Published online Feb 26, 2017. doi: 10.4330/wjc.v9.i2.92
Peer-review started: July 16, 2016
First decision: September 2, 2016
Revised: October 27, 2016
Accepted: December 1, 2016
Article in press: December 3, 2016
Published online: February 26, 2017
Processing time: 223 Days and 17.4 Hours
Coronary artery disease (CAD) is a leading cause of death and disability worldwide. Cardiovascular magnetic resonance (CMR) is established in clinical practice guidelines with a growing evidence base supporting its use to aid the diagnosis and management of patients with suspected or established CAD. CMR is a multi-parametric imaging modality that yields high spatial resolution images that can be acquired in any plane for the assessment of global and regional cardiac function, myocardial perfusion and viability, tissue characterisation and coronary artery anatomy, all within a single study protocol and without exposure to ionising radiation. Advances in technology and acquisition techniques continue to progress the utility of CMR across a wide spectrum of cardiovascular disease, and the publication of large scale clinical trials continues to strengthen the role of CMR in daily cardiology practice. This article aims to review current practice and explore the future directions of multi-parametric CMR imaging in the investigation of stable CAD.
Core tip: Coronary artery disease (CAD) is a leading cause of death worldwide. Cardiovascular magnetic resonance (CMR) is established in clinical practice guidelines with a growing evidence base supporting its use to aid diagnosis and management of patients with suspected or established CAD. CMR is a multi-parametric imaging modality that yields high spatial resolution images that can be acquired in any plane for assessment of global and regional cardiac function, myocardial perfusion and viability, tissue characterisation and coronary artery anatomy, all within a single study protocol and without exposure to ionising radiation.
- Citation: Foley JRJ, Plein S, Greenwood JP. Assessment of stable coronary artery disease by cardiovascular magnetic resonance imaging: Current and emerging techniques. World J Cardiol 2017; 9(2): 92-108
- URL: https://www.wjgnet.com/1949-8462/full/v9/i2/92.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i2.92
Coronary artery disease (CAD) is a leading cause of death and disability worldwide[1]. Despite major advances in the treatment of CAD resulting in significantly decreased mortality rates, CAD remains the single most common cause of death in the European Union, leading to 19% of deaths in men and 20% of deaths in women[2]; in the United States, CAD causes 1 in every 7 deaths, accounting for 370213 deaths in 2013[3]. The economic health burden of CAD is substantial with an estimated cost of CAD management at €60 billion in the European Union[4], and $182 billion in the United States[3]. Cardiovascular medicine benefits from a myriad of diagnostic methods that can guide intervention and clinical decision-making. Invasive coronary X-ray angiography delineates coronary anatomy in patients presenting with stable chest pain, however there is a low yield of obstructive CAD in those referred, and there are associated risks, albeit low, from major complications and ionising radiation[5]. Furthermore unless measurement of fractional flow reserve (FFR) is performed, routine coronary angiography does not give information on ischaemia burden, which according to current guidelines, is required to guide revascularisation decisions. Non-invasive functional imaging modalities such as single-photon emission computed tomography (SPECT), dobutamine stress echocardiography (DSE), cardiovascular magnetic resonance (CMR) or positron emission tomography (PET) are used to diagnose CAD, guide clinical decision making and confer prognostic information and consequently are well established for these roles in clinical practice guidelines[6,7].
CMR is a unique multi-parametric imaging modality producing high spatial resolution images that can be acquired in any plane for the assessment of global and regional cardiac function, myocardial perfusion and viability, tissue characterisation and proximal coronary artery anatomy, all within a single study and without exposure to ionising radiation (Figure 1). Historically, long scanning times, limited scanner availability and narrow bore sizes restricted the use of CMR, but these issues have been largely resolved, such that CMR has become a first line investigation for suspected stable angina in many centres in the United Kingdom and Europe. Consequently CMR is part of international clinical practice guidelines for the assessment of known and unknown stable CAD and for the identification of those who may benefit from revascularisation[6-9]. This review aims to focus on the current utility of CMR for the diagnosis of suspected stable CAD and potential future developments and applications of CMR in this role.
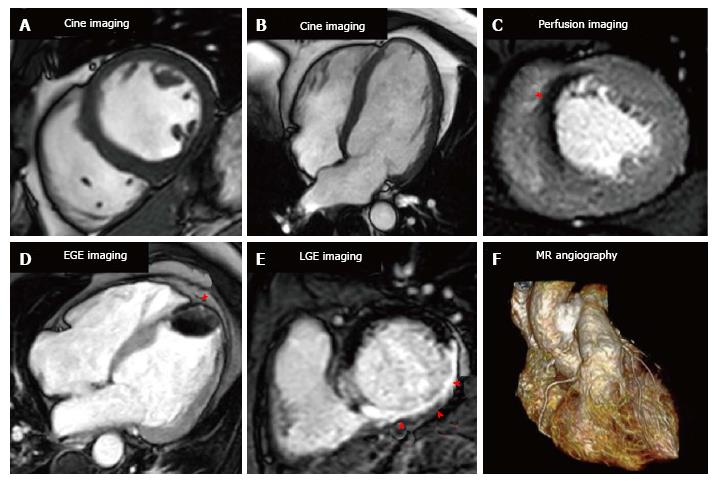
A CMR protocol for the investigation of stable CAD will typically take between 30-60 min and involves the acquisition of cine images in multiple planes for the assessment of left ventricular function and volumes, stress and rest myocardial perfusion imaging and late gadolinium enhancement (LGE) imaging for the assessment of myocardial viability and scar quantification (Figure 2).
CMR is the reference standard non-invasive technique for the measurement of left ventricular (LV) and right ventricular (RV) volumes, and ejection fraction, with high intra- and inter-observer reproducibility[10,11]. Steady state free precession cine imaging is typically performed for the assessment of LV function to enable visual assessment of global and regional myocardial function in a similar manner to echocardiography; however there are no limitations due to poor acoustic windows or large body habitus degrading image quality. CMR volumetric analysis is performed by acquiring a stack of contiguous breath held cine images from the base of the heart to the apex; the endocardial and epicardial borders are subsequently contoured giving mass, volumes and function. Thus CMR provides a true 3D analysis of LV and RV function unlike 2D echocardiography that relies on geometric assumptions for volumetric calculations. Furthermore specific myocardial tagging pulse sequences can be performed that enable more detailed assessment of intra-myocardial mechanics beyond ejection fraction, including torsion, twist, strain and strain rates[12]. Additionally, feature tracking is a novel post-processing method of quantitatively assessing strain and strain rate using standard cine images without having to acquire further imaging sequences as is the case with standard CMR tissue tagging[12,13].
Ischaemia detection by CMR is performed using either vasodilator or inotropic stress. Ischaemia detection by CMR is recommended as a first line strategy for investigating suspected angina in patients with an intermediate pre-test likelihood of CAD in both the current European Society of Cardiology (ESC) and National Institute for Health and Care Excellence (NICE) guidelines (Table 1)[6,14], whilst the United States guidelines are more conservative and give a grade IIa recommendation for stress perfusion CMR in patients with uninterpretable ECGs or unable to exercise[7].
| ESC guidelines | |
| Suspected/stable coronary artery disease[6] | |
| In patients with suspected stable coronary artery disease and pretest probability of 15%-85% stress imaging is preferred as the initial test option if local expertise and availability permit | Class I |
| An imaging stress test is recommended in patients with resting ECG abnormalities, which prevent accurate interpretation of ECG changes during stress | Class I |
| CMR should be considered in symptomatic patients with prior revascularisation (PCI or CABG) | Class IIa |
| Risk stratification is recommended based on clinical assessment and the results of the stress test initially employed for making a diagnosis of stable coronary artery disease | Class I |
| CMR is recommended in the presence of recurrent or new symptoms once instability has been ruled out | Class I |
| In symptomatic patients with revascularised stable coronary artery disease, CMR is indicated rather than stress ECG | Class I |
| CMR is recommended for risk stratification in patients with known stable coronary artery disease and a deterioration in symptoms if the site and extent of ischemia would influence clinical decision making Recommendations for imaging to determine ischemia to plan revascularisation[6,144] | Class I |
| An imaging stress test should be considered to assess the functional severity of intermediate lesions on coronary arteriography | Class IIa |
| To achieve a prognostic benefit by revascularisation in patients with coronary artery disease, ischemia has to be documented by non-invasive imaging | Class I |
| Following MI with multivessel disease, or in whom revascularisation of other vessels is considered, CMR for ischaemia and viability is indicated before or after discharge | Class I |
| Heart failure[8] | |
| CMR should be considered in patients with HF thought to have CAD, and who are considered suitable for coronary revascularization, to determine whether there is reversible myocardial ischaemia and viable myocardium | Class IIa |
| AHA guidelines | |
| Diagnosis and management of stable coronary artery disease[7] | |
| CMR can be used for patients with an intermediate (10%-90%) to high (> 90%) pretest probability of obstructive IHD who have an uninterpretable ECG and at least moderate physical functioning or no disabling comorbidity | Class IIa |
| CMR is reasonable for patients with an intermediate to high pretest probability of IHD who are incapable of at least moderate physical functioning or have disabling comorbidity | Class IIa |
| Pharmacological stress CMR is reasonable for risk assessment in patients with SIHD who are unable to exercise to an adequate workload regardless of interpretability of ECG | Class IIa |
| CMR is reasonable in patients with known SIHD who have new or worsening symptoms (not unstable) and who are incapable of at least moderate physical functioning or have disabling comorbidity | Class IIa |
Stress perfusion CMR requires the induction of hyperaemia by a vasodilating agent, and then observation of the passage of a gadolinium based contrast agent (GBCA) through the myocardium to identify perfusion defects. Typically the vasodilating agent used is adenosine though regadenason and less commonly dipyridamole and nicorandil are also used. Adenosine produces vasodilatation in most vascular beds, including the coronary circulation, via A2A and A2B receptors[15]. Adenosine is given as an intravenous infusion typically at a rate of 140 mcg/kg per minute, though this can be increased if there is no haemodynamic response; the main side effects of adenosine are transient heart block, and bronchospasm can be caused in those with reversible airways disease[15]. Regadenason is a new selective A2A adenosine receptor agonist that is given via an intravenous bolus, has less respiratory side effects than adenosine, and has recently been approved by both the FDA and in Europe for this indication[16,17]. The coronary micro-vasculature can dilate up to 4 or 5 times from the resting state to ensure adequate tissue perfusion for example during exercise. However the microvasculature distal to a stenosed coronary artery is already near-maximally vasodilated at rest and consequently when hyperaemia is provoked a coronary steal effect is caused. GBCAs increase the signal intensity in T1 weighted images and the passage of GBCAs through the myocardium causes healthy myocardium to become brighter while regions of hypoperfusion (“ischaemia”) remain dark (Figure 3).
The diagnostic accuracy of stress perfusion CMR for the detection of CAD is well validated. A meta-analysis of 37 studies demonstrated a combined sensitivity of 89% (95%CI: 88%-91%) and specificity of 76% (95%CI: 73%-78%) for perfusion CMR for the diagnosis of CAD[18]. The CE-MARC study (n = 752), the largest prospective randomised single-centre trial of CMR in this context showed superiority of perfusion CMR over SPECT, with a higher sensitivity (87% vs 67%, P < 0.0001) and negative predictive value (91% vs 79%, P < 0.0001) but similar specificity (83% vs 83% P = 0.916) and positive predictive values (77% vs 71%, P = 0.061)[19,20]. Furthermore in a pre-specified gender sub analysis of CE-MARC, CMR showed similar sensitivity for CAD detection in both males and females, whilst SPECT had significantly lower sensitivity in females compared to males[21].
The multi-centre, multi-vendor MR-IMPACT II trial (n = 515) also confirmed CMR’s superior sensitivity compared to SPECT (67% vs 59%, P = 0.024) but with a lower specificity (61% vs 72%, P = 0.038)[22]; however unlike CE-MARC only the stress/rest perfusion component of the CMR protocol was analysed. CE-MARC included analysis of LGE for scar detection, cine imaging for regional ventricular function and magnetic resonance angiography (MRA) for coronary artery anatomy, and a subsequent sub-analysis of CE-MARC demonstrated the additive diagnostic accuracy of the summation of these components of the multi-parametric protocol[23].
Stress perfusion CMR has also been validated against FFR in a recent meta-analysis with a pooled sensitivity and specificity of 0.90 (95%CI: 0.86-0.93) and 0.87 (95%CI: 0.82-0.90) at the patient level and 0.89 (95%CI: 0.83-0.92) and 0.86 (95%CI: 0.77-0.92) at the coronary artery and territory levels[24]. Furthermore CMR stress perfusion had comparable sensitivity and specificity to cardiac CT and PET in a recent meta-analysis of non-invasive imaging modalities, and was superior to both SPECT and DSE when using FFR as the reference standard[25]. Most trials thus far have excluded patients with arrhythmia amid concerns regarding ECG gating, however the diagnostic accuracy of stress perfusion CMR remains high in suspected CAD patients with AF or frequent ectopy (sensitivity 80%, specificity 74%)[26].
Although 1.5T is remains the standard field strength used in clinical CMR, imaging at a higher field strength of 3.0T offers increased signal to noise and contrast to noise ratios thereby giving improved spatial and temporal enhancement[27]. Consequently the diagnostic accuracy of perfusion imaging at 3.0T may be improved, and in a small direct comparison of CMR perfusion at 1.5T, 3.0T (n = 61) showed greater diagnostic accuracy in both single vessel (AUC: 0.89 vs 0.70; P < 0.05) and multi-vessel disease (AUC: 0.95 vs 0.82, P < 0.05)[28]. Furthermore, 3.0T has been compared to 1.5T using FFR as reference standard, corroborating it’s superior diagnostic accuracy[29,30]. The higher 3.0T field strength does however pose challenges with greater field inhomogeneity, susceptibility artefacts and higher local energy deposition. Also, many implants deemed “MR compatible” at 1.5T cannot be scanned at 3.0T[31]. These issues are however being overcome with improved technology and the use of multi-transmit radiofrequency CMR techniques improving field homogeneity[32].
Currently typical CMR perfusion imaging acquires 3 short axis slices of the left ventricle with an in-plane spatial resolution of 2-3 mm. Developments in CMR technology however now allow faster scan speeds; these novel acquisition techniques allow accelerated data acquisition based on spatio-temporal undersampling (k-t SENSE or k-t BLAST and highly constrained back projection HYPR, compressed sensing and others)[33]. These faster data acquisition techniques have been applied to achieve in-plane spatial resolution < 2 mm or full-coverage of the LV using 3D whole-heart perfusion imaging. High spatial-resolution imaging offers benefits by significantly reducing dark rim artefacts, as these are directly proportional to voxel size[34]. Moreover there is improved ability to detect sub-endocardial ischaemia which is critical in multi-vessel disease where there is a lack of reference healthy myocardium for comparison[35,36]. High spatial-resolution perfusion CMR has been validated at both 1.5T and 3.0T against quantitative coronary angiography (QCA) with improved diagnostic accuracy at both field strengths compared to standard resolution perfusion imaging[27,36,37]. Furthermore validation against FFR gave sensitivity and specificity to detect stenoses at a threshold of FFR < 0.75 of 0.82 and 0.94 (P < 0.0001) respectively, and an area under the curve of 0.92 (P < 0.0001)[38].
Conventional stress perfusion CMR is typically acquired in 3 short-axis slices, and thus unlike SPECT does not truly calculate global ischaemia burden. Accelerated acquisition techniques can also be employed to achieve full LV coverage using a 3D whole-heart single shot acquisition. Such 3D acquisitions can overcome the assumptions made about “missing” myocardium between the slices from conventional 2D multi-slice perfusion imaging. Two studies have validated the feasibility and diagnostic accuracy of 3D stress perfusion CMR against FFR; at 1.5T 3D perfusion demonstrated a sensitivity, specificity and diagnostic accuracy of 90%, 82% and 87% respectively and 91%, 90% and 91% respectively at 3.0T[39,40]. Furthermore in a recent multicentre trial of 3D stress perfusion at 3.0T, sensitivity and specificity were 84.7% and 90.8% relative to the FFR reference[41]. The main motivation for 3D perfusion is to give a more accurate quantification of total myocardial ischaemia burden; evidence from SPECT suggests a prognostic benefit for revascularisation in those with an ischaemia burden > 10%, with an ischaemia burden of 10% conferring a risk of approximately 5% for death or MI per year[42,43]. Ischaemia burden as measured by 3D stress perfusion CMR has been compared to SPECT and showed good correlation (rs = 0.70, P < 0.001)[44]. Intriguingly a recent pilot study compared ischaemia burden by high-resolution perfusion (using 3 short axis slices) and 3D perfusion imaging (providing whole heart coverage) suggesting that there was also a good correlation between the techniques (r = 0.72; P = 0.001), and that therefore the two methods are potentially interchangeable[45].
CMR stress perfusion studies are normally reported in a qualitative manner; however this can prove challenging in diffuse or multi-vessel disease where there is no healthy reference myocardium to use as a visual comparator. These situations can introduce subjectivity into the analysis and consequently quantitative measurement techniques have been developed to provide an objective assessment of myocardial blood flow. A number of different methods of quantitative analysis have been assessed with the Fermi deconvolution method showing most accuracy when compared to microspheres in an explanted porcine model at 1.5T and mice at 3.0T[46,47], and when compared to SPECT and with QCA[48]. When compared to angiography with FFR, an MPR threshold of 1.58 detected a stenosis with an FFR < 0.75 with a sensitivity of 0.80, specificity of 0.89 (P < 0.0001), and area under the curve of 0.89 (P < 0.0001)[38]. Myocardial perfusion reserve derived from quantitative CMR perfusion has also shown good correlation to PET imaging, the imaging modality that is widely regarded as the reference standard non-invasive measure of myocardial blood flow[49,50]. Currently, time consuming post-processing has limited quantitative perfusion methods to a research tool, but automated methods are being developed that may potentially overcome this[51].
GBCAs have an excellent safety profile[52], but in patients with poor renal clearance (e.g., on dialysis) there is a risk of nephrogenic systemic fibrosis[53]. In those patients unable to have GBCAs inotropic stress CMR is an alternative. Inotropic stress CMR is typically performed with dobutamine in a similar manner to DSE with inducible regional wall motion abnormalities identified in territories supplied by a stenosed coronary artery at peak stress. Unlike DSE however, DSMR’s accuracy is not limited by body habitus or in those with poor acoustic windows and in a single centre study DSMR was shown to have significantly greater diagnostic performance to DSE in this context[54]. However echocardiography in this study was performed without harmonic imaging and contrast agents, so that the performance of DSE is likely to be underreported compared with contemporaneous methods. DSMR has a comparable safety profile to DSE with an event rate of 0.1% for sustained VT and 0.4% for non-sustained VT, and 1.6% for atrial fibrillation; patients thus require close monitoring during scanning and resuscitation equipment needs to be available[55]. DSMR has been shown to have high diagnostic accuracy for the detection of CAD with one meta-analysis of 14 trials showing a pooled sensitivity of 0.83 (95%CI: 0.79-0.88) and specificity of 0.86 (95%CI: 0.81-0.91)[56]; furthermore a single centre trial of DSMR vs perfusion CMR showed similar diagnostic accuracy[57]. First-pass perfusion can be performed additionally at peak dobutamine stress to provide incremental diagnostic accuracy[58], and can be a useful adjunct in challenging patient groups such as those with pre-existing wall motion abnormalities or dyssynchrony from left bundle branch block[59].
Exercise is commonly used rather than pharmacological agents as the stressor in echocardiography, and gives useful prognostic information such as workload in metabolic equivalent (METs) in addition to ischaemia testing[60,61]. CMR is limited in this respect due to the need for supine scanning and consistent positioning within the scanner. Recent studies however have assessed the feasibility of exercise stress CMR and showed comparable accuracy to echocardiography, though it has yet to reach mainstream clinical use[62,63]. Promising developments are “steppers” and cycle ergometers that can attach directly to the MRI scanner, and thereby eliminate the need to transfer the patient from the exercise equipment into the scanner[64,65].
Both perfusion CMR and DSMR provide excellent prognostic information, and this has recently been shown in two large meta-analyses. One meta-analysis of 14 studies including 12178 patients showed that a negative stress CMR was associated with a 1.03% annualised event rate, comparable to the normal population[66]. A further meta-analysis of 19 studies including 11636 patients showed a similar annualised event rate of 0.8% for a negative stress CMR over a mean follow up of 32 mo[67]. In a large prospective study of 1229 patients undergoing adenosine stress with a mean follow-up period of 4.2 ± 2.1 years, patients with reversible perfusion deficits had a 3-fold increased risk of major adverse cardiovascular events, with significantly more cardiac deaths (P < 0.0001) and nonfatal myocardial infarctions (P < 0.001)[68]. Similarly the data from DSMR mirrors the results of first-pass perfusion CMR with a negative study conferring an equally low annual event rate of 1.3%[66,69]. Recently the five-year outcome data from CE-MARC were published with prognostic data for both CMR and SPECT in the same patient population. The analysis showed that although an abnormal result from both tests was a strong indicator of future major adverse cardiovascular events (MACE), CMR was superior at predicting time to MACE in this population[70]. Furthermore CMR remained the only independent predictor of outcome after adjustment for major cardiovascular risk factors, stratification for initial patient treatment and coronary angiographic findings[70]. These findings likely reflect CMR’s overall greater diagnostic accuracy, combined with CMR’s higher spatial resolution enabling greater identification of subendocardial scar compared to SPECT[71]; a feature known to confer prognostic significance beyond ejection fraction, and clinical or angiographic features[72].
GBCAs have a large molecular weight and cannot penetrate an intact cell membrane; consequently GBCAs are constrained to the extracellular space. In healthy myocardium the extracellular space is limited and contrast enters and clears rapidly. The extracellular space in infarcted myocardium however is substantially increased compared to normal myocardium and is less vascular. Thus in chronic myocardial infarction scar tissue composed of a matrix of collagen fibres has significantly increased extracellular space, leading to GBCA accumulation (slow washout), whilst in acute infarction GBCAs passively diffuse across disrupted myocardial cell membranes and into the intracellular space (greater volume of distribution)[73]. Thus both acute and chronic myocardial infarction retain more GBCAs. Imaged with T1 sensitive acquisition methods, this results in a higher signal.
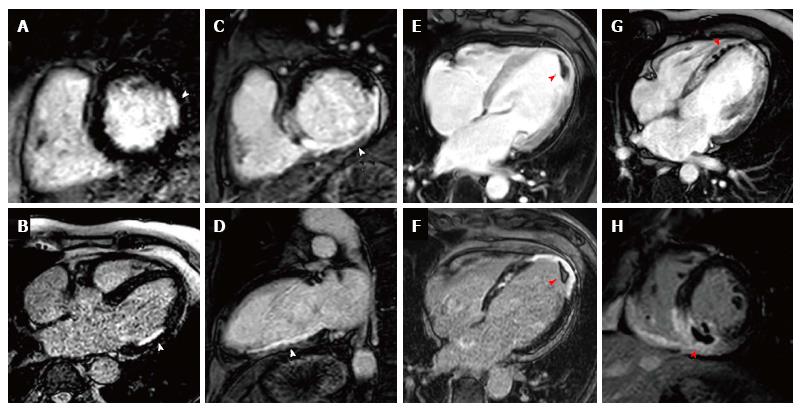
Early gadolinium enhancement imaging is performed immediately following contrast administration; this allows mainly the visualisation of ventricular thrombi that appear “dark/black” due to a lack of contrast uptake as they are non-vascular (Figure 4). CMR has been shown to be superior to both trans-thoracic echocardiography and trans-oesophageal echocardiography for the identification of ventricular thrombi[74,75]. LGE imaging is performed between 10-20 min after contrast administration, an appropriate inversion time is set to null the normal myocardium and the areas where gadolinium is retained enhances (Figure 4). Typically a stack of short axis slices, a 4-chamber view and VLA are acquired. Alternatively, 3D LGE CMR imaging enables whole heart quantification of scar burden to be acquired in a shorter time period (although with a reduction in image quality), which may provide an alternative for patients that struggle to breath-hold[76,77].
CMR viability assessment using LGE enables the accurate detection, and extent and trans-murality of previous myocardial infarction to be determined, and identifies regions with potential to recover function following revascularisation. Hibernating myocardium is dysfunctional myocardium that has been down-regulated through a process of chronic/repetitive ischaemia and which has the potential for functional recovery when blood flow is restored. LGE imaging detects replacement of normal viable myocytes by focal necrosis or fibrosis with high spatial resolution, and has excellent correlation to histopathology[73]. Furthermore the degree of transmural extent of hyper-enhancement on LGE imaging has a direct association to the potential for functional recovery following revascularisation; Kim et al[78] demonstrated that segments with less than 25% hyper-enhancement were most likely to attain functional recovery whilst segments with over 75% hyper-enhancement were unlikely to improve, notably this was irrespective of whether the region was initially hypokinetic, dyskinetic or akinetic. A meta-analysis of 331 patients using 50% trans-murality of hyper-enhancement reported a sensitivity of 95% (95%CI: 93%-97%) and specificity of 51% (40%-62%) for predicting functional recovery[79].
CMR viability assessment is not however limited to just LGE imaging; whilst LGE identifies the transmural extent of scarring, the use of low-dose dobutamine (LDD) identifies the contractile reserve. Myocardium is considered viable if there is a 2 mm or more increase in systolic wall thickening within a segment following administration of LDD (5-10 mcg/kg per minute)[80]. While scar burden on LGE has been shown to be most sensitive method for assessment for functional recovery compared to LDD and diastolic wall thickness[81], LDD CMR offers higher specificity and PPV for prediction of functional recovery (91% and 93%, respectively)[79]. Consequently a stepwise approach utilising LGE first followed by LDD if the trans-mural extent of LGE in the territory of the diseased coronary is between 1%-50% has been proposed[82]. Recently both tissue tagging and feature tracking have been used to give quantitative viability assessment with LDD and have been suggested as possible methods to reduce reliance on operator experience in what is currently a qualitative method of assessment[83-85].
LGE imaging has a grade A recommendation to determine myocardial viability prior to revascularisation in the ACCF/AHA/SCMR appropriate use guidelines[86], though viability assessment by LGE is currently not recommended for this indication in ESC or US practice guidelines for management of stable CAD or coronary revascularisation[6,7,9,87]. The utility of viability assessment has been questioned recently following the results of the STICH trial and the subsequently published viability sub-study that showed no mortality benefit from revascularisation following viability assessment[88,89]. This is contrary to prior observational data in large meta-analyses including over 3000 patients with viability; revascularisation was associated with 79.6% reduction in annual mortality (P < 0.0001) compared with medical treatment[90,91] and presence of dysfunctional viable myocardium by LGE-CMR without revascularisation is an independent predictor of mortality in patients with ischemic LV dysfunction[92]. Questions have been asked however whether the STICH sub-study results would have been different if CMR had been used rather than SPECT, and consequently in Europe the third highest indication for CMR remains the assessment of viability[93].
In addition to identifying viable myocardium, the presence and extent of LGE provides valuable prognostic information, and the extent of scar burden by LGE is readily quantified and reproducible on CMR[94]. Impairment of left ventricular ejection fraction is well recognized as an independent risk factor in those with coronary artery disease[8,95]; LGE can provide additive prognostication in these patients and a recent study of 1560 patients established that the presence of scar by LGE irrespective of LVEF identified those at risk of increased mortality[96]. Furthermore a meta-analysis showed that the presence of LGE increases the risk of death by 4.77% and MACE by 3.9% and that each gram of scar measured by LGE increased the hazards of death and MACE by 4% and 5%, respectively[97]. Additionally the identification of previously unrecognized MI by LGE confers a significantly increased risk of both mortality and MACE[72,98].
The extent of scar burden by LGE in patients with ischaemic heart disease has also been identified in a number of studies to be an independent predictor of ventricular arrhythmias in patients with internal cardiac defibrillators (ICD)[99-101], and a recent meta-analysis of 1105 patients with ICDs determined that the extent of LGE was predictive of ventricular arrhythmia whilst LVEF was not[102]. Additionally in a high risk cohort of patients with a mean LVEF of 35% being considered for ICD implantation, LGE demonstrated that significant scarring (> 5% LV) in patients with LVEF > 30%, conferred a risk similar to those with LVEF ≤ 30%[103]. Equally, in patients with LVEF ≤ 30%, minimal or no scar burden established a lower risk cohort similar to those with LVEF > 30%[103]. Other studies have identified the presence of a “grey zone” on LGE imaging, a heterogeneous region of viable and non-viable myocardium at the infarct periphery, as predictive of VT[104,105].
LGE and quantification of scar burden has also been used to predict responsiveness to cardiac resynchronization therapy (CRT)[106], and identification of scarring in the pacing region of the LV lead has been associated with non-response to device therapy[107,108]. In a similar method to imaging the coronary artery anatomy, coronary venous anatomy can be reliably demonstrated using GBCAs, which can potentially aid planning of device implantation[109]. The combination of coronary venous imaging, assessment of ventricular function and LGE may be a useful adjunct in the management of patients with ischaemic cardiomyopathy being considered for CRT, as well as risk stratifying those being considered for defibrillator therapy.
The economic burden of CAD is enormous with £6.8 billion spent in 2012 in the United Kingdom; in the United States over 15 million people have CAD costing the US economy $108.9 billion/year[110,111]. Cost effectiveness analyses help to inform optimal management pathways in order to maximise health care benefit within the constraints of limited resources. In the United States a low yield has been reported at diagnostic angiography with just over 40% of patients referred having obstructive CAD[5]. CMR can act as a potential gatekeeper to invasive coronary angiography in order to reduce downstream costs as well as reduce risk from unnecessary invasive assessments.
Health economic analyses based on the CE-MARC dataset identified that despite the higher initial cost of CMR to SPECT, the superior diagnostic accuracy of CMR lead to an overall greater cost effectiveness in models of the United Kingdom, German and Swiss healthcare systems[112-114]. A study of 1158 German patients being investigated for suspected CAD were randomised to either DSMR prior to angiography or direct to angiography; DSMR prior to invasive angiography led to a saving of 12466€ of hospital costs per life year, furthermore this cost saving was maintained through a median period of 7.9 years follow-up[115].
In a cost analysis comparing CMR and X-ray angiography vs angiography and FFR to determine the need for revascularisation, CMR and angiography was more cost-effective below a CAD prevalence of 62%, 65%, 83% and 82% for the Swiss, German, United Kingdom, and the United States health care systems, respectively[116]. These studies confirm that as well as the established high diagnostic accuracy, CMR is also a financially advantageous investigative strategy in patients with CAD.
Studies thus far have predominantly focused on the diagnostic accuracy of CMR; forthcoming multi-centre clinical effectiveness trials are however focused on evaluating clinical pathways to improve patient outcomes. The recently published CE-MARC 2 trial is a prospective, multi-centre, 3-arm parallel group, randomised controlled trial comparing multi-parametric CMR vs UK NICE CG95 guidance[14] vs AHA/ACCF SPECT appropriate-use criteria[117] to investigate patients with suspected CAD (pre-test likelihood 10%-90%) requiring further investigation[118,119]. The primary outcome measure was FFR defined unnecessary angiography (FFR > 0.8) with the important safety secondary outcome measure of MACE at 1 and 3 years. CE-MARC 2 showed overall that CMR guided care resulted in significantly reduced rates of unnecessary angiography at 12 mo compared to routine guideline directed care[119].
Contemporary registry data from the United States suggests roughly 12%-26% of elective PCI are deemed inappropriate with considerable variation in practice between sites[120,121]. Both FAME and DEFER showed improved outcomes using FFR guided revascularisation based on ischaemia detection, compared to reliance on visual assessment at angiography[122,123]. These trials would suggest that a better way of selecting patients prior to invasive revascularisation procedures is required. CMR offers a non-invasive ischaemia assessment and the MR-INFORM trial aims to establish if perfusion CMR could act as a non-invasive surrogate to FFR to determine the need for revascularisation in patients with stable CAD[124]. MR-INFORM is a multi-centre, non-inferiority study comparing adenosine perfusion CMR vs angiography with FFR measurement to guide revascularisation decisions in patients with stable angina and moderate to high probability of CAD; the primary endpoint is the occurrence of MACE at one year. The trial has completed recruitment and is expected to report in 2017.
The prognostic benefit of revascularisation in stable coronary artery disease is a topic of debate; both the COURAGE trial and BARI-2D failed to show any prognostic benefit of revascularisation over optimal medical therapy (OMT) in patients with stable CAD[125,126]. Determination of extent of ischaemia in both these 2 trials was however limited; in COURAGE only 33% of patients had moderate/severe ischaemia and moreover around 40% had < 5% ischaemia[127]. In both trials however those with a higher residual ischaemia burden had a worse prognosis[127-129]. The ISCHEMIA trial aims to test the hypothesis that a routine invasive strategy with early cardiac catheterisation and revascularisation plus OMT is superior to a conservative management strategy of OMT for patients with moderate or severe ischemia[42]. The trial aims to recruit over 8000 patients worldwide with ischaemia determined by non-invasive imaging (CMR, stress echocardiography, SPECT) with a primary endpoint of time to cardiovascular death or non-fatal myocardial infarction.
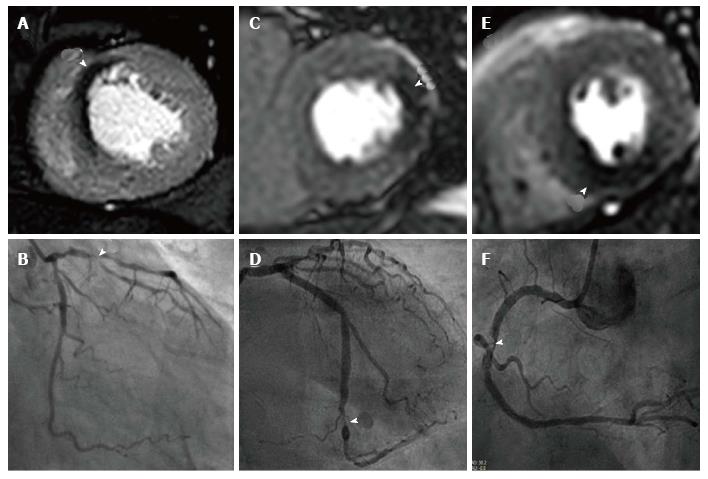
Coronary MRA (CMRA) allows the non-invasive anatomical assessment of coronary arteries; currently clinical indications are limited to the detection of aberrant origin of coronary arteries, coronary ectasia and/or aneurysms (class I indication) and evaluation of bypass grafts (class II indication)[130,131]. CMRA for diagnosis of CAD is not presently part of routine clinical practice. The initial multi-centre experience using CMRA in this context showed interpretable image quality in 84% of proximal and middle coronary artery segments, though with a specificity of 42%; CMRA did however exclude triple-vessel disease and left main coronary artery stenosis with a negative predictive value of 100%[132]. Progress in CMRA techniques have improved significantly however, and a recent multi-centre study showed that CMRA at 1.5T detects significant CAD with a sensitivity of 88% and specificity of 72% and a negative predictive value of 88%[133]. Furthermore one study showed in a direct comparison between CMRA and CT coronary angiography (CTCA) there was no significant difference between coronary imaging at 3.0T and 64-slice CTCA for the detection of CAD with a sensitivity of 87% vs 90% (P = 0.16) and specificity of 77% vs 83% (P = 0.06) respectively[134].
Currently CMRA techniques are time consuming and there are questions over the incremental diagnostic merit they provide in addition to established perfusion protocols; the CE-MARC study found no additional diagnostic benefit by including CMRA into a full multi-parametric protocol vs the perfusion/LV function/LGE combination (overall accuracy 84.6% vs 84.2% (P = 0.5316)[23]. Moreover there was no significant improvement in diagnostic accuracy when CMRA was added to perfusion imaging at 1.5T and compared to FFR as the reference standard[135].
Native T1, T1 mapping, and extra cellular volume fraction quantification are novel methods for CMR tissue characterisation. These techniques are currently research tools that have shown promise for diagnosis and prognostication in acute coronary syndromes and other rare disease processes (e.g., Amyloid and Fabry’s disease), presently however they do not have an established role in the diagnosis or management of stable ischaemic heart disease[136,137]. Post myocardial infarction however a role for these imaging “biomarkers” is being established in predicting both prognosis and adverse LV remodelling[138,139].
CMR uses the paramagnetic properties of deoxyhaemoglobin as an endogenous contrast agent; increasing deoxyhaemoglobin content leads to a reduction of signal intensity on T2 or T2* weighted images[140]. The magnitude of the BOLD effect depends on the static magnetic field strength, with an exponential increase at 3.0T from 1.5T; consequently most studies have used 3.0T. Thus far BOLD has shown good correlation with QCA and conventional CMR perfusion imaging, but studies are generally small and single centre, limiting its clinical validation[141,142].
Finally hyperpolarised CMR is making the transition from animal studies to human applications. Hyperpolarisation methods artificially increase the number of molecules in one orientation resulting in a significant increase in MR signal; combined with 13C enriched metabolic tracers enable real time imaging of in vivo substrate metabolism, coronary angiography and quantitative perfusion imaging[143]. The results of human hyperpolarisation studies are eagerly awaited.
Over the last decade the evidence base for the diagnostic accuracy of CMR for the investigation of stable coronary artery disease has been confirmed through the publication of large-scale clinical trials and meta-analyses, and CMR is now firmly established in clinical practice guidelines. CMR enables assessment of cardiac dimensions, function, ischaemia, scar burden and tissue viability in a single study without exposure to ionising radiation. CMR also offers prognostic information with a normal stress CMR associated with a < 1% risk of death or MI at 2 years, whilst the presence of LGE confers added prognostication above and beyond simple LV ejection fraction. New technical developments continue apace and ongoing large clinical trials will further clarify the role of CMR in routine clinical practice and guide the future development of international guidelines.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A, A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chello M, Falconi M, Satoh H S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
| 1. | World Health Organization. The top 10 causes of death [Internet]. [cited 2015 Nov 3]. Available from: http: //www.who.int/mediacentre/factsheets/fs310/en/. |
| 2. | Townsend N, Nichols M, Scarborough P, Rayner M. Cardiovascular disease in Europe--epidemiological update 2015. Eur Heart J. 2015;36:2696-2705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 394] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 3. | Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2843] [Cited by in RCA: 3816] [Article Influence: 381.6] [Reference Citation Analysis (0)] |
| 4. | Moschetti K, Petersen SE, Pilz G, Kwong RY, Wasserfallen JB, Lombardi M, Korosoglou G, Van Rossum AC, Bruder O, Mahrholdt H. Cost-minimization analysis of three decision strategies for cardiac revascularization: results of the “suspected CAD” cohort of the european cardiovascular magnetic resonance registry. J Cardiovasc Magn Reson. 2016;18:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1067] [Cited by in RCA: 1227] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 6. | Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949-3003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2772] [Cited by in RCA: 2989] [Article Influence: 249.1] [Reference Citation Analysis (0)] |
| 7. | Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44-e164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 1255] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 8. | McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3411] [Cited by in RCA: 3549] [Article Influence: 273.0] [Reference Citation Analysis (0)] |
| 9. | Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541-2619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3295] [Cited by in RCA: 3377] [Article Influence: 307.0] [Reference Citation Analysis (0)] |
| 10. | Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, Pennell DJ. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1033] [Cited by in RCA: 1074] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 11. | Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J. 2004;147:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 541] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 12. | Ibrahim el-SH. Myocardial tagging by cardiovascular magnetic resonance: evolution of techniques--pulse sequences, analysis algorithms, and applications. J Cardiovasc Magn Reson. 2011;13:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 187] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 13. | Claus P, Omar AM, Pedrizzetti G, Sengupta PP, Nagel E. Tissue Tracking Technology for Assessing Cardiac Mechanics: Principles, Normal Values, and Clinical Applications. JACC Cardiovasc Imaging. 2015;8:1444-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 336] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 14. | Skinner JS, Smeeth L, Kendall JM, Adams PC, Timmis A; Chest Pain Guideline Development Group. NICE guidance. Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin. Heart. 2010;96:974-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | Layland J, Carrick D, Lee M, Oldroyd K, Berry C. Adenosine: physiology, pharmacology, and clinical applications. JACC Cardiovasc Interv. 2014;7:581-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Al Jaroudi W, Iskandrian AE. Regadenoson: a new myocardial stress agent. J Am Coll Cardiol. 2009;54:1123-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 17. | Nguyen KL, Bandettini WP, Shanbhag S, Leung SW, Wilson JR, Arai AE. Safety and tolerability of regadenoson CMR. Eur Heart J Cardiovasc Imaging. 2014;15:753-760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Jaarsma C, Leiner T, Bekkers SC, Crijns HJ, Wildberger JE, Nagel E, Nelemans PJ, Schalla S. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2012;59:1719-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 336] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 19. | Greenwood JP, Maredia N, Radjenovic A, Brown JM, Nixon J, Farrin AJ, Dickinson C, Younger JF, Ridgway JP, Sculpher M. Clinical evaluation of magnetic resonance imaging in coronary heart disease: the CE-MARC study. Trials. 2009;10:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, Bijsterveld P, Ridgway JP, Radjenovic A, Dickinson CJ. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet. 2012;379:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 803] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 21. | Greenwood JP, Motwani M, Maredia N, Brown JM, Everett CC, Nixon J, Bijsterveld P, Dickinson CJ, Ball SG, Plein S. Comparison of cardiovascular magnetic resonance and single-photon emission computed tomography in women with suspected coronary artery disease from the Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease (CE-MARC) Trial. Circulation. 2014;129:1129-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 22. | Schwitter J, Wacker CM, Wilke N, Al-Saadi N, Sauer E, Huettle K, Schönberg SO, Luchner A, Strohm O, Ahlstrom H. MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. Eur Heart J. 2013;34:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 317] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 23. | Ripley DP, Motwani M, Brown JM, Nixon J, Everett CC, Bijsterveld P, Maredia N, Plein S, Greenwood JP. Individual component analysis of the multi-parametric cardiovascular magnetic resonance protocol in the CE-MARC trial. J Cardiovasc Magn Reson. 2015;17:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Li M, Zhou T, Yang LF, Peng ZH, Ding J, Sun G. Diagnostic accuracy of myocardial magnetic resonance perfusion to diagnose ischemic stenosis with fractional flow reserve as reference: systematic review and meta-analysis. JACC Cardiovasc Imaging. 2014;7:1098-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Takx RA, Blomberg BA, El Aidi H, Habets J, de Jong PA, Nagel E, Hoffmann U, Leiner T. Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ Cardiovasc Imaging. 2015;8:pii: e002666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 290] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 26. | Greulich S, Steubing H, Birkmeier S, Grün S, Bentz K, Sechtem U, Mahrholdt H. Impact of arrhythmia on diagnostic performance of adenosine stress CMR in patients with suspected or known coronary artery disease. J Cardiovasc Magn Reson. 2015;17:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Plein S, Schwitter J, Suerder D, Greenwood JP, Boesiger P, Kozerke S. k-Space and time sensitivity encoding-accelerated myocardial perfusion MR imaging at 3.0 T: comparison with 1.5 T. Radiology. 2008;249:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Cheng AS, Pegg TJ, Karamitsos TD, Searle N, Jerosch-Herold M, Choudhury RP, Banning AP, Neubauer S, Robson MD, Selvanayagam JB. Cardiovascular magnetic resonance perfusion imaging at 3-tesla for the detection of coronary artery disease: a comparison with 1.5-tesla. J Am Coll Cardiol. 2007;49:2440-2449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Bernhardt P, Walcher T, Rottbauer W, Wöhrle J. Quantification of myocardial perfusion reserve at 1.5 and 3.0 Tesla: a comparison to fractional flow reserve. Int J Cardiovasc Imaging. 2012;28:2049-2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Ebersberger U, Makowski MR, Schoepf UJ, Platz U, Schmidtler F, Rose J, Kessel A, Roth P, Antoni D, Schnackenburg B. Magnetic resonance myocardial perfusion imaging at 3.0 Tesla for the identification of myocardial ischaemia: comparison with coronary catheter angiography and fractional flow reserve measurements. Eur Heart J Cardiovasc Imaging. 2013;14:1174-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Bernstein MA, Huston J, Ward HA. Imaging artifacts at 3.0T. J Magn Reson Imaging. 2006;24:735-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 32. | Rajiah P, Bolen MA. Cardiovascular MR imaging at 3 T: opportunities, challenges, and solutions. Radiographics. 2014;34:1612-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Motwani M, Jogiya R, Kozerke S, Greenwood JP, Plein S. Advanced cardiovascular magnetic resonance myocardial perfusion imaging: high-spatial resolution versus 3-dimensional whole-heart coverage. Circ Cardiovasc Imaging. 2013;6:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Maredia N, Radjenovic A, Kozerke S, Larghat A, Greenwood JP, Plein S. Effect of improving spatial or temporal resolution on image quality and quantitative perfusion assessment with k-t SENSE acceleration in first-pass CMR myocardial perfusion imaging. Magn Reson Med. 2010;64:1616-1624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Motwani M, Maredia N, Fairbairn TA, Kozerke S, Greenwood JP, Plein S. Assessment of ischaemic burden in angiographic three-vessel coronary artery disease with high-resolution myocardial perfusion cardiovascular magnetic resonance imaging. Eur Heart J Cardiovasc Imaging. 2014;15:701-708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Plein S, Kozerke S, Suerder D, Luescher TF, Greenwood JP, Boesiger P, Schwitter J. High spatial resolution myocardial perfusion cardiac magnetic resonance for the detection of coronary artery disease. Eur Heart J. 2008;29:2148-2155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Motwani M, Maredia N, Fairbairn TA, Kozerke S, Radjenovic A, Greenwood JP, Plein S. High-resolution versus standard-resolution cardiovascular MR myocardial perfusion imaging for the detection of coronary artery disease. Circ Cardiovasc Imaging. 2012;5:306-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Lockie T, Ishida M, Perera D, Chiribiri A, De Silva K, Kozerke S, Marber M, Nagel E, Rezavi R, Redwood S. High-resolution magnetic resonance myocardial perfusion imaging at 3.0-Tesla to detect hemodynamically significant coronary stenoses as determined by fractional flow reserve. J Am Coll Cardiol. 2011;57:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 39. | Jogiya R, Kozerke S, Morton G, De Silva K, Redwood S, Perera D, Nagel E, Plein S. Validation of dynamic 3-dimensional whole heart magnetic resonance myocardial perfusion imaging against fractional flow reserve for the detection of significant coronary artery disease. J Am Coll Cardiol. 2012;60:756-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 40. | Manka R, Paetsch I, Kozerke S, Moccetti M, Hoffmann R, Schroeder J, Reith S, Schnackenburg B, Gaemperli O, Wissmann L. Whole-heart dynamic three-dimensional magnetic resonance perfusion imaging for the detection of coronary artery disease defined by fractional flow reserve: determination of volumetric myocardial ischaemic burden and coronary lesion location. Eur Heart J. 2012;33:2016-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 41. | Manka R, Wissmann L, Gebker R, Jogiya R, Motwani M, Frick M, Reinartz S, Schnackenburg B, Niemann M, Gotschy A. Multicenter evaluation of dynamic three-dimensional magnetic resonance myocardial perfusion imaging for the detection of coronary artery disease defined by fractional flow reserve. Circ Cardiovasc Imaging. 2015;8:pii: e003061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 42. | Shaw LJ, Berman DS, Picard MH, Friedrich MG, Kwong RY, Stone GW, Senior R, Min JK, Hachamovitch R, Scherrer-Crosbie M. Comparative definitions for moderate-severe ischemia in stress nuclear, echocardiography, and magnetic resonance imaging. JACC Cardiovasc Imaging. 2014;7:593-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 149] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 43. | Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107:2900-2907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1163] [Cited by in RCA: 1112] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 44. | Jogiya R, Morton G, De Silva K, Reyes E, Hachamovitch R, Kozerke S, Nagel E, Underwood SR, Plein S. Ischemic burden by 3-dimensional myocardial perfusion cardiovascular magnetic resonance: comparison with myocardial perfusion scintigraphy. Circ Cardiovasc Imaging. 2014;7:647-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 45. | McDiarmid AK, Ripley DP, Mohee K, Kozerke S, Greenwood JP, Plein S, Motwani M. Three-dimensional whole-heart vs. two-dimensional high-resolution perfusion-CMR: a pilot study comparing myocardial ischaemic burden. Eur Heart J Cardiovasc Imaging. 2016;17:900-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Schuster A, Zarinabad N, Ishida M, Sinclair M, van den Wijngaard JP, Morton G, Hautvast GL, Bigalke B, van Horssen P, Smith N. Quantitative assessment of magnetic resonance derived myocardial perfusion measurements using advanced techniques: microsphere validation in an explanted pig heart system. J Cardiovasc Magn Reson. 2014;16:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 47. | Jogiya R, Makowski M, Phinikaridou A, Patel AS, Jansen C, Zarinabad N, Chiribiri A, Botnar R, Nagel E, Kozerke S. Hyperemic stress myocardial perfusion cardiovascular magnetic resonance in mice at 3 Tesla: initial experience and validation against microspheres. J Cardiovasc Magn Reson. 2013;15:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Biglands JD, Magee DR, Sourbron SP, Plein S, Greenwood JP, Radjenovic A. Comparison of the Diagnostic Performance of Four Quantitative Myocardial Perfusion Estimation Methods Used in Cardiac MR Imaging: CE-MARC Substudy. Radiology. 2015;275:393-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 49. | Morton G, Chiribiri A, Ishida M, Hussain ST, Schuster A, Indermuehle A, Perera D, Knuuti J, Baker S, Hedström E. Quantification of absolute myocardial perfusion in patients with coronary artery disease: comparison between cardiovascular magnetic resonance and positron emission tomography. J Am Coll Cardiol. 2012;60:1546-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 50. | Miller CA, Naish JH, Ainslie MP, Tonge C, Tout D, Arumugam P, Banerji A, Egdell RM, Clark D, Weale P. Voxel-wise quantification of myocardial blood flow with cardiovascular magnetic resonance: effect of variations in methodology and validation with positron emission tomography. J Cardiovasc Magn Reson. 2014;16:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Weng AM, Ritter CO, Lotz J, Beer MJ, Hahn D, Köstler H. Automatic postprocessing for the assessment of quantitative human myocardial perfusion using MRI. Eur Radiol. 2010;20:1356-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Bruder O, Schneider S, Pilz G, van Rossum AC, Schwitter J, Nothnagel D, Lombardi M, Buss S, Wagner A, Petersen S. 2015 Update on Acute Adverse Reactions to Gadolinium based Contrast Agents in Cardiovascular MR. Large Multi-National and Multi-Ethnical Population Experience With 37788 Patients From the EuroCMR Registry. J Cardiovasc Magn Reson. 2015;17:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 53. | Kribben A, Witzke O, Hillen U, Barkhausen J, Daul AE, Erbel R. Nephrogenic systemic fibrosis: pathogenesis, diagnosis, and therapy. J Am Coll Cardiol. 2009;53:1621-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 54. | Nagel E, Lehmkuhl HB, Bocksch W, Klein C, Vogel U, Frantz E, Ellmer A, Dreysse S, Fleck E. Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation. 1999;99:763-770. [PubMed] |
| 55. | Wahl A, Paetsch I, Gollesch A, Roethemeyer S, Foell D, Gebker R, Langreck H, Klein C, Fleck E, Nagel E. Safety and feasibility of high-dose dobutamine-atropine stress cardiovascular magnetic resonance for diagnosis of myocardial ischaemia: experience in 1000 consecutive cases. Eur Heart J. 2004;25:1230-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 56. | Nandalur KR, Dwamena BA, Choudhri AF, Nandalur MR, Carlos RC. Diagnostic performance of stress cardiac magnetic resonance imaging in the detection of coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2007;50:1343-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 375] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 57. | Manka R, Jahnke C, Gebker R, Schnackenburg B, Paetsch I. Head-to-head comparison of first-pass MR perfusion imaging during adenosine and high-dose dobutamine/atropine stress. Int J Cardiovasc Imaging. 2011;27:995-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 58. | Gebker R, Frick M, Jahnke C, Berger A, Schneeweis C, Manka R, Kelle S, Klein C, Schnackenburg B, Fleck E. Value of additional myocardial perfusion imaging during dobutamine stress magnetic resonance for the assessment of intermediate coronary artery disease. Int J Cardiovasc Imaging. 2012;28:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 59. | Mordi I, Stanton T, Carrick D, McClure J, Oldroyd K, Berry C, Tzemos N. Comprehensive dobutamine stress CMR versus echocardiography in LBBB and suspected coronary artery disease. JACC Cardiovasc Imaging. 2014;7:490-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Roger VL, Jacobsen SJ, Pellikka PA, Miller TD, Bailey KR, Gersh BJ. Prognostic value of treadmill exercise testing: a population-based study in Olmsted County, Minnesota. Circulation. 1998;98:2836-2841. [PubMed] |
| 61. | Fleischmann KE, Hunink MG, Kuntz KM, Douglas PS. Exercise echocardiography or exercise SPECT imaging? A meta-analysis of diagnostic test performance. JAMA. 1998;280:913-920. [PubMed] |
| 62. | Thavendiranathan P, Dickerson JA, Scandling D, Balasubramanian V, Pennell ML, Hinton A, Raman SV, Simonetti OP. Comparison of treadmill exercise stress cardiac MRI to stress echocardiography in healthy volunteers for adequacy of left ventricular endocardial wall visualization: A pilot study. J Magn Reson Imaging. 2014;39:1146-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 63. | Sukpraphrute B, Drafts BC, Rerkpattanapipat P, Morgan TM, Kirkman PM, Ntim WO, Hamilton CA, Cockrum RL, Hundley WG. Prognostic utility of cardiovascular magnetic resonance upright maximal treadmill exercise testing. J Cardiovasc Magn Reson. 2015;17:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 64. | Gusso S, Salvador C, Hofman P, Cutfield W, Baldi JC, Taberner A, Nielsen P. Design and testing of an MRI-compatible cycle ergometer for non-invasive cardiac assessments during exercise. Biomed Eng Online. 2012;11:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Forouzan O, Flink E, Warczytowa J, Thate N, Hanske A, Lee T, Roldan-Alzate A, François C, Wieben O, Chesler NC. Low Cost Magnetic Resonance Imaging-Compatible Stepper Exercise Device for Use in Cardiac Stress Tests. J Med Device. 2014;8:0450021-0450028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 66. | Gargiulo P, Dellegrottaglie S, Bruzzese D, Savarese G, Scala O, Ruggiero D, D’Amore C, Paolillo S, Agostoni P, Bossone E. The prognostic value of normal stress cardiac magnetic resonance in patients with known or suspected coronary artery disease: a meta-analysis. Circ Cardiovasc Imaging. 2013;6:574-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 67. | Lipinski MJ, McVey CM, Berger JS, Kramer CM, Salerno M. Prognostic value of stress cardiac magnetic resonance imaging in patients with known or suspected coronary artery disease: a systematic review and meta-analysis. J Am Coll Cardiol. 2013;62:826-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 68. | Buckert D, Dewes P, Walcher T, Rottbauer W, Bernhardt P. Intermediate-term prognostic value of reversible perfusion deficit diagnosed by adenosine CMR: a prospective follow-up study in a consecutive patient population. JACC Cardiovasc Imaging. 2013;6:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 69. | Korosoglou G, Elhmidi Y, Steen H, Schellberg D, Riedle N, Ahrens J, Lehrke S, Merten C, Lossnitzer D, Radeleff J. Prognostic value of high-dose dobutamine stress magnetic resonance imaging in 1,493 consecutive patients: assessment of myocardial wall motion and perfusion. J Am Coll Cardiol. 2010;56:1225-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 70. | Greenwood JP, Herzog BA, Brown JM, Everett CC, Nixon J, Bijsterveld P, Maredia N, Motwani M, Dickinson CJ, Ball SG. Prognostic Value of Cardiovascular Magnetic Resonance and Single-Photon Emission Computed Tomography in Suspected Coronary Heart Disease: Long-Term Follow-up of a Prospective, Diagnostic Accuracy Cohort Study. Ann Intern Med. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 71. | Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, Klocke FJ, Bonow RO, Kim RJ, Judd RM. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003;361:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 891] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 72. | Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, Davis RB. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733-2743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 539] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 73. | Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1774] [Cited by in RCA: 1744] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 74. | Mollet NR, Dymarkowski S, Volders W, Wathiong J, Herbots L, Rademakers FE, Bogaert J. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation. 2002;106:2873-2876. [PubMed] |
| 75. | Srichai MB, Junor C, Rodriguez LL, Stillman AE, Grimm RA, Lieber ML, Weaver JA, Smedira NG, White RD. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J. 2006;152:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 301] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 76. | Goetti R, Kozerke S, Donati OF, Sürder D, Stolzmann P, Kaufmann PA, Lüscher TF, Corti R, Manka R. Acute, subacute, and chronic myocardial infarction: quantitative comparison of 2D and 3D late gadolinium enhancement MR imaging. Radiology. 2011;259:704-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 77. | Pierce IT, Keegan J, Drivas P, Gatehouse PD, Firmin DN. Free-breathing 3D late gadolinium enhancement imaging of the left ventricle using a stack of spirals at 3T. J Magn Reson Imaging. 2015;41:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 78. | Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2376] [Cited by in RCA: 2211] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 79. | Romero J, Xue X, Gonzalez W, Garcia MJ. CMR imaging assessing viability in patients with chronic ventricular dysfunction due to coronary artery disease: a meta-analysis of prospective trials. JACC Cardiovasc Imaging. 2012;5:494-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 80. | Baer FM, Theissen P, Schneider CA, Voth E, Sechtem U, Schicha H, Erdmann E. Dobutamine magnetic resonance imaging predicts contractile recovery of chronically dysfunctional myocardium after successful revascularization. J Am Coll Cardiol. 1998;31:1040-1048. [PubMed] |
| 81. | Shah DJ, Kim HW, James O, Parker M, Wu E, Bonow RO, Judd RM, Kim RJ. Prevalence of regional myocardial thinning and relationship with myocardial scarring in patients with coronary artery disease. JAMA. 2013;309:909-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 82. | Nagel E, Schuster A. Myocardial viability: dead or alive is not the question! JACC Cardiovasc Imaging. 2012;5:509-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 83. | Bree D, Wollmuth JR, Cupps BP, Krock MD, Howells A, Rogers J, Moazami N, Pasque MK. Low-dose dobutamine tissue-tagged magnetic resonance imaging with 3-dimensional strain analysis allows assessment of myocardial viability in patients with ischemic cardiomyopathy. Circulation. 2006;114:I33-I36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 84. | Schuster A, Paul M, Bettencourt N, Morton G, Chiribiri A, Ishida M, Hussain S, Jogiya R, Kutty S, Bigalke B. Cardiovascular magnetic resonance myocardial feature tracking for quantitative viability assessment in ischemic cardiomyopathy. Int J Cardiol. 2013;166:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 85. | Schuster A, Paul M, Bettencourt N, Hussain ST, Morton G, Kutty S, Bigalke B, Chiribiri A, Perera D, Nagel E. Myocardial feature tracking reduces observer-dependence in low-dose dobutamine stress cardiovascular magnetic resonance. PLoS One. 2015;10:e0122858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 86. | Hendel RC, Patel MR, Kramer CM, Poon M, Hendel RC, Carr JC, Gerstad NA, Gillam LD, Hodgson JM, Kim RJ. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48:1475-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1092] [Cited by in RCA: 948] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 87. | Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 Appropriate use criteria for coronary revascularization focused update: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, American Society of Nuclear Cardiology, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2012;59:857-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 392] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 88. | Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607-1616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1012] [Cited by in RCA: 950] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 89. | Bonow RO, Maurer G, Lee KL, Holly TA, Binkley PF, Desvigne-Nickens P, Drozdz J, Farsky PS, Feldman AM, Doenst T. Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med. 2011;364:1617-1625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 696] [Cited by in RCA: 624] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 90. | Allman KC, Shaw LJ, Hachamovitch R, Udelson JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol. 2002;39:1151-1158. [PubMed] |
| 91. | Schinkel AF, Bax JJ, Poldermans D, Elhendy A, Ferrari R, Rahimtoola SH. Hibernating myocardium: diagnosis and patient outcomes. Curr Probl Cardiol. 2007;32:375-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 256] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 92. | Gerber BL, Rousseau MF, Ahn SA, le Polain de Waroux JB, Pouleur AC, Phlips T, Vancraeynest D, Pasquet A, Vanoverschelde JL. Prognostic value of myocardial viability by delayed-enhanced magnetic resonance in patients with coronary artery disease and low ejection fraction: impact of revascularization therapy. J Am Coll Cardiol. 2012;59:825-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 93. | Bruder O, Wagner A, Lombardi M, Schwitter J, van Rossum A, Pilz G, Nothnagel D, Steen H, Petersen S, Nagel E. European Cardiovascular Magnetic Resonance (EuroCMR) registry--multi national results from 57 centers in 15 countries. J Cardiovasc Magn Reson. 2013;15:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 180] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 94. | Flett AS, Hasleton J, Cook C, Hausenloy D, Quarta G, Ariti C, Muthurangu V, Moon JC. Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc Imaging. 2011;4:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 488] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 95. | Nicolosi GL, Latini R, Marino P, Maggioni AP, Barlera S, Franzosi MG, Geraci E, Santoro L, Tavazzi L, Tognoni G. The prognostic value of predischarge quantitative two-dimensional echocardiographic measurements and the effects of early lisinopril treatment on left ventricular structure and function after acute myocardial infarction in the GISSI-3 Trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico. Eur Heart J. 1996;17:1646-1656. [PubMed] |
| 96. | Klem I, Shah DJ, White RD, Pennell DJ, van Rossum AC, Regenfus M, Sechtem U, Schvartzman PR, Hunold P, Croisille P. Prognostic value of routine cardiac magnetic resonance assessment of left ventricular ejection fraction and myocardial damage: an international, multicenter study. Circ Cardiovasc Imaging. 2011;4:610-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 97. | Zemrak F, Petersen SE. Late gadolinium enhancement CMR predicts adverse cardiovascular outcomes and mortality in patients with coronary artery disease: systematic review and meta-analysis. Prog Cardiovasc Dis. 2011;54:215-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 98. | Schelbert EB, Cao JJ, Sigurdsson S, Aspelund T, Kellman P, Aletras AH, Dyke CK, Thorgeirsson G, Eiriksdottir G, Launer LJ. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA. 2012;308:890-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 234] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 99. | Scott PA, Morgan JM, Carroll N, Murday DC, Roberts PR, Peebles CR, Harden SP, Curzen NP. The extent of left ventricular scar quantified by late gadolinium enhancement MRI is associated with spontaneous ventricular arrhythmias in patients with coronary artery disease and implantable cardioverter-defibrillators. Circ Arrhythm Electrophysiol. 2011;4:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 100. | Gao P, Yee R, Gula L, Krahn AD, Skanes A, Leong-Sit P, Klein GJ, Stirrat J, Fine N, Pallaveshi L. Prediction of arrhythmic events in ischemic and dilated cardiomyopathy patients referred for implantable cardiac defibrillator: evaluation of multiple scar quantification measures for late gadolinium enhancement magnetic resonance imaging. Circ Cardiovasc Imaging. 2012;5:448-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 172] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 101. | Demirel F, Adiyaman A, Timmer JR, Dambrink JH, Kok M, Boeve WJ, Elvan A. Myocardial scar characteristics based on cardiac magnetic resonance imaging is associated with ventricular tachyarrhythmia in patients with ischemic cardiomyopathy. Int J Cardiol. 2014;177:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 102. | Scott PA, Rosengarten JA, Curzen NP, Morgan JM. Late gadolinium enhancement cardiac magnetic resonance imaging for the prediction of ventricular tachyarrhythmic events: a meta-analysis. Eur J Heart Fail. 2013;15:1019-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 103. | Klem I, Weinsaft JW, Bahnson TD, Hegland D, Kim HW, Hayes B, Parker MA, Judd RM, Kim RJ. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J Am Coll Cardiol. 2012;60:408-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 257] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 104. | Roes SD, Borleffs CJ, van der Geest RJ, Westenberg JJ, Marsan NA, Kaandorp TA, Reiber JH, Zeppenfeld K, Lamb HJ, de Roos A. Infarct tissue heterogeneity assessed with contrast-enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator. Circ Cardiovasc Imaging. 2009;2:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 349] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 105. | Zeidan-Shwiri T, Yang Y, Lashevsky I, Kadmon E, Kagal D, Dick A, Laish Farkash A, Paul G, Gao D, Shurrab M. Magnetic resonance estimates of the extent and heterogeneity of scar tissue in ICD patients with ischemic cardiomyopathy predict ventricular arrhythmia. Heart Rhythm. 2015;12:802-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 106. | White JA, Yee R, Yuan X, Krahn A, Skanes A, Parker M, Klein G, Drangova M. Delayed enhancement magnetic resonance imaging predicts response to cardiac resynchronization therapy in patients with intraventricular dyssynchrony. J Am Coll Cardiol. 2006;48:1953-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 272] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 107. | Wong JA, Yee R, Stirrat J, Scholl D, Krahn AD, Gula LJ, Skanes AC, Leong-Sit P, Klein GJ, McCarty D. Influence of pacing site characteristics on response to cardiac resynchronization therapy. Circ Cardiovasc Imaging. 2013;6:542-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 108. | Taylor RJ, Umar F, Panting JR, Stegemann B, Leyva F. Left ventricular lead position, mechanical activation, and myocardial scar in relation to left ventricular reverse remodeling and clinical outcomes after cardiac resynchronization therapy: A feature-tracking and contrast-enhanced cardiovascular magnetic resonance study. Heart Rhythm. 2016;13:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 109. | Younger JF, Plein S, Crean A, Ball SG, Greenwood JP. Visualization of coronary venous anatomy by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2009;11:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 110. | Bhatnagar P, Wickramasinghe K, Williams J, Rayner M, Townsend N. The epidemiology of cardiovascular disease in the UK 2014. Heart. 2015;101:1182-1189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 250] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 111. | Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2162] [Cited by in RCA: 2279] [Article Influence: 162.8] [Reference Citation Analysis (0)] |
| 112. | Walker S, Girardin F, McKenna C, Ball SG, Nixon J, Plein S, Greenwood JP, Sculpher M. Cost-effectiveness of cardiovascular magnetic resonance in the diagnosis of coronary heart disease: an economic evaluation using data from the CE-MARC study. Heart. 2013;99:873-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 113. | Boldt J, Leber AW, Bonaventura K, Sohns C, Stula M, Huppertz A, Haverkamp W, Dorenkamp M. Cost-effectiveness of cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary artery disease in Germany. J Cardiovasc Magn Reson. 2013;15:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 114. | Pletscher M, Walker S, Moschetti K, Wasserfallen JB, Greenwood JP, Schwitter J, Girardin FR. Cost-effectiveness of functional cardiac imaging in the diagnostic work-up of coronary heart disease. Eur Hear J - Qual Care Clin Outcomes. 2016;In press. |
| 115. | Petrov G, Kelle S, Fleck E, Wellnhofer E. Incremental cost-effectiveness of dobutamine stress cardiac magnetic resonance imaging in patients at intermediate risk for coronary artery disease. Clin Res Cardiol. 2015;104:401-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 116. | Moschetti K, Favre D, Pinget C, Pilz G, Petersen SE, Wagner A, Wasserfallen JB, Schwitter JJ. Comparative cost-effectiveness analyses of cardiovascular magnetic resonance and coronary angiography combined with fractional flow reserve for the diagnosis of coronary artery disease. J Cardiovasc Magn Reson. 2014;16:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 117. | Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, Pohost GM, Williams KA. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 Appropriate Use Criteria for Cardiac Radionuclide Imaging: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. J Am Coll Cardiol. 2009;53:2201-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 378] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 118. | Ripley DP, Brown JM, Everett CC, Bijsterveld P, Walker S, Sculpher M, McCann GP, Berry C, Plein S, Greenwood JP. Rationale and design of the Clinical Evaluation of Magnetic Resonance Imaging in Coronary heart disease 2 trial (CE-MARC 2): a prospective, multicenter, randomized trial of diagnostic strategies in suspected coronary heart disease. Am Heart J. 2015;169:17-24.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 119. | Greenwood JP, Ripley DP, Berry C, McCann GP, Plein S, Bucciarelli-Ducci C, Dall’Armellina E, Prasad A, Bijsterveld P, Foley JR. Effect of Care Guided by Cardiovascular Magnetic Resonance, Myocardial Perfusion Scintigraphy, or NICE Guidelines on Subsequent Unnecessary Angiography Rates: The CE-MARC 2 Randomized Clinical Trial. JAMA. 2016;316:1051-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 206] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 120. | Desai NR, Bradley SM, Parzynski CS, Nallamothu BK, Chan PS, Spertus JA, Patel MR, Ader J, Soufer A, Krumholz HM. Appropriate Use Criteria for Coronary Revascularization and Trends in Utilization, Patient Selection, and Appropriateness of Percutaneous Coronary Intervention. JAMA. 2015;314:2045-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 189] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 121. | Chan PS, Patel MR, Klein LW, Krone RJ, Dehmer GJ, Kennedy K, Nallamothu BK, Weaver WD, Masoudi FA, Rumsfeld JS. Appropriateness of percutaneous coronary intervention. JAMA. 2011;306:53-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 122. | Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2974] [Cited by in RCA: 3052] [Article Influence: 190.8] [Reference Citation Analysis (0)] |
| 123. | Bech GJ, De Bruyne B, Pijls NH, de Muinck ED, Hoorntje JC, Escaned J, Stella PR, Boersma E, Bartunek J, Koolen JJ. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation. 2001;103:2928-2934. [PubMed] |
| 124. | Hussain ST, Paul M, Plein S, McCann GP, Shah AM, Marber MS, Chiribiri A, Morton G, Redwood S, MacCarthy P. Design and rationale of the MR-INFORM study: stress perfusion cardiovascular magnetic resonance imaging to guide the management of patients with stable coronary artery disease. J Cardiovasc Magn Reson. 2012;14:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 125. | Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3259] [Cited by in RCA: 3202] [Article Influence: 177.9] [Reference Citation Analysis (0)] |
| 126. | Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503-2515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1539] [Cited by in RCA: 1391] [Article Influence: 86.9] [Reference Citation Analysis (0)] |
| 127. | Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, Weintraub WS, O’Rourke RA, Dada M, Spertus JA. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1215] [Cited by in RCA: 1162] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 128. | Shaw LJ, Weintraub WS, Maron DJ, Hartigan PM, Hachamovitch R, Min JK, Dada M, Mancini GB, Hayes SW, O’Rourke RA. Baseline stress myocardial perfusion imaging results and outcomes in patients with stable ischemic heart disease randomized to optimal medical therapy with or without percutaneous coronary intervention. Am Heart J. 2012;164:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 129. | Shaw LJ, Cerqueira MD, Brooks MM, Althouse AD, Sansing VV, Beller GA, Pop-Busui R, Taillefer R, Chaitman BR, Gibbons RJ. Impact of left ventricular function and the extent of ischemia and scar by stress myocardial perfusion imaging on prognosis and therapeutic risk reduction in diabetic patients with coronary artery disease: results from the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. J Nucl Cardiol. 2012;19:658-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 130. | Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, Friedrich MG, Ho VB, Jerosch-Herold M, Kramer CM, Manning WJ. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55:2614-2662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 531] [Cited by in RCA: 474] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 131. | Ripley DP, Saha A, Teis A, Uddin A, Bijsterveld P, Kidambi A, McDiarmid AK, Sivananthan M, Plein S, Pennell DJ. The distribution and prognosis of anomalous coronary arteries identified by cardiovascular magnetic resonance: 15 year experience from two tertiary centres. J Cardiovasc Magn Reson. 2014;16:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 132. | Kim WY, Danias PG, Stuber M, Flamm SD, Plein S, Nagel E, Langerak SE, Weber OM, Pedersen EM, Schmidt M. Coronary magnetic resonance angiography for the detection of coronary stenoses. N Engl J Med. 2001;345:1863-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 577] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 133. | Kato S, Kitagawa K, Ishida N, Ishida M, Nagata M, Ichikawa Y, Katahira K, Matsumoto Y, Seo K, Ochiai R. Assessment of coronary artery disease using magnetic resonance coronary angiography: a national multicenter trial. J Am Coll Cardiol. 2010;56:983-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 134. | Hamdan A, Asbach P, Wellnhofer E, Klein C, Gebker R, Kelle S, Kilian H, Huppertz A, Fleck E. A prospective study for comparison of MR and CT imaging for detection of coronary artery stenosis. JACC Cardiovasc Imaging. 2011;4:50-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 135. | Bettencourt N, Ferreira N, Chiribiri A, Schuster A, Sampaio F, Santos L, Melica B, Rodrigues A, Braga P, Teixeira M. Additive value of magnetic resonance coronary angiography in a comprehensive cardiac magnetic resonance stress-rest protocol for detection of functionally significant coronary artery disease: a pilot study. Circ Cardiovasc Imaging. 2013;6:730-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 136. | Ripley DP, Motwani M, Plein S, Greenwood JP. Established and emerging cardiovascular magnetic resonance techniques for the assessment of stable coronary heart disease and acute coronary syndromes. Quant Imaging Med Surg. 2014;4:330-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 137. | Garg P, Underwood SR, Senior R, Greenwood JP, Plein S. Noninvasive cardiac imaging in suspected acute coronary syndrome. Nat Rev Cardiol. 2016;13:266-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 138. | Carrick D, Haig C, Rauhalammi S, Ahmed N, Mordi I, McEntegart M, Petrie MC, Eteiba H, Hood S, Watkins S. Prognostic significance of infarct core pathology revealed by quantitative non-contrast in comparison with contrast cardiac magnetic resonance imaging in reperfused ST-elevation myocardial infarction survivors. Eur Heart J. 2016;37:1044-1059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 139. | Carberry J, Carrick D, Haig C, Rauhalammi SM, Ahmed N, Mordi I, McEntegart M, Petrie MC, Eteiba H, Hood S. Remote Zone Extracellular Volume and Left Ventricular Remodeling in Survivors of ST-Elevation Myocardial Infarction. Hypertension. 2016;68:385-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 140. | Friedrich MG, Karamitsos TD. Oxygenation-sensitive cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2013;15:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 141. | Jahnke C, Gebker R, Manka R, Schnackenburg B, Fleck E, Paetsch I. Navigator-gated 3D blood oxygen level-dependent CMR at 3.0-T for detection of stress-induced myocardial ischemic reactions. JACC Cardiovasc Imaging. 2010;3:375-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 142. | Arnold JR, Karamitsos TD, Bhamra-Ariza P, Francis JM, Searle N, Robson MD, Howells RK, Choudhury RP, Rimoldi OE, Camici PG. Myocardial oxygenation in coronary artery disease: insights from blood oxygen level-dependent magnetic resonance imaging at 3 tesla. J Am Coll Cardiol. 2012;59:1954-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 143. | Olsson LE, Chai CM, Axelsson O, Karlsson M, Golman K, Petersson JS. MR coronary angiography in pigs with intraarterial injections of a hyperpolarized 13C substance. Magn Reson Med. 2006;55:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 144. | Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3540] [Cited by in RCA: 3702] [Article Influence: 284.8] [Reference Citation Analysis (0)] |