Published online May 26, 2013. doi: 10.4331/wjbc.v4.i2.30
Revised: March 26, 2013
Accepted: April 10, 2013
Published online: May 26, 2013
Processing time: 86 Days and 13 Hours
AIM: To investigate whether caspase-1 activation/intracellular processing of pro-interleukin-1β (pro-IL-1β) and extracellular release of mature IL-1β from activated monocytes are separable events.
METHODS: All experiments were performed on fresh or overnight cultured human peripheral blood monocytes (PBMCs) that were isolated from healthy donors. PBMCs were activated by lipopolysaccharide (LPS) stimulation before being treated with Adenosine triphosphate (ATP, 1 mmol/L), human α-defensin-5 (HD-5, 50 μg/mL), and/or nigericin (Nig, 30 μmol/L). For each experiment, the culture supernatants were collected separately from the cells. Cell lysates and supernatants were both subject to immunoprecipitation with anti-IL-1β antibodies followed by western blot analysis with anti-caspase-1 and anti-IL-1β antibodies.
RESULTS: We found that pro-IL-1β was processed to mature IL-1β in LPS-activated fresh and overnight cultured human monocytes in response to ATP stimulation. In the presence of HD-5, this release of IL-1β, but not the processing of pro-IL-1β to IL-1β, was completely inhibited. Similarly, in the presence of HD-5, the release of IL-1β, but not the processing of IL-1β, was significantly inhibited from LPS-activated monocytes stimulated with Nig. Finally, we treated LPS-activated monocytes with ATP and Nig and collected the supernatants. We found that both ATP and Nig stimulation could activate and release cleaved caspase-1 from the monocytes. Interestingly, and contrary to IL-1β processing and release, caspase-1 cleavage and release was not blocked by HD-5. All images are representative of three independent experiments.
CONCLUSION: These data suggest that caspase-1 activation/processing of pro-IL-1β by caspase-1 and the release of mature IL-1β from human monocytes are distinct and separable events.
Core tip: Activated macrophages release large amounts of interleukin-1β (IL-1β) and macrophages deficient in caspase-1 expression have undetectable IL-1β secretion. This suggests that IL-1β release and caspase-1 activation are closely related events. We found that human α-defensin 5 (HD-5) inhibited the release of IL-1β, but not the processing of pro-IL-1β to IL-1β in lipopolysaccharide-activated monocytes stimulated with Adenosine triphosphate or nigericin. Different from IL-1β processing and release, the activation and release of caspase-1 from stimulated monocytes was not blocked by HD-5. These data suggest that caspase-1 activation/processing of pro-IL-1β by caspase-1 and the release of mature IL-1β from human monocytes are distinct and separable events.
- Citation: Galliher-Beckley AJ, Lan LQ, Aono S, Wang L, Shi J. Caspase-1 activation and mature interleukin-1β release are uncoupled events in monocytes. World J Biol Chem 2013; 4(2): 30-34
- URL: https://www.wjgnet.com/1949-8454/full/v4/i2/30.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v4.i2.30
Interleukin-1β (IL-1β) is an important acute response factor of host defense against microbial infections and a key mediator of inflammation in multiple organs[1,2]. Viral and bacterial pathogens trigger inflammasome formation and subsequent caspase-1 activation and IL-1β maturation[3]. IL-1β is synthesized as a biologically inactive 31 kDa pro-IL-1β polypeptide and must be post-translationally processed by caspase-1 to generate the mature 17 kDa pro-inflammatory cytokine IL-1β[4]. Overproduction of IL-1β is associated with multiple autoimmune diseases and septic shock and animals deficient in IL-1β are highly susceptible to microbial infections[5,6].
Activated monocytes and macrophages rapidly release large amounts of mature IL-1β and inflammasome components including caspase-1[7]; macrophages deficient in caspase-1 expression have impaired processing of pro-IL-1β and undetectable IL-1β secretion[8]. This suggests that IL-1β release and caspase-1 activation are closely related events. Because most known inhibitors of IL-1β production block caspase-1 activation, previous studies are not able to determine whether caspase-1 activation/pro-IL-1β processing and IL-1β release are separate or linked processes. We have previously demonstrated that human defensin peptide human α-defensin-5 (HD-5) can block the release of IL-1β, but not tumor necrosis factor-α (TNF-α), from lipopolysaccharide (LPS)-activated human monocytes stimulated with Adenosine triphosphate (ATP)[9]. Here, using HD-5 as a molecular tool, we explored whether IL-1β release is an indivisible process from caspase-1 activation/pro-IL-1β processing in human monocytes.
Synthetic human defensin HD-5 was prepared by t-Boc solid-phase synthesis as described previously[9]. All peptides were folded and purified to homogeneity by reversed-phase-high-performance liquid chromatography and their molecular weights verified by electrospray ionization mass spectrometry.
Peripheral blood monocytes (PBMC) from healthy adult donors were prepared as described previously[9]. Briefly, blood was diluted with RPMI 1640, overlaid on lymphocyte separation medium (Mediatech), and centrifuged at 400 ×g for 35 min. The mononuclear cell layer was washed with PBS and centrifuged at 250 ×g for 10 min. The cells were then resuspended in 25 mL of PBS plus citrate solution and overlaid on Percoll (GE Healthcare) prediluted 9/1 with 1.5 mol/L NaCl. After a 35-min centrifugation at 400 ×g, the PBMC were washed with PBS again. Cells were counted using trypan blue exclusion and cells were resuspended in monocyte medium [RPMI 1640, 5%FBS, 20 mmol/L HEPES (pH 7.3), 1%streptomycin/penicillin] and incubated at 37 °C for 2 h to allow for adherence, after which medium supernatants were discarded. Attached cells were rinsed twice with monocyte medium and used immediately or incubated in monocyte medium overnight at 37 °C in a 5%CO2 environment.
Fresh or overnight cultured PBMCs were treated with 20 ng/mL LPS for 2 h at 37 °C. In some experiments, the media was removed then replaced with RPMI (without Met, Cys, or Glu), + 1% FBS + 25 mmol/L HEPES pH 7.4 + 300 mg/L Glutamine + 83 μCi/mL 35S-Met/Cys and incubated at 37 °C for 1 h. Cells were then rinsed and media was replaced with RPMI (+ Glu). Adenosine triphosphate (ATP, 1 mmol/L), HD-5 (50 μg/mL), and/or nigericin (Nig, 30 μmol/L) was added to culture media and incubated at 37 °C for 1.5 h. The supernatants were collected, 1%Triton X-100 and protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, United States) added, then spun down. Cells were lysed in 500 μL lysis buffer (25 nmol/L HEPES pH 7.4 + 150 mmol/L NaCl + 0.1%Triton X-100 + Protease Inhibitor cocktail) then spun down. Cell lysates and supernatants were subject to IL-1β immunoprecipitation and western blot analysis of caspase-1 as described previously[9]. Results shown are a representative from three independent experiments.
All experiments were repeated 3 times to ensure reproducibility. Image J software (NIH) was used to quantitate protein bands. Proteins bands between two different treatment groups were considered statistically significant when P < 0.05, by t-test analysis.
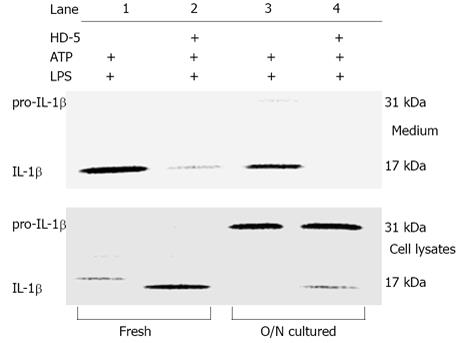
Our previous studies have shown that HD-5 can block the release of pro-IL-1β and mature IL-1β from ATP stimulated human monocytes that were cultured overnight prior to LPS exposure[9]. Since culturing monocytes in vitro can lead to further differentiation into macrophage-like cells[10,11], we determined whether the subcultured monocytes behaved similarly to freshly isolated cells. As shown in Figure 1 (upper panel) the extracellular release of mature IL-1β from both freshly isolated and overnight cultured monocytes was blocked by HD-5. Interestingly, we also observed that freshly isolated monocytes had enhanced intracellular processing of pro-IL-1β to IL-1β (Figure 1, lower panel, lanes 1-2 vs 3-4) suggesting that increased time in culture can decrease the ability of monocytes to process IL-1β. Most importantly, it was found that the intracellular processing of pro-IL-1β to mature IL-1β was not inhibited by HD-5 as evidenced by the presence of intracellular mature IL-1β in cell lysates from monocytes treated with HD-5 (Figure 1, lower panel).
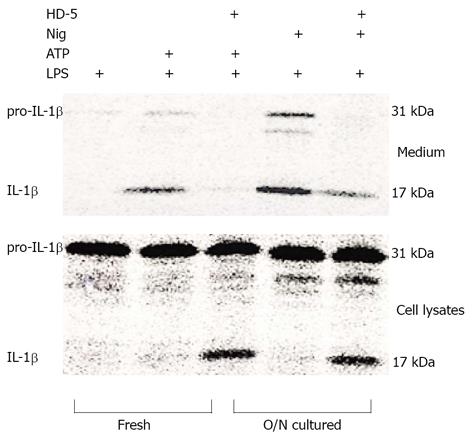
To determine whether the ability of HD-5 to block IL-1β release in overnight-cultured, LPS-activated monocytes was specific to ATP stimulation, we treated LPS-activated monocytes with ATP or Nig. Similar to ATP, Nig is a microbial toxin that acts as an inflammasome inducer, leading to caspase-1 maturation and IL-1β processing and release[12,13]. We found that, similar to its effect on ATP-mediated IL-1β release, HD-5 treatment was also able to significantly block IL-1β release from monocytes stimulated with Nig (Figure 2, upper panel). Furthermore, HD-5 did not block Nig-induced intracellular processing of pro-IL-1β to mature IL-1β as indicated by the presence of mature IL-1β in the cell lysate (Figure 2, lower panel).
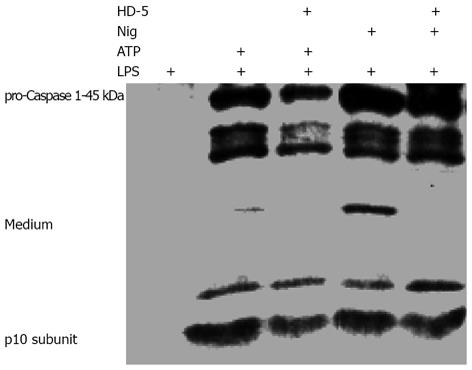
Caspase-1 activation has been implicated in both the processing and release of IL-1β[14,15]. Because our current studies have shown that HD-5 selectively blocked the release but not the processing of pro-IL-1β to mature IL-1β, it is important to know whether HD-5 has any effect on caspase-1 activation and release. As shown in Figure 3, ATP/Nig-mediated caspase-1 activation and extracellular release from LPS-activated monocytes were not affected by HD-5. This observation is consistent with the finding that HD-5 did not block the intracellular processing of pro-IL-1β to IL-1β (Figures 1 and 2).
Current research suggests that once caspase-1 becomes active it leads to both the processing and release of IL-1β[16-18]. Although the processing and release of IL-1β are rapid and probably concurrent events, it has been suggested previously that the cleavage of pro-IL-1β and release of mature IL-1β are likely independent of each other[19]. However, this speculation remains hypothetical because, due to the technical limitations, the presence of mature IL-1β inside LPS-primed, ATP-stimulated monocytes have not been documented prior to this report. Here, under certain conditions we have clearly shown that IL-1β release but not its processing from pro-IL-1β by caspase-1 in ATP/Nig-stimulated monocytes can be blocked by HD-5. To our knowledge, this report provides the first direct evidence that the processing of pro-IL-1β to mature IL-1β and extracellular release of mature IL-1β are two divisible events in human monocytes.
Our results also indicate that the majority of pro-IL-1β is processed intracellularly by activated caspase-1 in freshly isolated monocytes (Figure 1A). This observation is consistent with the report that human blood monocytes release processed IL-1β after a one-time stimulation with toll-like receptor 4 ligands due to the resulting constitutively activated caspase-1[16]. Furthermore, because the release of IL-1β, but not the externalization of caspase-1 was affected by HD-5, our studies suggest that IL-1β seems to be released directly to the extracellular environment without the involvement of caspase-1 inflammasome.
The mechanisms by which pro-IL-1β and mature IL-1β are released from cytokine producing cells have been an intriguing and unsolved question of IL-1β biology for decades. Known activators of caspase-1 and IL-1β release include ATP, adjuvants, and various microbial molecules[20]. Using different experimental systems, studies on ATP-induced IL-1β maturation have posited four different and conflicting models of IL-1β secretion, including secretory lysosome exocytosis[21,22], microvesicle shedding[23-25], direct transport across the plasma membrane[26], and exocytosis of exosome-containing multivesicular bodies[7]. The multiple models of IL-1β secretion reflect the confusion in this area. It is true that although ATP-induced caspase-1 activation is followed by the processing of pro-IL-1β to IL-1β in the cytosol and secretion of IL-1β, other protein release mechanisms, such as externalization of caspase-1, secretory lysosomes and microvesicle shedding, do occur. However, externalization of caspase-1 and other inflammasome components, secretory lysosomes and microvesicle shedding may be cell-type specific processes after ATP stimulation and may not be involved in IL-1β secretion from monocytes. Here, we provide direct evidence that IL-1β secretion is a separable process from inflammasome activation/caspase-1 externalization and IL-1β processing in human monocytes. Inflammasome activation and caspase-1 activity are required but not sufficient for the release of IL-1β.
Taken together, our studies clearly demonstrated that intracellular processing of pro-IL-1β to mature IL-1β and the externalization of mature IL-1β are divisible events in human monocytes. Although the molecular mechanisms by which HD-5 blocks the release of mature IL-1β has yet to be revealed in future studies, a better understanding of IL-1β processing and release may lead to the discovery of novel molecular targets for IL-1β blockade and the development of new therapeutic approaches to treat life-threatening microbial infections and inflammatory diseases.
Interleukin-1β (IL-1β) is secreted by monocytes and macrophages and is an important acute response factor of host defense against microbial infections. IL-1β is synthesized as a biologically inactive 31 kDa pro-IL-1β polypeptide and must be post-translationally processed by caspase-1 to generate the mature 17 kDa IL-1β that is released into the extracellular space.
Macrophages deficient in caspase-1 expression have undetectable IL-1β secretion. This suggests that IL-1β release and caspase-1 activation are closely related events. Because most known inhibitors of IL-1β production block caspase-1 activation, previous studies are not able to determine whether caspase-1 activation/pro-IL-1β processing and IL-1β release are separate or linked processes. This report shows that processing of pro-IL-1β by caspase-1 and the release of mature IL-1β from human monocytes are distinct and separable events.
Recent reports suggest IL-1β secretion involves the formation of the inflammasome, leading to the cleavage and activation of caspase-1, which in turn proteolytically processes pro-IL-1β. Biologically active IL-1β is subsequently secreted by the cell. Here authors propose that IL-1β secretion involves a more complex regulatory mechanism.
Standard therapy for patients with autoimmune diseases or lymphomas involves blocking IL-1β activity. By knowing how IL-1β is processed and released, this study may lead to the development of novel therapeutics that can block IL-1β release and prevent or enhance treatment for these debilitating pro-inflammatory disorders.
The inflammasome is a multi-protein complex responsible for the activation of caspase-1, an enzyme that cleaves and activates downsteam targets such as IL-1β. IL-1β is a pro-inflammatory cytokine secreted by immune cells to aid in the defense of microbial infection. Human α-defensin 5 (HD-5) is an anti-microbial peptide that normally functions by binding to the microbial cell membrane to form a lethal pore.
The authors examined the ability of HD-5 to block the release, but not the activation, of IL-1β from Adenosine triphosphate and nigericin stimulation human monocytes. It revealed that while HD-5 can block IL-1β release, caspase-1 activation is not affected. The results are interesting and may represent a new molecular mechanism in IL-1β secretion.
P- Reviewers Holan V, Cairo G S- Editor Gou SX L- Editor A E- Editor Lu YJ
| 1. | Dinarello CA. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur J Immunol. 2011;41:1203-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 602] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 2. | van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1β and IL-18 processing during infection. Trends Immunol. 2011;32:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 538] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 3. | Broz P, Monack DM. Molecular mechanisms of inflammasome activation during microbial infections. Immunol Rev. 2011;243:174-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 200] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 4. | Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galán JE, Askenase PW. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 792] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 5. | Levine JS, Pugh BJ, Hartwell D, Fitzpatrick JM, Marshak-Rothstein A, Beller DI. Interleukin-1 dysregulation is an intrinsic defect in macrophages from MRL autoimmune-prone mice. Eur J Immunol. 1993;23:2951-2958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Dinarello CA. Interleukin-1 and tumor necrosis factor: effector cytokines in autoimmune diseases. Semin Immunol. 1992;4:133-145. [PubMed] |
| 7. | Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol. 2007;179:1913-1925. [PubMed] |
| 8. | Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1091] [Cited by in RCA: 1108] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 9. | Shi J, Aono S, Lu W, Ouellette AJ, Hu X, Ji Y, Wang L, Lenz S, van Ginkel FW, Liles M. A novel role for defensins in intestinal homeostasis: regulation of IL-1beta secretion. J Immunol. 2007;179:1245-1253. [PubMed] |
| 10. | Namiki M, Hara H. Enhancement of colony-forming activity of granulocyte-macrophage colony-stimulating factor by monocytes in vitro. Blood. 1989;74:918-924. [PubMed] |
| 11. | Hammerstrøm J. Human macrophage differentiation in vivo and in vitro. A comparison of human peritoneal macrophages and monocytes. Acta Pathol Microbiol Scand C. 1979;87C:113-120. [PubMed] |
| 12. | Cheneval D, Ramage P, Kastelic T, Szelestenyi T, Niggli H, Hemmig R, Bachmann M, MacKenzie A. Increased mature interleukin-1beta (IL-1beta) secretion from THP-1 cells induced by nigericin is a result of activation of p45 IL-1beta-converting enzyme processing. J Biol Chem. 1998;273:17846-17851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Stout-Delgado HW, Vaughan SE, Shirali AC, Jaramillo RJ, Harrod KS. Impaired NLRP3 inflammasome function in elderly mice during influenza infection is rescued by treatment with nigericin. J Immunol. 2012;188:2815-2824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1274] [Cited by in RCA: 1306] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 15. | Lopez-Castejon G, Luheshi NM, Compan V, High S, Whitehead RC, Flitsch S, Kirov A, Prudovsky I, Swanton E, Brough D. Deubiquitinases regulate the activity of caspase-1 and interleukin-1β secretion via assembly of the inflammasome. J Biol Chem. 2013;288:2721-2733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 16. | Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009;113:2324-2335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 649] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 17. | Gavrilin MA, Mitra S, Seshadri S, Nateri J, Berhe F, Hall MW, Wewers MD. Pyrin critical to macrophage IL-1beta response to Francisella challenge. J Immunol. 2009;182:7982-7989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Perregaux DG, Gabel CA. Human monocyte stimulus-coupled IL-1beta posttranslational processing: modulation via monovalent cations. Am J Physiol. 1998;275:C1538-C1547. [PubMed] |
| 19. | Mehta VB, Hart J, Wewers MD. ATP-stimulated release of interleukin (IL)-1beta and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J Biol Chem. 2001;276:3820-3826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 226] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 20. | Weber A, Wasiliew P, Kracht M. Interleukin-1beta (IL-1beta) processing pathway. Sci Signal. 2010;3:cm2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Andrei C, Dazzi C, Lotti L, Torrisi MR, Chimini G, Rubartelli A. The secretory route of the leaderless protein interleukin 1beta involves exocytosis of endolysosome-related vesicles. Mol Biol Cell. 1999;10:1463-1475. [PubMed] |
| 22. | Andrei C, Margiocco P, Poggi A, Lotti LV, Torrisi MR, Rubartelli A. Phospholipases C and A2 control lysosome-mediated IL-1 beta secretion: Implications for inflammatory processes. Proc Natl Acad Sci USA. 2004;101:9745-9750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 325] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 23. | Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185:579-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 374] [Cited by in RCA: 379] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 24. | MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity. 2001;15:825-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 688] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 25. | Pizzirani C, Ferrari D, Chiozzi P, Adinolfi E, Sandonà D, Savaglio E, Di Virgilio F. Stimulation of P2 receptors causes release of IL-1beta-loaded microvesicles from human dendritic cells. Blood. 2007;109:3856-3864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 218] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 26. | Brough D, Rothwell NJ. Caspase-1-dependent processing of pro-interleukin-1beta is cytosolic and precedes cell death. J Cell Sci. 2007;120:772-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 187] [Article Influence: 10.4] [Reference Citation Analysis (0)] |