Published online Jun 15, 2025. doi: 10.4239/wjd.v16.i6.99602
Revised: March 5, 2025
Accepted: April 9, 2025
Published online: June 15, 2025
Processing time: 190 Days and 2.9 Hours
Managing sudden deafness (SD) in patients with diabetes mellitus (DM) is particularly challenging due to the heightened risk of adverse effects associated with systemic drug administration. This study explores the potential of retroauricular subperiosteal injection as a localized drug delivery method for a more effective and safe treatment.
To compare the efficacy of retroauricular subperiosteal injection vs systemic intravenous glucocorticoid (GC) administration for SD in patients with DM and assess the effects on blood glucose levels.
A total of 128 cases of type 2 DM (T2DM) with SD diagnosed and treated in Zibo Central Hospital from February 2021 to July 2023 were divided into two groups: An observation group (66 cases receiving retroauricular subperiosteal injection of methylprednisolone) and a control group (62 cases receiving systemic intravenous administration of methylprednisolone). The two groups were compared in terms of therapeutic efficacy, hearing recovery, blood glucose level changes, and incidence of adverse reactions. Binary logistic regression was used to analyze the factors affecting therapeutic efficacy.
The observation group showed a significantly higher total effective rate (90.91%) compared with the control group (75.81%, P < 0.05). Additionally, pure-tone hearing threshold, fasting plasma glucose, and 2-hour postprandial blood glucose were significantly lower in the observation group compared with the control group (P < 0.05). The incidence of adverse reactions was also lower in the observation group than in the control group (7.58% vs 22.58%, P < 0.05). A T2DM course longer than 5 years and systemic intravenous GC administration were identified as independent risk factors for treatment inefficacy (P < 0.05).
In treating patients with diabetes and SD, retroauricular subperiosteal injection of methylprednisolone offers superior therapeutic efficacy and lower incidence of adverse reactions compared with systemic intravenous GC administration, with minimal impact on blood glucose.
Core Tip: Managing sudden deafness in patients with diabetes poses significant therapeutic challenges, particularly due to the limitations of systemic drug administration, prompting the exploration of retroauricular subperiosteal methylprednisolone injection as a potentially superior clinical approach. Our comprehensive analysis revealed that retroauricular subperiosteal injection of methylprednisolone yields significantly better therapeutic outcomes compared with systemic intravenous infusion and demonstrates enhanced safety profiles and minimal impact on blood glucose levels. This finding underscores its potential as a safer and more effective treatment strategy, offering substantial clinical value and practical significance.
- Citation: Long J, Zuo HW. Retroauricular subperiosteal vs systemic intravenous glucocorticoid administration on efficacy and blood glucose in diabetic patients with sudden deafness. World J Diabetes 2025; 16(6): 99602
- URL: https://www.wjgnet.com/1948-9358/full/v16/i6/99602.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i6.99602
Sudden deafness (SD) is a clinical emergency characterized by rapid-onset hearing loss that is often accompanied by clinical symptoms such as tinnitus and vertigo[1]. Although its pathogenesis remains unclear, it is supposedly associated with factors, including inner ear microcirculation disorders, autoimmune diseases, and viral infections[2,3]. Patients with diabetes are particularly susceptible to microvascular complications due to poor long-term glycemic control, affecting the ear’s microcirculation, subsequently increasing the risk of SD[4].
Type 2 diabetes mellitus (T2DM), a chronic metabolic disease, causes multiple microvascular damages throughout the body, complicating SD treatment in patients with diabetes[5,6]. Inner ear microcirculation disturbances in patients with diabetes may exacerbate the risk of SD, and its pathological process may be related to vascular endothelial dysfunction, inflammatory reactions, and hemorrhological changes caused by diabetes mellitus (DM)[7].
Current SD treatments include glucocorticoids (GCs), vasodilators, and hyperbaric oxygen therapy[8]. GCs are widely used due to their anti-inflammatory and immunosuppressive effects[9]. However, systemic GCs may cause blood glucose (BG) fluctuations and even increase the risk of complications in patients with DM, limiting their clinical use in this population[10]. Therefore, there is a compelling and immediate need for a local alternative solution that minimally affects the metabolic mechanisms.
In recent years, retroauricular subperiosteal injection has emerged as a localized administration modality for treating SD[11]. This method allows drugs to directly act on the inner ear, avoiding the side effects of systemic administration and having a minor impact on BG levels, providing a potentially effective treatment for patients with diabetes[12]. However, there is limited clinical evidence to compare the efficacy and glycaemic effects of retroauricular subperiosteal injection vs systemic intravenous GC administration in patients with SD and diabetes.
This study aimed to explore the efficacy of retroauricular subperiosteal injection and systemic intravenous GC administration for treating patients with SD and DM and their effects on BG, providing a safer and more effective clinical treatment approach.
This retrospective study enrolled 128 patients diagnosed with T2DM and SD who were treated at Zibo Central Hospital between February 2021 and July 2023. Based on their treatment protocols, the patients were divided into two groups: An observation group of 66 patients who received retroauricular subperiosteal injection of GCs and a control group of 62 patients who underwent systemic intravenous GC therapy. The Medical Ethics Committee approved this study, ensuring its ethical soundness. Each group’s sample size complied with the minimum sample size requirement (110 cases).
Inclusion criteria: All patients were diagnosed with T2DM according to the T2DM American Diabetes Association diagnostic criteria[13], and were complicated by SD, confirmed through oto-endoscopy and internal auditory canal magnetic resonance imaging. The SD was a first-time occurrence, affecting one ear with symptom onset less than 2 weeks prior. Their clinical medical records were complete.
Exclusion criteria: Abnormal ear canal structure, acoustic neuroma, internal auditory canal tumor, otitis media, or space-occupying lesions. SD caused by trauma, drugs, or infections. Allergic reactions to the therapeutic drugs. Pregnant or lactating patients. Mental illness or communication disorders.
Control group: Patients received a systemic intravenous infusion of 40 mg of methylprednisolone dissolved in 100 mL of normal saline. The initial dose was 0.8 mg/kg/day. Starting from Day 6 of treatment, the dose was reduced by 8 mg until the completion of the 10-day treatment.
Observation group: Patients received retroauricular methylprednisolone injection: (1) Before treatment, the patients were seated with the affected ear facing upward, and the injection area was thoroughly disinfected; (2) The injection point was the posterior junction of one-third of the retroauricular sulcus; (3) Routine injections were performed subperiosteally after ensuring that the needle contacted the bone surface without blood backflow; and (4) After injection, pressure was applied to the injection area using a sterile cotton swab for approximately 10 minutes, and the affected ear remained upward for approximately 30 minutes. The injections were administered once every 2 days at a dose of 40 mg each time over a 10-day treatment cycle.
Both groups followed the dietary guidelines for DM management and maintained their existing hypoglycemic drug regimens.
An inter-group comparison of patient baseline data [age, sex, body mass index (BMI), affected ear, smoking history, vertigo, hypertension, hyperlipidemia, course of T2DM, and place of residence] was conducted.
Therapeutic efficacy was also evaluated and compared. Cure meant that the hearing of the affected ear has returned to normal or premorbid levels. Marked efficacy referred to an increase of > 30 dB in the hearing of the affected ear. Efficacy corresponded to an increased hearing of the affected ear between 15 and 30 dB. Inefficacy referred to a hearing improvement of < 15 dB. Total effective cases = the number of cure cases + the number of marked efficacy cases + the number of efficacy cases.
Pre and posttreatment pure-tone hearing thresholds (PTHTs) were measured and compared.
Pre- and post-treatment fasting plasma glucose (FPG) and 2-hour postprandial BG (2hPG) levels were measured using a BG meter.
The occurrence of treatment-related adverse reactions, including dizziness, nausea, insomnia, and eye swelling and pain, in both groups was monitored and compared.
Factors affecting therapeutic efficacy were determined using binary logistic regression analysis.
This study used SPSS 20.0 for statistical analysis. Categorical data, expressed as rates (%), were analyzed using χ2 tests. Continuous data, all normally distributed, were analyzed using the independent-sample Student’s t-test (comparisons between groups) and the paired t-test (comparisons between two time periods within the same group), and are expressed using mean ± SD. Univariate and multivariate analyses were conducted using binary logistic regression. P < 0.05 was considered statistically significant.
Inter-group comparisons of baseline characteristics (age, sex, BMI, affected ear, smoking history, dizziness, hypertension, hyperlipidemia, T2DM course, and place of residence) showed no statistically significant difference (P > 0.05; Table 1).
| Observation group (n = 66) | Control group (n = 62) | χ2 | P value | |
| Age, years old | 0.183 | 0.669 | ||
| ≤ 60 | 38 (57.58) | 38 (61.29) | ||
| > 60 | 28 (42.42) | 24 (38.71) | ||
| Sex | 0.32 | 0.572 | ||
| Male | 46 (69.70) | 46 (74.19) | ||
| Female | 20 (30.30) | 16 (25.81) | ||
| Body mass index, kg/m² | 0.183 | 0.669 | ||
| ≤ 24 | 28 (42.42) | 24 (38.71) | ||
| > 24 | 38 (57.58) | 38 (61.29) | ||
| Affected ear | 0.125 | 0.724 | ||
| Left | 32 (48.48) | 32 (51.61) | ||
| Right | 34 (51.52) | 30 (48.39) | ||
| History of smoking | 0.847 | 0.357 | ||
| With | 7 (10.61) | 10 (16.13) | ||
| Without | 59 (89.39) | 52 (83.87) | ||
| Dizziness | 0.484 | 0.486 | ||
| With | 9 (13.64) | 6 (9.68) | ||
| Without | 57 (86.36) | 56 (90.32) | ||
| Hypertension | 1.002 | 0.317 | ||
| With | 20 (30.30) | 24 (38.71) | ||
| Without | 46 (69.70) | 38 (61.29) | ||
| Hyperlipidemia | 0.671 | 0.413 | ||
| With | 14 (21.21) | 17 (27.42) | ||
| Without | 52 (78.79) | 45 (72.58) | ||
| Course of T2DM, years | 0.426 | 0.514 | ||
| ≤ 5 | 25 (37.88) | 27 (43.55) | ||
| > 5 | 41 (62.12) | 35 (56.45) | ||
| Residence | 0.157 | 0.692 | ||
| Urban | 55 (83.33) | 50 (80.65) | ||
| Rural | 11 (16.67) | 12 (19.35) |
The total effective rates in the observation and control groups were 90.91% and 75.81%, respectively, demonstrating significantly higher therapeutic efficacy for retroauricular subperiosteal methylprednisolone injections (P < 0.05; Table 2).
| Observation group (n = 66) | Control group (n = 62) | χ2 | P value | |
| Cure | 20 (30.30) | 16 (25.81) | ||
| Marked effectiveness | 26 (39.39) | 18 (29.03) | ||
| Effectiveness | 14 (21.21) | 13 (20.97) | ||
| Ineffectiveness | 6 (9.09) | 15 (24.19) | ||
| Total effectiveness | 60 (90.91) | 47 (75.81) | 5.317 | 0.021 |
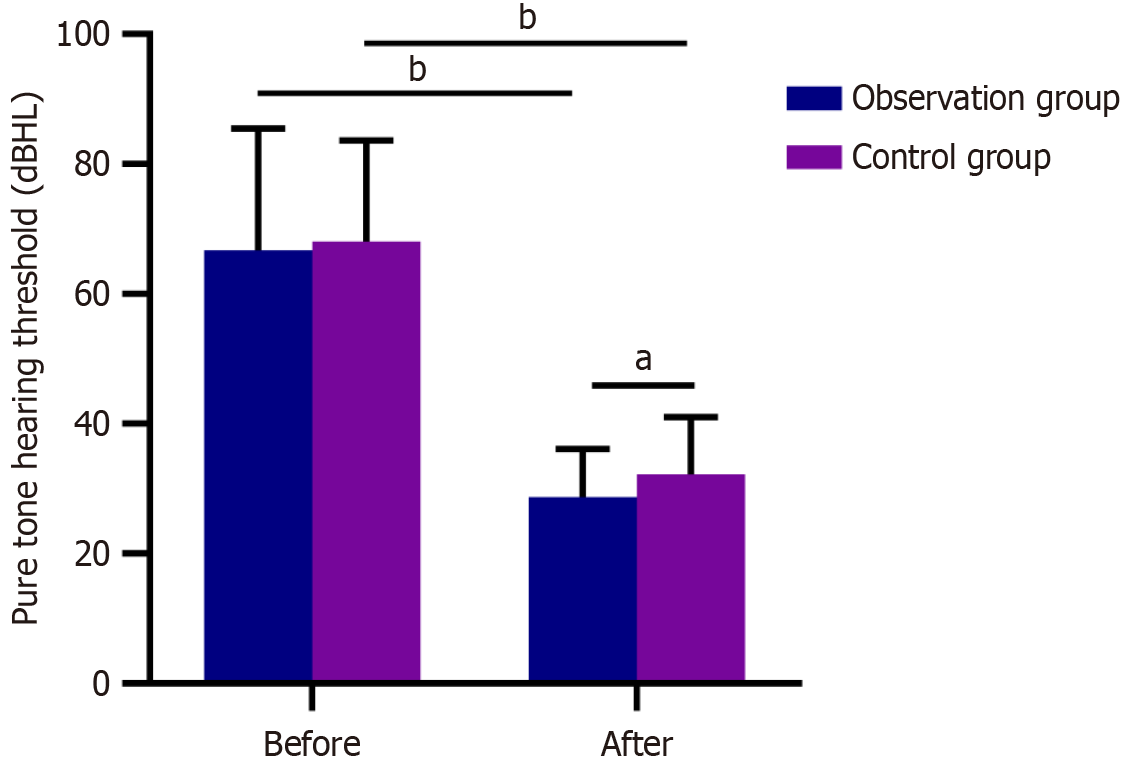
Both groups had similar post-treatment PTHT (P > 0.05); Both groups had significantly reduced PTHT after treatment (P < 0.05). Figure 1 shows that the post-treatment PTHT was lower in the observation group than in the control group (P < 0.05).
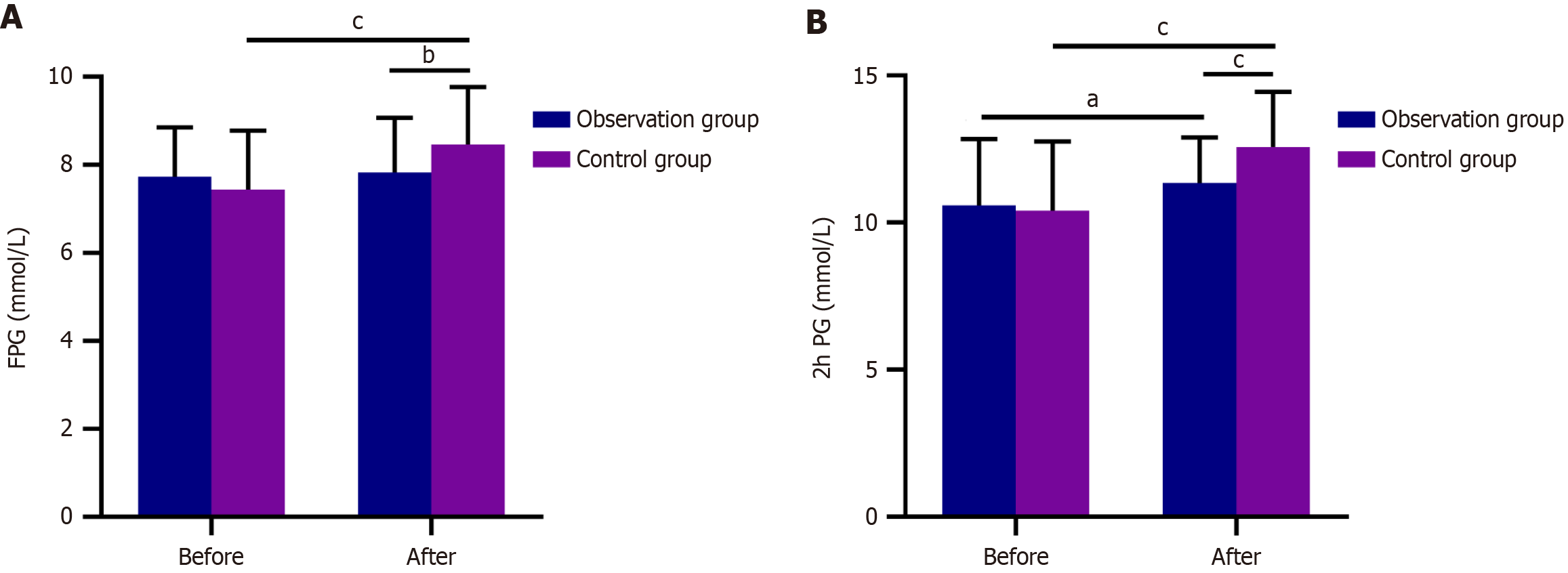
Pretreatment FPG and 2hPG levels were statistically similar (P > 0.05). After treatment, the 2hPG levels increased significantly in the observation group, while both increased significantly in the control group (P < 0.05). Posttreatment FPG and 2hPG levels were significantly lower in the observation group than in the control group (P < 0.05; Figure 2).
The total incidence of adverse reactions was 7.58% in the observation group and 22.58% in the control group, indicating a significantly lower incidence of adverse reactions in the observation group (P < 0.05; Table 3).
| Observation group (n = 66) | Control group (n = 62) | χ2 | P value | |
| Dizziness | 3 (4.55) | 4 (6.45) | ||
| Nausea | 1 (1.52) | 3 (4.84) | ||
| Insomnia | 1 (1.52) | 3 (4.84) | ||
| Eye swelling and pain | 0 (0.00) | 4 (6.45) | ||
| Total adverse reactions | 5 (7.58) | 14 (22.58) | 5.694 | 0.017 |
Univariate and multivariate analysis of 21 ineffective and 107 non-ineffective cases revealed that T2DM duration > 5 years [odds ratio (OR): 0.171, P = 0.008] and systemic intravenous GC treatment (OR: 0.267, P = 0.014) were independent risk factors for treatment efficacy (Table 4).
| Ineffective (n = 21) | Non-ineffective (n = 107) | Univariate analysis | Multivariate analysis | |||
| OR | P value | OR | P value | |||
| Age, years old | 0.565 (0.217-1.453) | 0.234 | ||||
| ≤ 60 | 10 (47.62) | 66 (61.68) | ||||
| > 60 | 11 (52.38) | 41 (38.32) | ||||
| Sex | 0.974 (0.358-2.943) | 0.960 | ||||
| Male | 15 (71.43) | 77 (71.96) | ||||
| Female | 6 (28.57) | 30 (28.04) | ||||
| BMI, kg/m² | 0.530 (0.178-1.415) | 0.224 | ||||
| ≤ 24 | 6 (28.57) | 46 (42.99) | ||||
| > 24 | 15 (71.43) | 61 (57.01) | ||||
| Affected ear | 1.121 (0.437-2.903) | 0.811 | ||||
| Left | 11 (52.38) | 53 (49.53) | ||||
| Right | 10 (47.62) | 54 (50.47) | ||||
| History of smoking | 1.701 (0.439-5.499) | 0.399 | ||||
| With | 4 (19.05) | 13 (12.15) | ||||
| Without | 17 (80.95) | 94 (87.85) | ||||
| Dizziness | 1.319 (0.280-4.675) | 0.690 | ||||
| With | 3 (14.29) | 12 (11.21) | ||||
| Without | 18 (85.71) | 95 (88.79) | ||||
| Hypertension | 1.952 (0.746-5.072) | 0.167 | ||||
| With | 10 (47.62) | 34 (31.78) | ||||
| Without | 11 (52.38) | 73 (68.22) | ||||
| Hyperlipidemia | 0.974 (0.296-2.766) | 0.962 | ||||
| With | 5 (23.81) | 26 (24.30) | ||||
| Without | 16 (76.19) | 81 (75.70) | ||||
| Course of T2DM, years | 0.197 (0.044-0.626) | 0.013 | 0.171 (0.038-0.557) | 0.008 | ||
| ≤ 5 | 3 (14.29) | 49 (45.79) | ||||
| > 5 | 18 (85.71) | 58 (54.21) | ||||
| Residence | 0.647 (0.220-2.177) | 0.448 | ||||
| Urban | 16 (76.19) | 89 (83.18) | ||||
| Rural | 5 (23.81) | 18 (16.82) | ||||
| Therapeutic method | 0.313 (0.105-0.835) | 0.026 | 0.267 (0.086-0.738) | 0.014 | ||
| Retroauricular subperiosteal injection of glucocorticoids injection | 6 (28.57) | 60 (56.07) | ||||
| Systemic intravenous administration of glucocorticoids | 15 (71.43) | 47 (43.93) | ||||
This study demonstrated that retroauricular subperiosteal methylprednisolone injections were more effective than systemic intravenous GC administration in treating SD in patients with T2DM. The total effective rate of the observation group was 90.91%, which was significantly higher than that of the control group (75.81%). Wang et al[14] showed that retroauricular subperiosteal methylprednisolone injections to patients with diabetes and SD had superior efficacy compared with systemic administration and significantly improved patients’ pure-tone audiometry thresholds, consistent with the results observed in the present study. Additionally, post-treatment PTHT and incidence of adverse reactions were significantly reduced in the observation group compared with the control group, highlighting the significant efficacy and safety of retroauricular subperiosteal methylprednisolone injections.
SD is a clinical emergency requiring rapid hearing restoration[15]. In this study, the observation group exhibited significantly better hearing recovery than the control group, possibly related to the potent anti-inflammatory, immunosuppressive, and neuroprotective properties of methylprednisolone, which enhance cochlear microcirculation and stabilize endolymphatic fluid[16-18]. Mechanistically, methylprednisolone administered via retroauricular injection can rapidly reach the sigmoid sinus through the retroauricular and mastoid veins. Subsequently, it diffuses into the endolymphatic sac while maintaining a high concentration. It then returns to the inner ear through the minute veins surrounding the endolymphatic sac, attaining an effective therapeutic concentration and maximizing its curative potential[19]. Song et al[20] showed that treating SD with retroauricular dexamethasone injections improves the hearing threshold, consistent with our findings. The local injection method allows the drug to act directly on the lesion site, avoiding the possible side effects of systemic medication, which is especially important in patients with diabetes.
When treating SD in patients with diabetes, special attention should be paid to the impact of drugs on BG levels. This study identified significantly lower post-treatment FPG and 2hPG levels in the observation group than in the control group, indicating that retroauricular subperiosteal methylprednisolone injection insignificantly affects BG levels. This is crucial for patients with diabetes because a stable BG level helps to reduce the risk of diabetic complications and improve the overall prognosis of patients[21].
Furthermore, the incidence of adverse reactions was significantly lower in the observation group (7.58%) than in the control group (22.58%). This may be attributed to the local administration mode of retroauricular subperiosteal methylprednisolone injection, which reduces the occurrence of systemic side effects. Systemic intravenous GCs may cause various adverse reactions, including dizziness, nausea, insomnia, and eye swelling and pain, which are significantly reduced under local injections[22]. A meta-analysis by Deng et al[23] showed that for patients with SD, retroauricular injections were associated with better hearing improvement and a higher safety profile than systemic steroid therapy. A prospective investigation by Xie et al[24] revealed that the use of retroauricular methylprednisolone injections in patients with sudden sensorineural hearing loss yielded no notable complications, strongly implying its satisfactory clinical safety profile. The above research provides an important reference for clinicians when choosing treatment plans, especially when dealing with patients with diabetes, emphasizing more on treatment safety and patient tolerance.
Furthermore, binary logistic regression analysis showed that a T2DM course of > 5 years and systemic intravenous GC administration were independent risk factors for treatment inefficacy. This finding suggests that the DM course should be considered when treating SD in patients with T2DM and that the use of systemic GC therapy should be avoided as much as possible. Patients with a long-term DM course may have more severe microangiopathy and inner ear microcirculation disorders, thereby adversely affecting their treatment outcomes. Therefore, topical administration may be more appropriate for these patients.
Although this study provides valuable clinical information, it also has some limitations. First, the relatively small sample size may affect the result’s generalizability. Second, given the short follow-up period, the long-term efficacy and safety warrant further investigation. Third, sensitivity analyses were not conducted. Incorporating such analyses would strengthen the robustness of the findings, address potential confounding variables or biases, and improve overall transparency. Fourth, the study did not cover the effects of retroauricular subperiosteal methylprednisolone injection on other complications in patients with diabetes, thereby requiring attention in future research. We hope that multi-center, large-scale, long-term prospective clinical trials will be conducted in the future to validate this study’s findings and explore other factors that may influence treatment outcomes.
In conclusion, when it comes to the treatment of SD in patients with DM, retroauricular subperiosteal methylprednisolone injection exhibits superior efficacy compared with systemic intravenous GC administration, with a smaller impact on BG levels and a lower incidence of adverse reactions.
| 1. | Zou T, Xu J, Lu H, Yu M. The Relationship between the Characteristics of Tinnitus and the Hearing Curative Effect of Sudden Deafness. Audiol Neurootol. 2023;28:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Kaneko H, Ozono Y, Iwakiri H, Hatada H, Uchiyama N, Komaki Y, Nakamura K, Hasuike S, Nagata K, Kawakami H. Reactivation of hepatitis C virus caused by steroid monotherapy for sudden deafness. Clin J Gastroenterol. 2024;17:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (1)] |
| 3. | Boulassel MR, Deggouj N, Tomasi JP, Gersdorff M. Inner ear autoantibodies and their targets in patients with autoimmune inner ear diseases. Acta Otolaryngol. 2001;121:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Chen HQ, Peng Y, Feng Y, Jin TL. Clinical Observations on the Combined Use of Hyperbaric Oxygenation and Conventional Medications in the Management of Type 2 Diabetes Mellitus Concurrent With Sudden Deafness. Ear Nose Throat J. 2024;1455613241254433. [PubMed] [DOI] [Full Text] |
| 5. | Zeng GH, Su YJ, Liu HY, Lu DH. Is sudden deafness in non-diabetic patients affected by their glycosylated hemoglobin levels? Eur Rev Med Pharmacol Sci. 2022;26:1668-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Eid SA, Rumora AE, Beirowski B, Bennett DL, Hur J, Savelieff MG, Feldman EL. New perspectives in diabetic neuropathy. Neuron. 2023;111:2623-2641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 89] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 7. | Godinho I, Gameiro J, Jorge S, Abreu F, Neves M, Lopes JA, Gomes da Costa A. Diabetes, deafness and renal disease. Clin Kidney J. 2017;10:487-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Sun H, Hu J, Mao Z, Ma Z. Efficacy of combination therapy in adolescent and adult patients with total-deafness sudden sensorineural hearing loss. Acta Otolaryngol. 2019;139:258-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 9. | Takeda T, Takeda S, Kakigi A. Effects of Glucocorticoids on the Inner Ear. Front Surg. 2020;7:596383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Kuo T, McQueen A, Chen TC, Wang JC. Regulation of Glucose Homeostasis by Glucocorticoids. Adv Exp Med Biol. 2015;872:99-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 431] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 11. | Li DB, Zhou S, Xu WJ. [Intratympanic dexamethasone vesus post-auricular subperiosteal injection of methylprednisolone treatment for sudden hearing loss]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017;31:1265-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Chen Y, Pei T, Sun Y, Jiang J, Zhang H. [New progress of IGF-1 and allosteroid injection in the treatment of sudden deafness complicated with type 2 diabetes]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020;34:569-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45:S17-S38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 1418] [Article Influence: 472.7] [Reference Citation Analysis (1)] |
| 14. | Wang H, Zhao Z, Chen S. Local vs Systemic Use of Steroids for Sudden Deafness with Diabetes: A Systematic Review and Meta-Analysis. Ear Nose Throat J. 2023;1455613231170090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Waissbluth S, Sepúlveda V, Urzúa P. Sudden sensorineural hearing loss: Recovery rates according to audiometric patterns. Acta Otorrinolaringol Esp (Engl Ed). 2022;73:346-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Zhou M, Xu Z, Feng L, Zhong H, Yang H, Ning G, Feng S. 50 years of methylprednisolone application in spinal cord injury: a bibliometric analysis. Acta Neurochir (Wien). 2025;167:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Bhandari A, Jain S. Early Intratympanic Methylprednisolone in Sudden SNHL: A Frequency-wise Analysis. Indian J Otolaryngol Head Neck Surg. 2019;71:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Xu F, Feng E, Su L, Xu G. Doxofylline and methylprednisolone sodium succinate are stable and compatible under normal injection conditions. Pak J Pharm Sci. 2013;26:261-265. [PubMed] |
| 19. | Li X, Zhang XY, Wang QJ, Wang DY. Efficacy of methylprednisolone sodium succinate for injection (postotic injection) on the auditory threshold and speech recognition rate of sudden deafness patients. Int J Clin Exp Med. 2015;8:14110-14114. [PubMed] |
| 20. | Song J, Zhang L, Chen Y. [Analysis of the treatment effects of refractory sudden total frequency deafness with steroid from different topical administration routes]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2018;32:1897-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Jiang K, Li S, Cheng L, Yang J. Intratympanic methylprednisolone administration promotes the recovery of idiopathic sudden sensorineural hearing loss: a retrospective case-control study. Acta Otolaryngol. 2018;138:998-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Wang D, Zhao Y, Zhao F, Zong L, Han B, Lan L, Zhang Q, Qi Y, Wang Q. [Clinical analysis of in-patients with large vestibular aqueduct syndrome]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013;27:1063-1067. [PubMed] |
| 23. | Deng HS, Hou YW, Zhang JN, Yang T. Postauricular versus systemic use of steroids for sudden hearing loss: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2023;102:e34494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | Xie W, Karpeta N, Liu J, Peng H, Li C, Zhang Z, Liu Y, Duan M. Efficacy of intratympanic or postauricular subperiosteal corticosteroid injection combined with systemic corticosteroid in the treatment of sudden sensorineural hearing loss: A prospective randomized study. Front Neurol. 2023;14:1138354. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |