Published online Jun 26, 2025. doi: 10.4330/wjc.v17.i6.107386
Revised: April 11, 2025
Accepted: May 13, 2025
Published online: June 26, 2025
Processing time: 90 Days and 14.3 Hours
Atrial fibrillation (AF) is a growing global health burden, with a prevalence of over 52.55 million cases. Rising disability-adjusted life-years, increasing age, and disparities in care have contributed to the worsening severity and mortality of AF. Modifiable risk factors, such as hypertension, obesity, and diabetes mellitus, are associated with alterations in gut microbiota, making the gut-heart axis a potential therapeutic target. Gut dysbiosis influences AF pathogenesis through inflammation, metabolic disruption, and autonomic dysfunction. Key mechanisms include gut barrier dysfunction, short-chain fatty acid (SCFA) depletion, lipopolysaccharides (LPS)-induced inflammation, and ferroptosis-mediated atrial remodeling. Trimethylamine N-oxide, bile acids, and tryptophan metabolites contribute to arrhythmogenic remodeling. Emerging evidence suggests that dietary interventions, including prebiotics and probiotics, as well as gut surveillance, may help mitigate AF progression. Clinical implications of gut modulation in AF include personalized dietary strategies, microbiome assessment through metagenomic sequencing, and targeted interventions such as SCFA-based therapies and ferroptosis inhibition. Metabolite surveillance, including LPS and indoxyl sulfate monitoring, may influence the effectiveness of anticoagulant and antiarrhythmic therapy. Despite growing mechanistic evidence linking gut dysbiosis to AF, clinical applications remain unexplored. This review summarizes the current understanding of the gut microbiome's role in AF.
Core Tip: Gut microbiome alteration contributes to the generation of harmful metabolites, loss of gut integrity, and cardiac remodeling. Gut dysbiosis interacts with modifiable risk factors to promote cardiac tissue remodeling. Animal models and clinical studies indicate that microbial composition is associated with arrhythmogenesis, atrial fibrillation (AF) recurrence, and modulates medication response. Integrating microbiome surveillance and gut microbiome modulation therapy into AF management may slow disease progression and reduce the arrhythmia burden. However, clinical trials are needed to establish causality and support the incorporation of gut modulation into clinical care.
- Citation: Brar AS, Vemula SL, Yanamaladoddi V, Sodhi S, Hatwal J, Sohal A, Batta A. Impact of gut microbiome on atrial fibrillation: Mechanistic insights and future directions in individualized medicine. World J Cardiol 2025; 17(6): 107386
- URL: https://www.wjgnet.com/1949-8462/full/v17/i6/107386.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i6.107386
Atrial fibrillation (AF) affects an estimated 52.55 million people worldwide[1]. The rising number of disability-adjusted life-years (DALY), an indicator of disability, highlights the widening care gap in AF management and disease burden[1]. DALY rose from 3.3 million cases in 1990 to 8.3 million in 2021[1]. Over the past 30 years, increasing age, higher rates of modifiable risk factors, greater public awareness, and disparities in care have contributed to the growing severity and mortality of AF[1].
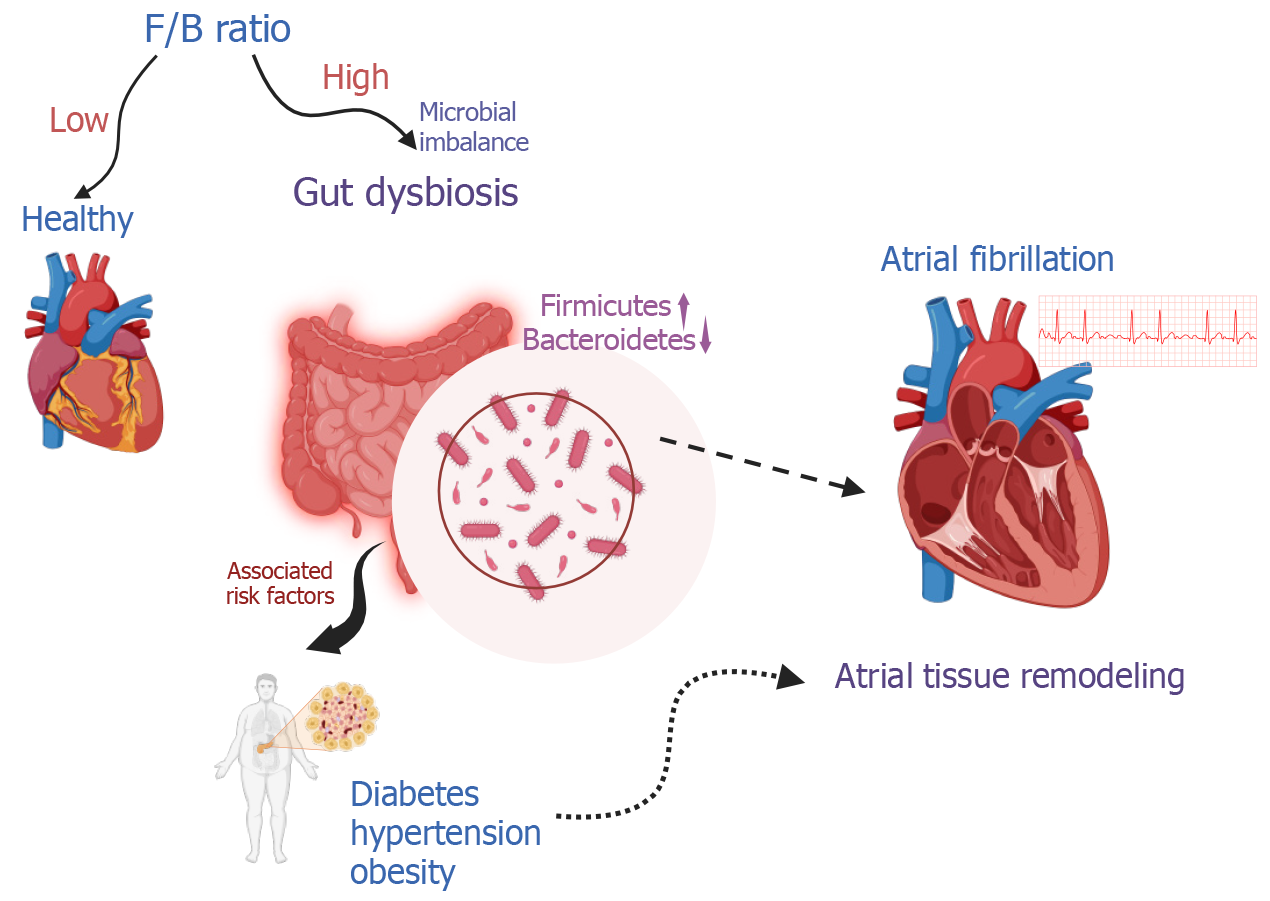
Gut dysbiosis is characterized by a high Firmicutes/Bacteroidetes (F/B) ratio and contributes to atrial tissue remodeling. Microbial imbalance (dysbiosis) interacts with modifiable AF risk factors, such as hypertension, obesity, and diabetes mellitus, which are linked to dietary factors (Figure 1)[2]. Chen et al[3] emphasizes that intestinal microbiome imbalance plays a significant role in the emergence and progression of AF[3]. The gut microbiota, a complex community of bacteria residing in the intestines, is directly influenced by dietary intake and can, when imbalanced, impact cardiovascular health[4]. Gut microbiome-targeted therapies are key areas for intervention.
This review examines the clinical significance of gut microbiota in AF, with a focus on current evidence and future implications. It reviews the mechanisms through which intestinal flora and their metabolites contribute to the onset of AF. We will also examine the bidirectional relationship between gut health and cardiac function. By correlating gut dysbiosis to established risk factors, this review underscores the potential for targeted interventions, the need for clinical evidence, and randomized controlled trials to characterize this association further.
Despite growing evidence linking gut dysbiosis to AF, a gap remains in clinical studies connecting gut microbiome-modulating therapies with improved AF outcomes[5]. This review discusses individualized therapeutic interventions to optimize AF management, including dietary modification, microbiome surveillance, and pharmacological strategies. Finally, we will highlight future research directions in this evolving field.
The gut microbiota is a complex community of bacteria, viruses, and fungi in our intestines[6]. It is primarily composed of Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteria. The F/B ratio is a relative estimate of intestinal microbial health (lower F/B ratio) or disease state (higher F/B ratio)(Figure 1)[7]. This ecosystem develops from birth and changes in response to diet, medication use, and overall health[8]. It produces substances crucial for the host's immune system development and regulation. Imbalances in the gut microbiota have been linked to various disorders, including cardiovascular diseases[9,10].
The gut microbiome is involved in cardiometabolic disease pathogenesis[11]. Diabetes, obesity, and hypertension increase AF risk and demonstrate gut dysbiosis[12–14]. However, the role of gut microbiota in AF within specific patient populations needs to be explored, and its potential as a therapeutic target warrants further investigation. Gut dysbiosis has been observed in individuals with AF through observational and small cohort studies. Zuo et al[15] found that patients with paroxysmal and persistent AF have distinct gut microbiota and metabolite profiles compared to non-AF individuals[15].
Additionally, the composition of gut bacteria varies with AF duration and treatment modality. Persistent AF is linked with a decreased abundance of Butyricicoccus and Paraprevotella and an increased abundance of Blautia, Dorea, and Coprococcus. However, the exact nature of the causal relationship between AF and gut microbiota requires further exploration.
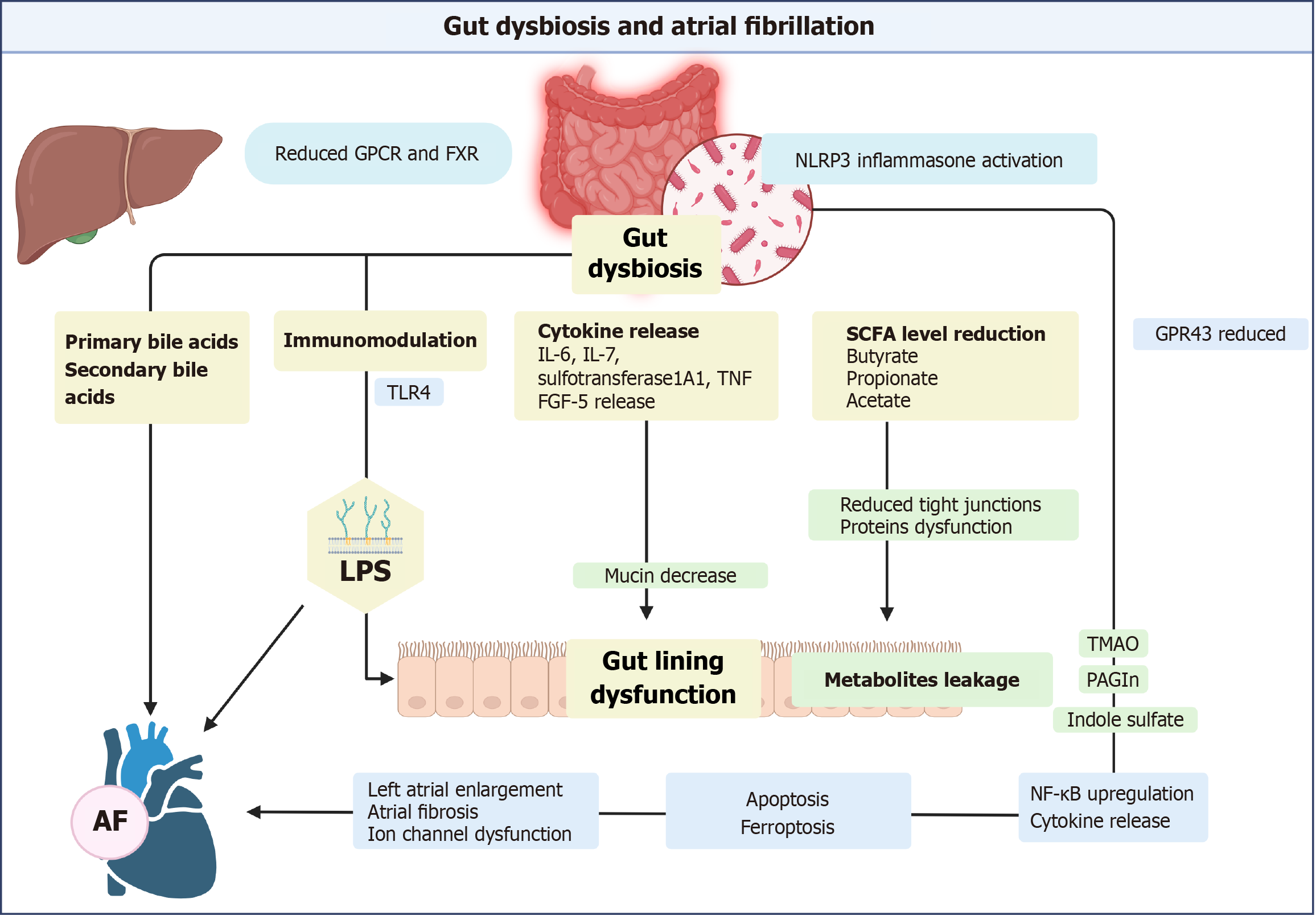
Gut dysbiosis contributes to AF through multiple interconnected pathways. Dysbiosis leads to a reduction in beneficial short-chain fatty acid (SCFA), loss of gut integrity, and leakage of harmful metabolites[10]. These disruptions trigger systemic inflammation, ferroptosis, and atrial remodeling, ultimately promoting AF (Figure 2). In the section below, we will discuss the various components involved in these pathways and their clinical relevance.
Gut dysbiosis damages the gut lining by triggering an inflammatory response and reducing the production of SCFAs. Low SCFA butyrate levels weaken the gut barrier, as butyrate increases tight junction proteins in the gut lining[10]. Harmful metabolites enter the circulation through dysfunctional mucosa. Reduced mucin production promotes AF through immunomodulation[16].
The endocannabinoid system dose-dependently improves gut integrity in mouse models[17]. It acts similarly on human coronary endothelial cell models. Enhanced integrity due to the endocannabinoid system prevents endothelial damage in response to hyperglycemia[18]. Dietary fibers and probiotics enhance gut integrity.
Ferroptosis is a cell death mediated by iron-dependent lipid peroxidation and is involved in AF pathogenesis[19]. It is regulated by glutathione peroxidase 4 (GPX4), an enzyme that inhibits lipid peroxidation[20]. Decreased GPX4 promotes iron accumulation, leading to phospholipid membrane dysfunction[21]. Membrane lipid peroxidation releases reactive oxygen species (ROS) and harmful metabolites, damaging organelles[20]. Iron buildup in the body upregulates cardiac ferroptosis by increasing the availability of availability of activated iron. Additionally, damage-associated molecular patterns cause immunomodulation.
Gut bacteria upregulate ferroptosis in response to cell damage or inflammation[22]. They regulate ferroptosis locally by modulating iron absorption and storage[23]. Additionally, gut microbiota influences hepcidin[24].
Ferroptosis causes mitochondrial dysfunction, oxidative stress, electrical remodeling, ion channel dysregulation, and impaired autophagy, contributing to AF[19]. Loss of mitochondria depletes the energy available for contraction. Iron-dependent Fenton reactions generate ROS that remodel atria[25,26]. Cytoplasmic calcium leakage, rectifier potassium channel upregulation, and alterations in connexin propagate electrical remodeling. Cellular changes reduce conduction velocity and shorten the atrial refractory period[27]. Additionally, it triggers the interleukin (IL)-6/signal transducer and activator of transcription 3 pathway and plays a role in hypercoagulability[28].
Clinical implication: Probiotics can regulate iron absorption. Probiotics containing Lactobacillus alimentarus NKU556 have improved iron absorption[29]. Bifidobacteria and Lactobacillus may enhance iron bioavailability by decreasing pondus hydrogenii. Conversely, Escherichia coli (E. coli) downregulates the Fenton reaction to reduce inflammation[30]. Lactobacillus rhamnosis reduces ROS generation by influencing NADPH oxidase 1 (NOX1), and Butyrate-producing bacteria reduce ROS by inhibiting NOX2.
Gut bacteria contribute to the AF substrate through their influence on metabolite production. These metabolites cross the intestinal lining, modulate immune responses, and function as signaling molecules within the host[11]. Nod-like receptor protein 3 (NLRP3) inflammasome activation generates a substrate for AF both directly and indirectly[31,32]. Inflammasome activation leads to the release of inflammatory cytokines and the development of AF[33]. Gut dysbiosis activates signaling pathways nuclear factor kappa B (NF-κB), toll-like receptor (TLR)-4, and NLRP3[34]. These pathways interact with CD40L, leukemia inhibitory factor receptor, IL-2 receptor subunit Beta, and Fms-related tyrosine kinase-3 ligand, resulting in the release of IL-6, IL-7, sulfotransferase 1A1, tumor necrosis factor (TNF), and fibroblast growth factor 5. These molecules potentiate AF risk through complex interactions. Additionally, the autonomic nervous system sustains AF and is a target for research focusing on pulmonary vein and atrial tissue changes[35,36].
The gut microbiota plays a crucial role in the production of trimethylamine N-oxide (TMAO). It converts dietary choline or L-carnitine into trimethylamine. This gaseous product enters the circulation and is oxidized into TMAO by hepatic flavin-containing monooxygenases[37]. MAO triggers inflammatory pathways, increasing autonomic activity and inducing AF. Yu et al[38] injected TMAO into specific nerve clusters in canine hearts, which resulted in rapid electrical changes compared to controls. Increased pro-inflammatory molecules, such as IL-1β, IL-6, and TNF-α, accompanied this. Similarly, elevated TMAO levels increased cardiac dysfunction, heart weight gain, and cardiac fibrosis in mice[39]. Cardiac changes were attributed to collagen accumulation, higher profibrotic markers, elevated inflammatory factors, and activation of the NLRP3 inflammasome.
While TMAO is linked to cardiovascular disease, its specific role in AF remains contested due to inconsistent findings across studies[40]. Svingen et al[41] noted that plasma TMAO levels and AF have a consistent association after adjusting for traditional AF risk factors and dietary choline intake. Conversely, Büttner et al[42] and Florea et al[43] observed no significant difference in plasma TMAO levels between individuals with and without AF when patients with coronary artery disease were removed from the analysis. The selection of the patients, the number of comorbidities, and the adjustment of confounders could explain the differences between the findings mentioned above.
Clinical implications: Due to the common risk factors shared by AF and many cardiac diseases, it is currently challenging to determine whether TMAO is a direct cause of AF or an incidental factor. Further clinical evidence is needed to validate its potential as a therapeutic target in the future.
Colonic microbiota ferment glucose and dietary fiber, producing SCFAs. SCFA is an energy source utilized by the gut, resulting in secondary byproducts. The byproducts differentially modulate the host's immune system. Acetate, butyrate, and propionate are the most active and important, accounting for 95% of the SCFA[44]. Sodium butyrate exhibits anti-inflammatory properties against lipopolysaccharides (LPS), an endotoxin found in Gram-negative bacteria[45]. Butyrate lowers blood pressure by increasing the levels of nitric oxide synthase.
SCFA enhances T-cell differentiation into effector T cells, such as T helper (Th) 1 and Th17 cells, while also promoting the coexistence of anti-inflammatory IL-10+ regulatory T cells. SCFAs bind to G-protein-coupled receptor 43 (GPR43), significantly modulating inflammatory responses[46,47]. G-protein coupled receptor activation reduces the expression of genes implicated in cardiac hypertrophy, cardiorenal fibrosis, and inflammation[48–50]. Animal studies have demonstrated that stimulation of GPR43 by SCFAs is essential for reducing inflammation. Conversely, GPR43-deficient immune cells, particularly leukocytes, exhibit increased production of inflammatory mediators and enhanced immune cell recruitment[51].
AF, aging, and gut dysbiosis reduce SCFA production. AF lowers SCFA-producing bacterial species (prevotella), Kyoto Encyclopedia of Genes and Genomes orthologues, and enzymes involved in SCFA synthesis. Leukocyte GPR43/NLRP3 interactions may be utilized for microbiome evaluation, as they mediate the effect of SCFAs on AF[52,53]. Fang et al[54] demonstrated that reduced acetic acid disrupted GPR43 and NLRP3 expression in peripheral blood leukocytes and contributed to left atrial enlargement. GPR43 mRNA levels correlate positively with acetic acid levels in the fecal samples and negatively with NLRP3 mRNA expression. Crucially, GPR43 levels are inversely correlated with the diameter of the left atrium.
Clinical implication: Direct evidence linking SCFAs to AF is limited. Indirectly, low SCFA levels are associated with traditional AF risk factors such as hypertension, obesity, heart failure (HF), and atherosclerosis[55]. Gut surveillance through a constructed GPR43–NLRP3 score demonstrates the potential for predicting AF.
LPS are an endotoxin found in the outer layer of gram-negative bacteria. LPS-producing microbes are more prevalent in the gut of AF patients. They are implicated in AF development due to their role in immunomodulation, inflammation, and loss of gut integrity. Kong et al[56] noted that a high-fat diet alters gut microbiota and increases LPS production.
LPS decreases the expression of L-type calcium channel subunits (α1C and β2) and increases the expression of connexin 43. TLR-4 activation upregulates NF-κB activation, which increases pro-inflammatory cytokines in the atria[10]. These changes shorten the effective refractory period and induce AF[57,58]. Aging exerts a similar effect on the atrial tissue as a rise in LPS concentration[34].
Incorporating LPS measurements into risk models significantly enhances their ability to predict new-onset AF (NOAF) in specific sub-groups. Ren et al[57] observed that elevated LPS levels in patients with ST-elevation myocardial infarction (MI) predicted the development of NOAF. Similarly, Xu et al[59] correlated the risk of NOAF after lung cancer surgery with LPS elevation.
Clinical implication: Incorporating LPS measurement into standard risk assessment models may improve NOAF prediction. Following a Mediterranean diet rich in fruits and legumes reduces circulating LPS levels and warrants further clinical inquiry[60].
Bile acids (BAs) are systemic signaling molecules involved in fat and vitamin absorption[61]: Taurine and glycine conjugation in the liver results in primary BAs (cholic and chenodeoxycholic acid). Gut microbiota deconjugation produces secondary BAs, including deoxycholic and lithocholic acid, which are then reabsorbed[62]. Farnesoid X receptor (FXR), a nuclear transcription factor, regulates BAs. It prevents BA overload, reducing bile acid-mediated adverse effects on atrial cardiomyocytes[63].
Gut dysbiosis decreases secondary BAs and increases primary BAs. AF patients have elevated chenodeoxycholic acid levels, which are associated with atrial enlargement and electrical abnormalities. BA ratio alteration promotes atrial cardiomyocyte apoptosis[64–66]. Additionally, primary BA induces structural remodeling, cardiac fibrosis, and NLRP3 inflammasome activation[66]. In contrast, secondary BAs, such as ursodeoxycholic acid, exert antiarrhythmic effects through stabilization of the cell membrane potential[67,68]. However, specific secondary BAs (glycolithocholate sulfate and glycocholenate sulfate) are linked with AF risk in African-American cohorts, suggesting a complex interaction[69].
Clinical implication: Steroidal and Nonsteroidal FXR agonists are currently the subject of pre-clinical and clinical trials and may have a potential therapeutic role[63].
Tryptophan is an amino acid involved in obesity-mediated inflammation[70]. The gut microbiome differentially metabolizes tryptophan into either beneficial or harmful metabolites, depending on the available substrate[71]. Clostridium sporogenes metabolized tryptophan into beneficial indoleacetic acid and indole propionic acid (IPA). Conversely, E. coli metabolizes tryptophan into the harmful indole through the tnaA-encodedtryptophanasee enzyme. The microbial composition determines whether tryptophan metabolism is detrimental or beneficial by regulating the metabolites formed. IPA maintains the gut lining by regulating the TLR pathway[72]. Downstream IPA inhibits atherosclerosis and exhibits an anti-inflammatory effect[73,74].
Indole sulfate is a harmful metabolite formed from indole in the liver and excreted by the kidneys[75]. It is implicated in arrhythmogenesis. In rabbit models, indoxyl sulfate (IS) promoted arrhythmogenesis in the arrhythmogenesis in the pulmonary veins and left atrium[76]. Arrhythmias are attributed to disruptions in cardiomyocyte calcium handling, profibrotic signaling, and oxidative stress[76,77]. However, discrepantly high IS plasma concentrations identified in animal models require contextual consideration, as humans have significantly lower circulating levels. Finally, kidney function regulates IS clearance; its accumulation in chronic kidney disease patients may create a proarrhythmic environment, as observed in animal models[75].
Clinical implication: The gut microbiome differentially metabolizes tryptophan into beneficial or harmful metabolites based on substrate availability.
Post-MI mouse models with gut dysfunction demonstrated Phenylacetylglutamine (PAGln) elevation and correlated it with the risk of NOAF in the cohort. PAGln-producing bacteria impair claudin-1 and occludin gut barrier proteins, resulting in PAGln translocation across the gut. PAGln activates the NLRP3 inflammasome in cardiac tissue, increasing atrial ectopy through the release of IL-1β, IL-6, and TNF-α[78]. Ferroptosis and PAGln-mediated collagen deposition impair electrical conduction through fibrosis and loss of ion channels[78].
Clinical implication: Improvement in gut integrity can reduce the risk of AF in post-MI patients.
Therapy targeting gut dysbiosis can decrease atrial tissue degeneration[55]. Compared to other cardiovascular diseases, gut microbiome surveillance, dietary alteration, and physical activity are underutilized in AF care. The lack of robust clinical evidence limits their use in clinical practice. Gut modulation therapy includes prebiotics, probiotics, dietary modification, supplementation, weight loss, and fecal microbiota transplantation. However, recent advances based on animal, clinical, and trial data highlight the potential of gut health in the care of AF (Table 1)[53,54,57,59,71,78,79].
| Ref. | Study year | Category | Index | Key findings |
| Ren et al[57] | 2024 | Observational study | 57 | LPS levels associated with NOAF in ST-elevation MI patients |
| Xu et al[59] | 2024 | Observational study | 59 | Serum LPS linked to NOAF in cancer patients |
| Sinha et al[71] | 2024 | In vivo and in vitro | 71 | Gut microbiome alters tryptophan metabolism |
| Fang et al[54] | 2024 | Humans | 54 | SCFA-dependent G-protein-coupled receptor 43/Nod-like receptor protein 3 score is associated with AF risk |
| Liu et al[53] | 2024 | Animal model | 53 | Relationship between gut dysbiosis, aging, and AF risk. Use of SCFA and fecal microbiota transplant to mitigate damage |
| Shi et al[79] | 2025 | Animal model | 126 | Lactobacillus gasseri prevents ibrutinib-associated AF |
| Wang et al[78] | 2025 | Animal model | 78 | Phenylacetylglutamine is associated with increased AF risk in post-MI mice |
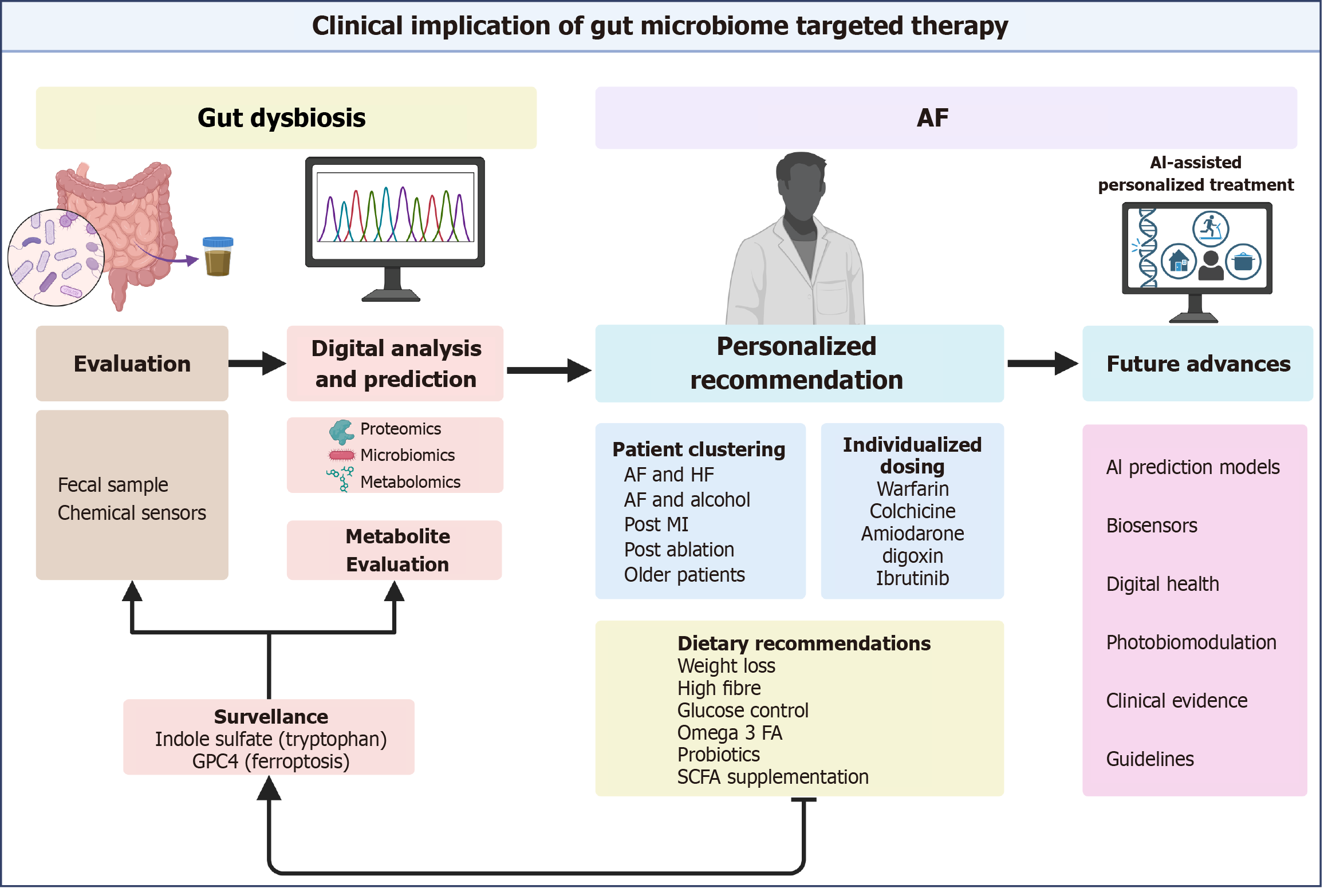
Microbiome evaluation can be done through fecal sampling. Organism culture, 16S ribosomal sequencing, and mouse models have traditionally been used for assessment but are limited by their precision. Shotgun metagenomic sequencing has revolutionized microbiome assessment[80]. It leverages computing algorithms for data analysis and can identify species that cannot be cultured in the laboratory. Additionally, metaproteomics and metabolomics help us identify proteins involved and byproducts formed in the gut[81,82].
Metabolite surveillance is central to effective gut modulation. Human studies have noted a decrease in IS after successful catheter ablation. Higher IS levels increase the risk of AF recurrence after ablation[83]. Notably, kidney function influences IS levels. Future research warrants further investigation in groups stratified by kidney function[84]. The complex interactions of metabolites with the human body contribute to inaccuracies and limit their use.
GPX4, a regulator of ferroptosis, can predict AF recurrence. Adding GPX4 levels and TGF-β levels to the Left atrial diameter measurements enhances the predictive ability to detect AF recurrence, based on a study of 249 patients[85]. Recent advances can help design wearable sensors that continuously monitor the gut microbiome. Ingestible capsules are implantable chemical sensors being investigated to monitor gut health[86,87]. These sensors are independent circuits with bioreceptors that can analyze body fluids and provide continuous feedback remotely. A similar wearable biosensor was recently used to monitor C-reactive protein continuously through sweat[88]. However, no agreement exists on assessing the microbiota composition or dietary patterns. In the future, prediction models may leverage existing clinical evidence, epidemiologic data, and metabolite evaluation to provide recommendations.
Personalized AF management identifies patients who benefit from gut modulation based on genetic information and comorbidity clustering (Figure 3). Therefore, identifying high-risk or high-benefit groups through microbiome evaluation enables physicians to provide individualized care. Additionally, patients may be divided into subgroups such as older patients, HF patients, post-MI patients, alcohol users, post-ablation patients, or those on certain drugs.
Age-mediated gut dysbiosis increases the risk of AF in older patients[34]. Aging contributes to elevated LPS levels, loss of gut integrity, and increased serum levels of harmful metabolites. These patients may benefit from glucose management, tryptophan supplementation, a high-fiber diet, and monitoring of indole-sulfate levels.
In two recent nested case-control studies, tryptophan supplementation increased the (1) Kynurenine: Tryptophan ratio; and (2) Reduced AF risk[89,90]. In mouse models, tryptophan has been shown to protect against age-mediated dysbiosis[91]. Enhanced gut integrity, improved microbiome diversity, and decreased pro-inflammatory cytokines may explain its beneficial effects[92].
Indole sulfate monitoring can guide tryptophan requirements. The oral tryptophan challenge test (OTCT) categorizes patients into low IS or high IS producers by measuring IS levels 48 hours after tryptophan administration[93]. High IS producers are at an increased AF risk and could benefit from low-tryptophan diets. A high-fiber diet in high IS producers shifts tryptophan metabolism to reduce levels of IS. Further research is needed to explore the clinical applications of OTCT in precision nutrition.
HF-driven intestinal congestion damages the gut mucosa and alters the gut microbiome[94,95]. This congestion gives rise to cardiac cachexia and is associated with poor outcomes[96]. Gut modulation can reduce gut inflammation and improve integrity. Patients with concomitant AF and HF may benefit from gut microbiome-altering therapy.
Alcohol displays a strong link with increased AF risk[97,98]. In these patients, autonomic nervous system dysfunction, ion channel damage, and fibrosis contribute to atrial remodeling[99]. SIRT3 signaling and ferroptosis have been identified as potential therapeutic targets for the treatment of various diseases. Ferroptosis inhibition is a novel therapy to reduce the risk of AF in populations with high alcohol consumption[100].
To effectively implement dietary recommendations, healthcare providers must be grounded in clearly defined biochemical pathways that are tailored to specific patient subgroups and supported by evidence[101]. Clinical evidence suggests that weight loss, physical activity, increased fiber intake, and supplementation can improve gut health[102-104]. Clustering patients based on comorbidities and microbiome phenotypes enables targeted modulation of the gut microbiome for therapeutic benefit.
Weight loss in obese patients restores a healthier F/B ratio and increases beneficial Rikenellaceae levels[102]. The increase in Rikenellaceae is crucial, as it mediates IL-6, linking the gut microbiome alteration to obesity-related inflammation. Animal models of Metabolic dysfunction-associated steatotic liver disease consistently demonstrate that a high-fat diet correlates with reduced Rikenellaceae levels, whereas low body mass index correlates with increased Rikenellaceae levels[103,104].
Moreover, improved glucose control suppresses LPS-driven NLRP3-inflammasome activation, a mechanism central to gut dysbiosis[34]. Aging-related gut barrier dysfunction permits increased LPS translocation, underscoring the importance of tight glycemic control in mitigating gut dysbiosis, particularly in older adults.
Fibre slows colon transit time, improves carbohydrate availability, and promotes the microbiome to produce beneficial SCFA-producing metabolites. In contrast, rapid transition limits carbohydrate availability and shifts the substrate to protein, which generates harmful metabolites[105]. Qi et al[106] followed 9290 patients for 5.7 years and reported that a high-fiber diet shifted tryptophan metabolism toward the beneficial IPA.
A high-fiber diet offers distinct benefits for AF and HF and positively influences tryptophan metabolism[71]. At a microbial level, HF is associated with low SCFA producers, such as Bifidobacterium and Lachnospiraceae[107]. Dietary Fibre indirectly boosts SCFA-producing species. Higher SCFA consequently counters atherogenesis, dyslipidemia, and neurohormonal activation[108]. Omega-3 fatty acid (FA), found in fish oil, reduces the harmful metabolites and increases levels of SCFA, such as butyrate[109,110]. A pooled analysis of 54799 patients showed a reduced incidence of AF with omega-3 FA supplementation[111]. Omega-3 FA increased beneficial Akkermansia, restored a healthier F/B ratio, and reduced TMAO, collectively improving endothelial function in a randomized controlled trial[112]. Additionally, mouse models have shown that Akkermansia reduces AF by lowering TMAO[113].
EFA includes FAs such as omega-3 and omega-6 precursors, alpha-linolenic acid (ALA), and linoleic acid (LA). An omega-6/omega-3 ratio of 10/1 to 3/1 is optimal[114,115]. However, Western diets commonly exceed this ratio, with a 15:1 ratio. This results in an omega-3 deficiency because omega-3 and omega-6 compete for the same enzyme, delta-6 desaturase.
ALA supplementation of up to 2.5 g/day reduced AF risk in a cohort of 54260 over a 16-year follow-up[116]. Despite the substrate’s preference for ALA, ALA and LA compete for delta-6-saturation reactions. Low ALA, compared to LA, increases anti-inflammatory Arachidonic acid production. Conversely, high ALA forms cell membranes and exhibits anti-inflammatory effects[117]. These findings highlight the gut microbiome's role in balancing the omega-3/omega-6 ratio and the benefits of ALA supplementation.
Harm from drugs with narrow therapeutic indexes and arrhythmogenic side effects of medications can be mitigated through gut modulation.
Vitamin K production by gut bacteria potentiates the effect of warfarin. Warfarin has a narrow therapeutic index requiring close monitoring. Enterococcus is associated with an increased response to warfarin[118]. Conversely, Bacteroides, Shigella, and Klebsiella are associated with a reduced response. Further exploration of warfarin's relationship with bacteria prevalent in patients with AF can improve individualized treatment regimens and enhance patient safety.
Omega-3 FAs are being investigated for their potential to improve gastrointestinal (GI) tolerability by influencing the gut microbiome[119]. Colchicine prevents AF recurrence after cardiac ablation; however, GI intolerance has limited its use[120,121]. GI side effects can be mitigated by using probiotics.
Actinobacterium Egerthella lenta metabolizes digoxin into inactive metabolite dihydro-digoxin through its action at the cardiac glycoside reductase (CGR) operon[122]. Digoxin has a narrow therapeutic index, with high concentrations correlated with mortality in AF patients[123,124]. The CGR protein to Eggerthella lenta ratio can predict the amount of digoxin lowered. Enhanced prediction of digoxin levels is critical to patients with HF and AF and requires continued inquiry[122].
Based on mouse models, the Probiotic E. coli-induced cytochrome p450 2C activation alters amiodarone metabolism[125]. Conversely, amiodarone exhibits antibacterial activity against E. coli, highlighting a bidirectional relationship[126]. Amiodarone is frequently prescribed in AF, and gut modulation may influence its effectiveness in the future.
SCFA-producer Lactobacillus gasseri reduces the risk of Ibrutinib-induced AF[79]. Ibrutinib is an anti-cancer drug that increases AF risk via the C-terminal Src kinase-Src pathway[127]. It also damages gut integrity. As a result, the gut microbiome mediates Irutinib's effect on atrial tissue and electrophysiology.
Gut modulation therapy is not yet well defined or integrated into standard care. Animal and clinical studies suggest that dysbiosis contributes to AF progression. However, several important issues must be addressed before gut microbiota can be considered a viable target for AF management.
Specifically, despite the correlation between gut dysbiosis and AF, the safety and efficacy of gut-modulating therapy are yet to be tested robustly in randomized controlled trials.
Most current studies on gut microbiota and AF have focused on bacteria, overlooking the potential involvement of other microorganisms such as fungi, viruses, archaea, and protists. The contribution of non-bacterial microorganisms, individually or as part of microbial communities, to AF development warrants further exploration. Additionally, extracting, sequencing, and identifying microorganisms with smaller genomes, such as bacteriophages, is challenging[128]. Instead of focusing on individual microbes, it is essential to explore the combined effects of microbial communities on gut microbiota.
Dietary habits significantly contribute to long-term changes in gut microbiota, but our understanding of this relationship remains limited. However, research indicates that nutritional patterns modulate the stability of gut microbiota over a six-month period[129]. More research is needed to determine the duration of dietary interventions required to produce lasting effects on gut microbiota.
Future studies should also consider whether certain probiotics and postbiotics can influence AF in humans and whether their use could be incorporated into risk factor modification programs for both primary and secondary prevention of AF[128,130].
Studies of association should be investigated through well-designed, randomized clinical trials that incorporate standardized, prospective microbiota analyses to establish causation. Future research should elucidate and characterize both the direct and indirect benefits of gut-modulating therapy in patients with AF.
Human research exploring the role of dysbiosis management in AF care is minimal[131]. The endocannabinoid system enhances gut integrity in mouse models in a dose-dependent manner[17]. It acts on tight junctions[132]. Human coronary endothelial cell models exhibit similar effects on membrane permeability at high glucose levels[18]. Decreased permeability mitigates endothelial damage in response to hyperglycemia. However, this needs human research.
Photobiomodulation altered the gut microbiome in obese mouse models[133]. The infrared and red light projected on mice’s abdomens over 2 weeks increased Allobaculum levels. Allobaculum improves gut integrity. Interestingly, exercise also increased Allobaculum levels in obese mice. However, this study was limited in number and requires further inquiry.
Gut dysbiosis plays a pivotal role in AF pathogenesis through inflammatory, metabolic, and autonomic pathways. Despite growing evidence linking gut microbiome alterations to the risk and progression of AF, clinical applications remain underexplored. Targeted interventions—such as dietary modifications, microbiome surveillance, and pharmacological strategies—offer promise for the prevention and management of AF.
Technological advancements in microbiome assessment, including metagenomics and wearable biosensors, offer new opportunities for personalized AF care. However, robust clinical trials are essential for validating gut-modulating therapies and establishing their role in routine AF management. Future research should focus on integrating microbiome-based precision medicine into AF care to bridge the evidence gap and improve patient outcomes.
| 1. | Cheng S, He J, Han Y, Han S, Li P, Liao H, Guo J. Global burden of atrial fibrillation/atrial flutter and its attributable risk factors from 1990 to 2021. Europace. 2024;26:euae195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
| 2. | Lau DH, Nattel S, Kalman JM, Sanders P. Modifiable Risk Factors and Atrial Fibrillation. Circulation. 2017;136:583-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 445] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 3. | Chen J, Wang Y, Wang K, Mei Z, Wang L. Exploring the Axis of Gut Microbiota-Inflammatory Cytokine-Atrial Fibrillation in the Pathogenesis of Atrial Fibrillation. J Cell Mol Med. 2025;29:e70379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Martins D, Silva C, Ferreira AC, Dourado S, Albuquerque A, Saraiva F, Batista AB, Castro P, Leite-Moreira A, Barros AS, Miranda IM. Unravelling the Gut Microbiome Role in Cardiovascular Disease: A Systematic Review and a Meta-Analysis. Biomolecules. 2024;14:731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 5. | Williams EG, Alissa M, Alsugoor MH, Albakri GS, Altamimi AA, Alabdullateef AA, Almansour NM, Aldakheel FM, Alessa S, Marber M. Integrative approaches to atrial fibrillation prevention and management: Leveraging gut health for improved cardiovascular outcomes in the aging population. Curr Probl Cardiol. 2025;50:102952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the human microbiome. Nutr Rev. 2012;70 Suppl 1:S38-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 714] [Article Influence: 54.9] [Reference Citation Analysis (1)] |
| 7. | De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691-14696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3584] [Cited by in RCA: 4034] [Article Influence: 268.9] [Reference Citation Analysis (0)] |
| 8. | Hasan N, Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019;7:e7502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 442] [Article Influence: 73.7] [Reference Citation Analysis (3)] |
| 9. | Emoto T, Yamashita T, Sasaki N, Hirota Y, Hayashi T, So A, Kasahara K, Yodoi K, Matsumoto T, Mizoguchi T, Ogawa W, Hirata K. Analysis of Gut Microbiota in Coronary Artery Disease Patients: a Possible Link between Gut Microbiota and Coronary Artery Disease. J Atheroscler Thromb. 2016;23:908-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 10. | Suresh MG, Mohamed S, Yukselen Z, Hatwal J, Venkatakrishnan A, Metri A, Bhardwaj A, Singh A, Bush N, Batta A. Therapeutic Modulation of Gut Microbiome in Cardiovascular Disease: A Literature Review. Heart Mind. 2025;9:68-79. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Vinjé S, Stroes E, Nieuwdorp M, Hazen SL. The gut microbiome as novel cardio-metabolic target: the time has come! Eur Heart J. 2014;35:883-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Yang G, Wei J, Liu P, Zhang Q, Tian Y, Hou G, Meng L, Xin Y, Jiang X. Role of the gut microbiota in type 2 diabetes and related diseases. Metabolism. 2021;117:154712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 226] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 13. | Maruvada P, Leone V, Kaplan LM, Chang EB. The Human Microbiome and Obesity: Moving beyond Associations. Cell Host Microbe. 2017;22:589-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 341] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 14. | Marques FZ, Mackay CR, Kaye DM. Beyond gut feelings: how the gut microbiota regulates blood pressure. Nat Rev Cardiol. 2018;15:20-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 281] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 15. | Zuo K, Li J, Li K, Hu C, Gao Y, Chen M, Hu R, Liu Y, Chi H, Wang H, Qin Y, Liu X, Li S, Cai J, Zhong J, Yang X. Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. Gigascience. 2019;8:giz058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 151] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 16. | DeJong EN, Surette MG, Bowdish DME. The Gut Microbiota and Unhealthy Aging: Disentangling Cause from Consequence. Cell Host Microbe. 2020;28:180-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 237] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 17. | Alhamoruni A, Lee AC, Wright KL, Larvin M, O'Sullivan SE. Pharmacological effects of cannabinoids on the Caco-2 cell culture model of intestinal permeability. J Pharmacol Exp Ther. 2010;335:92-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Rajesh M, Mukhopadhyay P, Bátkai S, Haskó G, Liaudet L, Drel VR, Obrosova IG, Pacher P. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am J Physiol Heart Circ Physiol. 2007;293:H610-H619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 19. | Fan S, Hu Y, Shi J. Role of ferroptosis in atrial fibrillation: a review. Front Pharmacol. 2025;16:1362060. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Conrad M, Pratt DA. The chemical basis of ferroptosis. Nat Chem Biol. 2019;15:1137-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 661] [Article Influence: 110.2] [Reference Citation Analysis (0)] |
| 21. | Liu LL, Liu ZK, Zhang L, Li N, Fang T, Zhang DL, Xu GZ, Zhan SY. [Epidemiological and etiological characteristics of hand, foot and mouth disease among children aged 5 years and younger in Ningbo (2016 to 2019)]. Beijing Da Xue Xue Bao Yi Xue Ban. 2021;53:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Yao T, Li L. The influence of microbiota on ferroptosis in intestinal diseases. Gut Microbes. 2023;15:2263210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 23. | Das NK, Schwartz AJ, Barthel G, Inohara N, Liu Q, Sankar A, Hill DR, Ma X, Lamberg O, Schnizlein MK, Arqués JL, Spence JR, Nunez G, Patterson AD, Sun D, Young VB, Shah YM. Microbial Metabolite Signaling Is Required for Systemic Iron Homeostasis. Cell Metab. 2020;31:115-130.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 199] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 24. | Shanmugam NK, Trebicka E, Fu LL, Shi HN, Cherayil BJ. Intestinal inflammation modulates expression of the iron-regulating hormone hepcidin depending on erythropoietic activity and the commensal microbiota. J Immunol. 2014;193:1398-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Clark A, Mach N. The Crosstalk between the Gut Microbiota and Mitochondria during Exercise. Front Physiol. 2017;8:319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 234] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 26. | Wang S, Li F, Qiao R, Hu X, Liao H, Chen L, Wu J, Wu H, Zhao M, Liu J, Chen R, Ma X, Kim D, Sun J, Davis TP, Chen C, Tian J, Hyeon T, Ling D. Arginine-Rich Manganese Silicate Nanobubbles as a Ferroptosis-Inducing Agent for Tumor-Targeted Theranostics. ACS Nano. 2018;12:12380-12392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 286] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 27. | Lenaerts I, Bito V, Heinzel FR, Driesen RB, Holemans P, D'hooge J, Heidbüchel H, Sipido KR, Willems R. Ultrastructural and functional remodeling of the coupling between Ca2+ influx and sarcoplasmic reticulum Ca2+ release in right atrial myocytes from experimental persistent atrial fibrillation. Circ Res. 2009;105:876-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 28. | Yaegashi T, Kato T, Usui S, Kanamori N, Furusho H, Takashima SI, Murai H, Kaneko S, Takamura M. Short-term rapid atrial pacing alters the gene expression profile of rat liver: Cardiohepatic interaction in atrial fibrillation. Heart Rhythm. 2016;13:2368-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Zhao N, Liu JM, Yang FE, Ji XM, Li CY, Lv SW, Wang S. A Novel Mediation Strategy of DSS-Induced Colitis in Mice Based on an Iron-Enriched Probiotic and In Vivo Bioluminescence Tracing. J Agric Food Chem. 2020;68:12028-12038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Pilarczyk-Zurek M, Strus M, Adamski P, Heczko PB. The dual role of Escherichia coli in the course of ulcerative colitis. BMC Gastroenterol. 2016;16:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Yao C, Veleva T, Scott L Jr, Cao S, Li L, Chen G, Jeyabal P, Pan X, Alsina KM, Abu-Taha I Dr, Ghezelbash S, Reynolds CL, Shen YH, LeMaire SA, Schmitz W, Müller FU, El-Armouche A, Tony Eissa N, Beeton C, Nattel S, Wehrens XHT, Dobrev D, Li N. Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation. 2018;138:2227-2242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 475] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 32. | Heijman J, Muna AP, Veleva T, Molina CE, Sutanto H, Tekook M, Wang Q, Abu-Taha IH, Gorka M, Künzel S, El-Armouche A, Reichenspurner H, Kamler M, Nikolaev V, Ravens U, Li N, Nattel S, Wehrens XHT, Dobrev D. Atrial Myocyte NLRP3/CaMKII Nexus Forms a Substrate for Postoperative Atrial Fibrillation. Circ Res. 2020;127:1036-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 211] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 33. | Wang Y, Liu X, Shi H, Yu Y, Yu Y, Li M, Chen R. NLRP3 inflammasome, an immune-inflammatory target in pathogenesis and treatment of cardiovascular diseases. Clin Transl Med. 2020;10:91-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 34. | Zhang Y, Zhang S, Li B, Luo Y, Gong Y, Jin X, Zhang J, Zhou Y, Zhuo X, Wang Z, Zhao X, Han X, Gao Y, Yu H, Liang D, Zhao S, Sun D, Wang D, Xu W, Qu G, Bo W, Li D, Wu Y, Li Y. Gut microbiota dysbiosis promotes age-related atrial fibrillation by lipopolysaccharide and glucose-induced activation of NLRP3-inflammasome. Cardiovasc Res. 2022;118:785-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 155] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 35. | Lau DH, Linz D, Schotten U, Mahajan R, Sanders P, Kalman JM. Pathophysiology of Paroxysmal and Persistent Atrial Fibrillation: Rotors, Foci and Fibrosis. Heart Lung Circ. 2017;26:887-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 36. | Al-Kaisey AM, Kalman JM. Obesity and Atrial Fibrillation: Epidemiology, Pathogenesis and Effect of Weight Loss. Arrhythm Electrophysiol Rev. 2021;10:159-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Yang S, Li X, Yang F, Zhao R, Pan X, Liang J, Tian L, Li X, Liu L, Xing Y, Wu M. Gut Microbiota-Dependent Marker TMAO in Promoting Cardiovascular Disease: Inflammation Mechanism, Clinical Prognostic, and Potential as a Therapeutic Target. Front Pharmacol. 2019;10:1360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 227] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 38. | Yu L, Meng G, Huang B, Zhou X, Stavrakis S, Wang M, Li X, Zhou L, Wang Y, Wang M, Wang Z, Deng J, Po SS, Jiang H. A potential relationship between gut microbes and atrial fibrillation: Trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int J Cardiol. 2018;255:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 39. | Li X, Geng J, Zhao J, Ni Q, Zhao C, Zheng Y, Chen X, Wang L. Trimethylamine N-Oxide Exacerbates Cardiac Fibrosis via Activating the NLRP3 Inflammasome. Front Physiol. 2019;10:866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 40. | Verhaar BJH, Prodan A, Nieuwdorp M, Muller M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients. 2020;12:2982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 245] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 41. | Svingen GFT, Zuo H, Ueland PM, Seifert R, Løland KH, Pedersen ER, Schuster PM, Karlsson T, Tell GS, Schartum-Hansen H, Olset H, Svenningsson M, Strand E, Nilsen DW, Nordrehaug JE, Dhar I, Nygård O. Increased plasma trimethylamine-N-oxide is associated with incident atrial fibrillation. Int J Cardiol. 2018;267:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 42. | Büttner P, Okun JG, Hauke J, Holzwirth E, Obradovic D, Hindricks G, Thiele H, Kornej J. Trimethylamine N-oxide in atrial fibrillation progression. Int J Cardiol Heart Vasc. 2020;29:100554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Florea CM, Rosu RO, Minciuna IA, Cismaru G, Pop D, Vlase AM, Nenu I, Filip GA. The Impact of Trimethylamine N-Oxide on Atrial Fibrillation Presence in Patients with Cardiovascular Disease. J Xenobiot. 2025;15:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 44. | Tang WHW, Li DY, Hazen SL. Dietary metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol. 2019;16:137-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 511] [Article Influence: 102.2] [Reference Citation Analysis (0)] |
| 45. | Huuskonen J, Suuronen T, Nuutinen T, Kyrylenko S, Salminen A. Regulation of microglial inflammatory response by sodium butyrate and short-chain fatty acids. Br J Pharmacol. 2004;141:874-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 200] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 46. | Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, Kim CH. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8:80-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 575] [Cited by in RCA: 871] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 47. | Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, Parmentier M, Detheux M. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481-25489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1107] [Cited by in RCA: 1234] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 48. | Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan NA, Berkowitz DE, Pluznick JL. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol Genomics. 2016;48:826-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 316] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 49. | Park J, Wang Q, Wu Q, Mao-Draayer Y, Kim CH. Bidirectional regulatory potentials of short-chain fatty acids and their G-protein-coupled receptors in autoimmune neuroinflammation. Sci Rep. 2019;9:8837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 50. | Marques FZ, Nelson E, Chu PY, Horlock D, Fiedler A, Ziemann M, Tan JK, Kuruppu S, Rajapakse NW, El-Osta A, Mackay CR, Kaye DM. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation. 2017;135:964-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 737] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 51. | Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2007] [Cited by in RCA: 2399] [Article Influence: 149.9] [Reference Citation Analysis (0)] |
| 52. | Zhang J, Zuo K, Fang C, Yin X, Liu X, Zhong J, Li K, Li J, Xu L, Yang X. Altered synthesis of genes associated with short-chain fatty acids in the gut of patients with atrial fibrillation. BMC Genomics. 2021;22:634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 53. | Liu L, Yi Y, Yan R, Hu R, Sun W, Zhou W, Zhou H, Si X, Ye Y, Li W, Chen J. Impact of age-related gut microbiota dysbiosis and reduced short-chain fatty acids on the autonomic nervous system and atrial fibrillation in rats. Front Cardiovasc Med. 2024;11:1394929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 54. | Fang C, Zuo K, Liu Z, Xu L, Yang X. Disordered GPR43/NLRP3 expression in peripheral leukocytes of patients with atrial fibrillation is associated with intestinal short chain fatty acids levels. Eur J Med Res. 2024;29:233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 55. | Gawałko M, Agbaedeng TA, Saljic A, Müller DN, Wilck N, Schnabel R, Penders J, Rienstra M, van Gelder I, Jespersen T, Schotten U, Crijns HJGM, Kalman JM, Sanders P, Nattel S, Dobrev D, Linz D. Gut microbiota, dysbiosis and atrial fibrillation. Arrhythmogenic mechanisms and potential clinical implications. Cardiovasc Res. 2022;118:2415-2427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 56. | Kong B, Fu H, Xiao Z, Zhou Y, Shuai W, Huang H. Gut Microbiota Dysbiosis Induced by a High-Fat Diet Increases Susceptibility to Atrial Fibrillation. Can J Cardiol. 2022;38:1962-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 57. | Ren H, Wang Z, Li Y, Liu J. Association of lipopolysaccharide with new-onset atrial fibrillation in ST-segment elevation myocardial infarction. Heliyon. 2024;10:e27552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 58. | Chen YY, Sun ZW, Jiang JP, Kang XD, Wang LL, Shen YL, Xie XD, Zheng LR. α-adrenoceptor-mediated enhanced inducibility of atrial fibrillation in a canine system inflammation model. Mol Med Rep. 2017;15:3767-3774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | Xu H, Zhou J, Ye F, Gao Y. Serum lipopolysaccharide associated with new-onset atrial fibrillation in patients with non-small-cell lung cancer a retrospective observational study. Front Surg. 2024;11:1404450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 60. | Pastori D, Carnevale R, Nocella C, Novo M, Santulli M, Cammisotto V, Menichelli D, Pignatelli P, Violi F. Gut-Derived Serum Lipopolysaccharide is Associated With Enhanced Risk of Major Adverse Cardiovascular Events in Atrial Fibrillation: Effect of Adherence to Mediterranean Diet. J Am Heart Assoc. 2017;6:e005784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 61. | Desai MS, Penny DJ. Bile acids induce arrhythmias: old metabolite, new tricks. Heart. 2013;99:1629-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 62. | Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1641] [Cited by in RCA: 1695] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 63. | Fleishman JS, Kumar S. Bile acid metabolism and signaling in health and disease: molecular mechanisms and therapeutic targets. Signal Transduct Target Ther. 2024;9:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 100] [Article Influence: 100.0] [Reference Citation Analysis (0)] |
| 64. | Wang XH, Li Z, Zang MH, Yao TB, Mao JL, Pu J. Circulating primary bile acid is correlated with structural remodeling in atrial fibrillation. J Interv Card Electrophysiol. 2020;57:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Pu J, Yuan A, Shan P, Gao E, Wang X, Wang Y, Lau WB, Koch W, Ma XL, He B. Cardiomyocyte-expressed farnesoid-X-receptor is a novel apoptosis mediator and contributes to myocardial ischaemia/reperfusion injury. Eur Heart J. 2013;34:1834-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 66. | Gong Z, Zhou J, Zhao S, Tian C, Wang P, Xu C, Chen Y, Cai W, Wu J. Chenodeoxycholic acid activates NLRP3 inflammasome and contributes to cholestatic liver fibrosis. Oncotarget. 2016;7:83951-83963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 67. | Zhu Y, Shui X, Liang Z, Huang Z, Qi Y, He Y, Chen C, Luo H, Lei W. Gut microbiota metabolites as integral mediators in cardiovascular diseases (Review). Int J Mol Med. 2020;46:936-948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 68. | Lofthouse EM, Torrens C, Manousopoulou A, Nahar M, Cleal JK, O'Kelly IM, Sengers BG, Garbis SD, Lewis RM. Ursodeoxycholic acid inhibits uptake and vasoconstrictor effects of taurocholate in human placenta. FASEB J. 2019;33:8211-8220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 69. | Alonso A, Yu B, Qureshi WT, Grams ME, Selvin E, Soliman EZ, Loehr LR, Chen LY, Agarwal SK, Alexander D, Boerwinkle E. Metabolomics and Incidence of Atrial Fibrillation in African Americans: The Atherosclerosis Risk in Communities (ARIC) Study. PLoS One. 2015;10:e0142610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 70. | Favennec M, Hennart B, Caiazzo R, Leloire A, Yengo L, Verbanck M, Arredouani A, Marre M, Pigeyre M, Bessede A, Guillemin GJ, Chinetti G, Staels B, Pattou F, Balkau B, Allorge D, Froguel P, Poulain-Godefroy O. Erratum: The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity (Silver Spring). 2016;24:1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 71. | Sinha AK, Laursen MF, Brinck JE, Rybtke ML, Hjørne AP, Procházková N, Pedersen M, Roager HM, Licht TR. Dietary fibre directs microbial tryptophan metabolism via metabolic interactions in the gut microbiota. Nat Microbiol. 2024;9:1964-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 58] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 72. | Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, Fleet JC, Kortagere S, Mukherjee P, Fasano A, Le Ven J, Nicholson JK, Dumas ME, Khanna KM, Mani S. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity. 2014;41:296-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 733] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 73. | Xue H, Chen X, Yu C, Deng Y, Zhang Y, Chen S, Chen X, Chen K, Yang Y, Ling W. Gut Microbially Produced Indole-3-Propionic Acid Inhibits Atherosclerosis by Promoting Reverse Cholesterol Transport and Its Deficiency Is Causally Related to Atherosclerotic Cardiovascular Disease. Circ Res. 2022;131:404-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 124] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 74. | Zhang B, Jiang M, Zhao J, Song Y, Du W, Shi J. The Mechanism Underlying the Influence of Indole-3-Propionic Acid: A Relevance to Metabolic Disorders. Front Endocrinol (Lausanne). 2022;13:841703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 75. | Leong SC, Sirich TL. Indoxyl Sulfate-Review of Toxicity and Therapeutic Strategies. Toxins (Basel). 2016;8:358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 184] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 76. | Chen WT, Chen YC, Hsieh MH, Huang SY, Kao YH, Chen YA, Lin YK, Chen SA, Chen YJ. The uremic toxin indoxyl sulfate increases pulmonary vein and atrial arrhythmogenesis. J Cardiovasc Electrophysiol. 2015;26:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 77. | Aoki K, Teshima Y, Kondo H, Saito S, Fukui A, Fukunaga N, Nawata T, Shimada T, Takahashi N, Shibata H. Role of Indoxyl Sulfate as a Predisposing Factor for Atrial Fibrillation in Renal Dysfunction. J Am Heart Assoc. 2015;4:e002023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 78. | Wang G, He Q, Shuai W, Yang H, Kong B, Lu S, Gong Y. The gut microbial metabolite phenylacetylglutamine increases susceptibility to atrial fibrillation after myocardial infarction through ferroptosis and NLRP3 inflammasome. Apoptosis. 2025;30:210-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 79. | Shi L, Duan Y, Fang N, Zhang N, Yan S, Wang K, Hou T, Wang Z, Jiang X, Gao Q, Zhang S, Li Y, Zhang Y, Gong Y. Lactobacillus gasseri prevents ibrutinib-associated atrial fibrillation through butyrate. Europace. 2025;27:euaf018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 80. | Zaheer R, Noyes N, Ortega Polo R, Cook SR, Marinier E, Van Domselaar G, Belk KE, Morley PS, McAllister TA. Impact of sequencing depth on the characterization of the microbiome and resistome. Sci Rep. 2018;8:5890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 81. | Verberkmoes NC, Russell AL, Shah M, Godzik A, Rosenquist M, Halfvarson J, Lefsrud MG, Apajalahti J, Tysk C, Hettich RL, Jansson JK. Shotgun metaproteomics of the human distal gut microbiota. ISME J. 2009;3:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 394] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 82. | Vojinovic D, Radjabzadeh D, Kurilshikov A, Amin N, Wijmenga C, Franke L, Ikram MA, Uitterlinden AG, Zhernakova A, Fu J, Kraaij R, van Duijn CM. Relationship between gut microbiota and circulating metabolites in population-based cohorts. Nat Commun. 2019;10:5813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 186] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 83. | Yamagami F, Tajiri K, Yumino D, Ieda M. Uremic Toxins and Atrial Fibrillation: Mechanisms and Therapeutic Implications. Toxins (Basel). 2019;11:597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 84. | Yang J, Li H, Zhang C, Zhou Y. Indoxyl sulfate reduces Ito,f by activating ROS/MAPK and NF-κB signaling pathways. JCI Insight. 2022;7:e145475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 85. | Liu T, Zhou D, Liu F, Long D, Yang Y, Li M, Zhao X, Li C, Wang W, Jiang C, Tang R. Glutathione peroxidase 4 as a potential biomarker for atrial fibrosis and recurrence of atrial fibrillation. Heart Rhythm O2. 2025;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 86. | Brasier N, Wang J, Gao W, Sempionatto JR, Dincer C, Ates HC, Güder F, Olenik S, Schauwecker I, Schaffarczyk D, Vayena E, Ritz N, Weisser M, Mtenga S, Ghaffari R, Rogers JA, Goldhahn J. Applied body-fluid analysis by wearable devices. Nature. 2024;636:57-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 87. | Hu C, Wang L, Liu S, Sheng X, Yin L. Recent Development of Implantable Chemical Sensors Utilizing Flexible and Biodegradable Materials for Biomedical Applications. ACS Nano. 2024;18:3969-3995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 88. | Tu J, Min J, Song Y, Xu C, Li J, Moore J, Hanson J, Hu E, Parimon T, Wang TY, Davoodi E, Chou TF, Chen P, Hsu JJ, Rossiter HB, Gao W. A wireless patch for the monitoring of C-reactive protein in sweat. Nat Biomed Eng. 2023;7:1293-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 135] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 89. | Purton T, Staskova L, Lane MM, Dawson SL, West M, Firth J, Clarke G, Cryan JF, Berk M, O'Neil A, Dean O, Hadi A, Honan C, Marx W. Prebiotic and probiotic supplementation and the tryptophan-kynurenine pathway: A systematic review and meta analysis. Neurosci Biobehav Rev. 2021;123:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 90. | Razquin C, Ruiz-Canela M, Toledo E, Hernández-Alonso P, Clish CB, Guasch-Ferré M, Li J, Wittenbecher C, Dennis C, Alonso-Gómez A, Fitó M, Liang L, Corella D, Gómez-Gracia E, Estruch R, Fiol M, Lapetra J, Serra-Majem L, Ros E, Aros F, Salas-Salvadó J, Hu FB, Martínez-González MA. Metabolomics of the tryptophan-kynurenine degradation pathway and risk of atrial fibrillation and heart failure: potential modification effect of Mediterranean diet. Am J Clin Nutr. 2021;114:1646-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 91. | Yusufu I, Ding K, Smith K, Wankhade UD, Sahay B, Patterson GT, Pacholczyk R, Adusumilli S, Hamrick MW, Hill WD, Isales CM, Fulzele S. A Tryptophan-Deficient Diet Induces Gut Microbiota Dysbiosis and Increases Systemic Inflammation in Aged Mice. Int J Mol Sci. 2021;22:5005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 92. | Sorgdrager FJH, Naudé PJW, Kema IP, Nollen EA, Deyn PP. Tryptophan Metabolism in Inflammaging: From Biomarker to Therapeutic Target. Front Immunol. 2019;10:2565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 249] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 93. | Lin TY, Wu WK, Hung SC. High interindividual variability of indoxyl sulfate production identified by an oral tryptophan challenge test. NPJ Biofilms Microbiomes. 2025;11:15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 94. | Mocan D, Jipa R, Jipa DA, Lala RI, Rasinar FC, Groza I, Sabau R, Sulea Bratu D, Balta DF, Cioban ST, Puschita M. Unveiling the Systemic Impact of Congestion in Heart Failure: A Narrative Review of Multisystem Pathophysiology and Clinical Implications. J Cardiovasc Dev Dis. 2025;12:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 95. | Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, Schroedl W, Karhausen T, Doehner W, Rauchhaus M, Poole-Wilson P, Volk HD, Lochs H, Anker SD. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1561-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 477] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 96. | Anderson K, Schmitt A, Weintraub W, Scally R, Steen T. Cardiac Cachexia and the Associations to the Microbiome: State‐of‐the‐Science Review. JCSM Communications. 2025;8. [DOI] [Full Text] |
| 97. | Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, Alasady M, Hanley L, Antic NA, McEvoy RD, Kalman JM, Abhayaratna WP, Sanders P. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. 2014;64:2222-2231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 713] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 98. | Voskoboinik A, Kalman JM, De Silva A, Nicholls T, Costello B, Nanayakkara S, Prabhu S, Stub D, Azzopardi S, Vizi D, Wong G, Nalliah C, Sugumar H, Wong M, Kotschet E, Kaye D, Taylor AJ, Kistler PM. Alcohol Abstinence in Drinkers with Atrial Fibrillation. N Engl J Med. 2020;382:20-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 292] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 99. | Zhang H, Ruan H, Rahmutula D, Wilson E, Marcus GM, Vedantham V, Olgin JE. Effect of acute and chronic ethanol on atrial fibrillation vulnerability in rats. Heart Rhythm. 2020;17:654-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 100. | Yu LM, Dong X, Xu YL, Zhou ZJ, Huang YT, Zhao JK, Xu DY, Xue XD, Zhao QS, Liu T, Yin ZT, Jiang H, Wang HS. Icariin attenuates excessive alcohol consumption-induced susceptibility to atrial fibrillation through SIRT3 signaling. Biochim Biophys Acta Mol Basis Dis. 2022;1868:166483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 101. | Heymsfield SB, Shapses SA. Guidance on Energy and Macronutrients across the Life Span. N Engl J Med. 2024;390:1299-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 102. | Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA, Hunault G, Oberti F, Calès P, Diehl AM. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 763] [Cited by in RCA: 1052] [Article Influence: 116.9] [Reference Citation Analysis (0)] |
| 103. | Dai XC, Yu Y, Zhou SY, Yu S, Xiang MX, Ma H. Assessment of the causal relationship between gut microbiota and cardiovascular diseases: a bidirectional Mendelian randomization analysis. BioData Min. 2024;17:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 104. | Oki K, Toyama M, Banno T, Chonan O, Benno Y, Watanabe K. Comprehensive analysis of the fecal microbiota of healthy Japanese adults reveals a new bacterial lineage associated with a phenotype characterized by a high frequency of bowel movements and a lean body type. BMC Microbiol. 2016;16:284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 105. | Roager HM, Hansen LB, Bahl MI, Frandsen HL, Carvalho V, Gøbel RJ, Dalgaard MD, Plichta DR, Sparholt MH, Vestergaard H, Hansen T, Sicheritz-Pontén T, Nielsen HB, Pedersen O, Lauritzen L, Kristensen M, Gupta R, Licht TR. Colonic transit time is related to bacterial metabolism and mucosal turnover in the gut. Nat Microbiol. 2016;1:16093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 313] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 106. | Qi Q, Li J, Yu B, Moon JY, Chai JC, Merino J, Hu J, Ruiz-Canela M, Rebholz C, Wang Z, Usyk M, Chen GC, Porneala BC, Wang W, Nguyen NQ, Feofanova EV, Grove ML, Wang TJ, Gerszten RE, Dupuis J, Salas-Salvadó J, Bao W, Perkins DL, Daviglus ML, Thyagarajan B, Cai J, Wang T, Manson JE, Martínez-González MA, Selvin E, Rexrode KM, Clish CB, Hu FB, Meigs JB, Knight R, Burk RD, Boerwinkle E, Kaplan RC. Host and gut microbial tryptophan metabolism and type 2 diabetes: an integrative analysis of host genetics, diet, gut microbiome and circulating metabolites in cohort studies. Gut. 2022;71:1095-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 156] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 107. | Mayerhofer CCK, Kummen M, Holm K, Broch K, Awoyemi A, Vestad B, Storm-Larsen C, Seljeflot I, Ueland T, Bohov P, Berge RK, Svardal A, Gullestad L, Yndestad A, Aukrust P, Hov JR, Trøseid M. Low fibre intake is associated with gut microbiota alterations in chronic heart failure. ESC Heart Fail. 2020;7:456-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 108. | Liu J, An N, Ma C, Li X, Zhang J, Zhu W, Zhang Y, Li J. Correlation analysis of intestinal flora with hypertension. Exp Ther Med. 2018;16:2325-2330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 109. | Liu Y, Li Z, Lee SC, Chen S, Li F. Akkermansia muciniphila: promises and pitfallsfor next-generation beneficial microorganisms. Arch Microbiol. 2025;207:76. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 110. | Costantini L, Molinari R, Farinon B, Merendino N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int J Mol Sci. 2017;18:2645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 474] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 111. | Qian F, Tintle N, Jensen PN, Lemaitre RN, Imamura F, Feldreich TR, Nomura SO, Guan W, Laguzzi F, Kim E, Virtanen JK, Steur M, Bork CS, Hirakawa Y, O'Donoghue ML, Sala-Vila A, Ardisson Korat AV, Sun Q, Rimm EB, Psaty BM, Heckbert SR, Forouhi NG, Wareham NJ, Marklund M, Risérus U, Lind L, Ärnlöv J, Garg P, Tsai MY, Pankow J, Misialek JR, Gigante B, Leander K, Pester JA, Albert CM, Kavousi M, Ikram A, Voortman T, Schmidt EB, Ninomiya T, Morrow DA, Bayés-Genís A, O'Keefe JH, Ong KL, Wu JHY, Mozaffarian D, Harris WS, Siscovick DS; Fatty Acids and Outcomes Research Consortium (FORCE). Omega-3 Fatty Acid Biomarkers and Incident Atrial Fibrillation. J Am Coll Cardiol. 2023;82:336-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 112. | Tsutsumi R, Yamasaki Y, Takeo J, Miyahara H, Sebe M, Bando M, Tanba Y, Mishima Y, Takeji K, Ueshima N, Kuroda M, Masumoto S, Harada N, Fukuda D, Yoshimoto R, Tsutsumi YM, Aihara KI, Sata M, Sakaue H. Long-chain monounsaturated fatty acids improve endothelial function with altering microbial flora. Transl Res. 2021;237:16-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 113. | Luo Y, Zhang Y, Han X, Yuan Y, Zhou Y, Gao Y, Yu H, Zhang J, Shi Y, Duan Y, Zhao X, Yan S, Hao H, Dai C, Zhao S, Shi J, Li W, Zhang S, Xu W, Fang N, Gong Y, Li Y. Akkermansia muciniphila prevents cold-related atrial fibrillation in rats by modulation of TMAO induced cardiac pyroptosis. EBioMedicine. 2022;82:104087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 114. | Bertoni C, Abodi M, D'Oria V, Milani GP, Agostoni C, Mazzocchi A. Alpha-Linolenic Acid and Cardiovascular Events: A Narrative Review. Int J Mol Sci. 2023;24:14319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 115. | Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood). 2008;233:674-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1774] [Cited by in RCA: 1765] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 116. | Bork CS, Larsen JM, Lundbye-Christensen S, Olsen A, Dahm CC, Riahi S, Overvad K, Schmidt EB. Plant Omega-3 Fatty Acids May Lower Risk of Atrial Fibrillation in Individuals with a Low Intake of Marine Omega-3 Fatty Acids. J Nutr. 2024;154:2827-2833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 117. | Mejía-Zepeda R, Pérez-Hernández IH. Effect of alpha linolenic acid on membrane fluidity and respiration of liver mitochondria in normoglycemic and diabetic Wistar rats. J Bioenerg Biomembr. 2020;52:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 118. | Wang L, Liu L, Liu X, Xiang M, Zhou L, Huang C, Shen Z, Miao L. The gut microbes, Enterococcus and Escherichia-Shigella, affect the responses of heart valve replacement patients to the anticoagulant warfarin. Pharmacol Res. 2020;159:104979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 119. | Fan ZX, Xu XF, Yang CJ. Combination of colchicine and omega-3 fatty acids for reducing the recurrence of atrial fibrillation: A promising approach? Int J Cardiol. 2025;426:133100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 120. | Al-Sadawi M, Aslam F, Henriques MD, Alsaiqali M, Gier C, Kim P, Almasry I, Singh A, Fan R, Rashba E. Effect of low dose colchicine on long term recurrence after atrial fibrillation ablation. Int J Cardiol. 2025;423:132972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 121. | Mohanty S, Mohanty P, Kessler D, Gianni C, Baho KK, Morris T, Yildiz T, Quintero Mayedo A, MacDonald B, Della Rocca DG, Al-Ahmad A, Bassiouny M, Gallinghouse GJ, Horton R, Burkhardt JD, di Biase L, Natale A. Impact of Colchicine Monotherapy on the Risk of Acute Pericarditis Following Atrial Fibrillation Ablation. JACC Clin Electrophysiol. 2023;9:1051-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 122. | Haiser HJ, Gootenberg DB, Chatman K, Sirasani G, Balskus EP, Turnbaugh PJ. Predicting and manipulating cardiac drug inactivation by the human gut bacterium Eggerthella lenta. Science. 2013;341:295-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 499] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 123. | Lopes RD, Rordorf R, De Ferrari GM, Leonardi S, Thomas L, Wojdyla DM, Ridefelt P, Lawrence JH, De Caterina R, Vinereanu D, Hanna M, Flaker G, Al-Khatib SM, Hohnloser SH, Alexander JH, Granger CB, Wallentin L; ARISTOTLE Committees and Investigators. Digoxin and Mortality in Patients With Atrial Fibrillation. J Am Coll Cardiol. 2018;71:1063-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 124. | Virgadamo S, Charnigo R, Darrat Y, Morales G, Elayi CS. Digoxin: A systematic review in atrial fibrillation, congestive heart failure and post myocardial infarction. World J Cardiol. 2015;7:808-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 125. | Matuskova Z, Anzenbacherova E, Vecera R, Tlaskalova-Hogenova H, Kolar M, Anzenbacher P. Administration of a probiotic can change drug pharmacokinetics: effect of E. coli Nissle 1917 on amidarone absorption in rats. PLoS One. 2014;9:e87150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 126. | Ittzes B, Szentkiralyi E, Szabo Z, Batai IZ, Gyorffy O, Kovacs T, Batai I, Kerenyi M. Amiodarone that has antibacterial effect against human pathogens may represent a novel catheter lock. Acta Microbiol Immunol Hung. 2020;67:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 127. | Yan S, Xu W, Fang N, Li L, Yang N, Zhao X, Hao H, Zhang Y, Liang Q, Wang Z, Duan Y, Zhang S, Gong Y, Li Y. Ibrutinib-induced pulmonary angiotensin-converting enzyme activation promotes atrial fibrillation in rats. iScience. 2024;27:108926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 128. | Sutton TDS, Hill C. Gut Bacteriophage: Current Understanding and Challenges. Front Endocrinol (Lausanne). 2019;10:784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 129. | Roager HM, Licht TR, Poulsen SK, Larsen TM, Bahl MI. Microbial enterotypes, inferred by the prevotella-to-bacteroides ratio, remained stable during a 6-month randomized controlled diet intervention with the new nordic diet. Appl Environ Microbiol. 2014;80:1142-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 130. | Żółkiewicz J, Marzec A, Ruszczyński M, Feleszko W. Postbiotics-A Step Beyond Pre- and Probiotics. Nutrients. 2020;12:2189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 331] [Cited by in RCA: 388] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 131. | Rahman MM, Islam F, -Or-Rashid MH, Mamun AA, Rahaman MS, Islam MM, Meem AFK, Sutradhar PR, Mitra S, Mimi AA, Emran TB, Fatimawali, Idroes R, Tallei TE, Ahmed M, Cavalu S. The Gut Microbiota (Microbiome) in Cardiovascular Disease and Its Therapeutic Regulation. Front Cell Infect Microbiol. 2022;12:903570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 134] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 132. | Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121:2126-2132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 686] [Cited by in RCA: 592] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 133. | Bicknell B, Liebert A, Johnstone D, Kiat H. Photobiomodulation of the microbiome: implications for metabolic and inflammatory diseases. Lasers Med Sci. 2019;34:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |