EPIDEMIOLOGY AND CLINICAL PRESENTATIONS
Epidemiology and risk factors
ICS is predominantly seen in women (72%-74%) in their second to fourth decade of life; females also tend to develop more severe compression than males[3-6]. The exact prevalence of ICS is not known, especially since most patients are asymptomatic. Nonetheless, growing evidence suggests that it is not as uncommon as previously thought[1,6]. Significant venous obstruction, defined as a stenosis diameter or cross sectional area (CSA) greater than 50% on computed tomography/magnetic resonance imaging (CT/MRI) venography and intravascular ultrasound (IVUS) respectively[7], was seen in 25%-37% of patients with advanced venous diseases (C4-6)[7,8]. Iliac vein compression is also prevalent among asymptomatic patients; a study on MTS incidence among patients admitted for non-venous complaints showed that almost a quarter of the cohort had significant compression of the left iliac vein[9]. Generally, patients would remain asymptomatic until exposure to stress such as surgery and prolonged bed rest[5]. Other factors associated with symptomatic ICS include spinal deformities such as exaggerated lordosis and scoliosis, oral contraceptive use, post-partum and multiparous[10-12]. When symptoms do emerge, they may present as deep vein thrombosis and chronic venous disease.
Clinical presentations
Prolonged venous stenosis causes venous stasis which in turn predisposes individuals to acute deep vein thrombosis and chronic venous diseases[3]. Patients with ICS are more likely to develop deep vein thrombosis (OR = 9.5, P < 0.001), typically after an inciting prothrombotic event such as prolonged immobility and pregnancy which may worsen the venous compression[13,14]. Standard systemic anticoagulation is usually inadequate in these patients due to extensive clot burden, warranting more aggressive intervention such as intravascular thrombolysis[15]. In spite of prompt and adequate treatment, they are more likely to develop post-treatment sequalae such as re-thrombosis (RR = 2.8, P = 0.04) and post-thrombotic syndrome if the underlying anatomic variant is not corrected[16,17].
Besides deep vein thrombosis, patients may suffer with symptoms of leg pain, heaviness and swelling usually worse by the end of the day. Chronic venous disease develops from complex processes such as venous reflux and obstruction, with varying clinical severity depending on the presence of telangiectasia (C1), varicose veins (C2), edema (C3), pigmentation (C4a), lipodermatosclerosis (C4b) and venous ulcers (C5-6)[18]. Although reflux from incompetent valves is the predominant pathophysiology of chronic venous disease, venous obstruction is a common and aggravating factor that should be addressed[19]. In a cohort of 4026 patients with chronic venous disease, significant (greater than 50%) venous obstruction warranting stent placement was noted in 879 patients, of which almost half (n = 319) were due to extrinsic iliac venous compression[20]. Not only is venous compression a common contributor of chronic venous disease, it is also associated with more severe disease, in part due to the raised venous ambulatory pressure[21]. Class C4-6 disease occurred in 35% of patients with venous obstruction[20], compared to 3%-6% of the general population (i.e., patients with chronic venous disease unselected for a specific pathophysiology)[22,23]. In addition, disease with untreated venous compression is more resistant to conventional treatment such as vein ligation and stripping[5].
Given the poor disease and treatment outcomes associated with ICS, early recognition and prompt treatment are essential. Making this diagnosis requires a high index of suspicion, particularly in young to middle-aged women presenting with acute deep vein thrombosis and unilateral stigmata of chronic venous disease[24]. Other features that may require further investigations include presence of varicosities on the abdominal wall, groin or thigh, persistence or recurrence of condition despite appropriate treatment.
DIAGNOSTIC IMAGING STUDIES
Investigations are targeted at the identification of the site and severity of the stenosis. Sites of compression in ICS include inferior vena cava (50%), common iliac (32%), external iliac (7%) and common femoral veins (11%)[25]. Another common site is the confluence of external and internal iliac veins but the exact prevalence is not known. Severity of the stenosis can be represented in relation to the reference diameter and CSA. Traditionally, stenosis greater than 50% has been considered as significant stenosis requiring stenting[19]. This cut-off was recently corroborated by a prospective study which reported CSA stenosis greater than 54% to be the best predictor of post-treatment response[26]. Reference diameter and CSA are determined from the non-stenosed segments proximal or distal to the stenosis, or from the contralateral non-stenosed vein[27]. Reference diameters from the literature include 24 mm for the inferior vena cava[28], 16 mm for the CIV[29], 14 mm for the external iliac vein (EIV)[30], and 10-12 mm for the common femoral vein[27,31,32]. Reference areas can be calculated from the diameters or directly measured from the axial cross-sectional images on IVUS.
Ultrasonography
Duplex ultrasound is the best initial test because it is fast, easily accessible and non-invasive. With the patient supine, the external iliac, common iliac and inferior vena cava are visualized with a 2 to 5 MHz curvilinear transducer[25]. The iliocaval vessels are imaged using different views at the paraumbilical, midline and adjacent to the line connecting the superior iliac spine to the umbilicus[25]. Site of stenosis is identified by the presence of abnormal Doppler signal and flow patterns such as post-stenotic turbulence and slow flow[25]. Indirect signs of stenosis include pre-stenotic vein dilation, absent respiratory variations, flow reversal, and poor response to both flow augmentation and Valsalva maneuver[8,33]. Degree of stenosis is then determined based on the peak vein velocity of the post-stenotic and pre-stenotic segments, and the percent reduction in vein diameter when compared with the normal lumen[14]. Significant stenosis is defined as a peak vein velocity ratio greater than 2.0 or vein diameter greater than 50%[25]. Transvaginal ultrasound has been reliably used for evaluating the internal iliac veins[34]. In comparison, transabdominal ultrasound is unsuitable for proximal iliocaval vessels which are deep in the pelvis and may be obscured by the intraabdominal fats and bowel gas[33]. Moreover, indirect signs are often absent; the sensitivity of flow reversal and absent respiratory variations in the detection of significant iliocaval obstruction are only 7.9% and 23.7% respectively while both have a specificity of 100%[7]. Hence, patients with a negative ultrasound study should be referred for further imaging such as CT and MRI before confirming the diagnosis[7,8]. Whilst Duplex ultrasound is operator dependent, it remains a reliable initial investigation in high volume centres.
CT venography
CT venogram of the pelvis is used in the diagnosis of ICS. Patients are given intravenous contrast at a rate of 4 to 5 mL/s followed by 20-50 mL of saline and venous phase imaging is obtained generally after 60-80 s delay[8,22]. Timing of the imaging sequence may require optimization to obtain maximal contrast opacification of the target veins. The scan coverage extends from the infrarenal segment of the vena cava to the common femoral veins. The degree of stenosis is then derived either by comparing the smallest diameter of the vein with the normal diameter caudal to the obstruction, or with the corresponding contralateral healthy vein segment[7].
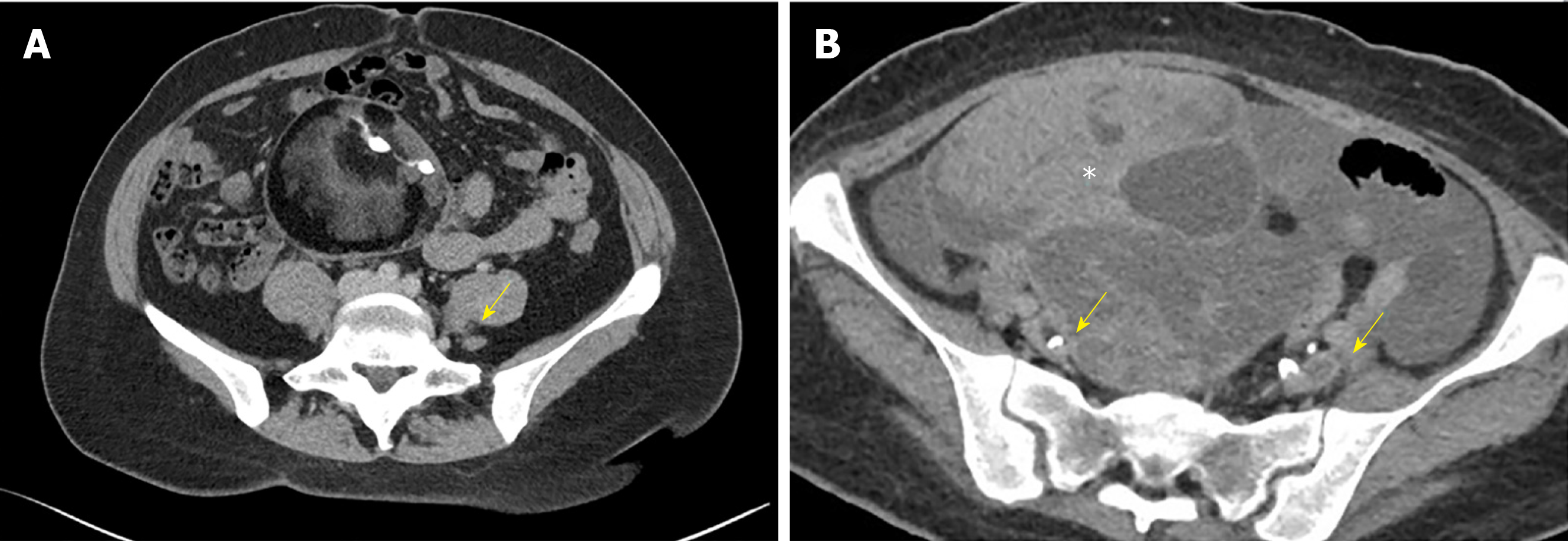
Unlike Duplex ultrasound, CT venogram allows a clearer visualization of the anatomic compression sites and collaterals, which indicates the hemodynamic significance of the stenosis (Figure 1A)[27,35]. CT also serves to rule out other causes of the compression such as pelvic tumors and ascites (Figure 1B)[35]. CT is relatively less operator dependent compared to ultrasound. Significant reduction in vessel calibre or complete venous occlusion can be visualized on CT imaging. Indirect signs such as enlarged paravertebral and internal iliac veins resultant of prominent collateral drainage, also support the diagnosis of iliocaval venous compression. Unfortunately, these anatomical changes represent late stage disease. In two small retrospective studies, CT venography (CTV) was reported to have both high sensitivity and specificity in detecting iliac vein compression[29,36]. However, patients in these studies presented with acute deep vein thrombosis of the lower limbs, which is an advanced stage of iliocaval venous compression where the veins are severely stenosed or completely occluded. Earlier signs such as intraluminal webs or scarring, vein wall thickening and partial luminal occlusion cannot be seen on CT imaging. Spurs and webs can serve as nidus for thrombosis. Early detection of these changes is crucial given that patients with such changes would benefit most from thrombolytic therapy[37,38]. Left undetected and untreated, these would progress and further impair venous drainage, eventually manifesting as symptoms of venous incompetence.
Figure 1 Axial Computed tomography venography image.
A: Axial computed tomography venography image showing compression of the left common iliac vein (arrow) between the right common iliac artery and the lumbar vertebral body; B: Extraluminal compression of the external iliac veins (arrows) from an ovarian tumor (asterixis).
Hence in our opinion, CT has limited sensitivity in the diagnosis of early ICS. Its main utility is to diagnose advanced disease and to exclude compressive pelvic masses prior to the use of invasive imaging and therapy. The diagnostic performance of CTV in patients with IVCS leading to chronic venous insufficiency remains unknown.
CT is readily available in most centres and can be used in patients with metallic implants and iliac vascular stents. Unfortunately, CT imaging requires administration of contrast and radiation, which may be contraindicated in patients with chronic renal failure and pregnancy. In these instances, MRV may be considered. An alternative is the dual energy CT which offers the advantage of better contrast opacification at relatively lower contrast dose compared to conventional single energy CT, which may be acceptable to patients with chronic renal failure. dual energy CT also reduces streak/beam hardening artefacts from iodinated contrast and metal implants such as previous stents. Hence, it is useful in assessing the patency of previously stented veins[39].
MR venography
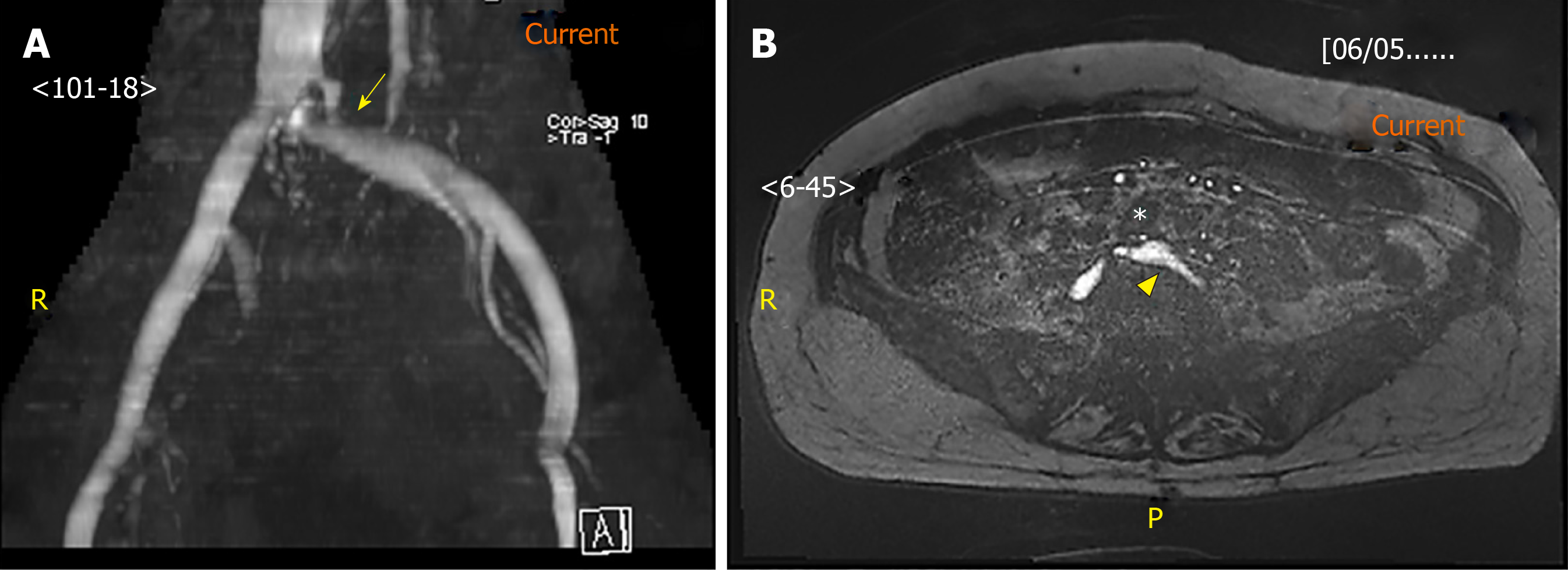
Similar to CT, MRV is non-invasive, operator-independent and allows identification of venous stenosis or occlusion, and collaterals[19]. MRV can be performed either with injection of Gadolinium contrast to opacify the veins or using non contrast techniques such as two- or three-dimensional time of flight sequence. An example of stenosis demonstrated on non-contrast MRV is seen in Figure 2. Unlike CTV, contrast-enhanced MRV can demonstrate intraluminal webs, partial occlusion or venous wall thickening due to superior soft tissue resolution[40]. Increasingly, studies have advocated using MRV to confirm the diagnosis of iliocaval compression[4,41-43], in part due to its success in diagnosing pelvic venous thrombosis[44,45]. Without direct comparison with catheter-based venography, the sensitivity and specificity of MRV in the diagnosis of ICS are not yet known and MRV should not be used in place of catheter-based venography[24]. Furthermore, the degree of venous compression on MRV can vary over time; a retrospective study on 36 MTS patients revealed that the venous compression vary significantly from 12% increase to 69% decrease between the index and follow-up MRV studies[30]. This variability might be explained by the dependence of compression on the hydration status and study position of the patient. In most centers, the CTV/MRV protocol requires patients to fast overnight. Consequently, the patient is dehydrated and the veins are collapsed, which may be misinterpreted as stenotic. The supine position adopted during the scans further contributes to extrinsic narrowing of the veins. Behzadi et al[46] demonstrated an increase in the common femoral vein area on MRV (59 ± 21 mm2 when supine and dehydrated) with a prone/decubitus position (83 ± 35 mm2) and hydration (123 ± 44 mm2)[46]. To avoid false positive findings, the patient may be hydrated intravenously prior to the study and kept prone/decubitus during the study.
Figure 2 Magnetic resonance venography image.
A: Magnetic resonance venography image of the stenosed left common iliac vein (arrow); B: Axial image of the left common iliac artery (asterisk) compressing against the left common iliac vein (arrowhead). Under magnetic resonance venography, venous blood generates high signal intensity (hyperintense) while arterial blood is suppressed (hypointense).
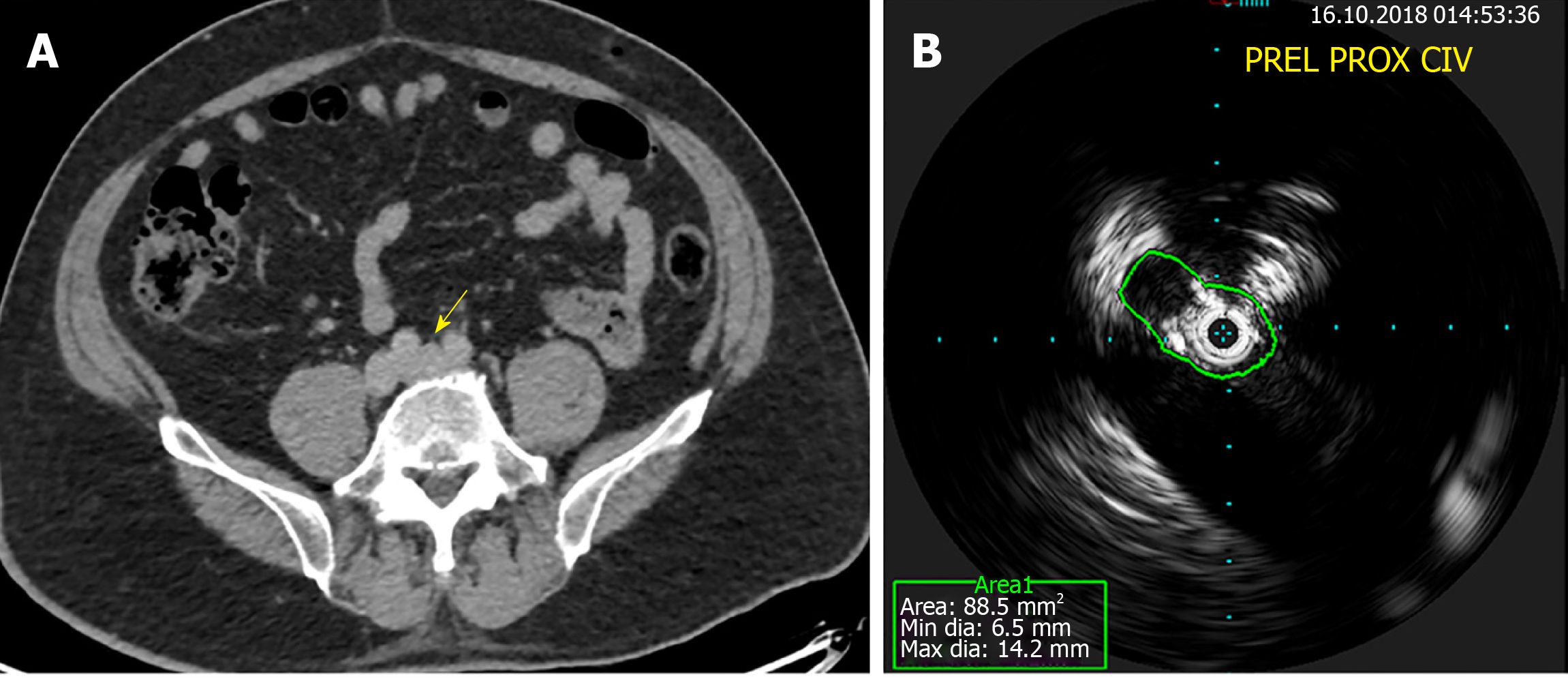
Practical limitations of MRV include the cost, availability and the contraindications in patients with metallic spinal fixation devices in the lower lumbar spine or iliac vein stents due to image degradation by artefacts from the metal implants. Non contrast MRV images are also easily degraded, by arterial pulsation artefacts and breathing movement artefacts. In our experience, a negative MRV or CTV study should still be confirmed with invasive imaging (catheter-based venography and IVUS) if the clinical suspicion of ICS is high due to potential missed diagnoses on CTV/MRV (Figure 3A, 3B).
Figure 3 Computed tomography venography and intravascular ultrasound.
A: Normal filling of the left common iliac vein (arrow) on computed tomography venography; B: Obvious stenosis on intravascular ultrasound for a patient with May-Thurner syndrome.
Catheter-based venography
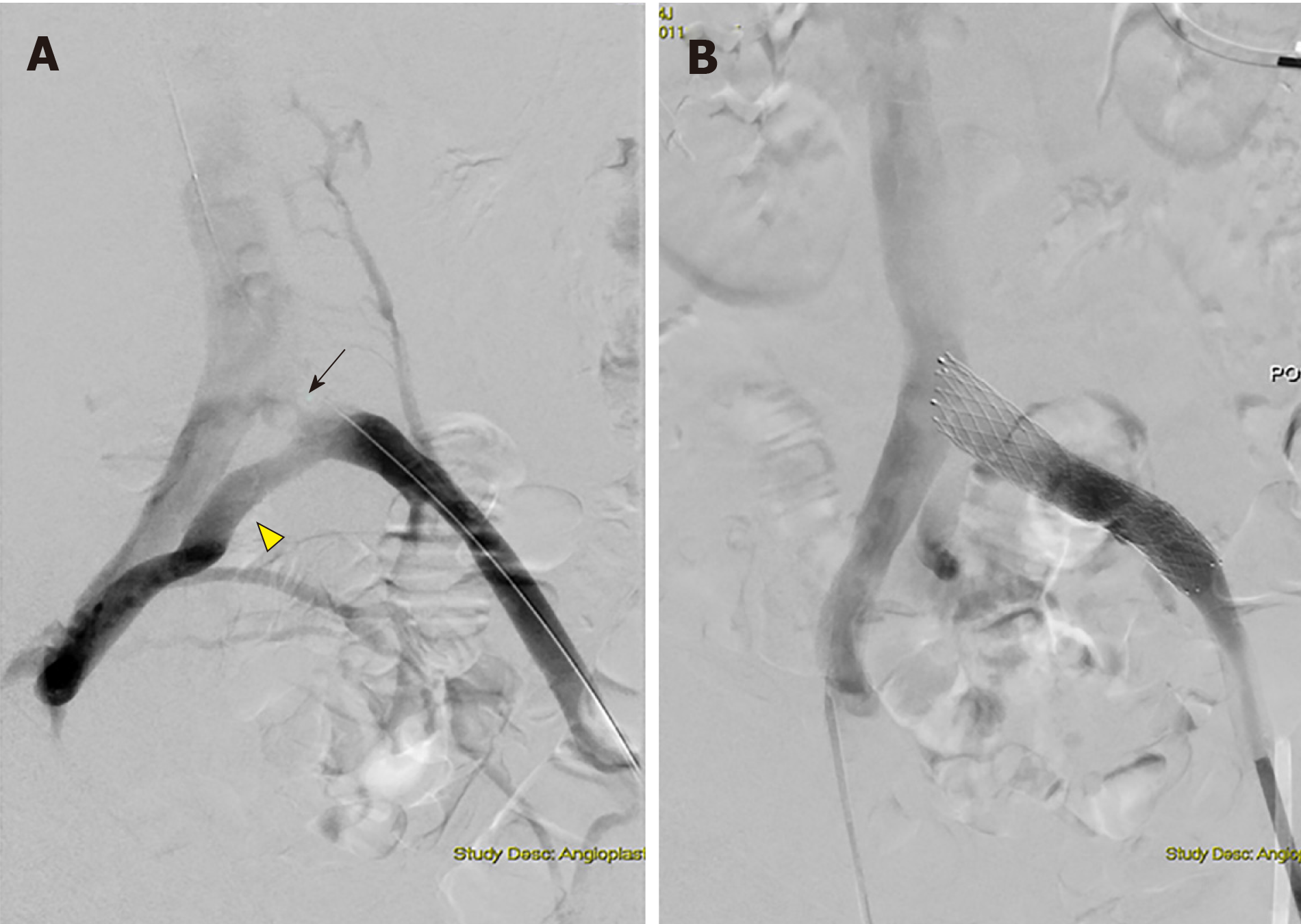
Catheter-based venography is generally regarded as the gold-standard diagnostic test for ICS, as it has been around for the longest period and is used for planning the treatment[24]. Venous access is via the ipsilateral popliteal or femoral vein and the imaged venous segments include the inferior vena cava, CIV, EIV and common femoral vein[27]. Location and degree of narrowing can be assessed directly (Figure 4A). To improve the diagnostic accuracy, venography is performed in at least two orthogonal views beyond the standard anteroposterior, such as left, right anterior oblique and cranial caudal views[1]; single plane venography has low sensitivity (45%) in detecting venous stenosis above 70%[47]. However, the acquisition of additional views will require higher doses of radiation and contrast, thus this is not routinely done in clinical practice[47]. Instead, the diagnosis of ICS can be inferred in the presence of other supporting signs such as collaterals (Figure 4B) and raised pressure gradients (greater than 2 mmHg) across the stenosed sites[25,47]. Unlike the previous diagnostic modalities discussed, digital subtraction venography is usually performed as part of the planned endovascular intervention, given its invasiveness and the significant amount of iodinated contrast and radiation administered.
Figure 4 Digital subtraction angiography images.
A: Digital subtraction angiography images obtained during revascularization showing left proximal common iliac vein stenosis (arrow) with formation of collaterals (arrowhead); B: Digital subtraction angiography images obtained during revascularization showing dilation post-stenting.
IVUS
IVUS is usually performed after catheter based venography to improve the diagnostic accuracy[47]. After venography, the lesions are crossed with a diagnostic 4 French (F) catheter and hydrophilic guide wire (usually 035” Glide wire, Terumo, Japan) under fluoroscopic guidance into guide the distal inferior vena cava (IVC). After exchanging for a stiffer guidewire (HI-TORQUE SupracoreTM Abbott Medical, United States) the access sheath is upsized to 9F to accomadate the IVUS catheter. A 10 MHz IVUS transducer catheter (VolcanoTM, Philips Healthcare, Netherlands) is inserted over the guide wire into the distal IVC. A preliminary pull back scan of the iliac and femoral veins is performed to get a quick overview of the lesions under Xray fluoroscopy. The IVUS catheter is reintroduced into the IVC and crosss sectional measurements of the distal IVC, proximal CIV, mid CIV, distal CIV at the confluence, proximal, mid and distal EIV and the common femoral veins are measured using the IVUS. In our experience, IVUS provides the most accurate estimate of the severity and extent of the venous lesions as well as the diameters of the normal iliac veins. The combination of high probe frequency and close luminal distance facilitates high-resolution imaging of the stenosis (Figure 5A)[25]. This improves the detection of finer endoluminal venous lesions and features such as webs, trabeculations and spur morphologies, which might be missed on catheter-based venography[47]. Up to one-third of venous compressions detected on IVUS were missed on initial CT venography[24,27]. Besides having a greater sensitivity, IVUS allows for a more accurate measurement of the stenosed vein diameter and cross-sectional area[1,27,45]; a study comparing IVUS and single-plane catheter-based venography found the stenotic area directly measured in IVUS was more severe than that derived in venography (80% vs 50%)[47]. Similarly, another study comparing IVUS and multiplanar venography reported 33% underestimation of stenosis diameter by venography[27]. This underestimation was attributed to the non-circular geometry of the stenosed lumens which are only seen on certain views. Since IVUS examines each segment with a full 360-degree field of view, it is better able to identify these lesions than multiplanar venography[47]. The recent VIDIO trial comparing multiplanar venography (MPV) with IVUS for the diagnosis and treatment of iliofemoral stenosis showed that IVUS was more sensitive than MPV and that clinical improvement post stenting was best predicted by IVUS baseline measurement of the venous CSA[26].
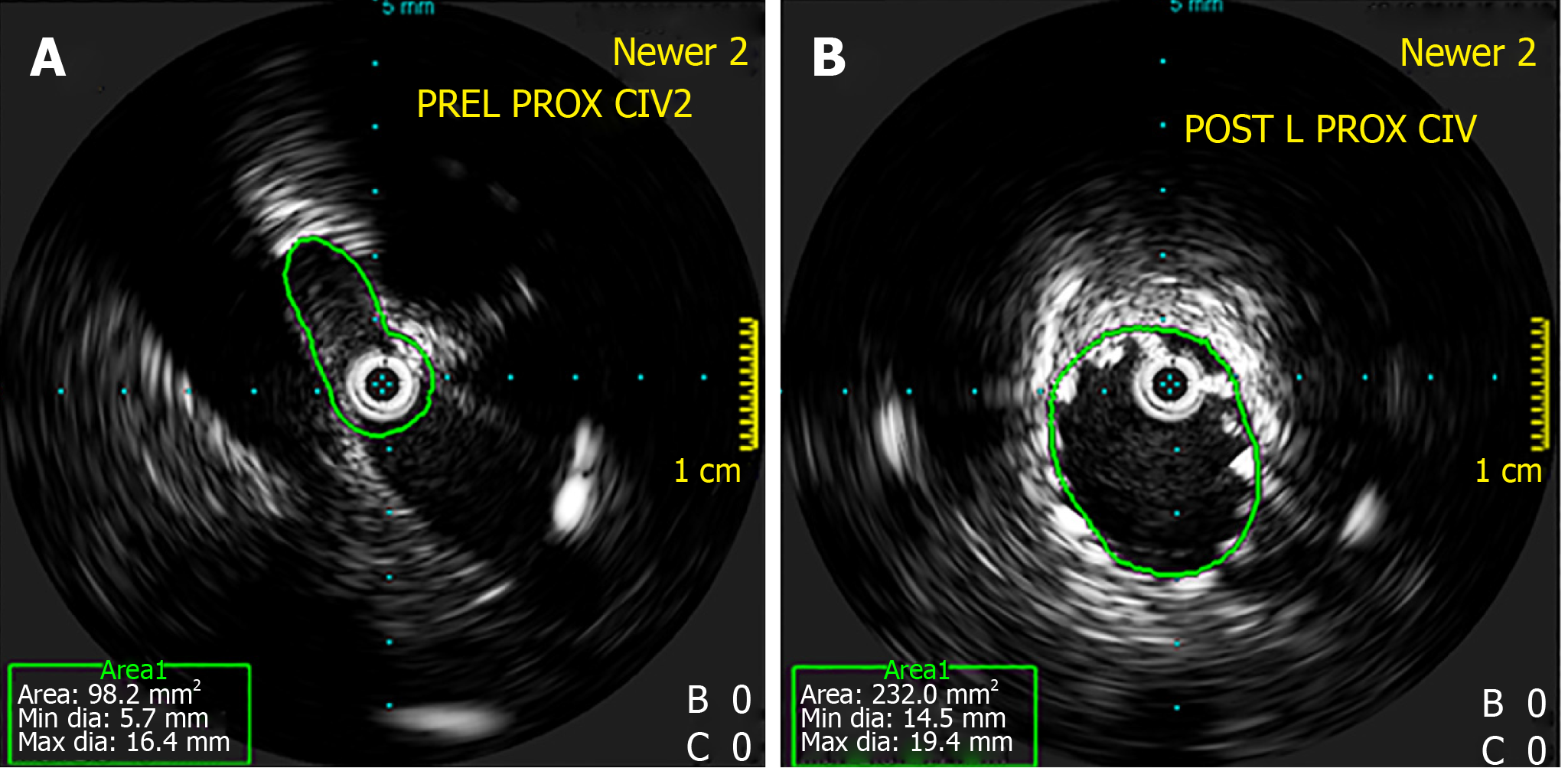
Figure 5 Intravascular ultrasound.
A: Intravascular ultrasound showing stenosis at the left proximal common iliac vein; B: Intravascular ultrasound showing dilatation post-stenting. Intravascular ultrasound allows for direct imaging of the stenosis and evaluation of post-stenting vein diameter.
Routine use of IVUS may allow for better detection hence treatment of iliocaval stenotic lesions (Figure 5B). Precise vessel diameter and lesion length measurement facilitate the choice of stents with the appropriate diameter and length. IVUS also guides the precise deployment of the stent to ensure adequate coverage of the lesion. This is especially important for lesions near the iliac vein confluence, which may be difficult to ascertain on venography alone. IVUS helps to avoid unwanted “jailing” of the origin of the contralateral CIV and undercoverage of lesion, which risks early restenosis. Once the stent is deployed, IVUS can be used to ensure adequate stent apposition to the venous wall. However, IVUS is limited by the image artefacts such as acoustic shadowing from calcification and stent struts which can obscure the underlying venous walls. Image quality in IVUS is also impaired by rotational distortion artefacts created during manoeuvres in tortuous vessels, and multiple circular echoes from reverberating ultrasound beams[48].
MANAGEMENT
Endovascular intervention has become the established treatment for IVCS. Intervention is usually performed during the same session as venography with IVUS. An appropriately sized balloon is placed across the lesion and pre-dilatation of the vessel is performed to allow stent deployment. Stents are crucial for deep venous work due to the high tendency of stenotic fibrotic veins to recoil after balloon dilation. Stenotic veins managed with balloon angioplasty alone have high rates of reocclusion[49,50], with a 5-year cumulative patency rate of only 18%[51]. An oversized stent (usually 20%) is then deployed across with combined fluoroscopic and IVUS guidance to ensure adequate coverage of the lesion. Alternatives to endovascular interventions include open surgical approaches such as dissection and reconstruction of the diseased veins. However, these procedures are associated with higher morbidity and only indicated in patients with long-segment venous obstruction (e.g., extended femorocaval occlusions) or endovascular angioplasty failure[52].
Following the endovascular interventions, most patients would experience prompt and sustained relief from symptoms/signs of pain and swelling (Figure 6). In a study by Raju et al[53] on 304 limbs with symptomatic chronic venous insufficiency, the endovascular interventions of stenting and ballooning achieved complete pain relief and ulcer resolution in 62%-71% of the patients at 24 mo of follow up[53]. In another large cohort study by Peter et al[6] on 982 limbs, 5-year cumulative rates of complete relief of pain and swelling were 62% and 58% respectively[54]. In most centers, the diseased veins would remain patent for sustained periods. In a recent meta-analysis of stent efficacy in iliofemoral venous compression, patency rates at 1 year were 96% for non-thrombotic and 79%-87% for thrombotic lesions[55]. In the longer term, patency rate at 5 years ranges from 70%-84%[56]. Predominant factors associated with higher failure rates include the presence of thrombosis (26.7, P < 0.001), degree of occlusion (OR = 8.3, P < 0.001) and stent extension to below the inguinal ligament (OR = 5.5, P < 0.001)[54,56,57].
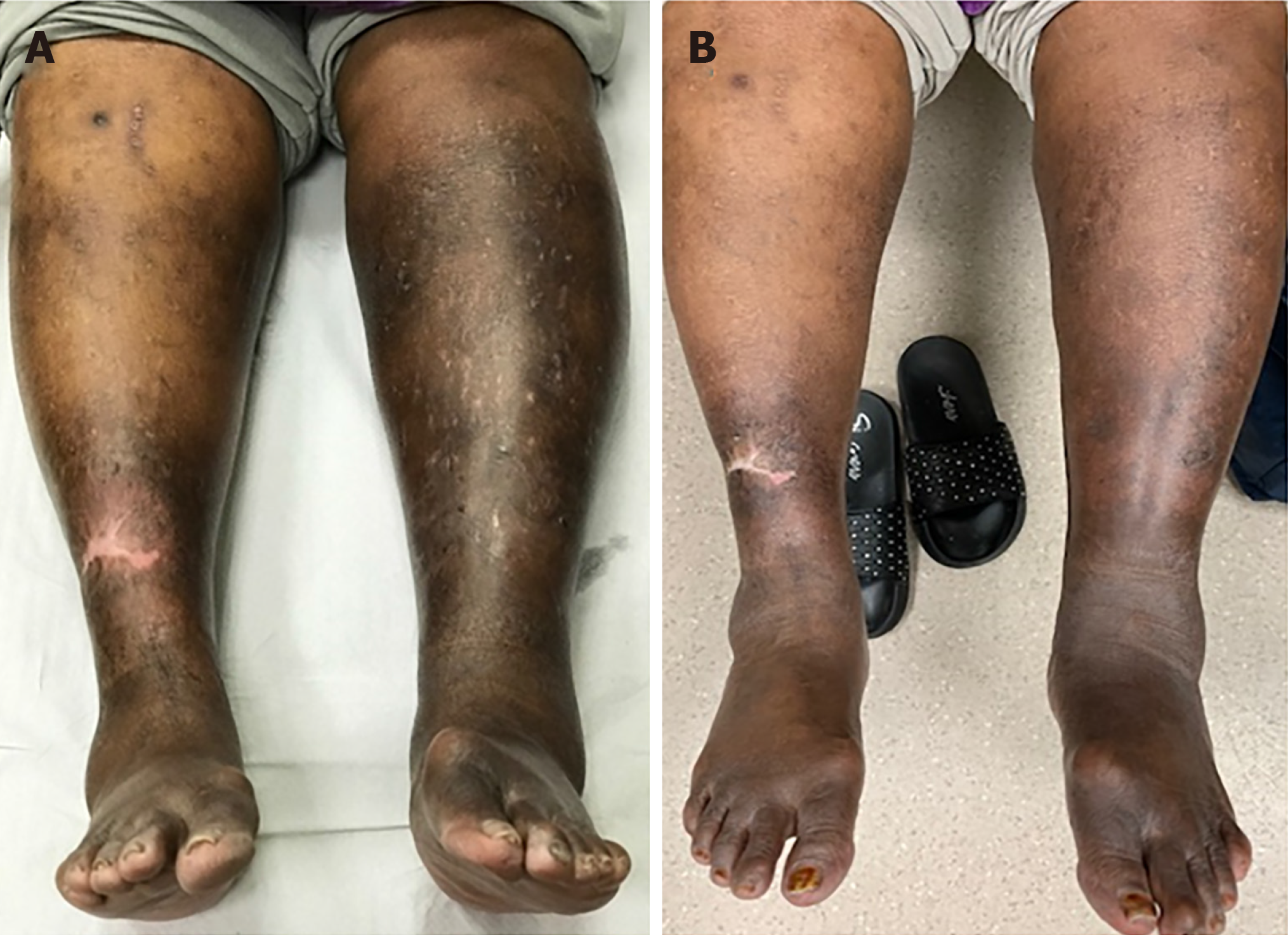
Figure 6 Marked hyperpigmentation and swelling of the legs in a patient with May-Thurner syndrome which showed significant resolution as early as three months after endovascular stenting was performed.
A: Pre-Stenting; B: Three months post-stenting.