Published online Mar 28, 2019. doi: 10.4329/wjr.v11.i3.27
Peer-review started: November 23, 2018
First decision: December 10, 2018
Revised: February 28, 2019
Accepted: March 12, 2019
Article in press: March 12, 2019
Published online: March 28, 2019
Processing time: 127 Days and 11.2 Hours
In the new era of functional magnetic resonance imaging (MRI), the utility of chest MRI is increasing exponentially due to several advances, including absence of ionizing radiation, excellent tissue contrast and high capability for lesion characterization and treatment monitoring. The application of several of these diagnostic weapons in a multiparametric fashion enables to better characterize thymic epithelial tumors and other mediastinal tumoral lesions, accurate assessment of the invasion of adjacent structures and detection of pathologic lymph nodes and metastasis. Also, “do not touch lesions” could be identified with the associated impact in the management of those patients. One of the hot-spots of the multiparametric chest MR is its ability to detect with acuity early response to treatment in patients with mediastinal malignant neoplasms. This has been related with higher rates of overall survival and progression free survival. Therefore, in this review we will analyze the current functional imaging techniques available (18F-Fluorodeoxiglucose positron emission tomography/computed tomography, diffusion-weighted imaging, dynamic contrast-enhanced MRI, diffusion tensor imaging and MR spectroscopy) for the evaluation of mediastinal lesions, with a focus in their correct acquisition and post-processing. Also, to review the clinical applications of these techniques in the diagnostic approach of benign and malignant conditions of the mediastinum.
Core tip: With the past improvements on magnetic resonance hardware, gradients and advanced sequences, the interest of magnetic resonance application in the chest is exponentially growing. In this review, we show the evidence in the literature of its application in mediastinal malignancies. In addition, we explore the advantages of applying new imaging techniques for lesion characterization, helping to differentiate with acuity benign and malignant etiologies. The use of several advanced sequences together yields specificity in identifying false positives from 18F-Fluorodeoxiglucose positron emission tomography/computed tomography. Also, due to its precision in defining invasion and variation in tissue properties through the time, magnetic resonance enhances accurate staging and treatment monitoring.
- Citation: Broncano J, Alvarado-Benavides AM, Bhalla S, Álvarez-Kindelan A, Raptis CA, Luna A. Role of advanced magnetic resonance imaging in the assessment of malignancies of the mediastinum. World J Radiol 2019; 11(3): 27-45
- URL: https://www.wjgnet.com/1949-8470/full/v11/i3/27.htm
- DOI: https://dx.doi.org/10.4329/wjr.v11.i3.27
Morphological evaluation of mediastinal tumors are traditionally performed using computed tomography (CT). Magnetic resonance (MR) is considered a second-line test. However, other imaging methods are used to study tumor ultrastructure characteristics. Of them, the most validated and widely used is 18F-Fluorodeoxiglucose positron emission tomography/CT (18F-FDG-PET/CT) for assessing cell metabolism. Functional MR sequences, such as diffusion-weighted imaging (DWI) and perfusion-weighted imaging (PWI), are gradually becoming more available in daily clinical practice for assessment of mediastinal tumors. In addition to the absence of ionizing radiation, one of its significant advantages is the possibility of studying several physiological characteristics of tumors in the same protocol. Therefore, although technically very demanding, chest MR imaging (MRI) allows an integral evaluation of tumor and accurate differentiation from the non-neoplastic tissue.
Although there is scarce evidence in the literature, DWI helps to differentiate non tumoral thymic entities from thymo-epithelial neoplasms[1-3]. Moreover, by means of apparent diffusion coefficient (ADC) values, it could identify well-differentiated from more aggressive thymomas. DWI has a great value in the diagnosis, characterization and staging of central lung cancer and lymphoma[4]. Also, helps to characterize thyroid nodules and neurogenic tumors. By contrast, PWI is useful to characterize anterior mediastinal neoplasms. When a threshold time to peak of more than 120 seconds is used, it could separate non-invasive thymomas from invasive thymo-epithelial tumors, lymphoma and germ cell neoplasms[5]. The combination of DWI and PWI has demonstrated and increased in the diagnostic performance of MR in lung neoplasms[6]. Also, increases the precision of lung cancer staging by means of better identification of local invasion, metastatic lymph nodes (LN) and distant metastasis. By acquiring PWI and real time steady state free precession real time cine sequences (RT-SSFP), a higher conspicuity of mediastinal, pleural, chest wall and diaphragmatic invasion is obtained[7]. Finally, current data suggests a promising role of functional MRI in treatment monitoring of some neoplasms, like lung and esophageal cancer. The identification of early response has been liked to higher overall and progression free survival rates[8].
Different imaging methods can study several tumor ultrastructure characteristics. The most widely available and validated molecular method is 18F-FDG-PET/CT for evaluating mainly, but not only, tumor glucose metabolism. Using other radiotracers, hypoxia and proliferation of mediastinal tumors, as long as the total tumor burden, could be analyzed. Recently, chest MR has become more widely available, allowing the assessment of different functional and morphological neoplastic characteristics in a one – stop – shop examination, with the advantage of avoiding the use of ionizing radiation.
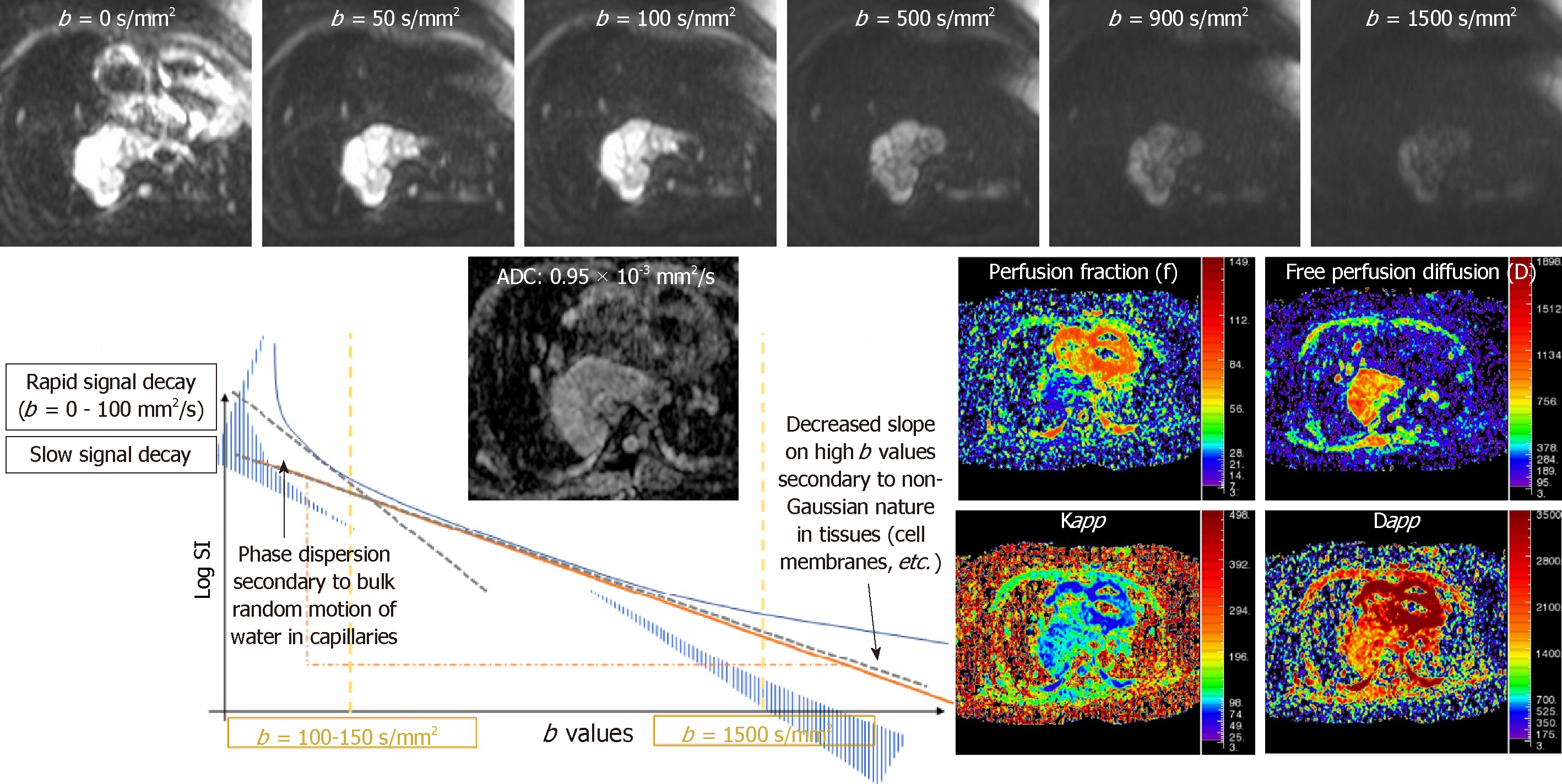
DWI interrogates the Brownian motion of water molecules of the tissues and lesions, particularly in the extracellular-extravascular space. DWI is a consolidated oncological biomarker in clinical practice, indirectly representing the occupancy of the interstitial space. For that purpose, several motion probe gradients are placed surrounding a 180° radiofrequency pulse. At least, two b values are necessary for analyzing the signal intensity decay of the tissues and calculating its ADC, following a mono-exponential model of diffusion signal decay[9]. In this manner, the higher the magnitude of signal intensity decay, the higher the ADC would be, indicating no restriction of water molecules. But, when a tissue shows restricted movement of water molecules, the signal intensity decay is lower and, therefore, ADC has a smaller value[10].
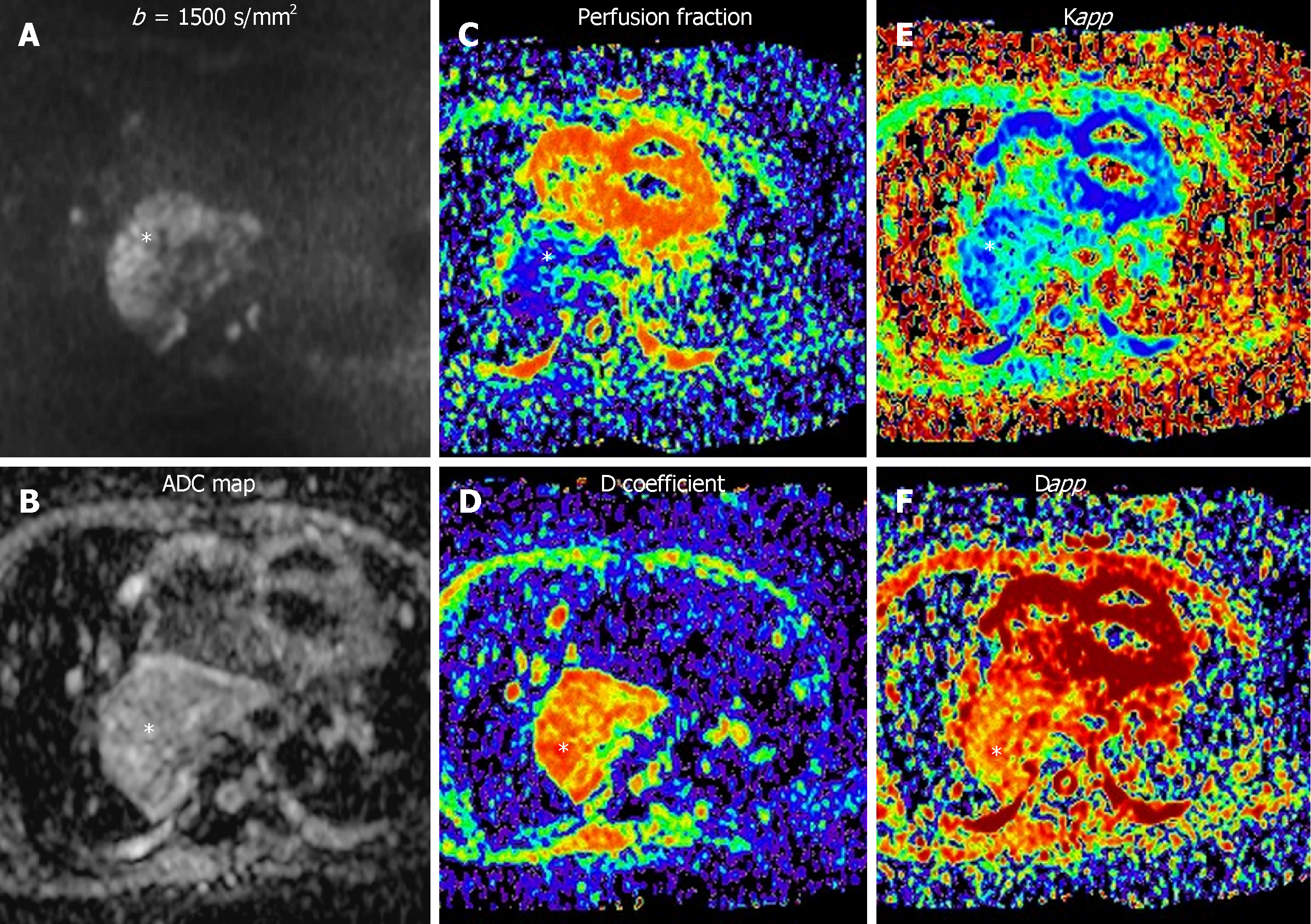
Intra-voxel incoherent motion (IVIM) model of diffusion signal decay is better than mono-exponential analysis for assessment of well-vascularized organs such as kidney, liver, pancreas, and prostate. For this model, it is necessary to acquire several b values. The diffusion signal decay splits into two components. At low b values (< 100-150 s/mm2) the slope of signal decay is higher due to the bulk motion of water molecules inside randomly oriented capillaries (perfusion related decay of diffusion signal). But at higher b values (> 150 s/mm2) the diffusion signal decay curve is related to the true diffusion of water molecules inside the tissue being evaluated (Figure 1)[9].
At very high b values (b > 1500 s/mm2), there is a deviation of the theoretically mono-exponential based signal decay of DWI, that does not follow any Gaussian distribution. Diffusion kurtosis imaging explains this decreased slope of signal decay following a non–Gaussian distribution and relates it to tissue heterogeneity (Figure 1). In addition, these advanced models of quantification of DWI provide several derived parameters (Table 1).
| Mono-exponential model | ||
| ADC | Apparent diffusion coefficient | Exponential signal decay of water molecules in a voxel by voxel basis. |
| IVIM-derived parameters | ||
| D | True diffusion of H2O molecules | Not influenced by movement of water molecules within capillaries |
| f | Perfusion contribution to diffusion signal | Fractional volume of flowing water molecules within capillaries |
| D | Perfusion contribution to diffusion signal decay | Amount of non-diffusional random movements of water molecules |
| DKI-derived parameters | ||
| Dapp | Apparent diffusion | Estimation of diffusion coefficient in the direction parallel to the orientation of diffusion sensitizing agents |
| Kapp | Apparent diffusional kurtosis | Measures the deviation of the true diffusion from a Gaussian pattern. |
| DTI-derived parameters | ||
| MD | Mean diffusivity | Reflects the average diffusion of water molecules in all three directions. |
| FA | Fractional anisotropy | Measures the extent to which diffusion is non-uniform in the three orthogonal directions. |
Some cellular structures impede the diffusion of water molecules in one preferential direction. By the application of at least 6 non-collinear DWI gradients, a 3D diffusion profile of motion of water molecules could be plotted (diffusion tensor model) at a given voxel, which is an ellipsoid[11]. Therefore, when water molecules in a specified tissue move freely in all directions, the diffusion is isotropic. Contrarily, when this motion occurs mainly in one axis or axes due to structural properties of the tissue, this diffusion is anisotropic. Two metric measurements are derived. Mean diffusivity constitute the average diffusion in all three directions. Fractional anisotropy (FA) measures the degree of non-uniform diffusion in the three orthogonal directions[12].
Tumor angiogenesis is essential for the development and behavior of solid tumors. Vasculature formation, growth patterns and vascular permeability are affected by antiangiogenic factors. Therefore, they modulate host response and weights tumor invasion, metastasis and outcome[13]. PWI is a functional technique focused on the evaluation of tumor neoangiogenesis[14,15]. It is a non-invasive and sensitive technique to neoplastic perfusion parameters such as blood volume, blood flow and vascular permeability[16].
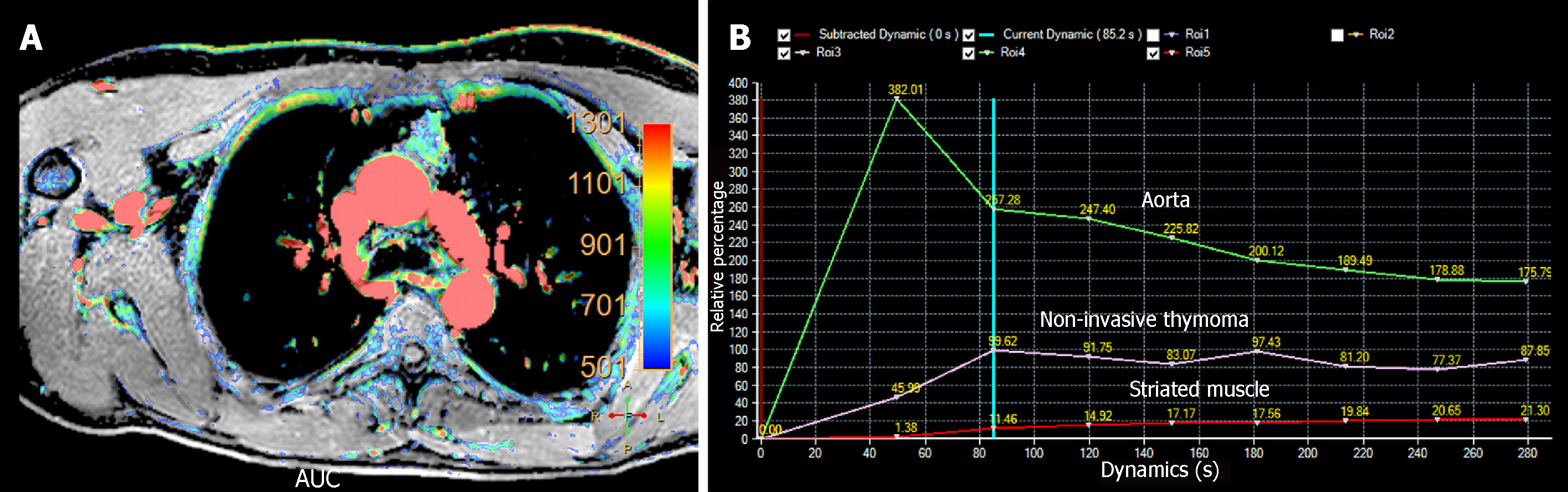
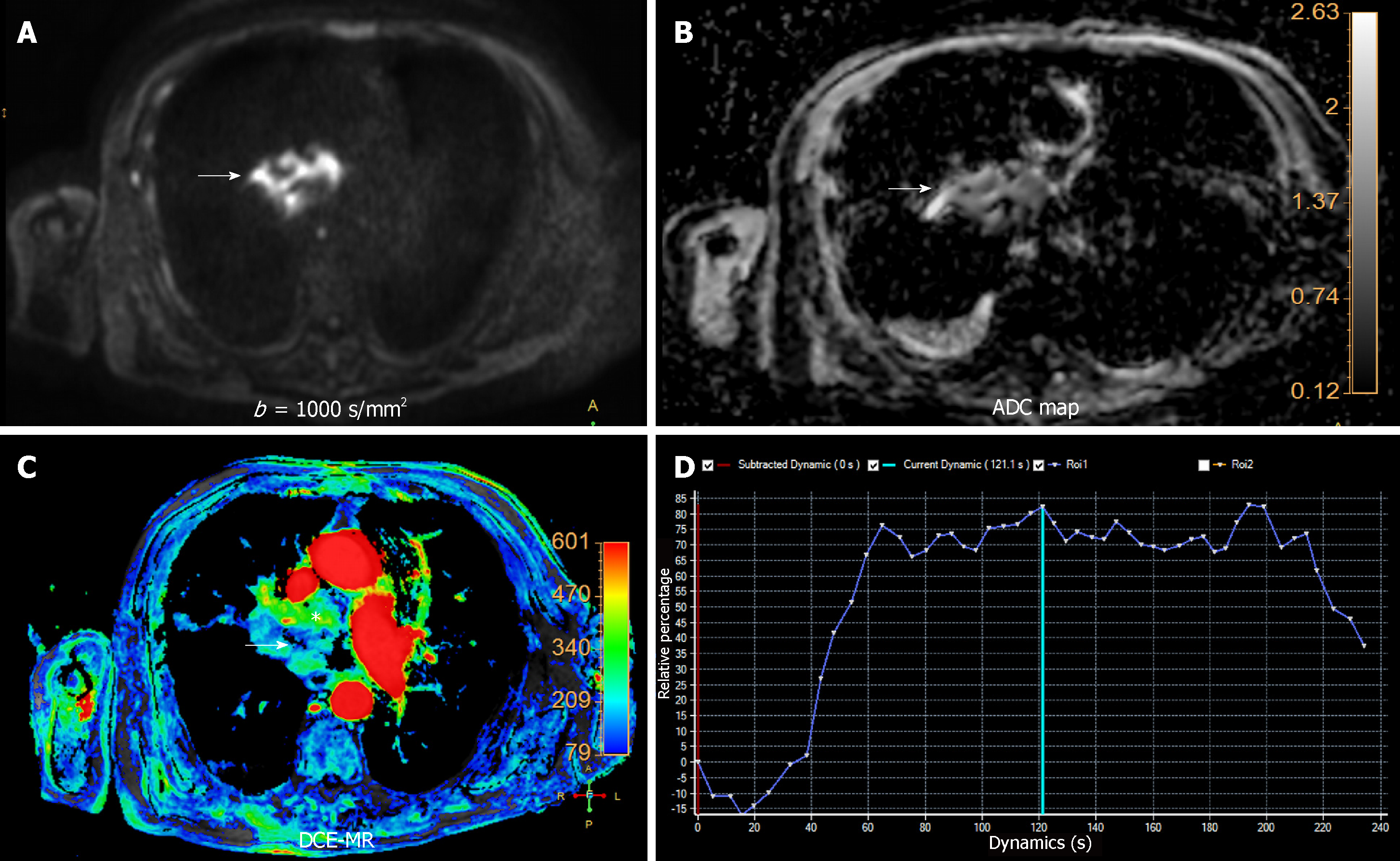
There are two main technical approaches in the evaluation of tumor perfusion with MRI. Dynamic contrast-enhanced MRI (DCE-MRI) uses 2D or 3D gradient echo dynamic acquisitions, acquired during breath-holding. The temporal resolution of each dynamic acquisition is low (13-15 s), but with an excellent spatial resolution. Contrarily, PWI is based on ultrafast (2 to 3 s) 2D or 3D gradient echo dynamic sequences acquired during free-breathing. In order to achieve this very high temporal resolution, it is necessary to employ acceleration techniques such as non-Cartesian parallel imaging and compressed sensing, with the shortest available echo time[17]. Both of them show limited coverage, being higher in DCE-MR compared to PWI. Movement and respiratory artifacts are significant limitations of these techniques requiring the use of motion correction during post-processing[14]. Subtraction images are useful for adjacent structures infiltration detection. Semi-quantitative analysis can be performed with both types of acquisition, which benefits of the use of parametric color-coded maps. Because, these sequences track the variation of signal intensity secondary to gadolinium infusion per second, time-intensity curves (TIC) could be plotted (Figure 2).
PWI has the advantage to allow quantitative analysis using a mono-compartmental or bicompartmental approach. The mono-compartmental approach does not distinguish the intravascular and extravascular–extracellular compartments. Conversely, in the bicompartmental model, both spaces and the exchange of contrast agent between both of them are explored. For its quantification, a region of interest is placed in a principal artery, so the arterial input function is calculated. Additionally, it is possible to differentiate the transfer rates between both compartments. Mono and bicompartmental derived parameters are useful in tissue characterization and lesion management, and some of them have also demonstrated to be prognostic biomarkers of chest malignancies (Table 2)[16].
| Semiquantitative parameters | |
| Initial area under the curve | Include information of blood flow, blood volume, permeability, extravascular–extracellular space volume and microvessel density. |
| Time to peak | Depends on tissue perfusion |
| Wash in | Represents velocity of enhancement |
| Wash out | Represents velocity of enhancement loss |
| Quantitative parameters | |
| Ktrans | Influx volume transfer constant of a contrast agent from the vascular compartment to the interstitial space |
| Ve | Volume of extravascular–extracellular space per unit of tumor volume |
| Vp | Blood plasma volume |
| Kep | Rate constant between extravascular-extracelular space and plasma |
Proton-based MR spectroscopy helps to depict the mi-croscopic metabolic microenvironment of tissues and lesions. The presence of a choline peak has been related to excessive cellular turnover in tumors outside the brain, helping to differentiate benign from malignant lesions[18]. To the best of our knowledge, there is no evidence in the literature about its utility in mediastinal tumors.
Blood oxygen level dependent imaging (BOLD) evaluates changes in the concentration of paramagnetic molecules, being a surrogate marker of tumor hypoxia, a well-known cause of radioresistance of malignancies. BOLD acquisition is based on a multi-echoT2*GE sequence, which is able to depict small reductions in T2* relaxation in tissues, secondary to increases in deoxyhemoglobin content during physiological gaseous exchange. Both, hyperoxia or hypercapnia challenges can be applied to depict changes in oxygen content within the tissues. Tissues with rising oxygen consumption would decrease T2* in those sequences. The rate of spin dephasing (R2* = 1/T2*) is related to the index of tissular oxygenation. Therefore, hypoxic tumors with high blood volume will show greater ΔR2* to physiological changes being, as a consequence, are less suitable to radiotherapy treatment[19].
The mediastinum is a complex segment of the chest, since it contains multiple vital structures in a reduced space. Traditional classification schemes have divided the mediastinum into three or four compartments. The 3-compartment models describe anterior, middle, and posterior divisions, and most 4-compartment models include superior, anterior, middle, and posterior divisions. The International Thymic Malignancy Interest Group (ITMIG) propose a 3-compartment model of the mediastinum based on boundaries identifiable on routine cross-sectional imaging. These segments include prevascular (anterior), visceral (middle), and paravertebral (posterior) compartments[20].
Thymic hyperplasia: Thymic hyperplasia manifests as diffuse symmetric enlargement of the thymus. There are two histologic types of thymic hyperplasia: lymphofollicular hyperplasia and true thymic hyperplasia. Lymphofollicular hyperplasia refers to the presence of hyperplastic lymphoid germinal centers with lymphocytic and plasma cell infiltrate. It is most commonly associated with myasthenia gravis (50% of patients) and other autoimmune conditions such as thyrotoxicosis, systemic lupus erythematosus (SLE), rheumatoid arthritis, scleroderma, among others. True thymic hyperplasia is an enlargement of the thymus, without histologic abnormalities. Rebound hyperplasia occurs after chemotherapy, steroids, and radiotherapy[21].
In patients with diffuse thymic enlargement, it may be difficult to differentiate between hyperplasia and tumor. Fat infiltration is present within the normal thymus and thymic hyperplasia[22]. Chemical shift imaging (CSI) is a fat-suppression technique that enables the identification of microscopic or intravoxel fat[1]. It allows the detection of an intravoxel mixture of water and fat by showing a signal loss on the opposed-phase image relative to the in-phase image in the thymic tissue compared to paraspinal muscle. CSI can be used to distinguish thymo-epithelial tumor from thymic hyperplasia, by detecting microscopic fat in the latter. A chemical shift ratio (CSR) can be calculated for quantifying the signal suppression on opposed-phase divided by the signal intensity in-phase of the thymic tissue related to the paraspinal muscle. A CSR value of less than or equal to 0.7 is suggestive of thymic hyperplasia, depending on sex and age[23]. When the CSR is over 1.0, it is indicative of tumoral origin. Between 0.8 and 0.9 the lesion is unclear and a control examination is needed. It is essential to take into account that not all young thymus suppress fat in out of phase imaging[24]. Also, the thymus of young women had lower CSR than the thymus of young men[25]. On DWI, thymic hyperplasia shows no significant restriction of free water molecules, revealing higher ADC values compared to tumoral lesions. Also, on DCE-MRI, it shows minimal (type D) or no enhancement after gadolinium intake. Meanwhile, thymic tumors show increased perfusion.
Thymic epithelial neoplasms: Thymic epithelial neoplasms are rare malignant tumors, with a described prevalence of 0.13/100000 individual in the United States[3]. Thymic epithelial neoplasms include thymoma, thymic neuroendocrine tumors (NETs), and thymic carcinoma. Up to 15 different staging systems have been described for thymo-epithelial neoplasms. The Masaoka system and its variant, the Masaoka–Koga staging system, are the most commonly used in clinical practice (Table 3). The radiologist has a crucial role in the staging, because the identification of advanced stages (III and IV) directs the patient towards neoadjuvant therapy[2]. In fact, the ITMIG recommended this staging system due to its correlation with patient survival[26]. Recently, the ITMIG in association with the International Association for the Study of Lung Cancer proposed a TNM staging system according to conclusions obtained from the retrospective inclusion of more than 10000 patients (Table 4)[27].
| Stage | Degree of invasion | 5 yr survival rate (%) |
| I | Tumor completely encapsulated | 96-100 |
| IIa | Microscopic tumor invasion into the capsule | 86-95 |
| IIb | Tumor invasion into the surrounding fat | |
| III | Tumor invasion into surrounding organ such as the pericardium, great vessel or lung | 56-69 |
| IVa | Pleural or pericardial dissemination | 11-50 |
| IVb | Lymphatic or hematogeneous metastasis |
| TNM staging | |||||||
| Tumor (T) descriptor | I | II | IIIA | IIIB | IVA | IVB | |
| T1a | Encapsulated or unencapsulated tumor, with or without extension into fat | X | X | X | |||
| T1b | Invasion of mediastinal pleura | X | X | X | |||
| T2 | Invasion of pericardium | X | X | X | |||
| T3 | Involvement of lung, chest wall, phrenic nerve, brachiocephalic vein, superior vena cava, or hilar (extrapericardial) pulmonary vessels | X | X | X | |||
| T4 | Invasion of thoracic aorta, arch vessels, main pulmonary artery, trachea, esophagus, or myocardium | X | X | X | |||
| Node (N) descriptor | I | II | IIIA | IIIB | IVA | IVB | |
| N0 | No lymph node metastasis | X | X | X | X | X | X |
| N1 | Involvement of anterior (perithymic) lymph nodes | X | X | ||||
| N2 | Involvement of deep intrathoracic or cervical lymph nodes | X | |||||
| Metastasis (M) descriptor | I | II | IIIA | IIIB | IVA | IVB | |
| M0 | No Metastasis | X | X | X | X | X (N1) | X (N2) |
| M1a | Pleural or pericardial metastatic nodule or lesions | X (N0,1) | X (N2) | ||||
| M1b | Pulmonary intraparenchymal metastastic nodule or distant organ metastasis | X (any T, N) | |||||
Thymoma is the most common histologic type of thymic epithelial neoplasms of the prevascular mediastinum. Men and women are affected equally. Has a peak incidence in middle age (40-60 years) and is rare in children and young adults[2]. About 20% of thymomas coexist with other neoplasms such as lymphoma, lung cancer, and thyroid cancer. Thymomas are slow-growing neoplasms and, although an aggressive behavior is rare, they may present pleural, pericardial, and more unusually, hematogenous spread[28]. The association of thymoma with myasthenia gravis is common. Between 30 to 50% of thymomas coexist with myasthenia gravis, being more frequent this relation in women, and up to 10% to 15% of patients with myasthenia gravis have a thymoma[1,2]. Other associated conditions are hypogammaglobulinemia (10%), pure red cell aplasia (5%), Good’s syndrome (B and T cell immunodeficiency) and autoimmune disorders (SLE, polymyositis, and myocarditis)[29].
On MRI, thymomas have low to intermediate signal intensity on T1 and high signal intensity on T2-weighted sequences. They may present with cystic changes, necrosis, fibrous septa, nodules, and hemorrhage[4]. DWI is useful for differentiating between benign and malignant tumors by using ADC values. The study by Razek et al[4] showed ADC values of 2.38 ± 0.65 × 10-3 mm2/s and 1.09 ± 0.25 × 10-3 mm2/s, for benign and malignant tumors. They defined a threshold ADC value of 1.56 × 10-3 mm2/s for differentiating malignant from benign mediastinal neoplasms, with a sensitivity, specificity, and accuracy of 96%, 94%, and 95%, respectively. Additionally, well-differentiated tumors showed higher ADC values (1.20 ± 0.22 × 10-3 mm2/s) compared to poor differentiated tumors (0.98 ± 0.18 × 10-3 mm2/s). Nevertheless, there was an overlap in the ADC values of invasive thymoma, lymphoma and lung cancer. Consequently, the solely use of ADC values cannot differentiate them with accuracy[4]. On the other hand, Yabuuchi et al[5] found no significant difference in ADC values between thymic epithelial tumors, lymphomas and malignant germ cell tumors. Furthermore, Carter et al[3] stated that ADC could not differentiate between low-grade thymomas, high-grade thymomas and thymic carcinomas.
DCE-MRI can be used to differentiate anterior mediastinal masses. Yabuuchi et al[5] described 3 types of time-signal intensity curves: (1) Persistent, with a time-to-peak (TTP) > 120 s; (2) Plateau, with a TTP < 120 s and a wash-out < 30%; and (3) Wash-out, with a TTP < 120 s and wash-out > 30%. In their population, the washout pattern was seen only in thymic epithelial tumors (Figure 2). Also, they added the use of 18FDG-PET for this differentiation. The combination of TIC pattern (washout or persistent/plateau pattern), maximal diameter (< 6.8 cm vs ≥ 6.8 cm), and a maximum standardized uptake value (SUVmax) cut off value of 11.6 could help differentiate between thymoma (SUVmax < 11.6) and thymic carcinoma (SUVmax > 11.6). They proposed a flow diagram combining the TIC pattern, SUVmax, and maximal diameter for characterization of anterior mediastinal solid tumors. With this scheme, they obtained a sensitivity and specificity of 93.9% and 77.8%, respectively, for differentiating anterior mediastinal tumors (Figure 3)[5].
On the other hand, Sakai et al[30] found a correlation between histologic grade and DCE-MRI parameters. Low-risk thymomas (Masaoka stage I/II) demonstrated rapid TTP with a mean time of 1.5 min; Masaoka stage III showed a TTP of 2.5 minutes, and other lesions such as thymic carcinoma, NETs, lymphoma, and malignant germ cell tumor demonstrated progressive enhancement, with a TTP of 3.2 min. The differentiation of thymoma from other mediastinal masses showed a sensitivity, specificity, and accuracy of 79%, 84%, and 81% respectively, when a cut-off value of TTP < 2 min was used (Figure 4).
NETs or thymic carcinoid are the least common type of thymic epithelial neoplasms (incidence 525 cases per 100000 people per year in the United States)[31]. Thymic NETs primarily affect males (male to female ratio of 3:1) in their sixth decade of life. The median age at presentation is approximately 57 years. Thymic NETs originate from neural crest cells and are associated with poor prognosis as they are aggressive, locally invasive with distant metastases and resistant to chemotherapy[32]. Nearly 25% of thymic NETs arise in patients with multiple endocrine neoplasia type 1, Cushing syndrome and inappropriate antidiuretic hormone secretion syndrome[33].
Imaging findings are similar to those of thymomas at CT and conventional MR, although, they tend to be larger, lobulated and locally invasive masses. As well as thymic carcinomas, suggestive findings include: (1) Irregular contour and cystic component; (2) metastasis and vascular invasion; and (3) heterogeneous enhancement and lymphadenopathy. They may present with low ADC values, which represents aggressiveness, hypercellularity, and poor differentiation. Additionally, a DCE-MRI with a TTP > 2 min suggests a high-grade tumor[4,5,30].
Thymic carcinomas are the second most common type of thymic epithelial neoplasms, representing up to 20% of those tumors[34]. Middle age men are more commonly affected. Thymic carcinomas are more aggressive and invasive than thymomas, with a larger size, lobulated contour, and invasion of adjacent structures. They may also present with cystic changes and necrosis. Poor prognosis is related with infiltrating tumor margin, the absence of a lobular growth pattern, high-grade atypia and necrosis, and more than ten mitoses per High-Power Field[35,36].
Thymic carcinomas present similar findings at CT and conventional MR as thymomas. Suggestive signs of thymic carcinomas are the presence of metastases, vascular invasion, irregular contour, cystic component, heterogeneous enhancement, and lymphadenopathy[35,36]. As well as thymic NETs, low ADC values suggest aggressiveness and poor differentiation, and DCE-MRI with a TTP > 2 min indicates a high-grade tumor (Figure 3). High 18FDG uptake is another characteristic that may help differentiate between thymoma and thymic carcinoma, with the latter presenting with higher SUVmax values (Figure 4)[4,5,30].
Lymphoma: Lymphoma comprises a heterogeneous group of neoplasms involving the LN. Mediastinal involvement is frequently a part of the systemic disease. Primary mediastinal lymphomas are rare (10%). The majority of lymphomas affecting the mediastinum are Hodgkin lymphoma (HL)[37]. There were 66000 new cases of non-Hodgkin lymphoma (NHL) and 8800 new cases of HL diagnosed in the United States in 2011[38-40]. The value of imaging techniques relies on the capacity to differentiate reactive from malignant lymph node, but also to evaluate tumor extension, treatment response, and relapse. Additionally, the ability to discriminate lymphoma subtypes allows treatment assessment, outcome, and differentiation between indolent and aggressive NHL[41].
It usually presents as an anterior mediastinal soft-tissue mass or a conglomerate of LN with mild enhancement. The association of lymphadenopathy affecting different lymph node stations in the chest without mediastinal mass suggests a secondary involvement of NHL arising from other location[27]. When a mediastinal mass shows an infiltrative nature with encasement or encirclement of vascular structures without involving it, a lymphoma should be the first entity to keep in mind over thymo-epithelial or germ cell tumors[27].
18FDG-PET/CT is better than 18FDG-PET or CT alone for evaluating lymphoma as it helps to discriminate indolent from aggressive NHL. In 2016, the International Workshop on NHL approved the new response evaluation criteria in lymphoma (RECIL). RECIL criteria are aligned with response evaluation criteria in solid tumors, in as much as it suggests a uni-dimensional measurement method, but introduce tumor metabolic evaluation with 18FDG-PET/CT. SUVmax is useful for evaluating treatment response and outcome in 18FDG-avid lymphoma. There is an excellent correlation between high SUVmean values and progression-free survival (PFS)[42].
DWI reveals inherent tissue properties, such as hypercellularity, nuclear hyperchromatism and an increase in the number of macromolecular proteins. Koşucu et al[43] described the utility of ADC values in the differentiation of malignant and benign mediastinal LN. They found the lowest ADC values in metastatic LN compared to benign LN (1.02 ± 0.19 × 10-3 mm2/s vs 1.51 ± 0.07 × 10-3 mm2/s, for malignant and benign LN respectively). Also, an overlap in ADC values was present with invasive thymoma and bronchogenic carcinoma. The study by Mosavi et al[44] showed a difference in ADC values between indolent NHL, aggressive NHL and HL using whole-body DWI, with ADC values being lower in indolent NHL (597 ± 115 mm2/s) rather than aggressive NHL (822 ± 266 mm2/s) and HL (1020 ± 547 mm2/s) (Figure 5). The explanation for lower values of ADC in indolent NHL could be explained by the higher cell density when compared to HL and aggressive NHL. Additionally, an increased ADC value has been related to longer overall survival (OS). Whole body DWI had a similar diagnostic performance to 18FDG-18FDG-PET/CT. Therefore, in the assessment of early treatment response, usually at after one week, could be an alternative radiation-free surveillance method. PET/MR could be a promising alternative method for staging and follow up of patients with lymphoma. In fact, ADCs and SUVs demonstrate to be independent biomarkers in lymphoma, with significant correlation between them in follicular lymphoma[17].
Germ cell tumors: They comprise neoplasms arising from primitive germ cells, miss-migrated along the urogenital ridge. There are two types, of seminomatous (seminoma and dysgerminoma) or non-seminomatous (NSGCT) origin (embryonal carcinoma, choriocarcinoma, Yolk-Salk tumor, and teratoma). Malignant germ cell tumors usually have a male predominance. Mature teratoma constitutes the most common mediastinal germ cell tumor, demostrating a varying amount of intralesional fluid, fat (present in up to 50% of cases), calcification or soft tissue components. Frequently, teratomas manifest as large unilocular or multilocular thin-walled cystic masses[3]. Occasionally, bones or tooth-like elements could be identified[45,46]. Fat-fluid levels are highly specific to this entity, which typically affect young patients, with a described prevalence of 25% of the prevascular masses in patients of 10-19 years-old, 10%-15% in individuals of 20-49 years old and less than 5% in subjects of more than 50 years-old[47]. Differential diagnosis includes other fat-containing anterior mediastinal lesions like thymolipoma, lipoma or liposarcoma.
Non-teratomatous germ cell tumors usually manifest as large soft-tissue prevascular mediastinal masses. Clinical and serological information is useful in the differentiation of these lesions with lymphoma. Seminomas generally affect patients between 10 to 39 years old. In up to 10% of patients, there is an elevation of serum levels of beta-human chorionic gonadotropin (b-HCG)[48]. Elevated levels of lactate dehydrogenase could be present in seminomas but also in lymphoma patients[49,50]. Contrarily, NSGCT present with high levels of serum b-HCG and alpha-fetoprotein in 90% of them[51,52]. Seminomatous tumors manifest as hypointense masses on T2-weighted images, with homogeneous enhancement after gadolinium uptake. By contrast, NSGCT are large and heterogeneous masses. Internal foci of hyperintensity on T1-weighted images are related to intralesional hemorrhage. Although, the presence of pleural effusion is rare, pulmonary metastasis may help to distinguish seminomatous germ cell tumors from lymphoma. An NSGCT is firstly suspected when a large and heterogeneous prevascular mass with pulmonary nodules is identified on a male patient below 40 years-old[45,53]. DWI could help to differentiate a benign from a malignant origin, but also, may increase our specificity to detect mature fibrosis or cystic components. At DCE-MR, they usually show a persistent or plateau pattern of TIC, with a TTP over 120 s. On 18FDG-PET/CT, they show a high radiotracer uptake, often with maximum SUVs higher than 11.6 (Figure 6). The combination of size, DCE-MR derived TIC, and 18FDG-PET/CT along with serologic markers could help to differentiate germ cell tumors from other anterior mediastinal masses[5].
Mediastinal goiter: It has a described incidence of 1%-15% of patients undergoing thyroidectomy. On a CT exam, when a hyperattenuating (70-85 Hounsfield units) prevascular mediastinal mass with intense and sustained enhancement is identified in continuity with the cervical thyroid gland, a mediastinal goiter should be the most preferred diagnosis. Cystic changes and calcifications may be present. Additional findings favouring a malignant transformation are loss of mediastinal tissue planes and cervical or mediastinal lymphadenopathy[27,47].
DWI could help to differentiate benign from malignant thyroid nodules. Using a b value of 500 s/mm2 and an ADC cut-off value of 1.704 × 10-3 mm2/s, a sensitivity, specificity, and accuracy of 92%, 88%, and 87%, respectively has been described. Additionally, using a DW-sequence with a b value of 1000 s/mm2, significant differences on ADC values have been described between benign and malignant nodules (2.75 ± 0.6 × 10-3 mm2/s vs 0.69 ± 0.35 × 10-3 mm2/s). The decrease in ADC values in malignant lesions is due to the presence of increased cell density and relatively severe desmoplastic response. Contrarily, the cause of elevated ADC in thyroid adenomas and hyperplastic nodular goiter is the predominance of abundant cellular follicles, extracellular fluid and reduced cell density[54]. Furthermore, the presence of a delayed wash-out pattern is also suggestive of thyroid carcinoma, with higher diagnostic performance compared to fine needle aspiration (sensitivity 100% vs 50%-85.7%; accuracy 90% vs 70% to 87.5%, respectively)[55].
Parathyroid adenoma: It usually presents in a patient with a history of primary hyperparathyroidism, hypercalcemia and/or elevated serum parathormone levels, with or without parathyrodectomy and a soft-tissue nodule in the anterior–prevascular mediastinum[56]. 20% of parathyroid adenomas are ectopic, and 80% of ectopic parathyroid adenomas showed an anterior mediastinal location. Possible ectopic locations include the thymus, tracheoesophageal groove, retrosternal region and posterosuperior mediastinum[36]. On imaging, they usually present as small (< 3 cm) rounded and well-defined nodules, with high radiotracer uptake at 99mTecnetium and 201Talium scans[27]. Four dimensional (4D) CT provides both functional (perfusion) and highly detailed anatomic knowledge about parathyroid lesions. At 4D CT imaging, they present strong enhancement after intravenous contrast infusion with significant washout at the delayed phase[57,58]. Although there is no reference in the literature about the utility of DWI and DCE-MR in parathyroid adenoma, in the authors’ experience, these lesions demonstrate high ADC values and, and on PWI, a TIC with a steep slope and significant washout, in a similar fashion to the findings described on 4D CT[27].
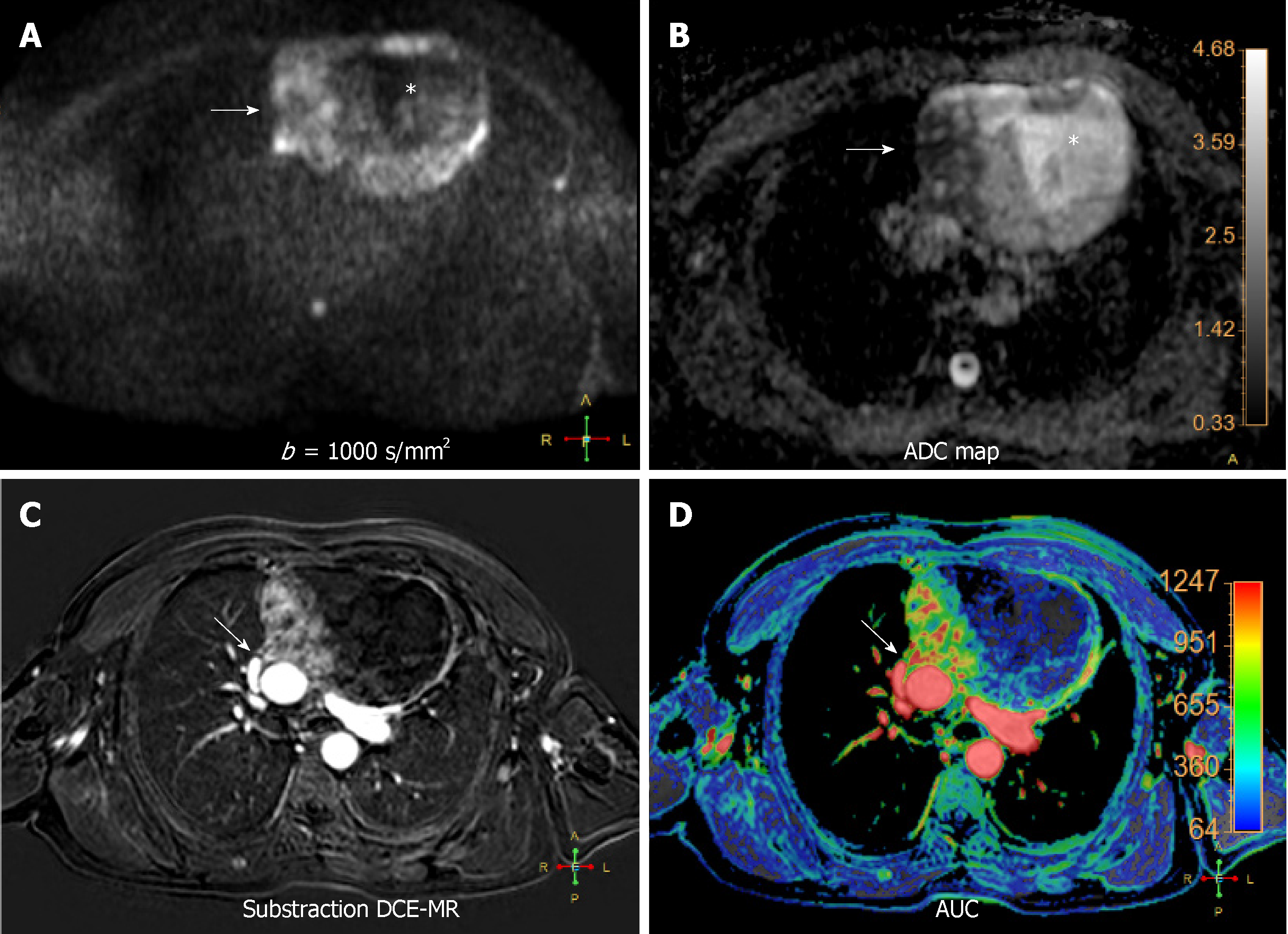
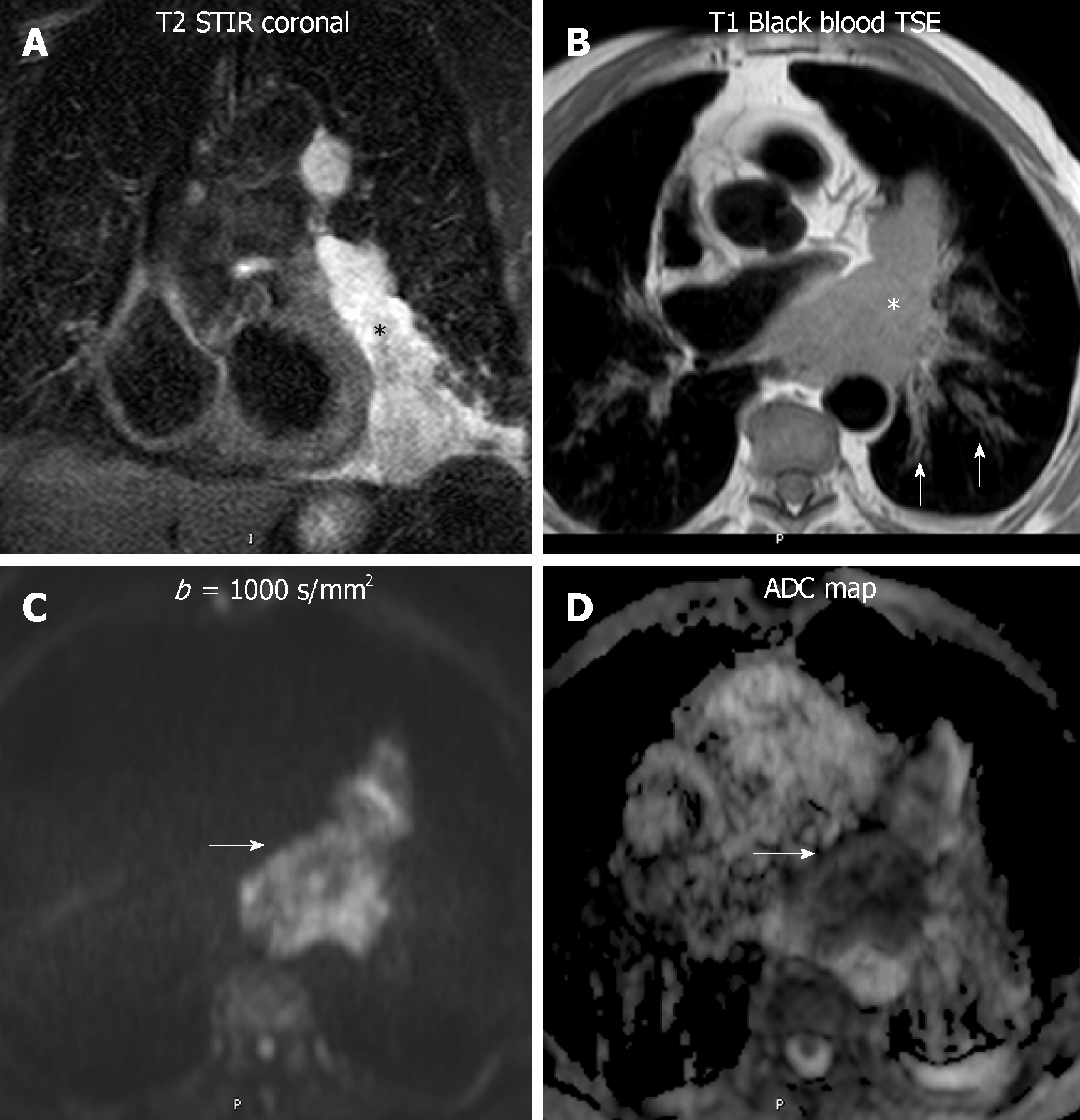
Central lung cancer: Functional MR has shown great utility in patients with lung cancer, enabling to differentiate with accuracy benign from malignant nodules. The combination of DWI and DCE-MR showed similar sensitivity to 18FDG-PET-CT but higher specificity and accuracy (up to 94%), thanks to the reduction of the number of false positives from the latter technique, such as active inflammatory and tuberculous nodules[6]. In addition, DWI has a potential impact on lung cancer differentiation. Well-differentiated adenocarcinomas display higher ADC values than aggressive adenocarcinomas, with certain overlap with small cell lung cancer and squamous cell lung cancer[59]. The amount of tumor cells and its distribution is related to signal intensity at high b value. A signal intensity lesion-to-spinal cord ratio (LSR) on high b value has been explored in the differentiation of malignant and benign ones. Malignant tumors demonstrated higher values, having more specificity and accuracy than ADC and IVIM derived values in this differentiation[60,61]. Furthermore, aggressive subtypes of adenocarcinoma showed higher signal intensity at high b value with a heterogeneous pattern. However, other authors did not show significant differences in the LSR of benign and malignant pulmonary lesions (Figure 7)[62].
Different patterns of enhancement have been described in primary mediastinal neoplasms, although some overlap is also present[63-66]. Some authors obtained higher relative signal enhancement and steeper slope in active inflammatory lesions[66]. Coolen et al[63] described a flowchart , where the use of DWI in lesions with TIC type B curves could distinguish benign (non-restrictive) from malignant (restrictive) entities (Figure 7). Therefore, functional multiparametric chest MRI constitutes a valuable one-stop-shop radiation-free diagnostic modality for lung cancer characterization.
Neurogenic tumors group a certain type of lesions that arise from peripheral nerves, sympathetic and parasympathetic ganglia. Correspond to 75% of all posterior mediastinal lesions, 20% of adult posterior mediastinal lesions and 25% of pediatric mediastinal lesions[3]. There are significant differences in the ADC of benign and malignant peripheral nerve tumors (1.848 ± 0.40 × 10-3 mm2/s vs 0.900 ± 0.25 × 10-3 mm2/s; for benign and malignant tumors, respectively, P < 0.001)[67]. Also, the FA derived from diffusion tensor imaging of involved nerves was lower compared to normal ones[67]. There is limited evidence about the application of functional MRI in other mediastinal lesions.
Functional MR has shown great utility in the staging of lung cancer by allowing an accurate assessment of invasion of vascular, bronchial and other mediastinal and chest wall structures. DWI can differentiate, without the need for any radiotracer intake, central tumor (restrictive) from post-obstructive pneumonitis (non-restrictive), which is vital for cancer staging and radiotherapy planning. DCE-MR is superior to contrast enhanced CT and morphological MR in the identification of vascular and mediastinal invasion[12]. Free breathing RT-SSFP sequences can distinguish those peripheral tumors invading only the visceral pleura (mobile with respiratory motion) rather than those that invade parietal pleura and beyond (static with respiratory motion). Therefore, the combination of DCE-MR and RT-SSFP could be an optimized strategy for assessing pleural, mediastinal, diaphragmatic and chest wall invasion.
For nodal staging (N-staging) 18FDG-PET/CT constitute the primary modality in lung cancer. The primary objective is to identify occult metastatic LN and distant lesions. It could discriminate N3 from M1 stages, which require neoadjuvant therapy. By contrast, DWI has also shown great utility in the differentiation of benign from malignant LN, distinguishing false positive 18FDG-PET/CT targets due to reactive – inflammatory LN. In DWI, false positives are due to granulomatous LN, and false negatives are secondary to microscopic cancer deposits, mainly. Nomori et al[68] detected higher ADC values in benign lymph nodes compared to metastatic ones, using an ADC threshold value of 1.63 × 10-3 mm2/s (accuracy: 89%, specificity: 99%). Finally, Chhabra et al[67] applied a semiquantitative approach. They referred that short tau inversion recovery was more sensitive and accurate for N staging compared to DWI and 18FDG-PET/CT.
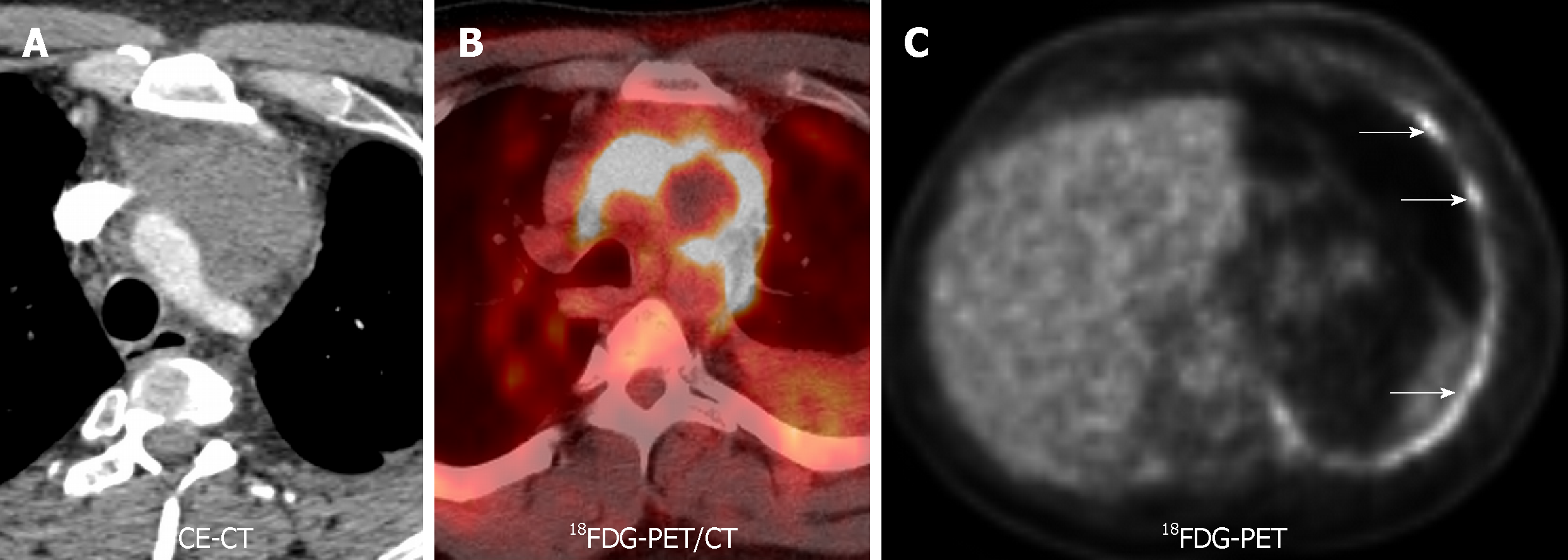
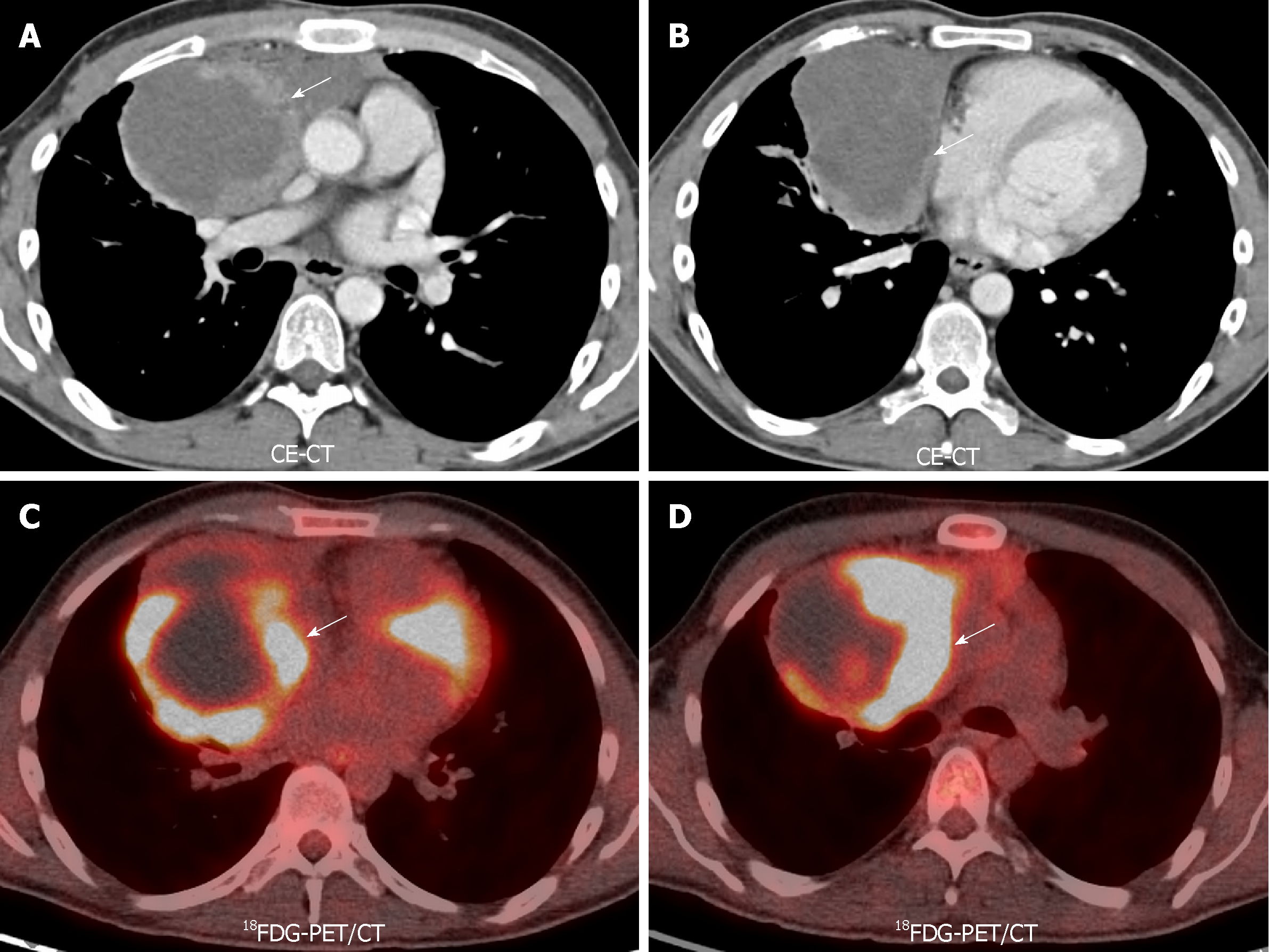
18FDG-PET/CT is the current standard technique for staging esophageal cancer, with an overall accuracy of 90%-92%. False negatives are due to early stage (Tin situ, T1 and T2). False positives of this technique are secondary to leiomyomas and esophagitis (Figure 8). Another limitation is the poor spatial resolution, which explains its limited role in defining the depth of invasion. MRI has no role in current staging guidelines. MR also has poor detection rates in the early stages. The detection rates using a combination of high resolution T2 and DWI are 33%, 58%, 96% and 100% for T1, T2, T3, and T4 stages, respectively[69]. Contrarily to lung cancer LN, in esophageal carcinoma the ADC of malignant LN is higher than benign LN (1.46 ± 0.35 × 10-3 mm2/s vs 1.15 ± 0.25 × 10-3 mm2/s, P < 0.0001), due to their mucinous content[70].
Whole body–DWI (WB-DWI) is a promising tool for M staging, which globally reflects similar results to 18FDG-PET/CT. In detail, it is superior to bone scintigraphy, 18FDG-PET/CT and CT in the identification of bone metastasis, and has substantial advantages in the detection of bone, liver, brain, and kidney metastasis[71]. Contrarily, compared to a WB-DWI scheme, 18FDG-PET/CT has better results in LN and soft-tissue metastasis, probably due to the non-selective fat saturation technique applied for background signal suppression in WB-DWI[72,73].
One of the most promising applications of functional MR in the chest is treatment monitoring and detection of recurrence (Table 5). An increase in ADC in patients of non-small cell lung cancer during or after chemotherapy or radiotherapy has been related to good response, being associated to higher OS and PFS. This increase in ADC is due to cell death, necrosis, apoptosis, and cell lysis. DWI has better results for detection of early response than DCE-MR and 18FDG-PET/CT. Also, a low ADC value at the pre-treatment stage has been predictive of proper response to che-motherapy[8,74,75].
| Good response | Poor response | |
| T2WI | No tumor ↑ SI in bone marrow | Residual/↑soft tissue mass ↑ extent bone marrow invasion |
| DWI | ↑ ADC | ↓ ADC |
| DCE-MRI | ↓ slope/absent enhancement | Persistent / ↑ enhancement |
| MRS | ↑ Choline peak | ↓ Choline peak |
Another important application of DWI is the treatment monitoring of new therapeutic agents (vascular disrupting agents and immunotherapy). Because of the different mechanism of action in cancerous cells compared to conventional drugs, the radiologist must be conscious of the paradigmatic behavior of the lesions during treatment surveillance. Lung tumors with high ADC value are predictive of good response to vascular disrupting agents, and during the follow-up, a decrease of ADC is a sign of treatment effectiveness[8].
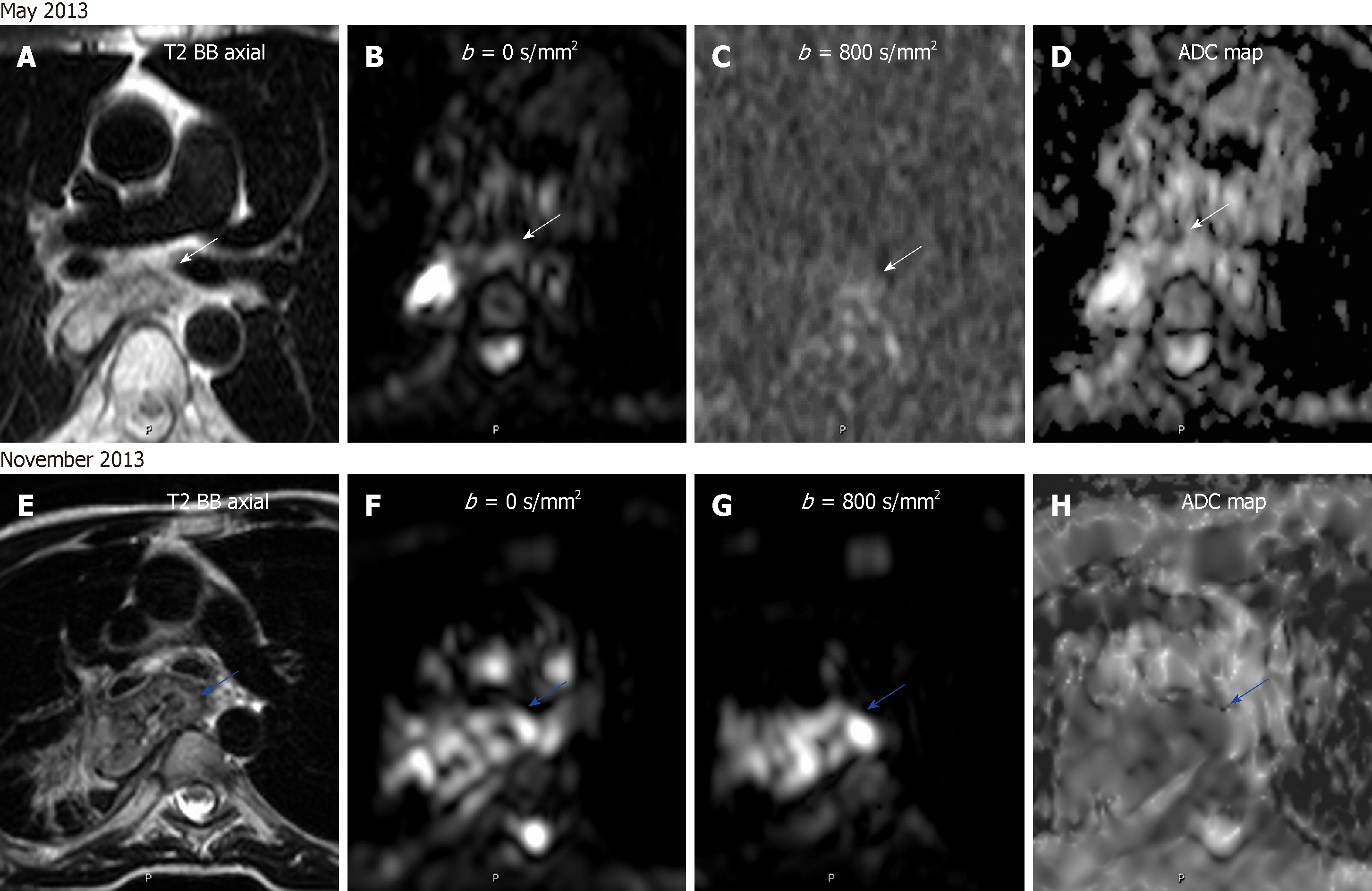
As well as in staging, the treatment response of esophageal cancer is made usually by 18FDG-PET/CT. A decrease of more than 50% of the SUVmax/SUVmean in the post-treatment follow-up compared to pre-treatment examination is in keeping with a good response. This method has low sensitivity and specificity (67%-70%), mainly due to chemotherapy and radiotherapy induced esophagitis. Also, the identification of a good response behavior precludes the detection of local recurrence, being present only in 42% of PET-based clinical responders[69]. On MRI, a difference in post-treatment ADC compared to pre-treatment value has been correlated with histopathological regression grade. This difference early on the treatment onset has a 100% predictive value on responders[76-78]. Therefore, the assessment of treatment effect with functional MR has a great potential utility in the differentiation of early response, with a possible impact in the prognosis of these patients (Figure 9).
Functional imaging of the mediastinum enhances an accurate assessment of mediastinal masses. It allows to distinguish with accuracy benign from malignant lesions. DWI and DCE-MRI are functional techniques which can be used in clinical protocols, providing an alternative to 18FDG-PET/CT in the evaluation of mediastinal lesions. It allows a complete characterization of them in a one-stop-shop procedure, while avoiding the use of ionizing radiation. Functional techniques have a great potential impact in the staging of lung and esophageal cancers, increasing its precision, and in the therapy monitoring of mediastinal neoplasms. Quantitative functional MR-derived parameters provide unique information, which makes them authentic biomarkers with potential prognostic implications.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Battal B, Kumar J, Xiao EH, Yang L S-Editor: Yan JP L-Editor: A E-Editor: Wu YXJ
| 1. | Ackman JB. MR Imaging of Mediastinal Masses. Magn Reson Imaging Clin N Am. 2015;23:141-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Carter BW, Benveniste MF, Truong MT, Marom EM. State of the Art: MR Imaging of Thymoma. Magn Reson Imaging Clin N Am. 2015;23:165-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Carter BW, Betancourt SL, Benveniste MF. MR Imaging of Mediastinal Masses. Top Magn Reson Imaging. 2017;26:153-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Razek AA, Elmorsy A, Elshafey M, Elhadedy T, Hamza O. Assessment of mediastinal tumors with diffusion-weighted single-shot echo-planar MRI. J Magn Reson Imaging. 2009;30:535-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Yabuuchi H, Matsuo Y, Abe K, Baba S, Sunami S, Kamitani T, Yonezawa M, Yamasaki Y, Kawanami S, Nagao M, Okamoto T, Nakamura K, Yamamoto H, Sasaki M, Honda H. Anterior mediastinal solid tumours in adults: Characterisation using dynamic contrast-enhanced MRI, diffusion-weighted MRI, and FDG-PET/CT. Clin Radiol. 2015;70:1289-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Broncano J, Luna A, Sánchez-González J, Alvarez-Kindelan A, Bhalla S. Functional MR Imaging in Chest Malignancies. Magn Reson Imaging Clin N Am. 2016;24:135-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Ciliberto M, Kishida Y, Seki S, Yoshikawa T, Ohno Y. Update of MR Imaging for Evaluation of Lung Cancer. Radiol Clin North Am. 2018;56:437-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Bains LJ, Zweifel M, Thoeny HC. Therapy response with diffusion MRI: An update. Cancer Imaging. 2012;12:395-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 9. | Le Bihan D. Apparent diffusion coefficient and beyond: What diffusion MR imaging can tell us about tissue structure. Radiology. 2013;268:318-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 298] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 10. | Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2349] [Cited by in RCA: 2447] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 11. | Filippi M, Agosta F. Diffusion tensor imaging and functional MRI. Handb Clin Neurol. 2016;136:1065-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Huston JM, Field AS. Clinical applications of diffusion tensor imaging. Magn Reson Imaging Clin N Am. 2013;21:279-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Barrett T, Brechbiel M, Bernardo M, Choyke PL. MRI of tumor angiogenesis. J Magn Reson Imaging. 2007;26:235-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 197] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 14. | Buonaccorsi GA, Roberts C, Cheung S, Watson Y, O'Connor JP, Davies K, Jackson A, Jayson GC, Parker GJ. Comparison of the performance of tracer kinetic model-driven registration for dynamic contrast enhanced MRI using different models of contrast enhancement. Acad Radiol. 2006;13:1112-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Runge VM, Clanton JA, Herzer WA, Gibbs SJ, Price AC, Partain CL, James AE. Intravascular contrast agents suitable for magnetic resonance imaging. Radiology. 1984;153:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 41] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Rijpkema M, Kaanders JH, Joosten FB, van der Kogel AJ, Heerschap A. Method for quantitative mapping of dynamic MRI contrast agent uptake in human tumors. J Magn Reson Imaging. 2001;14:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 136] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Luna A, Pahwa S, Bonini C, Alcalá-Mata L, Wright KL, Gulani V. Multiparametric MR Imaging in Abdominal Malignancies. Magn Reson Imaging Clin N Am. 2016;24:157-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Vilanova JC, Baleato-Gonzalez S, Romero MJ, Carrascoso-Arranz J, Luna A. Assessment of Musculoskeletal Malignancies with Functional MR Imaging. Magn Reson Imaging Clin N Am. 2016;24:239-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Vaupel P, Mayer A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1540] [Cited by in RCA: 1691] [Article Influence: 93.9] [Reference Citation Analysis (0)] |
| 20. | Carter BW, Tomiyama N, Bhora FY, Rosado de Christenson ML, Nakajima J, Boiselle PM, Detterbeck FC, Marom EM. A modern definition of mediastinal compartments. J Thorac Oncol. 2014;9:S97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Nishino M, Ashiku SK, Kocher ON, Thurer RL, Boiselle PM, Hatabu H. The thymus: A comprehensive review. Radiographics. 2006;26:335-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | Priola AM, Priola SM, Ciccone G, Evangelista A, Cataldi A, Gned D, Pazè F, Ducco L, Moretti F, Brundu M, Veltri A. Differentiation of rebound and lymphoid thymic hyperplasia from anterior mediastinal tumors with dual-echo chemical-shift MR imaging in adulthood: Reliability of the chemical-shift ratio and signal intensity index. Radiology. 2015;274:238-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Inaoka T, Takahashi K, Mineta M, Yamada T, Shuke N, Okizaki A, Nagasawa K, Sugimori H, Aburano T. Thymic hyperplasia and thymus gland tumors: Differentiation with chemical shift MR imaging. Radiology. 2007;243:869-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 24. | Ackman JB, Mino-Kenudson M, Morse CR. Nonsuppressing normal thymus on chemical shift magnetic resonance imaging in a young woman. J Thorac Imaging. 2012;27:W196-W198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Ackman JB, Kovacina B, Carter BW, Wu CC, Sharma A, Shepard JA, Halpern EF. Sex difference in normal thymic appearance in adults 20-30 years of age. Radiology. 2013;268:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Falkson CB, Bezjak A, Darling G, Gregg R, Malthaner R, Maziak DE, Yu E, Smith CA, McNair S, Ung YC, Evans WK; Lung Cancer Disease Site Group of Cancer Care Ontario's Program in Evidence-Based Care. The management of thymoma: A systematic review and practice guideline. J Thorac Oncol. 2009;4:911-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 189] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 27. | Carter BW, Benveniste MF, Madan R, Godoy MC, de Groot PM, Truong MT, Rosado-de-Christenson ML, Marom EM. ITMIG Classification of Mediastinal Compartments and Multidisciplinary Approach to Mediastinal Masses. Radiographics. 2017;37:413-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 28. | Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol. 2010;5:S260-S265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 396] [Cited by in RCA: 411] [Article Influence: 27.4] [Reference Citation Analysis (1)] |
| 29. | Levy Y, Afek A, Sherer Y, Bar-Dayan Y, Shibi R, Kopolovic J, Shoenfeld Y. Malignant thymoma associated with autoimmune diseases: A retrospective study and review of the literature. Semin Arthritis Rheum. 1998;28:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Sakai S, Murayama S, Soeda H, Matsuo Y, Ono M, Masuda K. Differential diagnosis between thymoma and non-thymoma by dynamic MR imaging. Acta Radiol. 2002;43:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 31. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after "carcinoid": Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3246] [Article Influence: 190.9] [Reference Citation Analysis (0)] |
| 32. | Gaur P, Leary C, Yao JC. Thymic neuroendocrine tumors: A SEER database analysis of 160 patients. Ann Surg. 2010;251:1117-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 33. | Gibril F, Chen YJ, Schrump DS, Vortmeyer A, Zhuang Z, Lubensky IA, Reynolds JC, Louie A, Entsuah LK, Huang K, Asgharian B, Jensen RT. Prospective study of thymic carcinoids in patients with multiple endocrine neoplasia type 1. J Clin Endocrinol Metab. 2003;88:1066-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 34. | Nasseri F, Eftekhari F. Clinical and radiologic review of the normal and abnormal thymus: Pearls and pitfalls. Radiographics. 2010;30:413-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 35. | Nishino M, Ashiku SK, Kocher ON, Thurer RL, Boiselle PM, Hatabu H. The Thymus: A Comprehensive Review-Erratum. Radiographics. 2017;37:1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Ried M, Marx A, Götz A, Hamer O, Schalke B, Hofmann HS. State of the art: Diagnostic tools and innovative therapies for treatment of advanced thymoma and thymic carcinoma. Eur J Cardiothorac Surg. 2016;49:1545-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 37. | Duwe BV, Sterman DH, Musani AI. Tumors of the mediastinum. Chest. 2005;128:2893-2909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 282] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 38. | Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6112] [Cited by in RCA: 5989] [Article Influence: 332.7] [Reference Citation Analysis (0)] |
| 39. | Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3032] [Cited by in RCA: 3123] [Article Influence: 223.1] [Reference Citation Analysis (0)] |
| 40. | Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, Schwartz LH, Zucca E, Fisher RI, Trotman J, Hoekstra OS, Hicks RJ, O'Doherty MJ, Hustinx R, Biggi A, Cheson BD. Role of imaging in the staging and response assessment of lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32:3048-3058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 961] [Cited by in RCA: 1151] [Article Influence: 115.1] [Reference Citation Analysis (0)] |
| 41. | Kulkarni NM, Pinho DF, Narayanan S, Kambadakone AR, Abramson JS, Sahani DV. Imaging for Oncologic Response Assessment in Lymphoma. AJR Am J Roentgenol. 2017;208:18-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Younes A, Hilden P, Coiffier B, Hagenbeek A, Salles G, Wilson W, Seymour JF, Kelly K, Gribben J, Pfreunschuh M, Morschhauser F, Schoder H, Zelenetz AD, Rademaker J, Advani R, Valente N, Fortpied C, Witzig TE, Sehn LH, Engert A, Fisher RI, Zinzani PL, Federico M, Hutchings M, Bollard C, Trneny M, Elsayed YA, Tobinai K, Abramson JS, Fowler N, Goy A, Smith M, Ansell S, Kuruvilla J, Dreyling M, Thieblemont C, Little RF, Aurer I, Van Oers MHJ, Takeshita K, Gopal A, Rule S, de Vos S, Kloos I, Kaminski MS, Meignan M, Schwartz LH, Leonard JP, Schuster SJ, Seshan VE. International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017). Ann Oncol. 2017;28:1436-1447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 242] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 43. | Koşucu P, Tekinbaş C, Erol M, Sari A, Kavgaci H, Oztuna F, Ersöz S. Mediastinal lymph nodes: Assessment with diffusion-weighted MR imaging. J Magn Reson Imaging. 2009;30:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Mosavi F, Wassberg C, Selling J, Molin D, Ahlström H. Whole-body diffusion-weighted MRI and (18)F-FDG PET/CT can discriminate between different lymphoma subtypes. Clin Radiol. 2015;70:1229-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Rosado-de-Christenson ML, Templeton PA, Moran CA. From the archives of the AFIP. Mediastinal germ cell tumors: Radiologic and pathologic correlation. Radiographics. 1992;12:1013-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 81] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Molinari F, Bankier AA, Eisenberg RL. Fat-containing lesions in adult thoracic imaging. AJR Am J Roentgenol. 2011;197:W795-W813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 47. | Carter BW, Okumura M, Detterbeck FC, Marom EM. Approaching the patient with an anterior mediastinal mass: A guide for radiologists. J Thorac Oncol. 2014;9:S110-S118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 48. | Strollo DC, Rosado-de-Christenson ML. Primary mediastinal malignant germ cell neoplasms: Imaging features. Chest Surg Clin N Am. 2002;12:645-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Lemarié E, Assouline PS, Diot P, Regnard JF, Levasseur P, Droz JP, Ruffié P. Primary mediastinal germ cell tumors. Results of a French retrospective study. Chest. 1992;102:1477-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Economou JS, Trump DL, Holmes EC, Eggleston JE. Management of primary germ cell tumors of the mediastinum. J Thorac Cardiovasc Surg. 1982;83:643-649. [PubMed] |
| 51. | Kesler KA, Rieger KM, Ganjoo KN, Sharma M, Fineberg NS, Einhorn LH, Brown JW. Primary mediastinal nonseminomatous germ cell tumors: The influence of postchemotherapy pathology on long-term survival after surgery. J Thorac Cardiovasc Surg. 1999;118:692-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 52. | Wright CD, Kesler KA, Nichols CR, Mahomed Y, Einhorn LH, Miller ME, Brown JW. Primary mediastinal nonseminomatous germ cell tumors. Results of a multimodality approach. J Thorac Cardiovasc Surg. 1990;99:210-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Lee KS, Im JG, Han CH, Han MC, Kim CW, Kim WS. Malignant primary germ cell tumors of the mediastinum: CT features. AJR Am J Roentgenol. 1989;153:947-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Shi HF, Feng Q, Qiang JW, Li RK, Wang L, Yu JP. Utility of diffusion-weighted imaging in differentiating malignant from benign thyroid nodules with magnetic resonance imaging and pathologic correlation. J Comput Assist Tomogr. 2013;37:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Schob S, Voigt P, Bure L, Meyer HJ, Wickenhauser C, Behrmann C, Höhn A, Kachel P, Dralle H, Hoffmann KT, Surov A. Diffusion-Weighted Imaging Using a Readout-Segmented, Multishot EPI Sequence at 3 T Distinguishes between Morphologically Differentiated and Undifferentiated Subtypes of Thyroid Carcinoma-A Preliminary Study. Transl Oncol. 2016;9:403-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | Juanpere S, Cañete N, Ortuño P, Martínez S, Sanchez G, Bernado L. A diagnostic approach to the mediastinal masses. Insights Imaging. 2013;4:29-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 57. | Kukar M, Platz TA, Schaffner TJ, Elmarzouky R, Groman A, Kumar S, Abdelhalim A, Cance WG. The use of modified four-dimensional computed tomography in patients with primary hyperparathyroidism: An argument for the abandonment of routine sestamibi single-positron emission computed tomography (SPECT). Ann Surg Oncol. 2015;22:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 58. | Rodgers SE, Hunter GJ, Hamberg LM, Schellingerhout D, Doherty DB, Ayers GD, Shapiro SE, Edeiken BS, Truong MT, Evans DB, Lee JE, Perrier ND. Improved preoperative planning for directed parathyroidectomy with 4-dimensional computed tomography. Surgery. 2006;140:932-40; discussion 940-1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 277] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 59. | Matoba M, Tonami H, Kondou T, Yokota H, Higashi K, Toga H, Sakuma T. Lung carcinoma: Diffusion-weighted mr imaging--preliminary evaluation with apparent diffusion coefficient. Radiology. 2007;243:570-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 60. | Koyama H, Ohno Y, Seki S, Nishio M, Yoshikawa T, Matsumoto S, Maniwa Y, Itoh T, Nishimura Y, Sugimura K. Value of diffusion-weighted MR imaging using various parameters for assessment and characterization of solitary pulmonary nodules. Eur J Radiol. 2015;84:509-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 61. | Gümüştaş S, Inan N, Akansel G, Ciftçi E, Demirci A, Ozkara SK. Differentiation of malignant and benign lung lesions with diffusion-weighted MR imaging. Radiol Oncol. 2012;46:106-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 62. | Liu H, Liu Y, Yu T, Ye N. Usefulness of diffusion-weighted MR imaging in the evaluation of pulmonary lesions. Eur Radiol. 2010;20:807-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 63. | Coolen J, Vansteenkiste J, De Keyzer F, Decaluwé H, De Wever W, Deroose C, Dooms C, Verbeken E, De Leyn P, Vandecaveye V, Van Raemdonck D, Nackaerts K, Dymarkowski S, Verschakelen J. Characterisation of solitary pulmonary lesions combining visual perfusion and quantitative diffusion MR imaging. Eur Radiol. 2014;24:531-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Schaefer JF, Vollmar J, Schick F, Vonthein R, Seemann MD, Aebert H, Dierkesmann R, Friedel G, Claussen CD. Solitary pulmonary nodules: Dynamic contrast-enhanced MR imaging--perfusion differences in malignant and benign lesions. Radiology. 2004;232:544-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 65. | Donmez FY, Yekeler E, Saeidi V, Tunaci A, Tunaci M, Acunas G. Dynamic contrast enhancement patterns of solitary pulmonary nodules on 3D gradient-recalled echo MRI. AJR Am J Roentgenol. 2007;189:1380-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Kono R, Fujimoto K, Terasaki H, Müller NL, Kato S, Sadohara J, Hayabuchi N, Takamori S. Dynamic MRI of solitary pulmonary nodules: Comparison of enhancement patterns of malignant and benign small peripheral lung lesions. AJR Am J Roentgenol. 2007;188:26-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 67. | Chhabra A, Thakkar RS, Andreisek G, Chalian M, Belzberg AJ, Blakeley J, Hoke A, Thawait GK, Eng J, Carrino JA. Anatomic MR imaging and functional diffusion tensor imaging of peripheral nerve tumors and tumorlike conditions. AJNR Am J Neuroradiol. 2013;34:802-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 68. | Nomori H, Mori T, Ikeda K, Kawanaka K, Shiraishi S, Katahira K, Yamashita Y. Diffusion-weighted magnetic resonance imaging can be used in place of positron emission tomography for N staging of non-small cell lung cancer with fewer false-positive results. J Thorac Cardiovasc Surg. 2008;135:816-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 69. | van Rossum PS, van Lier AL, Lips IM, Meijer GJ, Reerink O, van Vulpen M, Lam MG, van Hillegersberg R, Ruurda JP. Imaging of oesophageal cancer with FDG-PET/CT and MRI. Clin Radiol. 2015;70:81-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 70. | Sakurada A, Takahara T, Kwee TC, Yamashita T, Nasu S, Horie T, Van Cauteren M, Imai Y. Diagnostic performance of diffusion-weighted magnetic resonance imaging in esophageal cancer. Eur Radiol. 2009;19:1461-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 71. | Takenaka D, Ohno Y, Matsumoto K, Aoyama N, Onishi Y, Koyama H, Nogami M, Yoshikawa T, Matsumoto S, Sugimura K. Detection of bone metastases in non-small cell lung cancer patients: Comparison of whole-body diffusion-weighted imaging (DWI), whole-body MR imaging without and with DWI, whole-body FDG-PET/CT, and bone scintigraphy. J Magn Reson Imaging. 2009;30:298-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 72. | Ohno Y, Koyama H, Onishi Y, Takenaka D, Nogami M, Yoshikawa T, Matsumoto S, Kotani Y, Sugimura K. Non-small cell lung cancer: Whole-body MR examination for M-stage assessment--utility for whole-body diffusion-weighted imaging compared with integrated FDG PET/CT. Radiology. 2008;248:643-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 73. | Yi CA, Shin KM, Lee KS, Kim BT, Kim H, Kwon OJ, Choi JY, Chung MJ. Non-small cell lung cancer staging: Efficacy comparison of integrated PET/CT versus 3.0-T whole-body MR imaging. Radiology. 2008;248:632-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 74. | Yabuuchi H, Hatakenaka M, Takayama K, Matsuo Y, Sunami S, Kamitani T, Jinnouchi M, Sakai S, Nakanishi Y, Honda H. Non-small cell lung cancer: Detection of early response to chemotherapy by using contrast-enhanced dynamic and diffusion-weighted MR imaging. Radiology. 2011;261:598-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 75. | Ohno Y, Koyama H, Yoshikawa T, Matsumoto K, Aoyama N, Onishi Y, Sugimura K. Diffusion-weighted MRI versus 18F-FDG PET/CT: Performance as predictors of tumor treatment response and patient survival in patients with non-small cell lung cancer receiving chemoradiotherapy. AJR Am J Roentgenol. 2012;198:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 76. | van Rossum PS, van Lier AL, van Vulpen M, Reerink O, Lagendijk JJ, Lin SH, van Hillegersberg R, Ruurda JP, Meijer GJ, Lips IM. Diffusion-weighted magnetic resonance imaging for the prediction of pathologic response to neoadjuvant chemoradiotherapy in esophageal cancer. Radiother Oncol. 2015;115:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 77. | Wang L, Liu L, Han C, Liu S, Tian H, Li Z, Ren X, Shi G, Wang Q, Wang G. The diffusion-weighted magnetic resonance imaging (DWI) predicts the early response of esophageal squamous cell carcinoma to concurrent chemoradiotherapy. Radiother Oncol. 2016;121:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 78. | Giganti F, Salerno A, Ambrosi A, Chiari D, Orsenigo E, Esposito A, Albarello L, Mazza E, Staudacher C, Del Maschio A, De Cobelli F. Prognostic utility of diffusion-weighted MRI in oesophageal cancer: Is apparent diffusion coefficient a potential marker of tumour aggressiveness? Radiol Med. 2016;121:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |