Published online Aug 15, 2025. doi: 10.4239/wjd.v16.i8.104371
Revised: April 8, 2025
Accepted: July 10, 2025
Published online: August 15, 2025
Processing time: 239 Days and 2.8 Hours
Type 2 diabetes (T2D) is a major health concern globally and its prevalence is expected to continue to escalate. Lifestyle intervention is an integral part of T2D management. Meal replacements are often used as part of lifestyle intervention programs in T2D and weight management programs. There are various trials being carried out to date; however, a thorough review regarding the usage of meal replacement on its types, dosage and associated outcomes and adverse events is still lacking.
To provide a comprehensive overview on existing studies regarding meal re
This scoping review is conducted based on Arksey and O’Malley’s seminal framework for scoping reviews. A systematic search has been done for studies published between January 2020 and January 2024 across six online databases (Cochrane Library, PubMed, Science Direct, Scopus, Web of Science and Eb
The initial search resulted in an initial count of 53922 articles from which 133 articles were included in this review after eligibility screening. Included studies were categorized based on meal replacement type into low calo
The results suggest that meal replacements, especially when combined with lifestyle intervention programs and counseling, are an effective and safe strategy in glycemic and weight management among patients with T2D.
Core Tip: This scoping review systematically evaluated meal replacement interventions in type 2 diabetes, focusing on their effects on glycemic and weight management, as well as associated risks. Key findings included significant improvements in hemoglobin A1c and body mass index after meal replacement interventions, with notable efficacy when combined with lifestyle programs. Most reported adverse events were mild. The review categorized existing studies by meal replacement type and dosage, providing comprehensive insights for tailoring interventions in clinical practice. These results emphasize meal replacements as a promising tool in diabetes management, with implications for improving patient outcomes and reducing healthcare burdens.
- Citation: Lew LC, Mat Ludin AF, Abdul Manaf Z, Mohd Tohit N, Shahar S. Mapping evidence and identifying risks: A systematic scoping review of meal replacements in type 2 diabetes. World J Diabetes 2025; 16(8): 104371
- URL: https://www.wjgnet.com/1948-9358/full/v16/i8/104371.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i8.104371
According to the International Diabetes Foundation, about 451 million individuals across the globe are currently living with type 2 diabetes (T2D), and this figure is projected to rise to 693 million by 2045[1,2]. T2D with suboptimal glycemic management can lead to a series of comorbidities, such as nephropathy, retinopathy, atherosclerosis, hypertension, osteoarthritis, cardiovascular disease and higher mortality risk[3-7]. T2D has also severely affected the quality of life for the patients[8,9].
T2D and its comorbidities progression have led to a heavy burden on economic and healthcare cost[10-14]. Hence, a cost-effective intervention for the management of T2D is essential to reduce the economic burden of T2D on an already straining healthcare sector. Studies have demonstrated that weight loss positively impacts fasting plasma glucose and hemoglobin A1c (HbA1c) levels in individuals with T2D[15]. Glycemic management among patients with T2D has been closely linked to weight and body mass index (BMI)[16]. The American Diabetes Association (ADA) advises a weight reduction of over 5% for individuals with T2D and overweight or obesity[17]. This guideline is reinforced by findings from the DiRECT study, which revealed that 46% of 306 participants who lost up to 15 kg experienced a clinically significant remission of T2D[18].
Meal replacement represents one strategy for weight management. These products, which include pre-packaged or commercially prepared foods and beverages, are designed to substitute one or more regular meals[19]. As a form of medical nutrition therapy, meal replacement is frequently employed in diabetes care and weight management programs due to its convenience and high retention rates. The 2025 Standards of Care in Diabetes by the ADA endorsed the use of short-term meal replacements for individuals with T2D and overweight or obesity, aimed at achieving an energy deficit and facilitating weight loss[17]. Diabetes United Kingdom also stated in their evidence-based nutrition guidelines that total diet replacement plans providing 800-1200 kcal were effective for weight loss in T2D[20]. Similarly, Diabetes Canada Clinical Practice Guidelines also suggested that partial meal replacement replacing one or two meals could be used for patients with diabetes undergoing weight loss programs[21]. Meal replacement is also recommended by the Malaysia Clinical Practice Guidelines 2020 and the Malaysian Dietitians Association for weight loss and weight maintenance in patients with T2D[22,23].
Numerous trials on meal replacements are ongoing; however, a comprehensive review focusing on their types, compositions, dosages, and delivery methods remains insufficient. This scoping review aimed to consolidate existing research on meal replacements for managing T2D. We explored the types of meal replacements available for adults with T2D and evaluated their respective impacts on glucose regulation/HbA1c, weight loss, and other health outcomes. Additionally, potential risks or side effects associated with these meal replacements were identified. The findings were systematically organized by categorizing and summarizing the reviewed studies. The specific objectives of this scoping review included the following: (1) To present a comprehensive overview of existing research on the use of self-administered meal replacements in adult patients with T2D; (2) To systematically map the effects of self-administered meal replacements on specific parameters, including HbA1c levels, glucose regulation, and weight reduction in participants; and (3) To identify and categorize the adverse effects associated with the use of self-administered meal replacements among adults with T2D.
A previously published study protocol outlined the search strategy and provided detailed explanations of all methodological steps[24]. This scoping review adopted the methodological framework proposed by Arksey and O’Malley[25]. Reporting of the review followed the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA) checklist, which is available in Supplementary material[26].
The key stages in conducting this scoping review included: (1) Formulating the research question; (2) Identifying relevant studies; (3) Selecting studies for inclusion; (4) Extracting and organizing information from the selected studies; (5) Synthesizing and reporting the findings; and (6) Engaging in consultations with stakeholders.
(1) What are the various types, compositions, dosages, delivery methods, and durations of meal replacement plans utilized for the management of patients with T2D? (2) How do meal replacements affect glycemic management (HbA1c), weight reduction, and other health parameters in patients with T2D?; and (3) What adverse effects, if any, are reported by patients with T2D using meal replacements?
A systematic and extensive search was conducted for studies published between January 2000 and January 2024. In our previously published protocol, the search period was limited to January 2000 through August 2020[24]. We updated the search up to January 2024 to include more recent studies. The detailed search strategy is available in our previously published protocol[24]. Keywords and search terms were developed using the PICO framework, with synonyms identified for the key terms. These keywords (e.g., meal replacement, T2D, glycemic management, weight reduction, and risks) and their synonyms were combined to create multiple search strings. These strings were applied by the research team across various databases to capture all relevant resources (Supplementary material).
The study identification process was conducted across six online databases: Cochrane Library, PubMed, Science Direct, Scopus, Web of Science, and EBSCOhost Discovery. Additionally, manual searches were performed on reference lists of included studies and reviews to enhance coverage. Review articles were excluded from our analysis. Two independent researchers, Lew LC and Mat Ludin AF, carried out the searches simultaneously, employing a comprehensive search strategy.
Studies were selected based on predefined inclusion and exclusion criteria as detailed in our previously published protocol paper. The screening and selection process was conducted in three steps.
In the first step, two researchers, Lew LC and Mat Ludin AF, reviewed all titles retrieved from the database searches. All articles that involved meal replacements and patients with T2D were considered.
In the second step, the researchers independently assessed the selected titles and abstracts from step one to identify studies potentially relevant to the objectives.
In the third step, the researchers independently reviewed the full-text articles identified in step two. If full-text articles were not accessible online, they were obtained through the university library. Each full-text article was thoroughly examined to ensure alignment with the study objectives. Regular meetings were held among all researchers to discuss results and address any disagreements. The PRISMA flow chart was utilized to guide and document the study selection process.
Two researchers, Lew LC and Mat Ludin AF, carried out data extraction and charting. The primary outcomes extracted included the types and dosage of meal replacements and their effects on HbA1c, glucose levels, and weight reduction. Additionally, the side effects of meal replacements were recorded. Other significant findings were documented as 'other health status'. Other researchers, Shahar S, Abdul Manaf Z and Mohd Tohit N subsequently reviewed the extracted data for accuracy. Any discrepancies were addressed and resolved during group discussions.
Following data extraction, the information was categorized and summarized based on the types of meal replacements, including low-calorie/energy meal replacements, low glycemic index (GI) meal replacements, diabetes-specific formulas, protein-rich meal replacements, low-fat meal replacements, and combined meal replacements integrated with lifestyle intervention programs.
The complete data is presented in the results section and detailed in Supplementary material.
This scoping review was exclusively based on literature and did not involve any patient or public participation.
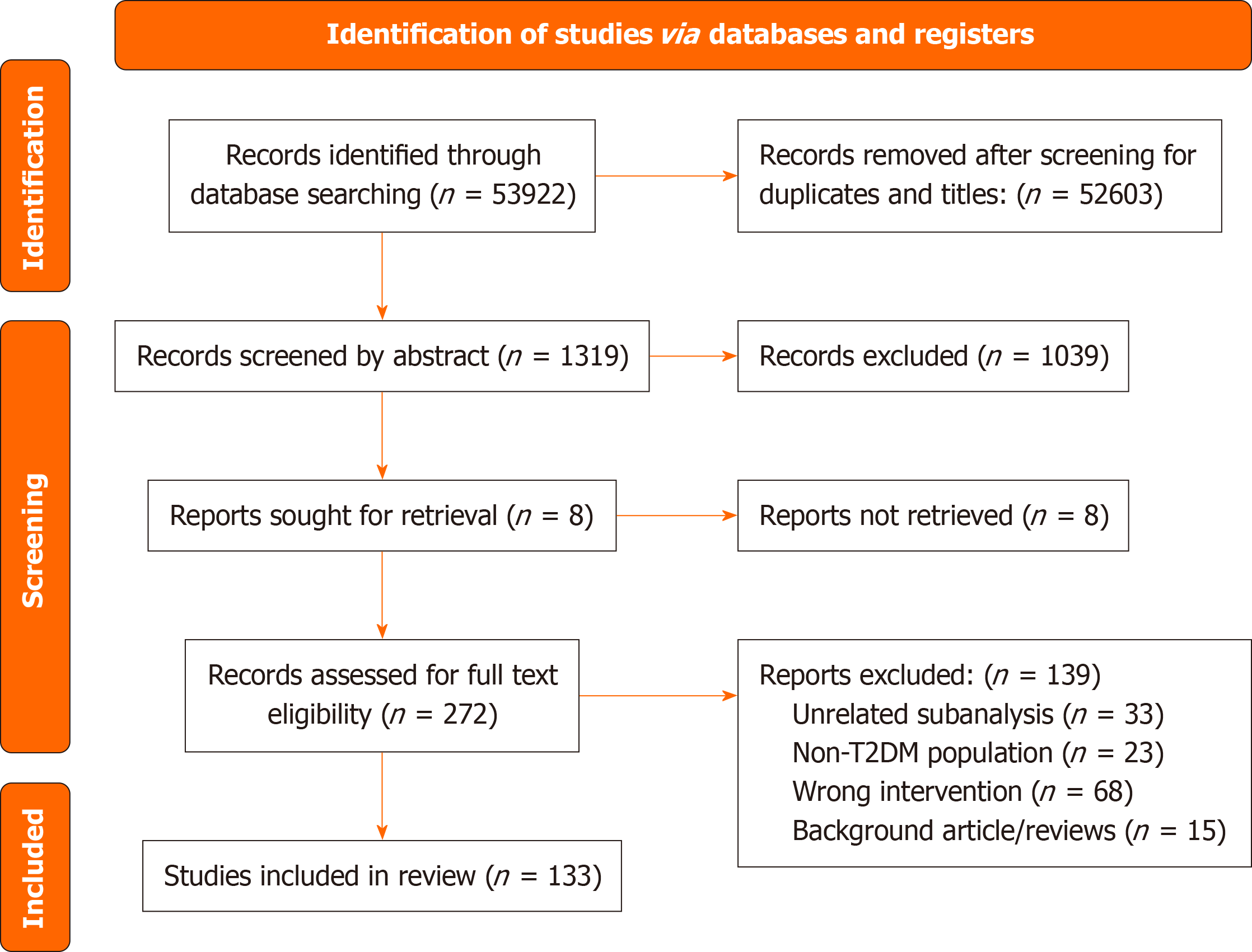
A total of 53922 articles were obtained from our search from six different databases. After removing the duplicates and screening through the titles, most of the records searched were excluded due to our overinclusive search strategy, leaving only 1326 articles to be screened subsequently for their abstract. Of the abstract screened, 286 records were included for in-depth full text review. Finally, 133 articles were selected to be included in the review. The PRISMA flow diagram in Figure 1 outlines the search and selection procedures implemented during the systematic literature search for this scoping review.
The included publications (n = 133) in the data extraction were published between the year 2001 to 2024. Most of the studies were published after year 2010 (n = 106) with only 27 studies published by 2010. Among the 114 studies selected, 27 studies were from the Look Ahead study, three were from the WAIT study, four were from the DiRECT study, two were from the Malaysian tDNA study, five were from the POWER study, and two were from the Diadem study. We have combined and summarized the articles of each study under one entry in the results section for easier interpretation. Hence, there were 96 articles presented in the table. The 133 studies selected involved 49 studies from the United States, involving 3301 participants; 47 studies from Europe, involving 2872 participants; 22 studies from Asia, involving 1401 participants; four studies from Canada, involving 514 participants; one study from Brazil, involving 34 participants and 10 studies from Australia and New Zealand, involving 416 participants. Detailed distribution of studies according to the geographical location is as shown in Table 1.
| Country | Number of studies | Participants in meal replacement arm (n) | Ref. |
| Asia | |||
| China | 7 | 465 | [33,37,44,47,86,103,104] |
| India | 4 | 303 | [63,82,92,93] |
| Japan | 1 | 119 | [91] |
| Malaysia | 3 | 193 | [24,45,141] |
| Singapore | 1 | 19 | [85] |
| Indonesia | 1 | 30 | [117] |
| Taiwan | 1 | 30 | [46] |
| Thailand | 2 | 172 | [39,118] |
| Qatar | 2 | 70 | [40] |
| Oceania | |||
| Australia | 9 | 396 | [36,52,55,56,61,90,95,106,116] |
| New Zealand | 1 | 20 | [70] |
| America | |||
| Canada | 4 | 514 | [48,54,62,94] |
| United States | 49 | 3301 | [30,34,38,43,49,50,72-76,80,84,88,89,96,101,107,115,121,122] |
| Brazil | 1 | 34 | [100] |
| Europe | |||
| Czech Republic | 1 | 22 | [102] |
| Denmark | 1 | 11 | [83] |
| Germany | 8 | 700 | [57,64-68,81,120] |
| Greece | 1 | 43 | [59] |
| Italy | 3 | 77 | [32,41,97] |
| Netherlands | 8 | 147 | [28,31,42,87] |
| Spain | 1 | 17 | [29] |
| Sweden | 2 | 392 | [109,114] |
| Ireland | 1 | 20 | [113] |
| United Kingdom | 21 | 1443 | [18,35,51,58,60,69,71,77-79,99,105,106,108,110-112,119] |
Among the studies included, 116 completed intervention studies while another 17 were study protocols. Among the intervention studies, most studies were randomized-controlled trials (RCT). The included studies comprised a total of 8543 participants undergoing meal replacement intervention. Only one study protocol did not report the number of participants. T2D was an inclusion criterion for all the included studies while overweight and obesity were an inclusion criterion for 62.4% of the studies (n = 83).
Majority of the participants were recruited from clinical establishments which included hospitals, clinics, lifestyle intervention centers, university research centers and other healthcare facilities while approximately 7% of participants (n = 601) were recruited from community settings. The age of the participants ranged from 18 years old to approximately 75 years old, with majority being in the 50-60 years age group, which is in line with the higher prevalence of T2D in the group aged 50 years and above based on the data from the Global Burden of Disease[27].
More than 60% of the included studies incorporated diet education or counseling sessions delivered by trained personnel. Specifically, 33 studies involved a research dietitian, four involved a physician, and 15 utilized both a physician and a dietitian. Additionally, two studies worked with nurses, three involved a combination of dietitians and nurses, five worked with trained counselors or diabetes educators, and a study reported the involvement of a pharmacist. The duration of these sessions typically ranged from 15 minutes to an hour, although one study reported a 90-minute session and another extended to 2 hours. The session most often occurred at baseline and continued weekly or biweekly throughout the intervention.
Clinical outcomes extracted and assessed included HbA1c, glucose levels, body weight/BMI, lipid profiles and some other indicators related to glycemic management, such as insulin levels if reported.
HbA1c was measured in 60 of the studies. Of these, 59 studies (98.3%) reported improvements in patient’s HbA1c levels. For example, a study by Lean et al[18] demonstrated a reduction in mean HbA1c levels of -0.9% among 149 intervention group participants compared to baseline after 12 months of meal replacement intervention. Only one study showed no significant changes in HbA1c levels[28].
Glucose level was measured in 48 of the included studies. Among them, 42 studies showed improvements in glucose levels. The reduction in glucose levels was often accompanied by a reduction in HbA1c levels among participants in most of the studies. Some studies (n = 6) have reported no changes in fasting glucose levels after meal replacement interventions[29-34].
Changes in weight or BMI were the most common outcome measured in 71 of the studies; 70 of them reported positive improvements while only one of them reported no changes after intervention[28].
Lipid profiles, such as cholesterol and high-density lipoprotein (HDL) levels, were evaluated as outcome measures in 42 studies. Among these, 36 studies reported positive effects, while six observed no significant changes[28,29,35-38]. One study noted an increase in triglycerides[39] and another reported elevated total cholesterol and low-density lipoprotein (LDL) levels[40].
Meal replacement interventions were grouped into low calorie/energy, low GI, protein rich, low fat meal replacements, diabetes specific formulas as well as combined meal replacements with lifestyle intervention programs. There was a series of commercially available meal replacements used in the trials, including Glucerna SR (13 trials)[28,37,38,41-50], Optifast (14 trials)[36,50-62], Almased Vitalkost (6 trials)[63-68], Cambridge Weight Plan (4 trials)[35,40,69,70], Slimfast (5 trials)[50,71-74], HMR (3 trials)[50,75,76], Counterweight Pro 800 (4 trials)[18,77-79], ONCE-PRO[39], Medifast[80], Modifast[81], Prototal Whey[82], Nutriletts[83], Nutrimed[84], Kicstart[85], Maide Technology co[86], Prodimed[87], Nutrisystem D[88,89], Probiotec Formula WL[90], Enerzona[29], Microdiet[91], Glucaeffect[32], Prohance D[92,93], Diasip[31], Ultra Glucose Control[38], Ideal Protein[94], Quaker Oats[38], KicStartä[95], BOOST Glucose Control[96], Therascience[97], Metabolic Sauver[98], LighterLife[99], and Nutren Control[100].
Table 2 provides comprehensive information on meal replacement types and dosage for the included studies. Detailed mapping of each study with outcome is presented in Table 3.
| Ref. | Country | Trial type & setting | Setting | Meal replacement arm (n) | Meal replacement type | Dosage/calorie | Combination | Length of intervention/follow up |
| Low calorie/energy MR (n = 35) | ||||||||
| Rothberg et al[75] | United States | Observational study | Clinical | 66 | HMR®, Boston, MA, United States | TDR; 160-170 kcal per packet, total 800 kcal per day; > 160 kg additional 160-170 kcal/day per 23 kg | / | 12 weeks |
| Tatti et al[41] | Italy | Non-randomised controlled trial | Clinical | 38 | Glucerna® SR, Abbott Nutrition | PMR; 206 calories per 230 mL for one main meal; Blend with low calorie frozen yoghurt into 250-270 kcal | / | 12 weeks |
| Steven et al[51] | United Kingdom | Longitudinal study | Clinical | 30 | Optifast (Nestle Nutrition, Croydon, United Kingdom) | TDR; 3 shakes per day + 240 g non starchy vegetables; total Cal intake 624-700 kcal/day | / | 8 weeks VLCD; + 24 weeks wight maintenance phase |
| Shantha et al[101] | United States | Cohort Study | Clinical | 72 | Calorie-restricted diet using meal replacements | 1000 kcal/day energy deficit; Individualized | Behaviour modification plan; Aerobic and strength training | Individualized 3 months to 1.5 years |
| Astbury et al[35] | United Kingdom | Randomized controlled trial | Clinical | 138 | TDR with four formula products daily (LCD) provided by Cambridge Weight Plan United Kingdom | TDR; First 2 weeks, liquid MR. Week 3 onwards MR bars option; Energy intake comprised 810 kcal/day (3389 kJ/day) | / | 8 weeks LCD, gradual food reintroduction, weekly behavioural support for 24 weeks - one MR is consumed per day |
| Baker et al[52]; | Australia | Non-randomized case-control trial | Clinical | 37 | Optifast (Nestle Nutrition, Croydon, United Kingdom) | TDR; 3 sachets daily combined with serving of vegetable once daily (800 kcal/day) | After week 12, 8-week transition phase to calorie restriction diet based on Australian Commonwealth Scientific and Industrial Research Organization Total Wellbeing diet (1350 kcal) for another 4 weeks | 12 weeks VLCD + 8 weeks transition to calorie restriction diet + 4 weeks 1350 kcal diet |
| Bishay[53] | Australia | RCT Protocol | Clinical | Not reported | Optifast (Nestle Nutrition, Croydon, United Kingdom) | TDR; 5-months of complete VLCD (820 kcal/day) | / | 32 weeks |
| Bhatt et al[82] | India | Clinical Audit | Clinical | 12 | Protein formula (Prototal Whey) | TDR; 1000 kcal/day diet | / | 12 weeks |
| Berk et al[42] | Netherlands | Randomized controlled trial | Clinical | 64 | Glucerna® SR, Abbott Nutrition | PMR; Replace breakfast and lunch; Light dinner for a combined 750 kcal/day | / | 8 weeks + 12 weeks LCD reintroduction (1300 kcal/day) |
| Shiau et al[54] | Canada | Retrospective cohort study | Clinical | 317 | OPTIFAST® 900 | TDR; 4 MR shakes per day for a total of 900 kcal per day | First 6 months - weekly diet session, behavioural and therapy; Next 6 months - optional monthly support session | BMI > 33 kg/m2 - 12 weeks of full MRs; BMI < 33 kg/m2 - 6 weeks of full MRs with option to increase to up to 12 weeks of full MRs; 5-week transition to maintenance diet |
| Cinkajzlová et al[102] | Czech Republic | Intervention study | Clinical | 22 | VLCD | 2500 kJ per day | / | / |
| Taheri et al[40] | Qatar | Non-blinded, randomised controlled, parallel-group trial | Clinical | 70 | LCD from Cambridge Weight Plan | 800 kcal; First 12 weeks: TDR supplemented by low-fat milk; Month 4 to month 6: PMR; Gradual introduction to three meal per day eating pattern | Individual dietetic and activity appointments. Unsupervised physical activity of at least 150 minutes per week | PHASE 1: 12 weeks LED + physical activity; PHASE 2: 12 weeks partial LED + physical activity; PHASE 3: 6 months own food, physical activity, and lifestyle change. PHASE 4: 12 months follow-up |
| Harder et al[83] | Denmark | Single arm study | Clinical | 11 | Nutriletts (NutriPharma, Oslo, Norway) | TDR; 850 kcal/day | / | 8 weeks |
| Friedman et al[84] | United States | Proof-of-concept pilot study | Clinical | 6 | Nutrimed (Robard Corporation, Mount Laurel, NJ, United States) | PMR; 800 kcal/day | / | 12 weeks |
| Lean and Leslie[77] | United Kingdom | RCT protocol | Clinical | Total N = 25 | Counterweight Pro 800 | TDR; Initial Total Diet Replacement phase (0-12 weeks); 825-853 kcal/day | Counter weight-Plus’ weight management programme; Structured food reintroduction (12-20 weeks).; -Replacing TDR with meals which contain 30% of energy from fat | 20 weeks with initial 12 weeks TDR |
| Wong et al[103] | China | Randomized, non-blinded, single center trial | Clinical | Total N = 37 | LED | PMR; Replace at least one meal with less than 300 kcal | / | 24 weeks |
| Leader et al[55] | Australia | RCT | Clinical | 36 | Optifast VR | PMR; 1 or 2 PMR per day | / | 12 months |
| Overl et al[104] | China | Conference paper; RCT | NA | Total N = 10 | MR shakes | Three meal replacement shakes (600 kcal/day) for two 24-hour periods/week | / | 12 weeks |
| Gulsin et al[69] | United Kingdom | Single-center, prospective, randomized, open-label, blinded end point trial with a nested case-control study | Clinical | 24 | Low energy MRP from Cambridge Weight Plan | TDR approximately 810 kcal/day (Cambridge Weight Plan) | / | 12 weeks |
| Farrer and Golley[36] | Australia | Non-randomized intervention | Clinical | 19 | Optifast VLCD programme | Three phases of 4 weeks each: Intensive (3 Optifast VLCD per day, < 3360 kJ/day), Transition (2 Optifast VLCD per day, approximately 5040 kJ/day) and Maintenance (1 Optifast VLCD per day, approximately 5040 kJ/day); 12th week: Full normal meals were resumed to meet energy deficit requirements of 50% total energy | Traditional diabetes and weight management education | 12 weeks |
| Sumithran and Proietto[56] | Australia | Case study | Clinical | 1 | Optifast VLCD | TDR; 5 months VLCD 3 times a day (1908 kJ); Transition phase 7 months: Two VLCD meals per day and one low fat meal per day | Daily exercise program; Weight control clinic | 12 months |
| Rolland et al[105] | United Kingdom | Case study | Community | 355 | VLCD in the form of food packs (soups, shakes, textured meals and bars) | TDR; 550 kcal daily | Commercial weight-management programme (Lighter Life Total); Group support; Cognitive behaviour therapy | 12 weeks |
| Dhindsa et al[71] | United Kingdom | Intervention | Clinical | 44 | VLCD Slimfast, liquid MR | PMR; 3 Slimfast MR per day; 750 cal | Follow-up phase (week 8 to week 52) with a standard low-calorie weight-maintenance diet | (1) 8 weeks of VLCD therapy; and (2) Follow-up phase (week 8 to week 52) with a standard low-calorie weight-maintenance diet |
| Khoo et al[85] | Singapore | Randomized Trial | Clinical | 19 | Liquid meal replacement (Kicstart, Pharmacy Health Solutions, Sydney, Australia) | PMR; Maximum 450 kcal, 0.8 g/kg ideal body weight of protein and one other small meal, for a total of approximately 900 kcal/day for 8 weeks | Switch to or continue high protein diet for the remaining 44 weeks | 8 weeks LCD/52 weeks |
| Moriconi et al[97] | Italy | Retrospective study | Clinical | 15 | Very-low-calorie ketogenic diet (VLCKD) (Therascience, New Penta SRL or Pronokal Group) | First phase (45 days) TEI was < 800 kcal with 4/5 MR per day. Second phase (45 days), one and subsequently two replacement meals were replaced with conventional food | Caloric intake gradually increases; Full carb reintroduction - 6 months | Phase 1 - 45 days; Phase 2 - 45 days; Phase 3 - until 12 months |
| Tang and Lin[86] | China | RCT | Clinical | 50 | Fasting-mimicking diet (FMD) MR (Maide Technology Co., Ltd., Wuhan, China) | PMR; FMD MR from Monday to Friday in the second week of a month. Eat normally for the rest of the month. Energy for first day and the second to fifth days was 1196 and 805 kcal respectively | / | First 3 months - MR; Last month - normal diet |
| Maher et al[106] | Ireland | Case study | Clinical | 1 | Low-energy liquid diet | TDR; 1012 kcal per day with 2.2 L of semi-skimmed milk | / | 8 weeks LELD; + 16 weeks of phased reintroduction of normal diet |
| Nori Janosz et al[34] | United States | Retrospective chart review | Clinical | 33 | LCD MR | PMR; 1000-1200 kcal/day | Behavioural treatment plan | At least 4 weeks |
| Storck et al[57] | Germany | Prospective, interventional study | Clinical | 36 | OPTIFAST® II Short program, Nestlé Health Science, Germany) | TDR; 5 sachets per day of 800 kcal for 6 weeks. 4-week refeeding phase.; Last 5 weeks, energy intake will be gradually increased to between 1200 and 1500 kcal | Standardized weight-loss program (OPTIFAST® II Short program, Nestlé Health Science, Germany); Weekly visit for exercise training. Diet counseling | 12 weeks |
| Schwasinger-Schmidt et al[107] | United States | Retrospective analysis | Community | 44 | LCD in form of shakes, soups, cereal, and entrees | TDR; Consume at least five meal replacements with a minimum of 800 kcal per day | Weekly behavioural education classes | 12 weeks |
| Steven and Taylor[58] | United Kingdom | Intervention study | Clinical | 30 | VLCD liquid MR (Optifast) | TDR; Replaced all meals with Optifast 624 kcal/day | Discontinued all diabetic medications | 8 weeks |
| Redmon et al[72] | United States | RCT | Clinical | 30 | LCD MR (meal shakes or meal bars) (Slim Fast Foods Company) | PMR; Repetitive intermittent LCD weeks: LCD of 900-1300 kcal per day (220 kcal/serving, four to six servings daily) for 7 consecutive days every 2 months; On normal days, use one MR and one snack bar (120 kcal/snack bar) daily to replace one usual meal and snack to reach 500 to 1000 kcal per day reduction goal | Combination therapy group; Individual counseling by a registered dietitian; Individualized diet of 500-1000 kcal reduction in daily energy; Individualized exercise prescription | 12 months |
| Lips et al[87] | Netherlands | Controlled nonrandomized observational trial | Clinical | 12 | Commercially available Prodimed (Prodimed Benelux BV, Val-Kenswaard, The Netherlands) | TDR; 4-5 sachets a day with 90 kcal each sachet, average 600 kcal/day | / | 3 weeks |
| Elizabeth O Beale[76] | United States | RCT protocol | Clinical | 15 | TDR first 3 months; PMR 3-6 months; HMR70 Plus products; HMR Boston, MA, United States | Phase 1: TDR 3-months 1200-1400 cal diet. Phase 2: Regular meals and 1 MR/day. Phase 3: 6-month weight loss maintenance period with 1 MR/day | Usual care at Roybal Diabetes Management Clinic | 12 months |
| Abi-Chahine et al[108] | United Kingdom | Abstract for poster presentation for a person-centred intervention study | Community | 23 | TDR | TDR of 800 kcal daily supplementation | Food reintroduction after 12 weeks; Culturally sensitive diet and lifestyle support through 26 e-learning modules | 24 weeks |
| Rafey et al[113] | Ireland | Prospective observational cohort study | Clinical | 20 | Milk-based LELD | TDR; Week 1-8: Approximately 1200 kcal/day, or 130 g of carbohydrates and 40 g of fat intake per day. Week 9-16: Gradual reintroduction of low calorie meals.; Week 17-24: Stopped milk replacement and start fully isocaloric meal plan | / | 24 weeks |
| Reynolds[70] | New Zealand | RCT protocol | Clinical | 20 | Cambridge Weight Plan products (VLED) | TDR; Consumption of 3-4 meal replacements per day providing approximately 3600 kJ/day | Structured programme with monthly visits for long-term weight loss maintenance | 12 weeks |
| Scragg et al[112] | United Kingdom | RCT protocol | Clinical | 254 | Low-energy low-carbohydrate diet | TDR; 800-1000 kcal with a maximum of 40-60 g carbohydrate/day, compared to usual intake of 200-250 g | / | 6 months |
| Tsompanaki et al[111] | United Kingdom | RCT protocol | Community | 28 | TDR with LED | TDR; LED with package of soups, shakes, bars (4 per day providing approximately 850 kcal/day) for first 12 weeks | Stepped food reintroduction starting from 12th weeks and weight maintenance phase | 12 months |
| Shirmann[110] | United Kingdom | RCT protocol | Clinical | 36 | TDR with Low-calorie Diet Programme | TDR; approximately 850 cal per day through four TDR products daily for 12 weeks; Followed by a 6-week food reintroduction period and weight maintenance support for 8 months | 12 weeks | |
| Hocking et al[61] | United Kingdom | Single arm intervention trial | Clinical | 155 | TDR with LED; Optifast; Nestlé Health Science | TDR; 3 MRP per day (800 kcal per day). If BMI more than 40, 950 kcal per day | Dieitian visits every 2-4 weeks | 13-week TDR; 8 weeks structured food reintroduction; 31-week supported weight maintainence |
| Ekberg[109] | Sweden | RCT Protocol | Not stated | 286 | Low Carb or low calorie diet | Not stated | / | 15 months |
| Low glycemic index MR (n = 5) | ||||||||
| Stenvers et al[28] | Netherlands | Randomized, controlled, cross over trial | Clinical | 29 | Glucerna SR (Abbott Nutrition) | PMR; Baseline breakfast intake mean 292 kcal | / | 12 weeks |
| Foster et al[88] | United States | Randomized controlled trial | Clinical | 50 | Pre-packaged, Portion Controlled Diet (Nutrisystem D, Fort Washington, PA, United States) | PMR; Women approximately 1250 and men 1550 kcal per day, with approximately 55% of total energy from the packaged foods and 45% from supplemental grocery items | / | 6 months |
| Boonyavarakul et al[39] | Thailand | Randomized controlled trial | Clinical | 60 | ONCE-PRO | PMR; Replace one meal per day to provide 30% of energy intake; Aim: 25-30 kcal/kg/day | / | 12 weeks |
| Li et al[33] | China | randomized, open label, interventional study | Clinical | 47 | Multi-nutrient powdered supplements (LEHEL Company, Guangzhou, China) | PMR; Provides 346 kcal energy in place of breakfast | Diabetic health education organized by nutritionists every 2 weeks | 12 weeks |
| Eliana and Pranoto[117] | Indonesia | Randomized, controlled, crossover, open-labelled study | Clinical | 30 | Carbohydrate mix-fortified liquid meal replacement nutrition (LMRN) | TDR; 4008 kcal | / | 4 days × 2 (crossover) |
| Santen et al[89] | United States | Intervention Pilot study | Clinical | 11 | TDR with Nutrisystem®D® Meal Replacements, Nutrisystem Inc. | TDR; 1450 to 1550 kcal/day for men; 1200 to 1250 kcal/day for women | Telcare with glucometer, weighing scale and cloud data assessment.; Education sessions | 6 months |
| Otten[114] | Sweden | RCT Protocol | Clinical | 106 | TDR | TDR for 850 kcal/day | Cognitive behavioural therapy programs through face to face or ehealth application | Total diet replacement for 3 months. Weight maintenance for 21 months |
| Anyiam et al[99] | United Kingdom | RT | Clinical | 18 | TDR VLCD; LighterLife® total meal replacement | TDR VLCD with less than 800 kilocalories per day | / | 12 weeks |
| De Freitas et al[115] | United States | Case study | Clinical | 1 | TDR VLCD with a formula liquid diet meal replacement | TDR 800 kcal/day; Twelve weeks of VLCD were followed by 4 weeks of low-calorie diet (incorporating 3 meal replacements and 1 meal with ad libitum non-starchy vegetables) | / | 16 weeks |
| Khoo et al[116] | AUS | Retrospective cohort study | Clinical | 51 | PMR with LCD | PMR Two meal replacement shakes plus a healthy meal | / | 3 months PMR + 21 months reduced calorie meal |
| Diabetes Specific Formulas (n = 16) | ||||||||
| Belcaro et al[32] | Italy | Single blinded RCT | Clinical | 24 | Glucaffect™ (Reliv Inc., Chesterfield, MO, United States) | PMR; Substitute up to two meals a day for 6 days a week. Dinner following regular choices | Personal Exercise Program with 60 minutes each day | 8 weeks |
| Fonda et al[43] | United States | Prospective, 3-way, cross-over design | Clinical | 18 | Glucerna Weight Loss Shake, Slim-Fast Shake, and Ensure with Fiber Shake | Subjects consumed the meal replacement; beverages after an overnight fast, in random sequence on; different weeks, 1 week apart | / | 1 week |
| Garvey et al[119] | United Kingdom | Multi-center, single arm, unblinded study | Clinical | 147 | / | PMR; Two meal replacement shakes and snack bars daily | Diet and lifestyle counseling | 12 weeks + 12 weeks sustainability |
| Sun et al[37] | China | Unblinded, randomized, controlled clinical trial | Clinical | 100 | Glucerna SR (Abbot Nutrition) | PMR; Replace breakfast, providing 200 kcal | Weekly sessions on: Diet consultation; Review of blood glucose measurements | 24 weeks |
| Peng et al[44] | China | RCT | Clinical | 62 | Glucerna SR (Abbot Nutrition) | PMR; Replacing breakfast providing 220.5 kcal | Lifestyle education; Individualized meal plan | 4 weeks |
| Chee et al[45] | Malaysia | RCT | Clinical | 115 | Glucerna SR (Abbot Nutrition) | PMR; Structured low-calorie meal plan - 1200 or 1500 kcal/day; Normal foods + one or two diabetes-specific formula servings | tDNA group receives physical activity at least 150 minutes/week; Education using tDNA toolkit: Flipchart on healthy eating, 14-day meal plans, information on physical activity; Subgroup receives motivational interviewing or conventional interviewing | 6 + 6 months follow-up |
| Patel[92] | India | RCT Protocol | Clinical | 100 | Prohance D Vanilla Flavour (Nutraveutical Product) | PMR; Once a day | / | 12 weeks |
| Hwu[46] | Taiwan | RCT protocol | Clinical | 30 | Glucerna SR (Abbot Nutrition) | PMR; To replace one meal (breakfast) and one pre-sleep snack for 24 weeks | Diet plan with 500-800 kcal/day less than their estimated daily maintenance energy requirement | 24 weeks |
| Bao[47] | China | Single-centre, Randomized, Open-label, Parallel Group Study Protocol | Clinical | 66 | Glucerna SR (Abbot Nutrition) | To replace breakfast | / | 4 weeks |
| Lansink et al[31] | Netherlands | Randomized, controlled, double-blind, parallel-group study | Clinical | 22 | Diasip® (Nutricia N.V., Zoetermeer, The Netherlands) | PMR; Two 200 mL portions (200 kcal per portion) per day for 4 weeks, one for breakfast and one for snack in the afternoon or evening | / | 4 weeks |
| Mottalib et al[30] | United States | A prospective, randomized, three-arm study | Clinical | 72 | DSNF | PMR; Group B and C - hypocaloric dietary plan (1500 kcal/day for women, 1800 kcal/day for men) with a commercially available DSNF (220 kcal/serving) 1-3 times per day | / | 16 weeks |
| Otto et al[48] | Canada | Retrospective cohort study | Clinical | 47 | Glucerna SR (Abbot Nutrition) | PMR; 2 cans of Glucerna per day (230 kcal/serving as part of a 1200 to 1400 kcal diet) | / | At least 3 months |
| Yip et al[73] | United States | RCT | Clinical | 41 | Liquid MR preparation containing lactose, fructose, and sucrose (Slim-Fast; MR1); Liquid MR in which sucrose and fructose were replaced by nonsugar-containing glucose oligosaccharides (sugar-free Slim-Fast; MR2) | MR1 (Slim-Fast Foods, New York, NY, United States): 11 g lactose, 13 g fructose, 8.5 g sucrose, and 14 g protein. MR2: Identical to MR1, fructose and sucrose were replaced with equivalent levels of maltodextrins. (250 kcal each); Replaced their three meals with MRs for the first 5 days. Replaced two meals for remainder of the study | / | 12 weeks |
| Mottalib et al[38] | United States | Cross-over, three-way and open-label clinical study | Clinical | 22 | Three types of meal replacement served on each visit on breakfast: Glucerna (Abbott Nutrition Inc., Columbus, OH, United States); Ultra Glucose Control (Metagenics Inc., Aliso Viejo, CA, United States); OM (Quaker Oats Co., Chicago, IL, United States) | PMR for breakfast; 200 kcal/meal | / | Three visits with crossover of different MR |
| Cheskin et al[80] | United States | Controlled clinical trial | Community | 54 | Medifast Plus for Diabetics (Medifast, Inc, Owings Mills, MD, United States) | PMR; 25% of energy calorie deficit at weight-loss-phase diet; 10% calorie deficit at weight-maintenance-phase diet. PCD group received 50% to 60% of their prescribed calories from meal replacements | An initial 34-week weight loss period, then PCD participants were rerandomized for 52-week maintenance phase to either 26 weeks of PCD followed by 26 weeks of Standard Diet (PCD1) or vice versa (PCD2) | 34-week weight loss period and 52-week maintenance phase (86 weeks) |
| Mustad et al[49] | United States | Randomized, open-label, three-group parallel study design | Clinical | 49 | Glucerna Hunger Smart (Abbott Nutrition, Columbus, OH, United Sates) | PMR; One meal supplies 180 kcal. DSNS breakfast and afternoon snack (Bkfst/AS); DSNS breakfast and bedtime snack (Bkfst/PBS); Self-selected diets for 7 days, then MR for 7 days | / | 7 SSD + 7 days MR |
| Lew et al[98] | Malaysia | RCT Protocol | Clinical | 78 | PMR with Diabetes specific meal replacement - Metabolic Sauver, Powerlife (M) Sdn Bhd, Kuala Lumpur, Malaysia | PMR for 5 days a week replacing 1 meal per day, providing 327 kcal per comsumption | Dietary Consultation | 12 weeks intervention + 12 weeks follow up |
| Wichansawakun[118] | Thailand | RCT Protocol | Not stated | 76 | PMR with Diabetes specific meal replacement | Replace 1 meal per day based on weight based calculation at 25-30 kcal of energy per ideal body weight per day, and the ratio of carbohydrates to protein to fat is 45-50:20:30-35 of total energy | / | 12 weeks |
| Dharmalingam et al[93] | India | RCT | Clinical | 71 | PMR with DSNS (Prohance-D® Vanilla flavored powder); (Sun Pharmaceutical Industries Limited, Mumbai, India) | One serving of DSNS used as breakfast/evening snack replacement, providing 16.8% of the recommended daily allowance (RDA) of protein, 454 kcal energy | Oviva Diabetes Remission Insulin (ODR-I) programme: Expert dietitian coaching; Oviva app (with a 12-month weight prediction chart); Capilar Blood Glucose meters; BodyTrace weight scales | 12 weeks |
| Zagury et al[100] | Brazil | Randomized control crossover trial | Clinical | 34 | Glycemia targeted specialized supplement PMR; Nutren Control®, Nestle | Replaced breakfast to provide 208 kcal | / | 7 days |
| Protein rich MR (n = 13) | ||||||||
| Keogh and Clifton[90] | Australia | Randomized controlled trial | Community | 60 | Probiotec Formula WL (Probiotec Limited, Laverton North, VIC, Australia 3026) | PMR; 2 MR (880 kJ each) and low-fat evening meal per day first 12 weeks + at least 5 serves of fruit and vegetables/day (total approximately 5000 kJ); 1 MR for further 12 weeks | / | 24 weeks |
| Navas-Carretero et al[29] | Spain | Single group, sequential, longitudinal design | Clinical | 17 | 4 weeks Structured meal replacements: Breakfast, morning snack and afternoon snack, were exchanged by specific products, with a moderately high protein content and low glycemic index (55); Enerzona© (Equipe Enervit) | TDR; approximately 1800 kcal | / | 4 + 4 weeks |
| Manjunath[63] | India | Randomized, Parallel Group, Multiple Arm Trial Protocol | Clinical | 120 | Almased Soya protein powder with yogurt | PMR; First 6 months, Almased substituting one major meal/day; Dosage will be defined individually according to body weight. Next 6 months, Almased (50 g/day) added to the diet before one meal | / | 6 + 6 months follow-up |
| Kempf et al[64] | Germany | Proof of principle study | Clinical | 22 | Almased-Vitalkost; Almased-Wellness-GmbH, Bienenbüttel, Germany | First week, breakfast, lunch and dinner replaced with Almased (50 g per meal = 150 g per day = 2223 kJ) + 45 g oil (1717 kJ) + 750 mL vegetable juice (544 kJ), Total 4903 kJ per day. 2-4 week: Breakfast and dinner (1465 kJ) + regular lunch (2093 kJ) + 45 g oil, total 4600-5300 kJ; 5-12 week, only dinner is replaced with 50 g Almased | / | 12 weeks |
| Kempf et al[65] | Germany | RCT | Clinical | Strict diet regime N = 37; Moderate group N = 43 | Almased®, Almased-Wellness-GmbH, Germany | PMR; Strict diet group replaced three meals in week 1, two meals in weeks 2-4 and one meal in weeks 5-12 with 1 g PMR (Almased®) per kg normal body weight.; Moderate group replaced two meals in weeks 1-4 and one meal in weeks 5-12 | / | 12 weeks + 9 months follow up |
| Kempf et al[120] | Germany | Conference paper; RCT | Clinical | 55 | Protein-rich meal replacement | PMR | / | 12 weeks |
| Martin et al[66] | Germany | RCT | Clinical | Stringent diet regime (n = 40); moderate diet regime (n = 37) | Almased-Vitalkost, Almased Wellness GmbH, Bienenbüttel, Germany | 1st week: Replaced 3 main meals by 50 g PMR = 1100 kcal/day; 2-4th week: 2 meals were replaced, and a protein-rich lunch was allowed; 5-12th week: Only dinner was replaced.; Moderate Group: Replaced breakfast and dinner for 5 weeks and then only dinner during the next 7 weeks | 12 weeks | |
| Li et al[74] | United States | RCT | Clinical | 46 | SlimFast Food Company, Inc. West Palm Beach, FL 33401, United States | Replace 3 meals per day for first 5 days of the study. Replace 2 meals for three additional months. After three months, replace one to two meals per day with MR | / | 12 months |
| Kempf et al[68] | Germany | Single-blind, active comparator, intervention study | Clinical | 102 | Almased-Vitalkost, Almased Wellness GmbH, Bienenbüttel, Germany | 1st week: Replaced 3 main meals by 50 g PRMR = 1100 kcal/day; 2-4th week: 2 meals were replaced, and a low carb protein-rich lunch was allowed; 5-12th week: Only dinner was replaced | Weekly care calls (planned duration 20 minutes) from trained diabetes coaches. Received a weighing scale, and a step counter; TeLiPro group additionally received a blood glucose meter | 12 weeks; 26 weeks and 56 weeks follow up without intervention |
| Shirai et al[91] | Japan | Randomized Trial | Clinical | 119 | Protein Sparing Formula Diet (Microdiet, Sunny Health Co. Ltd) | PMR; One pack of MR in the morning providing 240 kcal/meal; 2 conventional Japanese meal in noon and evening | / | 24 weeks |
| Durrer et al[94] | Canada | RCT Protocol | Clinical | 100 | Commercial diet plan (Ideal Protein) | Commercial diet plan with pre-packaged foods used for two meals and one snack each day. The third meal prepared from lower-fat protein sources and low-carbohydrate vegetables.; Meal plan: 850-1100 kcal per day | / | 12 weeks |
| Kempf et al[67] | Germany | RCT | Clinical | M: Moderate diet group 146; S: Stringent diet group 139 | Almased-Vitalkost; Almased-Wellness-GmbH, Bienenbüttel, Germany | PMR; Contained 30.6 g carbohydrates and 1507 kJ (360 kcal) energy per 100 g powder | / | 12 weeks of intervention, and 52 weeks of follow-up |
| Papakonstantinou et al[59] | Greece | Randomized, crossover Protocol | Clinical | 17 | Optifast by Novartis Hellas, S.A.C.I., Metamorfossi, Greece | PMR; Replaced breakfast and lunch and made up 26% of their energy intake | / | / |
| Low fat MR (n = 1) | ||||||||
| Barbosa-Yañez et al[81] | Germany | randomized, parallel group, intervention study | Clinical | 43 | Flavoured meal replacement powder [MODIFAST® (OTC Siebenhandl GmbH) Ulm, Germany] | TDR; 1000-1200 kcal/day, and less than 30% of the total energy intake (E%) is fat; 200 g of raw or steamed vegetables | / | 3 weeks of intensive low-fat diet, 49 weeks of eucaloric diet under DGE guidelines |
| MR + lifestyle intervention programme (n = 10) | ||||||||
| Delahanty et al[121] | United States | Randomized, assessor-blinded, practice-based clinical trial | Clinical | 69 | Shakes, bars, and pre-packaged entrees | PMR; Use of meal replacements was recommended (but not supplied) for 1-2 meals per day starting in week 3 based on the Look AHEAD protocol. Meal replacement use was not formally tracked | Lifestyle intervention delivered by registered dietitian with 37 session identical contents in in-person and telephone arm.; Medical nutrition therapy participants were referred to a dietitian at their health center or preferred location as per usual care | 12 months |
| Wycherley et al[95] | Australia | Randomized clinical trial | Community | 37 | KicStartä, Pharmacy Health Solutions, New SouthWales, Australia | PMR; High-protein, energy-restricted diet (5500 kJ/day) | A group of only diet. Another group of diet + exercise; Walking/jogging exercise programme comprising four to five exercise sessions per week | 12 weeks |
| McDiarmid et al[60] | United Kingdom | RCT | Clinical | 79 | Optifast 820 | TDR; 8 weeks of Optifast 820 kcal/3430 kJ formula diet, followed by 4 weeks of food reintroduction. Both groups were asked to complete 56 days during their active weight loss phase | After active weight loss phase, participants will be separated into two groups with CLED following a portion-controlled Mediterranean diet 7 days per week while ILED follows a MR diet for 1-2 days and portion-controlled Mediterranean diet for 5-6 days a week | 12 months |
| Reynolds et al[122] | United States | RCT | Clinical | 21 | Pre-packaged entrees and low-calorie shakes (Health Management Resources, Boston, MA, United States) | PMR; Replace two meals with at least 1500 kcal per day | Placebo or Rosiglitazone 4 mg/day; Lifestyle programme with weekly behavioural education classes for 6 weeks then bi-weekly classes for remainder of the study | 6 months |
| Hamdy and Carver[96] | United States | Intervention study | Clinical | 85 | BOOST Glucose Control (Nestlé HealthCare Nutrition, Inc., Minneapolis, MN, United States) | PMR; A meal plan with a 500-cal reduction rounded to the nearest 1200-, 1500-, or 1800-cal level | Weekly cognitive behavioural support; Weekly group education; Intensive and interactive diabetes medication adjustment; Individualized exercise plan | 12 weeks |
| Pi-Sunyer et al[50] | United States | RCT | 16 Clinical Centres | 2496 (97.1%) ILI; 2463 (95.7%) DSE | 4 meal replacements to choose: SlimFast (SlimFast Foods), Glucerna (Ross Laboratories), OPTIFAST (Novartis Nutrition) and HMR (HMR, Inc.) | First 3 weeks: Self-selected diet with energy goal for persons < 114 kg is 1200-1500 kcal/day and is 1500-1800 kcal/day for individuals ≥ 114 kg. First 6 months: Replace two meals and one snack a day with liquid shakes and meal bars (1200-1500 kcal/day); Months 7 onwards to year 4: Replace one meal and one snack per day; Calorie targets personalized based on participants weight loss goals | 1 hour diabetes education class on first visit + Three group educational/social support sessions each year for 4 years; Physical activity + Behavioural techniques | Intensive intervention for first 4 years, with an average of 10.25 years follow up |
| Lean et al[18] | United Kingdom | RCT | Clinical | 149 | Counterweight-Plus MR | TDR phase using a low energy formula diet (825-853 kcal/day) | Counterweight-Plus weight management programme | Total diet replacement of 3-5 months with stepped food reintroduction of 2-8 weeks and long-term weight maintenance program until month 12 |
| Sattar et al[78] | United Kingdom | RCT | Clinical | 25 | TDR with Low energy liquid formula diet (Counterweight Pro 800) | Initial Total Diet Replacement phase (3-5 months); A commercial micronutrient-replete 825-853 kcal/day LELD is provided (Counterweight Pro 800) to replace normal foods | Counterweight-Plus’ weight management programme; Structured food reintroduction (6-8 weeks) | 3 months minimum |
| Marples et al[79] | United Kingdom | Intervention study/service evaluation | Clinical | 37 | Phased LED TDR and PMR with Counterweight TDR products | TDR phase: 825-853 kcal/day; Food reintroduction phase: Gradual reduction in the formula product and the incorporation of nutritionally dense and energy-restricted meals (360-400 cal per meal). Weight Loss Maintenance phase | Behhaviour change techniques | TDR phase: 12 weeks; Food reintroduction phase: 9 weeks; Weight Loss Maintenance phase: 31 weeks |
| Dasgupta et al[62] | United Kingdom and Canada | RCT protocol | Clinical | 50 | Phased TDR and PMR with Optifast products (Nestlé) | First 2 weeks: Optifast products (Nestlé), totalling 800-900 kcal/day (30% protein, 50% carbohydrate and 20% fat).; Week 3-12: PMR with 800-900 kcal daily on non-exercise days and an additional 150-200 kcal from meal replacement products on exercise days. Week 12-24: Maintainence phase, individualized meal plan | Exercise training | 24 weeks: 12 weeks MR and 12 week maintainence |
| Ref. | Year | HbA1c | Glucose | Weight (kg) | Lipid profile | Other health status | Adverse effects |
| Low calorie/energy MR (n = 32) | |||||||
| Rothberg et al[75] | 2014 | 1R | / | 1R | / | / | Not reported |
| Tatti et al[41] | 2010 | 1R | 1R | 1R | Total cholesterol - 1R; Triglycerides - 1R; HDL - 2I | SBP - 1R; DBP - 1R | Not reported |
| Steven et al[51] | 2016 | 1R | 1R | 1R | 1R | Plasma insulin levels - 1R | Not reported |
| Shantha et al[101] | 2012 | 1R | / | 1R | / | / | Not reported |
| Astbury et al[35] | 2018 | 1R | 1R | 1R | NC | HOMA-IR - 2I | Constipation (1 in 7), fatigue (1 in 12), Headache (1 in 17), dizziness (1 in 22) |
| Baker et al[52] | 2011 | / | / | 1R | / | Plasma insulin levels - 1R | Not reported |
| Bhatt et al[82] | 2017 | 1R | 1R | 1R | Triglycerides - 1R; HDL - NC; LDL - NC | / | Not reported |
| Berk et al[42] | 2016 | 1R | 1R | 1R | Total cholesterol - 1R; Triglycerides - 1R; HDL - 2I; LDL - 1R; Non-HDL Cho - 1R | / | Not reported |
| Shiau et al[54] | 2017 | 1R | / | 1R | / | / | Not reported |
| Cinkajzlová et al[102] | 2017 | / | 1R | 1R | Total cholesterol - 1R; Triglycerides - 1R; HDL - 2I; LDL - 1R | / | Not reported |
| Taheri et al[40] | 2019 | 1R | / | 1R | Total cholesterol - 2I; Triglycerides - 1R; HDL - 2I; LDL - 2I | Diabetes remission% - 1R; Quality of life - 2I | Dizziness, constipation and other gastrointestinal; symptoms, hair loss, and fatigue |
| Harder et al[83] | 2003 | 1R | 1R | 1R | Total cholesterol - 1R; Triglycerides - 1R; LDL - 1R | / | Not reported |
| Friedman et al[84] | 2013 | 1R | 1R | 1R | / | Serum creatinine, cystatin C and estimated glomerular filtration rate- 1R; Albuminuria - 1R | Elevations in BUN and serum creatinine early in the diet, resolved after reducing doses of antihypertensive medications |
| Lean and Leslie[77] | 2017 | 1R | / | 1R | / | / | Not reported |
| Leader et al[55] | 2012 | 1R | / | 1R | / | / | Not reported |
| Overl et al[104] | 2017 | 1R | / | 1R | / | / | Not reported |
| Gulsin et al[69] | 2020 | 1R | / | 1R | / | Blood pressure - 1R | Not reported |
| Farrer and Golley[36] | 2013 | 1R | / | 1R | Cholesterol - NC | / | Not reported |
| Sumithran and Proietto[56] | 2008 | 1R | 1R | 1R | / | / | Not reported |
| Rolland et al[105] | 2013 | / | / | 1R | / | / | Not reported |
| Dhindsa et al[71] | 2003 | 1R | / | 1R | 1R | BP - 1R | Not reported |
| Khoo et al[85] | 2011 | / | 1R | 1R | LDL - 1R | Quantitative Insulin Sensitivity check - 2I | Not reported |
| Moriconi et al[97] | 2021 | 1R | / | 1R | Total cholesterol - 1R | SBP - 1R | Not reported |
| Tang and Lin[86] | 2020 | 1R | 1R | 1R | Total cholesterol - 1R; Triglycerides - 1R; HDL - 2I; LDL - 1R | / | Not reported |
| Maher et al[106] | 2019 | 1R | / | 1R | / | / | Not reported |
| Nori Janosz et al[34] | 2008 | 1R | NC | 1R | / | / | Not reported |
| Storck et al[57] | 2021 | 1R | 1R | 1R | Total cholesterol - 1R; Triglycerides - 1R | WC - 1R; Liver profile - 1R; Insulin and HOMAIR - 2I | Constipation (n = 5) |
| Schwasinger-Schmidt et al[107] | 2020 | 1R | 1R | 1R | / | / | Not reported |
| Steven and Taylor[58] | 2015 | 1R | 1R | 1R | 1R | / | Not reported |
| Redmon et al[72] | 2003 | 1R | 1R | 1R | Total cholesterol - 1R; Triglycerides - 1R; LDL - 1R | / | Dry mouth; Constipation; Mild hypoglycemia |
| Lips et al[87] | 2014 | / | 1R | 1R | / | HOMA-IR - 1R | Not reported |
| Abi-Chahine et al[108] | 2021 | 1R | / | 1R | / | Quality of life - 2I | Not reported |
| Rafey et al[113] | 2022 | 1R | / | / | / | Leptin - 1R; Adiponectin - 2I | Not reported |
| Hocking et al[61] | 2023 | 1R | / | 1R | / | / | Two serious adverse events - hypotension |
| Anyiam et al[99] | 2023 | / | / | R | / | / | / |
| De Freitas et al[115] | 2023 | R | R | R | Total cholesterol - R; Triglycerides - R; HDL - I; LDL - R | HOMA-IR - R | / |
| Khoo et al[116] | 2023 | 1R | / | 1R | / | / | / |
| Low Glycemic Index MR (n = 5) | |||||||
| Stenvers et al[28] | 2014 | NC | 1R | NC | NC | / | Altered defecation pattern and/or flatulence - n = 8; Nausea - n = 1; Mild attack of gout - n = 1 |
| Foster et al[88] | 2013 | 1R | 1R | 1R | Total cholesterol - 1R; Triglycerides - 1R; HDL - 2I; LDL - 1R | BP - 1R | No related adverse effects |
| Boonyavarakul et al[39] | 2018 | 1R | 1R | 1R | Total cholesterol - 1R; Triglycerides - 2I; LDL - 1R | / | Not reported |
| Li et al[33] | 2014 | 1R | NC | 1R | / | / | Not reported |
| Eliana and Pranoto[117] | 2018 | / | 1R | / | / | / | Vomiting - n = 1; Soft stools - n = 1 |
| Santen et al[89] | 2023 | 1R | / | 1R | Total cholesterol - R | / | / |
| Diabetes specific formulas (n = 13) | |||||||
| Belcaro et al[32] | 2009 | 1R | 1R | 1R | / | / | No adverse effects |
| Fonda et al[43] | 2010 | / | 1R | / | / | / | Not reported |
| Garvey et al[119] | 2006 | 1R | 1R | 1R | HDL-C - 2I | Insulin sensitivity - 2I; SBP and DBP - 1R; Quality of life - 2I | Not reported |
| Sun et al[37] | 2008 | 1R | 1R | 1R | NC | / | Not reported |
| Peng et al[44] | 2019 | 1R | 1R | 1R | Triglycerides - 1R | SBP - 2I; HOMA-IR - 2I | Not reported |
| Chee et al[45] | 2017 | 1R | 1R | 1R | HDL - 2I; LDL - 1R | SBP - 1R | Not reported |
| Lansink et al[31] | 2011 | / | NC | 1R | / | / | Low incidence and mild intensity of reported abdominal pain |
| Mottalib et al[30] | 2018 | 1R | NC | 1R | Total cholesterol - NC; HDL - 2I; LDL - NC | / | Not reported |
| Otto et al[48] | 2009 | 1R | / | 1R | / | Not reported | |
| Yip et al[73] | 2001 | 1R | 1R | 1R | Total cholesterol - 1R; LDL - 1R | Insulin levels - 1R | Not reported |
| Mottalib et al[38] | 2016 | / | 1R | / | NC | / | Not reported |
| Cheskin et al[80] | 2008 | 1R | 1R | 1R | HDL: 34 weeks - 2I; 86 weeks - 2I | SBP and DBP - 1R | Not reported |
| Mustad et al[49] | 2020 | / | 1R | / | / | / | Not reported |
| Dharmalingam et al[93] | 2022 | 1R | 1R | 1R | Total cholesterol - R; Triglycerides - R; HDL - I; LDL - I | / | No serious adverse events; Six mild adverse effects: Loss of appetite, stomach bloating, peripheral leg edema, burning micturition, and urinary retention |
| Zagury et al[100] | 2022 | / | 1R | / | / | / | No serious adverse events. 4 participants (13%) had mild diarrhea and 3 participants (10%) had mild nausea |
| Protein rich MR (n = 10) | |||||||
| Keogh and Clifton[90] | 2012 | 1R | 1R | 1R | Total cholesterol - 1R; Triglycerides - 1R; LDL - 1R | SBP and DBP - 1R | Not reported |
| Navas-Carretero et al[29] | 2011 | / | NC | 1R | NC | / | Not reported |
| Kempf et al[64] | 2014 | 1R | 1R | 1R | Triglycerides - 1R; HDL - 2I | / | Not reported |
| Kempf et al[65] | 2014 | / | / | 1R | / | SBP and DBP - 1R | Not reported |
| Kempf et al[120] | 2015 | 1R | / | 1R | / | / | Not reported |
| Martin et al[66] | 2014 | 1R | / | 1R | / | / | Not reported |
| Li et al[74] | 2005 | 1R | 1R | 1R | / | High-sensitivity C-reactive protein - 1R | Not reported |
| Kempf et al[68] | 2017 | 1R | 1R | 1R | / | SBP - 1R; Quality of life and eating behaviour - 2I | Not reported |
| Shirai et al[91] | 2013 | 1R | 1R | 1R | HDL - 2I | Insulin and HOMAIR - 1R; Leptin - 1R; Adiponectin - 2I; Lipoprotein lipase mass - 2I | Not reported |
| Kempf et al[67] | 2018 | 1R | 1R | 1R | / | / | Not reported |
| Low fat MR (n = 1) | |||||||
| Barbosa-Yañez et al[81] | 2018 | 1R | / | 1R | 1R | / | Not reported |
| MR + lifestyle intervention programme (n = 9) | |||||||
| Delahanty et al[121] | 2020 | 1R | / | 1R | / | / | Not reported |
| Wycherley et al[95] | 2008 | / | 1R | 1R | / | / | Not reported |
| Reynolds et al[122] | 2002 | 1R | / | 1R | Total cholesterol - 1R; LDL - 1R | WC- 1R; BP - 1R | Not reported |
| Hamdy and Carver[96] | 2008 | 1R | / | 1R | 1R | / | Not reported |
| Pi-Sunyer et al[50] | 2009 | 1R | 1R | 1R | Triglycerides - 1R; HDL - 2I | WC - 1R | Not reported |
| Lean et al[18] | 2018 | 1R | / | 1R | / | Quality of life - 2I | 65% reported constipation, 57% reported sensitivity to cold and 53% reported headache |
| Sattar et al[78] | 2023 | R | R | 1R | Total cholesterol - R; HDL - R | WC - R | / |
| McDiarmid et al[142] | 2021 | R | / | 1R | / | / | / |
| Marples et al[79] | 2022 | 1R | / | 1R | Total cholesterol - R; Triglycerides - 1R; HDL - I; LDL - R | / | No serious adverse events were reported.; Mild side-effects: Constipation, diarrhoea, nausea, fatigue and feeling cold |
A total of 46 studies reported low calorie meal replacements interventions with intervention durations lasting from three weeks to 15 months[34-36,40-42,51-58,61,69-72,75-77,82-87,97,99,101-116]. Among the studies, twenty two studies re
Among the low-calorie meal replacement interventions, 32 studies reported reduction in HbA1c levels which corresponded to reduction in fasting plasma glucose levels reported in 17 studies[35,41,42,51,56-58,72,82-87,102,107,115] and improvements in plasma insulin level in four studies[51,52,87,115]. Only one study reported no changes in fasting plasma glucose level after a short-term meal replacement intervention of 4 weeks[34].
Thirty-six studies reported significant weight loss after low-calorie meal replacement interventions. This also corelated well with the improvement of lipid profiles as reported in 15 studies[40-42,51,57,58,71,72,82,83,85,86,97,102,115]. Even in a short-term low-calorie meal replacement as brief as 3 weeks, the participants have reduced their mean body weight from 112 kg to 105.3 kg[87].
Six studies were reported with an intervention using low GI meal replacements[28,33,39,88,89,117]. The low GI meal replacement was categorized according to the specification by the authors and had the GI of the meal replacement products stated. The utilized meal replacement products had GI ranging from 19 to 35. Among the six studies, two study utilized full meal replacements[89,117] while four studies utilized partial meal replacement[28,33,39,88] with an intervention period of 8 days to 6 months.
Among five studies which reported HbA1c outcomes, three studies had improved HbA1c levels from 0.2% to 1.83%[33,39,88,89] while one had no changes in the HbA1c levels after low GI meal replacement intervention[28]. Four studies reported a reduction in glucose level after meal replacement consumption[28,39,88,117]. Only three studies reported a significant weight loss after the intervention in which the lipid profile was also improved in the same study[39,88,89].
The studies included in this category were those in which the meal replacements were specified by the authors as diabetes-specific formulas. All the studies utilized the diabetes-specific formulas as partial meal replacements, with 12 studies providing a single meal replacement per day[37,38,43,44,47,49,80,92,93,98,100,118] and another eight providing two or more replacements per day[30-32,45,46,48,73,119]. Intervention period for these studies was 1 week to 34 weeks.
Of the 10 studies that reported HbA1c outcomes, all of them reported reduction in HbA1c levels which correlated with nine studies that reported improvement in glucose levels[30,32,37,44,45,73,80,93,119]. All the studies that reported body weight demonstrated improvements in body weight[30-32,37,44,45,48,73,80,93,119]. Six out of nine studies which re
Thirteen studies reported utilizing protein-rich meal replacements in their intervention with duration of intervention ranging from 4 to 24 weeks[29,59,63-68,74,90,91,94,120]. Nine studies utilized the meal replacement as a structured meal replacement plan[29,63-66,68,74,90,94] while four used them as partial meal replacements[59,67,91,120].
Eight studies which reported HbA1c outcome showed improvement in HbA1c levels[64,66-68,74,91,120] while six out of seven studies that reported glucose levels showed positive improvements[64,67,68,74,90,91]. All studies have shown a reduction in body weight after the protein-rich meal replacement intervention[29,64-68,74,90,91,120]. Lipid profile improved in three of the included studies[64,90,91].
There was only one study under the category of low-fat meal replacements which involved utilizing a 3 weeks’ intensive total diet replacement with a target of 1200 kcal per day and less than 30% of total energy intake was fat[81]. HbA1c was reduced by 0.1% but not significant while body weight and lipid profile improved significantly.
Ten of the reported studies were not exclusively nutritional based. Instead, the intervention focused on a combination of meal replacements and lifestyle intervention programs, from exercise sessions to intensive lifestyle interventions (ILI) consisting of behavioral classes, diet modifications and supervised physical activities as described in the Look AHEAD and DIRECT studies[18,50,60,62,78,79,95,96,121,122]. The ILIs were delivered by trained physicians, nurses, and die
Overall, the reported HbA1c outcome decreased significantly compared to baseline ranging from -0.64% to -3.6%. All the studies also had reported significant reduction in body weight, with four studies demonstrating improved lipid profile levels[50,79,96,122].
Only 13 studies reported the occurrence of mild adverse effects throughout the intervention[18,28,31,35,40,57,61,72,79,84,93,100,117]. However, most of the incidents were mild events, such as constipation and other altered defecation patterns, sensitivity to cold, headache, abdominal pain, nausea, stomach bloating, peripheral leg edema, dizziness and fatigue. Among them, constipation was the most reported adverse effect[18,35,40,57,72,100].
This scoping review compiled existing literature to explore the use of meal replacements in the dietary management of patients with T2D. It examined the types of meal replacements available for T2D and evaluated their impact on glucose/HbA1C, weight/BMI, and other health outcomes. Additionally, the review identified the potential adverse effects associated with meal replacements.
According to the classification of studies by continent or country, the utilization of meal replacements was predominantly studied in higher income settings, with 47 studies conducted in United states and Europe, including 12 studies from the United Kingdom. However, as shown in a study by Kaiser et al[123], it was estimated that in 2018, the prevalence of T2D to be similar across high- and low-income countries. The prevalence of diabetes has risen more rapidly in low- and middle-income countries compared to high-income nations[124]. While lifestyle interventions have been found to have an ethnic nonspecific benefits, their implementation in low- and middle-income countries remains underrepresented in the literature[125]. From our review, there is a lack of meal replacement intervention trials from Southeast Asia, South Asia, and Africa.
Among the few studies included in our review that were conducted in low- and middle-income countries, all but one demonstrated significant improvements in HbA1c, blood glucose, and weight or BMI. These suggest clinical effectiveness across different populations. However, the generalizability of findings from high-income settings to low- and middle-income countries may be limited. Differences in food preference and availability, dietary practices, and nutrition status, can influence the safety, acceptability, and efficacy of meal replacement interventions.
In addition, barriers, such as limited access to dietitians and lack of structured diabetes care, can further challenge the real-world implementation of meal replacement in the region. Many commercially available meal replacements are costly or unavailable in these settings. Despite being crucial for public health adoption, limited studies have evaluated their cost-effectiveness in low- and middle-income countries[126].
Through this review, we found that over 60% of the studies were performed together with diet education commonly administered via a dietitian or physician. These studies often involved weekly follow-ups and visits to encourage consistent participation in the intervention. This observation reflected the real-world feasibility in an outpatient clinical setting. Individuals that participated in a meal replacement program have cited that health coaching support facilitated them in the weight loss maintenance phase[127]. A review by Maula et al[128] highlighted that meal replacements, when combined with education, were among the most effective strategies for achieving significant reductions in weight and BMI for individuals with T2D. Wan Rohimi and Mohd Tahir[129] also found that most education approaches were cost-effective and effective in improving self-efficacy in managing T2D.
While detailed comparisons between professional roles and outcomes were limited, the consistent involvement of dietitians, either independently or alongside physicians, was common among studies that demonstrated positive metabolic outcomes. The variation in counseling duration and frequency suggests that regular sessions as brief as 15 minutes may be effective, highlighting the importance of accessible, multidisciplinary support. These findings underscore the potential benefit of multidisciplinary approaches into future interventions for people with T2D.
The increasing use of GLP-1 receptor agonists, such as semaglutide and liraglutide, has significantly advanced pharmacologic options for glycemic management and weight reduction in T2D. However, their real-world application is often constrained by high costs, limited accessibility, and gastrointestinal side effects, contributing to discontinuation in a considerable proportion of users[130,131]. In contrast, meal replacements offer a structured, non-pharmacologic alternative with demonstrated benefits on weight and glycemic outcomes. Economic evaluations highlight the cost-effectiveness of meal replacements in some of the past studies. For example, the OPTI program reported substantial cost savings of $24487 per person compared to no intervention and $18034 compared to liraglutide 3 mg, while also generating 1.133 and 0.734 additional quality-adjusted life years (QALYs), respectively[132]. Similarly, a diabetes specific meal replacement-based intervention demonstrated an incremental cost-effectiveness ratio of $47917 per QALY gained, positioning it well within commonly accepted cost-effectiveness thresholds[133]. Given these factors, meal replacements may serve as a viable alternative for individuals unable to sustain long-term GLP-1 therapy, warranting further in
However, despite favorable economic evaluations, the cost of meal replacements may remain a barrier for individuals with limited financial resources, highlighting the need for studies to explore more affordable alternatives and the integration of meal replacements into subsidized healthcare or public nutrition programs.
The present findings demonstrate that 95% of the studies have reported effective weight reduction even after 3 weeks of intervention. One of the largest diabetes weight management interventions, the Look AHEAD study, has reported a significant weight loss of approximately 8.6% as compared to 0.7% in control group with similar calorie intake per day[50]. Another study by Astbury et al[134] compared two studies with similar behavioral program and found that incorporating meal replacement enhanced their effectiveness among participants with overweight, with 2.22 kg greater weight loss at 1 year. Similarly, the OPTIWIN study on participants with obesity has found that total meal replacement has generated more effective and sustainable weight loss of 10.5% ± 0.6% compared to 5.5% ± 0.6% for participants on food-based diet plans at 52 weeks[135]. The weight reduction in meal replacement group might be caused by the reduced energy intake. This is common in our findings in which participants consuming low-calorie meal replacements experienced weight reduction. A systematic review on nine VLEDs studies has also shown that VLEDs are effective for substantial weight loss among participants with T2D[136].
The amount of weight loss might also result in significant improvement in diabetes control with reductions in HbA1c and glucose levels. American Heart Association/American College of Cardiology also reported that weight loss of more than 5% can lower HbA1c level by 0.6%-1.0%. A review by Churuangsuk et al[137] found that the main contributor to HbA1c reduction and remission was weight loss. From our findings, 98% of the studies in this review reported a reduction in HbA1c levels.
Among the meal replacements used, all 10 studies that utilized diabetes-specific formulas solely as intervention have shown significant improvements in HbA1c levels[30,32,37,44,45,48,73,80,93,119]. This is probably due to the presence of high fat, high fiber, and low carbohydrate content normally present in the diabetes-specific formulas, leading to lower GI as well as better glycemic management as compared to other formula diets. The Look AHEAD study has demonstrated a change of -0.64% in HbA1c in the ILI utilizing a diabetes-specific meal replacement vs -0.14% in the standard diet group from a baseline of approximately 7.3% in both groups (P < 0.001) at 1 year[50]. This was also followed by the subsequent reduction in glucose-lowering medication used in the intensive lifestyle intervention group.
The reduced energy intake from meal replacement interventions, along with their low GI nature, may contribute to improved glycemic management by attenuating postprandial glucose spikes, decreasing insulin secretion, and enhancing insulin sensitivity. Meal replacement interventions have also been associated with increased adiponectin levels, which promote glucose uptake and fatty acid oxidation[138,139]. Improved leptin sensitivity, linked with meal replacement usage, may aid in appetite regulation[113]. These hormonal changes may reduce insulin resistance and contribute to sustained improvements in glycemic and weight outcomes in individuals with T2D.
Reductions in biochemical values of lipid profile (cholesterol, triglycerides, LDL and HDL) were also observed. For example, in an RCT with low-fat meal replacement, 3 weeks of total diet replacement was able to improve the lipid profile significantly[81]. In the other intervention utilizing diabetes-specific meal replacements, six out of nine studies also reported a reduction in lipid profile. Participants with obesity undergoing partial meal replacement intervention also reported that the levels of total cholesterol, triglycerides and LDL significantly decreased with a significant increase in HDL levels after 8 weeks of intervention.
Only 13 studies reported occurrence of adverse events[18,28,31,35,40,57,61,72,79,84,93,100,117]. The most reported adverse effect was constipation which might be due to the inadequate fiber consumption during meal replacement interventions[18,35,40,57,72,79]. Another review of meal replacement utilization on weight loss programs reported that the overall nutritional quality of the diet may be further improved[134].
The findings from the review can help provide new evidence to supplement the existing clinical guidelines regarding utilization of meal replacement for management of T2D as well as obesity. This scoping review has some strengths that provide validity and reliability as a literary map or overview for rapid analysis in the field. Sixty-six articles in this review are protocols or reports from randomized controlled trial, improving the credibility of the reported outcomes. The total sample size included in this review was 8543 participants with T2D. This review has also included studies over 12 months of follow up as T2D is a progressive chronic disease. Lifestyle interventions of < 3 months often report beneficial effects, but it is also important to understand the effects of the longer-term interventions as well as their sustainability.
There are also some limitations in this review that should be noted. Despite employing a broad search strategy without language restrictions, most of the included studies involved European participants, which may limit the generalizability of the findings to other ethnic groups or communities. Secondary analyses of several large studies have been excluded from the review because they could not satisfy the study inclusion criteria.
Another limitation on the current body of evidence is the lack of homogeneity across studies. The included studies varied significantly in design, ranging from randomized controlled trials to observational studies, which differed in methodological strength and possible bias. Intervention durations were also inconsistent, with some lasting only a few weeks while others extending up to 2 years, making direct comparisons difficult. Furthermore, the definition and reporting of adverse effects lacked standardization, which challenges the ability to draw firm conclusions about safety profiles.
There were also considerable variations in the composition of meal replacement used, including differences in macronutrient content, GI, and presence of functional ingredients. These factors complicate cross-study comparisons. Furthermore, retention rates and dropout data were inconsistently reported, making it difficult to evaluate real-world feasibility. This scoping review approach allowed for the inclusion of a broader range of studies, regardless of study heterogeneity. However, more definitive conclusions on effectiveness and implementations should be further evaluated through meta-analyses.
After evaluating the studies included in this review, there are several recommendations that can be suggested for future research. First, there is a lack of meal replacement studies in Africa and Asia, apart from China. The population in Africa and South Asia is under-represented in the study pool. Attention needs to be given to these regions especially in the Southeast Asia as more than half (56%) of all people with diabetes were found to be residing in the Southeast Asian or Western Pacific regions[140]. Genetic variations and ethnic differences should be considered when incorporating nutritional strategies in lifestyle intervention programs. There is also a lack of study on assessing the underlying mechanisms of meal replacement on T2D. More studies on the underlying changes of hormones related to glycemic management and weight control such as adiponectin and leptin should be conducted. Some other meaningful and significant outcomes should also be assessed in future studies, such as quality of life and cost effectiveness to further justify the utilization of meal replacement in clinical practice settings.
Future meal replacement studies should also focus on methods to measure retention rate and nutrient intake more accurately. Although meal replacements with standard formulas like VLEDs have been used extensively for management in T2D and yield positive results in weight reduction and glycemic management, there are still adverse effects, especially constipation as shown in some of the studies included in our review. Diabetes-specific formulas are developed to provide a more complete nutrient package for patients with T2D, but it is not extensively studied worldwide and sometimes too costly for populations in low-income countries. Hence, efforts to develop an ideal nutritional formula for patients with different backgrounds and regions should be continued.
This review has enabled us to map and summarize the types and dosage of meal replacements utilized, along with findings from existing studies to highlight the limitations and propose recommendations for future research in nutritional interventions for diabetes. In conclusion, the review provides evidence that meal replacements, particularly when combined with lifestyle intervention programs and education or counseling, serve as an effective and safe strategy for glycemic management and promote weight reduction in patients with T2D. Therefore, incorporating meal replacements as part of lifestyle intervention programs is a viable recommendation for managing T2D.
The authors would like to thank the National University of Malaysia (UKM) for the provision of resources towards this review and the Centre for Healthy Ageing and Wellness (H-CARE) UKM for training and technical support.
| 1. | Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3709] [Cited by in RCA: 4382] [Article Influence: 626.0] [Reference Citation Analysis (0)] |
| 2. | Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5345] [Cited by in RCA: 5922] [Article Influence: 987.0] [Reference Citation Analysis (8)] |
| 3. | Carrillo-Larco RM, Barengo NC, Albitres-Flores L, Bernabe-Ortiz A. The risk of mortality among people with type 2 diabetes in Latin America: A systematic review and meta-analysis of population-based cohort studies. Diabetes Metab Res Rev. 2019;35:e3139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Iglay K, Hannachi H, Joseph Howie P, Xu J, Li X, Engel SS, Moore LM, Rajpathak S. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr Med Res Opin. 2016;32:1243-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 315] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 5. | Yang JJ, Yu D, Wen W, Saito E, Rahman S, Shu XO, Chen Y, Gupta PC, Gu D, Tsugane S, Xiang YB, Gao YT, Yuan JM, Tamakoshi A, Irie F, Sadakane A, Tomata Y, Kanemura S, Tsuji I, Matsuo K, Nagata C, Chen CJ, Koh WP, Shin MH, Park SK, Wu PE, Qiao YL, Pednekar MS, He J, Sawada N, Li HL, Gao J, Cai H, Wang R, Sairenchi T, Grant E, Sugawara Y, Zhang S, Ito H, Wada K, Shen CY, Pan WH, Ahn YO, You SL, Fan JH, Yoo KY, Ashan H, Chia KS, Boffetta P, Inoue M, Kang D, Potter JD, Zheng W. Association of Diabetes With All-Cause and Cause-Specific Mortality in Asia: A Pooled Analysis of More Than 1 Million Participants. JAMA Netw Open. 2019;2:e192696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 6. | Mat S, Shi Rui S, Jun Jie T, Yusup AM, Rajab NF, Shahar S, Ajit Singh D. Diabetes induced Osteoarthritis: A Narrative Review on Key Concepts and its Implications. Buletin Sains Kesihatan. 2022;6:17-23. |
| 7. | Zarkasi KA, Abdul Murad NA, Ahmad N, Jamal R, Abdullah N. Coronary Heart Disease in Type 2 Diabetes Mellitus: Genetic Factors and Their Mechanisms, Gene-Gene, and Gene-Environment Interactions in the Asian Populations. Int J Environ Res Public Health. 2022;19:647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Lu Y, Wang N, Chen Y, Nie X, Li Q, Han B, Chen Y, Xia F, Cang Z, Lu M, Meng Y, Lu Y. Health-related quality of life in type-2 diabetes patients: a cross-sectional study in East China. BMC Endocr Disord. 2017;17:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Prajapati VB, Blake R, Acharya LD, Seshadri S. Assessment of quality of life in type II diabetic patients using the modified diabetes quality of life (MDQoL)-17 questionnaire. Braz J Pharm Sci. 2017;53. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Cannon A, Handelsman Y, Heile M, Shannon M. Burden of Illness in Type 2 Diabetes Mellitus. J Manag Care Spec Pharm. 2018;24:S5-S13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 11. | Nath B, Gupta SD, Kumari R. Effect of comorbidities on direct cost among type 2 diabetes mellitus (T2DM) patients in tertiary care government hospital in Uttarakhand, India: A primary data analysis of out of pocket expenditure. Diabetes Metab Syndr. 2020;14:2153-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Ismail A, Suddin LS, Sulong S, Ahmed Z, Kamaruddin NA, Sukor N. Economic burden of managing Type 2 diabetes mellitus: Analysis from a Teaching Hospital in Malaysia. Indian J Public Health. 2017;61:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Feisul IM, Azmi S, Mohd Rizal AM, Zanariah H, Nik Mahir NJ, Fatanah I, Aizuddin AN, Goh A. What are the direct medical costs of managing Type 2 Diabetes Mellitus in Malaysia? Med J Malaysia. 2017;72:271-277. [PubMed] |
| 14. | Ganasegeran K, Hor CP, Jamil MFA, Loh HC, Noor JM, Hamid NA, Suppiah PD, Abdul Manaf MR, Ch'ng ASH, Looi I. A Systematic Review of the Economic Burden of Type 2 Diabetes in Malaysia. Int J Environ Res Public Health. 2020;17:5723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Boden G, Sargrad K, Homko C, Mozzoli M, Stein TP. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med. 2005;142:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 357] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 16. | Hassan MR, Jamhari MN, Hayati F, Ahmad N, Zamzuri MIA, Nawi AM, Sharif KY, Sufri M, Ahmad SB, Ismail N, Rahim SSSA, Jeffree MS. Determinants of glycaemic control among type 2 diabetes mellitus patients in Northern State of Kedah, Malaysia: a cross-sectional analysis of 5 years national diabetes registry 2014-2018. Pan Afr Med J. 2021;39:206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 17. | American Diabetes Association Professional Practice Committee. 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Care in Diabetes-2025. Diabetes Care. 2025;48:S167-S180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 18. | Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, Peters C, Zhyzhneuskaya S, Al-Mrabeh A, Hollingsworth KG, Rodrigues AM, Rehackova L, Adamson AJ, Sniehotta FF, Mathers JC, Ross HM, McIlvenna Y, Stefanetti R, Trenell M, Welsh P, Kean S, Ford I, McConnachie A, Sattar N, Taylor R. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1230] [Article Influence: 175.7] [Reference Citation Analysis (0)] |
| 19. | Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, Hoogwerf BJ, Lichtenstein AH, Mayer-Davis E, Mooradian AD, Wheeler ML. Nutrition recommendations and interventions for diabetes--2006: a position statement of the American Diabetes Association. Diabetes Care. 2006;29:2140-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Dyson PA, Twenefour D, Breen C, Duncan A, Elvin E, Goff L, Hill A, Kalsi P, Marsland N, McArdle P, Mellor D, Oliver L, Watson K. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2018;35:541-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 176] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 21. | Sievenpiper JL, Chan CB, Dworatzek PD, Freeze C, Williams SL; Diabetes Canada Clinical Practice Guidelines Expert Committee. Nutrition Therapy. Can J Diabetes. 2018;42 Suppl 1:S64-S79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 22. | Kementerian Kesihatan Malaysia. Malaysia Clinical Practice Guidelines for T2DM Management. Kuala Lumpur. 2020. [cited 23 June 2025]. Available from: https://www.moh.gov.my/moh/resources/Penerbitan/CPG/Endocrine. |
| 23. | Malaysian Dietitians' Association. Medical nutrition therapy guidelines for type 2 diabetes mellitus. 2nd ed. Kuala Lumpur, Malaysia: Malaysian Dietitians’ Association, 2013. |
| 24. | Chen LL, Mat Ludin AF, Shahar S, Manaf ZA, Tohit NM. Meal replacement in dietary management of type-2 diabetes mellitus: a scoping review protocol. Syst Rev. 2020;9:265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Arksey H, O'malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19-32. [DOI] [Full Text] |
| 26. | Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, Hempel S, Akl EA, Chang C, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson MG, Garritty C, Lewin S, Godfrey CM, Macdonald MT, Langlois EV, Soares-Weiser K, Moriarty J, Clifford T, Tunçalp Ö, Straus SE. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22118] [Cited by in RCA: 18433] [Article Influence: 2633.3] [Reference Citation Analysis (1)] |
| 27. | Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of Type 2 Diabetes - Global Burden of Disease and Forecasted Trends. J Epidemiol Glob Health. 2020;10:107-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 1646] [Article Influence: 411.5] [Reference Citation Analysis (2)] |
| 28. | Stenvers DJ, Schouten LJ, Jurgens J, Endert E, Kalsbeek A, Fliers E, Bisschop PH. Breakfast replacement with a low-glycaemic response liquid formula in patients with type 2 diabetes: a randomised clinical trial. Br J Nutr. 2014;112:504-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Navas-Carretero S, Abete I, Zulet MA, Martínez JA. Chronologically scheduled snacking with high-protein products within the habitual diet in type-2 diabetes patients leads to a fat mass loss: a longitudinal study. Nutr J. 2011;10:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Mottalib A, Salsberg V, Mohd-Yusof BN, Mohamed W, Carolan P, Pober DM, Mitri J, Hamdy O. Effects of nutrition therapy on HbA1c and cardiovascular disease risk factors in overweight and obese patients with type 2 diabetes. Nutr J. 2018;17:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | Lansink M, van Laere KM, Vendrig L, Rutten GE. Lower postprandial glucose responses at baseline and after 4 weeks use of a diabetes-specific formula in diabetes type 2 patients. Diabetes Res Clin Pract. 2011;93:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Belcaro G, Cesarone M, Silvia E, Ledda A, Stuard S, G V, Dougall M, Cornelli U, Hastings C, Schönlau F. Daily consumption of Reliv Glucaffect for 8 weeks significantly lowered blood glucose and body weight in 50 subjects. Phytother Res. 2009;23:1673-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Li D, Zhang P, Guo H, Ling W. Taking a low glycemic index multi-nutrient supplement as breakfast improves glycemic control in patients with type 2 diabetes mellitus: a randomized controlled trial. Nutrients. 2014;6:5740-5755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Nori Janosz KE, Koenig Berris KA, Leff C, Miller WM, Yanez J, Myers S, Vial C, Vanderlinden M, Franklin BA, Mccullough PA. Clinical Resolution of Type 2 Diabetes with Reduction in Body Mass Index Using Meal Replacement Based Weight Loss. Vasc Dis Prev. 2008;5:17-23. [DOI] [Full Text] |
| 35. | Astbury NM, Aveyard P, Nickless A, Hood K, Corfield K, Lowe R, Jebb SA. Doctor Referral of Overweight People to Low Energy total diet replacement Treatment (DROPLET): pragmatic randomised controlled trial. BMJ. 2018;362:k3760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 36. | Farrer O, Golley R. Feasibility study for efficacy of group weight management programmes achieving therapeutic weight loss in people with type 2 diabetes. Nutr Diet. 2014;71:16-21. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Sun J, Wang Y, Chen X, Chen Y, Feng Y, Zhang X, Pan Y, Hu T, Xu J, Du L, Zhou W, Zhao H, Riley RE, Mustad VA. An integrated intervention program to control diabetes in overweight Chinese women and men with type 2 diabetes. Asia Pac J Clin Nutr. 2008;17:514-524. [PubMed] |
| 38. | Mottalib A, Mohd-Yusof BN, Shehabeldin M, Pober DM, Mitri J, Hamdy O. Impact of Diabetes-Specific Nutritional Formulas versus Oatmeal on Postprandial Glucose, Insulin, GLP-1 and Postprandial Lipidemia. Nutrients. 2016;8:443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Boonyavarakul A, Leelawattana R, Pongchaiyakul C, Buranapin S, Phanachet P, Pramyothin P. Effects of meal replacement therapy on metabolic outcomes in Thai patients with type 2 diabetes: A randomized controlled trial. Nutr Health. 2018;24:261-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Taheri S, Chagoury O, Zaghloul H, Elhadad S, Ahmed SH, Omar O, Payra S, Ahmed S, El Khatib N, Amona RA, El Nahas K, Bolton M, Chaar H, Suleiman N, Jayyousi A, Zirie M, Janahi I, Elhag W, Alnaama A, Zainel A, Hassan D, Cable T, Charlson M, Wells M, Al-Hamaq A, Al-Abdulla S, Abou-Samra AB. Diabetes Intervention Accentuating Diet and Enhancing Metabolism (DIADEM-I): a randomised controlled trial to examine the impact of an intensive lifestyle intervention consisting of a low-energy diet and physical activity on body weight and metabolism in early type 2 diabetes mellitus: study protocol for a randomized controlled trial. Trials. 2018;19:284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Tatti P, di Mauro P, Neri M, Pipicelli G, Mussad VA. Effect of a low-calorie high nutritional value formula on weight loss in type 2 diabetes mellitus. Mediterr J Nutr Metab. 2010;3:65-69. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Berk KA, Vongpromek R, Jiang M, Schneider WJ, Timman R, Verhoeven AJ, Bujo H, Sijbrands EJ, Mulder MT. Levels of the soluble LDL receptor-relative LR11 decrease in overweight individuals with type 2 diabetes upon diet-induced weight loss. Atherosclerosis. 2016;254:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Fonda SJ, Jain A, Vigersky RA. A head-to-head comparison of the postprandial effects of 3 meal replacement beverages among people with type 2 diabetes. Diabetes Educ. 2010;36:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Peng J, Lu J, Ma X, Ying L, Lu W, Zhu W, Bao Y, Zhou J. Breakfast replacement with a liquid formula improves glycaemic variability in patients with type 2 diabetes: a randomised clinical trial. Br J Nutr. 2019;121:560-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Chee WSS, Gilcharan Singh HK, Hamdy O, Mechanick JI, Lee VKM, Barua A, Mohd Ali SZ, Hussein Z. Structured lifestyle intervention based on a trans-cultural diabetes-specific nutrition algorithm (tDNA) in individuals with type 2 diabetes: a randomized controlled trial. BMJ Open Diabetes Res Care. 2017;5:e000384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 46. | Hwu CM. A Study to Evaluate the Postprandial Metabolic Response After Use of Glucerna SR in Obese Type 2 Diabetes. [accessed 2025 Jun 23]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT00631774 ClinicalTrials.gov Identifier: NCT00631774. |
| 47. | Bao Y. The Efficacy of Glucerna SR in Chinese Drug-naïve Subjects With Type 2 Diabetes. [accessed 2025 Jun 23]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT02248714 ClinicalTrials.gov Identifier: NCT02248714. |
| 48. | Otto R, Mullan Y, Gerstein H. The effect of partial meal replacement therapy on weight loss and glycaemic control in obese individuals with type 2 diabetes. Can J Diabetes. 2009;33:269. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 49. | Mustad VA, Hegazi RA, Hustead DS, Budiman ES, Rueda R, Maki K, Powers M, Mechanick JI, Bergenstal RM, Hamdy O. Use of a diabetes-specific nutritional shake to replace a daily breakfast and afternoon snack improves glycemic responses assessed by continuous glucose monitoring in people with type 2 diabetes: a randomized clinical pilot study. BMJ Open Diabetes Res Care. 2020;8:e001258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 50. | Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, Curtis JM, Espeland MA, Foreyt JP, Graves K, Haffner SM, Harrison B, Hill JO, Horton ES, Jakicic J, Jeffery RW, Johnson KC, Kahn S, Kelley DE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montgomery B, Nathan DM, Patricio J, Peters A, Redmon JB, Reeves RS, Ryan DH, Safford M, Van Dorsten B, Wadden TA, Wagenknecht L, Wesche-Thobaben J, Wing RR, Yanovski SZ; Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374-1383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1197] [Cited by in RCA: 1058] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 51. | Steven S, Hollingsworth KG, Al-Mrabeh A, Avery L, Aribisala B, Caslake M, Taylor R. Very Low-Calorie Diet and 6 Months of Weight Stability in Type 2 Diabetes: Pathophysiological Changes in Responders and Nonresponders. Diabetes Care. 2016;39:808-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 269] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 52. | Baker ST, Jerums G, Prendergast LA, Panagiotopoulos S, Strauss BJ, Proietto J. Less fat reduction per unit weight loss in type 2 diabetic compared with nondiabetic obese individuals completing a very-low-calorie diet program. Metabolism. 2012;61:873-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Bishay R. TWO BIRDS - Intensive lifestyle and medication to help improve diabetes and fatty liver disease through weight loss [Internet]. 2019. [cited 23 June 2025]. Available from: http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12619000931178. |
| 54. | Shiau JY, So DYF, Dent RR. Effects on Diabetes Medications, Weight and Glycated Hemoglobin Among Adult Patients With Obesity and Type 2 Diabetes: 6-Month Observations From a Full Meal Replacement, Low-Calorie Diet Weight Management Program. Can J Diabetes. 2018;42:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 55. | Leader NJ, Ryan L, Molyneaux L, Yue DK. How best to use partial meal replacement in managing overweight or obese patients with poorly controlled type 2 diabetes. Obesity (Silver Spring). 2013;21:251-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Sumithran P, Proietto J. Safe year-long use of a very-low-calorie diet for the treatment of severe obesity. Med J Aust. 2008;188:366-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Storck LJ, Meffert PJ, Rausch J, Gärtner S, Aghdassi AA, Kühn JP, Kraft M, Pietzner M, Lerch MM, Steveling A. Efficiency of a 15-Week Weight-Loss Program, Including a Low-Calorie Formula Diet, on Glycemic Control in Patients with Type 2 Diabetes Mellitus and Overweight or Obesity. Obes Facts. 2021;14:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Steven S, Taylor R. Restoring normoglycaemia by use of a very low calorie diet in long- and short-duration Type 2 diabetes. Diabet Med. 2015;32:1149-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 59. | Papakonstantinou E, Triantafillidou D, Panagiotakos DB, Koutsovasilis A, Saliaris M, Manolis A, Melidonis A, Zampelas A. A high-protein low-fat diet is more effective in improving blood pressure and triglycerides in calorie-restricted obese individuals with newly diagnosed type 2 diabetes. Eur J Clin Nutr. 2010;64:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 60. | McDiarmid S, Harvie M, Johnson R, Vyas A, Aglan A, Moran J, Ruane H, Hulme A, Sellers K, Issa B. Intermittent Versus Continuous Low-Energy Diet in Patients With Type 2 Diabetes: Protocol for a Pilot Randomized Controlled Trial. JMIR Res Protoc. 2021;10:e21116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | Hocking SL, Markovic TP, Lee CMY, Picone TJ, Gudorf KE, Colagiuri S. Intensive Lifestyle Intervention for Remission of Early Type 2 Diabetes in Primary Care in Australia: DiRECT-Aus. Diabetes Care. 2024;47:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 62. | Dasgupta K, Boulé N, Henson J, Chevalier S, Redman E, Chan D, McCarthy M, Champagne J, Arsenyadis F, Rees J, Da Costa D, Gregg E, Yeung R, Hadjiconstantinou M, Dattani A, Friedrich MG, Khunti K, Rahme E, Fortier I, Prado CM, Sherman M, Thompson RB, Davies MJ, McCann GP, Yates T. Remission of type 2 diabetes and improved diastolic function by combining structured exercise with meal replacement and food reintroduction among young adults: the RESET for REMISSION randomised controlled trial protocol. BMJ Open. 2022;12:e063888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 63. | Manjunath R. Evaluation of Almased on glycemic control and metabolic effects in patients with type 2 diabetes ‐ AMDIT.2011 2022; (2019 Issue 3). Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01830750/full.. |
| 64. | Kempf K, Schloot NC, Gärtner B, Keil R, Schadewaldt P, Martin S. Meal replacement reduces insulin requirement, HbA1c and weight long-term in type 2 diabetes patients with >100 U insulin per day. J Hum Nutr Diet. 2014;27 Suppl 2:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 65. | Kempf K, Gärtner B, Keil R, Ullmann S, Martin S. Long-term reduction of weight and antidiabetic medication by protein-rich meal replacement in type 2 diabetes patients-a randomized, controlled trial. Proceedings of the 74th Scientific Sessions of the American Diabetes Association; 2014 Jun 13-17; San Francisco, CA, United States, 2014. |
| 66. | Behavioral Medicine, Clinical Nutrition, Education, and Exercise. Diabetes. 2013;62 (Supplement_1):A172-A217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 67. | Kempf K, Röhling M, Niedermeier K, Gärtner B, Martin S. Individualized Meal Replacement Therapy Improves Clinically Relevant Long-Term Glycemic Control in Poorly Controlled Type 2 Diabetes Patients. Nutrients. 2018;10:1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 68. | Kempf K, Altpeter B, Berger J, Reuß O, Fuchs M, Schneider M, Gärtner B, Niedermeier K, Martin S. Efficacy of the Telemedical Lifestyle intervention Program TeLiPro in Advanced Stages of Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care. 2017;40:863-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 69. | Gulsin GS, Swarbrick DJ, Athithan L, Brady EM, Henson J, Baldry E, Argyridou S, Jaicim NB, Squire G, Walters Y, Marsh AM, McAdam J, Parke KS, Biglands JD, Yates T, Khunti K, Davies MJ, McCann GP. Effects of Low-Energy Diet or Exercise on Cardiovascular Function in Working-Age Adults With Type 2 Diabetes: A Prospective, Randomized, Open-Label, Blinded End Point Trial. Diabetes Care. 2020;43:1300-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 70. | Reynolds A. A primary care-led weight management intervention for adults with type 2 diabetes and obesity: diRECT to Aotearoa New Zealand [Internet]. 2022. [cited 23 June 2025]. Available from: https://trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12622000151730. |
| 71. | Dhindsa P, Scott AR, Donnelly R. Metabolic and cardiovascular effects of very-low-calorie diet therapy in obese patients with Type 2 diabetes in secondary failure: outcomes after 1 year. Diabet Med. 2003;20:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | Redmon JB, Raatz SK, Reck KP, Swanson JE, Kwong CA, Fan Q, Thomas W, Bantle JP. One-year outcome of a combination of weight loss therapies for subjects with type 2 diabetes: a randomized trial. Diabetes Care. 2003;26:2505-2511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 73. | Yip I, Go VL, DeShields S, Saltsman P, Bellman M, Thames G, Murray S, Wang HJ, Elashoff R, Heber D. Liquid meal replacements and glycemic control in obese type 2 diabetes patients. Obes Res. 2001;9 Suppl 4:341S-347S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 74. | Li Z, Hong K, Saltsman P, DeShields S, Bellman M, Thames G, Liu Y, Wang HJ, Elashoff R, Heber D. Long-term efficacy of soy-based meal replacements vs an individualized diet plan in obese type II DM patients: relative effects on weight loss, metabolic parameters, and C-reactive protein. Eur J Clin Nutr. 2005;59:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 75. | Rothberg AE, McEwen LN, Kraftson AT, Fowler CE, Herman WH. Very-low-energy diet for type 2 diabetes: an underutilized therapy? J Diabetes Complications. 2014;28:506-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 76. | Elizabeth O Beale M. A Trial of Meal Replacement at a Community Diabetes Clinic Serving a Low Socioeconomic Hispanic Population. [accessed 2025 Jun 23]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT01729117 ClinicalTrials.gov Identifier: NCT01729117. |
| 77. | Lean M, Leslie W. Study to determine the feasibility and acceptability of an intensive weight management programme, to achieve remission of diabetes, in patients of South Asian Ethnicity. Feb 14, 2023. [cited 23 June 2025]. Available from: https://www.isrctn.com/ISRCTN10720065. |
| 78. | Sattar N, Welsh P, Leslie WS, Thom G, McCombie L, Brosnahan N, Richardson J, Gill JMR, Crawford L, Lean MEJ. Dietary weight-management for type 2 diabetes remissions in South Asians: the South Asian diabetes remission randomised trial for proof-of-concept and feasibility (STANDby). Lancet Reg Health Southeast Asia. 2023;9:100111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 79. | Marples O, Resca L, Plavska J, Hassan S, Mistry V, Mallik R, Brown A. Real-World Data of a Group-Based Formula Low Energy Diet Programme in Achieving Type 2 Diabetes Remission and Weight Loss in an Ethnically Diverse Population in the UK: A Service Evaluation. Nutrients. 2022;14:3146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 80. | Cheskin LJ, Mitchell AM, Jhaveri AD, Mitola AH, Davis LM, Lewis RA, Yep MA, Lycan TW. Efficacy of meal replacements versus a standard food-based diet for weight loss in type 2 diabetes: a controlled clinical trial. Diabetes Educ. 2008;34:118-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 81. | Barbosa-Yañez RL, Dambeck U, Li L, Machann J, Kabisch S, Pfeiffer AFH. Acute Endothelial Benefits of Fat Restriction over Carbohydrate Restriction in Type 2 Diabetes Mellitus: Beyond Carbs and Fats. Nutrients. 2018;10:1859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 82. | Bhatt AA, Choudhari PK, Mahajan RR, Sayyad MG, Pratyush DD, Hasan I, Javherani RS, Bothale MM, Purandare VB, Unnikrishnan AG. Effect of a Low-Calorie Diet on Restoration of Normoglycemia in Obese subjects with Type 2 Diabetes. Indian J Endocrinol Metab. 2017;21:776-780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 83. | Harder H, Dinesen B, Astrup A. The effect of a rapid weight loss on lipid profile and glycemic control in obese type 2 diabetic patients. Int J Obes Relat Metab Disord. 2004;28:180-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 84. | Friedman AN, Chambers M, Kamendulis LM, Temmerman J. Short-term changes after a weight reduction intervention in advanced diabetic nephropathy. Clin J Am Soc Nephrol. 2013;8:1892-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 85. | Khoo J, Piantadosi C, Duncan R, Worthley SG, Jenkins A, Noakes M, Worthley MI, Lange K, Wittert GA. Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med. 2011;8:2868-2875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 86. | Tang F, Lin X. Effects of Fasting-Mimicking Diet and Specific Meal Replacement Foods on Blood Glucose Control in Patients with Type 2 Diabetes: A Randomized Controlled Trial. Oxid Med Cell Longev. 2020;2020:6615295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 87. | Lips MA, de Groot GH, van Klinken JB, Aarts E, Berends FJ, Janssen IM, Van Ramshorst B, Van Wagensveld BA, Swank DJ, Van Dielen F, Willems van Dijk K, Pijl H. Calorie restriction is a major determinant of the short-term metabolic effects of gastric bypass surgery in obese type 2 diabetic patients. Clin Endocrinol (Oxf). 2014;80:834-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 88. | Foster GD, Wadden TA, Lagrotte CA, Vander Veur SS, Hesson LA, Homko CJ, Maschak-Carey BJ, Barbor NR, Bailer B, Diewald L, Komaroff E, Herring SJ, Vetter ML. A randomized comparison of a commercially available portion-controlled weight-loss intervention with a diabetes self-management education program. Nutr Diabetes. 2013;3:e63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 89. | Santen RJ. Patients with diabetes in rural underserved areas. Open Access Gov. 2023;40:118-119. [DOI] [Full Text] |
| 90. | Keogh JB, Clifton PM. Meal replacements for weight loss in type 2 diabetes in a community setting. J Nutr Metab. 2012;2012:918571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 91. | Shirai K, Saiki A, Oikawa S, Teramoto T, Yamada N, Ishibashi S, Tada N, Miyazaki S, Inoue I, Murano S, Sakane N, Satoh-Asahara N, Bujo H, Miyashita Y, Saito Y. The effects of partial use of formula diet on weight reduction and metabolic variables in obese type 2 diabetic patients--multicenter trial. Obes Res Clin Pract. 2013;7:e43-e54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 92. | Patel N. Evaluate Partial Meal Replacement with Prohance D (Nutraceutical Product) in type II Diabetic Population [Internet]. 2019. [cited 23 June 2025]. Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02066666/full. |
| 93. | Dharmalingam M, Das R, Jain S, Gupta S, Gupta M, Kudrigikar V, Bachani D, Mehta S, Joglekar S. Impact of Partial Meal Replacement on Glycemic Levels and Body Weight in Indian Patients with Type 2 Diabetes (PRIDE): A Randomized Controlled Study. Diabetes Ther. 2022;13:1599-1619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 94. | Durrer C, McKelvey S, Singer J, Batterham AM, Johnson JD, Wortman J, Little JP. Pharmacist-led therapeutic carbohydrate restriction as a treatment strategy for type 2 diabetes: the Pharm-TCR randomized controlled trial protocol. Trials. 2019;20:781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 95. | Wycherley TP, Brinkworth GD, Noakes M, Buckley JD, Clifton PM. Effect of caloric restriction with and without exercise training on oxidative stress and endothelial function in obese subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10:1062-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 96. | Hamdy O, Carver C. The Why WAIT program: improving clinical outcomes through weight management in type 2 diabetes. Curr Diab Rep. 2008;8:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 97. | Moriconi E, Camajani E, Fabbri A, Lenzi A, Caprio M. Very-Low-Calorie Ketogenic Diet as a Safe and Valuable Tool for Long-Term Glycemic Management in Patients with Obesity and Type 2 Diabetes. Nutrients. 2021;13:758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 98. | Lew LC, Mat Ludin AF, Shahar S, Abdul Manaf Z, Mohd Tohit N. Efficacy and Sustainability of Diabetes-Specific Meal Replacement on Obese and Overweight Type-2 Diabetes Mellitus Patients: Study Approaches for a Randomised Controlled Trial and Impact of COVID-19 on Trial Progress. Int J Environ Res Public Health. 2022;19:4188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 99. | Anyiam O, Phillips BE, Wilkinson DJ, Smith K, Atherton PJ, Idris IR. 278-LB: The Effect of Combining Very-Low-Calorie Diet with Semaglutide on Beta-Cell Function in Individuals with Type 2 Diabetes. Diabetes. 2023;72. [DOI] [Full Text] |
| 100. | Zagury RL, Lacativa P, Gregório LH, Rosenfeld VAS, Campos LF, Pinheiro RAC, Ferreira da Costa S, Russo LAT. Randomized clinical trial to evaluate the effect on postprandial glycemia of Nutren Control®, a glycemia-targeted specialized supplement, compared to standardized breakfast in patients with type-2 diabetes: the CONTROL DIABETES study. Nutr Hosp. 2023;40:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 101. | Shantha GP, Kumar AA, Kahan S, Cheskin LJ. Association between glycosylated hemoglobin and intentional weight loss in overweight and obese patients with type 2 diabetes mellitus: a retrospective cohort study. Diabetes Educ. 2012;38:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 102. | Cinkajzlová A, Lacinová Z, Kloučková J, Kaválková P, Trachta P, Kosák M, Krátký J, Kasalický M, DoleŽalová K, Mráz M, Haluzík M. An alternatively activated macrophage marker CD163 in severely obese patients: the influence of very low-calorie diet and bariatric surgery. Physiol Res. 2017;66:641-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 103. | Wong TW, Ching KM, Chan WS, Lee H, Wong SC, S A, Leung JYY. A 24-week structured multidisciplinary weight reduction program in overweight type 2 diabetic patients in a hospital outpatient setting: clinical diabetes [Internet]. 2016. [cited 23 June 2025]. Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01267370/full. |
| 104. | Overl J, Griffiths C, Gauld A, Gibson A, Franklin J, Toth K, Sainsbury A, Wong J. Intermittent Fasting vs. Continuous energy restriction: a pilot study in people with type 2 diabetes and overweight and obesity. 77th Scientific Sessions of the American Diabetes Association. 2017. [cited 23 June 2025]. Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01724333/full. |
| 105. | Rolland C, Lula S, Jenner C, Dyson L, Macdonald I, Johnston KL, Broom I. Weight loss for individuals with type 2 diabetes following a very-low-calorie diet in a community-based setting with trained facilitators for 12 weeks. Clin Obes. 2013;3:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 106. | Maher M, Rafey MF, Griffin H, Cunningham K, Finucane FM. Utilising a milk-based meal replacement programme in a bariatric patient with poorly controlled type 2 diabetes mellitus. Endocrinol Diabetes Metab Case Rep. 2019;2019:19-0008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 107. | Schwasinger-Schmidt TE, Elhomsy G, Paull-Forney BG. Impact of a Community-Based Weight Loss Program on Renal Function. Cureus. 2020;12:e8101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 108. | Abi-Chahine T, Chatterjee S, Cooper C, Willis T, Morais M, Hadfield W. Community-based pilot study of type 2 diabetes remission through weight loss achieved by total diet replacement based on direct trial. Proceedings of Diabetes UK Professional Conference 2021; 2021 Apr 13-30; 2021. |
| 109. | Ekberg NR. Normalized Glucose Levels in Type 2 Diabetes With Carbohydrate or Caloric Restriction. [accessed 2025 Jun 23]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT05801614 ClinicalTrials.gov Identifier: NCT05801614. |
| 110. | Shirmann F. SAFE-LCD A Randomised Controlled Trial for Insulin-treated Adults Living With Type 2 Diabetes. [accessed 2025 Jun 23]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT00417417 ClinicalTrials.gov Identifier: NCT06119204. |
| 111. | Tsompanaki E, Aveyard P, Park RJ, Koutoukidis DA. The Impact of Low-Energy Total Diet Replacement with Behavioural Support for Remission of Type 2 Diabetes on Disordered Eating (Ariadne): Protocol for a Non-Inferiority Randomised Controlled Trial. [DOI] [Full Text] |
| 112. | Scragg J, Morris E, Wane S, Noreik M, Jerome D, Yu LM, Galal U, Dyson P, Tan GD, Fox R, Breeze P, Thomas C, Jebb SA, Aveyard P. Dietary Approaches to the Management Of type 2 Diabetes (DIAMOND) in primary care: A protocol for a cluster randomised trial. Contemp Clin Trials. 2023;129:107199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 113. | Rafey MF, Abdalgwad R, O'Shea PM, Foy S, Claffey B, Davenport C, O'Keeffe DT, Finucane FM. Changes in the Leptin to Adiponectin Ratio Are Proportional to Weight Loss After Meal Replacement in Adults With Severe Obesity. Front Nutr. 2022;9:845574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 114. | Otten J. The eHealth Diabetes Remission Trial. [accessed 2025 Jun 23]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT05491005 ClinicalTrials.gov Identifier: NCT05491005. |
| 115. | De Freitas MF, Neidert A, Nay C, Chenevert T, Oral E, Rothberg A. RF24 | PSUN126 A Very-Low Calorie Diet Can Reduce Insulin Resistance and Cause Remission of Diabetes Mellitus and Hypertriglyceridemia in a Patient With Familial Partial Lipodystrophy Type 2. J Endocr Soc. 2022;6:A34-A34. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 116. | Khoo CL, Chimoriya R, Simmons D, Piya MK. Partial meal replacement for people with type 2 diabetes: 2-year outcomes from an Australian general practice. Aust J Prim Health. 2023;29:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 117. | Eliana F, Pranoto BA. A randomized controlled clinical trial of carbohydrate mix-fortified nutrition in type 2 diabetes mellitus patients. Med J Indones. 2020;29:275-82. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 118. | Wichansawakun S. Efficacy of using diabetic specific formula as a meal replacement to control blood sugar level. 2023. [cited 23 June 2025]. Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02521734/full. |
| 119. | Garvey WT, Baumgartner CJ, Fern, es JK, Fernstrom MH, Lausch MJ. A Diabetes Management Program Using Diabetes-Specific Meal Replacements and Snack Bars Improves Weight Loss, Metabolic Parameters, and Quality of Life (QOL): 2577-PO. Diabetes. 2006;55:A596-A. |
| 120. | Kempf K, Niedermeier K, Gärtner B, Keil R, Martin S. Elevated fasting insulin levels at baseline predict a poorer hba1c outcome long-term after protein-rich meal replacement in poorly controlled type 2 diabetes patients-a randomized controlled trial. Proceedings of the 75th Scientific Sessions of the American Diabetes Association; 2015 Jun 5-9; Boston, MA, United States, 2015. |
| 121. | Delahanty LM, Levy DE, Chang Y, Porneala BC, Goldman V, McCarthy J, Bissett L, Rodriguez AR, Chase B, LaRocca R, Wheeler A, Wexler DJ. Effectiveness of Lifestyle Intervention for Type 2 Diabetes in Primary Care: the REAL HEALTH-Diabetes Randomized Clinical Trial. J Gen Intern Med. 2020;35:2637-2646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 122. | Reynolds LR, Konz EC, Frederich RC, Anderson JW. Rosiglitazone amplifies the benefits of lifestyle intervention measures in long-standing type 2 diabetes mellitus. Diabetes Obes Metab. 2002;4:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 123. | Kaiser AB, Zhang N, Der Pluijm WV. Global Prevalence of Type 2 Diabetes over the Next Ten Years (2018-2028). Diabetes. 2018;67. [DOI] [Full Text] |
| 124. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513-1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2594] [Cited by in RCA: 2527] [Article Influence: 280.8] [Reference Citation Analysis (0)] |
| 125. | Lam DW, LeRoith D. The worldwide diabetes epidemic. Curr Opin Endocrinol Diabetes Obes. 2012;19:93-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 222] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 126. | Lum KY, Oppong R, Kigozi J. Economic Evaluation of Type 2 Diabetes Mellitus Interventions in Low- and Middle-Income Countries: A Systematic Review of the Literature. Asia Pac J Public Health. 2022;34:752-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 127. | Kleine HD, McCormack LA, Drooger A, Meendering JR. Barriers to and Facilitators of Weight Management in Adults Using a Meal Replacement Program That Includes Health Coaching. J Prim Care Community Health. 2019;10:2150132719851643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 128. | Maula A, Kai J, Woolley AK, Weng S, Dhalwani N, Griffiths FE, Khunti K, Kendrick D. Educational weight loss interventions in obese and overweight adults with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabet Med. 2020;37:623-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 129. | Wan Rohimi WNLH, Mohd Tahir NA. The cost-effectiveness of different types of educational interventions in type II diabetes mellitus: A systematic review. Front Pharmacol. 2022;13:953341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 130. | Sikirica MV, Martin AA, Wood R, Leith A, Piercy J, Higgins V. Reasons for discontinuation of GLP1 receptor agonists: data from a real-world cross-sectional survey of physicians and their patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2017;10:403-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 131. | Weiss T, Carr RD, Pal S, Yang L, Sawhney B, Boggs R, Rajpathak S, Iglay K. Real-World Adherence and Discontinuation of Glucagon-Like Peptide-1 Receptor Agonists Therapy in Type 2 Diabetes Mellitus Patients in the United States. Patient Prefer Adherence. 2020;14:2337-2345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 132. | Nuijten M, Dainelli L, Rasouli B, Araujo Torres K, Perugini M, Marczewska A. A Meal Replacement Program for the Treatment of Obesity: A Cost-Effectiveness Analysis from the Swiss Payer's Perspective. Diabetes Metab Syndr Obes. 2021;14:3147-3160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 133. | Randolph S, Mustad VA, Lee J, Sun J. Economic analysis of a diabetes-specific nutritional meal replacement for patients with type 2 diabetes. Asia Pac J Clin Nutr. 2010;19:1-7. [PubMed] |
| 134. | Astbury NM, Piernas C, Hartmann-Boyce J, Lapworth S, Aveyard P, Jebb SA. A systematic review and meta-analysis of the effectiveness of meal replacements for weight loss. Obes Rev. 2019;20:569-587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 135. | Ard JD, Lewis KH, Rothberg A, Auriemma A, Coburn SL, Cohen SS, Loper J, Matarese L, Pories WJ, Periman S. Effectiveness of a Total Meal Replacement Program (OPTIFAST Program) on Weight Loss: Results from the OPTIWIN Study. Obesity (Silver Spring). 2019;27:22-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 136. | Rehackova L, Arnott B, Araujo-Soares V, Adamson AA, Taylor R, Sniehotta FF. Efficacy and acceptability of very low energy diets in overweight and obese people with Type 2 diabetes mellitus: a systematic review with meta-analyses. Diabet Med. 2016;33:580-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 137. | Churuangsuk C, Hall J, Reynolds A, Griffin SJ, Combet E, Lean MEJ. Diets for weight management in adults with type 2 diabetes: an umbrella review of published meta-analyses and systematic review of trials of diets for diabetes remission. Diabetologia. 2022;65:14-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 138. | Zhong Y, Chen X, Huang C, Chen Y, Zhao F, Hao R, Wang N, Liao W, Xia H, Yang L, Wang S, Sun G. The effects of a low carbohydrate diet combined with partial meal replacement on obese individuals. Nutr Metab (Lond). 2023;20:18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 139. | Sheng T, Yang K. Adiponectin and its association with insulin resistance and type 2 diabetes. J Genet Genomics. 2008;35:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 140. | Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2306] [Cited by in RCA: 2505] [Article Influence: 313.1] [Reference Citation Analysis (0)] |
| 141. | Gilcharan Singh HK, Chee WSS, Hamdy O, Mechanick JI, Lee VKM, Barua A, Mohd Ali SZ, Hussein Z. Eating self-efficacy changes in individuals with type 2 diabetes following a structured lifestyle intervention based on the transcultural Diabetes Nutrition Algorithm (tDNA): A secondary analysis of a randomized controlled trial. PLoS One. 2020;15:e0242487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 142. | McDiarmid S, Harvie M, Johnson R, Vyas A, Aglan A, Moran J, Ruane H, Hulme A, Sellers K, Issa BG. Manchester Intermittent versus Daily Diet App Study (MIDDAS): A pilot randomized controlled trial in patients with type 2 diabetes. Diabetes Obes Metab. 2022;24:432-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |