Published online Sep 27, 2021. doi: 10.4240/wjgs.v13.i9.1012
Peer-review started: February 24, 2021
First decision: May 13, 2021
Revised: June 3, 2021
Accepted: August 2, 2021
Article in press: August 2, 2021
Published online: September 27, 2021
Processing time: 206 Days and 0.2 Hours
Optimal surveillance strategies for stage III colorectal cancer (CRC) are lacking, and intensive surveillance has not conferred a significant survival benefit.
To examine the association between surveillance intensity and recurrence and survival rates in patients with stage III CRC.
Data from patients with pathologic stage III CRC who underwent radical surgery between January 2005 and December 2012 at Asan Medical Center, Seoul, Korea were retrospectively reviewed. Surveillance consisted of abdominopelvic computed tomography (CT) every 6 mo and chest CT annually during the 5 year follow-up period, resulting in an average of three imaging studies per year. Patients who underwent more than the average number of imaging studies annually were categorized as high intensity (HI), and those with less than the average were categorized as low intensity (LI).
Among 1888 patients, 864 (45.8%) were in HI group. Age, sex, and location were not different between groups. HI group had more advanced T and N stage (P = 0.002, 0.010, each). Perineural invasion (PNI) was more identified in the HI group (21.4% vs 30.3%, P < 0.001). The mean overall survival (OS) and recurrence-free interval (RFI) was longer in the LI group (P < 0.001, each). Multivariate analysis indicated that surveillance intensity [odds ratio (OR) = 1.999; 95% confidence interval (CI): 1.680–2.377; P < 0.001], pathologic T stage (OR = 1.596; 95%CI: 1.197–2.127; P = 0.001), PNI (OR = 1.431; 95%CI: 1.192–1.719; P < 0.001), and circumferential resection margin (OR = 1.565; 95%CI: 1.083–2.262; P = 0.017) in rectal cancer were significantly associated with RFI. The mean post-recurrence survival (PRS) was longer in patients who received curative resection (P < 0.001). Curative resection rate of recurrence was not different between HI (29.3%) and LI (23.8%) groups (P = 0.160). PRS did not differ according to surveillance intensity (P = 0.802).
Frequent surveillance with CT scan do not improve OS in stage III CRC patients. We need to evaluate role of other surveillance method rather than frequent CT scans to detect recurrence for which curative treatment was possible because curative resection is the important to improve post-recurrence survival.
Core Tip: This is a retrospective study to evaluate the association between surveillance intensity and recurrence and survival rates in patients with stage III colorectal cancer (CRC). The overall survival (OS) and recurrence-free interval (RFI) was longer in the low intensity group. Post-recurrence survival (PRS) did not change according to surveillance intensity. Therefore, frequent postoperative imaging studies do not improve OS or RFI in patients with stage III CRC. However, in high-risk patients, early detection of recurrence improves the chance of curative resection, which may improve PRS.
- Citation: Park MY, Park IJ, Ryu HS, Jung J, Kim M, Lim SB, Yu CS, Kim JC. Optimal postoperative surveillance strategies for stage III colorectal cancer. World J Gastrointest Surg 2021; 13(9): 1012-1024
- URL: https://www.wjgnet.com/1948-9366/full/v13/i9/1012.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i9.1012
In patients who undergo surgery for colorectal cancer (CRC), ongoing surveillance is recommended to detect and treat recurrences early, which improves the chances of curative treatment and thus overall survival (OS)[1]. Surveillance also provides an opportunity to assess the quality of the primary surgery and detect metachronous tumors at an earlier stage.
CRC is the second most common cancer among Korean males and the fourth most common among females, and the third leading cause of cancer-related death in South Korea[2]. The 5 year trend from 2013 to 2017 indicates that approximately 78% of CRC patients in Korea have resectable tumors with localized or regional disease is similar with that in United States[3]. Despite high prevalence and mortality rates, patients with CRC represent the second largest group of 5 year cancer survivors. More than 90% of local recurrences appear within the first 5 years after surgery, and the most of them appear within 3 years after surgery[4,5]. After radical surgery with curative intent, surveillance is recommended with the goal of improving OS and disease-specific survival by detecting recurrence or metachronous cancer at an early stage. Hypothetically intensive surveillance during recurrence-prone period could be useful to detect recurrence in early phase and thus improve the prognosis of these patients[6-8] especially in patients with high risk of recurrence by early onset of proper treatment.
Although many clinical guidelines recommended surveillance method and sche
The purpose of the current study was to determine the association between surveillance intensity, the detection of recurrence, and survival rates. Additionally, this study investigated the effect of intensive surveillance on the outcome of curative treatment in patients with recurrent disease.
Data from patients with pathologic stage III CRC who underwent radical surgery between January 2005 and December 2012 at Asan Medical Center, Seoul, Korea were retrospectively reviewed. Patients who underwent radical resection and elective surgery for primary CRC, as well as those treated with preoperative chemoradiotherapy (PCRT) followed by radical resection, were included. Patients with syn
Patient characteristics analyzed included age, sex, pathologic differentiation, lymphovascular invasion (LVI), perineural invasion (PNI), circumferential resection margin (CRM) of rectal cancer (involving < 1 mm), PCRT, recurrence, treatment after recurrence, and survival. Postoperative surveillance included abdomino-pelvic CT (APCT) and chest CT (CCT).
This study was approved by the Institutional Review Board of Asan Medical Center, No. 2017-0955.
The objectives of surgical treatment for colon cancer were ligation of feeding vessels at their roots, principal node removal, and achieving a sufficient resection margin for both proximal and distal margins. Surgery was performed according to the principle of total mesorectal excision for rectal cancer. Patients who received PCRT underwent surgical resection at 6–10 wk after completion of the chemoradiotherapy course. The majority of surgical procedures were carried out by one of seven experienced colomajority of surgical procedures were carried out by one of seven experiencedrectal surgeons, and the remaining procedures were performed by colorectal fellows.
Adjuvant chemotherapy was recommended for pathologic stage III colon cancer patients and for stage II patients with risk factors such as preoperative obstruction, LVI, PNI, high tumor budding, and < 12 resected lymph nodes. In patients with rectal cancer, adjuvant chemotherapy was recommended for pathologic stage II and III patients or for those treated with PCRT regardless of pathologic stage. PCRT was indicated for patients who had clinical stage II or III cancer and for those with clinical stage I who were eligible for sphincter-saving surgery due to low lying rectal cancer and those who were not candidates for major surgery because of medical comorbidities.
All patients received postoperative follow-up examination consisting of a physical examination and serum carcinoembryonic antigen measurements every 3–6 mo. Abdominal, pelvic, and chest CT scans were performed every 6–12 mo. Patients with obstructive lesions underwent colonoscopy within 6 mo after surgical resection and every 2–3 years thereafter.
All patients were followed-up for approximately 5 years after surgery with APCT and CCT. Patients underwent surveillance every 6 mo at the outpatient clinic, including APCT every 6 mo and CCT every 12 mo on average. The number of expected imaging studies was two for APCT and one for CCT, with a total of three studies per year.
The average number of studies for each patient was calculated as the number of examinations during 5 years/60 mo of follow-up without recurrence, or the number of examinations until the first recurrence for patients who experienced recurrence. Pa
Continuous variables were compared using a t-test and expressed as the mean and range. Categorical variables were compared using Pearson’s χ2 test or Fisher’s exact test and expressed as numbers and percentages. Univariate analyses were performed to identify factors associated with survival. Factors with P < 0.1 on univariate analysis were included in a multivariate binary logistic regression analysis. OS, recurrence-free interval (RFI), and post-recurrence survival (PRS) were calculated using the Ka
Of 1888 patients, 1024 were included in the LI group and 864 were included in the HI group. The demographic characteristics of the patients and the clinicopathological features of the tumors are shown in Table 1. Demographic characteristics did not differ between the LI group and the HI group. In terms of pathologic features, patients in the HI group had a higher T and N stage and included more risk factors such as a high degree of malignant differentiation, PNI, or positive CRM. The average number of APCT studies performed per year was 1.8-fold higher in the HI group than in the LI group, and CCT was performed at a 2.4-fold higher rate in the HI group than in the LI group (P < 0.001) (Table 1). In patients with rectal cancer, positive CRM was higher in the HI group than in the LI group (Supplementary Table 1).
| Variables | Surveillance intensity | P value | |
| Lower intensity (n = 1024) | Higher intensity (n = 864) | ||
| Age, mean (IQR) | 60.0 (52.0–68.0) | 58.0 (50.3–67.0) | 0.178 |
| Gender, n (%) | 0.502 | ||
| Male | 607 (59.3) | 528 (61.1) | |
| Female | 417 (40.7) | 336 (38.9) | |
| Cancer site, n (%) | 0.795 | ||
| Colon | 365 (35.6) | 303 (35.1) | |
| Rectum | 659 (64.4) | 561 (64.9) | |
| Differentiation, n (%) | 0.027 | ||
| WD/MD | 945 (92.3) | 781 (90.4) | |
| PD/SRC/MUC | 72 (7.0) | 82 (9.5) | |
| Unknown | 7 (0.7) | 1 (0.1) | |
| Total lymph nodes, n (%) | 0.001 | ||
| < 12 | 129 (12.6) | 49 (5.7) | |
| ≥ 12 | 895 (87.4) | 815 (94.3) | |
| (y) pT, n (%) | 0.002 | ||
| 0 | 12 (1.2) | 6 (0.7) | |
| 1 | 66 (6.4) | 36 (4.2) | |
| 2 | 126 (12.3) | 89 (10.3) | |
| 3 | 770 (75.2) | 660 (76.4) | |
| 4 | 50 (4.9) | 73 (8.4) | |
| (y) pN, n (%) | 0.010 | ||
| 1c | 14 (1.4) | 8 (0.9) | |
| 1 | 735 (71.8) | 570 (66.0) | |
| 2 | 275 (26.8) | 286 (33.1) | |
| Perineural invasion, n (%) | 219 (21.4) | 262 (30.3) | < 0.001 |
| Lymphovascular invasion, n (%) | 371 (36.2) | 344 (39.8) | 0.110 |
| Resection margin, n (%) | 0.004 | ||
| Positive | 18 (1.7) | 41 (4.7) | |
| Unknown | 7 (0.7) | 8 (0.9) | |
| APCT, mean ± SD | 1.49 ± 0.47 | 2.67 ± 1.31 | < 0.001 |
| CCT, mean ± SD | 0.62 ± 0.41 | 1.48 ± 0.91 | < 0.001 |
| Total imaging studies, mean ± SD | 2.11 ± 0.58 | 4.14 ± 1.64 | < 0.001 |
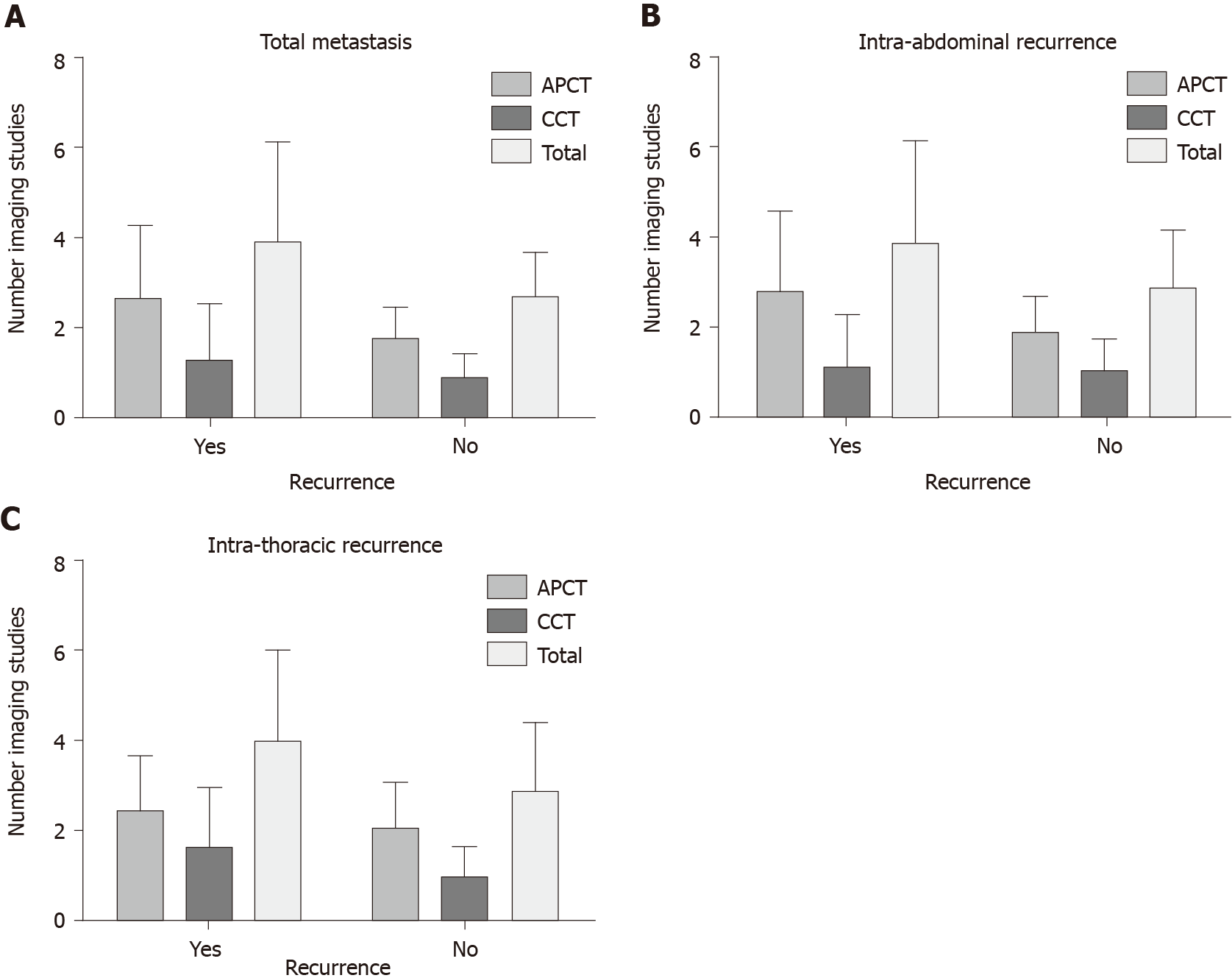
The number of APCT and CCT studies was significantly higher in patients who expe
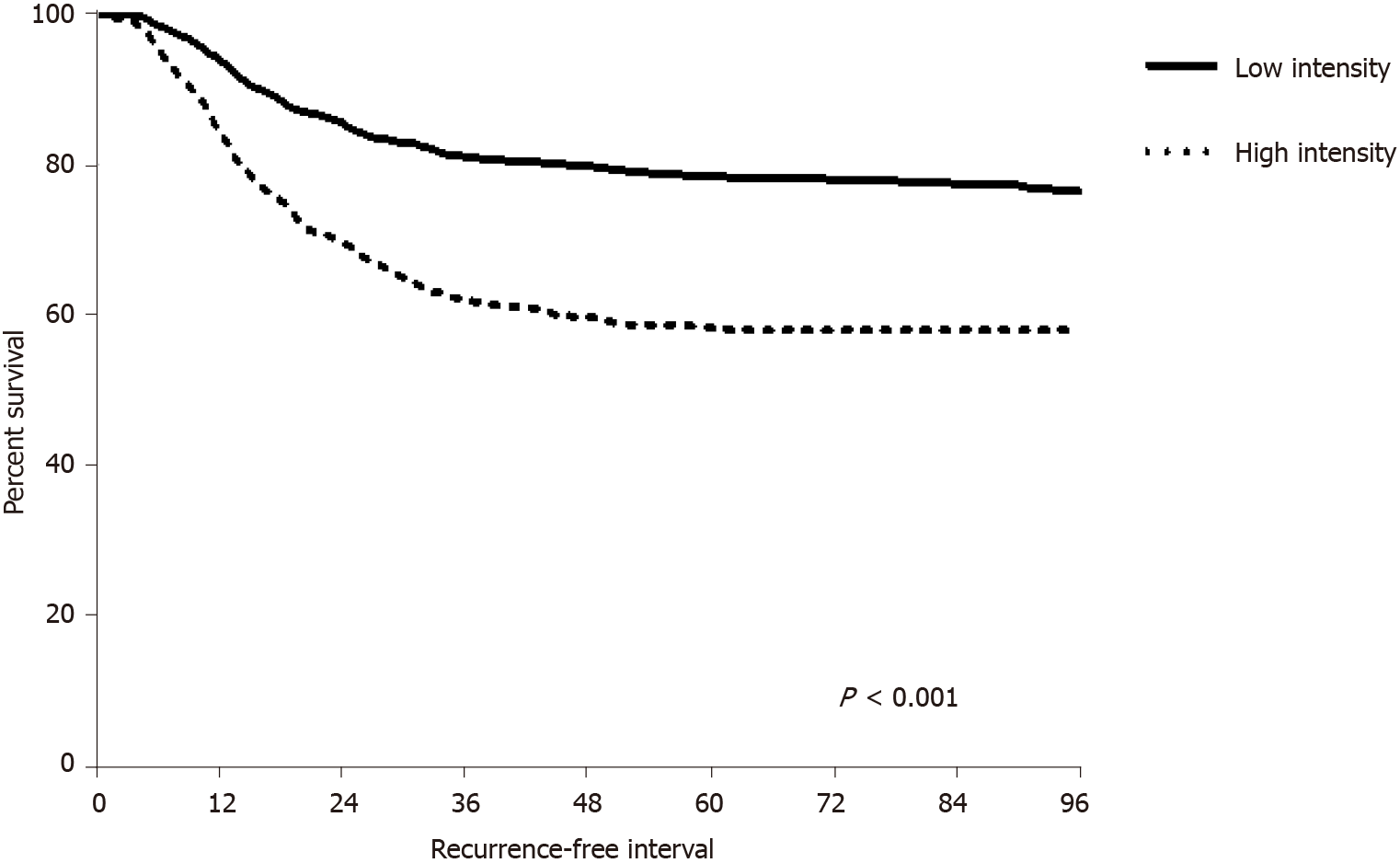
The RFI was longer in the LI group than in the HI group (61 ± 33.95 mo vs 45 ± 28.35 mo, P < 0.001). In patients who experienced recurrence, the mean RFI remained longer in the LI group than in the HI group (23 ± 16.09 mo vs 19 ± 11.86 mo, P = 0.001). Both intra-abdominal RFI according to APCT intensity and intra-thoracic RFI according to CCT intensity were longer in the LI group than in the HI group (abdomen, 23 ± 16.38 mo vs 17 ± 11.39 mo, P < 0.001; chest, 26 ± 15.36 mo vs 20 ± 13.79 mo, P = 0.004) (Figure 2). The mean RFI in recurred patients did not differ significantly according to tumor location (colon, 22 ± 11.21 mo vs rectum, 20 ± 14.41 mo, P = 0.059).
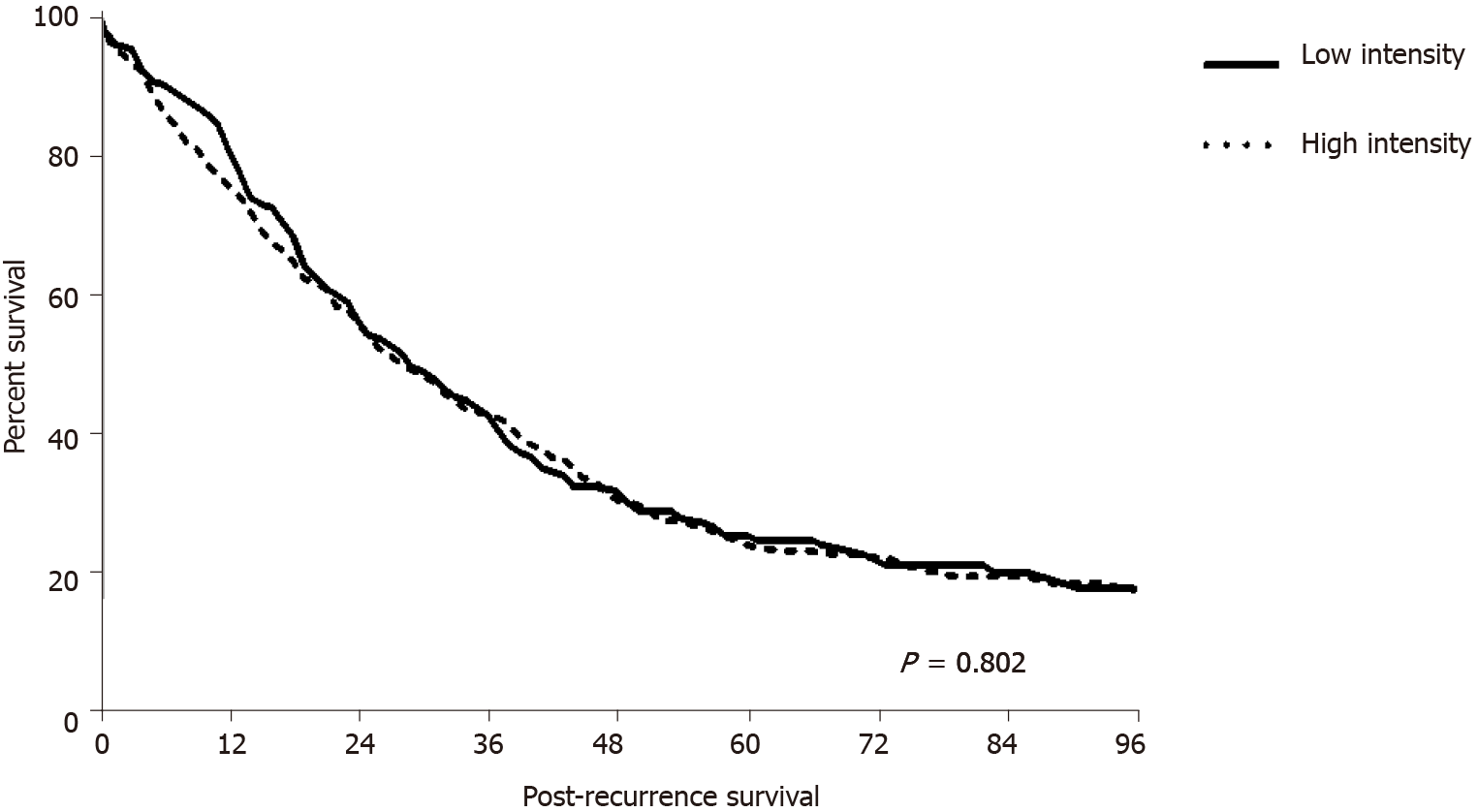
Among patients who experienced recurrence, the mean PRS time did not differ according to surveillance intensity (35 ± 31.94 mo in the LI group and 34 ± 29.28 mo in the HI group; P = 0.802) (Figure 3). There was no difference in the PRS according to tumor location (colon, 29 ± 29.65 mo vs 37 ± 30.08 mo, P = 0.250; rectum, 36 ± 32.20 mo vs 33 ± 28.94 mo, P = 0.415). Curative resection was possible in 152 of all recurred patients, of which 51 (23.8%) were in the LI group and 101 (29.3%) were in the HI group (P = 0.160). Of the 51 patients in the LI group, seven (13.7%) had colon cancer and 44 (86.3%) had rectal cancer. In the HI group, 35 (34.6%) patients had colon cancer and 66 (55.4%) had rectal cancer. There was no difference in the rate of curative resection between surveillance intensity groups according to tumor location (colon, P = 0.673; rectum, P = 0.318). PRS according to the curative intent after recurrence was significantly longer in patients who underwent curative resection (54 ± 30.96 mo vs 27 ± 26.82 mo, P < 0.001).
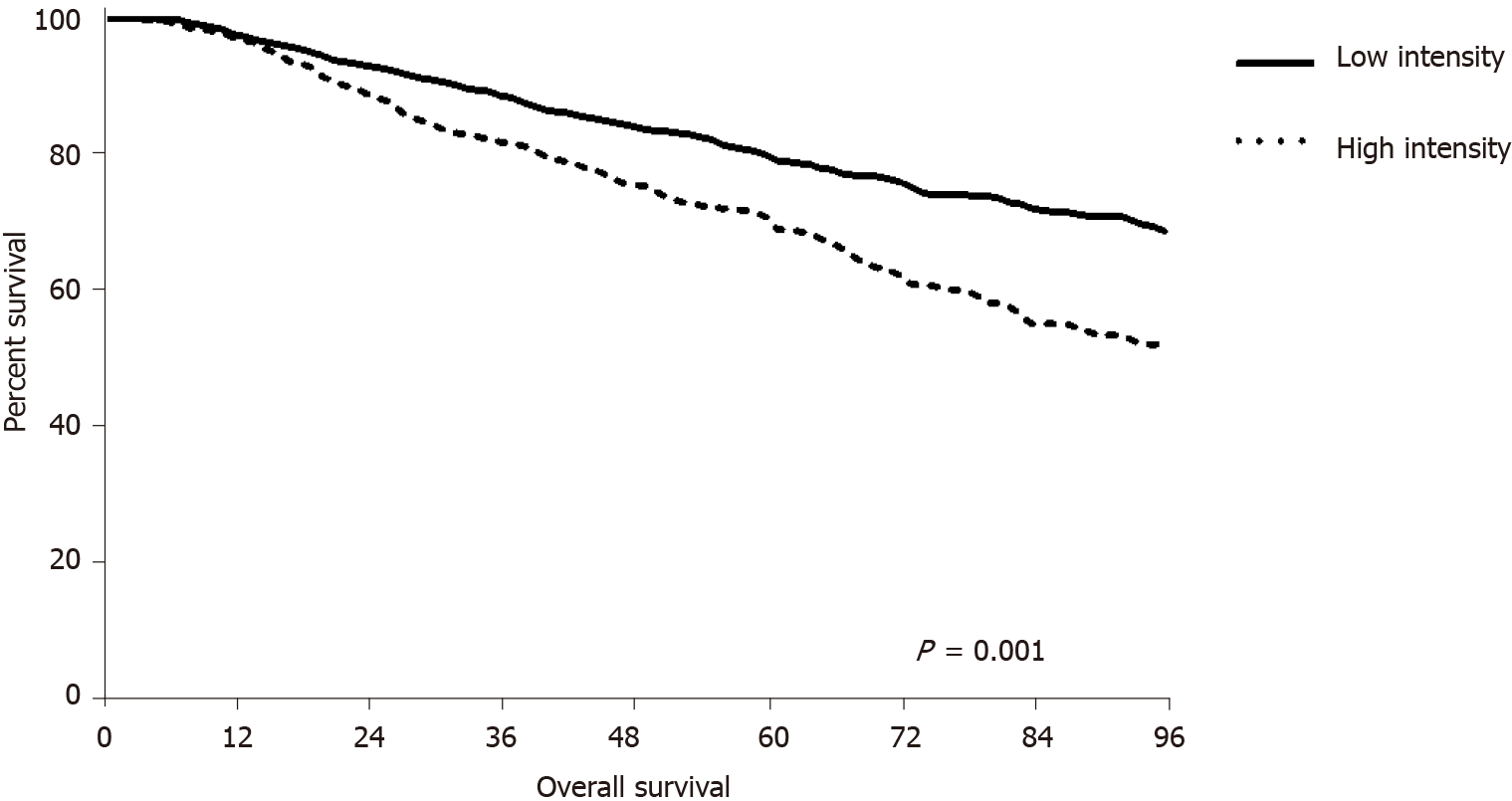
The mean OS was significantly longer in the LI group (68 ± 31.89 mo) than in the HI group (58 ± 27.35 mo, P < 0.001) (Figure 4). Analysis of survival according to tumor location showed that OS was longer in the LI group regardless of tumor location (colon, 74 ± 27.84 mo vs 56 ± 23.66 mo, P < 0.001; rectum, 65 ± 33.58 mo vs 59 ± 29.12 mo, P = 0.001).
Univariate analysis identified factors affecting OS. Age, sex, surveillance intensity, pathologic differentiation, pathologic T and N stages, LVI, PNI, and CRM in rectal cancer significantly affected OS (P < 0.05). In the multivariate analysis, age, sex, surveillance intensity, differentiation, pathologic T stage, LVI, PNI, and CRM in rectal cancer were significantly associated with OS (Table 2).
| Factors | Univariate | Multivariate | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age (yr) | 1.027 (1.019–1.035) | < 0.001 | 1.031 (1.023–1.039) | < 0.001 |
| Sex | 0.704 (0.592–0.836) | < 0.001 | 0.711 (0.598–0.845) | < 0.001 |
| Surveillance intensity | 1.650 (1.400–1.945) | < 0.001 | 1.531 (1.295–1.808) | < 0.001 |
| Differentiation | ||||
| WD/MD | Ref. | Ref. | ||
| PD/SRC/MUC | 1.832 (1.424–2.356) | < 0.001 | 1.660 (1.285–2.143) | < 0.001 |
| (y) pT stage | ||||
| 0–2 | Ref. | Ref. | ||
| 3–4 | 1.937 (1.491–2.516) | < 0.001 | 1.461 (1.111–1.921) | 0.007 |
| (y) pN stage | ||||
| 1c | Ref. | Ref. | ||
| 1 | 5.136 (0.721–36.571) | 0.102 | 4.754 (0.667–33.906) | 0.12 |
| 2 | 9.322 (1.308–66.457) | 0.026 | 7.067 (0.988–50.556) | 0.051 |
| Lymphovascular invasion | 1.607 (1.365–1.891) | < 0.001 | 1.256 (1.057–1.491) | 0.01 |
| Perineural invasion | 1.818 (1.535–2.154) | < 0.001 | 1.466 (1.224–1.755) | < 0.001 |
| Resection margin1 | 1.972 (1.360–2.860) | < 0.001 | 1.603 (1.097–2.341) | 0.015 |
Univariate analysis of factors affecting RFI indicated that surveillance intensity, differentiation, pathologic T stage, pathologic N stage, LVI, PNI, and CRM in rectal cancer significantly affected RFI (P < 0.05). In the multivariate analysis, surveillance intensity, pathologic T stage, PNI, and CRM in rectal cancer were significantly associated with RFI. Among patients who experienced intra-abdominal recurrence, APCT intensity, differentiation, pathologic T stage, PNI, and CRM in rectal cancer were significantly associated with RFI. In patients with intra-thoracic recurrence, CCT intensity, differentiation, pathologic T stage, LVI, PNI, and CRM in rectal cancer were significantly associated with RFI (Table 3).
| Factors | Univariate | Multivariate | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age (yr) | 0.995 (0.987–1.002) | 0.165 | 0.999 (0.991–1.006) | 0.715 |
| Sex | 0.907 (0.765–1.076) | 0.262 | ||
| Surveillance intensity | 2.218 (1.870–2.632) | < 0.001 | 1.999 (1.680–2.377) | < 0.001 |
| Differentiation | ||||
| WD/MD | Ref. | Ref. | ||
| PD/SRC/MUC | 1.507 (1.151–1.974) | 0.003 | 1.287 (0.979–1.694) | 0.071 |
| (y) pT stage | ||||
| 0–2 | Ref. | Ref. | ||
| 3–4 | 2.118 (1.610–2.785) | < 0.001 | 1.596 (1.197–2.127) | 0.001 |
| (y) pN stage | ||||
| 1c | Ref. | Ref. | ||
| 1 | 2.737 (0.682–10.989) | 0.156 | 2.501 (0.621–10.063) | 0.197 |
| 2 | 5.260 (1.308–21.156) | 0.019 | 3.813 (0.943–15.413) | 0.060 |
| Lymphovascular invasion | 1.460 (1.236–1.724) | < 0.001 | 1.143 (0.957–1.364) | 0.140 |
| Perineural invasion | 1.949 (1.641–2.313) | < 0.001 | 1.431 (1.192–1.719) | < 0.001 |
| Resection margin1 | 2.192 (1.529–3.144) | < 0.001 | 1.565 (1.083–2.262) | 0.017 |
Univariate analysis of patients who experienced recurrence to identify factors affecting PRS showed that age, differentiation, LVI, PNI, and curative resection were significantly associated with PRS. Multivariate analysis showed that age, differentiation, PNI, and curative resection were significantly associated with PRS. In patients with intra-abdominal recurrence, age, differentiation, PNI, and curative resection were associated with PRS, whereas in patients with intra-thoracic recurrence, only sex and curative resection affected PRS (Table 4). The results of univariate and multivariate analyses of patients with rectal cancer were comparable to the results for all patients (Supplementary Table 1).
| Factors | Univariate | Multivariate | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age (yr) | 1.015 (1.007–1.024) | < 0.001 | 1.015 (1.006–1.024) | 0.001 |
| Sex | 0.824 (0.676–1.004) | 0.054 | 0.842 (0.688–1.032) | 0.098 |
| Image intensity | 0.971 (0.799–1.179) | 0.767 | ||
| Differentiation | ||||
| WD/MD | Ref. | Ref. | ||
| PD/SRC/MUC | 2.632 (1.779–3.137) | < 0.001 | 2.072 (1.553–2.766) | < 0.001 |
| (y) pT stage | ||||
| 0–2 | Ref. | |||
| 3–4 | 1.146 (0.833–1.576) | 0.401 | ||
| (y) pN stage | ||||
| 1c | Ref. | |||
| 1 | 2.139 (0.300–15.256) | 0.448 | ||
| 2 | 3.363 (0.471–24.009) | 0.226 | ||
| Lymphovascular invasion | 1.456 (1.204–1.760) | < 0.001 | 1.152 (0.940–1.412) | 0.174 |
| Perineural invasion | 1.384 (1.141–1.677) | 0.001 | 1.284 (1.045–1.579) | 0.018 |
| Resection margin1 | 1.416 (0.966–2.075) | 0.075 | 1.266 (0.856–1.871) | 0.237 |
| Curative resection | 0.296 (0.229–0.381) | < 0.001 | 0.331 (0.255–0.428) | < 0.001 |
Existing guidelines recommend surveillance after primary surgery with a curative intent for CRC[22-26], although consistent guidelines are lacking. The European Society of Medical Oncology recommends abdominal and chest CT every 6 to 12 mo for 3 years, and then yearly for 2 years for patients with colon cancer; however, there are no imaging recommendations for patients with rectal cancer. The American Society of Clinical Oncology guidelines recommend abdominal and chest CT annually for 3 years, and every 6 to 12 mo for the first 3 years for high-risk patients. The National Comprehensive Cancer Network guidelines suggest an abdominal CT scan for high-risk patients with poorly differentiated cancer or those with perineural or venous invasion, although there are no guidelines regarding frequency. The American Society of Colorectal Surgeons guidelines recommend chest and abdominopelvic imaging annually for 5 years.
The Gruppo Italiano Lavoro per la Diagnosi Anticipata trial launched in 1998 found that an intensive surveillance program after curative treatment for CRC detects asymptomatic local or distant recurrences but does not affect OS[27]. Similarly, the Follow-up After Colorectal Surgery randomized trial, the results of which were recently publi
In this study, patients were divided into LI and HI groups according to the number of imaging studies during the follow-up period. The average number of imaging studies was higher in patients with recurrence regardless of the location of recurrence. Patients in the HI group had higher pathologic T and N stages and were more likely to have risk factors such as LVI and PNI. This suggests a tendency to perform survei
Survival analysis showed that OS and RFI were longer in the LI group than in the HI group, whereas PRS did not differ between the two groups. The shorter OS and RFI could be related to the higher aggressive biology of the HI group. Analysis of patients who did not experience recurrence showed that OS was approximately 10 mo shorter in the HI group than in the LI group. Although not statistically significant, the proba
This study has several limitations. First, it was a retrospective, observational cohort study, and patients were not randomized. Surveillance intensity can vary according to the patient’s condition at the time of treatment, which may have resulted in selection bias. Second, the average surveillance schedule may have differed depending on the physician. Additional research is needed to determine the standard routine surve
In conclusion, in patients with stage III CRC, frequent postoperative image studies alone do not improve OS and RFI. Curative resection is the most important factors to improve PRS and we need to find a way to increase curative treatment of recurrent disease via optimal surveillance. Therefore, role of other imaging modalities according to risk of recurrence would be evaluated rather than increasing surveillance frequency to improve oncologic outcomes.
Optimal surveillance strategies for stage III colorectal cancer (CRC) are lacking, and intensive surveillance has not conferred a significant survival benefit.
Evaluating appropriate surveillance intensity would be helpful to improve oncologic outcomes or decrease un-necessary imaging studies during surveillance.
We examined the association between surveillance intensity and recurrence and survival rates in patients with stage III CRC.
Data from patients with pathologic stage III CRC who underwent radical surgery between January 2005 and December 2012 at Asan Medical Center, Seoul, Korea were retrospectively reviewed. Surveillance consisted of abdominopelvic computed tomography (CT) every 6 mo and chest CT annually during the 5 year follow-up pe
Among 1888 patients, 864 (45.8%) were in HI group. The HI group had more advanced T and N stage (P = 0.002, 0.010, each). A high degree of malignant differentiation was more common in the HI group than in the LI group (P = 0.027). Perineural invasion (PNI) was significantly more identified in the HI group (21.4% vs 30.3%, P < 0.001).
The mean overall survival (OS) and Recurrence-free interval (RFI) was longer in the LI group (P < 0.001, each). Multivariate analysis indicated that surveillance intensity was negatively associated with RFI [odds ratio (OR) = 1.999; 95% confidence interval (CI): 1.680–2.377; P < 0.001] and OS [OR = 1.531, 95%CI: 1.295–1.808; P < 0.001]. The mean post-recurrence survival (PRS) was significantly longer in patients who received curative resection (P < 0.001). Curative resection rate of recurrence was not different between HI (29.3%) and LI (23.8%) groups (P = 0.160). PRS did not differ according to surveillance intensity (P = 0.802).
Frequent postoperative surveillance with CT scan alone do not improve OS and RFI. Curative resection is the most important factors to improve PRS and we need to find a way to increase curative treatment of recurrent disease via optimal surveillance.
Role of other imaging modalities according to risk of recurrence would be evaluated rather than increasing surveillance frequency to improve oncologic outcomes.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hazafa A S-Editor: Fan JR L-Editor: A P-Editor: Wu RR
| 1. | Taylor I. Quality of follow-up of the cancer patient affecting outcome. Surg Oncol Clin N Am. 2000;9:21-25, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Lee BI, Hong SP, Kim SE, Kim SH, Kim HS, Hong SN, Yang DH, Shin SJ, Lee SH, Park DI, Kim YH, Kim HJ, Yang SK, Jeon HJ; Multi-Society Task Force for Development of Guidelines for Colorectal Polyp Screening, Surveillance and Management. Korean guidelines for colorectal cancer screening and polyp detection. Clin Endosc. 2012;45:25-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2417] [Cited by in RCA: 3074] [Article Influence: 512.3] [Reference Citation Analysis (0)] |
| 4. | Böhm B, Schwenk W, Hucke HP, Stock W. Does methodic long-term follow-up affect survival after curative resection of colorectal carcinoma? Dis Colon Rectum. 1993;36:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Kjeldsen BJ, Kronborg O, Fenger C, Jørgensen OD. The pattern of recurrent colorectal cancer in a prospective randomised study and the characteristics of diagnostic tests. Int J Colorectal Dis. 1997;12:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Holm T, Cedermark B, Rutqvist LE. Local recurrence of rectal adenocarcinoma after 'curative' surgery with and without preoperative radiotherapy. Br J Surg. 1994;81:452-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 79] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Secco G, Fardelli R, Campora E, Rovida S, Martinoli C, Motta G. Results of postoperative follow-up vs no follow-up in colorectal cancer. Coloproctology. 1990;6:362-368. |
| 8. | Törnqvist A, Ekelund G, Leandoer L. The value of intensive follow-up after curative resection for colorectal carcinoma. Br J Surg. 1982;69:725-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 111] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Renehan AG, Egger M, Saunders MP, O'Dwyer ST. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ. 2002;324:813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 450] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 10. | Figueredo A, Rumble RB, Maroun J, Earle CC, Cummings B, McLeod R, Zuraw L, Zwaal C; Gastrointestinal Cancer Disease Site Group of Cancer Care Ontario's Program in Evidence-based Care. Follow-up of patients with curatively resected colorectal cancer: a practice guideline. BMC Cancer. 2003;3:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 261] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 11. | Mant D, Gray A, Pugh S, Campbell H, George S, Fuller A, Shinkins B, Corkhill A, Mellor J, Dixon E, Little L, Perera-Salazar R, Primrose J. A randomised controlled trial to assess the cost-effectiveness of intensive vs no scheduled follow-up in patients who have undergone resection for colorectal cancer with curative intent. Health Technol Assess. 2017;21:1-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Rosati G, Ambrosini G, Barni S, Andreoni B, Corradini G, Luchena G, Daniele B, Gaion F, Oliverio G, Duro M, Martignoni G, Pinna N, Sozzi P, Pancera G, Solina G, Pavia G, Pignata S, Johnson F, Labianca R, Apolone G, Zaniboni A, Monteforte M, Negri E, Torri V, Mosconi P, Fossati R; GILDA working group. A randomized trial of intensive vs minimal surveillance of patients with resected Dukes B2-C colorectal carcinoma. Ann Oncol. 2016;27:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 13. | Wille-Jørgensen P, Syk I, Smedh K, Laurberg S, Nielsen DT, Petersen SH, Renehan AG, Horváth-Puhó E, Påhlman L, Sørensen HT; COLOFOL Study Group. Effect of More vs Less Frequent Follow-up Testing on Overall and Colorectal Cancer-Specific Mortality in Patients With Stage II or III Colorectal Cancer: The COLOFOL Randomized Clinical Trial. JAMA. 2018;319:2095-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 14. | Smoragiewicz M, Lim H, Peixoto RD. Surveillance for asymptomatic recurrence in resected stage III colon cancer: does it result in a more favorable outcome? J Gastrointest Oncol. 2015;6:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2019;9:CD002200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 16. | Primrose JN, Perera R, Gray A, Rose P, Fuller A, Corkhill A, George S, Mant D; FACS Trial Investigators. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA. 2014;311:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 346] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 17. | Wang T, Cui Y, Huang WS, Deng YH, Gong W, Li CJ, Wang JP. The role of postoperative colonoscopic surveillance after radical surgery for colorectal cancer: a prospective, randomized clinical study. Gastrointest Endosc. 2009;69:609-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Tjandra JJ, Chan MK. Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum. 2007;50:1783-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 247] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 19. | Augestad KM, Rose J, Crawshaw B, Cooper G, Delaney C. Do the benefits outweigh the side effects of colorectal cancer surveillance? World J Gastrointest Oncol. 2014;6:104-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Adams K, Higgins L, Beazley S, Papagrigoriadis S. Intensive surveillance following curative treatment of colorectal cancer allows effective treatment of recurrence even if limited to 4 years. Int J Colorectal Dis. 2015;30:1677-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35:1-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5822] [Cited by in RCA: 5950] [Article Influence: 124.0] [Reference Citation Analysis (0)] |
| 22. | Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Gurski L, Freedman-Cass DA. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:874-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 683] [Article Influence: 113.8] [Reference Citation Analysis (0)] |
| 23. | Benson AB 3rd, Venook AP, Cederquist L, Chan E, Chen YJ, Cooper HS, Deming D, Engstrom PF, Enzinger PC, Fichera A, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wu CS, Gregory KM, Freedman-Cass D. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 568] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 24. | Argilés G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T, Laurent-Puig P, Quirke P, Yoshino T, Taieb J, Martinelli E, Arnold D; ESMO Guidelines Committee. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1291-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 807] [Article Influence: 161.4] [Reference Citation Analysis (0)] |
| 25. | Meyerhardt JA, Mangu PB, Flynn PJ, Korde L, Loprinzi CL, Minsky BD, Petrelli NJ, Ryan K, Schrag DH, Wong SL, Benson AB 3rd; American Society of Clinical Oncology. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2013;31:4465-4470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 243] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 26. | Steele SR, Chang GJ, Hendren S, Weiser M, Irani J, Buie WD, Rafferty JF; Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. Practice Guideline for the Surveillance of Patients After Curative Treatment of Colon and Rectal Cancer. Dis Colon Rectum. 2015;58:713-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 27. | Johnson F, Virgo K, Grossmann E, Longo W, Fossati R. Colorectal cancer patient follow-up following surgery with curative intent: the GILDA trial. J Clin Oncol. 2004;22:3645-3645. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Laubert T, Bader FG, Oevermann E, Jungbluth T, Unger L, Roblick UJ, Bruch HP, Mirow L. Intensified surveillance after surgery for colorectal cancer significantly improves survival. Eur J Med Res. 2010;15:25-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Rodríguez-Moranta F, Saló J, Arcusa A, Boadas J, Piñol V, Bessa X, Batiste-Alentorn E, Lacy AM, Delgado S, Maurel J, Piqué JM, Castells A. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: a prospective, multicenter, randomized, controlled trial. J Clin Oncol. 2006;24:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 206] [Article Influence: 10.8] [Reference Citation Analysis (0)] |