Published online Jun 15, 2023. doi: 10.4239/wjd.v14.i6.656
Peer-review started: December 26, 2022
First decision: February 28, 2023
Revised: March 13, 2023
Accepted: April 17, 2023
Article in press: April 17, 2023
Published online: June 15, 2023
Processing time: 170 Days and 16.2 Hours
Diabetes mellitus is a complicated disease characterized by a complex interplay of genetic, epigenetic, and environmental variables. It is one of the world's fastest-growing diseases, with 783 million adults expected to be affected by 2045. Devastating macrovascular consequences (cerebrovascular disease, cardiovascular disease, and peripheral vascular disease) and microvascular complications (like retinopathy, nephropathy, and neuropathy) increase mortality, blindness, kidney failure, and overall quality of life in individuals with diabetes. Clinical risk factors and glycemic management alone cannot predict the development of vascular problems; multiple genetic investigations have revealed a clear hereditary component to both diabetes and its related complications. In the twenty-first century, technological advancements (genome-wide association studies, next-generation sequencing, and exome-sequencing) have led to the identification of genetic variants associated with diabetes, however, these variants can only explain a small proportion of the total heritability of the condition. In this review, we address some of the likely explanations for this "missing heritability", for diabetes such as the significance of uncommon variants, gene-environment interactions, and epigenetics. Current discoveries clinical value, management of diabetes, and future research directions are also discussed.
Core Tip: Diabetes pathogenesis encompasses genetic, epigenetic, and environmental variables and their interactions. To date, the examined common variations can explain just a small portion of the heritability of diabetes. Furthermore, the technique of integrating the associated variants as a type of genetic risk score does not accurately predict diabetes risk. As a result, the trend for genetic risk factors for diabetes is shifting from common to rare variants. Aside from genetic variables, systemic data from other transomics such as epigenomics, transcriptomics, proteomics, metabolomics, and metagenomics will contribute to a better understanding of genetic determinants in the progression of metabolic illnesses like diabetes. Technological, computational, and collaborative developments continue to uncover novel genetic diabetes risk factors. There are high prospects for tailored diabetes treatment in the future, based on increased knowledge of the molecular genetic profile of the patients.
- Citation: Goyal S, Rani J, Bhat MA, Vanita V. Genetics of diabetes. World J Diabetes 2023; 14(6): 656-679
- URL: https://www.wjgnet.com/1948-9358/full/v14/i6/656.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i6.656
Diabetes mellitus (DM) is a set of diverse metabolic illnesses characterized by disturbances in the metabolism of glucose, resulting in hyperglycemia and glucose intolerance. Diabetes can occur either by the failure of the body to produce insulin, resistance to the action of insulin, or both[1,2]. DM is one of the most common endocrinological disorders worldwide. Its prevalence is rising because of phy
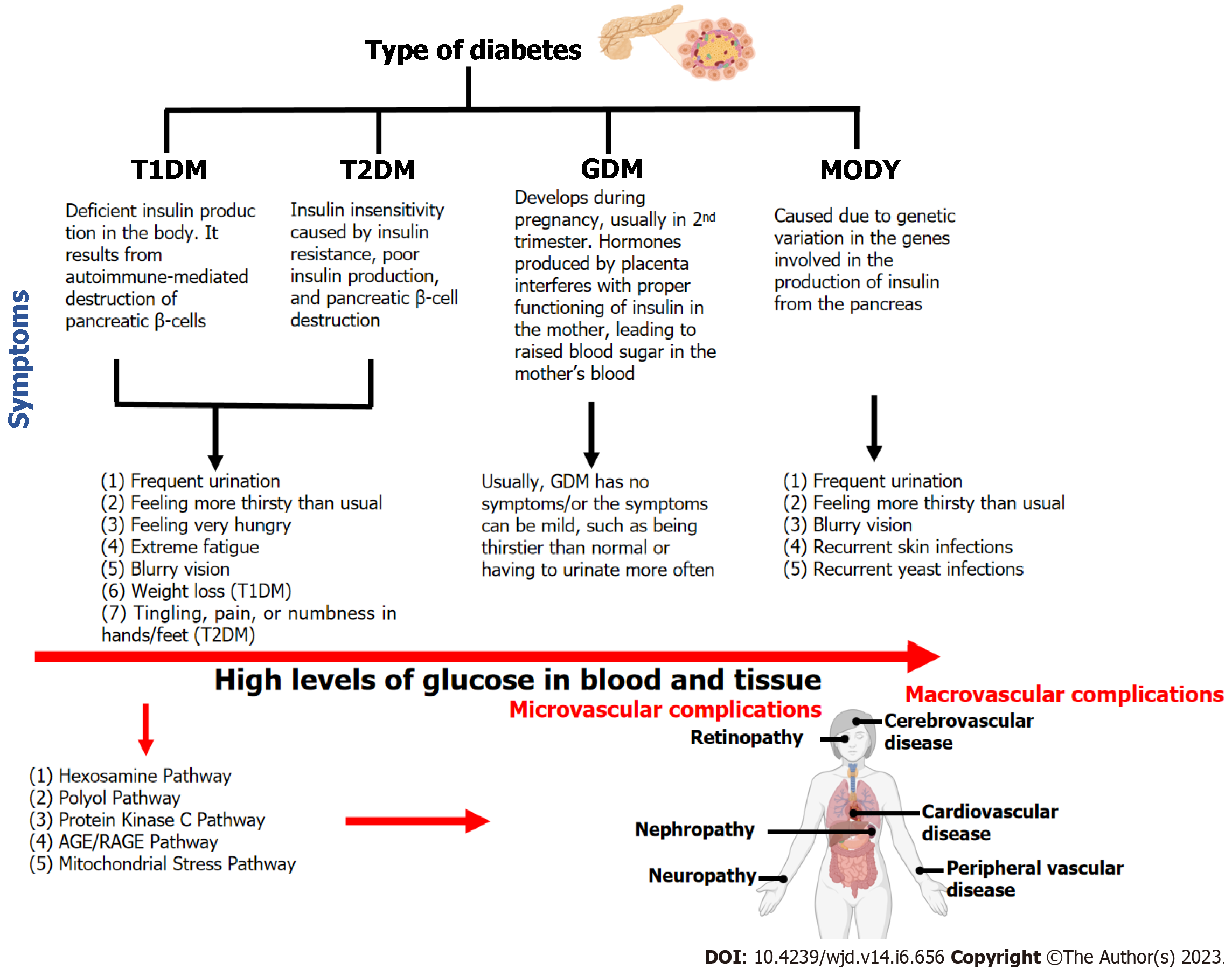
DM can be broadly classified into four types (Figure 1) i.e., type 1 DM (T1DM), type 2 DM (T2DM), gestational DM (GDM), and maturity-onset diabetes of young (MODY)[7]. Of these, T2DM is the most prevalent form of diabetes accounting for 90% of all cases worldwide.
T1DM is also known as insulin-dependent DM (IDDM) or juvenile-onset diabetes. T1DM is caused by the autoimmune destruction of pancreatic beta cells by a T-cell-mediated inflammatory response, resulting in reduced insulin production. T1DM accounts for around 5%-10% of the individuals diagnosed with diabetes and approximately 80%-90% of cases with diabetes among children and adolescents[8]. The interaction between T-lymphocytes and autoantigens causes beta-cell death. In newborns and children, the rate of beta cell loss is relatively variable with rapid progression. Adults are more likely to develop the slowly progressive form, commonly known as latent autoimmune diabetes in adults (LADA). At this stage, the body secretes little or no insulin, and patients frequently become dependent on insulin for survival[2,9].
T2DM is the most common type of diabetes, accounting for almost 90% of all cases globally. T2DM is characterized by insulin insensitivity caused by insulin resistance, poor insulin production, and pancreatic beta-cell destruction. The increased demand for insulin in the target tissues caused by insulin resistance could not be met due to beta cell abnormalities, resulting in hyperglycemia[10]. T2DM is a complex condition characterized by a combination of genetic as well as environmental variables, such as stress, obesity, and lack of physical activity[11].
Gestational diabetes is most common in pregnant women and accounts for about 7% of all pregnancy cases. Females having a history of GDM are 10 times more likely to develop postpartum T2DM, cardiovascular disease, and metabolic perturbation in the future[12]. Furthermore, children of pregnant women with gestational diabetes are at risk of anomalies related to glucose metabolism and have a 40 to 60 percent chance of getting diabetes in adulthood[13]. Women with a family history of diabetes and obese women are more likely to develop gestational diabetes[14].
MODY, a monogenic variant of type 2 diabetes, has an autosomal dominant inheritance pattern and is characterized mostly by insulin secretion abnormalities, however, with normal insulin action[15]. MODY generally occurs before the age of 25 years or during childhood[2]. Roughly 2%-5% of type 2 diabetes patients have been estimated to have MODY. Different types of MODY are classified based on underlying genetic defect: MODY1 (HNF4A); MODY2 (GCK); MODY3 (HNF1A); MODY4 (PDX1); MODY5 (HNF1B); MODY6 (NEUROD1); MODY12 (ABCC8), and MODY13 (KCNJ11).
There are two atypical types of DM: LADA and ketosis-prone DM (KPDM), both of which are prone to misdiagnosis, leading to ineffective management.
LADA is a kind of autoimmune diabetes that resembles T1DM, but the onset is during adulthood, and it progresses slowly toward absolute insulin insufficiency than classical childhood-onset T1DM, which requires prompt exogenous insulin therapy[16]. Approximately 2%-12% of all DM patients may have LADA[17]. Most LADA patients do not require insulin at the time of diagnosis; nevertheless, they do have diabetes-specific autoantibodies. As a result, they have characteristics of both T1DM and T2DM and are at risk of being misdiagnosed as having T2DM[18]. According to studies from China, Korea, India, and the United Arab Emirates, the prevalence of LADA is 5.7%, 4.4% to 5.3%, 2.6% to 3.2%, and 2.6%, respectively[19]. Usage of clinical risk tools (age of onset of diabetes < 50 years, acute symptoms of hyperglycemia at the time of onset, body mass index < 25 kg/m2, family history or personal history of autoimmune disease), and evaluation of C-peptide level can help identify individuals at higher risk of LADA in adults[19].
Diabetic ketoacidosis is a potentially fatal but treatable complication of DM that is characterized by hyperglycemia, metabolic acidosis, and ketonemia as a result of absolute or relative insulin insufficiency[20]. Although the actual prevalence of KPDM is unknown, men have a higher prevalence than women[21]. Patients with KPDM typically show acute and very recent history (mostly < 4 wk) of hyperglycemic symptoms such as polyuria, polydipsia, and weight-loss[22,23].
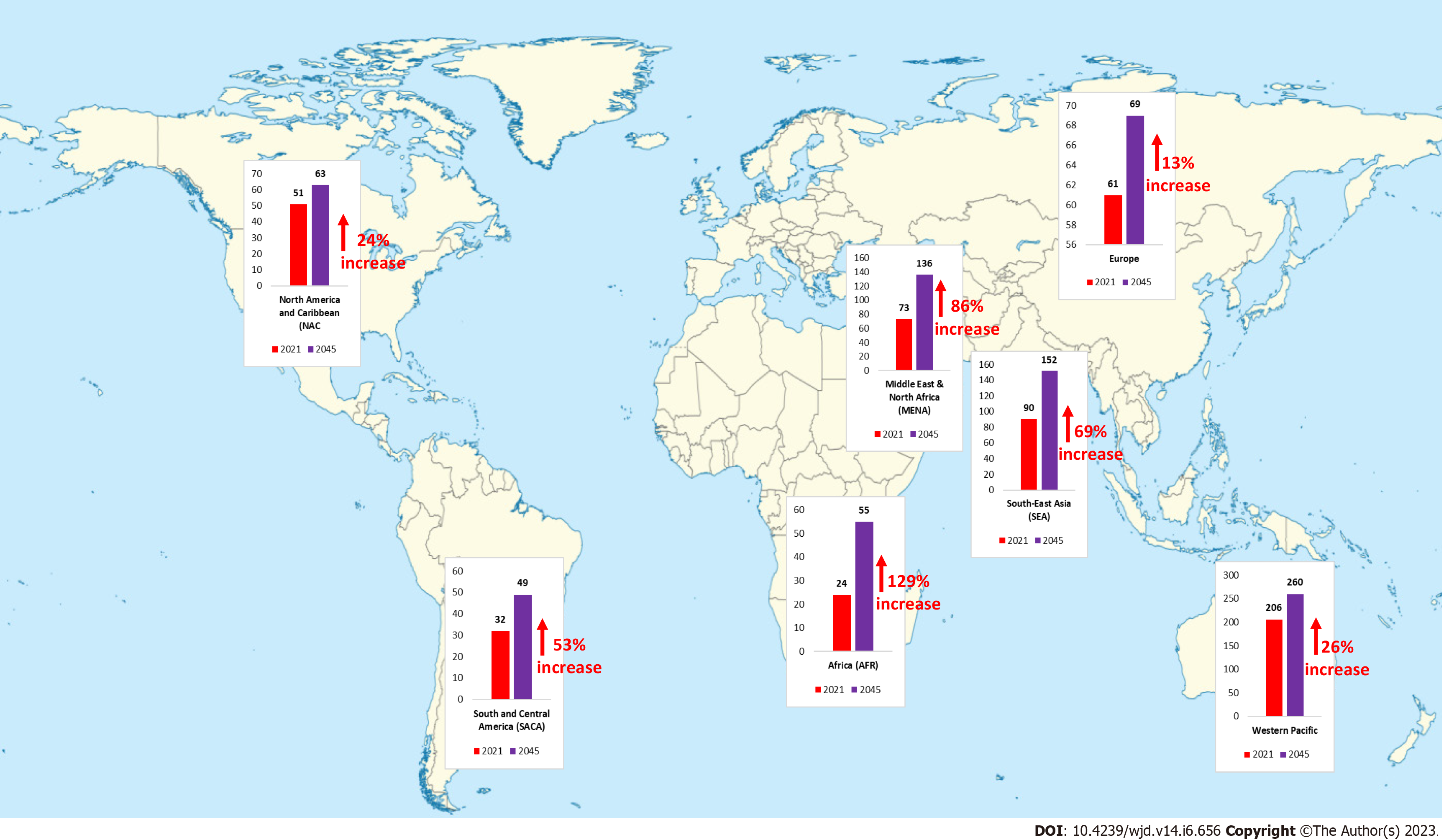
Diabetes is one of the fastest-growing global health emergencies of the 21st century (Figure 2). Diabetes affected around 537 million people in 2021, and this number is projected to reach 643 million by 2030 and 783 million by 2045, which is a nearly 46% increase in its prevalence[24]. Middle-income countries are expected to see the greatest percentage increase in the prevalence of diabetes, followed by high- and low-income countries. In 2021, there were approximately 8.4 million individuals worldwide with T1DM, of which 1.5 million were younger than 20 years of age. In 2040 the prevalence of T1DM has been predicted to increase to 13.5-17.4 million (60%-107% higher than in 2021)[25]. The frequency of the most common type of DM i.e., T2DM varies substantially by region, with low and middle-income countries accounting for almost 80% of all T2DM cases[26]. This variance in diabetes incidence across the globe may be attributable to environmental as well as lifestyle factors apart from underlying genetic components. Globally, the prevalence of GDM varies greatly (from 1% to 28%) depending on demo
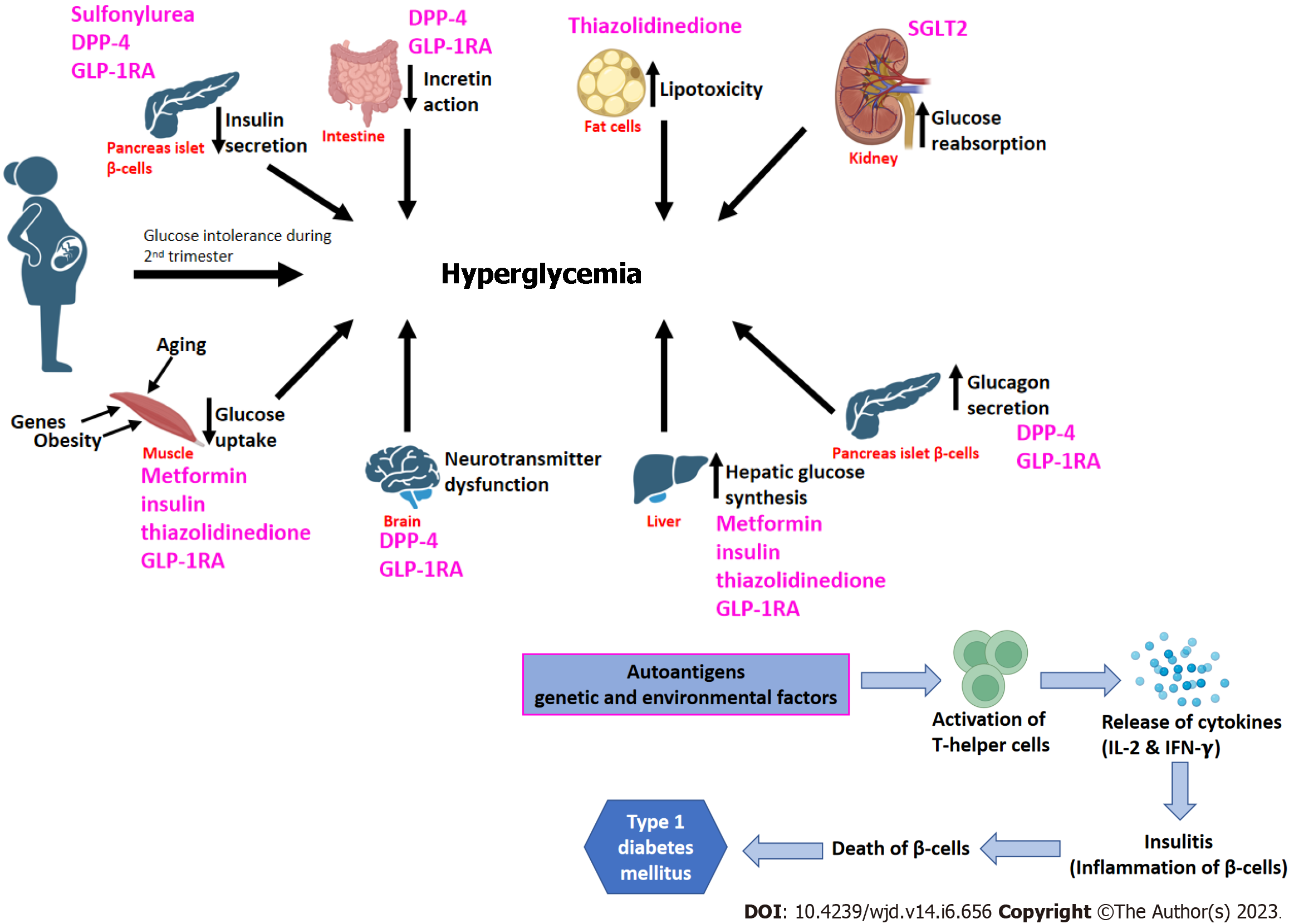
The pathogenesis of type 2 DM is influenced by eight key abnormalities described collectively as "the ominous octet"[27] (Figure 3). Reduced insulin secretion, decreased incretin action, increased lipolysis, increased glucose reabsorption, decreased glucose uptake, neurotransmitter dysfunction, increased hepatic glucose synthesis, and increased glucagon secretion are examples of these[27,28]. Therapy options for T2DM should target these documented pathophysiological abnormalities while also using a patient-centered approach that incorporates aspects other than glycemic control, such as lowering overall cardiovascular risk[29,30]. Recent research has indicated that during the progression of T2DM, pancreatic β-cells undergo dynamic compensation and decompensation processes, with metabolic stressors such as endoplasmic reticulum stress, oxidative stress, and apoptosis acting as major regulators of the β-cell dynamics[31].
T1DM is characterized by the autoimmune death of pancreatic beta cells produced by a T-cell-mediated inflammatory response, which results in decreased insulin production (Figure 3). On the other hand, in GDM, glucose intolerance develops usually in the second trimester which results in adverse impacts on both mother and offspring (Figure 3). MODY is caused by mutations in the GCK, HNF, and NEUROD1 genes, which are involved in glucose metabolism, insulin control, glucose transport, and fetal pancreas development.
Several pathways play a significant role in causing the microvascular and macrovascular complications associated with T2DM. Hexosamine biosynthetic pathway is implicated in the development of insulin resistance and diabetic vascular problems. It has been reported that hyperglycemia increases the production of transforming growth factor-beta, a prosclerotic cytokine implicated in the development of diabetic nephropathy[32]. The polyol pathway is a two-step metabolic mechanism that converts glucose to sorbitol and then to fructose[33,34]. It has long been assumed that the polyol pathway is almost silent under normal physiological conditions but becomes active and detrimental under hyperglycemic conditions. The protein kinase C pathway in diabetes promotes vascular contractility in an endo-thelium-independent way through K+ channel inactivation and Ca2+ sensitization of myofilaments in vascular smooth muscle cells[35]. The binding of advanced glycation end products to its receptor activates a range of signaling pathways, which further enhances oxidative stress, hence leading to nerve cell damage and apoptosis[36].
Family and twin studies have reported 20%-80% of heritability in diabetes. First-degree relatives of people with T2DM are three times more likely to get the disease than people without a positive family history[37]. Even though diabetes from both the maternal and paternal side increases the risk of acquiring diabetes, the Framingham Offspring research reported that offspring with maternal diabetes had a slightly higher risk of impaired glucose tolerance than those with paternal diabetes[24]. Multiple twin concordance studies in T2DM found that monozygotic twins had a greater concordance rate than dizygotic twins, indicating that the condition has a significant genetic component[37]. On the other hand for T1DM, monozygotic twins have a concordance rate of 40%-50% in population-based twin studies[38]. The following methods have been used to identify the diabetes risk gene.
Linkage analysis is based on the principle that genetic sequences located on the same chromosome tend to be inherited together and are not separated during meiotic homologous recombination. It is typically used in family studies to determine the position of an associated variant(s)[39,40]. Linkage studies have successfully uncovered genetic variations that cause monogenic diseases such as MODY[41]. In 1996, using linkage analysis, major histocompatibility complex loci (HLA) on chromosome 6 were identified as the genetic susceptibility loci for T1DM[42]. In 2004, the calpain-10 gene (CAPN10) on chromosome 2 was identified as the cause of T2DM using genome-wide screening and positional cloning[43,44]. TCF7L2, the now well-known T2DM gene, was mapped to chromosome 10 in a Mexican-American group in the year 1999 and has been replicated several times in T2DM genome-wide association studies (GWAS)[45,46]. TCF7L2 plays an important role in the Wnt/β-catenin signaling pathway and helps in regulating the expression of genes in lipid metabolism in adipocytes and glucose-induced insulin exocytosis.
It is a hypothesis-driven method in which candidate genes are chosen based on prior knowledge such as a gene's biological function, position, or probable significance about a given phenotype[47]. This method is usually more suitable in studies where individuals are unrelated[48]. Candidate gene studies revealed an association between T2DM and insulin receptor substrate 1 (IRS1), peroxisome proliferator-activated receptor gamma (PPARG), and insulin receptor substrate 2 (IRS2), Wolfram syndrome 1 (wolframin) (WFS1), potassium inwardly-rectifying channel, subfamily J, member 11 (KCNJ11), HNF1 homeobox A (HNF1A), and HNF1 homeobox B (HNF1B)[49]. By association studies for T1DM, four non-HLA genes with established risk loci [HLA, INS (insulin), CTLA4 (cytotoxic T-lymphocyte antigen 4), PTPN22][50] could be identified. Of all the genes identified for gestational DM; TCF7L2, MTNR1B, CDKAL1, IRS1, and KCNQ1 candidate genes are the most common, whereas other identified genes are ethnic-specific. On the other hand, MODY is inherited in an autosomal dominant pattern and manifests itself as a result of mutations in transcription factor genes such as HNF4 (hepatocyte nuclear factor), HNF1, IPF1 (insulin promoter factor), and neuro-D1[51,52].
GWAS are large-scale hypothesis-free investigations that entail the fast scanning of genetic variants (SNPs on genotyping arrays) across the complete human genome to uncover unique genetic associations with a certain trait[53]. The initial T2DM-related GWAS studies identified hematopoietic expressed homeobox (HHEX), solute carrier family 30 member 8 (SLC30A8), cyclin-dependent kinase inhibitor 31 2A/2B (CDKN2A/2B), insulin-like growth factor 2 mRNA binding protein 2 (IGF2BP2), CDK5 regulatory subunit associated protein 1 Like 1 (CDKAL1), and FTO alpha-ketoglutarate (FTO)[54-58]. Approximately 250 significant susceptibility loci for T2DM have been identified to date (https://www.ebi.ac.uk/gwas/efotraits/MONDO_0005148). On the other hand, for T1DM by GWAS more than 60 loci have so far been discovered (https://www.ebi.ac.uk/gwas/efotraits/MONDO_0005147), revealing the pathways underlying the disease, and overlaps with autoimmune diseases[59]. GWAS in T1DM has not only verified the previously reported T1DM loci but also uncovered several novel variations, such as those near the KIAA0350 (CLEC16A approved symbol)[60] gene and with UBASH3A (ubiquitin-associated and SH3 containing A)[61]. To our knowledge, to date, only three GWAS have been conducted for GDM[62-64]. Kwak et al[62] identified two significant GDM variants, rs7754840 and rs10830962 in the intronic region of CDKAL1, and upstream of MTNR1B, respectively. On the other hand, Wu et al[63] identified 23 SNPs in four genes: CTIF, CDH18, PTGIS, and SYNPR to be associated with GDM. Recently, Pervjakova et al[64] through multi-ancestry meta-analysis reported five loci (mapping to/near MTNR1B, TCF7L2, CDKAL1, CDKN2A-CDKN2B, and HKDC1) through genome-wide association studies for GDM. Using a meta-analysis approach, the genetic architecture of T1DM and T2DM has been determined in many populations with different ethnic backgrounds[65-74].
There are many challenges to the GWAS approach. The current GWAS genotyping arrays are based on HapMap and the 1000 genome project dataset, and these are designed to target common SNPs (MAF > 5%). As a result, the prior GWAS did not directly investigate rare variants for an association with the trait[75]. Also, the observed variants that are linked to the trait may not be the causal variations, but rather be in linkage disequilibrium with the causal variants. Furthermore, since the variant is often located outside the coding regions and may affect genes and regulatory elements at a distance, it is usually difficult to understand how the variant affects the trait.
The 'common disease, rare variant' hypothesis, in contrast to the standard 'common disease, common variant' paradigm, says that many rare genetic variations with relatively high penetrance play a significant influence in the elevated risk of common diseases[76]. Huyghe et al[77] for the first time in 2013 investigated the significance of low-frequency variants (minor allele frequency < 5%) associated with the risk of T2DM or T2DM-related traits using the Illumina exome array technique. Two low-frequency variants in SGSM2 and MADD were reported to be associated with fasting proinsulin concentrations and three novel variants in TBC1D30, KANK1, and PAM genes were reported with proinsulin or insulinogenic index. Later in 2014, Steinthorsdottir et al[68] using an exome sequencing technique in the Icelandic population, reported three more T2DM-associated low-frequency variants in CCND2, PAM, and PDX1. In the following years, rare variants in MTNR1B, HNF1, and G6PC2 genes were also reported to be associated with T2DM or T2D-related traits[78]. Nejentsev et al[79] reported four rare variants (rs35667974, rs35337543, rs35732034, and rs35744605) in IFIH1, a gene previously discovered in T1DM GWAS. Additionally, a cluster of rare detrimental variations in PTPN22 was identified for T1DM, comprising two novel frameshift mutations (rs538819444 and rs371865329) and two missense variants (rs74163663 and rs56048322)[80].
The term "epigenetics" refers to heritable alterations in gene function that occur without a change in the nucleotide sequence. Epigenetic changes can be inherited from one cell generation to the next and in some cases, can be inherited through the generations. Epigenetic changes can also develop during life, either randomly or in response to environmental stimuli, impacting the effects of genetic variants and so acting as a gene-environment interaction mechanism. Both DNA methylation and histone modifications can amend the response of our genome to the environment during life. The involvement of intrauterine DNA methylation and imprinting in the programming of diabetogenic effects later in life has received significant interest in the etiology of the T2DM[81]. An intriguing study by Dabelea et al[82] found that intrauterine diabetes exposure increased the incidence of diabetes and obesity in offspring compared to siblings born before their mothers' diabetes onset. However, the precise mechanism underlying this maternal impact is unknown. Some studies have suggested a role of epigenetic regulation of genes involved in energy metabolism, appetite control, and -cell function, such as PPARA[83], LEP[84], and pancreatic and duodenal homeobox 1 (PDX1)[85].
MicroRNAs (miRNAs) have emerged as promising novel biomarkers for T2DM and related problems due to their metabolic stability and abundance in various body fluids including blood and cerebrospinal fluid. miRNAs are a class of endogenous, small (18-25 nucleotide) RNA that regulates many cellular activities by suppressing gene expression[86]. According to recent research, differential concentrations of circulating miRNAs (Table 1)[87-128] may offer the intriguing potential for diabetes (T1DM, T2DM, MODY, and GDM) diagnosis, prognosis, and treatment monitoring.
| Mechanism/pathway (diabetes type) | Expression of miRNAs | Ref. |
| Endothelial dysfunction (T2DM) | ↑miR-28-3p | [87] |
| ↓miR-24 | ||
| ↓miR-21 | ||
| ↓miR-20b | ||
| ↓miR-15a | ||
| ↓miR-126 | ||
| ↓miR-191 | ||
| ↓miR-197 | ||
| ↓miR-223 | ||
| ↓miR-320 | ||
| ↓miR-486 | ||
| ↓miR-150 | ||
| ↓miR-29b | ||
| ↓miR-107 | ||
| ↓miR-132 | ||
| ↓miR-144 | ||
| Glucose metabolism (T2DM) | ↑miR-9 | [88] |
| ↑miR-29a | ||
| ↑miR-30d | ||
| ↑miR-34a | ||
| ↑miR-124a | ||
| ↑miR-146a | ||
| ↑miR-375 | ||
| Inflammation (T2DM) | ↓miR-146a | [89] |
| Glucose metabolism (T2DM) | ↑miR-27a | [90] |
| ↑miR-320a | ||
| Glucose metabolism (T2DM) | ↓miR-126 | [91-93] |
| Inflammation (T2DM) | ↓miR-103b | [94] |
| Inflammation (T2DM) | ↓miR-126-3p | [95] |
| ↓miR-21-5p | ||
| Inflammation (T2DM) | ↓miR-126 | [96] |
| Endothelial dysfunction (T2DM) | ↓miR-126 | [97] |
| ↓miR-26a | ||
| Glucose metabolism (T2DM) | ↓miR-21 | [98] |
| Inflammation (T2DM) | ↓miR-126-3p | [99] |
| Endothelial dysfunction (T2DM) | ↓miR-24 | [100] |
| Platelet reactivity (T2DM) | ↓miR-223 | [101] |
| ↓miR-26b | ||
| ↓miR-126 | ||
| ↓miR-140 | ||
| Glucose metabolism (T2DM) | ↑miR-375 | [102] |
| ↑miR-9 | ||
| Glucose metabolism (T2DM) | ↑miR-30a-5p | [103] |
| ↑miR-150 | ||
| ↓miR-103 | ||
| ↓miR-28-3p | ||
| ↓miR-29a | ||
| ↓miR-9 | ||
| ↓miR-15a | ||
| ↓miR-126 | ||
| ↓miR-145 | ||
| ↓miR-375 | ||
| ↓miR-223 | ||
| ↓miR-133 | ||
| ↓miR-107 | ||
| Endothelial dysfunction (miR-126); hypoxia (miR-210) (T2DM) | ↓miR-126 | [104] |
| ↑miR-210 | ||
| Angiogenesis (T2DM) | ↑miR-193b-3p | [105] |
| ↑let-7i-5p | ||
| ↑miR-199a-3-5p | ||
| ↑miR-26b-5p | ||
| ↑miR-30b-5p | ||
| ↑miR-374a-5p | ||
| ↑miR-20a-3p | ||
| ↑miR-26a-5p | ||
| ↑miR-30c-5p | ||
| ↓miR-409-3p | ||
| ↓miR-95-3p | ||
| Apoptosis (T1DM) | ↑miR-21 | [106,107] |
| ↓miR-23a-3p | [108] | |
| ↓miR-23b-3p | ||
| ↓miR-149-5p | ||
| Inflammation (T1DM) | ↑miR-101a | [109] |
| ↑miR-30b | ||
| -cell dysfunction (T1DM) | ↑miR-106b-5p | [110,111] |
| ↑miR-222-3p | ||
| ↑miR-181a | ||
| T-cell dysfunction (T1DM) | ↑miR-26a | [112] |
| ↑miR-98 | [113] | |
| ↑miR-23b | ||
| ↑miR-590-5p | ||
| -cell lymphopoiesis (T1DM) | ↑miR-34a | [114] |
| DNA damage checkpoint (T1DM) | ↑miR-200 | [115] |
| Apoptosis (T1DM) | ↓miR-144 | [116] |
| Autoimmune imbalance (T1DM) | ↓miR-146a | [117] |
| MODY | ↑miR-103 | [118] |
| MODY | ↑miR-224 | |
| Glucose metabolism (GDM) | ↑miR-222 | [119] |
| ↑miR-98 | [120] | |
| ↑miR-518d | [121] | |
| ↑miR-340 | [122] | |
| ↑miR-130b, miR148a | [123] | |
| -cell dysfunction (GDM) | ↑miR-33a-5p | [124] |
| ↑miR-330-3p | [125] | |
| ↓miR-494 | [126] | |
| ↓miR-96 | [127] | |
| ↓miR-221 | [128] |
Since, T2DM is the most common form of diabetes, hence most of the polygenic risk scores (PRSs) studies have been performed on T2DM. GWAS investigations have enabled the development of PRSs or genetic risk score (GRS) that assess an individual's lifetime genetic risk for various diseases. Several studies on coronary artery disease have been reported[129-132], however, there is a scarcity of reports on the prediction models for diabetes (T1DM, T2DM, and GDM). The area under the receiver operating characteristics curve is a measure of the prediction accuracy of the constructed PRS[133]. One of the first research estimated a T2DM GRS using a combination of 18 loci and reported that genetic information only marginally improved risk prediction when paired with standard clinical risk factors such as age, gender, or diabetes family history[134-136] (Table 2). There has been a rise of interest in GRS in recent years, utilizing many more loci reported from large-scale, multi-ancestry cohorts. T2DM GRS studies from large datasets[137-139] reported that GRS constructed from multi-ethnic computed weights indicated a marginal increase in predictive power as compared to single-ancestry computed weights, the reason might be heterogeneity across different ancestries (Table 2)[140-149].
| Diabetes type | SNPs | AUC for PRS | Ethnicity | Ref. |
| T1DM | 41 | 0.87 | Caucasian | [140] |
| T1DM | 30 | 0.88 | Caucasian | [141] |
| T1DM + T2DM | 99 | 0.89 | Caucasian | |
| T1DM | 32 | 0.86 | Caucasian | [142] |
| T1DM | 32 | 0.90 | Caucasian Hispanic | |
| T1DM | 32 | 0.75 | African-American | |
| T1DM | 32 | 0.92 | Asian-American | |
| T1DM | 67 | 0.93 | Caucasian | [143] |
| T2DM | 3 | 0.58 | Caucasian | [144] |
| T2DM | 18 | 0.80 | Caucasian | [136] |
| T2DM | 16 | 0.75 | Caucasian | [134] |
| T2DM | 18 | 0.91 | Caucasian | [135] |
| T2DM | 22 | 0.74 | Caucasian | [145] |
| T2DM | 62 | 0.91 | Caucasian United States population | [146] |
| T2DM | 1000 | 0.79 | Caucasian | [147] |
| T2DM | 4 | 0.67 | African | [148] |
| T2DM | 7 million | 0.73 | Caucasian | [149] |
PRSs have also been demonstrated to predict pre-diabetes and T2DM in women with a history of GDM (Table 3)[150-153]. Some studies have found that using a PRS in conjunction with traditional T2DM risk factors improves discrimination of the risk of pre-diabetes in women with prior GDM, potentially giving more accurate tools for the prediction of future T2DM.
GRS, on the other hand, may have a role in recognizing high-risk patients before clinical risk markers become apparent. It needs to be shown whether GRS data can drive preventive therapy to meaningfully reduce rates of future incident T2DM.
In the long term, the pharmacological strategy for treating diabetes may be only partially effective. Major changes in patients' lifestyles (change in physical activity, dietary alteration, stress management, and improved sleeping patterns), along with treatments through pharmacological techniques, are required to ensure optimal disease management. Self-monitoring of blood glucose is an excellent tool for monitoring glycemic status. Current American Diabetes Association (ADA) guidelines urge its use in all patients with T1DM, T2DM, or any other form of diabetes (e.g., gestational diabetes) that requires numerous subcutaneous insulin injections[154]. Continuous glucose monitoring systems i.e., Dexcom G6, Frestyle Libre 1 and 2, GlucoMen day, Eversense, Eversense XL, S7 EasySense, Guardian, and Connect have been reported to be of great use to diabetics. Insulin pens are the most often utilized method of insulin administration in T2DM patients[155]. Users can track boluses, calculate remaining insulin, check insulin temperature, and receive dosage reminders using Bluetooth-enabled insulin pen caps and attachments that connect to smartphone apps[156]. The integration of insulin pumps with other diabetes technologies developed over the last decade has paved the way for techniques of optimally regulating blood glucose while minimizing user stress. For the management of LADA C-peptide levels should be monitored every 6 mo. For KPDM patients lifestyle modifications as stated above have been proposed to successfully treat the disease.
In addition to the above-mentioned methods, the following steps can be taken to control blood sugar levels.
Physical exercise is positively associated with controlled hyperglycemia levels among T2DM patients. Moderate physical activity (walking, gardening, regular household chores) on a regular basis has been shown to be an effective method to reducing the long-term symptoms of diabetes[157]. In women with type 2 diabetes, yoga practice is more beneficial than the same course of aerobic exercise in enhancing sleep quality, hence, yoga activity can thus be recommended to these patients[158]. The identification of cytokines such as irisin, osteocalcin, and adiponectin has led to the assumption that they may be important hormonal mediators of exercise therapy for diabetes and metabolic illnesses, although the precise mechanism remains unknown[159-161].
Strict adherence to a restricted diet combined with adequate physical exercise is strongly linked to a lower incidence of diabetes[162]. The incorporation of a Paleolithic diet (a diet rich in lean meat, fish, fruits, and vegetables) into the daily routine of diabetic patients resulted in a significant improvement in glucose management[163]. Foods that are naturally abundant in dietary fiber also contain a variety of chemicals that may help decrease glycemia. For example, bioactive proteins, polyphenolic compounds, and other phytochemicals[164]. Additionally, according to current research, meal timing and frequency, missing meals, and fasting are all linked to metabolic syndrome. Eating frequently and in the morning may help to prevent metabolic syndrome. Understanding the impact of dietary choices on health is just as important as understanding the impact of nutrients on health.
The bulk of T2DM and T1DM-related parameters, including the release of glucose (and lipids) in circulation, the development of inflammatory cytokines, and raised blood pressure, are heavily influenced by psychological stress[165]. The underlying mechanisms entail a complex neuroendocrine structure that includes both the central nervous system and the peripheral nervous system. In one study, when type 2 diabetes patients were subjected to acute stress during the postprandial period, significant increases in blood glucose levels were seen[166]. Treatment options, including stress management therapies, appear to be a promising approach for effectively preventing or reducing type 2 diabetes incidence.
Another modifiable lifestyle choice that has been shown to influence metabolic health and energy status is sleep. Sleeping pattern optimization is critical in the diabetes management[167]. According to a population-based study, short sleep (less than 5 h) or insomnia is related to an elevated risk of T2DM[168]. Poor sleep was linked to increased glycated hemoglobin (HbA1c) levels (> 7%) and insulin resistance in T2DM patients in previous research[167]. Similar results has been observed for T1DM also, where persons with T1DM who reported sleeping more than 6 h had 0.24% lower A1C values than those who slept less than 6 h[169].
The one step or two step techniques are used to diagnose gestational DM. The one step method consists of a 2-h oral glucose tolerance test with a 75-g glucose overload that examines plasma glucose concentration at fasting, 1 h, and 2 h following glucose delivery. A positive result is characterized as a number more than 92, 180, or 153 mg/dL[170-172]. The two-step method comprises a nonfasting oral 50-g glucose load followed by a glucose blood measurement 1 h later. A positive result is defined as a blood glucose level greater than 130, 135, or 140 mg/dL; the most used number is 135 mg/dL. A diagnostic test is performed after a positive screening test[173].
Pharmacogenomics is the process of developing a genetically personalized therapy strategy to obtain the best optimal individual response. Several polymorphisms in the genes i.e., ABCC8, KCNJ11, TCF7L2, CYP2C9, IRS1, CDKAL1, CDKN2A, CDKN2B, KCNQ1, NOS1AP, and CAPN10 have been explored in recent years in relation to the therapeutic response of various anti-diabetic medicines[174]. The American Association of Clinical Endocrinologists/American College of Endocrinology and the ADA in addition to metformin had proposed four oral options (sulfonylurea, thiazolidinedione, dipeptidyl peptidase-4 inhibitor, sodium-glucose cotransporter 2 inhibitor) and injectable agents (glucagon-like peptide-1 receptor agonist or basal insulin) for lowering blood glucose levels (Figure 3). Although these drugs have important therapeutic effects on diabetes, their long-term impact has not been accomplished, and their responses in individuals also display variances[175,176]. Moreover, some agents produce adverse side effects, such as hypoglycemia, weight gain, gastrointestinal discomfort, urogenital infections, discomfort at the injection site, and in some cases heart failure[177].
It is important to identify and develop novel targets to improve the therapeutic efficacy of present anti-diabetic medications, reduce the risk of side effects, and even reverse the development of diabetes. Many potential antidiabetic drugs i.e., Dorzagliatin (glucokinase activators), BI 135585 [b-hydroxysteroid dehydrogenase-1 inhibitors (11-b-HSD1 inhibitors)], DS-8500a (G-protein-coupled receptor 119 agonists), and PF-06291874/LGD-6972 (glucagon receptor antagonists) with new targets are currently undergoing clinical trials. These drugs may become new diabetes treatment options and provide more therapeutic alternatives for diabetes patients.
There is growing evidence that vitamin D insufficiency may play a critical role in the T2DM etiology[178]. Thus, in a randomized controlled study, the oral daily doses of vitamin D supplementation with metformin significantly reduced HbA1c levels after 3 and 6 mo of supplementation, compared to the metformin alone[179].
In diabetic patients, monotherapies combined with herbal extracts or phytoconstituents demonstrated significant improvements in blood glucose levels. Plant-derived chemical compounds have also proven to be potential alternatives. Table 4[180-194] shows the known effects of various phytoconstituents on diabetes. Diabetes can be managed using either nonpharmacological (reasonable diet and exercise) or pharmacological (drugs or insulin) techniques. However, T2DM medication is expensive for patients and has substantial adverse effects. Plants appear to offer an appealing alternative to traditional diabetes treatment. They comprise complex compounds including many natural bioactive principles with less adverse effects.
| Phytochemical | Source | Outcomes | Ref. |
| Curcumin | Curcuma longa | ↑Insulin sensitivity, ↓blood glucose levels, and hypoglycemia | [180] |
| Rutin | Buckwheat (Fagopyrum esculentum) | ↓Hepatic glucose production, ↑glucose tolerance | [181] |
| Resveratrol | Grapes, plums, peanuts, nuts, red wine | Improved insulin signaling, ↑glucose-mediated insulin secretion | [182] |
| Quercetin | Apples, black tea, berries, capers, red wine, onions | ↑Glucose uptake, ↓hepatic glucose production | [182,183] |
| Genistein | Legumes | Improved lipid glucose metabolism and ↓fasting glucose | [184] |
| Hesperidin | Orange, lemon | ↑Glucose uptake, ↓HbA1c, ↓oxidative stress | [185] |
| Naringin | Skin of grapefruit and orange | ↓Hepatic glucose production, ↓oxidative stress, ↑glucose uptake | [185] |
| Naringenin | Citrus fruits, tomatoes, cherries, grapefruit, cocoa | ↑Glucose uptake, ↓glucose intolerance and reduced blood glucose levels | [186] |
| Vitamin A, D, and E | Eggs, yellow, red, and green (leafy) vegetables, such as spinach, carrots, sweet potatoes and red peppers. yellow fruit, such as mango, papaya and apricots | ↓Glucose intolerance, ↓hyperglycemia | [182] |
| Fisetin | Strawberry, apple, persimmon, grape, onion, and cucumber | ↓Hepatic glucose and ↑glucose metabolism | [187] |
| Flavonoids | Coffee, guava tea, whortleberry, olive oil, propolis, chocolate, and cocoa | ↓Glucose absorption, inhibition of advanced glycation end products | [188] |
| Isoflavones | Soybean | Improves glucose metabolism | [189] |
| Catechins | Tea leaves and red wine | Promote insulin sensitivity | [190] |
| Hydroxycinnamic acids | Fruits and vegetables, especially the outer part of ripe fruits | Promote glucokinase activity | [191] |
| Caffeoylquinic | Potatoes, eggplants, peaches, prunes, and coffee beans | Promote insulin response | [192] |
| Anthocyanins and anthocyanidins | Berries, eggplants, avocado, oranges, olives, red onion, fig, sweet potato, mango, and purple corn | Promote blood glucose regulation | [193] |
| Stillbenoids | Grapevine, berries, and peanuts | Promote pancreatic -cell and hepatoprotective activity | [194] |
Diabetes pathogenesis encompasses genetic, epigenetic, and environmental variables and their interactions. To date, the examined common variations can explain just a small portion of the heritability of diabetes. Furthermore, the technique of integrating the associated variants as a type of GRS does not accurately predict diabetes risk. As a result, the trend for genetic risk factors for diabetes is shifting from common to rare variants. Aside from genetic variables, systemic data from other trans-omics such as epigenomics, transcriptomics, proteomics, metabolomics, and metagenomics will contribute to a better understanding of genetic determinants in the progression of metabolic illnesses like diabetes. Technological, computational, and collaborative developments continue to uncover novel genetic diabetes risk factors. There are high prospects for tailored diabetes treatment in the future, based on increased knowledge of the molecular genetic profile of the patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cai L, United States; Nagamine T, Japan; Vorobjova T, Estonia; Zeng Y, China S-Editor: Li L L-Editor: A P-Editor: Guo X
| 1. | Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3804] [Cited by in RCA: 3688] [Article Influence: 153.7] [Reference Citation Analysis (0)] |
| 2. | American Diabetes Association. Standards of medical care in diabetes--2009. Diabetes Care. 2009;32 Suppl 1:S13-S61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1215] [Cited by in RCA: 1237] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 3. | Kutsuma A, Nakajima K, Suwa K. Potential Association between Breakfast Skipping and Concomitant Late-Night-Dinner Eating with Metabolic Syndrome and Proteinuria in the Japanese Population. Scientifica (Cairo). 2014;2014:253581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes 1. 2008;26:77-82. [RCA] [DOI] [Full Text] [Cited by in Crossref: 947] [Cited by in RCA: 1055] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 5. | Kwak SH, Park KS. Genetic Studies on Diabetic Microvascular Complications: Focusing on Genome-Wide Association Studies. Endocrinol Metab (Seoul). 2015;30:147-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Kirigia JM, Sambo HB, Sambo LG, Barry SP. Economic burden of diabetes mellitus in the WHO African region. BMC Int Health Hum Rights. 2009;9:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (35)] |
| 7. | Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5513] [Cited by in RCA: 5461] [Article Influence: 195.0] [Reference Citation Analysis (0)] |
| 8. | Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, Bell R, Badaru A, Talton JW, Crume T, Liese AD, Merchant AT, Lawrence JM, Reynolds K, Dolan L, Liu LL, Hamman RF; SEARCH for Diabetes in Youth Study. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1078] [Cited by in RCA: 1018] [Article Influence: 92.5] [Reference Citation Analysis (1)] |
| 9. | Sena CM, Bento CF, Pereira P, Seiça R. Diabetes mellitus: new challenges and innovative therapies. EPMA J. 2010;1:138-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (2)] |
| 10. | Druet C, Tubiana-Rufi N, Chevenne D, Rigal O, Polak M, Levy-Marchal C. Characterization of insulin secretion and resistance in type 2 diabetes of adolescents. J Clin Endocrinol Metab. 2006;91:401-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Wolfs MG, Hofker MH, Wijmenga C, van Haeften TW. Type 2 Diabetes Mellitus: New Genetic Insights will Lead to New Therapeutics. Curr Genomics. 2009;10:110-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Lemos Costa TMR, Detsch JM, Pimazoni-Netto A, de Almeida ACR, Sztal-Mazer S, de Oliveira LMT, Nascimento DJ, Réa RR. Glycemic variability and mean weekly glucose in the evaluation and treatment of blood glucose in gestational diabetes mellitus; evidence for lower neonatal complications. J Diabetes Metab. 2011;2. [DOI] [Full Text] |
| 13. | Philips JC, Emonts P, Pintiaux A, Kirkpatrick C, Scheen AJ. [Management of gestational diabetes]. Rev Med Liege. 2013;68:489-496. [PubMed] |
| 14. | Buchanan TA, Xiang AH, Page KA. Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol. 2012;8:639-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 488] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 15. | McDonald TJ, Colclough K, Brown R, Shields B, Shepherd M, Bingley P, Williams A, Hattersley AT, Ellard S. Islet autoantibodies can discriminate maturity-onset diabetes of the young (MODY) from Type 1 diabetes. Diabet Med. 2011;28:1028-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Guglielmi C, Palermo A, Pozzilli P. Latent autoimmune diabetes in the adults (LADA) in Asia: from pathogenesis and epidemiology to therapy. Diabetes Metab Res Rev. 2012;28 Suppl 2:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Naik RG, Brooks-Worrell BM, Palmer JP. Latent autoimmune diabetes in adults. J Clin Endocrinol Metab. 2009;94:4635-4644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Buzzetti R, Tuomi T, Mauricio D, Pietropaolo M, Zhou Z, Pozzilli P, Leslie RD. Management of Latent Autoimmune Diabetes in Adults: A Consensus Statement From an International Expert Panel. Diabetes. 2020;69:2037-2047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 134] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 19. | Patil SP. Atypical Diabetes and Management Considerations. Prim Care. 2022;49:225-237. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Nyenwe EA, Kitabchi AE. The evolution of diabetic ketoacidosis: An update of its etiology, pathogenesis and management. Metabolism. 2016;65:507-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 21. | Kitabchi AE. Ketosis-prone diabetes--a new subgroup of patients with atypical type 1 and type 2 diabetes? J Clin Endocrinol Metab. 2003;88:5087-5089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Umpierrez GE, Casals MM, Gebhart SP, Mixon PS, Clark WS, Phillips LS. Diabetic ketoacidosis in obese African-Americans. Diabetes. 1995;44:790-795. [PubMed] [DOI] [Full Text] |
| 23. | Vellanki P, Umpierrez GE. Diabetic ketoacidosis: A common debut of diabetes among african americans with type 2 diabetes. Endocr Pract. 2017;23:971-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3033] [Cited by in RCA: 4762] [Article Influence: 1587.3] [Reference Citation Analysis (36)] |
| 25. | Gregory GA, Robinson TIG, Linklater SE, Wang F, Colagiuri S, de Beaufort C, Donaghue KC; International Diabetes Federation Diabetes Atlas Type 1 Diabetes in Adults Special Interest Group, Magliano DJ, Maniam J, Orchard TJ, Rai P, Ogle GD. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol. 2022;10:741-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 498] [Article Influence: 166.0] [Reference Citation Analysis (0)] |
| 26. | Deshmukh C, Jain A, Nahata BR. Diabetes Mellitus: A Review. Indian J. Pure Appl. Biosci. 2015;3:224-230. |
| 27. | Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1971] [Cited by in RCA: 1952] [Article Influence: 122.0] [Reference Citation Analysis (0)] |
| 28. | Schwartz SS, Epstein S, Corkey BE, Grant SF, Gavin JR 3rd, Aguilar RB. The Time Is Right for a New Classification System for Diabetes: Rationale and Implications of the β-Cell-Centric Classification Schema. Diabetes Care. 2016;39:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 208] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 29. | Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, DeFronzo RA, Einhorn D, Fonseca VA, Garber JR, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez GE. Consensus statement by the american association of clinical endocrinologists and american college of endocrinology on the comprehensive type 2 diabetes management algorithm - 2017 executive summary. Endocr Pract. 2017;23:207-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 333] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 30. | Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1872] [Cited by in RCA: 1899] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 31. | Lv C, Sun Y, Zhang ZY, Aboelela Z, Qiu X, Meng ZX. β-cell dynamics in type 2 diabetes and in dietary and exercise interventions. J Mol Cell Biol. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 32. | Schleicher ED, Weigert C. Role of the hexosamine biosynthetic pathway in diabetic nephropathy. Kidney Int Suppl. 2000;77:S13-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 174] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | HERS HG. The mechanism of the transformation of glucose in fructose in the seminal vesicles. Biochim Biophys Acta. 1956;22:202-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 130] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6145] [Cited by in RCA: 6193] [Article Influence: 258.0] [Reference Citation Analysis (0)] |
| 35. | Kizub IV, Klymenko KI, Soloviev AI. Protein kinase C in enhanced vascular tone in diabetes mellitus. Int J Cardiol. 2014;174:230-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Kay AM, Simpson CL, Stewart JA Jr. The Role of AGE/RAGE Signaling in Diabetes-Mediated Vascular Calcification. J Diabetes Res. 2016;2016:6809703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 230] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 37. | Ali O. Genetics of type 2 diabetes. World J Diabetes. 2013;4:114-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 188] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (23)] |
| 38. | Kyvik KO, Green A, Beck-Nielsen H. Concordance rates of insulin dependent diabetes mellitus: a population based study of young Danish twins. BMJ. 1995;311:913-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 205] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 39. | Ott J, Wang J, Leal SM. Genetic linkage analysis in the age of whole-genome sequencing. Nat Rev Genet. 2015;16:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 192] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 40. | Hattersley AT, Turner RC, Permutt MA, Patel P, Tanizawa Y, Chiu KC, O'Rahilly S, Watkins PJ, Wainscoat JS. Linkage of type 2 diabetes to the glucokinase gene. Lancet. 1992;339:1307-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 247] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 41. | Vaxillaire M, Froguel P. Genetic basis of maturity-onset diabetes of the young. Endocrinol Metab Clin North Am. 2006;35:371-384, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | She JX, Marron MP. Genetic susceptibility factors in type 1 diabetes: linkage, disequilibrium and functional analyses. Curr Opin Immunol. 1998;10:682-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Song Y, Niu T, Manson JE, Kwiatkowski DJ, Liu S. Are variants in the CAPN10 gene related to risk of type 2 diabetes? A quantitative assessment of population and family-based association studies. Am J Hum Genet. 2004;74:208-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Tripathy D, Eriksson KF, Orho-Melander M, Fredriksson J, Ahlqvist G, Groop L. Parallel manifestation of insulin resistance and beta cell decompensation is compatible with a common defect in Type 2 diabetes. Diabetologia. 2004;47:782-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Duggirala R, Blangero J, Almasy L, Dyer TD, Williams KL, Leach RJ, O'Connell P, Stern MP. Linkage of type 2 diabetes mellitus and of age at onset to a genetic location on chromosome 10q in Mexican Americans. Am J Hum Genet. 1999;64:1127-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 243] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 46. | Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, Styrkarsdottir U, Magnusson KP, Walters GB, Palsdottir E, Jonsdottir T, Gudmundsdottir T, Gylfason A, Saemundsdottir J, Wilensky RL, Reilly MP, Rader DJ, Bagger Y, Christiansen C, Gudnason V, Sigurdsson G, Thorsteinsdottir U, Gulcher JR, Kong A, Stefansson K. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1578] [Cited by in RCA: 1580] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 47. | Teare MD. Candidate gene association studies. Methods Mol Biol. 2011;713:105-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 48. | Kwon JM, Goate AM. The candidate gene approach. Alcohol Res Health. 2000;24:164-168. [PubMed] |
| 49. | Barroso I, Luan J, Middelberg RP, Harding AH, Franks PW, Jakes RW, Clayton D, Schafer AJ, O'Rahilly S, Wareham NJ. Candidate gene association study in type 2 diabetes indicates a role for genes involved in beta-cell function as well as insulin action. PLoS Biol. 2003;1:E20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 205] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 50. | Rich SS. Genetics and its potential to improve type 1 diabetes care. Curr Opin Endocrinol Diabetes Obes. 2017;24:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27 Suppl 1:S5-S10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 955] [Cited by in RCA: 1010] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 52. | Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4004] [Cited by in RCA: 3876] [Article Influence: 84.3] [Reference Citation Analysis (1)] |
| 53. | Bush WS, Moore JH. Chapter 11: Genome-wide association studies. PLoS Comput Biol. 2012;8:e1002822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 738] [Cited by in RCA: 666] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 54. | Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJ, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CN, Doney AS, Morris AD, Smith GD, Hattersley AT, McCarthy MI. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889-894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3537] [Cited by in RCA: 3201] [Article Influence: 177.8] [Reference Citation Analysis (0)] |
| 55. | Dina C, Meyre D, Gallina S, Durand E, Körner A, Jacobson P, Carlsson LM, Kiess W, Vatin V, Lecoeur C, Delplanque J, Vaillant E, Pattou F, Ruiz J, Weill J, Levy-Marchal C, Horber F, Potoczna N, Hercberg S, Le Stunff C, Bougnères P, Kovacs P, Marre M, Balkau B, Cauchi S, Chèvre JC, Froguel P. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39:724-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1174] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 56. | Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshuler D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson Boström K, Isomaa B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, Orho-Melander M, Råstam L, Speliotes EK, Taskinen MR, Tuomi T, Guiducci C, Berglund A, Carlson J, Gianniny L, Hackett R, Hall L, Holmkvist J, Laurila E, Sjögren M, Sterner M, Surti A, Svensson M, Tewhey R, Blumenstiel B, Parkin M, Defelice M, Barry R, Brodeur W, Camarata J, Chia N, Fava M, Gibbons J, Handsaker B, Healy C, Nguyen K, Gates C, Sougnez C, Gage D, Nizzari M, Gabriel SB, Chirn GW, Ma Q, Parikh H, Richardson D, Ricke D, Purcell S, Diabetes Genetics Initiative of Broad Institute of Harvard and MIT, Lund University, and Novartis Institutes of BioMedical Research. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2243] [Cited by in RCA: 2124] [Article Influence: 118.0] [Reference Citation Analysis (0)] |
| 57. | Sanghera DK, Ortega L, Han S, Singh J, Ralhan SK, Wander GS, Mehra NK, Mulvihill JJ, Ferrell RE, Nath SK, Kamboh MI. Impact of nine common type 2 diabetes risk polymorphisms in Asian Indian Sikhs: PPARG2 (Pro12Ala), IGF2BP2, TCF7L2 and FTO variants confer a significant risk. BMC Med Genet. 2008;9:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 214] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 58. | Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS; Wellcome Trust Case Control Consortium (WTCCC), McCarthy MI, Hattersley AT. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336-1341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1762] [Cited by in RCA: 1652] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 59. | Cerosaletti K, Hao W, Greenbaum CJ. Erratum. Genetics coming of age in type 1 diabetes. Diabetes Care 2019;42:189-191. Diabetes Care. 2019;42:987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 60. | Hakonarson H, Grant SF, Bradfield JP, Marchand L, Kim CE, Glessner JT, Grabs R, Casalunovo T, Taback SP, Frackelton EC, Lawson ML, Robinson LJ, Skraban R, Lu Y, Chiavacci RM, Stanley CA, Kirsch SE, Rappaport EF, Orange JS, Monos DS, Devoto M, Qu HQ, Polychronakos C. A genome-wide association study identifies KIAA0350 as a type 1 diabetes gene. Nature. 2007;448:591-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 399] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 61. | Concannon P, Onengut-Gumuscu S, Todd JA, Smyth DJ, Pociot F, Bergholdt R, Akolkar B, Erlich HA, Hilner JE, Julier C, Morahan G, Nerup J, Nierras CR, Chen WM, Rich SS; Type 1 Diabetes Genetics Consortium. A human type 1 diabetes susceptibility locus maps to chromosome 21q22.3. Diabetes. 2008;57:2858-2861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 62. | Kwak SH, Kim SH, Cho YM, Go MJ, Cho YS, Choi SH, Moon MK, Jung HS, Shin HD, Kang HM, Cho NH, Lee IK, Kim SY, Han BG, Jang HC, Park KS. A genome-wide association study of gestational diabetes mellitus in Korean women. Diabetes. 2012;61:531-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 63. | Wu NN, Zhao D, Ma W, Lang JN, Liu SM, Fu Y, Wang X, Wang ZW, Li Q. A genome-wide association study of gestational diabetes mellitus in Chinese women. J Matern Fetal Neonatal Med. 2021;34:1557-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 64. | Pervjakova N, Moen GH, Borges MC, Ferreira T, Cook JP, Allard C, Beaumont RN, Canouil M, Hatem G, Heiskala A, Joensuu A, Karhunen V, Kwak SH, Lin FTJ, Liu J, Rifas-Shiman S, Tam CH, Tam WH, Thorleifsson G, Andrew T, Auvinen J, Bhowmik B, Bonnefond A, Delahaye F, Demirkan A, Froguel P, Haller-Kikkatalo K, Hardardottir H, Hummel S, Hussain A, Kajantie E, Keikkala E, Khamis A, Lahti J, Lekva T, Mustaniemi S, Sommer C, Tagoma A, Tzala E, Uibo R, Vääräsmäki M, Villa PM, Birkeland KI, Bouchard L, Duijn CM, Finer S, Groop L, Hämäläinen E, Hayes GM, Hitman GA, Jang HC, Järvelin MR, Jenum AK, Laivuori H, Ma RC, Melander O, Oken E, Park KS, Perron P, Prasad RB, Qvigstad E, Sebert S, Stefansson K, Steinthorsdottir V, Tuomi T, Hivert MF, Franks PW, McCarthy MI, Lindgren CM, Freathy RM, Lawlor DA, Morris AP, Mägi R. Multi-ancestry genome-wide association study of gestational diabetes mellitus highlights genetic links with type 2 diabetes. Hum Mol Genet. 2022;31:3377-3391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 65. | Strawbridge RJ, Dupuis J, Prokopenko I, Barker A, Ahlqvist E, Rybin D, Petrie JR, Travers ME, Bouatia-Naji N, Dimas AS, Nica A, Wheeler E, Chen H, Voight BF, Taneera J, Kanoni S, Peden JF, Turrini F, Gustafsson S, Zabena C, Almgren P, Barker DJ, Barnes D, Dennison EM, Eriksson JG, Eriksson P, Eury E, Folkersen L, Fox CS, Frayling TM, Goel A, Gu HF, Horikoshi M, Isomaa B, Jackson AU, Jameson KA, Kajantie E, Kerr-Conte J, Kuulasmaa T, Kuusisto J, Loos RJ, Luan J, Makrilakis K, Manning AK, Martínez-Larrad MT, Narisu N, Nastase Mannila M, Ohrvik J, Osmond C, Pascoe L, Payne F, Sayer AA, Sennblad B, Silveira A, Stancáková A, Stirrups K, Swift AJ, Syvänen AC, Tuomi T, van 't Hooft FM, Walker M, Weedon MN, Xie W, Zethelius B; DIAGRAM Consortium; GIANT Consortium; MuTHER Consortium; CARDIoGRAM Consortium; C4D Consortium, Ongen H, Mälarstig A, Hopewell JC, Saleheen D, Chambers J, Parish S, Danesh J, Kooner J, Ostenson CG, Lind L, Cooper CC, Serrano-Ríos M, Ferrannini E, Forsen TJ, Clarke R, Franzosi MG, Seedorf U, Watkins H, Froguel P, Johnson P, Deloukas P, Collins FS, Laakso M, Dermitzakis ET, Boehnke M, McCarthy MI, Wareham NJ, Groop L, Pattou F, Gloyn AL, Dedoussis GV, Lyssenko V, Meigs JB, Barroso I, Watanabe RM, Ingelsson E, Langenberg C, Hamsten A, Florez JC. Genome-wide association identifies nine common variants associated with fasting proinsulin levels and provides new insights into the pathophysiology of type 2 diabetes. Diabetes. 2011;60:2624-2634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 269] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 66. | Ahlqvist E, Ahluwalia TS, Groop L. Genetics of type 2 diabetes. Clin Chem. 2011;57:241-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 67. | Tabassum R, Chauhan G, Dwivedi OP, Mahajan A, Jaiswal A, Kaur I, Bandesh K, Singh T, Mathai BJ, Pandey Y, Chidambaram M, Sharma A, Chavali S, Sengupta S, Ramakrishnan L, Venkatesh P, Aggarwal SK, Ghosh S, Prabhakaran D, Srinath RK, Saxena M, Banerjee M, Mathur S, Bhansali A, Shah VN, Madhu SV, Marwaha RK, Basu A, Scaria V, McCarthy MI; DIAGRAM; INDICO, Venkatesan R, Mohan V, Tandon N, Bharadwaj D. Genome-wide association study for type 2 diabetes in Indians identifies a new susceptibility locus at 2q21. Diabetes. 2013;62:977-986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 68. | Steinthorsdottir V, Thorleifsson G, Sulem P, Helgason H, Grarup N, Sigurdsson A, Helgadottir HT, Johannsdottir H, Magnusson OT, Gudjonsson SA, Justesen JM, Harder MN, Jørgensen ME, Christensen C, Brandslund I, Sandbæk A, Lauritzen T, Vestergaard H, Linneberg A, Jørgensen T, Hansen T, Daneshpour MS, Fallah MS, Hreidarsson AB, Sigurdsson G, Azizi F, Benediktsson R, Masson G, Helgason A, Kong A, Gudbjartsson DF, Pedersen O, Thorsteinsdottir U, Stefansson K. Identification of low-frequency and rare sequence variants associated with elevated or reduced risk of type 2 diabetes. Nat Genet. 2014;46:294-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 229] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 69. | Go MJ, Lee Y, Park S, Kwak SH, Kim BJ, Lee J. Genetic-risk assessment of GWAS-derived susceptibility loci for type 2 diabetes in a 10 year follow-up of a population-based cohort study. J Hum Genet. 2016;61:1009-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 70. | Fuchsberger C, Flannick J, Teslovich TM, Mahajan A, Agarwala V, Gaulton KJ, Ma C, Fontanillas P, Moutsianas L, McCarthy DJ, Rivas MA, Perry JRB, Sim X, Blackwell TW, Robertson NR, Rayner NW, Cingolani P, Locke AE, Tajes JF, Highland HM, Dupuis J, Chines PS, Lindgren CM, Hartl C, Jackson AU, Chen H, Huyghe JR, van de Bunt M, Pearson RD, Kumar A, Müller-Nurasyid M, Grarup N, Stringham HM, Gamazon ER, Lee J, Chen Y, Scott RA, Below JE, Chen P, Huang J, Go MJ, Stitzel ML, Pasko D, Parker SCJ, Varga TV, Green T, Beer NL, Day-Williams AG, Ferreira T, Fingerlin T, Horikoshi M, Hu C, Huh I, Ikram MK, Kim BJ, Kim Y, Kim YJ, Kwon MS, Lee S, Lin KH, Maxwell TJ, Nagai Y, Wang X, Welch RP, Yoon J, Zhang W, Barzilai N, Voight BF, Han BG, Jenkinson CP, Kuulasmaa T, Kuusisto J, Manning A, Ng MCY, Palmer ND, Balkau B, Stančáková A, Abboud HE, Boeing H, Giedraitis V, Prabhakaran D, Gottesman O, Scott J, Carey J, Kwan P, Grant G, Smith JD, Neale BM, Purcell S, Butterworth AS, Howson JMM, Lee HM, Lu Y, Kwak SH, Zhao W, Danesh J, Lam VKL, Park KS, Saleheen D, So WY, Tam CHT, Afzal U, Aguilar D, Arya R, Aung T, Chan E, Navarro C, Cheng CY, Palli D, Correa A, Curran JE, Rybin D, Farook VS, Fowler SP, Freedman BI, Griswold M, Hale DE, Hicks PJ, Khor CC, Kumar S, Lehne B, Thuillier D, Lim WY, Liu J, van der Schouw YT, Loh M, Musani SK, Puppala S, Scott WR, Yengo L, Tan ST, Taylor HA Jr, Thameem F, Wilson G Sr, Wong TY, Njølstad PR, Levy JC, Mangino M, Bonnycastle LL, Schwarzmayr T, Fadista J, Surdulescu GL, Herder C, Groves CJ, Wieland T, Bork-Jensen J, Brandslund I, Christensen C, Koistinen HA, Doney ASF, Kinnunen L, Esko T, Farmer AJ, Hakaste L, Hodgkiss D, Kravic J, Lyssenko V, Hollensted M, Jørgensen ME, Jørgensen T, Ladenvall C, Justesen JM, Käräjämäki A, Kriebel J, Rathmann W, Lannfelt L, Lauritzen T, Narisu N, Linneberg A, Melander O, Milani L, Neville M, Orho-Melander M, Qi L, Qi Q, Roden M, Rolandsson O, Swift A, Rosengren AH, Stirrups K, Wood AR, Mihailov E, Blancher C, Carneiro MO, Maguire J, Poplin R, Shakir K, Fennell T, DePristo M, de Angelis MH, Deloukas P, Gjesing AP, Jun G, Nilsson P, Murphy J, Onofrio R, Thorand B, Hansen T, Meisinger C, Hu FB, Isomaa B, Karpe F, Liang L, Peters A, Huth C, O'Rahilly SP, Palmer CNA, Pedersen O, Rauramaa R, Tuomilehto J, Salomaa V, Watanabe RM, Syvänen AC, Bergman RN, Bharadwaj D, Bottinger EP, Cho YS, Chandak GR, Chan JCN, Chia KS, Daly MJ, Ebrahim SB, Langenberg C, Elliott P, Jablonski KA, Lehman DM, Jia W, Ma RCW, Pollin TI, Sandhu M, Tandon N, Froguel P, Barroso I, Teo YY, Zeggini E, Loos RJF, Small KS, Ried JS, DeFronzo RA, Grallert H, Glaser B, Metspalu A, Wareham NJ, Walker M, Banks E, Gieger C, Ingelsson E, Im HK, Illig T, Franks PW, Buck G, Trakalo J, Buck D, Prokopenko I, Mägi R, Lind L, Farjoun Y, Owen KR, Gloyn AL, Strauch K, Tuomi T, Kooner JS, Lee JY, Park T, Donnelly P, Morris AD, Hattersley AT, Bowden DW, Collins FS, Atzmon G, Chambers JC, Spector TD, Laakso M, Strom TM, Bell GI, Blangero J, Duggirala R, Tai ES, McVean G, Hanis CL, Wilson JG, Seielstad M, Frayling TM, Meigs JB, Cox NJ, Sladek R, Lander ES, Gabriel S, Burtt NP, Mohlke KL, Meitinger T, Groop L, Abecasis G, Florez JC, Scott LJ, Morris AP, Kang HM, Boehnke M, Altshuler D, McCarthy MI. The genetic architecture of type 2 diabetes. Nature. 2016;536:41-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 809] [Cited by in RCA: 778] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 71. | Wang X, Strizich G, Hu Y, Wang T, Kaplan RC, Qi Q. Genetic markers of type 2 diabetes: Progress in genome-wide association studies and clinical application for risk prediction. J Diabetes. 2016;8:24-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 72. | Scott RA, Scott LJ, Mägi R, Marullo L, Gaulton KJ, Kaakinen M, Pervjakova N, Pers TH, Johnson AD, Eicher JD, Jackson AU, Ferreira T, Lee Y, Ma C, Steinthorsdottir V, Thorleifsson G, Qi L, Van Zuydam NR, Mahajan A, Chen H, Almgren P, Voight BF, Grallert H, Müller-Nurasyid M, Ried JS, Rayner NW, Robertson N, Karssen LC, van Leeuwen EM, Willems SM, Fuchsberger C, Kwan P, Teslovich TM, Chanda P, Li M, Lu Y, Dina C, Thuillier D, Yengo L, Jiang L, Sparso T, Kestler HA, Chheda H, Eisele L, Gustafsson S, Frånberg M, Strawbridge RJ, Benediktsson R, Hreidarsson AB, Kong A, Sigurðsson G, Kerrison ND, Luan J, Liang L, Meitinger T, Roden M, Thorand B, Esko T, Mihailov E, Fox C, Liu CT, Rybin D, Isomaa B, Lyssenko V, Tuomi T, Couper DJ, Pankow JS, Grarup N, Have CT, Jørgensen ME, Jørgensen T, Linneberg A, Cornelis MC, van Dam RM, Hunter DJ, Kraft P, Sun Q, Edkins S, Owen KR, Perry JRB, Wood AR, Zeggini E, Tajes-Fernandes J, Abecasis GR, Bonnycastle LL, Chines PS, Stringham HM, Koistinen HA, Kinnunen L, Sennblad B, Mühleisen TW, Nöthen MM, Pechlivanis S, Baldassarre D, Gertow K, Humphries SE, Tremoli E, Klopp N, Meyer J, Steinbach G, Wennauer R, Eriksson JG, Mӓnnistö S, Peltonen L, Tikkanen E, Charpentier G, Eury E, Lobbens S, Gigante B, Leander K, McLeod O, Bottinger EP, Gottesman O, Ruderfer D, Blüher M, Kovacs P, Tonjes A, Maruthur NM, Scapoli C, Erbel R, Jöckel KH, Moebus S, de Faire U, Hamsten A, Stumvoll M, Deloukas P, Donnelly PJ, Frayling TM, Hattersley AT, Ripatti S, Salomaa V, Pedersen NL, Boehm BO, Bergman RN, Collins FS, Mohlke KL, Tuomilehto J, Hansen T, Pedersen O, Barroso I, Lannfelt L, Ingelsson E, Lind L, Lindgren CM, Cauchi S, Froguel P, Loos RJF, Balkau B, Boeing H, Franks PW, Barricarte Gurrea A, Palli D, van der Schouw YT, Altshuler D, Groop LC, Langenberg C, Wareham NJ, Sijbrands E, van Duijn CM, Florez JC, Meigs JB, Boerwinkle E, Gieger C, Strauch K, Metspalu A, Morris AD, Palmer CNA, Hu FB, Thorsteinsdottir U, Stefansson K, Dupuis J, Morris AP, Boehnke M, McCarthy MI, Prokopenko I; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium. An Expanded Genome-Wide Association Study of Type 2 Diabetes in Europeans. Diabetes. 2017;66:2888-2902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 520] [Cited by in RCA: 519] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 73. | Onengut-Gumuscu S, Chen WM, Burren O, Cooper NJ, Quinlan AR, Mychaleckyj JC, Farber E, Bonnie JK, Szpak M, Schofield E, Achuthan P, Guo H, Fortune MD, Stevens H, Walker NM, Ward LD, Kundaje A, Kellis M, Daly MJ, Barrett JC, Cooper JD, Deloukas P; Type 1 Diabetes Genetics Consortium, Todd JA, Wallace C, Concannon P, Rich SS. Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat Genet. 2015;47:381-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 520] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 74. | Spracklen CN, Horikoshi M, Kim YJ, Lin K, Bragg F, Moon S, Suzuki K, Tam CHT, Tabara Y, Kwak SH, Takeuchi F, Long J, Lim VJY, Chai JF, Chen CH, Nakatochi M, Yao J, Choi HS, Iyengar AK, Perrin HJ, Brotman SM, van de Bunt M, Gloyn AL, Below JE, Boehnke M, Bowden DW, Chambers JC, Mahajan A, McCarthy MI, Ng MCY, Petty LE, Zhang W, Morris AP, Adair LS, Akiyama M, Bian Z, Chan JCN, Chang LC, Chee ML, Chen YI, Chen YT, Chen Z, Chuang LM, Du S, Gordon-Larsen P, Gross M, Guo X, Guo Y, Han S, Howard AG, Huang W, Hung YJ, Hwang MY, Hwu CM, Ichihara S, Isono M, Jang HM, Jiang G, Jonas JB, Kamatani Y, Katsuya T, Kawaguchi T, Khor CC, Kohara K, Lee MS, Lee NR, Li L, Liu J, Luk AO, Lv J, Okada Y, Pereira MA, Sabanayagam C, Shi J, Shin DM, So WY, Takahashi A, Tomlinson B, Tsai FJ, van Dam RM, Xiang YB, Yamamoto K, Yamauchi T, Yoon K, Yu C, Yuan JM, Zhang L, Zheng W, Igase M, Cho YS, Rotter JI, Wang YX, Sheu WHH, Yokota M, Wu JY, Cheng CY, Wong TY, Shu XO, Kato N, Park KS, Tai ES, Matsuda F, Koh WP, Ma RCW, Maeda S, Millwood IY, Lee J, Kadowaki T, Walters RG, Kim BJ, Mohlke KL, Sim X. Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature. 2020;582:240-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 341] [Cited by in RCA: 306] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 75. | Visscher PM, Wray NR, Zhang Q, Sklar P, McCarthy MI, Brown MA, Yang J. 10 Years of GWAS Discovery: Biology, Function, and Translation. Am J Hum Genet. 2017;101:5-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2025] [Cited by in RCA: 2152] [Article Influence: 269.0] [Reference Citation Analysis (0)] |
| 76. | Schork NJ, Murray SS, Frazer KA, Topol EJ. Common vs. rare allele hypotheses for complex diseases. Curr Opin Genet Dev. 2009;19:212-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 462] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 77. | Huyghe JR, Jackson AU, Fogarty MP, Buchkovich ML, Stančáková A, Stringham HM, Sim X, Yang L, Fuchsberger C, Cederberg H, Chines PS, Teslovich TM, Romm JM, Ling H, McMullen I, Ingersoll R, Pugh EW, Doheny KF, Neale BM, Daly MJ, Kuusisto J, Scott LJ, Kang HM, Collins FS, Abecasis GR, Watanabe RM, Boehnke M, Laakso M, Mohlke KL. Exome array analysis identifies new loci and low-frequency variants influencing insulin processing and secretion. Nat Genet. 2013;45:197-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 78. | Estrada K, Aukrust I, Bjørkhaug L, Burtt NP, Mercader JM, García-Ortiz H, Huerta-Chagoya A, Moreno-Macías H, Walford G, Flannick J, Williams AL, Gómez-Vázquez MJ, Fernandez-Lopez JC, Martínez-Hernández A, Jiménez-Morales S, Centeno-Cruz F, Mendoza-Caamal E, Revilla-Monsalve C, Islas-Andrade S, Córdova EJ, Soberón X, González-Villalpando ME, Henderson E, Wilkens LR, Le Marchand L, Arellano-Campos O, Ordóñez-Sánchez ML, Rodríguez-Torres M, Rodríguez-Guillén R, Riba L, Najmi LA, Jacobs SB, Fennell T, Gabriel S, Fontanillas P, Hanis CL, Lehman DM, Jenkinson CP, Abboud HE, Bell GI, Cortes ML, Boehnke M, González-Villalpando C, Orozco L, Haiman CA, Tusié-Luna T, Aguilar-Salinas CA, Altshuler D, Njølstad PR, Florez JC, MacArthur DG; SIGMA Type 2 Diabetes Consortium. Association of a low-frequency variant in HNF1A with type 2 diabetes in a Latino population. JAMA. 2014;311:2305-2314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 191] [Article Influence: 17.4] [Reference Citation Analysis (1)] |
| 79. | Nejentsev S, Walker N, Riches D, Egholm M, Todd JA. Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science. 2009;324:387-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 792] [Cited by in RCA: 739] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 80. | Ge Y, Onengut-Gumuscu S, Quinlan AR, Mackey AJ, Wright JA, Buckner JH, Habib T, Rich SS, Concannon P. Targeted Deep Sequencing in Multiple-Affected Sibships of European Ancestry Identifies Rare Deleterious Variants in PTPN22 That Confer Risk for Type 1 Diabetes. Diabetes. 2016;65:794-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 81. | Simmons RA. Developmental origins of beta-cell failure in type 2 diabetes: the role of epigenetic mechanisms. Pediatr Res. 2007;61:64R-67R. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 82. | Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, Roumain J, Bennett PH, Knowler WC. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49:2208-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 856] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 83. | Tateishi K, Okada Y, Kallin EM, Zhang Y. Role of Jhdm2a in regulating metabolic gene expression and obesity resistance. Nature. 2009;458:757-761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 351] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 84. | Milagro FI, Campión J, García-Díaz DF, Goyenechea E, Paternain L, Martínez JA. High fat diet-induced obesity modifies the methylation pattern of leptin promoter in rats. J Physiol Biochem. 2009;65:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 85. | Park JH, Stoffers DA, Nicholls RD, Simmons RA. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Invest. 2008;118:2316-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 273] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 86. | Eyileten C, Wicik Z, De Rosa S, Mirowska-Guzel D, Soplinska A, Indolfi C, Jastrzebska-Kurkowska I, Czlonkowska A, Postula M. MicroRNAs as Diagnostic and Prognostic Biomarkers in Ischemic Stroke-A Comprehensive Review and Bioinformatic Analysis. Cells. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 87. | Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, Shah A, Willeit J, Mayr M. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107:810-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1196] [Cited by in RCA: 1132] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 88. | Kong L, Zhu J, Han W, Jiang X, Xu M, Zhao Y, Dong Q, Pang Z, Guan Q, Gao L, Zhao J, Zhao L. Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: a clinical study. Acta Diabetol. 2011;48:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 410] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 89. | Balasubramanyam M, Aravind S, Gokulakrishnan K, Prabu P, Sathishkumar C, Ranjani H, Mohan V. Impaired miR-146a expression links subclinical inflammation and insulin resistance in Type 2 diabetes. Mol Cell Biochem. 2011;351:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 191] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 90. | Karolina DS, Tavintharan S, Armugam A, Sepramaniam S, Pek SL, Wong MT, Lim SC, Sum CF, Jeyaseelan K. Circulating miRNA profiles in patients with metabolic syndrome. J Clin Endocrinol Metab. 2012;97:E2271-E2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 336] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 91. | Zhang T, Lv C, Li L, Chen S, Liu S, Wang C, Su B. Plasma miR-126 is a potential biomarker for early prediction of type 2 diabetes mellitus in susceptible individuals. Biomed Res Int. 2013;2013:761617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 92. | Liu Y, Gao G, Yang C, Zhou K, Shen B, Liang H, Jiang X. The role of circulating microRNA-126 (miR-126): a novel biomarker for screening prediabetes and newly diagnosed type 2 diabetes mellitus. Int J Mol Sci. 2014;15:10567-10577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 93. | Zhang T, Li L, Shang Q, Lv C, Wang C, Su B. Circulating miR-126 is a potential biomarker to predict the onset of type 2 diabetes mellitus in susceptible individuals. Biochem Biophys Res Commun. 2015;463:60-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 94. | Luo M, Li R, Deng X, Ren M, Chen N, Zeng M, Yan K, Xia J, Liu F, Ma W, Yang Y, Wan Q, Wu J. Platelet-derived miR-103b as a novel biomarker for the early diagnosis of type 2 diabetes. Acta Diabetol. 2015;52:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 95. | Olivieri F, Spazzafumo L, Bonafè M, Recchioni R, Prattichizzo F, Marcheselli F, Micolucci L, Mensà E, Giuliani A, Santini G, Gobbi M, Lazzarini R, Boemi M, Testa R, Antonicelli R, Procopio AD, Bonfigli AR. MiR-21-5p and miR-126a-3p levels in plasma and circulating angiogenic cells: relationship with type 2 diabetes complications. Oncotarget. 2015;6:35372-35382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 96. | Witkowski M, Weithauser A, Tabaraie T, Steffens D, Kränkel N, Witkowski M, Stratmann B, Tschoepe D, Landmesser U, Rauch-Kroehnert U. Micro-RNA-126 Reduces the Blood Thrombogenicity in Diabetes Mellitus via Targeting of Tissue Factor. Arterioscler Thromb Vasc Biol. 2016;36:1263-1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 97. | Jansen F, Wang H, Przybilla D, Franklin BS, Dolf A, Pfeifer P, Schmitz T, Flender A, Endl E, Nickenig G, Werner N. Vascular endothelial microparticles-incorporated microRNAs are altered in patients with diabetes mellitus. Cardiovasc Diabetol. 2016;15:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 98. | Ghorbani S, Mahdavi R, Alipoor B, Panahi G, Nasli Esfahani E, Razi F, Taghikhani M, Meshkani R. Decreased serum microRNA-21 level is associated with obesity in healthy and type 2 diabetic subjects. Arch Physiol Biochem. 2018;124:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 99. | Giannella A, Radu CM, Franco L, Campello E, Simioni P, Avogaro A, de Kreutzenberg SV, Ceolotto G. Circulating levels and characterization of microparticles in patients with different degrees of glucose tolerance. Cardiovasc Diabetol. 2017;16:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 100. | Deng X, Liu Y, Luo M, Wu J, Ma R, Wan Q. Circulating miRNA-24 and its target YKL-40 as potential biomarkers in patients with coronary heart disease and type 2 diabetes mellitus. Oncotarget. 2017;8:63038-63046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 101. | Fejes Z, Póliska S, Czimmerer Z, Káplár M, Penyige A, Gál Szabó G, Beke Debreceni I, Kunapuli SP, Kappelmayer J, Nagy B Jr. Hyperglycaemia suppresses microRNA expression in platelets to increase P2RY12 and SELP levels in type 2 diabetes mellitus. Thromb Haemost. 2017;117:529-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 102. | Al-Muhtaresh HA, Al-Kafaji G. Evaluation of Two-Diabetes Related microRNAs Suitability as Earlier Blood Biomarkers for Detecting Prediabetes and type 2 Diabetes Mellitus. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 103. | Jiménez-Lucena R, Rangel-Zúñiga OA, Alcalá-Díaz JF, López-Moreno J, Roncero-Ramos I, Molina-Abril H, Yubero-Serrano EM, Caballero-Villarraso J, Delgado-Lista J, Castaño JP, Ordovás JM, Pérez-Martinez P, Camargo A, López-Miranda J. Circulating miRNAs as Predictive Biomarkers of Type 2 Diabetes Mellitus Development in Coronary Heart Disease Patients from the CORDIOPREV Study. Mol Ther Nucleic Acids. 2018;12:146-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 104. | Amr KS, Abdelmawgoud H, Ali ZY, Shehata S, Raslan HM. Potential value of circulating microRNA-126 and microRNA-210 as biomarkers for type 2 diabetes with coronary artery disease. Br J Biomed Sci. 2018;75:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 105. | Stępień EŁ, Durak-Kozica M, Kamińska A, Targosz-Korecka M, Libera M, Tylko G, Opalińska A, Kapusta M, Solnica B, Georgescu A, Costa MC, Czyżewska-Buczyńska A, Witkiewicz W, Małecki MT, Enguita FJ. Circulating ectosomes: Determination of angiogenic microRNAs in type 2 diabetes. Theranostics. 2018;8:3874-3890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 106. | Ruan Q, Wang T, Kameswaran V, Wei Q, Johnson DS, Matschinsky F, Shi W, Chen YH. The microRNA-21-PDCD4 axis prevents type 1 diabetes by blocking pancreatic beta cell death. Proc Natl Acad Sci U S A. 2011;108:12030-12035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 107. | Sims EK, Lakhter AJ, Anderson-Baucum E, Kono T, Tong X, Evans-Molina C. MicroRNA 21 targets BCL2 mRNA to increase apoptosis in rat and human beta cells. Diabetologia. 2017;60:1057-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 108. | Grieco FA, Sebastiani G, Juan-Mateu J, Villate O, Marroqui L, Ladrière L, Tugay K, Regazzi R, Bugliani M, Marchetti P, Dotta F, Eizirik DL. MicroRNAs miR-23a-3p, miR-23b-3p, and miR-149-5p Regulate the Expression of Proapoptotic BH3-Only Proteins DP5 and PUMA in Human Pancreatic β-Cells. Diabetes. 2017;66:100-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 109. | Zheng Y, Wang Z, Tu Y, Shen H, Dai Z, Lin J, Zhou Z. miR-101a and miR-30b contribute to inflammatory cytokine-mediated β-cell dysfunction. Lab Invest. 2015;95:1387-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 110. | Tsukita S, Yamada T, Takahashi K, Munakata Y, Hosaka S, Takahashi H, Gao J, Shirai Y, Kodama S, Asai Y, Sugisawa T, Chiba Y, Kaneko K, Uno K, Sawada S, Imai J, Katagiri H. MicroRNAs 106b and 222 Improve Hyperglycemia in a Mouse Model of Insulin-Deficient Diabetes via Pancreatic β-Cell Proliferation. EBioMedicine. 2017;15:163-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 111. | Nabih ES, Andrawes NG. The Association Between Circulating Levels of miRNA-181a and Pancreatic Beta Cells Dysfunction via SMAD7 in Type 1 Diabetic Children and Adolescents. J Clin Lab Anal. 2016;30:727-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 112. | Zhang Y, Feng ZP, Naselli G, Bell F, Wettenhall J, Auyeung P, Ellis JA, Ponsonby AL, Speed TP, Chong MM, Harrison LC. MicroRNAs in CD4(+) T cell subsets are markers of disease risk and T cell dysfunction in individuals at risk for type 1 diabetes. J Autoimmun. 2016;68:52-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 113. | de Jong VM, van der Slik AR, Laban S, van 't Slot R, Koeleman BP, Zaldumbide A, Roep BO. Survival of autoreactive T lymphocytes by microRNA-mediated regulation of apoptosis through TRAIL and Fas in type 1 diabetes. Genes Immun. 2016;17:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 114. | Berry GJ, Budgeon LR, Cooper TK, Christensen ND, Waldner H. The type 1 diabetes resistance locus B10 Idd9.3 mediates impaired B-cell lymphopoiesis and implicates microRNA-34a in diabetes protection. Eur J Immunol. 2014;44:1716-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 115. | Bhatt S, Gupta MK, Khamaisi M, Martinez R, Gritsenko MA, Wagner BK, Guye P, Busskamp V, Shirakawa J, Wu G, Liew CW, Clauss TR, Valdez I, El Ouaamari A, Dirice E, Takatani T, Keenan HA, Smith RD, Church G, Weiss R, Wagers AJ, Qian WJ, King GL, Kulkarni RN. Preserved DNA Damage Checkpoint Pathway Protects against Complications in Long-Standing Type 1 Diabetes. Cell Metab. 2015;22:239-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 116. | Yu M, Liu Y, Zhang B, Shi Y, Cui L, Zhao X. Inhibiting microRNA-144 abates oxidative stress and reduces apoptosis in hearts of streptozotocin-induced diabetic mice. Cardiovasc Pathol. 2015;24:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 117. | Yang M, Ye L, Wang B, Gao J, Liu R, Hong J, Wang W, Gu W, Ning G. Decreased miR-146 expression in peripheral blood mononuclear cells is correlated with ongoing islet autoimmunity in type 1 diabetes patients 1miR-146. J Diabetes. 2015;7:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 118. | Bonner C, Nyhan KC, Bacon S, Kyithar MP, Schmid J, Concannon CG, Bray IM, Stallings RL, Prehn JH, Byrne MM. Identification of circulating microRNAs in HNF1A-MODY carriers. Diabetologia. 2013;56:1743-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 119. | Shi Z, Zhao C, Guo X, Ding H, Cui Y, Shen R, Liu J. Differential expression of microRNAs in omental adipose tissue from gestational diabetes mellitus subjects reveals miR-222 as a regulator of ERα expression in estrogen-induced insulin resistance. Endocrinology. 2014;155:1982-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 120. | Cao JL, Zhang L, Li J, Tian S, Lv XD, Wang XQ, Su X, Li Y, Hu Y, Ma X, Xia HF. Up-regulation of miR-98 and unraveling regulatory mechanisms in gestational diabetes mellitus. Sci Rep. 2016;6:32268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 121. | Zhao C, Zhang T, Shi Z, Ding H, Ling X. MicroRNA-518d regulates PPARα protein expression in the placentas of females with gestational diabetes mellitus. Mol Med Rep. 2014;9:2085-2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 122. | Stirm L, Huypens P, Sass S, Batra R, Fritsche L, Brucker S, Abele H, Hennige AM, Theis F, Beckers J, Hrabě de Angelis M, Fritsche A, Häring HU, Staiger H. Maternal whole blood cell miRNA-340 is elevated in gestational diabetes and inversely regulated by glucose and insulin. Sci Rep. 2018;8:1366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 123. | Tryggestad JB, Vishwanath A, Jiang S, Mallappa A, Teague AM, Takahashi Y, Thompson DM, Chernausek SD. Influence of gestational diabetes mellitus on human umbilical vein endothelial cell miRNA. Clin Sci (Lond). 2016;130:1955-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 124. | Feng Y, Qu X, Chen Y, Feng Q, Zhang Y, Hu J, Li X. MicroRNA-33a-5p sponges to inhibit pancreatic β-cell function in gestational diabetes mellitus LncRNA DANCR. Reprod Biol Endocrinol. 2020;18:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 125. | Sebastiani G, Guarino E, Grieco GE, Formichi C, Delli Poggi C, Ceccarelli E, Dotta F. Circulating microRNA (miRNA) Expression Profiling in Plasma of Patients with Gestational Diabetes Mellitus Reveals Upregulation of miRNA miR-330-3p. Front Endocrinol (Lausanne). 2017;8:345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 126. | He Y, Bai J, Liu P, Dong J, Tang Y, Zhou J, Han P, Xing J, Chen Y, Yu X. miR-494 protects pancreatic β-cell function by targeting PTEN in gestational diabetes mellitus. EXCLI J. 2017;16:1297-1307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 127. | Li L, Wang S, Li H, Wan J, Zhou Q, Zhou Y, Zhang C. microRNA-96 protects pancreatic β-cell function by targeting PAK1 in gestational diabetes mellitus. Biofactors. 2018;44:539-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 128. | Zhao H, Tao S. MiRNA-221 protects islet β cell function in gestational diabetes mellitus by targeting PAK1. Biochem Biophys Res Commun. 2019;520:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 129. | Mujwara D, Henno G, Vernon ST, Peng S, Di Domenico P, Schroeder B, Busby GB, Figtree GA, Bottà G. Integrating a Polygenic Risk Score for Coronary Artery Disease as a Risk-Enhancing Factor in the Pooled Cohort Equation: A Cost-Effectiveness Analysis Study. J Am Heart Assoc. 2022;11:e025236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 130. | Natarajan P, Young R, Stitziel NO, Padmanabhan S, Baber U, Mehran R, Sartori S, Fuster V, Reilly DF, Butterworth A, Rader DJ, Ford I, Sattar N, Kathiresan S. Polygenic Risk Score Identifies Subgroup With Higher Burden of Atherosclerosis and Greater Relative Benefit From Statin Therapy in the Primary Prevention Setting. Circulation. 2017;135:2091-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 387] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 131. | Howe LJ, Dudbridge F, Schmidt AF, Finan C, Denaxas S, Asselbergs FW, Hingorani AD, Patel RS. Polygenic risk scores for coronary artery disease and subsequent event risk amongst established cases. Hum Mol Genet. 2020;29:1388-1395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 132. | Elliott J, Bodinier B, Bond TA, Chadeau-Hyam M, Evangelou E, Moons KGM, Dehghan A, Muller DC, Elliott P, Tzoulaki I. Predictive Accuracy of a Polygenic Risk Score-Enhanced Prediction Model vs a Clinical Risk Score for Coronary Artery Disease. JAMA. 2020;323:636-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 322] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 133. | Padilla-Martínez F, Collin F, Kwasniewski M, Kretowski A. Systematic Review of Polygenic Risk Scores for Type 1 and Type 2 Diabetes. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 134. | Lyssenko V, Jonsson A, Almgren P, Pulizzi N, Isomaa B, Tuomi T, Berglund G, Altshuler D, Nilsson P, Groop L. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med. 2008;359:2220-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 625] [Article Influence: 36.8] [Reference Citation Analysis (1)] |
| 135. | Meigs JB, Shrader P, Sullivan LM, McAteer JB, Fox CS, Dupuis J, Manning AK, Florez JC, Wilson PW, D'Agostino RB Sr, Cupples LA. Genotype score in addition to common risk factors for prediction of type 2 diabetes. N Engl J Med. 2008;359:2208-2219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 610] [Cited by in RCA: 560] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 136. | Lango H; UK Type 2 Diabetes Genetics Consortium, Palmer CN, Morris AD, Zeggini E, Hattersley AT, McCarthy MI, Frayling TM, Weedon MN. Assessing the combined impact of 18 common genetic variants of modest effect sizes on type 2 diabetes risk. Diabetes. 2008;57:3129-3135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 240] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 137. | Mahajan A, Taliun D, Thurner M, Robertson NR, Torres JM, Rayner NW, Payne AJ, Steinthorsdottir V, Scott RA, Grarup N, Cook JP, Schmidt EM, Wuttke M, Sarnowski C, Mägi R, Nano J, Gieger C, Trompet S, Lecoeur C, Preuss MH, Prins BP, Guo X, Bielak LF, Below JE, Bowden DW, Chambers JC, Kim YJ, Ng MCY, Petty LE, Sim X, Zhang W, Bennett AJ, Bork-Jensen J, Brummett CM, Canouil M, Ec Kardt KU, Fischer K, Kardia SLR, Kronenberg F, Läll K, Liu CT, Locke AE, Luan J, Ntalla I, Nylander V, Schönherr S, Schurmann C, Yengo L, Bottinger EP, Brandslund I, Christensen C, Dedoussis G, Florez JC, Ford I, Franco OH, Frayling TM, Giedraitis V, Hackinger S, Hattersley AT, Herder C, Ikram MA, Ingelsson M, Jørgensen ME, Jørgensen T, Kriebel J, Kuusisto J, Ligthart S, Lindgren CM, Linneberg A, Lyssenko V, Mamakou V, Meitinger T, Mohlke KL, Morris AD, Nadkarni G, Pankow JS, Peters A, Sattar N, Stančáková A, Strauch K, Taylor KD, Thorand B, Thorleifsson G, Thorsteinsdottir U, Tuomilehto J, Witte DR, Dupuis J, Peyser PA, Zeggini E, Loos RJF, Froguel P, Ingelsson E, Lind L, Groop L, Laakso M, Collins FS, Jukema JW, Palmer CNA, Grallert H, Metspalu A, Dehghan A, Köttgen A, Abecasis GR, Meigs JB, Rotter JI, Marchini J, Pedersen O, Hansen T, Langenberg C, Wareham NJ, Stefansson K, Gloyn AL, Morris AP, Boehnke M, McCarthy MI. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. 2018;50:1505-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1280] [Cited by in RCA: 1216] [Article Influence: 173.7] [Reference Citation Analysis (1)] |
| 138. | Vujkovic M, Keaton JM, Lynch JA, Miller DR, Zhou J, Tcheandjieu C, Huffman JE, Assimes TL, Lorenz K, Zhu X, Hilliard AT, Judy RL, Huang J, Lee KM, Klarin D, Pyarajan S, Danesh J, Melander O, Rasheed A, Mallick NH, Hameed S, Qureshi IH, Afzal MN, Malik U, Jalal A, Abbas S, Sheng X, Gao L, Kaestner KH, Susztak K, Sun YV, DuVall SL, Cho K, Lee JS, Gaziano JM, Phillips LS, Meigs JB, Reaven PD, Wilson PW, Edwards TL, Rader DJ, Damrauer SM, O'Donnell CJ, Tsao PS; HPAP Consortium; Regeneron Genetics Center; VA Million Veteran Program, Chang KM, Voight BF, Saleheen D. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat Genet. 2020;52:680-691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 497] [Article Influence: 99.4] [Reference Citation Analysis (0)] |
| 139. | Polfus LM, Darst BF, Highland H, Sheng X, Ng MCY, Below JE, Petty L, Bien S, Sim X, Wang W, Fontanillas P, Patel Y; 23andMe Research Team; DIAMANTE Hispanic/Latino Consortium; MEta-analysis of type 2 DIabetes in African Americans Consortium, Preuss M, Schurmann C, Du Z, Lu Y, Rhie SK, Mercader JM, Tusie-Luna T, González-Villalpando C, Orozco L, Spracklen CN, Cade BE, Jensen RA, Sun M, Joo YY, An P, Yanek LR, Bielak LF, Tajuddin S, Nicolas A, Chen G, Raffield L, Guo X, Chen WM, Nadkarni GN, Graff M, Tao R, Pankow JS, Daviglus M, Qi Q, Boerwinkle EA, Liu S, Phillips LS, Peters U, Carlson C, Wikens LR, Marchand LL, North KE, Buyske S, Kooperberg C, Loos RJF, Stram DO, Haiman CA. Genetic discovery and risk characterization in type 2 diabetes across diverse populations. HGG Adv. 2021;2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 140. | Winkler C, Krumsiek J, Buettner F, Angermüller C, Giannopoulou EZ, Theis FJ, Ziegler AG, Bonifacio E. Feature ranking of type 1 diabetes susceptibility genes improves prediction of type 1 diabetes. Diabetologia. 2014;57:2521-2529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 141. | Oram RA, Patel K, Hill A, Shields B, McDonald TJ, Jones A, Hattersley AT, Weedon MN. A Type 1 Diabetes Genetic Risk Score Can Aid Discrimination Between Type 1 and Type 2 Diabetes in Young Adults. Diabetes Care. 2016;39:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 234] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 142. | Perry DJ, Wasserfall CH, Oram RA, Williams MD, Posgai A, Muir AB, Haller MJ, Schatz DA, Wallet MA, Mathews CE, Atkinson MA, Brusko TM. Application of a Genetic Risk Score to Racially Diverse Type 1 Diabetes Populations Demonstrates the Need for Diversity in Risk-Modeling. Sci Rep. 2018;8:4529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 143. | Sharp SA, Rich SS, Wood AR, Jones SE, Beaumont RN, Harrison JW, Schneider DA, Locke JM, Tyrrell J, Weedon MN, Hagopian WA, Oram RA. Development and Standardization of an Improved Type 1 Diabetes Genetic Risk Score for Use in Newborn Screening and Incident Diagnosis. Diabetes Care. 2019;42:200-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 233] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 144. | Weedon MN, McCarthy MI, Hitman G, Walker M, Groves CJ, Zeggini E, Rayner NW, Shields B, Owen KR, Hattersley AT, Frayling TM. Combining information from common type 2 diabetes risk polymorphisms improves disease prediction. PLoS Med. 2006;3:e374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 213] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 145. | Chatterjee N, Wheeler B, Sampson J, Hartge P, Chanock SJ, Park JH. Projecting the performance of risk prediction based on polygenic analyses of genome-wide association studies. Nat Genet. 2013;45:400-405, 405e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 276] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 146. | Vassy JL, Hivert MF, Porneala B, Dauriz M, Florez JC, Dupuis J, Siscovick DS, Fornage M, Rasmussen-Torvik LJ, Bouchard C, Meigs JB. Polygenic type 2 diabetes prediction at the limit of common variant detection. Diabetes. 2014;63:2172-2182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 147. | Läll K, Mägi R, Morris A, Metspalu A, Fischer K. Personalized risk prediction for type 2 diabetes: the potential of genetic risk scores. Genet Med. 2017;19:322-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 148. | Chikowore T, van Zyl T, Feskens EJ, Conradie KR. Predictive utility of a genetic risk score of common variants associated with type 2 diabetes in a black South African population. Diabetes Res Clin Pract. 2016;122:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 149. | Khera AV, Chaffin M, Aragam KG, Haas ME, Roselli C, Choi SH, Natarajan P, Lander ES, Lubitz SA, Ellinor PT, Kathiresan S. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet. 2018;50:1219-1224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1251] [Cited by in RCA: 1925] [Article Influence: 275.0] [Reference Citation Analysis (0)] |
| 150. | Kawai VK, Levinson RT, Adefurin A, Kurnik D, Collier SP, Conway D, Stein CM. A genetic risk score that includes common type 2 diabetes risk variants is associated with gestational diabetes. Clin Endocrinol (Oxf). 2017;87:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 151. | Lauenborg J, Grarup N, Damm P, Borch-Johnsen K, Jørgensen T, Pedersen O, Hansen T. Common type 2 diabetes risk gene variants associate with gestational diabetes. J Clin Endocrinol Metab. 2009;94:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 152. | Powe CE, Nodzenski M, Talbot O, Allard C, Briggs C, Leya MV, Perron P, Bouchard L, Florez JC, Scholtens DM, Lowe WL Jr, Hivert MF. Genetic Determinants of Glycemic Traits and the Risk of Gestational Diabetes Mellitus. Diabetes. 2018;67:2703-2709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 153. | Perišić MM, Vladimir K, Karpov S, Štorga M, Mostashari A, Khanin R. Polygenic Risk Score and Risk Factors for Gestational Diabetes. J Pers Med. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 154. | Standards of Medical Care in Diabetes-2016: Summary of Revisions. Diabetes Care. 2016;39 Suppl 1:S4-S5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 325] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 155. | Klonoff DC, Kerr D. Smart Pens Will Improve Insulin Therapy. J Diabetes Sci Technol. 2018;12:551-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 156. | Sangave NA, Aungst TD, Patel DK. Smart Connected Insulin Pens, Caps, and Attachments: A Review of the Future of Diabetes Technology. Diabetes Spectr. 2019;32:378-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 157. | Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, Horton ES, Castorino K, Tate DF. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1608] [Cited by in RCA: 1615] [Article Influence: 179.4] [Reference Citation Analysis (1)] |
| 158. | Ebrahimi M, Guilan-Nejad TN, Pordanjani AF. Effect of yoga and aerobics exercise on sleep quality in women with Type 2 diabetes: a randomized controlled trial. Sleep Sci. 2017;10:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 159. | Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3682] [Cited by in RCA: 3517] [Article Influence: 270.5] [Reference Citation Analysis (0)] |
| 160. | Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456-469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2030] [Cited by in RCA: 1803] [Article Influence: 100.2] [Reference Citation Analysis (0)] |
| 161. | Wang ZV, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol. 2016;8:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 425] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 162. | Barnard RJ, Jung T, Inkeles SB. Diet and exercise in the treatment of NIDDM. The need for early emphasis. Diabetes Care. 1994;17:1469-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 85] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 163. | Nicholson AS, Sklar M, Barnard ND, Gore S, Sullivan R, Browning S. Toward improved management of NIDDM: A randomized, controlled, pilot intervention using a lowfat, vegetarian diet. Prev Med. 1999;29:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 119] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 164. | Moreno-Valdespino CA, Luna-Vital D, Camacho-Ruiz RM, Mojica L. Bioactive proteins and phytochemicals from legumes: Mechanisms of action preventing obesity and type-2 diabetes. Food Res Int. 2020;130:108905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (1)] |
| 165. | Hackett RA, Steptoe A. Type 2 diabetes mellitus and psychological stress - a modifiable risk factor. Nat Rev Endocrinol. 2017;13:547-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 278] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 166. | Faulenbach M, Uthoff H, Schwegler K, Spinas GA, Schmid C, Wiesli P. Effect of psychological stress on glucose control in patients with Type 2 diabetes. Diabet Med. 2012;29:128-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 167. | Arora T, Taheri S. Sleep Optimization and Diabetes Control: A Review of the Literature. Diabetes Ther. 2015;6:425-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 168. | Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: A population-based study. Diabetes Care. 2009;32:1980-1985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 376] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 169. | Reutrakul S, Thakkinstian A, Anothaisintawee T, Chontong S, Borel AL, Perfect MM, Janovsky CC, Kessler R, Schultes B, Harsch IA, van Dijk M, Bouhassira D, Matejko B, Lipton RB, Suwannalai P, Chirakalwasan N, Schober AK, Knutson KL. Sleep characteristics in type 1 diabetes and associations with glycemic control: systematic review and meta-analysis. Sleep Med. 2016;23:26-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 168] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 170. | Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva Ad, Hod M, Kitzmiler JL, Lowe LP, McIntyre HD, Oats JJ, Omori Y, Schmidt MI; International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2777] [Cited by in RCA: 3167] [Article Influence: 211.1] [Reference Citation Analysis (1)] |
| 171. | American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care. 2012;35 Suppl 1:S11-S63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 969] [Cited by in RCA: 1365] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 172. | Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Res Clin Pract. 2014;103:341-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 566] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 173. | Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet Gynecol. 2013;122:406-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 459] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 174. | Gloyn AL, Drucker DJ. Precision medicine in the management of type 2 diabetes. Lancet Diabetes Endocrinol. 2018;6:891-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 175. | Fodor A, Cozma A, Suharoschi R, Sitar-Taut A, Roman G. Clinical and genetic predictors of diabetes drug's response. Drug Metab Rev. 2019;51:408-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 176. | Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G; ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2205] [Cited by in RCA: 2108] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 177. | American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S98-S110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 683] [Article Influence: 136.6] [Reference Citation Analysis (0)] |
| 178. |
Martin T, Campbell RK.
Vitamin D and diabetes |
| 179. | Cojic M, Kocic R, Klisic A, Kocic G. The Effects of Vitamin D Supplementation on Metabolic and Oxidative Stress Markers in Patients With Type 2 Diabetes: A 6-Month Follow Up Randomized Controlled Study. Front Endocrinol (Lausanne). 2021;12:610893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 180. | Zhang DW, Fu M, Gao SH, Liu JL. Curcumin and diabetes: a systematic review. Evid Based Complement Alternat Med. 2013;2013:636053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 181. | Ghorbani A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed Pharmacother. 2017;96:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 284] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 182. | Li R, Zhang Y, Rasool S, Geetha T, Babu JR. Effects and Underlying Mechanisms of Bioactive Compounds on Type 2 Diabetes Mellitus and Alzheimer's Disease. Oxid Med Cell Longev. 2019;2019:8165707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 183. | Youl E, Bardy G, Magous R, Cros G, Sejalon F, Virsolvy A, Richard S, Quignard JF, Gross R, Petit P, Bataille D, Oiry C. Quercetin potentiates insulin secretion and protects INS-1 pancreatic β-cells against oxidative damage via the ERK1/2 pathway. Br J Pharmacol. 2010;161:799-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 184. | Ae Park S, Choi MS, Cho SY, Seo JS, Jung UJ, Kim MJ, Sung MK, Park YB, Lee MK. Genistein and daidzein modulate hepatic glucose and lipid regulating enzyme activities in C57BL/KsJ-db/db mice. Life Sci. 2006;79:1207-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 185. | Mahmoud AM, Ashour MB, Abdel-Moneim A, Ahmed OM. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J Diabetes Complications. 2012;26:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 186. | Singh AK, Raj V, Keshari AK, Rai A, Kumar P, Rawat A, Maity B, Kumar D, Prakash A, De A, Samanta A, Bhattacharya B, Saha S. Isolated mangiferin and naringenin exert antidiabetic effect via PPAR(γ)/GLUT4 dual agonistic action with strong metabolic regulation. Chem Biol Interact. 2018;280:33-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 187. | Prasath GS, Pillai SI, Subramanian SP. Fisetin improves glucose homeostasis through the inhibition of gluconeogenic enzymes in hepatic tissues of streptozotocin induced diabetic rats. Eur J Pharmacol. 2014;740:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 188. | Loureiro G, Martel F. The effect of dietary polyphenols on intestinal absorption of glucose and fructose: Relation with obesity and type 2 diabetes. Food Rev Int. 2019;35:390-406. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 189. | Liu CL, Yan L, Cai KR, Sun K, Qi Y, Han YL, Zhang XD, Sun XD. Effects of soybean isoflavones on Wnt/β-catenin and the TGF-β1 signaling pathway in renal tissue of type 2 diabetic rats. J Biol Regul Homeost Agents. 2018;32:455-464. [PubMed] |
| 190. | Li F, Gao C, Yan P, Zhang M, Wang Y, Hu Y, Wu X, Wang X, Sheng J. EGCG Reduces Obesity and White Adipose Tissue Gain Partly Through AMPK Activation in Mice. Front Pharmacol. 2018;9:1366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 191. | Pei K, Ou J, Huang J, Ou S. p-Coumaric acid and its conjugates: dietary sources, pharmacokinetic properties and biological activities. J Sci Food Agric. 2016;96:2952-2962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 368] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 192. | Reis CEG, Dórea JG, da Costa THM. Effects of coffee consumption on glucose metabolism: A systematic review of clinical trials. J Tradit Complement Med. 2019;9:184-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 193. | Shi M, Loftus H, McAinch AJ, Su XQ. Blueberry as a source of bioactive compounds for the treatment of obesity, type 2 diabetes and chronic inflammation. J Funct Foods. 2017;30:pp 16-29. [RCA] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 194. | Bahmanzadeh M, Goodarzi MT, Rezaei Farimani A, Fathi N, Alizadeh Z. Resveratrol supplementation improves DNA integrity and sperm parameters in streptozotocin-nicotinamide-induced type 2 diabetic rats. Andrologia. 2019;51:e13313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |