosis level (Figure 3B). The association between the localization of RL and RNRs according to the stenotic segment and CSA of the dural sac was examined. On sagittal plane MR images of the patients with RNRs of the cauda equina, the disc herniation type was classified based on Poureisa et al[11] study’s as soft margin when the disc causing stenosis in the intervertebral disc space on the midsagittal image was indented into the dural sac with a wide angle, while it was classified as sharp margin when it was indented with an acute angle (Figure 4). In patients with RNRs of the cauda equina, the presence of spondylolisthesis and its association with the CSA of the dural sac were investigated.
The study was approved by the Ethics Committee of the Tokat Gaziosmanpasa University Medical School (No: 19-KAEK-099).
Data for continuous variables are shown as mean and standard deviation, whereas data for categorical variables are expressed as frequency and percentage. Independent samples t-test or one-way ANOVA test were used to compare the variable means between/among the groups. Receiver operating characteristic (ROC) analysis was employed to determine the power of CSA of the dural sac of stenotic segments in predicting RNRs of the cauda equina. P values < 0.05 were considered significant. Analyses were performed using SPSS 22.0 (Chicago, IL, United States).
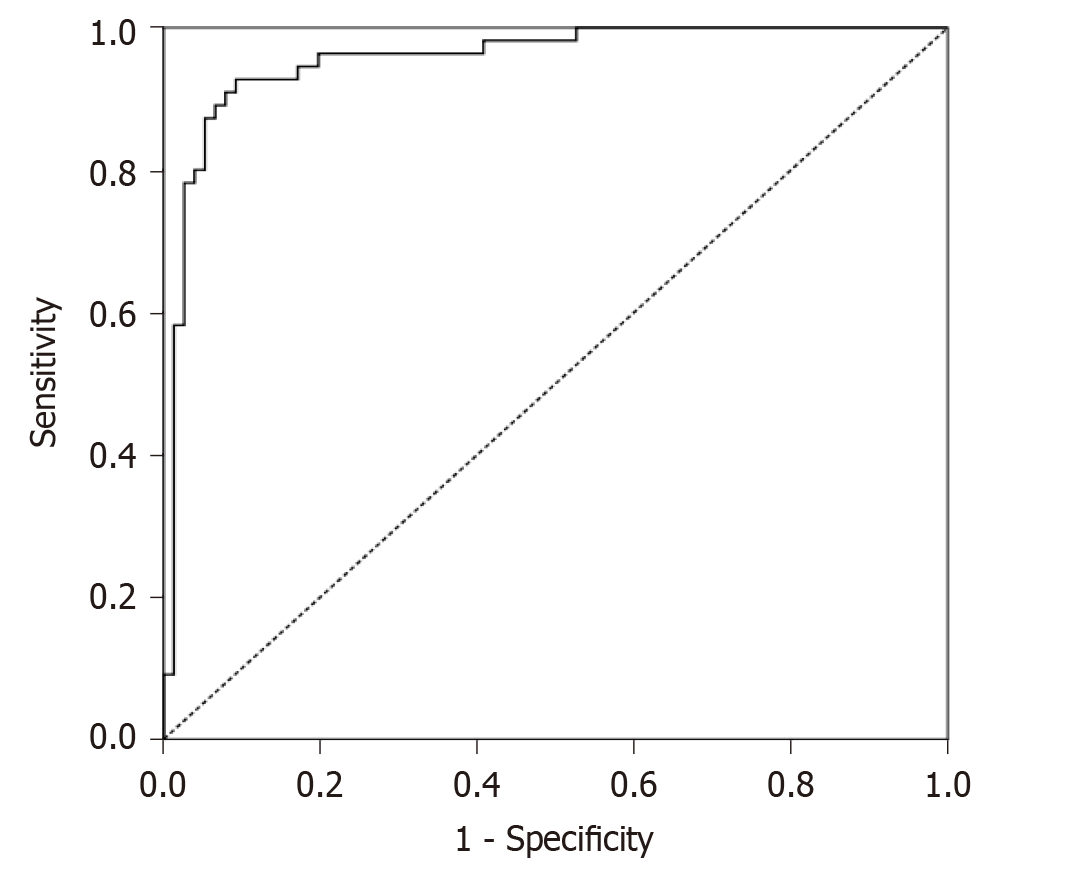
On lumbar MRI examination of the 131 patients (90 females and 41 males) included in the study, central spinal canal stenosis was detected at one or more levels. In 76 of these patients (58.0%), cauda equina fibers were found with normal appearance, while 55 (42.0%) were found to have RNRs of the cauda equina. The mean age of patients with RNRs of the cauda equina was 62.38 ± 10.37 years (range: 37-80), while patients without RNRs had an average age of 59.26 ± 10.97 years (range: 40-90). There was no significant difference in average age between the patients with RNRs of the cauda equina and the spinal stenosis patients without RNRs (P = 0.103). CSA ranged from 14.94 to 77.83 mm2 (mean 40.99 ± 12.76) in patients with RNRs of the cauda equina and from 17.57 to 99.22 mm2 (mean 66.83 ± 19.32) in the stenosis group without RNRs. The difference in CSA values between the two groups was significant (P < 0.001). CSAs of dural sacs according to disc space levels in the stenotic patients without RNRs and stenotic patients with RNRs of the cauda equina are shown in Table 2. Using a cut-off value of ≤ 55.22 mm2 based on ROC analysis for CSA of the dural sac that could lead to RNRs of the cauda equina in stenotic segments, the area under the curve (AUC) was 0.96, sensitivity was 0.92, and specificity was 0.91, while the positive predictive value was 0.88 and the negative predictive value was 0.94 (P < 0.001) (Figure 5).
| Intervertebral discal space levels | Cross-sectional area without redundant nerve roots of the cauda equina, mean ± SD dev (range) mm2 | Cross-sectional area with redundant nerve roots of the cauda equina, mean ± SD dev (range) mm2 |
| L2-L3 | 130.85 ± 38.56 (48.68-240.56) | 93.84 ± 34.63 (39.40-194.50) |
| L3-L4 | 100.90 ± 31.50 (38.86-176.00) | 68.87 ± 31.23 (25.42-164.59) |
| L4-L5 | 78.92 ± 22.69 (21.03-126.02) | 61.05 ± 35.76 (14.94-163.92) |
| L5-S1 | 102.56 ± 43.27 (17.57-251.53) | 97.11 ± 41.90 (15.05-211.13) |
RL of RNRs varied from 0.93 to 6.01 (mean: 3.39 ± 1.31). In terms of the extension of RNRs to superior and/or inferior spinal canal stenosis levels, 30 patients (54.5%) had superior, 18 patients (32.8%) had both superior and inferior, and seven patients (12.7%) had inferior extension only. As CSA decreased at the level of stenosis in the spinal canal (i.e., as stenosis became apparent), the RNRs were more prevalently observed at the superior of the stenosis level (P < 0.001). RL of RNRs increased significantly in redundant roots extending to both superior and inferior compared to those extending only to superior or inferior (P < 0.001). However, there was no significant relationship between CSA values and RL that led to the cauda equina (P = 0.305). Table 3 shows the statistical relationship of the localization level (superior, inferior, and both superior and inferior) of RNRs with RL and CSA measurements of the dural sac at extension levels of RNRs.
| Localization level of redundant nerve roots | P value | |||
| Inferior (n = 7), mean ± SD | Superior (n = 30), mean ± SD | Inferior + Superior (n = 18), mean ± SD | ||
| Relative length of redundant nerve roots | 2.07 ± 0.67 (a)1 | 2.95 ± 1.09 (a) | 4.66 ± 0.73 (b) | < 0.001 |
| Cross sectional area (mm2) | 49.27 ± 8.06 (a) | 35.61 ± 9.78 (b) | 46.77 ± 14.73 (a) | 0.001 |
There were 29 disc herniations of soft margins and 26 disc herniations of sharp margins to the dural sac at the RNRs of the cauda equina levels. Disc herniation types were not significantly associated with CSAs or RL of RNRs of the cauda equina. The relationships of the disc herniation type at the stenosis levels causing RNRs with the CSAs and RL of the RNRs of the cauda equina are shown in Table 4. Spondylolisthesis was detected in 12 patients with RNRs of the cauda equina. However, these spondylolistheses were not significantly associated with CSA of the dural sac in patients with RNRs of the cauda equina (P = 0.280).
| Type of disc herniation | P value | ||
| Soft margin (n = 29), mean ± SD | Sharp margin (n = 26), mean ± SD | ||
| Relative length of RNRs | 3.3 ± 1.42 | 3.5 ± 1.2 | 0.562 |
| CSA of RNRs of the cauda equina (mm2) | 39.62 ± 12.02 | 42.54 ± 13.62 | 0.401 |
RNRs of the cauda equina are characterized by the presence of enlarged, elongated, and tortuous nerve roots at the subarachnoid distance adjacent to the stenosis area of the spinal canal[1-14]. Redundancy of nerve roots is probably a pathological consequence of chronic pressure force at the spinal canal stenosis zone level[2,9]. Basic pathological findings in patients with RNRs of the cauda equina are demyelination, damage to and reduction in the number of nerve fibers, and the proliferation of Schwann cells and endoneural fibrosis[2,9,10]. In the study by Savarese et al[4], the CSA cut-off value that led to RNRs of the cauda equina was found to be 55 mm2. In our study, the cut-off value for the CSA of the dural sac leading to RNRs of the cauda equina (55.22 mm2) was very close to the reported value in that study. RNRs could also be observed as inferior or superior to the stenosis level but were usually superior to the spinal canal stenosis level. Kawasaki et al[12] found that RNRs were superior to the stenosis level in all cases. Poureisa et al[11], on the other hand, reported that in 84% of cases RNRs were superior to the stenosis level, while in 16% they were inferior to the stenosis. In the present study, 54.5% of RNRs were superior to the stenosis level, while in 12.7% of cases RNRs were inferior to the stenosis level and 32.8% of the cases had both configurations. The different results in previous studies in terms of the localizations of the RNRs could be due to the differences in study populations. Similar to the study by Poureisa et al[11], we observed a significant relationship between the stenosis level in the spinal canal and the frequency of RNRs superior to the level of stenosis. In addition, similar to Poureisa et al[11], the degree of stenosis in the spinal canal was not associated with the RL of RNRs. The data in the literature and the findings of our study indicate that the frequency of RNRs superior to the stenosis was associated with the degree of stenosis. This suggested that RNRs develop more easily with the fixation of nerve roots between the narrow segment and conus medullaris due to limitation of the nerve roots by conus medullaris in the superior direction.
Poureisa et al[11] investigated the relationship between the RNRs of the cauda equina and the disc herniation with soft or sharp configuration into the dural sac and found that 85.3% of the cases with RNRs of the cauda equina had sharp margin type disc herniation, and this association was significant. However, only 47.3% of patients with RNRs of the cauda equina in the present study had sharp margin type herniation and the type of disc herniation was not significantly associated with CSAs and RL of RNRs of the cauda equina. Due to these contradictory results, it would be beneficial to carry out further studies with broader series.
In recent years, MRI findings of RNRs of the cauda equina have been identified and the frequency of RNRs of the cauda equina in patients with lumbar canal stenosis was reported to be in the range of 33.8%-69.3%, while a frequency of 8.2% was reported in elderly Japanese cadavers[2,4,5,10,11,13]. In our study, the frequency of RNRs of the cauda equina was 42.0% in 131 patients with lumbar spinal stenosis, and this rate was within the limits specified in the literature.
In an anatomical study carried out by Suzuki et al[10], RNRs were observed in fibers passing through the spinal canal stenosis area but no redundancy was found in roots not passing through that area. Demyelination and axonal loss are thought to be the results of constant mechanical compression of nerve roots trapped in the spinal stenosis area[10]. Suzuki et al[10] examined the topographic distribution of levels where RNRs of the cauda equina were observed and found that 33.3% were at S1 level, 33.3% at S2 level, 16% at L5, and 17.3% were inferior to S2 roots. Min et al[6], on the other hand, reported that RNRs of the cauda equina were most commonly observed at L4-L5 (78.2%) followed by L3-L4 levels (17.4%). In contrast, Poureisa et al[11] reported L3-L4 level as the most common localization for RNRs of the cauda equina (38.7%) followed by L2-L3 level (30.7%). Similar to Min et al[6], RNRs of the cauda equina were most common at the L4-L5 level with 45.4% and at the L3-L4 level with 32.7% in the present study. Different frequencies of RNRs of the cauda equina at different levels of intervertebral disc spaces in the literature could reflect the ethnic structural differences in the study populations.
In a study based on the RL of RNRs measurements on the midsagittal image on sagittal lumbar MR images, a statistically significant relationship was reported between the length of the affected nerve roots and clinical findings[6]. RL of RNRs was also calculated in the present study, but its relationship with clinical findings could not be evaluated as our study was based solely on radiological findings.
There is also a study in the literature that assessed the relationship between spondylolisthesis and RNRs of the cauda equina[4]. In that study, Savarese et al[4] found that spondylolisthesis increases the risk of cauda equina and is an independent risk factor for RNRs of the cauda equina. Nevertheless, no significant relationship was determined between spondylolisthesis and RNRs of the cauda equina in the present study. Therefore, it might be useful to perform large series studies that explore the relationship between spondylolisthesis and RNRs.
Suzuki et al[10] found that patients with RNRs of the cauda equina are more likely to be older, have longer symptom duration, and have more intense neurological findings and symptoms compared to patients with spinal canal stenosis without RNRs. Similarly, Min et al[6] and Poureisa et al[11] reported that patients with RNRs of the cauda equina were significantly older. Min et al[6] found no difference between the patients with and without RNRs of the cauda equina in terms of the duration of symptoms. However, they noted that better postoperative results were achieved in the patient group without RNRs[6]. Similarly, the average age of patients with RNRs of the cauda equina was higher than the patients without RNRs, but the difference was not significant.
In patients with RNRs of the cauda equina, serpentine-shaped lesions and/or loop-shaped lesions that cause filling defects are observed on conventional myelography. In their studies, Ono et al[5] found that in 97.6% of loop-shaped lesions detected on conventional myelography, positive findings were found on MRI examination, while only 23.5% of the serpentine-shaped lesions turned out to have positive findings on MRI. Serpiginous filling defects on myelography have been defined in dural or intradural arteriovenous malformations (AVM), and they constitute one of the important differential diagnoses[2,14]. Although less frequently, plexiform neurofibroma or neurinoma can also lead to thickening and redundancy in nerve roots. Diseases such as arachnoiditis, chronic inflammatory demyelinating polyneuropathy, and some hereditary neuropathies can lead to hypertrophic neuropathy, but no relationship was reported between such entities and the serpiginous nerve roots of the cauda equina[2].
RNRs of the cauda equina should be considered first in the presence of enlarged, elongated, and tortuous or serpiginous nerve roots, which do not contain prominent pathological signals on MRI in the area adjacent to lumbar spinal canal stenosis in patients with spondyloarthrosis[2-6]. However, it is essential to distinguish between AVM and arteriovenous fistula (AVF) on MRI. In AVM or AVF, intradural serpiginous veins and coronal venous plexus ectasia are generally observed on MRI. AVMs may appear with signs of subarachnoid hemorrhage or medullary ischemia on imaging[2,8,14]. On MRI of dural AVFs, abnormal signals are usually observed in the spinal cord on the T2W series. Another important MRI finding in most patients with AVF is excessive contrast-enhancement of coronal venous plexus on contrast-enhanced series[2,14].
RNRs of the cauda equina are typically associated with spinal canal stenosis, and clinically neurological claudication is observed in the patient[2]. However, the literature has controversial findings on the association of RNRs of the cauda equina with the clinic and its treatment[5,9,10,12]. Some authors noted that since the damage to affected nerve roots is irreversible, neurological healing cannot be achieved and decompressive surgery will not contribute to recovery[2,9,10]. It was reported that the decline of stenosis symptoms after surgical decompression was rare in patients with typical RNRs of the cauda equina and that complaints of dysesthesia and paresthesia often persisted[2,13]. However, a recent study reported that intermittent claudication disappeared in all patients after decompression surgery[12]. Ono et al[5] mentioned that the severity of the disease was greater in patients for whom RNRs of the cauda equine were diagnosed with MRI compared to those for whom the diagnosis was made clinically only and that this difference negatively affected surgical outcomes. Kawasaki et al[12], on the other hand, reported that in 84% of patients undergoing surgical decompression, MRI findings of RNRs of the cauda equina disappeared two weeks later.
The present study has some limitations. The first is that the radiological and clinical findings of the patients cannot be correlated due to the retrospective and radiological basis of the study. As the examination of the patient during MRI is performed in a neutral position, it was reported that spinal stenosis patients could get over the disease in cases of mild intensity[2,5]. The second limitation was that lumbar MRI examinations performed in the supine (neutral) position rather than standing or axial loading might have led to lower stenosis measurements than the actual degree of stenosis. A third limitation was that since the narrowest level of CSA of the dural sac level was considered the level that caused RNRs of the cauda equina in patients with multiple levels of spinal stenosis, the effects of the narrow segments at other levels had to be ignored.
In conclusion, the present study showed that RNRs of the cauda equina are not uncommon in patients with lumbar spinal canal stenosis. RNRs of the cauda equina are frequently observed in the superior of the stenosis level but can also be observed in both inferior and superior, and less frequently in inferior localizations only. Patients who undergo lumbar MRI and are found to have dural sac CSA of 55 mm2 or lower should be carefully evaluated for RNRs of the cauda equina, and when present, the findings of the RNRs of the cauda equina should definitely be reported.
Redundant nerve roots (RNRs) of the cauda equina are often defined as the development of elongated, enlarged, and tortuous nerve roots at the superior and/or inferior of the lumbar canal stenosis and as secondary to it due to degenerative processes. Clinically, they can lead to lower back and leg pain, paresthesia, and neurogenic claudication in patients.
The radiological diagnosis of RNRs of the cauda equina was previously made with conventional myelography, while magnetic resonance imaging (MRI) findings have been more commonly defined in recent years. Nevertheless, this condition has been relatively under-recognized in radiological practice. Therefore, there is a need to keep this issue on the agenda by discussing it in light of the literature.
In this study, lumbar MRI findings of RNRs of the cauda equina were evaluated in spinal stenosis patients. Cross-sectional area (CSA) of the dural sac at the stenosis level that could lead to RNRs of the cauda equina and how the cauda equina nerve roots are affected by this stenosis (redundant segment length and extensions, etc.) were investigated.
On lumbar MRI of patients with stenosis, dural sac CSA levels of less than 100 mm2 at the intervertebral disc space were considered stenosis, and levels leading to lumbar stenosis were determined. Statistical differences between the CSA levels that led to RNRs of the cauda equina and those that did not lead to RNRs were investigated. Relative length (RL) was calculated by dividing the length of RNRs on sagittal T2-weighted images by the vertebrae corpus height adjacent to the stenotic segment superior. The relationships of herniation type into the dural sac (soft or sharp margins) and spondylolisthesis with CSA and RL were investigated.
RNRs of the cauda equina were observed in 42% of patients with spinal stenosis. Mean CSA was 40.99 ± 12.76 mm2 in patients with RNRs of the cauda equina and 66.83 ± 19.32 mm2 in patients without RNRs (P < 0.001). Using a cut-off value of 55.22 mm2 for CSA leading to RNRs of the cauda equina, the sensitivity was 96.4%, specificity 96.1%, positive predictive value (PPV) 89.4%, and negative predictive value (NPV) 98.7%. RL varied from 0.93 to 6.01 (mean: 3.39 ± 1.31). Of all RNRs, 54.5% were at the superior of stenosis level, 32.8% at both superior and inferior of stenosis level, and 7% at inferior of stenosis. Soft margin disc type was observed in 29 and sharp margin type was found in 26 of the disc herniations at the stenosis levels that led to RNRs of the cauda equina. Disc herniation type and spondylolisthesis were not significantly associated with RL or CSA of the dural sac with stenotic levels (P > 0.05). As the CSA of the dural sac decreased, the frequency of RNRs at the superior of the stenosis level increased (P < 0.001).
RNRs of the cauda equina are not uncommon in patients with lumbar spinal canal stenosis. Although RNRs of the cauda equina are frequently observed at the superior of stenosis level, a considerable percentage of them can also be found at both superior and inferior, and at a lower rate at the inferior localization. The possibility of RNRs of the cauda equina is high in patients with dural sac CSA of 55 mm2 or less.
Although clinical and treatment outcomes are controversial, lumbar stenosis patients with marked reductions in CSA of the dural sac on MRI should be carefully evaluated for RNRs of the cauda equina. In these patients, tortuosity, elongation, and extension findings indicating redundancy in nerve roots should be reported as this could contribute to efficient treatment of the patients.
We thank Demir O for his help with the statistical analyses.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Peng BG, Xie Q S-Editor: Gao CC L-Editor: Webster JR P-Editor: Wang LYT
| 1. | Cressman MR, Pawl RP. Serpentine myelographic defect caused by a redundant nerve root. Case report. J Neurosurg. 1968;28:391-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Nogueira-Barbosa MH, Savarese LG, Herrero CFPS, Defino HLA. Redundant nerve roots of the cauda equina: review of the literature. Radiol Bras. 2012;45:155-159. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Hakan T, Celikoğlu E, Aydoseli A, Demir K. The redundant nerve root syndrome of the Cauda equina. Turk Neurosurg. 2008;18:204-206. [PubMed] |
| 4. | Savarese LG, Ferreira-Neto GD, Herrero CF, Defino HL, Nogueira-Barbosa MH. Cauda equina redundant nerve roots are associated to the degree of spinal stenosis and to spondylolisthesis. Arq Neuropsiquiatr. 2014;72:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Ono A, Suetsuna F, Irie T, Yokoyama T, Numasawa T, Wada K, Toh S. Clinical significance of the redundant nerve roots of the cauda equina documented on magnetic resonance imaging. J Neurosurg Spine. 2007;7:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Min JH, Jang JS, Lee SH. Clinical significance of redundant nerve roots of the cauda equina in lumbar spinal stenosis. Clin Neurol Neurosurg. 2008;110:14-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Duncan AW, Kido DK. Serpentine cauda equina nerve roots. Radiology. 1981;139:109-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Hacker DA, Latchaw RE, Yock DH Jr, Ghosharjura K, Gold LH. Redundant lumbar nerve root syndrome: myelographic features. Radiology. 1982;143:457-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Suzuki K, Takatsu T, Inoue H, Teramoto T, Ishida Y, Ohmori K. Redundant nerve roots of the cauda equina caused by lumbar spinal canal stenosis. Spine (Phila Pa 1976). 1992;17:1337-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Suzuki K, Ishida Y, Ohmori K, Sakai H, Hashizume Y. Redundant nerve roots of the cauda equina: clinical aspects and consideration of pathogenesis. Neurosurgery. 1989;24:521-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Poureisa M, Daghighi MH, Eftekhari P, Bookani KR, Fouladi DF. Redundant nerve roots of the cauda equina in lumbar spinal canal stenosis, an MR study on 500 cases. Eur Spine J. 2015;24:2315-2320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Kawasaki Y, Seichi A, Zhang L, Tani S, Kimura A. Dynamic Changes of Cauda Equina Motion Before and After Decompressive Laminectomy for Lumbar Spinal Stenosis With Redundant Nerve Roots: Cauda Equina Activation Sign. Global Spine J. 2019;9:619-623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Tsuji H, Tamaki T, Itoh T, Yamada H, Motoe T, Tatezaki S, Noguchi T, Takano H. Redundant nerve roots in patients with degenerative lumbar spinal stenosis. Spine (Phila Pa 1976). 1985;10:72-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 44] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Gilbertson JR, Miller GM, Goldman MS, Marsh WR. Spinal dural arteriovenous fistulas: MR and myelographic findings. AJNR Am J Neuroradiol. 1995;16:2049-2057. [PubMed] |