Published online May 27, 2025. doi: 10.4240/wjgs.v17.i5.105783
Revised: March 9, 2025
Accepted: April 9, 2025
Published online: May 27, 2025
Processing time: 105 Days and 6.7 Hours
Combined hepatocellular cholangiocarcinoma (cHCC-CCA) is a rare and ag
Core Tip: Combined hepatocellular cholangiocarcinoma is a rare and aggressive liver malignancy with diagnostic and therapeutic challenges. Although surgical resection remains the primary curative approach, liver transplantation is emerging as a potential option for carefully selected patients, particularly those with early-stage tumors or cirrhosis. Adherence to stringent selection criteria, such as the Milan criteria, is crucial for optimizing post-transplant outcomes. Ongoing research aims to refine the selection framework and to improve prognostic accuracy.
- Citation: Zhou RQ, Yang PJ, Liu TT, Han DD, Liu XL, Liu LG, Si S, Yang SW, Xu SS, Guo YW, Tan HD. Liver transplantation for combined hepatocellular cholangiocarcinoma: Current evidence, selection criteria, and therapeutic controversies. World J Gastrointest Surg 2025; 17(5): 105783
- URL: https://www.wjgnet.com/1948-9366/full/v17/i5/105783.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i5.105783
Combined hepatocellular cholangiocarcinoma (cHCC-CCA) is an extremely rare primary liver cancer (PLC). cHCC-CCA is defined as a solitary tumor containing both hepatocellular components and cholangiocellular components within the same tumor. cHCC-CCA accounts for 2%-5% of all PLCs according to the World Health Organization (WHO)[1,2]. The prevalence reported in previous studies may be misinterpreted because of confusion regarding histological definitions. cHCC-CCA is often misdiagnosed preoperatively as hepatocellular carcinoma (HCC) or intrahepatic cholangiocarcinoma (iCCA) owing to its heterogeneous morphological characteristics and imaging features[3,4]. Thus, cHCC-CCA is diagnosed based on proper multisite sampling of surgical specimens.
Liver resection (LR) remains an accepted curative treatment for cHCC-CCA and appears to lead to longer survival compared with non-surgical treatment[5,6]. This suggests that the biological and clinical behaviors of cHCC-CCAs are similar to those of iCCA. The role of liver transplantation (LT) in patients with cHCC-CCA remains controversial because of the high recurrence rate and poor prognosis. However, several previous studies have suggested that LT may be feasible for select patients with cHCC-CCA and should be considered for these patients[7]. This review provided an overview of the current knowledge of the definition, clinical characteristics, diagnosis, surgical treatment, and prognosis of cHCC-CCA and attempted to answer the following questions: Is LT an indication for selected patients with cHCC-CCA? and what LT criteria should be adopted for patients with cHCC-CCA?
Since the first case of cHCC-CCA was reported in 1903[8], there has been under-recognition and misdiagnosis of cHCC-CCA owing to several different definition criteria. In 1949, Allen and Lisa[9] described the first classification of cHCC-CCA and proposed three subtypes based on the histology of five cases: Type 1, separate nodules of hepatocellular and cholangiocarcinoma (double); type 2, contiguity with intermingling (combined); and type 3, strongly associated with an origin from the same focus (mixed). In 1985, Goodman et al[10] reviewed 24 cases of cHCC-CCAs and suggested a modified three subtype classification: Type I (collision tumors), the presence of both HCC and iCCA separately in the same patient; type II (transitional tumors), the presence of the same nodule in an area with intermediate differentiation and an identifiable transition between HCC and iCCA; and type III (fibrolamellar tumors), resembled fibrolamellar HCC containing mucin-producing pseudoglands.
The definition recommended by the WHO has evolved in recent decades. In 2000, the WHO classified cHCC-CCA as a tumor containing both HCC and iCCA elements in the presence of both bile and mucin. Several studies have subsequently suggested the existence of cHCC-CCAs with stem cell features and an intermediate morphology between HCC and iCCA[11-13]. Thus, the fourth WHO classification modified the classification in 2010 into classical type, tumors containing unequivocal, intimately mixed elements of both HCC and iCCA, and three subtypes with stem/progenitor cell features, which include typical, intermediate cell, and cholangiocellular.
However, a series of challenges emerged regarding the fourth edition of the WHO classification, and the three subtypes with stem/progenitor cell features were particularly problematic because these subtypes can be found in different types of hepatic tumors[14-16]. In 2008, an international group composed of pathologists, radiologists, and clinicians proposed a consensus and standardized the nomenclature for cHCC-CCA[17]. These recommendations were incorporated into the fifth edition of the WHO classification in 2019: cHCC-CCA is a primary hepatic carcinoma with unequivocal existence of both hepatocytic and cholangiocytic differentiation within a single tumor, classified as a type 3 tumor by Allen and Lisa[9] and type II tumor by Goodman et al[10]. Distinct multifocal HCC and iCCA in separate nodules, the so-called collision tumors of HCC and iCCA arising separately, should be no longer considered as cHCC-CCA; the intermediate cell subtype is classified as a separate category of intermediate carcinoma, and the cholangiocellular type is currently classified as iCCA.
The incidence of cHCC-CCA (2%-5% of PLCs) is markedly lower than that of HCC (75%-85%) and iCCA (10%-15%)[1]. cHCC-CCA is more commonly diagnosed in male patients, accounting for 65%-80% of cases, and typically affects adults aged between 60 and 65 years[18-20]. The epidemiology and clinical presentation of cHCC-CCAs exhibit marked geographical disparities that significantly influence risk stratification and management strategies. In Asian cohorts, HBV infection dominates as the primary risk factor (> 50%), often arising from a background of cirrhosis or chronic hepatitis[21,22]. In contrast, Western populations demonstrate a distinct profile of hepatitis C virus infection, metabolic dysfunction associated with steatohepatitis, and alcohol-related steatohepatitis, which play larger roles[23-25].
The clinical symptoms of cHCC-CCA are analogous to those of HCC and iCCA, most of which are non-specific and include fatigue, abdominal pain, fever, weight loss, jaundice, etc. Although HCC predominantly arises in cirrhotic livers (70%-90% of cases), and iCCA often occurs in non-cirrhotic livers (60%-70%), cHCC-CCA occupies an intermediate position, with 35%-60% of cases associated with cirrhosis[23]. Prognostically, cHCC-CCA demonstrates more aggressive behavior than HCC but has slightly better survival than iCCA[3,21]. Synchronous elevation of alpha-fetoprotein (AFP) and carbohydrate antigen 19-9 (CA19-9) levels suggests a diagnosis of cHCC-CCA. However, elevated AFP and CA19-9 levels are observed only in a subset of patients[20,26,27]. The imaging features of cHCC-CCA depend on the proportion of HCC or iCCA components and display a mixed enhancement pattern: Arterial phase hyperenhancement, washout appearance, and enhancing capsule when HCC components are predominant; and peripheral rim arterial enhancement, possibly peripheral washout, and central enhancement when iCCA components are predominant[28-30].
Several retrospective studies have evaluated the performance of the Liver Imaging Reporting and Data System (LI-RADS) for the assessment of cHCC-CCA[31-33]. However, Jeon et al[34] reported that LI-RADS misdiagnosed approximately half of all cHCC-CCA nodules. Therefore, an accurate preoperative diagnosis of cHCC-CCA is difficult, although a few studies have proposed new diagnostic criteria and improved diagnostic efficacy by combining LI-RADS with serum biomarkers[35,36].
The gold standard for diagnosing cHCC-CCA is a thorough histological evaluation of hematoxylin and eosin-stained sections at low-power magnification. Immunohistochemical markers are helpful but not essential for the diagnosis of cHCC-CCA[37]. A comprehensive examination of the resected specimens is critical for an accurate diagnosis. The true incidence of cHCC-CCA may be underestimated because most patients do not undergo surgical resection, and a substantial number of cases may consequently be misdiagnosed as HCC or iCCA[3].
The role of needle biopsy is controversial considering the limited tissue volume and histological heterogeneity of cHCC-CCA. Jung et al[38] suggested that preoperative biopsy should be avoided in patients planning to undergo LR because of the risk of tumor spread and because its diagnosis usually does not change the surgical plan. In contrast, they suggested that systematic multiple-site biopsy is necessary for patients awaiting LT because large-sized cHCC-CCA and iCCA cases would be excluded from LT due to the poor prognosis and high recurrence rates.
For an accurate diagnosis, Gigante et al[30] proposed a two-step strategy combining imaging features and serum biomarker levels, followed by an extended tissue biopsy performed in different areas of the tumor. Recently, a deep learning-based artificial intelligence model was developed as a promising tool for the diagnosis and reclassification of cHCC-CCAs, thereby assisting in treatment decision-making and ultimately improving clinical outcomes[39].
Recent advances in molecular profiling have unraveled the genomic complexity of cHCC-CCAs, revealing distinct molecular signatures that bridge hepatocellular and cholangiocellular differentiation. Although most cHCC-CCAs lack actionable mutations, emerging data highlight divergent molecular drivers between HCC-dominant and iCCA-dominant components. HCC-like subtypes frequently harbor alterations in CTNNB1 (β-catenin pathway), TERT promoter mu
The current clinical treatment patterns for patients with cHCC-CCAs reveal significant heterogeneity. A population-based study reported that 17.6% of patients underwent LR, 13.1% underwent LT, and 65.2% were managed nonsurgically because of advanced disease or comorbidities[3]. LR is currently considered a preferable curative treatment option for patients with cHCC-CCA and is indicated for patients with no distant metastasis and adequate liver function based on surgical strategies for HCC and iCCA[5,42]. cHCC-CCA is similar to HCC in terms of vascular involvement and to iCCA in terms of lymph node (LN) metastasis. The goal of LR is to remove the tumor with adequate margins while preserving sufficient liver remnant volume. The presence of underlying liver disease, tumor size, and extent of vascular invasion are factors that influence the feasibility of surgical intervention.
Ma and Chok[43] proposed that a resection margin greater than 10 mm correlates with prolonged disease-free survival (DFS) in patients with multiple tumor nodules. Minor hepatectomy is generally preferred in patients with liver cirrhosis because of the increased risk of postoperative liver failure associated with major hepatectomy although major resection tends to confer a superior survival rate[6].
LR appears to be one of the predominant prognostic factors for patients with cHCC-CCA, while other risk factors include large tumor size (> 5 cm), presence of satellite nodules, LN involvement, multifocality, vascular invasion, portal vein invasion, high tumor stage, high levels of CA19-9, incomplete capsule formation, and a resection margin of less than 2 cm[44-48]. The prognosis of patients who underwent LR for cHCC-CCA was less favorable than that of patients with HCC with a mean DFS and overall survival (OS) of 13 and 31 months, respectively[21]. The survival rates for cHCC-CCA were more similar to those for iCCA than to those for HCC.
The role of LT in cHCC-CCA remains debatable in the surgical and transplant communities. Many patients with cHCC-CCA have been preoperatively misdiagnosed with HCC and subsequently underwent LT. Most studies concerning LT for cHCC-CCA have been retrospective and of limited size due to the rarity of the condition. Consequently, most surgeons have a limited understanding of the feasibility of LT for cHCC-CCA.
Due to the poor prognosis associated with iCCA components, cHCC-CCA has traditionally been considered a contraindication for LT. In a systematic review on cHCC-CCA, the DFS was 14.2 months, and the median OS was 37.1 months, both of which were significantly worse than those of HCC[21]. Other studies have assessed the outcomes of patients with cHCC-CCA undergoing LT and reported a high recurrence rate of approximately 40%[49,50]. Consistent with previous findings, Park et al[51] examined the outcomes of 15 patients who underwent LT for cHCC-CCA and observed that LT was associated with high recurrence and mortality rates, especially during the first year post-LT.
However, several studies have shown promising results in patients with cHCC-CCA who underwent LT. Dageforde et al[52] suggested that patients with cHCC-CCAs who underwent LT had significantly better OS and DFS than those who underwent LR, regardless of the tumor burden. Jaradat et al[53] conducted a multicenter analysis of 19 patients with cHCC-CCA and concluded that individuals with early-stage cHCC-CCA should be considered for transplant evaluation. De Martin et al[25] conducted a comparative analysis of patients with iCCA or cHCC-CCA who underwent LT and LR. The LT group had significantly higher 5-year recurrence-free survival and lower recurrence rate regardless of the tumor size (≤ 2 cm and > 2 cm but ≤ 5 cm) (5-year recurrence-free survival of patients with iCCA > 2 but ≤ 5 cm: 74%) and suggested that LT may offer a benefit for highly selected patients with cirrhosis and unresectable cHCC-CCA tumors of 5 cm or less.
Owing to the small sample size and confusion regarding diagnostic criteria previously, it is challenging to obtain robust and persuasive results. In recent years, researchers have increasingly relied on large-size databases to achieve more credible conclusions. Based on the Surveillance, Epidemiology, and End Results (SEER) database, several studies have indicated promising outcomes for patients with cHCC-CCA receiving LT compared to LR (Table 1)[3,7,42,54-57]. An analysis of 465 patients revealed that LT, minor hepatectomy, and major hepatectomy had similar hazard ratios and 5-year OS[3]. Wang et al[42] and Groeschl et al[54] reported that LT conferred a survival benefit similar to that of LR for cHCC-CCA. More recent studies concluded that LT provides a survival advantage over LR in patients with cHCC-CCA[7,55-57]. These results indicated that patients with cHCC-CCA should also be considered potential candidates for LT, particularly selected cases with early-stage disease or small tumor size.
| Ref. | Study interval | Number of cases (LR:LT) | Outcomes | |
| LR | LT | |||
| Groeschl et al[54], 2013 | 1973-2007 | 35:19 | 1-year OS: 71%; 3-year OS: 46% | 1-year OS: 89%; 3-year OS: 48% |
| Garancini et al[3], 2014 | 1988-2009 | 32:42:551 | 5-year OS of minor/major resection: 28.1/27.1 | 5-year OS: 41.1 |
| Wang et al[42], 2021 | 2004-2015 | 122:38/65:26 (AJCC stage I + II) | Median OS: 13 months/31 months (AJCC stage I+II) | Median OS: 19 months/57 months (AJCC stage I + II) |
| Chen et al[7], 2021 | 2004-2018 | 32:32 (after PSM) | 1-year CSD: 12.8%; 3-year CSD: 52.7%; 5-year CSD: 60.9% | 1-year CSD: 10.2%; 3-year CSD: 21.1%; 5-year CSD: 35.4% |
| Chen et al[55], 2022 | 2004-2015 | 37:37 (after PSM) | 5-year OS: 38.6%; 5-year CSS: 45.2% | 5-year OS: 62.8%; 5-year CSS: 73.1% |
| Mi et al[56], 2023 | 2000-2018 | 74:49 | 1 year OS: 70.3%; 3-year OS: 44.4%; 5-year OS: 34.5% | 1 year OS: 86.2%; 3-year OS: 72.4%; 5-year OS: 60.3% |
| Peng et al[57], 2023 | 2002-2018 | 119:49 | 1-year CSS: 76.1%; 3-year CSS: 44.5%; 5-year CSS: 42.7% | 1-year CSS: 86.3%; 3-year CSS: 73%; 5-year CSS: 61.4% |
The ethical dilemma of organ allocation in patients with cHCC-CCA who are LT candidates remains particularly acute, given the persistent global donor liver shortage. Some studies have suggested that LT should be avoided in the treatment of cHCC-CCA to allocate organs to more appropriate diseases[58]. Current allocation systems prioritizing maximal survival benefit naturally favor patients with HCC, considering that patients with cHCC-CCA seem to have a poorer prognosis than those with HCC[54]. This prognostic discrepancy fuels ongoing debates about equitable organ distribution, particularly when considering that 35%-60% of patients with cHCC-CCA present with underlying cirrhosis, a critical determinant of therapeutic options[49,50].
In patients with cirrhosis, LR has dual limitations: (1) Technical constraints from portal hypertension; and (2) Failure to eliminate the pro-oncogenic cirrhotic microenvironment responsible for approximately 40% of tumor recurrence rates even after R0 resection[53,59]. These findings emphasize that hepatic background evaluation (cirrhosis severity and functional reserve) must inform risk-benefit analyses when comparing LR morbidity and mortality against potential LT outcomes[60]. Consequently, LT merits consideration for patients with cirrhotic cHCC-CCA where resection proves anatomically or functionally prohibitive[53,61,62].
As an intermediate form between HCC and iCCA, cHCC-CCA has not been listed as a standard indication for LT by the United Network for Organ Sharing. There are three possible reasons for this: (1) Historically inferior prognostic outcomes compared with pure HCC; (2) Persistent diagnostic challenges stemming from evolving histopathological criteria; and (3) Insufficient high-quality prospective data attributable to both diagnostic ambiguity and relative rarity of the tumor. Nevertheless, the evolving landscape of oncological transplantation and the success reported in other malignancies, such as iCCA and hilar cholangiocarcinoma, are typical contraindications for LT[63-65]. Recent ad
Evolving evidence challenges the traditional conclusion that cHCC-CCA is a contraindication for LT. Although historical contraindications stem from poorer survival outcomes compared to HCC and diagnostic uncertainties, contemporary large-scale analyses based on databases have demonstrated comparable survival benefits between LT and LR in selected cohorts. These results necessitate a paradigm re-evaluation, positioning LT as a viable therapeutic alternative rather than an absolute contraindication for carefully selected patients with cHCC-CCA meeting stringent LT criteria.
The establishment of evidence-based selection criteria for LT in patients with combined cHCC-CCA and CCA remains a critical yet unresolved challenge. Historically, LT has been contraindicated owing to poor outcomes; however, emerging evidence suggests that stringent patient selection could optimize post-transplant survival. Although iCCA has traditionally been considered an absolute contraindication for LT, recent studies have demonstrated that early-stage iCCA (e.g., solitary tumors ≤ 2 cm) may achieve post-LT survival comparable to HCC[66]. This paradigm shift underscores the fact that patients with cHCC-CCA, despite hybrid clinicopathological features, should not be categorically excluded from LT considerations when stringent criteria are applied.
Several LT criteria have been adopted for clinical management at different centers (Table 2). Current practice usually empirically adopts the Milan criteria, originally designed for HCC, owing to frequent preoperative misdiagnosis and diagnostic ambiguity. Retrospective multicenter analyses revealed that patients with cHCC-CCAs who received LT based on the Milan criteria achieved 5-year OS rates comparable to patients with HCC (70.1% vs 73.4%, P = 0.806), albeit with significantly higher 5-year recurrence rates (23.1% vs 11.5%, P < 0.001)[52]. Similarly, a large-scale comparative study confirmed that LT outcomes surpass those of hepatectomy for tumors based on the Milan criteria, whereas no survival advantage existed for tumors that exceeded these limits[7].
| Criteria | Tumor size | Nodules | Vascular invasion | Risk stratification |
| Milan | ≤ 5 cm | ≤ 3 | Excluded | None |
| UCSF | ≤ 6.5 cm | ≤ 3 (total tumor burden ≤ 8 cm) | Excluded | None |
| SEER-derived RSM[55] | > 2 cm (+ 1 point) | ≥ 3 (+ 2 points) | + 1 point | Low-risk: ≤ 2 points; high-risk: > 2 points or extrahepatic metastasis |
| “Very early” stage cHCC-CCA[38] | ≤ 2 cm | 1-2 | Excluded | None |
Growing evidence supports the implementation of refined LT criteria for cHCC-CCA, with multiple studies proposing risk stratification tools or novel thresholds to optimize outcomes. Notably, a SEER-derived risk-scoring model stratified patients with HCC into low-risk (≤ 2 points) and high-risk (> 2 points or extrametastasis) cohorts[55]. LT was de
The pragmatic utility of the Milan criteria for cHCC-CCAs should be emphasized, particularly given the persistent challenges of preoperative diagnosis and organ scarcity. Although post-LT recurrence rates remain higher than those of HCC, adherence to the Milan criteria minimizes organ misallocation risks while offering curative potential for selected patients.
Optimal strategies for addressing LN involvement in cHCC-CCA present complex clinical dilemmas, balancing diagnostic uncertainty, therapeutic risks, and prognostic implications. LN involvement occurs in 20%-40% of patients with cHCC-CCA, positioning its incidence between that of HCC (< 10%) and iCCA (approximately 50%)[20,21,69,70]. Several studies demonstrated that LN metastasis is a strong predictor of poor outcomes[47,71].
The hepatoduodenal ligament is the most common site of LN metastasis, necessitating regional lymphadenectomy encompassing the portocaval, pancreaticoduodenal, and common hepatic artery nodes up to the celiac axis[5,72]. Anatomical resection with lymphadenectomy is recommended to address the dual metastatic pathways of cHCC-CCA because HCC tends to invade via the portal veins, whereas iCCA primarily spreads through the lymphatics[19]. However, whether lymphadenectomy improves prognosis has not been demonstrated[73,74]. Extensive dissection (e.g., para-aortic nodes) is discouraged owing to unsubstantiated survival benefits and increased morbidity risks, particularly in patients with cirrhosis prone to postoperative ascites.
The precise role of lymphadenectomy in LT for cHCC-CCA remains unclear, necessitating prospective studies to define standardized protocols. Considering the poor prognosis of patients with cHCC-CCA and LN metastasis, rigorous preoperative LN evaluation is imperative. We recommend intraoperative frozen-section analysis of suspicious LNs during diagnostic laparoscopy to guide real-time transplant eligibility decisions.
Vascular invasion is a pivotal prognostic determinant in cHCC-CCA, occurring in 9%-85% of cases[75-78]. Its presence correlates with dismal outcomes, reducing 3-year OS from 56.8% to 36.8% and independently increasing mortality risk by 1.6-fold to 5.2-fold[75,79,80]. The iCCA component of cHCC-CCA predisposes patients to vascular infiltration, including hepatic vein invasion and portal vein involvement. Gross invasion of the inferior vena cava (IVC) or hepatic veins is frequent in cHCC-CCA, necessitating complex surgical strategies to achieve R0 margins[5].
Vascular resection may offer curative potential for locally advanced cHCC-CCAs with vascular involvement. Retrospective data suggest that hepatic vein resection achieves a 3-year survival rate of 50% for HCC, whereas IVC resection yields a 5-year survival rate of 33% in carefully selected patients with iCCA[81]. However, specific data on cHCC-CCA is scarce. Major vascular resections (e.g., IVC reconstruction) carry elevated morbidity and mortality, mandating referral to high-volume centers[82].
Historically, vascular invasion has been an absolute contraindication for LT due to the high risk of recurrence. However, Chen et al[55] reported that 16.7% of LT recipients with cHCC-CCA and vascular invasion achieved 5-year OS and cancer-specific survival rates of 70% and 80%, respectively, likely reflecting favorable tumor biology (e.g., small tumor burden, HCC-dominant subtype). This finding underscores the limitations of the conventional criteria (Milan/University of California, San Francisco) in stratifying patients with cHCC-CCA and vascular invasion.
Transarterial chemoembolization (TACE) is the most common locoregional therapy for unresectable or recurrent cHCC-CCA. Na et al[83] explored the efficacy of TACE and found that treatment response, and consequently prognosis, is closely related to tumor vascularity. In a Korean cohort of 50 patients with unresectable cHCC-CCA, TACE induced partial response or stable disease in 70% of cases, with a median OS of 12.3 months[84]. Poor outcomes were associated with tumor vascularity, Child-Pugh B/C cirrhosis, and portal vein invasion. Thus, conventional TACE did not de
Transarterial radioembolization (TARE) using yttrium-90 microspheres has emerged as a promising alternative therapy for unresectable cHCC-CCA. Studies have reported partial response rates ranging from 55% to 60% and disease control rates between 60% and 65%, with a median OS exceeding 9 months after the first TARE treatment[87,88]. TARE has shown potential to downstage 22% of initially unresectable iCCA for surgery, a finding that may extend to cHCC-CCA with iCCA-dominant features[89]. A retrospective analysis of 18 patients with inoperable cHCC-CCA (from a cohort of 79) undergoing transarterial therapies, including TACE, hepatic arterial infusion chemotherapy, and TARE, de
Radiofrequency ablation (RFA) has been explored as a locoregional treatment option for small cHCC-CCA tumors, although the evidence remains limited compared with that for HCC. A SEER database analysis found no significant survival difference among RFA, LR, and LT for cHCC-CCA of 3.0 cm or less without distant metastases, though the sample size was small[57]. However, cHCC-CCA frequently exhibits occult microvascular invasion and intrahepatic micrometastases that are not radiologically detectable, and RFA cannot address because of its localized ablation zone[78,91].
Locoregional therapies play a critical role in bridging therapy for patients undergoing LT. TACE and TARE have been used preoperatively to stabilize the disease, reduce tumor burden, and improve transplant candidacy. De Martin et al[25] suggested that the survival was comparable between LT and LR cohorts with iCCA and/or cHCC-CCA, although nearly half of the LT recipients received neoadjuvant TACE. Panayotova et al[92] highlighted that post-bridging disease stability was correlated with improved post-LT survival, suggesting prognostic utility. Nevertheless, current guidelines lack a consensus on optimal bridging strategies, with selection often dictated by tumor vascularity, hepatic reserve, and institutional expertise.
The absence of standardized regimens for cHCC-CCA has led clinicians to extrapolate their approaches for HCC and iCCA. Platinum-based combinations, particularly gemcitabine/cisplatin, have demonstrated superior efficacy over sorafenib monotherapy, with a retrospective study reporting a median OS of 15.5 months vs 5.3 months[93]. Furthermore, a multicenter analysis of platinum-containing regimens demonstrated partial response rates of 29% and median OS of up to 16.2 months[94]. However, heterogeneity in chemotherapy protocols (platinum-based vs fluoropyrimidine-based) and selection bias in retrospective data limit definitive conclusions[95].
Tyrosine kinase inhibitors showed limited activity against cHCC-CCA. In several retrospective studies, sorafenib failed to demonstrate superior outcomes compared with cytotoxic chemotherapy regimens[93,95]. The lack of robust clinical validation restricts the use of targeted agents. Early evidence suggests immune checkpoint inhibitors (ICIs) may benefit patients[96]. Programmed cell death protein 1 and its ligand inhibitors achieved a 20% overall response rate with an 11.6-month response duration in a small cohort[97]. A 72-year-old male patient achieved stable disease for 18 months with tumor regression (54% reduction) and normalized liver volume[98]. Several case reports have demonstrated the potential of pembrolizumab in treating refractory cHCC-CCA. A 53-year-old female patient showed rapid AFP decline (1790 to 52 μg/L) and pulmonary metastasis resolution within 6 months, though treatment was halted due to immune-mediated hepatitis requiring immunosuppressants[99]. Both patients, who were refractory to multiagent chemotherapy, achieved durable responses exceeding 6 months with ICIs, highlighting the role of programmed cell death protein 1 inhibitors in salvage therapy despite the risk of hepatotoxicity.
Preclinical models demonstrated that FGFR2-altered tumors may respond to FGFR inhibitors (e.g., pemigatinib)[100]. Early phase trials such as FIGHT-302 evaluated pemigatinib vs gemcitabine/cisplatin in FGFR2-rearranged biliary tumors, with implications for iCCA-dominant cHCC-CCA. Intriguingly, TP53 mutations, present in 49% of cHCC-CCAs, may synergize with ICIs in sarcomatoid variants exhibiting programmed cell death protein 1 and its ligand expression[101,102]. Neoadjuvant and bridging therapies may be utilized pre-LT to enhance OS outcomes[6]. However, the absence of standardized bridging protocols necessitates individualized selections based on the tumor biology and patient comorbidities.
cHCC-CCA is a diagnostically and therapeutically complex malignancy characterized by a hybrid histopathology and aggressive clinical behavior. Evolving definitions from classical subtypes to the emphasis by the WHO on stem cell features reflect persistent diagnostic challenges[9]. LR remains the cornerstone of curative therapy. In this context, LT has emerged as a contentious but potentially transformative option. Although historically deemed contraindicated due to poor prognosis, contemporary analyses of large databases challenge this dogma, demonstrating that LT using the Milan criteria achieves comparable outcomes to LR.
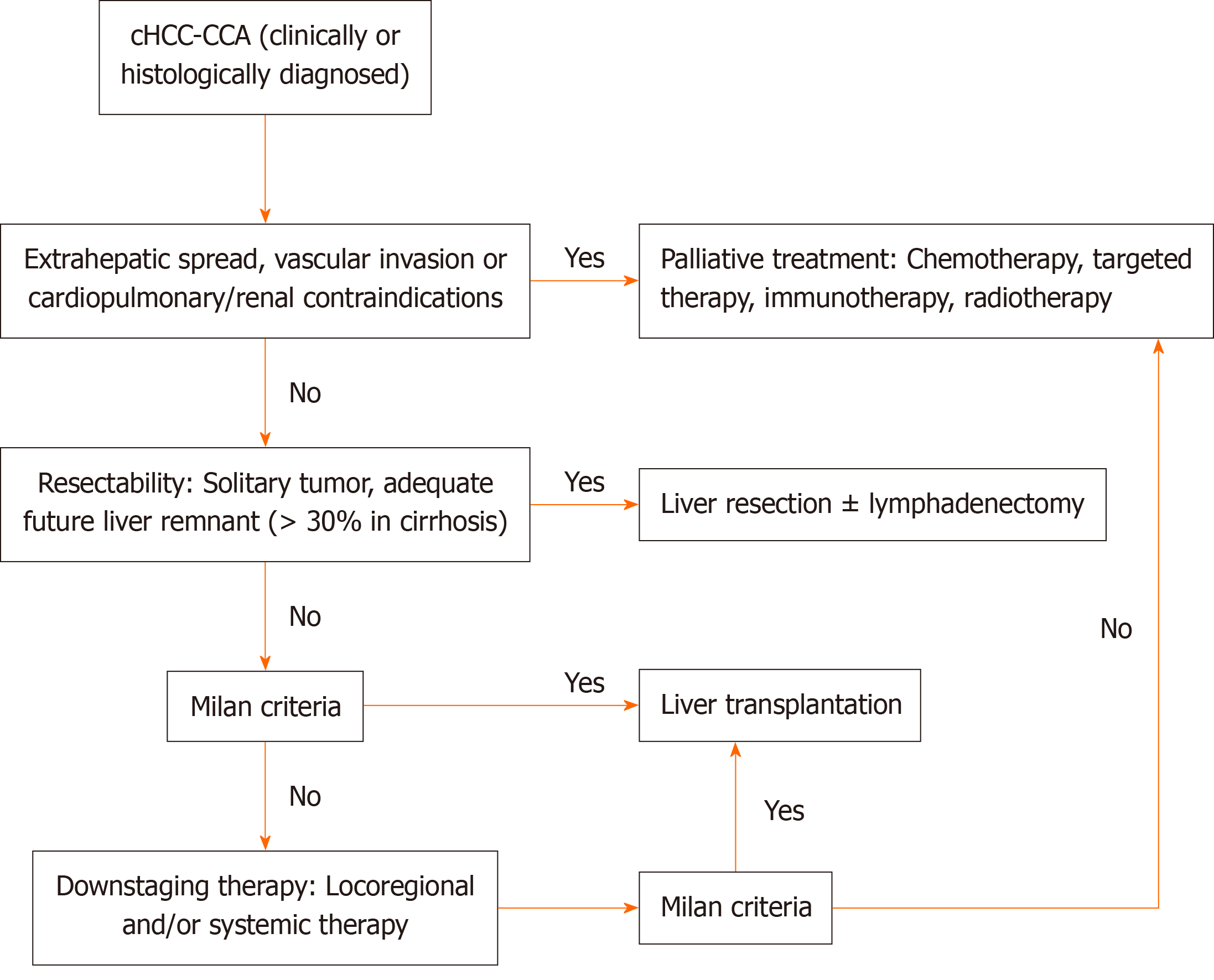
Stringent patient selection remains paramount for LT candidates, with the Milan criteria serving as the current pragmatic benchmark in clinical practice. Emerging selection frameworks incorporating small tumor size, absence of vascular invasion, and multifocality-biomarker-integrated risk stratification models hold promise for optimizing prognostic outcomes. For patients with cirrhosis and unresectable early-stage tumors, LT achieves two therapeutic objectives: Complete oncological eradication and elimination of the carcinogenic hepatic microenvironment, thereby attenuating the inherent recurrence risks associated with LR. Nevertheless, persistent ethical challenges in organ allocation stem from the significantly inferior survival of patients with cHCC-CCA compared with that of patients with HCC. Neoadjuvant locoregional therapies and systemic regimens, although primarily palliative for advanced diseases, play critical adjunctive roles as bridging or downstaging modalities for potential curative interventions. We summarized a treatment protocol based on the suggestions of the literature and clinical experience of surgeons worldwide (Figure 1).
This review had some inherent limitations that warrant further investigation. First, the predominance of retrospective studies and heterogeneous patient cohorts introduced an unavoidable selection bias and confounders, particularly given the historical evolution of the diagnostic criteria for cHCC-CCA. Many earlier studies misclassified tumors owing to outdated histological definitions, complicating cross-study comparisons. Second, the lack of prospective trials specifically addressing cHCC-CCA limits evidence-based recommendations for systemic therapies, with current data extrapolated from HCC and iCCA studies. Finally, geographic disparities in risk factors (e.g., HBV prevalence in Asia vs alcohol and hepatitis C virus in Western cohorts) and diagnostic resource availability may have biased the outcomes, underscoring the need for globally harmonized protocols. Future studies integrating multiomics profiling and prospective trial designs are essential to refine therapeutic algorithms and validate risk-stratified LT criteria.
| 1. | Wege H, Campani C, de Kleine R, Meyer T, Nault JC, Pawlik TM, Reig M, Ricke J, Sempoux C, Torzilli G, Zucman-Rossi J. Rare primary liver cancers: An EASL position paper. J Hepatol. 2024;S0168-8278(24)02305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2428] [Article Influence: 485.6] [Reference Citation Analysis (3)] |
| 3. | Garancini M, Goffredo P, Pagni F, Romano F, Roman S, Sosa JA, Giardini V. Combined hepatocellular-cholangiocarcinoma: a population-level analysis of an uncommon primary liver tumor. Liver Transpl. 2014;20:952-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 4. | Chu KJ, Kawaguchi Y, Wang H, Jiang XQ, Hasegawa K. Update on the Diagnosis and Treatment of Combined Hepatocellular Cholangiocarcinoma. J Clin Transl Hepatol. 2024;12:210-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 5. | Bahra M, Yahyazadeh A. Surgical Strategies for Combined Hepatocellular-Cholangiocarcinoma (cHCC-CC). Cancers (Basel). 2023;15:774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | Goodwin B, Lou J, Butchy M, Wilson T, Atabek U, Spitz F, Hong Y. Hepatocellular-Cholangiocarcinoma Collision Tumors: An Update of Current Management Practices. Am Surg. 2023;89:2685-2692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Chen X, Lu Y, Shi X, Chen X, Rong D, Han G, Zhang L, Ni C, Zhao J, Gao Y, Wang X. Morbidity, Prognostic Factors, and Competing Risk Nomogram for Combined Hepatocellular-Cholangiocarcinoma. J Oncol. 2021;2021:3002480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Sciarra A, Park YN, Sempoux C. Updates in the diagnosis of combined hepatocellular-cholangiocarcinoma. Hum Pathol. 2020;96:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Allen RA, Lisa JR. Combined liver cell and bile duct carcinoma. Am J Pathol. 1949;25:647-655. [PubMed] |
| 10. | Goodman ZD, Ishak KG, Langloss JM, Sesterhenn IA, Rabin L. Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer. 1985;55:124-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 11. | Falkowski O, An HJ, Ianus IA, Chiriboga L, Yee H, West AB, Theise ND. Regeneration of hepatocyte 'buds' in cirrhosis from intrabiliary stem cells. J Hepatol. 2003;39:357-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Theise ND, Yao JL, Harada K, Hytiroglou P, Portmann B, Thung SN, Tsui W, Ohta H, Nakanuma Y. Hepatic 'stem cell' malignancies in adults: four cases. Histopathology. 2003;43:263-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 154] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Kim H, Park C, Han KH, Choi J, Kim YB, Kim JK, Park YN. Primary liver carcinoma of intermediate (hepatocyte-cholangiocyte) phenotype. J Hepatol. 2004;40:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Akiba J, Nakashima O, Hattori S, Tanikawa K, Takenaka M, Nakayama M, Kondo R, Nomura Y, Koura K, Ueda K, Sanada S, Naito Y, Yamaguchi R, Yano H. Clinicopathologic analysis of combined hepatocellular-cholangiocarcinoma according to the latest WHO classification. Am J Surg Pathol. 2013;37:496-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Sasaki M, Sato H, Kakuda Y, Sato Y, Choi JH, Nakanuma Y. Clinicopathological significance of 'subtypes with stem-cell feature' in combined hepatocellular-cholangiocarcinoma. Liver Int. 2015;35:1024-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Brunt EM, Paradis V, Sempoux C, Theise ND. Biphenotypic (hepatobiliary) primary liver carcinomas: the work in progress. Hepat Oncol. 2015;2:255-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Brunt E, Aishima S, Clavien PA, Fowler K, Goodman Z, Gores G, Gouw A, Kagen A, Klimstra D, Komuta M, Kondo F, Miksad R, Nakano M, Nakanuma Y, Ng I, Paradis V, Nyun Park Y, Quaglia A, Roncalli M, Roskams T, Sakamoto M, Saxena R, Sempoux C, Sirlin C, Stueck A, Thung S, Tsui WMS, Wang XW, Wee A, Yano H, Yeh M, Zen Y, Zucman-Rossi J, Theise N. cHCC-CCA: Consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology. 2018;68:113-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 260] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 18. | Wang J, Li E, Yang H, Wu J, Lu HC, Yi C, Lei J, Liao W, Wu L. Combined hepatocellular-cholangiocarcinoma: a population level analysis of incidence and mortality trends. World J Surg Oncol. 2019;17:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Ramai D, Ofosu A, Lai JK, Reddy M, Adler DG. Combined Hepatocellular Cholangiocarcinoma: A Population-Based Retrospective Study. Am J Gastroenterol. 2019;114:1496-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Teufel A, Rodriguez I, Winzler C, Kokh D, Ebert MP, Surovtsova I, Morakis P. Clinical Characterization of HCC/CCA Mixed Cancers in a Population-based Cohort. J Gastrointestin Liver Dis. 2023;32:190-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Gentile D, Donadon M, Lleo A, Aghemo A, Roncalli M, di Tommaso L, Torzilli G. Surgical Treatment of Hepatocholangiocarcinoma: A Systematic Review. Liver Cancer. 2020;9:15-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 22. | Zhou YM, Sui CJ, Zhang XF, Li B, Yang JM. Influence of cirrhosis on long-term prognosis after surgery in patients with combined hepatocellular-cholangiocarcinoma. BMC Gastroenterol. 2017;17:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Gigante E, Paradis V, Ronot M, Cauchy F, Soubrane O, Ganne-Carrié N, Nault JC. New insights into the pathophysiology and clinical care of rare primary liver cancers. JHEP Rep. 2021;3:100174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Nguyen CT, Caruso S, Maille P, Beaufrère A, Augustin J, Favre L, Pujals A, Boulagnon-Rombi C, Rhaiem R, Amaddeo G, di Tommaso L, Luciani A, Regnault H, Brustia R, Scatton O, Charlotte F, Brochériou I, Sommacale D, Soussan P, Leroy V, Laurent A, Le VK, Ta VT, Trinh HS, Tran TL, Gentien D, Rapinat A, Nault JC, Allaire M, Mulé S, Zucman-Rossi J, Pawlotsky JM, Tournigand C, Lafdil F, Paradis V, Calderaro J. Immune Profiling of Combined Hepatocellular- Cholangiocarcinoma Reveals Distinct Subtypes and Activation of Gene Signatures Predictive of Response to Immunotherapy. Clin Cancer Res. 2022;28:540-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | De Martin E, Rayar M, Golse N, Dupeux M, Gelli M, Gnemmi V, Allard MA, Cherqui D, Sa Cunha A, Adam R, Coilly A, Antonini TM, Guettier C, Samuel D, Boudjema K, Boleslawski E, Vibert E. Analysis of Liver Resection Versus Liver Transplantation on Outcome of Small Intrahepatic Cholangiocarcinoma and Combined Hepatocellular-Cholangiocarcinoma in the Setting of Cirrhosis. Liver Transpl. 2020;26:785-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 26. | Li R, Yang D, Tang CL, Cai P, Ma KS, Ding SY, Zhang XH, Guo DY, Yan XC. Combined hepatocellular carcinoma and cholangiocarcinoma (biphenotypic) tumors: clinical characteristics, imaging features of contrast-enhanced ultrasound and computed tomography. BMC Cancer. 2016;16:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 27. | Maximin S, Ganeshan DM, Shanbhogue AK, Dighe MK, Yeh MM, Kolokythas O, Bhargava P, Lalwani N. Current update on combined hepatocellular-cholangiocarcinoma. Eur J Radiol Open. 2014;1:40-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Park SH, Lee SS, Yu E, Kang HJ, Park Y, Kim SY, Lee SJ, Shin YM, Lee MG. Combined hepatocellular-cholangiocarcinoma: Gadoxetic acid-enhanced MRI findings correlated with pathologic features and prognosis. J Magn Reson Imaging. 2017;46:267-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Mao Y, Xu S, Hu W, Huang J, Wang J, Zhang R, Li S. Imaging features predict prognosis of patients with combined hepatocellular-cholangiocarcinoma. Clin Radiol. 2017;72:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Gigante E, Ronot M, Bertin C, Ciolina M, Bouattour M, Dondero F, Cauchy F, Soubrane O, Vilgrain V, Paradis V. Combining imaging and tumour biopsy improves the diagnosis of combined hepatocellular-cholangiocarcinoma. Liver Int. 2019;39:2386-2396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 31. | Potretzke TA, Tan BR, Doyle MB, Brunt EM, Heiken JP, Fowler KJ. Imaging Features of Biphenotypic Primary Liver Carcinoma (Hepatocholangiocarcinoma) and the Potential to Mimic Hepatocellular Carcinoma: LI-RADS Analysis of CT and MRI Features in 61 Cases. AJR Am J Roentgenol. 2016;207:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 32. | Granata V, Fusco R, Venanzio Setola S, Sandomenico F, Luisa Barretta M, Belli A, Palaia R, Tatangelo F, Grassi R, Izzo F, Petrillo A. Major and ancillary features according to LI-RADS in the assessment of combined hepatocellular-cholangiocarcinoma. Radiol Oncol. 2020;54:149-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Bao J, Nie Z, Wang Q, Chen Y, Wang K, Liu X. Evaluation of combined hepatocellular-cholangiocarcinoma using CEUS LI-RADS: correlation with pathological characteristics. Abdom Radiol (NY). 2025;50:646-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 34. | Jeon SK, Joo I, Lee DH, Lee SM, Kang HJ, Lee KB, Lee JM. Combined hepatocellular cholangiocarcinoma: LI-RADS v2017 categorisation for differential diagnosis and prognostication on gadoxetic acid-enhanced MR imaging. Eur Radiol. 2019;29:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 35. | Wen R, Lin P, Wu Y, Yin H, Huang W, Guo D, Peng Y, Liu D, He Y, Yang H. Diagnostic value of CEUS LI-RADS and serum tumor markers for combined hepatocellular-cholangiocarcinoma. Eur J Radiol. 2022;154:110415. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 36. | Zhou Y, Yin S, Zhao L, Zhang X, Li M, Ding J, Yan K, Jing X. CEUS and CT/MRI LI-RADS in Association With Serum Biomarkers for Differentiation of Combined Hepatocellular-Cholangiocarcinoma From Hepatocellular Carcinoma. Front Oncol. 2022;12:897090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Beaufrère A, Calderaro J, Paradis V. Combined hepatocellular-cholangiocarcinoma: An update. J Hepatol. 2021;74:1212-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 130] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 38. | Jung DH, Hwang S, Song GW, Ahn CS, Moon DB, Kim KH, Ha TY, Park GC, Hong SM, Kim WJ, Kang WH, Kim SH, Yu ES, Lee SG. Longterm prognosis of combined hepatocellular carcinoma-cholangiocarcinoma following liver transplantation and resection. Liver Transpl. 2017;23:330-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 39. | Calderaro J, Ghaffari Laleh N, Zeng Q, Maille P, Favre L, Pujals A, Klein C, Bazille C, Heij LR, Uguen A, Luedde T, Di Tommaso L, Beaufrère A, Chatain A, Gastineau D, Nguyen CT, Nguyen-Canh H, Thi KN, Gnemmi V, Graham RP, Charlotte F, Wendum D, Vij M, Allende DS, Aucejo F, Diaz A, Rivière B, Herrero A, Evert K, Calvisi DF, Augustin J, Leow WQ, Leung HHW, Boleslawski E, Rela M, François A, Cha AW, Forner A, Reig M, Allaire M, Scatton O, Chatelain D, Boulagnon-Rombi C, Sturm N, Menahem B, Frouin E, Tougeron D, Tournigand C, Kempf E, Kim H, Ningarhari M, Michalak-Provost S, Gopal P, Brustia R, Vibert E, Schulze K, Rüther DF, Weidemann SA, Rhaiem R, Pawlotsky JM, Zhang X, Luciani A, Mulé S, Laurent A, Amaddeo G, Regnault H, De Martin E, Sempoux C, Navale P, Westerhoff M, Lo RC, Bednarsch J, Gouw A, Guettier C, Lequoy M, Harada K, Sripongpun P, Wetwittayaklang P, Loménie N, Tantipisit J, Kaewdech A, Shen J, Paradis V, Caruso S, Kather JN. Deep learning-based phenotyping reclassifies combined hepatocellular-cholangiocarcinoma. Nat Commun. 2023;14:8290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 40. | Gurzu S, Szodorai R, Jung I, Banias L. Combined hepatocellular-cholangiocarcinoma: from genesis to molecular pathways and therapeutic strategies. J Cancer Res Clin Oncol. 2024;150:270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 41. | Sasaki M, Sato Y, Nakanuma Y. Expression of fibroblast growth factor receptor 2 (FGFR2) in combined hepatocellular-cholangiocarcinoma and intrahepatic cholangiocarcinoma: clinicopathological study. Virchows Arch. 2024;484:915-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 42. | Wang J, Li Z, Liao Y, Li J, Dong H, Peng H, Xu W, Fan Z, Gao F, Liu C, Liu D, Zhang Y. Prediction of Survival and Analysis of Prognostic Factors for Patients With Combined Hepatocellular Carcinoma and Cholangiocarcinoma: A Population-Based Study. Front Oncol. 2021;11:686972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Ma KW, Chok KSH. Importance of surgical margin in the outcomes of hepatocholangiocarcinoma. World J Hepatol. 2017;9:635-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Tang Y, Wang L, Teng F, Zhang T, Zhao Y, Chen Z. The clinical characteristics and prognostic factors of combined Hepatocellular Carcinoma and Cholangiocarcinoma, Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma after Surgical Resection: A propensity score matching analysis. Int J Med Sci. 2021;18:187-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 45. | Lv TR, Hu HJ, Ma WJ, Liu F, Jin YW, Li FY. Meta-analysis of prognostic factors for overall survival and disease-free survival among resected patients with combined hepatocellular carcinoma and cholangiocarcinoma. Eur J Surg Oncol. 2024;50:107279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 46. | Sempokuya T, Wien EA, Pattison RJ, Ma J, Wong LL. Factors associated with 5-year survival of combined hepatocellular and cholangiocarcinoma. World J Hepatol. 2020;12:1020-1030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Tian MX, Luo LP, Liu WR, Deng W, Yin JC, Jin L, Jiang XF, Zhou YF, Qu WF, Tang Z, Wang H, Tao CY, Fang Y, Qiu SJ, Zhou J, Liu JF, Fan J, Shi YH. Development and validation of a prognostic score predicting recurrence in resected combined hepatocellular cholangiocarcinoma. Cancer Manag Res. 2019;11:5187-5195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Chu KJ, Lu CD, Dong H, Fu XH, Zhang HW, Yao XP. Hepatitis B virus-related combined hepatocellular-cholangiocarcinoma: clinicopathological and prognostic analysis of 390 cases. Eur J Gastroenterol Hepatol. 2014;26:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 49. | Sapisochin G, Fidelman N, Roberts JP, Yao FY. Mixed hepatocellular cholangiocarcinoma and intrahepatic cholangiocarcinoma in patients undergoing transplantation for hepatocellular carcinoma. Liver Transpl. 2011;17:934-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 50. | Vilchez V, Shah MB, Daily MF, Pena L, Tzeng CW, Davenport D, Hosein PJ, Gedaly R, Maynard E. Long-term outcome of patients undergoing liver transplantation for mixed hepatocellular carcinoma and cholangiocarcinoma: an analysis of the UNOS database. HPB (Oxford). 2016;18:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 51. | Park YH, Hwang S, Ahn CS, Kim KH, Moon DB, Ha TY, Song GW, Jung DH, Park GC, Namgoong JM, Park CS, Park HW, Kang SH, Jung BH, Lee SG. Long-term outcome of liver transplantation for combined hepatocellular carcinoma and cholangiocarcinoma. Transplant Proc. 2013;45:3038-3040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 52. | Dageforde LA, Vachharajani N, Tabrizian P, Agopian V, Halazun K, Maynard E, Croome K, Nagorney D, Hong JC, Lee D, Ferrone C, Baker E, Jarnagin W, Hemming A, Schnickel G, Kimura S, Busuttil R, Lindemann J, Florman S, Holzner ML, Srouji R, Najjar M, Yohanathan L, Cheng J, Amin H, Rickert CA, Yang JD, Kim J, Pasko J, Chapman WC, Majella Doyle MB. Multi-Center Analysis of Liver Transplantation for Combined Hepatocellular Carcinoma-Cholangiocarcinoma Liver Tumors. J Am Coll Surg. 2021;232:361-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 53. | Jaradat D, Bagias G, Lorf T, Tokat Y, Obed A, Oezcelik A. Liver transplantation for combined hepatocellular-cholangiocarcinoma: Outcomes and prognostic factors for mortality. A multicenter analysis. Clin Transplant. 2021;35:e14094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Groeschl RT, Turaga KK, Gamblin TC. Transplantation versus resection for patients with combined hepatocellular carcinoma-cholangiocarcinoma. J Surg Oncol. 2013;107:608-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 55. | Chen X, Sun S, Lu Y, Shi X, Wang Z, Chen X, Han G, Zhao J, Gao Y, Wang X. Promising role of liver transplantation in patients with combined hepatocellular-cholangiocarcinoma: a propensity score matching analysis. Ann Transl Med. 2022;10:434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 56. | Mi S, Hou Z, Qiu G, Jin Z, Xie Q, Huang J. Liver transplantation versus resection for patients with combined hepatocellular cholangiocarcinoma: A retrospective cohort study. Heliyon. 2023;9:e20945. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 57. | Peng S, Dong SC, Bai DS, Zhang C, Jin SJ, Jiang GQ. Radiofrequency ablation versus liver resection and liver transplantation for small combined hepatocellular-cholangiocarcinoma stratified by tumor size. Langenbecks Arch Surg. 2023;408:119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 58. | Magistri P, Tarantino G, Serra V, Guidetti C, Ballarin R, Di Benedetto F. Liver transplantation and combined hepatocellular-cholangiocarcinoma: Feasibility and outcomes. Dig Liver Dis. 2017;49:467-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 59. | Sapisochin G, Rodríguez de Lope C, Gastaca M, Ortiz de Urbina J, Suarez MA, Santoyo J, Castroagudín JF, Varo E, López-Andujar R, Palacios F, Sanchez Antolín G, Perez B, Guiberteau A, Blanco G, González-Diéguez ML, Rodriguez M, Varona MA, Barrera MA, Fundora Y, Ferron JA, Ramos E, Fabregat J, Ciria R, Rufian S, Otero A, Vazquez MA, Pons JA, Parrilla P, Zozaya G, Herrero JI, Charco R, Bruix J. "Very early" intrahepatic cholangiocarcinoma in cirrhotic patients: should liver transplantation be reconsidered in these patients? Am J Transplant. 2014;14:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 60. | Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Extended hepatic resection for hepatocellular carcinoma in patients with cirrhosis: is it justified? Ann Surg. 2002;236:602-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 139] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 61. | Kassahun WT, Hauss J. Management of combined hepatocellular and cholangiocarcinoma. Int J Clin Pract. 2008;62:1271-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 62. | Sapisochin G, de Lope CR, Gastaca M, de Urbina JO, López-Andujar R, Palacios F, Ramos E, Fabregat J, Castroagudín JF, Varo E, Pons JA, Parrilla P, González-Diéguez ML, Rodriguez M, Otero A, Vazquez MA, Zozaya G, Herrero JI, Antolin GS, Perez B, Ciria R, Rufian S, Fundora Y, Ferron JA, Guiberteau A, Blanco G, Varona MA, Barrera MA, Suarez MA, Santoyo J, Bruix J, Charco R. Intrahepatic cholangiocarcinoma or mixed hepatocellular-cholangiocarcinoma in patients undergoing liver transplantation: a Spanish matched cohort multicenter study. Ann Surg. 2014;259:944-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 63. | Machairas N, Kostakis ID, Tsilimigras DI, Prodromidou A, Moris D. Liver transplantation for hilar cholangiocarcinoma: A systematic review. Transplant Rev (Orlando). 2020;34:100516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 64. | Huang G, Song W, Zhang Y, Yu J, Lv Y, Liu K. Liver transplantation for intrahepatic cholangiocarcinoma: a propensity score-matched analysis. Sci Rep. 2023;13:10630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 65. | Andraus W, Ochoa G, de Martino RB, Pinheiro RSN, Santos VR, Lopes LD, Arantes Júnior RM, Waisberg DR, Santana AC, Tustumi F, D'Albuquerque LAC. The role of living donor liver transplantation in treating intrahepatic cholangiocarcinoma. Front Oncol. 2024;14:1404683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 66. | Sapisochin G, Ivanics T, Heimbach J. Liver Transplantation for Intrahepatic Cholangiocarcinoma: Ready for Prime Time? Hepatology. 2022;75:455-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 67. | Lunsford KE, Court C, Seok Lee Y, Lu DS, Naini BV, Harlander-Locke MP, Busuttil RW, Agopian VG. Propensity-Matched Analysis of Patients with Mixed Hepatocellular-Cholangiocarcinoma and Hepatocellular Carcinoma Undergoing Liver Transplantation. Liver Transpl. 2018;24:1384-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 68. | Gupta R, Togashi J, Akamatsu N, Sakamoto Y, Kokudo N. Impact of incidental/misdiagnosed intrahepatic cholangiocarcinoma and combined hepatocellular cholangiocarcinoma on the outcomes of liver transplantation: an institutional case series and literature review. Surg Today. 2017;47:908-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 69. | de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, Pulitano C, Barroso E, Clary BM, Aldrighetti L, Ferrone CR, Zhu AX, Bauer TW, Walters DM, Gamblin TC, Nguyen KT, Turley R, Popescu I, Hubert C, Meyer S, Schulick RD, Choti MA, Gigot JF, Mentha G, Pawlik TM. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140-3145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 559] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 70. | Amory B, Goumard C, Laurent A, Langella S, Cherqui D, Salame E, Barbier L, Soubrane O, Farges O, Hobeika C, Kawai T, Regimbeau JM, Faitot F, Pessaux P, Truant S, Boleslawski E, Herrero A, Mabrut JY, Chiche L, Di Martino M, Rhaiem R, Schwarz L, Resende V, Calderaro J, Augustin J, Caruso S, Sommacale D, Hofmeyr S, Ferrero A, Fuks D, Vibert E, Torzilli G, Scatton O, Brustia R; AFC-ICC-2009, AFC-LLR-2018, and PRS-2019 Study Group. Combined hepatocellular-cholangiocarcinoma compared to hepatocellular carcinoma and intrahepatic cholangiocarcinoma: Different survival, similar recurrence: Report of a large study on repurposed databases with propensity score matching. Surgery. 2024;175:413-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 71. | Wakizaka K, Yokoo H, Kamiyama T, Ohira M, Kato K, Fujii Y, Sugiyama K, Okada N, Ohata T, Nagatsu A, Shimada S, Orimo T, Kamachi H, Taketomi A. Clinical and pathological features of combined hepatocellular-cholangiocarcinoma compared with other liver cancers. J Gastroenterol Hepatol. 2019;34:1074-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 72. | Bagante F, Spolverato G, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Soubrane O, Martel G, Groot Koerkamp B, Guglielmi A, Itaru E, Ruzzenente A, Pawlik TM. Surgical Management of Intrahepatic Cholangiocarcinoma in Patients with Cirrhosis: Impact of Lymphadenectomy on Peri-Operative Outcomes. World J Surg. 2018;42:2551-2560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 73. | Sasaki A, Kawano K, Aramaki M, Ohno T, Tahara K, Takeuchi Y, Yoshida T, Kitano S. Clinicopathologic study of mixed hepatocellular and cholangiocellular carcinoma: modes of spreading and choice of surgical treatment by reference to macroscopic type. J Surg Oncol. 2001;76:37-46. [PubMed] [DOI] [Full Text] |
| 74. | Ercolani G, Grazi GL, Ravaioli M, Grigioni WF, Cescon M, Gardini A, Del Gaudio M, Cavallari A. The role of lymphadenectomy for liver tumors: further considerations on the appropriateness of treatment strategy. Ann Surg. 2004;239:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 75. | Tang YY, Zhao YN, Zhang T, Chen ZY, Ma XL. Comprehensive radiomics nomogram for predicting survival of patients with combined hepatocellular carcinoma and cholangiocarcinoma. World J Gastroenterol. 2021;27:7173-7189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 76. | Yin X, Zhang BH, Qiu SJ, Ren ZG, Zhou J, Chen XH, Zhou Y, Fan J. Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features, treatment modalities, and prognosis. Ann Surg Oncol. 2012;19:2869-2876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 77. | Zhan Q, Shen BY, Deng XX, Zhu ZC, Chen H, Peng CH, Li HW. Clinical and pathological analysis of 27 patients with combined hepatocellular-cholangiocarcinoma in an Asian center. J Hepatobiliary Pancreat Sci. 2012;19:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 78. | Shibahara J, Hayashi A, Misumi K, Sakamoto Y, Arita J, Hasegawa K, Kokudo N, Fukayama M. Clinicopathologic Characteristics of Hepatocellular Carcinoma With Reactive Ductule-like Components, a Subset of Liver Cancer Currently Classified as Combined Hepatocellular-Cholangiocarcinoma With Stem-Cell Features, Typical Subtype. Am J Surg Pathol. 2016;40:608-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 79. | Chi CT, Chau GY, Lee RC, Chen YY, Lei HJ, Hou MC, Chao Y, Huang YH. Radiological features and outcomes of combined hepatocellular-cholangiocarcinoma in patients undergoing surgical resection. J Formos Med Assoc. 2020;119:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 80. | Holzner ML, Tabrizian P, Parvin-Nejad FP, Fei K, Gunasekaran G, Rocha C, Facciuto ME, Florman S, Schwartz ME. Resection of Mixed Hepatocellular-Cholangiocarcinoma, Hepatocellular Carcinoma, and Intrahepatic Cholangiocarcinoma. Liver Transpl. 2020;26:888-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 81. | Hemming AW, Reed AI, Langham MR Jr, Fujita S, Howard RJ. Combined resection of the liver and inferior vena cava for hepatic malignancy. Ann Surg. 2004;239:712-9; discussion 719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 82. | Azoulay D, Andreani P, Maggi U, Salloum C, Perdigao F, Sebagh M, Lemoine A, Adam R, Castaing D. Combined liver resection and reconstruction of the supra-renal vena cava: the Paul Brousse experience. Ann Surg. 2006;244:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 83. | Na SK, Choi GH, Lee HC, Shin YM, An J, Lee D, Shim JH, Kim KM, Lim YS, Chung YH, Lee YS. The effectiveness of transarterial chemoembolization in recurrent hepatocellular-cholangiocarcinoma after resection. PLoS One. 2018;13:e0198138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 84. | Kim JH, Yoon HK, Ko GY, Gwon DI, Jang CS, Song HY, Shin JH, Sung KB. Nonresectable combined hepatocellular carcinoma and cholangiocarcinoma: analysis of the response and prognostic factors after transcatheter arterial chemoembolization. Radiology. 2010;255:270-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 85. | Liu WR, Tian MX, Tao CY, Tang Z, Zhou YF, Song SS, Jiang XF, Wang H, Zhou PY, Qu WF, Fang Y, Ding ZB, Zhou J, Fan J, Shi YH. Adjuvant Transarterial chemoembolization does not influence recurrence-free or overall survival in patients with combined hepatocellular carcinoma and Cholangiocarcinoma after curative resection: a propensity score matching analysis. BMC Cancer. 2020;20:642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 86. | Ayyub J, Dabhi KN, Gohil NV, Tanveer N, Hussein S, Pingili S, Makkena VK, Jaramillo AP, Awosusi BL, Nath TS. Evaluation of the Safety and Efficacy of Conventional Transarterial Chemoembolization (cTACE) and Drug-Eluting Bead (DEB)-TACE in the Management of Unresectable Hepatocellular Carcinoma: A Systematic Review. Cureus. 2023;15:e41943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 87. | Chan LS, Sze DY, Poultsides GA, Louie JD, Abdelrazek Mohammed MA, Wang DS. Yttrium-90 Radioembolization for Unresectable Combined Hepatocellular-Cholangiocarcinoma. Cardiovasc Intervent Radiol. 2017;40:1383-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 88. | Malone CD, Gibby W, Tsai R, Kim SK, Lancia S, Akinwande O, Ramaswamy RS. Outcomes of Yttrium-90 Radioembolization for Unresectable Combined Biphenotypic Hepatocellular-Cholangiocarcinoma. J Vasc Interv Radiol. 2020;31:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 89. | Edeline J, Touchefeu Y, Guiu B, Farge O, Tougeron D, Baumgaertner I, Ayav A, Campillo-Gimenez B, Beuzit L, Pracht M, Lièvre A, Le Sourd S, Boudjema K, Rolland Y, Boucher E, Garin E. Radioembolization Plus Chemotherapy for First-line Treatment of Locally Advanced Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2020;6:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 90. | Fowler K, Saad NE, Brunt E, Doyle MB, Amin M, Vachharajani N, Tan B, Chapman WC. Biphenotypic Primary Liver Carcinomas: Assessing Outcomes of Hepatic Directed Therapy. Ann Surg Oncol. 2015;22:4130-4137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 91. | Koh KC, Lee H, Choi MS, Lee JH, Paik SW, Yoo BC, Rhee JC, Cho JW, Park CK, Kim HJ. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg. 2005;189:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 92. | Panayotova G, Lunsford KE, Latt NL, Paterno F, Guarrera JV, Pyrsopoulos N. Expanding indications for liver transplantation in the era of liver transplant oncology. World J Gastrointest Surg. 2021;13:392-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 93. | Pomej K, Balcar L, Shmanko K, Welland S, Himmelsbach V, Scheiner B, Mahyera A, Mozayani B, Trauner M, Finkelmeier F, Weinmann A, Vogel A, Pinter M. Clinical characteristics and outcome of patients with combined hepatocellular-cholangiocarcinoma-a European multicenter cohort. ESMO Open. 2023;8:100783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 94. | Salimon M, Prieux-Klotz C, Tougeron D, Hautefeuille V, Caulet M, Gournay J, Matysiak-Budnik T, Bennouna J, Tiako Meyo M, Lecomte T, Zaanan A, Touchefeu Y. Gemcitabine plus platinum-based chemotherapy for first-line treatment of hepatocholangiocarcinoma: an AGEO French multicentre retrospective study. Br J Cancer. 2018;118:325-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 95. | Kim EJ, Yoo C, Kang HJ, Kim KP, Ryu MH, Park SR, Lee D, Choi J, Shim JH, Kim KM, Lim YS, Lee HC, Ryoo BY. Clinical outcomes of systemic therapy in patients with unresectable or metastatic combined hepatocellular-cholangiocarcinoma. Liver Int. 2021;41:1398-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 96. | Pinter M, Scheiner B, Pinato DJ. Immune checkpoint inhibitors in hepatocellular carcinoma: emerging challenges in clinical practice. Lancet Gastroenterol Hepatol. 2023;8:760-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 53] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 97. | Jang YJ, Kim EJ, Kim HD, Kim KP, Ryu MH, Park SR, Choi WM, Lee D, Choi J, Shim JH, Kim KM, Lim YS, Lee HC, Ryoo BY, Yoo C. Clinical outcomes of immune checkpoint inhibitors in unresectable or metastatic combined hepatocellular-cholangiocarcinoma. J Cancer Res Clin Oncol. 2023;149:7547-7555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 98. | Saint A, Benchetrit M, Novellas S, Ouzan D, Falk AT, Leysalle A, Barriere J. Prolonged efficacy of pembrolizumab in a patient presenting a multi-treated metastatic hepatocholangiocarcinoma. Therap Adv Gastroenterol. 2020;13:1756284820935189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 99. | Rizell M, Åberg F, Perman M, Ny L, Stén L, Hashimi F, Svanvik J, Lindnér P. Checkpoint Inhibition Causing Complete Remission of Metastatic Combined Hepatocellular-Cholangiocarcinoma after Hepatic Resection. Case Rep Oncol. 2020;13:478-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 100. | Frampton JE. Pemigatinib: A Review in Advanced Cholangiocarcinoma. Target Oncol. 2024;19:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 101. | Xue R, Chen L, Zhang C, Fujita M, Li R, Yan SM, Ong CK, Liao X, Gao Q, Sasagawa S, Li Y, Wang J, Guo H, Huang QT, Zhong Q, Tan J, Qi L, Gong W, Hong Z, Li M, Zhao J, Peng T, Lu Y, Lim KHT, Boot A, Ono A, Chayama K, Zhang Z, Rozen SG, Teh BT, Wang XW, Nakagawa H, Zeng MS, Bai F, Zhang N. Genomic and Transcriptomic Profiling of Combined Hepatocellular and Intrahepatic Cholangiocarcinoma Reveals Distinct Molecular Subtypes. Cancer Cell. 2019;35:932-947.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 209] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 102. | Yoshuantari N, Jeng YM, Liau JY, Lee CH, Tsai JH. Hepatic Sarcomatoid Carcinoma Is an Aggressive Hepatic Neoplasm Sharing Common Molecular Features With Its Conventional Carcinomatous Counterparts. Mod Pathol. 2023;36:100042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |