Published online Mar 15, 2023. doi: 10.4239/wjd.v14.i3.209
Peer-review started: August 17, 2022
First decision: December 12, 2022
Revised: January 5, 2023
Accepted: February 15, 2023
Article in press: February 15, 2023
Published online: March 15, 2023
Processing time: 210 Days and 10.4 Hours
Diabetes is a chronic metabolic disease, and a variety of miRNA are involved in the occurrence and development of diabetes. In clinical studies, miR-124 is highly expressed in the serum of patients with diabetes and in pancreatic islet β-cells. However, few reports exist concerning the role and mechanism of action of miR-124 in diabetes.
To investigate the expression of miR-124 in diabetic mice and the potential mechanism of action in islet β-cells.
The expression levels of miR-124 and enhancer of zeste homolog 2 (EZH2) in pancreatic tissues of diabetic mice were detected. The targeted relationship between miR-124 and EZH2 was predicted by Targetscan software and verified by a double luciferase reporter assay. Mouse islet β-cells Min6 were grown in a high glucose (HG) medium to mimic a diabetes model. The insulin secretion, proliferation, cell cycle and apoptosis of HG-induced Min6 cells were detected after interference of miR-124a and/or EZH2.
The expression of miR-124 was upregulated and EZH2 was downregulated in the pancreatic tissue of diabetic mice compared with control mice, and the expression of miR-124 was negatively correlated with that of EZH2. miR-124 was highly expressed in HG-induced Min6 cells. Inhibition of miR-124 promoted insulin secretion and cell proliferation, induced the transition from the G0/G1 phase to the S phase of the cell cycle, and inhibited cell apoptosis in HG-induced Min6 cells. EZH2 was one of the targets of miR-124. Co-transfection of miR-124 inhibitor and siRNA-EZH2 could reverse the effects of the miR-124 inhibitor in HG-induced Min6 cells.
miR-124 is highly expressed in diabetic mice and HG-induced Min6 cells and regulates insulin secretion, proliferation and apoptosis of islet β-cells by targeting EZH2.
Core Tip: In clinical studies, miR-124 is highly expressed in the serum of patients with diabetes and in pancreatic islet β-cells. In this study, the role and mechanism of miR-124 in diabetes was explored. miR-124 was highly expressed in the diabetic mice, suggesting that the miR-124 was involved in the pathological process of diabetes. In high glucose-induced Min6 cells, miR-124 regulated the insulin secretion, proliferation and apoptosis of islet β-cells by targeting enhancer of zeste homolog 2 (EZH2), indicating that miR-124 was involved in the pathological process of diabetes by targeting EZH2, which might provide a new idea and target for the diagnosis and treatment of diabetes.
- Citation: Duan XK, Sun YX, Wang HY, Xu YY, Fan SZ, Tian JY, Yu Y, Zhao YY, Jiang YL. miR-124 is upregulated in diabetic mice and inhibits proliferation and promotes apoptosis of high-glucose-induced β-cells by targeting EZH2. World J Diabetes 2023; 14(3): 209-221
- URL: https://www.wjgnet.com/1948-9358/full/v14/i3/209.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i3.209
Diabetes is a chronic metabolic disease characterized by hyperglycemia and clinical manifestations including polydipsia, polyuria and weight loss. In China, the incidence of diabetes is increasing, and about 90% of patients with diabetes have type 2 diabetes mellitus (T2DM)[1]. At present, it is widely accepted that hyperglycemia plays a vital role in the apoptosis and insulin secretion of pancreatic islet β-cells, and dysfunctional islet β-cells contribute to the serious clinical symptoms of T2DM[2,3]. However, the exact mechanisms of T2DM remain poorly understood because of its complex etiology and pathogenesis. It is, therefore, important to explore the pathogenesis of T2DM in depth.
Evidence exists that a variety of micro RNAs (miRNAs), such as miR-375, miR-29 and miR-146 negatively regulate the synthesis and secretion of insulin, and that this process is associated with the development of T2DM[4,5]. Several studies have shown that miR-124 is highly expressed in uninephrectomized diabetic rats treated with streptozotocin (STZ). Furthermore, overexpression of miR-124a in the Min6 cell line decreases glucose-stimulated insulin secretion by directly affecting the levels of Rab27A[6,7]. At present, evidence is lacking concerning the role and mechanism of miR-124 in diabetes.
Enhancer of zeste homolog 2 (EZH2) is an enzymatic catalytic subunit of polycomb repressive complex 2 that can mediate gene silencing by coordinating with other epigenetic modifying enzymes[8]. Many studies have shown that miR-124 targets EZH2 to regulate the cell proliferation and apoptosis in a variety of cancer cells and is involved in the occurrence and development of cancers[9,10]. Previous studies have shown that with the aging of pancreatic islet cells, the expression of EZH2 gradually decreases, along with a decline in proliferative ability, an increase in apoptosis, and a decrease in insulin levels. These findings suggest that EZH2 plays an important role in the proliferation and apoptosis of pancreatic islet β-cells and the pathogenesis and progression of diabetes[11]. However, whether miR-124 targeting EZH2 is involved in the occurrence and development of diabetes remains unclear.
This study explored the expression of miR-124 and EZH2 in the pancreatic tissue of diabetic mice and the effect of miR-124 on insulin secretion and the proliferation and apoptosis of pancreatic islet β-cells. The aim was to provide new insights and targets for the diagnosis and treatment of diabetes.
Twenty male, specified-pathogen free BALB/c mice aged 6-8 wk, were obtained from Sanxia University (Hubei, China). All mice were fed at 25℃, with relative humidity between 60% and 75%, and a 12-h light/dark cycle with free access to food and water. The mice were randomly divided into a normal control group (NC group) and diabetes group (DM group), with 10 mice in each group.
After acclimation for 1 wk, mice in the DM group were fed with a high sucrose/high-fat diet, whilst mice in the NC group were fed with standard chow for 4 wk. All mice were fasted for 12 h and then the mice in the DM group were intraperitoneally injected with STZ solution (45 mg/kg, dissolved in 0.1 mol/L citrate buffer solution; pH4.4) for 3 d to induce T2DM[12,13]. Mice in the NC group were injected with an equal volume of the same citrate buffer solution for 3 d. Fasting blood glucose levels were measured by the glucose oxidase method at 72 h after the last injection. Mice with fasting blood glucose levels above 16.7 mmol/L were regarded as having T2DM[14,15]. One week after this measurement, the mice were killed under deep anesthesia with excessive ether. Pancreases were immediately dissected and washed with 0.9% cold saline solution and fixed in 10% formalin solution for subsequent experiments.
Part of the pancreatic tissue was fixed in 4% paraformaldehyde for 24 h. After dehydration by ethanol gradients, the pancreatic tissue was embedded in paraffin and sliced into 4 μm sections. Then after dewaxing and rehydration, the sections were stained with hematoxylin and eosin (HE). The histopathological changes of the pancreatic tissue were observed under a light microscope.
Paraffin sections of pancreatic tissue were dewaxed with xylene, rehydrated with ethanol gradients and treated with 3% H2O2 solution at 37℃ for 15 min. Rabbit anti-mouse EZH2 and insulin antibody (Abcam, 1:1000) was incubated overnight at 4℃. After being washed by phosphate buffer saline, horse radish peroxidase-labeled goat anti-rabbit IgG secondary antibody (Abcam, 1:1000) was incubated at 37℃ for 30 min. DAB solution was added to provide the staining for 6-8 min. Next, the sections were counterstained with hematoxylin and observed under a light microscope. The positive cells of EZH2 or insulin were characterized by brown granules in the nucleus. The expression levels of EZH2 and insulin were scored semi-quantitatively based on staining intensity and the percentage of positive cells using Image J software [National Institutes of Health, The United States (USA)]. Briefly, Immunohistochemistry score = staining intensity × percentage of positive cells. Four score categories in immunostaining intensity: negative (0), weak (1), moderate (2), and strongly (3). Percentage of positive defined as 0 = 0%-25%; 1 = 26%-50%; 3 = 51%-75%; 4 = 76%-100%[16]. Five fields were randomly selected in each section.
Mice pancreatic islet β-cells lines Min6 were purchased from Shanghai Meilian Biotechnology Co. Ltd, and the cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM, HyClone) containing 10% fetal bovine serum and 50 mmol/L β-mercaptoethanol in an incubator at 37℃ with 5% CO2. The medium was changed once every other day. When reached 80%-90% confluence, the cells were passaged. And the Min6 cells were incubated in DMEM medium containing 25 mmol/L glucose for 24 h to mimic diabetes model for subsequent experiments.
Small interference RNA targeting EZH2 (si-EZH2) and its negative control (si-NC), miR-124 inhibitor and its negative control (inhibitor-NC), and miR-124 mimic and its negative control (mimic-NC) were purchased from Shanghai GenePharma. The cells were divided into a blank control (BC) group, high glucose culture (HG) medium group (Min6 cells were grown in medium with 25 mmol/L glucose), miR-124 inhibitor group (miR-124 inhibitor was transfected into HG-induced Min6 cells), inhibitor NC group (inhibitor-NC was transfected into HG-induced Min6 cells), miR-124 inhibitor + si-EZH2 group (miR-124 inhibitor and si-EZH2 were co-transfected into HG-induced Min6 cells), and a miR-124 inhibitor + si-NC group (miR-124 inhibitor and si-NC were co-transfected into HG-induced Min6 cells). The cells at logarithmic phase were seeded into a 6-well plate. When the cells reached 70% confluence, the cells were transfected according to the instructions of Lipofectamine 2000. After transfection for 24 h, the following experiments were conducted.
The cells were seeded into 24 well plates at a density of 5 × 103/well and cultured with 1mL Krebs-Ringer bicarbonate buffer containing 25 mmol/L glucose for 2 h at 37℃ and 5% CO2. Then the supernatants were collected and insulin content was measured using enzyme linked immunosorbent assay (ELISA) according to the instructions of the mouse insulin ELISA kit (Millipore, USA).
Cell viability was detected by the cell counting kit-8 (CCK-8) method. The transfected cells were seeded into 96-well plates with five multiple wells in each group. After cell were cultured for 12 h, 24 h, 36 h, and 48 h, 10 μL of CCK-8 solution was added into each well and the cells were incubated for 4 h. Then, the optical density (OD) value at 450 nm of each well was detected by a microplate reader. The cell proliferation curve was depicted according to time as abscissa and OD value as coordinate.
After transfection for 48 h, the cells were washed with PBS and fixed overnight with 70% pre-cooled ethanol at 4 ℃. For detection of cell cycle distribution, 2 μL of RNase A (0.25 mg/mL) and 500 μL of propidium iodide (PI) dye solution (50 μg/mL) were added to the cells and they were incubated for 30 min in the dark at room temperature before cell cycle distribution was assessed by flow cytometry.
For the detection of cell apoptosis, cell density was adjusted to 1 × 105 /mL. For a 100 μL cell suspension, 5 μL Annexin V- Fluorescein isothiocyanate was added, and the cells were incubated for 15min at room temperature. Then, 10 μL of PI was added and the cells were incubated for 5 min in the dark before cell apoptosis was detected by CytoFLEX flow cytometry.
The expression levels of miR-124 and EZH2 in pancreatic tissues and cells were detected by Real-time quantitative polymerase chain reaction (RT-qPCR). The tissue samples were ground under liquid nitrogen and the total RNA from tissues and cells were extracted by Trizol (Invitrogen, USA). The transcriptor First Strand cDNA Synthesis kit (Takara, Japan) was used for the reverse transcription of RNA into cDNA. The SYBR Green Master Mix (Takara, Japan) was used for RT-qPCR. The primers were as follows: miR-124, forward: 5'-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACGGCATTCA-3'; U6, forward: 5'-CTCGCTTCGGCAGCACA-3'; and reverse: 5'-AACGCTTCACGAATTTGCGT-3'; EZH2, forward: 5'-GTAGACACTC CTCCAAGAA-3', and reverse: 5'-GGTCACAGGGTTGATAGTT-3'; β-actin, forward: 5'-CACGAAACTACCTTCAACTCCAT-3', and reverse: 5'-ATCTTGATCTTCATTGTG CTGGG-3'. The reaction conditions: 95 ℃ 3 min, 95 ℃ 15 s, 58 ℃ 30 s, a total of 40 cycles. The relative expression of miR-124 and EZH2 were calculated by 2-ΔΔCt, with U6 and β-actin as the internal references.
The total proteins in tissues and cells were extracted by ProteoPrep® total protein extraction kit (Millipore, USA), and the protein concentration was detected by the bicinchoninic acid method. The protein samples were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and transferred onto polyvinylidene fluoride membranes. The membranes were blocked using Tris-Hydrochloride buffer saline + Tween solution with 5% skim milk for 1h at room temperature. Next, the samples were incubated with first antibodies: EZH2 (Abcam, ab186006, 1:1000), caspase-3 (Abcam, ab13847, 1:500), Bax (Abcam, ab32503, 1:2000) and Bcl-2 (Abcam, ab182858, 1:2000) were incubated overnight at 4℃. After washing with TBST solution, the samples were incubated with the second antibody HRP-IgG (Abcam, ab7090, 1:5000) for 1 h at room temperature. Images were developed by an enhanced chemiluminescence reagent (Thermo-Fisher Scientific, USA) and photographed using a gel imaging system (BioRad, USA). The gray value ratios were analyzed using Image J software to represent the relative expression levels.
Targetscan (http://www.targetscan.org/vert_71/) was used to predict the target gene of miR-124. According to the sequence of pmirGLO and the EZH2 gene in PubMed, the wild-type and mutant primers (containing cleavage sites of Nhe I and Sal I) were synthesized. The wild-type and mutant fragment of the EZH2 gene 3'-untranslated region (UTR) were amplified to construct the recombinant plasmid pmirGLO-EZH2-wild type (WT) and pmirGLO-EZH2-mutant (MUT). Min6 cells were seeded into 24-well plates. After the cells were cultured for 24 h, pmirGLO-EZH2-WT or pmirGLO-EZH2-MUT plasmids and miR-124 mimic or mimic-NC were co-transfected into the cells. After transfection for 48 h, luciferase activity was detected by the double luciferase reporter assay kit (Promega).
All data were analyzed by SPSS 21.0. Data with a normal distribution are presented as mean ± SD. The comparison among multiple groups was analyzed using one-way analysis of variance, followed by a Tukey post hoc test. In the case of small sample sizes or non-parametric distribution, the difference among groups was analyzed by the Kruskal-Wallis test. The correlation between miR-124 and EZH2 was tested by Pearson correlation analysis. Statistical significance was considered to be P < 0.05.
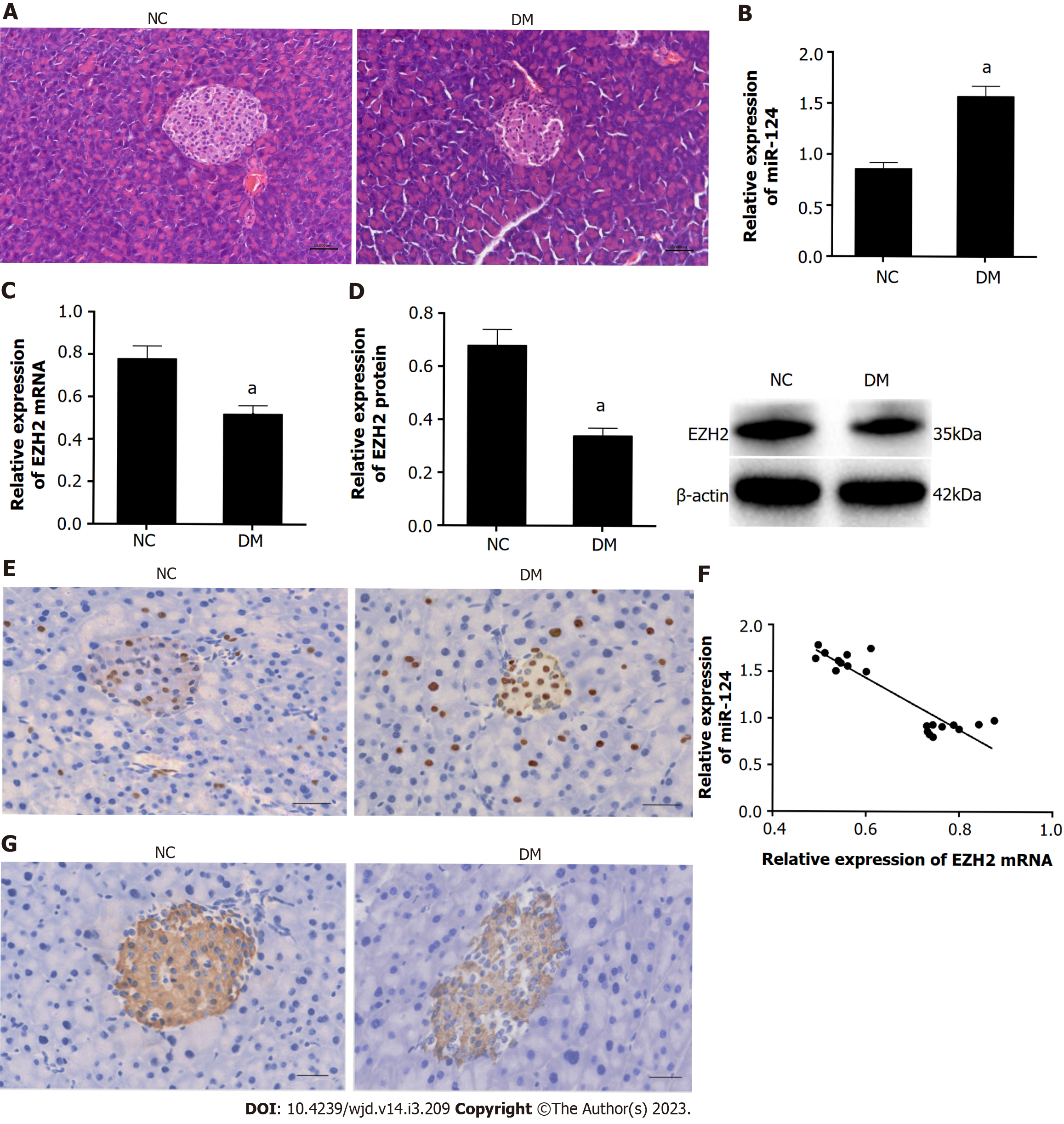
After 3 d of STZ injection, the fasting blood glucose level of mice in the DM group was more than 16.7 mmol/L. Furthermore, HE staining showed that the pancreatic islets of diabetic mice were atrophic and irregular with a fuzzy boundary and fewer cells, which were disordered and swelling. By contrast, the pancreatic islets of NC mice were oval with a clear boundary and many cells, which were evenly distributed and arranged in the islets (Figure 1A). These findings demonstrated that the diabetic mice models had been successfully established.
RT-qPCR was used to detect the expression of miR-124 in pancreatic tissue of mice. The expression of miR-124 in the DM group was significantly higher than that in the NC group (Figure 1B). By contrast, the expression of EZH2 in the DM group was significantly lower than that in the NC group, as detected by RT-qPCR, western blot and immunohistochemistry (Figure 1C-E). Pearson correlation analysis showed that the expression of miR-124 was significantly negatively correlated with EZH2 in pancreatic tissue (r = -0.911, P < 0.05) (Figure 1F). Furthermore, immunohistochemical staining showed that insulin expression in the DM group was lower than that of the NC group (Figure 1G).
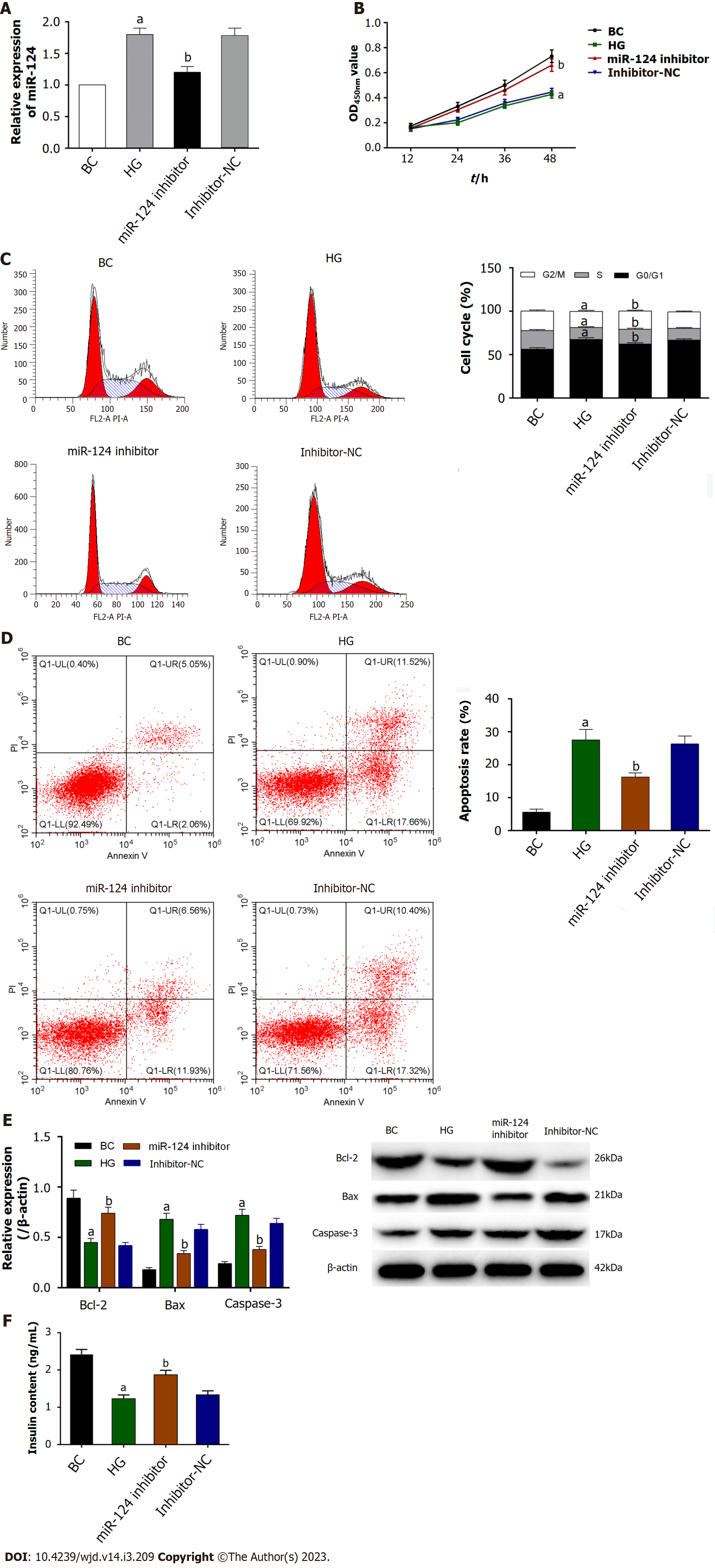
Min6 cells were induced by HG to mimic glucolipotoxicity and establish the diabetes cell model[17]. Firstly, the expression of miR-124 was upregulated in HG-induced Min6 cells detected by RT-qPCR (Figure 2A). Moreover, the expression of miR-124 was downregulated by miR-124 inhibitor in HG-induced Min6 cells, suggesting that miR-124 was highly expressed in the diabetes cell model and was downregulated by miR-124 inhibitor.
The CCK-8 assay was used to detect the proliferation activity of Min6 cells to explore the effect of miR-124 on the proliferation of HG-induced Min6 cells (Figure 2B). The results showed that the proliferation viability of HG-induced Min6 cells was inhibited, whereas knockdown of miR-124 significantly improved the proliferation of HG-induced Min6 cells. These findings suggested that silencing of miR-124 promoted the proliferation of HG-induced Min6 cells.
The cell cycle was detected by flow cytometry to study the effect of miR-124 on the cell cycles. As shown in Figure 2C, HG-induced Min6 cells were arrested at the transition from the G0/G1 phase to the S phase of the cells cycle. In the miR-124 inhibitor group, the proportion of G0/G1 phase cells was significantly reduced and the proportion of S phase cells was significantly increased. This finding suggested that miR-124 inhibitor promoted the cells at the transition from the G0/G1 phase to the S phase of the cell cycle in HG-induced Min6 cells.
As shown in Figure 2D, the apoptosis rate of HG-induced Min6 cells was increased. By contrast, knockdown of miR-124 inhibited the apoptosis of HG-induced Min6 cells. In addition, the expression levels of the apoptosis-related proteins Bax, Bcl-2 and caspase-3 were detected by western blot (Figure 2E). In the HG group, the expression of Bax and caspase-3 were increased, while the expression of Bcl-2 was decreased. In the miR-124 inhibitor group, the opposite effect was shown. miR-124 inhibitor inhibited the increase in expression of Bax and caspase-3 and the decrease in expression of Bcl-2 induced by HG. All results emphasized the pro-apoptotic effect of miR-124 in HG-induced Min6 cells.
To further explore the potential function of miR-124, insulin secretion was detected by ELISA (Figure 2F). The levels of insulin secretion decreased in HG-induced Min6 cells. In addition, insulin content was increased in the miR-124 inhibitor group in response to HG stimulus, suggesting that miR-124 inhibitor promoted the insulin secretion in HG-induced Min6 cells.
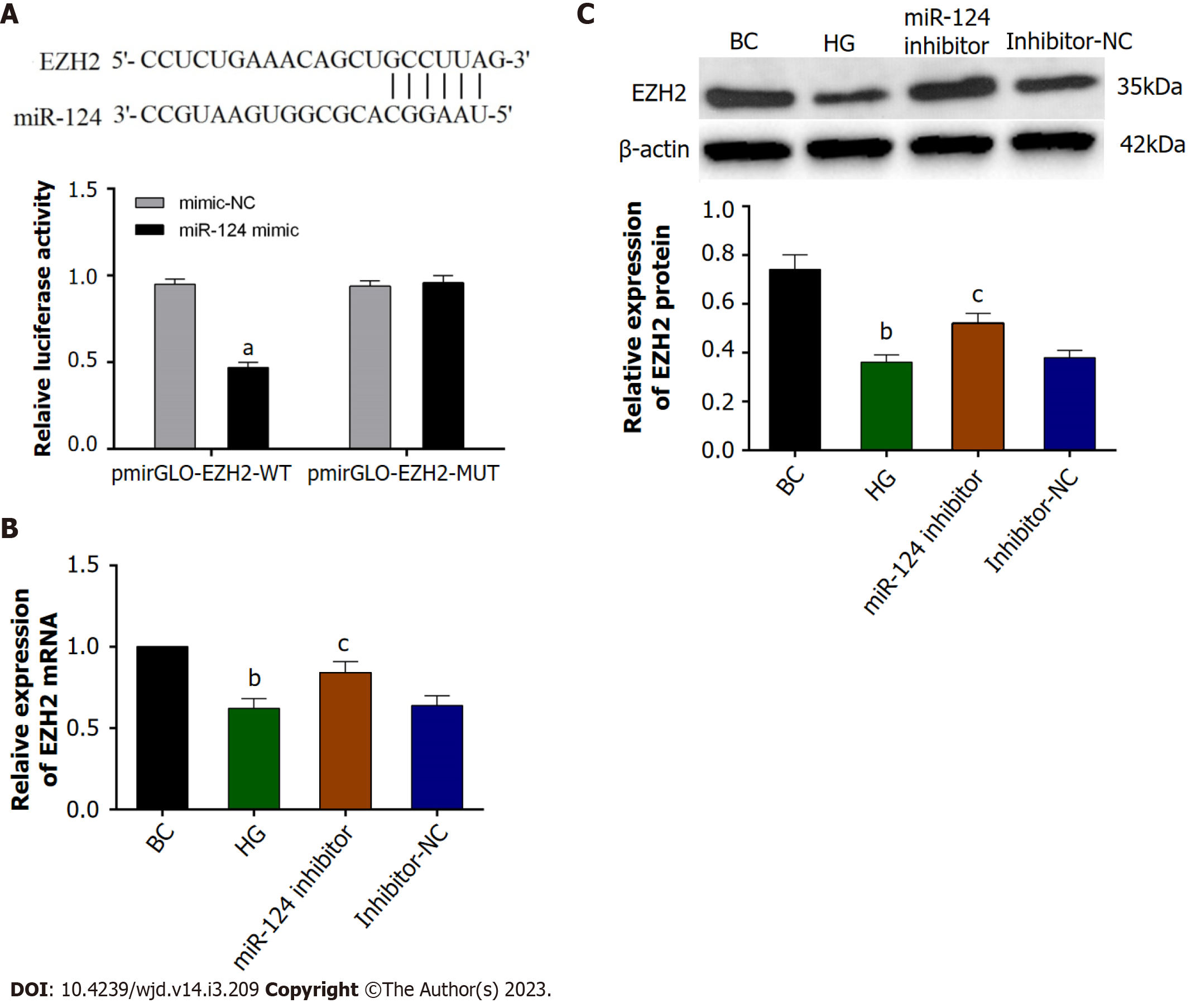
TargetScan predicted that there was a binding site for miR-124 in the 3'-UTR region of the EZH2 gene (Figure 3A). Furthermore, a double luciferase reporter gene assay showed that in Min6 cells transfected with the pmirGLO-EZH2-WT plasmid, the miR-124 mimic reduced the fluorescence intensity (P < 0.05), This result suggested that miR-124 could bind to the 3'-UTR of the EZH2 gene and inhibit luciferase activity. The results indicated that EZH2 was a target gene of miR-124.
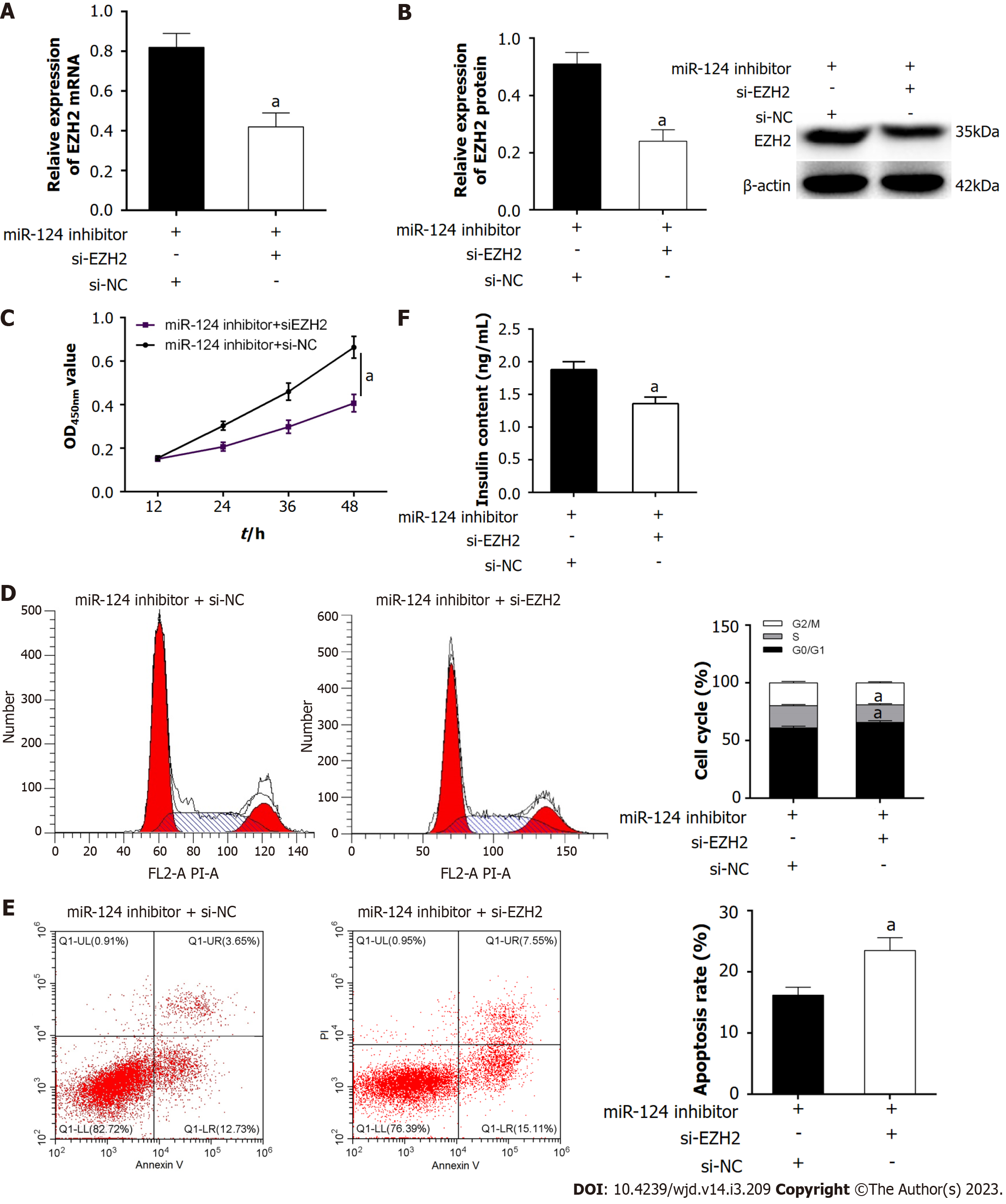
Firstly, RT-qPCR and western blot were used to detect the expression of EZH2 in Min6 cells. The expression of EZH2 was downregulated in HG-induced Min6 cells, and the miR-124 inhibitor could upregulate EZH2 expression in HG-induced Min6 cells (Figure 3B and C). To further investigate whether the miR-124 inhibitor protected pancreatic β-cell function by targeting EZH2, the si-EZH2 and miR-124 inhibitor were co-transfected into HG-induced Min6 cells. In this experiment, the elevation of EZH2 expression induced by the miR-124 inhibitor was inhibited by si-EZH2 (Figure 4A and B). In addition, effect on the proliferation, insulin secretion, cell cycle and apoptosis were investigated (Figure 4C-F). si-EZH2 reduced proliferation activity and insulin secretion in HG-induced Min6 cells transfected with miR-124 inhibitor. The result of flow cytometry showed that si-EZH2 inhibited the promotional effect of miR-124 inhibitor on the cell cycle. Moreover, si-EZH2 reversed the inhibitory effect of the miR-124 inhibitor on apoptosis of HG-induced Min6 cells. Together, the results indicated that silencing of miR-124 promoted the proliferation, insulin secretion, induced the transition from the G0/G1 phase to the S phase of the cell cycle, and suppressed the apoptosis of HG-induced Min6 cells by targeting EZH2.
Diabetes is a metabolic disorder disease characterized by chronic hyperglycemia. The pathological features of diabetes include insulin resistance and a secretory deficiency in pancreatic islets β-cell. Hyperglycemia is the direct clinical manifestation of diabetes, which can induce pancreatic β-cell apoptosis and decrease β-cell proliferation[18]. Dysfunctional β-cells result in low insulin secretion and subsequently accelerated development of diabetes[19]. In this study, mice were fed with a high sucrose/high-fat diet combined with a low-dose STZ injection to establish the mouse model of T2DM[12]. To model diabetic mouse islets β-cells, Min6 cells were grown in HG medium to mimic glucolipotoxicity. These cells exhibited more suppressive effects on β-cell viability and proliferation compared with cells grown in normal medium, which is consistent with the findings of previous studies[20]. Insulin secretion also decreased in HG-induced Min6 cells. This decrease might result from hypofunction of HG-induced Min6 cells, leading to decreased glucose uptake and, therefore, decreased insulin secretion[21].
A variety of miRNAs were abnormally expressed in patients with diabetes and affected insulin secretion by regulating the differentiation of islet β-cells, or affecting insulin synthesis and secretion[22]. Kong et al[23] found that miR-9, miR-29a, miR-30d, miR-34a, miR-124a, miR-146a, and miR-375 Levels were significantly elevated in the serum of individuals with T2DM. Guo et al[24] also found that miR-124 was highly expressed in T2DM patients and involved in glucose and lipid metabolism in diabetes.
In this study, miR-124 was upregulated in the pancreatic tissue of diabetic mice and in HG-induced Min6 cells. Inhibition of miR-124 expression in HG-induced Min6 cells promoted proliferation activity and insulin secretion and also inhibited cell apoptosis. The results suggested that miR-124 was involved in the occurrence and development of diabetes by regulating insulin secretion, proliferation and apoptosis of pancreatic islets β-cells.
Previous studies of miR-124 have found that miR-124 regulates the expression of SNAP25, Rab3A, Noc2 or other target genes to mediate insulin secretion of islets β-cells[25]. Weng et al[26] found that insulin secretion levels were decreased in INS-1 cells with impaired insulin secretion and speculated that upregulation of miR-124 was one mechanisms by which insulin secretion was reduced in INS-1 cells. Another study also reported that miR-124 could negatively target forkhead box A2 to regulate the function of islet β-cells[27]. Parsa et al[28] pointed out that the levels of miR-124a in plasma were significantly reduced in patients with diabetes compared with individuals without diabetes and that miR-124a may affect the pathogenesis and progression of diabetes by regulating TRIB3. In the current study, EZH2 was downregulated in the pancreatic tissue of diabetic mice, which was negatively correlated with the level of miR-124, consistent with the results of Lu et al[11]. Furthermore, bioinformatics and a double luciferase reporter gene assay confirmed that EZH2 was one of the target genes of miR-124 and was negatively regulated by miR-124. Neo et al[29] showed that EZH2 was downregulated in neural P19 cells and negatively correlated with the expression of miR-124, and miR-124 promoted the differentiation of P19 by targeting EZH2. Ma et al[30] found that miR-124 played an anti-tumor role in cholangiocarcinoma by regulating the EZH2- signal transducer and activator of transcription 3 signal axis and inducing autophagy. Tian et al[31] showed that miR-124 negatively regulated the expression of EZH2 to inhibit the proliferation, migration and invasion of esophageal cancer cells. In order to explore whether miR-124 regulated the proliferation and apoptosis of Min6 cells by targeting EZH2, the miR-124 inhibitor and si-EZH2 were co-transfected into Min6 cells and the biological characteristics were detected. The results showed that miR-124 could target EZH2, to inhibit insulin secretion and proliferation activity and induce apoptosis in HG-induced Min6 cells.
In conclusion, this study found that miR-124 was highly expressed in diabetic mice and HG-induced Min6 cells. miR-124 inhibited insulin secretion and cell proliferation, promoted apoptosis of islet β-cells via targeting EZH2 and was involved in the pathological process of diabetes. This study indicates a new target for the treatment or research into the pathogenesis of diabetes. However, the study had some limitations: First, how EZH2 promotes insulin secretion was not studied. Furthermore, one miRNA can potentially target several genes. Therefore, miR-124 might target other genes to regulate islet βcells function and the development of diabetes. Further experiments are needed to elaborate the role and mechanism of miR-124 in diabetes. Currently, long non-coding RNAs (lncRNAs) are another important type of RNA involved in the pathogenesis of several diseases in cooperation with miRNAs. Several lncRNAs cooperate with miR-124 to regulate diseases, including cancer or stroke. Therefore, the lncRNA that regulates miR-124 in T2DM should be investigated.
Diabetes is a metabolic disorder disease characterized by chronic hyperglycemia. The pathological features of diabetes include insulin resistance and a secretory deficiency in pancreatic islets β-cell. In clinical studies, a variety of miRNA are involved in the occurrence and development of diabetes. And miR-124 is highly expressed in the serum of patients with diabetes and in pancreatic islet β-cells. So, the role and mechanism of action of miR-124 in diabetes was explored in the diabetes mellitus type 2 (T2DM) mice and high glucose (HG) -induced Min6 cells.
A variety of miRNAs were abnormally expressed in patients with diabetes and affected insulin secretion by regulating the differentiation of islet β-cells, or affecting insulin synthesis and secretion. So, what is the role and mechanism of miR-124 in diabetes.
The present study aimed to investigate the expression of miR-124 in diabetic mice and the potential mechanism of action in islet β-cells.
Mice were fed with a high sucrose/high-fat diet combined with a low-dose streptozotocin injection to establish the mouse model of T2DM. The expression levels of miR-124 and enhancer of zeste homolog 2 (EZH2) in pancreatic tissues of diabetic mice were detected. The targeted relationship between miR-124 and EZH2 was predicted by Targetscan software and verified by a double luciferase reporter assay. To model diabetic mouse islets β-cells, Min6 cells were grown in HG medium to mimic glucolipotoxicity. The insulin secretion, proliferation, cell cycle and apoptosis of HG-induced Min6 cells were detected after interference of miR-124a and/or EZH2.
The expression of miR-124 was upregulated and EZH2 was downregulated in the pancreatic tissue of diabetic mice compared with control mice, and the expression of miR-124 was negatively correlated with that of EZH2. miR-124 was highly expressed in HG-induced Min6 cells. Inhibition of miR-124 promoted insulin secretion and cell proliferation, induced the transition from the G0/G1 phase to the S phase of the cell cycle, and inhibited cell apoptosis in HG-induced Min6 cells. EZH2 was one of the targets of miR-124. Co-transfection of miR-124 inhibitor and siRNA-EZH2 could reverse the effects of the miR-124 inhibitor in HG-induced Min6 cells.
miR-124 is highly expressed in diabetic mice and HG-induced Min6 cells and regulates insulin secretion, proliferation and apoptosis of islet β-cells by targeting EZH2.
miR-124-EZH2 axis might be one of the pathogenesis mechanisms of diabetes, providing a new target for the treatment of diabetes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Seetharaman RV, India; Zhang Z, China S-Editor: Liu GL L-Editor: A P-Editor: Guo X
| 1. | Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Type 2 Diabetes and its Impact on the Immune System. Curr Diabetes Rev. 2020;16:442-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 517] [Article Influence: 103.4] [Reference Citation Analysis (0)] |
| 2. | Aguayo-Mazzucato C, van Haaren M, Mruk M, Lee TB Jr, Crawford C, Hollister-Lock J, Sullivan BA, Johnson JW, Ebrahimi A, Dreyfuss JM, Van Deursen J, Weir GC, Bonner-Weir S. β Cell Aging Markers Have Heterogeneous Distribution and Are Induced by Insulin Resistance. Cell Metab. 2017;25:898-910.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 3. | Ashcroft FM, Rohm M, Clark A, Brereton MF. Is Type 2 Diabetes a Glycogen Storage Disease of Pancreatic β Cells? Cell Metab. 2017;26:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Massart J, Sjögren RJO, Lundell LS, Mudry JM, Franck N, O'Gorman DJ, Egan B, Zierath JR, Krook A. Altered miR-29 Expression in Type 2 Diabetes Influences Glucose and Lipid Metabolism in Skeletal Muscle. Diabetes. 2017;66:1807-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 5. | Zhuang P, Muraleedharan CK, Xu S. Intraocular Delivery of miR-146 Inhibits Diabetes-Induced Retinal Functional Defects in Diabetic Rat Model. Invest Ophthalmol Vis Sci. 2017;58:1646-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Li D, Lu Z, Jia J, Zheng Z, Lin S. MiR-124 is related to podocytic adhesive capacity damage in STZ-induced uninephrectomized diabetic rats. Kidney Blood Press Res. 2013;37:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Dehwah MA, Xu A, Huang Q. MicroRNAs and type 2 diabetes/obesity. J Genet Genomics. 2012;39:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Duan R, Du W, Guo W. EZH2: a novel target for cancer treatment. J Hematol Oncol. 2020;13:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 435] [Cited by in RCA: 596] [Article Influence: 119.2] [Reference Citation Analysis (0)] |
| 9. | Tan JZ, Yan Y, Wang XX, Jiang Y, Xu HE. EZH2: biology, disease, and structure-based drug discovery. Acta Pharmacol Sin. 2014;35:161-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 169] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 10. | Chang CJ, Hung MC. The role of EZH2 in tumour progression. Br J Cancer. 2012;106:243-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 11. | Lu D, Lv LL, Liu D, Wu JL, Wang XX. Relationship between enhancer of zeste homolog 2 (EZH2) and age-related islet cell function. Zhongguo Tangnianbing Zazhi. 2014;22:548-552. [DOI] [Full Text] |
| 12. | Xiang Z, Xie H, Tong Q, Pan J, Wan L, Fang J, Chen J. Revealing hypoglycemic and hypolipidemic mechanism of Xiaokeyinshui extract combination on streptozotocin-induced diabetic mice in high sucrose/high fat diet by metabolomics and lipidomics. Biomed Pharmacother. 2021;135:111219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Ren Z, Yang Z, Lu Y, Zhang R, Yang H. Antiglycolipid disorder effect of epigallocatechin3gallate on highfat diet and STZinduced T2DM in mice. Mol Med Rep. 2020;21:2475-2483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Li D, Jiang C, Mei G, Zhao Y, Chen L, Liu J, Tang Y, Gao C, Yao P. Quercetin Alleviates Ferroptosis of Pancreatic β Cells in Type 2 Diabetes. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 196] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 15. | Xu L, Li Y, Yin L, Qi Y, Sun H, Sun P, Xu M, Tang Z, Peng J. miR-125a-5p ameliorates hepatic glycolipid metabolism disorder in type 2 diabetes mellitus through targeting of STAT3. Theranostics. 2018;8:5593-5609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 16. | Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, Liu Q, Dou R, Xiong B. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer. 2019;18:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 572] [Article Influence: 95.3] [Reference Citation Analysis (1)] |
| 17. | Deng S, Yang L, Ma K, Bian W. Astragalus polysaccharide improve the proliferation and insulin secretion of mouse pancreatic β cells induced by high glucose and palmitic acid partially through promoting miR-136-5p and miR-149-5p expression. Bioengineered. 2021;12:9872-9884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Wang S, Yang Z, Gao Y, Li Q, Su Y, Wang Y, Zhang Y, Man H, Liu H. Pyruvate kinase, muscle isoform 2 promotes proliferation and insulin secretion of pancreatic β-cells via activating Wnt/CTNNB1 signaling. Int J Clin Exp Pathol. 2015;8:14441-14448. [PubMed] |
| 19. | Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6:456-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 649] [Cited by in RCA: 768] [Article Influence: 76.8] [Reference Citation Analysis (10)] |
| 20. | Wang M. miR-433 protects pancreatic β cell growth in high-glucose conditions. Mol Med Rep. 2017;16:2604-2610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Song Z, Ma J, Lu Y, Zhou C, Zhao T, Ai X, Wei X, Lin J, Wang W, Yan W, Jiao P. The protective role of the MKP-5-JNK/P38 pathway in glucolipotoxicity-induced islet β-cell dysfunction and apoptosis. Exp Cell Res. 2019;382:111467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Lovis P, Gattesco S, Regazzi R. Regulation of the expression of components of the exocytotic machinery of insulin-secreting cells by microRNAs. Biol Chem. 2008;389:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 214] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 23. | Kong L, Zhu J, Han W, Jiang X, Xu M, Zhao Y, Dong Q, Pang Z, Guan Q, Gao L, Zhao J, Zhao L. Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: a clinical study. Acta Diabetol. 2011;48:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 410] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 24. | Guo DG, Tian WZ, Liu SY, Lu XY, Li HM. Expression of miR-9 and miR-124 in peripheral blood and their clinical significance in primary type 2 diabetes mellitus. Shiyong Tangnianbing Zazhi. 2012;8:49-51. |
| 25. | de Siqueira KC, de Lima FM, Lima FS, Taki MS, da Cunha CF, de Lima Reis SR, Camargo RL, Batista TM, Vanzela EC, Nardelli TR, Carneiro EM, Bordin S, Ignácio-Souza LM, Latorraca MQ. miR-124a expression contributes to the monophasic pattern of insulin secretion in islets from pregnant rats submitted to a low-protein diet. Eur J Nutr. 2018;57:1471-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Weng SY, Mao ZJ, Chai KF. Effects of Yiqihuazhuo decoction on improvement of insulin secretion in ISD-INS-1 cells by down-regulating miR-124-3p. Zhonghua Zhongyiyao Xuekan. 2019;37:563-568. [DOI] [Full Text] |
| 27. | Jing G, Westwell-Roper C, Chen J, Xu G, Verchere CB, Shalev A. Thioredoxin-interacting protein promotes islet amyloid polypeptide expression through miR-124a and FoxA2. J Biol Chem. 2014;289:11807-11815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Parsa E, Doustimotlagh AH, Rezaeinejad F, Alipoor S, Esmaeeli M, Sharifi A, Alipoor B. Decreased Plasma Level of TRIB3 is Associated with Circulating miR-124a in Patients with Type 2 Diabetes. Clin Lab. 2020;66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Neo WH, Yap K, Lee SH, Looi LS, Khandelia P, Neo SX, Makeyev EV, Su IH. MicroRNA miR-124 controls the choice between neuronal and astrocyte differentiation by fine-tuning Ezh2 expression. J Biol Chem. 2014;289:20788-20801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 30. | Ma J, Weng L, Wang Z, Jia Y, Liu B, Wu S, Cao Y, Sun X, Yin X, Shang M, Mao A. MiR-124 induces autophagy-related cell death in cholangiocarcinoma cells through direct targeting of the EZH2-STAT3 signaling axis. Exp Cell Res. 2018;366:103-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Tian Z, Li Z, Zhu Y, Meng L, Liu F, Sang M, Wang G. Hypermethylation-mediated inactivation of miR-124 predicts poor prognosis and promotes tumor growth at least partially through targeting EZH2/H3K27me3 in ESCC. Clin Exp Metastasis. 2019;36:381-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |