Published online Sep 5, 2025. doi: 10.4331/wjbc.v16.i3.108045
Revised: May 13, 2025
Accepted: August 5, 2025
Published online: September 5, 2025
Processing time: 150 Days and 18.8 Hours
Cellular communication is required for the normal function and maintenance of homeostasis. The extracellular communications are mediated by cell surface receptors, which transmit signals for various cell functions. Cell defense also relies on distinguishing between self and non-self. The integrins belong to the trans
Core Tip: In the current review, we discussed multifunctional roles (apoptosis, phagocytosis, metabolism, activation, hematopoietic stem cell migration, and malignancies) and molecular mechanisms of CD47/signal regulatory protein α receptors in various cells.
- Citation: Bhardwaj N, Chandra H, Singh A, Babu R. CD47/SIRPα pathways: Functional diversity and molecular mechanisms. World J Biol Chem 2025; 16(3): 108045
- URL: https://www.wjgnet.com/1949-8454/full/v16/i3/108045.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v16.i3.108045
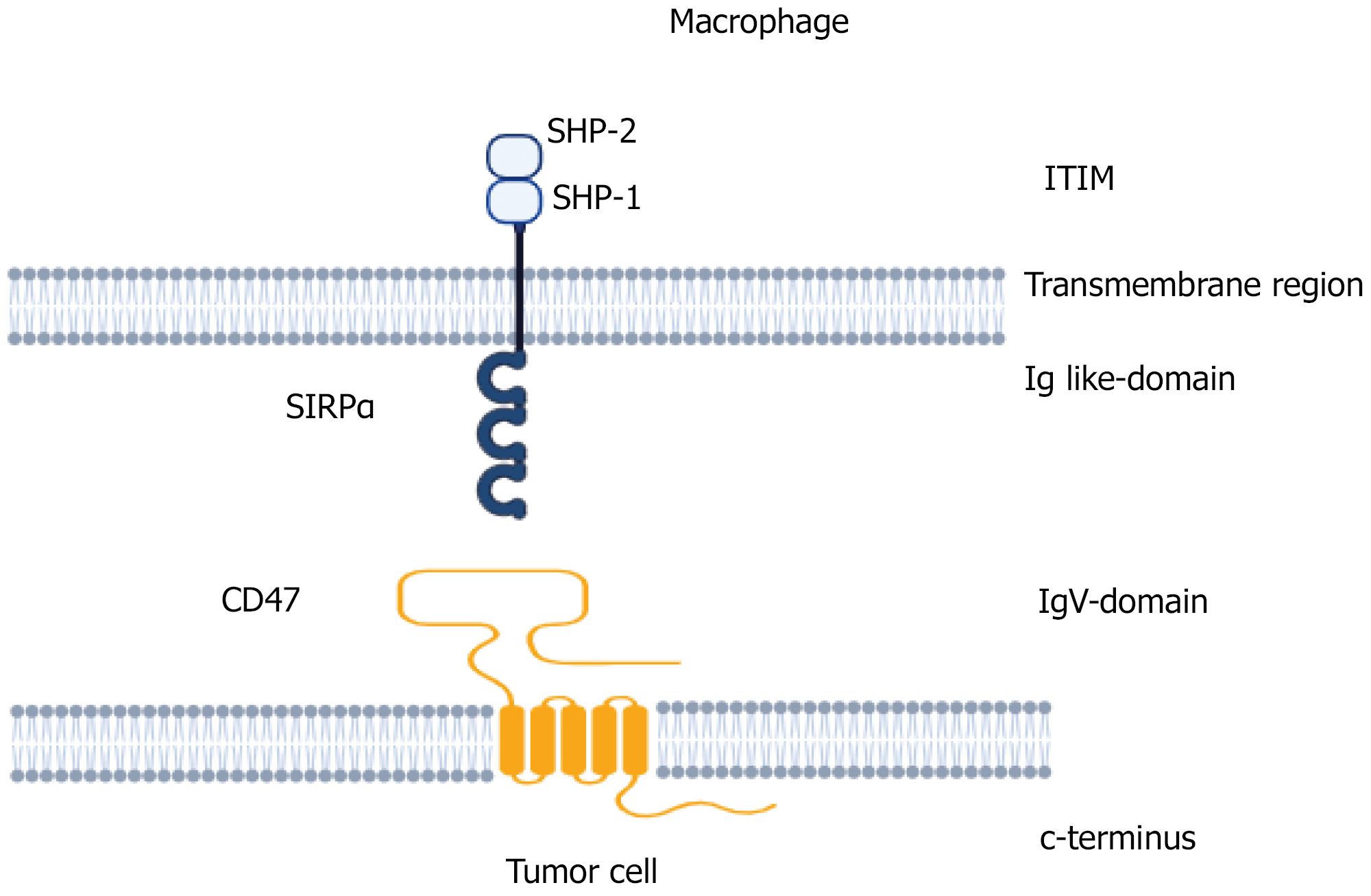
CD47, or integrin-associated protein, is a 50 kDa heavily glycosylated plasma membrane transmembrane protein ubiquitously expressed in various organs and tissues[1]. It is expressed on erythrocytes and lymphocytes and a variety of cells having β3 integrin expression[1,2]. It contains an extracellular immunoglobulin domain, five transmembrane domains, and a short intracellular tail (Figure 1). It was initially recognised as a protein associated with α3β3 integrin in the placenta and neutrophils and later shown to modulate integrin function and leukocyte responsiveness to RGD-containing extracellular matrix protein[3]. Various isoforms of CD47 proteins with different functional profiles have been recognized[4]. These isoforms are generated either by post-translational changes, like glycosylation, pyroglutamate and glycosaminoglycan modification, and proteolytic cleavage, or by a pre-translational alternative cleavage, and polyadeny
SIRPα is expressed on various cells, most abundantly on neurons and myeloid-lineage hematopoietic cells, polymor
CD47/SIRPα signaling performs a wide range of functions. CD47-mediated signaling modulates metabolism and mitochondrial homeostasis in various cells[13]. CD47/SIRPα signaling performs a crucial role in immune defense, lymphocyte homeostasis, bone remodeling, hematopoietic stem cell (HSCs) engraftment, atherosclerosis, and tumor immune surveillance by activation of macrophage phagocytic response and cells of the immune system[14]. The inter
| Cell | Physiological function |
| Erythrocytes | Serves as a marker of self. Maintain membrane integrity and erythrophagocytosis |
| Macrophages | Regulation of phagocytosis |
| Dendritic cells | Regulation of phagocytosis. Activation. Mediates immune-inhibitory signals. Transendothelial migration |
| Neutrophils | Adhesion. Phagocytosis. Transendothelial migration. Inflammatory response. Apoptosis |
| T-lymphocyte | T-cell survival and differentiation. Activates T-cell receptor-mediated pathways |
| Monocytes | Transendothelial migration |
| Endothelial cells | Insulin growth factor signaling |
| Platelets | Integrins α2β1 and αIIbβ3 expression |
| Cancer cell | Migration. Invasion. Immune resistance |
| Natural killer cells | NK cell homeostasis. Graft rejection |
| Osteoclast cells | Proliferation |
| Hematopoietic stem cells | Migration |
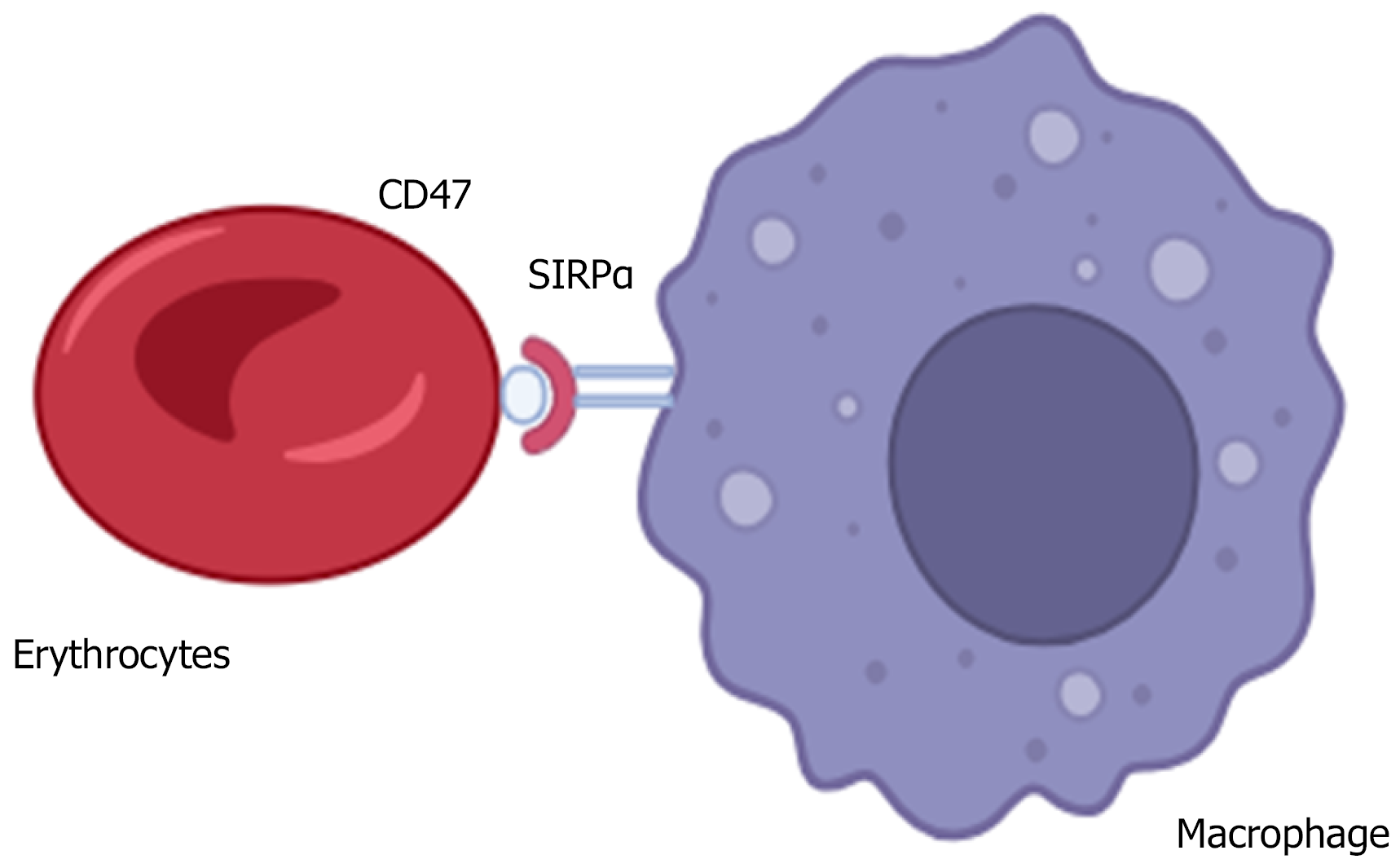
Although mature erythrocytes lack integrin expression, CD47 is extensively expressed on their surfaces. It is coupled with the erythrocyte cytoskeleton and the non-cytoskeleton portion and performs different functions[15]. CD47 regulates the erythrocyte membrane integrity by creating an important connection between the band 3 complex and the Rh complex[16]. It serves as a marker of self on erythrocytes[17]. CD47-lacking erythrocytes have reduced survival in circulation. Spleen- or bone marrow-derived macrophages phagocytose CD47-deficient erythrocytes at a much faster rate than control[17]. CD47/SIRPα interaction sends a negative signal to macrophages to prevent erythrophagocytosis[17,18] (Figure 2). The negative signalling through the CD47-SIRPα interaction is mediated by the SHP-1, which forms a complex with the SIRPα[19]. CD47/SHPS-1 interaction causes the phosphorylation of SHPS-1 tyrosine and prevents the Fc-R from breaking down the SHPS-1-SHP-1 complex and inhibits the erythrophagocytosis[20]. CD47-SIRPα signalling activates CDC42, a GTP-binding protein that causes cytoskeleton movement[21]. This also leads to the induction of myosin IIA and induces a phagocytic response.
The loss of the CD47/SHPS-1 interaction facilitates erythrocyte removal, as seen in the pathogenesis of autoimmune hemolytic anemia or immune thrombocytopenia[22]. CD47 expression decreases with the maturation of reticulocytes, erythrocyte aging, and in vitro-stored erythrocytes[23,24]. The downregulation of CD47 expression on erythrocytes in sickle cell disease, Gaucher disease, smokers, and obese patients has also been shown[25-28]. The concentrated ambient PM2.5 also suppresses the CD47 expression of erythrocyte membranes[28]. CD47 also plays a crucial role in hematoma clearance after intracerebral hemorrhage[29]. The paraquat and carbon nanotube administration induces the CD47 expression on erythrocytes as a defensive mechanism[30,31]. Experimental aging changes the confirmation of the CD47 receptor from an inhibitory into an activating signal, and it also acts as an “eat me signal” in a subset of the aged erythrocyte population[32]. Recently it has been seen that CD47 monomers are distributed randomly on the erythrocyte membrane, and 14% of them are found attached to the cytoskeleton[33].
The discrimination between self and non-self-elicits the immune responses. CD47 regulates innate and adaptive immune responses. It performs two important functions in the immune system. It interacts with the SIRPα on macrophages and dendritic cells (DC) and regulates the phagocytic responses[19]. The CD47–SIRPα interaction also regulates the neutrophil adhesion, phagocytosis, transendothelial migration, and inflammatory response[34].
CD47 expression is upregulated during infection, and anti-CD47 antibody increases DC activation and CD8+ T cell activation to chronic viral infection[35]. This interaction is also important in the development of CD11+ DC and secondary lymphoid organs. It is a ligand for SIRPα, a protein found in dendritic and macrophage cells, and a self-marker on various cells. CD47–SIRPα pathways mediate immune-inhibitory signals to DCs and reduce the cytokine interleukin-12 pro
The CD47 expression also regulates transendothelial migration of monocytes and DC. The interaction between lym
CD47 receptors intercommunicate with VEGFR2 and integrins in endothelial cells. It plays a crucial role in SHP2-dependent insulin growth factor signaling. Extracellular vesicles that originate from T-cell-mediated endothelial cell responses to vascular endothelial growth factor depend on CD47 expression[41].
CD47 regulates the integrins α2β1 and αIIbβ3 on platelets. Transfused CD47-deficient platelets cleared rapidly in wild-type recipients. CD47-deficient mice exhibit experimental thrombocytopenia. Platelet-derived exosomes also express CD47 receptors on their surface[42].
Cancer cells can be recognised and killed by the immune cells. Cancer cells exhibit immune resistance by altering various immunoregulatory pathways. CD47 expression is increased in ovarian carcinoma cell lines and various solid tumors, including non-Hodgkin lymphoma, murine myeloid leukemia, gastric and breast cancer, lung adenocarcinoma, osteo
CD47-SIRPα-based immunotherapies have been implicated in the treatment of various cancers. Acute myeloid leukemia and solid tumor patients with increased CD47 expression have reduced survival[47]. CD47/SIRPα signalling causes cancer immune escape by triggering a ‘don’t eat me’ signal by inhibiting the phagocytosis of cancerous cells. CD47-SIRPα blockage and SHPS-1 activation trigger Syk activation, which induces macrophage-mediated phagocytosis[48]. It also increases antigen presentation and induces the CD8+ T cell response and neutrophil-mediated apoptosis of tumor cells. The neutrophil-mediated removal of tumor cells caused by IgA or IgG-mediated response[49]. CD47 expre
Anti-CD47 antibodies have proven to have a significant effect in cancer treatment. The therapy targeting CD47/SIRPα has been prescribed for the treatment of various cancers. CD47 and PD1/PDL1 are involved in the T-lymphoblastic lymphoma/Leukemia (T-LBL/ALL) progression. T-LBL/ALL patients have increased CD47, PDL1, and PD1 expression levels. The targeting of CD47 and PD1/PDL1 could be a new therapeutic target for the treatment of T-LBL/ALL[51]. CD47 expression is increased in intestinal non-GCB-type diffuse large B-cell lymphoma[52].
CD47 is overexpressed on myeloma cells and is a potential therapeutic target for myeloma therapies. The anti-CD47 blocking antibodies increased phagocytosis of myeloma cells and non-Hodgkin lymphoma cells and inhibited the tumor growth[53]. Exosomes with high levels of CD47 expression may facilitate tumor translocation and aid in the spread of malignancies in the microenvironment[54].
The CD47 agonist peptides produced from the C-terminal domain of TSP1 activate phospholipase C gamma-1, which induces Ca2+-mediated, caspase-independent programmed cell death in chronic lymphocytic leukemia B cells[55]. The antitumor response mediated by anti-CD47 antibodies depends on the FcγRs. The antibody FCγ induces macrophage and T-cell responses and increases antitumor immunity.
Anti-CD47 antibody induces NK cell death in head-and-neck squamous carcinoma cells in vitro. Increased CD47 expression on HNSCC cell lines have reduced NK cell-mediated cytotoxicity as compared with the low CD47 expression[56]. In CD47−/− NK cells, proapoptotic gene expression was linked to stress-mediated elevations in reactive oxygen species, mitochondrial proton leak, and apoptosis. CD47 regulates NK cell homeostasis and functions in both a positive and negative manner in response to a viral infection[57]. The recruitment of NK cells into the tumor microenvironment is induced by increased CD47 expression. Melanoma-bearing CD47−/− mice were shown in a study to have a decreased number of splenic NK cells. TSP1/CD47 signalling negatively regulates NK cell functions, which can improve NK immune surveillance[58]. CD47 blocking activates DC, which trigger the NK cells, which induce antitumor activity in murine hepatocellular carcinoma[59].
CD47/SIRPα interaction regulates bone homeostasis by the production of osteoclast cells[60]. CD47-/- mice had decreased body weight and areal bone mineral density and have a decreased number of osteoclast cells[61]. The femurs are shorter and have lower trabecular bone[61]. CD47/SIRPα signalling induces osteoclastogenesis by the dephosphorylation of myosin protein[60]. Another study in SIRPα mutant mice suggests that SIRPα negatively regulates this function in osteoclasts[62]. The role of CD47 in bone injuries in murine fracture model was recently investigated[63]. There was reduced callus bone formation and decreased mesenchymal progenitor proliferation in CD47-/- mice[63].
CD47 and integrins αVβ3 play a crucial role in osteoarthritis[64]. Osteoarthritic joints have increased CD47 expression and ligand attachment capability of integrin αVβ3. The triggering of αVβ3 and CD47 promotes inflammation and joint injury in osteoarthritis[64].
CD47/SIRPα interaction plays a crucial role in macrophage-mediated graft transplantation. CD47 overexpression reduces NK cell response and macrophage-mediated phagocytosis in cell transplantation[65,66]. The role of CD47/CD274 expression in transplanted hepatocyte graft rejection has been described[67]. CD47/CD274 overexpression suppressed the macrophage and T cell responses in vitro as well as reduced innate and adaptive immune responses during hepato
The CD47 expression up-regulates with the maturation of HSCs[69]. It also helps in the migration of HSCs in response to inflammation[70]. The aged muscular stem cells have increased CD47 expression[71].
The prevention of the CD47/SIRPα interaction between tumor cells and macrophages causes tumor cells to die in several ways. The macrophages can phagocytose the tumor cells by antibody-dependent cellular cytotoxicity (ADCC) or com
The major therapeutic challenge with anti-CD47 therapy includes on-target toxicity, as CD47 is expressed ubiquitously on all cells. Since erythrocytes express CD47, anti-CD47 antibody induces anemia and other blood-related disorders by non-selectively phagocytizing erythrocytes by macrophages. The Fc regions of antibodies activate ADCC and CDC pathways and induce toxicity of normal cells. Another challenge in cancer therapeutics is the development of resistance in tumor cells by reducing the CD47 expression or by increasing the immune response, which will reduce the extended therapeutic effects response.
CD47/SIRPα interactions accomplish a wide range of physiological functions. This pathway-mediated downstream signaling is crucial in immune defense, lymphocyte homeostasis, bone remodeling, HSCs engraftment, atherosclerosis, and tumor immune surveillance. It activates macrophages' phagocytic response and modulates metabolism and mitochondrial homeostasis in various cells. Cancerous cells in various tumors increased CD47 expression, which helps the migration and invasion and helps to evade the innate immune system. The therapy utilizing the CD47 target offers a new approach for the treatment of cancer. The phase1 trial using antibodies have shown significant effects on tumors. The dose for phase II trials has not been measured yet. However, challenges like on-target and off-target toxicity, nonselective phagocytosis, anemia, and resistance development are the major concerns that require further research. There is also a need to identify potential biomarkers and examine biosafety, efficacy, and side effects and develop guidelines for therapeutic approaches.
| 1. | Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 683] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 2. | Anniss AM, Sparrow RL. Expression of CD47 (integrin-associated protein) decreases on red blood cells during storage. Transfus Apher Sci. 2002;27:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Lindberg FP, Lublin DM, Telen MJ, Veile RA, Miller YE, Donis-Keller H, Brown EJ. Rh-related antigen CD47 is the signal-transducer integrin-associated protein. J Biol Chem. 1994;269:1567-1570. [PubMed] |
| 4. | Zhang T, Wang F, Xu L, Yang YG. Structural-functional diversity of CD47 proteoforms. Front Immunol. 2024;15:1329562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 5. | Parthasarathy R, Subramanian S, Boder ET, Discher DE. Post-translational regulation of expression and conformation of an immunoglobulin domain in yeast surface display. Biotechnol Bioeng. 2006;93:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Kaur S, Kuznetsova SA, Pendrak ML, Sipes JM, Romeo MJ, Li Z, Zhang L, Roberts DD. Heparan sulfate modification of the transmembrane receptor CD47 is necessary for inhibition of T cell receptor signaling by thrombospondin-1. J Biol Chem. 2011;286:14991-15002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Berkovits BD, Mayr C. Alternative 3' UTRs act as scaffolds to regulate membrane protein localization. Nature. 2015;522:363-367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 364] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 8. | Murata Y, Kotani T, Ohnishi H, Matozaki T. The CD47-SIRPα signalling system: its physiological roles and therapeutic application. J Biochem. 2014;155:335-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 9. | van den Berg TK, van Beek EM, Bühring HJ, Colonna M, Hamaguchi M, Howard CJ, Kasuga M, Liu Y, Matozaki T, Neel BG, Parkos CA, Sano S, Vignery A, Vivier E, Wright M, Zawatzky R, Barclay AN. A nomenclature for signal regulatory protein family members. J Immunol. 2005;175:7788-7789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Barclay AN, Brown MH. The SIRP family of receptors and immune regulation. Nat Rev Immunol. 2006;6:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 319] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 11. | Lowell CA. Src-family and Syk kinases in activating and inhibitory pathways in innate immune cells: signaling cross talk. Cold Spring Harb Perspect Biol. 2011;3:a002352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 12. | Brooke G, Holbrook JD, Brown MH, Barclay AN. Human lymphocytes interact directly with CD47 through a novel member of the signal regulatory protein (SIRP) family. J Immunol. 2004;173:2562-2570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Roberts DD, Isenberg JS. CD47 and thrombospondin-1 regulation of mitochondria, metabolism, and diabetes. Am J Physiol Cell Physiol. 2021;321:C201-C213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Oronsky B, Carter C, Reid T, Brinkhaus F, Knox SJ. Just eat it: A review of CD47 and SIRP-α antagonism. Semin Oncol. 2020;47:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 15. | van den Akker E, Satchwell TJ, Williamson RC, Toye AM. Band 3 multiprotein complexes in the red cell membrane; of mice and men. Blood Cells Mol Dis. 2010;45:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Oldenborg PA. Role of CD47 in erythroid cells and in autoimmunity. Leuk Lymphoma. 2004;45:1319-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051-2054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1241] [Cited by in RCA: 1446] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 18. | Bian Z, Shi L, Guo YL, Lv Z, Tang C, Niu S, Tremblay A, Venkataramani M, Culpepper C, Li L, Zhou Z, Mansour A, Zhang Y, Gewirtz A, Kidder K, Zen K, Liu Y. Cd47-Sirpα interaction and IL-10 constrain inflammation-induced macrophage phagocytosis of healthy self-cells. Proc Natl Acad Sci U S A. 2016;113:E5434-E5443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 19. | Matozaki T, Murata Y, Okazawa H, Ohnishi H. Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway. Trends Cell Biol. 2009;19:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 369] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 20. | Okazawa H, Motegi S, Ohyama N, Ohnishi H, Tomizawa T, Kaneko Y, Oldenborg PA, Ishikawa O, Matozaki T. Negative regulation of phagocytosis in macrophages by the CD47-SHPS-1 system. J Immunol. 2005;174:2004-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 244] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 21. | Soto-Pantoja DR, Kaur S, Roberts DD. CD47 signaling pathways controlling cellular differentiation and responses to stress. Crit Rev Biochem Mol Biol. 2015;50:212-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 22. | Olsson M, Nilsson A, Oldenborg PA. Target cell CD47 regulates macrophage activation and erythrophagocytosis. Transfus Clin Biol. 2006;13:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Lutz HU, Bogdanova A. Mechanisms tagging senescent red blood cells for clearance in healthy humans. Front Physiol. 2013;4:387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 24. | Saxena RK, Bhardwaj N, Sachar S, Puri N, Khandelwal S. A Double in vivo Biotinylation Technique for Objective Assessment of Aging and Clearance of Mouse Erythrocytes in Blood Circulation. Transfus Med Hemother. 2012;39:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Eldakhakhny B, Al Sadoun H, Taleb NB, Nori DA, Helmi N, Ahmed IM, Bakhrebah MA, Abdulaal WH. Evaluation of the role of CD47 in sickle cell disease. J Hematopathol. 2021;14:31-39. [RCA] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 26. | Eltayeb MM, Waggiallah HA, Hakami NY, Elmosaad YM. Correlation between smoking and downregulation of red cell CD47 as eryptosis marker. Eur Rev Med Pharmacol Sci. 2023;27:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Wiewiora M, Piecuch J, Sedek L, Mazur B, Sosada K. The effects of obesity on CD47 expression in erythrocytes. Cytometry B Clin Cytom. 2017;92:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Asplund H, Dreyer HH, Singhal R, Rouchka EC, O'Toole TE, Haberzettl P, Conklin DJ, Sansbury BE. Exposure to Fine Particulate Matter Air Pollution Disrupts Erythrocyte Turnover. Circ Res. 2024;134:1224-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Ni W, Mao S, Xi G, Keep RF, Hua Y. Role of Erythrocyte CD47 in Intracerebral Hematoma Clearance. Stroke. 2016;47:505-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 30. | Bhardwaj N, Singh A. Paraquat treatment modulates integrin associated protein (CD47) and basigin (CD147) expression and mitochondrial potential on erythroid cells in mice. Environ Toxicol Pharmacol. 2018;58:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Bhardwaj N, Chauhan P, Chandra H, Singh A, Gupta NJ. Polydispersed Acid-Functionalized Single-Walled Carbon Nanotubes Induced the Integrin-Associated Protein (CD47) and Basigin (CD147) Expression and Modulated the Antioxidant Gene Expression in Erythroid Cells in Mice. BioNanoSci. 2023;13:695-703. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Burger P, Hilarius-Stokman P, de Korte D, van den Berg TK, van Bruggen R. CD47 functions as a molecular switch for erythrocyte phagocytosis. Blood. 2012;119:5512-5521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 247] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 33. | Yang J, Xing F, Hu F, Hou M, Dong H, Cheng J, Li W, Yan R, Xu J, Xu K, Pan L. Super-resolution microscopy unveils the nanoscale organization and self-limiting clustering of CD47 in human erythrocytes. J Mol Cell Biol. 2025;16:mjae041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Liu Y, Bühring HJ, Zen K, Burst SL, Schnell FJ, Williams IR, Parkos CA. Signal regulatory protein (SIRPalpha), a cellular ligand for CD47, regulates neutrophil transmigration. J Biol Chem. 2002;277:10028-10036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 169] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 35. | Nath PR, Pal-Nath D, Kaur S, Gangaplara A, Meyer TJ, Cam MC, Roberts DD. Loss of CD47 alters CD8+ T cell activation in vitro and immunodynamics in mice. Oncoimmunology. 2022;11:2111909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | Liu Q, Wen W, Tang L, Qin CJ, Lin Y, Zhang HL, Wu H, Ashton C, Wu HP, Ding J, Dong W, Yu LX, Yang W, Huang DD, Wu MC, Wang HY, Yan HX. Inhibition of SIRPα in dendritic cells potentiates potent antitumor immunity. Oncoimmunology. 2016;5:e1183850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Cham LB, Rosas-Umbert M, Lin L, Tolstrup M, Søgaard OS. Single-Cell Analysis Reveals That CD47 mRNA Expression Correlates with Immune Cell Activation, Antiviral Isgs, and Cytotoxicity. Cell Physiol Biochem. 2024;58:322-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | Pettersen RD, Hestdal K, Olafsen MK, Lie SO, Lindberg FP. CD47 Signals T Cell Death. J Immunol. 1999;162:7031-7040. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 39. | Barrera L, Montes-Servín E, Hernandez-Martinez JM, García-Vicente MLÁ, Montes-Servín E, Herrera-Martínez M, Crispín JC, Borbolla-Escoboza JR, Arrieta O. CD47 overexpression is associated with decreased neutrophil apoptosis/phagocytosis and poor prognosis in non-small-cell lung cancer patients. Br J Cancer. 2017;117:385-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 40. | Graf R, Freyberg M, Kaiser D, Friedl P. Mechanosensitive induction of apoptosis in fibroblasts is regulated by thrombospondin-1 and integrin associated protein (CD47). Apoptosis. 2002;7:493-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Kaur S, Elkahloun AG, Singh SP, Arakelyan A, Roberts DD. A function-blocking CD47 antibody modulates extracellular vesicle-mediated intercellular signaling between breast carcinoma cells and endothelial cells. J Cell Commun Signal. 2018;12:157-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Sadallah S, Eken C, Martin PJ, Schifferli JA. Microparticles (ectosomes) shed by stored human platelets downregulate macrophages and modify the development of dendritic cells. J Immunol. 2011;186:6543-6552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 43. | Hayat SMG, Bianconi V, Pirro M, Jaafari MR, Hatamipour M, Sahebkar A. CD47: role in the immune system and application to cancer therapy. Cell Oncol (Dordr). 2020;43:19-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 44. | Huang CY, Ye ZH, Huang MY, Lu JJ. Regulation of CD47 expression in cancer cells. Transl Oncol. 2020;13:100862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 45. | Ye ZH, Jiang XM, Huang MY, Xu YL, Chen YC, Yuan LW, Huang CY, Yu WB, Chen X, Lu JJ. Regulation of CD47 expression by interferon-gamma in cancer cells. Transl Oncol. 2021;14:101162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 46. | Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, Weiskopf K, Willingham SB, Raveh T, Park CY, Majeti R, Weissman IL. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010;2:63ra94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 587] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 47. | Pan J, Zhang YY, Jiao XX, Song LN, Lin CQ, Wang SL, Zhu B, Pan SY, Ding ZY, Zhao WL. [Correlation between Expression of CD47 Molecule in Patients with Newly Diagnosed Adult Acute Myeloid Leukemia and Clinical Prognosis]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2022;30:1071-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Shi L, Bian Z, Kidder K, Liang H, Liu Y. Non-Lyn Src Family Kinases Activate SIRPα-SHP-1 to Inhibit PI3K-Akt2 and Dampen Proinflammatory Macrophage Polarization. J Immunol. 2021;207:1419-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Brandsma AM, Bondza S, Evers M, Koutstaal R, Nederend M, Jansen JHM, Rösner T, Valerius T, Leusen JHW, Ten Broeke T. Potent Fc Receptor Signaling by IgA Leads to Superior Killing of Cancer Cells by Neutrophils Compared to IgG. Front Immunol. 2019;10:704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 50. | Yang K, Xu J, Liu Q, Li J, Xi Y. Expression and significance of CD47, PD1 and PDL1 in T-cell acute lymphoblastic lymphoma/leukemia. Pathol Res Pract. 2019;215:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 51. | Cho J, Yoon SE, Kim SJ, Ko YH, Kim WS. CD47 overexpression is common in intestinal non-GCB type diffuse large B-cell lymphoma and associated with 18q21 gain. Blood Adv. 2022;6:6120-6130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Kim D, Wang J, Willingham SB, Martin R, Wernig G, Weissman IL. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia. 2012;26:2538-2545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 53. | Luan Y, Zhang Y, Li S, Gao C, Ying X, Zhao S, Zhang B. CD47 is a tumor cell-derived exosomal signature and regulates tumor immune microenvironment and immunotherapy responses. Transl Oncol. 2025;53:102291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 54. | Martinez-Torres AC, Quiney C, Attout T, Boullet H, Herbi L, Vela L, Barbier S, Chateau D, Chapiro E, Nguyen-Khac F, Davi F, Le Garff-Tavernier M, Moumné R, Sarfati M, Karoyan P, Merle-Béral H, Launay P, Susin SA. CD47 agonist peptides induce programmed cell death in refractory chronic lymphocytic leukemia B cells via PLCγ1 activation: evidence from mice and humans. PLoS Med. 2015;12:e1001796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 55. | Kim MJ, Lee JC, Lee JJ, Kim S, Lee SG, Park SW, Sung MW, Heo DS. Association of CD47 with natural killer cell-mediated cytotoxicity of head-and-neck squamous cell carcinoma lines. Tumour Biol. 2008;29:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 56. | Nath PR, Gangaplara A, Pal-Nath D, Mandal A, Maric D, Sipes JM, Cam M, Shevach EM, Roberts DD. CD47 Expression in Natural Killer Cells Regulates Homeostasis and Modulates Immune Response to Lymphocytic Choriomeningitis Virus. Front Immunol. 2018;9:2985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 57. | Nath PR, Pal-Nath D, Mandal A, Cam MC, Schwartz AL, Roberts DD. Natural Killer Cell Recruitment and Activation Are Regulated by CD47 Expression in the Tumor Microenvironment. Cancer Immunol Res. 2019;7:1547-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 58. | Wang S, Wu Q, Chen T, Su R, Pan C, Qian J, Huang H, Yin S, Xie H, Zhou L, Zheng S. Blocking CD47 promotes antitumour immunity through CD103(+) dendritic cell-NK cell axis in murine hepatocellular carcinoma model. J Hepatol. 2022;77:467-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 100] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 59. | Lundberg P, Koskinen C, Baldock PA, Löthgren H, Stenberg A, Lerner UH, Oldenborg PA. Osteoclast formation is strongly reduced both in vivo and in vitro in the absence of CD47/SIRPalpha-interaction. Biochem Biophys Res Commun. 2007;352:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 60. | Uluçkan O, Becker SN, Deng H, Zou W, Prior JL, Piwnica-Worms D, Frazier WA, Weilbaecher KN. CD47 regulates bone mass and tumor metastasis to bone. Cancer Res. 2009;69:3196-3204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 61. | van Beek EM, de Vries TJ, Mulder L, Schoenmaker T, Hoeben KA, Matozaki T, Langenbach GE, Kraal G, Everts V, van den Berg TK. Inhibitory regulation of osteoclast bone resorption by signal regulatory protein alpha. FASEB J. 2009;23:4081-4090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 62. | Zondervan RL, Capobianco CA, Jenkins DC, Reicha JD, Fredrick L, Lam C, Schmanski JT, Isenberg JS, Ahn J, Marcucio RS, Hankenson KD. CD47 is required for mesenchymal progenitor proliferation and fracture repair. Bone Res. 2025;13:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 63. | Wang Q, Onuma K, Liu C, Wong H, Bloom MS, Elliott EE, Cao RR, Hu N, Lingampalli N, Sharpe O, Zhao X, Sohn DH, Lepus CM, Sokolove J, Mao R, Cisar CT, Raghu H, Chu CR, Giori NJ, Willingham SB, Prohaska SS, Cheng Z, Weissman IL, Robinson WH. Dysregulated integrin αVβ3 and CD47 signaling promotes joint inflammation, cartilage breakdown, and progression of osteoarthritis. JCI Insight. 2019;4:e128616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 64. | Waern JM, Yuan Q, Rüdrich U, Becker PD, Schulze K, Strick-Marchand H, Huntington ND, Zacher BJ, Wursthorn K, DiSanto JP, Guzman CA, Manns MP, Ott M, Bock M. Ectopic expression of murine CD47 minimizes macrophage rejection of human hepatocyte xenografts in immunodeficient mice. Hepatology. 2012;56:1479-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Deuse T, Hu X, Gravina A, Wang D, Tediashvili G, De C, Thayer WO, Wahl A, Garcia JV, Reichenspurner H, Davis MM, Lanier LL, Schrepfer S. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol. 2019;37:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 503] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 66. | Germani G, Rodriguez-Castro K, Russo FP, Senzolo M, Zanetto A, Ferrarese A, Burra P. Markers of acute rejection and graft acceptance in liver transplantation. World J Gastroenterol. 2015;21:1061-1068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 67. | Ma C, Cao H, Sun Z, Deng Q, Liu W, Xin Y, Qiao S, Cen J, Shu Y, Qi K, Han L, Zhang L, Pan G. CD47 and PD-L1 overexpression in proliferating human hepatocytes attenuated immune responses and ameliorated acute liver injury in mice. Am J Transplant. 2023;23:1832-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Bhardwaj N, Singh A, Babu R, Ahmed MZ. Modulation of Redox Metabolism, CD147, and CD47 Expression with the Maturation of Hematopoietic Stem Cells in Bone Marrow. Indian J Hematol Blood Transfus. 2024;. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 69. | Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1313] [Cited by in RCA: 1237] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 70. | Porpiglia E, Mai T, Kraft P, Holbrook CA, de Morree A, Gonzalez VD, Hilgendorf KI, Frésard L, Trejo A, Bhimaraju S, Jackson PK, Fantl WJ, Blau HM. Elevated CD47 is a hallmark of dysfunctional aged muscle stem cells that can be targeted to augment regeneration. Cell Stem Cell. 2022;29:1653-1668.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 71. | Maute R, Xu J, Weissman IL. CD47-SIRPα-targeted therapeutics: status and prospects. Immunooncol Technol. 2022;13:100070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 72. | Jiang C, Sun H, Jiang Z, Tian W, Cang S, Yu J. Targeting the CD47/SIRPα pathway in malignancies: recent progress, difficulties and future perspectives. Front Oncol. 2024;14:1378647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 73. | Kaur S, Cicalese KV, Banerjee R, Roberts DD. Preclinical and Clinical Development of Therapeutic Antibodies Targeting Functions of CD47 in the Tumor Microenvironment. Antib Ther. 2020;3:179-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |