Published online Aug 15, 2023. doi: 10.4239/wjd.v14.i8.1323
Peer-review started: April 24, 2023
First decision: May 8, 2023
Revised: May 16, 2023
Accepted: July 14, 2023
Article in press: July 14, 2023
Published online: August 15, 2023
Processing time: 109 Days and 3.1 Hours
Diabetic foot ulcers (DFUs) are common in patients with diabetes, especially those undergoing hemodialysis. In severe cases, these ulcers can cause damage to the lower extremities and lead to amputation. Traditional treatments such as flap transposition and transfemoral amputation are not always applicable in all cases. Therefore, there is a need for alternative treatment methods.
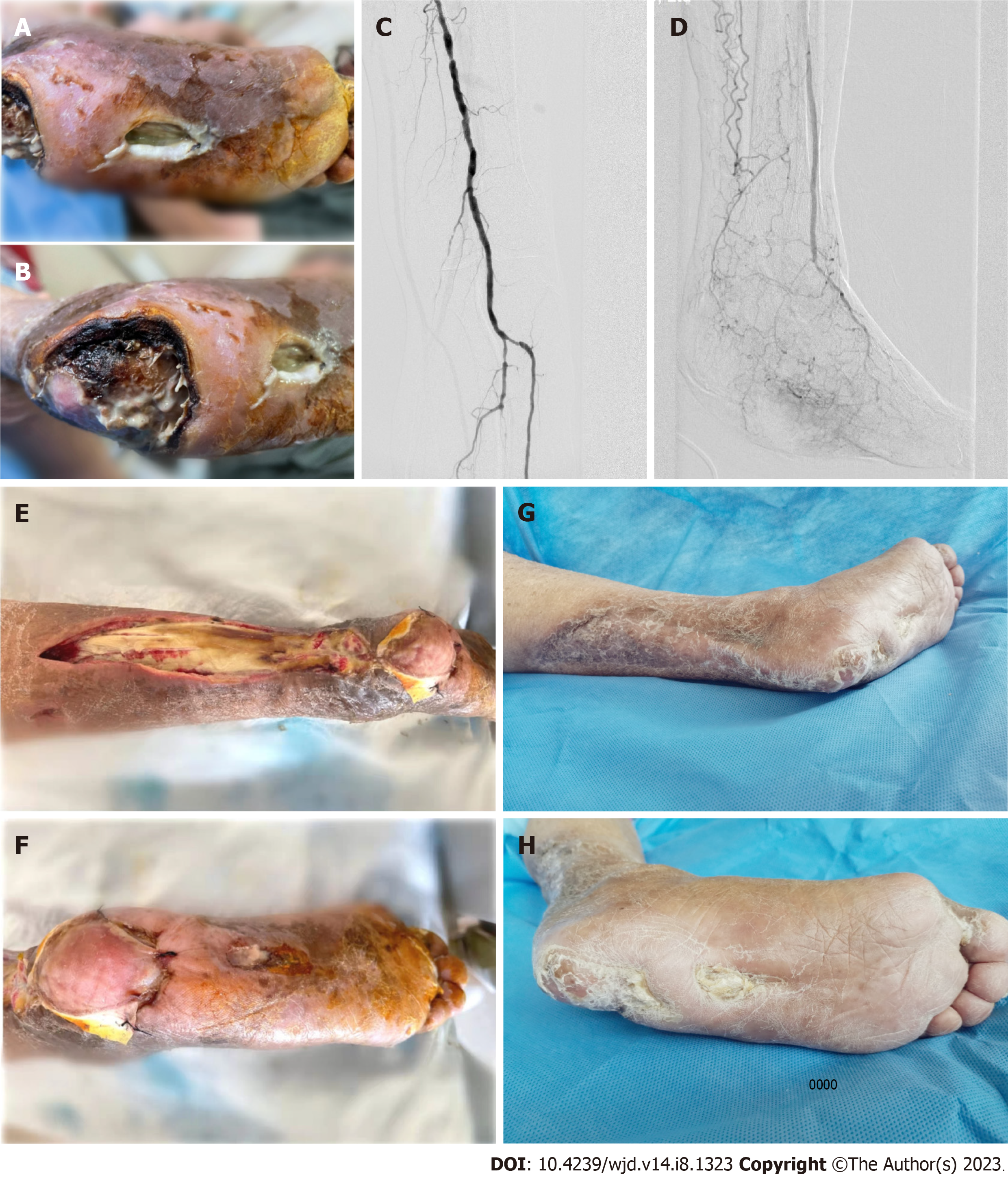
This report describes a 62-year-old female patient who was admitted to the hospital with plantar and heel ulcers on her left foot. The patient had a history of renal failure and was undergoing regular hemodialysis. Digital subtraction angiography showed extensive stenosis and occlusion in the left superficial femoral artery, left peroneal artery and left posterior tibial artery. Following evaluation by a multidisciplinary team, the patient was diagnosed with type 2 DFUs (TEXAS 4D). Traditional treatments were deemed unsuitable, and the patient was treated with endovascular surgery in the affected area, in addition to supportive medical treatment, local debridement, and sequential repair using split-thickness skin and tissue-engineered skin grafts combined with negative pressure treatment. After four months, the wound had completely healed, and the patient was able to walk with a walking aid.
This study demonstrates a new treatment method for DFUs was successful, using angioplasty, skin grafts, and negative pressure.
Core Tip: Diabetic foot ulcers can be a serious and common complication of diabetes. In severe cases, they can lead to lower extremity damage and amputation. Traditional treatments such as flap transposition and transfemoral amputation are not always applicable in all cases. This report describes the successful treatment of ischemic diabetic plantar and heel ulcers in a patient undergoing hemodialysis using sequential treatment involving percutaneous transluminal angioplasty, tissue-engineered skin grafts, and negative pressure wound therapy. This treatment method may be a viable alternative for patients who are unsuitable for traditional treatments and could help prevent the need for amputation.
- Citation: Wang JJ, Yu YY, Wang PY, Huang XM, Chen X, Chen XG. Sequential treatment for diabetic foot ulcers in dialysis patients: A case report. World J Diabetes 2023; 14(8): 1323-1329
- URL: https://www.wjgnet.com/1948-9358/full/v14/i8/1323.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i8.1323
Diabetic foot is one of the most serious and painful chronic complications in diabetic patients, especially in elderly patients, with an annual incidence of 8.1% in China[1]. Although the rate of major amputation caused by diabetic foot has been reduced to 2.14% nationwide, China still lags far behind the developed countries in Europe and America in this respect[2]. The increased complications of diabetic nephropathy and advanced renal replacement therapies have significantly enhanced the proportion of diabetic patients treated with dialysis for end-stage renal diseases, leading to an increasing number of hemodialysis patients with diabetic foot ulcers (DFUs). These patients cannot always be cured and usually have a poor prognosis, particularly when they have complications such as arterial occlusion of the lower limbs, which, to a certain extent, explains the high amputation and mortality rates of diabetic foot patients in China[3]. Therefore, it is essential to formulate affordable and effective treatment schemes in clinical practice to reduce the amputation and mortality rates among hemodialysis patients with diabetic foot.
The patient described in this report is the first to undergo open debridement, percutaneous transluminal angioplasty (PTA), split-thickness skin graft, tissue-engineered skin graft, and negative pressure wound therapy to treat ischemic diabetic plantar and heel ulcers, with no relapses during the 6-mo follow-up period.
The 62-year-old female was admitted to hospital in February 2021 due to left foot ulcer for one month, aggravated and painful for one week.
The patient had a left foot ulcer for one month, aggravated and painful for one week.
The patient had a history of chronic renal failure, uremia, and hemodialysis for 5 years. Four years ago, she underwent left forearm arteriovenous fistula formation. In July 2019, the patient underwent balloon angioplasty and stenting for lower extremity arterial sclerosis with ulceration in the left leg. In November 2019, she underwent balloon angioplasty and stenting for lower extremity arterial sclerosis with ulceration in the right leg.
The patient denied any family history of diseases.
Physical examination at admission showed a body temperature of 38.3℃, pulse rate of 103 bpm, a respiratory rate of 21 breaths/min, and blood pressure of 162/83 mmHg. According to a specialized medical check-up, the left foot, with toenail hypertrophy, was dark in color, with the fourth toe absent, and fine hair had fallen out. An ulcer 4 cm × 2 cm in size was observed in the middle of the planta pedis, and the base was yellow and rotten, with the plantar fascia exposed. In addition, the peripheral skin showed wound undermining, which had spread to the heel ulcer. There was a large area of absent skin on the heel, exposing the calcaneus, and the base was yellow and rotten. A dark scab was found on part of the wound, and around the area where the pus percolated, the skin was red and swollen, with a blurry boundary and symptoms of wound undermining. The vascular lacuna had spread towards the lower leg via the proximal end.
Laboratory examinations were performed at admission. As shown by routine blood examination, the red blood cell and white blood cell counts were 3.12 × 1012/L and 18.23 × 109/L, respectively, and the hemoglobin concentration was 83 g/L. The following blood biochemistry indices were obtained: K+ mmol/L, serum albumin 24.0 g/L, serum creatinine 468.0 μmol/L, plasma brain natriuretic peptide > 35 000 pg/mL, erythrocyte sedimentation rate 65.0 mm/h, interleukin-6 89.5 pg/mL, C-reactive protein 83.7 mg/L, procalcitonin 6.6 ng/mL, and glycosylated hemoglobin 8.1.
Echocardiography showed an ejection fraction of 34%; the ankle-brachial index (ABI) was 0.6 on the left side and 0.7 on the right side. According to digital subtraction angiography, the patient suffered from severe stenosis of the left superficial femoral artery at the proximal and distal ends of the stent, extensive stenosis and occlusion of the left peroneal artery, and occlusion of the left posterior tibial artery.
Based on clinical symptoms, physical signs, as well as laboratory and auxiliary examinations, the patient was diagnosed with: (1) Type 2 diabetic foot combined with peripheral vascular disease, peripheral neuropathy, and retinopathy; (2) Chronic renal failure treated with dialysis for uremia; (3) Hypertension (Grade 3) defined as a very high risk; (4) Coronary atherosclerotic heart disease with cardiac function Level III; (5) Anemia; (6) Hypoproteinemia; (7) Arteriosclerosis obliterans of the upper limbs with gangrene; and (8) Hyperkalemia. In addition, this patient had undergone PTA of both lower limbs and resection of toes on both feet.
The final diagnosis was type 2 DFU.
On admission, the patient underwent debridement in addition to routine treatment (including control of blood glucose, blood pressure, and blood lipids, systemic antibiotic treatment, vascular dilation, pain relief, neurotrophic supplement, etc.).
PTA was performed in the first week. Restenosis of the superficial femoral arterial stent as well as the occlusion of peroneal and posterior tibial arteries were alleviated by endovascular drug-coated balloon dilatation to ensure that the blood could flow from the main artery to the affected foot. In the second week, the necrotic tissues on the patient’s planta pedis and heel were removed by surgery, during which the area of debridement was expanded on the back of the lower leg to resect part of the tendo calcaneus. Negative pressure treatment was then initiated. In the fourth week, a split-thickness skin graft combined with negative pressure treatment was performed on the back of the lower leg, and the patient also underwent tissue-engineered skin graft and negative pressure treatment for the heel ulcer. During surgery, the tissue-engineered skin was soaked in sterile normal saline for 3-4 min, and then trimmed to fit the shape of the wound. The stent was sutured along the edge of the wound under tension-free conditions, with the collagen layer appressed to the wound. The silica gel layer was then covered by sterile Vaseline gauze, which was followed by negative pressure treatment and regular postoperative dressing changes. Following adequate vascularization in the collagen layer, the silica gel layer was removed using tweezers[4]. The wound bed was kept moist with the application of artificial dermis, and regular dressing changes were performed to evaluate the extent of epithelialization. The decision to perform a secondary graft using split-thickness skin or reapply artificial skin was made, particularly in cases involving exposed tendons or areas subjected to mechanical stress. Following split-thickness skin grafting, an optimal level of moisture was maintained, and the need for additional grafting, or even multiple grafting procedures, was determined based on the viability of the skin grafts. The tissue-engineered skin was then grafted repeatedly until the wound healed (Figure 1).
The patient was successfully cured of ischemic diabetic plantar heel ulcer.
It is reported that 39.3% of diabetic foot patients also have chronic renal diseases[5]. As the glomerular filtration rate reduces, there is a fold increase in nonhealing ulcers, major amputation, and mortality risk[6]. Massive proteinuria is not only a crucial risk factor causing nonhealing ulcers and amputation, but also an independent risk factor for cardiovascular events and death, as well as all-cause mortality[7]. Both hemodialysis and peritoneal dialysis can increase the risk of DFU by more than four times among patients with uremia. On average it takes diabetic patients 7 mo (2-40 mo) to progress from hemodialysis to amputation[8,9]. Since 2017, our department has received and cured 115 dialysis patients with DFUs, with the limb salvage rate reaching 83.1%.
This report describes a female patient with multiple complications and comorbidities, who had undergone hemodialysis treatment for 3 years. Although the blood supply in both lower limbs seemed sufficient according to the ABI which was measured to be 0.6 on the left side and 0.7 on the right side, the results of X-ray, color Doppler ultrasound and computed tomography angiography indicated that the patient had severe arterial calcification in her lower limbs. Therefore, the ABI was considered to be too “impractically high” to evaluate the degree of foot ischemia accurately. Under such circumstances, endovascular drug-coated balloon dilatation was performed to improve the blood supply to the distal end of the left lower limb.
Considering that the heel ulcer was located in the weight-bearing area, a sural neurovascular flap or free flap can be used to repair the wound to ensure that the healed skin is extremely hard-wearing. However, our patient who had a large area of ulcer in the flap donor site on the back of her lower leg, was intolerant to the anesthesia used during the free flap operation, and the therapeutic effect could not be guaranteed. Therefore, the tissue-engineered skin containing an artificial dermal matrix was grafted. After treatment, the heel ulcer healed with good abrasive resistance, and the patient was able to walk with the help of a walking aid.
The artificial composite dermis prepared by Yannas et al[10] in 1982 using collagen matrix and a medical silicone rubber membrane was successfully used as a dermal regeneration template to repair a deep burn wound. In 2017, China developed the first double-layer tissue-engineered skin[4], which achieved good results in various departments, such as the Department of Burn, Department of Plastic Surgery, Department of Hand and Foot Surgery, etc. The tissue-engineered skin has now been widely applied to deep burns, traumatic skin defects, chronic skin ulcers, wound repair after tumor resection, and scar plastic surgery, with the therapeutic effect highly recognized by clinicians at home and abroad[11].
In domestic and foreign literature, the dermal substitute is also known as tissue-engineered skin, tissue-engineered skin matrix, artificial skin, and artificial dermis in accordance with different structures, materials, and preparation methods. However, in essence, it is used to induce the regeneration of dermis through a dermal stent template, thus substituting the defective dermal tissues and optimizing the appearance and function of the healed wound. The primary methods for wound repair involve skin grafting and flap procedures. For patients with lower limb chronic ischemia, both pedicle flaps and free flaps can result in significant trauma. Pure split-thickness skin grafts, especially in weight-bearing areas, have poor durability. Mid-thickness skin grafts pose challenges in terms of graft survival, particularly in this patient population. The use of artificial dermis alone is associated with high costs and increased patient burden. Therefore, a combination of split-thickness skin grafting and artificial dermis is employed for such patients.
In this report, the double-layer tissue-engineered skin was adopted. The upper layer was a semipermeable silicone rubber membrane, which acted like the epidermis to control the evaporation of water and inhibit the invasion of microorganisms; the lower layer, namely the spongy dermal stent layer constructed by collagen-chondroitin sulfate, has high biocompatibility and low immunogenicity, and acts as a cell proliferation stent to promote the intrusive growth of vascular endothelial cells and fibroblasts in the graft site, thus forming a composite constituted by the stent, new capillaries, and cells. After adequate vascularization for 2-3 wk, the autologous split-thickness skin can be grafted[12]. The dermal stent is then gradually degraded and substituted by new dermal tissues.
National and international research has shown that the tissue-engineered skin, which can promote and accelerate wound healing of chronic ulcers[13], has been successfully used to treat diabetic, vascular and pressure ulcers. When the wound caused by chronic ulcers is repaired with tissue-engineered skin, the debridement must be repeated to avoid infection, and the graft cannot be performed until the wound is clean and the basal blood supply is sufficient. It may take two or more weeks to realize adequate vascularization of tissue-engineered skin on the chronic ulcer wound, so the autologous skin should be grafted according to the vascularization status.
In the case of deep wounds, the tissue-engineered skin can be overlaid repeatedly to thicken the new dermis, which was the method used in this case, in which the tissue-engineered skin was repeatedly grafted to the heel, and the wound healed before skin grafting. There are many clinical reports on the treatment of DFUs using tissue-engineered skin[14,15], but the application to weight-bearing areas and dialysis patients with ischemic DFUs has not previously been reported (Table 1).
| No. | TEXA grade | Complication | Diseased region | Application method | Outcome | Healing time |
| 1 | 2B | Peripheral neuropathy | Heel | Sural neurovascular flap transposition under epidural anesthesia | Partly healed | 4 wk |
| 2 | 2B | Peripheral neuropathy; hypertension | Heel | Sural neurovascular flap transposition under epidural anesthesia | Healed | 6 wk |
| 3 | 2A | Peripheral neuropathy; coronary heart disease | Non-weight-bearing area | Tissue-engineered skin | Healed | 2 mo |
| 3 | 2A | Hypertension | Non-weight-bearing area | Tissue-engineered skin | Healed | 2 mo |
| 4 | 2A | Peripheral neuropathy | Non-weight-bearing area | Tissue-engineered skin | Healed | 3 mo |
| 5 | 4D | Peripheral neuropathy; peripheral vascular disease; retinopathy; dialysis for uremia; hypertension; coronary heart disease; anemia; hypoproteinemia; arteriosclerosis obliterans of upper limbs with gangrene | Planta pedis, heel, and back of the lower leg | PTA, negative pressure treatment, autologous split-thickness skin graft, and tissue-engineered skin | Healed | 4 mo |
Additionally, Blood glucose levels play a critical role in diabetes management and can affect wound healing and susceptibility to infection. In diabetic patients with ischemic ulcers, maintaining optimal glycemic control is vital for successful wound healing. Similarly, creatinine levels reflect renal function and can provide insight into the patient's overall health status and the potential impact of hemodialysis on wound healing. In future studies, we recommend the inclusion of these parameters to provide a more comprehensive assessment of treatment outcomes. By examining the relationship between blood glucose levels, renal function, and wound healing in similar patient populations, researchers can gain further insights into the effectiveness of the sequential treatment approach described in our case report.
Diabetic nephropathy is a high-risk factor leading to DFU and amputation. Diabetic foot patients who have undergone amputation usually have a poor prognosis, with the median survival time being 3.12 years (minor amputation: 5.5 years; major amputation: 1.9 years) and the 5-year postoperative survival rate is approximately 40%. The independent risk factors for postoperative death include age and major amputation. Therefore, whether to perform amputation should be thoroughly discussed in clinical practice, especially in diabetic foot patients.
Ischemia is another tough issue for dialysis patients. Although endovascular surgery is preferred to bypass surgery during revascularization in these patients, they still face significant challenges. First, abnormal calcium-phosphorus metabolism and severe vascular calcification cause considerable difficulties during endovascular revascularization. Second, dialysis patients are less tolerant to anticoagulants and contrast agents, which will prolong the process of endovascular revascularization. Third, dialysis patients with diabetes, in poor physical condition, are usually complicated by hypoproteinemia and renal anemia.
The tissue-engineered skin, containing natural extracellular matrices, protogenous growth factors and living cells, can transfer growth and cell factors to the wound to accelerate the healing process. This will promote dermal regeneration and inhibit scar hyperplasia, thus restoring wound elasticity and flexibility and improving the appearance and function of the skin. Furthermore, traditional flap transposition can be replaced by this skin graft technique as the exposed bones and tendons can be directly covered using the tissue-engineered skin during wound repair.
Research indicates that tissue-engineered skin substitutes can promote the healing of refractory foot ulcers with high safety. According to a network meta-analysis, these substitutes have a low failure rate, but there are few relevant studies and the overall sample size is small. Therefore, more studies are needed to verify the safety, effectiveness, and failure rate of these substitutes in the treatment of DFUs. Based on the existing evidence, the tissue-engineered skin graft can be used as an auxiliary therapy for treating refractory DFUs.
At present, there are few domestic and foreign clinical reports on the repair of nonhealing diabetic foot wounds, especially in hemodialysis patients or in weight-bearing areas such as the planta pedis and heel, and the combination of tissue-engineered skin graft with negative pressure treatment and autologous split-thickness skin graft is not mentioned in the available literature. Therefore, it is necessary to conduct further clinical research based on this case report to explore the proper use and amount of tissue-engineered skin in weight-bearing areas of dialysis patients with ischemic diabetic foot.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Maranta F, Italy; Millman JR, United States S-Editor: Wang JL L-Editor: A P-Editor: Ji MX
| 1. | Jiang Y, Wang X, Xia L, Fu X, Xu Z, Ran X, Yan L, Li Q, Mo Z, Yan Z, Ji Q. A cohort study of diabetic patients and diabetic foot ulceration patients in China. Wound Repair Regen. 2015;23:222-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 2. | Xu Z, Ran X. Diabetic foot care in China: challenges and strategy. Lancet Diabetes Endocrinol. 2016;4:297-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Al-Thani H, El-Menyar A, Koshy V, Hussein A, Sharaf A, Asim M, Sadek A. Implications of foot ulceration in hemodialysis patients: a 5-year observational study. J Diabetes Res. 2014;2014:945075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Writing group of experts consensus on clinical application of bilayer artificial dermis (2019 version). [Experts consensus on clinical application of bilayer artificial dermis (2019 version)]. Zhonghua Shao Shang Za Zhi. 2019;35:705-711. [PubMed] |
| 5. | He Y, Qian H, Xu L, Zhang S, Gu X, Gu J, Shi J, Shen Y, Liu J, Tang Z. Association between estimated glomerular filtration rate and outcomes in patients with diabetic foot ulcers: a 3-year follow-up study. Eur J Endocrinol. 2017;177:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Pollard J, Hamilton GA, Rush SM, Ford LA. Mortality and morbidity after transmetatarsal amputation: retrospective review of 101 cases. J Foot Ankle Surg. 2006;45:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Chronic Kidney Disease Prognosis Consortium; Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073-2081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3261] [Cited by in RCA: 3067] [Article Influence: 204.5] [Reference Citation Analysis (0)] |
| 8. | He J, Li Z, Xia P, Shi A, FuChen X, Zhang J, Yu P. Ferroptosis and ferritinophagy in diabetes complications. Mol Metab. 2022;60:101470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 100] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 9. | Ali MK, Pearson-Stuttard J, Selvin E, Gregg EW. Interpreting global trends in type 2 diabetes complications and mortality. Diabetologia. 2022;65:3-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 187] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 10. | Yannas IV, Burke JF, Orgill DP, Skrabut EM. Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science. 1982;215:174-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 388] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Zhao X, Li X, Wang Y, Guo Y, Huang Y, Lv D, Lei M, Yu S, Luo G, Zhan R. Stability and biosafety of human epidermal stem cell for wound repair: preclinical evaluation. Stem Cell Res Ther. 2023;14:4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 12. | Qiu X, Wang J, Wang G, Wen H. Vascularization of Lando(®) dermal scaffold in an acute full-thickness skin-defect porcine model. J Plast Surg Hand Surg. 2018;52:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Weng T, Wu P, Zhang W, Zheng Y, Li Q, Jin R, Chen H, You C, Guo S, Han C, Wang X. Regeneration of skin appendages and nerves: current status and further challenges. J Transl Med. 2020;18:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 14. | Mendoza-Marí Y, García-Ojalvo A, Fernández-Mayola M, Rodríguez-Rodríguez N, Martinez-Jimenez I, Berlanga-Acosta J. Epidermal growth factor effect on lipopolysaccharide-induced inflammation in fibroblasts derived from diabetic foot ulcer. Scars Burn Heal. 2022;8:20595131211067380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Raepsaet C, Alves P, Cullen B, Gefen A, Lázaro-Martínez JL, Lev-Tov H, Najafi B, Santamaria N, Sharpe A, Swanson T, Woo K, Beeckman D. Clinical research on the use of bordered foam dressings in the treatment of complex wounds: A systematic review of reported outcomes and applied measurement instruments. J Tissue Viability. 2022;31:514-522. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |