Published online Aug 15, 2025. doi: 10.4239/wjd.v16.i8.107779
Revised: April 21, 2025
Accepted: June 25, 2025
Published online: August 15, 2025
Processing time: 138 Days and 23.8 Hours
Diabetic gastroparesis (DGP) disrupts gastric motility. Electroacupuncture (EA) at Zusanli (ST36) may alleviate DGP symptoms via neural pathways.
To investigate how EA current intensities at ST36 regulate neural pathways and improve gastric motility in DGP models.
A DGP model was established using intraperitoneal injection of streptozotocin. Gastrointestinal motility was measured in rats after 2 weeks of continuous EA at ST36. Current intensity was selected as 0.5 mA, 1 mA, and 3 mA. Gastric electrodynamics were detected by recording and analyzing the number of gastric discharges. The gastric emptying rate and propulsion rate of the small intestine were measured to assess dynamic gastrointestinal function. Hematoxylin-eosin staining was conducted to measure histopathological changes in the gastric sinus. Reverse transcription-polymerase chain reaction was conducted to determine mRNA levels of Rho guanine nucleotide-binding protein A and Rho-associated coiled-coil forming protein kinase. Western blotting was conducted to determine the expression levels of choline acetyltransferase, tyrosine hydroxylase, Rho guanine nucleotide-binding protein A, and Rho-associated coiled-coil forming protein kinase. Immunofluorescence staining in the stomach was conducted to detect the distribution of C-kit, an interstitial cell of Cajal marker. An enzyme-linked immunosorbent assay was conducted to detect serum levels of acetyl
Treatment with EA improved gastric emptying and gastric smooth muscle disorders in rats with DGP, mitigated pathological damage, and restored the function of interstitial cells of Cajal. In addition, different current intensities of EA affected gastrointestinal function of rats with DGP. The 0.5 mA, 1 mA, and 3 mA EA groups all improved gastrointestinal function. 0.5 mA EA increased acetylcholine levels by increasing protein expression of choline acetyltransferase (P < 0.05), thereby upregulating vagus nerve activity and enhancing parasympathetic nerve regulation. 3 mA EA increased norepinephrine levels (P < 0.05) by increasing protein expression of tyrosine hydroxylase, thereby activating the sympathetic nervous pathway. 1 mA coordinated the function of the vagus and sympathetic nerves to improve gastrointestinal motility.
EA with ST36 improved gastric motility in rats with DGP. 0.5 mA EA activated the vagus nerve, while 3 mA EA regulated gastrointestinal motility by activating the sympathetic nerves.
Core Tip: Electroacupuncture (EA) repairs the interstitial cells of Cajal network by upregulating smooth muscle-related factors Rho guanine nucleotide-binding protein A and Rho-associated coiled-coil forming protein kinase, thereby sig
- Citation: Tang YW, Zhang Y, Zhou J, Peng YT, Zi Y, Wei YR, Yue ZH. Electroacupuncture with different current intensities can improve gastrointestinal motility in diabetic gastroparesis via vagal and sympathetic pathways. World J Diabetes 2025; 16(8): 107779
- URL: https://www.wjgnet.com/1948-9358/full/v16/i8/107779.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i8.107779
Diabetic gastroparesis (DGP) is a severe complication of diabetes mellitus, characterized by delayed gastric emptying in the absence of mechanical obstruction[1]. Common clinical manifestations of DGP encompass postprandial fullness, nausea, vomiting, abdominal distension, and epigastric pain[2]. These symptoms not only impair the quality of life of patients but also lead to malnutrition and glycemic dysregulation, increasing healthcare burdens and disease-related risks[2].
Epidemiological studies report a global DGP prevalence of approximately 9.3%, with incidence rates rising annually, making DGP a major public health challenge[3]. Mechanistic studies reveal that gastrointestinal motility relies on the rhythmic contractions of smooth muscle cells, a process orchestrated by the interstitial cells of Cajal (ICC) through slow-wave potentials and modulated by the C-kit receptor tyrosine kinase signaling pathway[4]. Concurrently, the Rho guanine nucleotide-binding protein A (RhoA)/Rho-associated coiled-coil forming protein kinase (ROCK) signaling pathway enhances smooth muscle contractility by regulating myosin light chain phosphorylation[5]. Preclinical studies have demonstrated that targeting the RhoA/ROCK pathway can significantly improve gastric emptying in animal models of DGP[6], highlighting its potential as a therapeutic target.
Currently, the clinical management of DGP relies on prokinetic agents, such as metoclopramide[7], which provide transient symptomatic relief. However, the use of prokinetic agents is limited due to adverse effects (e.g., tardive dyskinesia) and diminished efficacy with prolonged use. Therefore, the development of safe, sustainable, and minimally invasive non-pharmacological treatment has emerged as a priority in the treatment of DGP.
Electroacupuncture (EA) exhibits unique therapeutic advantages in regulating gastrointestinal motility, including sustained efficacy and low recurrence rates[8,9]. It acts through both the traditional meridian theory and modern neuroregulatory principles, precisely modulating gastrointestinal function. Accumulating evidence suggests that EA can enhance gastrointestinal motility by promoting coordinated contraction and relaxation of smooth muscle cells[10]. For instance, EA stimulation at Zusanli (ST36) accelerates gastric emptying and improves motility through two mechanisms: (1) Releasing gastrointestinal hormones such as motilin and ghrelin[11]; and (2) Restoring ICC network integrity via C-kit signaling[12]. These findings not only support the use of EA for treating gastrointestinal disorders but also highlight its potential as a targeted therapy for motility dysfunction in DGP.
The nervous system plays a pivotal role in regulating gastrointestinal motility[13]. Stimulation of the vagus nerve can enhance gastrointestinal smooth muscle contraction and motility by activating cholinergic pathways[14]. Conversely, sympathetic pathways originating from the spinal cord release norepinephrine (NE) and epinephrine, which inhibit smooth muscle contraction via β- and α2-adrenergic receptors, thereby delaying gastric emptying[15]. Liu et al[16] demonstrated that EA at distinct current intensities can selectively activate specific neural pathways; 0.5 mA EA predominantly engages the vagus nerve-adrenal axis to exert anti-inflammatory and prokinetic effects, whereas 3 mA EA activates the spinal-sympathetic pathway and induces systemic adrenaline release. These findings suggest that the efficacy of EA in DGP treatment is modulated by several factors, with current intensity emerging as a critical determinant of neuromodulation specificity. Thus, this study aimed to systematically investigate whether EA at ST36 can alleviate DGP symptoms and whether its effects on gastrointestinal function in DGP rats (across 0.5 mA, 1 mA, and 3 mA intensities) are mediated via vagal/sympathetic neural modulation.
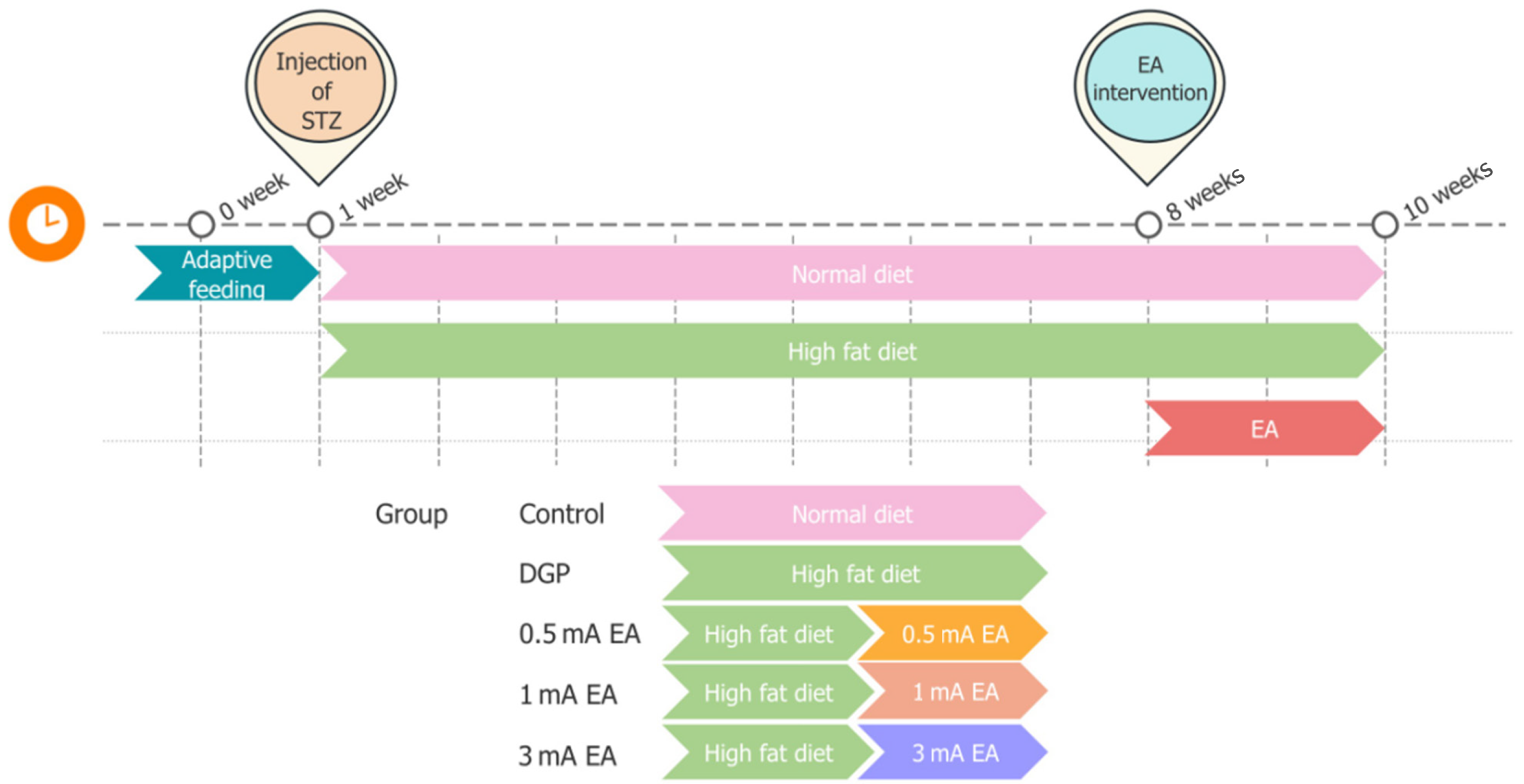
A rat model of DGP was established[17] as follows. After 12 hours of fasting, streptozotocin (STZ, 55 mg/kg) dissolved in 0.1 mol/L sodium citrate buffer (pH = 4.5) was injected into rats. After injection, the animals received a high-fat, high-sugar diet (composition by weight: 58% standard chow, 15% lard, 20% sucrose, 5% milk powder, and 2% egg yolk) for 8 weeks to induce metabolic and gastrointestinal dysfunction. An equal volume of sodium citrate buffer (0.1 mmol) was injected into rats, and the animals received a normal diet. The criteria for the successful establishment of the DGP rat model were as follows: (1) Non-fasting blood sugar ≥ 16.7 mmol/L; and (2) Reduced gastric peristalsis and gastric emptying rate compared with the control group. Rats in the control group were injected with an equal volume of sodium citrate buffer (0.1 mmol) and fed an ordinary diet. DGP was induced in 60 normal rats. Blood glucose level was measured for 8 consecutive weeks after injecting STZ, and non-fasting blood glucose values ≥ 16.7 mmol/L were regarded as the model maintenance. The model was successfully sustained in 54 rats. We randomly selected 48 rats from successfully modeled individuals for subsequent studies to ensure the reliability of the experimental results.
Male Sprague-Dawley rats (6-8 weeks old, 200-220 g) were purchased from Hunan Sileike Jingda Laboratory Animal Co., Ltd. (Hunan, China) via the Experimental Animal Center of Hunan University of Chinese Medicine. The rats were housed under controlled environmental conditions, with a temperature of 21 ± 2 °C, a humidity of 50% ± 10%, and a 12-hour light/dark cycle. The animals were acclimatized for 7 days with ad libitum access to water and standard chow. All experimental protocols were approved by the Animal Ethics Committee of the Hunan University of Chinese Medicine (No. HNUCM21-2311-08) and conducted strictly following institutional guidelines for laboratory animal welfare and the ARRIVE guidelines. Using the Excel random number table method, 48 rats with successful modeling of DGP were randomly divided into four groups (n = 12 per group): The DGP group, the 0.5 mA EA group, the 1 mA EA group, and the 3 mA EA group. In addition, 12 healthy adult male-specific pathogen-free-grade Sprague-Dawley rats were selected as the blank control group (Figure 1).
EA stimulation was applied to the bilateral ST36 acupoints on the hind limbs of rats using an EA instrument (model: HANS-200A; manufacturer: Nanjing Jisheng Medical Technology Co., Ltd, Nanjing, China). Acupuncture needles with a diameter of 0.3 mm were employed to vertically penetrate the bilateral ST36 acupoints to a depth of 2 mm. The EA stimulation parameter waveform was selected as a sparse wave (sparse wave at 20 Hz and dense wave at 100 Hz), and the current intensity was set to 0.5 mA, 1 mA, or 3 mA, respectively, to correspond to the intervention requirements of each experimental group.
During the experiment, the weight of rats was measured using an electronic balance at regular times each week. Data were recorded and the weight change was calculated until just before sample collection. At the same time, blood glucose levels were monitored via tail vein sampling using a glucometer with compatible test strips. The rats were placed in the holder, their tails were exposed and disinfected with an alcohol swab. When alcohol had evaporated, a lancet was used to lightly prick the tip of the tail to collect blood samples. The blood drops entered the test strip, and the glucometer showed blood glucose values. After blood sample collection, a cotton ball was wiped to avoid infection.
Following a 12-hour fasting period (with ad libitum access to water), the rats were anesthetized using isoflurane and fixed in a supine position on a surgical platform. The abdominal skin was disinfected with 75% ethanol, and a midline laparotomy was conducted to expose the gastric corpus. Bipolar needle electrodes (stainless steel, diameter 0.1 mm) were implanted into the gastric antrum smooth muscle layer 0.5 cm proximal to the pylorus, aligned parallel to the longitudinal muscle axis. The electrodes were placed 0.3 cm apart and anchored with absorbable sutures. The abdominal incision was closed in layers, and the electrode leads were externalized subcutaneously. After a 10-minute stabilization period, the electrodes were connected to an MP160 multichannel physiological recorder (Biopac Systems, Goleta, CA, United States). For sensitivity selection, gain amplification was set to 1000, sampling frequency was set to 500 Hz, high-pass filter was set to DC, and low-pass filter was set to 1 Hz. Gastric slow-wave activity was continuously recorded for 30 minutes. Slow wave discharge in the gastric antrum of rats was calculated within 5 minutes according to Tomita et al[18]. All five consecutive 5-minute gastric myoelectric activity graphs were selected to calculate the number of slow wave discharges.
Following a 24-hour fast and a 2-hour water restriction, rats were administered 2 mL of a phenol red solution containing 50 mg/dL of the dye via oral gavage. Fifteen minutes later, anaesthesia was induced by intraperitoneal injection of 10% sodium pentobarbital (3 mL/kg). The abdominal cavity was then opened, and the entire stomach was excised and cut along the greater curvature. The stomach was then thoroughly rinsed with 20 mL of 0.9% sodium chloride solution, after which the contents were collected into a clean beaker. The contents were then mixed with 20 mL of 0.5 mol/L sodium hydroxide (NaOH) solution and left to stand for 1 hour. Thereafter, 5 mL of the resulting mixture was collected and mixed with 0.5 mL of a 20% trichloroacetic acid solution. The mixture was then subjected to centrifugation at 3500 rpm for a period of 10 minutes. Thereafter, 2 mL of the resulting interface was analyzed for its optical density (OD) at 560 nm using ultraviolet spectrophotometer. A standard phenol red solution (2 mL phenol red solution, 18 mL distilled water, 20 mL 0.5 mol/L NaOH, and 4 mL 20% trichloroacetic acid) was prepared, and its OD value was measured using the same method. The gastric emptying rate was calculated as follows: Gastric emptying rate (%) = (1 - sample OD value/standard phenol red OD value) × 100%. The small intestine was meticulously dissected and positioned in a horizontal position on ice to ascertain its total length. The distal end, which had been stained with phenol red, was identified, and a small incision was made using ophthalmic scissors. A 0.5 mol/L NaOH solution was then added to the incision site, and a pinkish-purple color change indicated the presence of phenol red. Additional drops of NaOH were applied proximal and distal to this point to determine the farthest point reached by phenol red. The intestinal propulsive rate (IPR) was calculated as follows: IPR (in percentage form) = (farthest distance reached by phenol red/total length of the small intestine) × 100%[19].
After the experiment, the rats were euthanized via intraperitoneal injection of sodium pentobarbital (50 mg/kg). The abdominal cavity was rapidly opened to expose the abdominal aorta, and whole blood was collected into sterile tubes. Blood samples were allowed to clot at room temperature for 30 minutes, followed by centrifugation at 3000 g (4 °C) for 15 minutes to isolate serum samples. Serum aliquots were stored at -80 °C for subsequent quantification of acetylcholine (Ach) and NE levels using enzyme-linked immunosorbent assay (ELISA). Gastric tissues were immediately excised and rinsed with ice-cold 0.9% saline to remove residual blood. For histopathological analysis, tissue samples were fixed in 4% paraformaldehyde for 24 hours before paraffin embedding and hematoxylin-eosin (HE) staining. For molecular analyses, tissue samples were snap-frozen in liquid nitrogen and stored at -80 °C until western blotting and quantitative polymerase chain reaction (PCR) were conducted. All procedures were conducted on ice to preserve tissue integrity.
Following fixation in 4% paraformaldehyde for 24 hours, gastric tissue samples were dehydrated through a graded ethanol series (70%, 80%, 90%, 95%, and 100%, 1 hour per concentration) and cleared in two changes of xylene (15 minutes each). Tissue samples were then embedded in paraffin, sectioned at 4-5 μm thickness, and mounted on glass slides. For staining, the sections were deparaffinized in xylene and rehydrated through a descending ethanol series (100% to 70%). Cell nuclei were stained with Harris hematoxylin (5-10 minutes), differentiated in 1% acid alcohol (1% HCl in 70% ethanol, 5-10 seconds), and blued in 0.5% ammonia water (30 seconds). Cytoplasm was counterstained with eosin Y (1-2 minutes), followed by dehydration through an ascending ethanol series and final clearing in xylene. Tissue sections were cover-slipped using a neutral mounting medium and images were captured using an optical microscope.
The gastric tissue was harvested, and total RNA was extracted following the kit instructions. The concentration was measured using a spectrophotometer. RNA was reverse-transcribed into cDNA using the kit. A PCR amplification program was run on a thermocycler with the following conditions: 95 °C for 30 seconds for pre-denaturation; 40 cycles of 95 °C for 15 seconds, and 60 °C for 30 seconds. The relative mRNA expression levels were calculated using the 2-∆∆Ct method. The internal reference gene for the primers was β-actin. The primer sequences are listed in Table 1.
| Gene | Primer sequence (5’ to 3’) |
| ROCK1 | F: 5’-ATCTTCCAGTTGGTTCTGCC-3’ |
| R: 5’-ACATACTGTTTCTTCCAGCC-3’ | |
| RhoA | F: 5’-AGAGGTTTATGTGCCCACGG-3’ |
| R: 5’-TGTCTGGGTAGGAGAGAGGC-3’ | |
| β-actin | F: 5’-TCGTGCGTGACATTAAAGAG-3’ |
| R: 5’-TGCCACAGGATTCCATACC-3’ |
Frozen gastric tissues were collected and lysed in radioimmunoprecipitation assay buffer containing 1% protease inhibitor and 1% phosphatase inhibitor. The tissue was minced and lysed on ice for 30 minutes. After centrifugation at 12000 rpm for 15 minutes at 4 °C, the supernatant was collected, and protein concentration was determined using a bicinchoninic acid protein assay kit (Beyotime, Shanghai, China). The protein sample was mixed with 5 × loading buffer and boiled at 100 °C for 5 minutes. 70 μg protein was loaded onto a 10% sodium-dodecyl sulfate gel and electrophoresed at 80 V until the sample entered the separating gel, then electrophoresed at 120 V until the bromophenol blue reached the bottom of the gel. The protein was transferred to a polyvinylidene fluoride membrane (Millipore, MA, United States) at a constant current of 250 mA for 90 minutes. The membrane was blocked with 5% bovine serum albumin (Elabscience, Hubei, China) at room temperature for 1 hour. The membrane was subsequently incubated overnight at 4 °C with primary antibodies against ROCK1 (1:1000, ab134181, Abcam, United Kingdom), Ach transferase (1:1000, ab178850, Abcam, United Kingdom), tyrosine hydroxylase (TH) (1:1000, GB11181-100, Servicebio, Hubei, China), RhoA (1:5000, ab187027, Abcam, United Kingdom), and β-actin (1:1000, 4970S, Cell Signaling Technology, MA, United States). The membrane was washed three times for 10 minutes each with tris-buffered saline with Tween buffer. Next, the membrane was incubated with goat anti-rabbit (1:2000, A-1003, Elabscience, Hubei, China), as the secondary antibody at room temperature for 1 hour. After three tris-buffered saline with Tween washes (10 minutes each), the bands were detected using an enhanced chemiluminescence reagent, and signals were captured using a chemiluminescence imaging system. Image J software was employed to analyze the gray value of the bands.
The tissue sections were removed from the refrigerator and immersed in 0.01 mol/L sodium citrate buffer (pH = 6.0). The sections were heated at 95 °C for 20 minutes for antigen retrieval, then allowed to cool to room temperature. The sections were then rinsed with phosphate-buffered saline (PBS) and blocked with 5% bovine serum albumin at room temperature for 1 hour. The sections were incubated with a primary antibody against C-kit (1:400, 3074T, Cell Signaling Technology, MA, United States) at 4 °C overnight, and washed three times with PBS for 5 minutes each. Next, the samples were incubated in the dark with the corresponding secondary antibody at room temperature for 1 hour. After another round of washing with PBS, the sections were stained with DAPI (1 μg/mL) for 5 minutes in the dark. An anti-fluorescence quenching sealing agent was applied, covered with a coverslip, and stored in the dark. The sections were observed under a fluorescence microscope, and images were captured and analyzed using K-Viewer software.
After anesthesia, the rat’s abdominal cavity was opened, and a blood sample was collected from the abdominal aorta. The blood sample was left for 30 minutes and centrifuged at 3000 rpm for 15 minutes to separate the serum. The serum concentrations of ACh (AF3392-A, Ai Fang Biology, Changsha, China) and NE (AF3643-A, Ai Fang Biology, Changsha, China) were measured using an ELISA kit following the manufacturer’s instructions. OD was recorded at 450 nm using a microplate reader (Thermo Fisher, MA, United States).
Experimental data are expressed as mean ± SD. Statistical analysis was conducted using SPSS 27.0, and data visualization was conducted using GraphPad Prism 10. One-way ANOVA was used to compare the groups. The least significant difference method was applied for multiple comparisons when ANOVA indicated a significant difference (P < 0.05). The Kruskal-Wallis H test was applied when the variances were uneven. P < 0.05 was considered statistically significant.
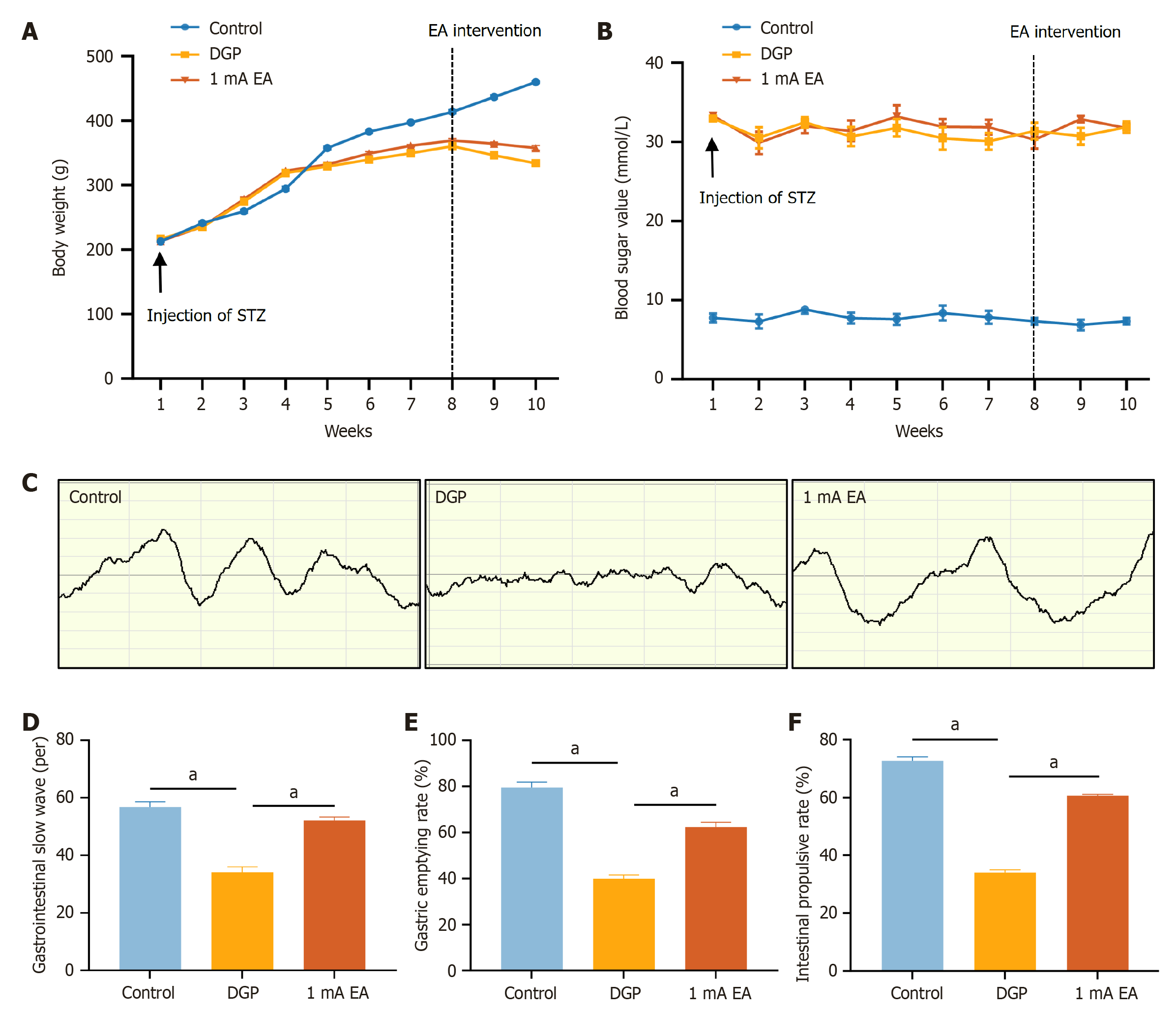
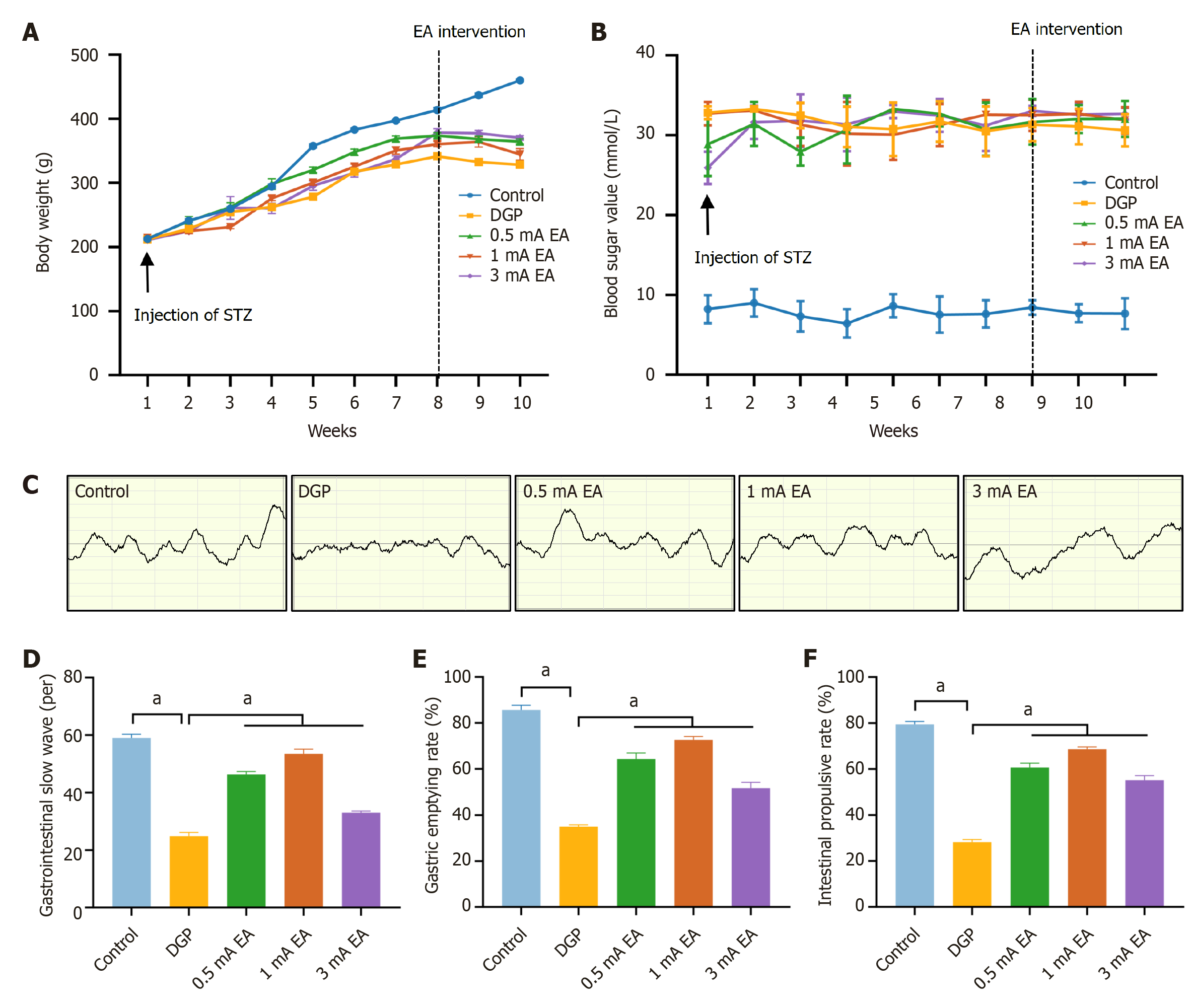
We systematically monitored changes in blood glucose levels and body weight of rats to determine whether the intraperitoneal STZ injection directly destroyed pancreatic β-cells to successfully establish a rat model of DGP. Following STZ injection, body weight growth rate decreased in both the DGP and EA groups (Figure 2A). EA was initiated from the 9th week, and the EA group body weight was significantly higher than that of the DGP group at week 10 (P < 0.05), suggesting that EA mitigated weight loss in rats with DGP. Additionally, compared to the control group, blood glucose levels exceeded 16.7 mmol/L in the DGP and EA groups (Figure 2B). Nevertheless, there was no significant difference in blood glucose levels between the DGP and EA groups. These findings suggest that a 14-day EA regimen may selectively stabilize body weight, whereas chronic hyperglycemia, a hallmark of advanced diabetes, may necessitate prolonged therapeutic interventions to achieve normoglycemia. We then conducted gastric electromyography to detect and quantify discharges (Figure 2C and D). Compared to the control group, the number of slow-wave discharges in the stomach of the DGP group significantly decreased. In contrast, the number of discharges increased after EA (P < 0.05). Additionally, gastric emptying rate and small intestinal propulsion rate (Figure 2E and F) were significantly lower in the DGP group compared to the control group (P < 0.05), while the EA group exhibited significantly higher values than the DGP group (P < 0.05). We measured the gastrointestinal motility of rats with DGP based on gastric electromyography, gastric emptying rate, and small intestinal propulsion rate. The results demonstrated that EA significantly improved gastric emptying in rats with DGP, evidenced by increased gastric emptying and small intestinal propulsion rates, along with enhanced gastric motor activity. EA may significantly relieve gastrointestinal motility in DGP rats by restoring the slow-wave rhythm of the gastric antrum and improving gastric emptying.
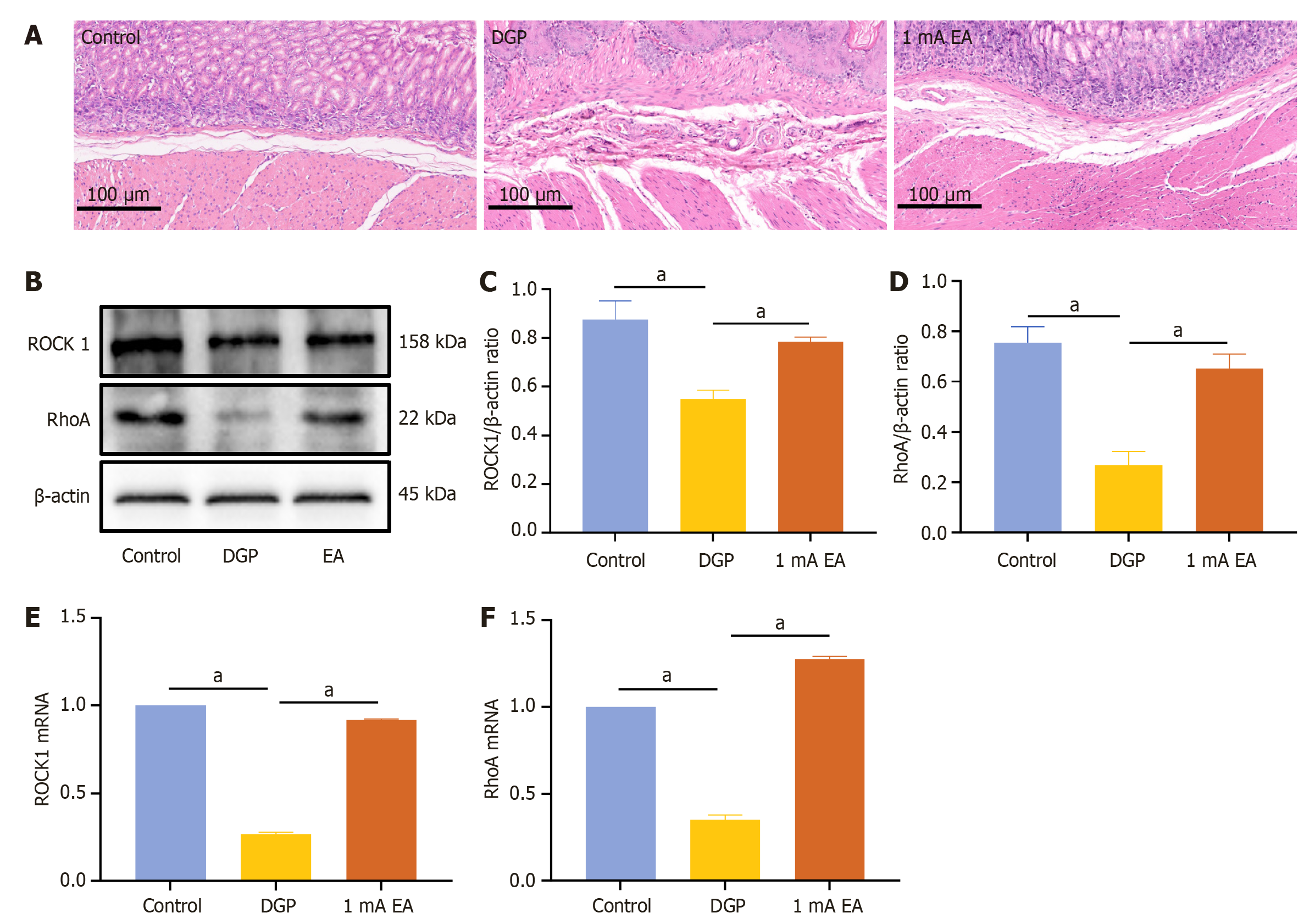
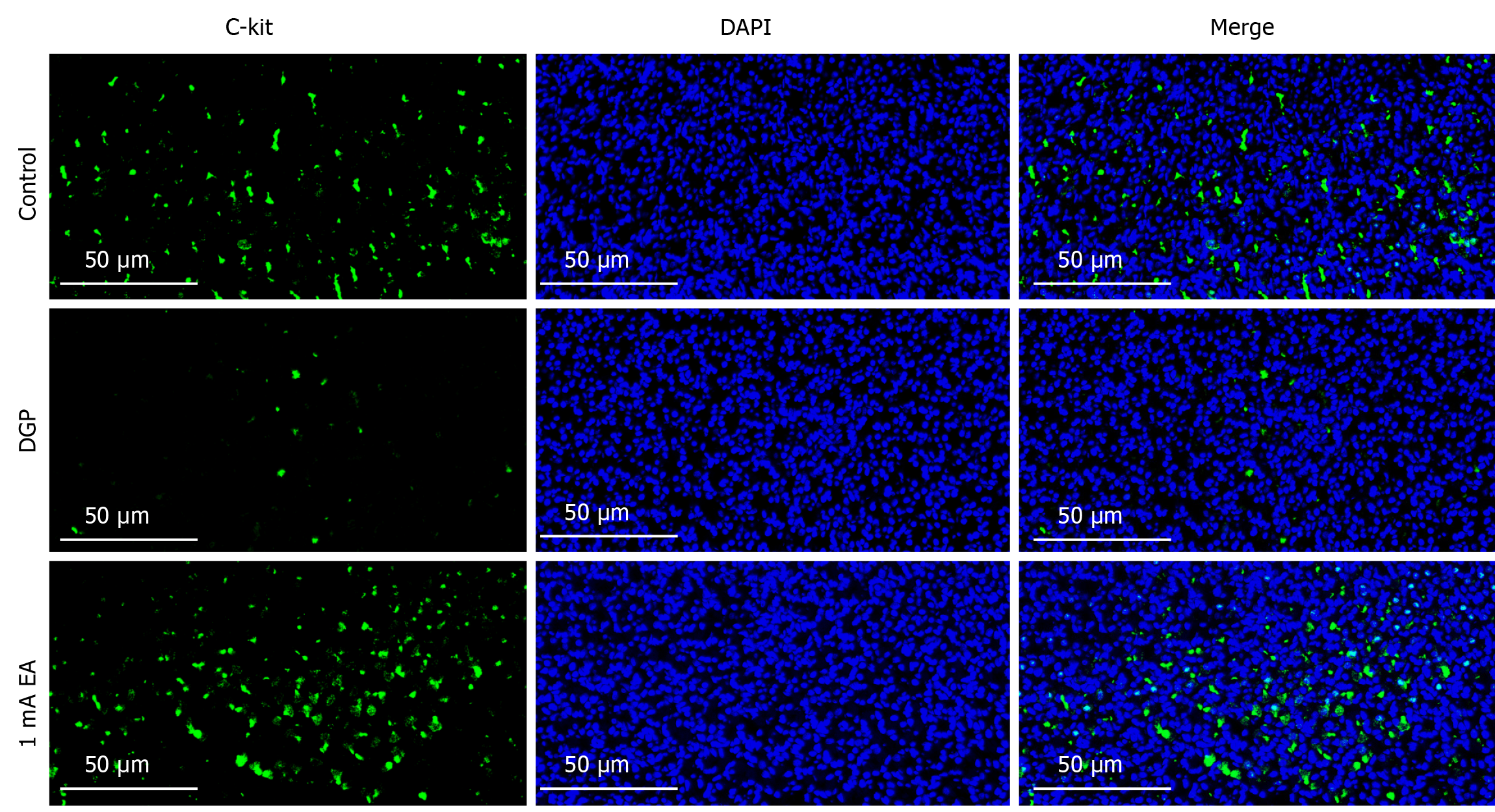
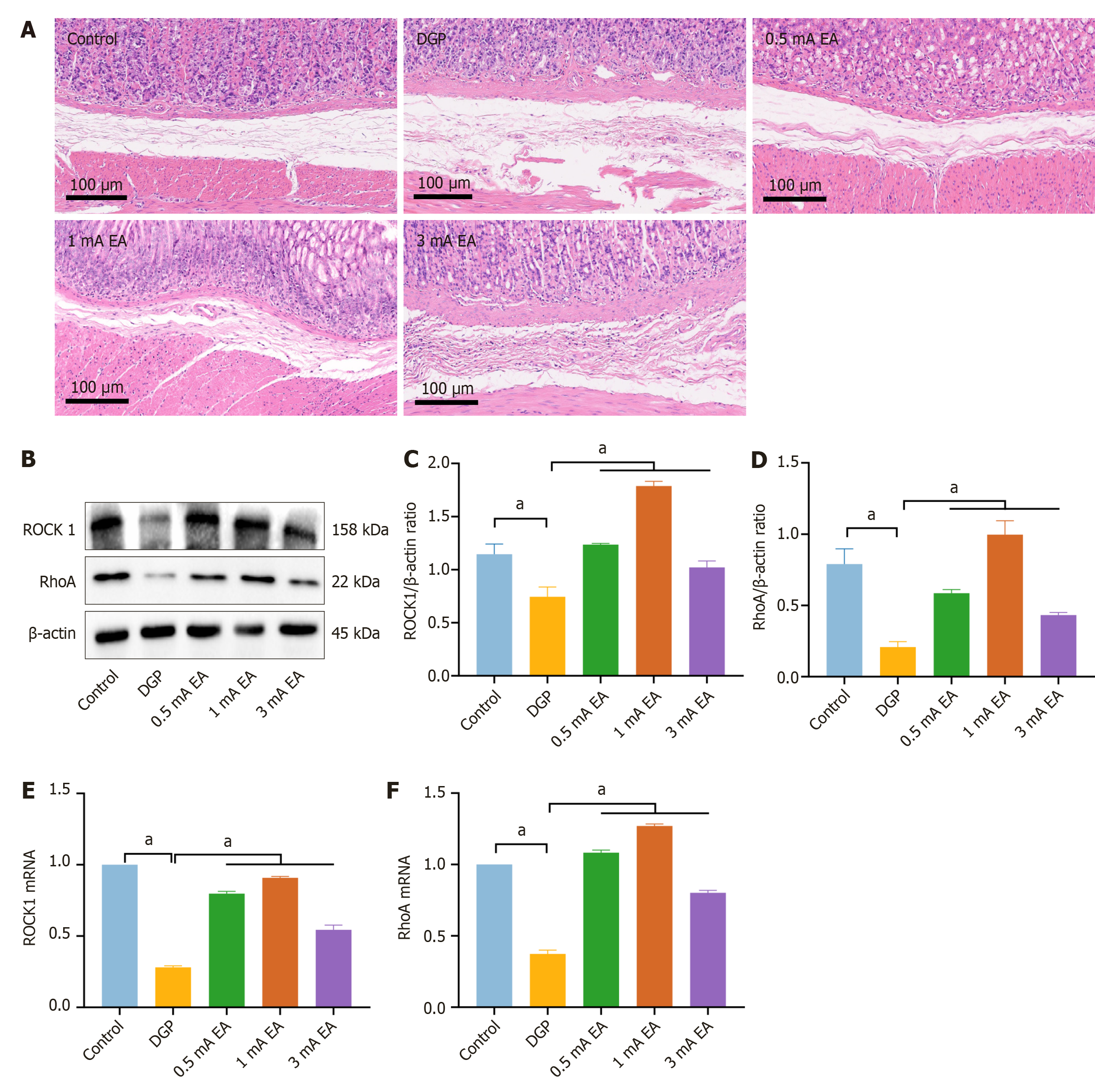
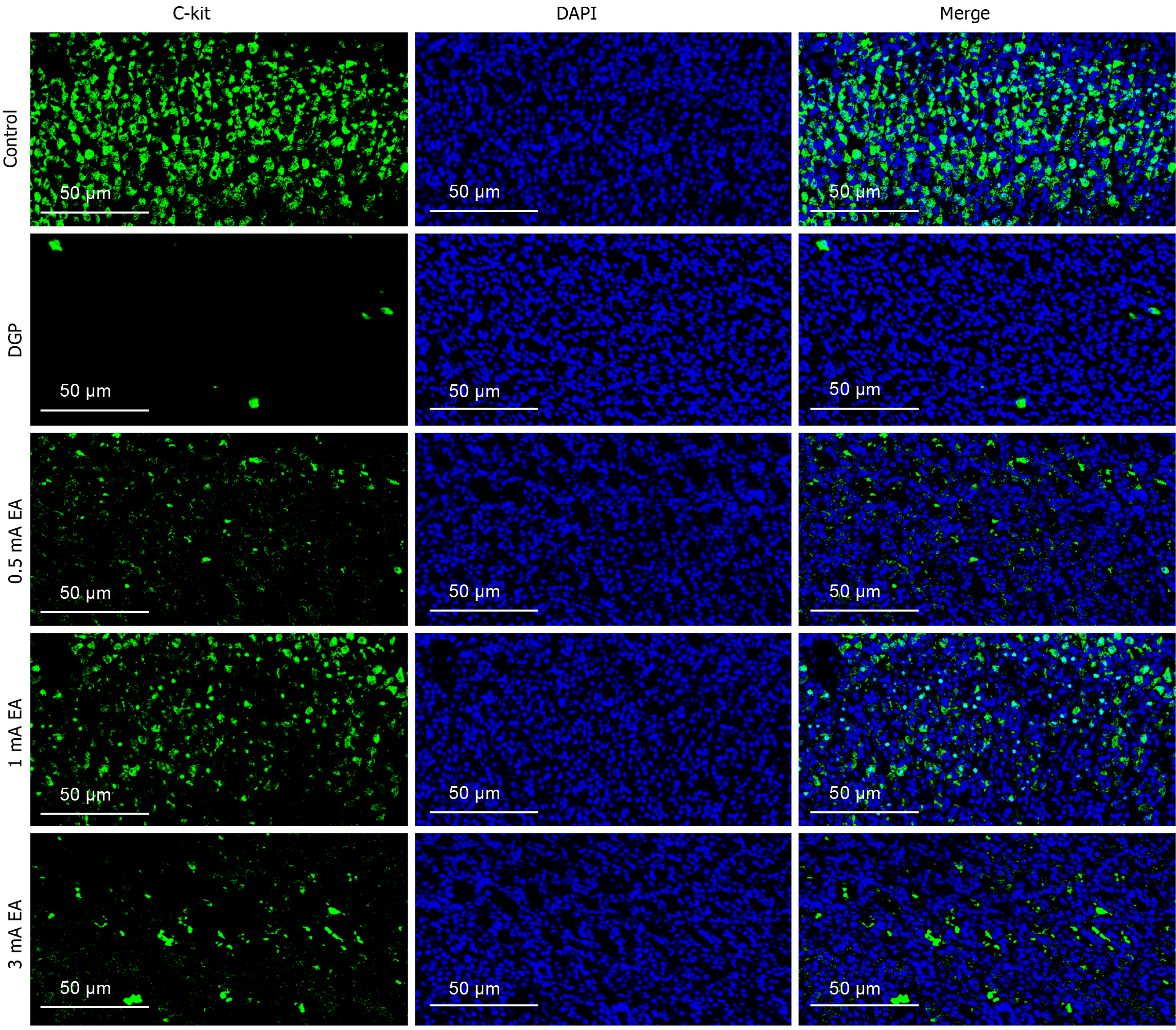
The experimental results demonstrated that EA can promote gastrointestinal motility in rats with DGP, a process that relies on the rhythmic contraction of smooth muscles. We conducted HE staining on gastric tissue samples to measure pathological changes in the gastric smooth muscle of rats with DGP (Figure 3A). The control group exhibited no obvious pathological damage, whereas the DGP group displayed abnormal gastric mucosal structure, inflammatory cell infiltration, and a loosely arranged muscular layer. The pathological changes were significantly less severe in the EA group than in the DGP group, with a more intact gastric mucosal structure, reduced inflammatory cell infiltration, and a more compact muscular layer. Next, we measured the RNA and protein levels of RhoA and ROCK1, which are associated with gastrointestinal motility (Figure 3B-F). Compared to the control group, the mRNA and protein levels of RhoA and ROCK1 were significantly lower in the DGP group (P < 0.05), and EA upregulated their expression (P < 0.05). The expression level of C-kit, a marker of ICCs, is closely associated with ICC number and function in gastric tissue[20]. Therefore, we conducted immunofluorescence staining to detect C-kit expression in the stomach (Figure 4). Compared to the control group, the number of C-kit-positive cells was reduced in the DGP group, and EA significantly increased C-kit expression compared to the DGP group.
The potential of EA in improving gastrointestinal dysfunction has attracted widespread attention; however, the differential effects of varying current intensities of EA on gastric emptying in DGP remain unclear. To address this gap, this study included 0.5 mA and 3 mA EA groups to compare the effects of different current intensities on gastric emptying in rats with DGP. Body weight monitoring and blood glucose measurement (Figure 5A and B) indicated that the trends in the 0.5 mA and 3 mA EA groups were consistent with those observed in the 1 mA EA group (P < 0.05). Electromyogram (Figure 5C and D) revealed that compared to the control group, the number of gastric slow-wave discharges significantly decreased in the DGP group, and EA intervention significantly increased the number of gastric slow-wave discharges (P < 0.05). Notably, the 1 mA EA group exhibited a significantly higher number of gastric slow-wave discharges than the other two EA groups (P < 0.05), and the 0.5 mA EA group showed a higher number than the 3 mA EA group (P < 0.05). A similar trend was observed in the gastric emptying rate and small intestinal propulsion rate tests (Figure 5E and F). The gastric emptying and small intestinal propulsion rates in all three EA groups were significantly higher than those in the DGP group (P < 0.05). Among them, the 1 mA EA group exhibited the highest rates (P < 0.05), followed by the 0.5 mA EA group and the 3 mA EA group, respectively (P < 0.05). These findings suggest that EA treatment effectively restored rhythmic gastric electrical activity and enhanced gastrointestinal motility. Among the different current intensities, 1 mA EA had the greatest effect on gastric emptying in rats with DGP. Additionally, compared to 3 mA, 0.5 mA EA more effectively enhanced gastrointestinal motility.
A similar trend was observed regarding the gastric emptying rate and small intestine propulsion rate (Figure 5E and F). The gastric emptying rate and small intestine propulsion rate of the three EA groups were significantly higher than those of the DGP group (P < 0.05). The 1 mA EA group showed significantly higher values compared to the other two EA groups (P < 0.05), followed by the 0.5 mA EA group and the 3 mA EA group (P < 0.05). These results suggest that EA treatment effectively restored rhythmic gastric electrical activity and enhanced gastrointestinal motility. EA treatment at 1 mA more effectively promoted gastric emptying in rats. At the same time, 0.5 mA exhibited a more prominent effect in promoting gastrointestinal motility compared to 3 mA.
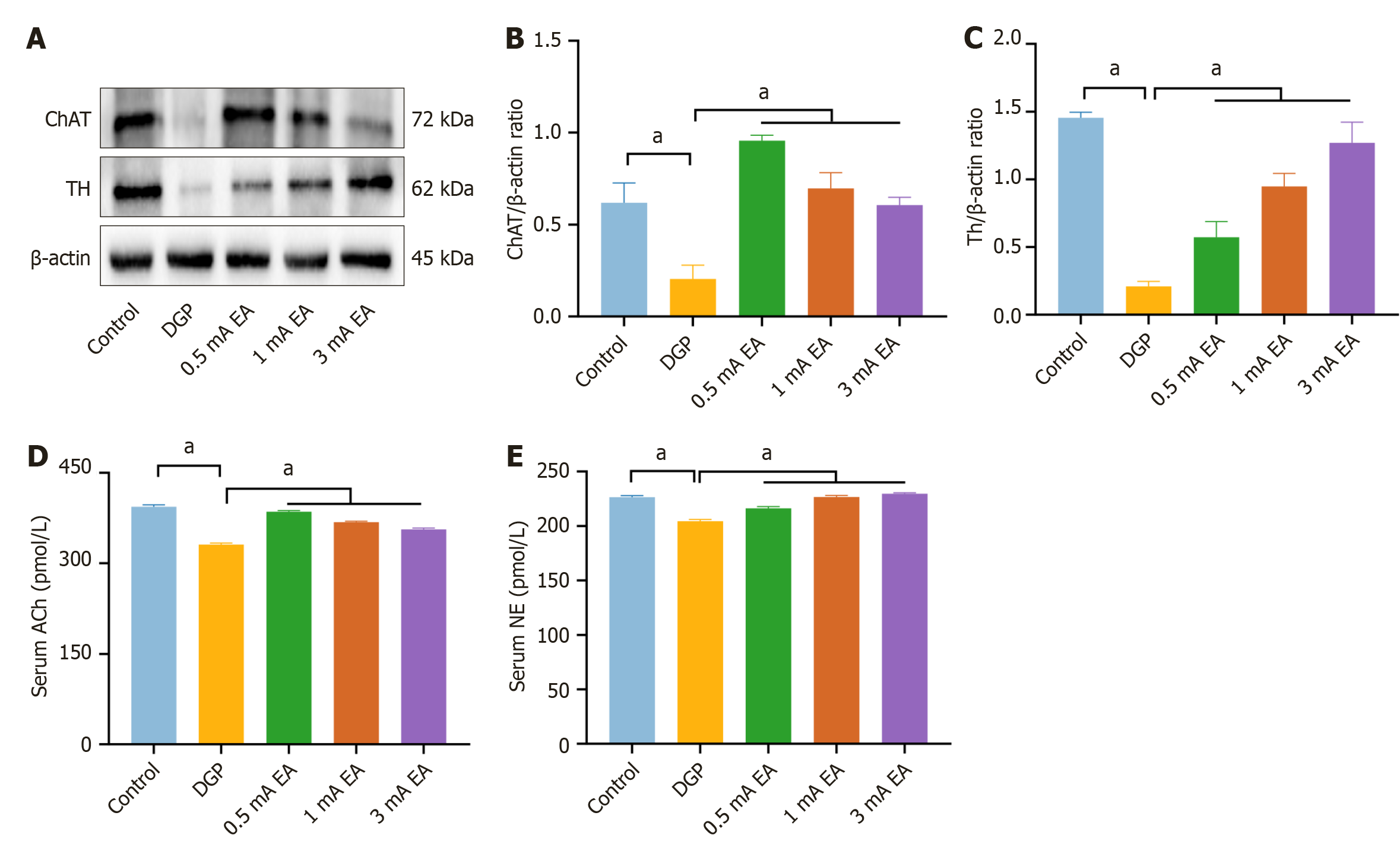
To investigate whether EA with different current intensities activates distinct neural pathways, we measured the protein expression of choline acetyltransferase (ChAT) and TH, which are associated with vagus nerve and sympathetic nerve activity, respectively (Figure 6A-C). The results showed that compared to the DGP group, the protein expression of ChAT and TH was significantly increased in all three EA groups (P < 0.05). Notably, the 0.5 mA EA group exhibited a significant increase in ChAT expression (P < 0.05), while the 3 mA EA group displayed a significant increase in TH expression (P < 0.05). Because increased ChAT expression directly promotes ACh synthesis, and TH is the rate-limiting enzyme for NE synthesis, ELISA results followed the same trend (Figure 6D and E). Compared to the DGP group, serum levels of ACh and NE were significantly elevated in all three EA groups (P < 0.05). Specifically, the 0.5 mA EA group exhibited the highest serum levels of ACh (P < 0.05), whereas the 3 mA EA group showed the greatest increase in NE levels (P < 0.05). These findings suggest that 0.5 mA EA preferentially enhanced vagus nerve activity, while 3 mA EA predominantly activated the sympathetic nervous system. Meanwhile, 1 mA EA facilitated a comprehensive recovery of gastrointestinal motility in rats with DGP by regulating gastric tissue repair. This study unveiled that EA with different current intensities can modulate gastrointestinal motility by differentially activating the vagus and sympathetic nerves.
Based on the above experimental results, we investigated the regulatory effects of EA with different current intensities on smooth muscle by activating distinct neural pathways. HE staining (Figure 7A) revealed that the gastric tissue structure was intact in the control group and the mucosal, submucosal, and muscular layers were neatly arranged, with no apparent pathological changes. In contrast, the DGP group exhibited significant structural abnormalities, including mucosal layer damage, inflammatory cell infiltration, and a loosely arranged smooth muscle layer, confirming the successful establishment of the DGP model. The most evident improvement in gastric tissue structure was observed in the 1 mA EA group, characterized by reduced mucosal damage, decreased inflammatory cell infiltration, and a well-organized smooth muscle layer. The 0.5 mA EA group showed moderate improvement, with partial restoration of gastric tissue structure compared to the DGP group. The 3 mA EA group exhibited the weakest improvement, and gastric tissue structure showed partial recovery compared to the DGP group, but notable mucosal damage and disorganized arrangement of the muscle layer were still observed. In the previous section, we confirmed that EA at different current intensities exerts distinct regulatory effects on gastrointestinal smooth muscle. To determine the efficacy of EA in promoting gastrointestinal motility, we analyzed RhoA and ROCK1 RNA and protein levels. RhoA and ROCK1 expression was significantly increased in all three EA groups (P < 0.05) (Figure 7B-F), with the most pronounced upregulation observed in the 1 mA EA group (P < 0.05). This trend was consistent with the histopathological repair observed in HE staining and the improvement in gastrointestinal motility. Additionally, immunofluorescence staining (Figure 8) indicated that C-kit expression in the 1 mA EA group was significantly higher than in other EA groups. These findings suggest that different current intensities of EA promoted gastrointestinal motility through distinct molecular me
In this study, we confirmed the efficacy of EA in treating DGP. EA significantly mitigated weight loss in rats but had no direct regulatory effect on the symptoms of hyperglycemia. In addition, phenol red gastric emptying and small intestinal propulsion rate experiments demonstrated that EA effectively promoted the recovery of gastric emptying in rats with DGP. Gastric electrical detection also revealed that EA significantly increased the frequency of gastric slow waves and improved the rhythm of gastrointestinal peristalsis. Additionally, HE staining showed that EA markedly reduced structural disorders in the gastric mucosal layer, suppressed inflammatory cell infiltration, and mitigated the loose arrangement of the smooth muscle layer. These findings suggest that EA alleviated gastric motility disorders by regulating gastrointestinal smooth muscle function, providing a non-pharmacological strategy with translational potential for the treatment of DGP.
Recent studies have demonstrated that smooth muscle is the primary driver of gastrointestinal motility, regulating functions such as contraction and relaxation[21]. RhoA and ROCK play crucial roles in regulating the function of gastrointestinal smooth muscle. RhoA, a member of the Ras superfamily, is a G protein with GTPase activity that is critical for actin filament formation and myosin-actin interactions[22]. ROCK, a key downstream signaling molecule in the Rho kinase pathway, is involved in the contractile response of smooth muscle. A recent study reported that low-intensity pulsed ultrasound combined with ST36 stimulation can significantly enhance the expression of gastric smooth muscle contraction markers by modulating the RhoA/ROCK signaling pathway, thereby improving gastrointestinal motility in rats with DGP[6]. Consistent with these findings, our study revealed that RhoA and ROCK1 mRNA and protein expression levels were significantly reduced in the gastric tissue of rats with DGP, whereas EA significantly upregulated their expression, consistent with improved gastric emptying function. ICCs are closely associated with gastrointestinal smooth muscle function. As key regulators of gastrointestinal motility, ICCs are widely distributed in the muscular layer and around the nervous plexus of the gastrointestinal tract. They play a central role in peristalsis by generating slow wave potentials and mediating nervous signal transmission[23]. Clinical studies have shown that ICC levels are significantly decreased in patients with DGP, while animal studies have demonstrated that EA stimulation can restore the ICC network[24,25], consistent with our immunofluorescence results. We employed immunofluorescence staining to detect the expression of C-kit, an ICC marker, to assess changes in ICC number and morphology. EA significantly increased the number of C-kit-positive cells and enhanced the structural integrity of the ICC network, consistent with the observed morphological improvements. These findings suggest that EA may improve gastrointestinal motility by promoting ICC proliferation and restoring its network function. Furthermore, improved gastrointestinal motility cannot be solely explained by the structural repair of smooth muscle and ICC network reconstruction. The autonomic nervous system dynamically regulates smooth muscle function and controls gastrointestinal motility.
The vagus nerve is the primary branch of the parasympathetic nervous system and promotes gastrointestinal peristalsis by releasing ACh, which activates smooth muscle M3 receptors, thereby increasing the frequency and amplitude of gastrointestinal contractions[26]. Upregulation of ChAT expression is a readout of vagus nerve activation. Recent studies have shown that EA can activate cholinergic neurons in the dorsal motor nucleus of the vagus nerve, driving the anti-inflammatory effects of the vagus nerve and enhancing gastrointestinal motility[16,27]. In contrast, the sympathetic nervous system modulates gastrointestinal function by releasing NE, which acts on smooth muscle β2 receptors to reduce smooth muscle excitability and delay gastric emptying[28]. These two systems regulate gastro
In this study, 0.5 mA EA significantly upregulated ChAT expression in the gastric tissue of rats with DGP, accompanied by increased ACh serum levels, suggesting that EA improves gastrointestinal motility by activating the vagus nerve-cholinergic pathway. The sympathetic nervous system is another key component of autonomic regulation, and excessive sympathetic activation in DGP may lead to autonomic imbalance and exacerbate gastrointestinal motility disorders[30]. As a rate-limiting enzyme in NE synthesis[31], TH plays a crucial role in sympathetic activation by promoting NE production. Our results showed that 3 mA EA significantly upregulated TH expression, a marker of sympathetic nerve activity, and simultaneously increased NE serum levels. This finding aligns with the “current intensity-dependent neuromodulation” theory proposed by Liu et al[16]. The theory suggests that high-intensity (3 mA) EA preferentially activates the sympathetic-adrenal medulla axis, modulating systemic physiological responses through NE release. Specifically, we revealed that 3 mA EA enhances NE release by activating the sympathetic-adrenal medulla axis via ST36 stimulation. Further analyses revealed an antagonistic relationship between the sympathetic-adrenal pathway and the vagus-cholinergic pathway: ACh levels in the 3 mA EA group were significantly lower than those in the 0.5 mA EA group, suggesting that high-intensity EA may indirectly suppress vagal nerve function through “sympathetic dominance”.
In summary, EA stimulation at ST36 enhanced gastrointestinal smooth muscle contraction and improved gastric emptying in rats with DGP. Mechanistically, EA may activate the vagus nerve-cholinergic pathway at a current intensity of 0.5 mA and regulate the sympathetic-adrenergic pathway at 3 mA, promoting gastrointestinal motility.
| 1. | Schol J, Huang IH, Carbone F, Fernandez LMB, Gourcerol G, Ho V, Kohn G, Lacy BE, Colombo AL, Miwa H, Moshiree B, Nguyen L, O'Grady G, Siah KTH, Stanghellini V, Tack J. Rome Foundation and international neurogastroenterology and motility societies' consensus on idiopathic gastroparesis. Lancet Gastroenterol Hepatol. 2025;10:68-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 2. | Schol J, Wauters L, Dickman R, Drug V, Mulak A, Serra J, Enck P, Tack J; ESNM gastroparesis consensus group. United European Gastroenterology (UEG) and European Society for Neurogastroenterology and Motility (ESNM) consensus on gastroparesis. United European Gastroenterol J. 2021;9:883-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Li L, Wang L, Long R, Song L, Yue R. Prevalence of gastroparesis in diabetic patients: a systematic review and meta-analysis. Sci Rep. 2023;13:14015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Komuro T. Structure and organization of interstitial cells of Cajal in the gastrointestinal tract. J Physiol. 2006;576:653-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Wu Z, Yang W, Wu T, Liu Y, Pu Y, Hu W, Jiang Y, Zhang J, Zhu H, Li X, Feng S. Long term Coptidis Rhizoma intake induce gastrointestinal emptying inhibition and colon barrier weaken via bitter taste receptors activation in mice. Phytomedicine. 2025;136:156292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Han N, Cheng S, Jin Y, Li G, Wang H, Jin L. Low-intensity pulsed ultrasound combined with ST36 modulate gastric smooth muscle contractile marker expression via RhoA/Rock and MALAT1/miR-449a/DLL1 signaling in diabetic rats. Neurogastroenterol Motil. 2024;36:e14843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Shakhatreh M, Jehangir A, Malik Z, Parkman HP. Metoclopramide for the treatment of diabetic gastroparesis. Expert Rev Gastroenterol Hepatol. 2019;13:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Chen H, He M, Cao J, Zhang Y, Zhou Y, Yu Q, Wang A, Xuan J, Li T. Acupuncture and moxibustion intervention in functional dyspepsia: Gastric and duodenal regulation. Heliyon. 2024;10:e35696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Yin J, Chen J, Chen JD. Ameliorating effects and mechanisms of electroacupuncture on gastric dysrhythmia, delayed emptying, and impaired accommodation in diabetic rats. Am J Physiol Gastrointest Liver Physiol. 2010;298:G563-G570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Jin Z, Shen Z, Yan S, Chen G, Yin Y, Zhang Y, Wu X. Electroacupuncture ameliorates gastrointestinal dysfunction by modulating DMV cholinergic efferent signals to drive the vagus nerve in p-MCAO rats. Heliyon. 2024;10:e29426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 11. | Yu H, Deng H, Zhou W, Liang Z. Effects of electroacupuncture combined with acupoint catgut embedding on gastrointestinal motility and gastrointestinal hormones in rats with functional dyspepsia. Chin J Physiol. 2023;66:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 12. | Wang H, Zhao K, Shi N, Niu Q, Liu C, Chen Y. Electroacupuncture Regularizes Gastric Contraction and Reduces Apoptosis of Interstitial Cells of Cajal in Diabetic Rats. Front Physiol. 2021;12:560738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Marathe CS, Jones KL, Wu T, Rayner CK, Horowitz M. Gastrointestinal autonomic neuropathy in diabetes. Auton Neurosci. 2020;229:102718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Li S, Ye F, Zhang S, Liu Y, Chen JDZ. Effect and Mechanism of Vagal Nerve Stimulation on Gastric Motility: A Preliminary Rodent Study. Neuromodulation. 2025;S1094-7159(25)00003. [PubMed] [DOI] [Full Text] |
| 15. | Lu MJ, Yu Z, He Y, Yin Y, Xu B. Electroacupuncture at ST36 modulates gastric motility via vagovagal and sympathetic reflexes in rats. World J Gastroenterol. 2019;25:2315-2326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 16. | Liu S, Wang Z, Su Y, Qi L, Yang W, Fu M, Jing X, Wang Y, Ma Q. A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature. 2021;598:641-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 390] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 17. | Huang H, Peng Y, Xiao L, Wang J, Xin YH, Zhang TH, Li XY, Wei X. Electroacupuncture Promotes Gastric Motility by Suppressing Pyroptosis via NLRP3/Caspase-1/GSDMD Signaling Pathway in Diabetic Gastroparesis Rats. Chin J Integr Med. 2025;31:448-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Tomita R. Regulation of the peptidergic nerves (substance P and vasoactive intestinal peptide) in the colon of women patients with slow transit constipation: an in vitro study. Hepatogastroenterology. 2008;55:500-507. [PubMed] |
| 19. | Wei X, Lin Y, Zhao D, Xiao X, Chen Q, Chen S, Peng Y. Electroacupuncture Relieves Suppression of Autophagy in Interstitial Cells of Cajal of Diabetic Gastroparesis Rats. Can J Gastroenterol Hepatol. 2020;2020:7920715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Wu L, Niu Y, Ren B, Wang S, Song Y, Wang X, Zhao K, Yue Z, Li Y, Gao J. Naringenin Promotes Gastrointestinal Motility in Mice by Impacting the SCF/c-Kit Pathway and Gut Microbiota. Foods. 2024;13:2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Fernandes SQ, Kothare MV, Mahmoudi B. A novel compartmental approach for modeling stomach motility and gastric emptying. Comput Biol Med. 2024;181:109035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Kim JG, Islam R, Cho JY, Jeong H, Cap KC, Park Y, Hossain AJ, Park JB. Regulation of RhoA GTPase and various transcription factors in the RhoA pathway. J Cell Physiol. 2018;233:6381-6392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 23. | Hirst GD, Edwards FR. Role of interstitial cells of Cajal in the control of gastric motility. J Pharmacol Sci. 2004;96:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Moraveji S, Bashashati M, Elhanafi S, Sunny J, Sarosiek I, Davis B, Torabi A, McCallum RW. Depleted interstitial cells of Cajal and fibrosis in the pylorus: Novel features of gastroparesis. Neurogastroenterol Motil. 2016;28:1048-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Lin G, Zhang J, Li L, Zou Z, Chen C, Xue L, Zhao L. Effect of electroacupuncture on gastric interstitial cells of Cajal in a rat model of diabetic gastroparesis. Exp Ther Med. 2016;11:2489-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Lin J, Shen Y, Xia Y, Li Y, Jiang T, Shen X, Fu Y, Zhang D, Yang L, Xu H, Xu Z, Wang L. Vagotomy suppresses food intake by increasing GLP-1 secretion via the M3 AChR-AMPKα pathway in mice. Mol Cell Endocrinol. 2025;599:112464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Yang NN, Yang JW, Ye Y, Huang J, Wang L, Wang Y, Su XT, Lin Y, Yu FT, Ma SM, Qi LY, Lin LL, Wang LQ, Shi GX, Li HP, Liu CZ. Electroacupuncture ameliorates intestinal inflammation by activating α7nAChR-mediated JAK2/STAT3 signaling pathway in postoperative ileus. Theranostics. 2021;11:4078-4089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 28. | Song J, Zheng L, Zhang X, Feng X, Fan R, Sun L, Hong F, Zhang Y, Zhu J. Upregulation of β1-adrenoceptors is involved in the formation of gastric dysmotility in the 6-hydroxydopamine rat model of Parkinson's disease. Transl Res. 2014;164:22-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Liu S, Wang ZF, Su YS, Ray RS, Jing XH, Wang YQ, Ma Q. Somatotopic Organization and Intensity Dependence in Driving Distinct NPY-Expressing Sympathetic Pathways by Electroacupuncture. Neuron. 2020;108:436-450.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 229] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 30. | Ouyang X, Li S, Foreman R, Farber J, Lin L, Yin J, Chen JD. Hyperglycemia-induced small intestinal dysrhythmias attributed to sympathovagal imbalance in normal and diabetic rats. Neurogastroenterol Motil. 2015;27:406-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Sakamoto K, Butera MA, Zhou C, Maurizi G, Chen B, Ling L, Shawkat A, Patlolla L, Thakker K, Calle V, Morgan DA, Rahmouni K, Schwartz GJ, Tahiri A, Buettner C. Overnutrition causes insulin resistance and metabolic disorder through increased sympathetic nervous system activity. Cell Metab. 2025;37:121-137.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |