Published online Aug 15, 2013. doi: 10.4239/wjd.v4.i4.135
Revised: June 4, 2013
Accepted: June 19, 2013
Published online: August 15, 2013
Processing time: 218 Days and 8.5 Hours
AIM: To analyze a large population of patients with diabetes and peripheral neuropathy (PN) to determine other meaningful comorbid etiologies for PN.
METHODS: Peripheral Neuropathy is a common complication of type 1 and 2 diabetes mellitus; however, other potential causes for PN may be co-existing in patients with diabetes. A prospective cohort study was performed to assess patients with diabetes and PN. We compared patients having PN due solely to diabetes with patients possessing co-existing comorbidities, performing clinical (Toronto Clinical Scoring System and the Utah Early Neuropathy Scale), laboratory and electrophysiological assessments in all patients.
RESULTS: Patients with either type 1 or 2 diabetes mellitus and co-existing comorbidities did not have more severe clinical or electrophysiological PN phenotypes overall. However, in patients with type 1 diabetes, presence of a lipid disorder was associated with greater PN severity. In type 2 diabetes patients, both a lipid disorder and cobalamin deficiency were associated with greater PN severity. There was no additive effect upon PN severity with presence of three or more comorbid etiologies.
CONCLUSION: The presence of specific, and not general, comorbidities in patients with type 1 or 2 diabetes corresponds with greater PN severity.
Core tip: The cause of diabetic peripheral neuropathy (DPN) has remained elusive. Comorbid conditions may contribute to the severity of DPN. We studied patients with type 1 or 2 diabetes and concurrent DPN in order to identify potential comorbid conditions associated with greater neuropathic deficit. Concurrent lipidemia was associated with worse DPN in either type 1 or 2 diabetes. A concurrent vitamin B12 deficiency increased severity of DPN in type 2 diabetes. Although our results were potentially confounded by higher HbA1C values in patients with comorbid conditions, vigilance should occur for other causes of PN when diabetes is present.
- Citation: Sachedina S, Toth C. Association of comorbidities with increasing severity of peripheral neuropathy in diabetes mellitus. World J Diabetes 2013; 4(4): 135-144
- URL: https://www.wjgnet.com/1948-9358/full/v4/i4/135.htm
- DOI: https://dx.doi.org/10.4239/wjd.v4.i4.135
Peripheral neuropathy (PN) is a prevalent condition in the general population[1]. While the most common cause of PN is diabetes mellitus, of both type 1 and type 2 forms, there are many other proven etiologies and forms of PN. Patients with diabetes are subject to comorbid conditions, either by association or coincidence. As such, patients with PN due to diabetes [termed diabetic peripheral neuropathy (DPN)] may manifest other conditions capable of exacerbating or initiating PN. Although different etiologies of PN possess various pathophysiologies, the presence of PN and its increasing severity greatly reduces quality of life[2]. Clinically, patients presenting with symptoms of PN in the presence of already or newly diagnosed diabetes are often subsequently concluded to have only DPN without further laboratory investigations performed. This may preclude investigations to determine other potential, and sometimes treatable, causes of PN. The aim of this study was to identify those patients with the presence of multiple conditions capable of causing PN other than diabetes to determine if multiple comorbidities increases PN severity. We hypothesized that the presence of comorbidity capable of leading to PN occurring in conjunction with either type 1 or 2 diabetes would lead to an increase in severity of PN. Further, we hypothesized that the presence of multiple comorbidities would have an additive effect upon the severity of PN.
Particular comorbidities have shown relationship to greater severity of DPN, and have included elevated triglycerides, smoking, hypertension, and obesity[3]. Hyperlipidemia[4] and statin medication use[5,6] are both exceedingly common in patients with diabetes, and may also be implicated as causative for PN. Another recent association is that of metformin use, which was associated with elevation of fasting methylmalonic acid levels and greater presence of DPN[7]; this association may relate to a resulting vitamin B12 (cobalamin) deficiency. At present, we are not aware of other potential comorbidities important in the assessment of DPN patients. Therefore, in the current study, we sought for any additional comorbidities capable of contributing to the greater impact and severity of DPN.
We designed this prospective study to examine our hypotheses and to detect any clinically meaningful synergistic effects of comorbid conditions in patient populations with diabetes mellitus. We assessed for presence of both general and specific comorbidities, including alcoholism, thyroid disease, monoclonal gammopathy of uncertain significance, autoimmune antibody presence, uremia, and cobalamin or other vitamin deficiencies with or without associated high fasting methylmalonic acid levels. We concurrently examined hypercholesterolemia and hyperlipidemia (grouped as a lipid disorder), and hypertension, all of which are potential risk factors for the development of DPN[3].
This study was ethically approved by the University of Calgary Centre for Advancement of Health. Recruitment of subjects occurred from December 2008 until July 2010 at the Neuromuscular and Neuropathic Pain Clinics at the University of Calgary. Subjects were recruited prospectively upon initial evaluation at the tertiary care clinics. Inclusion criteria consisted of the following: (1) all subjects provided informed written consent prior to involvement; and (2) a diagnosis of pre-existing type 1 or 2 diabetes was provided based upon laboratory testing-two prior fasting glucose results of ≥ 7.1 mmol/L (126 mg/dL) [or random glucose of ≥ 11.1 mmol/L (200 mg/dL) with symptoms of hyperglycemia for type 1 diabetes] or two oral glucose tolerance tests leading to a 2 h serum glucose of ≥ 11.1 mmol/L (200 mg/dL) (based on Canadian Diabetes Association guidelines). Exclusion criteria included: (1) subjects with impaired fasting glucose or impaired glucose tolerance; and (2) absence of discernible PN or presence of questionable PN (see below). The age of diagnosis of diabetes and the duration of symptoms of PN were recorded.There was no specific sample size calculation performed and no pre-specified cohort patient number was determined for this study.
Clinically, each patient was examined for PN and scored using the Toronto Clinical Scoring System (TCSS)[8] and the Utah Early Neuropathy Scale (UENS)[9]. The TCSS is a validated method for evaluation of PN with higher scores correlated with greater sural nerve pathology on biopsy[8]. The TCSS has greater emphasis upon sensory deficits related to PN as compared with other comparable scales. All clinical examination was performed prior to knowledge of blood testing results. The UENS has greater applicability to determining clinical progression of PN than TCSS, and also places emphasis upon sensory abnormalities. After clinical scales were completed, subjects with TCSS ≤ 5 and UENS ≤ 6 were excluded due to uncertainty regarding the presence of PN. Subjects receiving known neurotoxic medications or chemotherapy, or with a history of carcinoma were excluded. Although laboratory testing was performed after clinical evaluation, the evaluator was not blinded to the form of diabetes mellitus or the presence of previously diagnosed comorbid conditions.
Laboratory testing (Calgary Laboratory Services) was performed after clinical evaluation, and consisted tests listed in Table 1. When abnormalities were identified with blood testing, those particular abnormal tests were repeated using new blood samples for verification. Past or present alcoholism was also taken into account and diagnosed based upon Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV criteria. Seated blood pressure measurements were performed twice-hypertension was based upon two measurements ≥ 130/80 using the Canadian Diabetes Association criteria or based upon pre-existing diagnosis made prior to evaluation.
| Blood test | Normative range |
| Red blood cell counts | |
| Women | 4.0-5.6 × 1012/L |
| Men | 4.5-6.0 × 1012/L |
| White blood cell counts | 4.0-11.0 × 109/L |
| Platelet counts | 150-400 × 109/L |
| Electrolytes (mmol/L) | |
| Sodium | 133-145 |
| Potassium | 3.3-5.1 |
| Chloride | 98-111 |
| Bicarbonate | 21-31 |
| Calcium | 2.10-2.55 |
| Magnesium | 0.65-1.05 |
| Urea (mmol/L) | 3.0-8.5 |
| Creatinine (μmol/L) | 50-120 |
| Aspartate transaminase (U/L) | 8-40 |
| Alanine transaminase (U/L) | 1-60 |
| Gamma glutamyl-transferase (U/L) | 8-40 |
| Albumin (g/L) | 33-48 |
| Total bilirubin (μmol/L | 0-20 |
| Total cholesterol (mmol/L) | 3.8-5.2 |
| Low density lipoproteins (mmol/L) | 2.2-3.4 |
| High density lipoproteins (mmol/L) | > 0.9 |
| Triglycerides (mmol/L) | 0.6-2.3 |
| Cobalamin (measured by immunoassay), (pmol/L) | > 155 |
| Thiamine (μg/L) | 33-110 |
| Thyroid stimulating hormone (mU/L) | 0.2-6.0 |
| Total thyroxine (nmol/L) | 59-154 |
| Antinuclear autoantibody detection | ≤ 1:80 |
| Extractable nuclear antigens | Absent |
| Serum protein electrophoresis | The sensitivity for detection of gammopathy was 2 g/L with serum protein electrophoresis, and samples with peaks of 2-4 g/L were subjected to immunofixation for verification |
| Serum copper (μmol/L) | 11-24 |
| Fasting methylmalonic acid (measured using high performance liquid chromatography), (μmol/L) | < 0.15 |
| Hemoglobin A1C | 4.3%-6.1% |
Diagnosis of comorbidity was provided based upon an identified laboratory abnormality or previously identified comorbidity diagnosed prior to this evaluation. Previously diagnosed comorbidities were verified using electronic or paper patient chart information. All abnormalities were determined using the population normal values as determined by Calgary Laboratory Services.
Once the presence of underlying comorbidities was determined, subjects were categorized into four groups: patients solely with type 1 diabetes (DM1 only); patients with type 1 diabetes and an existing comorbidity or comorbidities (DM1 plus comorbidity); patients solely with type 2 diabetes (DM2 only); and patients with type 2 diabetes and an existing comorbidity or comorbidities (DM2 plus comorbidity). Sub-categorization based upon individual comorbidities and number of comorbidities was performed subsequently. The following comorbidities were used for categorization: lipid disorder (elevated low density lipoprotein or triglycerides or both), cobalamin deficiency (depressed cobalamin level or elevated fasting MMA level or both), monoclonal gammopathy of uncertain significance (MGUS), thyroid disorder, renal dysfunction (elevated creatinine), autoimmune disorder [prior diagnosis or detection of positive extractable nuclear antigen (ENA) status of significance], alcoholism, or hypertension. We defined an autoimmune disorder as the presence of an inappropriate immune response with detectable auto-antibodies having potential for leading to a neurological disease including rheumatoid arthritis, sjogren syndrome, systemic lupus erythematosus, or systemic vasculitis. Other potential comorbidities were recorded for consideration of potential impact.
All patients received electrophysiological evaluation for PN severity after clinical evaluation and prior to receipt of laboratory testing results. Cadwell Sierra Wave (Cadwell Laboratories, Kennewick, WA) electromyography machines were used. Both motor and sensory testing was performed on the non-dominant upper and lower limb; in the case of ambidextrous patients, the left upper and lower limbs were studied. Motor nerve conduction studies (NCS) were performed using stimulation of the median nerve (wrist, elbow), ulnar nerve (wrist, below elbow, above elbow), peroneal (ankle, below fibular head and above fibular head locations) and tibial (ankle, popliteal fossa locations) nerves. For each motor nerve, distal motor latencies, compound motor action potentials, and conduction velocities were obtained or calculated. F wave latencies were obtained from median, ulnar, peroneal and tibial nerves. Sensory antidromic NCS were performed using the median (digits 2 and 4), ulnar (digits 4 and 5), superficial radial, superficial peroneal and sural nerves, with sensory nerve action potentials (SNAP), onset latency, and conduction velocity obtained or calculated. Temperatures were maintained at ≥ 32 °C for the upper extremities, and ≥ 30 °C for the lower extremities during NCS testing. Absent electrophysiological responses were used to calculate amplitude values, but latency and velocity values were not entered in the analysis for absent responses in order to not obscure data analysis. Patients were excluded if they refused electrophysiological testing or laboratory testing.
Our primary objective was to determine the impact of presence of any comorbidities associated with development of PN upon the severity of PN in patients with either type 1 or 2 diabetes. Secondarily, we also analyzed specific individual comorbidities, and presence of multiple comorbidities for determination of impact upon PN severity. Analysis was performed for the subject categorizations described above. Group equivalence for age, duration of diabetes, duration of PN symptoms, A1C, and alcohol exposure were compared by independent samples t-test; gender was compared by chi-square testing. In all cases, type 1 and 2 diabetes were considered separately and the two forms of diabetes were not directly compared. Other elements of the past medical history not specified above were not statistically compared due to their heterogeneity. Our primary outcome measures were clinical neuropathy severity (TCSS, UENS) and electrophysiological markers of neuropathy; for the latter we chose to test sensory NCS of the lower extremity (conduction velocity and SNAP for superficial peroneal and sural nerves) as we hypothesized these would most likely to demonstrate exacerbation due to progression of PN. Secondary outcome variables included the other sensorimotor electrophysiological parameters for motor responses of the lower limbs and sensorimotor studies of the upper limbs. We determined that these data did not follow a normal distribution (performed with Shapiro-Wilk testing) so comparisons were made using Mann-Whitney U test. Bivariate correlations of primary outcomes and numbers of comorbidities were calculated using Spearman rho test. In addition, we performed a post-hoc linear regression analysis for determination of any potential associations with worsening diabetic status (using HbA1C). We used HbA1C scores as the dependent variable, while explaining variables were chosen to be fasting Methylmalonic acid (MMA) levels, triglycerides, total cholesterol levels, low density cholesterol, and high density cholesterol. Furthermore, a post-hoc linear regression analysis was performed for the type 2 diabetes patient cohort to determine any potential association between cobalamin and fasting MMA levels with greater severity of PN-for this, we used TCSS and UENS scores as the dependent variables, while explaining variables were chosen to be fasting MMA levels. Lastly, a linear regression analysis was performed using TCSS amd UENS total scores as the dependent variable and age, duration of diabetes, A1C and presence of comorbidities and number of comorbities as explaining variables. We set α to be 0.05, and we utilized Bonferroni corrections for analysis of secondary outcome measures, applied whenever multiple comparisons for the same cohorts were performed. Values are presented as mean ± SE throughout.
Demographics and individual comorbidities for each cohort are presented in Table 2. We prospectively enrolled a total of 369 patients. A total of 32 patients (3 type 1 diabetes, 29 type 2 diabetes) declined participation based upon personal choice. DM1 only and DM1 plus comorbidity cohorts were similar with respect to age, gender, duration of diabetes, and HbA1C. However, DM2 plus comorbidity cohorts had longer durations of diabetes and higher HbA1C levels as compared to the DM2 only cohort. We excluded a total of 10 patients for unwillingness to perform testing. Another 17 patients were excluded due to presence of impaired fasting glucose or impaired glucose tolerance rather than strict diabetes.
| Type 1 diabetes only | Type 1 diabetesplus comorbidity | Type 2 diabetesonly | Type 2diabetes plus comorbidity | |
| Patients | 31 | 19 | 228 | 91 |
| Duration of disease (mo) | 316 ± 26 | 310 ± 25 | 107 ± 7 | 150 ± 12a |
| HbA1c level (%) | 11.2 ± 1.4 | 11.7 ± 1.6 | 9.8 ± 0.2 | 11.2 ± 0.3a |
| Age (yr) | 50 ± 7 | 54 ± 8 | 62.0 ± 1.8 | 61.0 ± 1.4 |
| Sex (male) | 17 (55) | 12 (63) | 91 (40) | 36 (40) |
| Nature of comorbidities | 15 (79) | 63 (69) | ||
| lipid disorder | ||||
| Low cobalamin/elevated MMA | 9 (47) | 36 (40) | ||
| Monoclonal gammopathy | 2 (11) | 4 (4) | ||
| Thyroid disease | 5 (26) | 11 (12) | ||
| Uremia | 7 (37) | 13 (14) | ||
| Autoimmune diseases | 4 (21) | 8 (9) | ||
| Alcoholism | 3 (16) | 10 (11) | ||
| Hypertension | 13 (68) | 74 (81) | ||
| Medications | ||||
| Insulin | 31 (100) | 19 (100) | 55 (23) | 24 (26) |
| Metformin | 198 (86) | 80 (88) | ||
| Glyburide | 155 (68) | 63 (69) | ||
| Gliclazide | 41 (18) | 11 (12) | ||
| Statins/Ezetemide | 21 (68) | 9 (47) | 146 (64) | 48 (53) |
| Blood pressure medications | 22 (71) | 14 (74) | 118 (52) | 57 (62) |
| Thyroid replacement | 12 (39) | 5 (26) | 38 (17) | 14 (15) |
| SSRIs | 4 (13) | 2 (11) | 28 (12) | 16 (18) |
| Anxiolytics/Sedatives | 8 (26) | 3 (16) | 48 (21) | 13 (14) |
| NSAIDs | 12 (39) | 9 (47) | 104 (46) | 55 (60) |
| Acetaminophen | 6 (19) | 3 (16) | 37 (16) | 17 (19) |
| Gabapentin | 5 (16) | 4 (21) | 41 (18) | 15 (16) |
| Pregabalin | 4 (13) | 3 (16) | 36 (16) | 11 (12) |
| Codeine | 3 (10) | 4 (21) | 31 (13) | 8 (9) |
| Amitriptyline | 3 (10) | 3 (16) | 25 (11) | 6 (7) |
| Oxycocet | 1 (3) | 2 (11) | 18 (8) | 4 (4) |
| Nortriptyline | 1 (3) | 1 (5) | 12 (5) | 4 (4) |
| Duloxetine | 2 (6) | 3 (16) | 22 (10) | 5 (5) |
| Venlafaxine | 3 (10) | 2 (11) | 25 (11) | 4 (4) |
| Fentanyl | 1 (3) | 2 (11) | 6 (3) | 3 (3) |
| Tramadol | 1 (3) | 0 (0) | 8 (3) | 2 (2) |
| Morphine | 0 (0) | 2 (11) | 7 (3) | 3 (3) |
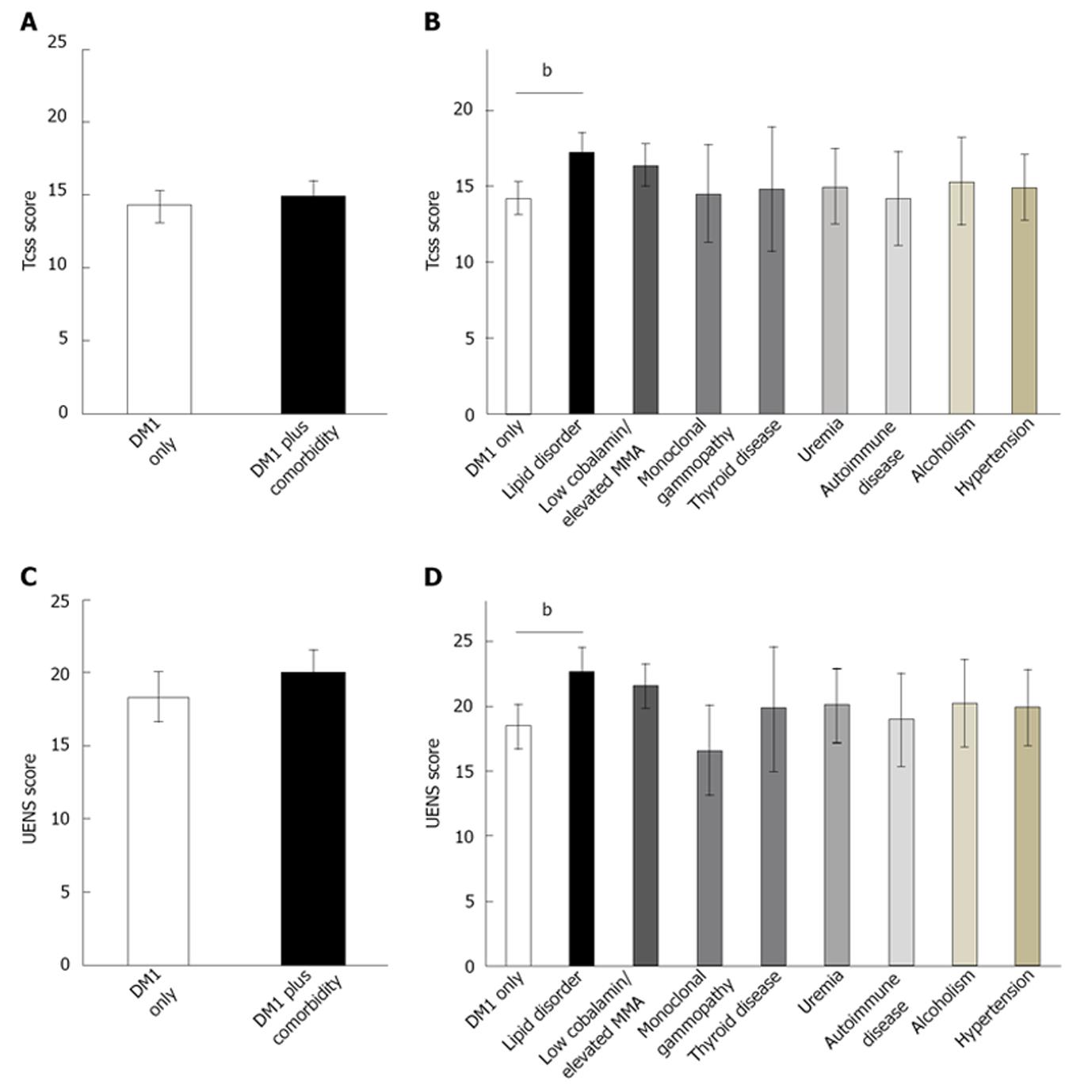
The presence of an identified comorbidity (Table 2) in patients with type 1 diabetes did not increase the TCSS (P = NS, F = 3.1) or UENS (P = NS, F = 1.4) scores (Figure 1). In addition, primary electrophysiological outcomes for sensory electrophysiological testing of the lower limbs were also not different between DM1 only and DM1 plus comorbidity cohorts (P = NS, F = 0.00-1.2).
For secondary outcome measures, after Bonferroni corrections were applied. Analysis showed DM1 plus comorbidity subjects had increased onset latency for the sensory conduction study at the ulnar nerve at digits 4 and 5 (3.3 ± 0.1 ms vs 3.6 ± 0.1 ms, P < 0.001, F = 8.9 and 3.2 ± 0.1 ms vs 3.6 ± 0.1 ms, P < 0.001, F = 10.6 respectively).
For individual comorbidities, type 1 diabetes patients[6,10] with presence of triglyceridemia or lipid disorder had greater TCSS (ANOVA, P < 0.007, F = 8.4) and UENS (ANOVA, P < 0.007, F = 13.7) scores (Figure 1) than type 1 diabetes patients without comorbidities. Other individual comorbidities did not impact upon severity of PN. Finally, the presence of multiple (≥ 3) comorbidities in type 1 diabetes patients did not have a compounding effect for the severity of PN (P = NS, F = 1.2).
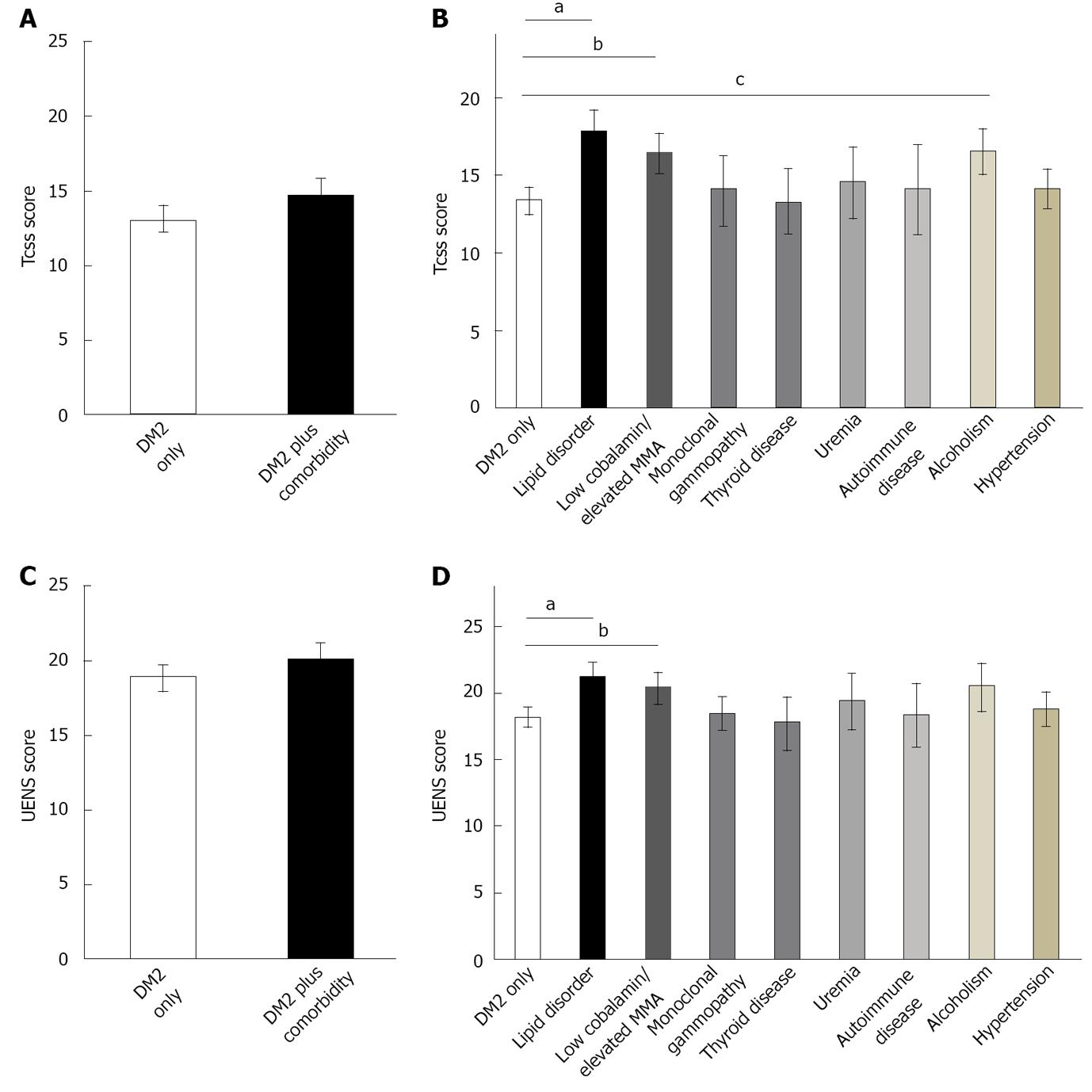
The presence of an acknowledged comorbidity in patients with Type 2 diabetes did not increase the TCSS (P = NS, F = 2.3) or UENS (P = NS, F = 2.2) scores (Figure 2). Similarly, there were no electrophysiological differences between DM2 only and DM2 plus comorbidity for sural and superficial peroneal parameters (P = NS, F = 0.0-0.9).
For secondary outcome measures, there were significant differences for other electrophysiological parameters. DM2 plus comorbidity subjects did have prolonged onset latency for the median motor studies (5.4 ± 0.1 vs 4.2 ± 0.1, P < 0.001, F = 4.6), peroneal motor studies (5.5 ± 0.1 vs 5.0 ± 0.1, P < 0.001, F = 7.6), and tibial motor studies (5.5 ± 0.2 vs 4.9 ± 0.1, P < 0.001, F = 6.2) when compared to the DM2 only cohort. In addition, there was greater slowing of conduction velocities for the median nerve across the forearm (44.3 ± 0.7 vs 50.0 ± 0.5, P < 0.001, F = 11.5), for the ulnar nerve across the forearm (49.3 ± 1.3 vs 54.2 ± 0.8, P < 0.001, F = 7.7), and for the peroneal nerve across the lower leg (35.3 ± 1.1 vs 39.3 ± 0.6, P < 0.001, F = 5.5) as compared to the DM2 only cohort.
Examination of individual comorbidities again identified the presence of a lipid disorder to contribute to greater PN severity based upon TCSS (ANOVA, P < 0.007, F = 5.7) and UENS (ANOVA, P < 0.007, F = 2.5) scores (Figure 2) in type 2 diabetes subjects. In addition, the presence of cobalamin deficiency or elevated fasting MMA levels were associated with higher TCSS (ANOVA, P < 0.007,
F = 3.9) and UENS (ANOVA, P < 0.007, F = 3.3) scores in type 2 diabetes subjects as compared to DM2 only subjects. Finally, the presence of alcoholism was associated with a higher TCSS (ANOVA, P < 0.007, F = 2.1) but not UENS (ANOVA, P = NS, F = 1.6) score in type 2 diabetes cohorts relative to DM2 only subjects.
As with type 1 diabetes, the presence of multiple comorbidities in type 2 diabetes patients did not have an additive effect upon severity of PN.
We used linear regression to determine the impact of multiple comorbidities upon severity of PN. In type 1 diabetes, there was no significant linear relationship between multiple comorbidities and severity of PN using TCSS (R2 = 0.21) or UENS (R2 = 0.33) scores. Likewise, no additive effect upon PN severity could be shown for type 2 diabetes subjects for multiple comorbidities using TCSS (R2 = 0.04) or UENS (R2 = 0.02) scores.
We examined for association of potentially important comorbid factors with severity of diabetes mellitus using HbA1C levels. There were no significant associations between HbA1C for either of type 1 or 2 diabetes with any of fasting MMA, triglycerides, total cholesterol, low density cholesterol, or high density cholesterol levels (R2 = 0.08-0.26). However, it should be noted that statin medication use was very common in both type 1 and type 2 diabetic cohorts.
In type 2 diabetes patients, there was a significant association between TCSS and UENS scores with fasting MMA levels (R2 = 0.48 and R2 = 0.52 respectively, P < 0.025), and a less robust, but still significant association with cobalamin levels (R2 = 0.42 and R2 = 0.44 respectively, P < 0.025). Greater elevation of MMA levels and greater depression of cobalamin levels were associated with greater severity of PN.
Although the presence of a coexisting comorbidity did not increase the severity of PN overall in patient cohorts with type 1 or 2 diabetes, particular comorbidities were associated with a more severe phenotype of PN. The presence of a lipid disorder in either type 1 or 2 diabetes was associated with greater neuropathy severity. The presence of cobalamin deficiency and/or elevated fasting MMA levels was correlated with greater presence of neuropathy in type 2 diabetes subjects. While these associations are not necessarily causative, they suggest that greater attention should be afforded to potentially correctable comorbid lipid disorders and for cobalamin deficiencies and/or elevated fasting MMA levels in patients with diabetes. In our patient populations, we have initiated management of cobalamin deficiencies and elevated fasting MMA levels with continuous monthly intramuscular cobalamin therapy; patients with lipid disorders have simultaneously started on appropriate management. Follow-up data for these interventions is not yet available. The worsening of PN was detected by clinical scoring and not electrophysiological measures for peripheral nerves in the lower extremities, suggesting that worsening of PN may relate to additional small fibre dysfunction, or dorsal column dysfunction in case of cobalamin deficiency, that is undetectable with nerve conduction studies. Another interesting and unexpected finding was the presence of electrophysiological prolongation of latencies and slowing of conduction velocities in DM2 plus comorbidity subjects.
Progression and severity of DPN has been reported to depend upon a number of factors including elevated triglycerides, smoking, hypertension, and obesity[3]. Hyperlipidemia may contribute to oxidative stress at the dorsal root ganglia, contributing to greater diabetes-induced neurodegeneration[11]. This may also relate to the presence of oxidized low density lipoprotein and its receptor, the lectin-like oxLDL (LOX-1) receptor[12]. Possibly toxic to dorsal root ganglia on its own, oxLDL presence, not quantified in this study, is known to be elevated in patients with diabetes and may contribute to other diabetic complications, including retinopathy[13]. Previously, there has been speculation about hyperlipidemia leading to peripheral neuropathy irrespective to diabetic status[4], but its occurrence in idiopathic peripheral neuropathy does not appear to be different from that of control subjects[14]. However, hypertriglyceridemia is more common in patients with PN due to diabetes, impaired glucose tolerance, or alcoholism[14] so its co-existence is not unexpected. As effective treatments do exist for the management of lipid disorders, future research should assess the potential for intervention in patient populations with DPN. The role of co-existing treatments of lipid modulating drugs, such as statins[6], in the presence of concurrent diabetes requires further investigation as well. Although controversially implicated in peripheral neuropathy[5,6], the role of statins is unclear but they may have played a confounding role in the present study. Lastly, it is possible that patients with concurrent lipid disorders have less rigorous care of their diabetes-patients with DM2 plus comorbidities had a greater duration of diabetes as well as a higher HbA1C value.
Recently, the presence of elevated MMA levels in patients with DPN has been related to metformin use contributing to greater presence of DPN[7]. Moreover, while higher MMA levels are generally related to vitamin B12 (cobalamin) deficiency, this may result from renal dysfunction or elderly age as well[15]. Although cobalamin deficiency is most classically associated with subacute combined degeneration, an exclusive peripheral neuropathy (PN) presentation occurs, typically manifesting as an axonal polyneuropathy with additional small fiber dysfunction[16-18]. As accumulating evidence suggests that the cobalamin-deficiency-associated metabolite MMA is more sensitive and specific than serum cobalamin itself[19], its use for detection of potential cobalamin deficiency has been recommended as an investigations with high diagnostic yield in patients with distal symmetric polyneuropathy[20]. This concurrent deficiency was found in patients with type 2 diabetes, many of whom were taking metformin, with more severe neuropathy phenotypes. In this work, higher levels of fasting MMA or lower levels of cobalamin were also associated with greater severity of PN, as we showed previously in a separate cohort of type 2 diabetes patients[7]. However, our findings support the additional and often overlooked assessment for the concurrent presence of cobalamin deficiency, a potentially treatable contribution, in patients with DPN and type 2 diabetes.
Alcoholic polyneuropathy is another form or PN which can be associated with concomitant neuropathic pain. This may be due to the direct toxic effects of ethanol or its metabolites upon peripheral nerve fibers[21] or may be related to a subsequent thiamine deficiency[22]. Its potential for worsening existing DPN was possibly a factor in the type 2 diabetes population (using the TCSS but not the UENS scale). The presence of alcoholism in type 1 diabetes influences the presence of PN with a U-shaped associative curve[23]. This may be true in the type 2 diabetic patient also[24]. There were a number of other comorbidities that were not associated with greater severity of PN in type 1 or type 2 diabetes patients. Peripheral neuropathy associated with MGUS[25] was rarely coincidental in DPN patients. Thyroid disorders (both hyperthyroidism[26] and hypothyroidism)[27], frequently treated early after discovery, were not associated with further worsening of PN. Somewhat surprisingly, renal dysfunction, a potent cause of peripheral neuropathy[28] with potential relationship to diabetes[29] was not additive for DPN severity. Autoimmune disorders may have been too uncommon to contribute significantly to exacerbation of PN severity. Although speculated to impact upon DPN[3,30], hypertension was not a significant contributor to DPN severity in our cohorts of type 1 or 2 diabetes patients. We did not examine other potential factors implicated in progression of DPN, such as smoking or body mass index due to incomplete data acquisition during assessments. It is possible that some of the above comorbidities were not associated with greater severities of PN due to insufficient sample sizes.
We present these findings with limitations. Although we identified patients prospectively, they were not randomly selected from a population with type 1 or 2 diabetes with or without DPN; instead, these patients were referred for tertiary care. Our sample size was not based upon a pre-determined power analysis. We did not identify a separate cohort of patients with asymptomatic DPN. Higher HbA1C levels and longer durations of diabetes in patients with type 2 diabetes and comorbidities would certainly be anticipated to contribute to greater PN severity and may have impacted upon presented findings. Investigators were blinded to the laboratory results until clinical and electrophysiological studies were completed, but were not blinded to presence of type of diabetes or presence of comorbidities previously diagnosed. We did not use an established comorbidity burden tool to assess the studied comorbidities studied. All of our results were based upon TCSS and UENS scores-these are clinically relevant scales easily performed at the bedside, but have subjective components, and may not have the sensitivity of epidermal nerve fiber densities with skin biopsy[31] or confocal corneal microscopy[32]. Patients were on numerous medications for diabetes and other conditions; the heterogeneity of these medications and the conditions they were intended to treat made their individual assessment for contribution impossible. As a result, we did not exclude patients based upon any medication used, and acknowledge that this may have impacted upon clinical and electrophysiological assessments. Finally, this study was conducted at a single centre by a single assessor which could introduce biases due to referral patterns and assessment protocols used.
This study identified potential contributing comorbidities in approximately 30% of patients with diabetes and PN. Although we identified two potentially treatable specific comorbidities (cobalamin deficiency and lipid disorder), we do not yet know if management of these conditions will slow progression of PN, as may occur with procedures such as pancreatic islet transplantation[33] for treatment of type 1 diabetes. However, the use of simple, routine laboratory testing by primary physicians can identify factors for potential intervention in the future for DPN patients which may prevent clinical progression of PN. Lastly, the identification of these comorbidities, should not be viewed as a replacement for symptomatic relief, but as a potentially identifiable and modifiable component of an already diagnosed PN in patients with diabetes.
The pathophysiology of diabetic peripheral neuropathy remains uncertain and complex. Studies of comorbid conditions capable of causing peripheral neuropathy may assist in determination of causation of diabetic peripheral neuropathy and assist the clinician in managing patients with multiple conditions capable of causing peripheral neuropathy.
The presence of multiple potentially causative conditions is not uncommon in patients with diabetic peripheral neuropathy. Those comorbid conditions capable of worsening diabetic peripheral neuropathy may be subject to intervention, slowing the progression of peripheral neuropathy in patients with diabetes mellitus. Future studies should address the management of lipid disorders and vitamin B12 deficiency in populations with diabetic peripheral neuropathy.
Although its isolated relationship to peripheral neuropathy is controversial, a lipid disorder was associated with greater severity of peripheral neuropathy in our patient populations with type 1 or 2 diabetes mellitus. However, lipidemia may have an additive effect when present with hyperglycemia present in diabetes mellitus. Also, prior studies have shown that metformin therapy is associated with impairment of vitamin B12 levels, a condition also associated with peripheral neuropathy, its management in patients with type 2 diabetes may also slow progression of diabetic peripheral neuropathy.
Greater vigilance for other comorbidities in patients with diabetic peripheral neuropathy may reveal potentially manageable conditions that may be contributing to worsening of peripheral neuropathy over time.
The authors examined the severity of peripheral neuropathy in patients with diabetes mellitus with and without comorbid conditions capable of causing peripheral neuropathy. In particular, two conditions (lipid disorder and vitamin B12 deficiency) were detected and associated with greater neuropathic deficit. The results suggest that greater vigilance for these conditions may help patients with diabetic peripheral neuropathy by slowing the process of peripheral neurodegeneration.
P- Reviewers Neumiller JJ, Uehara Y S- Editor Huang XZ L- Editor A E- Editor Lu YJ
| 1. | Martyn CN, Hughes RA. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1997;62:310-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 379] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 2. | Chen MY, Huang WC, Peng YS, Jong MC, Chen CY, Lin HC. Health status and health-related behaviors among type 2 diabetes community residents. J Nurs Res. 2011;19:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, Witte DR, Fuller JH. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 877] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 4. | McManis PG, Windebank AJ, Kiziltan M. Neuropathy associated with hyperlipidemia. Neurology. 1994;44:2185-2186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Gaist D, Jeppesen U, Andersen M, García Rodríguez LA, Hallas J, Sindrup SH. Statins and risk of polyneuropathy: a case-control study. Neurology. 2002;58:1333-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 165] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Chong PH, Boskovich A, Stevkovic N, Bartt RE. Statin-associated peripheral neuropathy: review of the literature. Pharmacotherapy. 2004;24:1194-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Wile DJ, Toth C. Association of metformin, elevated homocysteine, and methylmalonic acid levels and clinically worsened diabetic peripheral neuropathy. Diabetes Care. 2010;33:156-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 8. | Bril V, Perkins BA. Validation of the Toronto Clinical Scoring System for diabetic polyneuropathy. Diabetes Care. 2002;25:2048-2052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 329] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 9. | Singleton JR, Bixby B, Russell JW, Feldman EL, Peltier A, Goldstein J, Howard J, Smith AG. The Utah Early Neuropathy Scale: a sensitive clinical scale for early sensory predominant neuropathy. J Peripher Nerv Syst. 2008;13:218-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 10. | Vinik AI, Park TS, Stansberry KB, Pittenger GL. Diabetic neuropathies. Diabetologia. 2000;43:957-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 303] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 11. | Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst. 2009;14:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Vincent AM, Hayes JM, McLean LL, Vivekanandan-Giri A, Pennathur S, Feldman EL. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes. 2009;58:2376-2385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 203] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 13. | Lopes-Virella MF, Baker NL, Hunt KJ, Lyons TJ, Jenkins AJ, Virella G. High concentrations of AGE-LDL and oxidized LDL in circulating immune complexes are associated with progression of retinopathy in type 1 diabetes. Diabetes Care. 2012;35:1333-1340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Rajabally YA, Shah RS. Dyslipidaemia in chronic acquired distal axonal polyneuropathy. J Neurol. 2011;258:1431-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Morris MS, Jacques PF, Rosenberg IH, Selhub J. Elevated serum methylmalonic acid concentrations are common among elderly Americans. J Nutr. 2002;132:2799-2803. [PubMed] |
| 16. | Healton EB, Savage DG, Brust JC, Garrett TJ, Lindenbaum J. Neurologic aspects of cobalamin deficiency. Medicine (Baltimore). 1991;70:229-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 445] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 17. | Saperstein DS, Wolfe GI, Gronseth GS, Nations SP, Herbelin LL, Bryan WW, Barohn RJ. Challenges in the identification of cobalamin-deficiency polyneuropathy. Arch Neurol. 2003;60:1296-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Fine EJ, Soria E, Paroski MW, Petryk D, Thomasula L. The neurophysiological profile of vitamin B12 deficiency. Muscle Nerve. 1990;13:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 47] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Kumar N. Nutritional neuropathies. Neurol Clin. 2007;25:209-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | England JD, Gronseth GS, Franklin G, Carter GT, Kinsella LJ, Cohen JA, Asbury AK, Szigeti K, Lupski JR, Latov N. Practice parameter: the evaluation of distal symmetric polyneuropathy: the role of laboratory and genetic testing (an evidence-based review). Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PM R. 2009;1:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Koike H, Mori K, Misu K, Hattori N, Ito H, Hirayama M, Sobue G. Painful alcoholic polyneuropathy with predominant small-fiber loss and normal thiamine status. Neurology. 2001;56:1727-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 113] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Koike H, Iijima M, Sugiura M, Mori K, Hattori N, Ito H, Hirayama M, Sobue G. Alcoholic neuropathy is clinicopathologically distinct from thiamine-deficiency neuropathy. Ann Neurol. 2003;54:19-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Beulens JW, Kruidhof JS, Grobbee DE, Chaturvedi N, Fuller JH, Soedamah-Muthu SS. Alcohol consumption and risk of microvascular complications in type 1 diabetes patients: the EURODIAB Prospective Complications Study. Diabetologia. 2008;51:1631-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Bell DS. Alcohol and the NIDDM patient. Diabetes Care. 1996;19:509-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Latov N. Prognosis of neuropathy with monoclonal gammopathy. Muscle Nerve. 2000;23:150-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Ludin HP, Spiess J, Koenig MP. Neuropathy and hyperthyroidism. Electroencephalogr Clin Neurophysiol. 1969;27:107. [PubMed] |
| 27. | Misiunas A, Niepomniszcze H, Ravera B, Faraj G, Faure E. Peripheral neuropathy in subclinical hypothyroidism. Thyroid. 1995;5:283-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Bolton CF. Electrophysiologic changes in uremic neuropathy after successful renal transplantation. Neurology. 1976;26:152-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 38] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Bolton CF, McKeown MJ, Chen R, Toth B, Remtulla H. Subacute uremic and diabetic polyneuropathy. Muscle Nerve. 1997;20:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Jarmuzewska EA, Ghidoni A, Mangoni AA. Hypertension and sensorimotor peripheral neuropathy in type 2 diabetes. Eur Neurol. 2007;57:91-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Lauria G, Cornblath DR, Johansson O, McArthur JC, Mellgren SI, Nolano M, Rosenberg N, Sommer C. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol. 2005;12:747-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 406] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 32. | Edwards K, Pritchard N, Vagenas D, Russell A, Malik RA, Efron N. Utility of corneal confocal microscopy for assessing mild diabetic neuropathy: baseline findings of the LANDMark study. Clin Exp Optom. 2012;95:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 33. | Lee TC, Barshes NR, O’Mahony CA, Nguyen L, Brunicardi FC, Ricordi C, Alejandro R, Schock AP, Mote A, Goss JA. The effect of pancreatic islet transplantation on progression of diabetic retinopathy and neuropathy. Transplant Proc. 2005;37:2263-2265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |