Published online May 27, 2025. doi: 10.4240/wjgs.v17.i5.107579
Revised: April 8, 2025
Accepted: April 25, 2025
Published online: May 27, 2025
Processing time: 58 Days and 0.4 Hours
Gastric cancer (GC) is a major global health challenge, and the treatment of proximal GC in particular presents unique clinical and surgical complexities. Currently, there is no consensus on whether proximal gastrectomy (PG) or total gastrectomy (TG) should be used for advanced proximal GC, and the choice of postoperative gastrointestinal reconstruction method remains controversial.
To compare the short-term efficacy, long-term survival, and postoperative reflux outcomes of PG with tubular stomach reconstruction vs TG with Roux-en-Y re
A multicenter retrospective cohort study was conducted at two Chinese medical centers between December, 2012 and December, 2022. Patients with histologically confirmed proximal GC who received NACT followed by either PG with tubular stomach reconstruction or TG with Roux-en-Y reconstruction were included. Propensity score matching (PSM) was performed to balance baseline characteristics, and the primary endpoint was 5-year overall survival (OS). Se
After PSM, 244 patients (122 PG, 122 TG) were finally included and all baseline characteristics were comparable between groups. The PG group had a significantly shorter operation time compared to the TG group (189.50 vs 215.00 minutes, P < 0.001), with no differences in intraoperative blood loss or postoperative complications (19.68% vs 14.75%, P = 0.792). The 5-year OS rates were 52.7% vs 45.5% (P = 0.330), and 5-year RFS rates were 54.3% vs 47.6% (P = 0.356) for the PG and TG groups, respectively. Reflux symptoms (18.0% vs 31.1%, P = 0.017) and clinically significant reflux based on gastroesophageal reflux disease questionnaire scores ≥ 8 (7.4% vs 21.3%, P < 0.001) were significantly less frequent in the PG group. Multivariate analysis identified histological differentiation (HR = 2.98, 95%CI: 2.03-4.36, P < 0.001) and tumor size (HR = 0.26, 95%CI: 0.17-0.41 for tumors ≤ 4 cm, P < 0.001) as independent prognostic factors.
PG with tubular stomach reconstruction is comparable to TG in terms of surgical safety and long-term oncological outcomes for proximal GC patients following NACT. Additionally, PG has the advantages of shorter operation time and lower rates of postoperative reflux, suggesting potential benefits for patient quality of life. Notably, the analysis of postoperative prognostic factors, including histological differentiation and tumor size, further informs clinical decision-making and highlights the importance of individualized treatment strategies.
Core Tip: This multicenter retrospective study compares proximal gastrectomy (PG) with tubular stomach reconstruction vs total gastrectomy (TG) for proximal gastric cancer following neoadjuvant chemotherapy. Our analysis of 244 propensity score-matched patients reveals that PG achieves comparable survival outcomes and surgical safety to TG, while offering significant advantages in operation time and postoperative reflux prevention. These findings support PG with tubular stomach reconstruction as a viable alternative to TG, potentially improving quality of life without compromising oncological efficacy in this patient population.
- Citation: Lu YM, Jin P, Wang HK, Shao XX, Hu HT, Jiang YJ, Li WY, Tian YT. Proximal gastrectomy with tubular stomach reconstruction vs total gastrectomy for proximal gastric cancer following neoadjuvant chemotherapy: A multicenter retrospective study. World J Gastrointest Surg 2025; 17(5): 107579
- URL: https://www.wjgnet.com/1948-9366/full/v17/i5/107579.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i5.107579
Gastric cancer (GC) continues to present a significant global health challenge despite its decline in incidence during the past two decades. In 2022, GC accounted for 968784 new diagnoses and 660175 fatalities worldwide, positioning it as the fifth-ranking cancer in both incidence and mortality on a global scale, and third in mortality in China[1]. According to a comprehensive 2018 analysis of global cancer registry data on gastric and esophageal cancers, East Asia represented 67.1% of all new cardia cancer cases reported globally[2], though the incidence of proximal GC has shown an increasing trend both in Western and Asian countries[3,4]. Proximal GC management presents distinctive clinical and surgical complexities that require specialized therapeutic approaches. Clinicians must develop customized treatment strategies specifically designed to address these challenges in order to achieve optimal clinical outcomes.
Neoadjuvant chemotherapy (NACT) aims to reduce tumor volume, downstage the disease’s clinical stage, improve R0 resection rate, eliminate potential micro-metastases, and assess tumor sensitivity to treatment through prior systemic therapy[5]. Both the NCCN guidelines and the ESMO guidelines recommend neoadjuvant therapy for resectable GC with clinical staging of cT2 or higher, or any N stage (including proximal GC)[6,7]. In contrast, Japanese Gastric Cancer Association (JGCA) guidelines are more cautious in recommending preoperative neoadjuvant therapy, especially for resectable proximal GC with relatively early clinical stage[8]. Overall, NACT has become an indispensable component of the comprehensive treatment of advanced proximal GC, and with the gradual introduction of immunotherapeutic agents, such as PD-1/PD-L1 inhibitors, neoadjuvant therapy is likely to play an even more significant role in the future.
Current international surgical guidelines for proximal GC remain strict, with the ideal indication being early-stage upper GC, where an R0 resection can be achieved while preserving more than half of the distal stomach[8]. Clinical opinions on the tendency to choose proximal gastrectomy (PG) or total gastrectomy (TG) are influenced by a variety of factors, including tumor stage and histological type. For patients who respond well to NACT, some have suggested that PG may result in better overall survival (OS)[9]. For Siewert II esophagogastric junction (EGJ) tumors, PG combined with distal esophagectomy is considered adequate[10,11]. At present, the choice of surgical procedure is often a decision made collaboratively by the surgical team after considering both the likely oncological and functional outcomes as well as patient preferences. Nonetheless, the use of PG or TG for advanced proximal GC still requires further validation.
Another controversy surrounding PG is the method of gastrointestinal reconstruction. The resection of the esophagogastric junction disrupts the original anti-reflux anatomic structure, making the choice of postoperative gastrointestinal reconstruction crucial for patient recovery[12]. Different reconstruction methods exhibit significant differences in preventing reflux, improving nutritional status, and enhancing quality of life. Currently, there are several different techniques for GI reconstruction after PG, such as esophagogastrostomy (EG), jejunal pouch interposition, double tract reconstruction, and tube-like stomach EG (gastric tubular reconstruction)[12-14]. However, there is no widely accepted "gold standard" reconstruction technique, which is a reflection of the complexity and ongoing development of this field. In response to this research gap, we conducted a multicenter retrospective cohort study and utilized propensity score matching (PSM) to analyze and compare the outcomes of PG with tube-like stomach EG and TG with Roux-en-Y reconstruction in patients with locally advanced proximal GC who received NACT. The purpose of our study was to evaluate short-term clinical outcomes, long-term survival, and postoperative reflux conditions in these patients, with the objective of providing valuable insights into the selection of the most appropriate surgical approach.
This retrospective cohort study was conducted at two medical centers: The Pancreatic and Gastric Surgery Department of Cancer Hospital of the Chinese Academy of Medical Sciences and the Gastric Surgery Department of Tianjin Medical University Cancer Institute & Hospital. The eligibility criteria were as follows: (1) Histologic confirmation of GC; (2) Tumor location in the upper third of the stomach without esophagus invasion; (3) Computed tomography indicating locally advanced GC without evidence of distant metastasis; (4) American Society of Anesthesiology (ASA) score of class I-III; (5) NACT prior to surgery; and (6) Gastric tubular reconstruction. The exclusion criteria were: (1) Previous treatment for GC (including surgery, chemotherapy, targeted therapy etc.); (2) Emergency surgery; (3) Prior or co-occurrence of other malignancies; (4) Other types of anastomoses; and (5) Incomplete data.
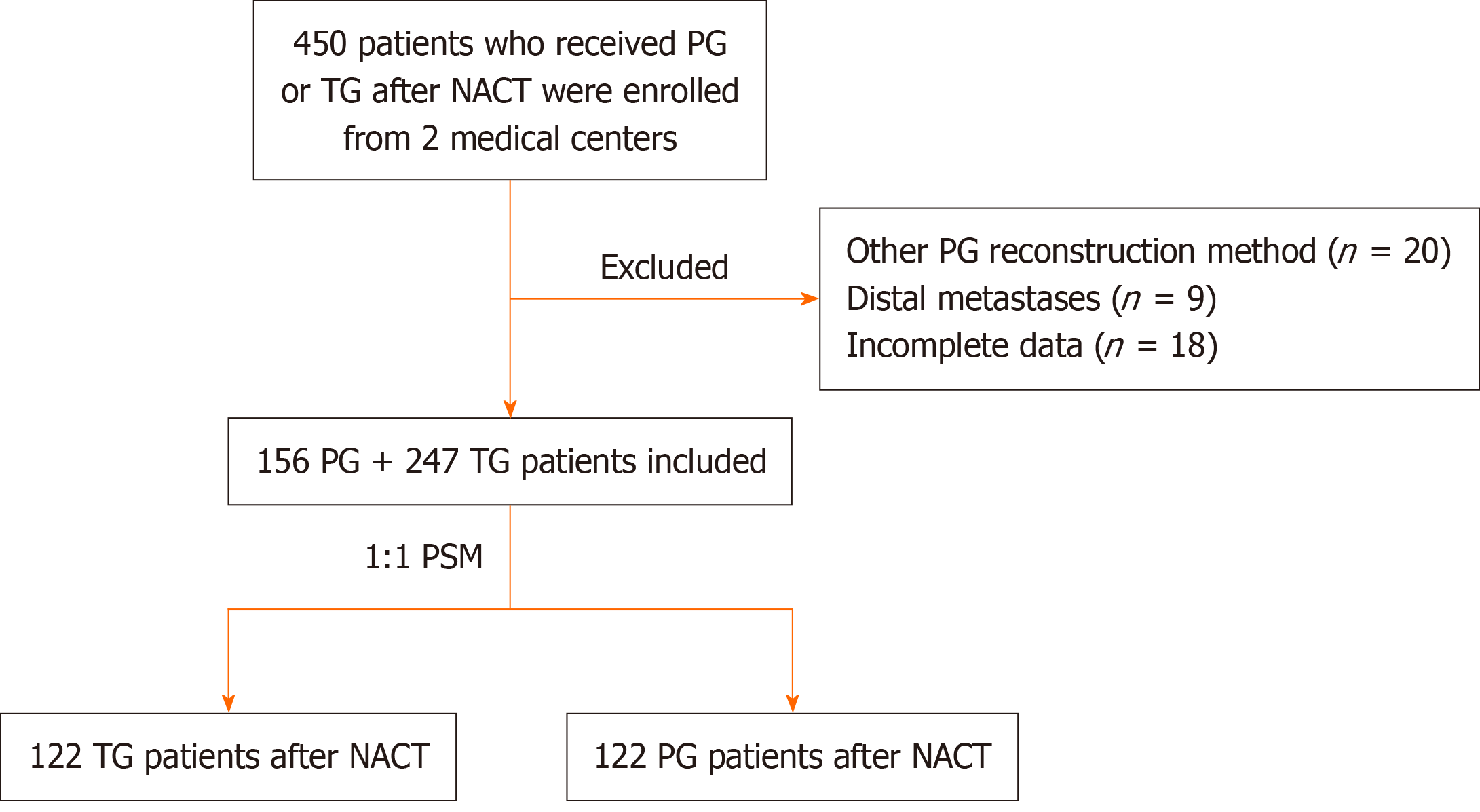
The flowchart shows in Figure 1. Between December 2012, and December 2022, we identified 284 patients at the Cancer Hospital of the Chinese Academy of Medical Sciences who received PG or TG after NACT. We also enrolled 166 patients with the same criteria at Tianjin Medical University Cancer Institute & Hospital. After applying the exclusion criteria, 156 PG patients and 247 TG patients with previous NACT from the two medical centers were included in the final cohort. All patients and their families provided written informed consent to participate in the study and acknowledged and consenting to the potential use of clinical data collected during their treatment for future research. This study received approval from the Ethics Committee of the Cancer Hospital, Chinese Academy of Medical Sciences (21/489-3160). All patients were followed until the date of last contact or until death, and follow-up was conducted through December 15, 2024. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the institutional review boards of both participating hospitals.
All patients received NACT before PG or TG. The NACT regimens included SOX (118/244, 48.4%), CAPEOX (30/244, 12.3%), DOS (35/244, 14.3%), FLOT (25/244, 10.2%), and other regimens (36/244, 14.8%), and the selection of chemotherapy regimen and the number of treatment cycles were determined by experienced medical oncologists or through multidisciplinary team (MDT) discussion after taking into consideration each patient’s individual tolerability to chemotherapy.
The decision to perform TG or PG with D2 lymphadenectomy was made based on a comprehensive evaluation of multiple factors, including tumor regression grade (TRG), tumor size, tumor location, and patient preference. After a thorough discussion of the potential benefits and risks of the treatment options, patients and their families decided whether to proceed with PG or TG. All surgeries were performed by four experienced surgical teams within our department in which each lead surgeon had experience of over 1000 cases of open or laparoscopic/robotic gastrectomy. The extent of D2 lymphadenectomy was performed according to the JGCA guidelines[8]. In the PG group, a side-to-side anastomosis was performed between the esophagus and the residual tubular stomach along the greater curvature to preserve approximately half of the distal stomach, whereas in the TG group, gastrointestinal reconstruction was achieved using the Roux-en-Y anastomosis technique.
The primary endpoint was 5-year OS, and secondary endpoints included 5-year recurrence-free survival (RFS), short-term postoperative complications, TRG, complete response (CR) rate, and postoperative reflux status. OS was calculated from the date of surgery to the date of death from any cause or to the last follow-up, and RFS was defined as the interval from surgery to the date of disease recurrence or last follow-up. TRG following NACT was assessed using the Becker grading system. Postoperative complications were classified according to the Clavien-Dindo system, and pathological staging (ypTNM) was determined based on the 8th edition of the AJCC/UICC classification. Histologic differentiation was categorized as “well”, “moderate”, “poor”, or “other” types, including signet ring cell carcinoma, mucinous adenocarcinoma, and other specific histopathologic variants. During the follow-up period, postoperative quality of life, as well as the frequency and severity of reflux symptoms, were assessed through patient interviews. Additionally, postoperative endoscopic findings were collected to evaluate the presence of esophageal reflux according to The Los Angeles classification by experienced endoscopists. The gastroesophageal reflux disease questionnaire (GERD-Q) was also ad
PSM was performed to minimize potential bias from confounding variables between the two groups. Matching factors included sex, age, surgical approach, ASA score, Charlson comorbidity index (CCI), body mass index (BMI), ypT stage, ypN stage, overall ypTNM stage, tumor size, histological differentiation, resection type, number of NACT cycles, NACT regimen, and the presence of perineural and vascular invasion. A 1:1 nearest-neighbor matching algorithm without replacement was applied, using a caliper width of 0.02 to optimize the use of available data.
Comparisons of categorical variables between the PG and TG groups were conducted using the χ2 test or Fisher’s exact test, as appropriate. Continuous variables were analyzed using either student’s t-test or the Wilcoxon rank-sum test, depending on the data distributions. Survival outcomes, including OS and RFS, were estimated using the Kaplan-Meier method, and HR and 95%CI were calculated using Cox proportional hazards models. Both univariate and multivariate Cox analyses were performed to identify factors independently associated with OS, where variables with a P value < 0.05 in the univariate analysis were included in the multivariate model. All data analyses were conducted using R software version 4.4.0 (http://www.r-project.org/).
Following PSM, 244 patients (122 in the PG group and 122 in the TG group) were included in the final analysis. As shown in Table 1, there were no significant differences in baseline characteristics between the two groups, including gender distribution (80.3% male in both groups), median age (62.0 vs 63.0 years, P = 0.453), BMI (23.40 vs 24.21 kg/m², P = 0.673), ASA score (P = 0.697), and CCI score (P = 0.494). The pathological characteristics were also comparable between groups, with similar distributions in tumor size (median 3.00 cm in both groups, P = 0.771), histological differentiation (P = 0.324), number of retrieved lymph nodes (32.50 vs 31.00, P = 0.455), and lymph node metastasis (median 2.00 in both groups, P = 0.381). ypTNM staging was also balanced across both groups, with no statistically significant differences in ypT stage (P = 0.269), ypN stage (P = 0.509), or overall ypTNM stage distribution. Finally, The R0 resection rate was similarly high in both groups (98.36% in PG vs 99.18% in TG, P = 1.000).
| TG (n = 122) | PG (n = 122) | P value | |
| Gender | 1.000 | ||
| Male | 98 (80.3) | 98 (80.3) | |
| Female | 24 (19.7) | 24 (19.7) | |
| Age1, years | 63.00 (59.50-68.25) | 62.00 (58.00-67.00) | 0.453 |
| BMI1, kg/m2 | 24.11 (21.44-25.91) | 23.40 (21.50-25.95) | 0.673 |
| ASA score | 0.697 | ||
| 1-2 | 108 (88.5) | 106 (86.9) | |
| 3 | 14 (11.5) | 16 (13.1) | |
| CCI score | 0.494 | ||
| ≤ 2 | 80 (65.6) | 85 (69.7) | |
| > 2 | 42 (34.4) | 37 (30.3) | |
| Tumor size1, cm | 3.00 (2.00-4.50) | 3.00 (2.00-4.00) | 0.771 |
| Histologic differentiation | 0.324 | ||
| Poor/other types | 72 (59.02) | 61 (50.00) | |
| Moderate | 45 (36.89) | 53 (43.44) | |
| Well | 13 (5.33) | 8 (6.56) | |
| Number of lymph nodes retrieved1 | 31.00 (22.75-4100) | 32.50 (23.75-43.00) | 0.455 |
| Number of lymph node metastasis1, n | 2.00 (0.00-8.00) | 2.00 (0.00-5.25) | 0.381 |
| ypT stage | 0.269 | ||
| ypT0 | 16 (13.11) | 19 (15.57) | |
| ypT1a | 9 (7.38) | 4 (3.28) | |
| ypT1b | 10 (8.20) | 5 (4.10) | |
| ypT2 | 12 (9.84) | 18 (14.75) | |
| ypT3 | 33 (27.05) | 25 (20.49) | |
| ypT4a | 40 (32.79) | 50 (40.98) | |
| ypT4b | 2 (1.64) | 1 (0.82) | |
| ypN stage | 0.509 | ||
| ypN0 | 49 (40.16) | 48 (39.34) | |
| ypN1 | 21 (17.21) | 22 (18.03) | |
| ypN2 | 21 (17.21) | 30 (24.59) | |
| ypN3a | 24 (19.67) | 18 (14.75) | |
| ypN3b | 7 (5.74) | 4 (3.28) | |
| ypTNM stage | |||
| 0 | 20 (16.4) | 19 (15.6) | |
| I | 9 (7.4) | 15 (12.3) | |
| II | 40 (32.8) | 30 (24.6) | |
| III | 53 (43.4) | 58 (47.5) | |
| Vascular invasion | 0.328 | ||
| No | 82 (67.2) | 89 (73.0) | |
| Yes | 40 (32.8) | 33 (27.0) | |
| Perineural invasion | 0.419 | ||
| No | 77 (63.1) | 83 (68.0) | |
| Yes | 45 (36.9) | 39 (32.0) | 1.000 |
| Radical resection | |||
| R0 | 121 (99.18) | 120 (98.36) | |
| R1/R2 | 1 (0.82) | 2 (1.64) |
Table 2 presents the NACT details and outcomes. The distribution of NACT regimens was similar between the two groups (P = 0.778), with SOX being the most common regimen in both the PG (50.00%) and TG (46.72%) groups, followed by CAPEOX, DOS, and FLOT. The majority of patients received 3-4 cycles of NACT (67.21% in TG vs 75.41% in PG), with no significant difference in the distribution of NACT cycles between groups (P = 0.251). Notably, the TRG following NACT showed significant differences between the two groups (P < 0.001). The PG group demonstrated higher rates of TRG 1a (17.21% vs 4.92%) and TRG 3 (47.54% vs 20.49%), and the TG group had a higher proportion of TRG 2 (59.02% vs 24.59%). CR was achieved in significantly more patients in the PG group compared to the TG group as well (13.11% vs 3.28%, P = 0.005), but the proportion of patients who received additional immunotherapy alongside NACT was similar between groups (24.59% in PG vs 18.85% in TG, P = 0.797).
| TG (n = 122) | PG (n = 122) | P value | |
| NACT regimen | 0.778 | ||
| SOX | 57 (46.72) | 61 (50.00) | |
| CAPEOX | 15 (12.30) | 15 (12.30) | |
| DOS | 20 (16.39) | 15 (12.30) | |
| FLOT | 10 (8.20) | 15 (12.30) | |
| FOLFOX | 5 (4.10) | 3 (2.46) | |
| Other | 15 (12.30) | 13 (10.66) | |
| NACT cycles | 0.251 | ||
| 1 | 4 (3.28) | 7 (5.74) | |
| 2 | 18 (14.75) | 14 (11.48) | |
| 3 | 37 (30.33) | 36 (29.51) | |
| 4 | 46 (37.70) | 56 (45.90) | |
| 6 | 13 (10.06) | 9 (7.38) | |
| > 6 | 4 (3.28) | 7 (5.74) | |
| TRG (Becker) | < 0.001 | ||
| 1a | 6 (4.92) | 21 (17.21) | |
| 1b | 19 (15.57) | 13 (10.66) | |
| 2 | 72 (59.02) | 30 (24.59) | |
| 3 | 25 (20.49) | 58 (47.54) | |
| CR response | 0.005 | ||
| CR | 4 (3.28) | 16 (13.11) | |
| Non-CR | 118 (96.72) | 106 (86.89) | |
| Plus immunotherapy | 0.797 | ||
| Yes | 23 (18.85) | 30 (24.59) | |
| No | 99 (81.15) | 92 (75.41) |
The surgical approach distribution was comparable between groups (P = 0.915), with laparoscopic surgery being predominant in both the PG (66.39%) and TG (68.85%) groups, followed by open surgery and the robotic approach (Table 3). The median operation time was significantly shorter in the PG group compared to the TG group (189.50 vs 215.00 minutes, P < 0.001), however, but there were no significant differences in intraoperative blood loss (median 100 mL in both groups, P = 0.445) or postoperative hospitalization time (11.00 vs 10.00 days, P = 0.314). The overall incidence of postoperative complications was similar between the PG and TG groups as well (19.68% vs 14.75%, P = 0.792), and individual complications, including anastomotic leakage (1.64% in both groups), bleeding (2.46% vs 1.64%), intrabdominal infection (3.28% in both groups), and pleural effusion (6.56% vs 4.92%), showed no significant differences either. The distribution of complications according to the Clavien-Dindo classification was also similar between groups (P = 0.543), with the majority of complications being grade II or lower (12.30% in PG vs. 7.38% in TG), as was the proportion of patients who received adjuvant chemotherapy (80.3% in PG vs 72.1% in TG, P = 0.133).
| TG (n = 122) | PG (n = 122) | P value | |
| Surgical approach | 0.915 | ||
| Laparoscopic | 84 (68.85) | 81 (66.39) | |
| Open | 30 (24.59) | 32 (26.23) | |
| Robotic | 8 (6.56) | 9 (7.38) | |
| Operation time1, minutes | 215 (195.00-248.75) | 189.50 (169.25-227.25) | < 0.001 |
| Blood loss1, mL | 100.00 (100.00-200.00) | 100.00 (100.00-200.00) | 0.445 |
| Hospitalization time1, days | 10.00 (9.00-12.00) | 11.00 (8.00-13.75) | 0.314 |
| Postoperative complications | 0.792 | ||
| No complications | 104 (85.25) | 98 (80.32) | 0.186 |
| Anastomotic leakage | 2 (1.64) | 2 (1.64) | 1.000 |
| Anastomotic stricture | 0 (0.00) | 0 (0.00) | 1.000 |
| Bleeding | 2 (1.64) | 3 (2.46) | 1.000 |
| Intrabdominal infection | 4 (3.28) | 4 (3.28) | 1.000 |
| Gastroparesis | 1 (0.82) | 1 (0.82) | 1.000 |
| Intestinal obstruction | 2 (1.64) | 3 (2.46) | 1.000 |
| Pneumonia | 1 (0.82) | 2 (1.64) | 0.209 |
| Pleural effusion | 6 (4.92) | 8 (6.56) | 0.518 |
| Respiratory failure | 0 (0.00) | 1 (0.82) | 1.000 |
| Clavien_Dindo classification | 0.543 | ||
| ≤ Grade II | 9 (7.38) | 15 (12.30) | |
| Grade IIIa/IIIb | 8 (6.56) | 10 (8.20) | |
| Grade IVa/IVb | 1 (0.82) | 1 (0.82) | |
| Grade V | 104 (85.25) | 95 (77.87) | |
| Adjuvant chemotherapy | 0.133 | ||
| No | 34 (27.9) | 24 (19.7) | |
| Yes | 88 (72.1) | 98 (80.3) |
Postoperative reflux symptoms were significantly less frequent in the PG group compared to the TG group (18.0% vs 31.1%, P = 0.017), as shown in Table 4. The GERD-Q scores also favored the PG group, with significantly fewer patients having scores ≥ 8 indicative of clinically significant reflux (7.4% vs 21.3%, P < 0.001). The Los Angeles Classification of reflux esophagitis further confirmed these findings, with a higher proportion of patients without reflux in the PG group (82.0% vs 66.4%), and notably fewer patients with severe (grade C) reflux esophagitis (0.8% vs 9.0%) compared to the TG group (P < 0.001).
| TG (n = 122) | PG (n = 122) | P value | |
| Reflux symptoms | 0.017 | ||
| No | 84 (68.9) | 100 (82.0) | |
| Yes | 38 (31.1) | 22 (18.0) | |
| Gerd-Q score | < 0.001 | ||
| < 8 | 96 (78.7) | 113 (92.6) | |
| ≥ 8 | 26 (21.3) | 9 (7.4) | |
| Reflux esophagitis (Los Angeles Classification) | < 0.001 | ||
| No reflux | 81 (66.4) | 100 (82.0) | |
| A | 14 (11.5) | 15 (12.3) | |
| B | 11 (9.0) | 6 (4.9) | |
| C | 16 (9.0) | 1 (0.8) |
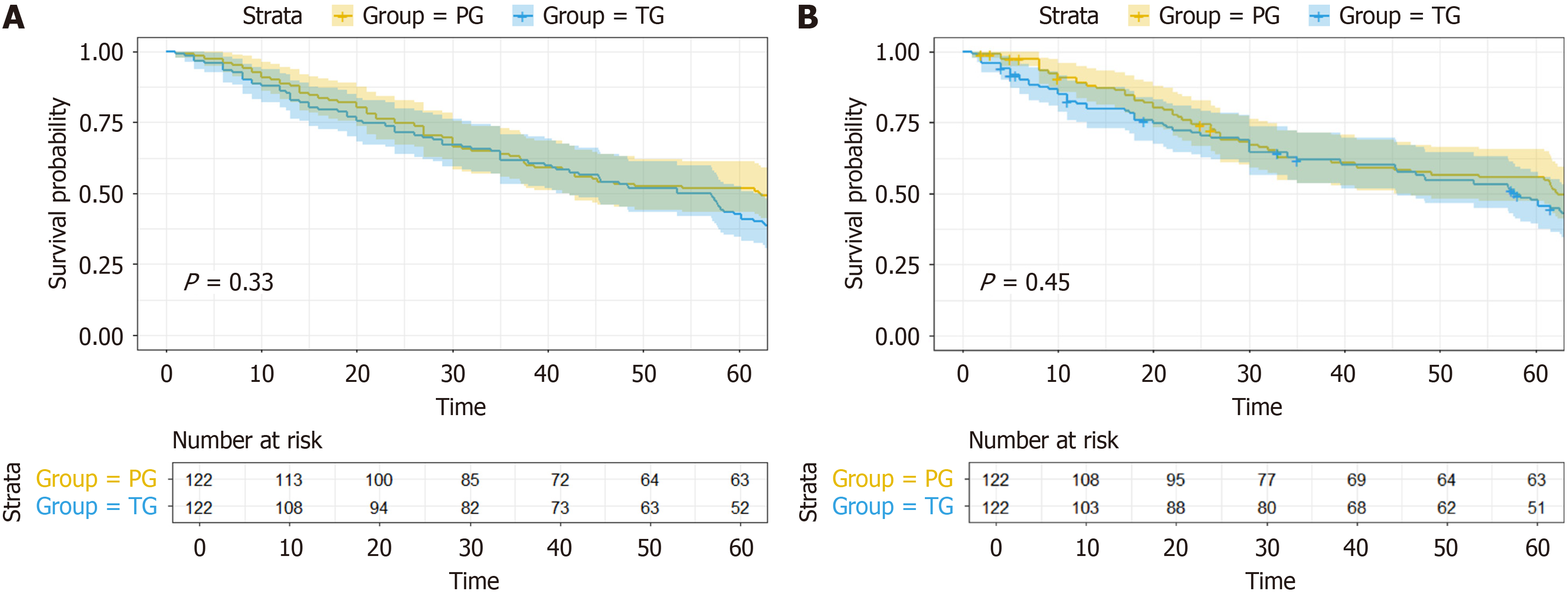
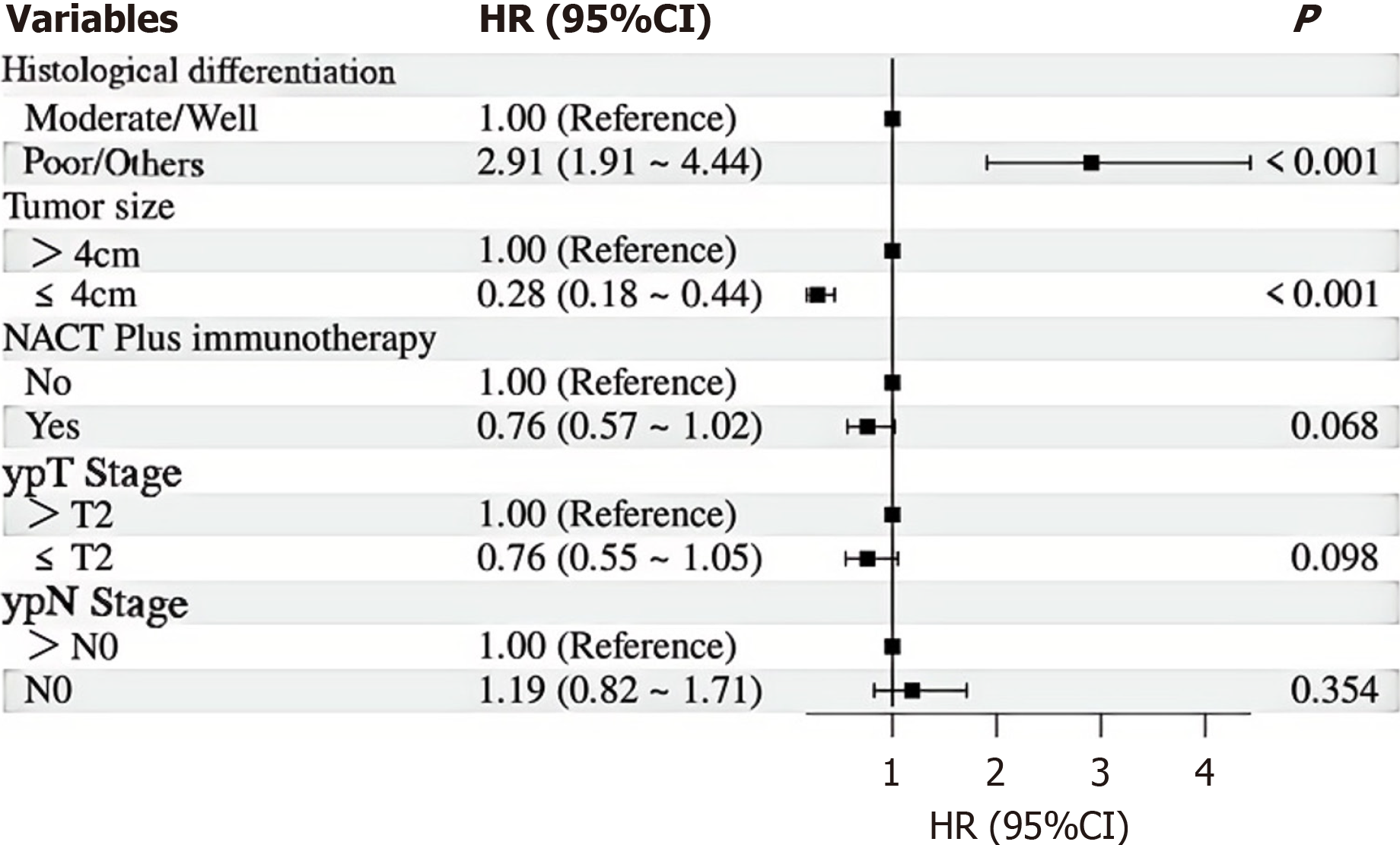
With a median follow-up of 36.8 months, the 5-year OS rates were 52.7% for the PG group and 45.5% for the TG group (Figure 2A), with no statistical difference between the groups (P = 0.330). Similarly, the 5-year RFS rates were 54.3% and 47.6% for the PG and TG groups, respectively, also showing no statistical difference (P = 0.356) (Figure 2B). Univariate Cox regression analysis for OS (Table 5) identified several prognostic factors, including histological differentiation (HR = 3.60, 95%CI: 2.52-5.14, P < 0.001), tumor size (HR = 0.18, 95%CI: 0.12-0.27 for tumors ≤ 4cm, P < 0.001), NACT plus immunotherapy (HR = 0.71, 95%CI: 0.53-0.94, P = 0.019), ypT stage (HR = 0.58, 95%CI: 0.43-0.79 for ypT ≤ 2, P < 0.001), and ypN stage (HR = 0.68, 95%CI: 0.48-0.96 for ypN0, P = 0.027). Neither TG nor PG was identified as a significant prognostic factor for OS (HR = 1.15, 95%CI: 0.87-1.54, P = 0.330). Multivariate Cox regression analysis (Figure 3) confirmed 2 independent prognostic factors for OS: Histological differentiation (HR = 2.98, 95%CI: 2.03-4.36, P < 0.001), and tumor size (HR = 0.26, 95%CI: 0.17-0.41 for tumors ≤ 4 cm, P < 0.001).
| Variables | Hazard ratio (95%CI) | P value |
| Group | ||
| PG | 1.00 (Reference) | |
| TG | 1.15 (0.87-1.54) | 0.330 |
| Histological differentiation | ||
| Moderate/Well | 1.00 (Reference) | |
| Poor/others | 3.60 (2.52-5.14) | < 0.001 |
| Tumor size | ||
| > 4 cm | 1.00 (Reference) | |
| ≤ 4 cm | 0.18 (0.12-0.27) | < 0.001 |
| Gender | ||
| Female | 1.00 (Reference) | |
| Male | 0.88 (0.62-1.26) | |
| Age | ||
| > 70 | 1.00 (Reference) | |
| ≤ 70 | 0.77 (0.53-1.13) | |
| BMI | ||
| > 24 kg/m2 | 1.00 (Reference) | |
| ≤ 24 kg/m2 | 1.10 (0.83-1.47) | |
| Clavien-Dindo Classification | ||
| > Grade II | 1.00 (Reference) | |
| ≤ Grade II | 0.68 (0.42-1.11) | |
| TRG | ||
| > 1 | 1.00 (Reference) | |
| ≤ 1 | 0.95 (0.68-1.32) | |
| NACT plus immunotherapy | ||
| No | 1.00 (Reference) | |
| Yes | 0.71 (0.53-0.94) | |
| NACT regimens | ||
| Others | 1.00 (Reference) | |
| SOX | 0.97 (0.73-1.29) | |
| NACT cycles | ||
| > 4 | 1.00 (Reference) | |
| ≤ 4 | 0.83 (0.56-1.25) | |
| Vascular invasion | ||
| No | 1.00 (Reference) | |
| Yes | 1.19 (0.87-1.63) | |
| Perineural invasion | ||
| No | 1.00 (Reference) | |
| Yes | 1.34 (1.00-1.81) | |
| CR response | ||
| CR | 1.00 (Reference) | |
| Non-CR | 1.25 (0.72-2.16) | |
| Radical resection | ||
| R0 | 1.00 (Reference) | |
| R1 | 0.81 (0.20-3.29) | |
| ypT Stage | ||
| > T2 | 1.00 (Reference) | |
| ≤ T2 | 0.58 (0.43-0.79) | |
| ypN Stage | ||
| > N0 | 1.00 (Reference) | |
| N0 | 0.68 (0.48-0.96) |
PG and TG are two major surgical approaches to treating proximal GC. Traditionally, due to concerns about insufficient resection margins and inadequate lymph node dissection, TG has often been considered the standard procedure for treating proximal GC[15]. However, patients who undergo TG experience significant impacts on their quality of life, such as malnutrition and dumping syndrome[16]. With advances in surgical techniques and a deeper understanding of the biological behavior of GC, PG, which preserves some gastric function, has gradually gained attention, especially for early proximal GC[17,18]. As for advanced proximal GC, most previous studies have suggested that there is no difference in short-term and long-term efficacy between PG and TG for patients with proximal GC undergone surgical treatment. A meta-analysis that included three studies involving a total of 4815 patients with locally advanced proximal GC indicated that compared to TG, PG was significantly associated with improved OS (HR = 1.15, 95%CI: 1.05-1.25)[19], and Rosa et al[20] reported that postoperative complication rates for PG and TG were 25.3% and 28%, respectively, in patients with adenocarcinoma of the upper third of the stomach (P = 0.084). Additionally, 5-year OS rates have been reported to be 56.7% for PG patients and 46.5% for TG patients (P = 0.07). However, most of the current clinical studies that compare PG and TG have not incorporated NACT. However, Yuan et al[9] reported that, when combined with NACT, the PG group achieved a satisfactory 5-year OS rate of 68.4% and demonstrated a significantly better 5-year OS in patients with pathological stage I after NACT (91.0% vs 85.7%, P = 0.036) compared to the TG group.
Our results show that, following NACT, the PG group achieved comparable 5-year OS rates (52.7% vs 45.5%, P = 0.33) and RFS rates (54.3% vs 47.6%, P = 0.45) to those of the TG group, without a significant increase in the incidence of short-term postoperative complications. Importantly, multivariate analysis identified histological differentiation and tumor size as independent prognostic factors for OS, underscoring the need for a comprehensive evaluation of these factors in clinical decision-making. We believe that for patients with proximal GC who have well-differentiated histology and a tumor size of no more than 4 cm, NACT combined with PG and tubular stomach reconstruction could be considered as a treatment option to achieve better survival benefits. Of course, the varying sensitivity of different patients to che
Preoperative NACT for advanced GC has been widely recommended in the United States and Europe, and the results of the FLOT4 trial provide strong evidence supporting the implementation of NACT in locally advanced, resectable gastric or gastro-esophageal junction adenocarcinoma[21]. In Asian countries, especially Japan, both PG and NACT recommendations are more conservative[8]. Possible reasons may include inconsistency in chemotherapy regimens, possible poor chemotherapy responses in some patients, and emphasis on D2 lymph node dissection. Although the FLOT regimen has become the first-line regimen in both the NCCN and ESMO guidelines, its superiority over other regimens, such as cisplatin-based combination chemotherapy, in the neoadjuvant treatment of proximal GC remains under investigation. The choice between triplet and doublet chemotherapy regimens is also a matter of ongoing debate[22]. In addition, S-1–based regimens have demonstrated favorable efficacy in Asian populations[23-25], but their applicability in Western populations requires further evaluation. Therefore, identifying the optimal NACT strategy tailored to individual patient characteristics and regional differences remains an important direction for future research. According to current guidelines and clinical studies, the recommended duration of NACT for GC typically ranges from 2 to 4 cycles[6,8,22-25], and determining the optimal number of NACT cycles for each patient requires MDT discussion and an individualized treatment plan that takes into account the clinical stage, performance status, comorbidities, and tolerability of the selected regimen.
We also observed significant differences in TRG between the PG and TG groups after NACT. This discrepancy likely stems from the varying sensitivity of tumors to chemotherapy, which can depend on tumor biology, chemotherapy regimens, and patient-specific factors. Some tumors respond well to chemotherapy and show significant regression (TRG 1a), but others may only experience partial regression (TRG 1b or 2) or minimal response (TRG 3). However, after PSM, we ensured that the baseline characteristics, such as tumor size, stage, and histological differentiation, were balanced between the two groups. Therefore, despite the differences in TRG, the groups were still comparable in terms of clinical and pathological factors. This suggests that the observed differences in TRG may not significantly affect the comparison of surgical outcomes. The heterogeneity in chemotherapy response highlights the need for individualized treatment strategies based on tumor characteristics and treatment response. Future studies should explore tools to predict chemotherapy efficacy in order to tailor treatment for patients with advanced proximal GC.
Immunotherapy for GC has also been gaining popularity based on recent findings. For example, the results of the DRAGON IV/CAP 05 trial indicated that the addition of camrelizumab (anti–PD-1) and low-dose rivoceranib (VEGFR-2 inhibitor) to the SOX chemotherapy regimen significantly improved pathologic outcomes in patients with locally advanced gastric or gastroesophageal junction adenocarcinoma[25]. Moreover, the KEYNOTE-585 trial[26] demonstrated that the pCR rate was significantly higher in the pembrolizumab group compared to the placebo group (cisplatin-based regimen: 12.9% vs 2.0%; FLOT regimen: 17.0% vs 7.0%, P < 0.05). Similarly, the DANTE trial[27] reported superior tumor downstaging with immunotherapy, with higher proportions of patients achieving ypT0 (23% vs 15%, one-sided P = 0.044), ypT0-T2 (61% vs 48%, one-sided P = 0.015), and ypN0 (68% vs 54%, one-sided P = .012). Additionally, the pCR rate or TRG1a rate was significantly higher in the NACT plus immunotherapy group (24% vs 15%, P = 0.032)[27]. Notably, patients with microsatellite instability-high status derived greater benefit from immunotherapy in terms of pCR[26,27]. These findings showcase the promising potential of immunotherapy in the treatment of advanced GC. However, although immunotherapy may increase the pCR rate, the answer to the question of whether the addition of immunotherapy to NACT can improve OS in advanced proximal GC patients remains unclear.
The choice of gastrointestinal reconstruction method following PG is also critically important. Gastric tubular reconstruction involves the creation of a tube-like stomach on the greater curvature side of the remaining stomach, which is then anastomosed end-to-side with the esophagus[14]. This technique may reduce acid reflux by lengthening the distance between and by removing a portion of the antrum[14]. In addition, the operation requires the completion of only one anastomosis, which may reduce the incidence of postoperative anastomotic leakage compared to reconstruction with multiple anastomoses. Moreover, this approach preserves the function of the duodenum and more of the proximal jejunum, which is essential for digestion and absorption of nutrients, thereby improve postoperative nutritional status[14,28]. However, this type of anastomosis also has several drawbacks. A previous review[29] highlighted various potential complications, including excessive cutting and suturing, which can lead to gastric hemorrhage, poor healing, and the formation of gastric fistulas. Additionally, the arterial blood supply and venous return to the gastric tube may be compromised. The smaller gastric lumen and more direct food passage may, in some cases, increase the incidence of abnormal gastric emptying and reflux esophagitis. Moreover, the tube-shaped stomach tends to increase anastomotic tension, which can result in postoperative anastomotic leakage and stricture. In our study, the right gastroepiploic vessels and right gastric vessels were preserved to ensure adequate arterial blood supply and venous return to the remnant stomach. A side-to-side anastomosis was then performed between the anterior wall of the tubular remnant stomach and the posterior wall of the esophagus using a linear stapler with a 45-mm cartridge, thereby enlarging the anastomotic lumen and reducing the risk of postoperative stricture. Finally, the common hole was sutured to prevent anastomotic leakage and bleeding. The anastomotic leakage rate was 1.64% in both PG and TG group, the bleeding rate was 2.46% in the PG group and 1.64% in the TG group, and no anastomotic stricture was observed. These results provide valuable evidence to support the feasibility and safety of gastric tubular reconstruction.
TG has a negative impact on the nutritional status of patients due to the complete removal of the stomach. Patients are more likely to experience dietary restrictions, nutritional deficiencies, and weight loss after TG than after subtotal gastrectomy[30]. Although patients who undergo PG may still experience various nutritional challenges, the preservation of partial gastric function is associated with less weight loss compared to TG[31]. Thus, PG may have potential ad
To the best of our knowledge, this is the first study to evaluate and compare the surgical outcomes of PG and TG in the context of NACT while also investigating the tubular stomach reconstruction method and assessing postoperative quality of life. Our study highlights the potential clinical benefits of PG with tubular stomach reconstruction for patients with advanced proximal GC following NACT. This surgical approach not only maintains oncological safety but also offers advantages in terms of shorter operation time and lower rates of postoperative reflux, which can significantly improve patients' quality of life. Based on these findings, we suggest that patients with well-differentiated tumors or smaller tumors (≤ 4 cm) may benefit the most from PG, as it preserves some gastric function and may also offer better oncological outcomes. As NACT continues to evolve, the integration of novel therapies such as PD-1 inhibitors may further improve outcomes for these patients, warranting future studies to explore the synergistic effects of such treatments in combination with PG.
This study has certain limitations, however. First, its retrospective design may have introduced bias despite PSM, as the choice of surgical approach was influenced by various clinical factors, including tumor stage and location. Second, although we assessed short-term and long-term outcomes, the postoperative nutritional status of patients was not fully addressed, which is critical for evaluating the impact on postoperative quality of life, particularly for those undergoing TG. Finally, both PG and TG procedures may affect function in distinct ways, underscoring the need to conduct prospective studies with patient-reported outcomes in order to evaluate the associated trade-offs more comprehensively.
To conclude, the above results indicate that PG with tubular stomach reconstruction is comparable to TG in terms of surgical safety and long-term oncological outcomes for patients with locally advanced proximal GC following NACT. Additionally, PG has advantages in shorter operation time and lower rates of postoperative reflux, suggesting potential benefits for patient quality of life.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 8000] [Article Influence: 8000.0] [Reference Citation Analysis (2)] |
| 2. | Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69:1564-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 362] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 3. | Wei J, Yang P, Huang Q, Chen Z, Zhang T, He F, Hu H, Zhong J, Li W, Wei F, Wang Q, Cao J. Proximal versus total gastrectomy for proximal gastric cancer: a Surveillance, Epidemiology, and End Results Program database analysis. Future Oncol. 2021;17:1185-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Zhang Y, Zhang PS, Rong ZY, Huang C. One stomach, two subtypes of carcinoma-the differences between distal and proximal gastric cancer. Gastroenterol Rep (Oxf). 2021;9:489-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Newton AD, Datta J, Loaiza-Bonilla A, Karakousis GC, Roses RE. Neoadjuvant therapy for gastric cancer: current evidence and future directions. J Gastrointest Oncol. 2015;6:534-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 6. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 954] [Article Influence: 318.0] [Reference Citation Analysis (0)] |
| 7. | Eom SS, Ryu KW, Han HS, Kong SH. A Comprehensive and Comparative Review of Global Gastric Cancer Treatment Guidelines: 2024 Update. J Gastric Cancer. 2025;25:153-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer. 2023;26:1-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 593] [Article Influence: 296.5] [Reference Citation Analysis (2)] |
| 9. | Yuan Z, Cui H, Xu Q, Gao J, Liang W, Cao B, Lin X, Song L, Huang J, Zhao R, Li H, Yu Z, Du J, Wang S, Chen L, Cui J, Zhao Y, Wei B. Total versus proximal gastrectomy for proximal gastric cancer after neoadjuvant chemotherapy: a multicenter retrospective propensity score-matched cohort study. Int J Surg. 2024;110:1000-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 10. | Kolozsi P, Varga Z, Toth D. Indications and technical aspects of proximal gastrectomy. Front Surg. 2023;10:1115139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Kano Y, Ohashi M, Nunobe S. Laparoscopic Function-Preserving Gastrectomy for Proximal Gastric Cancer or Esophagogastric Junction Cancer: A Narrative Review. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Li L, Cai X, Liu Z, Mou Y, Wang Y. Digestive tract reconstruction after laparoscopic proximal gastrectomy for Gastric cancer: A systematic review. J Cancer. 2023;14:3139-3150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Shibamoto J, Kubota T, Nishibeppu K, Ohashi T, Konishi H, Shiozaki A, Fujiwara H, Otsuji E. Clinical Relevance of Proximal Gastrectomy With Double-flap Esophagogastrostomy Reconstruction With Glycemic Profile and Postgastrectomy Syndromes. Anticancer Res. 2023;43:857-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Fu J, Li Y, Liu X, Jiao X, Wang Y, Qu H, Niu Z. Clinical outcomes of proximal gastrectomy with gastric tubular reconstruction and total gastrectomy for proximal gastric cancer: A matched cohort study. Front Surg. 2022;9:1052643. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Solsky I, In H. Surgical Treatment for Gastric Cancer. Gastrointest Endosc Clin N Am. 2021;31:581-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Hirata Y, Kim HI, Grotz TE, Matsuda S, Badgwell BD, Ikoma N. The role of proximal gastrectomy in gastric cancer. Chin Clin Oncol. 2022;11:39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Tsekrekos A, Okumura Y, Rouvelas I, Nilsson M. Gastric Cancer Surgery: Balancing Oncological Efficacy against Postoperative Morbidity and Function Detriment. Cancers (Basel). 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Xu Y, Tan Y, Wang Y, Xi C, Ye N, Xu X. Proximal versus total gastrectomy for proximal early gastric cancer: A systematic review and meta-analysis. Medicine (Baltimore). 2019;98:e15663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Su PJ, Huang YT, Liao TK, Lu WH, Wang CJ, Chao YJ, Shan YS. Comparing survival after proximal gastrectomy vs. total gastrectomy in advanced gastric cancer: A systematic review and metaanalysis. Oncol Lett. 2024;28:427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 20. | Rosa F, Quero G, Fiorillo C, Bissolati M, Cipollari C, Rausei S, Chiari D, Ruspi L, de Manzoni G, Costamagna G, Doglietto GB, Alfieri S. Total vs proximal gastrectomy for adenocarcinoma of the upper third of the stomach: a propensity-score-matched analysis of a multicenter western experience (On behalf of the Italian Research Group for Gastric Cancer-GIRCG). Gastric Cancer. 2018;21:845-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp HG, Mayer F, Haag GM, Luley K, Lindig U, Schmiegel W, Pohl M, Stoehlmacher J, Folprecht G, Probst S, Prasnikar N, Fischbach W, Mahlberg R, Trojan J, Koenigsmann M, Martens UM, Thuss-Patience P, Egger M, Block A, Heinemann V, Illerhaus G, Moehler M, Schenk M, Kullmann F, Behringer DM, Heike M, Pink D, Teschendorf C, Löhr C, Bernhard H, Schuch G, Rethwisch V, von Weikersthal LF, Hartmann JT, Kneba M, Daum S, Schulmann K, Weniger J, Belle S, Gaiser T, Oduncu FS, Güntner M, Hozaeel W, Reichart A, Jäger E, Kraus T, Mönig S, Bechstein WO, Schuler M, Schmalenberg H, Hofheinz RD; FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 974] [Cited by in RCA: 1639] [Article Influence: 273.2] [Reference Citation Analysis (0)] |
| 22. | Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, Vogel A, Smyth EC; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1005-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 670] [Article Influence: 223.3] [Reference Citation Analysis (0)] |
| 23. | Terashima M, Iwasaki Y, Mizusawa J, Katayama H, Nakamura K, Katai H, Yoshikawa T, Ito Y, Kaji M, Kimura Y, Hirao M, Yamada M, Kurita A, Takagi M, Boku N, Sano T, Sasako M; Stomach Cancer Study Group, Japan Clinical Oncology Group. Randomized phase III trial of gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer, the short-term safety and surgical results: Japan Clinical Oncology Group Study (JCOG0501). Gastric Cancer. 2019;22:1044-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 24. | Wang X, Lu C, Wei B, Li S, Li Z, Xue Y, Ye Y, Zhang Z, Sun Y, Liang H, Li K, Zhu L, Zheng Z, Zhou Y, He Y, Li F, Wang X, Liang P, Huang H, Li G, Shen X, Ji J, Tang Y, Xu Z, Chen L; RESONANCE study group. Perioperative versus adjuvant S-1 plus oxaliplatin chemotherapy for stage II/III resectable gastric cancer (RESONANCE): a randomized, open-label, phase 3 trial. J Hematol Oncol. 2024;17:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 25. | Li C, Tian Y, Zheng Y, Yuan F, Shi Z, Yang L, Chen H, Jiang L, Wang X, Zhao P, Zhang B, Wang Z, Zhao Q, Dong J, Lian C, Xu S, Zhang A, Zheng Z, Wang K, Dang C, Wu D, Chen J, Xue Y, Liang B, Cheng X, Wang Q, Chen L, Xia T, Liu H, Xu D, Zhuang J, Wu T, Zhao X, Wu W, Wang H, Peng J, Hou Z, Zheng R, Chen Y, Yin K, Zhu Z. Pathologic Response of Phase III Study: Perioperative Camrelizumab Plus Rivoceranib and Chemotherapy Versus Chemotherapy for Locally Advanced Gastric Cancer (DRAGON IV/CAP 05). J Clin Oncol. 2025;43:464-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 26. | Shitara K, Rha SY, Wyrwicz LS, Oshima T, Karaseva N, Osipov M, Yasui H, Yabusaki H, Afanasyev S, Park YK, Al-Batran SE, Yoshikawa T, Yanez P, Dib Bartolomeo M, Lonardi S, Tabernero J, Van Cutsem E, Janjigian YY, Oh DY, Xu J, Fang X, Shih CS, Bhagia P, Bang YJ; KEYNOTE-585 investigators. Neoadjuvant and adjuvant pembrolizumab plus chemotherapy in locally advanced gastric or gastro-oesophageal cancer (KEYNOTE-585): an interim analysis of the multicentre, double-blind, randomised phase 3 study. Lancet Oncol. 2024;25:212-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 139] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 27. | Lorenzen S, Götze TO, Thuss-Patience P, Biebl M, Homann N, Schenk M, Lindig U, Heuer V, Kretzschmar A, Goekkurt E, Haag GM, Riera-Knorrenschild J, Bolling C, Hofheinz RD, Zhan T, Angermeier S, Ettrich TJ, Siebenhuener AR, Elshafei M, Bechstein WO, Gaiser T, Loose M, Sookthai D, Kopp C, Pauligk C, Al-Batran SE; AIO and SAKK Study Working Groups. Perioperative Atezolizumab Plus Fluorouracil, Leucovorin, Oxaliplatin, and Docetaxel for Resectable Esophagogastric Cancer: Interim Results From the Randomized, Multicenter, Phase II/III DANTE/IKF-s633 Trial. J Clin Oncol. 2024;42:410-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 83] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 28. | Toyomasu Y, Ogata K, Suzuki M, Yanoma T, Kimura A, Kogure N, Yanai M, Ohno T, Mochiki E, Kuwano H. Restoration of gastrointestinal motility ameliorates nutritional deficiencies and body weight loss of patients who undergo laparoscopy-assisted proximal gastrectomy. Surg Endosc. 2017;31:1393-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Lu S, Ma F, Zhang Z, Peng L, Yang W, Chai J, Liu C, Ge F, Ji S, Luo S, Chen X, Hua Y. Various Kinds of Functional Digestive Tract Reconstruction Methods After Proximal Gastrectomy. Front Oncol. 2021;11:685717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Akad F, Filip B, Preda C, Zugun-Eloae F, Peiu SN, Akad N, Crauciuc DV, Vatavu R, Gavril LC, Sufaru RF, Mocanu V. Assessing Nutritional Status in Gastric Cancer Patients after Total versus Subtotal Gastrectomy: Cross-Sectional Study. Nutrients. 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | Park SC, Jeong O, Kang JH, Jung MR. Longitudinal Change in Health-Related Quality of Life after Total Gastrectomy: Approach Based on the Minimally Important Difference. Ann Clin Nutr Metab. 2021;13:43-51. [DOI] [Full Text] |