Published online Jun 26, 2025. doi: 10.4330/wjc.v17.i6.106295
Revised: April 8, 2025
Accepted: May 21, 2025
Published online: June 26, 2025
Processing time: 119 Days and 2.7 Hours
Women with adult congenital heart disease (CHD) face unique challenges during pregnancy, as gestational cardiovascular (CV) and hemodynamic changes can exacerbate underlying cardiac conditions. While these adaptations are well tole
Core Tip: Managing adult congenital heart disease (ACHD) in pregnancy requires a multidisciplinary approach. Preconception counseling, risk stratification with the modified World Health Organization classification, and tailored monitoring optimize maternal and fetal outcomes. Hemodynamic changes can worsen ACHD complications, necessitating specialized cardio-obstetric team care. Individualized delivery plans, vigilant postpartum surveillance, and post-delivery contraception counseling are essential. Advances in imaging and interventions improve outcomes, but high-risk cases demand tertiary center expertise to address cardiac decompensation, arrhythmia, or heart failure, ensuring safe pregnancy management. Future directions emphasize AI-driven risk prediction to further enhance outcomes.
- Citation: Das BB, Aggarwal V, Deshpande SR. Navigating women with congenital heart disease during pregnancy: Management strategies and future directions. World J Cardiol 2025; 17(6): 106295
- URL: https://www.wjgnet.com/1949-8462/full/v17/i6/106295.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i6.106295
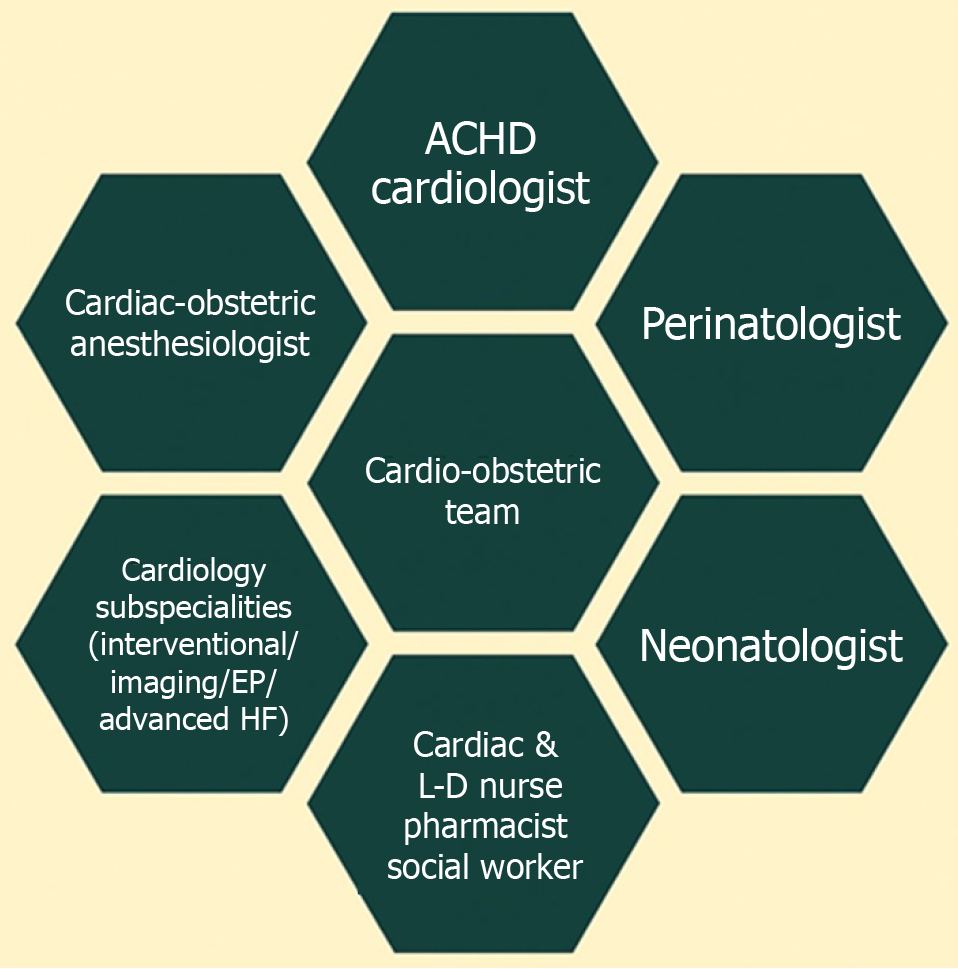
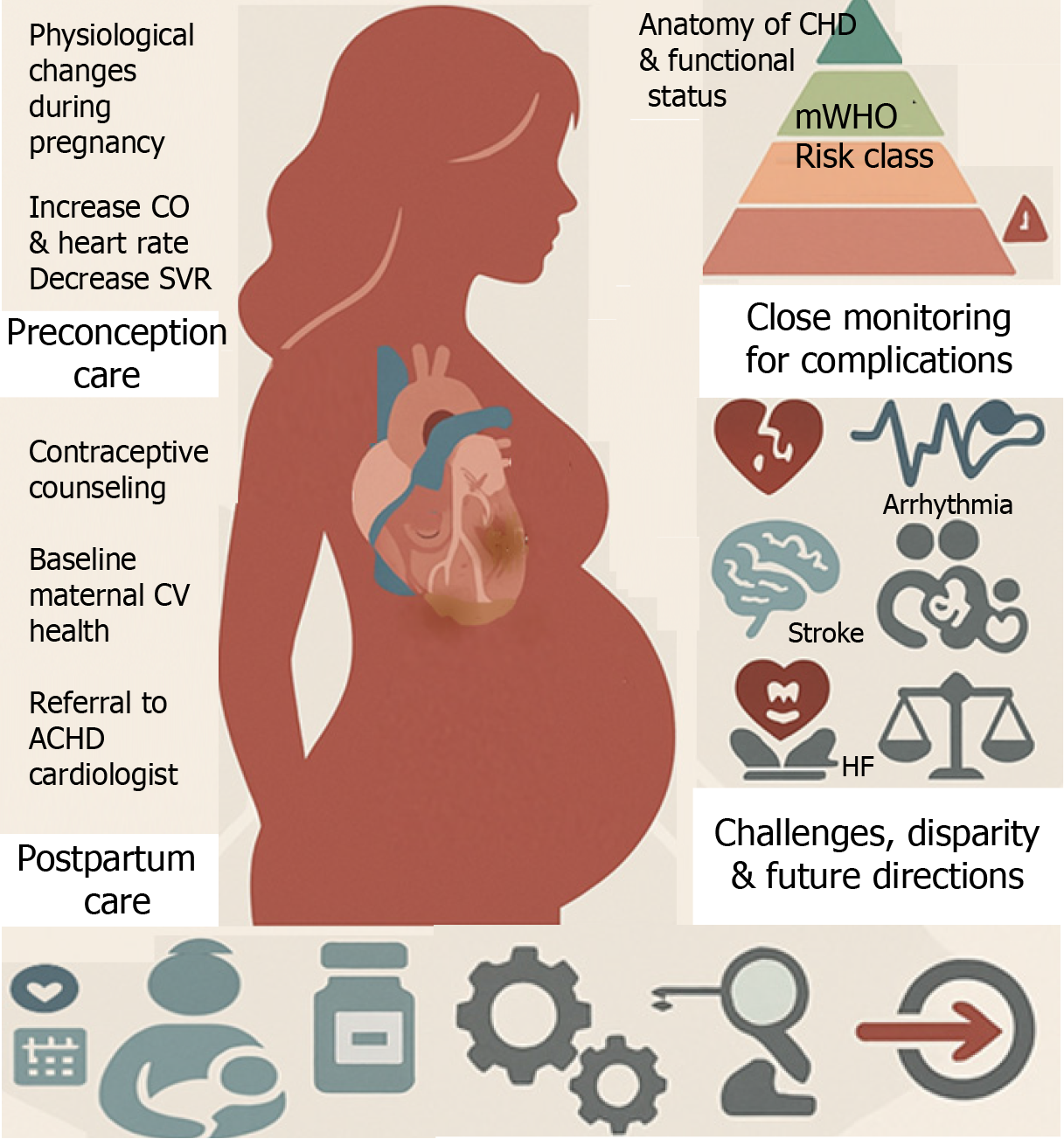
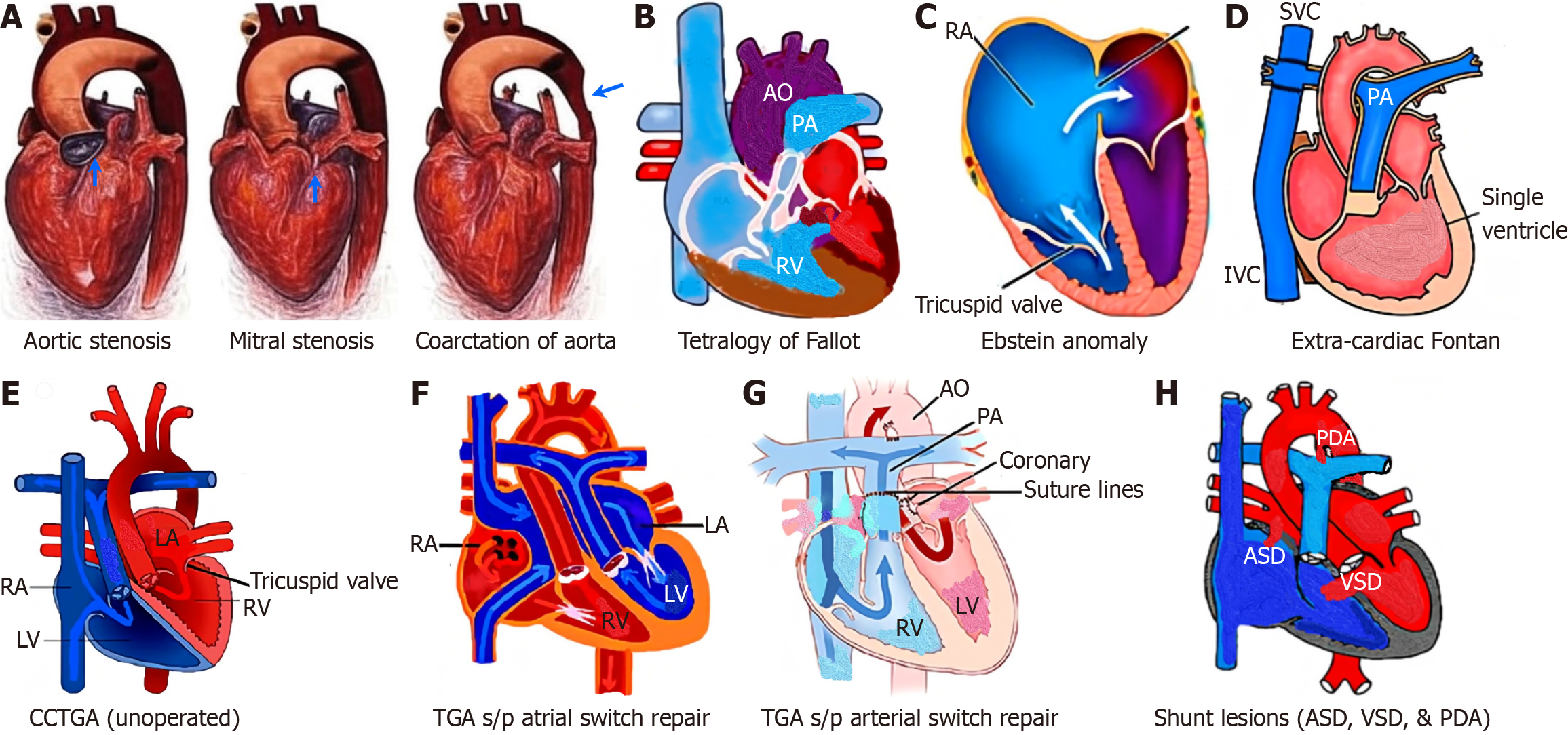
Advances in managing congenital heart disease (CHD) have led to an increased number of women with adult CHD (ACHD) reaching childbearing age. Pregnancy is a unique and complex physiological state that presents numerous challenges to women with pre-existing ACHD, whether unrepaired, repaired with residual defects, or palliated[1]. Currently, about 8 in every 10000 pregnant individuals have CHD[2]. Cardiovascular (CV) conditions, including ACHD, significantly contribute to maternal morbidity and mortality, stillbirths, premature births, and neonatal deaths, posing a growing public health concern[2,3]. Guideline-directed medical therapy (GDMT) for acquired heart diseases is not directly applicable to pregnant women who require special care to ensure optimal maternal and fetal outcomes. Several factors, including the complexity of CHD, unrepaired defects, and socioeconomic disparities, predict higher complication rates for pregnant women and their offspring. Most of the complications during pregnancy can be prevented through enhanced provider education and proper risk stratification, as recommended by leading societal guidelines, including those from the American College of Cardiology (ACC)/American Heart Association (AHA), European Society of Cardiology (ESC), Canadian Cardiovascular Society, International Society for Adult Congenital Heart Disease (ISACHD), and American College of Obstetricians and Gynecologists (ACOG)[4-9]. The evolving field of cardio-obstetrics (Figure 1) is a beacon of hope, involving a multidisciplinary team to surveil patients' CV health for preconception, antenatal, and postpartum care[10,11]. The cardio-obstetrics team includes perinatologists (maternal-fetal medicine physicians), ACHD cardiologists, heart failure (HF) cardiologists, cardiac and obstetric anesthesiologists, pharmacists, neonatologists, nurses, and social workers, and offers a comprehensive approach tailored to each patient's specific needs. This review emphasizes understanding pregnancy physiology, evaluating maternal and fetal risks during labor and delivery, management of CV complications such as stroke, HF, and arrhythmia, postpartum care, identifying areas for practice improvement, and exploring future directions (Figure 2).
During pregnancy, the CV system undergoes significant changes (Table 1), characterized by an increase in cardiac output (CO), reaching its height between 16 and 28 weeks and rises an additional 30% during labor, due to an increased heart rate (HR) of 10-20 beats/minute and expanded blood volume[12]. Blood volume increases by 30% to 50%, and systemic vascular resistance (SVR) decreases due to higher levels of estradiol and prostacyclin, leading to lower blood pressure (BP) that peaks at 24 weeks of gestation before returning to preconception levels by term[13,14]. Pulmonary vascular resistance decreases by approximately 24% by the eighth week of gestation, accommodating a 47% increase in pulmonary flow[15,16]. Venous pressure, especially in the lower extremities, rises due to the growing uterus's pressure on pelvic veins and increased blood volume, leading to dependent edema, which can be confused with signs of HF[17]. Additionally, the dilutional effect of increased plasma volume can result in physiological anemia. These physiological changes are exacerbated in twin pregnancies[18]. The above physiologic adaptations ensure adequate fetal blood flow and are generally well-tolerated in healthy pregnant women. However, in patients with preexisting ACHD, these hemodynamic changes during pregnancy pose many challenges.
| Changes | Variables | First trimester | Second trimester | Third trimester | Delivery |
| Hemodynamic | CO | Small increase | Moderate increase | Moderate increase | Significant increase |
| SVR | Mild decrease | Moderate decrease | Moderate decrease | - | |
| PVR | Mild decrease | Mild decrease | Mild decrease | Mild increase | |
| HR | Small increase | Moderate increase | Significant increase | Very significant increase | |
| BP | Mild decrease | Mild decrease | No change | Mild increase | |
| WBC | WBC count | - | - | - | High |
| RBC | RBC mass | Small increase | Moderate increase | Moderate increase | - |
| Blood volume | Plasma volume | Moderate increase | Moderate increase | Very significant increase | Extremely significant increase |
| Remodeling in heart | LV mass | Small increase | Small increase | Small increase | - |
| Chamber sizes | - | - | 4-chamber enlargement | - | |
| Aorta | Distensibility | Increase | - | - | - |
| Respiratory minute ventilation | O2 saturation1 | Small increase | Moderate increase | Moderate increase | - |
| Cardiac biomarkers | BNP | No change | - | - | Mild increase |
| cTn | No change | - | - | - | |
| CK-MB | Increase | - | - | - | |
| D-dimer | Small increase | Moderate increase | Significant increase | - | |
| ECG changes | P wave | Small increase | Plateau | - | - |
| Q wave | - | - | Prominent Q wave in inferior and anterolateral leads | - | |
| QTc | - | - | Mild increase | - | |
| ST changes | - | - | - | ST depression after cesarean section delivery | |
| Arrhythmia | APC | Common | - | - | - |
| PVC | Common | - | - | - |
Highlighting the significance of preconception counseling for women with ACHD is essential due to the potential risks involved. These include hemodynamic deterioration with unplanned pregnancy, the risk of CHD in the offspring of parents with ACHD, and the potential teratogenicity of cardiac medications. A comprehensive evaluation, encompassing medical history for prior cardiac surgeries, history of arrhythmias, interventional procedures, physical examination, electrocardiogram, echocardiogram, and other relevant diagnostic tests such as serial monitoring of brain natriuretic peptide (BNP)/N-terminal fragment of the BNP precursor (NT-proBNP), forms the basis of effective care. Risk stratification, considering CHD's anatomic complexity and physiological stage[1], New York Heart Association (NYHA) functional class, and associated comorbidities, is the key to proactive management. Cardiopulmonary exercise testing (CPET) is a valuable tool for assessing functional capacity, evaluating cardiac and pulmonary pathology, and providing guidance on prognosis and interventional recommendations before pregnancy[19]. For parents with inherited cardiac conditions, a formal genetic evaluation is recommended to discuss the transmission of the disease to offspring[20].
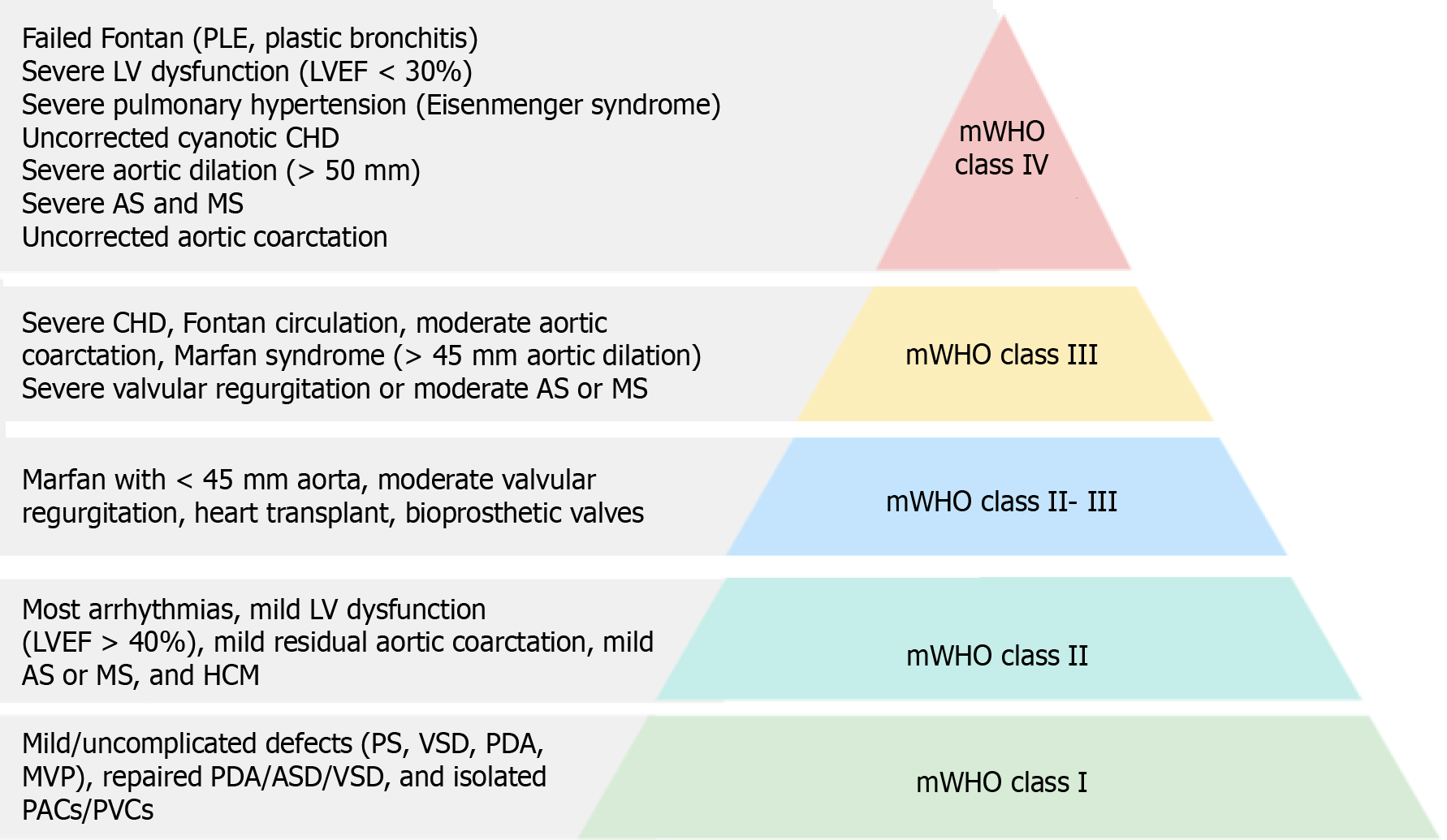
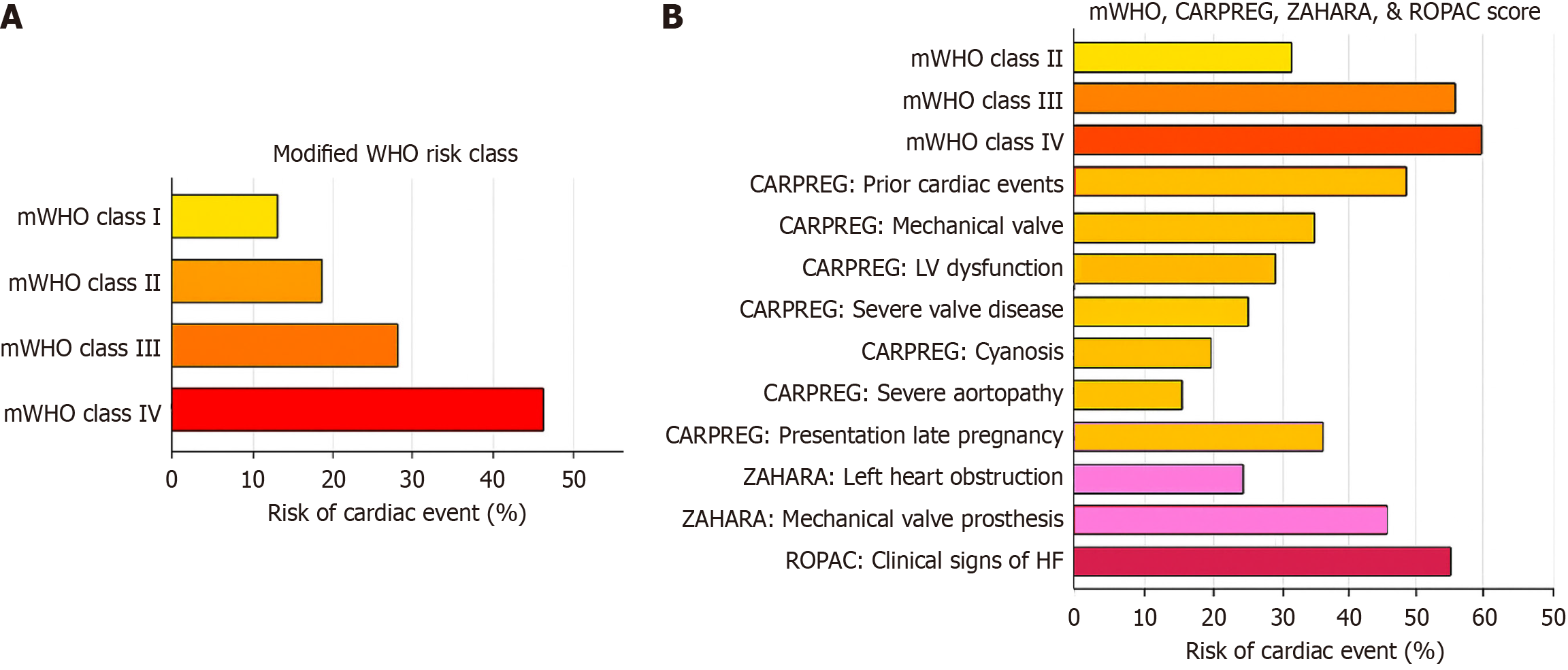
Risk scoring systems, such as the modified World Health Organization (WHO) classification (adopted by the 2018 ESC guidelines)[6], Harris score[21], CARPREG II risk score[22], and ZAHARA risk score[23], estimate the risk of adverse maternal and fetal outcomes in pregnant women with ACHD. The modified WHO (mWHO) classification system for maternal CV risk offers the best approach to implementing risk assessment[24] and guiding management decisions (Figure 3). Patients are classified into minimal risk (class I), low to moderate risk (class II), high risk (class III), and extremely high risk (class IV), where pregnancy is contraindicated. Women in classes I and II can be cared for in a peripheral hospital, whereas those in classes II-III and III should be managed in a tertiary center with a cardio-obstetric team. Figure 4A illustrates the risk of cardiac events per mWHO class, and Figure 4B describes the composite risk of adverse effects by the integration of mWHO, CARPREG, ZAHARA, and ROPAC risk scores.
The modified WHO classification emphasizes primary anatomic pathology rather than functional status, offering broader predictions about expected maternal outcomes. However, there is substantial diversity in ACHD patient anatomy, types of previous intervention/reconstruction, and physiology (including residual hemodynamically significant lesions and/or cyanosis), all of which collectively affect individual management. Table 2 describes key clinical features of different ACHD types.
| ACHD type | Maternal risk | Fetal risk | Key clinical considerations |
| ASD | Low risk (< 5% arrhythmia, endocarditis, TE) | Low fetal mortality | DVT prophylaxis; consider anticoagulation if high-risk; aspirin in select cases |
| VSD | Similar to ASD | Low fetal mortality, CHD recurrence 27% | Standard management; low risk overall |
| Tetralogy of Fallot (repaired) | Low cardiac event rate, arrhythmia (2%-6%) | Low fetal risk | Elective PVR if RV dysfunction or dilation |
| CoA | HTN (5%-30%), rare dissection | Low fetal mortality, CHD recurrence 4% | Avoid pregnancy in severe CoA (mWHO IV); control BP carefully |
| Ebstein anomaly | HF 3%, arrhythmia 4% | Preterm 22% | Assess cyanosis, degree of TR, and RV function |
| d-transposition of great arteries s/p atrial switch | HF 10%, arrhythmia 15% | Preterm 34%-38%, low CHD recurrence | Assess systemic ventricular function and TR |
| ccTGA/l-TGA | HF 10%, cardiac event 2% | Preterm 9%, CHD recurrence 36% | Assess systemic RV, TR, and heart block risk |
| Cyanotic CHD (unrepaired) | High maternal risk (HF 19%, TE 3.6%) | Fetal mortality 12%, preterm 45% | Contraindicated for pregnancy; require thromboembolism prophylaxis, iron support |
| Eisenmenger syndrome | Very high maternal mortality (33%), TE 18% | Fetal mortality up to 30%, preterm 65% | Pregnancy is contraindicated; PDE-5i/prostanoids may be used, endothelin antagonists contraindicated |
| Fontan circulation | HF 3%-11%, arrhythmia up to 37% | Preterm 28%-59%, live birth only 45% of evidence of Fontan failure, postpartum hemorrhage 14% | Avoid pregnancy in complicated Fontan; anticoagulation recommended |
| Severe mitral stenosis | Mortality 3%, HF 37%, arrhythmia 16% | Fetal mortality 6%, preterm 18% | Severe MS = mWHO IV (contraindicated); moderate = mWHO III |
| Severe aortic stenosis | Mortality 2%, HF 9%, arrhythmia 4% | Fetal mortality 5%, preterm 4% | Severe symptomatic AS = mWHO IV; assisted delivery may be considered |
| Severe pulmonary stenosis | Generally well tolerated; worsening function possible | No significant fetal effects observed | Monitor for worsening symptoms; limited data |
| Moderate/severe AV valve regurgitation | Mortality < 1%, HF 8%-11%, arrhythmia 6%-8% | Fetal mortality 0%-1%, preterm 12%-15% | Worse prognosis with pulmonary hypertension or LV dysfunction |
| Moderate/severe semilunar valve regurgitation | Mortality < 1%, HF 1%-3%, arrhythmia 0%-3% | Fetal mortality 1%-8%, preterm 5%-10% | Same considerations as AV regurgitation |
Left heart stenotic ACHD includes aortic stenosis (AS), mitral stenosis (MS), and the coarctation of the aorta (CoA; Figure 5A).
AS: Pregnancy is contraindicated in symptomatic AS or severe asymptomatic AS with severely impaired left ventricular (LV) function [LV ejection fraction (LVEF) < 30%, mWHO-IV] due to high maternal morbidity and mortality risks[6,25,26]. It is important to highlight that, according to the CARPREG II[21] and ZAHARA[22] scores, a peak gradient ≥ 50 mmHg across the aortic valve, a subaortic gradient ≥ 30 mmHg, or an aortic valve area ≤ 1.5 cm² is deemed high risk during pregnancy[27]. Mild to moderate AS lesions are usually well-tolerated unless associated with LV dysfunction or aortic regurgitation (AR). Continuous invasive hemodynamic monitoring is required for severe AS. Diuretics should be used cautiously. Balloon dilatation or transcatheter aortic valve replacement (TAVR) may be considered in advanced pregnancy stages[27]. Surgery, if needed, is recommended to be done between the 13th and 28th weeks of gestation or should be considered after early cesarean delivery. Patients with LV outflow tract obstruction and CoA are at higher risk of small-for-gestational-age (SGA) infants and premature births[28]. Regional anesthesia is suitable, but single-shot spinal anesthesia should be avoided due to sudden hemodynamic changes. Postpartum care includes monitoring for HF and deterioration of LV function.
MS: MS poses a significant risk during pregnancy, with pulmonary edema being the most common complication[29]. Pre-pregnancy severe MS (< 1.5 cm2 valve area) should be corrected before pregnancy. Anticoagulation is indicated for atrial fibrillation, left atrial (LA) thrombosis, prior embolism, spontaneous echo contrast in the LA, LA volume index ≥ 60 mL/m2, or when associated with HF. Mild MS is usually well-tolerated, while moderate or severe MS often leads to HF symptoms, especially in the second trimester. HR control is crucial, with beta blocker (BB; metoprolol) and calcium channel blocker (CCB; diltiazem), which are safe during pregnancy[30]. Diuretics should be used cautiously. Physical activity should be limited; bed rest is advised in moderate to severe cases. MS can cause atrial fibrillation, which is cha
CoA: Women with unrepaired CoA, classified as mWHO class IV, are at risk for aortic dissection at the site of narrowing, placental hypoperfusion, and IUGR; therefore, pregnancy is contraindicated[6]. Before conception, it is recommended that patients undergo special imaging studies, such as computed tomography or cardiac magnetic resonance imaging (CMR), to assess aortic structure and dimensions. Consequently, unrepaired CoA or aortic aneurysm (aortic diameter > 5 cm) should be repaired before proceeding with pregnancy. According to the mWHO stratification, those with repaired CoA but having residual coarctation (> 20 mmHg gradient or aortic lumen < 1.2 cm) and aneurysm at the prior coarctation site fall into mWHO classes II and III. The primary concern for women with a history of prior CoA, even after repair, is the development of hypertension (30%) and preeclampsia during pregnancy[32,33]. Aspirin 81 mg daily beginning in the second trimester given increased risk of pre-eclampsia is recommended. During pregnancy, close BP monitoring should be conducted at least every trimester with careful measurement of all four extremities. Labetalol, nifedipine, and methyldopa are the preferred medications for managing hypertension[30]. During the second trimester, it may be necessary to reduce the dosage as a 5-10 mmHg decrease in systolic BP commonly occurs due to pregnancy-related physiological changes. Diuretics should be used cautiously as they can lead to placental hypoperfusion. Salt restrictions for pregnancy-related hypertension are not recommended because of concerns for poor fetal growth[34]. Hypertensive crises may require intravenous sodium nitroprusside or nitroglycerin. For cases of refractory BP control or maternal or fetal hemodynamic compromise, invasive interventions such as percutaneous stenting of re-coarctation sites may be considered[35].
Mechanical valve for valvular ACHD: Patients with a history of valve replacement who are hospitalized for delivery face a significant risk of adverse maternal and fetal events, irrespective of the valve type. Studies indicate that outcomes for mechanical heart valves and bioprosthetic heart valves are comparable[36]. Managing anticoagulation in women with mechanical valves is challenging due to the risk of valve thrombosis, and warfarin can cause teratogenic effects, particu
In pulmonary stenosis (PS), the increased CO during pregnancy leads to a rise in the transvalvular gradient but is typically well tolerated during pregnancy[38]. Medical optimization with diuretics to control right-sided HF is some
For patients with unrepaired tetralogy of Fallot (TOF; Figure 5B), surgical repair is recommended before pregnancy. Pregnancy is not advised for women with unrepaired TOF whose oxygen (O2) saturation is below 90%, due to the significant maternal and perinatal complications associated with cyanosis. However, women with repaired TOF generally tolerate pregnancy well (mWHO risk class II)[6]. However, up to 12% of these patients who had an initial repair with a transannular patch are particularly prone to severe pulmonary regurgitation (PR) and subsequent right ventricle (RV) dilation. RV dysfunction and/or moderate to severe PR are significant risk factors for right-sided HF, arrhythmia, and thromboembolism (TE)[40]. Treatment includes diuretics and afterload-reducing medications such as hydralazine. Pulmonary valve replacement may be required, although the best timing of this intervention is still a matter of debate[1]. Although uncommon, adverse maternal events can occur, often linked to LV dysfunction, severe pulmonary hyper
For pregnant women with mild to moderate mitral regurgitation (MR) and AR, it's important to focus on managing symptoms. Chronic mild to moderate AR and MR are typically well tolerated, but women with severe MR are at in
Pulmonic and tricuspid regurgitation (TR) are commonly associated with ACHD. TR is more frequent due to infective endocarditis or Ebstein anomaly. When there is no pre-existing right-sided HF before conception, women generally fare well during pregnancy. However, those who have undergone right-sided heart valve procedures are susceptible to atrial arrhythmias and right-sided HF, potentially necessitating diuretic therapy in the later stages of pregnancy and postpartum. Surgical intervention for regurgitant lesions is rarely required during pregnancy, except in cases of infective endocarditis complicated by valvular regurgitation due to the potentially life-threatening risks to the mother and fetus if it goes untreated. Maternal risk is largely determined by the presence of concomitant LV dysfunction or PH. Addi
In women with uncomplicated Ebstein’s anomaly (Figure 5C), pregnancy is often well-tolerated (mWHO risk class II)[6]. Ebstein anomaly is often associated with atrial septal defect (ASD), right atrial (RA) dilation, and Wolff-Parkinson-White syndrome. Consequently, pregnancy may lead to progressive cyanosis, TE and/or arrhythmias in women at risk. The prognosis is less favorable if RV dysfunction is already present before pregnancy. The fatal complication rate (3%-17%) correlates closely with the degree of HF and with associated left-sided valve disease or cyanosis[43-45]. It's crucial to advise against pregnancy for symptomatic patients with cyanosis and/or HF (mWHO risk class IV)[6]. Women with cyanosis (an arterial O2 saturation < 90%) and hematocrit > 60% are indicators of a poor prognosis. Women with cyanotic ACHD face an elevated risk of miscarriage, preterm birth, fetal distress, and congenital anomalies in their children.
Fontan circulation represents a complex form of ACHD in which systemic venous blood flows passively into the pulmonary arteries without a subpulmonary ventricle[46]. Over the past two decades, most centers have transitioned to performing lateral tunnel or extracardiac conduit Fontan procedures (Figure 5D), given the long-term complications of the atrio-pulmonary Fontan approach, such as significant RA dilation, atrial arrhythmias, and increased risk for TE[47-49]. Pregnancy in women with Fontan circulation poses substantial challenges due to increased volume and pressure demands on the circuit[50]. This often leads to complications such as hepatic congestion, gastric congestion, and cardio
A comprehensive pre-pregnancy evaluation of Fontan physiology is critical and should include echocardiography, CMR, cardiac catheterization, and liver biochemical and ultrasound assessments[52]. Pregnancy is contraindicated (mWHO class IV) in the presence of severe complications like cyanosis, impaired ventricular function, atrioventricular valve (AV) regurgitation, arrhythmia, elevated Fontan pressures, protein-losing enteropathy, or plastic bronchitis[6]. Also, women with Fontan circulation face heightened risks of HF, TE, and hemorrhage during pregnancy and the postpartum period[53,54]. Expert consensus recommends therapeutic LMWH and aspirin for those with high thromboe
Fetal outcomes, however, tend to be less favorable. The live birth rate among women with Fontan circulation is reported at approximately 40%-50%[56]. Common complications include prematurity, IUGR, and SGA, driven by adverse hemodynamics, maternal medication effects, and the neurohormonal environment of Fontan circulation[57,58]. Addi
The postpartum period introduces additional hemodynamic challenges as uterine blood is auto-transfused back into circulation, and pressure on the inferior vena cava from the baby’s mass is relieved. This 48-72-hour window is a critical time for monitoring, particularly in patients at risk of decompensation. Post-delivery, Fontan patients should remain in the ICU for at least 24-48 hours with close monitoring of fluid balance, HR, and BP. A thorough echocardiographic assessment before discharge is essential, focusing on Fontan conduit flow, thrombus detection, AV regurgitation, and ventricular function. TE prophylaxis should continue for at least six weeks postpartum[60]. Prolonged LMWH use is preferred to minimize the risk of severe bleeding, which can be unpredictable and difficult to manage with warfarin therapy.
Patients with systemic morphological RV (sRV), including congenitally corrected transposition of the great arteries (ccTGA) or also called L-transposition of great arteries (Figure 5E) and dextro-transposition of the great arteries (d-TGA) with a Mustard or Senning atrial baffle repair (Figure 5F), have a high likelihood of maternal and neonatal morbidity and IUGR[61]. Pregnancy is generally well tolerated, but an sRV ejection fraction < 40% and clinical signs of HF and severe TR before pregnancy are significant risk factors for maternal complications[62]. These include maternal death, supraven
Contrary to d-TGA after Senning and Mustard (which is no longer the preferred surgical procedure), women with a history of d-TGA and an arterial switch operation (Figure 5G), with normal LV function pre-pregnancy and normal CPET, pregnancy is tolerated relatively well (mWHO II)[64]. During preconception counseling, most women with a normal CPET should be reassured that the risk of pregnancy is low[65].
Left-to-right shunt lesions, such as ASD, ventricular septal defect (VSD), partial anomalous pulmonary venous return, and patent ductus arteriosus (PDA), result in increased pulmonary blood flow (Figure 5H) and are at risk of developing PH. ASD is the most common form of CHD observed during pregnancy. In contrast, undiagnosed moderate or large VSD and PDA are extremely rare. Most women with a small VSD or PDA, uncomplicated by PH, have a low risk (mWHO I) of hemodynamic deterioration during pregnancy, as these shunt lesions are highly resistant to flow[66]. Atrial arrhythmias are common in patients with a prior history of delayed ASD closure. In the rare case of marked clinical deterioration, catheter-based closure of the ASD is the first-line treatment. However, repaired atrioventricular septal defect (AVSD) with significant left AV valve regurgitation poses a higher risk of pregnancy complications (class III) and should be managed as described under MR[30,42]. Vaginal delivery is generally preferred, but cesarean delivery may be indicated in cases of severe ventricular dysfunction or hemodynamic instability. Close monitoring during the postpartum period is essential due to the significant hemodynamic changes after delivery.
Eisenmenger syndrome (ES) is a severe form of PH that arises in patients with AVSD, VSD, ASD, or PDA, leading to increased pulmonary blood flow and irreversible pulmonary vascular disease. Preconception counseling is essential for women with ES, emphasizing that pregnancy is contraindicated (mWHO class IV)[6]. This counseling involves a detailed discussion of the significant maternal and fetal risks associated with ES, including elevated maternal mortality, arrhythmias, HF, and fetal complications such as prematurity, IUGR, and stillbirth[67].
With advancements in PH treatment and new approaches to managing pregnant women during the peripartum period, maternal mortality has decreased but remains significant, ranging from 11% to 25%[68]. There have been reports of favorable pregnancy outcomes in women with ES[69]. Despite this, pregnancy remains associated with unpredictable risks and may accelerate the progression of PH. Women with PH can deteriorate at any time during or after pregnancy, necessitating that physicians inform patients about the risks so that women and their families can make informed decisions[70]. If pregnancy is continued, PH therapies need to be adjusted. It is recommended to discontinue endothelin receptor antagonists (ERA) such as bosentan, ambrisentan, macitentan, soluble guanylate cyclase stimulators such as riociguat, and oral prostaglandin receptor agonists such as selexipag due to potential or unknown teratogenicity[30,71]. Despite limited evidence, CCB (nifedipine), phosphodiesterase type 5 inhibitors (sildenafil, tadalafil), and parenteral and inhaled prostanoids are considered safe during pregnancy[30,72,73].
Delivery planning should be individualized based on the severity of PH, maternal and fetal status, and obstetric considerations. This individualized approach, which considers the unique circumstances of each patient, is a considerate and attentive way to manage the situation. Vaginal delivery is generally well-tolerated in women with well-compensated ES, but the mode of delivery should be determined in consultation with a multidisciplinary cardio-obstetrics team[74]. In some cases, cesarean section may be preferred to minimize the risk of hemodynamic changes during labor and delivery. Close monitoring should continue in the postpartum period to detect and manage any potential complications, such as HF exacerbation or arrhythmias[75]. Medications should be adjusted as the pharmacokinetics can change throughout gestation, and contraception should be discussed to prevent unplanned pregnancies in women with ongoing risks[76].
Marfan syndrome: Marfan syndrome is diagnosed based on revised Ghent criteria and is confirmed by genetic testing[77]. The risk of aortic dissection during pregnancy in women with Marfan syndrome is approximately 3%[78]. Aortic size significantly influences this risk, with even those with an aortic root diameter of < 4 cm facing a 1% dissection risk. Although data is limited, it is generally advised that women with an aortic root diameter > 4.5 cm avoid pregnancy due to the heightened risk of dissection[79]. For those with an aortic root size between 4 and 4.5 cm, additional factors such as the family history of dissection and the rate of aortic growth (≥ 0.3 cm/year) are considered high-risk. Distal aortic dissection and dissection of other vessels also pose risks. Consequently, even after successful aortic root replacement, patients remain susceptible to further events in the abdominal aorta[80]. Studies on aortic growth during pregnancy in Marfan patients have shown mixed results; some indicate no significant increase, while others report growth exceeding 0.3 cm, with a partial decrease in diameter postpartum[81]. Other significant cardiac complications include progressive MR due to mitral valve prolapse, new arrhythmias, and HF resulting from ventricular dysfunction[82].
Bicuspid aortic valve: The bicuspid aortic valve is more common (2% of the population), but aortopathy associated with bicuspid aortic valve accounts for 6% of type A dissections during pregnancy[83]. The current evidence supports that hemodynamic wall stress, in combination with an underlying connective tissue disorder or genetic abnormality of the ascending aortic media, leads to bicuspid aortopathy, but overall, the risk of aortic dissection is small. There is a need for close monitoring of aortic dimensions with echocardiography or other imaging modalities to assess for aortic dilation in women planning for pregnancy[84]. Optimization of BP control during pregnancy, labor and delivery is warranted with medications, such as BB, to reduce the risk of aortic complications. Pregnancy should be avoided when the aorta diameter is > 5 cm with a pre-existing bicuspid aortic valve[6]. Careful management of chronic or gestational hypertension should include labetalol, nifedipine, and methyldopa during pregnancy.
Loeys-Dietz syndrome: Loeys-Dietz syndrome (LDS) is an autosomal dominant connective tissue disorder caused by mutations in TGFβR1, TGFβR2, TGFβ2, TGFβ3, and SMAD3 genes[85]. It affects connective tissue and can lead to aortic dissection, with most dissections occurring in the third trimester (50%) or postpartum (33%)[86]. Vessel tortuosity in the head and neck is a hallmark finding for this disorder and may extend to the uterine vessels. Identification of women with LDS before or early in pregnancy is essential to allow for adequate surveillance of aortopathy and to ensure delivery at a tertiary care center with expertise in aortic surgery. Managing LDS during pregnancy requires a multidisciplinary approach to ensure both maternal and fetal safety. Optimization of BP control with medications, such as labetalol, nifedipine, and methyldopa, is needed to reduce the risk of aortic complications. Recent studies in LDS have not shown that vaginal delivery increases the risk of uterine rupture. Thus, vaginal delivery may be considered[87]. The ACC and ESC recommend CMR imaging for the aorta post-delivery and at six months postpartum to monitor aortopathy[6,88].
Turner syndrome: Turner syndrome (TS) is linked to a higher risk of CHD, aortic dilation, hypertension, diabetes, and atherosclerotic events[89]. Although aortic dissection is rare in TS, it occurs six times more frequently in younger individuals compared to the general population[90]. Pregnancies in women with TS, conceived with either autologous or donated oocytes, are considered high risk because of the risks of aortic dissection, especially when associated with bicuspid aortic valve and CoA. Pregnancy should be avoided if the aortic diameter index exceeds 2.5 cm/m². Even after aortic root surgery, patients remain at risk for type B dissection[91]. Effective BP control and diabetes management are crucial for all TS patients during pregnancy.
Vascular Ehlers-Danlos syndrome: Severe vascular complications are predominantly associated with type IV Ehlers-Danlos syndrome (EDS). Maternal mortality is notably high due to risks of uterine rupture and dissection of major arteries and veins. Consequently, pregnancy is considered extremely high risk and is generally not recommended. Women with EDS should participate in a shared decision-making process when considering pregnancy[92].
Ideally, severe AS is identified and treated before conception. Preconception treatment options for native and bioprosthetic aortic valves include surgical aortic valve replacement (SAVR), TAVR, or a Ross procedure. During pregnancy, percutaneous balloon valvuloplasty can alleviate symptoms in native AS and may temporarily improve hemodynamics in patients with severe symptoms who do not respond to medical management. Balloon angioplasty typically serves as a palliative measure, postponing surgery until after childbirth. However, it carries the risk of significant AR and related hemodynamic instability. TAVR offers an alternative method for aortic valve replacement with lower immediate risks to both the mother and the fetus compared to SAVR.
The role of TAVR in pregnant women with AS is generally limited due to the potential for greater fetal exposure to radiation and the more critical nature of aortic valve function for the mother's overall health during pregnancy. Orwat et al[26] found that the peak aortic gradient of ≥ 50 mmHg before pregnancy was an independent predictor of complications during pregnancy. The development of symptoms such as dyspnea, near syncope or syncope, and arrhythmias is also an indicator of a complicated course[93]. The decision to proceed with TAVR during pregnancy should be made on a case-by-case basis, considering the potential risks and benefits to both the mother and the fetus in consultation with a cardio-obstetrics team. Fetal radiation exposure can cause miscarriage, IUGR, mental retardation, and major malformations. The risk to the fetus depends on the radiation dose and gestational age. The highest risk is during organogenesis (weeks 2-8) and neuronal stem cell proliferation (weeks 8-14)[94]. There are anecdotal experiences of successful TAVR during pregnancy, but further research is needed to recommend TAVR as an option for the treatment of severe symptomatic AS during pregnancy[95-101]. There is limited data available in the literature on how women with a previous TAVR for severe AS or TAVR in prior SAVR will tolerate subsequent pregnancies[102].
Like TAVR, transcatheter pulmonary valve replacement (TPVR) during pregnancy is classified as a high-risk procedure. However, it can be performed in specific scenarios where the mother's cardiac health is severely compromised by a failing RV, particularly when there is a history of prior surgical repair or intervention for RV outflow tract, such as TOF with residual PS and PR. Recently, there have been a few reported cases of successful TPVR during pregnancy[103,104]. For pregnant women with significant PS and PR who exhibit symptoms or are at risk of maternal or fetal complications, TPVR may be considered a viable alternative to surgical intervention[93]. Duarte et al[105] reported no major adverse cardiac events, including endocarditis or mortality, in nine pregnancies among seven women with various forms of ACHD who had undergone TPVR before pregnancy. From an obstetric perspective, it is important to note that preterm birth was common in these pregnancies, occurring in five out of nine cases[105].
Maternal history of ACHD increases the risk of stroke, HF, and arrhythmia during pregnancy and the peripartum period[2].
Pregnant and postpartum women have a threefold increased risk of stroke compared to non-pregnant women of the same age, and this risk is further heightened by the presence of ACHD[106]. Physiological changes during pregnancy, including hemodynamic shifts, venous stasis, hypercoagulability, and immune modulation, contribute to this heightened risk. The immediate postpartum period presents the highest stroke risk for mothers[60,107]. Common causes of pregnancy-related strokes include TE associated with cyanotic ACHD and Fontan circulation, cervical artery dissection, cerebral venous thrombosis, cerebral vasospasm or subarachnoid hemorrhage from reversible cerebral vasoconstriction syndrome, and hypertensive intracerebral hemorrhage, often linked with posterior reversible encephalopathy syndrome[108]. Risk factors for stroke encompass migraines, hypertensive disorders of pregnancy, diabetes, infections, cerebro
HF is the most common complication among women with preexisting heart disease, regardless of the cause, whether related to valvular disorders, ventricular dysfunction, PH, or ACHD[5]. The ROPAC study identified several predictors for HF during pregnancy, including cardiomyopathy, left heart stenotic ACHD, d-TGA with Mustard or Senning operation, ccTGA, ES, Fontan, pre-existing HF, and PH[59,111]. Those with repaired TOF or pulmonary atresia with residual PS and PR, are more likely to develop right-sided HF[5,6]. Pregnancy is contraindicated in women with severe systemic ventricular dysfunction (LVEF < 30%), NYHA class III or IV)[6,112].
For pregnant women with stable HF on medications, monitoring during pregnancy includes echocardiograms every trimester and monthly after 24 weeks until delivery[113]. Drug levels should be monitored throughout pregnancy and postpartum due to changes in plasma volume. Medications such as angiotensin receptor-neprilysin inhibitors (ARNI), sodium-glucose cotransporter-2 inhibitors (SGLT2is), angiotensin converting enzyme inhibitors (ACEis), angiotensin receptor blockers (ARBs), carvedilol, statins, spironolactone, amiodarone, and atenolol should be discontinued during pregnancy due to potential or unknown fetotoxicity, and should be substituted with alternative afterload-reducing agents such as hydralazine and oral isosorbide dinitrate[30]. Selective BBs such as metoprolol and bisoprolol are favored[30]. Women with ventricular dysfunction require close monitoring throughout pregnancy and delivery at a specialized tertiary center with a cardio-obstetrics team. Patients should be instructed to rest in a lateral decubitus position. Sympto
Acute HF usually occurs in the late second or third trimester or early postpartum, often triggered by eclampsia or preeclampsia[113]. Differentiating between normal pregnancy symptoms and HF is vital. While avoiding radiation is preferable, a chest radiograph may be necessary to diagnose pulmonary edema. Regular transthoracic echocardiograms and serum NT-pro BNP help assess heart function. Treating potential triggers like hypertension or anemia is important. Loop diuretics like furosemide should be used carefully to avoid reducing placental flow[30]. Inotropic drugs like dopamine, dobutamine, or milrinone may be used if oral HF therapies are ineffective[114]. There is some concern for excessive vasodilation with milrinone in pregnancy in the setting of already decreased SVR. If HF is refractory, care should be provided at a center equipped for a ventricular assist device (VAD)[115-119]. Pregnancy is contraindicated for women with a left VAD (LVAD), but unplanned pregnancies can still occur. In a review of nine pregnancies while on LVAD, one maternal death was reported[120]. During pregnancy, LVAD speed may need to be increased to provide adequate CO. Specific guidelines for anticoagulation for LVAD during pregnancy are not well-defined.
In select cases, pregnancy after heart transplantation can be considered[121]. Contraindications include being less than one year post-heart transplant, reduced graft function, nonadherence, presence of donor-specific antibodies, significant cardiac allograft vasculopathy, poorly controlled hypertension, diabetes, renal dysfunction, and active infections[122]. Mycophenolate mofetil and mycophenolic acid are teratogenic and should be discontinued, with azathioprine being a suitable alternative[123].
Labor induction should be based on clinical conditions, ideally after 37 weeks unless early delivery is necessary. In cases of refractory HF, early cesarean section delivery may be required. Fetal lung maturity should be addressed with corticosteroids if needed. O2 saturation and BP should be monitored during labor, and Swan-Ganz catheterization may be used for acute HF or significant systemic ventricular dysfunction. Women should labor in the left lateral decubitus position to avoid fetal compression of the inferior vena cava, and intravenous fluids should be minimized to prevent volume overload. Post-delivery, volume status should be reassessed, and frequent clinical evaluations should continue in the postpartum period. The first 12 weeks postpartum are crucial for monitoring cardiac function, optimizing HF medications, anticoagulation, contraception, and transitioning care teams[113]. Since ARNI and SGLT2i, both carrying a class 1A recommendation in HF and offering mortality benefits[124], are unsafe for lactating women, it is important to discuss the potential cessation of breastfeeding to facilitate the rapid up-titration of GDMT for HF during the postpartum period.
According to the 2023 HRS expert consensus statement, pregnant patients with cardiac arrhythmias should be managed by a cardiac electrophysiologist[125]. Unstable arrhythmias in pregnancy should be managed with electrical cardiover
The complexity of the ACHD and whether it has been surgically repaired play crucial roles in determining the risk level of CHD in offspring[128]. Additionally, independent factors related to the cardiac and non-cardiac status of the patients, such as chromosomal anomalies in parents, cardiac treatments received during pregnancy, hypertensive disorders of pregnancy, smoking during pregnancy, gestational diabetes, and pre-pregnancy body mass index < 18.5 kg/m², also influence the outcomes[129]. First-trimester nuchal translucency screening is a significant step during routine obstetric ultrasound and can lead to a fetal echocardiogram to diagnose any CHD[130]. Babies whose parents have a chromosomal anomaly and mothers with CHD during pregnancy should have genetic counseling[131-133]. Cytogenetic fetal karyoty
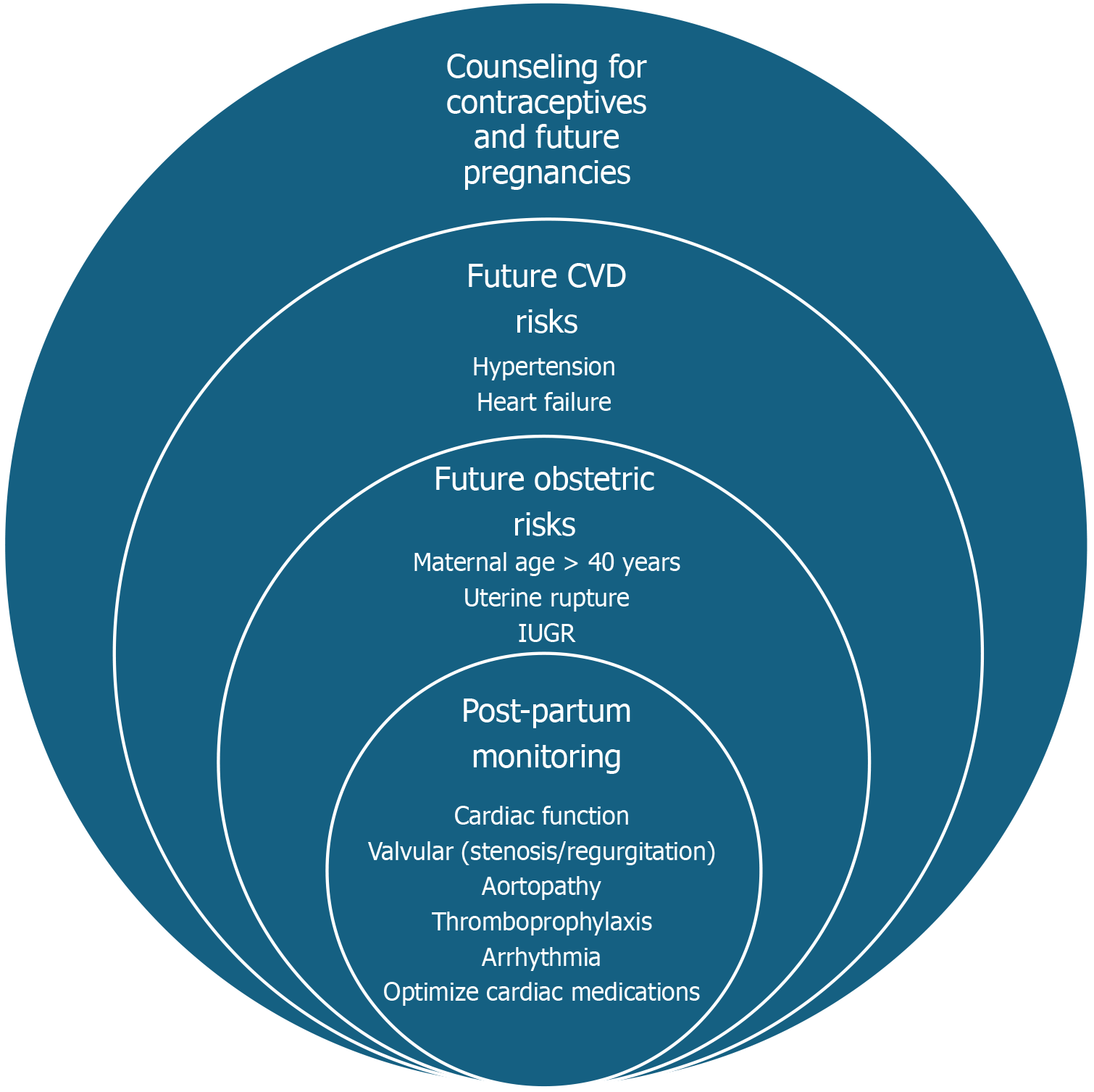
Figure 6 presents a holistic framework for postpartum care in women with ACHD. It highlights three domains of focus: (1) Postpartum monitoring, emphasizing the re-evaluation of cardiac function, arrhythmia surveillance, and medication optimization; (2) Obstetric risks, including considerations like maternal age, uterine rupture, prematurity, and fetal growth restrictions; and (3) Future CV risks, addressing potential complications such as hypertensive disorders, meta
Close cardiac monitoring should be continued in the postpartum period, with specific frequency (e.g., weekly for the first month, then bi-weekly for the next two months), to assess the mother's cardiac function, especially if she has a history of complex ACHD or has experienced any complications during pregnancy or delivery[138]. AHA statement recommends patient-centered holistic care and extending health coverage for one year postpartum to enhance maternal health outcomes and reduce disparities[139]. Women with ACHD should have a long-term care plan that includes regular cardiac evaluations and lifestyle modifications to reduce CV risks.
Medication management should be continued according to the mother's cardiac condition and the recommendations of her ACHD providers. This may involve adjusting or continuing medications for managing HF, arrhythmias, TE prophylaxis, and any potential drug interactions with breastfeeding[30,38,71]. Many medications, especially for HF, such as ACEi, ARB, ARNI, atenolol, statins, amiodarone, spironolactone, and NSAIDs, are contraindicated as these drugs are secreted in breast milk and can cause adverse effects in neonates[30]. A comprehensive resource for information on the safety of medications during lactation is available from the National Library of Medicine[140]. Discuss contraception options early in the postpartum period to prevent unintended pregnancies, which can pose significant risks for women with ACHD[141,142].
Emotional support: Women with ACHD often experience an increased prevalence of mental health issues such as depression, anxiety, or posttraumatic stress disorder related to complicated pregnancy or birth experiences[143]. Routine mental health screening, counseling, and support are essential components of care for women with ACHD[144]. If these mental health challenges go undetected and untreated, they can have significant consequences on the mother's well-being, her ability to parent, and consequently, on the cognitive and emotional development of her infant.
Managing contraception for women with ACHD requires careful consideration of the individual patient's cardiac anatomic and physiologic status, reproductive goals, and potential risks associated with different contraceptive methods[141,142]. A thorough assessment of the patient's cardiac function, exercise tolerance, and risk factors for TE should be performed to guide the selection of an appropriate contraceptive method[145]. Women with ACHD should receive com
Pfaller et al[148] have found that provider-related factors are responsible for nearly three-quarters of preventable events in maternal and fetal outcomes in women with ACHD. These included accurately identifying and appropriately stratifying ACHD, as well as promptly recognizing and responding to worsening clinical status. This finding indicates that enhanced education of healthcare providers on cardiac risk stratification during pregnancy can significantly reduce adverse events. Davis et al[149] highlighted the necessity for establishing the foundational elements of cardio-obstetrics training. Their proposed framework for training encompasses traditional levels I, II, and III, mirroring competency structures in other cardiology subspecialties. The significant gaps in knowledge regarding how pregnancy influences ACHD and vice versa emphasize the urgent need for more comprehensive research on how ACHD workforce availability influences outcomes for women during pregnancy[150]. A study analyzed the workforce for both pediatric and adult congenital cardiology, emphasizing the need for more trained providers from minority populations in the US to improve disparity in the ACHD workforce[151].
Pregnant women with ACHD are more likely to undergo cesarean delivery, which increases the risk of infection and hemorrhage. Easter et al[152] have reported that planned vaginal births for patients with maternal cardiac disease, offering vaginal delivery with assisted second stage, when necessary, resulted in similar cardiac outcomes but lower rates of postpartum hemorrhage compared to cesarean deliveries. These findings suggest that obstetricians should consider reducing cesarean delivery rates for women with ACHD.
Socioeconomic factors also contribute to adverse maternal outcomes[153]. In the United States, non-Hispanic Black pregnant women tend to experience worse outcomes compared to others[154]. In general, women with ACHD face many public health issues, such as disparities and inequity in care towards racial and ethnic groups, and increasing healthcare costs[155]. The AHA supports federal public policy and legislative actions to improve health outcomes for these vulnerable groups during pregnancy[156]. The Further Consolidated Appropriations Act of 2024 allocated additional funding to the CDC, HRSA, NIH, and SAMHSA to enhance maternal health and reduce the nation's high maternal mortality rate, which has been advocated by ACOG committee since 2021[157]. This includes financial support, health insurance coverage, and subsidies for healthcare services, medications, and medical equipment for women with ACHD during pregnancy[158,159].
Studies have shown that increased diversity in the healthcare workforce, including those who provide care for women with ACHD, leads to improved critical thinking and improved scientific research output[160]. However, like many medical specialties, the ACHD subspecialty has an underrepresentation of women and ethnic minorities relative to their proportions in the United States[161]. Also, there has been a wide disparity in the geographic distribution and access to care by ACHD providers[162].
Restrictions on abortion and abortion pills can have significant implications for pregnancy outcomes in women with ACHD and increase total maternal mortality in the United States[163,164]. On June 24, 2022, the Supreme Court of the United States ruled that the constitution does not confer a right to abortion in Dobbs vs Jackson Women’s Health Organization[165]. This landmark decision overturned the precedent set by United States Supreme Court[166], thus ending federal protection for abortion rights and allowing individual states to dictate abortion access for their residents. Under a complete abortion ban, one model predicts a 53.7% increase in single-ventricle cardiac defects, an additional 9 cases per 100000 Live births[167]. This increase would result in an extra 531 neonatal heart surgeries, 16 heart transplants, 77 extracorporeal membrane oxygenation utilizations, and 102 neonatal deaths annually in the United States. Women with hemodynamically significant ACHD (mWHO class III and IV) usually have high-risk pregnancies that pose significant health risks, including increased strain on the heart, risk of HF, arrhythmias, and death[168]. Overall, abortion bans will significantly affect both pregnancy-related and non-obstetric outcomes for pregnant women, such as mental health[169]. The inability to access abortion in high-risk situations could lead to significant psychological stress, anxiety, and trauma for women with ACHD, particularly if women are forced to continue a pregnancy that jeopardizes their health[170,171]. If the United States bans abortion, maternal mortality associated with pregnancy-related causes is expected to increase 21%, with Black women incurring a 33% increase compared with 13% among White women[172]. Findings demonstrate that black women at all educational levels and those with fewer years of education disproportionately experience adverse birth outcomes associated with restrictive abortion policies. Restrictive abortion policies may compound existing racial/ethnic, socioeconomic, and intersecting racial/ethnic and socioeconomic perinatal and infant health inequities[173]. Policymakers and healthcare systems would need to address these issues to mitigate the potential harm to women with ACHD[174]. This vulnerable population may face increased health risks if they are unable to make informed decisions about their pregnancies based on their individual health needs.
Emerging evidence underscores the importance of innovative therapies, such as targeted pharmacological agents and minimally invasive interventions, as well as the development of improved screening methods to identify high-risk patients before ACHD worsens[175].
Non-invasive imaging techniques, such as speckle-tracking echocardiography, strain imaging, three-dimensional echocardiography, and CMR, continue to advance and may provide more detailed information about the systemic ventricular function of pregnant women with ACHD, allowing for more accurate risk assessment and management decisions. There have also been notable advancements in fetal cardiac therapy, including transplacental pharmacologic treatments, enzyme replacement therapy, and fetal surgery for specific rare and severe CHD conditions such as hypoplastic left heart syndrome and others[176,177]. Advances in remote monitoring technologies, such as wearable devices and telehealth, provide opportunities for more frequent and convenient monitoring of women with ACHD during pregnancy[178]. Wearable devices, including mobile cardiac telemetry monitoring (MCT), enable earlier detection of potential arrhythmia and facilitate prompt intervention. Rodriguez and colleagues[179] researched to explore whether arrhythmia identified through 24-hour MCT monitoring, either before or early in pregnancy, was linked to negative pregnancy outcomes. Among the 141 pregnancies examined, 17% showed positive MCT findings, underscoring the high prevalence of arrhythmias in the ACHD population. Adverse cardiac outcomes were observed in 11% of the pregnancies, with clinically significant arrhythmia events occurring in 3.5%. Recent innovations in telemonitoring technology have expanded capabilities to assess critical metrics, including HR and its variability, O2 saturation, CO, and SVR[180]. These enhanced evaluations are particularly valuable for complex ACHD patients experiencing chronic low O2 levels and BP, as they allow for early detection of potential decompensation and timely intervention[181]. Furthermore, telehealth facilitates routine check-ups and specialist consultations, minimizing the need for in-person visits and improving access to care during pregnancy[182].
Greater emphasis on patient education and empowerment may become a cornerstone of care for women with ACHD, empowering them to take an active role in their care and make informed decisions. This may involve providing comprehensive education on ACHD, its impact on pregnancy, self-care strategies, and supporting patients in shared decision-making processes related to their care during pregnancy[183].
Men can significantly influence supporting and advocating for women during their pregnancy, as they have a critical responsibility in child development[184]. In the mother-and-child dyad, the father's role often takes a backseat. Fathers, however, can serve as essential allies in creating better outcomes for mother and baby, and addressing their needs along the way can improve a family's overall well-being. The importance of family for CV health promotion, focusing on (1) Mutual interdependence of the family system; (2) Shared environment; (3) Parenting style; (4) Caregiver perceptions; and (5) Genomics, contributes to overall improvement in CV health[185]. Family-based approaches that target caregivers and children encourage communication among the family unit and address the structural and environmental conditions in which families live and operate are likely the most effective approaches to promote women’s CV health[186].
Many women of childbearing age with ACHD now look forward to successful pregnancies and positive outcomes. However, these women still face a higher risk of cardiac complications during pregnancy and the postpartum period. It is crucial to tailor the management of these pregnancies based on the patient’s specific ACHD, current health status, and other existing comorbidities. Managing pregnancies in women with ACHD should involve a multidisciplinary cardio-obstetrics team, comprehensive patient education beginning with preconception counseling and contraception manage
| 1. | Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, Khairy P, Landzberg MJ, Saidi A, Valente AM, Van Hare GF. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e637-e697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 2. | Lammers AE, Diller GP, Lober R, Möllers M, Schmidt R, Radke RM, De-Torres-Alba F, Kaleschke G, Marschall U, Bauer UM, Gerß J, Enders D, Baumgartner H. Maternal and neonatal complications in women with congenital heart disease: a nationwide analysis. Eur Heart J. 2021;42:4252-4260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 3. | Messmer M, John AS. Improving Maternal Outcomes in Congenital Heart Disease: A National Call to Action. JACC Adv. 2024;3:101170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Mehta LS, Warnes CA, Bradley E, Burton T, Economy K, Mehran R, Safdar B, Sharma G, Wood M, Valente AM, Volgman AS; American Heart Association Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and Stroke Council. Cardiovascular Considerations in Caring for Pregnant Patients: A Scientific Statement From the American Heart Association. Circulation. 2020;141:e884-e903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 239] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 5. | Canobbio MM, Warnes CA, Aboulhosn J, Connolly HM, Khanna A, Koos BJ, Mital S, Rose C, Silversides C, Stout K; American Heart Association Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Functional Genomics and Translational Biology; and Council on Quality of Care and Outcomes Research. Management of Pregnancy in Patients With Complex Congenital Heart Disease: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2017;135:e50-e87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 266] [Article Influence: 33.3] [Reference Citation Analysis (1)] |
| 6. | Regitz-Zagrosek V. 'Ten Commandments' of the 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39:3269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Windram J, Grewal J, Bottega N, Sermer M, Spears D, Swan L, Siu SC, Silversides C. Canadian Cardiovascular Society: Clinical Practice Update on Cardiovascular Management of the Pregnant Patient. Can J Cardiol. 2021;37:1886-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 8. | Webb G, Mulder BJ, Aboulhosn J, Daniels CJ, Elizari MA, Hong G, Horlick E, Landzberg MJ, Marelli AJ, O'Donnell CP, Oechslin EN, Pearson DD, Pieper EP, Saxena A, Schwerzmann M, Stout KK, Warnes CA, Khairy P. The care of adults with congenital heart disease across the globe: Current assessment and future perspective: A position statement from the International Society for Adult Congenital Heart Disease (ISACHD). Int J Cardiol. 2015;195:326-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 9. | American College of Obstetricians and Gynecologists' Presidential Task Force on Pregnancy and Heart Disease and Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 212: Pregnancy and Heart Disease. Obstet Gynecol. 2019;133:e320-e356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 265] [Article Influence: 53.0] [Reference Citation Analysis (1)] |
| 10. | Davis MB, Walsh MN. Cardio-Obstetrics. Circ Cardiovasc Qual Outcomes. 2019;12:e005417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 11. | Hardee I, Wright L, McCracken C, Lawson E, Oster ME. Maternal and Neonatal Outcomes of Pregnancies in Women With Congenital Heart Disease: A Meta-Analysis. J Am Heart Assoc. 2021;10:e017834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 12. | Morton A. Physiological Changes and Cardiovascular Investigations in Pregnancy. Heart Lung Circ. 2021;30:e6-e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 13. | Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation. 2014;130:1003-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 654] [Article Influence: 59.5] [Reference Citation Analysis (1)] |
| 14. | ROVINSKY JJ, JAFFIN H. CARDIOVASCULAR HEMODYNAMICS IN PREGNANCY. I. BLOOD AND PLASMA VOLUMES IN MULTIPLE PREGNANCY. Am J Obstet Gynecol. 1965;93:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 67] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 15. | Clark SL, Cotton DB, Lee W, Bishop C, Hill T, Southwick J, Pivarnik J, Spillman T, DeVore GR, Phelan J. Central hemodynamic assessment of normal term pregnancy. Am J Obstet Gynecol. 1989;161:1439-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 284] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 16. | Robson SC, Hunter S, Boys RJ, Dunlop W. Serial changes in pulmonary haemodynamics during human pregnancy: a non-invasive study using Doppler echocardiography. Clin Sci (Lond). 1991;80:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Cutshall A, Gourdine A, Bender W, Karuppiah A. Trends in outcomes of pregnancy in patients with congenital heart disease. Curr Opin Anaesthesiol. 2023;36:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Veille JC, Morton MJ, Burry KJ. Maternal cardiovascular adaptations to twin pregnancy. Am J Obstet Gynecol. 1985;153:261-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 19. | Cifra B, Cordina RL, Gauthier N, Murphy LC, Pham TD, Veldtman GR, Ward K, White DA, Paridon SM, Powell AW; American Heart Association Council on Lifelong Congenital Heart Disease and Heart Health in the Young, Congenital Cardiac Defect Committee; the Council on Cardiovascular Radiology and Intervention; the Council on Clinical Cardiology; and the Council on Cardiovascular and Stroke Nursing. Cardiopulmonary Exercise Test Interpretation Across the Lifespan in Congenital Heart Disease: A Scientific Statement From the American Heart Association. J Am Heart Assoc. 2025;14:e038200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Burchill L, Greenway S, Silversides CK, Mital S. Genetic counseling in the adult with congenital heart disease: what is the role? Curr Cardiol Rep. 2011;13:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Harris IS. Management of pregnancy in patients with congenital heart disease. Prog Cardiovasc Dis. 2011;53:305-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 22. | Silversides CK, Grewal J, Mason J, Sermer M, Kiess M, Rychel V, Wald RM, Colman JM, Siu SC. Pregnancy Outcomes in Women With Heart Disease: The CARPREG II Study. J Am Coll Cardiol. 2018;71:2419-2430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 398] [Article Influence: 56.9] [Reference Citation Analysis (1)] |
| 23. | Balci A, Sollie-Szarynska KM, van der Bijl AG, Ruys TP, Mulder BJ, Roos-Hesselink JW, van Dijk AP, Wajon EM, Vliegen HW, Drenthen W, Hillege HL, Aarnoudse JG, van Veldhuisen DJ, Pieper PG; ZAHARA-II investigators. Prospective validation and assessment of cardiovascular and offspring risk models for pregnant women with congenital heart disease. Heart. 2014;100:1373-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (1)] |
| 24. | Bredy C, Deville F, Huguet H, Picot MC, De La Villeon G, Abassi H, Avesani M, Begue L, Burlet G, Boulot P, Fuchs F, Amedro P. Which risk score best predicts cardiovascular outcome in pregnant women with congenital heart disease? Eur Heart J Qual Care Clin Outcomes. 2023;9:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 25. | van Hagen IM, Roos-Hesselink JW. Pregnancy in congenital heart disease: risk prediction and counselling. Heart. 2020;106:1853-1861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 26. | Orwat S, Diller GP, van Hagen IM, Schmidt R, Tobler D, Greutmann M, Jonkaitiene R, Elnagar A, Johnson MR, Hall R, Roos-Hesselink JW, Baumgartner H; ROPAC Investigators. Risk of Pregnancy in Moderate and Severe Aortic Stenosis: From the Multinational ROPAC Registry. J Am Coll Cardiol. 2016;68:1727-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 27. | Lewey J, Andrade L, Levine LD. Valvular Heart Disease in Pregnancy. Cardiol Clin. 2021;39:151-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 28. | Vriend JW, Drenthen W, Pieper PG, Roos-Hesselink JW, Zwinderman AH, van Veldhuisen DJ, Mulder BJ. Outcome of pregnancy in patients after repair of aortic coarctation. Eur Heart J. 2005;26:2173-2178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 29. | van Hagen IM, Thorne SA, Taha N, Youssef G, Elnagar A, Gabriel H, ElRakshy Y, Iung B, Johnson MR, Hall R, Roos-Hesselink JW; ROPAC Investigators and EORP Team. Pregnancy Outcomes in Women With Rheumatic Mitral Valve Disease: Results From the Registry of Pregnancy and Cardiac Disease. Circulation. 2018;137:806-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (2)] |
| 30. | Halpern DG, Weinberg CR, Pinnelas R, Mehta-Lee S, Economy KE, Valente AM. Use of Medication for Cardiovascular Disease During Pregnancy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:457-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (1)] |
| 31. | Tromp CH, Nanne AC, Pernet PJ, Tukkie R, Bolte AC. Electrical cardioversion during pregnancy: safe or not? Neth Heart J. 2011;19:134-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 32. | Beauchesne LM, Connolly HM, Ammash NM, Warnes CA. Coarctation of the aorta: outcome of pregnancy. J Am Coll Cardiol. 2001;38:1728-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 150] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 33. | Gronningsaeter L, Langesaeter E, Sørbye IK, Quattrone A, Almaas VM, Skulstad H, Estensen ME. High prevalence of pre-eclampsia in women with coarctation of the aorta. Eur Heart J Open. 2023;3:oead072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (1)] |
| 34. | Sakuyama H, Katoh M, Wakabayashi H, Zulli A, Kruzliak P, Uehara Y. Influence of gestational salt restriction in fetal growth and in development of diseases in adulthood. J Biomed Sci. 2016;23:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 35. | Cherpak BV, Davydova YV, Kravchenko VI, Yaschuk NS, Siromakha SO, Lazoryshynets VV. Management of percutaneous treatment of aorta coarctation diagnosed during pregnancy. J Med Life. 2022;15:208-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 36. | Lester W, Walker N, Bhatia K, Ciantar E, Banerjee A, Trinder J, Anderson J, Hodson K, Swan L, Bradbury C, Webster J, Tower C. British Society for Haematology guideline for anticoagulant management of pregnant individuals with mechanical heart valves. Br J Haematol. 2023;202:465-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 37. | Ng AP, Verma A, Sanaiha Y, Williamson CG, Afshar Y, Benharash P. Maternal and Fetal Outcomes in Pregnant Patients With Mechanical and Bioprosthetic Heart Valves. J Am Heart Assoc. 2023;12:e028653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (1)] |
| 38. | Hameed AB, Goodwin TM, Elkayam U. Effect of pulmonary stenosis on pregnancy outcomes--a case-control study. Am Heart J. 2007;154:852-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Drenthen W, Pieper PG, Roos-Hesselink JW, Schmidt AC, Mulder BJ, van Dijk AP, Vliegen HW, Sollie KM, Voors AA, Ebels T, van Veldhuisen DJ; ZAHARA investigators. Non-cardiac complications during pregnancy in women with isolated congenital pulmonary valvar stenosis. Heart. 2006;92:1838-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 40. | Huang TT, Zhao WX, Lin JH. Risk Factors for Maternal and Perinatal Complications during Pregnancy among Women with Tetralogy of Fallot. Niger J Clin Pract. 2021;24:1138-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 41. | Blais S, Marelli A, Vanasse A, Dahdah N, Dancea A, Drolet C, Dallaire F. Comparison of Long-term Outcomes of Valve-Sparing and Transannular Patch Procedures for Correction of Tetralogy of Fallot. JAMA Netw Open. 2021;4:e2118141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 42. | Shapiro H, Alshawabkeh L. Valvular Heart Disease in Pregnancy. Methodist Debakey Cardiovasc J. 2024;20:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 43. | Nematollahi AR, Zamansaraei S, Safari F, Bahrami P. How to manage Ebstein's Anomaly in Pregnancy: A Literature Review with Case Study. J Health Sci Surveillance Sys. 2022;10:380-387. [DOI] [Full Text] |
| 44. | Van Der Zande JA, Tutarel O, Ramlakhan KP, Johson MR, Hall R, Roos-Hesselink JW. Pregnancy outcomes in women with Ebstein's anomaly: data from the EORP Registry of Pregnancy and Cardiac Disease (ROPAC). Eur Heart J. 2022;43. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 45. | Zhao W, Liu H, Feng R, Lin J. Pregnancy outcomes in women with Ebstein's anomaly. Arch Gynecol Obstet. 2012;286:881-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 46. | Rychik J, Atz AM, Celermajer DS, Deal BJ, Gatzoulis MA, Gewillig MH, Hsia TY, Hsu DT, Kovacs AH, McCrindle BW, Newburger JW, Pike NA, Rodefeld M, Rosenthal DN, Schumacher KR, Marino BS, Stout K, Veldtman G, Younoszai AK, d'Udekem Y; American Heart Association Council on Cardiovascular Disease in the Young and Council on Cardiovascular and Stroke Nursing. Evaluation and Management of the Child and Adult With Fontan Circulation: A Scientific Statement From the American Heart Association. Circulation. 2019;140:e234-e284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 554] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 47. | Canobbio MM, Cetta F, Silversides C, Warnes C, Aboulhosn J, Colman J. Pregnancy after Fontan operation: early and late outcomes. Am Coll Cardiol. 2013;61:E427. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 48. | Gouton M, Nizard J, Patel M, Sassolas F, Jimenez M, Radojevic J, Mathiron A, Amedro P, Barre E, Labombarda F, Vaksmann G, Chantepie A, Le Gloan L, Ladouceur M. Maternal and fetal outcomes of pregnancy with Fontan circulation: A multicentric observational study. Int J Cardiol. 2015;187:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 49. | Pieper PG, Balci A, Aarnoudse JG, Kampman MA, Sollie KM, Groen H, Mulder BJ, Oudijk MA, Roos-Hesselink JW, Cornette J, van Dijk AP, Spaanderman ME, Drenthen W, van Veldhuisen DJ; ZAHARA II investigators. Uteroplacental blood flow, cardiac function, and pregnancy outcome in women with congenital heart disease. Circulation. 2013;128:2478-2487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 50. | Ordoñez MV, Biglino G, Caputo M, Curtis SL. Pregnancy in the FONTAN palliation: physiology, management and new insights from bioengineering. J Congenit Heart Dis. 2021;5:8. [RCA] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 51. | Montanaro C, Boyle S, Wander G, Johnson MR, Roos-Hesselink JW, Patel R, Rafiq I, Silversides CK, Gatzoulis MA. Pregnancy in patients with the Fontan operation. Eur J Prev Cardiol. 2024;31:1336-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 52. | Wolfe NK, Sabol BA, Kelly JC, Dombrowski M, Benhardt AC, Fleckenstein J, Stout MJ, Lindley KJ. Management of Fontan circulation in pregnancy: a multidisciplinary approach to care. Am J Obstet Gynecol MFM. 2021;3:100257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 53. | Girnius A, Zentner D, Valente AM, Pieper PG, Economy KE, Ladouceur M, Roos-Hesselink JW, Warshak C, Partington SL, Gao Z, Ollberding N, Faust M, Girnius S, Kaemmerer H, Nagdyman N, Cohen S, Canobbio M, Akagi T, Grewal J, Bradley E, Buber Y, Palumbo J, Walker N, Aboulhosn J, Oechslin E, Baumgartner H, Kurdi W, Book WM, Mulder BJM, Veldtman GR. Bleeding and thrombotic risk in pregnant women with Fontan physiology. Heart. 2021;107:1390-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Breviario S, Krishnathasan K, Dimopoulos K, Gribaudo E, Constantine A, Li W, Kewada D, Patel D, Wander G, Patel RR, Johnson MR, Gatzoulis MA, Montanaro C, Rafiq I. Pregnancy in women with a Fontan circulation: Short and long-term outcomes. Int J Cardiol. 2024;415:132445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 55. | Alsaied T, Alsidawi S, Allen CC, Faircloth J, Palumbo JS, Veldtman GR. Strategies for thromboprophylaxis in Fontan circulation: a meta-analysis. Heart. 2015;101:1731-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (1)] |
| 56. | Garcia Ropero A, Baskar S, Roos Hesselink JW, Girnius A, Zentner D, Swan L, Ladouceur M, Brown N, Veldtman GR. Pregnancy in Women With a Fontan Circulation: A Systematic Review of the Literature. Circ Cardiovasc Qual Outcomes. 2018;11:e004575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 57. | Bonner SJ, Asghar O, Roberts A, Vause S, Clarke B, Keavney B. Corrigendum to "Cardiovascular, obstetric and neonatal outcomes in women with a previous Fontan repair'' [Eur. J. Obstet. Gynaecol. Reprod. Biol. 219 (2017) 53-56]. Eur J Obstet Gynecol Reprod Biol. 2018;221:209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 58. | Bartczak-Rutkowska A, Tomkiewicz-Pająk L, Kawka-Paciorkowska K, Bajorek N, Ciepłucha A, Ropacka-Lesiak M, Trojnarska O. Pregnancy Outcomes in Women after the Fontan Procedure. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 59. | Yokouchi-Konishi T, Ohta-Ogo K, Kamiya CA, Shionoiri T, Nakanishi A, Iwanaga N, Ohuchi H, Kurosaki K, Ichikawa H, Noguchi T, Ishibashi-Ueda H, Yoshimatsu J. Clinicopathologic Study of Placentas From Women With a Fontan Circulation. Circ J. 2021;86:138-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 60. | Bates SM, Rajasekhar A, Middeldorp S, McLintock C, Rodger MA, James AH, Vazquez SR, Greer IA, Riva JJ, Bhatt M, Schwab N, Barrett D, LaHaye A, Rochwerg B. American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2:3317-3359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 351] [Article Influence: 58.5] [Reference Citation Analysis (1)] |
| 61. | Pizula J, Devera J, Ng TMH, Yeung SL, Thangathurai J, Herrick N, Chatfield AJ, Mehra A, Elkayam U. Outcome of Pregnancy in Women With D-Transposition of the Great Arteries: A Systematic Review. J Am Heart Assoc. 2022;11:e026862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 62. | Tutarel O, Baris L, Budts W, Gamal Abd-El Aziz M, Liptai C, Majdalany D, Jovanova S, Frogoudaki A, Connolly HM, Johnson MR, Maggioni AP, Hall R, Roos-Hesselink JW; ROPAC Investigators Group. Pregnancy outcomes in women with a systemic right ventricle and transposition of the great arteries results from the ESC-EORP Registry of Pregnancy and Cardiac disease (ROPAC). Heart. 2022;108:117-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 63. | Jain VD, Moghbeli N, Webb G, Srinivas SK, Elovitz MA, Paré E. Pregnancy in women with congenital heart disease: the impact of a systemic right ventricle. Congenit Heart Dis. 2011;6:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 64. | Stoll VM, Drury NE, Thorne S, Selman T, Clift P, Chong H, Thompson PJ, Morris RK, Hudsmith LE. Pregnancy Outcomes in Women With Transposition of the Great Arteries After an Arterial Switch Operation. JAMA Cardiol. 2018;3:1119-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 65. | Dobson R, Danton M, Nicola W, Hamish W. The natural and unnatural history of the systemic right ventricle in adult survivors. J Thorac Cardiovasc Surg. 2013;145:1493-501; discussion 1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 66. | Zuber M, Gautschi N, Oechslin E, Widmer V, Kiowski W, Jenni R. Outcome of pregnancy in women with congenital shunt lesions. Heart. 1999;81:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 67. | Liu Y, Li Y, Zhang J, Zhao Y, Liu K, Li J, Zhao M, Gu H, Fan X, Wang J. Pregnancy outcomes of women with Eisenmenger syndrome: A single-center study. Int J Cardiol. 2023;374:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 68. | Ladouceur M, Benoit L, Radojevic J, Basquin A, Dauphin C, Hascoet S, Moceri P, Bredy C, Iserin L, Gouton M, Nizard J. Pregnancy outcomes in patients with pulmonary arterial hypertension associated with congenital heart disease. Heart. 2017;103:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 69. | Alsomali F, Mushtaq S, Bakir M, Almustanyir S. Fruitful Pregnancy Outcome in a Case of Eisenmenger Syndrome With Severe Pulmonary Hypertension: A Rare Case Report. Cureus. 2022;14:e21068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 70. | Dachlan EG, Amirah, Cininta N, Pranadyan R, Putri AY, Oktaviono YH, Akbar MIA. High Maternal Neonatal Mortality and Morbidity in Pregnancy with Eisenmenger Syndrome. J Pregnancy. 2021;2021:3248850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 71. | de Raaf MA, Beekhuijzen M, Bogaard HJ. Introduction on Pulmonary Arterial Hypertension and its relation to fetal development. Reprod Toxicol. 2015;56:6. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 72. | Brittain EL, Pugh ME, Wheeler LA, Robbins IM, Loyd JE, Newman JH, Austin ED, Hemnes AR. Prostanoids but not oral therapies improve right ventricular function in pulmonary arterial hypertension. JACC Heart Fail. 2013;1:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 73. | Zhang ZN, Jiang X, Zhang R, Li XL, Wu BX, Zhao QH, Wang Y, Dai LZ, Pan L, Gomberg-Maitland M, Jing ZC. Oral sildenafil treatment for Eisenmenger syndrome: a prospective, open-label, multicentre study. Heart. 2011;97:1876-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 74. | Banerjee D, Ventetuolo CE. Pulmonary Hypertension in Pregnancy. Semin Respir Crit Care Med. 2017;38:148-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 75. | Zhang J, Lu J, Zhou X, Xu X, Ye Q, Ou Q, Li Y, Huang J. Perioperative Management of Pregnant Women With Idiopathic Pulmonary Arterial Hypertension: An Observational Case Series Study From China. J Cardiothorac Vasc Anesth. 2018;32:2547-2559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 76. | Thorne S, MacGregor A, Nelson-Piercy C. Risks of contraception and pregnancy in heart disease. Heart. 2006;92:1520-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 268] [Article Influence: 14.1] [Reference Citation Analysis (1)] |
| 77. | Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, Devereux RB, Hilhorst-Hofstee Y, Jondeau G, Faivre L, Milewicz DM, Pyeritz RE, Sponseller PD, Wordsworth P, De Paepe AM. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47:476-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1234] [Cited by in RCA: 1368] [Article Influence: 91.2] [Reference Citation Analysis (1)] |
| 78. | Smith K, Gros B. Pregnancy-related acute aortic dissection in Marfan syndrome: A review of the literature. Congenit Heart Dis. 2017;12:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 79. | Pyeritz RE. Maternal and fetal complications of pregnancy in the Marfan syndrome. Am J Med. 1981;71:784-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 137] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 80. | Sayama S, Takeda N, Iriyama T, Inuzuka R, Maemura S, Fujita D, Yamauchi H, Nawata K, Bougaki M, Hyodo H, Shitara R, Nakayama T, Komatsu A, Nagamatsu T, Osuga Y, Fujii T. Peripartum type B aortic dissection in patients with Marfan syndrome who underwent aortic root replacement: a case series study. BJOG. 2018;125:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 81. | Rossiter JP, Repke JT, Morales AJ, Murphy EA, Pyeritz RE. A prospective longitudinal evaluation of pregnancy in the Marfan syndrome. Am J Obstet Gynecol. 1995;173:1599-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 169] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 82. | Goland S, Elkayam U. Cardiovascular problems in pregnant women with marfan syndrome. Circulation. 2009;119:619-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 83. | Januzzi JL, Isselbacher EM, Fattori R, Cooper JV, Smith DE, Fang J, Eagle KA, Mehta RH, Nienaber CA, Pape LA; International Registry of Aortic Dissection (IRAD). Characterizing the young patient with aortic dissection: results from the International Registry of Aortic Dissection (IRAD). J Am Coll Cardiol. 2004;43:665-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 290] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 84. | McKellar SH, MacDonald RJ, Michelena HI, Connolly HM, Sundt TM 3rd. Frequency of cardiovascular events in women with a congenitally bicuspid aortic valve in a single community and effect of pregnancy on events. Am J Cardiol. 2011;107:96-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 85. | Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, De Backer JF, Oswald GL, Symoens S, Manouvrier S, Roberts AE, Faravelli F, Greco MA, Pyeritz RE, Milewicz DM, Coucke PJ, Cameron DE, Braverman AC, Byers PH, De Paepe AM, Dietz HC. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355:788-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1212] [Cited by in RCA: 1143] [Article Influence: 60.2] [Reference Citation Analysis (1)] |
| 86. | Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, Kouchoukos NT, Lytle BW, Milewicz DM, Reich DL, Sen S, Shinn JA, Svensson LG, Williams DM; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American College of Radiology; American Stroke Association; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology; Society of Thoracic Surgeons; Society for Vascular Medicine. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology,American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons,and Society for Vascular Medicine. J Am Coll Cardiol. 2010;55:e27-e129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 1034] [Article Influence: 68.9] [Reference Citation Analysis (1)] |
| 87. | Jondeau G, Ropers J, Regalado E, Braverman A, Evangelista A, Teixedo G, De Backer J, Muiño-Mosquera L, Naudion S, Zordan C, Morisaki T, Morisaki H, Von Kodolitsch Y, Dupuis-Girod S, Morris SA, Jeremy R, Odent S, Adès LC, Bakshi M, Holman K, LeMaire S, Milleron O, Langeois M, Spentchian M, Aubart M, Boileau C, Pyeritz R, Milewicz DM; Montalcino Aortic Consortium. International Registry of Patients Carrying TGFBR1 or TGFBR2 Mutations: Results of the MAC (Montalcino Aortic Consortium). Circ Cardiovasc Genet. 2016;9:548-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 160] [Article Influence: 17.8] [Reference Citation Analysis (2)] |
| 88. | Lindley KJ, Bairey Merz CN, Asgar AW, Bello NA, Chandra S, Davis MB, Gomberg-Maitland M, Gulati M, Hollier LM, Krieger EV, Park K, Silversides C, Wolfe NK, Pepine CJ; American College of Cardiology Cardiovascular Disease in Women Committee and the Cardio-Obstetrics Work Group. Management of Women With Congenital or Inherited Cardiovascular Disease From Pre-Conception Through Pregnancy and Postpartum: JACC Focus Seminar 2/5. J Am Coll Cardiol. 2021;77:1778-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 89. | Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, Lin AE, Mauras N, Quigley CA, Rubin K, Sandberg DE, Sas TCJ, Silberbach M, Söderström-Anttila V, Stochholm K, van Alfen-van derVelden JA, Woelfle J, Backeljauw PF; International Turner Syndrome Consensus Group. Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol. 2017;177:G1-G70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 665] [Article Influence: 83.1] [Reference Citation Analysis (1)] |
| 90. | Carlson M, Silberbach M. Dissection of the aorta in Turner syndrome: two cases and review of 85 cases in the literature. J Med Genet. 2007;44:745-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 148] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 91. | Gravholt CH, Landin-Wilhelmsen K, Stochholm K, Hjerrild BE, Ledet T, Djurhuus CB, Sylvén L, Baandrup U, Kristensen BØ, Christiansen JS. Clinical and epidemiological description of aortic dissection in Turner's syndrome. Cardiol Young. 2006;16:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 178] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 92. | Murray ML, Pepin M, Peterson S, Byers PH. Pregnancy-related deaths and complications in women with vascular Ehlers-Danlos syndrome. Genet Med. 2014;16:874-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 93. | Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2438-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1379] [Article Influence: 125.4] [Reference Citation Analysis (1)] |
| 94. | Begano D, Söderberg M, Bolejko A. TO USE OR NOT USE PATIENT SHIELDING ON PREGNANT WOMEN UNDERGOING CT PULMONARY ANGIOGRAPHY: A PHANTOM STUDY. Radiat Prot Dosimetry. 2020;189:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 95. | Hodson R, Kirker E, Swanson J, Walsh C, Korngold EC, Ramelli S. Transcatheter Aortic Valve Replacement During Pregnancy. Circ Cardiovasc Interv. 2016;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 96. | Gandhi S, Ganame J, Whitlock R, Chu V, Natarajan MK, Velianou JL. Double Trouble: A Case of Valvular Disease in Pregnancy. Circulation. 2016;133:2206-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 97. | Berry N, Sawlani N, Economy K, Shook D, Nyman C, Singh MN, Pelletier M, Sobieszczyk P, Kaneko T, Shah P. Transcatheter Aortic Valve Replacement for Bioprosthetic Aortic Stenosis in Pregnancy. JACC Cardiovasc Interv. 2018;11:e161-e162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 98. | Herbert KA, Sheppard SM. Not Your Typical Dyspnea of Pregnancy: A Case Report of Transcatheter Valve-in-Valve Replacement During Pregnancy. A A Pract. 2019;12:202-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 99. | Zhong C, Rokey R, Rolak S, Mesa J. Pregnancy and transcatheter aortic valve replacement in a severely stenotic Freestyle full aortic root stentless bioprosthesis. Catheter Cardiovasc Interv. 2020;95:1225-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 100. | Hoover E, Corlin T, Lohr J, Sabol B, Yamamura Y, Jacobs K, March SK, Gorbaty B, Raveendran G. TAVR Beyond Fetal Viability: An Alternative to Preterm Delivery in Symptomatic Severe Aortic Stenosis. JACC Case Rep. 2023;27:102104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 101. | Panah LG, O'Leary J, Levack M, Brennan K, Osmundson S, Thompson J, Lindley K. Treatment of Severe Symptomatic Aortic Stenosis During Pregnancy: A Potential Role for TAVR? JACC Case Rep. 2023;28:102134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 102. | Rasmussen K, Rokey R, Rolak SC, Zhong C, Braxton JH, Hashimoto K. Repeat Pregnancy after Prior Aortic Valve-in-Valve Replacement: A Cautionary Tale. Clin Med Res. 2020;18:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 103. | Ormerod O, Newton JD, Westaby S, Wilson N. Percutaneous pulmonary valve replacement during pregnancy. Circulation. 2014;129:e373-e375. [PubMed] [DOI] [Full Text] |
| 104. | Detzner AA, Hopkins KA, Kay WA, Hoyer MH. Successful TPV Implantation in a Pregnant Patient With Right Ventricle to Pulmonary Artery Conduit Obstruction. JACC Case Rep. 2020;2:135-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 105. | Duarte VE, Graf JA, Marshall AC, Economy KE, Valente AM. Transcatheter Pulmonary Valve Performance During Pregnancy and the Postpartum Period. JACC Case Rep. 2020;2:847-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 106. | Swartz RH, Cayley ML, Foley N, Ladhani NNN, Leffert L, Bushnell C, McClure JA, Lindsay MP. The incidence of pregnancy-related stroke: A systematic review and meta-analysis. Int J Stroke. 2017;12:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 107. | Elgendy IY, Bukhari S, Barakat AF, Pepine CJ, Lindley KJ, Miller EC; American College of Cardiology Cardiovascular Disease in Women Committee. Maternal Stroke: A Call for Action. Circulation. 2021;143:727-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 108. | Miller EC. Maternal Stroke Associated With Pregnancy. Continuum (Minneap Minn). 2022;28:93-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 109. | Swartz RH, Ladhani NNN, Foley N, Nerenberg K, Bal S, Barrett J, Bushnell C, Chan WS, Chari R, Dowlatshahi D, Amrani ME, Gandhi S, Gubitz G, Hill MD, James A, Jeerakathil T, Jin A, Kirton A, Lanthier S, Lausman A, Leffert LR, Mandzia J, Menon B, Pikula A, Poppe A, Potts J, Ray J, Saposnik G, Sharma M, Smith EE, Bhogal S, Smitko E, Lindsay MP; Heart and Stroke Foundation Canadian Stroke Best Practice Advisory Committees. Canadian stroke best practice consensus statement: Secondary stroke prevention during pregnancy. Int J Stroke. 2018;13:406-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 110. | Yu AYX, Nerenberg KA, Diong C, Fang J, Chu A, Kapral MK, Edwards JD, Dancey SR, Austin PC, Auger N. Maternal Health Outcomes After Pregnancy-Associated Stroke: A Population-Based Study With 19 Years of Follow-Up. Stroke. 2023;54:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 111. | Ruys TP, Roos-Hesselink JW, Hall R, Subirana-Domènech MT, Grando-Ting J, Estensen M, Crepaz R, Fesslova V, Gurvitz M, De Backer J, Johnson MR, Pieper PG. Heart failure in pregnant women with cardiac disease: data from the ROPAC. Heart. 2014;100:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 112. | Bowater SE, Selman TJ, Hudsmith LE, Clift PF, Thompson PJ, Thorne SA. Long-term outcome following pregnancy in women with a systemic right ventricle: is the deterioration due to pregnancy or a consequence of time? Congenit Heart Dis. 2013;8:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 113. | DeFilippis EM, Bhagra C, Casale J, Ging P, Macera F, Punnoose L, Rasmusson K, Sharma G, Sliwa K, Thorne S, Walsh MN, Kittleson MM. Cardio-Obstetrics and Heart Failure: JACC: Heart Failure State-of-the-Art Review. JACC Heart Fail. 2023;11:1165-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 114. | Tapaskar N, Tremblay-Gravel M, Khush KK. Contemporary Management of Cardiogenic Shock During Pregnancy. J Card Fail. 2023;29:193-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 115. | LaRue S, Shanks A, Wang IW, Ewald G, Anderson D, Joseph S. Left ventricular assist device in pregnancy. Obstet Gynecol. 2011;118:426-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 116. | Makdisi G, Jan MY, Dungy-Poythress L, Wang IW, Caccamo MA. Successful Delivery in a Patient With Left Ventricular Assist Device and Unplanned Pregnancy. Ann Thorac Surg. 2017;104:e31-e33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 117. | Malik A, Winchester ML, Gorman K, Parrott J, Parrish M. Left ventricular assist device in pregnancy: Case report and review of the literature. J Obstet Gynaecol Res. 2021;47:1589-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 118. | Sims DB, Vink J, Uriel N, Cleary KL, Smiley RM, Jorde UP, Restaino SW. A successful pregnancy during mechanical circulatory device support. J Heart Lung Transplant. 2011;30:1065-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 119. | Vargas A, Armin S, Yeomans E. Successful pregnancy with left ventricular assist device failure in the setting of peripartum cardiomyopathy. Proc (Bayl Univ Med Cent). 2022;35:98-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 120. | Yadalam AK, Yoo BW, Horton JP, Krishna I, Vega JD, Bhatt KN, Gupta D, Abdou MH. Left Ventricular Assist Devices and Pregnancy: Systematic Review of Existing Literature and Case Report. Curr Probl Cardiol. 2023;48:101469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 121. | Defilippis EM, Kittleson MM. Pregnancy after Heart Transplantation. J Card Fail. 2021;27:176-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 122. | Kittleson MM, DeFilippis EM, Bhagra CJ, Casale JP, Cauldwell M, Coscia LA, D'Souza R, Gaffney N, Gerovasili V, Ging P, Horsley K, Macera F, Mastrobattista JM, Paraskeva MA, Punnoose LR, Rasmusson KD, Reynaud Q, Ross HJ, Thakrar MV, Walsh MN. Reproductive health after thoracic transplantation: An ISHLT expert consensus statement. J Heart Lung Transplant. 2023;42:e1-e42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 123. | Thai TN, Sarayani A, Wang X, Albogami Y, Rasmussen SA, Winterstein AG. Risk of pregnancy loss in patients exposed to mycophenolate compared to azathioprine: A retrospective cohort study. Pharmacoepidemiol Drug Saf. 2020;29:716-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 124. | Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW; ACC/AHA Joint Committee Members. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895-e1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1289] [Cited by in RCA: 1209] [Article Influence: 403.0] [Reference Citation Analysis (0)] |
| 125. | Joglar JA, Kapa S, Saarel EV, Dubin AM, Gorenek B, Hameed AB, Lara de Melo S, Leal MA, Mondésert B, Pacheco LD, Robinson MR, Sarkozy A, Silversides CK, Spears D, Srinivas SK, Strasburger JF, Tedrow UB, Wright JM, Zelop CM, Zentner D. 2023 HRS expert consensus statement on the management of arrhythmias during pregnancy. Heart Rhythm. 2023;20:e175-e264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 52] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 126. | Tamirisa KP, Elkayam U, Briller JE, Mason PK, Pillarisetti J, Merchant FM, Patel H, Lakkireddy DR, Russo AM, Volgman AS, Vaseghi M. Arrhythmias in Pregnancy. JACC Clin Electrophysiol. 2022;8:120-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 127. | Tfelt-Hansen J, Winkel BG, de Riva M, Zeppenfeld K. The '10 commandments' for the 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2023;44:176-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 43] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 128. | Majmundar M, Doshi R, Patel KN, Zala H, Kumar A, Kalra A. Prevalence, trends, and outcomes of cardiovascular diseases in pregnant patients in the USA: 2010-19. Eur Heart J. 2023;44:726-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 129. | Rahnama N, Ben Jemaa N, Colson A, Pasquet A, Houard De Castro L, Debieve F, Pierard S. Pregnancy in women with congenital heart disease : new insights into neonatal risk predictioc. Eur Heart J. 2024;45. [DOI] [Full Text] |
| 130. | Clur SA, Ottenkamp J, Bilardo CM. The nuchal translucency and the fetal heart: a literature review. Prenat Diagn. 2009;29:739-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 131. | Hopkins MK, Dugoff L, Kuller JA. Congenital Heart Disease: Prenatal Diagnosis and Genetic Associations. Obstet Gynecol Surv. 2019;74:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 132. | Ison HE, Griffin EL, Parrott A, Shikany AR, Meyers L, Thomas MJ, Syverson E, Demo EM, Fitzgerald KK, Fitzgerald-Butt S, Ziegler KL, Schartman AF, Stone KM, Helm BM. Genetic counseling for congenital heart disease - Practice resource of the National Society of Genetic Counselors. J Genet Couns. 2022;31:9-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 133. | Jenkins KJ, Correa A, Feinstein JA, Botto L, Britt AE, Daniels SR, Elixson M, Warnes CA, Webb CL; American Heart Association Council on Cardiovascular Disease in the Young. Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:2995-3014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 562] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 134. | Miller DT, Adam MP, Aradhya S, Biesecker LG, Brothman AR, Carter NP, Church DM, Crolla JA, Eichler EE, Epstein CJ, Faucett WA, Feuk L, Friedman JM, Hamosh A, Jackson L, Kaminsky EB, Kok K, Krantz ID, Kuhn RM, Lee C, Ostell JM, Rosenberg C, Scherer SW, Spinner NB, Stavropoulos DJ, Tepperberg JH, Thorland EC, Vermeesch JR, Waggoner DJ, Watson MS, Martin CL, Ledbetter DH. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet. 2010;86:749-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2071] [Cited by in RCA: 1917] [Article Influence: 127.8] [Reference Citation Analysis (0)] |
| 135. | Qin Y, Yao Y, Liu N, Wang B, Liu L, Li H, Gao T, Xu R, Wang X, Zhang F, Song J. Prenatal whole-exome sequencing for fetal structural anomalies: a retrospective analysis of 145 Chinese cases. BMC Med Genomics. 2023;16:262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 136. | Xiang J, Ding Y, Tang H, Zhang W, Mao J, He Q, Zhang Q, Wang T. Genetic analysis of pregnancy loss and fetal structural anomalies by whole exome sequencing. Orphanet J Rare Dis. 2024;19:330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 137. | Fu F, Li R, Yu Q, Wang D, Deng Q, Li L, Lei T, Chen G, Nie Z, Yang X, Han J, Pan M, Zhen L, Zhang Y, Jing X, Li F, Li F, Zhang L, Yi C, Li Y, Lu Y, Zhou H, Cheng K, Li J, Xiang L, Zhang J, Tang S, Fang P, Li D, Liao C. Application of exome sequencing for prenatal diagnosis of fetal structural anomalies: clinical experience and lessons learned from a cohort of 1618 fetuses. Genome Med. 2022;14:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 138. | Bock A. AHA Recommends Better Postpartum Care to Lower Heart Disease Risk. JAMA. 2024;332:189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 139. | Lewey J, Beckie TM, Brown HL, Brown SD, Garovic VD, Khan SS, Miller EC, Sharma G, Mehta LS; American Heart Association Cardiovascular Disease and Stroke in Women and Underrepresented Populations Committee of the Council on Clinical Cardiology; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; and Council on Cardiovascular and Stroke Nursing. Opportunities in the Postpartum Period to Reduce Cardiovascular Disease Risk After Adverse Pregnancy Outcomes: A Scientific Statement From the American Heart Association. Circulation. 2024;149:e330-e346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 50] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 140. | Sachs HC; Committee On Drugs. The transfer of drugs and therapeutics into human breast milk: an update on selected topics. Pediatrics. 2013;132:e796-e809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 262] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 141. | Lindley KJ, Bairey Merz CN, Davis MB, Madden T, Park K, Bello NA; American College of Cardiology Cardiovascular Disease in Women Committee and the Cardio-Obstetrics Work Group. Contraception and Reproductive Planning for Women With Cardiovascular Disease: JACC Focus Seminar 5/5. J Am Coll Cardiol. 2021;77:1823-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 142. | Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, Simmons KB, Pagano HP, Jamieson DJ, Whiteman MK. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 438] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 143. | Westhoff-Bleck M, Briest J, Fraccarollo D, Hilfiker-Kleiner D, Winter L, Maske U, Busch MA, Bleich S, Bauersachs J, Kahl KG. Mental disorders in adults with congenital heart disease: Unmet needs and impact on quality of life. J Affect Disord. 2016;204:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 144. | Freiberger A, Beckmann J, Freilinger S, Kaemmerer H, Huber M, Nagdyman N, Ewert P, Pieper L, Deppe C, Kuschel B, Andonian C. Psychosocial well-being in postpartum women with congenital heart disease. Cardiovasc Diagn Ther. 2022;12:389-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 145. | Abarbanell G, Tepper NK, Farr SL. Safety of contraceptive use among women with congenital heart disease: A systematic review. Congenit Heart Dis. 2019;14:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 146. | Lindley KJ, Teal SB. Contraception in Women With Cardiovascular Disease. JAMA. 2022;328:577-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 147. | Roos-Hesselink JW, Cornette J, Sliwa K, Pieper PG, Veldtman GR, Johnson MR. Contraception and cardiovascular disease. Eur Heart J. 2015;36:1728-1734, 1734a. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 148. | Pfaller B, Sathananthan G, Grewal J, Mason J, D'Souza R, Spears D, Kiess M, Siu SC, Silversides CK. Preventing Complications in Pregnant Women With Cardiac Disease. J Am Coll Cardiol. 2020;75:1443-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 149. | Davis MB, Bello NA, Berlacher K, Harrington CM, Lin JP, Lindley KJ, Panah LG, Park KE, Silversides CK, Walsh MN, Weissman G, DeFaria Yeh D, Damp JB. Cardiovascular Fellowship Training in Cardio-Obstetrics: JACC Review Topic of the Week. J Am Coll Cardiol. 2023;82:1792-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 150. | Sharma G, Lindley K, Grodzinsky A. Cardio-Obstetrics: Developing a Niche in Maternal Cardiovascular Health. J Am Coll Cardiol. 2020;75:1355-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 151. | Lopez KN, Allen KY, Baker-Smith CM, Bravo-Jaimes K, Burns J, Cherestal B, Deen JF, Hills BK, Huang JH, Lizano Santamaria RW, Lodeiro CA, Melo V, Moreno JS, Nuñez Gallegos F, Onugha H, Pastor TA, Wallace MC, Ansah DA. Health Equity and Policy Considerations for Pediatric and Adult Congenital Heart Disease Care among Minoritized Populations in the United States. J Cardiovasc Dev Dis. 2024;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 152. | Easter SR, Rouse CE, Duarte V, Hynes JS, Singh MN, Landzberg MJ, Valente AM, Economy KE. Planned vaginal delivery and cardiovascular morbidity in pregnant women with heart disease. Am J Obstet Gynecol. 2020;222:77.e1-77.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 153. | Malhamé I, Czuzoj-Shulman N, Abenhaim HA. Cardiovascular Severe Maternal Morbidity and Mortality at Delivery in the United States: A Population-Based Study. JACC Adv. 2022;1:100121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 154. | Cameron NA, Huang X, Petito LC, Ning H, Shah NS, Yee LM, Perak AM, Haas DM, Mercer BM, Parry S, Saade GR, Silver RM, Simhan HN, Reddy UM, Varagic J, Licon E, Greenland P, Lloyd-Jones DM, Kershaw KN, Grobman WA, Khan SS. Determinants of Racial and Ethnic Differences in Maternal Cardiovascular Health in Early Pregnancy. Circ Cardiovasc Qual Outcomes. 2025;18:e011217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 155. | Chowdhury D, Johnson JN, Baker-Smith CM, Jaquiss RDB, Mahendran AK, Curren V, Bhat A, Patel A, Marshall AC, Fuller S, Marino BS, Fink CM, Lopez KN, Frank LH, Ather M, Torentinos N, Kranz O, Thorne V, Davies RR, Berger S, Snyder C, Saidi A, Shaffer K. Health Care Policy and Congenital Heart Disease: 2020 Focus on Our 2030 Future. J Am Heart Assoc. 2021;10:e020605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 156. | Mehta LS, Sharma G, Creanga AA, Hameed AB, Hollier LM, Johnson JC, Leffert L, McCullough LD, Mujahid MS, Watson K, White CJ; American Heart Association Advocacy Coordinating Committee. Call to Action: Maternal Health and Saving Mothers: A Policy Statement From the American Heart Association. Circulation. 2021;144:e251-e269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 157. | Protecting and Expanding Medicaid to Improve Women's Health: ACOG Committee Opinion, Number 826. Obstet Gynecol. 2021;137:e163-e168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 158. | Wenger NK, Lloyd-Jones DM, Elkind MSV, Fonarow GC, Warner JJ, Alger HM, Cheng S, Kinzy C, Hall JL, Roger VL; American Heart Association. Call to Action for Cardiovascular Disease in Women: Epidemiology, Awareness, Access, and Delivery of Equitable Health Care: A Presidential Advisory From the American Heart Association. Circulation. 2022;145:e1059-e1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 109] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 159. | Eliason EL. Adoption of Medicaid Expansion Is Associated with Lower Maternal Mortality. Womens Health Issues. 2020;30:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 160. | Terlizzi EP, Connor EM, Zelaya CE, Ji AM, Bakos AD. Reported Importance and Access to Health Care Providers Who Understand or Share Cultural Characteristics With Their Patients Among Adults, by Race and Ethnicity. Natl Health Stat Report. 2019;1-12. [PubMed] |
| 161. | Mehta LS, Fisher K, Rzeszut AK, Lipner R, Mitchell S, Dill M, Acosta D, Oetgen WJ, Douglas PS. Current Demographic Status of Cardiologists in the United States. JAMA Cardiol. 2019;4:1029-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 162. | Lopez KN, Morris SA, Sexson Tejtel SK, Espaillat A, Salemi JL. US Mortality Attributable to Congenital Heart Disease Across the Lifespan From 1999 Through 2017 Exposes Persistent Racial/Ethnic Disparities. Circulation. 2020;142:1132-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 163. | Vilda D, Wallace ME, Daniel C, Evans MG, Stoecker C, Theall KP. State Abortion Policies and Maternal Death in the United States, 2015‒2018. Am J Public Health. 2021;111:1696-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 164. | Redd SK, Hall KS, Aswani MS, Sen B, Wingate M, Rice WS. Variation in Restrictive Abortion Policies and Adverse Birth Outcomes in the United States from 2005 to 2015. Womens Health Issues. 2022;32:103-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 165. | Romanis EC. The end of (reproductive) liberty as we know it: A note on Dobbs V. Jackson Women’s Health 597 USC __ (2022). Med Law Int. 2023;23:71-87. [DOI] [Full Text] |
| 166. | U.S. Supreme Court. Roe v. Wade. 22 Jan 1973. U S Rep U S Supreme Court. 1973;410:113-178. [PubMed] |
| 167. | Miller HE, Fraz F, Zhang J, Henkel A, Leonard SA, Maskatia SA, El-Sayed YY, Blumenfeld YJ. Abortion Bans and Resource Utilization for Congenital Heart Disease: A Decision Analysis. Obstet Gynecol. 2023;142:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 168. | Wander G, van der Zande JA, Patel RR, Johnson MR, Roos-Hesselink J. Pregnancy in women with congenital heart disease: a focus on management and preventing the risk of complications. Expert Rev Cardiovasc Ther. 2023;21:587-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 169. | Anderson MR, Burtch G, Greenwood BN. The impact of abortion restrictions on American mental health. Sci Adv. 2024;10:eadl5743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 170. | Curley M, Thornton JE. Mental Health Outcomes After Having or Being Denied an Abortion. JAMA Psychiatry. 2017;74:653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 171. | Biggs MA, Upadhyay UD, Foster DG. Mental Health Outcomes After Having or Being Denied an Abortion-Reply. JAMA Psychiatry. 2017;74:654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 172. | Stevenson AJ. The Pregnancy-Related Mortality Impact of a Total Abortion Ban in the United States: A Research Note on Increased Deaths Due to Remaining Pregnant. Demography. 2021;58:2019-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 173. | Redd SK, Rice WS, Aswani MS, Blake S, Julian Z, Sen B, Wingate M, Hall KS. Racial/ethnic and educational inequities in restrictive abortion policy variation and adverse birth outcomes in the United States. BMC Health Serv Res. 2021;21:1139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 174. | Xing E, Owda R, Loder C, Collins K. Abortion rights are health care rights. JCI Insight. 2023;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 175. | Ricci CA, Crysup B, Phillips NR, Ray WC, Santillan MK, Trask AJ, Woerner AE, Goulopoulou S. Machine learning: a new era for cardiovascular pregnancy physiology and cardio-obstetrics research. Am J Physiol Heart Circ Physiol. 2024;327:H417-H432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 176. | Barber N, Freud L. Advances in Fetal Cardiac Imaging and Intervention. CJC Pediatr Congenit Heart Dis. 2024;3:33-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 177. | Guseh S, Tworetzky W. Transforming congenital heart disease management: Advances in fetal cardiac interventions. Prenat Diagn. 2024;44:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 178. | Baumgartner H, De Backer J, Babu-Narayan SV, Budts W, Chessa M, Diller GP, Lung B, Kluin J, Lang IM, Meijboom F, Moons P, Mulder BJM, Oechslin E, Roos-Hesselink JW, Schwerzmann M, Sondergaard L, Zeppenfeld K; ESC Scientific Document Group. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021;42:563-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 1173] [Article Influence: 293.3] [Reference Citation Analysis (0)] |
| 179. | Rodriguez CP, Economy KE, Duarte VE, Mehta N, Duncan ME, Chandler S, Gauvreau K, Easter SR, Wu F, Lachtrupp C, Tedrow U, Valente AM, Tadros T. Mobile Cardiac Telemetry Use to Predict Adverse Pregnancy Outcomes in Patients With Congenital Heart Disease. JACC Adv. 2023;2:100593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 180. | Valencia SA, Barrientos Gómez JG, Gómez Ramirez MC, Luna IF, Caicedo HA, Torres-Silva EA, Díaz ES. Evaluation of a telehealth program for high-risk pregnancy in a health service provider institution. Int J Med Inform. 2023;179:105234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 181. | Borrelli N, Grimaldi N, Papaccioli G, Fusco F, Palma M, Sarubbi B. Telemedicine in Adult Congenital Heart Disease: Usefulness of Digital Health Technology in the Assistance of Critical Patients. Int J Environ Res Public Health. 2023;20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 182. | Kauw D, Koole MAC, Winter MM, Dohmen DAJ, Tulevski II, Blok S, Somsen GA, Schijven MP, Vriend JWJ, Robbers-Visser D, Mulder BJM, Bouma BJ, Schuuring MJ. Advantages of mobile health in the management of adult patients with congenital heart disease. Int J Med Inform. 2019;132:104011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 183. | Goldfarb MJ, Saylor MA, Bozkurt B, Code J, Di Palo KE, Durante A, Flanary K, Masterson Creber R, Ogunniyi MO, Rodriguez F, Gulati M; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Hypertension; Council on Lifestyle and Cardiometabolic Health; Council on Peripheral Vascular Disease; and Council on Quality of Care and Outcomes Research. Patient-Centered Adult Cardiovascular Care: A Scientific Statement From the American Heart Association. Circulation. 2024;149:e1176-e1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 184. | Alio AP, Lewis CA, Scarborough K, Harris K, Fiscella K. A community perspective on the role of fathers during pregnancy: a qualitative study. BMC Pregnancy Childbirth. 2013;13:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 185. | Vedanthan R, Bansilal S, Soto AV, Kovacic JC, Latina J, Jaslow R, Santana M, Gorga E, Kasarskis A, Hajjar R, Schadt EE, Björkegren JL, Fayad ZA, Fuster V. Family-Based Approaches to Cardiovascular Health Promotion. J Am Coll Cardiol. 2016;67:1725-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 186. | Gulati M. Saving women's hearts: Improving outcomes with prevention & policy. Am J Prev Cardiol. 2023;14:100504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |