Published online Jun 27, 2025. doi: 10.4240/wjgs.v17.i6.107866
Revised: April 19, 2025
Accepted: May 7, 2025
Published online: June 27, 2025
Processing time: 61 Days and 21.7 Hours
Liposarcomas (LPSs) are malignant mesenchymal tumors originating from adipocytes. Myxoid LPS (MLPS), a common subtype, predominantly arises in the extremities, retroperitoneum, and deep soft tissues, with a rare occurrence in the gastrointestinal tract. Primary mesenteric LPS is particularly uncommon, especi
This report describes the case of a 65-year-old female patient who presented with abdominal distension and was diagnosed with a giant mucinous LPS of the transverse colonic mesentery. Upon admission, the patient underwent a comprehensive evaluation. Contrast-enhanced computed tomography (CT) of the chest and abdomen revealed a large malignant tumor with aortic dissection, while colonoscopy identified rectal cancer. Given the patient's condition and surgical risk, an interventional procedure was first performed to manage the aortic coarc
While no standardized treatment exists for MLPS co-occurring with multiple diseases, operation remains the mainstay. However, recurrence, metastasis, and poor postoperative prognosis continue to pose serious threats to patient survival.
Core Tip: To the best of our knowledge, this is the first reported case of a giant mucinous liposarcomas (LPSs) of the transverse colonic mesentery coexisting with rectal cancer and aortic coarctation. This case contributes valuable clinical insight and highlights the unfavorable outcomes of recurrent and metastatic myxoid LPS.
- Citation: Wang M, Sun J, Song ZQ, Chen XQ, Xie GD, Zhu Y, Zhou YK. Giant transverse colonic mesenteric mucinous liposarcoma combined with rectal cancer and aortic coarctation: A case report and review of literature. World J Gastrointest Surg 2025; 17(6): 107866
- URL: https://www.wjgnet.com/1948-9366/full/v17/i6/107866.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i6.107866
Liposarcomas (LPSs) are soft-tissue sarcomas originating from adipose tissue. Their clinical presentation ranges from benign localized lesions to aggressive malignant tumors. LPSs are classified into four subtypes: (1) Hyperdifferentiated; (2) Dedifferentiated; (3) Pleomorphic; and (4) Mucinous-like[1-3]. Myxoid LPS (MLPS) accounts for approximately 5% of all soft-tissue sarcomas and 20%–30% of all LPS, with no sex predilection[4,5]. MLPS predominantly arises in the deep soft tissues of the extremities and retroperitoneum, with a high risk of recurrence even after complete resection. It is less common in the abdomen, accounting for approximately 20% of adult human mesenchymal malignancies[6,7]. It has been reported in the mesentery of the small intestine and transverse colon; however, cases involving the mesentery of the transverse colon are sparsely documented in the literature[8]. This report describes an exceptional case of giant mucinous LPS in the transverse colonic mesentery, combined with rectal cancer and aortic entrapment, presenting primarily as an abdominal mass. Intraoperative findings confirmed the tumor’s origin in the transverse colon mesentery. A literature review of related conditions is also included.
A 65-year-old woman presented to our general surgery department with an unexplained abdominal distension.
The patient experienced significant abdominal distension and fatigue but reported no abdominal pain, diarrhea, nausea, vomiting, or difficulty with defecation. There was noticeable weight loss, and the gas output was reduced. These symptoms persisted for over a month without any treatment.
The patient had a history of hypertension.
There was no family history of malignancy, psychological disorders, or genetic disorders.
Vital signs were stable. Physical examination revealed a distended abdomen with a large, ill-defined, smooth, and minimally mobile mass on palpation. Abdominal percussion elicited a drum sound.
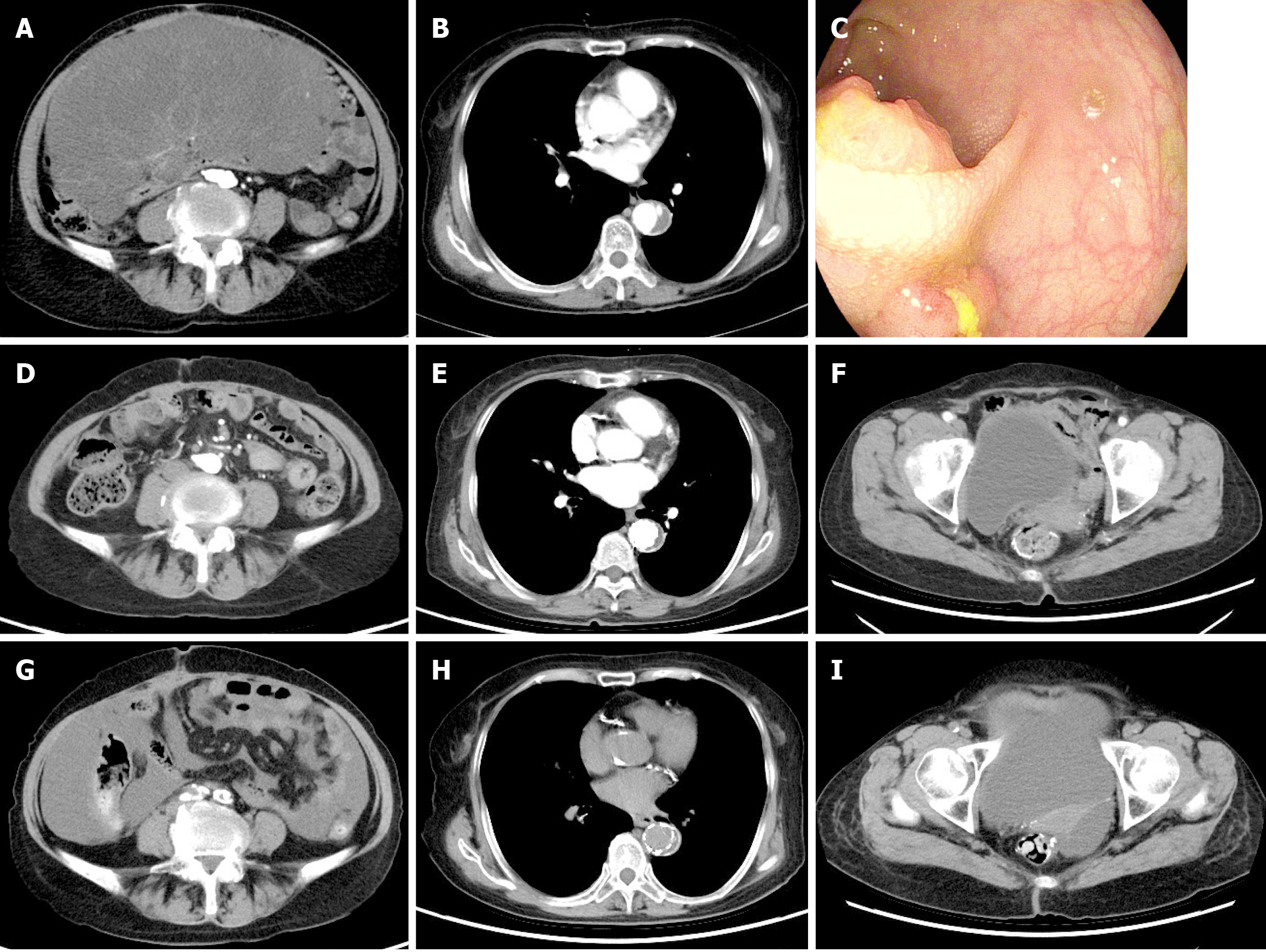
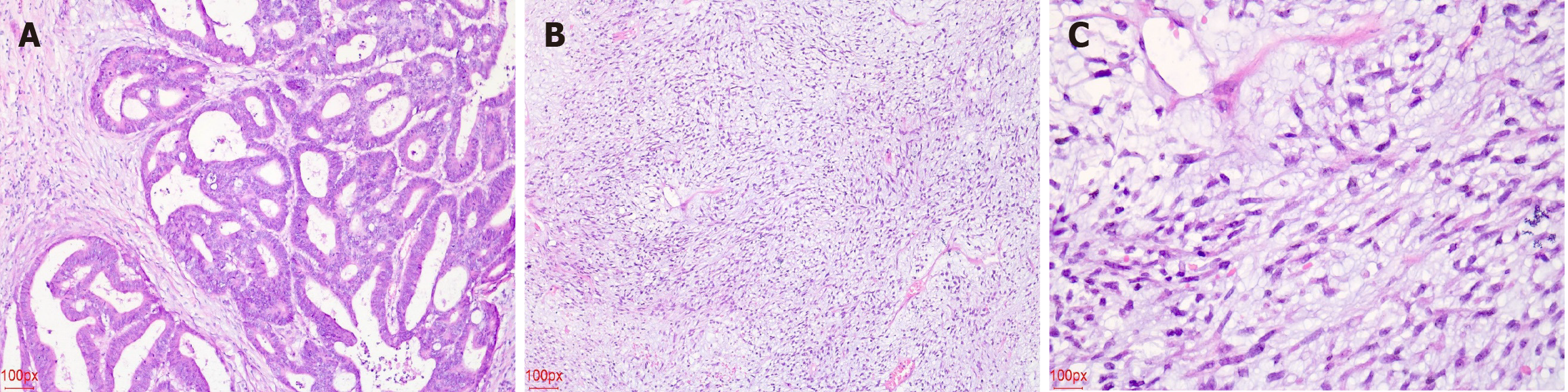
An abdominal ultrasound conducted at a local hospital indicated a large solid mass in the abdominal cavity, prompting further examination. Upon admission, the patient underwent contrast-enhanced computed tomography (CT) of the chest and abdomen, revealing a large, inhomogeneous hypoechoic mass with aortic coarctation (Figure 1). An electronic gastroenteroscopy was performed to determine the tumor’s origin. The first attempt failed due to the patient’s abdominal distension and poor physical condition. However, after repeated persuasion, a second attempt was successful, revealing a small elevated lesion 10 cm from the anal verge. A biopsy confirmed rectal cancer (Figure 2). The tumor was small and not detectable on CT. Other examination results indicated multiple comorbidities and poor physical condition.
The CT imaging could not determine the tumor’s nature, necessitating further clinical evaluation. The patient was diagnosed with a large abdominal tumor, rectal cancer, and aortic dissection (AD).
Cases involving abdominal tumors combined with aortic coarctation are rare in the literature and clinical practice. Given the patient's advanced age and multiple comorbidities, particularly aortic coarctation, which poses a high risk of vascular rupture, a multidisciplinary team opted to address the aortic condition primarily.
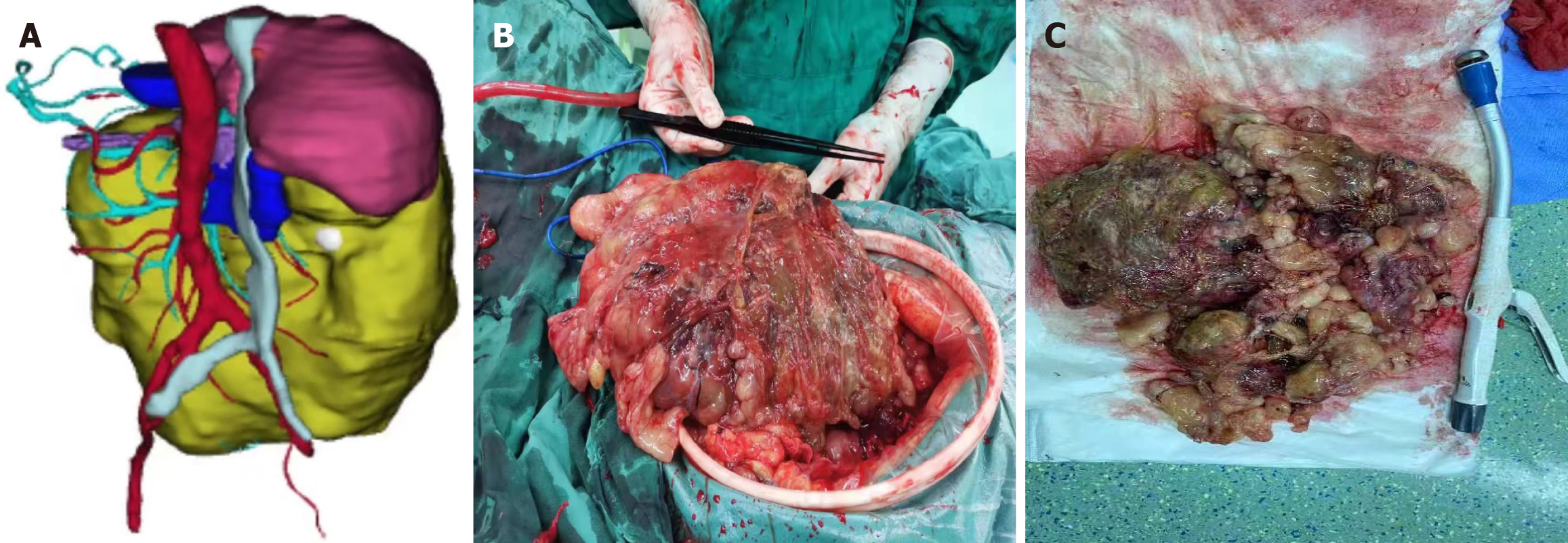
According to the literature, AD involves tearing the aortic intima, allowing blood to enter the aortic media, and separating the true and false lumens, accounting for approximately 80%–90% of acute aortic syndromes[9]. Based on the Debakey and Stanford classifications, as well as the expanded “non-A and non-B” categorization and time-based staging systems[10,11], this patient was diagnosed with acute, uncomplicated Stanford type B AD. In such cases, optimal medical therapy (OMT), primarily focused on blood pressure control, is the conventional initial treatment. OMT helps reduce the incidences of aneurysmal degeneration, early morbidity, and mortality[12]. However, long-term outcomes reveal a high complication rate, with 20%–40% of patients developing aneurysmal changes and up to 45% eventually requiring surgical intervention[13]. Recent studies support thoracic endovascular aortic repair as superior to medical therapy alone, offering improved aortic wall remodeling and reducing adverse event risks[14]. Considering the patient's condition, surgical risks, and recovery capacity, thoracic aortic stent placement was successfully performed in the interventional suite, followed by abdominal surgery after stabilization (Figure 3).
Abdominal surgery was performed in the operating room. Intraoperatively, a large abdominal mass, measuring approximately 25 cm × 20 cm, was identified, with an irregular shape, incompletely encapsulated by peritoneum, a brittle and friable texture, and coated with a mucoid substance (Figure 3). Further exploration revealed that the tumor originated from the mesentery of the transverse colon. No evidence of distant metastasis was observed in the abdominal cavity or peritoneum. Complete resection of the tumor and the involved colon segment was achieved, followed by anastomosis. The rectal tumor, located above the peritoneal reflection, was small and had not infiltrated the serosa. Segmental resection of the rectum and sigmoid colon was performed to ensure complete removal, followed by anastomosis. Gross examination revealed a 40 cm × 20 cm × 10 cm grayish-yellow mucoid mass on the cross-section (Figure 3). Postoperatively, both resected specimens were sent for pathological analysis, and histological examination by experienced pathologists confirmed a diagnosis of MLPS (Figure 2). The rectal tumor infiltrated the superficial muscularis propria without involving the deeper muscularis layer with negative margins, and no metastasis of the regional lymph nodes was identified (Figure 2). Based on to the tumor-node-metastasis staging criteria, the patient was diagnosed with stage I (pT2N0M0) rectal cancer.
The patient was discharged from the hospital 24 days postoperatively without any immediate complications. Based on oncology consultation, a FOLFOX chemotherapy regimen (consisting of 5-fluorouracil, oxaliplatin, and leucovorin) was initiated, alongside regular follow-up CT scans. However, abdominal CT and positron emission tomography-CT scans conducted 6 months postoperatively revealed multiple irregular low-density areas around the right margin of the liver and the right paracolic region, indicating a recurrence of MLPS. Given disease progression and with the consent of the patient's family, the chemotherapeutic regimen was changed to paclitaxel combined with platinum. However, the patient did not comply with the treatment. One year postoperatively, the tumor gradually enlarged, compressing the intestinal canal and narrowing the lumen, ultimately resulting in a mechanical intestinal obstruction that posed a severe life-threatening risk. A second cesarean section was urgently performed to relieve the obstruction. Due to the presence of multiple tumors, widespread metastasis, and tight adhesion to the abdominal wall, intestines, and surrounding tissues, radical resection was not possible. A substantial portion of the tumor was removed, successfully alleviating the intestinal obstruction. A postoperative CT scan showed a slight reduction in tumor volume compared to the previous scan (Figure 1). The patient survived for four months after the second surgery, with an overall survival of 19 months from the diagnosis.
LPS, a malignant tumor derived from adipocytes, includes MLPS as a common subtype. The MLPS is typically found in deep soft tissues of the extremities or retroperitoneum, with rare occurrences in the abdomen[15]. Clinical presentation is usually atypical, and tumors are frequently large at diagnosis[16]. Imaging tests such as CT or magnetic resonance imaging are essential for prompt diagnosis and treatment. Mucinous and non-mucinous tumors can be differentiated through puncture biopsy after tumor confirmation[17]. Moreover, DNA Damage Inducible Transcript 3 immunohistochemistry effectively distinguishes MLPS from other LPS subtypes[18].
Surgical resection is the primary treatment for MLPS. Limb-preserving resection is standard for tumors in the limbs and trunk, depending on the tumor’s location and size. There are no specific surgical guidelines for abdominal MLPS; this approach mirrors that for LPS, involving resection of extensive tissues with ≥ 10 mm margins of adjacent negative tissue[19]. A recent literature review indicates that this approach is applied to all primary mesenteric MLPS (Table 1)[20-38]. Due to the high recurrence rates of MLPS, adjuvant treatments such as postoperative radiotherapy and chemotherapy are used. MLPS is more sensitive to radiotherapy, with preoperative moderate-dose radiotherapy improving resection rates and postoperative radiotherapy reducing recurrence risk[39,40]. Chemotherapy’s effectiveness in MLPS, particularly in advanced or metastatic cases, is limited. Anthracycline-based regimens, such as “adriamycin + isocyclophosphamide”, are commonly used, though results are frequently unsatisfactory[41]. Recently, second-line chemotherapeutic agents such as trabectedin, pazopanib, and eribulin have shown some efficacy[42]. Trabectedin mechanistically inhibits the transcription of the oncogenic fusion protein fused in sarcoma-DNA damage-inducible transcript and is more radiosensitive, making it an effective combination with radiation for treating MLPS[43,44]. Moreover, the potential of immunotherapy and targeted therapies in MLPS treatment has garnered considerable attention. For advanced patients with unresectable tumors, immunotherapies, such as natural killer cell therapy or tumor microenvironment modulation, could provide promising new treatment options[45]. Following MLPS resection, circulating tumor DNA can be a non-invasive and cost-effective method for monitoring tumor activity, enabling the early detection and treatment of recurrence[46]. However, these approaches remain in the research phase and require further validation for clinical application.
| Ref. | Age/sex | Size (cm) | Wide excision | Pathological type | Location | Adjuvant therapy | Local recurrence/metastases | Follow-up time (months) |
| Rowe et al[20] | 76/F | 15 × 9 × 15 | Yes | D | Large intestine mesentery | No | No | 6 |
| Ahire et al[21] | 42/M | 20 × 15 | Yes | D | Jejunum mesentery | Chemotherapy | No | 6 |
| Mokfi et al[22] | 69/F | 29 × 25 × 12 | Yes | Myx | Small bowel mesentery | No | No | 6 |
| Park et al[23] | 47/M | 25.5 × 19.0 × 12.5 | Yes | D | Ascending colon mesentery | Chemotherapy | No | 21 |
| Dhakal et al[24] | 56/M | 12 × 10 × 7 | Yes | D | Small bowel mesentery | No | N/A | N/A |
| Poillucci et al[25] | 43/M | 12 × 12 × 9 | Yes | W | Small bowel mesentery | No | No | N/A |
| 60/M | Diameter 9, 10 and 7.5 | Yes | W | Small bowel mesentery | No | No | N/A | |
| Kuroda et al[26] | 67/F | 11 × 10 × 9 | Yes | D | Transverse colon mesentery | Chemotherapy | Dead (18 months) | 18 |
| Khanduri et al[27] | 55/M | 6 × 7 × 8 | Yes | D | Jejunum mesentery | Chemotherapy | No | 2 |
| Mori et al[28] | 71/M | Diameter is 3.10 | Yes | D | Small bowel mesentery | Chemotherapy | Recurrence (17days) | 6 |
| Innes et al[29] | 65/M | 8 × 7.5 × 3 | Yes | D | Cecum mesentery | No | Recurrence (2.5 months), dead (3 months) | 3 |
| Kim et al[30] | 60/M | 25 × 20 | Yes | Myx | Small bowel mesentery | Chemotherapy | No | 6 |
| Paasch et al[31] | 35/F | 14.6 × 10.1 × 12.4 | Yes | Myx | Small bowel mesentery | Chemotherapy | Dead (years) | 3 (years) |
| Zhang et al[32] | 65/M | 6 × 3 × 3 | Yes | D | Cecum mesentery | No | No | 6 |
| Jung et al[33] | 62/M | N/A | Yes | W | Small bowel mesentery | Chemotherapy | No | 2 (years) |
| Rosato et al[34] | 55/M | 5 × 5 × 6 | Yes | W | Small bowel mesentery | No | No | 144 |
| Matsuo et al[35] | 70/M | N/A | Yes | D | Small bowel mesentery | No | No | 5 (years) |
| Vats et al[36] | 36/F | 25 × 20 × 15 | Yes | D | Jejunum mesentery | Chemotherapy | No | 6 |
| Choi et al[37] | 73/M | 28 × 26 × 12 | Yes | Mix-type liposarcoma | Small bowel mesentery | No | No | 33 |
| Shen et al[38] | 49/F | 26 × 18 × 6 | Yes | Myx | Sigmoid mesentery | No | No | 17 |
This case highlights the rarity of primary mesenteric MPLS, primarily when originating from the transverse colonic mesentery, with few cases reported in the literature. Furthermore, aortic coarctation adds complexity and risk to the condition. The small size of rectal tumors, frequently undetectable on CT scans, was incidentally discovered during electronic colonoscopy, enabling timely diagnosis and treatment. Given the patient's advanced age and frailty, delayed diagnosis of rectal cancer could have necessitated a second surgery, significantly elevating the risk of complications. Therefore, we promptly adjusted the postoperative surveillance strategy to monitor both primary tumors. Although the initial surgery was successful, the tumor recurred rapidly, leading to malignant intestinal obstruction—the ultimate cause of death. Currently, clinical data and basic research on primary mesenteric MLPS remain scarce. More case reports and mechanistic studies are urgently needed to optimize individualized treatment strategies.
The diagnosis of primary mesenteric MLPS is frequently delayed, due to nonspecific clinical presentations, and surgical resection remains the only potentially curative treatment option. Comprehensive ancillary investigations and timely operation are essential for detecting surgically resectable lesions and improving prognosis. Comprehensive imaging and electronic gastroenteroscopy are recommended for all patients with abdominal masses to minimize the risk of missed diagnoses and maximize the chances of long-term disease-free survival. Future research should focus on optimizing comprehensive treatment strategies, implementing a multidisciplinary approach, and advancing early diagnosis and individualized treatment to improve clinical outcomes and prolong survival. There is a pressing need to refine clinical guidelines.
We sincerely appreciate the patients’ families for their cooperation with information acquisition, treatment, and follow-up.
| 1. | Doyle LA. Sarcoma classification: an update based on the 2013 World Health Organization Classification of Tumors of Soft Tissue and Bone. Cancer. 2014;120:1763-1774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 282] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 2. | Jundt G. [Updates to the WHO classification of bone tumours]. Pathologe. 2018;39:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Nili F, Baghai F, Aghai A, Etebarian A. Well-differentiated liposarcoma of the floor of the mouth: Report of a rare case and review of the literature. J Oral Maxillofac Pathol. 2016;20:312-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Kallen ME, Hornick JL. The 2020 WHO Classification: What's New in Soft Tissue Tumor Pathology? Am J Surg Pathol. 2021;45:e1-e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 214] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 5. | Lee ATJ, Thway K, Huang PH, Jones RL. Clinical and Molecular Spectrum of Liposarcoma. J Clin Oncol. 2018;36:151-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 240] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 6. | Orvieto E, Furlanetto A, Laurino L, Dei Tos AP. Myxoid and round cell liposarcoma: a spectrum of myxoid adipocytic neoplasia. Semin Diagn Pathol. 2001;18:267-273. [PubMed] |
| 7. | Karadayi K, Yildiz C, Karakus S, Kurt A, Bozkurt B, Soylu S, Cicekli AA, Egilmez R, Cetin A. Well-differentiated abdominal liposarcoma: experience of a tertiary care center. World J Surg Oncol. 2015;13:166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Sachidananda S, Krishnan A, Ramesh R, Kuppurao S. Primary multiple mesenteric liposarcoma of the transverse mesocolon. Ann Coloproctol. 2013;29:123-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Bossone E, Eagle KA. Epidemiology and management of aortic disease: aortic aneurysms and acute aortic syndromes. Nat Rev Cardiol. 2021;18:331-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 251] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 10. | Czerny M, Schmidli J, Adler S, van den Berg JC, Bertoglio L, Carrel T, Chiesa R, Clough RE, Eberle B, Etz C, Grabenwöger M, Haulon S, Jakob H, Kari FA, Mestres CA, Pacini D, Resch T, Rylski B, Schoenhoff F, Shrestha M, von Tengg-Kobligk H, Tsagakis K, Wyss TR, Document Reviewers, Chakfe N, Debus S, de Borst GJ, Di Bartolomeo R, Lindholt JS, Ma WG, Suwalski P, Vermassen F, Wahba A, Wyler von Ballmoos MC. Editor's Choice - Current Options and Recommendations for the Treatment of Thoracic Aortic Pathologies Involving the Aortic Arch: An Expert Consensus Document of the European Association for Cardio-Thoracic Surgery (EACTS) & the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2019;57:165-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 247] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 11. | Lombardi JV, Hughes GC, Appoo JJ, Bavaria JE, Beck AW, Cambria RP, Charlton-Ouw K, Eslami MH, Kim KM, Leshnower BG, Maldonado T, Reece TB, Wang GJ. Society for Vascular Surgery (SVS) and Society of Thoracic Surgeons (STS) reporting standards for type B aortic dissections. J Vasc Surg. 2020;71:723-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 406] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 12. | Sorber R, Hicks CW. Diagnosis and Management of Acute Aortic Syndromes: Dissection, Penetrating Aortic Ulcer, and Intramural Hematoma. Curr Cardiol Rep. 2022;24:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 13. | Arnaoutakis DJ, Khan TA, Scali ST, Neal D, Giles KA, Cooper MA, Beaver TM, Huber TS, Upchurch GR Jr, Arnaoutakis GJ, Back MR. Remodeling, Reintervention, and Survival After Endovascular Repair of Chronic Type B Dissection. Ann Thorac Surg. 2021;111:1560-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Lou X, Chen EP, Duwayri YM, Jordan WD, Keeling WB, Leshnower BG. Early Results of Thoracic Endovascular Aortic Repair for the Management of Acute Uncomplicated Type B Aortic Dissection. Semin Thorac Cardiovasc Surg. 2023;35:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Abaricia S, Hirbe AC. Diagnosis and Treatment of Myxoid Liposarcomas: Histology Matters. Curr Treat Options Oncol. 2018;19:64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Hoffman A, Ghadimi MP, Demicco EG, Creighton CJ, Torres K, Colombo C, Peng T, Lusby K, Ingram D, Hornick JL, Wang WL, Ravi V, Lazar AJ, Lev D, Pollock RE. Localized and metastatic myxoid/round cell liposarcoma: clinical and molecular observations. Cancer. 2013;119:1868-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Rohela H, Lee C, Yoo HJ, Kim HS, Kim Y, Cho HS, Han I. Comparison of the diagnostic performances of core needle biopsy in myxoid versus non-myxoid tumors. Eur J Surg Oncol. 2019;45:1293-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Scapa JV, Cloutier JM, Raghavan SS, Peters-Schulze G, Varma S, Charville GW. DDIT3 Immunohistochemistry Is a Useful Tool for the Diagnosis of Myxoid Liposarcoma. Am J Surg Pathol. 2021;45:230-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 19. | Gamboa AC, Gronchi A, Cardona K. Soft-tissue sarcoma in adults: An update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J Clin. 2020;70:200-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 350] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 20. | Rowe DA, Bowers WB, Mateja HL, Morgan A, Thomas D. Silent Giant: A Case of Dedifferentiated Retroperitoneal Liposarcoma Presenting as an Incidental Mesenteric Mass. Cureus. 2024;16:e72549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Ahire P, Myrthong AL, Mahankudo S, Tayade MB, Boricha S. A Rare Case of Primary Mesenteric Liposarcoma. Cureus. 2023;15:e38329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 22. | Mokfi R, Boutaggount F, Maskrout M, Rais G. Giant mesenteric myxoid liposarcoma: Challenges of diagnosis and treatment. Radiol Case Rep. 2022;17:4227-4231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 23. | Park N, Kuk JC, Shin EJ, Lim DR. Surgery of intraabdominal giant dedifferentiated liposarcoma of ascending colon mesentery: A rare case report. Int J Surg Case Rep. 2022;98:107482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | Dhakal S, Prajapati I. Dedifferentiated liposarcoma of small bowel mesentery: a rare case report. J Surg Case Rep. 2022;2022:rjab599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Poillucci G, Podda M, Pisanu A, Gomes CA, Gallo G, Di Saverio S, De Angelis R. Well-differentiated mesenteric liposarcoma: report of two cases. Acta Biomed. 2022;92:e2022121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Kuroda M, Yamada R, Tanaka T, Tsuboi J, Nakamura M, Katsurahara M, Hamada Y, Tanaka K, Horiki N, Nakagawa H. Dedifferentiated liposarcoma in the abdominal cavity: a case report. Clin J Gastroenterol. 2022;15:1094-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | Khanduri A, Bansal N, Singh A, Gupta J, Gupta R. Multifocal Dedifferentiated Liposarcoma of the Jejunal Mesentery. Cureus. 2021;13:e19780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Mori R, Ogino T, Fujino S, Takahashi H, Miyoshi N, Uemura M, Satoh T, Mizushima T, Doki Y, Eguchi H. An oncologic emergency case of massive dedifferentiated liposarcoma of the small bowel mesentery. Clin J Gastroenterol. 2021;14:759-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Innes R, Paulasir S, Combs L. An Aggressive Liposarcoma Presenting as a Perforated Colon Mass. Cureus. 2021;13:e13808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Kim DW, Jee YS. Solitary metastasis of myxoid liposarcoma from the thigh to intraperitoneum: a case report. World J Surg Oncol. 2019;17:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Paasch C, Isbruch A, Strik MW, Siegel R. Malignant intestinal obstruction during twin pregnancy: surgical resection of a myxoid liposarcoma without induction of labour. BMJ Case Rep. 2019;12:e229742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Zhang D, Williams WD, Lai J. An Unusual Case of Cecal Mesenteric Dedifferentiated Liposarcoma Involving the Ileocolic Artery Resected by Right Hemicolectomy. Anticancer Res. 2019;39:487-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Jung EE, Heinemann FS, Egelston CA, Wang J, Pollock RE, Lee PP, Tseng WW. Synchronous recurrence of concurrent colon adenocarcinoma and dedifferentiated liposarcoma. BMJ Case Rep. 2019;12:e228868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Rosato L, Panier Suffat L, Bertotti L, Perino P, Comello E, Mondini G. Retroperitoneal or mesenteric primary liposarcoma: clinical and prognostic evaluations on five cases. G Chir. 2018;39:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Matsuo K, Inoue M, Shirai Y, Kataoka T, Kagota S, Taniguchi K, Lee SW, Uchiyama K. Primary small bowel mesentery de-differentiated liposarcoma causing torsion with no recurrence for 5 years: A case report and review of the literature. Medicine (Baltimore). 2018;97:e13446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Vats M, Pandey D, Ahlawat H, Akhtar A, Singh N. Multiple Primary Dedifferentiated Liposarcoma of the Jejunal Mesentery: A Case Report and Review of Literature. J Clin Diagn Res. 2016;10:XD01-XD04. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Choi JY, Kim JE, Lee SM, Kang HJ, Sung JH, Koh BS, Park JS, Kim ID, Baik SY. [A case of pleomorphic liposarcoma originating from mesentery]. Korean J Gastroenterol. 2015;65:182-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Shen Z, Wang S, Fu L, Shi J, Yin M, Ye Y, Wang S. Therapeutic experience with primary liposarcoma from the sigmoid mesocolon accompanied with well-differentiated liposarcomas in the pelvis. Surg Today. 2014;44:1863-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Lansu J, Braam PM, van Werkhoven E, Scholten AN, Schrage Y, van Houdt WJ, van Langevelde K, Haas RL. A moderate dose of preoperative radiotherapy may improve resectability in myxoid liposarcoma. Eur J Surg Oncol. 2021;47:2633-2639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Chung PW, Deheshi BM, Ferguson PC, Wunder JS, Griffin AM, Catton CN, Bell RS, White LM, Kandel RA, O'Sullivan B. Radiosensitivity translates into excellent local control in extremity myxoid liposarcoma: a comparison with other soft tissue sarcomas. Cancer. 2009;115:3254-3261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 41. | Gronchi A, Ferrari S, Quagliuolo V, Broto JM, Pousa AL, Grignani G, Basso U, Blay JY, Tendero O, Beveridge RD, Ferraresi V, Lugowska I, Merlo DF, Fontana V, Marchesi E, Donati DM, Palassini E, Palmerini E, De Sanctis R, Morosi C, Stacchiotti S, Bagué S, Coindre JM, Dei Tos AP, Picci P, Bruzzi P, Casali PG. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017;18:812-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 344] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 42. | von Mehren M, Kane JM, Agulnik M, Bui MM, Carr-Ascher J, Choy E, Connelly M, Dry S, Ganjoo KN, Gonzalez RJ, Holder A, Homsi J, Keedy V, Kelly CM, Kim E, Liebner D, McCarter M, McGarry SV, Mesko NW, Meyer C, Pappo AS, Parkes AM, Petersen IA, Pollack SM, Poppe M, Riedel RF, Schuetze S, Shabason J, Sicklick JK, Spraker MB, Zimel M, Hang LE, Sundar H, Bergman MA. Soft Tissue Sarcoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:815-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 194] [Reference Citation Analysis (0)] |
| 43. | Di Giandomenico S, Frapolli R, Bello E, Uboldi S, Licandro SA, Marchini S, Beltrame L, Brich S, Mauro V, Tamborini E, Pilotti S, Casali PG, Grosso F, Sanfilippo R, Gronchi A, Mantovani R, Gatta R, Galmarini CM, Sousa-Faro JM, D'Incalci M. Mode of action of trabectedin in myxoid liposarcomas. Oncogene. 2014;33:5201-5210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 44. | Simoens C, Korst AE, De Pooter CM, Lambrechts HA, Pattyn GG, Faircloth GT, Lardon F, Vermorken JB. In vitro interaction between ecteinascidin 743 (ET-743) and radiation, in relation to its cell cycle effects. Br J Cancer. 2003;89:2305-2311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Minetto P, Guolo F, Pesce S, Greppi M, Obino V, Ferretti E, Sivori S, Genova C, Lemoli RM, Marcenaro E. Harnessing NK Cells for Cancer Treatment. Front Immunol. 2019;10:2836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 46. | Braig D, Becherer C, Bickert C, Braig M, Claus R, Eisenhardt AE, Heinz J, Scholber J, Herget GW, Bronsert P, Fricke A, Follo M, Stark GB, Bannasch H, Eisenhardt SU. Genotyping of circulating cell-free DNA enables noninvasive tumor detection in myxoid liposarcomas. Int J Cancer. 2019;145:1148-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |