Published online Feb 15, 2014. doi: 10.4239/wjd.v5.i1.69
Revised: October 21, 2013
Accepted: January 15, 2014
Published online: February 15, 2014
Processing time: 198 Days and 10 Hours
AIM: To evaluate the safety of four insulin titration algorithms in a homogeneous population of insulin-naïve type 2 diabetic patients.
METHODS: We conducted a 24-wk, open, single-center study with 92 insulin-naïve type 2 diabetes patients who failed treatment with one or two oral drugs. The patients were randomized to one of the four following algorithms: LANMET (n = 26) and LANMET PLUS (n = 22) algorithms, whose patients received a fixed initial insulin dose of 10 U, and DeGold (n = 23) and DeGold PLUS (n = 21) algorithms, whose patients’ initial insulin dose was based on their body mass index (BMI). In addition, patients in the PLUS groups had their insulin titrated twice a week from 2 to 8 U. In the other two groups, the titration was also performed also twice a week, but in a fixed increments of 2 U. The target fasting glucose levels for both groups was 100 mg/dL.
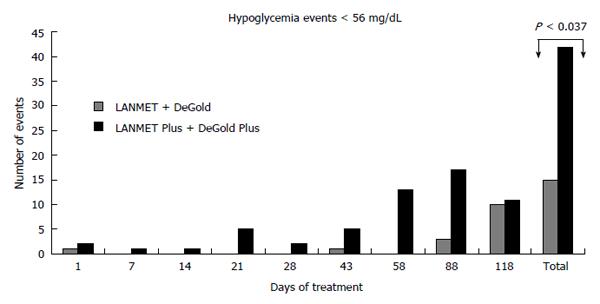
RESULTS: There was no significant difference in efficacy parameters. There was no significant difference when comparing moderate hypoglycemia events in algorithms starting with a 10 U fixed dose and algorithms based on BMI. However, there was a significant increase in moderate hypoglycemia events among the PLUS treated patients when the LANMET and DeGold algorithms were compared with the 2 fast-titration PLUS algorithms. We observed 12 hypoglycemia events in the first group, which corresponded to 0.94 events/patient per year, and we observed 42 events in the second group, which corresponded to 2.81 events/patient per year (P < 0.037). No further significant differences were observed when other comparisons between the algorithms were carried out.
CONCLUSION: Starting insulin glargine based on BMI is safe, but fast titration algorithms increase the risk of moderate hypoglycemia.
Core tip: To start insulin therapy in insulin naïve type 2 diabetes patients, a long-acting basal insulin, such as insulin glargine, is added once a day. The majority of algorithms determine insulin titration according to fasting plasma glucose levels, but the dosage differs at the initial dose, frequency and speed of adjustments. It is difficult to compare the different algorithms employed in trials with populations of different socio-economic strata and variable access to educational materials. Here, we compared the safety of different titration algorithms in a population that was homogeneous in terms of socio-economic strata and with the same degree of education in diabetes.
- Citation: Franco DR, Baptista J, Abreu FR, Batista RB, Eliaschewitz FG. Starting glargine in insulin-naïve type 2 diabetic patients based on body mass index is safe. World J Diabetes 2014; 5(1): 69-75
- URL: https://www.wjgnet.com/1948-9358/full/v5/i1/69.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i1.69
Type 2 diabetes is characterized by insulin resistance and is associated with the incremental loss of pancreatic beta cell mass and/or function[1]. Patients who are initially capable of maintaining a good metabolic control using oral anti-diabetes drugs (OADs) frequently need to add insulin to their treatment over time[2]. The simplest way to begin insulin therapy is to add a long-acting basal insulin, such as insulin glargine, once per day[3].
Basal insulin therapy is an efficient glycemia-lowering treatment, provided it is delivered in the appropriate doses. Therefore, it must be carefully titrated until patients achieve the established fasting plasma glucose goal (FPG)[4]. Several titration algorithms have been validated in clinical trials, and they can be used to guide basal insulin dose adjustments. Most algorithms determine insulin titration according to FPG levels, but differ in the initial insulin dose, frequency, and speed of dose adjustments[5-7]. A new algorithm (DeGold) has been recently described, and it considers the degree of insulin resistance due to obesity and recommends initial doses ranging from 0.2 to 0.35 U/kg according to the patient’s body mass index (BMI)[8].
The initial insulin dose is important for predicting whether a target can be reached and how long titration will take[4] before treatment is started. Treatment compliance may be jeopardized if the treatment period is too long and if patients do not see any significant changes in their FPG levels. The frequency and speed at which insulin doses are adjusted also vary according to the chosen algorithm. For example, in the AT.LANTUS trial with insulin Glargine, titration from 2 to 8 U weekly according to the FPG that was performed by physicians was compared to the increment of 2 U every 3 d until the FPG reached 100 mg/dL that was performed by the patients themselves. The results showed that titration performed by patients could be more effective in achieving A1C targets[6]. In the Canadian INSIGHT Trial, patients titrated their insulin Glargine dose by adding 1 U/d until they reached the target of 100 mg/dL FPG[5].
Provided it is employed correctly according to the “Treat to Target” concept, any algorithm can bring fasting glucose levels to normal and allow patients who are not in need of additional prandial therapy, like rapid acting insulin, to achieve the desired glycated hemoglobin (A1C) values[7].
Hypoglycemia may also be a factor in the achievement of a glycemic target. The occurrence of hypoglycemia events is not solely due to the effects caused by exogenous insulin[9] but is also fundamentally linked to other factors, including the level of education of diabetic patients, especially in regard to compliance to treatment and protective measures against hypoglycemia[10]. It is difficult to compare the efficacy and the safety of all different algorithms used in trials that have populations belonging to different socioeconomic levels and having different access to educative measures[4,7,10]. As such, we decided to compare the safety of different titration algorithms in a population that was homogeneous in terms of socioeconomic level and level of education in diabetes.
The main objective of the study was to evaluate the safety of four insulin glargine titration algorithms applied to a homogeneous sample of insulin-naïve type 2 diabetes patients and to compare the frequency of severe and moderate hypoglycemia (glycaemia < 56 mg/dL) events, the frequency of nocturnal symptomatic hypoglycemia, total number of hypoglycemic events, and serious adverse events. The efficacy parameters analyzed for each algorithm were the changes in A1C from baseline to study end, changes in FPG levels, weight variation during the study, insulin doses, time needed to reach the FPG target, and the proportion of patients who reached an A1C target between 7% and 7.5%, and below 7%.
This was a 24-wk, single-centered, randomized, open study. We screened 125 patients diagnosed with type 2 diabetes, > 18 years old and BMI < 40 kg/m2 who had been on stable treatment with one or two OADs for more than 3 mo, and A1C between 7% and 12%. The main criteria for exclusion were as follows: chronic kidney disease, liver disease with transaminases ≥ 2.5 times the normal value, and any pathology requiring systemic corticosteroid treatment. A total of 33 patients were excluded because their A1C was above threshold, their hepatic enzymes were above normal, or they had moderate renal failure.
The study was approved by the local institucional review board and was conducted according to the Helsinki Declaration and the GCP-ICH. Informed consent was obtained from all patients. All patients were living in the area outside of São Paulo city, had the same socioeconomic background and were insulin treatment naïve. All patients attended the same education sessions on diabetes, and lessons were always given by the same person.
Comparisons between the algorithms were made using ANOVA/Kruskal-Wallis and Student’s t tests. The data on patients who completed the protocol were used, and all patients who received at least one dose of insulin to evaluate data on safety parameters were included.
The demographic data of the randomized patients are shown on Table 1. Population homogeneity was tested and showed the groups were similar in terms of age, weight, BMI, time they have had diabetes for, initial A1C level, and previous treatment with OADs. However, the proportion of M/F gender was significantly different in the LANMET PLUS (P < 0.047) group.
| n | Gender | Age (yr) | Weight (kg) | BMI (kg/m2) | Duration (yr) | Baseline A1C | Baseline FPG (mg/dL) | Previous treatment | |
| Fixed titration 2/2 U | |||||||||
| LANMET | 26 | 8 M | 55.0 ± 10 | 78 ± 16.6 | 30.7 ± 4.95 | 8 ± 4.23 | 9.39% ± 1.67% | 193.0 ± 59.4 | 2 OAD (20) 1 OAD (6) |
| Variable titration | |||||||||
| LANMET PLUS | 22 | 6 M | 52.3 ± 7.7 | 70.6 ± 13 | 27.8 ± 4.7 | 7.8 ± 3.8 | 9.35% ± 1.34% | 179.4 ± 51.4 | 2 OAD (21) 1 OAD (1) |
| Fixed titration 2/2 U | |||||||||
| DeGold | 23 | 14 M | 54.6 ± 8 | 78.3 ± 13.5 | 28.8 ± 4.4 | 10.2 ± 7.1 | 9.21% ± 1.30% | 196.6 ± 54.8 | 2 OAD (19) 1 OAD (4) |
| Variable titration | |||||||||
| DeGold PLUS | 21 | 12 M | 53.8 ± 7.6 | 79.3 ± 15.9 | 29.5 ± 4.4 | 9.8 ± 5.4 | 9.61% ± 1.69% | 196.1 ± 53.4 | 2 OAD (19) 1 OAD (2) |
After 4 wk of a run-in period, 92 patients were randomly distributed to the four algorithms and were treated for the next 16 wk. During this period, 10 visits were scheduled and telephone monitoring was performed by the investigators between visits. A follow-up visit was performed 4 wk after the completion of the study. Three patients withdrew their informed consent. No patients dropped out due to hypoglycemia or any other adverse events.
Most patients were being treated with metformin and sulfonylurea, except for one patient in the DeGold PLUS group who received nateglinide and metformin, and another one in the LANMET PLUS group who received rosiglitazone and metformin. Thirteen patients were on monotherapy, of which seven were on sulfonylurea and six were on metformin. All patients were kept solely either on metformin 2 g/d or on the maximum tolerated dose during the treatment period.
LANMET and LANMET PLUS used the same initial Insulin Glargine dose of 10 U, while DeGold and DeGold PLUS used an initial insulin Glargine dose based on BMI, as shown on Table 2. For the LANMET and DeGold algorithms, the insulin doses were increased by 2 U, twice a week, to reach the FPG target of 100 mg/dL. For LANMET Plus and DeGold Plus, titration was performed by increasing insulin doses, from 2 to 8 U total, twice a week, according to the FPG.
| Initial dose | BMI | Algorithms | |||
| LANMET fixed | LANMETPlus | DeGold fixed | DeGoldPlus | ||
| Fixed initial dose in U | n.a. | 10 | 10 | ||
| Variable dose according to | < 26 | 0.2 | 0.2 | ||
| BMI (kg/m2) in U/kg | 26 < 30 | 0.25 | 0.25 | ||
| 30 < 35 | 0.3 | 0.3 | |||
| > 35 | 0.35 | 0.35 | |||
| Insulin adjustment | FPG | ||||
| Fixed Titration twice/week in U | 2 | 2 | |||
| Variable titration according to | < 100 | 0 | 0 | ||
| FPG (mg/dL) twice/week in U | 101 < 120 | -2 | -2 | ||
| 121 < 140 | 2 | 2 | |||
| 141 < 180 | 4 | 4 | |||
| > 180 | -2 | -2 | |||
Patients administered the insulin at bedtime and adjusted the doses under the supervision of a person over the phone. In all algorithms, the titration of insulin doses was delayed and an immediate reduction of the insulin dose was recommended if hypoglycemia < 70 mg/dL. Insulin titration continued in all algorithms until the targeted FPG, which was between 80 and 100 mg/dL, was reached. The insulin dose was then maintained and considered adequate when at least 50% of the subsequent FPG measurements corresponded to the aimed target.
Rescue therapy with rapid acting insulin was used on one patient who presented with persistent A1C > 8%, even though he had his FPG on target for more than 6 wk.
The patients measured their capillary FPG daily and were instructed to repeat the measurements if they started having symptoms suggestive of hypoglycemia. When necessary, the mean values of 3 d of capillary FPG were used to calculate a new insulin dose.
Severe hypoglycemia: Severe hypoglycemia was defined as an event requiring third party assistance and glucose levels below 30 mg/dL, or if the patient recovered after receiving oral carbohydrates, intravenous glucose, or glucagon.
Symptomatic hypoglycemia: Symptomatic hypoglycemia was defined as an event where the patient presented with symptoms of hypoglycemia, but responded to oral carbohydrate ingestion or had a glycemia < 70 mg/dL (mild) or < 56 mg/dL (moderate).
Asymptomatic hypoglycemia: Asymptomatic hypoglycemia was defined as an event without any hypoglycemia symptoms, but glucose levels below 70 mg/dL.
Asymptomatic nocturnal hypoglycemia: Asymptomatic nocturnal hypoglycemia was determined when glycemia under 70 mg/dL was detected before breakfast.
Symptomatic nocturnal hypoglycemia: Symptomatic nocturnal hypoglycemia was defined when hypoglycemia occurred during sleep, after the bedtime insulin dose and before wakening. In this case, hypoglycemia was classified as mild (plasma glucose > 56 mg/dL), moderate (36 mg/dL < plasma glucose < 56 mg/dL) or severe (plasma glucose < 36 mg/dL).
The evaluation of insulin titration was based on the patients’ diaries and glycemia levels at every visit. Treatment compliance was evaluated based on the aforementioned information.
Table 3 shows insulin glargine doses in U and U/kg, efficacy parameters, namely FPG and A1C at the end of the study, A1C decrease with respect to baseline value, proportion of patients reaching FPG target (A1C < 7.5% or < 7%), and mean titration time to reach the FPG target in the various groups.
| LANMET | LANMET PLUS | DeGold | DeGold PLUS | |
| Initial insulin dose (U) | 10.0 ± 0 | 10.0 ± 0 | 21.0 ± 7.3 | 18.3 ± 7.0 |
| Initial insulin dose (U/kg) | 0.13 ± 0.02 | 0.13 ± 0.03 | 0.26 ± 0.05 | 0.25 ± 0.05 |
| Final insulin dose (U) | 41.65 ± 14.00 | 87.00 ± 26.87 | 54.68 ± 21.63 | 48.19 ± 38.50 |
| Final insulin dose (U/kg) | 0.54 ± 0.20 | 0.59% ± 0.27% | 0.67% ± 0.24% | 0.65% ± 0.52% |
| Baseline A1C | 9.39% ± 1.67% | 9.35% ± 1.34% | 9.21% ± 1.30% | 9.61% ± 1.69% |
| Final A1C | 7.36% ± 1.32% | 7.32% ± 0.67% | 6.82% ± 0.70% | 7.38% ± 0.95% |
| Reduction in A1C | 2.02% ± 1.60% | 2.02% ± 1.17% | 2.48% ± 1.23% | 2.23% ± 1.69% |
| Proportion of patients reaching FPG target | 19/26 (73) | 16/20 (80) | 22/23 (95) | 20/21 (95) |
| Proportion of patients reaching A1C ≤ 7.5% | 17/26 (65) | 13/20 (65) | 20/23 (87) | 13/21 (62) |
| Proportion of patients reaching A1C ≤ 7.0% | 11/26 (42) | 5/20 (25) | 16/23 (69) | 7/21 (33) |
| Duration of titration to reach FPG target (d) | 28 ± 31 | 15 ± 19 | 22 ± 20 | 20 ± 17 |
| Weight variation (kg) | 0.276 ± 2.94 | 1.190 ± 2.430 | 0.954 ± 2.590 | 1.630 ± 2.500 |
| Final FPG (mg/dL) | 119.4 ± 36.2 | 109.0 ± 28.7 | 106.6 ± 18.0 | 107.6 ± 17.3 |
There was no significant difference between the groups in the time required to achieve the target. The safety parameters are shown in Table 4. A unique severe hypoglycemia event (glycaemia < 36 mg/dL) occurred after a prolonged fasting period in a patient randomized according to the DeGold PLUS algorithm. No other severe adverse events occurred.
| LANMET | LANMETPLUS | DeGold | DeGoldPLUS | LANMET andLANMET PLUS | DeGold andDeGold PLUS | LANMETand DeGold | LANMET PLUSand DeGold PLUS | |
| Fixed initial dose | Variable initial dose | Fixed titration | Variable titration | |||||
| Patients with moderate or severe hypoglycemia (n) | 7 (27) | 6 (30) | 5 (22) | 5 (23) | 13 (28) | 10 (23) | 12 (25) | 11 (27) |
| Number of moderate or severe hypoglycemia events | 10 | 22 | 5 | 20 | 32 | 25 | 15 | 42 |
| Patients with symptomatic night hypoglycemia (n) | 13 (50) | 4 (15) | 5 (22) | 4 (19) | 17 (37) | 9 (20) | 18 (37) | 8 (19) |
| Number of nocturnal symptomatic hypoglycemia events | 46 | 16 | 9 | 8 | 62 | 17 | 31 | 25 |
| Patients presenting any type of hypoglycemia (n) | 16 (61) | 14 (70) | 15 (68) | 12 (57) | 30 (65) | 27 (62) | 31 (64) | 26 (60) |
| Number of any type of hypoglycemia events | 113 | 107 | 48 | 111 | 220 | 159 | 157 | 155 |
In a pooled analysis, there was no significant difference when comparing moderate hypoglycemia events in algorithms starting with a 10 U fixed dose with algorithms with BMI variation. However, when we compared patients (n = 46) whose titration increment was 2 U twice a week with patients (n = 43) whose titration varied according to FPG, we observed a clear increase in the number of hypoglycemia events in the second group. We observed 12 hypoglycemia events in the first group, which corresponded to 0.94 events/patient per year, and we observed 42 events in the second group, which corresponded to 2.81 events/patient per year (P < 0.037, Figure 1).
There were no other significant differences in the further comparisons between the algorithms.
Titration algorithms are important tools for maximizing the benefits of insulin therapy for metabolic control. Many algorithms have been proposed as guides for achieving metabolic control with basal insulin therapy. These algorithms differ in their initial recommended doses, and in the frequency and speed of basal insulin dose adjustments[5,6,11,12]. All were conceived based on the treat-to-target concept, thus becoming comparable in efficiency if correctly used. However, because these algorithms are being used in different populations, it is difficult to compare their safety based on the risk of hypoglycemia because it is unclear whether differences in rates of hypoglycemia are truly due to the algorithm itself or to the patients’ varying levels of education. In this study, we evaluated the efficacy and safety of four insulin glargine titration algorithms in a highly homogeneous population to compare the impact of both the initial dose and the titration regimen on hypoglycemia events.
Titration was successfully performed in all groups. The DeGold and DeGold PLUS algorithms used a significantly higher initial insulin doses compared to the other two algorithms, which used a 10 U fixed initial dose. Nevertheless, at the end of the study, the doses were similar in all four groups. The doses were slightly higher (0.67 U/kg) in the DeGold groups, but were comparable to previously reported values (0.69 U/kg) in the LANMET study[11].
As expected, all four algorithms resulted in a decrease in FPG and A1C values, and 85% of all patients actually reached the FPG target and 39% of the patients achieved an A1C < 7% after 18 wk of treatment. This proportion is lower than the 60% reported in the Treat to Target study, where the introduced patient population had lower initial A1C levels (8.6% vs 9.5%) and results were reported after 36 wk of treatment. In our case, all groups presented a mean reduction of at least 2% in A1C values.
LANMET is a more conservative algorithm, as it recommends the smallest initial dose and slower titration, as opposed to the DeGold PLUS algorithm, which recommends the initial insulin dose based on BMI and a faster titration protocol. As such, the most important safety outcome to be compared is the frequency of moderate and severe hypoglycemia events, which is a barrier to the acceptance of insulin therapy among clinicians and patients[13,14].
In addition, hypoglycemia is currently acknowledged as risk factor that could lead to cardiovascular events and death[15-22]. Analysis on the incidence of mild, asymptomatic, or total hypoglycemia events showed no significant difference between the groups. However, when comparing the frequency of severe and moderate hypoglycemia events between the two groups on fixed titration and the other two groups using a variable regimen, a significant increase was observed in the latter groups (0.94 events/patient per year vs 2.81 events/patient per year, P < 0.037).
It has previously been reported that patients typically experience 3 events/patient per year, which is similar to what we observed in the patients who were subject to the titration regimen that varied according to FPG[12]. The frequency of symptomatic hypoglycemia events in the Treat to Target study was higher than in the LANMET trial (4.1 events/patient per year vs 13.9 events/patient per year) that used fixed titration, a finding that is in agreement with our observations[12,13].
The performance of the DeGold algorithm was especially notable, and it was recently proposed as an algorithm to guide the introduction of insulin glargine in replacement of OADs for inpatients[8]. We extended its use to outpatients currently being treated with OADs and as a result, after a mean titration period of 22 d, 95% of the individuals reached the FPG target and 69% reached values of A1C < 7%, without increase in any hypoglycemia categories.
Nevertheless, there was no significant difference between the algorithms regarding efficacy parameters, possibly due to a lack of statistical power because of the small sample size.
An increase in the risk of hypoglycemia was associated with the rapid titration algorithms, in comparison to patients receiving higher initial doses. A possible explanation for the observed discrepancy may be the extremely low number of events that occur in the beginning of treatment. Analysis of the distribution of occurrences throughout the study showed that only 14% of all events occurred during the first 4 wk of treatment (data not shown). After this period, the insulin doses in the titration regimens that varied according to FPG were higher, irrespective of the initial dose. Our data suggest that the initial dose is not important for achieving glycemic control, nor was it shown to affect the rates of hypoglycemia events, as long as titration was performed. However, forced and rapid titration did increase the rates of hypoglycemia events.
In conclusion, there is no increase in the risk of moderate/severe hypoglycemia events when treatment with insulin glargine is initiated on insulin-naïve type 2 diabetes patients using an algorithm where the initial insulin dose is calculated based on BMI, as observed in the DeGold algorithm. However, this risk is increased when a faster titration schedule was used, compared with a fixed 2-U increment twice a week.
Statistical analysis was conducted by Dr. Alves MRC from the School of Public Health, University of São Paulo.
To start insulin therapy in insulin naïve type 2 diabetes patients, a long-acting basal insulin, such as insulin glargine, is added once a day. The majority of algorithms determine insulin titration according to fasting plasma glucose levels, but the dosage differs at the initial dose, frequency and speed of adjustments.
It is difficult to compare the different algorithms employed in trials with populations of different socio-economic strata and variable access to educational materials.
Here, authors compared the safety of different titration algorithms in a population that was homogeneous in terms of socio-economic strata and with the same degree of education in diabetes.
Insulin algorithm titration: A guideline to modify the insulin dose after starting insulin therapy in a patient.
This is an interesting study. The authors tried to compare the safety and efficacy of four insulin glargine algorithms in insulin naive type 2 diabetes patients.
P- Reviewers: Georgescu A, Liu SH, Shafrir E S- Editor: Gou SX L- Editor: A E- Editor: Liu SQ
| 1. | Wright A, Burden AC, Paisey RB, Cull CA, Holman RR. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. Prospective Diabetes Study (UKPDS 57). Diabetes Care. 2002;25:330-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 392] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 2. | Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203. [PubMed] |
| 3. | Barnett A. Dosing of insulin glargine in the treatment of type 2 diabetes. Clin Ther. 2007;29:987-999. [PubMed] |
| 4. | Kadowaki T, Ohtani T, Odawara M. Potential formula for the calculation of starting and incremental insulin glargine doses: ALOHA subanalysis. PLoS One. 2012;7:e41358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Harris S, Yale JF, Dempsey E, Gerstein H. Can family physicians help patients initiate basal insulin therapy successfully: randomized trial of patient-titrated insulin glargine compared with standard oral therapy: lessons for family practice from the Canadian INSIGHT trial. Can Fam Physician. 2008;54:550-558. [PubMed] |
| 6. | Davies M, Storms F, Shutler S, Bianchi-Biscay M, Gomis R. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28:1282-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 226] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 7. | Unger J. Comparing the efficacy, safety, and utility of intensive insulin algorithms for a primary care practice. Diabetes Ther. 2011;2:40-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Sampaio CR, Franco DR, Goldberg DJ, Baptista J, Eliaschewitz FG. Glucose control in acute myocardial infarction: a pilot randomized study controlled by continuous glucose monitoring system comparing the use of insulin glargine with standard of care. Diabetes Technol Ther. 2012;14:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Moberg EA, Lins PE, Adamson UK. Variability of blood glucose levels in patients with type 1 diabetes mellitus on intensified insulin regimens. Diabete Metab. 1994;20:546-552. [PubMed] |
| 10. | Cryer PE. Elimination of hypoglycemia from the lives of people affected by diabetes. Diabetes. 2011;60:24-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Yki-Järvinen H, Kauppinen-Mäkelin R, Tiikkainen M, Vähätalo M, Virtamo H, Nikkilä K, Tulokas T, Hulme S, Hardy K, McNulty S. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia. 2006;49:442-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 295] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 12. | Riddle MC, Rosenstock J, Gerich J. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080-3086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1173] [Cited by in RCA: 1042] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 13. | Kunt T, Snoek FJ. Barriers to insulin initiation and intensification and how to overcome them. Int J Clin Pract Suppl. 2009;6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Polonsky WH, Fisher L, Earles J, Dudl RJ, Lees J, Mullan J, Jackson RA. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28:626-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 1040] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 15. | Johnston SS, Conner C, Aagren M, Smith DM, Bouchard J, Brett J. Evidence linking hypoglycemic events to an increased risk of acute cardiovascular events in patients with type 2 diabetes. Diabetes Care. 2011;34:1164-1170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3445] [Cited by in RCA: 3321] [Article Influence: 207.6] [Reference Citation Analysis (0)] |
| 17. | Desouza C, Salazar H, Cheong B, Murgo J, Fonseca V. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care. 2003;26:1485-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 283] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 18. | Hanefeld M, Duetting E, Bramlage P. Cardiac implications of hypoglycaemia in patients with diabetes - a systematic review. Cardiovasc Diabetol. 2013;12:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Goto A, Arah OA, Goto M, Terauchi Y, Noda M. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ. 2013;347:f4533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 363] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 20. | Bloomfield HE, Greer N, Newman D, MacDonald R, Carlyle M, Fitzgerald P, Rutks I, Wilt TJ. Washington (DC): Department of Veterans Affairs; 2012 Apr. VA Evidence-based Synthesis Program Reports. Predictors and Consequences of Severe Hypoglycemia in Adults with Diabetes - A Systematic Review of the Evidence [Internet]. . [PubMed] |
| 21. | Koshizaka M, Green JB, Alexander JH. Glycemic management in diabetes and the associated cardiovascular risk: are we helping or hurting our patients. Circ J. 2012;76:1572-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Snell-Bergeon JK, Wadwa RP. Hypoglycemia, diabetes, and cardiovascular disease. Diabetes Technol Ther. 2012;14 Suppl 1:S51-S58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |