Published online Dec 26, 2017. doi: 10.4330/wjc.v9.i12.853
Peer-review started: September 12, 2017
First decision: September 25, 2017
Revised: October 21, 2017
Accepted: November 8, 2017
Article in press: November 8, 2017
Published online: December 26, 2017
Processing time: 101 Days and 6.4 Hours
Pre-procedural planning is the key element of transcatheter aortic valve implantation (TAVI). Multislice computed tomography of the chest, abdomen and pelvis with the ability to perform a 3-dimensional reconstruction has become the cornerstone of pre-procedural planning. We would like to encourage TAVI operators (interventional cardiologist and surgeons) to get involved in imaging. All TAVI operators should know how to assess the annulus, the annular root, and the iliofemoral access. We strongly believe that this will improve outcomes of this evolving procedure.
Core tip: We have noticed that only a minority of interventional cardiologists and cardiac surgeons routinely look at their patients MDCTs and know how to perform a three dimensional multiplanar reconstruction. With this editorial, we would like to encourage all transcatheter aortic valve implantation (TAVI) operators to get involved in cardiac imaging. We do believe that this will improve outcomes. In case a complication occurs, TAVI operators will be more likely to understand the nature of the complication and learn from it. And this again will lead to improved outcomes in future.
- Citation: Brinkert M, Toggweiler S. Transcatheter aortic valve implantation operators - get involved in imaging! World J Cardiol 2017; 9(12): 853-857
- URL: https://www.wjgnet.com/1949-8462/full/v9/i12/853.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i12.853
Transcatheter aortic valve implantation (TAVI) is now routinely performed in inoperable, high-risk, and intermediate risk patients with low mortality- and complication rates[1,2]. Some of the key elements contributing to these impressive results are pre-procedural patient evaluation by the multidisciplinary HeartTeam, and pre-procedural imaging[3,4].
The important role of multislice computed tomography (MSCT). MSCT of the chest, abdomen and pelvis with 3-dimensional reconstruction has become the cornerstone of pre-procedural planning. MSCT is now routinely performed to assess the aortic annulus, the distance between the aortic annulus and the coronary ostia and the suitability for the transfemoral access[3,5]. Nowadays matured post-processing imaging software is widely available to perform these measurements automatically and create standardized reports[6]. However, automatic measurements may not include the degree and distribution of calcification and may not take into account all aspects of the anatomy. Most of the TAVI operators rely on such reports or on numbers and measurements reported by the radiologist[7-9]. Therefore, we would like to encourage all TAVI operators to get involved in imaging and learn how to perform a 3-dimensional multiplanar reconstruction.
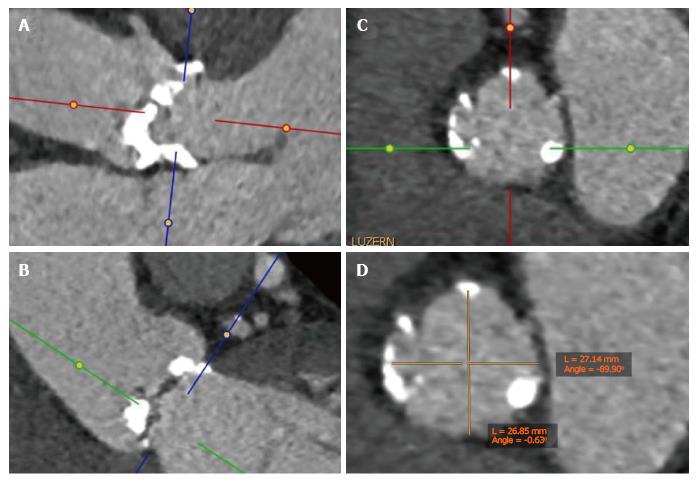
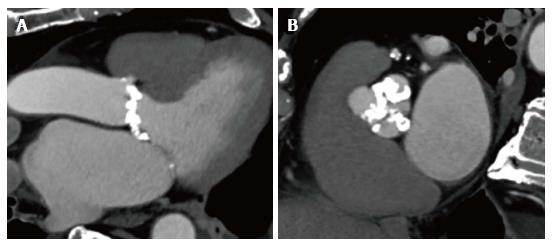
Choosing the valve type and size. It has been shown that left ventricular outflow tract (LVOT) calcification is associated with an increased risk for annular rupture during TAVI with balloon-expandable prostheses[10]. Extensive calcifications at the native aortic valve may increase the risk for paravalvular regurgitation or need for a permanent pacemaker[11,12]. As an interventional cardiologist or cardiac surgeon, we can easily perform multiplanar reconstructions of the aortic annulus not only to measure the dimensions of the annulus but also to get an impression of the distribution of calcification of the valve leaflets and the LVOT (Figure 1)[13]. Based on all information including the annular perimeter, area, distribution of calcification and anatomy of the aortic root, valve type and size can be chosen more specifically as part of a patient tailored therapy (Figure 2).
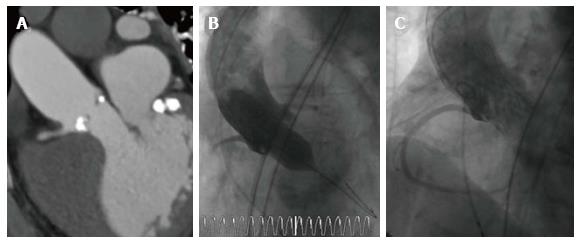
Assessment of the coronary artery height. The “Instructions for use” of different valves include specific recommendations for the minimal coronary artery height. However, the risk for coronary obstruction is greatly increased in patients with bulky atheroma or calcifications at the tip of the leaflets, a smaller sinus of valsalva diameter, narrow sinotubular junction and different patient characteristics like female gender or patients with previous surgical bioprosthesis[14]. Measuring the coronary artery height with MSCT is a great screening tool, but ‘”virtual implantation” by the operator comparing the length of the leaflets with the distance between annulus and coronary ostia and also assessing the distribution of calcifications may allow much better risk stratification (Figure 3). Radial strength depends largely on the valve type. Whereas the widely used balloon-expandable valves consist of cobalt chromium, self-expanding valves are composed of nitinol thus applying less radial force to the tissue[14]. Accordingly, a self-expandable and retrievable valve might be preferable in patients at risk for coronary obstruction. Moreover, in case of borderline anatomy, balloonvalvuloplasty with simultaneous contrast media injection may allow to estimate the risk for coronary obstruction during valve deployment. In patients considered at high risk for coronary obstruction placing a coaxial guiding catheter extension such as the GuideLiner catheter (Cascular Solutions Inc., Minneapolis, MN, United States) in the coronary artery during valve deployment may allow emergent percutaneous coronary intervention.
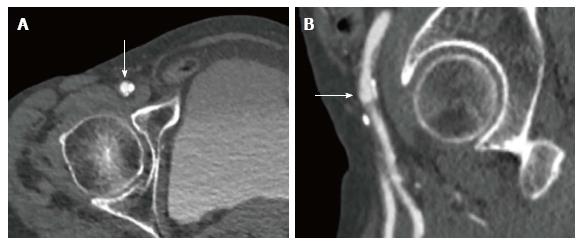
Choosing the ideal puncture site. Finally MSCT is routinely used to evaluate size, tortuosity and calcifications of the iliofemoral arteries and to determine the feasibility of transfemoral access[15]. MSCT provides detailed information about the height of the bifurcation of the common femoral artery in relationship to the femoral head. Furthermore, it allows visualization of the inferior epigastric artery which is located within the inguinal ligament. Finally, MSCT shows the extent of calcification at the level of the potential puncture site (Figure 4). Knowing your patients anatomy allows to perform a precise puncture under fluoroscopy guidance thus minimizing the risk for vascular injury[16,17].
How to get involved in imaging, and why? Potential TAVI candidates are discussed by the interdisciplinary HeartTeam consisting of non-invasive cardiologists specialized in cardiac imaging, interventional cardiologists and cardiac surgeon to define the best treatment option for the individual patient. Evaluation of associated comorbidities that may limit the life expectancy or the recovery after the procedure is of particular importance. Results from pre-procedural invasive angiogram, echocardiogram and MSCT are reviewed for each patient. We would like to encourage all TAVI operators to review their patients MSCT again immediately before the procedure. Look at the iliofemoral access to choose the better side with less calcification or tortuosity, and choose the ideal puncture site. Then, perform a three dimensional multiplanar reconstruction of the annulus, measure the annular diameters, perimeter, and the area. Look for calcification at the level of the annulus, but also at the level of the LVOT. Finally, review the root and the coronary arteries. With routine, this can be performed in 2-3 min in most patients. There are two potential advantages of being able to analyze your patient’s images. First, you may improve your patient’s outcomes. Second, if you have a complication, you are more likely to understand it and learn from it. And this will again lead to better outcomes in the future.
Manuscript source: Invited manuscript
Country of origin: Switzerland
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Amiya E, Chang ST, den Uil CA, Lin GM, Nunez-Gil NJ, Said SAM, Schoenhagen P S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Holmes DR Jr, Nishimura RA, Grover FL, Brindis RG, Carroll JD, Edwards FH, Peterson ED, Rumsfeld JS, Shahian DM, Thourani VH, Tuzcu EM, Vemulapalli S, Hewitt K, Michaels J, Fitzgerald S, Mack MJ; STS/ACC TVT Registry. Annual Outcomes With Transcatheter Valve Therapy: From the STS/ACC TVT Registry. Ann Thorac Surg. 2016;101:789-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 2. | Wenaweser P, Stortecky S, Heg D, Tueller D, Nietlispach F, Falk V, Pedrazzini G, Jeger R, Reuthebuch O, Carrel T. Short-term clinical outcomes among patients undergoing transcatheter aortic valve implantation in Switzerland: the Swiss TAVI registry. EuroIntervention. 2014;10:982-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Achenbach S, Delgado V, Hausleiter J, Schoenhagen P, Min JK, Leipsic JA. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J Cardiovasc Comput Tomogr. 2012;6:366-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 495] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 4. | Delgado V, Kapadia S, Schalij MJ, Schuijf JD, Tuzcu EM, Bax JJ. Transcatheter aortic valve implantation: implications of multimodality imaging in patient selection, procedural guidance, and outcomes. Heart. 2012;98:743-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Khalique OK, Pulerwitz TC, Halliburton SS, Kodali SK, Hahn RT, Nazif TM, Vahl TP, George I, Leon MB, D’Souza B. Practical considerations for optimizing cardiac computed tomography protocols for comprehensive acquisition prior to transcatheter aortic valve replacement. J Cardiovasc Comput Tomogr. 2016;10:364-374. [PubMed] [DOI] [Full Text] |
| 6. | Watanabe Y, Morice MC, Bouvier E, Leong T, Hayashida K, Lefèvre T, Hovasse T, Romano M, Chevalier B, Donzeau-Gouge P. Automated 3-dimensional aortic annular assessment by multidetector computed tomography in transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2013;6:955-964. [PubMed] [DOI] [Full Text] |
| 7. | Marwan M, Achenbach S. Role of Cardiac CT Before Transcatheter Aortic Valve Implantation (TAVI). Curr Cardiol Rep. 2016;18:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Leipsic J, Gurvitch R, Labounty TM, Min JK, Wood D, Johnson M, Ajlan AM, Wijesinghe N, Webb JG. Multidetector computed tomography in transcatheter aortic valve implantation. JACC Cardiovasc Imaging. 2011;4:416-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 219] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 9. | Cocchia R, D’Andrea A, Conte M, Cavallaro M, Riegler L, Citro R, Sirignano C, Imbriaco M, Cappelli M, Gregorio G. Patient selection for transcatheter aortic valve replacement: A combined clinical and multimodality imaging approach. World J Cardiol. 2017;9:212-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Barbanti M, Yang TH, Rodès Cabau J, Tamburino C, Wood DA, Jilaihawi H, Blanke P, Makkar RR, Latib A, Colombo A. Anatomical and procedural features associated with aortic root rupture during balloon-expandable transcatheter aortic valve replacement. Circulation. 2013;128:244-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 441] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 11. | Maeno Y, Abramowitz Y, Kawamori H, Kazuno Y, Kubo S, Takahashi N, Mangat G, Okuyama K, Kashif M, Chakravarty T. A Highly Predictive Risk Model for Pacemaker Implantation After TAVR. JACC Cardiovasc Imaging. 2017;10:1139-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 199] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 12. | Jilaihawi H, Makkar RR, Kashif M, Okuyama K, Chakravarty T, Shiota T, Friede G, Nakamura M, Doctor N, Rafique A. A revised methodology for aortic-valvar complex calcium quantification for transcatheter aortic valve implantation. Eur Heart J Cardiovasc Imaging. 2014;15:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 13. | Salgado RA, Leipsic JA, Shivalkar B, Ardies L, Van Herck PL, Op de Beeck BJ, Vrints C, Rodrigus I, Parizel PM, Bosmans J. Preprocedural CT evaluation of transcatheter aortic valve replacement: what the radiologist needs to know. Radiographics. 2014;34:1491-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Ribeiro HB, Webb JG, Makkar RR, Cohen MG, Kapadia SR, Kodali S, Tamburino C, Barbanti M, Chakravarty T, Jilaihawi H. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol. 2013;62:1552-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 465] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 15. | Toggweiler S, Gurvitch R, Leipsic J, Wood DA, Willson AB, Binder RK, Cheung A, Ye J, Webb JG. Percutaneous aortic valve replacement: vascular outcomes with a fully percutaneous procedure. J Am Coll Cardiol. 2012;59:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 246] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 16. | Toggweiler S, Leipsic J, Binder RK, Freeman M, Barbanti M, Heijmen RH, Wood DA, Webb JG. Management of vascular access in transcatheter aortic valve replacement: part 1: basic anatomy, imaging, sheaths, wires, and access routes. JACC Cardiovasc Interv. 2013;6:643-653. [PubMed] [DOI] [Full Text] |
| 17. | van der Boon RM, Marcheix B, Tchetche D, Chieffo A, Van Mieghem NM, Dumonteil N, Vahdat O, Maisano F, Serruys PW, Kappetein AP. Transapical versus transfemoral aortic valve implantation: a multicenter collaborative study. Ann Thorac Surg. 2014;97:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |