Published online Mar 15, 2015. doi: 10.4239/wjd.v6.i2.321
Peer-review started: August 19, 2014
First decision: September 16, 2014
Revised: October 15, 2014
Accepted: December 18, 2014
Article in press: December 19, 2014
Published online: March 15, 2015
Processing time: 212 Days and 18.2 Hours
Hepatic glycogenosis (HG) is characterized by excessive glycogen accumulation in hepatocytes and represents a hepatic complication of diabetes that particularly occurs in patients with longstanding poorly controlled type 1 diabetes (T1D). HG has been reported to be a very rare disease, although it is believed to be extremely underdiagnosed because it is not possible to distinguish it from non-alcoholic fatty liver disease (NAFLD) unless a liver biopsy is performed. In contrast to HG, NAFLD is characterized by liver fat accumulation and is the more likely diagnosis for patients with type 2 diabetes and metabolic syndrome. The pathogenesis of HG involves the concomitant presence of insulin and excess glucose, which increases glycogen storage in the liver. HG is characterized by a transient elevation in liver transaminases and hepatomegaly. Differentiating between these two conditions is of the utmost importance because HG is a benign disease that is potentially reversible by improving glycemic control, whereas NAFLD can progress to cirrhosis. Therefore, HG should be suspected when liver dysfunction occurs in patients with poorly controlled T1D. The aim of this article is to review the epidemiology, clinical characteristics, pathogenesis and histology of HG.
Core tip: Hepatic glycogenosis (HG) is a complication of diabetes mellitus that is often underdiagnosed. It is defined as pathological glycogen storage in hepatocytes with hepatomegaly and elevated liver enzymes and mainly occurs in patients with longstanding poorly controlled type 1 diabetes. HG cannot easily be distinguished from non-alcoholic fatty liver disease (NAFLD) by history, physical examination or ultrasound; only liver biopsy can provide a definitive diagnosis. The hallmark of this condition is its reversibility with improved glycemic control; in contrast, NAFLD can progress to fibrosis.
- Citation: Julián MT, Alonso N, Ojanguren I, Pizarro E, Ballestar E, Puig-Domingo M. Hepatic glycogenosis: An underdiagnosed complication of diabetes mellitus? World J Diabetes 2015; 6(2): 321-325
- URL: https://www.wjgnet.com/1948-9358/full/v6/i2/321.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i2.321
Diabetes mellitus (DM) is associated with various structural and functional liver abnormalities, including non-alcoholic fatty liver disease (NAFLD) and hepatic glycogenosis (HG). NAFLD represents the most common liver disease associated with DM, especially in patients with type 2 diabetes (T2D) and metabolic syndrome[1]. HG involves pathological glycogen storage in hepatocytes and is associated with poorly controlled DM, particularly type 1 diabetes (T1D). This condition is believed to be extremely underdiagnosed because it is indistinguishable from NAFLD in the absence of a liver biopsy. However, the distinction between these two diabetes-related complications is important: whereas NAFLD may progress to fibrosis and cirrhosis, HG is potentially reversible with sustained glycemic control[1,2]. This review aims to provide an overview of the clinical characteristics and pathological features of HG to improve recognition of this diabetes-related complication.
HG is defined as pathological excessive glycogen accumulation in hepatocytes and is characterized by hepatomegaly and a transient elevation in liver transaminases[2]. Glycogen accumulation in the liver was first described in children by Mauriac[3] in 1930 as a component of Mauriac’s syndrome. He observed glycogen accumulation in a child with T1D and poor diabetic control that was associated with hepatomegaly and abnormal liver enzymes as well as other features such as growth retardation and/or dwarfism, delayed puberty, cushingoid features and hypercholesterolemia. Currently, HG is a well-recognized disease that occurs at any age and can be present without the full spectrum of features described for Mauriac’s syndrome. Although numerous case reports and several series have been published[2,4-24], the exact prevalence of HG is unknown, but it is considered to be the primary cause of hepatomegaly in children and adolescents with T1D[24]. This condition has been given many labels, such as hepatic or liver glycogenosis[4,11,12,15,16], glycogenic hepatopathy[2,5,17,19,22,23], liver glycogen storage [6,13], and DM-associated glycogen storage hepatomegaly[10].
HG was first described in association with acute ketoacidosis or recurrent hypoglycemia[7-10,15] in cases presenting with excessive insulin and/or elevated glucose. However, hepatic glycogen accumulation also develops in diabetic patients with long-term poor control and several hospitalizations for diabetic ketoacidosis[4,5,11,16-21]. Although HG is more common in patients with T1D, it has also been described in insulin-dependent type 2 diabetic patients during ketosis or poor diabetic control requiring increasing amounts of insulin[25]. In addition, HG has also been reported in other clinical settings, such as in three children after short-term, high-dose steroid therapy without insulin treatment[26] and in a patient with dumping syndrome associated with gastrostomy[27].
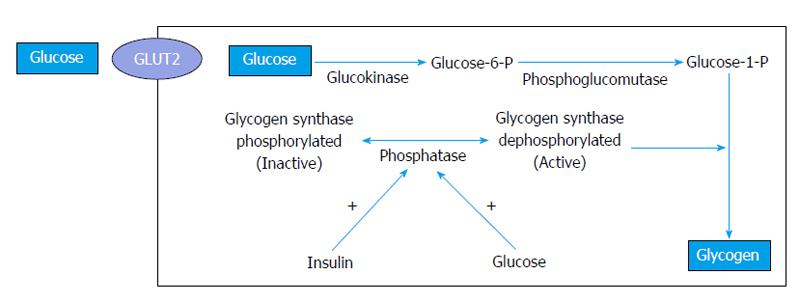
Although the underlying mechanisms by which HG develops have not yet been fully elucidated, wide fluctuations in glucose and insulin concentrations seem to be essential for its pathogenesis[28]. Blood glucose and insulin levels often fluctuate in diabetic patients with poor metabolic control, thereby promoting hepatic glycogen accumulation. High plasma glucose levels cause an insulin-independent glucose influx into hepatocytes by facilitated diffusion. In the cytoplasm of hepatocytes, glucose is irreversibly converted into glucose-6-phosphate by glucokinase, an enzyme regulated by glucose and insulin. Then, glucose-6-phosphate is converted into glycogen by glycogen synthase, which is converted from the inactive form into the activated form by a phosphatase. This phosphatase plays a key role in regulating this step in the pathway: its concentration is maintained by insulin, and its activity depends on the presence of glucose (Figure 1). Therefore, the synthesis of hepatic glycogen is the consequence of the combination of high blood glucose levels (which promote the flow of glucose into hepatocytes) and hyperinsulinemia (which stimulates the conversion of glucose to glycogen)[2,5,11,12]. This situation is frequently observed in patients with unstable diabetes who are treated with insulin for marked or prolonged hyperglycemia.
The clinical presentation is not specific and can include abdominal pain that is sometimes associated with nausea, vomiting and anorexia[2,11,12]. The key clinical features are hepatomegaly and a mild to moderate increase in transaminases, although in some cases, the transaminase levels can be dramatically elevated[2,5]. Alkaline phosphatase levels can be elevated, and liver synthetic function is usually normal. Ascites has rarely been reported[2,11]. The clinical and pathological features are similar in adults and children.
Torbenson et al[2] reported 14 patients (range, 8 to 25 years old) with HG. All had T1D with poor glycemic control. The clinical presentations included hepatomegaly, abdominal pain and elevated transaminases. Ascites was present in 1 patient. In 3 cases, the transaminase levels were markedly elevated to 10 times greater than the upper limit of normal. All the biopsies revealed excessive glycogen accumulation. Another large cohort was reported that included 11 patients (8 adults between 19 and 70 years of age and 3 children) with poorly controlled insulin-dependent diabetes (T1D or T2D was not specified)[11]. Nine patients (6 adults and 3 children) had hepatomegaly as evidenced by physical examination or ultrasonography. Ascites was present in 1 patient, and serum transaminases were markedly elevated in 4 patients.
HG cannot be distinguished from NAFLD by history, physical examination or laboratory blood tests. In addition, ultrasound cannot distinguish fatty liver from glycogen accumulation[2,12]. The usefulness of abdominal computed tomography (CT) in distinguishing HG from NAFLD was reported by Sweetser et al[19]. A low density liver is usually observed by CT in patients with fatty liver, whereas the liver density by CT is typically increased in patients with HG. However, a low liver density by CT may be not observed in the early stages of NAFLD[28], and CT only provides qualitative information. Recently, it has been reported that gradient dual-echo magnetic resonance imaging (MRI) can effectively discriminate glycogen from fat in the liver[28,29]. A gradient dual-echo MRI sequence, as well as magnetic resonance spectroscopy, can quantify the intrahepatic lipid content and detect even small amounts of fat accumulation[30].
The differential diagnosis of HG, as opposed to NAFLD, must consider several other potential causes of liver damage, such as infection (e.g., viral hepatitis), metabolic disorders (e.g., α1-antitrypsin deficiency and Wilson’s disease), obstruction, autoimmune liver disease and celiac disease[12]. On the other hand, there is an increasing evidence that focal, but sometimes also diffuse, HG is a potential preneoplastic lesion[31-33]. Investigations in animal’s models of chemical, viral and hormonal hepatocarcinogenesis and some observations in humans suggest that focal HG, represents a critical early event in the pathogenesis of benign and malignant hepatocellular neoplasm[34,35]. Although the exact mechanism remains elusive, recent data suggest that oncogenic agents have an early insulin-like effect[35-37]. It is noteworthy that a number of epidemiology studies have shown that DM is a risk factor for the development of hepatocellular carcinoma[38,39]. However, no relationship has been described between diabetes-related HG and hepatocellular neoplasms, but further studies are warranted in order to clarify this point.
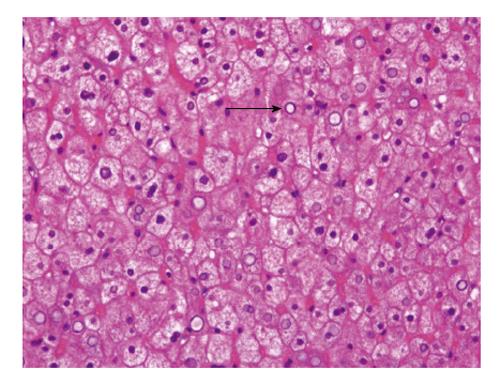
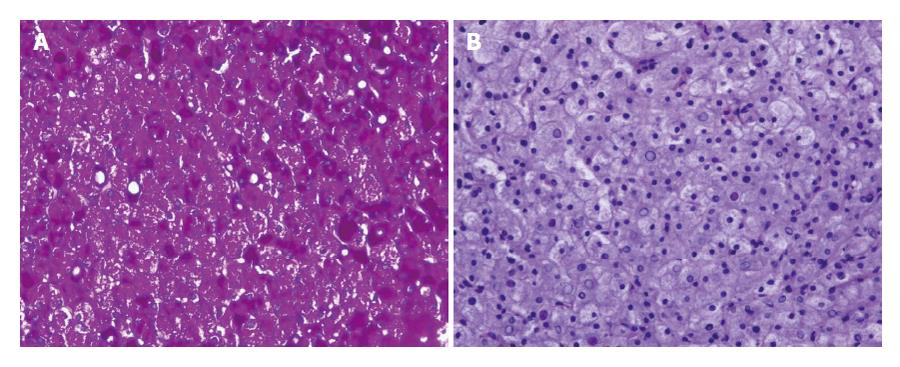
HG is only diagnosed by liver biopsy. In general, HG is characterized by several histological features: (1) marked glycogen accumulation. After conventional tissue preparation (fixation by formaldehyde-solution and staining with haematoxylin and eosin) the glycogen is usually removed from the hepatocytes. Thus, the hepatocytes are diffusely swollen with a pale cytoplasm and accentuation of the cell membranes, frequently with displacement of the nuclei to the cell periphery (Figure 2), the sinusoids are compressed by swollen hepatocytes, and glycogenated nuclei and giant mitochondria are present; glycogen accumulation within hepatocytes is demonstrated by periodic acid-Schiff staining (Figure 3A) which disappeared after digestion with diastase (Figure 3B); (2) no or a minimal change in fat content; (3) the absence of or minimal inflammation; (4) the absence or minimal presence of spotty lobular necrosis; and (5) intact liver architecture without or with minimal fibrosis[2,5,11].
In contrast to NAFLD, which can progress to cirrhosis, HG is potentially reversible with optimal diabetes control. The abnormal transaminase levels and hepatomegaly have been described to be reversible after improving glycemic control with insulin treatment, usually within 2 to 14 wk[11,12,16]. In a large series published by Chatila et al[11], hepatomegaly was resolved in all the patients within 2 wk of stabilizing the blood sugar levels. Aminotransferases rapidly decreased, but remained moderately elevated in some patients during the 14-wk follow-up. In 2011, our group published a case of HG in a 31-year-old woman with poorly controlled T1D. During admission for acute ketoacidosis, she presented with hepatomegaly and markedly elevated transaminases. Liver glycogen storage was diagnosed by biopsy. After optimal glycemic control, transaminase levels rapidly decreased, but the hepatomegaly remained after 6 mo[20]. In two previously published severe cases, pancreatic transplantation was reported to be effective[40].
HG most likely represents an underdiagnosed hepatic complication of diabetes that is difficult to distinguish from NAFLD. For this reason, a diagnosis of HG should be considered in diabetic patients, especially in those with T1D, who exhibit poor metabolic control and present with a transient elevation of liver transaminases and hepatomegaly. Although HG is definitively diagnosed histologically, a gradient dual-echo magnetic resonance imaging sequence combined with CT of the liver is a powerful methodology for distinguishing HG from NAFLD. The correct diagnosis of this disease is important given its potential resolution after improved glycemic control.
P- Reviewer: Bannasch P S- Editor: Gong XM L- Editor: A E- Editor: Liu SQ
| 1. | Krishnan B, Babu S, Walker J, Walker AB, Pappachan JM. Gastrointestinal complications of diabetes mellitus. World J Diabetes. 2013;4:51-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 120] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (2)] |
| 2. | Torbenson M, Chen YY, Brunt E, Cummings OW, Gottfried M, Jakate S, Liu YC, Yeh MM, Ferrell L. Glycogenic hepatopathy: an underrecognized hepatic complication of diabetes mellitus. Am J Surg Pathol. 2006;30:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Mauriac P. [Hepatomegaly, dwarfism, obesity and diabetes in children: Mauriac’s syndrome]. Vida Nueva. 1951;67:57-65. [PubMed] |
| 4. | Sayuk GS, Elwing JE, Lisker-Melman M. Hepatic glycogenosis: an underrecognized source of abnormal liver function tests? Dig Dis Sci. 2007;52:936-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Messeri S, Messerini L, Vizzutti F, Laffi G, Marra F. Glycogenic hepatopathy associated with type 1 diabetes mellitus as a cause of recurrent liver damage. Ann Hepatol. 2012;11:554-558. [PubMed] |
| 6. | Evans RW, Littler TR, Pemberton HS. Glycogen storage in the liver in diabetes mellitus. J Clin Pathol. 1955;8:110-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Manderson WG, McKiddie MT, Manners DJ, Stark JR. Liver glycogen accumulation in unstable diabetes. Diabetes. 1968;17:13-16. [PubMed] |
| 8. | Ruschhaupt DG, Rennert OM. Recurrent hepatomegaly and transient alteration of liver functions in an adolescent with diabetic acidosis. Clin Pediatr (Phila). 1970;9:122-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Knight AH, Williams DN, Spooner RJ, Goldberg DM. Serum enzyme changes in diabetic ketoacidosis. Diabetes. 1974;23:126-131. [PubMed] |
| 10. | Nakamuta M, Ohashi M, Goto K, Tanabe Y, Hiroshige K, Nawata H. Diabetes mellitus-associated glycogen storage hepatomegaly: report of a case and review of the Japanese literature. Fukuoka Igaku Zasshi. 1993;84:354-358. [PubMed] |
| 11. | Chatila R, West AB. Hepatomegaly and abnormal liver tests due to glycogenosis in adults with diabetes. Medicine (Baltimore). 1996;75:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 126] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 12. | Munns CF, McCrossin RB, Thomsett MJ, Batch J. Hepatic glycogenosis: reversible hepatomegaly in type 1 diabetes. J Paediatr Child Health. 2000;36:449-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Torres M, López D. Liver glycogen storage associated with uncontrolled type 1 diabetes mellitus. J Hepatol. 2001;35:538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Flotats Bastardas M, Miserachs Barba M, Ricart Cumeras A, Clemente León M, Gussinyer Canadell M, Yeste Fernández D, Albisu Aparicio MA, Carrascosa Lezcano A. [Hepatomegaly due to glycogen storage disease and type 1 diabetes mellitus]. An Pediatr (Barc). 2007;67:157-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Cuthbertson DJ, Brennan G, Walsh S, Henry E. Hepatic glycogenosis: abnormal liver function tests in Type 1 diabetes. Diabet Med. 2007;24:322-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Abaci A, Bekem O, Unuvar T, Ozer E, Bober E, Arslan N, Ozturk Y, Buyukgebiz A. Hepatic glycogenosis: a rare cause of hepatomegaly in Type 1 diabetes mellitus. J Diabetes Complications. 2008;22:325-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Hudacko RM, Manoukian AV, Schneider SH, Fyfe B. Clinical resolution of glycogenic hepatopathy following improved glycemic control. J Diabetes Complications. 2008;22:329-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | van den Brand M, Elving LD, Drenth JP, van Krieken JH. Glycogenic hepatopathy: a rare cause of elevated serum transaminases in diabetes mellitus. Neth J Med. 2009;67:394-396. [PubMed] |
| 19. | Sweetser S, Kraichely RE. The bright liver of glycogenic hepatopathy. Hepatology. 2010;51:711-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Julián MT, Olaizola I, Riu F, López R. [Hepatic glycogenosis in a patient with type 1 diabetes mellitus]. Rev Clin Esp. 2011;211:65-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Saadi T. Glycogenic hepatopathy: a rare disease that can appear and resolve rapidly in parallel with glycemic control. Isr Med Assoc J. 2012;14:269-270. [PubMed] |
| 22. | Imtiaz KE, Healy C, Sharif S, Drake I, Awan F, Riley J, Karlson F. Glycogenic hepatopathy in type 1 diabetes: an underrecognized condition. Diabetes Care. 2013;36:e6-e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Cha JH, Ra SH, Park YM, Ji YK, Lee JH, Park SY, Baik SK, Kwon SO, Cho MY, Kim MY. Three cases of glycogenic hepatopathy mimicking acute and relapsing hepatitis in type I diabetes mellitus. Clin Mol Hepatol. 2013;19:421-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Rubio-Rivas M, Montero-Alía P, Ordi-Ros J, Labrador M. [Hepatic glycogenosis and diabetes mellitus]. Med Clin (Barc). 2005;125:279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Tsujimoto T, Takano M, Nishiofuku M, Yoshiji H, Matsumura Y, Kuriyama S, Uemura M, Okamoto S, Fukui H. Rapid onset of glycogen storage hepatomegaly in a type-2 diabetic patient after a massive dose of long-acting insulin and large doses of glucose. Intern Med. 2006;45:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Iancu TC, Shiloh H, Dembo L. Hepatomegaly following short-term high-dose steroid therapy. J Pediatr Gastroenterol Nutr. 1986;5:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Resnick JM, Zador I, Fish DL. Dumping syndrome, a cause of acquired glycogenic hepatopathy. Pediatr Dev Pathol. 2011;14:318-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Murata F, Horie I, Ando T, Isomoto E, Hayashi H, Akazawa S, Ueki I, Nakamura K, Kobayashi M, Kuwahara H. A case of glycogenic hepatopathy developed in a patient with new-onset fulminant type 1 diabetes: the role of image modalities in diagnosing hepatic glycogen deposition including gradient-dual-echo MRI. Endocr J. 2012;59:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Saikusa M, Yatsuga S, Tonan T, Koga Y. Glycogenic hepatopathy and non-alcoholic fatty liver disease in type 1 diabetes patients. Pediatr Int. 2013;55:806-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Springer F, Machann J, Claussen CD, Schick F, Schwenzer NF. Liver fat content determined by magnetic resonance imaging and spectroscopy. World J Gastroenterol. 2010;16:1560-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 88] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 31. | Bannasch P, Mayer D, Hacker HJ. Hepatocellular glycogenosis and hepatocarcinogenesis. Biochim Biophys Acta. 1980;605:217-245. [PubMed] |
| 32. | Bannasch P, Hacker HJ, Klimek F, Mayer D. Hepatocellular glycogenosis and related pattern of enzymatic changes during hepatocarcinogenesis. Adv Enzyme Regul. 1984;22:97-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 81] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Terasaki S, Kaneko S, Kobayashi K, Nonomura A, Nakanuma Y. Histological features predicting malignant transformation of nonmalignant hepatocellular nodules: a prospective study. Gastroenterology. 1998;115:1216-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Bannasch P. Pathogenesis of hepatocellular carcinoma: sequential cellular, molecular, and metabolic changes. Prog Liver Dis. 1996;14:161-197. [PubMed] |
| 35. | Bannasch P. Glycogenotic hepatocellular carcinoma with glycogen-ground-glass hepatocytes: a heuristically highly relevant phenotype. World J Gastroenterol. 2012;18:6701-6708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Nehrbass D, Klimek F, Bannasch P. Overexpression of insulin receptor substrate-1 emerges early in hepatocarcinogenesis and elicits preneoplastic hepatic glycogenosis. Am J Pathol. 1998;152:341-345. [PubMed] |
| 37. | Aleem E, Nehrbass D, Klimek F, Mayer D, Bannasch P. Upregulation of the insulin receptor and type I insulin-like growth factor receptor are early events in hepatocarcinogenesis. Toxicol Pathol. 2011;39:524-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 38. | Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 514] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 39. | Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 765] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 40. | Fridell JA, Saxena R, Chalasani NP, Goggins WC, Powelson JA, Cummings OW. Complete reversal of glycogen hepatopathy with pancreas transplantation: two cases. Transplantation. 2007;83:84-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |