Published online Jun 27, 2025. doi: 10.4240/wjgs.v17.i6.106009
Revised: April 1, 2025
Accepted: April 25, 2025
Published online: June 27, 2025
Processing time: 98 Days and 20.5 Hours
At present, the concept of surgical treatment of gastric cancer (GC) has changed from “radical treatment” to “care for patients” to a certain extent. The recon
To evaluate the clinical advantages, feasibility, and safety of a modified Roux-en-Y digestive tract reconstruction in laparoscopy-assisted total gastrectomy for the treatment of GC compared with the traditional Roux-en-Y method.
Ninety-seven patients who underwent laparoscopy-assisted D2 radical gastrectomy (total gastrectomy) for GC were divided into two groups: fifty-four in the conventional Roux-en-Y reconstruction group (Orr group) and forty-three in the modified Roux-en-Y reconstruction group (the modified group). Perioperative and short-term outcomes were analyzed, including complications, postoperative weight loss, hemoglobin levels, and nutritional status.
The Orr group and the modified group showed no statistically significant differences in baseline characteristics. Compared with the Orr group, the modified group had shorter digestive tract reconstruction and operation times, less intraoperative bleeding, and shorter postoperative hospital stays compared to the Orr group. Although both groups had similar amounts of intraoperative blood loss, postoperative recovery times, and hospital expenses, the Orr group experienced longer operation times and digestive tract reconstruction times. Furthermore, the modified Roux-en-Y group demonstrated significantly fewer short-term and long-term complications, with a reduced incidence of reflux esophagitis and improved nutritional status.
The modified Roux-en-Y digestive tract reconstruction method after laparoscopy-assisted total gastrectomy for GC offers safety, simplicity, and a reduction in bile reflux. This method shortens operation times and minimizes postoperative complications, aligns with modern rapid rehabilitation surgery trends and potentially improves patient prognosis and overall survival. This method warrants further clinical application and promotion.
Core Tip: This study compared modified vs traditional Roux-en-Y reconstruction in 97 patients with gastric cancer undergoing laparoscopy-assisted total gastrectomy. The modified method (n = 43) demonstrated shorter operation (160.9 vs 211.7 minutes) and reconstruction times (21.46 vs 52.25 minutes), reduced intraoperative bleeding, and fewer complications (e.g., reflux esophagitis: 0% vs 11.1%). Postoperative recovery and nutritional outcomes (weight loss, hemoglobin levels) were superior in the modified group. Findings suggest that the modified technique improves safety, efficiency, and patient prognosis, aligning with rapid rehabilitation principles. Clinical adoption is recommended.
- Citation: Yu J, Li M, Qin XZ, Gong L, Qin L, Lv ZB, Guo W, Huang B, Tian YH. Application of modified Roux-en-Y digestive tract reconstruction in total gastrectomy for patients with gastric cancer. World J Gastrointest Surg 2025; 17(6): 106009
- URL: https://www.wjgnet.com/1948-9366/full/v17/i6/106009.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i6.106009
The latest global cancer statistics report shows that among the 18.1 million new cancer cases, the number of new cases in China is approximately 3.804 million, and the number of deaths is 2.296 million. The incidence and mortality of cancer in China are the highest in the world, and the incidence and mortality of gastric cancer (GC) are the third highest in China[1,2].
In recent years, with improvements in GC surgery technology and the promotion of standardized comprehensive treatment, at experienced medical institutions, the 5-year overall survival (OS) rate of patients undergoing radical gastrectomy is approximately 60%, and the 5-year OS rate of patients with early GC is approximately 90%-95%[3]. At present, the concept of surgical treatment for GC has changed from “radical treatment” to “care for patients” to a certain extent. The reconstruction method is the most likely to affect the postoperative life for the patient. Various techniques have been developed, and their outcomes have been reported[4]. Roux-en-Y gastrointestinal reconstruction has the advantages of high safety, relatively simple operation procedures, fewer postoperative complications, and partial physiological function in accordance with the stomach[5]. Currently, the traditional Roux-en-Y esophagojejunostomy anastomosis is a commonly used method for gastrointestinal reconstruction after total gastrectomy for GC[6]. However, more recent studies have shown that the traditional Roux-en-Y anastomosis is a complicated in operation procedure, with more reconstruction steps and longer reconstruction times, and the incidence of postoperative complications such as adhesive intestinal obstruction, internal abdominal hernia and volvulus is high[7,8]. Moreover, the incidence of Roux stasis syndrome is 10%-30% after traditional Roux-en-Y reconstruction[9,10].
Recently, there have been many reports on the improvements in digestive tract reconstruction based on the traditional Roux-en-Y have been published[11-14]. Laparoscopic total gastrectomy is a common technique of radical gastrectomy for GC, and currently, digestive tract reconstruction after laparoscopic gastrectomy mainly includes complete laparoscopic, laparoscopic assisted and hand-assisted laparoscopic reconstruction[15]. It is well known that the specimens from patients with GC after the laparoscopic surgery should to be removed through an abdominal small incision; the use of an abdominal auxiliary incision it can greatly reduce the difficulty of the operation, shorten the operation time, and enable safer and reliable anastomosis[16]. Therefore, laparoscopy-assisted gastrointestinal reconstruction is the most widely used method. The most commonly used traditional digestive tract reconstruction in total gastrectomy is Orr Roux-en-Y[17]. In this study, we modified the traditional Roux-en-Y alimentary tract reconstruction, and designed a new digestive tract reconstruction method for laparoscopy-assisted Roux-en-Y anastomosis for total gastrectomy in patients with GC.
In the present report, we utilized the Roux-en-Y reconstruction technique as originally described by Dr. Orr and accordingly designated this group the Roux-en-Y reconstruction group (Orr group). The group that underwent our improved reconstruction technique was called the modified Roux-en-Y reconstruction group (modified group). We retrospectively analyzed and compared the clinical indexes of the Orr Roux-en-Y group and the modified Roux-en-Y group in the perioperative period and the short-term efficacy after laparoscopic total gastrectomy, and evaluated the clinical advantages, practicability and safety of modified Roux-en-Y digestive tract reconstruction.
This study was approved by the Ethics Committee of the Nanchong Central Hospital Affiliated with North Sichuan Medical College (Nanchong city, Sichuan Province, China). Patients or their families signed informed consent forms.
A total of 97 patients underwent laparoscopic-assisted D2 radical gastrectomy (total gastrectomy) for GC from January 2016 to September 2021 at the Affiliated Hospital of North Sichuan Medical College and Nanchong Central Hospital Affiliated with North Sichuan Medical College in China, including 80 males and 17 females, were retrospectively enrolled in this study. The patients were retrospectively divided into two groups according to the digestive tract reconstruction method used: 54 patients in the Orr group and 43 patients in the modified group. There was no significant difference in sex, age, body mass index, tumor-node-metastasis (TNM) stage, tumor pathological type, tumor diameter, depth of tumor invasion or lymph node metastasis between the two groups (P > 0.05) (Table 1). All operations were performed by the same group of surgeons.
| Characteristics | Orr group (n = 54) | Modified group (n = 43) | χ2/F | P value |
| Sex | - | - | 0.001 | 0.998 |
| Male (cases) | 41 (75.9) | 39 (90.7) | - | - |
| Female (cases) | 13 (24.1) | 4 (8.7) | - | - |
| Age (year), mean ± SD | 62.38 ± 11.84 | 68.39 ± 10.11 | 2.272 | 0.110 |
| BMI | ||||
| TNM staging (cases) | - | - | 0.001 | 0.998 |
| I | 11 (20.4) | 4 (9.3) | - | - |
| II | 5 (9.3) | 6 (14.0) | - | - |
| III | 38 (70.4) | 33 (76.7) | - | - |
| Tumor pathological classification (cases) | - | - | 0.375 | 0.701 |
| Nodule type | 9 (16.7) | 4 (9.3) | - | - |
| Ulceration type | 31 (57.4) | 28 (65.1) | - | - |
| Diffuse invasive | 14 (25.9) | 11 (25.6) | - | - |
| Tumor diameter (cases) | - | - | 0.008 | 0.991 |
| ≤ 1 cm | 11 (20.4) | 7 (16.3) | - | - |
| 1-4 cm | 16 (29.6) | 11 (25.6) | - | - |
| > 4 cm | 27 (50.0) | 25 (58.1) | - | -- |
| Infiltrative depth (cases) | - | - | 0.001 | 0.998 |
| T1 | 11 (20.4) | 2 (4.7) | - | - |
| T2 | 2 (3.7) | 4 (9.3) | - | - |
| T3 | 7 (12.9) | 4 (9.3) | - | - |
| T4 | 34 (63.0) | 33 (76.7) | - | - |
| Lymphatic metastasis (cases) | - | - | 0.007 | 0.992 |
| N0 | 25 (46.3) | 17 (39.5) | - | - |
| N1 | 7 (13.0) | 6 (13.9) | - | - |
| N2 | 9 (16.7) | 9 (21.0) | - | - |
| N3 | 13 (24.0) | 11 (25.6) | - | - |
Inclusion criteria: (1) Age ≥ 18 years; (2) Preoperative gastrointestinal biopsy and computed tomography examination confirmed GC; (3) Preoperative computed tomography examination revealed that the clinical stage was T1-3N1-3M0 (7th edition of the Union for International Cancer Control - American Joint Committee on Cancer TNM classification of GC); (4) Zubrod-Eastern Cooperative Oncology Group-World Health Organization physical status score ≤ 2, Karnofsky functional status score ≥ 70; and (5) Deemed able to tolerate laparoscopic surgery based on preoperative examination.
Exclusion criteria: (1) Aged ≥ 80 years or with severe malnutrition; (2) Had a history of abdominal surgery; (3) Had liver, peritoneum, lung, or other adjacent organ invasion or metastasis; (4) Received neoadjuvant chemotherapy, radiotherapy, immunotherapy, targeted therapy or other special treatment before surgery; or (5) Had tumor recurrence, incomplete clinical data or missing follow-up data.
The nasogastric tube was placed routinely before the operation. All patients underwent total gastrectomy with D2 Lymphadenectomy according to the Japanese GC treatment guidelines[18]. Both the Orr Roux-en-Y group and the modified Roux-en-Y group underwent standard laparoscopic D2 radical gastrectomy for GC. Then, a mini-incision was made in the abdomen for digestive tract reconstruction.
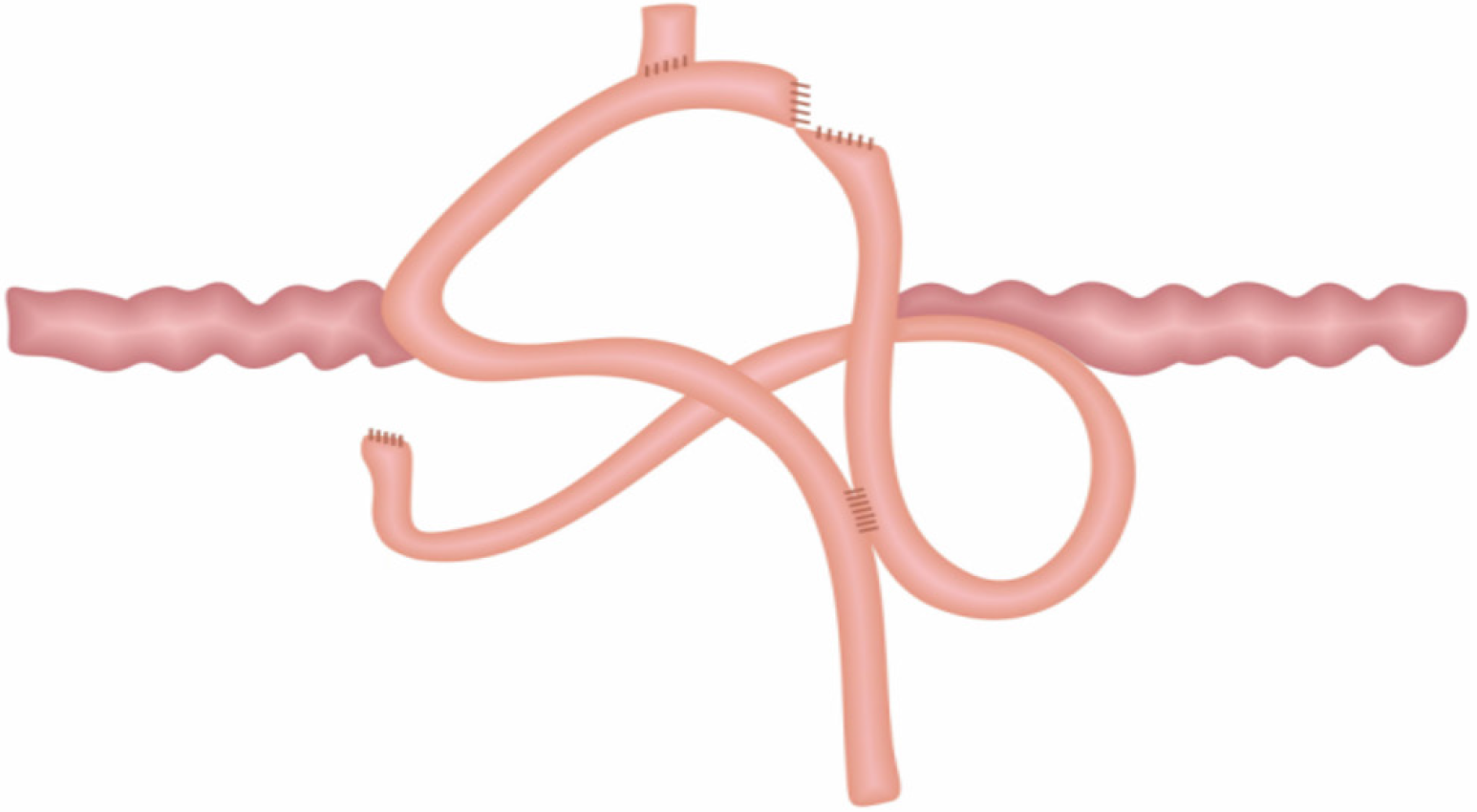
(1) A circular stapler buttress was embedded in the jejunum approximately 65-70 cm distal to the ligament of Treitz. Then the jejunum was fenestrated approximately 15 cm distal to the ligament of Treitz, where a circular stapler was inserted to perform the side - side anastomosis of the proximal jejunum (approximately 15 cm away distal to the ligament of Treitz) and the jejunum (approximately 65-70 cm distal to the ligament of Treitz); (2) The circular stapler buttress was inserted into the lower segment of the esophagus, and then the circular stapler was inserted from the jejunum “fenestration” to perform end - side anastomosis of the jejunum (approximately 25 cm away from the flexor ligament) and the lower esophagus; (3) The jejunum was closed with a linear stapler; and (4) All anastomoses were embedded intermittently with silk thread (Figure 1).
The observation indices were digestive tract reconstruction time (minute), intraoperative blood loss (mL), postoperative anal exhaust time (day), postoperative fasting time (day), postoperative hospital stay (day), total cost of hospitalization (10000 yuan), postoperative weight gain (kg), hemoglobin (g/L), and recent complications. Clinical data were collected, and patients were followed up for 6 months.
Statistical analysis software SPSS 20.0 (IBM SPSS Statistics, Armonk, NY, United States) was used for statistical processing. The measurement data with a normal distribution and homogeneous variance are presented as the mean ± SD, and if the difference was statistically significant (P < 0.05). Then the Bonferroni correction was further used to compare the two groups, and a test level of 0.05 was used. The χ2 test or Fisher’s exact probability method was used for count data, and differences were considered significant at P < 0.05.
There were no significant differences in sex, age, TNM stage, tumor pathological type, tumor diameter, tumor invasion depth, or number of lymph node metastases between the Orr group and the modified group (P > 0.05) (Table 1).
There were no significant differences in intraoperative blood loss, postoperative first anal exhaust time, postoperative fasting time, postoperative hospital stay, or hospital expenses between the two groups (P > 0.05). However, the operation time, digestive tract reconstruction time, and postoperative hospital stay of the Orr group were significantly longer than those of the modified group (P < 0.05) (Table 2).
| Characteristics | Orr group | Modified group | F value | P value |
| Operation time (minute) | 211.7 ± 19.66 | 160.9 ± 9.44 | 163.8 | 0.000 |
| Digestive tract reconstruction time (minute) | 52.25 ± 7.79 | 21.46 ± 2.77 | 502.3 | 0.000 |
| Intraoperative blood loss (mL) | 235.4 ± 79.12 | 215.42 ± 49.61 | 1.324 | 0.269 |
| Postoperative exhaust time (day) | 2.22 ± 1.30 | 2.15 ± 1.36 | 0.033 | 0.966 |
| Postoperative fasting time (day) | 2.25 ± 0.85 | 2.61 ± 0.49 | 0.086 | 0.916 |
| Hospital stay after operation (day) | 12.38 ± 5.77 | 10.04 ± 3.40 | 2.862 | 0.060 |
| Total cost of hospitalization (million RMB) | 6.286 ± 1.546 | 5.795 ± 1.338 | 1.755 | 0.176 |
| Cases | 54 | 43 | - | - |
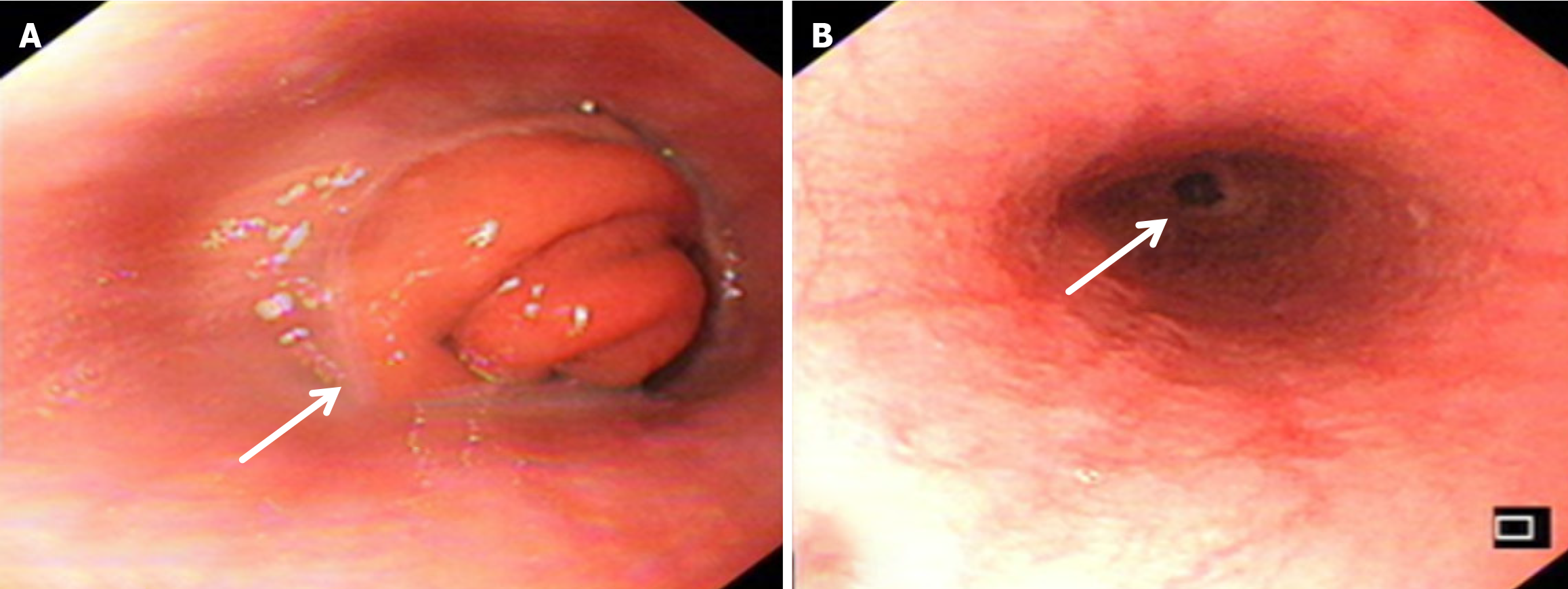
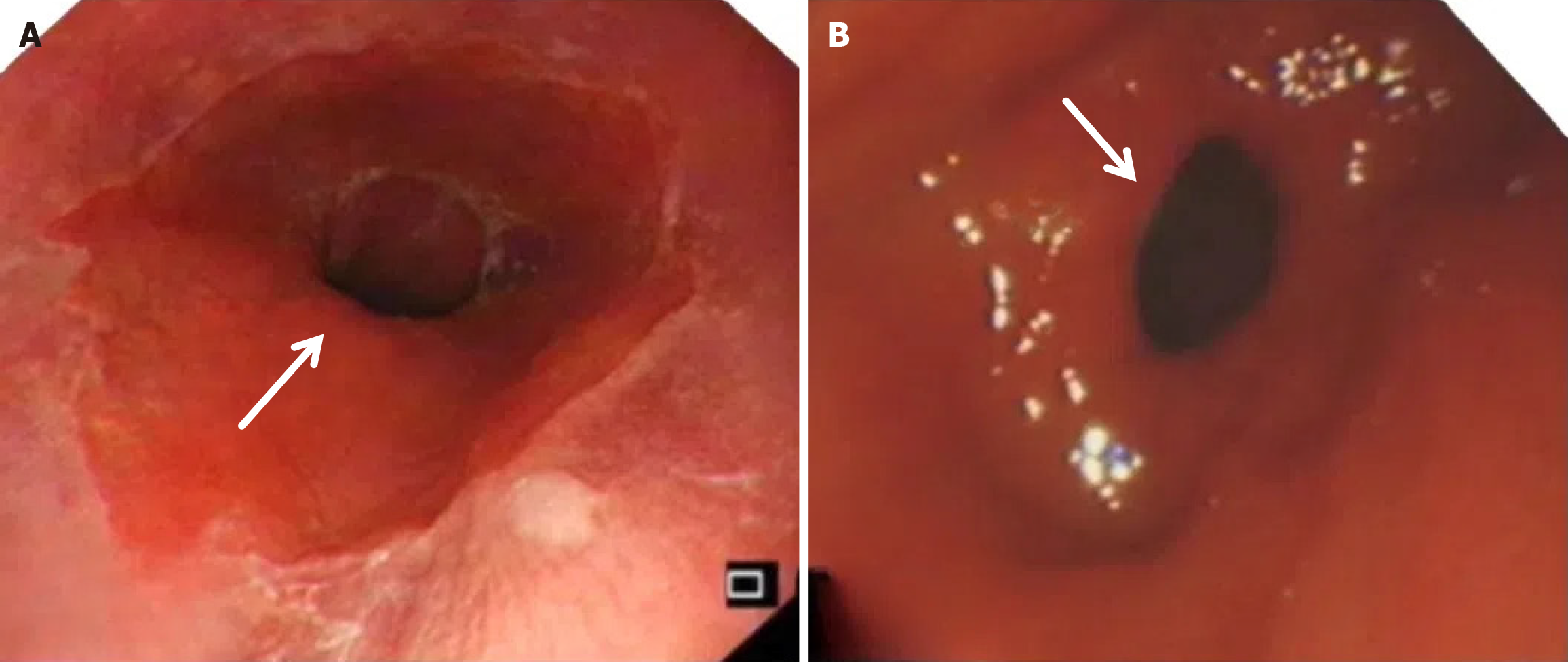
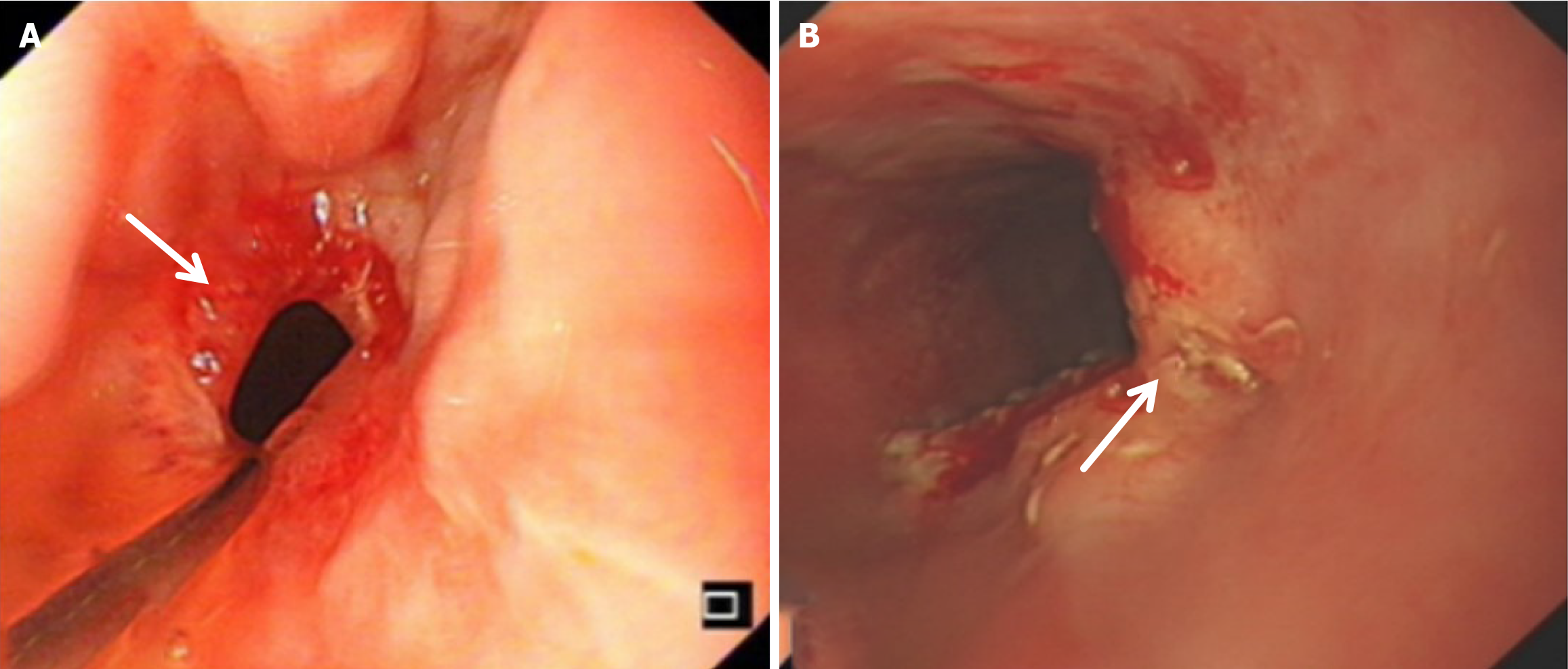
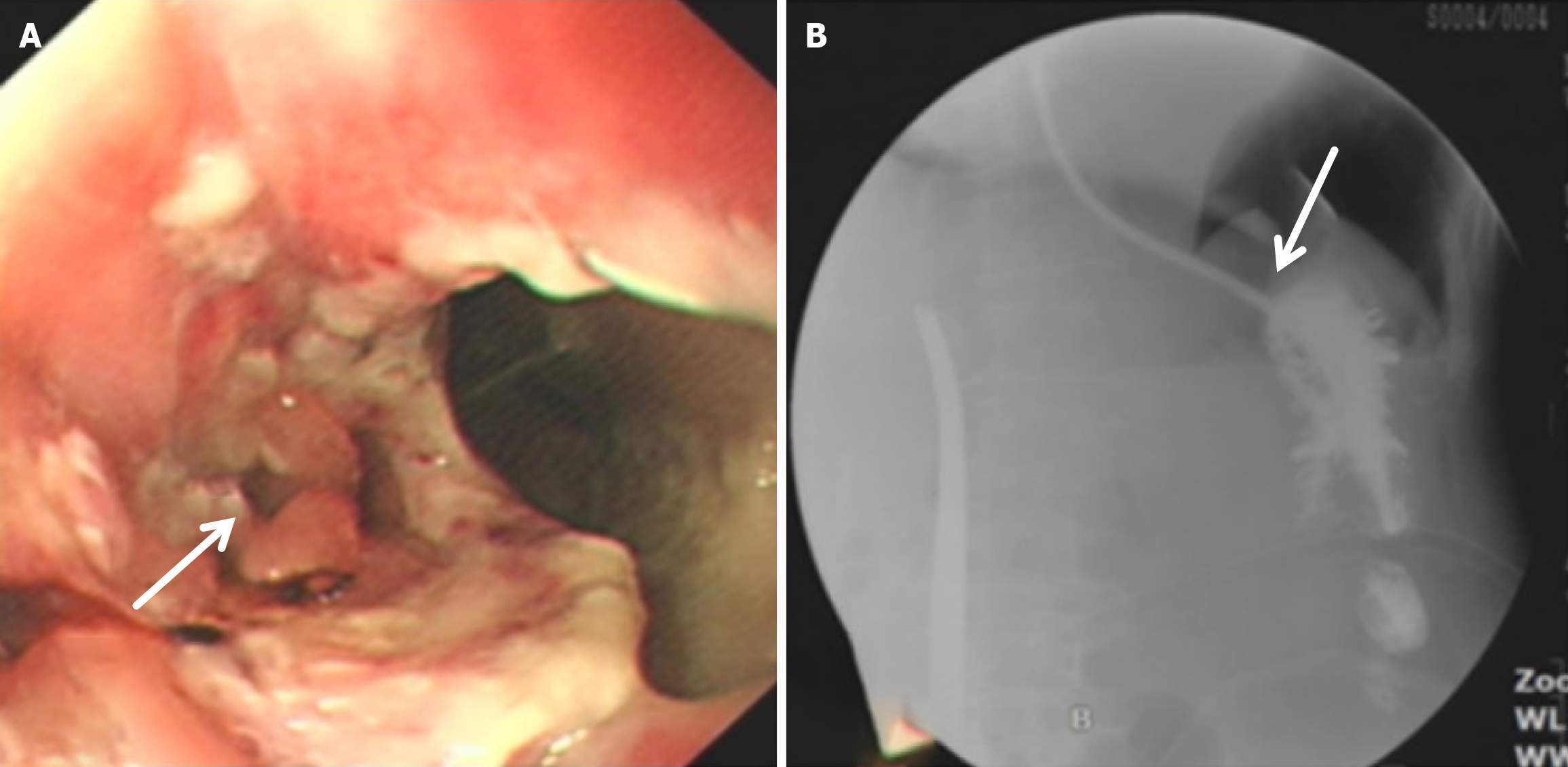
Compared with the modified group, the Orr group had a greater incidence of anastomotic leakage (AL) and short-term complications of anastomotic ischemia, although there was no significant difference between the two groups. Furthermore, Fisher’s exact probability analysis revealed that the incidence of reflux esophagitis, anastomotic ulcers by endoscopic examination, and intestinal obstruction in the Orr group was greater than that in the modified group at the 6-month postoperative follow-up period (P < 0.05) (Tables 3, 4 and 5).
| Characteristics | Orr group | Modified group | χ2 | P value |
| Anastomotic bleeding | 1 (1.8) | 1 (2.3) | 0.013 | 0.986 |
| Anastomotic leakage | 5 (9.3) | 1 (2.3) | 0.016 | 0.983 |
| Anastomotic stricture | 3 (5.5) | 2 (4.6) | 0.015 | 0.984 |
| Anastomotic ischemia | 5 (9.3) | 0 | 0.014 | 0.985 |
| Duodenal stump fistula | 2 (3.7) | 0 | 0.013 | 0.986 |
| Cases | 16 (29.6) | 4 (9.3) | 0.027 | 0.973 |
| Characteristics | Recent complications | Long-term complication |
| Orr group-modified group P value | 0.021 | 0.000 |
There was no significant difference in weight loss between the two groups at 1 month after the operation (P > 0.05). However, in the improved group, the weight of the improved group was less than that of the original group at 3 months and 6 months after the operation (P < 0.05). The hemoglobin in the Orr group decreased significantly at 1 and 3 months after the operation compared with that in the improved group (P < 0.05), but there was no significant difference between the two groups at 6 months after the operation (P > 0.05). There was no significant difference in total protein or serum albumin between the two groups at 1 month, 3 months, and 6 months after the operation (P > 0.05) (Figures 2, 3, 4, 5 and 6, Table 6).
| Characteristics | Orr group | Modified group | F value | P value |
| Weight loss (kg) | ||||
| 1 month after operation | 6 (2-12.5) | 4.5 (2-7.5) | 15.10 | 0.000 |
| 3 months after operation | 3.5 (1.5-4.5) | 1 (0.5-3) | 7.05 | 0.001 |
| 6 months after operation | 2 (1-2.5) | 1 (0.5-1.5) | 2.91 | 0.060 |
| Hemoglobin (g/L) | ||||
| 1 month after operation | 99.4 ± 14.40 | 112.1 ± 18.37 | 6.41 | 0.002 |
| 3 months after operation | 101.3 ± 15.73 | 110.0 ± 15.97 | 3.88 | 0.025 |
| 6 months after operation | 106.9 ± 14.62 | 116.3 ± 15.94 | 2.76 | 0.052 |
| Serum total protein (g/L) | ||||
| 1 month after operation | 61.4 ± 6.93 | 64.9 ± 7.05 | 1.87 | 0.161 |
| 3 months after operation | 64.8 ± 6.29 | 67.9 ± 5.45 | 2.25 | 0.112 |
| 6 months after operation | 67.7 ± 7.07 | 68.8 ± 5.89 | 0.55 | 0.575 |
| Serum albumin (g/L) | ||||
| 1 month after operation | 32.3 ± 5.44 | 34.3 ± 4.80 | 2.05 | 0.136 |
| 3 months after operation | 34.5 ± 4.53 | 34.9 ± 4.10 | 2.57 | 0.083 |
| 6 months after operation | 36.8 ± 4.31 | 36.0 ± 3.08 | 0.82 | 0.444 |
It has been reported that Roux-en-Y anastomosis has unique advantages, such as safety, simple procedures, fewer postoperative complications, and better fitness for the function of the stomach[19]. At present, researchers have focused mainly on preserving the duodenal pathway, performing jejunal storage bags or amputating the jejunum for Roux-en-Y anastomosis after total gastrectomy. However, there is no consensus among surgical experts for Roux-en-Y anastomosis after total gastrectomy. Based on the traditional Roux-en-Y gastrointestinal reconstruction, we designed a new gastrointestinal reconstruction method for total gastrectomy in laparoscopic radical gastrectomy for GC, named modified Roux-en-Y anastomosis. We evaluated the safety and efficacy by observing perioperative and postoperative complications and comparing the quality of life for 6 months between the modified Roux-en-Y group and the traditional Roux-en-Y group.
Our results showed that the modified Roux-en-Y group was better than the traditional Roux-en-Y group in terms of perioperative indicators, postoperative complications, and quality of life. As shown in Table 1, the digestive tract reconstruction time (21.46 ± 2.77 minutes) and operation time (160.9 ± 9.44 minutes) in the modified group were significantly shorter than those in the original group (62.25 ± 7.79 minutes and 211.7 ± 19.66 minutes, respectively) (P < 0.05). In addition, the amount of intraoperative bleeding in the improved group was significantly lower than that in the original group (P < 0.05). The reason may be the complexity and greater number of procedures, and the mesenteric vessels of the jejunum also need to be disconnected in the Orr group, whereas in the improved group, only one window needs to be opened in the jejunum to complete all gastrointestinal reconstruction.
There is no significant difference between duodenal pathway preservation and traditional Roux-en-Y gastrointestinal reconstruction in improving the long-term nutritional status of patients, and the duodenal pathway is not necessary for gastrointestinal reconstruction[10,20]. Compared with the preservation of the duodenal pathway, several patients with gallstones occurred in our two groups after gastrointestinal reconstruction, which may be the reason that no food passed through the duodenum and led to the obstruction of bile excretion in the gallbladder, resulting in the occurrence of gallstones. Therefore, we inferred that total gastrectomy combined with cholecystectomy may help reduce the probability of gallstones, which needs to be further explored.
After total gastrectomy, due to the loss of antireflux function of the cardia, the reverse peristaltic wave generated by the pacing cells of the duodenum leads to the reflux of food and bile into the esophagus, resulting in clinical symptoms such as reflux esophagitis and other gastrointestinal duct discomfort[21]. Previous studies have shown that the jejunal storage bag after total gastrectomy can effectively relieve the pressure of food passing through the esophagojejunostomy. When food flows back from the distal output loop, the storage bag has a similar “siphon effect” to play an antireflux role and reduce the incidence of reflux esophagitis[22]. The antireflux effect is positively proportional to the length of the jejunal storage bag; nevertheless, a storage bag that is too long increases the incidence of Roux stasis syndrome, and the ideal length is approximately 5 cm[23]. In our study, storage bags with a length of approximately 5 cm were constructed at the anastomosis of the esophagus and jejunum in both groups. Our results revealed that the incidence of reflux esophagitis in the Orr group was greater than that in the improved group (P < 0.05), which occurred because the jejunal storage bag was constructed after the jejunum and its mesentery were disconnected, and the residual end of the storage bag lacked traction of the mesentery in the Orr group; therefore, the “siphon effect” was weakened. By contrast, the storage bag of patients in the improved group only needed to disconnect the jejunum, and the jejunal mesentery was continuous, resulting in strong negative pressure suction.
In traditional Roux-en-Y gastrointestinal reconstruction, the jejunum must be disconnected, mainly considering the advantages of a simple operation and reducing the tension associated with esophagojejunostomy. However, subsequent studies have confirmed that the main influencing factor of safety and quality of life is the anastomosis mode. For example, the probability of AL and stenosis in end-to-end anastomosis of esophagojejunostomy is significantly greater than that in end-to-side anastomosis[24].
In traditional Roux-en-Y digestive tract reconstruction, the jejunum and its mesenteric vessels must be disconnected. Surgeons believe that this technique has the advantages of being simple and reducing the tension of esophagojejunostomy to reduce the incidence of AL, especially esophagojejunostomy leakage. However, follow-up studies confirmed that the main influencing factor of esophagojejunostomy leakage was the anastomosis mode. For example, the incidence of AL and stenosis in end-to-end esophagojejunostomy was significantly greater than that in end-to-side anastomosis. The findings of a study conducted by Xie et al[25] demonstrated that preserving mesenteric integrity yields superior clinical benefits than conventional Roux-en-Y gastrointestinal reconstruction, particularly in terms of postoperative recovery and complication rates[25]. Han et al[26] conducted a pioneering study that introduced a modified esophagojejunostomy technique involving selective single-vessel ligation while preserving the integrity of ileal vascular arcade. The prospective cohort analysis comparing open (n = 97) and laparoscopic (n = 97) approaches, this vessel-sparing mo
The nutritional parameters are likely affected by the progression of cancer or a patient’s performance status. During the follow-up periods of 1 month, 3 months, and 6 months after the operation, the weights of the two groups decreased significantly at 1 month after the operation, but there was no significant difference between the two groups. However, the weight loss of the improved group decreased less than that of the Orr group at 3 months and 6 months after the operation (P < 0.05). The hemoglobin level of the Orr group decreased significantly compared with that of the modified group at 1 month and 3 months after the operation (P < 0.05), but there was no significant difference between the two groups at 6 months after the operation (P > 0.05). Our results revealed that patients in the improved group had better nutritional status than those in the earlier group did, which may be related to more recent complications and poorer postoperative status in the former group; however, the underlying mechanism must be further explored. Research conducted by Yang et al[27] explored the impact of different gastrointestinal reconstruction methods on postoperative intestinal microbiota composition and diversity and nutrient absorption. This study highlighted the need to consider alterations to the gastrointestinal microflora when selecting a reconstruction approach for GC surgery. The authors also emphasized the need for further metabolomics analyses to elucidate these effects in the context of GC surgery.
In conclusion, compared with traditional Roux-en-Y digestive tract reconstruction after total gastrectomy, improved Roux-en-Y has the advantages of safety, simplicity and bile reflux reduction and can also shorten the operation time and reduce the occurrence of postoperative complications, which adapts to the development trend of modern rapid rehabilitation surgery and may lead to a better prognosis and longer OS time. It is an ideal method of digestive tract recon
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21370] [Article Influence: 2137.0] [Reference Citation Analysis (3)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55833] [Article Influence: 7976.1] [Reference Citation Analysis (132)] |
| 3. | Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, Wang C, Qiu MZ, Cai MY, Wu Q, Liu H, Guan WL, Zhou AP, Zhang YJ, Liu TS, Bi F, Yuan XL, Rao SX, Xin Y, Sheng WQ, Xu HM, Li GX, Ji JF, Zhou ZW, Liang H, Zhang YQ, Jin J, Shen L, Li J, Xu RH. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41:747-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 463] [Article Influence: 115.8] [Reference Citation Analysis (1)] |
| 4. | Rupp SK, Stengel A. Influencing Factors and Effects of Treatment on Quality of Life in Patients With Gastric Cancer-A Systematic Review. Front Psychiatry. 2021;12:656929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Liu YH, Meng R, Zhu B, Zhan QQ, Yang X, Ding GY, Jia CL, Xu WG. A meta-analysis of the efficacy of Roux-en-Y anastomosis and jejunal interposition after total gastrectomy. World J Surg Oncol. 2023;21:136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Shen J, Ma X, Yang J, Zhang JP. Digestive tract reconstruction options after laparoscopic gastrectomy for gastric cancer. World J Gastrointest Oncol. 2020;12:21-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Hoya Y, Mitsumori N, Yanaga K. The advantages and disadvantages of a Roux-en-Y reconstruction after a distal gastrectomy for gastric cancer. Surg Today. 2009;39:647-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Xu Z, Guo W. [Clinical research progress of mesenteric internal hernia after Roux-en-Y reconstruction]. Zhonghua Wei Chang Wai Ke Za Zhi. 2017;20:352-356. [PubMed] |
| 9. | Tu BL, Kelly KA. Surgical treatment of Roux stasis syndrome. J Gastrointest Surg. 1999;3:613-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Pan Y, Li Q, Wang DC, Wang JC, Liang H, Liu JZ, Cui QH, Sun T, Zhang RP, Kong DL, Hao XS. Beneficial effects of jejunal continuity and duodenal food passage after total gastrectomy: a retrospective study of 704 patients. Eur J Surg Oncol. 2008;34:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Mongin C, Zinzindohoue F. Jejunal J-pouch with Roux-en-Y esophagojejunostomy after total gastrectomy. J Visc Surg. 2013;150:341-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Chaiyasate K, Jacobs M, Brooks SE, del Rosario G, Andrus L, Kestenberg W, Mittal V. The uncut Roux-en-Y with jejunal pouch: a new reconstruction technique for total gastrectomy. Surgery. 2007;142:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Hangtian C, Huabing H, Tianhang L, Xiaoyi Y, Guoen F. Isoperistaltic versus antiperistaltic uncut Roux-en-Y anastomosis after distal gastrectomy for gastric cancer: a propensity score matched analysis. BMC Surg. 2020;20:274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Ye XS, Lin X, Liu JJ, Shi Y, Qian F, Yu PW, Zhao YL. [Comparison of clinical efficacy and quality of life between uncut Roux-en-Y and Billroth II with Braun anastomosis in laparoscopic distal gastrectomy for gastric cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2022;25:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Umemura A, Koeda K, Sasaki A, Fujiwara H, Kimura Y, Iwaya T, Akiyama Y, Wakabayashi G. Totally laparoscopic total gastrectomy for gastric cancer: literature review and comparison of the procedure of esophagojejunostomy. Asian J Surg. 2015;38:102-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Gastric Cancer Professional Committee; Chinese Anticancer Association; Oncogastroenterology Professional Committee, Chinese Anticancer Association. [Chinese experts consensus on standardized surgical management of specimens from radical gastrectomy (2022 edition)]. Zhonghua Wei Chang Wai Ke Za Zhi. 2022;25:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Nakane Y, Okumura S, Akehira K, Okamura S, Boku T, Okusa T, Tanaka K, Hioki K. Jejunal pouch reconstruction after total gastrectomy for cancer. A randomized controlled trial. Ann Surg. 1995;222:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 85] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1338] [Article Influence: 334.5] [Reference Citation Analysis (2)] |
| 19. | Liu F, Qin X. [Clinical research status of laparoscopic total gastrectomy in China]. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21:121-125. [PubMed] |
| 20. | Zhang JZ, Lu HS, Wu XY, Huang CM, Wang C, Guan GX, Zhang XF. [Influence of different procedures of alimentary tract reconstruction after total gastrectomy for gastric cancer on the nutrition and metabolism of patients: a prospective clinical study]. Zhonghua Yi Xue Za Zhi. 2003;83:1475-1478. [PubMed] |
| 21. | Tse G, Lai ET, Yeo JM, Tse V, Wong SH. Mechanisms of Electrical Activation and Conduction in the Gastrointestinal System: Lessons from Cardiac Electrophysiology. Front Physiol. 2016;7:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Ishigami S, Natsugoe S, Hokita S, Aoki T, Kashiwagi H, Hirakawa K, Sawada T, Yamamura Y, Itoh S, Hirata K, Ohta K, Mafune K, Nakane Y, Kanda T, Furukawa H, Sasaki I, Kubota T, Kitajima M, Aikou T. Postoperative long-term evaluation of interposition reconstruction compared with Roux-en-Y after total gastrectomy in gastric cancer: prospective randomized controlled trial. Am J Surg. 2011;202:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Picardi N, Santeusanio E, Tucci G. Study of the antireflux function of the Roux-en-y jejunal loop in reconstruction following gastrectomy. Ann Ital Chir. 2002;73:263-266. [PubMed] |
| 24. | Wang LJ, Wang S, Xu ZK. [History of digestive tract reconstruction after gastrectomy]. Zhonghua Wei Chang Wai Ke Za Zhi. 2024;27:1004-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Xie H, Wu F, Huang C, Chen Q, Ni Z, Wang S, Ge B, Liu L, Huang Q. Tranditional Roux-en-Y vs Uncut Roux-en-Y in Laparoscopic Distal Gastrectomy: a Randomized Controlled Study. J Gastrointest Surg. 2023;27:1098-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Han Y, Guo J, Huang Y, Xu D. Clinical comparison of total gastrectomy with single-vessel transection Roux-en-Y reconstruction vs total gastrectomy with conventional Roux-en-Y reconstruction for proximal gastric cancer. J Gastrointest Surg. 2024;28:1591-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Yang Y, Zhou HY, Zhou GM, Chen J, Ming R, Zhang D, Jiang HW. The impact of different gastrointestinal reconstruction techniques on gut microbiota after gastric cancer surgery. Front Microbiol. 2024;15:1494049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |