Published online Jun 27, 2025. doi: 10.4240/wjgs.v17.i6.104192
Revised: March 7, 2025
Accepted: May 7, 2025
Published online: June 27, 2025
Processing time: 107 Days and 3.3 Hours
Although surgery remains the primary treatment for proximal gastric cancer (PGC), ongoing refinements in surgical strategies are essential to improving clinical outcomes.
To investigate the effect of double-tract reconstruction (DTR) on immune function and stress response in patients undergoing laparoscopic proximal gastrectomy (LPG).
In total, 78 patients with PGC admitted between August 2020 and August 2024 were enrolled. The research group consisted of 39 patients who underwent DTR + LPG, whereas the control group comprised 39 patients who underwent laparoscopic total gastrectomy with Roux-en-Y esophagojejunostomy. Perioperative indices (intraoperative blood loss, digestive tract anastomosis time, and time to first postoperative flatus), postoperative complications (intestinal obstruction, anastomotic ulcer, diarrhea, dumping syndrome, and gastroesophageal reflux), nutritional parameters (serum albumin, hemoglobin, and body mass index), immune function [immunoglobulin (Ig) G, IgA, and IgM), and stress response indicators (C-reactive protein, interleukin-6, and tumor necrosis factor-α) were collected and analyzed for both groups.
The intraoperative blood loss was lower (P < 0.05), and the time to first posto
The combination of DTR and LPG in the treatment of patients with PGC is more effective in enhancing immune function and suppressing stress responses, showing more advantages over laparoscopic total gastrectomy.
Core Tip: This study focused on patients with proximal gastric cancer (PGC), enrolling 78 cases to compare the clinical outcomes of two digestive reconstruction techniques: Double-tract reconstruction following laparoscopic proximal gastrectomy vs Roux-en-Y esophagojejunostomy following laparoscopic total gastrectomy, and optimize therapeutic strategies for PGC. The results demonstrate that double-tract reconstruction + laparoscopic proximal gastrectomy offers superior clinical benefits to laparoscopic total gastrectomy + Roux-en-Y reconstruction, including reduced intraoperative blood loss, a shorter time to first postoperative flatus (indicating faster postoperative recovery), enhanced safety, improved preservation of nutritional status and immune function, and a mitigated postoperative stress response. This study provides critical insights for refining surgical protocols in PGC management.
- Citation: Qiu TH, Wen HY, Chen MM. Effect of double-tract reconstruction and laparoscopic proximal gastrectomy on immune function and stress. World J Gastrointest Surg 2025; 17(6): 104192
- URL: https://www.wjgnet.com/1948-9366/full/v17/i6/104192.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i6.104192
Gastric cancer (GC) represents a malignant neoplasm associated with a substantial disease burden and a relatively high lethality rate[1]. Although global cancer statistics indicate a reduction in the incidence and mortality risk of GC, the disease still accounted for nearly 1.1 million newly diagnosed cases and a staggering 800000 fatalities in 2020[2]. Depending on the anatomical location of the tumor, GC is classified into proximal GC (PGC) and distal GC (DGC). In PGC, the tumor is located in the upper one-third of the stomach (including gastric cardia cancer and gastric fundus cancer), whereas in DGC, the tumor is situated in the lower one-third of the stomach[3,4].
The clinical manifestations of GC vary depending on the tumor location. Patients with PGC typically present with retrosternal pain and progressive dysphagia, whereas those with DGC commonly exhibit reflux, eructation, or nausea[5]. Xue et al[6] noted that, compared with patients with DGC, those with PGC exhibited lower 1-year, 3-year, and 5-year overall survival rates, implying that this subset of patients may have a poorer prognosis than those with DGC. This study focused on patients with PGC to optimize therapeutic approaches for PGC management.
Surgery remains the primary treatment modality for patients with PGC and is classified as either laparoscopic total gastrectomy (LTG) or laparoscopic proximal gastrectomy (LPG) based on the extent of resection[7]. Despite the enhanced efficacy of surgery in recent years, there remains substantial room for improvement[8].
This study aimed to conduct a comparative analysis between LTG + Roux-en-Y esophagojejunostomy and double-tract reconstruction (DTR) + LPG. LTG + Roux-en-Y esophagojejunostomy represents a commonly employed surgical approach with advantages such as lymph node dissection and anti-reflux properties. However, this approach also harbors potential drawbacks, including protracted operative duration, increased blood loss, and a heightened risk of postoperative complications[9,10]. LPG, though a simple procedure, disrupts the lower esophageal sphincter and cardia structures, often leading to adverse postoperative reactions such as acid reflux and heartburn[11]. In contrast, DTR + LPG can precisely prevent reflux and offer advantages in enhancing nutritional status and quality of life[12]. Following DTR + LPG, food can enter the distal digestive tract via the remnant stomach or jejunum, which not only mitigates esophageal reflux after proximal gastrectomy but also preserves the storage and digestive functions of the remnant stomach[13,14]. Kim and Kim[15] elucidated that DTR + LPG is more conducive to the absorption of iron and vitamin B12 and is beneficial for the prevention of reflux esophagitis compared with LTG. Studies investigating the effects of DTR + LPG on immune function and stress response in patients with PGC remain relatively limited. We believe that supplementing relevant analyses will further augment the potential biomedical applications of this technique.
Inclusion criteria: All patients were confirmed to meet the diagnostic criteria for LPG following preoperative endoscopic biopsy pathological examination and were classified as stage I or II according to the 8th edition of the American Joint Committee on Cancer clinical staging system for GC. Preoperative imaging indicated that the lesions were located in the upper one-third of the stomach. All procedures were performed endoscopically, and the postoperative pathological diagnosis confirmed R0 resection. Patients had no contraindications for laparoscopic surgery and no history of abdominal surgery.
Exclusion criteria: Coexistence of other malignant tumors; pregnancy or lactation; presence of underlying diseases such as immune system diseases, coagulation disorders, and metabolic disorders; other types of GC that did not meet the indications for radical gastrectomy; severe cardiovascular, pulmonary, cerebral, and renal insufficiency and other diseases; and psychological ailments or mental disorders.
Based on these strict inclusion and exclusion criteria, 78 patients with PGC admitted to the Third Affiliated Hospital of Chengdu Medical College, Pidu District People’s Hospital, were meticulously screened and enrolled in the study. The enrollment period spanned from August 2020 to August 2024. Among them, 39 patients in the research group underwent DTR + LPG, whereas 39 in the control group underwent LTG + Roux-en-Y esophagojejunostomy. The hospital’s ethics committee approved this study without reservation. The intergroup comparison of general characteristics revealed no notable differences (P > 0.05), ensuring clinical comparability.
After admission, both groups underwent surgery performed by the same surgical team. The control group underwent LTG + Roux-en-Y esophagojejunostomy. After general anesthesia, the patient was placed in a supine position. Laparoscopic exploration was performed using the five-port method, the lymph nodes were cleaned, and the esophagus and gastric body were dissociated. Subsequently, a 5-cm incision was made in the midline of the upper abdomen. The duodenum was precisely transected at a site approximately 2 cm distal to the pylorus, and the lower esophagus was sutured approximately 3 cm from the cardia. Thereafter, the stomach and lower esophagus were ligated. An incision was then created on the right side of the esophagus above the ligation, and a small hole was made in the mesenteric margin of the jejunum approximately 30 cm from the ligament of Treitz. A side-to-side anastomosis was performed between the right end of the esophagus and proximal jejunum. The residual esophageal tissue was resected, and a side-to-side anastomosis of the jejunal Y-loop was performed approximately 40 cm from the jejunal anastomosis. Before completing these procedures, the bleeding condition was checked, and the common opening was closed.
The research group underwent DTR + LPG. The preoperative preparations and lymph node dissection procedures were identical to those implemented in the control group. For LPG, a 5-cm incision was made precisely in the midline of the upper abdomen to sever the esophagus. Subsequently, the proximal stomach was resected at a distance of 3 cm from the proximal tumor margin. For DTR, the jejunum and its mesenteric border were transected at a site 15 cm away from the ligament of Treitz, and the jejunum, colon, and esophageal stump were then anastomosed end-to-side. Thereafter, a side-to-side anastomosis between the remnant stomach and jejunum was performed at a distance ranging from 15 to 20 cm from the jejunoesophageal anastomosis site. In addition, the proximal and distal jejunum were anastomosed at a distance of approximately 30 cm from the remnant gastric-jejunal anastomosis site.
Perioperative indices: Intraoperative blood loss, digestive tract anastomosis time, and time to the first postoperative flatus in both groups were thoroughly observed and documented.
Postoperative complications: The occurrence of side effects, such as intestinal obstruction, anastomotic ulcer, diarrhea, dumping syndrome, and gastroesophageal reflux, was monitored and recorded in both groups following treatment. The overall incidence rate was subsequently computed.
Nutritional parameters: Five milliliters of fasting venous blood was collected from patients in the early morning before surgery and 1, 2, and 3 months postoperatively. After centrifugation, the serum was procured to quantify serum albumin and hemoglobin (Hb) levels using an automatic biochemical analyzer. The body mass index (BMI) was also calculated.
Immune function: The levels of immunoglobulin (Ig) G, IgA, and IgM in the serum were quantified using an enzyme-linked immunosorbent assay before surgery and on postoperative day 1.
Stress response indicators: Fasting blood samples were drawn in the early morning before surgery and on postoperative days 1, 3, and 5 to detect C-reactive protein (CRP), interleukin (IL)-6, and tumor necrosis factor-α (TNF-α).
Measurement data were expressed as the mean ± SEM. Between-group comparison of measurement data was conducted using an independent sample t-test, whereas comparisons before and after treatment were performed using a paired t-test. The count data are presented as rates (percentage), and intergroup comparisons were conducted using a χ2 test or Fisher’s exact probability test. The experimental data collected were analyzed with IBM SPSS Statistics version 20.0 (IBM Corp., Armonk, NY, United States). A P value < 0.05 indicated a significant difference.
No significant difference in general information such as age, sex, tumor diameter, lymphatic vessel infiltration, differentiation grade, and pathological stage was identified between the research and control groups (P > 0.05) (Table 1).
| Indicators | Research group (n = 39) | Control group (n = 39) | t/χ2 | P value |
| Age (years) | 58.31 ± 7.30 | 55.69 ± 6.52 | 1.672 | 0.099 |
| Sex (male/female) | 12/27 | 18/21 | 1.950 | 0.163 |
| Tumor diameter (cm) | 2.33 ± 0.99 | 1.95 ± 0.68 | 1.976 | 0.052 |
| Lymphatic vessel infiltration (positive/negative) | 8/31 | 9/30 | 0.075 | 0.784 |
| Differentiation degree (high differentiation/moderate-low differentiation) | 6/33 | 5/34 | 0.106 | 0.745 |
| Preoperative imaging staging (stage I/stage II) | 5/34 | 4/35 | 0.126 | 0.723 |
Compared with the control group, the research group exhibited less intraoperative blood loss (P < 0.05), comparable digestive tract anastomosis time (P > 0.05), and shorter time to the first postoperative flatus (P < 0.001) (Table 2).
| Indicators | Research group (n = 39) | Control group (n = 39) | t | P value |
| Intraoperative blood loss (mL) | 219.03 ± 53.68 | 245.08 ± 38.65 | 2.459 | 0.016 |
| Digestive tract anastomosis time (minutes) | 30.08 ± 5.64 | 33.51 ± 9.44 | 1.948 | 0.055 |
| Time to first postoperative anal exhaust (days) | 2.44 ± 0.85 | 3.10 ± 0.79 | 3.552 | < 0.001 |
The total complication incidence rate was significantly lower in the research group than in the control group based on the number of cases and incidence rates of intestinal obstruction, anastomotic ulcer, diarrhea, dumping syndrome, and gastroesophageal reflux (P = 0.042) (Table 3).
| Indicators | Research group (n = 39) | Control group (n = 39) | χ2 | P value |
| Intestinal obstruction | 0 (0.00) | 0 (0.00) | ||
| Anastomotic ulcer | 0 (0.00) | 0 (0.00) | ||
| Diarrhea | 1 (2.56) | 1 (2.56) | ||
| Dumping syndrome | 0 (0.00) | 4 (10.26) | ||
| Gastroesophageal reflux | 1 (2.56) | 3 (7.69) | ||
| Total | 2 (5.13) | 8 (20.51) | 4.129 | 0.042 |
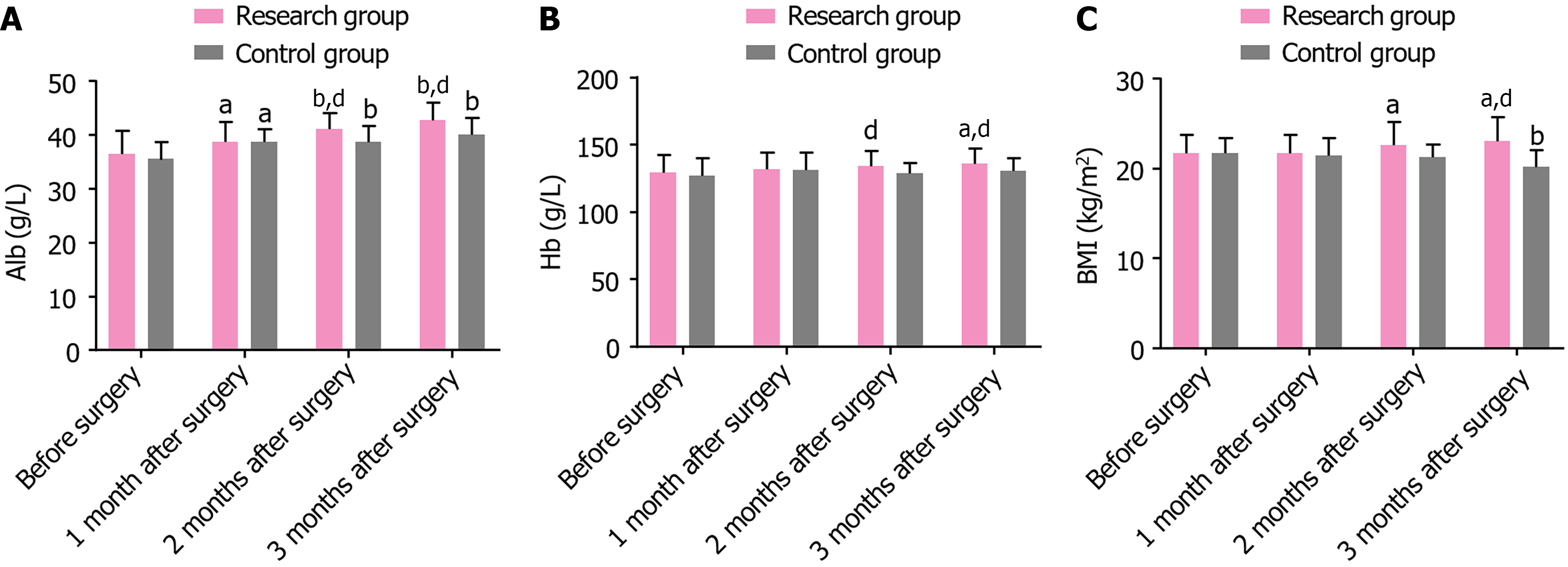
Preoperative albumin, Hb, and BMI were not significantly different between the two groups (P > 0.05). At 1, 2, and 3 months postoperatively, all indices in both groups (except for Hb and BMI in the control group) exhibited an upward trend. Moreover, at 2 and 3 months after the surgery, all indices were remarkably higher in the research group than in the control group (P < 0.05) (Table 4 and Figure 1).
| Indicators | Research group (n = 39) | Control group (n = 39) | t | P value |
| Alb (g/L) | ||||
| Before surgery | 36.51 ± 4.23 | 35.49 ± 3.20 | 1.201 | 0.234 |
| 1 month after surgery | 38.72 ± 3.68a | 38.74 ± 2.41a | 0.028 | 0.977 |
| 2 months after surgery | 41.15 ± 2.95b | 38.72 ± 2.99b | 3.613 | < 0.001 |
| 3 months after surgery | 42.79 ± 3.18b | 40.08 ± 3.14b | 3.787 | < 0.001 |
| Hb (g/L) | ||||
| Before surgery | 129.08 ± 13.70 | 127.18 ± 13.31 | 0.621 | 0.536 |
| 1 month after surgery | 131.56 ± 12.94 | 131.36 ± 13.13 | 0.068 | 0.946 |
| 2 months after surgery | 134.21 ± 11.08 | 128.95 ± 7.39 | 2.466 | 0.016 |
| 3 months after surgery | 135.87 ± 11.16a | 130.41 ± 9.57 | 2.319 | 0.023 |
| BMI (kg/m2) | ||||
| Before surgery | 21.69 ± 2.03 | 21.72 ± 1.70 | 0.071 | 0.944 |
| 1 month after surgery | 21.72 ± 2.01 | 21.46 ± 1.96 | 0.578 | 0.565 |
| 2 months after surgery | 22.62 ± 2.60 | 21.23 ± 1.46 | 2.911 | 0.005 |
| 3 months after surgery | 23.05 ± 2.69a | 20.15 ± 1.91b | 5.489 | < 0.001 |
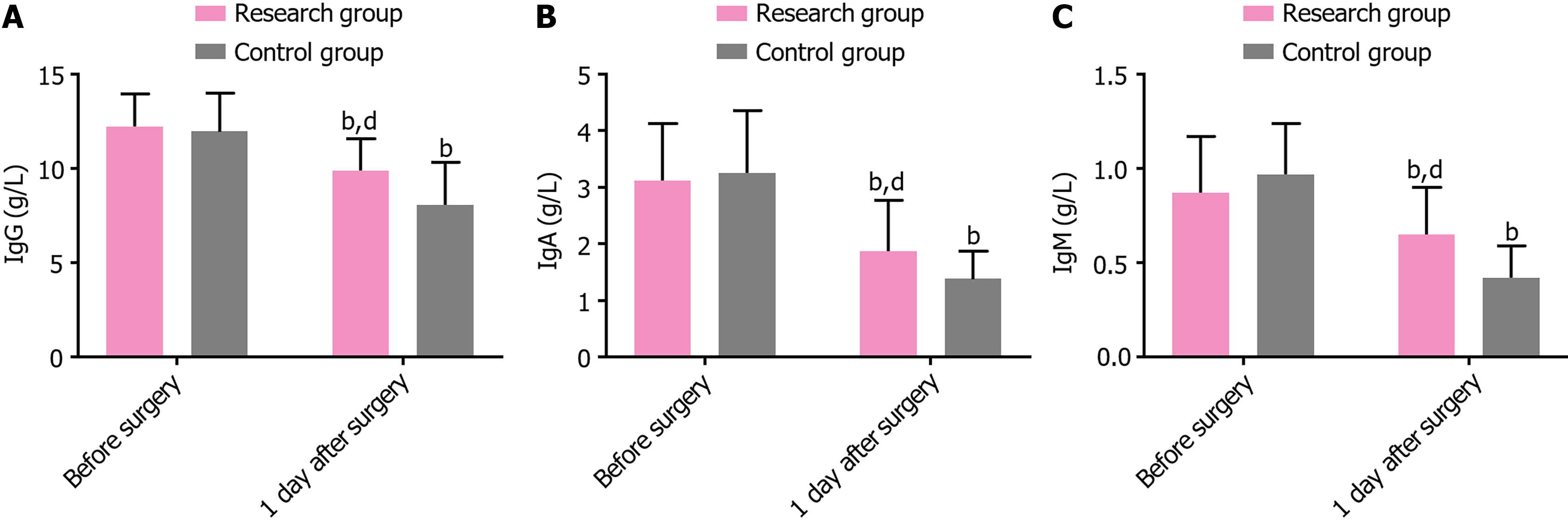
The two groups exhibited comparable preoperative IgG, IgA, and IgM levels (P > 0.05). Postoperatively, these indexes significantly decreased in both groups (P < 0.05); however, their levels remained higher in the research group than in the control group (P < 0.05) (Table 5 and Figure 2).
| Indicators | Research group (n = 39) | Control group (n = 39) | t | P value |
| IgG (g/L) | ||||
| Before surgery | 12.23 ± 1.72 | 11.97 ± 2.03 | 0.610 | 0.544 |
| 1 day after surgery | 9.90 ± 1.68b | 8.05 ± 2.28b | 4.079 | < 0.001 |
| IgA (g/L) | ||||
| Before surgery | 3.12 ± 1.01 | 3.26 ± 1.10 | 0.585 | 0.560 |
| 1 day after surgery | 1.87 ± 0.90b | 1.38 ± 0.49b | 2.986 | 0.004 |
| IgM (g/L) | ||||
| Before surgery | 0.87 ± 0.30 | 0.97 ± 0.27 | 1.547 | 0.126 |
| 1 day after surgery | 0.65 ± 0.25b | 0.42 ± 0.17b | 4.751 | < 0.001 |
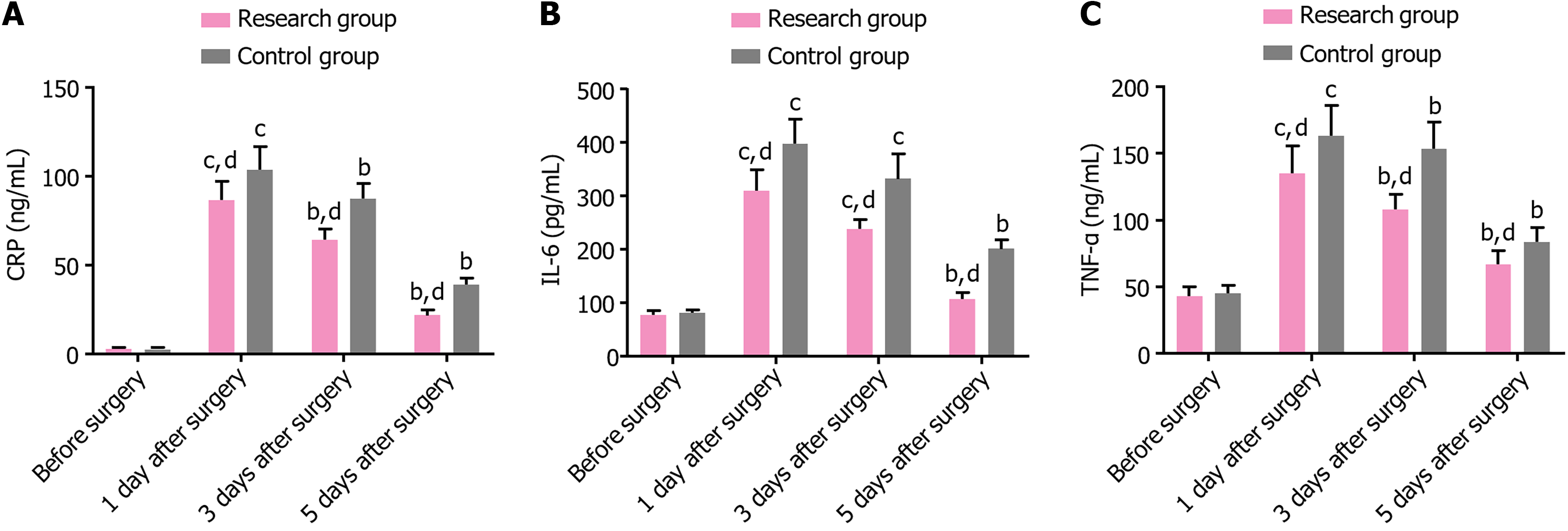
No significant differences were noted in the preoperative levels of CRP, IL-6, and TNF-α between the two groups (P > 0.05). On postoperative day 1, these indicators significantly increased in both groups. Subsequently, on postoperative days 3 and 5, both groups exhibited a gradual decline decrease in these indicators. In addition, all the indicators were considerably lower in the research group than in the control group at every time point postoperatively (P < 0.05) (Table 6 and Figure 3).
| Indicators | Research group (n = 39) | Control group (n = 39) | t | P value |
| CRP (ng/mL) | ||||
| Before surgery | 3.05 ± 0.69 | 2.95 ± 0.65 | 0.659 | 0.512 |
| 1 day after surgery | 86.64 ± 10.66c | 103.67 ± 12.71c | 6.411 | < 0.001 |
| 3 days after surgery | 64.36 ± 5.86b | 87.33 ± 8.58b | 13.806 | < 0.001 |
| 5 days after surgery | 21.77 ± 2.92b | 38.92 ± 3.72b | 22.647 | < 0.001 |
| IL-6 (pg/mL) | ||||
| Before surgery | 78.33 ± 6.69 | 81.05 ± 6.39 | 1.836 | 0.070 |
| 1 day after surgery | 310.77 ± 38.79c | 398.13 ± 45.78c | 9.092 | < 0.001 |
| 3 days after surgery | 238.00 ± 18.60c | 332.64 ± 45.73c | 11.972 | < 0.001 |
| 5 days after surgery | 106.97 ± 12.24b | 201.36 ± 17.14b | 27.988 | < 0.001 |
| TNF-α (ng/mL) | ||||
| Before surgery | 43.08 ± 7.19 | 45.44 ± 5.70 | 1.606 | 0.112 |
| 1 day after surgery | 134.85 ± 20.56c | 163.03 ± 22.68c | 5.749 | < 0.001 |
| 3 days after surgery | 107.92 ± 11.51b | 153.54 ± 20.08b | 12.309 | < 0.001 |
| 5 days after surgery | 66.72 ± 10.45b | 83.74 ± 10.45b | 7.192 | < 0.001 |
DTR + LPG demonstrated remarkable clinical advantages by reducing intraoperative blood loss and facilitating a quicker onset of the first postoperative flatus without affecting the time required for digestive tract anastomosis. This finding suggests that DTR + LPG confers significant clinical benefits in curtailing intraoperative blood loss and hastening the time to the first postoperative flatus in such patients.
In the study by Sun et al[16], performing DTR + LPG in patients with PGC and those with adenocarcinoma of the esophagogastric junction resulted in minimal blood loss without substantially extending operative time, which is consistent with the results of the present study. Xiao et al[17] reported that patients with PGC who underwent DTR + LPG experienced significantly shorter times to first postoperative flatus and postoperative hospitalization, corroborating our observations. DTR involves placing a jejunal segment between the esophagus and the remnant stomach. This not only preserves partial gastric function but also prevents severe postoperative gastroesophageal reflux[18]. With this technique, food reaches the jejunum directly and mixes thoroughly with bile, expediting the emptying of food in the remnant stomach and effectively curtailing the time to the first postoperative flatus[19].
From a safety perspective, the control group predominantly exhibited dumping syndrome (10.26%), followed by gastroesophageal reflux (7.69%) and diarrhea (2.56%). Conversely, the research group primarily presented with diarrhea (2.56%) and gastroesophageal reflux (2.56%), which was consistent with the findings of Hirata et al[20]. DTR + LPG proves to be more advantageous in reducing the overall incidence of postoperative complications among patients with PGC. This could be ascribed to the buffering effect of DTR on food, which aids in alleviating the stimulation of the gastric antrum by food and in circumventing gastric emptying impairments. This is conducive to mitigating the risks associated with gastroesophageal reflux and dumping syndrome[21].
As regards nutritional parameters, DTR + LPG more effectively improves the nutritional status of patients with PGC. After DTR + LPG, the duodenum and distal stomach are preserved, allowing food to enter the distal gastric cavity and distal jejunum through the esophagojejunostomy. This promotes full contact between food and digestive juices, thereby actively regulating gastrointestinal function and gastric hormone secretion and enhancing the postoperative nutritional status of patients[22]. The DTR + LPG technique is superior to LTG in preventing vitamin B12 deficiency among patients with early GC, implying that patients undergoing DTR + LPG intervention may experience better nutritional outcomes[23]. Wang et al[24] reported that patients with PGC who underwent DTR + LPG experienced fewer reflux symptoms and had higher BMI levels than those who underwent LTG + Roux-en-Y esophagojejunostomy, which concurs with the findings of the present study.
With regard to immune function, DTR + LPG has a relatively less pronounced effect on the immune function of patients with PGC. In terms of stress indexes, DTR + LPG exerts a relatively limited influence on CRP, IL-6, and TNF-α levels in patients with PGC, indicating that DTR + LPG contributes to a milder postoperative stress response and a more rapid recovery process. CRP, an acute-phase protein, is rapidly synthesized and secreted into the bloodstream by the liver following stress stimuli such as infection, inflammation, tissue injury, surgical trauma, and myocardial infarction. Elevated CRP levels are indicative of the body’s stress response. Similarly, IL-6, a versatile cytokine intimately associated with the advancement of inflammation, surges promptly in the face of stress triggers like infection, trauma, and ischemia, marking it as an early stress response indicator. Meanwhile, TNF-α, which is swiftly generated and discharged by immune cells upon encountering stress stimuli including infection, ischemia, and trauma, also denotes the body’s stress condition when found in elevated concentrations[25,26]. Consequently, this study evaluated the stress status of patients subjected to the two treatment approaches by monitoring these specific biomarkers. DTR + LPG helps reduce patient reflux symptoms, improve their nutritional status, preserve the function of the pylorus, decrease surgical trauma, and lower the risk of postoperative complications. These factors help minimize the effect on immune function and stress responses[27,28].
Numerous researchers have extensively explored and clinically analyzed various alternative treatment approaches. For instance, Huang et al[29] pointed out that the double-flap technique, when compared with DTR in patients with PGC, can result in a more significant improvement in postoperative nutritional status. However, it has higher requirements and is more time-consuming. Likewise, Yang et al[30] indicated that double-tract anastomosis and esophagogastric anastomosis in patients who underwent LPG could relieve the postoperative esophageal reflux symptoms more effectively and improve short-term nutritional status. Furthermore, Zhang et al[31] noted that a side overlap with fundoplication by the Yamashita technique performed in patients who underwent LPG further promoted postoperative recovery in terms of weight and eating function compared with LTG + Roux-en-Y esophagojejunostomy. Nevertheless, it also entailed a relatively high risk of postoperative reflux.
This study has some limitations that warrant further investigation. First, the time points for measuring immune function data are limited; immune parameters were evaluated only preoperatively and on postoperative day 1. To comprehensively assess the dynamic effect of surgical strategies on immune homeostasis, future studies should incorporate longitudinal data to clarify the temporal relationship between immune recovery and clinical outcomes. Second, as a single-center study with a modest sample size, the conclusions were derived from a single-center cohort of 78 patients. In subsequent analyses, analyzing clinical records of a wider range of patients from multiple centers will enhance the generalizability of the research results. Subsequent research efforts will be centered on these aspects to enhance the quality and scope of the study.
Compared with patients with PGC who underwent LTG + Roux-en-Y esophagojejunostomy, those who underwent DTR + LPG exhibited reduced intraoperative blood loss, shorter time to the first postoperative flatus, enhanced safety, remarkably improved nutritional status and immune function, and a milder postoperative stress response.
| 1. | López MJ, Carbajal J, Alfaro AL, Saravia LG, Zanabria D, Araujo JM, Quispe L, Zevallos A, Buleje JL, Cho CE, Sarmiento M, Pinto JA, Fajardo W. Characteristics of gastric cancer around the world. Crit Rev Oncol Hematol. 2023;181:103841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 165] [Reference Citation Analysis (3)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64672] [Article Influence: 16168.0] [Reference Citation Analysis (176)] |
| 3. | Zhang Y, Zhang PS, Rong ZY, Huang C. One stomach, two subtypes of carcinoma-the differences between distal and proximal gastric cancer. Gastroenterol Rep (Oxf). 2021;9:489-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2873] [Article Influence: 205.2] [Reference Citation Analysis (0)] |
| 5. | Gong S, Li X, Tian H, Song S, Lu T, Jing W, Huang X, Xu Y, Wang X, Zhao K, Yang K, Guo T. Clinical efficacy and safety of robotic distal gastrectomy for gastric cancer: a systematic review and meta-analysis. Surg Endosc. 2022;36:2734-2748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Xue J, Yang H, Huang S, Zhou T, Zhang X, Zu G. Comparison of the overall survival of proximal and distal gastric cancer after gastrectomy: a systematic review and meta-analysis. World J Surg Oncol. 2021;19:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Kano Y, Ohashi M, Nunobe S. Laparoscopic Function-Preserving Gastrectomy for Proximal Gastric Cancer or Esophagogastric Junction Cancer: A Narrative Review. Cancers (Basel). 2023;15:311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Tan L, Ran MN, Liu ZL, Tang LH, Ma Z, He Z, Xu Z, Li FH, Xiao JW. Comparison of the prognosis of four different surgical strategies for proximal gastric cancer: a network meta-analysis. Langenbecks Arch Surg. 2022;407:63-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Yamashita H, Seto Y, Sano T, Makuuchi H, Ando N, Sasako M; Japanese Gastric Cancer Association and the Japan Esophageal Society. Results of a nation-wide retrospective study of lymphadenectomy for esophagogastric junction carcinoma. Gastric Cancer. 2017;20:69-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 150] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 10. | Masuzawa T, Takiguchi S, Hirao M, Imamura H, Kimura Y, Fujita J, Miyashiro I, Tamura S, Hiratsuka M, Kobayashi K, Fujiwara Y, Mori M, Doki Y. Comparison of perioperative and long-term outcomes of total and proximal gastrectomy for early gastric cancer: a multi-institutional retrospective study. World J Surg. 2014;38:1100-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Peng W, Yan S, Huang Y, Cheng M, Liu T, Ren R, Chen Q, Zhang J, Gong W, Xing C, Wu Y. Laparoscopic proximal gastrectomy with right-sided overlap and single-flap valvuloplasty (ROSF): a case-series study. BMC Surg. 2023;23:90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (1)] |
| 12. | Irino T, Ohashi M, Hayami M, Makuuchi R, Ri M, Sano T, Yamaguchi T, Nunobe S. Updated Review of Proximal Gastrectomy for Gastric Cancer or Cancer of the Gastroesophageal Junction. J Gastric Cancer. 2025;25:228-246. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Aburatani T, Kojima K, Otsuki S, Murase H, Okuno K, Gokita K, Tomii C, Tanioka T, Inokuchi M. Double-tract reconstruction after laparoscopic proximal gastrectomy using detachable ENDO-PSD. Surg Endosc. 2017;31:4848-4856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Stegniy KV, Maslyantsev EV, Goncharuk RA, Krekoten AA, Kulakova TA, Dvoinikova ER. Double-tract reconstruction for oesofagocardial gastric cancer: A systematic review. Ann Med Surg (Lond). 2021;67:102496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Kim DJ, Kim W. Laparoscopy-assisted Proximal Gastrectomy with Double Tract Anastomosis Is Beneficial for Vitamin B12 and Iron Absorption. Anticancer Res. 2016;36:4753-4758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Sun Y, Chen C, Hou L, Zhao E. Short-term outcomes and quality of life of esophagogastrostomy versus the double-tract reconstruction after laparoscopic proximal gastrectomy. BMC Cancer. 2024;24:1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Xiao SM, Zhao P, Ding Z, Xu R, Yang C, Wu XT. Laparoscopic proximal gastrectomy with double-tract reconstruction for upper third gastric cancer. BMC Surg. 2021;21:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 18. | Li Y, Wu J, Han M, Li W, Bi Z. Modified Double-Tract Reconstruction in Gastrointestinal Reconstruction after Proximal Gastrectomy. J Coll Physicians Surg Pak. 2024;34:1374-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Liu BY, Wu S, Xu Y. Clinical efficacy and safety of double-channel anastomosis and tubular gastroesophageal anastomosis in gastrectomy. World J Gastrointest Surg. 2024;16:2012-2022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (5)] |
| 20. | Hirata Y, Kim HI, Grotz TE, Matsuda S, Badgwell BD, Ikoma N. The role of proximal gastrectomy in gastric cancer. Chin Clin Oncol. 2022;11:39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Zhu G, Jiao X, Zhou S, Zhu Q, Yu L, Sun Q, Li B, Fu H, Huang J, Lang W, Lang X, Zhai S, Xiong J, Fu Y, Liu C, Qu J. Can proximal gastrectomy with double-tract reconstruction replace total gastrectomy? a meta-analysis of randomized controlled trials and propensity score-matched studies. BMC Gastroenterol. 2024;24:230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Fujimoto D, Taniguchi K, Kobayashi H. Double-Tract Reconstruction Designed to Allow More Food Flow to the Remnant Stomach After Laparoscopic Proximal Gastrectomy. World J Surg. 2020;44:2728-2735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Park JY, Park KB, Kwon OK, Yu W. Comparison of laparoscopic proximal gastrectomy with double-tract reconstruction and laparoscopic total gastrectomy in terms of nutritional status or quality of life in early gastric cancer patients. Eur J Surg Oncol. 2018;44:1963-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Wang ZJ, Xu ZY, Huang ZJ, Li L, Guan D, Gao YH, Wang XX. Double tract reconstruction improves the quality of life and better maintain the BMI of patients with proximal gastric cancer. BMC Surg. 2024;24:171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Cusack B, Buggy DJ. Anaesthesia, analgesia, and the surgical stress response. BJA Educ. 2020;20:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 26. | Xu S, Hu S, Ju X, Li Y, Li Q, Wang S. Effects of intravenous lidocaine, dexmedetomidine, and their combination on IL-1, IL-6 and TNF-α in patients undergoing laparoscopic hysterectomy: a prospective, randomized controlled trial. BMC Anesthesiol. 2021;21:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Zhang Z, Zhao T, Wang Y, Xue F, Pu Y, Du Q, Wu Y. Comparison of proximal gastrectomy with tubular esophagogastric anastomosis and total gastrectomy with Roux-en-Y reconstruction in the treatment of adenocarcinoma of the esophagogastric junction of Siewert type II/III at stage II. BMC Surg. 2024;24:382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Zhang ZC, Wang WS, Chen JH, Ma YH, Luo QF, Li YB, Yang Y, Ma D. Perioperative outcomes of transvaginal specimen extraction laparoscopic total gastrectomy and conventional laparoscopic-assisted total gastrectomy. World J Gastrointest Surg. 2024;16:1527-1536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 29. | Huang QZ, Wang PC, Chen YX, Lin S, Ye K. Comparison of proximal gastrectomy with double-flap technique and double-tract reconstruction for proximal early gastric cancer: a meta-analysis. Updates Surg. 2023;75:2117-2126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 30. | Yang J, Zheng S, Li JJ, Li YL, Su R, Zheng X, Liu P, Zhao EH. Clinical application of laparoscopic continuous interposition jejunostomy with double-tract anastomosis and esophagogastric anastomosis: a retrospective study. Eur Rev Med Pharmacol Sci. 2023;27:9324-9332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 31. | Zhang H, Zheng Z, Liu X, Xin C, Huang Y, Li Y, Yin J, Zhang J. Safety and efficacy of laparoscopic proximal gastrectomy with SOFY versus laparoscopic total gastrectomy with Roux-en-Y for treating cT1-2 Siewert II/III adenocarcinoma of the esophagogastric junction: a single-center prospective cohort study. Langenbecks Arch Surg. 2023;408:69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |