Published online Jun 27, 2025. doi: 10.4240/wjgs.v17.i6.106069
Revised: March 17, 2025
Accepted: April 28, 2025
Published online: June 27, 2025
Processing time: 104 Days and 23.2 Hours
Gastric subepithelial lesions (SELs) are elevated lesions originating from the muscularis mucosa, submucosa, or muscularis propria, and may also include extraluminal lesions. For small SELs (less than 5 cm), complete endoscopic exci
To evaluate the efficacy of the interrupted closure technique compared to the traditional closure technique in EFTR for gastric SELs.
This single-center, prospective, randomized controlled trial was conducted at a tertiary hospital from September 2023 to September 2024. A total of 90 patients who underwent EFTR for gastric SELs were randomly allocated to either the in
All patients had complete resection and wound closure without any severe postoperative complications. The incidence of intraoperative gas-related complications was significantly lower in the interrupted closure group than in the traditional closure group (2.27% vs 26.09%, P = 0.001), demonstrating interrupted closure technique can reduce the incidence of gas-related issues. Statistical analysis revealed that the incidence of postoperative infection was significantly lower in the experimental group than in the control group (15.91% vs 41.30%, P = 0.008). Ad
The interrupted closure technique in EFTR for treating gastric SELs is safe and effective, reducing the incidence of intraoperative gas complications and postoperative infections.
Core Tip: The interrupted closure technique involves performing a two-thirds circumferential full-thickness incision around the diseased gastric wall, followed by the immediate closure of either the proximal or distal end of the defect using metallic clips. With minimal necessary exposure, lesion dissection and defect closure are performed alternately until complete tumor resection and wound closure are achieved. Our study findings demonstrate that this technique is an effective approach for the treatment of gastric subepithelial lesions, significantly reducing the incidence of intraoperative gas-related complications and postoperative infections compared to traditional endoscopic full-thickness resection.
- Citation: Zhang M, Liu J, Dong YP, Zhao Q, Lin ML, Gao TJ, Feng JL, Wang YF, Guo YF, Wang Z, Jia W, Yang Z. Comparison between interrupted closure technique and traditional closure technique in endoscopic full-thickness resection for treating gastric subepithelial lesions. World J Gastrointest Surg 2025; 17(6): 106069
- URL: https://www.wjgnet.com/1948-9366/full/v17/i6/106069.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i6.106069
Gastric subepithelial lesions (SELs) are elevated lesions originating from the muscularis mucosa, submucosa, or muscularis propria, and may also include extraluminal lesions[1,2]. The prevalence of SELs in patients undergoing gastroscopy is approximately 0.3% to 0.76%[3,4]. Common types of SELs including gastrointestinal stromal tumors, leiomyomas, ectopic pancreas, and lipomas. Although most SELs are benign, up to 13% may have malignant potential[5]. For SELs with malignant potential, such as gastrointestinal stromal tumors, guidelines recommend resection for lesions with a diameter of 2 cm or greater[6-8]. Benign lesions, should also be treated if they cause symptoms, or if the patient expresses a strong desire for treatment.
Complete endoscopic excision is the preferred treatment for small SELs (less than 5 cm)[9,10]. Endoscopic submucosal dissection (ESD), a common resection method for gastrointestinal lesions, enables complete resection of mucosal and submucosal lesions. However, SELs often originate from the muscularis propria layer, the perforation rate associated with ESD is significantly elevated (approximately 4%-13%)[11-13], which somewhat limits its applicability for SELs. Endoscopic full-thickness resection (EFTR) extends ESD by resecting the lesion along with the involved gastrointestinal wall, which has gradually emerged as a treatment option for refractory mucosal and submucosal lesions[14-16]. However, EFTR may lead to iatrogenic perforation, with the passage of gas and gastric contents into the peritoneal cavity potentially causing complications[17]. Consequently, strategies to reduce gas and fluid exudation, successfully close the wound, and minimize complications are critical considerations in EFTR.
Traditional EFTR techniques facilitate the complete excision of lesions; however, they can result in substantial gastric wall defects, allowing significant intragastric gas and fluid to enter the intraperitoneal space, making it difficult to ensure a good surgical field and increasing the risk of postoperative infection. To optimize the traditional EFTR, a novel technique, EFTR interrupted closure technique was developed, in this technique, after performing a full-thickness incision around the lesion's edge, proximal or distal of the wound is immediately closed with metallic clips, followed by alternating dissection of the lesion and wound closure. Retrospective studies[18,19] have demonstrated the safety and clinical utility of the EFTR interrupted closure technique, based on this, we conducted a prospective study to compare the efficacy of the EFTR interrupted closure technique with the traditional closure technique in the treatment of gastric SELs.
This single-center, prospective, randomized controlled trial was designed to assess the efficacy of interrupted closure vs traditional closure in EFTR for gastric SELs. The study population consisted of patients who underwent EFTR for gastric SELs in the Department of Endoscopy at Northern Theater General Hospital from September 2023 to September 2024. The study was approved by the ethics committee and registered on ClinicalTrials.gov (ChiCTR2400086500). Informed consent was obtained from all patients.
Inclusion criteria: (1) Age between 18 and 80 years; (2) SEL confirmed by endoscopic ultrasound; (3) No definitive metastasis detected on imaging; and (4) Maximum transverse diameter of the lesion ≤ 5.0 cm.
Exclusion criteria: (1) Submucosal lesion surface ulceration; (2) Multiple EFTR treatments were performed this time; (3) Allergy to lidocaine mucilage, pronase, and simethicone; (4) Pregnancy or lactation; (5) Known bleeding and coagulation disorders or use of anticoagulants or antiplatelet drugs cannot be discontinued; (6) Patients with other systemic malignancies; (7) Patients with psychoneurotic diseases, severe cardiopulmonary diseases, liver and kidney diseases, immune and blood system diseases, and electrolyte imbalance; and (8) American Society of Anesthesiologists classification ≥ IV before surgery.
Standard single-accessory-channel endoscopy (GIF-Q260J, GIF-H290T, Olympus) equipped with a transparent cap (D-201-11804; Olympus) and a high-frequency generator (VIO 200D; ERBE) and CO2 insufflator were used in these procedures. Other equipment included MFK knife [AMH-EK-O-2.4X1800(4)-N, Anrei], injection needle (NM-4 L-1, Olympus), hot biopsy forceps (FD-410 LR; Olympus), metal hemostatic clip (ROCC-D-26-195, Micro-Tech; AG-51044-1950-090-16, AGS MEDTECH), and nylon rope coil (Loop-30,loop-40; LeCamp™Endoloops).
Experienced endoscopists, each having performed at least 100 EFTR procedures, conducted all operations. Selective submucosal injection of succinylated gelatin containing methylene blue was administered, and an MFK knife was employed to incise the diseased mucosal layer for adequate tumor exposure, the serosal layer of the diseased gastric wall was incised for "artificial perforation", traction devices were selectively utilized during surgery.
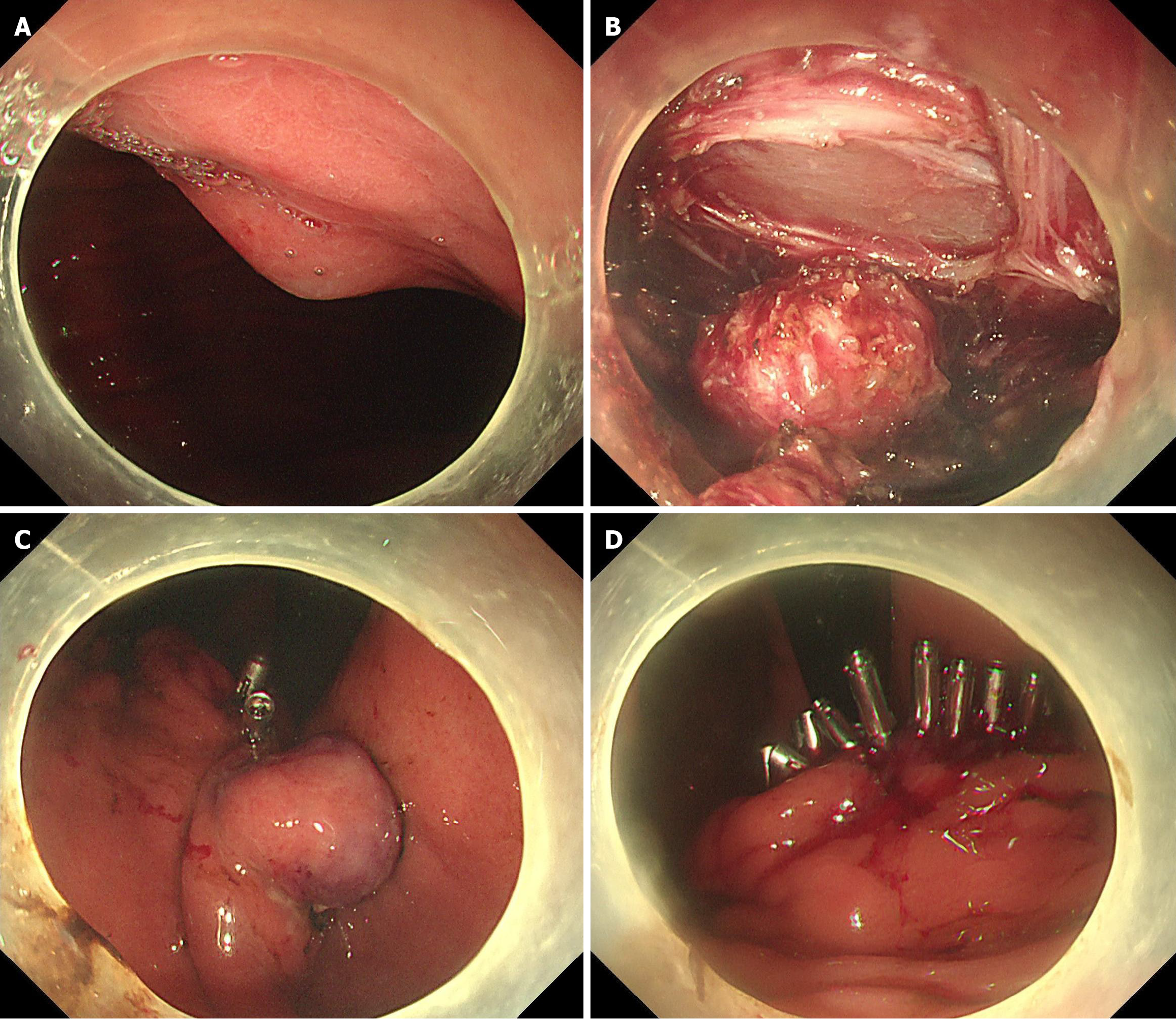
Experimental group (interrupted closure group): After performing a two-thirds circumferential full-thickness incision of the diseased gastric wall, the metal clips were performed at the proximal or distal to the wound. With minimal necessary exposure, the dissection and closure procedure were repeated until the tumor completely resected, then the defect was closed (Figure 1).
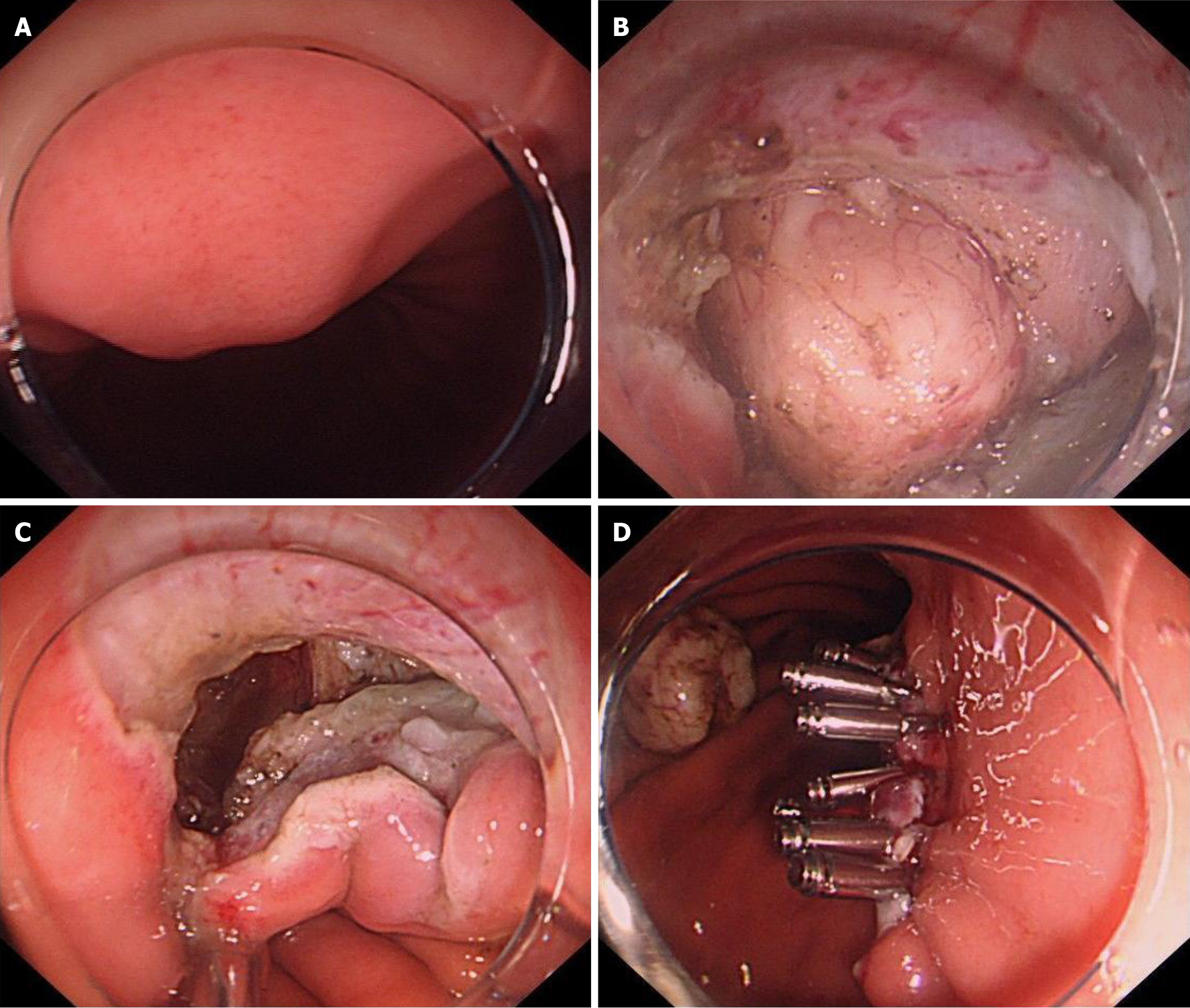
Control group (traditional closure group): Following the complete resection of the lesion, the wound was closed. Smaller wounds were closed with metal clips, larger wounds were managed using purse-string closures (Figure 2).
In cases of severe pneumoperitoneum during surgery, a syringe was used for puncture at McBurney's point. A nasogastric tube was routinely placed in the stomach.
Patients were closely monitored postoperatively, and abdominal computed tomography and endoscopic examination were performed promptly for patients suspected of having postoperative perforation or bleeding. Proton pump inhibitors were routinely administered postoperatively, while antibiotics were given only if infection appeared. Patients fasted for 3 to 5 days based on their condition and were started on a liquid diet after nasogastric tube removal. Discharge was allowed once they tolerated oral intake without abdominal pain, hematemesis, or other symptoms.
Three follow-up visits were scheduled for this study: on postoperative days 1, 4, and 28, to assess the occurrence of postoperative complications. On postoperative days 1 and 4, the physician evaluated EFTR-related complications through physical examination. On postoperative day 28, delayed complications were identified either through telephone follow-up or by consulting medical records.
Primary Outcome Measures: Incidence of postoperative complications and success rate of wound closure.
Secondary Outcome Measures: Incidence of intraoperative gas-related complications, incidence of intraoperative bleeding, complete resection rate, operation time, hospital stay, and associated costs.
Gas complications: The patient developed subcutaneous emphysema (manifested as emphysema on the face, neck, chest wall, and scrotum), pneumomediastinum (gastroscopy showed epiglottic swelling), severe pneumothorax and pneumoperitoneum. In this study, the occurrence of severe pneumoperitoneum requiring puncture and deflation via McBurney's point during surgery was included in the statistical analysis[1].
Intraoperative bleeding: Defined as significant active bleeding during surgery that is difficult to control endoscopically, requiring interruption of the procedure and/or blood transfusion. Minor bleeding controlled by endoscopic was not included in the assessment of intraoperative bleeding.
Severe postoperative complications: Delayed postoperative bleeding and delayed perforation.
Minor postoperative complications: Postoperative abdominal pain, fever, and infection.
Postoperative infection: Defined as the occurrence of fever (temperature > 37.3 °C) several hours to 3 days after EFTR, accompanied by localized abdominal pain [numerical rating scale (NRS) ≥ 3] and elevated inflammatory markers, with imaging excluding the possibility of obvious perforation.
Zero to ten NRS: Also known as a numerical analog scale, it allows patients to describe pain intensity using numbers from 0 to 10, with 0 representing no pain and 10 representing severe pain. NRS ≥ 3 is defined as abdominal pain.
After screening patients based on the inclusion and exclusion criteria, subjects were numbered using Microsoft Excel according to their order of enrollment, and randomized numbers were assigned. They were then allocated randomly to either the experimental or control group.
Previous literature suggests that the incidence rate of minor complications after conventional EFTR was 56.1%[20], 26.3% with interrupted closure technique[21], and 1:1 in the experimental group and the control group. A one-sided test assuming 5% significance level (α = 0.05) and 80% power (β = 0.2) was used. The required sample size was calculated as
Statistical analysis was performed using SPSS 27.0, continuous variables were expressed as mean ± SD for normally distributed data and median (Q25, Q75) for non-normally distributed data, with comparisons performed using the independent sample t-test and Wilcoxon rank-sum test, respectively. Categorical data were analyzed using frequency or percentage, and enumeration data were compared using the χ2 test or Fisher exact test for analysis. P < 0.05 was considered statistically significant. Logistic regression analysis was employed to evaluate the risk factors for complications in patients undergoing EFTR. Only variables with P < 0.05 in the univariable logistic regression model were included in multivariable analysis.
A total of 92 patients participated in this study between September 2023 and September 2024. Of these, 2 patients were excluded due to lesion diameter exceeding 5 cm, and 90 patients were finally analyzed. Patients were randomly divided into an experimental group (interrupted closure group, n = 44) and a control group (traditional closure group, n = 46).
There were no differences in the baseline characteristics of the patients between the two groups. Statistical analysis of lesion-related data revealed that the maximum lesion diameters were comparable between the two groups, with median lesion diameters of 29.5 mm. The majority of lesions in the experimental group were found in the body of the stomach (52.27%), whereas in the control group were located in the fundus of the stomach (56.52%). In both groups, lesions predominantly originated from the muscularis propria of the stomach, exhibiting both intraluminal and extraluminal growth patterns. Postoperative pathological analysis showed that the most common pathological types in both groups were stromal tumors. Detailed information is provided in Table 1.
| Experimental group (n = 44) | Control group (n = 46) | P value | |
| Age [M, (Q1, Q3), years] | 56.5 (49.0, 64.5) | 58.5 (49.0, 62.0) | 0.994 |
| Sex | 0.411 | ||
| Male | 21 (47.73) | 18 (39.13) | |
| Female | 23 (52.27) | 28 (60.87) | |
| Maximum lesion diameter [M, (Q1, Q3), mm] | 29.5 (20.0, 39.0) | 29.5 (20.0, 48.0) | 0.474 |
| Lesion location | 0.296 | ||
| Fundus of stomach | 18 (40.90) | 26 (56.52) | |
| Gastric body | 23 (52.27) | 18 (39.13) | |
| Antrum of stomach | 3 (6.82) | 2 (4.35) | |
| Level of origin of lesion | 0.072 | ||
| Not reaching muscularis propria | 3 (6.82) | 0 (0) | |
| Muscularis propria | 41 (93.18) | 46 (100) | |
| Growth pattern | 0.875 | ||
| Intraluminal growth | 3 (6.82) | 3 (6.52) | |
| Extraluminal growth | 2 (4.54) | 1 (2.17) | |
| Intraluminal and extraluminal growth | 39 (88.64) | 42 (91.30) | |
| Pathological type | 0.634 | ||
| Stromal tumor | 25 (56.82) | 33 (71.74) | |
| Leiomyoma | 10 (22.73) | 6 (13.04) | |
| Ectopia pancreas | 2 (4.54) | 1 (2.17) | |
| Neurofibroma | 3 (6.82) | 2 (4.35) | |
| Other | 3 (6.82) | 4 (8.70) |
In this study, all patients achieved complete excision of the lesion and successful wound closure. About gas complications, one patient in the experimental group required puncture at McBurney's point due to severe pneumoperitoneum, the gas-related complication rate was 2.27%, in contrast, in control group was 26.09%, with a total of 12 patients affected
| Experimental group (n = 44) | Control group (n = 46) | P value | |
| Complete wound closure | > 0.999 | ||
| Yes | 44 (100) | 46 (100) | |
| No | 0 (0) | 0 (0) | |
| En bloc resection | > 0.999 | ||
| Yes | 44 (100) | 46 (100) | |
| No | 0 (0) | 0 (0) | |
| Intraoperative bleeding | > 0.999 | ||
| Yes | 0 (0) | 0 (0) | |
| No | 44 (100) | 46 (100) | |
| Gas complication | |||
| Subcutaneous emphysema | 0 (0) | 0 (0) | > 0.999 |
| Pneumomediastinum | 0 (0) | 0 (0) | > 0.999 |
| Severe pneumothorax | 0 (0) | 0 (0) | > 0.999 |
| Severe pneumoperitoneum | 1 (2.27) | 12 (26.09) | 0.001 |
| Operative time [M, (Q1, Q3), minute] | 29.5 (20.0, 39.0) | 29.5 (20.0, 48.0) | 0.474 |
| Intraoperative traction | 0.673 | ||
| Yes | 8 (18.18) | 10 (21.74) | |
| No | 36 (81.82) | 36 (78.26) | |
| Complete lesion removal | 0.975 | ||
| Yes | 43 (97.73) | 45 (97.83) | |
| No | 1 (2.27) | 1 (2.17) |
The postoperative laboratory-related examination results showed that the postoperative C-reactive protein, white blood cell count, and neutrophil percentage in the experimental group were slightly lower than those in the control group, however, the differences had no statistical significance (P > 0.05). The median postoperative maximum body temperature was 36.8 °C in both groups. Regarding the degree of postoperative abdominal pain, the NRS scores at 1 and 4 days after the operation were lower in the experimental group compared to the control group. No cases of delayed complications were reported in either group. The incidence of postoperative infection was 15.91% in the experimental group and 41.30% in the control group (P = 0.008). In terms of antibiotic use, the duration was 3.5 days (1.3, 5) in the experimental group compared to 5 days (3.8, 6) in the control group (P = 0.013). The median number of fasting days was similar in both groups, at 5 days, with no statistically significant difference. Additionally, there was no statistical difference in postoperative hospital stays [6 (5, 7) days vs 6 (5, 7) days, P = 0.314] and total hospital costs [19843.3 (18361.6, 22260.5) China yuan (CNY) vs 20862.5 (19475.0, 24398.3) CNY, P = 0.075] between the experimental group and the control group. Detailed information is provided in Table 3.
| Experimental group (n = 44) | Control group (n = 46) | P value | |
| Postoperative CRP [M, (Q1, Q3), mg/L] | 17.98 (7.3, 34.2) | 30.8 (16.1, 47.1) | 0.066 |
| Postoperative white blood cell count [M, (Q1, Q3), 109/L] | 8.9 (7.9, 11.2) | 10.6 (9.0, 13.0) | 0.069 |
| Postoperative neutrophil percentage [M, (Q1, Q3), %] | 81.7 (75.7, 84.9) | 82.4 (76.7, 84.7) | 0.577 |
| Postoperative maximum body temperature [M, (Q1, Q3), °C] | 36.8 (36.7, 36.9) | 36.8 (36.8, 37.5) | 0.149 |
| NRS 1 day postoperative [M, (Q1, Q3), points] | 3 (2, 3.6) | 4 (3, 5) | < 0.001 |
| NRS 4 days postoperative [M, (Q1, Q3), points] | 1 (1, 2.0) | 2 (1, 2.3) | < 0.001 |
| Delayed postoperative bleeding | > 0.999 | ||
| Yes | 0 (0) | 0 (0) | |
| No | 44 (100) | 46 (100) | |
| Delayed postoperative perforation | > 0.999 | ||
| Yes | 0 (0) | 0 (0) | |
| No | 44 (100) | 46 (100) | |
| Postoperative infection | 0.008 | ||
| Yes | 7 (15.91) | 19 (41.30) | |
| No | 37 (84.09) | 27 (58.70) | |
| The days of use antibiotic [M, (Q1, Q3), day] | 3.5 (1.3, 5) | 5 (3.8, 6) | 0.013 |
| Postoperative hospital stays [M, (Q1, Q3), day] | 6 (5, 7) | 6 (5, 7) | 0.314 |
| Total hospitalization costs [M, (Q1, Q3), CNY] | 19843.3 (18361.6, 22260.5) | 20862.5 (19475.0, 24398.3) | 0.075 |
EFTR is appropriate for lesions that originate from deeper layers and exhibit significant fibrosis. Multiple clinical studies have demonstrated the safety and efficacy of EFTR for treating gastrointestinal submucosal lesions[14-16]. In this study, all patients who underwent EFTR achieved complete resection of the lesions.
A critical factor of EFTR is the complete closure of the defect. To achieve this objective, researchers have endeavored to develop suitable suturing techniques and devices for EFTR wound closure, including Over-the-scope clip[22,23], OverStitch[24], Full-thickness resection device devices[25,26], and X-Tack suturing systems[27]. However, the excessive costs and complex procedures associated limit their clinical application. Suturing with metal clips is a straightforward and widely used in clinical practice. In this study, after incising two-thirds of the gastric wall surrounding the lesion, metal clips were immediately applied to proximal or distal end of the wound, dissection and closure procedure were repeated until the tumor completely resected. The interrupted closure technique alleviates wound tension and prevents mucosal edge edema associated with prolonged surgery, thereby facilitating easier and more efficient suturing. It also prevents the tumor from falling into the abdominal. In this study, all patients in the experimental group achieved complete wound closure without delayed complications. Proper management of gastric wall vessels is crucial prior to using the interrupted closure technique, as residual metallic clips could hinder subsequent hemostasis in case of bleeding.
Traditional EFTR involves resecting the entire lesion and subsequently closing the defect, however, due to prolonged perforation, this approach may lead to insufficient gastric cavity distension, compromised intraoperative visibility, and an increased incidence of gas-related complications[28,29]. In contrast, the interrupted closure technique performs suturing during lesion resection, reducing the defect area, effectively decreasing intraoperative gas leakage into the abdominal, maintaining better visibility, and decreasing the incidence of gas complications[19]. In this study, the gas complications observed were limited to pneumoperitoneum. And only one patient in the experimental group experienced pneumoperitoneum, compared to 12 in the control group (P = 0.001), multivariate logistic analysis indicated that the interrupted closure technique served as a protective factor against gas complications (Supplementary materials). Notably, the lesions studied were larger in diameter compared to previous literature. In our clinical practice, smaller lesions derive limited benefit from the new technique. When lesions are small, even if they lead to active perforation, the perforation duration is short, the metallic clips placed in advance may hinder subsequent dissection. Therefore, this technique may be more suitable for larger lesions. Although EFTR has been reported for lesions > 5 cm, we limit its use to those < 5 cm based on guidelines[1] and clinical experience.
Previous studies have indicated a high possibility of postoperative infection following EFTR, attributed to several factors: (1) EFTR targets lesions originating from deeper layers, where thermal damage from intraoperative electrocautery directly affecting the muscularis mucosa, muscularis propria, and serosa[30]; and (2) Iatrogenic perforation results in communication between the abdominal and gastric cavities, increasing the risk of abdominal infection. Consequently, exploring methods to reduce the incidence of postoperative infection after EFTR is significant. In this study, the postoperative infection rate in control group was 41.30%, while the experimental group was only 15.91%. These results indicate that the interrupted closure technique can effectively reduce the incidence of postoperative infection (P = 0.008). Additionally, in multivariable analysis, interrupted closure serves as a protective factor against postoperative infection (Supplementary materials). Although the interrupted closure technique in EFTR can reduce postoperative infection rates, all patients remained hospitalized for 3-5 days postoperatively for close monitoring and to ensure their safety.
The use of traction techniques during lesion resection remains a prominent topic in endoscopic research. The application of traction during EFTR offers several advantages: (1) It increases cutting tension, enhances cutting efficiency, and shortens operative time[31]; (2) It provides a clear visual field allowing for lesion dissection under direct vision and reducing vascular injury[32]; (3) It pulls the lesion into the gastric lumen, reducing the risk of mucosal incision damage to abdominal organs[33]; and (4) It prevents the lesion from falling into the abdominal cavity, reducing the possibility of intraperitoneal spread. In this study, a total of 18 patients underwent intraoperative traction. Among these, one patient utilized snare traction technique, while the others used dental floss technique. Notably, the median operative time was significantly shorter in the traction group compared to the non-traction group (28.0 minutes vs 36.5 minutes, P = 0.019), indicating the effectiveness of selective traction in optimizing surgical efficiency.
This study has several limitations: (1) It is a single-center study; (2) NRS scoring for abdominal pain is subjective, and individual variability among patients may exist; (3) The study outcomes were short-term, with no long-term follow-up was conducted; and (4) The classification of lesion locations was imprecise, and no efficacy assessment was performed for each gastric wall segment.
Not all locations may benefit from the interrupted closure technique, particularly the lesser curvature of the upper gastric body. First, lesions in this area can be challenging to dissect from the anal side; initiating dissection from the oral side with partial wound closure may complicate subsequent operations. Second, the omentum covering the gastric wall somewhat reduces leakage of gastric contents[34], thereby decreasing the risk of severe pneumoperitoneum and postoperative infection. Further research is needed to evaluate the appropriateness of the interrupted closure technique for this specific location.
The interrupted closure technique is a safe and effective approach in EFTR for gastric SELs, reducing the incidence of intraoperative gas complications and postoperative infections.
| 1. | Zhou PH, Li ZS, Qin XY; Endoscopic Surgery Group, Chinese Society of Digestive Endoscopology, Chinese Medical Association; NOTES Group, Chinese Society of Digestive Endoscopology, Chinese Medical Association; Digestive Endoscopy Specialty Committee, Endoscopic Physicians Branch of Chinese Medical Doctor Association; Gastrointestinal Surgery Group, Chinese Society of Surgery, Chinese Medical Association. [Chinese consensus on endoscopic diagnosis and managment of gastrointestinal submucosal tumors (version 2023)]. Zhongguo Xiaohua Neijing Zazhi. 2023;40:253-263. [DOI] [Full Text] |
| 2. | Sharzehi K, Sethi A, Savides T. AGA Clinical Practice Update on Management of Subepithelial Lesions Encountered During Routine Endoscopy: Expert Review. Clin Gastroenterol Hepatol. 2022;20:2435-2443.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 3. | Shichijo S, Abe N, Takeuchi H, Ohata K, Minato Y, Hashiguchi K, Hirasawa K, Kayaba S, Shinkai H, Kobara H, Yamashina T, Ishida T, Chiba H, Ono H, Mori H, Uedo N. Endoscopic resection for gastric submucosal tumors: Japanese multicenter retrospective study. Dig Endosc. 2023;35:206-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Nishida T, Kawai N, Yamaguchi S, Nishida Y. Submucosal tumors: comprehensive guide for the diagnosis and therapy of gastrointestinal submucosal tumors. Dig Endosc. 2013;25:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 190] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 5. | Polkowski M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy. 2005;37:635-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Chinese Society of Digestive Endoscopy; Chinese Digestive Endoscopy Association; Beijing Digestive Endoscopy Society. [Consensus of Chinese experts on the endoscopic diagnosis and treatment of gastrointestinal stromal tumor (2020, Beijing)]. Zhonghua Weichang Neijing Dianzi Zazhi. 2020;7:176-185. [DOI] [Full Text] |
| 7. | Deprez PH, Moons LMG, OʼToole D, Gincul R, Seicean A, Pimentel-Nunes P, Fernández-Esparrach G, Polkowski M, Vieth M, Borbath I, Moreels TG, Nieveen van Dijkum E, Blay JY, van Hooft JE. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022;54:412-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 192] [Article Influence: 64.0] [Reference Citation Analysis (1)] |
| 8. | Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S, Sundar HM, Trent JC, Wayne JD. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8 Suppl 2:S1-41; quiz S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 820] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 9. | Wang K, Gao P, Cai M, Song B, Zhou P. Endoscopic full-thickness resection, indication, methods and perspectives. Dig Endosc. 2023;35:195-205. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Cai MY, Martin Carreras-Presas F, Zhou PH. Endoscopic full-thickness resection for gastrointestinal submucosal tumors. Dig Endosc. 2018;30 Suppl 1:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Białek A, Wiechowska-Kozłowska A, Pertkiewicz J, Polkowski M, Milkiewicz P, Karpińska K, Ławniczak M, Starzyńska T. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video). Gastrointest Endosc. 2012;75:276-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 12. | Bang CS, Baik GH, Shin IS, Suk KT, Yoon JH, Kim DJ. Endoscopic submucosal dissection of gastric subepithelial tumors: a systematic review and meta-analysis. Korean J Intern Med. 2016;31:860-871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Lee JS, Kim GH, Park DY, Yoon JM, Kim TW, Seo JH, Lee BE, Song GA. Endoscopic Submucosal Dissection for Gastric Subepithelial Tumors: A Single-Center Experience. Gastroenterol Res Pract. 2015;2015:425469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Tada N, Kobara H, Nishiyama N, Fujihara S, Masaki T, Uedo N. Current Status of Endoscopic Full-Thickness Resection for Gastric Subepithelial Tumors: A Literature Review Over Two Decades. Digestion. 2023;104:415-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Reference Citation Analysis (0)] |
| 15. | Tan Y, Tang X, Guo T, Peng D, Tang Y, Duan T, Wang X, Lv L, Huo J, Liu D. Comparison between submucosal tunneling endoscopic resection and endoscopic full-thickness resection for gastric stromal tumors originating from the muscularis propria layer. Surg Endosc. 2017;31:3376-3382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Kim SY, Kim KO. Management of gastric subepithelial tumors: The role of endoscopy. World J Gastrointest Endosc. 2016;8:418-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Kopelman Y, Siersema PD, Bapaye A, Kopelman D. Endoscopic full-thickness GI wall resection: current status. Gastrointest Endosc. 2012;75:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Li X, Zhang W, Gao F, Dong H, Wang J, Chai N, Linghu E. A modified endoscopic full-thickness resection for gastrointestinal stromal tumors: A new closure technique based on the instruction of super minimally invasive surgery. Endoscopy. 2023;55:E561-E562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Sun H, Cao T, Zhang F, Tao K, Xu H. Gastric defect closure after endoscopic full-thickness resection: the closing while dissecting technique. Surg Endosc. 2023;37:234-240. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Yang JP, Ren XM, Ni MH, Jin XY, Xu GF. [Comparison between endoscopic full-thickness resection and cap-assisted endoscopic full-thickness resection in the treatment of elderly patients with small gastric stromal tumors]. Zhonghua Xiaohua Neijing Zazhi. 2023;40:218-223. [DOI] [Full Text] |
| 21. | Ru N, Li LS, Bi YW, Gao F, Zhang B, Chai NL, Linghu EQ. A modified exposed endoscopic full-thickness resection: Traction-assisted resection while defect closing. J Dig Dis. 2023;24:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 22. | Hu J, Ge N, Wang S, Guo J, Liu X, Wang G, Sun S. Direct endoscopic full-thickness resection for submucosal tumors with an intraluminal growth pattern originating from the muscularis propria layer in the gastric fundus. BMC Gastroenterol. 2020;20:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Schlag C, Wilhelm D, von Delius S, Feussner H, Meining A. EndoResect study: endoscopic full-thickness resection of gastric subepithelial tumors. Endoscopy. 2013;45:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Stavropoulos SN, Modayil R, Friedel D. Current applications of endoscopic suturing. World J Gastrointest Endosc. 2015;7:777-789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 97] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 25. | Bauder M, Schmidt A, Caca K. Endoscopic full-thickness resection of duodenal lesions-a retrospective analysis of 20 FTRD cases. United European Gastroenterol J. 2018;6:1015-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Hajifathalian K, Ichkhanian Y, Dawod Q, Meining A, Schmidt A, Glaser N, Vosoughi K, Diehl DL, Grimm IS, James T, Templeton AW, Samarasena JB, Chehade NEH, Lee JG, Chang KJ, Mizrahi M, Barawi M, Irani S, Friedland S, Korc P, Aadam AA, Al-Haddad M, Kowalski TE, Smallfield G, Ginsberg GG, Fukami N, Lajin M, Kumta NA, Tang SJ, Naga Y, Amateau SK, Kasmin F, Goetz M, Seewald S, Kumbhari V, Ngamruengphong S, Mahdev S, Mukewar S, Sampath K, Carr-Locke DL, Khashab MA, Sharaiha RZ. Full-thickness resection device (FTRD) for treatment of upper gastrointestinal tract lesions: the first international experience. Endosc Int Open. 2020;8:E1291-E1301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Mahmoud T, Wong Kee Song LM, Stavropoulos SN, Alansari TH, Ramberan H, Fukami N, Marya NB, Rau P, Marshall C, Ghandour B, Bejjani M, Khashab MA, Haber GB, Aihara H, Antillon-Galdamez MR, Chandrasekhara V, Abu Dayyeh BK, Storm AC. Initial multicenter experience using a novel endoscopic tack and suture system for challenging GI defect closure and stent fixation (with video). Gastrointest Endosc. 2022;95:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 28. | Yamamoto Y, Uedo N, Abe N, Mori H, Ikeda H, Kanzaki H, Hirasawa K, Yoshida N, Goto O, Morita S, Zhou P. Current status and feasibility of endoscopic full-thickness resection in Japan: Results of a questionnaire survey. Dig Endosc. 2018;30 Suppl 1:2-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Hu JW, Ge L, Zhou PH, Li QL, Zhang YQ, Chen WF, Chen T, Yao LQ, Xu MD, Chu Y. A novel grasp-and-loop closure method for defect closure after endoscopic full-thickness resection (with video). Surg Endosc. 2017;31:4275-4282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Ni LJ, Zhu WX, Zou CT, Xu GT, Wang C, Wu AR. [Risk factors for complications of endoscopic full-thickness resection of upper gastrointestinal submucosal tumors]. Zhonghua Wei Chang Wai Ke Za Zhi. 2023;26:365-371. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | Li B, Shi Q, Qi ZP, Yao LQ, Xu MD, Lv ZT, Yalikong A, Cai SL, Sun D, Zhou PH, Zhong YS. The efficacy of dental floss and a hemoclip as a traction method for the endoscopic full-thickness resection of submucosal tumors in the gastric fundus. Surg Endosc. 2019;33:3864-3873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Shi Q, Li B, Qi ZP, Yao LQ, Xu MD, Cai SL, Sun D, Zhou PH, Zhong YS. Clinical Values of Dental Floss Traction Assistance in Endoscopic Full-Thickness Resection for Submucosal Tumors Originating from the Muscularis Propria Layer in the Gastric Fundus. J Laparoendosc Adv Surg Tech A. 2018;28:1261-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Zhang Q, Cai JQ, Wang Z, Xiao B, Bai Y. Snare combined with endoscopic clips in endoscopic resection of gastric submucosal tumor: a method of tumor traction. Endosc Int Open. 2019;7:E1150-E1162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Abe N, Takeuchi H, Ohki A, Hashimoto Y, Mori T, Sugiyama M. Comparison between endoscopic and laparoscopic removal of gastric submucosal tumor. Dig Endosc. 2018;30 Suppl 1:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |