Published online Sep 15, 2023. doi: 10.4239/wjd.v14.i9.1334
Peer-review started: April 14, 2023
First decision: June 13, 2023
Revised: June 18, 2023
Accepted: July 13, 2023
Article in press: July 13, 2023
Published online: September 15, 2023
Processing time: 151 Days and 22.2 Hours
Type 2 diabetes mellitus (T2DM) is a lifelong condition and a threat to human health. Thorough understanding of its pathogenesis is acutely needed in order to devise innovative, preventative, and potentially curative pharmacological interventions. MicroRNAs (miRNA), are small, non-coding, one-stranded RNA molecules, that can target and silence around 60% of all human genes through translational repression. MiR-155 is an ancient, evolutionarily well-conserved miRNA, with distinct expression profiles and multifunctionality, and a target repertoire of over 241 genes involved in numerous physiological and pathological processes including hematopoietic lineage differentiation, immunity, inflammation, viral infections, cancer, cardiovascular conditions, and particularly diabetes mellitus. MiR-155 Levels are progressively reduced in aging, obesity, sarcopenia, and T2DM. Thus, the loss of coordinated repression of multiple miR-155 targets acting as negative regulators, such as C/EBPβ, HDAC4, and SOCS1 impacts insulin signaling, deteriorating glucose homeostasis, and causing insulin resistance (IR). Moreover, deranged regulation of the renin angiotensin aldo-sterone system (RAAS) through loss of Angiotensin II Type 1 receptor downregulation, and negated repression of ETS-1, results in unopposed detrimental Angiotensin II effects, further promoting IR. Finally, loss of BACH1 and SOCS1 repression abolishes cytoprotective, anti-oxidant, anti-apoptotic, and anti-inflammatory cellular pathways, and promotes β-cell loss. In contrast to RAAS inhibitor treatments that further decrease already reduced miR-155 Levels, strategies to increase an ailing miR-155 production in T2DM, e.g., the use of metformin, mineralocorticoid receptor blockers (spironolactone, eplerenone, finerenone), and verapamil, alone or in various combinations, represent current treatment options. In the future, direct tissue delivery of miRNA analogs is likely.
Core Tip: MicroRNAs (miRNA) are small, non-coding, one-stranded RNA molecules that can target and silence over 60% of human genes thereby effectively regulating huge genetic networks. MiRNAs are abundantly found in every human cell and their production is tightly controlled. They play critical roles in regulating almost every cellular pathway, numerous human diseases, and have been linked to the development of diabetes mellitus (DM) and the regulation of blood pressure. In this minireview, we comment on crucial miR-155 effects in type 2 DM (T2DM). Deeper mechanistical understanding of this miRNA’s permeating action may lead to innovative therapeutic approaches in T2DM.
- Citation: Papadopoulos KI, Papadopoulou A, Aw TC. MicroRNA-155 mediates endogenous angiotensin II type 1 receptor regulation: implications for innovative type 2 diabetes mellitus management. World J Diabetes 2023; 14(9): 1334-1340
- URL: https://www.wjgnet.com/1948-9358/full/v14/i9/1334.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i9.1334
Diabetes mellitus (DM), until recently considered a lifelong and irreversible condition, is a devastating burden to over half a billion people worldwide[1]. The overwhelming majority of diabetic patients-over 90%-suffer from Type 2 DM (T2DM) caused by an intricate interaction between lifestyle and genetics that through insulin resistance (IR) lead to metabolic syndrome, pre-diabetes, failure of insulin-secreting pancreatic β-cells and ultimately overt disease[2,3]. Un-controlled T2DM eventually progresses to a myriad of severe health complications [among which cardiovascular disease (CVD), chronic renal failure, and hypertension (HT)], and to an early death[1]. The syndemic of coronavirus disease 2019 and T2DM has affirmed the latter’s lethal effect[4]. Ominous future predictions estimate the number of DM-afflicted individuals to be over 800 million by 2045, up from the current 500 million[1]. Increased understanding of the T2DM pathogenesis is, therefore, acutely needed in order to devise innovative, preventative and potentially curative pharmacological interventions[5].
The pathophysiological role of the renin angiotensin aldosterone system (RAAS) and its major effector, Angiotensin II (Ang II) through the Ang II Type 1 receptor (AT1R), in the development of IR in T2DM have long been recognized[6]. Furthermore, convincing evidence exists advocating the use of RAAS inhibition, ACE inhibitors (ACEi) or AT1 receptor antagonists/blockers (ARB), in patients with T2DM, not only for proteinuria and HT, but also as a means to improve IR and glucose homeostasis[6].
MicroRNAs (miRNAs or miRs) are small (21-25 nucleotides), non-coding RNAs, able to translationally repress and downregulate gene expression[7]. Present abundantly in all human cells, miRNAs are endogenously biosynthesized through a strictly regulated process that will ultimately result in a mature miRNA, with a 2-8 nucleotide long seed sequence in its 5’untranslated region (UTR), that will bind to a target messenger RNA (mRNA). If the miRNA seed sequence binds perfectly to the corresponding 3’UTR of a specific mRNA, the latter will be recruited to be degraded by an RNA silencing complex. If the binding is incomplete, mRNA translational machinery will be blocked, thereby inhibiting protein translational efficiency, and repressing (silencing) gene expression[7]. As a specific miRNA can target multiple mRNA molecules, and equally, a single mRNA molecule can bind to multiple miRNAs, the host can modulate response feedback, through regulatory gene networks, in a concerted effort to control diverse aspects of cellular processes[7]. In this minireview, we present additional miRNA- modulated pathways that can modulate AT1R and Ang II effects that are of importance for the pathogenesis of IR, T2DM, and the development of cardiovascular and renal diabetic complications.
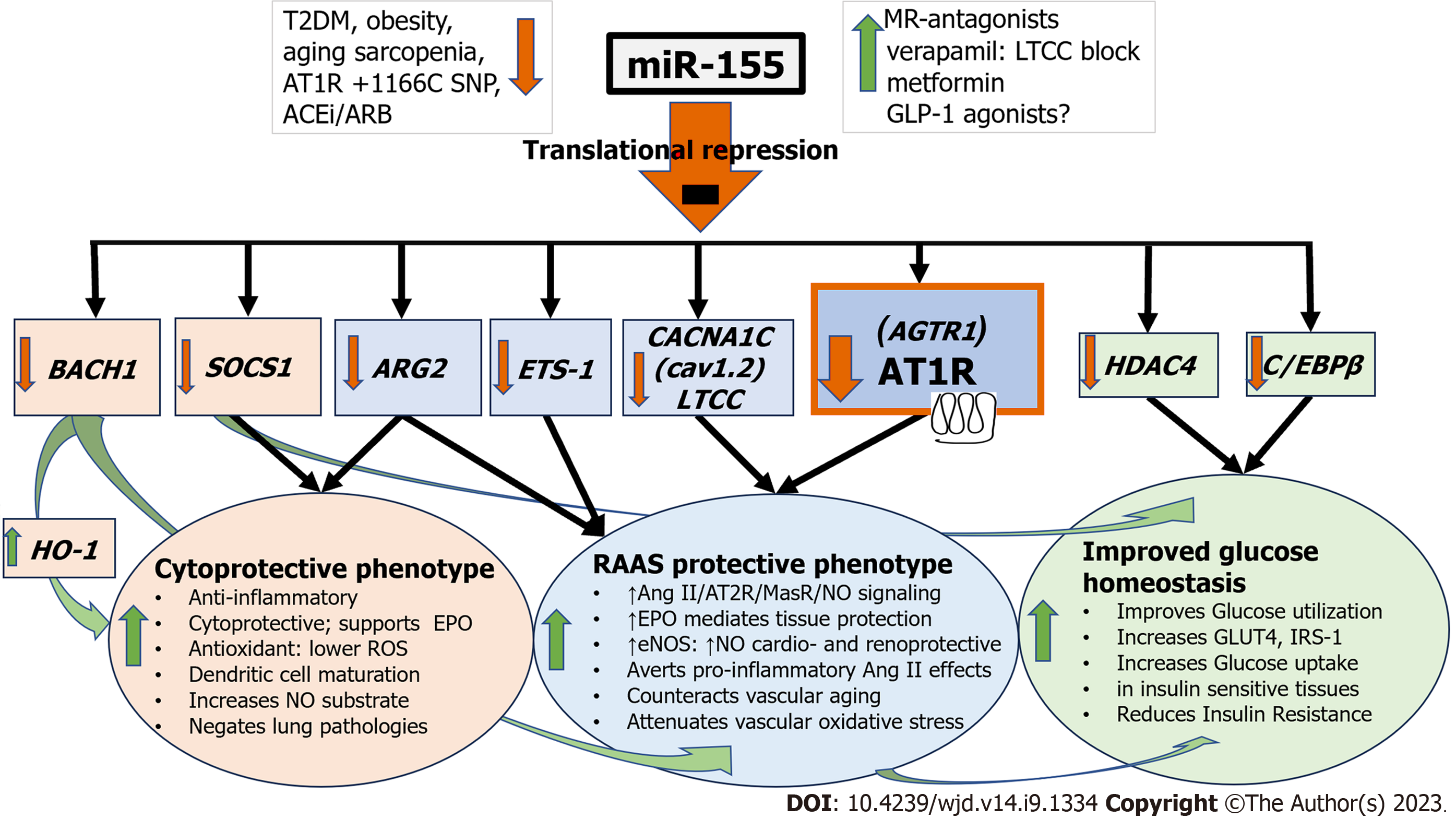
MiR-155 is of particular interest as it is intricately involved both in the pathogenesis of DM and the regulation of AT1R and Ang II effects (Figure 1)[6,8-12]. First identified in 1997, miR-155 is a highly conserved and ancient miRNA primarily expressed in the thymus and spleen. It exhibits unique expression profiles and multifunctionality but is minimally detected under normal physiological conditions[13]. With a target repertoire of over 241 genes, miR-155 plays critical roles in various physiological and pathological processes, such as hematopoietic cell line differentiation, inflammation, immunity (especially viral and parasitic infections), cancer, cardiovascular conditions, and notably, DM (Table 1)[5,8,12-25].
| Gene symbol | Full gene name | Action |
| AGTR1 | Angiotensin II type 1 receptor gene | Repressed translation downregulates gene expression mediating endogenous AT1R antagonism[9,10,21]. Human in-vitro and in-vivo studies |
| ARG2 | Arginase-2 | Repressed translation prevents L-arginine depletion, supports dendritic cell maturation, and negates lung pathologies[22,23]. Human and mouse in-vitro and in-vivo studies |
| BACH1 | BTB and CNC homology 1, basic leucine zipper transcription factor 1 | Translational repression of BACH1 leads to potent anti-inflammatory, cytoprotective, antioxidant programs through Heme Oxygenase-1[12]. Review of human in-vitro and in-vivo studies |
| C/EBPβ | CCAAT/enhancer-binding protein β | Repression downregulates Pyruvate Kinase 4 (PDK4) gene expression and negatively regulates Pyruvate kinase complex (PDC) activity, thereby improving glucose utilization [16]. Mouse in-vitro and human in-vivo studies |
| ETS-1 | E26 Transformation-specific Sequence-1 | Translational repression averts Ang II effects involving gene regulation of vascular remodeling, angiogenesis, and inflammation[9,10,24]. Review of human in-vitro and in-vivo studies. Mouse in-vitro and in-vivo studies |
| HDAC4 | Histone deacetylase 4 | Its repression increases GLUT4 and enhances glucose uptake in insulin-sensitive tissues, i.e., skeletal muscle[16]. Mouse in-vitro and human in-vivo studies |
| CACNA1C (Cav1.2) | L-type calcium channel subunit, LTCC | As a subunit of the L-type calcium channel, this pro-constrictive gene contributes to influx of calcium in vascular smooth muscle cells and reactive oxygen species production, thereby mediating the important components of vascular aging: Vasoconstriction and vascular oxidative stress[21]. Human in-vitro and in-vivo studies |
| SOCS1 | Suppressor of cytokine signaling 1 | Repression prevents the degradation of IRS-1 (Insulin Receptor Substrate-1) protein that mediates the effect of insulin in muscle, liver, and adipose tissue. Supports the JAK2/Y343/STAT5 pathway through which the protective effects of EPO against ischemic injury are mediated[16,25]. Human in-vivo study. Mouse in-vitro and in-vivo study |
In T2DM, miR-155 Levels in plasma, peripheral blood cells, platelets, and urine are significantly and consistently decreased, with surprising congruence between different ethnicities[8]. Ranging from obesity to IR to diabetic complications in T2DM, miR-155 Levels are progressively reduced[8,14,15,17]. MiR-155’s underlying molecular mechanism in enhancing insulin signaling, improving glucose homeostasis, and alleviating IR in T2DM, occurs partly through the coordinated repression of multiple negative regulators, such as CCAAT/enhancer-binding protein β (C/EBPβ), Histone Deacetylase 4 (HDAC4), and Suppressor of cytokine signaling 1 (SOCS1) (Table 1)[16]. MiR-155-mediated C/EBPβ repression downregulates Pyruvate Kinase 4 (PDK4) gene expression and negatively regulates Pyruvate kinase complex activity, thereby improving glucose utilization[16]. HDAC4 repression increases GLUT4 and enhances glucose uptake in insulin-sensitive tissues, i.e., skeletal muscle, while SOCS1 repression prevents the degradation of Insulin Receptor Substrate-1 (IRS-1) protein that mediates the effect of insulin in muscle, liver, and adipose tissue (Figure 1 and Table 1)[16].
Aging, obesity, sarcopenia, chronic RAAS activation, and IR, invariably predate the development of T2DM[26]. Shared miRNA signatures have been reported, highlighting the central role of miR-155 in the common pathogenesis of those conditions (Figure 1)[8,14,26]. One particularly important observation is the activation of the classical RAAS axis arm that involves Ang II/AT1R signaling in aging skeletal muscle and white adipose tissue (WAT), both fundamentally involved in T2DM pathogenesis[26,27]. In WAT, a chronically activated RAAS axis increases lipogenesis and reduces lipolysis, while in the aging skeletal musculature RAAS hyperactivity promotes protein degradation, and sarcopenia, altogether ultimately leading to oxidative stress, inflammation, fat accumulation, muscle atrophy, and IR[26,27]. In addition, RAAS’s protective arm, involving Ang 1-7/AT2R/MasR signaling, is inhibited at the same time, further augmenting an unfavorable AT1R/AT2R imbalance[26,27]. MiR-155, acting as a master regulator, is the key player in chronic RAAS/Ang II/AT1R activation, thereby, intricately associated with the development of IR[6]. Through its repression of the AGTR1 (the gene that codes for the AT1R) miR-155 regulates the homeostasis of the AT1R receptor, its membrane presence, and thus the biological activity of Ang II (Table 1)[9,10,28-30]. Moreover, its regulation of the E26 Transformation-specific Sequence-1 (ETS-1) averts several detrimental vascular Ang II effects involving gene regulation of inflammation, proliferation, remodeling, fibrosis, and angiogenesis (Table 1)[9,13,24]. Furthermore, its repressive effects on Arginase-2 (ARG2) prevent the depletion of l-arginine, the obligate substrate of endothelial nitric oxide (NO) synthase (eNOS), improving substrate availability and further increasing NO-production and NO-bioavailability that further support NO-dependent cardio- and renoprotection in T2DM (Table 1)[13,23]. From the sum of these actions, it is thus evident that the reported loss of miR-155 in T2DM has profound effects leading to persistent RAAS hyperactivity through chronic Ang II stimulation of the AT1R, thereby exerting its detrimental, pro-oxidant, pro-fibrotic, proliferative, and pro- inflammatory actions (Figure 1 and Table 1). Additional miR-155 effects through repressive actions on BTB and CNC homology 1, basic leucine zipper transcription factor 1 (BACH1) and SOCS1, synergistically enhance cytoprotective, anti-oxidant, anti-apoptotic, and anti-inflammatory cellular pathways and promote a protective cellular milieu, which is subsequently lost following miR-155 downregulation (Table 1)[12,13]. Genetic variants that perturb miR-155’s action (such as in carriers of AT1R + 1166C-allele) or that increase its synthesis (such as in trisomy 21 and the rs767649 polymorphism of miR-155) biochemically and molecularly demonstrate this central significance of miR in a plethora of DM-associated pathological conditions[11,18,31-33]. Moreover, clinical data in obese individuals demonstrate that miR-155 Levels correlate with improved insulin sensitivity post-bariatric surgery and are critical in mediating the effects of endurance exercise[34,35].
While miR-155 is consistently reduced in serum and tissues in T2DM, it is reported to be upregulated in Type 1 DM (T1DM), highlighting T1DM’s autoimmune pathogenesis and miR’s crucial and differential role in autoimmunity and innate and adaptive immunity[8,36]. However, even if robustly elevated in newly diagnosed T1DM, miR-155 strikingly diminishes within 5 years of diagnosis[32].
AT1R substrate modulation (ACEi) and/or receptor inhibition (ARBs) may improve glucose homeostasis[6]. However, strategies to increase an ailing miR-155 production in T2DM could prove to be a more appropriate course of action (Figure 1). Metformin with ACEi/ARB improves HbA1c goals[6]. Metformin and the newer Glucagon Like Peptide 1 (GLP-1) analogs have been shown to repress SOCS1 and 3 and increase IRS-1[37]. Metformin mediates miR-155 increases that repress SOCS1 and reduce NF-κB (nuclear factor κB), thereby disrupting NF-κB-mediated high-fat induced inflammatory effects in T2DM[38,39]. The clinical effects of GLP-1 analogs on miR-155 in humans are, to date, unknown, and additional research is needed, but miR-155 has been shown to promote GLP-1 production in the murine pancreas[40]. Moreover, in the resistance vessels of aging humans, elevated expression of mineralocorticoid receptor (MR) is accom-panied by a decrease in miR-155 Levels and an upregulation of miR-155 targets such as the CACNA1C (Cav1.2) gene [a subunit of the L-type calcium channel (LTCC)], and the AGTR1 gene. These alterations in gene expression play a role in promoting vasoconstriction and oxidative stress in aging mice (Table 1)[21]. MR inhibition reverses and reinstates the significantly low basal serum miR-155 Levels in the aging blood vessels and blocks two interactive steps involving LTCC and AGTR1 that underlie the pathogenesis of HT[13]. A correlation between improved blood pressure response to therapy with MR antagonists and changes in miR-155 Levels in older individuals has been reported[21]. Moreover, the use of MR-antagonists (spironolactone, eplerenone, finerenone) has shown renal and cardiovascular benefits in T2DM[41-43]. LTCC blockade per se, through verapamil alone, or in combination with MR antagonists/metformin, will offer additional therapeutic options in T2DM[44]. Besides improved blood pressure regulation and cardio-renal protection, verapamil demonstrates additional benefits while avoiding many of the common adverse effects associated with ACEi/ARB[45]. Verapamil’s mode of action is of particular interest in diabetes[46]. Apart from being present in cardiomyocytes, LTCCs are also present in pancreatic β-cells and participate in insulin homeostasis[47]. In the heart and the pancreas, effective pharmacological LTCC blockage can inhibit the expression of pro-apoptotic thioredoxin-interacting protein, a significant contributor to pancreatic β-cell dysfunction and a key gene regulated in response to hyperglycemia, thereby promoting the survival and proper functioning of β-cells and improving glucose homeostasis[46,48]. Verapamil has, thus, the potential not only to enhance β-cell survival and function, but also improve and even prevent overt diabetes of both types[48,49]. In a recent study, verapamil combined with metformin, significantly improved glycemic control in T2DM[49]. Finally, a drawback in the use of monotherapy as ACEi/ARBs (in conditions that already are associated with low miR-155 Levels) is that they significantly further decrease already reduced miR-155 Levels[50,51]. RAAS inhibition could, thus, theoretically deprive T2DM patients of additional miR-155-engendered favorable immunological and cytoprotective effects and potentially explain ACEi’s modest and ARBs’ non-existent effects in preventing CVD or improving glycemic indices in DM and HT (Figure 1)[13,50-53].
The data presented above strongly support the role of miR-155 as a major player in the pathogenesis of T2DM and complications, by triggering IR and β-cell loss as well as through RAAS modulatory effects (Figure 1)[5,8]. Large multicenter trials are required to establish this role of miRNA as a reliable biomarker and potential therapeutic target in DM. Then, as increased mechanistic knowledge regarding miR-155 becomes available, novel miRNA-modulating approaches with miR-155 as a target are likely in T2DM. Even though these therapeutic modalities are still in their infancy and might yet be far from the clinic, research must address this knowledge gap in order to devise how to effectively deliver specific, synthetic miRNA mimics (T2DM, aging, obesity, sarcopenia) or inhibitors-antagomiRs (T1DM, cancer), to a specific tissue, in the diabetic patient, as miR-155 actions are tissue-sensitive[54]. In addition, a better understanding is needed on how several miRNAs work synergistically on the same mRNA targets and how miRNA networks function. As disease-specific miRNA expression pattern is ubiquitous in all related tissues, it can prove challenging in a complex disease like DM to accomplish precise delivery to certain tissues/organs and avoid adverse off-target effects in others[5].
We are thankful to James T A Marshall for his invaluable editorial assistance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cai L, United States; Cen LS, China; Moreno-Gómez-Toledano R, Spain; Papadopoulos VP, Greece S-Editor: Li L L-Editor: A P-Editor: Ji MX
| 1. | Magliano DJ, Boyko EJ; IDF Diabetes Atlas 10th edition scientific committee. IDF Diabetes Atlas [Internet]. 10th ed. Brussels: International Diabetes Federation; 2021. [PubMed] |
| 2. | Taylor R, Al-Mrabeh A, Sattar N. Understanding the mechanisms of reversal of type 2 diabetes. Lancet Diabetes Endocrinol. 2019;7:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 175] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 3. | Cai L, Wheeler E, Kerrison ND, Luan J, Deloukas P, Franks PW, Amiano P, Ardanaz E, Bonet C, Fagherazzi G, Groop LC, Kaaks R, Huerta JM, Masala G, Nilsson PM, Overvad K, Pala V, Panico S, Rodriguez-Barranco M, Rolandsson O, Sacerdote C, Schulze MB, Spijkerman AMW, Tjonneland A, Tumino R, van der Schouw YT, Sharp SJ, Forouhi NG, Riboli E, McCarthy MI, Barroso I, Langenberg C, Wareham NJ. Genome-wide association analysis of type 2 diabetes in the EPIC-InterAct study. Sci Data. 2020;7:393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Papadopoulos KI, Sutheesophon W, Aw TC. Too hard to die: Exercise training mediates specific and immediate SARS-CoV-2 protection. World J Virol. 2022;11:98-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Palihaderu P, Mendis B, Premarathne J, Dias W, Yeap SK, Ho WY, Dissanayake AS, Rajapakse IH, Karunanayake P, Senarath U, Satharasinghe DA. Therapeutic Potential of miRNAs for Type 2 Diabetes Mellitus: An Overview. Epigenet Insights. 2022;15:25168657221130041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 6. | Lopez DL, Casillas OE, Jaramillo HJ, Romero-Garcia T, Vazquez-Jimenez JG. AT1 receptor downregulation: A mechanism for improving glucose homeostasis. World J Diabetes. 2023;14:170-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Papadopoulos KI, Wattanaarsakit P, Prasongchean W, Narain R. 10 - Gene therapies in clinical trials. In: Narain R, editor. Polymers and Nanomaterials for Gene Therapy. Cambridge: Woodhead Publishing, 2016: 231-256. [DOI] [Full Text] |
| 8. | Jankauskas SS, Gambardella J, Sardu C, Lombardi A, Santulli G. Functional Role of miR-155 in the Pathogenesis of Diabetes Mellitus and Its Complications. Noncoding RNA. 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 9. | Zhu N, Zhang D, Chen S, Liu X, Lin L, Huang X, Guo Z, Liu J, Wang Y, Yuan W, Qin Y. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis. 2011;215:286-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 247] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 10. | Elton TS, Selemon H, Elton SM, Parinandi NL. Regulation of the MIR155 host gene in physiological and pathological processes. Gene. 2013;532:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 372] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 11. | Sethupathy P, Borel C, Gagnebin M, Grant GR, Deutsch S, Elton TS, Hatzigeorgiou AG, Antonarakis SE. Human microRNA-155 on chromosome 21 differentially interacts with its polymorphic target in the AGTR1 3' untranslated region: a mechanism for functional single-nucleotide polymorphisms related to phenotypes. Am J Hum Genet. 2007;81:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 282] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 12. | Faraoni I, Antonetti FR, Cardone J, Bonmassar E. miR-155 gene: a typical multifunctional microRNA. Biochim Biophys Acta. 2009;1792:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 596] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 13. | Papadopoulos KI, Papadopoulou A, Aw TC. Beauty and the beast: host microRNA-155 versus SARS-CoV-2. Hum Cell. 2023;36:908-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Huang S, Xiang C, Song Y. Identification of the shared gene signatures and pathways between sarcopenia and type 2 diabetes mellitus. PLoS One. 2022;17:e0265221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Mahdavi R, Ghorbani S, Alipoor B, Panahi G, Khodabandehloo H, Esfahani EN, Razi F, Meshkani R. Decreased Serum Level of miR-155 is Associated with Obesity and its Related Metabolic Traits. Clin Lab. 2018;64:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Lin X, Qin Y, Jia J, Lin T, Lin X, Chen L, Zeng H, Han Y, Wu L, Huang S, Wang M, Xie R, Liang L, Liu Y, Liu R, Zhang T, Li J, Wang S, Sun P, Huang W, Yao K, Xu K, Du T, Xiao D. MiR-155 Enhances Insulin Sensitivity by Coordinated Regulation of Multiple Genes in Mice. PLoS Genet. 2016;12:e1006308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 17. | Corral-Fernández NE, Salgado-Bustamante M, Martínez-Leija ME, Cortez-Espinosa N, García-Hernández MH, Reynaga-Hernández E, Quezada-Calvillo R, Portales-Pérez DP. Dysregulated miR-155 expression in peripheral blood mononuclear cells from patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2013;121:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Latini A, Spallone V, D'Amato C, Novelli G, Borgiani P, Ciccacci C. A common polymorphism in MIR155 gene promoter region is associated with a lower risk to develop type 2 diabetes. Acta Diabetol. 2019;56:717-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Zhiping L, Chunli L, Shanna Z. Study on the correlation between PPARγ, Aβ1-42, miR-155 and the occurrence and development of diabetes. Cell Mol Biol (Noisy-le-grand). 2022;67:214-221. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Chatzopoulou F, Kyritsis KA, Papagiannopoulos CI, Galatou E, Mittas N, Theodoroula NF, Papazoglou AS, Karagiannidis E, Chatzidimitriou M, Papa A, Sianos G, Angelis L, Chatzidimitriou D, Vizirianakis IS. Dissecting miRNA-Gene Networks to Map Clinical Utility Roads of Pharmacogenomics-Guided Therapeutic Decisions in Cardiovascular Precision Medicine. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | DuPont JJ, McCurley A, Davel AP, McCarthy J, Bender SB, Hong K, Yang Y, Yoo JK, Aronovitz M, Baur WE, Christou DD, Hill MA, Jaffe IZ. Vascular mineralocorticoid receptor regulates microRNA-155 to promote vasoconstriction and rising blood pressure with aging. JCI Insight. 2016;1:e88942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | Dunand-Sauthier I, Irla M, Carnesecchi S, Seguín-Estévez Q, Vejnar CE, Zdobnov EM, Santiago-Raber ML, Reith W. Repression of arginase-2 expression in dendritic cells by microRNA-155 is critical for promoting T cell proliferation. J Immunol. 2014;193:1690-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Gambardella J, Khondkar W, Morelli MB, Wang X, Santulli G, Trimarco V. Arginine and Endothelial Function. Biomedicines. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 24. | Zhan Y, Brown C, Maynard E, Anshelevich A, Ni W, Ho IC, Oettgen P. Ets-1 is a critical regulator of Ang II-mediated vascular inflammation and remodeling. J Clin Invest. 2005;115:2508-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 185] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 25. | Breggia AC, Wojchowski DM, Himmelfarb J. JAK2/Y343/STAT5 signaling axis is required for erythropoietin-mediated protection against ischemic injury in primary renal tubular epithelial cells. Am J Physiol Renal Physiol. 2008;295:F1689-F1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Shou J, Chen PJ, Xiao WH. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetol Metab Syndr. 2020;12:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 27. | Frantz EDC, Prodel E, Braz ID, Giori IG, Bargut TCL, Magliano DC, Nobrega ACL. Modulation of the renin-angiotensin system in white adipose tissue and skeletal muscle: focus on exercise training. Clin Sci (Lond). 2018;132:1487-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Zheng L, Xu CC, Chen WD, Shen WL, Ruan CC, Zhu LM, Zhu DL, Gao PJ. MicroRNA-155 regulates angiotensin II type 1 receptor expression and phenotypic differentiation in vascular adventitial fibroblasts. Biochem Biophys Res Commun. 2010;400:483-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Cheng W, Liu T, Jiang F, Liu C, Zhao X, Gao Y, Wang H, Liu Z. microRNA-155 regulates angiotensin II type 1 receptor expression in umbilical vein endothelial cells from severely pre-eclamptic pregnant women. Int J Mol Med. 2011;27:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Yang Y, Zhou Y, Cao Z, Tong XZ, Xie HQ, Luo T, Hua XP, Wang HQ. miR-155 functions downstream of angiotensin II receptor subtype 1 and calcineurin to regulate cardiac hypertrophy. Exp Ther Med. 2016;12:1556-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Izmailova O, Shlykova O, Vatsenko A, Ivashchenko D, Dudchenko M, Koval T, Kaidashev I. Allele С (rs5186) of at1r is associated with the severity of COVID-19 in the Ukrainian population. Infect Genet Evol. 2022;98:105227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Assmann TS, Recamonde-Mendoza M, Puñales M, Tschiedel B, Canani LH, Crispim D. MicroRNA expression profile in plasma from type 1 diabetic patients: Case-control study and bioinformatic analysis. Diabetes Res Clin Pract. 2018;141:35-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | Assmann TS, Duarte GCK, Brondani LA, de Freitas PHO, Martins ÉM, Canani LH, Crispim D. Polymorphisms in genes encoding miR-155 and miR-146a are associated with protection to type 1 diabetes mellitus. Acta Diabetol. 2017;54:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Hubal MJ, Nadler EP, Ferrante SC, Barberio MD, Suh JH, Wang J, Dohm GL, Pories WJ, Mietus-Snyder M, Freishtat RJ. Circulating adipocyte-derived exosomal MicroRNAs associated with decreased insulin resistance after gastric bypass. Obesity (Silver Spring). 2017;25:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 35. | Soplinska A, Zareba L, Wicik Z, Eyileten C, Jakubik D, Siller-Matula JM, De Rosa S, Malek LA, Postula M. MicroRNAs as Biomarkers of Systemic Changes in Response to Endurance Exercise-A Comprehensive Review. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Xu WD, Feng SY, Huang AF. Role of miR-155 in inflammatory autoimmune diseases: a comprehensive review. Inflamm Res. 2022;71:1501-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 37. | Akarsu E, Sayiner ZA, Balcı SO, Demirel C, Bozdag Z, Korkmaz M, Yılmaz I. Effects of antidiabetics and exercise therapy on suppressors of cytokine signaling-1, suppressors of cytokine signaling-3, and insulin receptor substrate-1 molecules in diabetes and obesity. Rev Assoc Med Bras (1992). 2023;69:112-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 38. | Zhou Y, Ma XY, Han JY, Yang M, Lv C, Shao Y, Wang YL, Kang JY, Wang QY. Metformin regulates inflammation and fibrosis in diabetic kidney disease through TNC/TLR4/NF-κB/miR-155-5p inflammatory loop. World J Diabetes. 2021;12:19-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 39. | Gou L, Liu G, Ma R, Regmi A, Zeng T, Zheng J, Zhong X, Chen L. High fat-induced inflammation in vascular endothelium can be improved by Abelmoschus esculentus and metformin via increasing the expressions of miR-146a and miR-155. Nutr Metab (Lond). 2020;17:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 40. | Zhu M, Wei Y, Geißler C, Abschlag K, Corbalán Campos J, Hristov M, Möllmann J, Lehrke M, Karshovska E, Schober A. Hyperlipidemia-Induced MicroRNA-155-5p Improves β-Cell Function by Targeting Mafb. Diabetes. 2017;66:3072-3084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 41. | Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Nowack C, Schloemer P, Joseph A, Filippatos G; FIDELIO-DKD Investigators. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N Engl J Med. 2020;383:2219-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1696] [Cited by in RCA: 1502] [Article Influence: 300.4] [Reference Citation Analysis (1)] |
| 42. | Hu H, Zhao X, Jin X, Wang S, Liang W, Cong X. Efficacy and safety of eplerenone treatment for patients with diabetic nephropathy: A meta-analysis. PLoS One. 2022;17:e0265642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Lin M, Heizati M, Wang L, Nurula M, Yang Z, Wang Z, Abudoyreyimu R, Wu Z, Li N. A systematic review and meta-analysis of effects of spironolactone on blood pressure, glucose, lipids, renal function, fibrosis and inflammation in patients with hypertension and diabetes. Blood Press. 2021;30:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | McTavish D, Sorkin EM. Verapamil. An updated review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in hypertension. Drugs. 1989;38:19-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 126] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 45. | Lido P, Romanello D, Tesauro M, Bei A, Perrone MA, Palazzetti D, Noce A, Di Lullo L, Calò L, Cice G. Verapamil: prevention and treatment of cardio-renal syndromes in diabetic hypertensive patients? Eur Rev Med Pharmacol Sci. 2022;26:1524-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 46. | Xu G, Chen J, Jing G, Shalev A. Preventing β-cell loss and diabetes with calcium channel blockers. Diabetes. 2012;61:848-856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 47. | Borowiec AM, Właszczuk A, Olakowska E, Lewin-Kowalik J. TXNIP inhibition in the treatment of diabetes. Verapamil as a novel therapeutic modality in diabetic patients. Med Pharm Rep. 2022;95:243-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 48. | Forlenza GP, McVean J, Beck RW, Bauza C, Bailey R, Buckingham B, DiMeglio LA, Sherr JL, Clements M, Neyman A, Evans-Molina C, Sims EK, Messer LH, Ekhlaspour L, McDonough R, Van Name M, Rojas D, Beasley S, DuBose S, Kollman C, Moran A; CLVer Study Group. Effect of Verapamil on Pancreatic Beta Cell Function in Newly Diagnosed Pediatric Type 1 Diabetes: A Randomized Clinical Trial. JAMA. 2023;329:990-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 96] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 49. | Wang CY, Huang KC, Lu CW, Chu CH, Huang CN, Chen HS, Lee IT, Chen JF, Chen CC, Chen CS, Hsieh CH, Tien KJ, Chien HY, Huang YY, Hsu JP, Shane GT, Chang AC, Wu YC, Sheu WH. A Randomized Controlled Trial of R-Form Verapamil Added to Ongoing Metformin Therapy in Patients with Type 2 Diabetes. J Clin Endocrinol Metab. 2022;107:e4063-e4071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Weber M, Baker MB, Patel RS, Quyyumi AA, Bao G, Searles CD. MicroRNA Expression Profile in CAD Patients and the Impact of ACEI/ARB. Cardiol Res Pract. 2011;2011:532915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 51. | Zhu GF, Yang LX, Guo RW, Liu H, Shi YK, Ye JS, Yang ZH. microRNA-155 is inversely associated with severity of coronary stenotic lesions calculated by the Gensini score. Coron Artery Dis. 2014;25:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 52. | Fuchs FD, DiNicolantonio JJ. Angiotensin receptor blockers for prevention of cardiovascular disease: where does the evidence stand? Open Heart. 2015;2:e000236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Yao J, Fan S, Shi X, Gong X, Zhao J, Fan G. Angiotensin-converting enzyme inhibitors versus angiotensin II receptor blockers on insulin sensitivity in hypertensive patients: A meta-analysis of randomized controlled trials. PLoS One. 2021;16:e0253492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 54. | Mahesh G, Biswas R. MicroRNA-155: A Master Regulator of Inflammation. J Interferon Cytokine Res. 2019;39:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 248] [Article Influence: 41.3] [Reference Citation Analysis (0)] |