Published online Jun 26, 2025. doi: 10.4330/wjc.v17.i6.105452
Revised: April 11, 2025
Accepted: May 13, 2025
Published online: June 26, 2025
Processing time: 148 Days and 23.3 Hours
The persistent burden of cardiovascular (CV) disease in the United States requires innovative and cost-effective prognostic markers that can be relied upon.
To provide insights into how adiponectin can predict all-cause mortality and major adverse CV events (MACE) in patients with coronary artery disease (CAD) and to determine the prognostic value of adiponectin in predicting all-cause mortality and MACE in patients with stable CAD.
We conducted a systematic search on PubMed, Scopus, and Google Scholar to find relevant studies published through June 2023 evaluating the long-term prognostic role of adiponectin in patients with stable CAD. Using a random effects model with 95%CI, we estimated the odds ratio (OR) while assessing heterogeneity through I² statistics. To ensure robustness, we performed a sensitivity analysis using the leave-one-out approach.
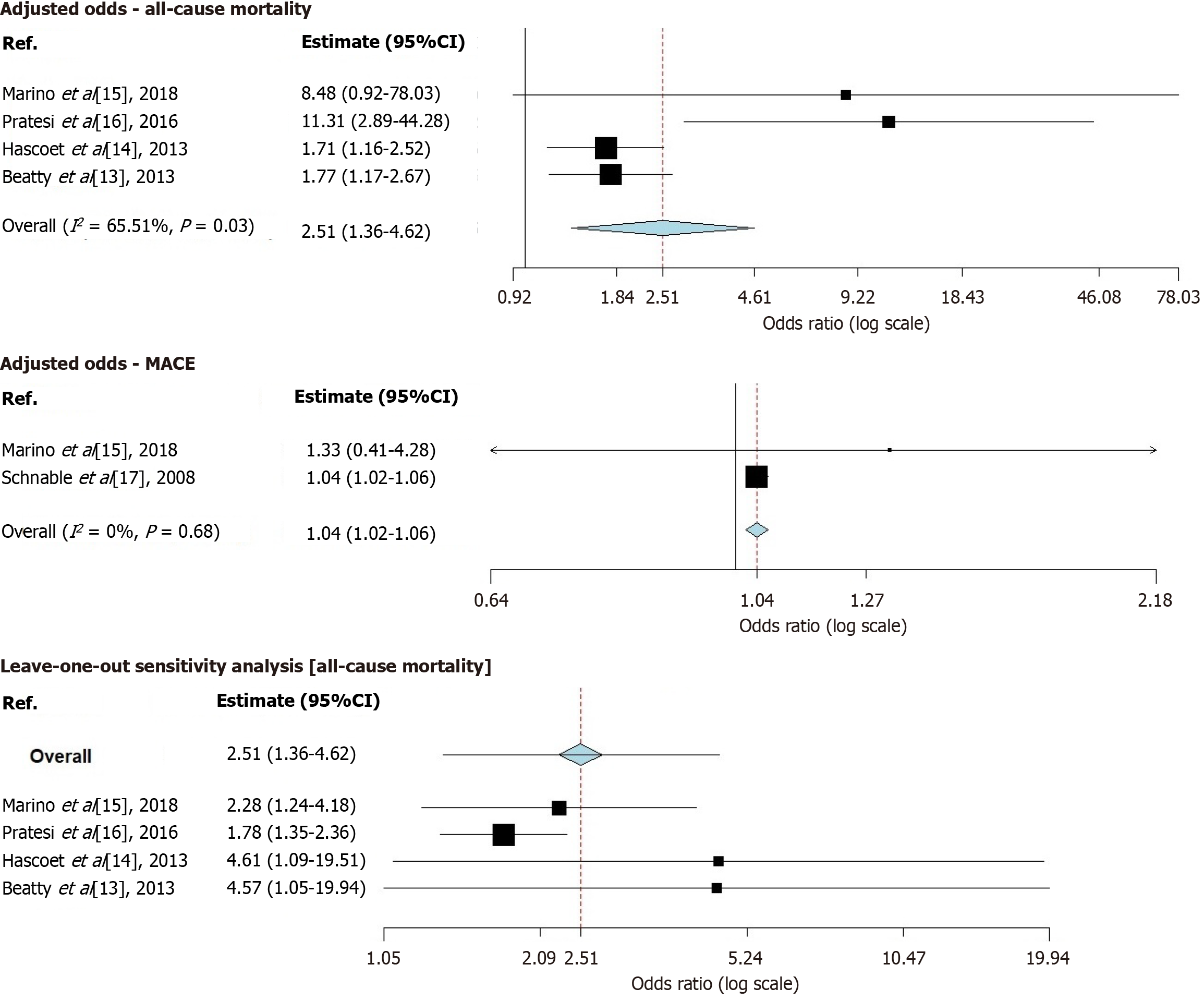
After screening, we included five prospective studies involving 3225 patients who were followed up for a median duration of 3.8 years. Within the study population, prevalent risk factors included hypertension, diabetes, hyperlipidemia, and smoking. The commonly prescribed medications were angiotensin-converting enzyme inhibitors, beta blockers, and statins. The combined adjusted OR for all-cause mortality was found to be 2.51 (95%CI: 1.36–4.62), showing heterogeneity (I² = 65.51%, P = 0.03). On the other hand, the combined adjusted OR for MACE was determined to be 1.04 (95%CI: 1.02–1.06) with no significant heterogeneity observed (I² = 0%, P = 0.68). Through a sensitivity analysis, it was discovered that none of the studies significantly impacted the overall results of the meta-analysis, thus indicating their robustness.
Higher levels of adiponectin were found to be associated with an increased risk of long-term mortality and MACE in patients with CAD, which highlights its potential as a cost-effective marker for risk assessment and guiding treatment strategies. Further research on the role of adiponectin could greatly influence decision-making and resource allocation in CV care.
Core Tip: The persistent burden of cardiovascular (CV) disease in the United States requires innovative and cost-effective prognostic markers that can be relied upon. Higher levels of adiponectin are associated with increased long-term mortality and major adverse CV events in patients with coronary artery disease. This highlights adiponectin as a potential cost-effective prognostic marker for risk assessment and treatment guidance.
- Citation: Jitta SR, Vatsavayi P, Tera CR, Krishnamurthy S, Adla Jala SR, Pasnoor DS, Dasari U, Farooq A, Maramreddy S, Jammula K, Kesani MR, Tripuraneni S, Jena N, Desai R. Long-term prognostic role of adiponectin in stable coronary artery disease: A meta-analysis of prospective studies. World J Cardiol 2025; 17(6): 105452
- URL: https://www.wjgnet.com/1949-8462/full/v17/i6/105452.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i6.105452
Adiponectin is a 244-amino acid protein hormone, first identified in 1995, with a molecular mass of approximately 28 kDa. In circulation, it primarily exists as a dimer[1] but it can also assemble into trimers, as well as higher molecular weight hexamers and multimers (90 kDa, 180 kDa, and > 400 kDa). Structurally, adiponectin forms a single-chain trimer composed of a variable N-terminal region, a collagen-like domain, and a globular C-terminal domain resembling complement component C1. The three globular regions are located at the N-termini and C-termini and are connected through the Pro104–Tyr109 region. This trimeric unit is encapsulated within a gong-shaped shell[2].
Adiponectin exerts its biological effects by modulating signaling pathways in various target cells, thereby counteracting inflammatory stimuli. Its anti-inflammatory properties are central to its beneficial effects on the cardiovascular (CV) system and metabolic conditions, including insulin resistance and atherosclerosis in the vascular endothelium[3,4], as well as obesity, type 2 diabetes mellitus, and coronary artery disease (CAD). Additionally, adiponectin maintains vascular homeostasis by regulating key signaling pathways in endothelial cells and modulating inflammatory processes within the subendothelial space[5].
The predictive role of adiponectin in long-term outcomes for patients with CAD is based on its complex involvement in metabolic and CV processes. Recent research has highlighted its importance as a biomarker for CV disease risk assessment. Higher levels of adiponectin have been associated with an increased risk of all-cause mortality in CAD patients, indicating its potential as a predictor of unfavorable results. However, the adiponectin paradox persists, as certain investigations suggest a protective role against CV events. Complexities arise from differences in study design, patient cohorts, and testing methods. This paradox highlights the intricate nature of adiponectin's involvement in CV health and its potential multifaceted impact[6,7].
The relationship between adiponectin levels and CV risk appears complex, as both higher and lower levels have been associated with adverse outcomes. Elevated adiponectin levels have been paradoxically linked to major adverse CV events (MACE), including ischemic stroke[8]. In contrast, reduced adiponectin levels have been implicated in increased CV complications in conditions such as obesity, insulin resistance, and diabetes[9]. This paradox underscores the intricate interplay between adiponectin's protective mechanisms and its potential role in promoting vascular dysfunction.
Previous research has delved into unraveling the adiponectin paradox. Some studies suggest that higher circulating adiponectin concentrations might serve as a marker of lower risk for myocardial infarction in men[10]. On the other hand, meta-analyses have indicated that both high and low adiponectin levels could be associated with increased CV mortality risk[11]. While adiponectin's anti-inflammatory and vasculoprotective properties could contribute to its beneficial effects, the paradoxical outcomes highlight the need for a deeper understanding of its complex mechanisms in CV pathophysiology[12]. In essence, the role of adiponectin, in predicting the risk of CAD, myocardial infarction, or stroke is complex. It highlights the connection between its levels and CV outcomes. The conventional understanding of how adiponectin exerts its effects on health is often derived from studies conducted on animal models or from early human research. However, these recent unexpected findings from genetic studies highlight the importance of rigorously examining and validating biomarkers, in real world clinical settings. Further research is necessary to elucidate its involvement in these diseases, especially in patients with stable CAD. This systematic review and meta-analysis aims to evaluate prospective trials with long-term follow-up.
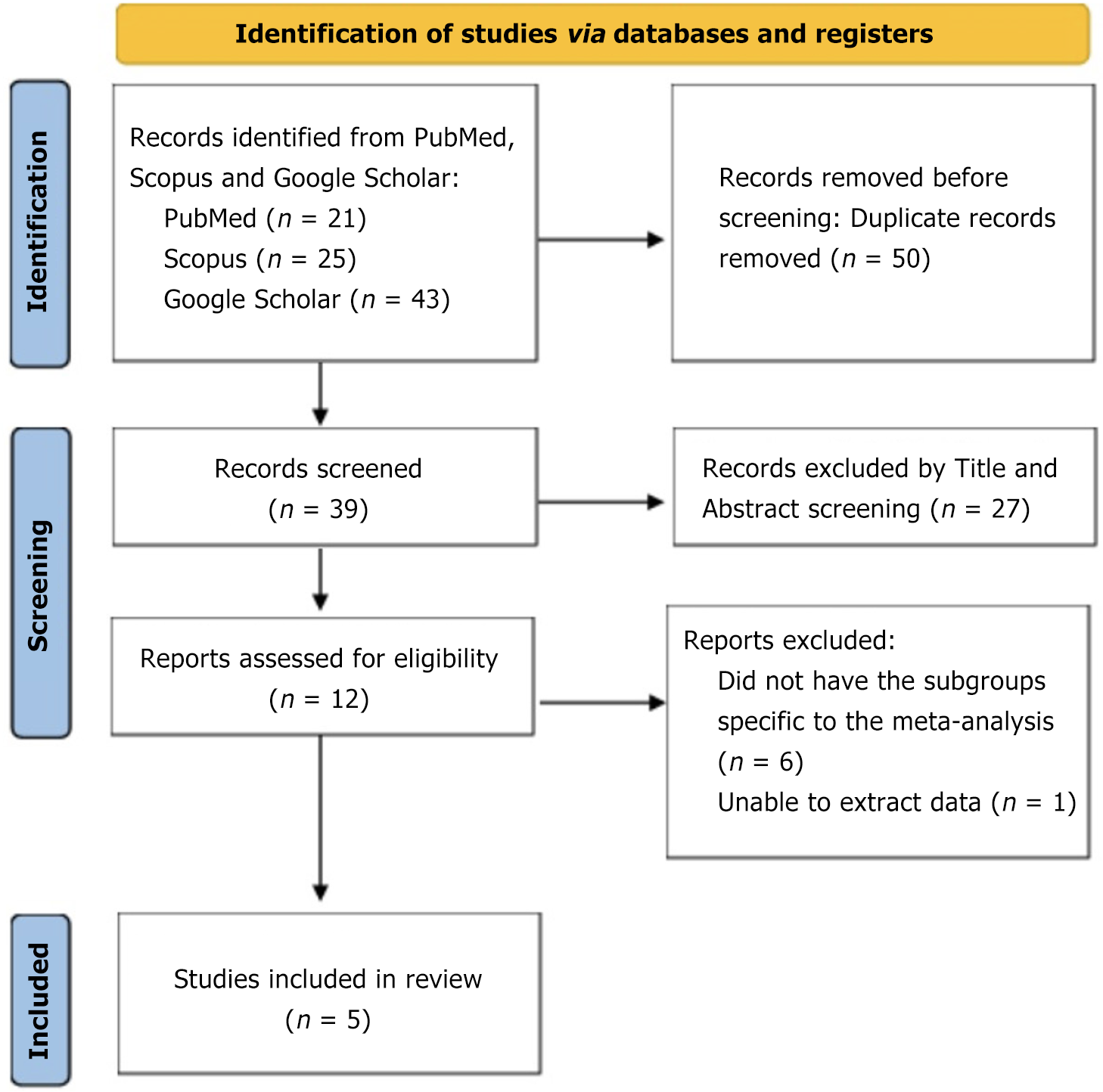
For this analysis, we followed the Preferred Reporting Items for Systematic Reviews and Meta Analyses guidelines (Figure 1). We conducted a search on PubMed, Scopus, and Google Scholar databases, including studies up until June 2023. To ensure a search, we used specific keywords such as "adiponectin", "stable coronary artery disease", "major adverse cardiac and cerebrovascular events", "mortality", "prospective studies" and "systematic review/meta-analysis". We excluded comments, editorials, case reports, reviews, proceedings, personal communications, and non-English publications. Only prospective studies that provided data on the predictive role of adiponectin and outcomes related to MACE (major adverse cardiac and cerebrovascular events) as well as all-cause mortality were included (Figure 2)[13-17].
Two reviewers independently screened the studies to determine their eligibility for inclusion. In cases where there were uncertainties or disagreements between the two reviewers, a third reviewer was consulted to reach a consensus. The extracted data included information such as author names, publication year, study design details, participant numbers, and their age, sex distribution, and comorbidities. Additionally, we collected data on adiponectin levels measured during the study period as well as information on follow-up duration and outcome measures related to all-cause mortality, or MACE.
We assessed the quality of each study using the Newcastle Ottawa Scale, which employs criteria to evaluate potential biases, in research design (Table 1). Two independent reviewers conducted the assessment process, and any discrepancies or disagreements were resolved through discussion until a consensus was reached.
| Criteria | Study 1 | Study 2 | Study 3 | Study 4 | Study 5 | |
| Selection | ||||||
| Representativeness of the exposed cohort | Were the included patient’s representative of the population with stable CAD | Yes | Yes | Yes | Yes | Yes |
| Selection of the non-exposed cohort | Were appropriate comparison groups selected to evaluate the association between adiponectin and outcomes in stable CAD patients | - | - | Yes | - | Yes |
| Ascertainment of exposure | Were adiponectin levels accurately measured and defined in the studies | Yes | Yes | Yes | Yes | Yes |
| Demonstration that outcome of interest was not present at the start of the study | Were patients with pre-existing conditions or adverse outcomes excluded or adequately accounted for at baseline | Yes | Yes | Yes | Yes | Yes |
| Comparability | ||||||
| Comparability of cohorts on the basis of the design or analysis | Did the studies control for only for main factor or all potential confounding factors or perform appropriate adjustments when examining the association between adiponectin and outcomes in stable CAD patients | Yes | Yes | Yes | Yes | Yes |
| Outcome | ||||||
| Assessment of outcome | Were the outcomes of interest (e.g., cardiovascular events, mortality) clearly defined and assessed using standardized criteria across the studies | Yes | Yes | Yes | Yes | Yes |
| Was follow-up long enough for outcomes to occur | Did the studies have a sufficient follow-up period to capture the occurrence of outcomes in stable CAD patients with adiponectin levels | Yes | Yes | Yes | Yes | Yes |
| Adequacy of follow-up of cohorts | Did the studies achieve a high follow-up rate for both the exposed and non-exposed cohorts throughout the follow-up period | - | - | Yes | - | Yes |
The study focused on two outcomes; MACE and all-cause mortality. To analyze the data, we used OpenMeta[Analyst] software and applied the random effects model to combine effect sizes. The results were visually presented using forest plots. We also measured heterogeneity using the I2 statistics. To examine the impact of studies, we conducted a sensitivity analysis by leaving out one study at a time. Additionally, we used funnel plots to assess publication bias, considering P values greater than 0.05, as statistically significant.
After screening, we included five prospective studies[13-17] involving 3225 patients who were followed up for a median duration of 3.8 years. Within the study population, prevalent risk factors included hypertension, diabetes, hyperlipidemia, and smoking. The commonly prescribed medications were angiotensin converting enzyme inhibitors, beta blockers, and statins. The baseline characteristics of the study population are reported in Table 2[13-17].
| Ref. | Location | Study design | Cohort | Sample size | Mean/median age (years) | Median follow-up (years) | Male participants | Hypertension | Hyperlipidemia | DM | Obesity (BMI > 25 kg/m2) | History of myocardial infarction | Smoking | Adiponectin cut offs | Outcomes reported (ACM, MACE) | Multivariable regression analysis was adjusted for which variables/confounders |
| Marino et al[15], 2018 | Netherlands | Prospective | Total: 570, ACS: 309, SAP: 261 | 261 | N/A | 1 | 203 (77.8) | 161(61.70) | Hypercholesterolemia: 180 (69) | 59 (22.60) | N/A | N/A | 49 (18.8) | In ACS patients-median IQR: 2.9 (1.8-4.1 μg/mL) μg/mL. In SAP patients-median IQR: 2.9 (1.9-3.9 μg/mL) μg/mL | ACM adjusted OR/HR = 8.48 (0.92-78.03), MACE adjusted OR/HR = 1.33 (0.41-4.28) | ACS and SAP-adjusted for age, gender, diabetes, hypertension, and CRP. Additionally, adjusted for indication for coronary angiography in the total cohort |
| Pratesi et al[16], 2016 | Italy | Prospective | Stable CAD | 138 | 69.3 ± 10.4 | 3.8 | 122 (89.7) | 98 (71) | 99 (71.70) | 61 (42.20) | Mean level of BMI was 26.8 kg/m2 ± 4.1 kg/m2 | 114 (82.60) | 91 (65.94) | 13.2 ng/mL | ACM adjusted OR/HR = 11.31 (2.89-44.28) | Model 1 (ACM), age, gender, BMI, Inflammatory Disease Score, previous percutaneous transluminal coronary angioplasty, atrial fibrillation, peripheral artery disease, NYHA class, EF, hemoglobin, NT-proBNP, eGFR. Model 2 (cardiovascular hospitalisation rate): age, gender, BMI, smoking, NYHA class, hemoglobin, NT-proBNP, EF |
| Hascoet et al[14], 2013 | France | Prospective | Stable CAD | 715 | 60.2 ± 8 | 8.1 | 715 (100) | 44.60% | 64.90% | 24.8% | N/A | N/A | 82.50% | 9.1 μg/mL | ACM adjusted OR/HR = 1.71 (1.16-2.52) | Diabetes mellitus, dyslipidaemia, and hypertension, systolic blood pressure, resting heart rate, tobacco consumption, hsCRP, HDL cholesterol, lipoprotein (a), ankle-arm index, and physical activity |
| Beatty et al[13], 2012 | United States | Prospective | Stable ischemic heart disease | 981 | 64 ± 10.5, 64.9 ± 10.2, 67.6 ± 10.9, 70.5 ± 11.2 | 7.1 | 800 (81.5) | 691 | N/A | 259 | N/A | N/A | N/A | Lowest detectable: 145.4 pg/mL. Median: 21.3 μg/mL | ACM adjusted OR/HR = 1.77 (1.12-2.67) | Model 1 adjusts for Demographics (age, sex, race). Model 2 adjusts for model 1 + clinical risk factors (diabetes, eGFR, beta-blocker, aspirin, statin). Model 3 adjusts for model 2 + metabolic markers (BMI, hemogloblin A1c, insulin, glucose, non-HDL cholesterol, HDL, triglycerides). Model 4 adjusts for model 3 + measures of baseline cardiac disease severity (left ventricle ejection fraction, diastolic dysfunction, inducible ischemia, log CRP, log NT-proBNP) |
| Schnabel et al[17], 2008 | Germany | Prospective | Total: 1890; stable CAD: 1130; ACS: 760 | 1130 | 63 | 2.5 | 906 (80.2) | 896 | Total cholesterol: 194; HDL: 48; low density lipoprotein: 121; triglycerides: 129 | DM: None or diet: 929; oral: 103; insulin: 98 | N/A | N/A | No: 413; past: 523; present: 194 | Not mentioned but concentrations were similar in patients presenting with SAP [9.03 μg/mL (6.7-13.45 μg/mL)] or ACS [(9.19 μg/mL (6.72-13.15 μg/mL)] | MACE adjusted OR/HR = 1.04 (1.008-1.062) | Model 1, univariate analysis. Model 2 is adjusted for age, sex, BMI, hypertension, diabetes mellitus, smoking, status, HDL cholesterol family history, ACS (only for total population). Model 3 is adjusted for age, sex, BMI, hypertension, diabetes mellitus, smoking, status, family history, ACS, statins, and beta blocker. Model 4 is adjusted for age, sex, BMI, hypertension, diabetes mellitus, smoking, status, family history, statins and beta blocker, BNP, and CRP |
The combined adjusted odds ratio (OR) for all-cause mortality was found to be 2.51 (95%CI: 1.36–4.62), showing heterogeneity (I2 = 65.51%, P = 0.03). On the other hand, the combined adjusted OR for MACE was determined to be 1.04 (95%CI: 1.02–1.06) with no significant heterogeneity observed (I2 = 0%, P = 0.68). Through a sensitivity analysis, it was discovered that none of the studies significantly impacted the overall results of the meta-analysis thus indicating their robustness. Concisely, higher levels of adiponectin were found to be associated with an increased risk of long-term mortality and MACE in patients with CAD, which highlights its potential as a cost-effective marker for risk assessment and guiding treatment strategies.
We acknowledge that Pratesi et al[16] reported adiponectin cut-off levels in ng/mL (13.2 ng/mL), whereas others, such as Hascoet et al[14], used µg/mL (9.1 µg/mL). Despite this unit discrepancy, our leave-one-out sensitivity analysis for all-cause mortality confirmed that the association between elevated adiponectin and increased mortality remained statistically significant even after excluding the study performed by Pratesi et al[16] [OR: 1.78 (1.35–2.36)]. This indicates that differences in units or cut-off values did not materially impact our findings. Additionally, the consistency of results across studies, despite slight methodological variations in adiponectin measurement, supports the robustness of our meta-analysis.
This systematic review and meta-analysis of prospective studies showed a link between higher levels of adiponectin and an increased likelihood of long-term mortality and adverse CV events in stable CAD patients. This finding supports the emerging evidence that suggests adiponectin could be a cost-effective marker for assessing risk and making management decisions[8,13]. The overall adjusted OR for all-cause mortality was 2.51 (95%CI: 1.36-4.62) with some variation among the studies (I² = 65.51%, P = 0.03). The pooled adjusted OR for events was 1.04 (95%CI: 1.02-1.06), showing no significant variation across the studies (I² = 0%, P = 0.68). Sensitivity analysis confirmed the reliability of these results, indicating that no individual study had an influence on the observed outcomes. Given the increased risk of mortality and adverse events associated with adiponectin levels, further research is warranted to understand its role in CAD pathophysiology.
The combined ORs for all-cause mortality and adverse CV events were determined based on data from five studies involving 3225 patients who were followed up for an average of 3.8 years. The study participants had risk factors such as hypertension, diabetes, hyperlipidemia, and smoking, while prescribed medications included angiotensin-converting enzyme inhibitors, beta blockers, and statins.
Adiponectin, first identified by Scherer P in 1995, is a hormone released by adipocytes. It exists in three oligomeric multimers in the body: (1) A low-molecular-weight trimer; (2) A medium-molecular-weight hexamer; and (3) A high-molecular-weight multimer. The high-molecular-weight form is the active form that exerts its effects on multiple organs. Adiponectin levels are inversely related to body fat content. While numerous studies have reported an association between low adiponectin levels and CV outcomes, our meta-analysis revealed a significant association between higher levels of adiponectin and the long-term MACE of CV origin. The discrepancy in the results can be explained by a requirement of intricate balance on the level of maintaining an equilibrium between body metabolism and excess active hormones. There is a similar adipokine, leptin, a nonglycosylated protein that plays a key role in maintaining the harmony of fat metabolism and working anti-synergistically with adiponectin.
In a post-hoc analysis of ATTEMPT-CVD by Kim-Mitsuyama et al[18], high total adiponectin levels were identified as an independent marker of CV and renal outcomes in hypertensive patients. A total of 1228 patients were enrolled, and subgroups were stratified into four quartiles based on total adiponectin levels. Patients with higher adiponectin levels exhibited a higher incidence of renal and CV events, with a statistically significant P value of 0.0135. Similarly, Beatty et al[13] followed 981 patients with existing ischemic heart disease for 7.1 years and observed an increased incidence of heart failure hospitalization (23%) and mortality (49%), but a lower incidence of myocardial infarction (12%), resulting in a combined CV event rate of 56% in groups with higher adiponectin levels. Another study by Cavusoglu et al[19] enrolled 324 patients, showing a higher incidence of CV and all-cause mortality in patients with higher adiponectin levels and existing CV risk factors.
According to a prior review, individuals with CVD who have higher levels of adiponectin in their plasma are at an increased risk of mortality. Additionally, another study found that patients with myocardial ischemia who had elevated levels of plasma adiponectin during their hospital discharge had a higher likelihood of all-cause mortality, after more than a decade of follow up period[14,16]. The results of our analysis align, with studies that suggest adiponectin could be a useful biomarker for evaluating CV risk[20,21]. However, there are some complexities in the field of adiponectin research, with studies indicating a protective role against CV events.
Prior reports also indicated that adiponectin possesses anti-inflammatory properties and metabolic benefits that could contribute to its protective role against CV disease. It may modulate insulin resistance, dyslipidemia, and non-alcoholic fatty liver disease[1,22]. Moreover, the impact of adiponectin on metabolic issues and its connection with controls who have the obesity level offer valuable understanding into its complex function among patients with CAD[1]. The intricate nature of adiponectin’s significance calls for investigations that take into account factors like gender-specific effects and correlations with particular CV results[23]. These conflicting findings may arise from differences in study design, patient groups, or testing methods. Our meta-analysis contributes by bringing together evidence from studies, enhancing our conclusions' reliability and applicability[24].
While our meta-analysis highlights the potential of adiponectin as a predictor of risk, it's important to recognize the gaps in our understanding. We still have limited knowledge about how exactly adiponectin influences the progression of CAD. The intriguing paradox surrounding the role of adiponectin in cardiometabolic health has triggered discussions, opening up fascinating avenues for future research. While initial preclinical studies indicate that adiponectin has effects on various aspects such as glucose regulation, inflammation, cell death, oxidative stress, and atherosclerosis, recent comprehensive human studies have presented a challenge to this traditional view. Surprisingly, these studies propose that adiponectin might primarily serve as a marker for insulin sensitivity and glucose regulation rather than directly influencing the risk of developing type 2 diabetes and CV disease[25].
Adiponectin is emerging as a powerful multidimensional biomarker for mortality risk prediction, particularly in individuals with cardiometabolic comorbidities. Unlike conventional markers such as N-terminal pro B-type Natriuretic Peptide (NT-proBNP), which reflects hemodynamic stress, or troponins, which signal acute myocardial injury, adiponectin captures a broader pathophysiological profile, including metabolic dysfunction, endothelial stress, and chronic inflammation[1,26]. This unique biological footprint enables identification of high-risk phenotypes often missed by cardiac-specific markers, especially in patients with diabetes, obesity, or subclinical vascular disease. Its inverse relationship with insulin resistance, atherogenesis, and systemic inflammation further positions it as a valuable tool for early prognostication and long-term risk stratification, even among asymptomatic individuals. Several studies have demonstrated its predictive value for CV and all-cause mortality, independent of traditional risk factors[27,28]. However, the standalone clinical utility of this method is restricted by a variety of challenges, such as the lack of standardized thresholds, assay heterogeneity, and paradoxically elevated levels in chronic illness and frailty. Consequently, adiponectin could prove to be particularly valuable when utilized in conjunction with NT-proBNP or troponins, providing enhanced understanding of residual CV risk. Furthermore, investigating cost-effective biomarkers in conjunc
The robustness and credibility of our findings, corroborated by sensitivity analysis, bolster the notion that assessing adiponectin levels may yield valuable insights into long-term mortality and MACE risk prediction. Incorporating adiponectin measurements into risk assessment models may improve their precision, aiding clinicians in identifying patients who would benefit from enhanced interventions and more rigorous monitoring. Irrespective of the outcomes, it is crucial to acknowledge that this meta-analysis possesses certain limitations. The quantity of studies and patients incorporated in our research may limit the applicability of our findings to a broader population. The discrepancies in characteristics and methodologies among studies may explain the variations in overall mortality outcomes. To substantiate our conclusions, it is imperative to conduct prospective randomized studies.
Based on our meta-analysis, we have discovered a link between rising adiponectin levels and higher risks of long-term mortality and MACE among stable CAD patients. These findings highlight the potential role of adiponectin as a cost-effective biomarker, supporting its integration into risk assessment and management strategies. It is crucial to investigate further the mechanisms by which adiponectin affects CAD pathophysiology, as it holds promise in shaping CV care. In conjunction with our study into the role of adiponectin, we underscore the significance of examining additional cost-effective biomarkers to augment risk assessment and enhance patient management.
| 1. | Fisman EZ, Tenenbaum A. Adiponectin: a manifold therapeutic target for metabolic syndrome, diabetes, and coronary disease? Cardiovasc Diabetol. 2014;13:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 2. | Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev. 2006;2:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 401] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 3. | Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007;380:24-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 664] [Cited by in RCA: 640] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 4. | Yoo JK, Hwang MH, Luttrell MJ, Kim HK, Meade TH, English M, Segal MS, Christou DD. Higher levels of adiponectin in vascular endothelial cells are associated with greater brachial artery flow-mediated dilation in older adults. Exp Gerontol. 2015;63:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Ebrahimi-Mamaeghani M, Mohammadi S, Arefhosseini SR, Fallah P, Bazi Z. Adiponectin as a potential biomarker of vascular disease. Vasc Health Risk Manag. 2015;11:55-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Lee SP, Youn SW, Cho HJ, Li L, Kim TY, Yook HS, Chung JW, Hur J, Yoon CH, Park KW, Oh BH, Park YB, Kim HS. Integrin-linked kinase, a hypoxia-responsive molecule, controls postnatal vasculogenesis by recruitment of endothelial progenitor cells to ischemic tissue. Circulation. 2006;114:150-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace AM, Danesh J, Whincup PH. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation. 2006;114:623-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 300] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 8. | Tu WJ, Qiu HC, Liu YK, Liu Q, Zeng X, Zhao J. Elevated levels of adiponectin associated with major adverse cardiovascular and cerebrovascular events and mortality risk in ischemic stroke. Cardiovasc Diabetol. 2020;19:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Woodward L, Akoumianakis I, Antoniades C. Unravelling the adiponectin paradox: novel roles of adiponectin in the regulation of cardiovascular disease. Br J Pharmacol. 2017;174:4007-4020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | Maya-ramos L, Gillette TG, Hill JA, Scherer PE. The Role of Adipose Tissue in Cardiovascular Pathophysiology. Cardiometab Syndr J. 2023;3:52-63. [DOI] [Full Text] |
| 11. | Scarale MG, Fontana A, Trischitta V, Copetti M, Menzaghi C. The Adiponectin-Mortality Paradox—A Systematic Review and Meta-analysis. Diabetes. 2018;67. [DOI] [Full Text] |
| 12. | Aljafary MA, Al-Suhaimi EA. Adiponectin System (Rescue Hormone): The Missing Link between Metabolic and Cardiovascular Diseases. Pharmaceutics. 2022;14:1430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 13. | Beatty AL, Zhang MH, Ku IA, Na B, Schiller NB, Whooley MA. Adiponectin is associated with increased mortality and heart failure in patients with stable ischemic heart disease: data from the Heart and Soul Study. Atherosclerosis. 2012;220:587-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Hascoet S, Elbaz M, Bongard V, Bouisset F, Verdier C, Vindis C, Genoux A, Taraszkiewicz D, Perret B, Galinier M, Carrié D, Ferrières J, Ruidavets JB. Adiponectin and long-term mortality in coronary artery disease participants and controls. Arterioscler Thromb Vasc Biol. 2013;33:e19-e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Marino BCA, Buljubasic N, Akkerhuis M, Cheng JM, Garcia-Garcia HM, Regar E, Geuns RV, Serruys PW, Boersma E, Kardys I. Adiponectin in Relation to Coronary Plaque Characteristics on Radiofrequency Intravascular Ultrasound and Cardiovascular Outcome. Arq Bras Cardiol. 2018;111:345-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Pratesi A, Di Serio C, Orso F, Foschini A, Bartoli N, Marella A, Fumagalli S, Di Bari M, Marchionni N, Tarantini F, Baldasseroni S. Prognostic value of adiponectin in coronary artery disease: Role of diabetes and left ventricular systolic dysfunction. Diabetes Res Clin Pract. 2016;118:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Schnabel R, Messow CM, Lubos E, Espinola-Klein C, Rupprecht HJ, Bickel C, Sinning C, Tzikas S, Keller T, Genth-Zotz S, Lackner KJ, Münzel TF, Blankenberg S. Association of adiponectin with adverse outcome in coronary artery disease patients: results from the AtheroGene study. Eur Heart J. 2008;29:649-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Kim-Mitsuyama S, Soejima H, Yasuda O, Node K, Jinnouchi H, Yamamoto E, Sekigami T, Ogawa H, Matsui K. Total adiponectin is associated with incident cardiovascular and renal events in treated hypertensive patients: subanalysis of the ATTEMPT-CVD randomized trial. Sci Rep. 2019;9:16589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, Clark LT, Pinsky DJ, Marmur JD. Adiponectin is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction in patients presenting with chest pain. Eur Heart J. 2006;27:2300-2309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 154] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 20. | Hui X, Lam KS, Vanhoutte PM, Xu A. Adiponectin and cardiovascular health: an update. Br J Pharmacol. 2012;165:574-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 21. | Barrios V, Gómez-Huelgas R, Rodríguez R, de Pablos-Velasco P. Adiponectina, un factor de riesgo cardiovascular emergente. Estudio REFERENCE [Adiponectin: an emerging cardiovascular risk factor. The REFERENCE Study]. Rev Esp Cardiol. 2008;61:1159-1167. [PubMed] |
| 22. | Yang L, Li B, Zhao Y, Zhang Z. Prognostic value of adiponectin level in patients with coronary artery disease: a systematic review and meta-analysis. Lipids Health Dis. 2019;18:227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Zhang M, Chen X, Zhu Y, Yin L, Quan Z, Ou Y, He B. Causal associations of circulating adiponectin with cardiometabolic diseases and osteoporotic fracture. Sci Rep. 2022;12:6689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Chiva-Blanch G, Badimon L. Benefits and Risks of Moderate Alcohol Consumption on Cardiovascular Disease: Current Findings and Controversies. Nutrients. 2019;12:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 25. | Menzaghi C, Trischitta V. The Adiponectin Paradox for All-Cause and Cardiovascular Mortality. Diabetes. 2018;67:12-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 128] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 26. | Cetinalp-Demircan P, Bekpinar S, Gurdol F, Orhan Y. Adiponectin is a link among inflammation, insulin resistance, and high-density lipoprotein cholesterol but is not associated with paraoxonase activity in premenopausal women. J Clin Hypertens (Greenwich). 2009;11:672-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1309] [Cited by in RCA: 1298] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 28. | Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1243] [Article Influence: 49.7] [Reference Citation Analysis (0)] |