Published online Feb 15, 2015. doi: 10.4239/wjd.v6.i1.109
Peer-review started: July 24, 2014
First decision: August 15, 2014
Revised: August 17, 2014
Accepted: November 27, 2014
Article in press: December 1, 2014
Published online: February 15, 2015
Processing time: 191 Days and 17.5 Hours
Type 2 diabetes (T2DM) is characterized by insulin resistance and β-cell dysfunction. Although, in contrast to type 1 diabetes, insulin resistance is assumed to be a major pathophysiological feature of T2DM, T2DM never develops unless β-cells fail to compensate insulin resistance. Recent studies have revealed that a deficit of β-cell functional mass is an essential component of the pathophysiology of T2DM, implying that β-cell deficit is a common feature of both type 1 and type 2 diabetes. β-cell dysfunction is present at the diagnosis of T2DM and progressively worsens with disease duration. β-cell dysfunction is associated with worsening of glycemic control and treatment failure; thus, it is important to preserve or recover β-cell functional mass in the management of T2DM. Since β-cell regenerative capacity appears somewhat limited in humans, reducing β-cell workload appears to be the most effective way to preserve β-cell functional mass to date, underpinning the importance of lifestyle modification and weight loss for the treatment and prevention of T2DM. This review summarizes the current knowledge on β-cell functional mass in T2DM and discusses the treatment strategy for T2DM.
Core tip: Recent studies have revealed that a deficit of β-cell functional mass is an essential component of the pathophysiology of type 2 diabetes (T2DM). β-cell dysfunction is present at the diagnosis of T2DM and progressively worsens with disease duration. β-cell dysfunction is associated with worsening of glycemic control and treatment failure; thus, it is important to preserve or recover β-cell functional mass in the management of T2DM. This review summarizes the current knowledge on β-cell functional mass in T2DM and discusses the treatment strategy for T2DM.
- Citation: Saisho Y. β-cell dysfunction: Its critical role in prevention and management of type 2 diabetes. World J Diabetes 2015; 6(1): 109-124
- URL: https://www.wjgnet.com/1948-9358/full/v6/i1/109.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i1.109
The number of patients with diabetes is countinuously increasing all over the world. Worldwide, there were 382 million patients with diabetes in 2013, which will rise to 592 million in 2035[1]. Diabetes is associated not only with diabetic microangiopathy such as retinopathy, nephropathy and neuropathy, but also with a 2- to 4-fold increase in risk of cardiovascular disease[2,3]. Among the people with diabetes, more than 90% have type 2 diabetes (T2DM). Therefore, optimal treatment and prevention strategies for T2DM are urgently needed.
T2DM is characterized by insulin resistance and β-cell dysfunction. Recent evidence suggests an important role of β-cell function in the development and management of T2DM. In this review, the current knowledge regarding β-cell dysfunction in T2DM is summarized and its critical role in the prevention and treatment of T2DM is discussed.
Disposition index: A true assessment ofβ-cell function
T2DM is characterized by insulin resistance and β-cell dysfunction[4,5]. However, since the development of an insulin radioimmunoassay, it was found that in people with T2DM, plasma insulin concentration is rather higher than that in those with normal glucose tolerance (NGT), indicating that insulin resistance rather than insulin deficiency is central in the pathogenesis of T2DM. Therefore, in contrast to type 1 diabetes, obesity, hyperinsulinemia and insulin resistance are often emphasized as characteristics of T2DM, and β-cell function in T2DM is often less emphasized or even ignored.
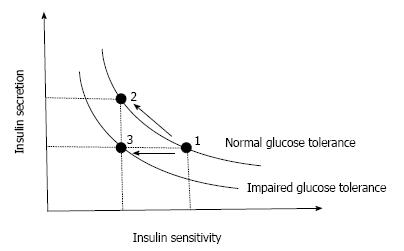
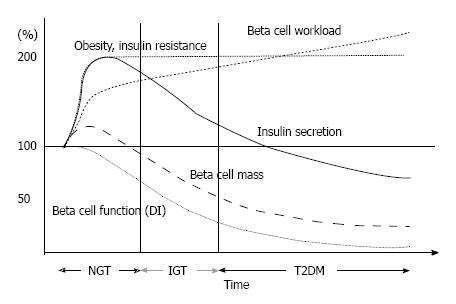
However, the higher plasma insulin concentration in patients with T2DM is often confounded by a higher plasma glucose level, which itself stimulates insulin secretion. Moreover, insulin sensitivity also affects insulin secretion. In normal physiological conditions, normoglycemia is maintained under a balance between insulin sensitivity and insulin secretion, and when insulin sensitivity decreases, insulin secretion increases to maintain normoglycemia. Thus, insulin secretion should always be assessed in relation to insulin sensitivity. Bergman and Cobelli have found that this relationship between insulin secretion and insulin sensitivity is expressed as a hyperbolic curve, and as a result the product of insulin sensitivity and insulin secretion is constant as long as normoglycemia is maintained[6,7] (Figure 1). The product of insulin sensitivity and insulin secretion, called the disposition index, refers to insulin secretion adjusted by insulin sensitivity and reflects true β-cell function in vivo.
Once insulin secretion is not able to sufficiently increase to compensate the decrease in insulin sensitivity, the insulin sensitivity-insulin secretion relationship is shifted to the left and abnormal glucose tolerance develops (Figure 1). In this case, the disposition index is decreased, indicating that abnormal glucose tolerance develops only when β-cells are no longer able to compensate decreased insulin sensitivity.
β-cell function in T2DM
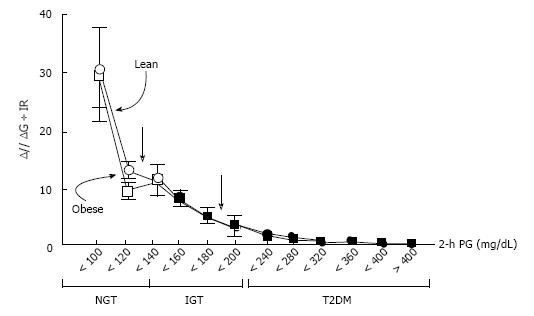
When β-cell function is assessed using the disposition index, a number of studies have consistently shown that β-cell function is diminished in people with T2DM[4,8,9]. Using the disposition index, DeFronzo et al[9] have shown that β-cell function is decreased by -80% in patients with impaired glucose tolerance (IGT) and is even less in patients with T2DM (Figure 2). Importantly, β-cell function starts to decline with higher plasma glucose levels, even within the range of normal plasma glucose levels[9], which suggests that β-cell function is already impaired prior to the development of IGT.
β-cell mass in T2DM
If β-cell function is impaired in patients with T2DM, what about the β-cell mass? β-cells are located in the islets of Langerhans, which are scattered within the exocrine pancreas. Each islet contains -1000 β-cells together with other endocrine cells such as alpha cells, delta cells, pancreatic polypeptide (PP) cells and epsilon cells, and a total of -1 million islets exist in the pancreas. β-cell mass refers to the total mass of β-cells and is approximately 1 g in humans.
Due to the anatomical characteristics of β-cells scattered throughout the whole pancreas, it is difficult to visualize β-cells in vivo, and direct measurement of β-cell mass in vivo in humans remains to be established[10,11]. Thus, to date, the measurement of β-cell mass inevitably relies on histological analysis of the pancreas obtained surgically or at autopsy.
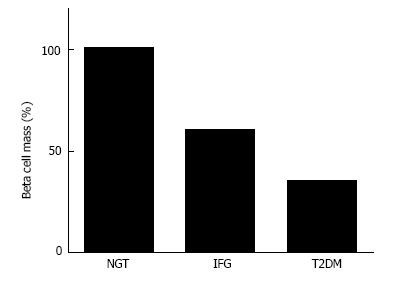
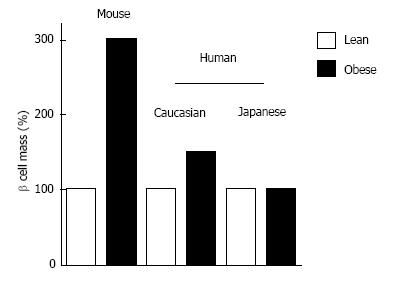
Since insulin resistance and hyperinsulinemia are often emphasized in people with T2DM, β-cell mass in people with T2DM is also often assumed to be increased or at least not decreased. However, based on histological analysis, Butler et al[12] have reported that β-cell mass is decreased by -40% and -65% in lean and obese people with T2DM, respectively, compared with non-diabetic controls matched for age and BMI. Other groups have also reported a significant (-30%-40%) decrease in β-cell mass in patients with T2DM[13-15]. These findings suggest that deficit of β-cell mass is a common pathophysiological feature of type 1 and T2DM (Figure 3), while the cause and degree of the deficit are different between type 1 and T2DM.
Mechanisms ofβ-cell deficit in T2DM
β-cell mass is regulated by the balance of newly formed β-cells and β-cell loss[16-18]. Butler et al[12] have shown that β-cell apoptosis is increased in patients with T2DM, whereas neither β-cell replication nor neogenesis is decreased, suggesting that increased β-cell loss is the main cause of reduced β-cell mass in T2DM. Various mechanisms that induce β-cell apoptosis have been proposed such as hyperglycemia (glucotoxicity)[19], fatty acids (lipotoxicity)[20], amyloid or islet amyloid polypeptide (IAPP, also called amylin)[21-24], oxidative stress[25], inflammatory cytokines[26], mitochondrial dysfunction[27], endoplasmic reticulum (ER) stress[28,29] and dysfunction of autophagy[30]. A recent study suggested that several mechanisms are simultaneously associated with β-cell failure in humans with T2DM[31].
Recently, transdifferentiation of β-cells to alpha cells has been suggested as a mechanism of β-cell loss in a rodent model of diabetes[32]. This mechanism could explain β-cell loss and the reciprocal increase in alpha cell mass observed in humans with T2DM[15,31], although whether an increase in alpha cell mass occurs in humans with T2DM remains controversial[33,34].
Association betweenβ-cell mass and glucose metabolism
The deficit of β-cell mass in patients with T2DM raises the next question of whether the change in β-cell mass is associated with the severity of glucose intolerance. It has been reported that there is a reciprocal relationship between β-cell mass and fasting plasma glucose level[35], suggesting that glucose intolerance develops when the β-cell mass decreases by -50% of the normal level. A similar relationship has been observed in rodents[36], pigs[37] and monkeys[38]. An increased risk of the development of IGT or diabetes after hemipancreatectomy has been reported in dogs and humans[39-43]. It has also been reported that β-cell mass was decreased by -20%-40% in patients with IGT and impaired fasting glycemia (IFG)[12,44]. We have also reported that there was a significant negative correlation between β-cell mass and glycated hemoglobin (HbA1c) level in non-diabetic individuals[45], suggesting that β-cell mass is related to glucose intolerance even prior to the development of T2DM. A significant correlation between β-cell mass and HbA1c was also observed in patients with T2DM[31].
Association betweenβ-cell mass andβ-cell function
The relationship between β-cell mass and β-cell function is more complicated. Whether β-cell dysfunction in T2DM is mainly due to a functional defect of each β-cell or due to a defect of β-cell mass has been extensively argued[46]. A close correlation between β-cell function assessed by maximum acute insulin response (AIRmax) induced by arginine infusion under a hyperglycemic state and β-cell mass of transplanted islets has been reported[47]. On the other hand, β-cell dysfunction was markedly improved after an overnight β-cell rest by somatostatin infusion[48]. Thus, it remains uncertain whether β-cell function in vivo sufficiently reflects β-cell mass in patients with T2DM.
Meier et al[49] assessed the relationship between β-cell mass and β-cell function in patients who had undergone pancreatic surgery and found that there was a significant positive correlation between β-cell mass and β-cell function, especially postprandial C-peptide level, suggesting that C-peptide measurement in clinical settings reflects β-cell mass.
Taken together, these results indicate that β-cell function and β-cell mass seem to be correlated with each other, although on some occasions they can be dissociated, and both β-cell function and mass seem to decrease during the development of glucose intolerance. Since β-cell function and mass are difficult to separate, currently they are referred to as “β-cell functional mass”, and it is now certain that β-cell functional mass decreases during the development of T2DM.
Progressive decline inβ-cell functional mass in T2DM
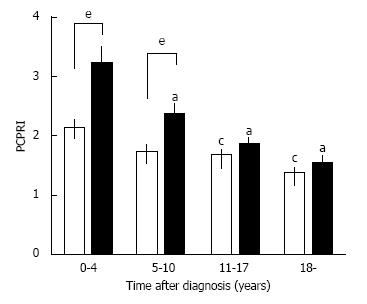
A deficit of β-cell functional mass is not only present in patients with T2DM, but it also progressively declines with disease duration. In the UK Prospective Diabetes Study (UKPDS), β-cell function assessed by homeostasis model assessment (HOMA) in patients with T2DM was already decreased by -50% at the time of diagnosis and progressively declined by -5% annually[50]. This also indicates that in patients with T2DM, β-cell function starts to decline -10 years prior to the onset of the disease. A gradual but significant decline in β-cell function assessed by C-peptide level has been confirmed in cross-sectional cohort studies of Japanese patients with T2DM[51,52] (Figure 4). Intriguingly, a significant negative correlation between β-cell mass and duration of T2DM has also been reported[13].
Limitedβ-cell regenerative capacity in humans
Since deficits of β-cell function and mass are now recognized as hallmarks of T2DM as well as type 1 diabetes, β-cell regeneration is considered to be an important therapeutic strategy for both types of diabetes.
Rodent studies show an adaptive change in β-cell mass in response to obesity or pregnancy[53-58], suggesting the presence of endogenous β-cell regenerative capacity in the postnatal period. However, recent observation of the human pancreas suggests that endogenous β-cell regenerative capacity is limited in humans.
We have reported that β-cell mass in obese non-diabetic individuals is -1.2 g compared with -0.8 g in lean non-diabetic individuals, an -50% increase[59], whereas β-cell mass increases 3- to 10-fold in response to obesity or insulin resistance in rodents[53,54] (Figure 5). This striking difference in β-cell regenerative capacity between humans and rodents suggests that the results of rodent studies are not necessarily applicable to humans[60,61].
In humans, β-cell mass increases from -37 mg to -1 g in the first five years of life, and during this period replicating β-cells are often observed[62,63]. However, after that, replicating β-cells are rarely seen and β-cell mass reaches a plateau. The β-cell mass then remains constant during adulthood[13,59,64], suggesting that β-cell turnover is limited in humans after the first five years of life. Estimation of β-cell life using either 14C measurement or cellular lipofuscin body content also suggests very slow turnover of β-cells in adult humans[65,66]. Recent studies have suggested that there is an increase in β-cell neogenesis in humans with obesity, pregnancy and IGT[67-70]; however, the extent of its contribution to β-cell mass remains unclear. Limited β-cell regenerative capacity has also been observed in monkeys[71,72]. Even in rodents, β-cell regenerative capacity significantly decreases with aging[54,73,74].
A hypothetical schema of the change in β-cell fun-ctional mass during the development of T2DM is shown in Figure 6. The magnitude of the increased demand for insulin due to insulin resistance caused by excess caloric intake and physical inactivity exceeds the magnitude of β-cell mass expansion, resulting in an increase in β-cell workload. In individuals who are susceptible to T2DM, increased β-cell workload may lead to β-cell failure and the development of T2DM. In addition, once hyperglycemia develops, it also causes β-cell dysfunction and apoptosis, which further exacerbate β-cell failure. Importantly, because insulin resistance persists, the β-cell workload continues to increase, with a reduction in β-cell mass. As a result, glucose metabolism progressively deteriorates in patients with T2DM. In our retrospective cohort, the progressive decline in β-cell function seemed to be exaggerated in the presence of obesity in Japanese patients with T2DM[51] (Figure 7). Another Japanese cohort showed a decreasing trend in fasting insulin level despite an increasing trend in BMI at the first clinic/hospital visit of patients with T2DM during the past ten years[75]. Recent studies have shown that even metabolically healthy obese individuals are at increased risk of future development of diabetes, cardiovascular events and all-cause mortality[76-78]. Thus, weight loss itself may be important to preserve β-cell function and improve clinical outcomes.
β-cell functional mass in Asian population
T2DM is characterized by obesity, but the degree of obesity differs between ethnic groups[79,80]. In Caucasians, most patients with T2DM are obese, and the mean BMI of patients with T2DM is -30 kg/m2. In contrast, the mean BMI of Asian patients with T2DM is -23 kg/m2, suggesting that about half of patients with T2DM are not even overweight (i.e., BMI ≥ 25 kg/m2, the definition of obesity in Asian countries).
The difference in adiposity between Caucasians and Asians has been postulated to explain this ethnic difference. Visceral adiposity is more apparent in Asians compared to Caucasians with the same BMI[81,82], indicating that Asians have a lower capacity for subcutaneous fat deposition and are more vulnerable to visceral fat accumulation compared with Caucasians. Nonetheless, a meta-analysis of studies examining the insulin sensitivity-insulin secretion relationship in individuals with NGT clearly showed that Asians have less insulin secretion with higher insulin sensitivity compared with Caucasians[83]. Direct comparison of insulin sensitivity and insulin secretion between Japanese and Caucasians showed that most of the difference in insulin secretion between the two ethnicities can be explained by the difference in BMI between the two[84]. Since the incidence of T2DM is comparable between the two ethnicities despite the different degree of obesity[1], it is plausible that the lower degree of obesity in Asians could be attributable to the lower β-cell functional capacity in this population.
We have recently examined the change in β-cell mass in Japanese obese nondiabetic individuals (mean BMI 20.4 kg/m2) compared to age- and sex-matched lean individuals (mean BMI 28.5 kg/m2)[85]. As a result, in contrast to the studies in Caucasians showing a significant increase in β-cell mass with obesity[13,59], there was no significant increase in β-cell mass in Japanese obese individuals (Figure 5). Another Japanese study also confirmed our findings[86]. These studies suggest that Asians have less β-cell regenerative capacity compared with Caucasians, which is probably derived from both genetic and environmental factors, and the lower β-cell functional capacity in Asians may contribute the different phenotype of T2DM between the two ethnicities. Because of the limited capacity of β-cell regeneration in Asians, excess β-cell workload could be induced in individuals with less obesity compared with Caucasians, which may lead to β-cell failure and the development of T2DM.
β-cell function and glycemic control
If a deficit of β-cell functional mass is a hallmark of T2DM, what is the clinical consequence? In UKPDS and A Diabetes Outcome Progression Trial (ADOPT), treatment failure was associated with a progressive decline in β-cell function[50,87,88]. In the Treatment Options for T2DM Adolescents and Youth (TODAY) study, similar results were observed in adolescents with T2DM, and in this study baseline β-cell function was associated with treatment efficacy[89]. In our retrospective cohort analysis, we found that a lower baseline C-peptide level was associated with poorer glycemic control and the need for insulin therapy thereafter[90-93] (Figure 8). In these studies, postprandial C-peptide index [i.e., postprandial serum C-peptide (ng/mL)/plasma glucose (mg/dL) × 100] was the best predictor of future insulin therapy among other C-peptide indices such as fasting C-peptide index and urinary C-peptide level. Since it was also significantly correlated with β-cell mass[49], postprandial C-peptide index may be a useful marker of β-cell function in clinical settings.
Thus, poorer β-cell function is associated with poorer glycemic control and treatment failure, indicating the important role of β-cell function in the treatment of T2DM.
β-cell function and glycemic variability
Furthermore, β-cell function is associated with glycemic variability. In patients with T1DM, it has been reported that lower β-cell functional capacity is associated with greater glycemic variability[94-96].
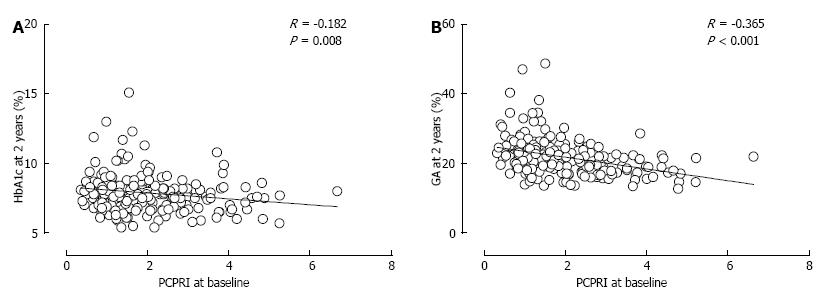
We and others have reported that serum and urinary C-peptide levels are negatively correlated with glycated albumin (GA) to HbA1c ratio in patients with T2DM[97-99] (Figure 9). Since albumin is more susceptable to glycation than is hemoglobin[100,101], GA more sensitively reflects glycemic variability than does HbA1c[97,102,103]. Thus, the inverse association between C-peptide level and GA to HbA1c ratio in patients with T2DM indicates that β-cell dysfunction is associated with greater glycemic variability in not only patients with type 1 diabetes but also those with T2DM.
Notably, we found that the relationship between postprandial C-peptide index and GA to HbA1c ratio in patients with T2DM was comparable to that in those with type 1 diabetes[97] (Figure 9B). This suggests that the impact of β-cell dysfunction on glycemic variability is irrespective of the type of diabetes, again indicating the central role of β-cell function in the pathogenesis of diabetes.
Recently, it has been reported that greater glycemic variability as well as poorer glycemic control is asso-ciated with the development of micro- and macro-angiopathy[104-107]. Thus, it should be stressed that greater glycemic variability and poorer glycemic control due to β-cell dysfunction may result in increased risk of diabetic complications.
Since β-cell dysfunction is associated with poor glycemic control in patients with T2DM, preservation and recovery of β-cell functional mass is an important therapeutic strategy for T2DM. Moreover, the current issues in the treatment of T2DM summarized in Table 1 are, to put it simply, all associated with either excess or insufficiency of insulin supplementation[108]. Thus, the recovery of physiological insulin secretion in patients with T2DM is also a key to resolving these issues.
| Issue | Cause |
| Hypoglycemia | Excess insulin |
| Weight gain | Excess insulin |
| Concern of increased risk of malignancy and/or atherosclerosis | Excess insulin, especially peripheral hyperinsulinemia |
| Postprandial hyperglycemia | Insufficient insulin in postprandial state, especially in portal vein |
To preserve or recover β-cell function, a reduction in excess β-cell workload appears to be the most effective strategy to date. In ADOPT, better glycemic control was obtained with metfomin or rosiglitazone monotherapy compared with glyburide in patients with T2DM[87]. Thus, therapy should be focused on improving insulin sensitivity to reduce β-cell workload.
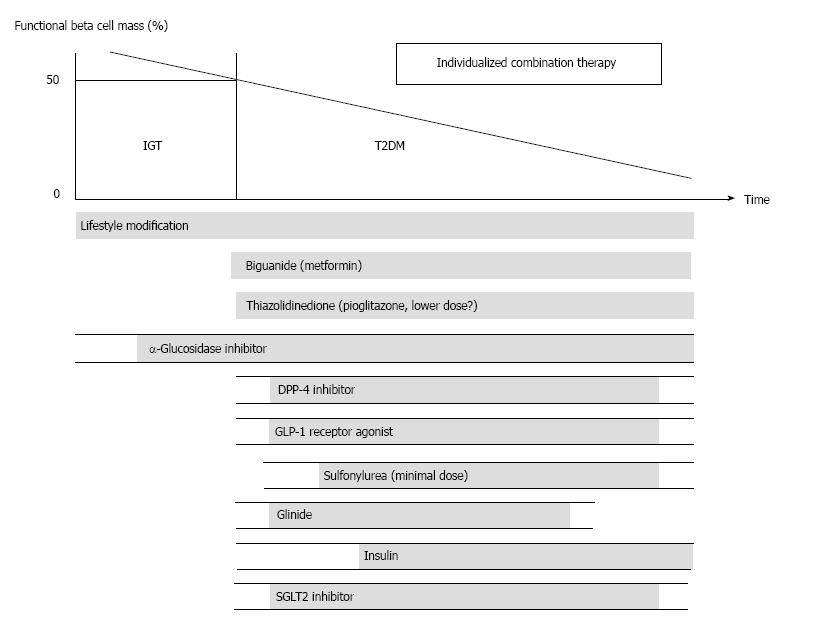
A proposed treatment strategy for T2DM is shown in Figure 10, as also described previously[108,109]. It is emphasized that, to reduce β-cell workload, lifestyle modification and weight reduction remain the most important therapy at any stage of T2DM. Although lifestyle modification failed to reduce the incidence of cardiovascular disease in the Action for Health in Diabetes (Look AHEAD) trial[110], it has been reported that lifestyle modification improved cardiovascular risk factors, reduced the need for and cost of medication, reduced the rate of sleep apnea and urinary incontinence, improved well-being and depression symptoms, and increased the rate of diabetes remission[111-116]. In a cohort analysis of the ADDITION-Cambridge study, it has been reported that healthy behavioral changes after the diagnosis of T2DM were associated with a significant reduction in risk of cardiovascular events[117], suggesting that early lifestyle intervention may be important to improve cardiovascular outcome.
Metformin is currently positioned as first-line therapy in most guidelines for the treatment of T2DM[118,119]. Since metformin is effective in lean patients as well as obese patients with T2DM[120,121], it should be used in both lean and obese individuals unless contraindicated. Its efficacy in reducing HbA1c (by -1.5%), low risk of hypoglycemia, favorable effect on body weight and low cost also support metformin as a first-line drug.
Thiazolidinediones (TZDs) have also been shown to reduce β-cell workload and maintain glycemic control in the long term[87,88]. Rosiglitazone has been shown to increase low-density lipoprotein (LDL) cholesterol and the risk of coronary heart disease in patients with T2DM[122], and its use has been suspended or strictly restricted in Europe and United States[123,124], although recently the US Food and Drug Administration has lifted most of its restrictions[125]. On the other hand, pioglitazone has been shown to suppress the progression of atherosclerosis and reduce the risk of cardiovascular disease[126-129]. However, TZDs often induce weight gain and edema due to fluid retention, and are contraindicated in patients with heart failure[118]. Recent studies have also shown an increase in risk of bone fracture in women[130] and risk of bladder cancer[131-133] in patients treated with pioglitazone. The risk of bladder cancer may be dose dependent. In addition, since low-dose pioglitazone also reduces the risk of weight gain and edema, it may be preferable to use pioglitazone at lower doses, especially in women. Pioglitazone should also be used with caution in postmenopausal women with osteoporosis because of the increased fracture risk.
α-glucosidase inhibitors (AGIs) delay the absorption of carbohydrate from the small intestine, and thereby reduce postprandial hyperglycemia, resulting in reduced β-cell workload in a postprandial state. AGIs have also been reported to reduce the progression to T2DM in patients with IGT[134,135]. Improving postprandial hyperglycemia by AGIs may also improve the cardio-vascular outcome[136-138]. Therefore, although the redu-ction in HbA1c by AGIs is relatively small (-0.5%), their use is also considered in patients with T2DM, especially those with postprandial hyperglycemia. The major side effect of AGIs is gastrointestinal disturbance such as flatulence, diarrhea and abdominal pain. In Japan, AGIs are the only medication indicated for patients with IGT. Thus, AGIs are also considered for the treatment of T2DM at the early stage of the disease, if tolerated.
On the other hand, the use of insulin secretagogues, which increase β-cell workload, may be somewhat limited. Sulfonylureas (SUs), while remaining among the most highly prescribed drugs for the treatment of T2DM, increase the risk of hypoglycemia and weight gain, resulting in a high rate of treatment failure[87]. These issues of SUs may be derived from their non-physiological augmentation of insulin secretion from β-cells.
Incretin drugs include dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 receptor agonists (GLP-1RAs). Both drug types reduce HbA1c mainly through an increase in insulin secretion, but also through suppression of glucagon secretion[139]. GLP-1RAs also slow gastric emptying and reduce appetite, resulting in weight loss. The most important characteristic of incretin drugs is probably that the enhancement of insulin secretion occurs in a glucose-dependent manner. Thus, the action of incretin drugs as insulin secretagogues is more physiological than that of SUs, thereby resulting in a low risk of hypoglycemia and weight gain with incretin therapy[140-142]. Whether this physiological enhancement of insulin secretion results in long-term maintenance of glycemic control remains to be elucidated. Although an increase in β-cell mass with incretin therapy has been reported in rodent studies[143,144], this effect has not been confirmed in humans[145-147]. Since incretin therapy is usually well tolerated without serious adverse effects, the use of incretin drugs is rapidly increasing[148].
Glinides, short-acting insulin secretagogues, enhance early-phase insulin secretion, thereby reducing post-prandial hyperglycemia[149]. Since a defect in early-phase insulin secretion is a hallmark of glucose intolerance[150], the enhancement of early-phase insulin secretion with-out prolonged hyperinsulinemia by glinides is more physiological, unlike the action of SUs, and is assumed to increase β-cell workload as well as the risk of hypo-glycemia to a lesser degree compared with SUs.
Thus, the use of insulin secretagogues may be limited because of an increase in β-cell workload as well as increased risk of hypoglycemia. Since incretin enhances insulin secretion in a more physiological manner and is also expected to improve β-cell function and/or mass, incretin drugs could be used at any stage of T2DM. On the other hand, SUs may be used rather to enhance incretin action at only a minimal dose. To recover physiological insulin secretion, a combination of an incretin drug and a glinide may also be useful.
Insulin has been shown to improve β-cell function in patients with IGT and T2DM[151-153]. Since initial intensive insulin therapy has been shown to preserve β-cell function thereafter[152], insulin therapy should be considered as early as possible in patients with T2DM. Insulin therapy is also the most effective medication to reduce HbA1c[118]. However, the increased risk of hypoglycemia, weight gain and non-physiological insulin delivery (i.e., systemic vs portal), in addition to the fear of injections, limit its use. Insulin therapy to overpower insulin resistance without eliminating excess calories may worsen ectopic lipid overload[154].
A sodium-glucose cotransporter 2 (SGLT2) inhibitor has recently been approved in several countries including United States, EU and Japan. SGLT2 inhibitors suppress reabsorption of glucose by SGLT2 in the proximal renal tubule and increase glucose excretion in urine (-60-80 g glucose/d)[155]. As a result, SGLT2 inhibitors not only decrease HbA1c, but also reduce body weight and blood pressure and improve the lipid profile. The action of SGLT2 inhibitors is independent of insulin. Thus, the efficacy of SGLT2 inhibitors seems to be regardless of β-cell function. SGLT2 inhibitors show a low risk of hypoglycemia but increase the incidence of bacterial urinary tract infections and fungal genital infections especially in women. A higher risk of hypotension has also been reported[156]. SGLT2 inhibitors may be suitable for obese patients with T2DM and metabolic syndrome; however, their longer term safety including cardiovascular and cancer risk and efficacy remain unknown[156,157].
Nonetheless, since currently no single therapy or agent can cure or even manage T2DM, an effective combination of current medications in addition to lifestyle modification aiming at reduction of β-cell workload is important to preserve or recover β-cell function.
Finally, marked weight reduction by bariatric surgery such as gastric bypass or sleeve gastrectomy has been reported to markedly improve glycemic control and even achieve remission of T2DM in severely obese T2DM patients[158,159]. This also suggests the importance of reducing β-cell workload, although change in incretin secretion has also been proposed as another mechanism by which glucose metabolism is improved after gastric bypass. On the other hand, it has been reported that gastric bypass markedly improved incretin’s effect on insulin secretion, but not insulin secretion induced by intravenous glucose infusion[160], suggesting limited recovery of β-cell function even with marked weight loss. Also, the remission of T2DM after bariatric surgery is associated with residual β-cell function[161,162], indicating the importance of residual β-cell function to manage and/or cure T2DM.
The progressive decline in β-cell functional capacity during the development of glucose intolerance also implies the important role of preservation or recovery of β-cell function to prevent T2DM.
Similarly to the treatment of T2DM, prevention strategies should focus on reducing β-cell workload or inducing β-cell rest. These include lifestyle modification and/or weight reduction, and use of metformin or TZD. Lifestyle modification, i.e., nutritional therapy and increase in physical activity, and weight reduction improve insulin sensitivity and thereby reduce β-cell workload. A number of studies have shown the efficacy of lifestyle intervention to prevent the development of T2DM in patients with IGT[163-166]. In the Diabetes Prevention Program (DPP), intensive lifestyle modification with more than 7% weight loss suppressed the progression to T2DM by -58% in patients with IGT[165]. In the same study, metformin therapy also reduced the progression to T2DM by -31%[165]. TZDs have also been shown to effectively suppress the progression from IGT to T2DM[167-169]. A significant reduction in the development of diabetes was also observed in patients with IGT treated with AGIs[134,135]. In the Outcome Reduction with Initial Glargine Intervention (ORIGIN) trial, adding basal insulin was also shown to suppress progression from IGT to T2DM[151], probably through inducing β-cell rest. On the other hand, in the Nateglinide And Valsartan in Impaired Glucose Tolerance Outcomes Research (NAVIGATOR) trial, nateglinide, a short-acting insulin secretagogue, failed to show a reduction in progression to T2DM in patients with IGT[170], suggesting that therapeutic strategies to increase β-cell workload may not be effective to prevent deterioration of glucose metabolism.
Although several anti-diabetic agents have been shown to effectively prevent the onset of T2DM, the importance of lifestyle modification remains unchanged, since the rapid increase in incidence of T2DM is certainly associated with the change in diet (i.e., westernization) and physical inactivity, resulting in increased incidence of obesity. In the NAVIGATOR trial, it has been reported that both baseline level and change in daily ambulatory activity were associated with a reduced risk of cardiovascular events in patients with IGT[171]. A six-year lifestyle intervention program for Chinese people with IGT showed a significant reduction in the incidence of cardiovascular and all-cause mortality as well as diabetes during 23 years of follow-up[172]. A combination of diet and exercise appears more beneficial than either alone in obese older adults[173]. Lifestyle modification may improve cardiovascular outcomes even after the onset of T2DM[117].
Nonetheless, it is difficult to continue lifestyle modifi-cation in most patients. Patients’ motivation is one of the most important factors in successful patient-centered management of T2DM[118]. Therefore, it is important to motivate and encourage them to improve their adherence to daily lifestyle modification. In this context, understanding the natural history of the development of T2DM and the importance of reducing β-cell workload to prevent or manage the disease may help to motivate or encourage patients to adhere to daily lifestyle changes.
Furthermore, as a whole society, not only patients with IGT or T2DM, but the healthy, general population should also be educated to motivate or encourage them to pursue a healthy lifestyle to prevent diseases associated with obesity and physical inactivity, resulting in improvement of quality of life (QOL). Changing our understanding of T2DM and a “modern” lifestyle may be needed to overcome this pandemic burden of T2DM all over the world.
This review summarizes the current knowledge of β-cell function and β-cell mass in T2DM. Recent evidence has emerged that a deficit of β-cell function along with β-cell mass is a hallmark of T2DM. Therefore, it is now acknowledged that a deficit of β-cell functional mass is a common characteristic of both type 1 and type 2 diabetes, indicating a core pathogenesis of diabetes. Genome-wide association studies have currently detected over 60 genetic loci associated with T2DM, most of which are assumed to relate to the β-cell, also indicating the imporance of β-cells in the pathogenesis of T2DM[174-178]. It is important to stress that diabetes never develops unless β-cells fail to compensate insulin resistance. In addition, β-cell function is related to treatment failure and glycemic control, suggesting its critical role in the management of T2DM. These findings suggest that recovery of β-cell functional mass is an important therapeutic strategy to manage or even cure T2DM. Although, unfortunately, currently no treatment strategy or medication to recover β-cell functional mass has been established, current evidence suggests that reducing β-cell workload is most effective to preserve β-cell functional mass. Thus, therapy or prevention of T2DM should focus on this point, and, therefore, lifestyle modification and weight loss remain the most important therapeutic strategy. Use of medication without lifestyle modification may even result in adverse outcomes. From the point of view of prevention, we need to tackle this pandemic burden of T2DM as a whole society, and correct understanding of the pathogenesis of T2DM may help motivate people to maintain a healthy lifestyle.
I apologize to the many authors of original research whose publications I could not cite owing to space restrictions.
P- Reviewer: Romani A, Traub M S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
| 1. | Aguiree F, Brown A, Cho NH, Dahlquist G, Dodd S, Dunning T, Hirst M, Hwang C, Magliano D, Patterson C. IDF Diabetes Atlas. IDF diabetes Atlas. 6th ed. Brussels, Belgium: International Diabetes Federation 2013; . |
| 2. | Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4846] [Cited by in RCA: 4474] [Article Influence: 165.7] [Reference Citation Analysis (1)] |
| 3. | Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829-841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2142] [Cited by in RCA: 2022] [Article Influence: 144.4] [Reference Citation Analysis (0)] |
| 4. | Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773-795. [PubMed] |
| 5. | Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383:1068-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1110] [Cited by in RCA: 1111] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 6. | Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68:1456-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1190] [Cited by in RCA: 933] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 7. | Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes. 2002;51 Suppl 1:S212-S220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 396] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 8. | Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE. Beta-cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U.S. Diabetes. 2002;51:2170-2178. [PubMed] |
| 9. | DeFronzo RA, Eldor R, Abdul-Ghani M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care. 2013;36 Suppl 2:S127-S138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 10. | Ichise M, Harris PE. Imaging of beta-cell mass and function. J Nucl Med. 2010;51:1001-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Tiedge M. Inside the pancreas: progress and challenges of human beta cell mass quantification. Diabetologia. 2014;57:856-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3031] [Cited by in RCA: 3041] [Article Influence: 138.2] [Reference Citation Analysis (0)] |
| 13. | Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10 Suppl 4:32-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 583] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 14. | Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia. 2002;45:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 15. | Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003;88:2300-2308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 488] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 16. | Butler PC, Meier JJ, Butler AE, Bhushan A. The replication of beta cells in normal physiology, in disease and for therapy. Nat Clin Pract Endocrinol Metab. 2007;3:758-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 17. | Manesso E, Toffolo GM, Saisho Y, Butler AE, Matveyenko AV, Cobelli C, Butler PC. Dynamics of beta-cell turnover: evidence for beta-cell turnover and regeneration from sources of beta-cells other than beta-cell replication in the HIP rat. Am J Physiol Endocrinol Metab. 2009;297:E323-E330. [PubMed] |
| 18. | Weir GC, Bonner-Weir S. Islet β cell mass in diabetes and how it relates to function, birth, and death. Ann N Y Acad Sci. 2013;1281:92-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 247] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 19. | Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 875] [Cited by in RCA: 807] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 20. | Kusminski CM, Shetty S, Orci L, Unger RH, Scherer PE. Diabetes and apoptosis: lipotoxicity. Apoptosis. 2009;14:1484-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 21. | Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev. 2008;29:303-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 470] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 22. | Costes S, Langen R, Gurlo T, Matveyenko AV, Butler PC. β-Cell failure in type 2 diabetes: a case of asking too much of too few? Diabetes. 2013;62:327-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 23. | Hull RL, Westermark GT, Westermark P, Kahn SE. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3629-3643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 428] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 24. | Jurgens CA, Toukatly MN, Fligner CL, Udayasankar J, Subramanian SL, Zraika S, Aston-Mourney K, Carr DB, Westermark P, Westermark GT. β-cell loss and β-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am J Pathol. 2011;178:2632-2640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 258] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 25. | Robertson RP. Antioxidant drugs for treating beta-cell oxidative stress in type 2 diabetes: glucose-centric versus insulin-centric therapy. Discov Med. 2010;9:132-137. [PubMed] |
| 26. | Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 262] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 27. | Supale S, Li N, Brun T, Maechler P. Mitochondrial dysfunction in pancreatic β cells. Trends Endocrinol Metab. 2012;23:477-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 28. | Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr Rev. 2008;29:317-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 443] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 29. | Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 892] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 30. | Masini M, Bugliani M, Lupi R, del Guerra S, Boggi U, Filipponi F, Marselli L, Masiello P, Marchetti P. Autophagy in human type 2 diabetes pancreatic beta cells. Diabetologia. 2009;52:1083-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 31. | Mizukami H, Takahashi K, Inaba W, Tsuboi K, Osonoi S, Yoshida T, Yagihashi S. Involvement of oxidative stress-induced DNA damage, endoplasmic reticulum stress, and autophagy deficits in the decline of β-cell mass in Japanese type 2 diabetic patients. Diabetes Care. 2014;37:1966-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 32. | Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell. 2012;150:1223-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 939] [Cited by in RCA: 1117] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 33. | Henquin JC, Rahier J. Pancreatic alpha cell mass in European subjects with type 2 diabetes. Diabetologia. 2011;54:1720-1725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 34. | Weir GC, Aguayo-Mazzucato C, Bonner-Weir S. β-cell dedifferentiation in diabetes is important, but what is it? Islets. 2013;5:233-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 35. | Ritzel RA, Butler AE, Rizza RA, Veldhuis JD, Butler PC. Relationship between beta-cell mass and fasting blood glucose concentration in humans. Diabetes Care. 2006;29:717-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 36. | Matveyenko AV, Butler PC. Beta-cell deficit due to increased apoptosis in the human islet amyloid polypeptide transgenic (HIP) rat recapitulates the metabolic defects present in type 2 diabetes. Diabetes. 2006;55:2106-2114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 37. | Kjems LL, Kirby BM, Welsh EM, Veldhuis JD, Straume M, McIntyre SS, Yang D, Lefèbvre P, Butler PC. Decrease in beta-cell mass leads to impaired pulsatile insulin secretion, reduced postprandial hepatic insulin clearance, and relative hyperglucagonemia in the minipig. Diabetes. 2001;50:2001-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Saisho Y, Butler AE, Manesso E, Galasso R, Zhang L, Gurlo T, Toffolo GM, Cobelli C, Kavanagh K, Wagner JD. Relationship between fractional pancreatic beta cell area and fasting plasma glucose concentration in monkeys. Diabetologia. 2010;53:111-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Matveyenko AV, Veldhuis JD, Butler PC. Mechanisms of impaired fasting glucose and glucose intolerance induced by an approximate 50% pancreatectomy. Diabetes. 2006;55:2347-2356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Kendall DM, Sutherland DE, Najarian JS, Goetz FC, Robertson RP. Effects of hemipancreatectomy on insulin secretion and glucose tolerance in healthy humans. N Engl J Med. 1990;322:898-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 156] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Robertson RP, Lanz KJ, Sutherland DE, Seaquist ER. Relationship between diabetes and obesity 9 to 18 years after hemipancreatectomy and transplantation in donors and recipients. Transplantation. 2002;73:736-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Kumar AF, Gruessner RW, Seaquist ER. Risk of glucose intolerance and diabetes in hemipancreatectomized donors selected for normal preoperative glucose metabolism. Diabetes Care. 2008;31:1639-1643. [PubMed] |
| 43. | Jin SM, Oh SH, Kim SK, Jung HS, Choi SH, Jang KT, Lee KT, Kim JH, Lee MS, Lee MK. Diabetes-free survival in patients who underwent islet autotransplantation after 50% to 60% distal partial pancreatectomy for benign pancreatic tumors. Transplantation. 2013;95:1396-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Meier JJ, Breuer TG, Bonadonna RC, Tannapfel A, Uhl W, Schmidt WE, Schrader H, Menge BA. Pancreatic diabetes manifests when beta cell area declines by approximately 65% in humans. Diabetologia. 2012;55:1346-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 45. | Kou K, Saisho Y, Sato S, Yamada T, Itoh H. Islet number rather than islet size is a major determinant of β- and α-cell mass in humans. J Clin Endocrinol Metab. 2014;99:1733-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Meier JJ, Bonadonna RC. Role of reduced β-cell mass versus impaired β-cell function in the pathogenesis of type 2 diabetes. Diabetes Care. 2013;36 Suppl 2:S113-S119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 47. | Robertson RP. Estimation of beta-cell mass by metabolic tests: necessary, but how sufficient? Diabetes. 2007;56:2420-2424. [PubMed] |
| 48. | Laedtke T, Kjems L, Pørksen N, Schmitz O, Veldhuis J, Kao PC, Butler PC. Overnight inhibition of insulin secretion restores pulsatility and proinsulin/insulin ratio in type 2 diabetes. Am J Physiol Endocrinol Metab. 2000;279:E520-E528. [PubMed] |
| 49. | Meier JJ, Menge BA, Breuer TG, Müller CA, Tannapfel A, Uhl W, Schmidt WE, Schrader H. Functional assessment of pancreatic beta-cell area in humans. Diabetes. 2009;58:1595-1603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 50. | [No authors listed]. U.K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes. 1995;44:1249-1258. [PubMed] |
| 51. | Saisho Y, Tanaka K, Abe T, Shimada A, Kawai T, Itoh H. Effect of obesity on declining beta cell function after diagnosis of type 2 diabetes: a possible link suggested by cross-sectional analysis. Endocr J. 2012;59:187-195. [PubMed] |
| 52. | Funakoshi S, Fujimoto S, Hamasaki A, Fujiwara H, Fujita Y, Ikeda K, Hamamoto Y, Hosokawa M, Seino Y, Inagaki N. Analysis of factors influencing pancreatic beta-cell function in Japanese patients with type 2 diabetes: association with body mass index and duration of diabetic exposure. Diabetes Res Clin Pract. 2008;82:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 53. | Brüning JC, Winnay J, Bonner-Weir S, Taylor SI, Accili D, Kahn CR. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell. 1997;88:561-572. [PubMed] |
| 54. | Tschen SI, Dhawan S, Gurlo T, Bhushan A. Age-dependent decline in beta-cell proliferation restricts the capacity of beta-cell regeneration in mice. Diabetes. 2009;58:1312-1320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 287] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 55. | Parsons JA, Brelje TC, Sorenson RL. Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology. 1992;130:1459-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 163] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 56. | Scaglia L, Smith FE, Bonner-Weir S. Apoptosis contributes to the involution of beta cell mass in the post partum rat pancreas. Endocrinology. 1995;136:5461-5468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 130] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 57. | Karnik SK, Chen H, McLean GW, Heit JJ, Gu X, Zhang AY, Fontaine M, Yen MH, Kim SK. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science. 2007;318:806-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 263] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 58. | Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, Mizukami H, Fujitani Y, Kawamori R, Miyatsuka T, Kosaka Y. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med. 2010;16:804-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 491] [Cited by in RCA: 449] [Article Influence: 29.9] [Reference Citation Analysis (1)] |
| 59. | Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC. β-cell mass and turnover in humans: effects of obesity and aging. Diabetes Care. 2013;36:111-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 309] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 60. | Jiao Y, Le Lay J, Yu M, Naji A, Kaestner KH. Elevated mouse hepatic betatrophin expression does not increase human β-cell replication in the transplant setting. Diabetes. 2014;63:1283-1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 61. | Stewart AF. Betatrophin versus bitter-trophin and the elephant in the room: time for a new normal in β-cell regeneration research. Diabetes. 2014;63:1198-1199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 62. | Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57:1584-1594. [PubMed] |
| 63. | Gregg BE, Moore PC, Demozay D, Hall BA, Li M, Husain A, Wright AJ, Atkinson MA, Rhodes CJ. Formation of a human β-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab. 2012;97:3197-3206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 263] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 64. | Menge BA, Tannapfel A, Belyaev O, Drescher R, Müller C, Uhl W, Schmidt WE, Meier JJ. Partial pancreatectomy in adult humans does not provoke beta-cell regeneration. Diabetes. 2008;57:142-149. [PubMed] |
| 65. | Perl S, Kushner JA, Buchholz BA, Meeker AK, Stein GM, Hsieh M, Kirby M, Pechhold S, Liu EH, Harlan DM. Significant human beta-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. J Clin Endocrinol Metab. 2010;95:E234-E239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 188] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 66. | Cnop M, Hughes SJ, Igoillo-Esteve M, Hoppa MB, Sayyed F, van de Laar L, Gunter JH, de Koning EJ, Walls GV, Gray DW. The long lifespan and low turnover of human islet beta cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia. 2010;53:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 67. | Mezza T, Muscogiuri G, Sorice GP, Clemente G, Hu J, Pontecorvi A, Holst JJ, Giaccari A, Kulkarni RN. Insulin resistance alters islet morphology in nondiabetic humans. Diabetes. 2014;63:994-1007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 68. | Butler AE, Cao-Minh L, Galasso R, Rizza RA, Corradin A, Cobelli C, Butler PC. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia. 2010;53:2167-2176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 328] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 69. | Yoneda S, Uno S, Iwahashi H, Fujita Y, Yoshikawa A, Kozawa J, Okita K, Takiuchi D, Eguchi H, Nagano H. Predominance of β-cell neogenesis rather than replication in humans with an impaired glucose tolerance and newly diagnosed diabetes. J Clin Endocrinol Metab. 2013;98:2053-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 70. | Gargani S, Thévenet J, Yuan JE, Lefebvre B, Delalleau N, Gmyr V, Hubert T, Duhamel A, Pattou F, Kerr-Conte J. Adaptive changes of human islets to an obesogenic environment in the mouse. Diabetologia. 2013;56:350-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 71. | Saisho Y, Manesso E, Butler AE, Galasso R, Kavanagh K, Flynn M, Zhang L, Clark P, Gurlo T, Toffolo GM. Ongoing beta-cell turnover in adult nonhuman primates is not adaptively increased in streptozotocin-induced diabetes. Diabetes. 2011;60:848-856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | Guardado-Mendoza R, Jimenez-Ceja L, Majluf-Cruz A, Kamath S, Fiorentino TV, Casiraghi F, Velazquez AO, DeFronzo RA, Dick E, Davalli A. Impact of obesity severity and duration on pancreatic β- and α-cell dynamics in normoglycemic non-human primates. Int J Obes (Lond). 2013;37:1071-1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 73. | Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes. 2005;54:2557-2567. [PubMed] |
| 74. | Rankin MM, Kushner JA. Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes. 2009;58:1365-1372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 265] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 75. | Matsuba I, Saito K, Takai M, Hirao K, Sone H. Fasting insulin levels and metabolic risk factors in type 2 diabetic patients at the first visit in Japan: a 10-year, nationwide, observational study (JDDM 28). Diabetes Care. 2012;35:1853-1857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 76. | Bell JA, Kivimaki M, Hamer M. Metabolically healthy obesity and risk of incident type 2 diabetes: a meta-analysis of prospective cohort studies. Obes Rev. 2014;15:504-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 327] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 77. | Heianza Y, Arase Y, Tsuji H, Fujihara K, Saito K, Hsieh SD, Tanaka S, Kodama S, Hara S, Sone H. Metabolically healthy obesity, presence or absence of fatty liver, and risk of type 2 diabetes in Japanese individuals: Toranomon Hospital Health Management Center Study 20 (TOPICS 20). J Clin Endocrinol Metab. 2014;99:2952-2960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 78. | Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Ann Intern Med. 2013;159:758-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 708] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 79. | Sone H, Ito H, Ohashi Y, Akanuma Y, Yamada N. Obesity and type 2 diabetes in Japanese patients. Lancet. 2003;361:85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 159] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 80. | Hsu WC, Boyko EJ, Fujimoto WY, Kanaya A, Karmally W, Karter A, King GL, Look M, Maskarinec G, Misra R. Pathophysiologic differences among Asians, native Hawaiians, and other Pacific Islanders and treatment implications. Diabetes Care. 2012;35:1189-1198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 81. | Gill TP. Cardiovascular risk in the Asia-Pacific region from a nutrition and metabolic point of view: abdominal obesity. Asia Pac J Clin Nutr. 2001;10:85-89. [PubMed] |
| 82. | Kadowaki T, Sekikawa A, Murata K, Maegawa H, Takamiya T, Okamura T, El-Saed A, Miyamatsu N, Edmundowicz D, Kita Y. Japanese men have larger areas of visceral adipose tissue than Caucasian men in the same levels of waist circumference in a population-based study. Int J Obes (Lond). 2006;30:1163-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 83. | Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013;36:1789-1796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 469] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 84. | Møller JB, Pedersen M, Tanaka H, Ohsugi M, Overgaard RV, Lynge J, Almind K, Vasconcelos NM, Poulsen P, Keller C. Body composition is the main determinant for the difference in type 2 diabetes pathophysiology between Japanese and Caucasians. Diabetes Care. 2014;37:796-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 85. | Kou K, Saisho Y, Satoh S, Yamada T, Itoh H. Change in β-cell mass in Japanese nondiabetic obese individuals. J Clin Endocrinol Metab. 2013;98:3724-3730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 86. | Mizukami H, Takahashi K, Inaba W, Osonoi S, Kamata K, Tsuboi K, Yagihashi S. Age-associated changes of islet endocrine cells and the effects of body mass index in Japanese. J Diabetes Investig. 2014;5:38-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 87. | Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2205] [Cited by in RCA: 2108] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 88. | Kahn SE, Lachin JM, Zinman B, Haffner SM, Aftring RP, Paul G, Kravitz BG, Herman WH, Viberti G, Holman RR. Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT. Diabetes. 2011;60:1552-1560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 89. | TODAY Study Group. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care. 2013;36:1749-1757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 243] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 90. | Saisho Y, Kou K, Tanaka K, Abe T, Kurosawa H, Shimada A, Meguro S, Kawai T, Itoh H. Postprandial serum C-peptide to plasma glucose ratio as a predictor of subsequent insulin treatment in patients with type 2 diabetes. Endocr J. 2011;58:315-322. [PubMed] |
| 91. | Saisho Y, Kou K, Tanaka K, Abe T, Shimada A, Kawai T, Itoh H. Postprandial serum C-peptide to plasma glucose ratio predicts future insulin therapy in Japanese patients with type 2 diabetes. Acta Diabetol. 2013;50:987-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 92. | Saisho Y, Kou K, Tanaka K, Abe T, Shimada A, Kawai T, Itoh H. Association between beta cell function and future glycemic control in patients with type 2 diabetes. Endocr J. 2013;60:517-523. [PubMed] |
| 93. | Saisho Y, Tanaka K, Abe T, Kawai T, Itoh H. Lower beta cell function relates to sustained higher glycated albumin to glycated hemoglobin ratio in Japanese patients with type 2 diabetes. Endocr J. 2014;61:149-157. [PubMed] |
| 94. | Fukuda M, Tanaka A, Tahara Y, Ikegami H, Yamamoto Y, Kumahara Y, Shima K. Correlation between minimal secretory capacity of pancreatic beta-cells and stability of diabetic control. Diabetes. 1988;37:81-88. [PubMed] |
| 95. | Nakanishi K, Kobayashi T, Inoko H, Tsuji K, Murase T, Kosaka K. Residual beta-cell function and HLA-A24 in IDDM. Markers of glycemic control and subsequent development of diabetic retinopathy. Diabetes. 1995;44:1334-1339. [PubMed] |
| 96. | Sassa M, Yamada Y, Hosokawa M, Fukuda K, Fujimoto S, Toyoda K, Tsukiyama K, Seino Y, Inagaki N. Glycemic instability in type 1 diabetic patients: Possible role of ketosis or ketoacidosis at onset of diabetes. Diabetes Res Clin Pract. 2008;81:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 97. | Saisho Y, Tanaka K, Abe T, Shimada A, Kawai T, Itoh H. Glycated albumin to glycated hemoglobin ratio reflects postprandial glucose excursion and relates to β-cell function in both type 1 and type 2 diabetes. Diabetol Int. 2011;2:146-153. |
| 98. | Tanaka C, Saisho Y, Tanaka K, Kou K, Tanaka M, Meguro S, Irie J, Jo R, Kawai T, Itoh H. Factors associated with glycemic variability in Japanese patients with diabetes. Diabetol Int. 2014;5:36-42. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 99. | Koga M, Murai J, Saito H, Kasayama S. Glycated albumin and glycated hemoglobin are influenced differently by endogenous insulin secretion in patients with type 2 diabetes. Diabetes Care. 2010;33:270-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 100. | Day JF, Ingebretsen CG, Ingebretsen WR, Baynes JW, Thorpe SR. Nonenzymatic glucosylation of serum proteins and hemoglobin: response to changes in blood glucose levels in diabetic rats. Diabetes. 1980;29:524-527. [PubMed] |
| 101. | Tahara Y, Shima K. Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care. 1995;18:440-447. [PubMed] |
| 102. | Yoshiuchi K, Matsuhisa M, Katakami N, Nakatani Y, Sakamoto K, Matsuoka T, Umayahara Y, Kosugi K, Kaneto H, Yamasaki Y. Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. Endocr J. 2008;55:503-507. [PubMed] |
| 103. | Imai T, Oikawa Y, Shimada A. Improved Monitoring of the Hyperglycemic State in Type 1 Diabetes Patients by Use of the Glycoalbumin/HbA1c Ratio. Rev Diabet Stud. 2007;4:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 104. | Takao T, Ide T, Yanagisawa H, Kikuchi M, Kawazu S, Matsuyama Y. The effect of fasting plasma glucose variability on the risk of retinopathy in type 2 diabetic patients: retrospective long-term follow-up. Diabetes Res Clin Pract. 2010;89:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 105. | Hsu CC, Chang HY, Huang MC, Hwang SJ, Yang YC, Lee YS, Shin SJ, Tai TY. HbA1c variability is associated with microalbuminuria development in type 2 diabetes: a 7-year prospective cohort study. Diabetologia. 2012;55:3163-3172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 106. | Sugawara A, Kawai K, Motohashi S, Saito K, Kodama S, Yachi Y, Hirasawa R, Shimano H, Yamazaki K, Sone H. HbA(1c) variability and the development of microalbuminuria in type 2 diabetes: Tsukuba Kawai Diabetes Registry 2. Diabetologia. 2012;55:2128-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 107. | Hirakawa Y, Arima H, Zoungas S, Ninomiya T, Cooper M, Hamet P, Mancia G, Poulter N, Harrap S, Woodward M. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: the ADVANCE trial. Diabetes Care. 2014;37:2359-2365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 278] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 108. | Saisho Y. Importance of Β-cell Function for the Treatment of Type 2 Diabetes. J Clin Med. 2014;3:923-943. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 109. | Saisho Y. Obesity, type 2 diabetes and β-cell failure: An Asian perspective. J Mol Genet Med. 2014;S1:8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 110. | Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2142] [Cited by in RCA: 1965] [Article Influence: 163.8] [Reference Citation Analysis (0)] |
| 111. | Williamson DA, Rejeski J, Lang W, Van Dorsten B, Fabricatore AN, Toledo K. Impact of a weight management program on health-related quality of life in overweight adults with type 2 diabetes. Arch Intern Med. 2009;169:163-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 112. | Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, Hill JO, Brancati FL, Peters A, Wagenknecht L. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1082] [Cited by in RCA: 1249] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 113. | Gregg EW, Chen H, Wagenknecht LE, Clark JM, Delahanty LM, Bantle J, Pownall HJ, Johnson KC, Safford MM, Kitabchi AE. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA. 2012;308:2489-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 500] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 114. | Breyer BN, Phelan S, Hogan PE, Rosen RC, Kitabchi AE, Wing RR, Brown JS; Look AHEAD Research Group. Intensive Lifestyle Intervention Reduces Urinary Incontinence in Overweight/Obese Men with Type 2 Diabetes: Results from the Look AHEAD Trial. J Urol. 2014;Jun 1; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 115. | Gerstein HC. Do lifestyle changes reduce serious outcomes in diabetes? N Engl J Med. 2013;369:189-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 116. | Wadden TA; Look AHEAD Research Group. Impact of Intensive Lifestyle Intervention on Depression and Health-Related Quality of Life in Type 2 Diabetes: The Look AHEAD Trial. Diabetes Care. 2014;37:1544-1553. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 117. | Long GH, Cooper AJM, Wareham NJ, Griffin SJ, Simmons RK. Healthy Behavior Change and Cardiovascular Outcomes in Newly Diagnosed Type 2 Diabetic Patients: A Cohort Analysis of the ADDITION-Cambridge Study. Diabetes Care. 2014;37:1712-1720. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 118. | Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2563] [Cited by in RCA: 2609] [Article Influence: 200.7] [Reference Citation Analysis (4)] |
| 119. | Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, Davidson MB, Einhorn D, Garvey WT. AACE comprehensive diabetes management algorithm 2013. Endocr Pract. 2013;19:327-336. [PubMed] |
| 120. | DeFronzo RA, Barzilai N, Simonson DC. Mechanism of metformin action in obese and lean noninsulin-dependent diabetic subjects. J Clin Endocrinol Metab. 1991;73:1294-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 257] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 121. | Kim CH, Han KA, Oh HJ, Tan KE, Sothiratnam R, Tjokroprawiro A, Klein M. Safety, tolerability, and efficacy of metformin extended-release oral antidiabetic therapy in patients with type 2 diabetes: an observational trial in Asia. J Diabetes. 2012;4:395-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 122. | Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3497] [Cited by in RCA: 3343] [Article Influence: 185.7] [Reference Citation Analysis (0)] |
| 123. | FDA places greater restrictions on access to rosiglitazone. BMJ. 2010;341:c5287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 124. | Blind E, Dunder K, de Graeff PA, Abadie E. Rosiglitazone: a European regulatory perspective. Diabetologia. 2011;54:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 126. | Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3109] [Cited by in RCA: 2958] [Article Influence: 147.9] [Reference Citation Analysis (0)] |
| 127. | Tzoulaki I, Molokhia M, Curcin V, Little MP, Millett CJ, Ng A, Hughes RI, Khunti K, Wilkins MR, Majeed A. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ. 2009;339:b4731. [PubMed] |
| 128. | Loke YK, Kwok CS, Singh S. Comparative cardiovascular effects of thiazolidinediones: systematic review and meta-analysis of observational studies. BMJ. 2011;342:d1309. [PubMed] |
| 129. | Nissen SE, Nicholls SJ, Wolski K, Nesto R, Kupfer S, Perez A, Jure H, De Larochellière R, Staniloae CS, Mavromatis K. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 2008;299:1561-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 661] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 130. | Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR. Use of thiazolidinediones and fracture risk. Arch Intern Med. 2008;168:820-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 244] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 131. | Lewis JD, Ferrara A, Peng T, Hedderson M, Bilker WB, Quesenberry CP, Vaughn DJ, Nessel L, Selby J, Strom BL. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 459] [Cited by in RCA: 470] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 132. | Neumann A, Weill A, Ricordeau P, Fagot JP, Alla F, Allemand H. Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study. Diabetologia. 2012;55:1953-1962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 218] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 133. | Colmers IN, Bowker SL, Majumdar SR, Johnson JA. Use of thiazolidinediones and the risk of bladder cancer among people with type 2 diabetes: a meta-analysis. CMAJ. 2012;184:E675-E683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 134. | Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1820] [Cited by in RCA: 1636] [Article Influence: 71.1] [Reference Citation Analysis (0)] |
| 135. | Kawamori R, Tajima N, Iwamoto Y, Kashiwagi A, Shimamoto K, Kaku K. Voglibose for prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet. 2009;373:1607-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 136. | Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1120] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 137. | Hanefeld M, Chiasson JL, Koehler C, Henkel E, Schaper F, Temelkova-Kurktschiev T. Acarbose slows progression of intima-media thickness of the carotid arteries in subjects with impaired glucose tolerance. Stroke. 2004;35:1073-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 190] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 138. | Hanefeld M, Cagatay M, Petrowitsch T, Neuser D, Petzinna D, Rupp M. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur Heart J. 2004;25:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 429] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 139. | Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2787] [Cited by in RCA: 2844] [Article Influence: 149.7] [Reference Citation Analysis (1)] |
| 140. | Kendall DM, Cuddihy RM, Bergenstal RM. Clinical application of incretin-based therapy: therapeutic potential, patient selection and clinical use. Am J Med. 2009;122:S37-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 141. | Karagiannis T, Paschos P, Paletas K, Matthews DR, Tsapas A. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ. 2012;344:e1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 302] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 142. | Aroda VR, Henry RR, Han J, Huang W, DeYoung MB, Darsow T, Hoogwerf BJ. Efficacy of GLP-1 receptor agonists and DPP-4 inhibitors: meta-analysis and systematic review. Clin Ther. 2012;34:1247-1258.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 143. | Rolin B, Larsen MO, Gotfredsen CF, Deacon CF, Carr RD, Wilken M, Knudsen LB. The long-acting GLP-1 derivative NN2211 ameliorates glycemia and increases beta-cell mass in diabetic mice. Am J Physiol Endocrinol Metab. 2002;283:E745-E752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 213] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 144. | Lamont BJ, Li Y, Kwan E, Brown TJ, Gaisano H, Drucker DJ. Pancreatic GLP-1 receptor activation is sufficient for incretin control of glucose metabolism in mice. J Clin Invest. 2012;122:388-402. [PubMed] |
| 145. | Farilla L, Bulotta A, Hirshberg B, Li Calzi S, Khoury N, Noushmehr H, Bertolotto C, Di Mario U, Harlan DM, Perfetti R. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144:5149-5158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 463] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 146. | Bunck MC, Cornér A, Eliasson B, Heine RJ, Shaginian RM, Taskinen MR, Smith U, Yki-Järvinen H, Diamant M. Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care. 2011;34:2041-2047. [PubMed] |
| 147. | Foley JE, Bunck MC, Möller-Goede DL, Poelma M, Nijpels G, Eekhoff EM, Schweizer A, Heine RJ, Diamant M. Beta cell function following 1 year vildagliptin or placebo treatment and after 12 week washout in drug-naive patients with type 2 diabetes and mild hyperglycaemia: a randomised controlled trial. Diabetologia. 2011;54:1985-1991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 148. | Kohro T, Yamazaki T, Sato H, Harada K, Ohe K, Komuro I, Nagai R. Trends in antidiabetic prescription patterns in Japan from 2005 to 2011. Int Heart J. 2013;54:93-97. [PubMed] |
| 149. | Kodani N, Saisho Y, Tanaka K, Kawai T, Itoh H. Effects of mitiglinide, a short-acting insulin secretagogue, on daily glycemic variability and oxidative stress markers in Japanese patients with type 2 diabetes mellitus. Clin Drug Investig. 2013;33:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 150. | Del Prato S, Tiengo A. The importance of first-phase insulin secretion: implications for the therapy of type 2 diabetes mellitus. Diabetes Metab Res Rev. 2001;17:164-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 158] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 151. | Gerstein HC, Bosch J, Dagenais GR, Díaz R, Jung H, Maggioni AP, Pogue J, Probstfield J, Ramachandran A, Riddle MC. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1318] [Cited by in RCA: 1177] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 152. | Weng J, Li Y, Xu W, Shi L, Zhang Q, Zhu D, Hu Y, Zhou Z, Yan X, Tian H. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371:1753-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 582] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 153. | Pennartz C, Schenker N, Menge BA, Schmidt WE, Nauck MA, Meier JJ. Chronic reduction of fasting glycemia with insulin glargine improves first- and second-phase insulin secretion in patients with type 2 diabetes. Diabetes Care. 2011;34:2048-2053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 154. | Unger RH. Reinventing type 2 diabetes: pathogenesis, treatment, and prevention. JAMA. 2008;299:1185-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 155. | Tahrani AA, Barnett AH, Bailey CJ. SGLT inhibitors in management of diabetes. Lancet Diabetes Endocrinol. 2013;1:140-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 247] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 156. | Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, Sarigianni M, Matthews DR, Tsapas A. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:262-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 663] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 157. | Riser Taylor S, Harris KB. The clinical efficacy and safety of sodium glucose cotransporter-2 inhibitors in adults with type 2 diabetes mellitus. Pharmacotherapy. 2013;33:984-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 158. | Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, Bucher HC, Nordmann AJ. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 936] [Cited by in RCA: 941] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 159. | Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, Aminian A, Pothier CE, Kim ES, Nissen SE. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014;370:2002-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1202] [Cited by in RCA: 1180] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 160. | Dutia R, Brakoniecki K, Bunker P, Paultre F, Homel P, Carpentier AC, McGinty J, Laferrère B. Limited recovery of β-cell function after gastric bypass despite clinical diabetes remission. Diabetes. 2014;63:1214-1223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 161. | Dixon JB, Chuang LM, Chong K, Chen SC, Lambert GW, Straznicky NE, Lambert EA, Lee WJ. Predicting the glycemic response to gastric bypass surgery in patients with type 2 diabetes. Diabetes Care. 2013;36:20-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 162. | Lee YC, Lee WJ, Liew PL. Predictors of remission of type 2 diabetes mellitus in obese patients after gastrointestinal surgery. Obes Res Clin Pract. 2013;7:e494-e500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 163. | Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2773] [Cited by in RCA: 2625] [Article Influence: 93.8] [Reference Citation Analysis (1)] |
| 164. | Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7148] [Cited by in RCA: 6609] [Article Influence: 275.4] [Reference Citation Analysis (0)] |
| 165. | Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13206] [Cited by in RCA: 12431] [Article Influence: 540.5] [Reference Citation Analysis (1)] |
| 166. | Yoon U, Kwok LL, Magkidis A. Efficacy of lifestyle interventions in reducing diabetes incidence in patients with impaired glucose tolerance: a systematic review of randomized controlled trials. Metabolism. 2013;62:303-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 167. | DeFronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, Clement SC, Henry RR, Hodis HN, Kitabchi AE. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011;364:1104-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 529] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 168. | Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 1062] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 169. | Knowler WC, Hamman RF, Edelstein SL, Barrett-Connor E, Ehrmann DA, Walker EA, Fowler SE, Nathan DM, Kahn SE. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes. 2005;54:1150-1156. [PubMed] |
| 170. | Holman RR, Haffner SM, McMurray JJ, Bethel MA, Holzhauer B, Hua TA, Belenkov Y, Boolell M, Buse JB, Buckley BM. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362:1463-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 335] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 171. | Yates T, Haffner SM, Schulte PJ, Thomas L, Huffman KM, Bales CW, Califf RM, Holman RR, McMurray JJ, Bethel MA. Association between change in daily ambulatory activity and cardiovascular events in people with impaired glucose tolerance (NAVIGATOR trial): a cohort analysis. Lancet. 2014;383:1059-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 172. | Li G, Zhang P, Wang J, An Y, Gong Q, Gregg EW, Yang W, Zhang B, Shuai Y, Hong J. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol. 2014;2:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 459] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 173. | Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, Napoli N, Qualls C, Shah K. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 761] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 174. | Pal A, McCarthy MI. The genetics of type 2 diabetes and its clinical relevance. Clin Genet. 2013;83:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 175. | Jonsson A, Ladenvall C, Ahluwalia TS, Kravic J, Krus U, Taneera J, Isomaa B, Tuomi T, Renström E, Groop L. Effects of common genetic variants associated with type 2 diabetes and glycemic traits on α- and β-cell function and insulin action in humans. Diabetes. 2013;62:2978-2983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 176. | Andersson EA, Allin KH, Sandholt CH, Borglykke A, Lau CJ, Ribel-Madsen R, Sparsø T, Justesen JM, Harder MN, Jørgensen ME. Genetic risk score of 46 type 2 diabetes risk variants associates with changes in plasma glucose and estimates of pancreatic β-cell function over 5 years of follow-up. Diabetes. 2013;62:3610-3617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 177. | Iwata M, Maeda S, Kamura Y, Takano A, Kato H, Murakami S, Higuchi K, Takahashi A, Fujita H, Hara K. Genetic risk score constructed using 14 susceptibility alleles for type 2 diabetes is associated with the early onset of diabetes and may predict the future requirement of insulin injections among Japanese individuals. Diabetes Care. 2012;35:1763-1770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 178. | Pasquali L, Gaulton KJ, Rodríguez-Seguí SA, Mularoni L, Miguel-Escalada I, Akerman I, Tena JJ, Morán I, Gómez-Marín C, van de Bunt M. Pancreatic islet enhancer clusters enriched in type 2 diabetes risk-associated variants. Nat Genet. 2014;46:136-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 388] [Cited by in RCA: 402] [Article Influence: 36.5] [Reference Citation Analysis (0)] |