Published online Aug 27, 2025. doi: 10.4240/wjgs.v17.i8.109213
Revised: May 21, 2025
Accepted: July 9, 2025
Published online: August 27, 2025
Processing time: 114 Days and 12.1 Hours
Ganglioneuroma is a rare, well-differentiated, slow-growing benign tumor of the peripheral nerves, with surgical resection being the only curative treatment. Sur
A 35-year-old woman was admitted to our hospital after the incidental discovery of a retroperitoneal space-occupying lesion. Imaging revealed a mass with the celiac axis and superior mesenteric artery passing through it. A neurogenic tumor was suspected, with ganglioneuroma being the most likely diagnosis. Following comprehensive preoperative preparation, the retroperitoneal tumor was resected using a three-dimensional laparoscopy combined with an organ suspension technique. The surgical approach involved incising the tumor along the vascular axis and conducting meticulous, vascular-preserving tumor excision. The ope
This case illustrates the feasibility of minimally invasive laparoscopic resection for retroperitoneal ganglioneuromas encasing major blood vessels.
Core Tip: Ganglioneuroma is a rare, well-differentiated, slow-growing benign tumor, with surgical resection being the only curative treatment. However, resecting ganglioneuromas that encase major blood vessels presents substantial technical challenges. Here, we present the first case of a retroperitoneal ganglioneuroma encasing the celiac axis and superior mesenteric artery, successfully resected with preservation of all vital vessels using three-dimensional laparoscopic surgery combined with organ suspension techniques. This case highlights the feasibility of minimally invasive laparoscopic approaches for managing complex retroperitoneal ganglioneuromas encasing major blood vessels.
- Citation: Wu GZ, Fang SZ, Yu SA, Yu M. Resection of a ganglioneuroma encasing major blood vessels using three-dimensional laparoscopy combined with organ suspension: A case report. World J Gastrointest Surg 2025; 17(8): 109213
- URL: https://www.wjgnet.com/1948-9366/full/v17/i8/109213.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i8.109213
Ganglioneuroma is a rare benign tumor originating from primitive neural crest cells surrounding the paravertebral nerve plexus. It can occur in different body parts, most commonly in the retroperitoneum (32%-52%), posterior mediastinum (39%-43%), and then cervical region (8%-9%)[1,2]. Histologically, it primarily comprises mature Schwann and ganglion cells within a fibrous stroma[3].
This disease typically begins insidiously, and many patients exhibit no obvious clinical symptoms. It is often detected during routine physical examinations or while undergoing tests for unrelated conditions. Symptoms may appear in a few patients due to local tumor expansion and compression of surrounding structures, such as interruption of venous blood flow, lower abdominal pain, vomiting, weight loss, and neurological deficits. In some cases, hormone secretion by the tumor can also cause clinical manifestations of autonomic nervous system dysfunction. In extremely rare cases, it may progress to a malignant peripheral nerve sheath tumor[4,5].
Currently, no effective medical therapies exist for retroperitoneal ganglioneuromas, making surgical resection the primary treatment option[6]. Before the refinement of laparoscopic techniques, most cases were managed through open surgery. However, advancements in minimally invasive surgery have enabled the laparoscopic resection of many tumors. Laparoscopic resection is typically considered simple for ganglioneuromas within the adrenal gland, with numerous successful cases reported for tumors closely abutting major blood vessels[7,8]. However, ganglioneuromas encasing critical vascular structures pose substantial surgical challenges, even when accessed via open techniques[9,10]. To date, reports on the successful laparoscopic resection of such complex cases are lacking, highlighting the requirement for further technical innovation and advanced surgical expertise in managing these challenging tumors. Herein, we report a novel surgical approach involving the resection of retroperitoneal ganglioneuromas encasing major blood vessels using three-dimensional (3D) laparoscopy combined with an organ suspension technique.
A 35-year-old woman was admitted to the hospital on April 28, 2024, following the incidental discovery of a retroperitoneal space-occupying lesion the previous day.
The patient presented to our outpatient department with a complaint of a cough the previous day. A non-contrast computed tomography (CT) scan revealed a space-occupying lesion in the left retroperitoneum, prompting further evaluation with contrast-enhanced CT. Currently, the patient exhibits no symptoms, including abdominal pain, abdo
The patient presents without history of abdominal surgery, smoking, alcohol use or hypertension.
All other personal and family medical histories were noncontributory.
No skin or sclera jaundice was observed. The abdomen was soft without tenderness or rebound tenderness. No abdo
Blood routine, biochemistry, tumor markers, glucocorticoids, renin, angiotensin II, aldosterone, adrenocorticotropic hormone, and other tests were all within normal ranges.
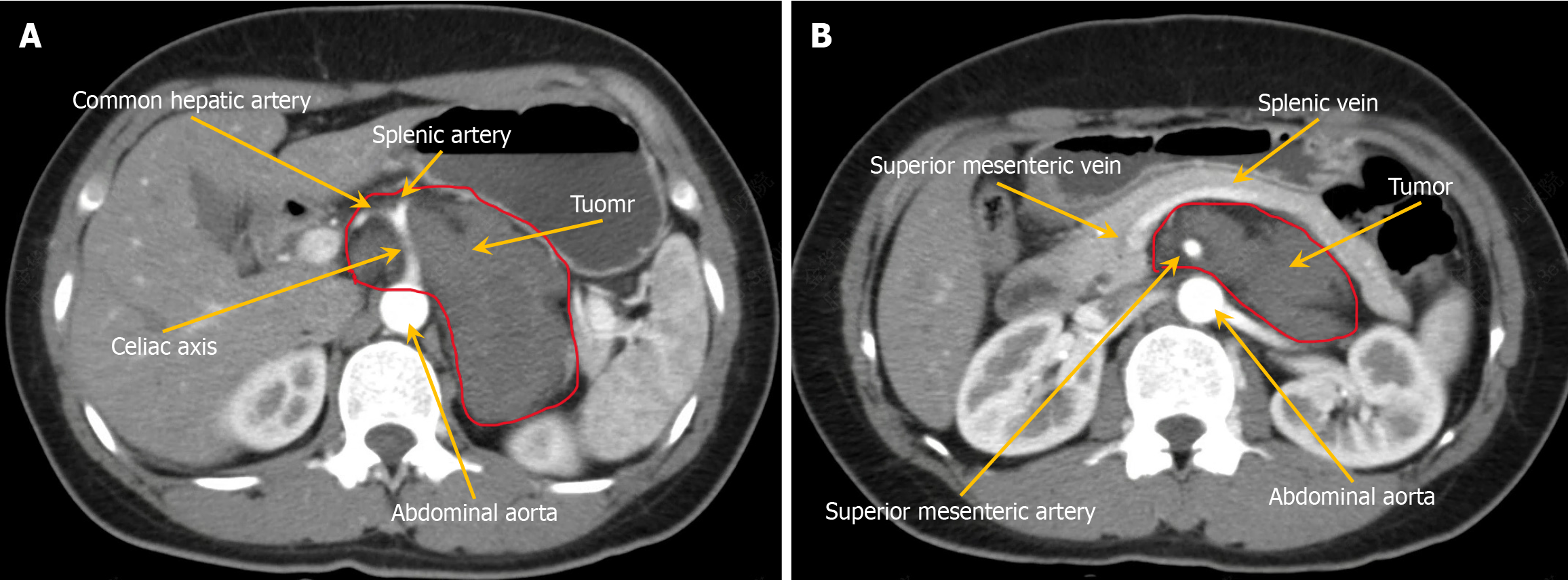
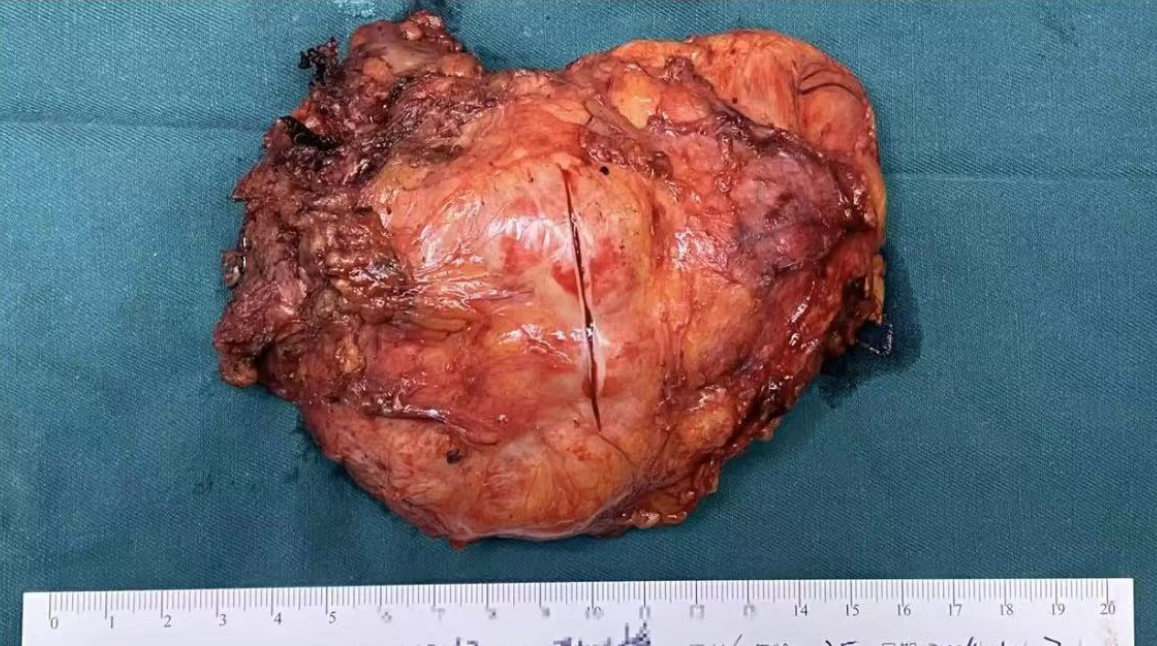
Abdominal contrast-enhanced CT revealed a slightly hypodense left retroperitoneum mass with heterogeneous internal density. The largest cross-sectional diameter measured approximately 8.5 cm × 5 cm. Progressive mild enhancement was observed on contrast-enhanced imaging. The mass laterally displaced the left adrenal gland and encased the celiac axis and superior mesenteric artery without considerable vascular lumen stenosis. A neurogenic tumor was suspected, with ganglioneuroma considered the most likely diagnosis (Figure 1A and B).
Contrast-enhanced magnetic resonance imaging revealed a solid-cystic mass in the left retroperitoneum. On diffusion-weighted imaging, the lesion exhibited slightly hyperintense signals. The mass measured approximately 8.6 cm × 5.6 cm, with progressive enhancement observed on contrast-enhanced sequences. A ganglioneuroma was suspected.
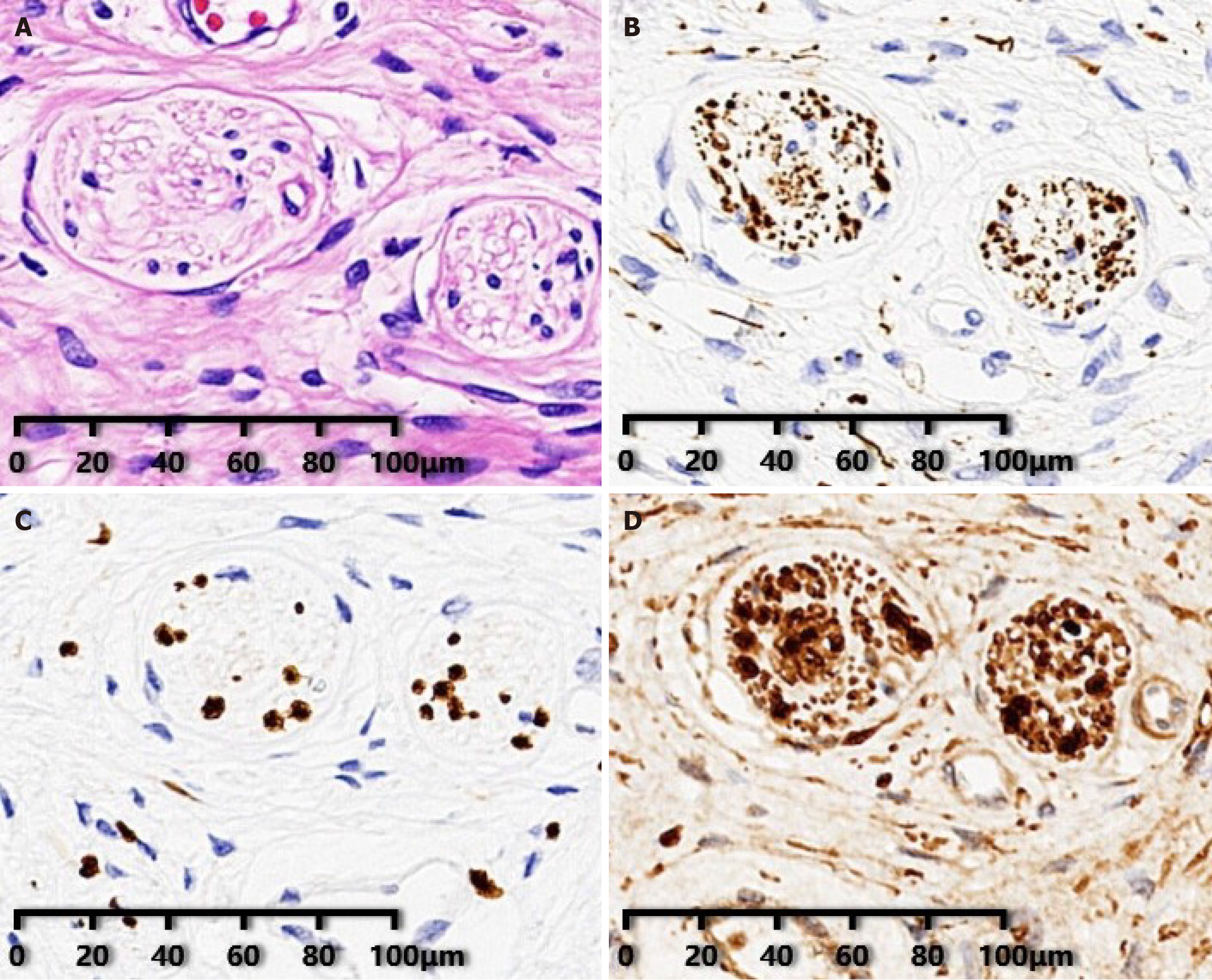
Postoperative pathological examination confirmed ganglioneuroma. Immunohistochemical analysis revealed positive tumor cells for neurofilament, SRY-related HMG-box 10 protein, and S-100 protein (Figure 2A-D).
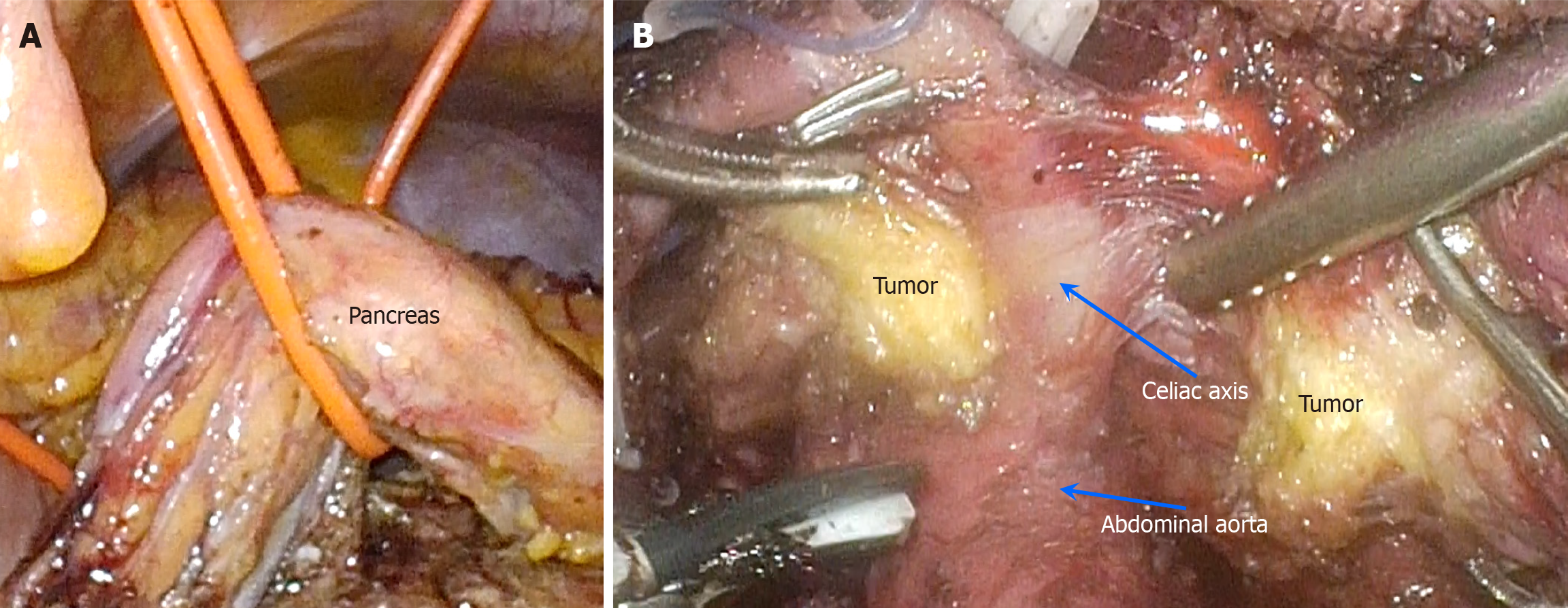
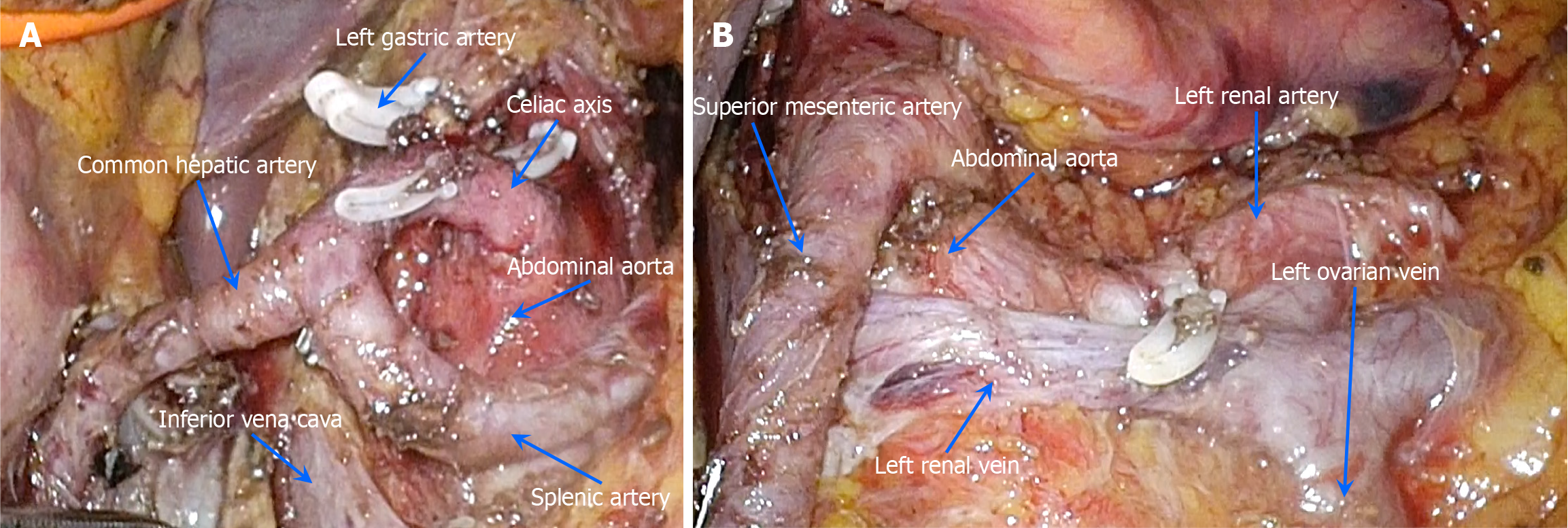
Following meticulous preoperative preparation, a retroperitoneal tumor resection was performed on May 7, 2024, utilizing 3D laparoscopy combined with organ suspension (Figure 3A). The procedure involved carefully incising the tumor along the vascular axis (Figure 3B) and performing a vascular-preserving excision (Figure 4A and B). The surgery lasted approximately 458 minutes, with an estimated blood loss of 50 mL. Postoperative pathological examination re
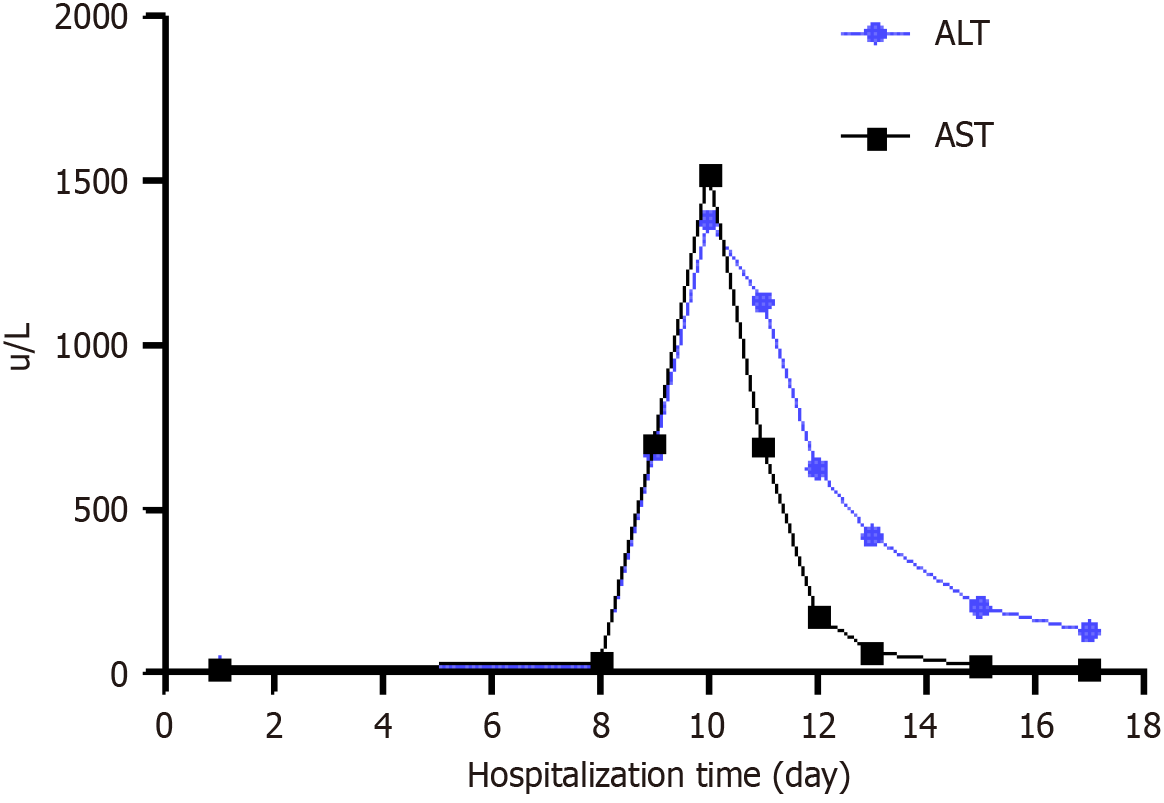
The patient was discharged on the 8th postoperative day. Although transient liver injury occurred after surgery, it improved rapidly (Figure 6). No pancreatic fistula, chylous fistula, or other complications were observed. After 11 months of postoperative follow-up, no complications or tumor recurrence were observed. The patient fully regained the ability to perform daily activities and work, expressing high satisfaction with the treatment outcomes. To monitor tumor status, we recommended enhanced abdominal CT scans every six months. Additionally, we will continue to monitor the patient's condition closely.
Ganglioneuroma is a rare, well-differentiated, slow-growing benign tumor of the peripheral nervous system. It typically grows along periorgan spaces, encasing adjacent blood vessels without causing vascular occlusion or stenosis. Ganglioneuromas can develop anywhere along the peripheral autonomic ganglia[11]. Given the nonspecific clinical presentation and limited diagnostic utility of laboratory tests, imaging modalities are currently the primary method of diagnosis. However, a definitive diagnosis requires pathological confirmation, and surgical resection remains the only curative approach, with complete excision offering the potential for cure and minimizing the risk of local recurrence. The prog
Ganglioneuromas are typically soft in texture due to the abundant mucinous matrix within the tumor. On CT, they often appear as low-density masses, with attenuation lower than that of adjacent muscle tissue at the same level[14]. Generally, ganglioneuromas possess a well-defined capsule and exhibit clear margins but tend to grow in a pseudopod-like or infiltrative manner along the spaces between surrounding organs and may compress and encase adjacent blood vessels. Ganglioneuromas are hypovascular tumors that typically exhibit no enhancement or only mild to moderate enhancement during the delayed phase of contrast-enhanced imaging. Approximately 10%–25% of ganglioneuromas may exhibit calcifications, usually in the form of pinpoint, punctate, or thin linear deposits along the[15]. This aligns with the CT features observed in the present case, although no calcifications were identified on imaging in this patient. Due to the characteristic of encircling blood vessels, ganglioneuromas are often misdiagnosed as malignant tumors before surgery, which may result in the tumor being deemed unresectable or unnecessary expansion of the resection scope. When needed, a puncture biopsy can be employed for a definitive diagnosis. However, in this case, given the tumor’s characteristic imaging features, both CT and magnetic resonance imaging provided a relatively definitive diagnosis suggestive of ganglioneuroma; therefore, a preoperative biopsy was not performed. Instead, an intraoperative biopsy was con
Vascular invasion is a hallmark of malignant tumors. Malignant tumors encase blood vessels and infiltrate vessel walls, resulting in luminal stenosis. No clear boundary typically exists between the tumor and the vessel wall. Radical resection of malignant tumors requires simultaneous removal of the involved blood vessels. However, the celiac axis and superior mesenteric artery are among the most critical arteries in the abdominal cavity, supplying blood to essential organs, such as the liver, stomach, pancreas, small intestine, and right half of the large intestine. Consequently, malignant tumors that invade the celiac axis or superior mesenteric artery are typically considered unresectable by radical surgery, with non
In contrast to malignant tumors, ganglioneuromas encase blood vessels but have a distinct boundary between the tumor and vessel walls, with no vascular infiltration. In this case, the tumor was incised along the vascular axis and meticulously dissected from the vessel walls, allowing complete removal while preserving vital abdominal blood vessels. Although this surgical technique carries a risk of tumor residue or dissemination, the benign nature of ganglioneuromas substantially reduces the likelihood of this concern. During the 11-month postoperative follow-up, enhanced CT scans were performed, with no evidence of tumor recurrence observed. The current follow-up period is still relatively short; therefore, continued monitoring of the patient is planned. It is recommended that contrast-enhanced abdominal CT scans be performed every six months. Although the tumor was completely resected, the possibility of postoperative recurrence cannot be ruled out.
Surgical resection of ganglioneuromas encasing major blood vessels remains technically challenging, with only a few cases reported. Early open surgical approaches often necessitated combined organ resection or vascular reconstruction with artificial grafts, causing substantial surgical trauma[16,17]. Subsequently, Wan et al[18] proposed a “staged resec
In 2016, Abe et al[19] described 24 cases of laparoscopic resection of paraspinal neurogenic tumors located adjacent to the abdominal aorta or inferior vena cava (IVC), noting that none of the tumors encased these vessels. The relatively large diameter and thick walls of the abdominal aorta and IVC reduced the risk of vascular injury, allowing for safe tumor removal through meticulous dissection along the tumor margins without the need for tumor incision or direct vascular exposure. Similarly, in 2012, Alimoglu et al[20] reported a case of ganglioneuroma located at the hepatic hilum, superior and posterior to the pancreas, measuring approximately 4.4 cm. Although the tumor was adjacent to the celiac trunk and its branches - including the left gastric artery, hepatic artery, and splenic artery - no vascular invasion was observed. In 2023, Felföldi et al[21] reported a ganglioneuroma in the hepatoduodenal ligament, closely abutting the IVC, portal vein, and hepatic artery. The tumor was safely resected along its margins without the need for tumor incision, as the vessels were not encased. In 2021, Nagano et al[22] presented a case of laparoscopic resection of a large (approximately 9 cm) extra-adrenal ganglioneuroma adherent to the abdominal aorta and inferior mesenteric artery. Only fibrous adhesions were observed intraoperatively, which were easily separated using an ultrasonic scalpel. Compared with the present case, the surgical complexity in these previously reported cases was significantly lower due to the absence of vascular en
Compared with two-dimensional laparoscopy, 3D laparoscopy offers higher magnification and 3D stereoscopic imaging. These features facilitate more precise intraoperative localization and optimal hand-eye coordination, consi
The laparoscopic suspension technique is analogous to the use of retractors in open abdominal surgeries. It allows the primary surgeon and assistant to keep their hands free from other surgical maneuvers. Suspension does not prolong the overall operative time; adequate suspension provides an excellent field of view and optimal exposure during the procedure[25,26]. Suspension of the liver and stomach is a widely used technique in hepatobiliary and pancreatic surgery. By employing sutures or specialized instruments to retract and stabilize these organs, the technique enables optimal exposure of complex anatomical regions, including the hepatic hilum, retrohepatic IVC, posterior segments of the right hepatic lobe, gastroesophageal junction, lesser curvature of the stomach, and pancreas. In contrast, pancreatic suspension is relatively rare. Adequate exposure of these critical areas facilitates the safe and effective execution of complex surgical procedures, thereby enhancing both surgical safety and oncological radicality.
In the case presented, a multilayer suspension of the stomach, liver, and pancreas was implemented. The gastrocolic and hepatogastric ligaments were first dissected using an ultrasonic scalpel, followed by a laparoscopic gauze strip to encircle the gastric body. The gauze was then sutured and fixed to the falciform ligament of the liver. To elevate the left lateral lobe of the liver, a purse-string suture was placed, with both ends passed through the abdominal wall on either side of the round ligament. A 10-Fr red rubber catheter was passed through the suture, secured at the root of the hepatogastric ligament with a Hem-o-lok clip, and tightened to establish the necessary suspension. A second catheter was introduced through the pancreatic base and suspended from the abdominal wall after the pancreatic base was separated. This comprehensive suspension method provided the necessary space for successful tumor resection. This technique, reported for the first time in retroperitoneal ganglioneuroma resection, was integral to the success of the procedure.
Postoperatively, the patient's transaminase levels rose sharply but improved rapidly thereafter. An early postoperative contrast-enhanced CT scan revealed ischemic changes in the liver's left lateral segment that resolved over time. Upon analysis, two potential causes were identified: (1) During the operation, bleeding from the left gastric artery required resection. However, the accessory left hepatic artery originated from the left gastric artery, impairing the blood supply to the left lateral segment of the liver; and (2) If the intraoperative suspension of the pancreas was too tight, it may have compromised the blood flow to the splenic, superior mesenteric, and portal veins, potentially leading to ischemia-reperfusion injury. Therefore, future surgeries should avoid unnecessary vascular resections and ensure the pancreatic suspension is not overly tight.
The operative time was 458 minutes, primarily due to the complexity of the procedure. Even when performed via an open approach, such surgeries typically require a relatively long duration. We anticipate that, with increased experience and greater proficiency in the surgical workflow, the operative time will gradually decrease. Similarly, after years of refinement, laparoscopic procedures such as pancreaticoduodenectomy and hepatectomy can now be performed within a timeframe comparable to that of open surgery. Moreover, laparoscopic surgery offers several well-established advan
Ganglioneuroma is a rare, well-differentiated, and slow-growing benign tumor arising from the peripheral nervous system, with surgical resection being the sole curative treatment. However, resecting ganglioneuromas that encase major blood vessels presents considerable technical challenges. These tumors have traditionally often required open abdominal surgery or combined organ resections and, in some cases, are deemed unresectable. This report presents the first case of a retroperitoneal ganglioneuroma encasing major vessels successfully resected using 3D laparoscopic surgery combined with organ suspension. At 11 months of postoperative follow-up, the patient exhibited neither complications nor evidence of tumor recurrence. This case highlights the feasibility of minimally invasive laparoscopic approaches for managing complex retroperitoneal ganglioneuromas encasing major blood vessels. However, further studies with larger cohorts and extended follow-up are needed to confirm these findings.
| 1. | Burroughs MA Jr, Urits I, Viswanath O, Kaye AD, Hasoon J. Adrenal Ganglioneuroma: A Rare Tumor of the Autonomic Nervous System. Cureus. 2020;12:e12398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Dinh HTP, Yamato Y, Hasegawa T, Yoshida G, Banno T, Arima H, Oe S, Ide K, Yamada T, Kurosu K, Matsuyama Y. Adolescent thoracic scoliosis due to giant ganglioneuroma: a two-case report and literature review. Nagoya J Med Sci. 2024;86:711-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 3. | Kirchweger P, Wundsam HV, Fischer I, Rösch CS, Böhm G, Tsybrovskyy O, Alibegovic V, Függer R. Total resection of a giant retroperitoneal and mediastinal ganglioneuroma-case report and systematic review of the literature. World J Surg Oncol. 2020;18:248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Farma JM, Porpiglia AS, Vo ET. Benign Neurogenic Tumors. Surg Clin North Am. 2022;102:679-693. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Mow TC, Navadgi S, Jackett L, Galloway S, Banting S. Malignant peripheral nerve sheath tumour arising de novo from ganglioneuroma. Pathology. 2015;47:595-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Othman BK, Badawy W. Intraparotid Ganglioneuroma: A rare case report. Indian J Otolaryngol Head Neck Surg. 2023;75:2613-2616. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Hemmati P, Ghanem O, Bingener J. Laparoscopic celiac plexus ganglioneuroma resection: A video case report. World J Gastrointest Surg. 2019;11:191-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Mohammadi A, Asgari F, Yar EZ, Rezayat M, Najarzadegan M, Kazem Aghamir SM. Synchronous Ipsilateral Adrenal and Retroperitoneal Ganglioneuroma: A Unique Case of Large Tumor Mass. Case Rep Med. 2025;2025:7440806. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Vasiliadis K, Papavasiliou C, Fachiridis D, Pervana S, Michaelides M, Kiranou M, Makridis C. Retroperitoneal extra-adrenal ganglioneuroma involving the infrahepatic inferior vena cava, celiac axis and superior mesenteric artery: A case report. Int J Surg Case Rep. 2012;3:541-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Kumar S, Singh S, Chandna A. Organ Preservation in a Case of Retroperitoneal Ganglioneuroma: A Case Report and Review of Literature. Case Rep Surg. 2016;2016:6597374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Crawford CK, Yasrab M, Chu LC, Fishman EK. Retroperitoneal ganglioneuroma simulating lymphoma: An unusual case presentation. Radiol Case Rep. 2025;20:2163-2166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 12. | Quinn R, Ellis-Clark J. Ganglioneuroma: a rare appendiceal tumour - case report and literature review. J Surg Case Rep. 2024;2024:rjae735. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Lameir Hussein M, Alkhateeb SO, Kolleri JJ, Saleem Abu-Dayeh A, Murshed K, Sherif Mahmood N. Mature Adrenal Ganglioneuroma With Lipomatous Content: A Radiological and Histopathological Diagnostic Challenge. Cureus. 2024;16:e75648. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Xiao J, Zhao Z, Li B, Zhang T. Primary Retroperitoneal Ganglioneuroma: A Retrospective Cohort Study of 32 Patients. Front Surg. 2021;8:642451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Ebrahimzadeh K, Mirahmadi Eraghi M, Bidari Zerehpoosh F, Tavasol HH, Abbaszdeh M, Dmytriw AA, Jahanshahi F. An intracranial odyssey: pediatric ganglioneuroma arising from the trigeminal ganglion: a case report and review of the literature. J Med Case Rep. 2024;18:600. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Fueglistaler P, Gurke L, Stierli P, Obeid T, Koella C, Oertli D, Kettelhack C. Major vascular resection and prosthetic replacement for retroperitoneal tumors. World J Surg. 2006;30:1344-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Liu Z, Xiao Y, Chen D, Wang Z. Surgical treatment of pediatric retroperitoneal tumors with invasion of major blood vessels: a single-center experience. Minerva Pediatr. 2016;68:162-166. [PubMed] |
| 18. | Wan Z, Yin T, Chen H, Li D. Surgical treatment of a retroperitoneal benign tumor surrounding important blood vessels by fractionated resection: A case report and review of the literature. Oncol Lett. 2016;11:3259-3264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Abe T, Sazawa A, Harabayashi T, Oishi Y, Miyajima N, Tsuchiya K, Maruyama S, Okada H, Shinohara N. Laparoscopic resection of paraaortic/paracaval neurogenic tumors: surgical outcomes and technical tips. Surg Endosc. 2016;30:4640-4645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Alimoglu O, Caliskan M, Acar A, Hasbahceci M, Canbak T, Bas G. Laparoscopic excision of a retroperitoneal ganglioneuroma. JSLS. 2012;16:668-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Felföldi T, Varga Z, Kolozsi P, Kovács DÁ, Tóth D. Laparoscopic resection of ganglioneuroma from the hepatoduodenal ligament: A case report. Int J Surg Case Rep. 2023;112:108914. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Nagano S, Miyoshi N, Fujino S, Mizusima T, Doki Y, Eguchi H. Laparoscopic resection of a large nonadrenal ganglioneuroma adhered to the aorta and inferior mesenteric artery: A case report and literature review. Int J Surg Case Rep. 2021;78:16-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 23. | Mizukami S, Shonaka T, Takeda T, Takahata H, Shimazaki R, Otani M, Ohara M, Tani C, Hasegawa K, Yokoo H. Comparative Evaluation of Laparoscopic Origami Crane Training With 3D and 2D Laparoscopy: Correlation With Fundamentals of Laparoscopic Surgery Scores. Surg Innov. 2025;32:262-269. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Tercan C, Gunes AC, Bastu E, Blockeel C, Aktoz F. The comparison of 2D and 3D systems in total laparoscopic hysterectomy: a systematic review and meta-analysis. Arch Gynecol Obstet. 2024;310:1811-1821. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Chen H, Jiang K, Li X, Ye Q, Wang J, Zhou S, Wang H, Qiu K. Application and advantage analysis of triangular gastric suspension technique in laparoscopic distal pancreatectomy. Surg Endosc. 2025;39:1372-1378. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Shan LY, Liang BQ, Wang JX, Wei RT, Zhang Y, Li ME, Yang CL, Dai YG. A mobile double-suspension technology for single-port D2 distal gastrectomy: A technical note with video vignette. Asian J Surg. 2024;S1015-9584(24)02725. [PubMed] [DOI] [Full Text] |
| 27. | Chen K, Liu XL, Pan Y, Maher H, Wang XF. Expanding laparoscopic pancreaticoduodenectomy to pancreatic-head and periampullary malignancy: major findings based on systematic review and meta-analysis. BMC Gastroenterol. 2018;18:102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Kasai M, Cipriani F, Gayet B, Aldrighetti L, Ratti F, Sarmiento JM, Scatton O, Kim KH, Dagher I, Topal B, Primrose J, Nomi T, Fuks D, Abu Hilal M. Laparoscopic versus open major hepatectomy: a systematic review and meta-analysis of individual patient data. Surgery. 2018;163:985-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |