INFLAMMATION IN DIABETIC KIDNEY DISEASE
Diabetes mellitus (DM) is one of the most significant health problems worldwide. According to the projections, the number of adult diabetic patients will be higher than 430 million in 2030. Diabetic kidney disease (DKD) is one of the most prevalent complications, and is now the leading cause of end-stage renal disease (ESRD) in developed countries[1,2]. In the general population, ESRD rate increases due to the rise of diabetes mellitus. However, a recent study by Burrows et al[3] found that the incidence of ESRD in the diabetic population had shown a reduction, suggesting that the strategies for controlling DKD, including early diagnosis, adequate control targets and follow-up, early initiation of therapy, and the use of effective renoprotective therapies, may be efficacious. However, it might be premature to state a real decline in ESRD in diabetes, since other reasons may be possible, such as the lack of enough time to develop ESRD in a large proportion of new diabetic subjects diagnosed in the last 20 years. In addition, the change of the diagnostic criteria for diabetes by the ADA in 1997, may have derived in the diagnosis of diabetes in a earlier stage of the disease, with a much less organ damage, and therefore, when diabetes have a more prolonged evolution, it is possible that this trends in the incidence of ESRD secondary to diabetes may reverse. Finally, another factor is the longer survival of diabetic patients, and thus, these subjects would have an increased risk of developing renal damage and ESRD.
Although kidney biopsy is required to definitively establish the diagnosis of DKD, in clinical practice this is unusual, since the careful screening of patients allow to identify people with DKD. The main criteria to diagnose DKD is the presence of an increased urinary albumin excretion (UAE), which is divided arbitrarily into microalbuminuria and macroalbuminuria, which is associated with an increased risk of decline in glomerular filtration rate (GFR) and a high risk of kidney failure.
DKD has been classically considered as the consequence from the interaction between hemodynamic and metabolic factors. However, renal damage is not completely explained by these factors. Current knowledge indicates that this represents only a partial view of a much more complex scenario. Clear evidence indicates that the pathogenesis of DKD is multifactorial, with the interaction of both genetic and environmental factors that trigger a complex network of pathophysiological events[4,5]. Clinical observations and epidemiological studies in different ethnic groups have indicated that there is familial aggregation of DKD. Although this information does not allow clearly establishing a model of transmission, diabetic nephropathy has been widely considered as a polygenic disease. There may be many genes, and each has a cumulative genetic effect and interacts with environmental factors in the development of DKD. The challenge in genetic studies of diabetic nephropathy is to dissect its genetic complexity. Researchers have searched for the genes involved in susceptibility, resistance or progression to DKD. The aim of genetic studies is to provide useful information for better understanding the pathogenesis and further developing novel therapeutic approach in this disease. Genome wide linkage analyses, candidate gene population association, family-based association and genome wide association studies have been used for the identification of the genes in DKD.
In this context, inflammation has become a cardinal pathophysiological mechanism in the development and progression of DKD. This review will focus on the implications of inflammation in DKD, with special attention to inflammatory cytokines.
INFLAMMATION IN DIABETES MELLITUS
Growing evidence indicates that pathogenesis of diabetes mellitus is widely related to the activation of the innate immune system and the presence of a chronic subclinical low-grade inflammatory state[6,7]. Many studies suggest that individuals who developed DM present characteristics of inflammation several years before the diagnosis of DM[8,9]. Population-based studies have shown that diverse inflammatory markers, such as cytokines, are strong predictors of the development of diabetes[10-12]. In addition, inflammatory cytokines have been involved in the pathogenesis of microvascular diabetic complications, including DKD[13-18].
DKD: AN INFLAMMATORY-BASED COMPLICATION
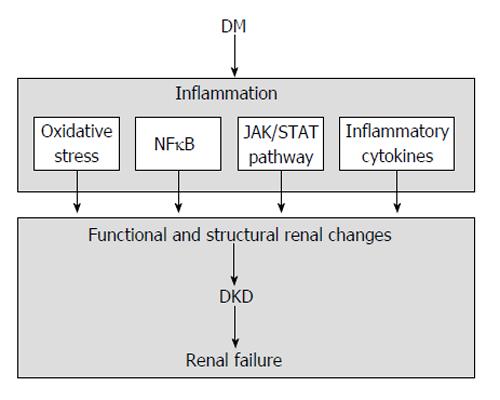
DM is associated with multiple deviations from normal homeostasis, including hemodynamic and metabolic alterations that produce the activation of diverse transduction pathways in the kidney. At the present time, inflammation is recognized as an important mechanism in the pathogenesis of this complication, through oxidative stress, transcription factors, including nuclear factor κB (NFκB), janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway, and inflammatory cytokines[13,14] (Figure 1).
Figure 1 Schematic representation of inflammatory-mediated renal injury in diabetic kidney disease.
DM: Diabetes mellitus; NFκB: Nuclear factor κB; DKD: Diabetic kidney disease; JAK/STAT: Janus kinase/signal transducers and activators of transcription.
OXIDATIVE STRESS
There is solid experimental evidence of a key role for reactive oxygen species (ROS) and oxidative stress and their interplay with the renin-angiotensin-aldosterone system (RAAS) and inflammation, in the pathogenesis of DKD. There is a disproportionate production of ROS secondary to hyperglycemia by different renal cells[19-25]. Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor that participates importantly in the regulation of the cellular antioxidant response[26,27]. Nrf2 appears to counteract renal damage in diabetes, possibly through inhibition of transforming growth factor-β1 (TGF-β1). In both in vitro and in vivo experimental studies, Nrf2 ameliorated streptozotocin-induced renal damage. Nrf2(-/-) mice produced greater amounts of ROS and suffered more severe oxidative renal damage compared with wild type mice[28].
NFκB
NFκB is a transcription factor that controls the expression of genes involved in different processes, such as the immune response, cell differentiation and development, apoptosis, cycle progression, inflammation, and tumorigenesis. Importantly, this factor is activated by many stimuli related to DKD[29]. Many of the signalling molecules that produce the activation of NFκB may be potential targets for the inhibition of this factor, some of them acting within a network of signals leading to the activation of NFκB.
NFκB is continuously present in cells in an inactive state. In resting cells, NFκB dimers are cloistered by inhibitors of NFκB (IκBs), which prevents the translocation of NFκB to the nucleus. Triggering of the NFκB signalling cascade results in degradation of IκBs, allowing the liberation of NFκB, and thus, this factor translocates to the nucleus and induces transcription. IκB can be classified into several groups: classical IκB (IκBα, IκBβ and IκBε), NFκB precursors (p105 and p100) and nuclear IκB (IκBζ, Bcl-3 and IκBNS). All of them have a central ankyrin repeat domain (ARD), which permits the interaction with NFκB. The activation process of NFκB needs the phosphorylation of IκB, which results in polyubiquitination, a sign for destruction of the IκB by proteasome. The Ser/Thr-specific IκB kinases (IKKs) are the main points for the activation of NFκB. The IKK holo-complex incorporates IKKα or IKKβ, and the protein NEMO (IKKγ or FIP-3). IKK turning on occurs with phosphorylation of the activation loop Ser residues in the canonical MAP kinase kinase consensus motif SxxxS in the kinase domain. NEMO is crucial for the turning on of IKK since in cells without this protein, IKKα and IKKβ cannot be activated by any of the conventional NFκB activators. IKKβ is a key factor for turning on of the canonical NFκB pathway secondary to inflammation, whereas IKKα has a critical function in the non-canonical NFκB pathway through the phosphorylation of p100.
Different extracellular signals initiate the activation of NFκB. After entering the nucleus, this factor interacts with specific sequence motifs (κB sites) on their target genes, resulting in transcriptional turning on. The particular DNA-binding site characteristics of diverse NFκB dimers for a group of related κB sites, and the specific protein-protein binding at target promoters explain the specificity of NFκB signaling. In the majority of instances, turning on of NFκB is temporary and cyclical under the existence of a continuous inducer. This cyclical characteristic is secondary to recurrent destruction and production of IκB and the resulting turning on and inactivation of NFκB, respectively.
NFκB regulates a huge variety of target genes, including those coding for adhesion molecules, chemokines, inflammatory cytokines, nitric oxide synthase, and other molecules related to inflammation and proliferation, all of them involved in the pathogenesis of DKD[30]. NFκB is activated by a wide variety of stimuli[31] such as cytokines, oxygen radicals, inhaled particles, ultraviolet irradiation, bacterial or viral products, and metabolic abnormalities. High glucose may produce the activation of NFκB in diverse cells, including endothelial and vascular smooth muscle cells, and cells of the proximal tubule[32,33]. NFκB is central in the interplay among the different factors, molecules and pathways resulting in structural alterations and functional abnormalities observed in DKD, such as activation of the RAAS, advanced glycation end-products accumulation, and NADPH-dependent oxidative stress[34]. In experimental models of DKD, it has been established the activation of NFκB in the renal cortical tissue[35]. Moreover, in human DKD, proteinuria itself, is an important activator of NFκB and it’s an important pro-inflammatory stimulus for tubular cells. Chemoattractants and adhesive molecules for inflammatory cells are upregulated by excess ultrafiltered protein load of proximal tubular cells via activation of NFκB-dependent and NFκB-independent pathways[36].
NFκB represents a central factor in inflammation, with the generation of intrincated regulatory circuits that include a huge variety of cellular mediators, such as adhesion molecules, intracellular second messengers, microRNA, growth and transcription factors, and cytokines. NFκB system is critical for the flow of biological messages from DNA information to protein synthesis. In addition, these elements have important pathogenic and pathophysiologic roles in human disease, including DKD.
JAK/STAT PATHWAY
In animal models and in clinical studies in DKD, it has been demonstrated the enhanced activation of JAK/STAT pathway in the glomeruli and tubulointerstitial cells. The JAK proteins are intracellular, non receptor tyrosine kinases that transduce cytokine-mediated signals. Secondary to the binding of the ligand to the cytokine receptor, the JAK proteins associated with the intracellular domain of the receptor, phosphorylate and activate each other. The autophosphorylation of the JAK proteins induces a conformational modification, allowing the transduction of the intracellular signal by further phosphorylating and activating the STAT transcription factors. The activated STAT molecules dissociated from the receptor and form dimers and translocate to the cell nucleus, where they activate many target genes. The JAK/STAT signaling route is a major connecting system between the receptors located at the cell surface and the transcriptional events occurring within the cell nucleus.
It has been demonstrated the great importance of the JAK/STAT pathway in the pathogenesis of DKD through its participation in several processes, such as the hypertrophy of mesangial cells induced by angiotensin II (Ang II), and the synthesis of TGF-β, collagen IV and fibronectin. In addition, the high levels of glucose stimulate the production of ROS within the cells, which in turn activates the JAK/STAT pathway.
Although there are several types of JAK proteins, the one primarily studied in renal and vascular tissue is JAK2[37]. Experimental studies in animal models of diabetic nephropathy have showed that hyperglycemia is able to turning on the JAK2/STAT pathway in renal cells[38-42]. Moreover, clinical studies in patients with early of advanced stages of DKD have showed an increased expression of JAK/STAT mRNAs and JAK2 protein in the glomerular and tubulointerstitial compartment, with an inverse correlation between JAK2 mRNA levels and estimated GFR in these patients[43].
The intimate mechanism by which hyperglycemia promotes JAK2 activation has been related to the interaction between JAK2 and ROS caused by high glucose. ROS enhance the activity of JAK2, whereas the use of an inhibitor of ROS formation (diphenylene iodonium) resulted in a marked inhibition of Ang II-induced activation of JAK2. These facts reveal that ROS act as an intracellular activator of the JAK-STAT pathway, and that ROS also act as a second messenger for the regulation of JAK2 activation by Ang II. One of the leading causes of the increased JAK2 tyrosine phosphorylation is the alteration of tyrosine phosphatases (SHP-1 and SHP-2). SHP-1 phosphorylation is abolished under hyperglycemia, whereas SHP-2 phosphorylation is increased under basal and Ang II stimulation, suggesting that JAK2 sustained activation under hyperglycemia is partly due to decreased SHP-1 and increased SHP-2 phosphorylation. In addition, these effects are due to hyperglycemia and not to hyperosmolarity, since no alterations in the tyrosine phosphorylation of both SHP-1 and SHP-2 have been observed under conditions with elevated osmolarity without hyperglycemia[38-41].
INFLAMMATORY CYTOKINES
Cytokines are low molecular weight polypeptides with autocrine, paracrine and juxtacrine effects, and very complex activities. The classic function of cytokines is related to the regulation of the inflammatory process, but they are also crucial effectors of the immune system. Cytokines often have multiple target cells and multiple pleiotropic actions, and thus a particular cytokine may activate diverse reactions based on the type of cell, the time of action, and the situation and ambience. Moreover, cytokines may share receptor subunits and intracellular signalling pathways, and they can act synergistically in many contexts[44].
The first studies suggesting that inflammatory cytokines were engaged in the pathogenesis of DKD were published more than 20 years ago by Hasegawa et al[45,46]. The authors reported that glomerular basement membranes (GBM) obtained from rats after the induction of diabetes, were able to induce the production of significantly higher quantity of the inflammatory cytokines tumor necrosis factor (TNF)-α and interleukin 1 (IL-1) when were incubated with peritoneal macrophages, as compared with the production of those cytokines when the macropages were cultured with membranes from normal rats. Later works showed that all types of resident renal cells, as well as infiltrating cells (monocytes, macrophages and lymphocytes) are able to synthesize proinflammatory cytokines[47,48]. Nowadays, the results of numerous studies support the notion that cytokines play a transcendent role in the pathogenesis of microvascular complications of DM[13,49,50]. The renal effects of cytokines in DKD are associated with different actions, including intrarenal hemodynamic alterations, modifications of the renal structure with changes in extracellular matrix and basement membranes, abnormalities in the expression of diverse molecules, cellular necrosis and apoptosis, modification in the permeability of glomerular endothelium, and increment in the production of ROS[50-54].
IL-1
In experimental models of DKD, renal expression of IL-1 is elevated[55,56], which has been associated with changes in the expression of molecules related to chemotaxis and cellular adhesion. Specifically, IL-1 augments the production of intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 by different renal cells, including endothelial, mesangial and tubular epithelial cells. In addition, IL-1 also stimulates the expression of endothelial-leukocyte adhesion molecule 1[57,58].
IL-1 produces abnormalities of intraglomerular hemodynamics. These effects are secondary to modifications in the synthesis of prostaglandins by mesangial cells. Experimental in vitro studies have shown that glomerular mesangial cells incubated with recombinant human IL-1 are stimulated to produce prostaglandin E2 and delivery phospholipase A2[51]. Futhermore, these cells present an increased secretion of prostaglandin E2 in response to Ang II[52], whereas the permeability of vascular endothelial cells is enhanced[59]. Finally, this cytokine raises the production of hyaluronan by epithelial cells of renal proximal tubule[60], which has been related with the development of hypercellularity in experimental models of diabetes[61].
IL-6
Clinical studies have shown that IL-6 levels are significantly higher in patients with DKD in comparison with DM patients without nephropathy[62]. In addition, the histopathological analysis of human renal samples by immunohistochemistry has demonstrated an increased expression of mRNA encoding IL-6 in cells infiltrating the mesangium, interstitium and tubules, with a positive relationship with the severity of mesangial expansion[63]. Other functional and structural abnormalities related to DKD and progression of renal damage have been associated with IL-6, including abnormalities in the permeability of glomerular endothelium, expansion of mesangial cells and enhanced expression of fibronectin[54] and increase in the thickness of the GBM[64,65]. Our experimental studies have demonstrated an increase in the mRNA levels of IL-6 in the renal cortex of diabetic rats, which is positively associated with the urinary concentration of this cytokine[56]. In addition, in animal models of diabetes, wet kidney weight, a marker of renal hypertrophy and an early phenomenon in kidney involvement in DM[66], has been reported to be enhanced, which was related to mRNA gene expression levels and urine concentration of this cytokine[56].
IL-6 signals through a cell surface receptor, which is formed by the ligand-binding IL-6 receptor (IL-6R)-α chain (CD126) and the signal-transducing component CD130, also called gp130. In addition to the membrane form of the IL-6R, there is a soluble form which is produced by cleavage of the membrane-bound form. These soluble form of the IL-6R comes to the circulation and is able to control the activity of this cytokine. Regarding this regulatory process, it is important to differentiate the actions of soluble CD126 and CD130. In plasma, soluble CD126 binds to IL-6 and results in the increase of the complex half-life, amplifying the bio-activity of this cytokine to tissues that express the membrane form of CD130. On the contrary, soluble form of CD130 in the circulation functions as an IL-6 antagonist. Recent studies have shown that the soluble form of the IL-6R is closely implicated in the evolution from the initial to the final stages of the inflammatory reaction. IL-6 has many biological properties, including the activation of the STAT3 transcription factor, and the induction of the expression of adhesion molecules and other inflammatory cytokines.
IL-18
IL-18, a potent inflammatory cytokine that belongs to the IL-1 superfamily[67,68], is implicated in different actions, including the release of interferon (IFN)-γ[69] (which stimulates functional chemokine receptor expression in human mesangial cells)[70], the synthesis of other molecules involved in the inflammatory reaction, such as IL-1 and TNF-α, the increase in the expression of ICAM-1, and the apoptotic process of endothelial cells[71-73]. Tubular renal cells show an increase in the expression of IL-18 in patients with DKD[74], which has been related to the triggering of mitogen-activated protein kinase (MAPK) pathways secondary to the action of TGF-β[75]. Many other cells may also produce this cytokine, such as infiltrating monocytes, macrophages and T cells[67,68]. High levels of IL-18 has been found in serum and urine of patients with DKD, with an independent relationship with UAE[76-78]. In addition, serum IL-18 levels are associated with the urine concentration of β-2 microglobulin, a low-weight protein that is used as a marker of tubular dysfunction[77]. In a recent longitudinal study in patients with type 2 diabetes, serum and urinary levels of IL-18 were direct and independently associated with UAE. In addition, the concentrations of this cytokine in serum and urine were also significantly associated with changes in albuminuria during the evolution of the study[77].
TNF-α
TNF-α is a cytokine with prominent proinflammatory effects. It is mainly produced by monocytes, macrophages and T cells, but also intrinsic kidney cells[47,79-81]. TNF-α exists in the cells as a precursor of the active form. This precursor is transformed in the active form through the action of the TNF-α-converting enzyme[82]. There are two specific TNF-α receptors: the TNF-α receptor 1 (TNFR1), an epithelial-cell receptor also named p55, and the TNFR2, which is an myeloid-cell receptor (p75). The exact roles of the receptors are not yet completely understood and may differ depending on the organ type[83]. While TNFR1 modulates the immune response (IL-6 synthesis) and apoptosis (apoptotic signaling kinase 1 and NFκB of mesangial cells), TNFR2 has been recognized as one of the proinflammatory mediators in glomerulonephritis[84,85]. After binding to these receptors, the intracellular transduction cascade is activated, leading to the final biological actions of this cytokine[86], with a potential role in the pathogenesis of DKD. Experimental studies in animal models of diabetes have showed that TNF-α levels and mRNA encoding TNF-α are enhanced in renal glomeruli and tubules[47,56,80,87-89].
TNF-α may cause direct cytotoxicity to renal cells, inducing direct renal injury[90], apoptosis and necrotic cell death[91,92]. It can also produce alterations of intraglomerular blood flow and reduction of glomerular filtration as consequence of the disequilibrium between factors promoting vasoconstriction and vasodilation[93], in addition to changes in the permeability of endothelial cells. Other actions of this cytokine are the modification in the location of molecules involved in the adhesion process among cells, such as the endothelial-cadherincatenin complexes, as well as the alteration of normal endothelial permeability due to alterations of cellular junctions secondary to the lack of F-actin stress fibers[94]. In addition, TNF-α is able to directly induce the formation of ROS by renal cells[95]. Experimental researches has shown that TNF-α induces the activation of NADPH oxidase in isolated rat glomeruli through the activation of the intracellular pathways protein kinase C/phosphatidylinositol-3 kinase and MAPK[96]. Thus, TNF-α prompts local ROS production, independent of hemodynamic mechanisms, resulting in alterations of the glomerular capillary wall and consequently increased albumin permeability[53].
An increase in renal size (kidney hypertrophy) and glomerular filtration rate (hyperfiltration) are early and relevant findings of DKD, which are significantly related to TNF-α[88,89]. In vitro studies demonstrated that TNF-α stimulates the solute uptake in proximal tubular cells secondary to the activation of sodium-dependent cotransporters[97], whereas in vivo studies in diabetic rats found an enhanced urinary excretion of TNF-α excretion, which was related to sodium retention and renal hypertrophy. All these effects could be blocked by the use of a soluble TNF-α receptor fusion protein[89,97]. In the renal distal tubule TNF-α activates the epithelial sodium channel resulting in an increased reabsorption of sodium, which can be abrogated by blockers of this renal channel, such as amiloride, and inhibitors of extracellular signal related protein kinase. The increment in renal sodium reabsorption might induce the expression of TFG-β, with the development of renal hypertrophy[98].
Expression mRNA levels in the renal cortex and urinary TNF-α excretion show a positive and independent correlation with albuminuria[56,87]. Moreover, microdialysis studies showed that the concentration of TNF-α in the kidney interstitial fluid is elevated, as well as in the urine, with no data of cellular renal infiltration. These findings are observed previously to the detection of an increase in UAE. In addition, there is an elevation in the levels of TNF-α in urine after the increase in UAE, which suggest that the rise of albuminuria has a stimulatory effect in the production of TNF-α by the kidney[99]. These findings support the intimate relationship between proteinuria and inflammation. Current data indicates that proteinuria per se is an important factor in the development of tubulointerstitial damage, but also by the capacity of activate an inflammatory cellular response via chemoattractants, adhesive molecules and proinflammatory cytokines. These changes lead to the renal infiltration by blood circulating cells, with the subsequent damage to renal cells, damage of tubular and interstitial structures, and finally, to the development of renal fibrosis and scarring[100].
Finally, many clinical studies in patients with DKD have reported that the serum and urinary concentrations of TNF-α are elevated as compared with non-diabetic individuals or with diabetic subjects and kidneys, and that these concentrations increase concomitantly with the progression of DKD. These findings indicate a potential relationship between the elevated levels of this inflammatory cytokine and the development and progression of renal injury in DM[76,101,102].
In addition to TNF-α, also TNF-α receptors have been related to DKD. In an observational study in type 1 diabetic patients, the serum levels of TNFR1 and TNFR2 were linked with renal function with independence of other variables, such as albuminuria, supporting the important participation of this cytokine in DKD[103]. In addition, this involvement has also been found in type 2 DM (T2DM). Thus, after more than 10 years of follow-up, the Nurses’ Health Study showed that increased concentrations of the soluble TNFR2 were a powerful predictor of the loss of renal function in these patients[104].
Finally, are also important the findings derived from studies focused on another cytokine within the TNF superfamily, the TNF-α-related apoptosis-inducing ligand (TRAIL). TRAIL participates in diverse cellular processes, including apoptosis, cell expansion and maturity[105]. Clinical studies in patients with diabetes have shown that the renal expression of this cytokine is enhanced, and more importantly, the grade of expression is directly related with the seriousness of kidney injury[106]. Regarding the cell types that express TRAIL, immunohistochemistry studies demonstrated that the renal expression of this cytokine was maximal in tubular epithelial cells. However, it is important to highlight that the expression of TRAIL has been also observed in podocytes[106,107]. It has been suggested the participation of TRAIL in the pathogenesis of DKD based on the finding that the magnitude of renal tissue staining for this cytokine was directly associated with the grade of tubulointerstitial inflammation, scarring and degeneration.
INFLAMMATION IN DKD: A THERAPEUTIC OPPORTUNITY
Established therapeutic strategies for prevention and treatment of DKD focus on blood pressure and glucose control, RAAS blockade and anti-thrombotic/-inflammatory treatment with aspirin. However, these therapies are insufficient[108] and new approaches are required[109].
Oxidative stress
In experimental models, the administration of different antioxidant drugs (tempol, thiol, kallistatin)[110-112] improved oxidative stress-induced renal injury, decreasing albuminuria and fibrosis. Triterpenoids, synthetic analogues of oleanolic acid with potent anti-inflammatory and antioxidant properties, activate the ARE-Keap1-Nrf2 pathway.
The renoprotective action of bardoxolone methyl, a triterpenoid that reduces oxidative stress and inflammation through Nrf2 activation and inhibition of NFκB, has been recently explored in humans. A large multicenter double-blind, randomized trial (BEAM study), including 227 patients with moderate-severe CKD and T2DM, showed that administration of bardoxolone was associated with significantly improvement of GFR at 24 wk, but some adverse events were found (mild reversible increase of albuminuria, decreased serum magnesium, muscle spasms, nausea and loss of body weight)[113]. Later, the BEACON trial, a multinational, multicentric and double-blind randomized, placebo-controlled Phase 3 trial, was designed to determine whether bardoxolone would have beneficial effects on the progression of renal injury and the hazard of ESRD in subjects with T2DM and severe stages of renal disease. Regrettably, the increased risk of heart failure and cardiovascular events observed in the bardoxolone arm of the BEACON study led to the premature ending of this trial[114].
The most commonly reported serious adverse event in the bardoxolone group was heart failure. The mechanism linking bardoxolone methyl to heart failure is unknown, although some aspects deserve consideration. Firstly, body weight declined significantly in the bardoxolone methyl group, which may suggest a situation of hemodilution secondary to fluid retention, since a reduction in the serum albumin and hemoglobin concentrations was observed. Secondly, it was observed an increase in blood pressure in the bardoxolone arm, which might result in an elevation of cardiac afterload. This fact, together with the increase in heart preload secondary to fluid retention, combined with a rise in heart rate, result in a situation likely to trigger heart failure. This hypothesis is congruent with the increase in the concentration of B-type natriuretic peptide with bardoxolone methyl, which may reflect an elevated left ventricular wall stress.
NFκB
The renoprotective effects conferred by blockade of RAAS, provides pleiotropic and anti-inflamatory issues through the suppression of NFκB-dependent pathways, beyond the control of blood pressure and proteinuria[115]. In addition, the beneficial effects on the kidney showed by other drugs, such as thiazolidinediones, have been also associated to a suppressive effect on the activation of this transcription factor[116,117]. In addition, recent experimental studies indicates that suppression of NFκB activation by various agents, such as 1,25-dihydroxyvitamin D3[118], cilostazol[119], and curcumin[120], could lead to amelioration of DKD, suggesting the importance of NFκB as a therapeutic target of DKD.
JAK/STAT pathway
Studies in experimental animal models of DKD have reported that the use of AG490, a specific tyrosine kinase inhibitor of JAK2, was able to abrogate the elevation of systolic blood pressure[121] and the increase of UAE[122]. On the other hand, recent studies have highlighted the role of suppressors of cytokine signaling (SOCS) proteins, a group of molecules that bind and interfere with initiating JAK proteins, and act as intracellular negative regulators of JAK/STAT activation in DKD[37]. Ortiz-Muñoz et al[123] demonstrated that high concentrations of glucose were associated in vitro with activated JAK/STAT/SOCS in human mesangial and tubular cells. Overexpression of SOCS reversed the glucose-induced activation of this pathway, expression of STAT-dependent genes and cell proliferation. On the other hand, the inoculation of recombinant SOCS1 and SOCS3 adenovirus to diabetic rats resulted in an improvement of renal function at 7 wk, and renal lesions such as mesangial expansion, fibrosis or influx of macrophages were also reduced. However, further research into JAK inhibitors, SOCS expression or SOCS mimetics is required, given the critical immunomodulatory role of this pathway, with possible adverse effects[37].
Inflammatory cytokines
Experimental works using animal models of both types of DM have revealed probable benefits from the use of immunosuppressive drugs. Mycophenolate mofetil (MMF), an immunosuppressive agent with anti-inflammatory properties, was able to avoid the initiation and progression of glomerular damage and albuminuria in rats with streptozotocin-induced diabetes[124]. Subsequent works demonstrated that MMF produced a marked reduction of proteinuria, as well as the amelioration of both renal glomerular and tubulointerstitial scarring[125]. All these renoprotective effects did not have any relationship with beneficial changes of hemodynamic or metabolic determinants, suggesting that the benefits probably resulted from its immunosuppressive and anti-inflammatory actions. Thus, it was demonstrated that MMF is able to reduce glomerular and tubulointerstitial inflammatory cell infiltration[126] and abrogate different processes related to the action of TNF-α, such as the expression of ICAM1, the adhesion of neutrophils to the endothelium, as well as the production and discharge of inflammatory cytokines (IL-6 and TNF-α)[127-129]. Despite these promising experimental results, immunosuppressive treatments actually are not a current clinical therapeutic option in patients with DKD.
Modulation of inflammatory cytokines, mainly TNF-α, has been evaluated in experimental works, as well as in studies with diabetic patients. In experimental studies, the use of etanercept, a recombinant human soluble TNF-α receptor, was associated with the reduction of the urinary excretion of this cytokine and the avoidance of initial kidney structural injury and renal hypertrophy in experimental models of DKD[88]. Similarly, the use of the monoclonal anti-TNF-α antibody infliximab on rats with DKD led to a significant reduction in the urine excretion of TNF-α and albuminuria[130]. At the present time, the use of soluble TNF-α receptors or monoclonal antibodies as therapy for DKD have been not tested in clinical trials. However, pentoxifylline (PTF), a drug used in the treatment of peripheral vascular disease, possesses modulating effects on TNF-α, with significant anti-inflammatory properties that has potential clinical applications as a therapy for DKD.
PTF, a methylxanthine derived with non-specific phosphodiesterase activity, possess significant anti-inflammatory properties: this drug is able to abrogate the transcription of the TNF-α gene and hamper the augmentation of TNF-α mRNA[131,132], regulate IL-1, IL-6 and IFN-γ, and lessen diverse cell actions related to inflammation, such as activation, adherence and phagocytosis[133,134]. PTF is able to reduce the generation of profibrotic factors (fibronectin and TGF-β) in human mesangial cells caused by elevated glucose levels, and also it protects these cells from the harmful effects of angiotensin II on matrix proteins[135]. Furthermore, in animal models of DKD, PTF significantly decreased the width of the GMB, the plastering of podocyte foot processes, and the disappearance of the fenestrations of glomerular endothelium[136]. In addition, PTF prevents the increased renal expression of the inflammatory cytokines TNF-α, IL-1 and IL-6 secondary to diabetes, resulting in a reduction of UAE, the urinary concentration of these cytokines, as well as a decrease of renal hypertrophy and sodium retention[56,87,88].
Beyond the results from experimental works, a number of clinical studies have showed that PTF is effective to reduce albuminuria and has potential beneficial effects on renal function in diabetic patients[137-143]. The antiproteinuric action of PTF has been straightly associated with its anti-TNF-α activity. This effect has been demonstrated to affect molecules with a high and a low molecular weight, such as IgG, ceruloplasmin, transferrin, albumin, and α 1-antitrypsine, lysozyme and β2-microglobulin, respectively[144]. The reduction of proteinuria after PTF administration has been confirmed in various prospective, controlled, randomized clinical studies[144-146]. Furthermore, PTF has showed beneficial effects on the urinary excretion of markers of tubular damage, such as N-acetylglucosaminidase[145]. The effectiveness of PTF to reduce urinary protein excretion has been compared with that of angiotensin-converting enzyme inhibitors (ACEI) in T2DM, and the results reveal that PTF is similar to captopril[144,145]. Moreover, the use of PTF on top of blockade of the RAAS with ACEI or angiotensin II receptor blockers, provide a supplementary and synergistic decrease of albuminuria[147,148], an effect not related to blood pressure and metabolic control, but positive and directly related with a lowering in the urinary concentration of TNF-α[147].
The capacity of PTF to reduce UAE in subjects with DKD has been confirmed by a recent meta-analysis, which highlighted that the anti-inflammatory properties of this drug, with a decrease in the generation of proinflammatory cytokines, was the main potential mechanism to explain its antiproteinuric effect[149]. A prospective, randomized clinical trial is now ongoing to evaluate the effects of PTF on the renal function of patients with DKD[150], and new definitive trials (multicentre, adequately powered, prospective, placebo controlled) are needed to give definitive evidence for the use of PTF as a real option for the treatment of DKD.
CONCLUSION
Diabetes mellitus is a major global health problem. DKD is one of the most important complications and constitutes a challenge for physicians. Conventional treatments provide incomplete protection for the development of renal failure. Therefore, new approaches and therapeutic targets are needed. Based on the results of recent studies, nowadays inflammation is acknowledged as a key factor in the development and progression of DKD. Future therapies will focus on modulation of inflammatory pathways, including targets such as inflammatory cytokines, oxidative stress, JAK/STAT pathway, or NFκB. In addition, further research is needed to understand how inflammatory pathways interact with other pathogenic factors in the context of diabetes.
P- Reviewer: Cui WP, Ozdemir S, Theilade S S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ