Published online Jun 27, 2025. doi: 10.4240/wjgs.v17.i6.103635
Revised: March 23, 2025
Accepted: April 21, 2025
Published online: June 27, 2025
Processing time: 185 Days and 20.9 Hours
Underwater endoscopic mucosal resection (UEMR) has been shown to be a good treatment option for the management of nonpedunculated polyps ≥ 10 mm since its introduction. However, there is a paucity of randomized controlled trials (RCTs) in Asia.
To compare the efficacy and safety of UEMR with those of conventional EMR (CEMR) in treating nonpedunculated colorectal lesions.
We carried out this RCT at a tertiary hospital from October 2022 to July 2024. Patients with nonpedunculated colorectal neoplasms ranging from 10 mm to 30 mm in size were randomly assigned to either the UEMR or CEMR group. The primary outcome was the curative resection (R0) rate. The secondary outcomes included en bloc resection, procedure time, adverse events, and the number of clips used for defect closure.
A total of 260 patients with 260 lesions (130 in each UEMR and CEMR group) were recruited. The median age was 58 (27-85) years, the male/female ratio was 1.74, and the median lesion size was 20 (10-30 mm) mm. Compared with CEMR, UEMR was associated with a significantly greater curative resection (R0) rate (98.4% vs 90.3%; P = 0.007), greater en bloc resection rate (100% vs 94.6%; P = 0.014), shorter procedure time (65 vs 185 seconds; P < 0.001), lower rate of bleeding complications (1.5% vs 10%; P = 0.003), and fewer clips used (2 vs 3; P < 0.001). No perforations were observed in either group.
Compared with CEMR, UEMR has a higher R0 rate, greater en bloc resection rate, shorter procedure time, fewer bleeding complications, and clips used in the management of nonpedunculated colorectal neoplasms.
Core Tip: This randomized controlled trial compared underwater endoscopic mucosal resection (UEMR) and conventional EMR for nonpedunculated colorectal neoplasms (10-30 mm). UEMR demonstrated superior outcomes, including a significantly higher curative resection (R0) rate (98.4% vs 90.3%; P = 0.007), greater en bloc resection (100% vs 94.6%; P = 0.014), shorter procedure time (65 vs 185 seconds; P < 0.001), reduced bleeding risk (1.5% vs 10%; P = 0.003), and fewer hemostatic clips used (2 vs 3; P < 0.001). These findings indicate that UEMR is a safer and more effective technique for managing nonpedunculated colorectal neoplasms.
- Citation: Le QD, Le NQ, Quach DT. Underwater vs conventional endoscopic mucosal resection for nonpedunculated colorectal neoplasms: A randomized controlled trial. World J Gastrointest Surg 2025; 17(6): 103635
- URL: https://www.wjgnet.com/1948-9366/full/v17/i6/103635.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i6.103635
Colorectal cancer (CRC) is a common type of cancer. According to GLOBOCAN 2020, the incidence of CRC ranks third among women and fourth among men in Vietnam[1]. CRC originates from colorectal neoplasms, and the risks of invasion and lymph node metastasis are greater for nonpedunculated lesions than for pedunculated lesions[2]. Furthermore, this risk increases with lesion size[3,4]. Data show that complete resection of these neoplasms can reduce the risk of developing CRC by 31%-71%[5]. On the other hand, incomplete resection of the lesion will lead to a 20% incidence of CRC related to the residual neoplasm[6]. Conventional endoscopic mucosal resection (CEMR) is recommended as the preferred choice for nonpedunculated neoplastic lesions with a size ≥ 10 mm and no signs of submucosal invasion[5,7], and it reduces the rate of surgical intervention and the cost of treatment for nonpedunculated colorectal neoplastic lesions[8]. However, CEMR has an incomplete resection rate and a recurrence rate of 15%-45%[9-11]. To overcome the draw
Therefore, we conducted this study to evaluate the efficacy and safety of UEMR compared with CEMR in managing nonpedunculated colorectal neoplastic lesions 10-30 mm in size in Vietnam.
The inclusion criteria were patients diagnosed with nonpedunculated colorectal neoplastic lesions with the following characteristics: (1) Age > 18 years; (2) Having one nonpedunculated lesion 10-30 mm in size; and (3) Having type 1 or type 2 lesions according to the narrow band imaging (NBI) international colorectal endoscopic (NICE) classification.
The exclusion criteria were as follows: (1) Lesions with signs of deep invasion (ulceration, hardness, fragility, poor mobility, positive “non-lifting” sign); (2) NICE type 3; (3) Advanced CRC; (4) ≥ 2 nonpedunculated lesions measuring 10 mm or more extensive; (5) Unstable chronic diseases (diabetes, hypertension, heart failure, renal failure, liver failure, chronic lung disease); and (6) Uncontrolled coagulation disorders (international normalized ratio > 1.5; platelets < 100000/mm3).
An RCT was conducted at the Department of Endoscopy, University Medical Center Ho Chi Minh City (Ho Chi Minh City, Vietnam) from October 2022 to July 2024. The study protocol followed the guidelines of the Helsinki Declaration 1975 and was approved by the Biomedical Research Ethics Committee of the University of Medicine and Pharmacy at Ho Chi Minh City in 10/2022 (No. 722/HĐĐĐ-ĐHYD). The study was registered at ClinicalTrials.gov with the identifier No. NCT05825664. All patients signed an informed consent form to participate in the study.
The primary outcome of the study was the curative resection rate. The sample size was calculated via the following formula: P1 and p2 are the curative resection (R0) rates of UEMR and CEMR, respectively, α = 0.05 and β = 0.2. On the basis of previous study data, the R0 rates of UEMRs and CEMRs were 69% and 50%, respectively[15]. With a 10% dropout rate, the minimum sample size for each group was 118 patients.
The data were randomly distributed into two groups via SPSS software at a 1:1 ratio. The results of the random allocation of the intervention methods were kept in sealed numbered envelopes. When a patient with a lesion meeting the inclusion criteria was encountered, the corresponding envelope was opened, and the CEMR or UEMR technique previously determined on the basis of a random distribution was chosen. Patients and the in-charge pathologists were blinded to the intervention method.
The endoscopists had more than 10 years of experience in colonoscopy and competently performed polypectomy and CEMR. Before the study, the endoscopists resected at least 30 nonpedunculated colorectal lesions via the UEMR technique.
All procedures were performed using the NBI CF-HQ190I colonoscope (Olympus Co., Tokyo, Japan). Other supporting equipment included a water irrigation and carbon dioxide insufflation system, an endoscope attachment D-201-14304 (Olympus Co.), and the VIO300D electrosurgical unit (ERBE, Tübingen, Germany) in Endocut Q mode (Effect 3, Duration 1, Interval 6) and Force Coagulation mode (Effect 2, 30 W) for both the UEMR and CEMR techniques.
The patients were under intravenous anesthesia and placed in the left lateral position. A complete examination of the entire colon was performed. When the target lesion was identified, it was evaluated by white light and NBI. The mor
The steps of performing CEMR include: (1) Submucosal injection of distilled water; (2) Snaring the entire lesion; and (3) Closing the snare with electrocautery. The steps of performing UEMR include: (1) Suctioning all the air from the colon; (2) Turning off the air and filling the colonic lumen with water at the lesion site; (3) Snaring the entire lesion; and (4) Closing the snare with electrocautery, similar to CEMR. After mucosal resection, the edge of the defect was carefully inspected via NBI mode, and any residual lesion was further resected via the same technique. The defect was subsequently closed with hemostatic clips. Patients with bleeding complications were managed endoscopically (injection of 1:20000 adrenaline, hemostatic clip application, and/or thermal coagulation).
Patients were followed up by the endoscopists, who performed the procedure to monitor for complications within 2 weeks after the intervention. In addition, all patients were instructed to self-monitor for symptoms of bloody stool, abdominal pain and fever.
All tissue samples obtained after resection were fixed on foam pieces, stretched with pins, immersed in 10% formalin solution, and transferred to the Department of Pathology, University Medical Center, Ho Chi Minh City. The pathologists were blinded to the resection method. The tissue samples were cut into 2-3 mm thick slices parallel to the long axis (no less than 2 mm). Hematoxylin and eosin staining was performed. The tissue samples were evaluated according to the 2019 World Health Organization classification[24] and the revised Vienna classification[25], and the resection margins were carefully assessed.
The primary outcome was the rate of curative resection (R0). R0 is when the resection margin has no neoplastic cells. Non-R0 is when the resection margin has neoplastic cells or the resection margin is indeterminate (RX).
The secondary outcomes included the following: (1) Rate of en bloc resection. In en bloc resection, the lesion is completely removed via a single resection; (2) The procedure time was calculated from the beginning of the intervention to the confirmation of complete resection via NBI assessment of the resection site with no residual tissue. For CEMR, the time is calculated from the beginning of needle insertion into the biopsy channel. For UEMR, the time is calculated from the beginning of water pumping into the lumen. The procedure time does not include time for the management of bleeding complications; (3) Bleeding complications, including intraoperative bleeding requiring hemostatic intervention, early bleeding ≤ 24 hours, and delayed bleeding > 24 hours. Bleeding complications are defined by the presence of hematochezia, signs of blood loss, or a decrease in hemoglobin > 2 g/dL after the procedure. Perforation is defined by visualization of the omentum and/or evidence of gas or fluid from the gastrointestinal tract on imaging. All compli
IBM SPSS Statistics for Windows (version 25.0; IBM Corp., Armonk, NY, United States) was used to analyze the data. Descriptive statistics were used to calculate the means for continuous variables and proportions for categorical variables. The normality of the quantitative data (procedure time and number of clips used) was assessed using the Shapiro-Wilk test. If the data were normally distributed, the independent t-test was applied; otherwise, the Mann-Whitney U test was used. Categorical data (R0 rate, en bloc resection rate, and complication rate) were compared via the χ2 test or Fisher’s exact test for small sample sizes. Missing data were addressed using intention-to-treat (ITT) and per-protocol analyses, with all randomized patients included in the ITT and nonneoplastic cases analyzed per protocol. Statistical significance was defined as P < 0.05.
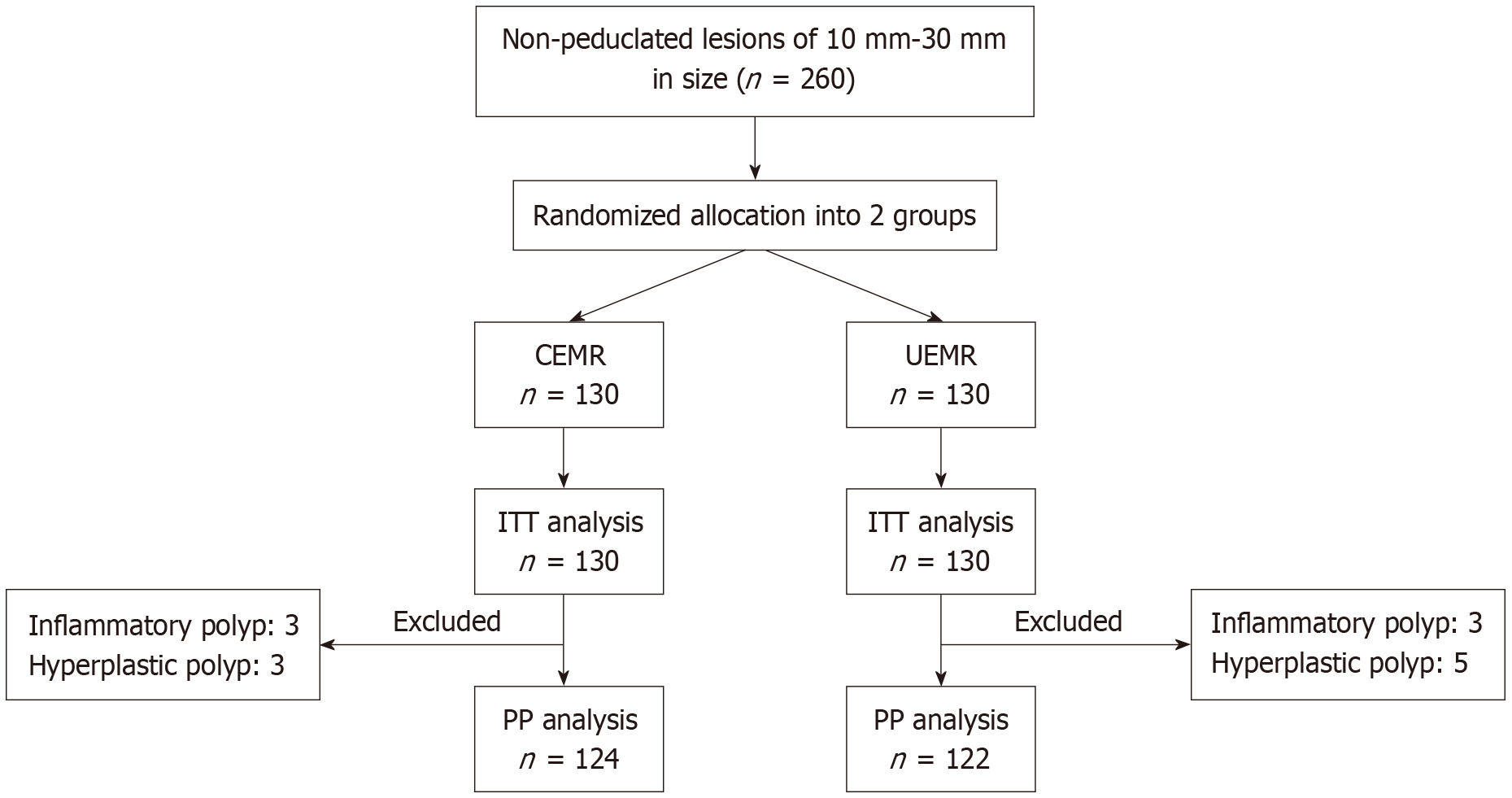
From October 2022 to July 2024, we collected 260 patients with 260 lesions who were randomly divided into CEMR (n = 130) and UEMR (n = 130) groups and analyzed them according to the ITT. We subsequently excluded 6 patients from the CEMR group and 8 patients from the UEMR group because the histopathology of these lesions was nonneoplastic. Therefore, 124 CEMR cases and 122 UEMR cases were analyzed per protocol (Figure 1).
The clinical characteristics of the patients in the two groups are presented in Table 1. There were no significant differences between the two groups in terms of age, sex, indications for colonoscopy, clinical presentations, or personal or family history. The endoscopic and pathological characteristics of the lesions are shown in Table 2. There were also no significant differences between the two groups regarding location, size, macroscopic appearance, NICE classification, or histopathology.
| Characteristics | Total (n = 260) | CEMR (n = 130) | UEMR (n = 130) | P value |
| Age, median (minimum-maximum) | 58 (27-85) | 60 (27-83) | 59 (32-85) | 0.9152 |
| Sex | 0.699 | |||
| Male | 165 (63.5) | 84 (64.6) | 81 (62.3) | |
| Female | 95 (36.5) | 46 (35.4) | 49 (37.7) | |
| Indication for colonoscopy | ||||
| Abdominal pain | 73 (28.1) | 42 (32.3) | 31 (23.8) | 0.129 |
| Screening | 60 (23.1) | 24 (18.5) | 36 (27.7) | 0.077 |
| Blood in stool | 34 (13.1) | 15 (11.5) | 19 (14.6) | 0.462 |
| Diarrhea | 33 (12.7) | 17 (13.1) | 16 (12.3) | 0.852 |
| Constipation | 33 (12.7) | 17 (13.1) | 16 (12.3) | 0.852 |
| Post-operative CRC | 23 (8.8) | 14 (10.8) | 9 (6.9) | 0.275 |
| Stool change | 2 (0.8) | 0 (0) | 2 (1.5) | 0.4981 |
| Weight loss | 2 (0.8) | 1 (0.8) | 1 (0.8) | 11 |
| Clinical symptoms | ||||
| No symptom | 85 (32.7) | 38 (29.3) | 47 (36.1) | 0.234 |
| Abdominal pain | 74 (28.5) | 40 (30.8) | 34 (26.2) | 0.410 |
| Diarrhea | 35 (13.5) | 19 (14.6) | 16 (12.3) | 0.586 |
| Blood in stool | 33 (12.7) | 17 (13.1) | 16 (12.3) | 0.852 |
| Constipation | 31 (11.9) | 15 (11.5) | 16 (12.3) | 0.848 |
| Weight loss | 2 (0.8) | 1 (0.8) | 1 (0.8) | 11 |
| Hypertension | 127 (48.8) | 62 (47.7) | 65 (50) | 0.710 |
| Diabetes | 20 (7.7) | 8 (6.2) | 12 (9.2) | 0.352 |
| Kidney failure | 3 (1.2) | 0 (0) | 3 (2.3) | 0.2471 |
| Personal history of CRC | 11 (4.2) | 5 (3.8) | 6 (4.6) | 0.758 |
| Family history of CRC | 4 (1.5) | 2 (1.5) | 2 (1.5) | 11 |
| Characteristics | Total (n = 260) | CEMR (n = 130) | UEMR (n = 130) | P value |
| Location of lesion | ||||
| Rectum | 67 (25.8) | 28 (21.5) | 39 (30.0) | 0.119 |
| Sigmoid colon | 98 (37.7) | 52 (40.0) | 46 (35.4) | 0.443 |
| Descending colon | 16 (6.2) | 10 (7.7) | 6 (4.6) | 0.302 |
| Transverse colon | 34 (12.3) | 17 (13.1) | 17 (13.1) | 1 |
| Ascending colon | 36 (13.8) | 18 (13.8) | 18 (13.8) | 1 |
| Cecum | 9 (3.5) | 5 (3.8) | 4 (3.1) | 11 |
| Size, median (minimum-maximum) (mm) | 20 (10-30) | 12 (10-30) | 12 (10-25) | 0.7582 |
| 10-19 | 233 (89.6) | 117 (90.0) | 116 (89.2) | 0.839 |
| 20-30 | 27 (10.4) | 13 (10.0) | 14 (10.8) | |
| Paris classification | 0.600 | |||
| 0-Is | 172 (66.1) | 88 (67.7) | 84 (64.6) | |
| 0-IIa/0-IIb | 88 (33.9) | 42 (32.3) | 46 (35.4) | |
| NICE classification | 0.656 | |||
| Type 1 | 22 (8.5) | 10 (7.7) | 12 (9.2) | |
| Type 2 | 238 (91.5) | 120 (92.3) | 118 (90.8) | |
| WHO classification | ||||
| Nonneoplastic polyp | 14 (5.4) | 6 (4.6) | 8 (6.2) | 0.583 |
| Tubular adenoma | 161 (61.9) | 76 (58.5) | 85 (65.4) | 0.250 |
| Tubulo-villous adenoma | 36 (13.8) | 21 (16.2) | 15 (11.5) | 0.281 |
| Serrated lesion | 49 (18.8) | 27 (20.8) | 22 (16.9) | 0.428 |
| Vienna classification | ||||
| No dysplasia | 21 (8.1) | 9 (6.9) | 12 (9.2) | 0.495 |
| Low-grade dysplasia | 209 (80.4) | 108 (83.1) | 101 (77.7) | 0.274 |
| High-grade dysplasia | 26 (10.0) | 10 (7.7) | 16 (12.3) | 0.215 |
| Superficial cancer | 4 (1.5) | 3 (2.3) | 1 (0.8) | 0.6221 |
Compared with the CEMR group, the UEMR group had significantly greater rates of R0 resection (98.4% vs 90.3%; P = 0.007) and en bloc resection (100% vs 94.6%; P = 0.014). The bleeding complication rate was significantly greater in the CEMR group than in the UEMR group (10.0% vs 1.5%; P = 0.003). There were 2 (1.5%) cases of immediate bleeding in the UEMR group, 11 (8.5%) cases of immediate bleeding, 1 (0.8%) case of bleeding within 24 hours, and 1 (0.8%) case of delayed bleeding after 24 hours in the CEMR group. No perforations were observed in either group. The procedure time was significantly shorter in the UEMR group than in the CEMR group (median time 65 seconds vs 185 seconds; P < 0.001), and the number of clips used to close the resection site was significantly lower in the UEMR group (P < 0.001) (Table 3 and Table 4).
Our study revealed significant differences in the R0 and en bloc resection rates between UEMR and CEMR. Additionally, UEMR was associated with a shorter procedure time, fewer bleeding complications, and a lower requirement for clips to close the resection site than CEMR was.
According to the recommendations of the United States Multi-Society Task Force and the European Society of Gastrointestinal Endoscopy, EMR is the preferred technique for nonpedunculated lesions ≥ 10 mm in length[5,7]. The CEMR technique is the most widely used technique. Lesions < 20 mm can be resected en bloc, whereas lesions ≥ 20 mm often require piecemeal resection[5]. Recently, UEMR has been introduced and has shown excellent en bloc resection capability, even for lesions 20-30 mm in size[14,26,27]. Data from the United States, some European countries and a few Asian countries suggest that UEMR has a higher en bloc resection rate with a similar complication rate to CEMR.
Concerning the primary outcome, we found that the R0 rate of UEMR was significantly greater than that of CEMR. This is similar to an RCT conducted at five endoscopy centers in Japan, where the R0 rate was significantly higher for UEMR than for CEMR (69% vs 50%; P = 0.011)[15]. In that study, 28 endoscopists performed most polypectomies with cap-assisted devices (98% in the UEMR group and 97% in the CEMR group)[15]. The rate in our study was 100%. Cap-assisted devices may help in the resection of difficult lesions in the colon more efficiently[28]. Another RCT conducted at 11 centers in Spain also revealed a trend toward a higher R0 rate with UEMR than with CEMR for lesions 20-30 mm in size[22]. R0 is an essential factor to consider in clinical practice. This approach may help reduce the risk of local recurrence and the development of CRC[29].
We found a higher en bloc resection rate with UEMR than with CEMR. This result was also similar to those of other studies. The results from a previous RCT conducted in the United States also revealed that the en bloc resection rate of nonpedunculated polyps 10-30 mm in size was 70.6% (48/68) in the UEMR group, which was higher than the 64% (32/50) in the CEMR group[30]. A larger RCT conducted at five endoscopy centers in Japan comparing 102 CEMRs and 108 UEMRs revealed that the en bloc resection rate was higher for UEMR than for CEMR (89% vs 75%; P = 0.007)[15]. Thus, in terms of the effectiveness of en bloc resection of lesions, UEMR tends to perform better than CEMR does. These findings suggest that UEMR may be able to replace CEMR to remove nonpedunculated lesions ≥ 10 mm in size.
Our study revealed that 7/130 (5.4%) lesions in the CEMR group required two resection attempts to achieve en bloc resection. Of these, 5/7 lesions were 20-30 mm in size. For lesions ≥ 20 mm, ESD has been used to achieve en bloc resection[7]. A systematic review revealed that ESD has an en bloc resection rate of 91% and an R0 rate of 82.9% for lesions with a mean size of 33 mm[31]. However, these results are only achieved when ESD is performed at centers with experienced endoscopists. Indeed, a cohort study evaluating the efficacy of ESD for 45 flat lesions > 10 mm at a French endoscopy center with limited ESD experience reported a low en bloc resection rate (64%), an R0 rate of only 53%, and a high complication rate (18% perforation, 13% bleeding and 7% recurrence)[32]. On the other hand, studies have shown that UEMR requires fewer resection attempts than CEMR does to achieve complete lesion resection (mean 1 vs 1.3 attempts,
Bleeding is an important complication that needs to be monitored after polypectomy. Our study revealed that CEMR had a higher rate of bleeding complications than did UEMR. Our results are similar to those of a retrospective study conducted in Taiwan, which revealed that UEMR had a lower rate of intraprocedure bleeding than CEMR (5.8% vs 15.7%, difference in proportion-9.9%, 95% confidence interval [CI]: -17.6% to -2.2%) and that there was no difference in the rate of delayed bleeding[34]. In contrast to our findings, an RCT revealed a higher rate of bleeding within 48 hours after UEMR than after CEMR, although the difference was not statistically significant (2.8% vs 2%)[15]. The differences between studies may be related to the cutting and coagulation settings and, more importantly, the endoscopist’s experience. Specifically, the study used the endo cut or pulse cut mode and was performed by 18 endoscopists with less experience (< 10 years of interventional endoscopy)[15], whereas our study was performed by endoscopists with > 10 years of polypectomy experience and only used the endo cut mode. Other data from an RCT of 303 lesions did not report a difference in the rate of complications between the UEMR and CEMR groups[35]. This result was also observed in a pooled study that did not find a statistically significant difference in the rates of bleeding and perforation when UEMR and CEMR were compared[17]. This difference may be related to the sample size of the studies. Although there are some minor differences between the studies, overall, the rate of bleeding complications did not differ between the UEMR and CEMR groups. All cases of bleeding were managed with endoscopic hemostasis.
Our study revealed that UEMR had a significantly shorter procedure time than CEMR did. This result is similar to those of other studies. Specifically, an RCT conducted in the United States revealed that the procedure time for UEMR was shorter than that for CEMR in both the 10-19 mm polyp group (2.9 vs 5.6 minutes) and the ≥ 20 mm polyp group (7.3 vs 9.5 minutes)[30]. Furthermore, a retrospective study in Taiwan that matched 112 UEMRs and 112 CEMRs revealed that UEMR had a shorter procedure time than CEMR did (mean time 8.6 vs 10.8 minutes; difference in mean -2.2; 95%CI: -4.1 to -0.3)[34]. Reducing the procedure time can help decrease the duration of anesthesia, reduce the anesthetic dose, and minimize the risks associated with prolonged anesthesia.
Our study also assessed the number of clips used to close the resected site. The results revealed that the number of clips used in the UEMR group was lower. The results of our study are similar to those of an RCT in the United States, which revealed that the number of clips used in the UEMR group was lower than that used in the CEMR group (2.41 vs 3 clips; P = 0.04)[30]. This may also be related to the intervention technique. The submucosal injection can increase the size of the lesion surface, whereas the lifting effect helps the lesion to be more easily and completely resected. Furthermore, UEMR does not require a submucosal injection needle, thereby reducing costs for the patient. This could provide cost benefits to patients and healthcare systems in countries with limited healthcare resources.
Compared with other studies, our study revealed a higher rate of en bloc and R0 resection[15,30,33]. Some contributing factors include lesion size, morphology, and the experience of the endoscopist[15]. In terms of endoscopist experience, an RCT conducted at five centers in Japan revealed that endoscopists with less experience (< 10 years of therapeutic endoscopy) had a lower R0 rate than did those with more experience (> 10 years of therapeutic endoscopy)[15]. In our study, all lesions were resected by an endoscopist with > 10 years of therapeutic endoscopy experience. Moreover, prior to this study, the physicians resected 30 nonpedunculated lesions via UEMR. In contrast, in other studies, the endoscopists had not previously performed UEMR and only learned this technique through training videos[34]. Additionally, when performing UEMR, we completely suctioned the air out of the lumen, turned off the air insufflation and started filling the lumen with water to achieve the best lifting effect to facilitate complete lesion resection. Thus, the endoscopist’s experience may have contributed to the higher rate of complete lesion resection with the UEMR technique. However, one study revealed that the group of less experienced endoscopists performing UEMR had a higher complete resection rate than did those performing CEMR (odds ratio = 2.07; 95%CI: 0.92 to 4.63)[15]. Data from a cohort study also suggested that UEMR can be safely performed by endoscopists experienced in CEMR without prior training in UEMR[36]. A multicenter retrospective study in Italy comparing 83 UEMRs and 86 CEMRs at public hospitals reported no difference in complete resection or complication rates[21]. These findings suggest that UEMR has potential for widespread implementation in clinical practice.
This study had several limitations. First, this single-center study with highly experienced endoscopists may limit generalizability to less experienced settings. Multicenter studies are needed to validate UEMR feasibility across diverse expertise levels. Second, the study was double-blinded to the patients and pathologists but not the endoscopists. Third, this study did not assess postoperative recurrence rates or the progression of residual lesions, thus failing to validate the long-term clinical significance of the R0 rate. Future studies are needed to assess recurrence rates, residual lesion progression, and recurrence factors after UEMR and CEMR.
In conclusion, UEMR achieved a significantly higher complete resection rate than did CEMR for nonpedunculated colorectal lesions of 10-30 mm, with a shorter procedure time, fewer clips for defect closure, and fewer bleeding complications. Therefore, UEMR should be considered a viable option for managing nonpedunculated colorectal lesions of this size.
The authors thank all staff at the Department of Endoscopy, University Medical Center at Ho Chi Minh City, Vietnam, for their support and Le HM at the Department of Pathology, University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam, for reviewing the sample.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64271] [Article Influence: 16067.8] [Reference Citation Analysis (174)] |
| 2. | Burgess NG, Hourigan LF, Zanati SA, Brown GJ, Singh R, Williams SJ, Raftopoulos SC, Ormonde D, Moss A, Byth K, Mahajan H, McLeod D, Bourke MJ. Risk Stratification for Covert Invasive Cancer Among Patients Referred for Colonic Endoscopic Mucosal Resection: A Large Multicenter Cohort. Gastroenterology. 2017;153:732-742.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (1)] |
| 3. | Nusko G, Mansmann U, Partzsch U, Altendorf-Hofmann A, Groitl H, Wittekind C, Ell C, Hahn EG. Invasive carcinoma in colorectal adenomas: multivariate analysis of patient and adenoma characteristics. Endoscopy. 1997;29:626-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3107] [Cited by in RCA: 3119] [Article Influence: 97.5] [Reference Citation Analysis (1)] |
| 5. | Kaltenbach T, Anderson JC, Burke CA, Dominitz JA, Gupta S, Lieberman D, Robertson DJ, Shaukat A, Syngal S, Rex DK. Endoscopic Removal of Colorectal Lesions-Recommendations by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2020;158:1095-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 202] [Article Influence: 40.4] [Reference Citation Analysis (3)] |
| 6. | Robertson DJ, Lieberman DA, Winawer SJ, Ahnen DJ, Baron JA, Schatzkin A, Cross AJ, Zauber AG, Church TR, Lance P, Greenberg ER, Martínez ME. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut. 2014;63:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 336] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 7. | Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau JM, Paspatis G, Jover R, Langner C, Bronzwaer M, Nalankilli K, Fockens P, Hazzan R, Gralnek IM, Gschwantler M, Waldmann E, Jeschek P, Penz D, Heresbach D, Moons L, Lemmers A, Paraskeva K, Pohl J, Ponchon T, Regula J, Repici A, Rutter MD, Burgess NG, Bourke MJ. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49:270-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 764] [Article Influence: 95.5] [Reference Citation Analysis (0)] |
| 8. | Jayanna M, Burgess NG, Singh R, Hourigan LF, Brown GJ, Zanati SA, Moss A, Lim J, Sonson R, Williams SJ, Bourke MJ. Cost Analysis of Endoscopic Mucosal Resection vs Surgery for Large Laterally Spreading Colorectal Lesions. Clin Gastroenterol Hepatol. 2016;14:271-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 160] [Article Influence: 17.8] [Reference Citation Analysis (2)] |
| 9. | Garg R, Singh A, Aggarwal M, Bhalla J, Mohan BP, Burke C, Rustagi T, Chahal P. Underwater Endoscopic Mucosal Resection for 10 mm or Larger Nonpedunculated Colorectal Polyps: A Systematic Review and Meta-Analysis. Clin Endosc. 2021;54:379-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Fukami N, Lee JH. Endoscopic treatment of large sessile and flat colorectal lesions. Curr Opin Gastroenterol. 2006;22:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 11. | Knabe M, Pohl J, Gerges C, Ell C, Neuhaus H, Schumacher B. Standardized long-term follow-up after endoscopic resection of large, nonpedunculated colorectal lesions: a prospective two-center study. Am J Gastroenterol. 2014;109:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Fujiya M, Tanaka K, Dokoshi T, Tominaga M, Ueno N, Inaba Y, Ito T, Moriichi K, Kohgo Y. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015;81:583-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 268] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 13. | Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M, Saitoh Y, Tsuruta O, Sugihara KI, Igarashi M, Toyonaga T, Ajioka Y, Kusunoki M, Koike K, Fujimoto K, Tajiri H. Japan Gastroenterological Endoscopy Society guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2020;32:219-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 267] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 14. | Binmoeller KF, Weilert F, Shah J, Bhat Y, Kane S. "Underwater" EMR without submucosal injection for large sessile colorectal polyps (with video). Gastrointest Endosc. 2012;75:1086-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 273] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 15. | Yamashina T, Uedo N, Akasaka T, Iwatsubo T, Nakatani Y, Akamatsu T, Kawamura T, Takeuchi Y, Fujii S, Kusaka T, Shimokawa T. Comparison of Underwater vs Conventional Endoscopic Mucosal Resection of Intermediate-Size Colorectal Polyps. Gastroenterology. 2019;157:451-461.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 16. | Tziatzios G, Gkolfakis P, Triantafyllou K, Fuccio L, Facciorusso A, Papanikolaou IS, Antonelli G, Nagl S, Ebigbo A, Probst A, Hassan C, Messmann H. Higher rate of en bloc resection with underwater than conventional endoscopic mucosal resection: A meta-analysis. Dig Liver Dis. 2021;53:958-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Choi AY, Moosvi Z, Shah S, Roccato MK, Wang AY, Hamerski CM, Samarasena JB. Underwater versus conventional EMR for colorectal polyps: systematic review and meta-analysis. Gastrointest Endosc. 2021;93:378-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 18. | Rodríguez Sánchez J, Uchima Koecklin H, González López L, Cuatrecasas M, de la Santa Belda E, Olivencia Palomar P, Sánchez García C, Sánchez Alonso M, Muñoz Rodríguez JR, Gómez Romero FJ, López Viedma B, Agarrabeitia AB, Olmedo Camacho J, Albéniz Arbizu E. Short and long-term outcomes of underwater EMR compared to the traditional procedure in the real clinical practice. Rev Esp Enferm Dig. 2019;111:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Nagl S, Ebigbo A, Goelder SK, Roemmele C, Neuhaus L, Weber T, Braun G, Probst A, Schnoy E, Kafel AJ, Muzalyova A, Messmann H. Underwater vs Conventional Endoscopic Mucosal Resection of Large Sessile or Flat Colorectal Polyps: A Prospective Randomized Controlled Trial. Gastroenterology. 2021;161:1460-1474.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 20. | Nomura H, Tsuji S, Utsunomiya M, Kawasaki A, Tsuji K, Yoshida N, Takemura K, Katayanagi K, Minato H, Doyama H. Resection depth and layer of underwater versus conventional endoscopic mucosal resection of intermediate-sized colorectal polyps: A pilot study. Endosc Int Open. 2022;10:E1037-E1044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 21. | Cadoni S, Liggi M, Gallittu P, Mura D, Fuccio L, Koo M, Ishaq S. Underwater endoscopic colorectal polyp resection: Feasibility in everyday clinical practice. United European Gastroenterol J. 2018;6:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Rodríguez Sánchez J, Alvarez-Gonzalez MA, Pellisé M, Coto-Ugarte D, Uchima H, Aranda-Hernández J, Santiago García J, Marín-Gabriel JC, Riu Pons F, Nogales O, Carreño Macian R, Herreros-de-Tejada A, Hernández L, Patrón GO, Rodriguez-Tellez M, Redondo-Cerezo E, Sánchez Alonso M, Daca M, Valdivielso-Cortazar E, Álvarez Delgado A, Enguita M, Montori S, Albéniz E. Underwater versus conventional EMR of large nonpedunculated colorectal lesions: a multicenter randomized controlled trial. Gastrointest Endosc. 2023;97:941-951.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 23. | Lenz L, Martins B, Andrade de Paulo G, Kawaguti FS, Baba ER, Uemura RS, Gusmon CC, Geiger SN, Moura RN, Pennacchi C, Simas de Lima M, Safatle-Ribeiro AV, Hashimoto CL, Ribeiro U, Maluf-Filho F. Underwater versus conventional EMR for nonpedunculated colorectal lesions: a randomized clinical trial. Gastrointest Endosc. 2023;97:549-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 24. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2412] [Article Influence: 482.4] [Reference Citation Analysis (3)] |
| 25. | Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 497] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 26. | Binmoeller KF, Hamerski CM, Shah JN, Bhat YM, Kane SD, Garcia-Kennedy R. Attempted underwater en bloc resection for large (2-4 cm) colorectal laterally spreading tumors (with video). Gastrointest Endosc. 2015;81:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Saito Y, Ono A. Underwater Endoscopic Mucosal Resection for Colorectal Lesions: A Bridge Between Conventional Endoscopic Mucosal Resection and Endoscopic Submucosal Dissection. Gastroenterology. 2021;161:1369-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Douglas SR, Rex DK, Repici A, Kelly M, Heinle JW, Spadaccini M, Moyer MT. Distal Cap-assisted Endoscopic Mucosal Resection for Non-lifting Colorectal Polyps: An International, Multicenter Study. Tech Innovat Gastroi. 2023;25:236-242. [DOI] [Full Text] |
| 29. | Parihar V, Sopena-Falco J, Leung E, Benz E, Cooney A, Keohane J, Sengupta S. R0 Resection Margin, A New Quality Measure in the Era of National Bowel Screening? Ir Med J. 2020;113:7. [PubMed] |
| 30. | Yen AW, Leung JW, Wilson MD, Leung FW. Underwater versus conventional endoscopic resection of nondiminutive nonpedunculated colorectal lesions: a prospective randomized controlled trial (with video). Gastrointest Endosc. 2020;91:643-654.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 31. | Fuccio L, Hassan C, Ponchon T, Mandolesi D, Farioli A, Cucchetti A, Frazzoni L, Bhandari P, Bellisario C, Bazzoli F, Repici A. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis. Gastrointest Endosc. 2017;86:74-86.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 206] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 32. | Rahmi G, Hotayt B, Chaussade S, Lepilliez V, Giovannini M, Coumaros D, Charachon A, Cholet F, Laquière A, Samaha E, Prat F, Ponchon T, Bories E, Robaszkiewicz M, Boustière C, Cellier C. Endoscopic submucosal dissection for superficial rectal tumors: prospective evaluation in France. Endoscopy. 2014;46:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Schenck RJ, Jahann DA, Patrie JT, Stelow EB, Cox DG, Uppal DS, Sauer BG, Shami VM, Strand DS, Wang AY. Underwater endoscopic mucosal resection is associated with fewer recurrences and earlier curative resections compared to conventional endoscopic mucosal resection for large colorectal polyps. Surg Endosc. 2017;31:4174-4183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Chien HC, Uedo N, Hsieh PH. Comparison of underwater and conventional endoscopic mucosal resection for removing sessile colorectal polyps: a propensity-score matched cohort study. Endosc Int Open. 2019;7:E1528-E1536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Hamerski C, Samarasena J, Lee DP, Wang AY, Strand D, Amato A, Watson R, Nett A, Calitis J, Binmoeller K. 125 Underwater Versus Conventional Endoscopic Mucosal Resection for the Treatment of Colorectal Laterally Spreading Tumors: Results From an International, Multicenter, Randomized Controlled Trial. Am J Gastroenterol. 2019;114:S75-S75. [DOI] [Full Text] |
| 36. | Curcio G, Granata A, Ligresti D, Tarantino I, Barresi L, Liotta R, Traina M. Underwater colorectal EMR: remodeling endoscopic mucosal resection. Gastrointest Endosc. 2015;81:1238-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |