Published online Dec 15, 2023. doi: 10.4239/wjd.v14.i12.1738
Peer-review started: September 2, 2023
First decision: September 29, 2023
Revised: October 10, 2023
Accepted: November 14, 2023
Article in press: November 14, 2023
Published online: December 15, 2023
Processing time: 102 Days and 23.3 Hours
Monogenic diabetes is caused by one or even more genetic variations, which may be uncommon yet have a significant influence and cause diabetes at an early age. Monogenic diabetes affects 1 to 5% of children, and early detection and gene-tically focused treatment of neonatal diabetes and maturity-onset diabetes of the young can significantly improve long-term health and well-being. The etiology of monogenic diabetes in childhood is primarily attributed to genetic variations affecting the regulatory genes responsible for beta-cell activity. In rare instances, mutations leading to severe insulin resistance can also result in the development of diabetes. Individuals diagnosed with specific types of monogenic diabetes, which are commonly found, can transition from insulin therapy to sulfonylureas, provided they maintain consistent regulation of their blood glucose levels. Scientists have successfully devised materials and methodologies to distinguish individuals with type 1 or 2 diabetes from those more prone to monogenic diabetes. Genetic screening with appropriate findings and interpretations is essential to establish a prognosis and to guide the choice of therapies and management of these interrelated ailments. This review aims to design a comprehensive literature summarizing genetic insights into monogenetic diabetes in children and adolescents as well as summarizing their diagnosis and mana-gement.
Core Tip: Monogenic diabetes, a rare yet impactful condition in childhood, results from genetic variations, causing early-onset diabetes. Affecting 1%-5% of children, early detection and tailored genetic treatments can enhance long-term health. Culprits include genetic variations in beta-cell regulatory genes and severe insulin resistance. Identifying specific types allows transitioning to sulfonylureas while maintaining glucose control. Tools to differentiate diabetes types underscore genetic screening's importance for prognosis and treatment guidance. This review delves into genetic insights into childhood monogenic diabetes, offering diagnosis and management guidance for affected youth's better health.
- Citation: Sun HY, Lin XY. Genetic perspectives on childhood monogenic diabetes: Diagnosis, management, and future directions. World J Diabetes 2023; 14(12): 1738-1753
- URL: https://www.wjgnet.com/1948-9358/full/v14/i12/1738.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i12.1738
Diabetes mellitus (DM) is a well-known metabolic syndrome characterized by elevated blood glucose levels and its frequently related symptoms, including polyuria and polyphagia. It has the potential to produce substantial medical issues, reducing life longevity and performance of life, and stands as a significant public health concern. For persons born in the United States, the lifetime chance of acquiring diabetes is predicted to be one in three[1]. Types of diabetes are commonly classified as autoimmune-mediated type 1 diabetes, which causes insulin insufficiency; diabetes caused by pancreatic injuries; diabetes caused by particular genetic abnormalities; and type 2 diabetes, characterized by decreased insulin production and resistance to insulin's activities[2,3]. Table 1 below shows the general classification of diabetes.
| Type of diabetes | Causes and characteristics |
| Type 1 | Insulin insufficiency is caused by the autoimmune destruction of β-cells |
| Type 2 | Insulin resistance and inadequate secretion of insulin |
| Gestational | Diagnosed in 2nd or 3rd trimester of pregnancy |
| Monogenic | Due to genetic variation in one or multiple genes leading to maternally-inherited diabetes and deafness, mature-onset diabetes of the young, and Neonatal diabetes |
The types of diabetes that are caused by monogenic alterations are the ones that are better suited to much more specific therapies. There are more than 50 genetic subgroups wherein the transmutation seems unaffected by behavioral or environmental variables. Since monogenic types of diabetes have a recognized origin, their pathophysiological mechanisms are more appreciated adequately than those of other diabetes types. Although these abnormalities constitute a minute percentage of overall diabetes cases (about 1 to 5% of findings in pediatric and young people), they provide a chance to display the practicality of accurate prognostic and treatment procedures[4-6]. Despite the necessity of a precise diagnosis, it is believed that about 80% of overall monogenic diabetes patients stay undiagnosed[7].
A single genetic mutation induces an uncommon kind of diabetes called monogenic diabetes. Gestational diabetes due to a mutation in the glucokinase (GCK) gene, maternally inherited diabetes and deafness (MIDD), mature-onset diabetes of the young (MODY), and other conditions are examples of such mutations. Early-onset diabetes and familial background of diabetes in several first-degree cousins are two characteristics of individuals with monogenic diabetes. Type 1 DM and type 2 DM are common misdiagnoses for monogenic diabetes. In some circumstances, the causal gene can guide the therapeutic strategy, and precise molecular and genetic identification of monogenic diabetes assists in the identification of affected members of the family. MODY is the least frequent subtype of monogenic diabetes among several forms. This is a medically diverse collection of illnesses characterized by cell malfunction, which results in early-onset diabetes and is inherited autosomally[8-10].
One of the primary challenges in diagnosing monogenic diabetes in pediatric patients lies in its clinical and genetic heterogeneity. Currently, the diagnosis often involves a combination of clinical presentation, family history, and genetic testing[11]. However, recent advancements in genetic testing methodologies have significantly improved our ability to identify specific genetic mutations associated with monogenic diabetes. Recent studies have shown promising results using next-generation sequencing (NGS) technologies in identifying monogenic forms of diabetes. These techniques allow for a more comprehensive analysis of the patient's genetic profile, enabling the detection of rare mutations that traditional methods might have missed. In addition to NGS, there is ongoing research into using machine learning algorithms to assist in interpreting genetic data. These algorithms can help clinicians pinpoint potential genetic mutations and streamline the diagnostic process[12-14].
The treatment of monogenic diabetes in pediatric patients is evolving to become more tailored and disease-specific. Understanding the genetic basis of the condition allows for targeted therapies that can address the root cause of the disease[15]. Recent therapeutic advancements include the development of gene-based therapies, such as gene editing techniques like CRISPR-Cas9, which hold promise in correcting genetic mutations responsible for monogenic diabetes. These therapies have succeeded in preclinical studies and may offer a potential cure for certain subtypes of monogenic diabetes[16]. International collaborations and data-sharing initiatives have also enabled researchers to collect valuable information on the global challenges of treating monogenic diabetes in pediatric populations. This collaborative approach fosters the sharing of best practices and the development of innovative treatment strategies[17].
This article aims to provide a comprehensive overview of the complex connections between genetic mutations, clinical symptoms, and treatment approaches in children and teenagers with monogenic diabetes. The article aims to improve the understanding of clinicians, researchers, and healthcare providers by exploring the genetic aspects of this condition. This will help them make informed diagnosis, treatment, and long-term care decisions. Moreover, with the ongoing progress in genetic research, this review article becomes crucial in laying the foundation for enhancing patient outcomes, developing personalized therapeutic strategies, and identifying potential areas for future research and intervention.
Monogenic diabetes encompasses a collection of infrequent hereditary variants of diabetes that arise from mutations occurring in a solitary gene. In pediatrics, monogenic diabetes, or MODY, has been comprehensively screened in many investigations with an estimated frequency of 1.1-4.2 percent[9]. A baseline MODY occurrence of 1.2 percent was found in the United States multicenter population-based study "SEARCH for Diabetes in Youth", and a further 0.2 percent had neonatal diabetes. Monogenic diabetes is 2.5 percent more common in individuals diagnosed in pediatric clinics in the United Kingdom than in patients diagnosed in general demographics over the age of 20 years[10,18]. The mutations could have occurred spontaneously, or they could have been transmitted predominately or recessively. Mutations in only one gene cause monogenic diabetes inherited either dominantly or recessively, or it could be a spontaneous case due to a de novo mutation. Most childhood cases of monogenic diabetes are caused by mutations in the genes that control beta-cell function.
In contrast, mutations causing severe insulin resistance can occasionally cause diabetes. Molecular genetic testing yields a diagnosis in about 1 or 2 percent of cases. Clinicians believed long ago that an abnormally significant heritable mutation could produce diabetes in some people. The observation was made on two primary clinical characteristics indicative of a putative monogenic origin, including diabetes in newborns or neonatal DM (NDM) and family having diabetes in teenagers or early adulthood from many generations, indicating an autosomal dominant inheritance pattern[18].
Monogenic β-cell malfunction is known as MODY and was first clinically diagnosed in the 1970s by examining numerous multigenerational families[19]. MODY is identified by:
Onset at an early age, dominant, autosomal inheritance, No signs of metabolic syndrome, Persistent synthesis of endogenous insulin, Not having β-cell autoimmunity.
MODY accounts for 1%-6% of all diabetes cases, and its prevalence is increasing among children and young individuals. However, it is believed that a significant portion, around 80%, of MODY cases are misdiagnosed as either type 1 or type 2 diabetes[7,20]. Some advanced nations offer molecular genetic diagnostics, utilizing mainly Sanger sequencing, which costs £350 per gene in the United Kingdom as of this writing. The ramifications of molecular diagnosis are significant for both the probands and their families, which will benefit from cascade monitoring and definitive diagnosis as well as from individualized care made possible by molecular diagnosis. GCK, hepatic nuclear factor 4α (HNF4A), hepatic nuclear factor 1b (HNF1B), and hepatic nuclear factor 1α (HNF1A) gene mutations are the leading causes of the most prevalent kinds of MODY (in order of frequency in the United Kingdom)[21,22].
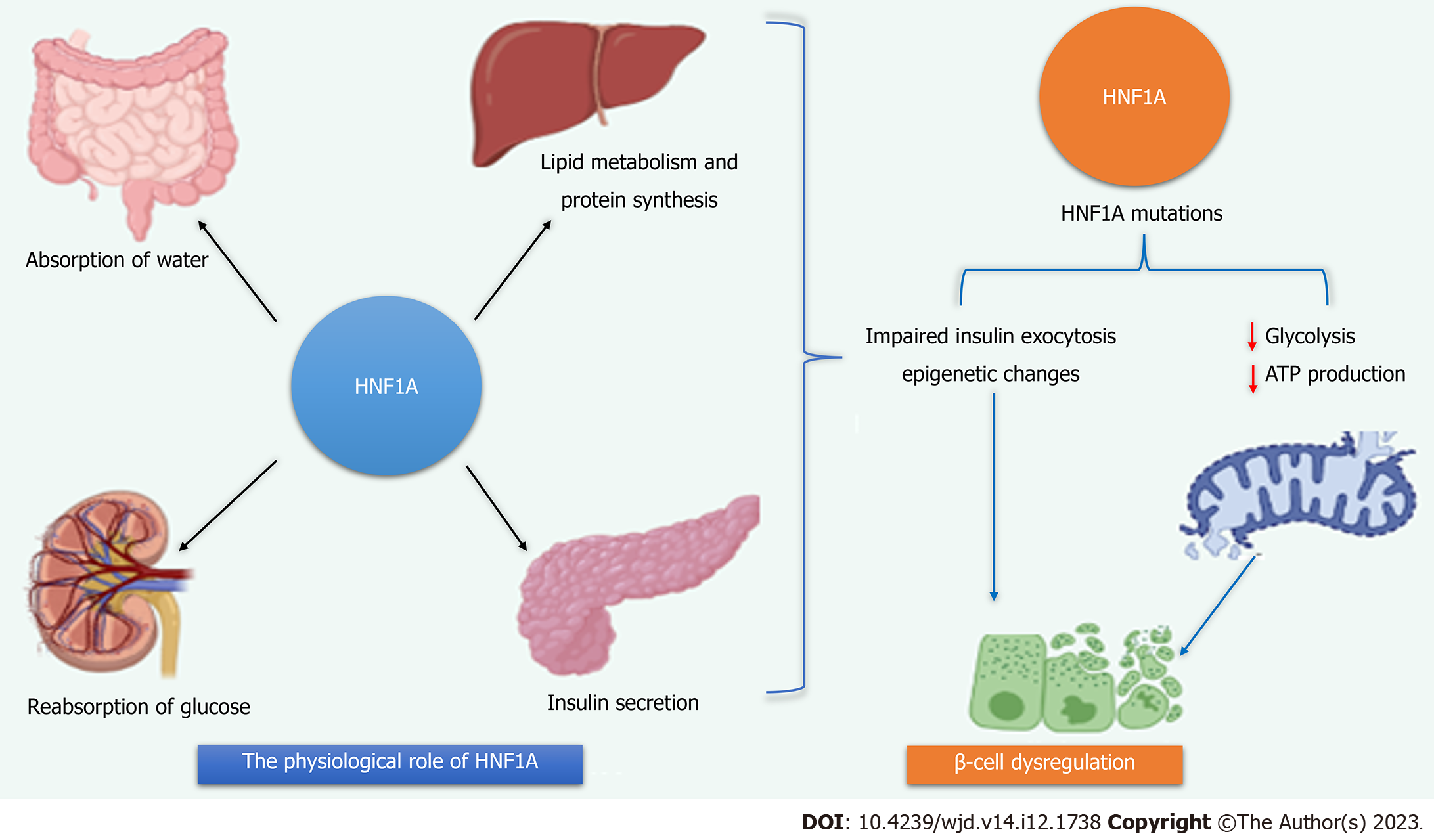
In the United Kingdom, 52% of all incidences of MODY fall into this category, making it the most prevalent type of monogenic diabetes. Hyperglycemia occurs in the 2nd and 4th decades of life due to genetic abnormalities in the transcription factor HNF1A, which promote increasing β-cell malfunction (Figure 1)[23]. Similar to type 1 and 2 diabetes, expert follow-up is advised since microvascular and macrovascular complications are frequent if glycemic objectives are not met. Sensitivity to sulfonylurea medications is among HNF1A-most MODY's significant characteristics[24]. The very first therapy is the administration of a small number of oral hypoglycemics, such as gliclazide (20-40 mg daily), which may typically be effectively replaced in patients who were previously treated with other medications, such as insulin, without a decline in glycemic control. However, it is important to note that therapy escalation is a common occurrence as individuals undergo treatment, particularly when it comes to insulin usage as β-cell dysfunction continues to deteriorate over time. Extra-pancreatic characteristics of HNF1A-MODY include reduced blood C-reactive protein concentration and a low renal glucose threshold. The latter characteristic, in particular, may serve as a useful diagnostic marker for identifying this condition[21,25].
Another form of MODY is caused by a defect in insulin gene (INS) promoter factor 1 (IPF1). PDX1 is a transcription factor that contains a homeobox and plays a role in pancreatic development and the expression of INSs. The NEUROD1 mutation is found in a basic-loop-helix transcription factor, which impacts the development of both the pancreas and neurons. Most patients must undergo insulin treatment[2].
The significant and frequent reason for monogenic diabetes, known as GCK-MODY or non-progressive hyperglycemia associated with GCK, is thought to affect as many as 1 in 1000 people[26]. GCK (β-cell glucose sensor) carries heterozygous inactivating mutations causing GCK-MODY[27,28]. The chain of processes leading to insulin production is initiated by glucose metabolism, triggered by GCK activity. However, when GCK activity is impaired, the threshold glucose level needed to start insulin secretion is raised, even though the β-cell function is relatively unaffected[29,30]. Disorders in such pathways are also brought on by GCK's crucial process in the storage and release of liver glucose. The end outcome is mild fasting hyperglycemia with an A1C of 5.8 to 7.6% (40 to 60 mmol/mol) and a range of 97 to 150 mg/dL (5.4 to 8.3 mmol/L) in most cases[31]. Even though there may occasionally be an age-associated elevation in A1C similar to that reported in elderly populations, this trend is present at birth (congenital). It has remained remarkably steady through time[32]. Patients are asymptomatic and are not detected with hyperglycemia until accidental lab testing or regular monitoring, frequently as pediatric accidental hyperglycemia, throughout pregnancy, or after an incidental illness results in the condition[33-36].
Diabetes and renal cysts are the two most common features of HNF1B-MODY. However, other developing abnormalities in many systems can also occur[37]. Since the etiology is a deficiency in β-cell growth, this type of diabetes commonly manifests in adolescence or early adulthood, is frequently insulin-dependent, and often requires insulin. There is a decreased pancreatic exocrine function, which may need to be treated. Exocrine pancreatic insufficiency can be diagnosed with the help of a smaller pancreatic tail or low fecal elastase levels. Several developmental kidney diseases have been reported, albeit renal cysts are typically present. The most frequent genetic cause of pediatric kidney disease, which accounts for 20%-30% of cases, is HNF1B-MODY[38].
The most frequent reason for permanent neonatal DM (PNDM) and a significant source of transient newborn (TNDM) is activated heterozygous abnormalities in either gene encoding the subunits of the β-cell ATP-sensitive potassium (KATP) channel (KCNJ11 or ABCC8)[39,40]. However, in the presence of acute hyperglycemia, mutant channels maintain membrane hyperpolarization. However, these deficiencies can be treated with large doses of sulfonylurea, allowing patients to transition from insulin and resume meal-stimulated insulin release with little to no hypoglycemia. However, after more than 10 years of therapy, effective glycemic control frequently lasts[41-43]. When a genetic diagnosis is made, initial sulfonylurea administration could, at least to some extent, alleviate a range of neurological impairments caused by more harmful variations. The medical phenotype is connected with the intensity of the mutation[44,45]. TNDM is commonly caused by gentle stimulatory mutations (ABCC8 more frequently than KCNJ11), or they may manifest as a specific type of MODY in people or families who develop later MODY-like diabetes, which is typically able to respond to a sulfonylurea and are not identified to have had neonatal hyperglycemia[46,47]. Bi-allelic moderately activated mutations (often homozygous) and compound heterozygous abnormalities, wherein one mutant is stimulating while the other is an impairment form, are two additional uncommon causes of neonatal diabetes from KATP mutations. Nevertheless, neonatal hyperinsulinism is caused by homozygous loss of function mutations in either gene[48,49].
A hereditary assessment may not alter the course of therapy for some varieties of monogenic diabetes, and that may nonetheless open the door to a precision-based strategy. For instance, the second most frequent etiology of PNDM is heterozygous abnormalities in the pro INS, which gradually deteriorate β-cell functioning capability due to the accumulation of improperly coiled proinsulin proteins[50]. Even though the only existing therapy is insulin, delaying the gradual decline of β-cell activity and improving long-term consequences may be possible by reducing the stimulation for increased synthesis of the genetically variant nutrients via minimizing blood glucose levels by initial intensive insulin administration[51]. Both permanent and transient neonatal diabetes are also caused by nonsense or promoter variations of the INS that inhibit or significantly reduce insulin production. The most effective treatment choices for these uncommon patients have not yet been determined[52-54]. Different types of monogenic diabetes in young are given in Table 2 below.
| Phenotypes | Responsible gene | Characteristics |
| HNF1A-MODY | HNF1A | Loss of function of the β-cell transcription factor, glucosuria, |
| GCK-MODY | GCK | Reduced glucokinase enzyme function, raising insulin secretion setpoint |
| HNF1B-MODY | HNF1B | Pancreatic/renal transcription factors' loss of functioning, genitourinary/renal malformations, exocrine pancreatic insufficiency, hypomagnesemia, variations in liver function tests, developmental delay, hyperuricemia |
| KCNJ11-NDM | KCNJ11 | Mutation in the β-cell KATP channels' Kir6.2 subunit leads to impaired neuro-developmental dysfunction of insulin secretion |
| ABCC8-MODY | ABCC8 | Mutation in the β-cell KATP channels' SUR1 subunit, leading to impaired insulin secretion and neurodevelopmental dysfunctions |
| INS-NDM and-MODY | INS | abnormalities in the proinsulin gene leading to a gradual deterioration of β-cell functioning capability due to the accumulation of improperly coiled proinsulin proteins |
Recent advancements in molecular genetics have provided us with a better understanding of the causes of diabetes at a young age. It has been discovered that these cases are often the result of monogenic abnormalities and mutations in a single gene. NDM is a condition that impacts approximately 1 in every 90000 to 160000 live births[55]. There are more than 20 genetic factors that might develop NDM. One of the most probable etiologies of diabetes is identified before the age of six months; hence, additional clinical factors should be investigated to help direct genetic testing. Medically, two categories could be distinguished: (1) TNDM, which is reversible after a median of 12 wk without the need for further treatment, although up to 50% of patients could recur over the pediatric age range[56,57]; and (2) in addition, lifetime insulin therapies are necessary for people with PNDM after their diagnosis[58]. Mutations in the KCNJ11 gene, which codes for the Kir6.2 subunit of the KATP channel, are the second most prevalent sources of mutations in people with diabetes who are reported well before the age of six months of childhood. These abnormalities might cause either TNDM (10%) or PNDM (5% of cases)[59,60].
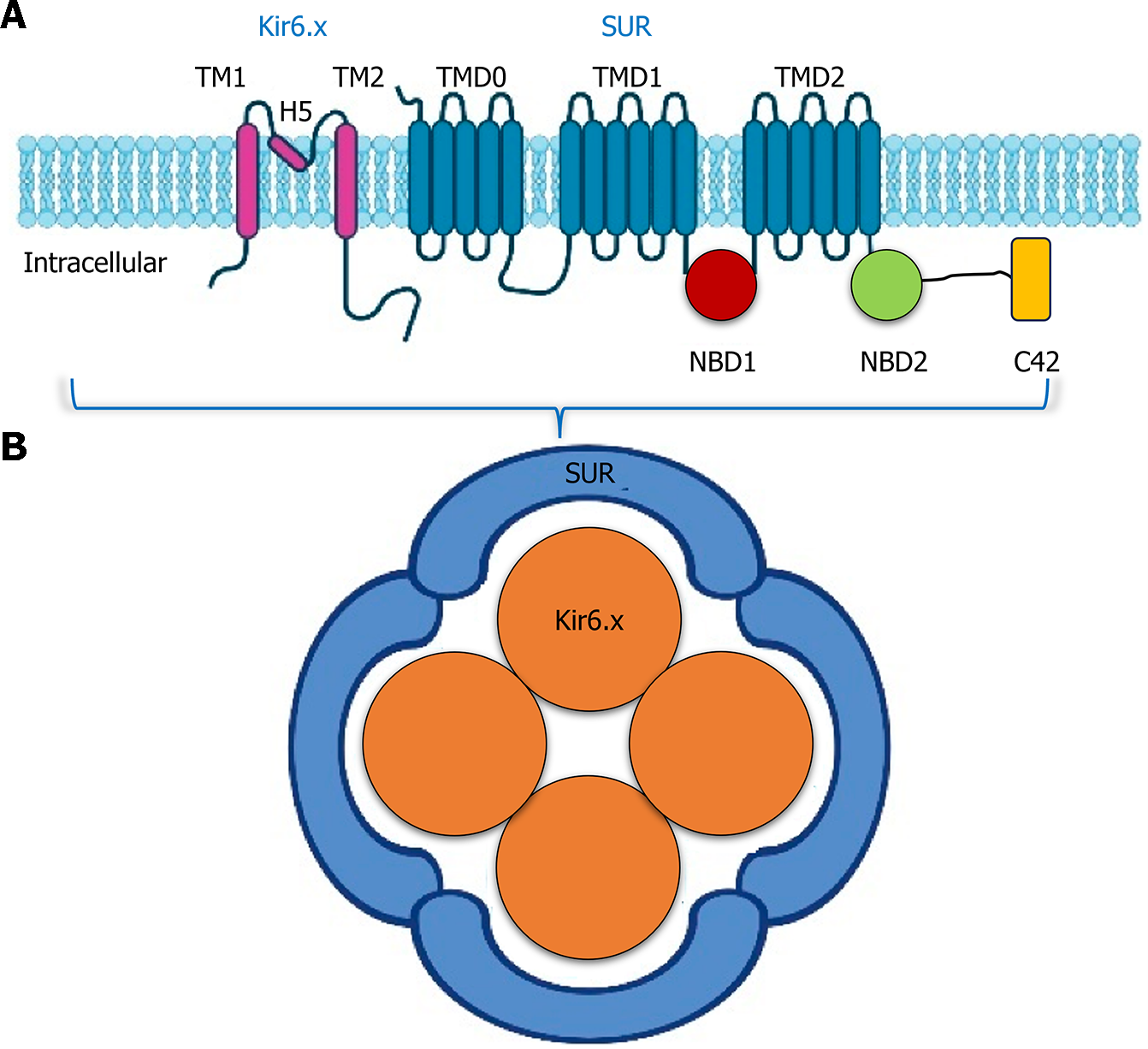
The KCNJ11 and ABCC8 genes, which code for four pore-forming Kir6.2 subunits and four SUR1 regulatory subunits, create the hetero-octameric complexes that comprise the KATP channels[61,62]. Every rise in intrinsic metabolic activities causes the cell's ATP /adenosine diphosphate ratio to rise and causes the KATP channels to shut. Depolarization of the cell membrane, as a result, eventually causes the release of insulin[63]. KCNJ11 or ABCC8 variants are discovered in about
Reduced newborn weight is widespread but lesser in individuals with 6q24 imprinting anomalies. Approximately 20% of probands with permanent neonatal diabetes have related neurological symptoms because the Kat p channel is expressed in nerves and musculature. Individuals sometimes develop a severe syndrome of epilepsy, neonatal diabetes, and developmental disorders [collectively known as or developmental delay, epilepsy and neonatal diabetes (ENDD)][67,68]. Nevertheless, an intermediary ENDD syndrome is more prevalent and is distinguished by DM and relatively developmental disorders without seizures. Like SUR1 neonatal diabetes, transient neonatal diabetes is more prevalent than permanent, and neurological symptoms are less frequent and typically include speech problems and aberrant breastfeeding behavior[48,69]. KATP-linked TNDM might reoccur early in adulthood, like in individuals with 6q24 imprinting anomalies. Since oral sulfonylureas (SU) are the most successful treatment for people with activating KATP channel mutations while being insulin dependent, it is critical to detect these patients. In an ATP-independent way, these attach with the SUR subunit and block the channels[42,70].
More than 90% of people with Kir6.2 diabetes and 85% with SUR1 diabetes can switch from insulin to oral hypo-glycemic pills and improve their blood sugar management without an increased glucose level. Furthermore, the quantity required is significantly greater than that used in type 2 diabetes (and slightly lesser in individuals with ABCC8 initiating genetic variation than in those with KCNJ11 mutations) or might result in temporary diarrhea[42]. KCNJ11-activated heterozygous mutations are linked to Kir6.2 DM. Since over 90% of alterations occur "de novo," individuals are typically born to parents without diabetes. Autosomal dominant transmission is evident in familial instances. This means there is a 50% chance of NDM for each subsequent child of an afflicted person. Similar to how few SUR1 DM patients have DM in their families. Most outbreaks also come from de novo heterozygous mutations, and those with mutations have a 50% probability of passing it on to their offspring.
Moreover, recessive inheritance is present in about 40% of PNDM individuals with ABCC8 mutations[71]. The probability of newborns' diabetes in these situations is 25% for every sibling of the children, but the affected child has a very minimal possibility of shifting the condition onto offspring. Nevertheless, since germline mosaicism (mutations involved in the germ cells but not identifiable in the blood) has been established in some individuals, healthy parents of a kid with a de novo mutation must be advised that the recurrent chance of affecting the next baby is insignificant[72] (Figure 2)[73].
The pancreatic KATP channels directly regulate insulin release. Multiple subunits of internal rectifying k+ channels 11 (Kir6.2, encoded by KCNJ11) and 4 subunits of the sulfonylurea receptor family (SUR1, encoded by ABCC8) combine to produce the hetero-octamer. GCK phosphorylates glucose to glucose-6-phosphate when it enters the cell, then glycolysis and the Calvin cycle decompose glucose to make ATP. The KATP channel closes due to the elevated ATP/MgATP ratio, depolarizing the cellular membranes and activating voltage-gated Ca2+ channels. Insulin is secreted from cells when calcium enters the cells via the active voltage-gated calcium channel. A system of transcriptional regulators, including HNF1A, NEUROD1, HNF4A, PDX1, and HNF1B, modulating the expression of insulin and the growth and division of beta cells. Red labels identify the genes linked to MODY[74,75].
The INS has been found to have heterogeneous mutations, which may contribute to approximately 10-13 percent of permanent neonatal diabetes occurrences[43,76,77]. Most mutations disrupt the insulin A or B chains. They are projected to prevent cysteine amino acids from forming disulfide bonds with one another by either adding an extra cysteine residue or transforming the existing one. Therefore, INS mutations cause a misfolded proinsulin molecule to be retained and aggregate in the endoplasmic reticulum, which causes the endoplasmic reticulum stress responses to be induced, inhibits protein production, and eventually results in β-cell destruction[78]. Inadequate birth weight, a characteristic of all subcategories of NDM, is the only extrapancreatic symptom present in patients with PNDM and an INS mutation.
Additionally, there is no variation in birth weight between INS mutation carriers and carriers of ABCC8 or activated KCNJ11 mutations. Children reported during the first 6 mo with persistent DM need molecular genetic screening to validate the chromosomal subtype, even though individuals with INS genetic variations are detected later since the range overlaps. Insulin is the sole medication option for individuals with monogenic diabetes because it causes the β-cells to progressively expire[79].
The overwhelming proportion of INS mutant individuals are spontaneous occurrences caused by denovo mutations. About 20% of incidences occur in families with an autosomal dominant transmission pattern. Therefore, 50% of afflicted people can transmit the illness to their offspring[50]. It is worth noting that between 6 and 12 mo, both INS and KCNJI1 mutants are an infrequent cause of irreversible diabetes. When dealing with diabetic newborns, particularly those who lack pancreatic autoantibodies or a high-threat human leukocyte antigens genotype for DM1, this must be considered[80].
The β-cell's sensor for glucose is the enzyme GCK, which catalyzes the rate-limiting reaction of glucose phosphorylation and allows the cells to react correctly to the level of glycemia[81]. Heterozygous GCK genetic mutations cause familial, moderate, non-progressive hyperglycemia. Nevertheless, the β-cells cannot secrete insulin in response to hyperglycemia if they have homozygous or compound heterozygous abnormalities in both genes that cause complete GCK insufficiency[82-84]. Only 4-5 percent of instances of PNDM are explained by this mechanism. Significant intrauterine developmental impairment and hyperglycemia can be seen as early as the first day of life (birth weight 1700 g). Individuals need to take insulin for a lifetime and don't have any significant additional pancreatic characteristics[85]. The diagnosis must be seriously investigated in consanguineous couples, particularly when both parents show moderate hyperglycemia. Monitoring fasting sugar levels in the parents of each newborn having NDM ought to be mandatory, particularly when there is no known family background of the condition because it is typically asymptomatic. Due to the recessive nature of this kind of diabetes, a patient's future siblings have a 25% chance of developing the condition[86].
Most monogenic diabetes in children is caused by gene abnormalities that alter insulin biosynthesis, packing, glucose sensing, or insulin release, resulting in β-cell depletion or malfunction[59,87]. The CD4+ CD25+ regulatory T lymphocytes, wherein overactivation leads to auto-immunity against β-cells, often leading to diabetes in the first three months of life, are the site of other alterations that influence insulin production and are not expressed in pancreatic beta cells. Decreased numbers of β-cells or granules and reduced insulin levels in these globules may be caused by mutations that impair the translational, breakdown, and packaging of insulin. Genes that control glucose sensing are affected by mutations that influence insulin release instead of the formation or degeneration of beta cells. They consist of mitochondrial DNA structural mutations. Most of these abnormalities decrease glucose sensitivity and metabolism, encouraging the open configuration of the K+ channel and preventing depolarization, which leads to insulin release. Monogenic diabetes, caused by mutations leading to extreme insulin resistance, rarely develops in children. These are primarily brought on by mutations in genes encoding the insulin receptor, which change the gene's biosynthesis and post-translational processing, promote receptor degradation, decrease insulin binding or receptor activation, and more. These result in Leprechauns, Rabson-Mendenhall syndrome, or type A severe insulin resistance. Alternately, hypertriglyceridemia linked to congenital generalized lipoatrophy or familial partial lipodystrophy may cause insulin resistance[88-91].
The other genetic origins of newborn DM are rare. While evaluating whether to test for additional genetic subtypes, related clinical information and understanding of kinship might be highly significant. About 5 to 10 percent of permanent neonatal diabetes cases are caused by pancreatic hypoplasia or aplasia. Whereas some of these individuals' mutations have already been discovered, the majority of these individuals still lack a genetic diagnosis. There have been two cases of pancreatic agenesis where the transcription factor IPF1 has completely failed due to homozygous or complex heterozygous alterations in the IPF1 gene[92,93]. Since it controls how midgut endodermic stem cells differentiate, IPF1 is crucial for the embryonic maturation of the pancreas. It also plays a role in INS transcription in adulthood. Therefore, IPF1 heterozygous mutations are responsible for a small number of incidences of inherited juvenile-stage diabetes[94]. Additionally, certain polymorphism variations of the gene increase the likelihood of getting type 2 diabetes[95]. Numerous individuals with pancreatic and cerebellar hypoplasia/agenesis from 2 consanguineous families had identical mutations in pancreas transcription factor 1α, which codes for pancreas transcription factor 1-α[96,97].
GLIS3, a transcriptional modulator with high levels of expression, has now been linked to a complicated syndrome that includes gestational hypothyroidism, neonatal diabetes, and dysmorphic traits. Neonatal glaucoma, liver cirrhosis, and glomerular cysts were also found in some cases. Four probands from three consanguineous families that were not linked to each other had homozygous mutations in the GL1S3 gene so far[98,99].
Multisystemic disorder, immune dysregulation, polyendocrinopathy, enteropathy, and X-linked syndrome manifest in homozygous recessive males with a mutation in the FOXP3 gene[100]. For regulating T cells to mature and perform properly, the genes that encode this protein must be present[101]. Its absence is linked to several autoimmune disorders with early development (enteropathy, DM, eczematous dermatitis, hypothyroidism, cytopenias, etc.), which frequently cause the patient to pass away during the initial few years of adulthood. Surprisingly, antibodies against β-cell antigens could be discovered, marking a significant distinction from other PNDM-causing factors. Therapeutic options include bone marrow transplants and immunosuppression. Female heterozygous carriers don't exhibit any symptoms[102].
Below 1% of kids seen in diabetic clinics have syndromic versions of the disease, making them uncommon. Most cases are either incorrectly or never diagnosed due to their rarity and complexity. It is crucial to appropriately diagnose these disorders in children so that difficulties can be anticipated, recognized, and treated. Parents may also choose to receive genetic counseling[103].
The condition known as MIDD is because of an A to G alteration at position m.3243A>G in the mitochondrial DNA that codes for the gene for tRNALeu and is thought to affect up to 1% of diabetics. Beta cell mass reduction, a steady decline in beta cell activity, and a reduction in glucose-induced insulin secretion are assumed to be the effects of mitochondrial malfunction in the extremely metabolically dynamic pancreatic islets. When compared to the percentage of diabetes induced by m.3243A>G, additional mitochondrial DNA genetic variations that have been linked to MIDD are incredibly rare[104,105].
Wolfram syndrome (WFS) is the most prevalent syndromic monogenic diabetes in kids and teenagers. The occurrence of WFS, commonly referred to as diabetes insipidus, DM, optic atrophy, and deafness, is thought to be 1 in 770000. Despite being a nonautoimmune type of diabetes, insulin insufficiency is a common complication in WFS patients due to the selective death of pancreatic beta cells and compromised insulin output. The latest reports link a missense alteration to nonsyndromic, autosomal dominant adult-onset diabetes[106-108].
Targeted therapy is made possible by the earlier diagnosis of monogenic diabetes in neonates and children. Improvements in glycemic control reduce comorbidities from diabetes, and a reduction in the expense and load of medication have all been linked to genetically-targeted therapy[65,109]. According to investigations, monogenic diabetes can be detected by affordable genetic analysis in the right individuals[66,110]. It is critical to differentiate between type 1 and type 2 diabetes and monogenic diabetes to monitor complications, identify extra-pancreatic illnesses that may be present, and identify afflicted and vulnerable members of the family[68,69]. To validate a clinical confirmation of monogenic diabetes, genetic screening must be conducted. Clinicians have various test methods and diagnostic strategies available as the set of genes linked to monogenic types of diabetes rises. Sanger sequencing is still the gold standard for finding single base changes and minor penetrations or removals. Still, it can only diagnose a small number of specific genes and requires previous knowledge of the probably afflicted gene. Carroll and Murphy[75] developed a diagnostic method in which doctors screen the most prevalent types of MODY (GCK, HNF1A, and HNF4A) first and only take into account the less common forms once those three have been ruled out[111].
WES, which focuses primarily on the human genome's protein-coding regions, is a potent method for identifying novel causal genes in monogenic illnesses. WES analysis has recently been a successful strategy for identifying the new genes in MODY-X cases. WES was performed on four Turkish patients from two families who were negative for the most prevalent MODY genes (HNF1A, HNF4A, GCK, and HNF4A). We detected disease-causing missense mutations in novel MODY candidate genes in two families after filtering pathologic variants. Two mutations (p.His307Gln in c-Myc and p.Gly107Ser in ARHGDIA) were not in any database and graded as probably detrimental by functional prediction software, while p.Asp129Asn in CDK4 was previously reported but not in 1000 genome, ESP6500, or ExAc databases[3,112].
NGS techniques have replaced Sanger sequencing in most industrial and clinical genomic labs. Several identified genes associated with diabetes can be simultaneously analyzed using next-generation targeted sequencing panels, which are about as expensive as Sanger sequencing to examine a few genes. Most crucially, specialized panels may find mutations in patients who don't have the disease's defining symptoms[113]. The likelihood that variations of ambiguous significance would appear in genetic testing findings is a significant side effect of employing panels. These variations are frequently challenging to interpret regarding illness risk or cause, necessitating additional patient medical data and testing of first-degree relatives to aid the assessment. When it relates to comprehending and explaining data to patients and making clinical care considerations, such situations present a unique difficulty for doctors. Whenever the cause of a variation is unclear, requesting physicians should speak with experts in monogenic diabetes.
The overall general opinion is that pharmaceutical intervention is not necessary, except for pregnancy, when management is based on fetal genotype, provided that the mild high blood sugar, the absence of long-term abnormalities, and the assessment that management with antidiabetic drugs or the insulin does not affect glycemia[114]. Thirty percent of GCK-MODY participants who received incorrect diagnosis and treatment with glucose-lowering medication, such as insulin, reported hypoglycemia and other negative consequences[115]. Vulnerability to SU is the first therapeutic option in HNF1A-MODY3, a significant and unique distinctiveness of HNF1A-MODY. It has an important implication, especially for individuals misdiagnosed with type 1 diabetes, because they might be able to stop insulin and receive SU medication even after receiving a lot of insulin[116]. Children on oral hypoglycemic drugs or sub-replacement insulin dosages can quit their insulin treatment and switch to low-dose SUs. The smallest quantity of sulfonylurea, such as glyburide (one-half to one 1.25 mg tab), must be used to start them. To get optimal blood glucose control, the dose can be increased. For those using replacement insulin doses, lowering basal insulin by at least 50% and ceasing bolus insulin at the start of SU are recommended. Meglitinides are among the additional therapy choices. In comparison to glibenclamide 1.25 mg, nateglinide 30 mg was demonstrated to produce reduced hypoglycemia in persons with HNF1A-MODY[117].
Repaglinide and nateglinide have been used in a case study of children with HNF1A-MODY. Meglitinides may be the first treatment for kids with HNF1A-MODY instead of SUs, according to this analysis of three teenagers, where the use of the medication was linked to little or infrequent hypoglycemia vs persistent hypoglycemia with SUs[118]. Compared to SU, glucagon-like peptide-1 (GLP-1) receptor analogs have been demonstrated to significantly decrease blood sugar concentrations in people with HNF1A-MODY[119]. SUs alone will not provide appropriate blood glucose control in certain people with HNF1A-MODY, or satisfactory control may worsen over time. This appears connected to gaining weight and latency in starting SUs[64]. Although the optimal replacement therapy plan is unknown, alternatives comprise supplementing SUs with metformin, basal insulin, or GLP-1 agonists. A study on the effects of SGLT2 inhibitors in HNF1A-MODY has been published, demonstrating an elevation in glycosuria[120].
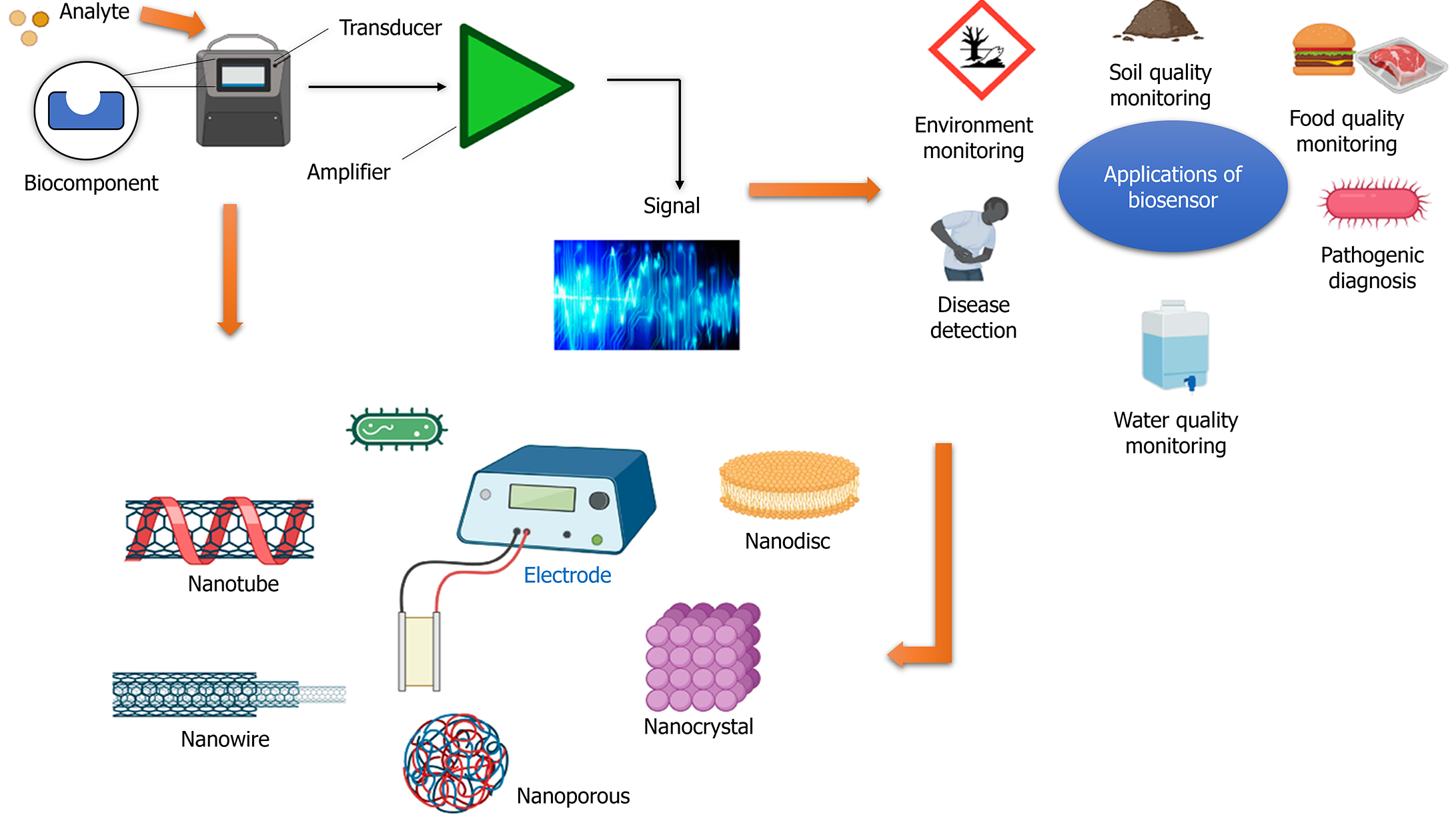
The identification of diabetes at an early stage and an assessment of its progression are critical components of diabetic care. Individuals diagnosed with diabetes must consistently check their blood glucose levels to manage and maintain their blood sugar levels effectively, mitigating the risk of developing diabetic complications[121]. The diagnostic tools commonly employed in clinical settings utilize the blood sample obtained by pricking the fingertip with a needle. However, there has been a recent trend towards implementing modern technology for continuous real-time monitoring of blood glucose levels. Glucose sensors are employed to monitor glucose concentrations in either the bloodstream or the interstitial fluid. A glucose sensor typically comprises three essential components: A detector, a transducer, and a reporter. A pressing requirement is to improve glucose sensors to enhance their accuracy and specificity and enable real-time detection[122-124].
The application of nanotechnology has been found to influence glucose sensors significantly. This is primarily due to nanotechnology's ability to enhance the sensors' surface area and improve the electrodes' catalytic activity. Moreover, nanotechnology has also played a crucial role in developing miniaturized nanoscale devices capable of detecting glucose. Recently, surface-enhanced raman spectroscopy-based biosensors have been widely studied to detect diabetes[125]. The utilization of carbon nanotubes (CNTs) has also been explored in the context of glucose detection in urine. The utilization of biopolymer chitosan (CS) aqueous solutions containing dissolved CNTs enables the monitoring of urine glucose levels without any interference[126]. The glucose detection in urine can be facilitated by employing ZnFe2O4 magnetic nanoparticles (NP) (MNPs) with inherent peroxidase-like activity. This research suggests these MNPs can be a colorimetric biosensor[127]. Another study devised a glucometer with flexibility, self-sustainability, and a skin-like appearance. This innovative device was designed to continuously monitor blood glucose levels within the human body, facilitating the proactive management and treatment of diabetes. The functioning mechanism relies on the interplay between piezoelectricity and enzyme processes within arrays of GOx@ZnO nanowires[128] (Figure 3)[129].
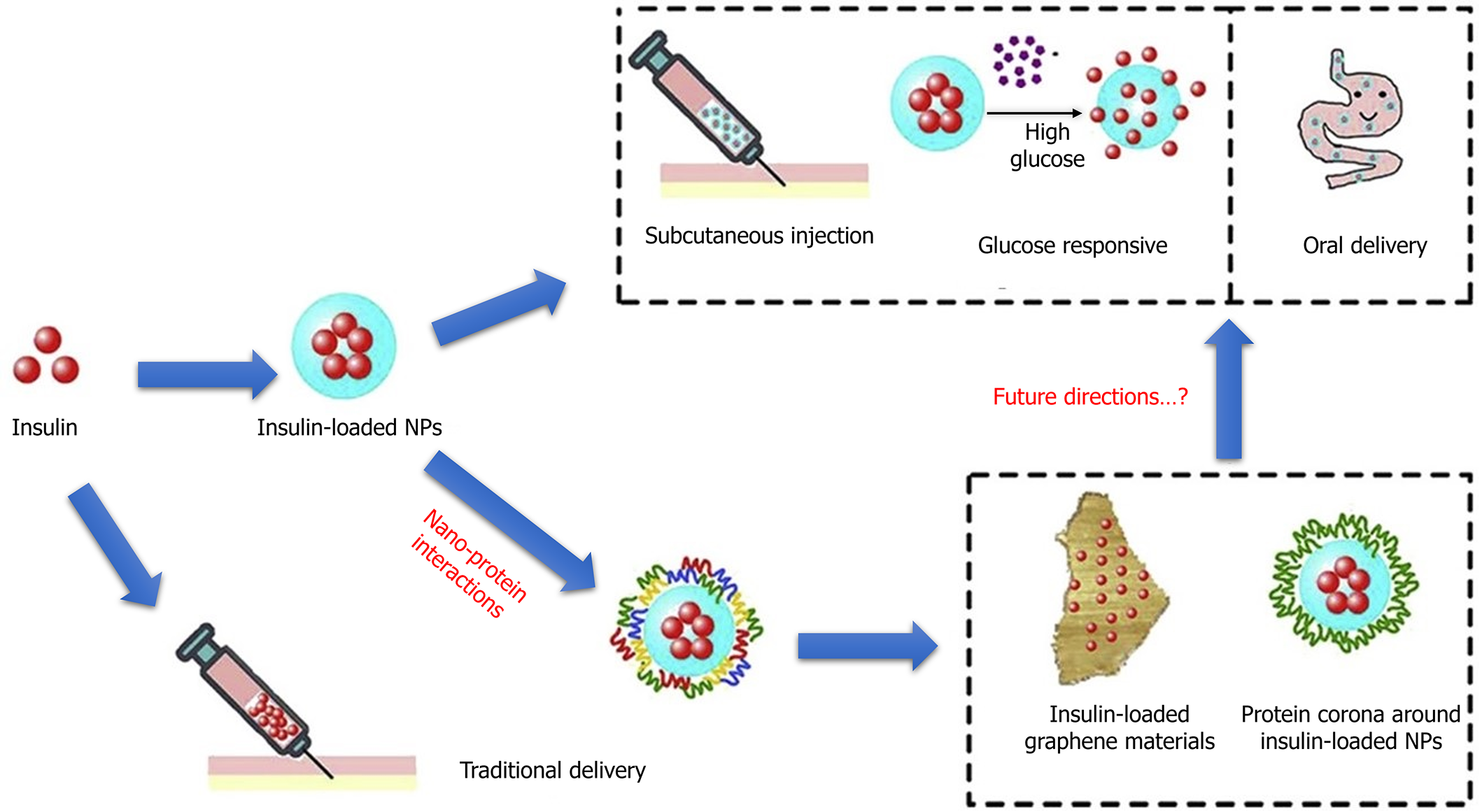
Diabetes is a chronic condition with no known cure; nevertheless, it can be effectively managed using many existing medical treatments. The efficacy of the treatment is dependent on the administration of insulin and other pharmacological medicines used to manage diabetes[130]. There is a notable scientific inclination towards advancing non-invasive techniques for administering insulin and/or extending its temporal efficacy through nanotechnology. The delivery of insulin through nanomedicine entails the utilization of polymeric NPs, micelles, metallic NPs, solid lipid nanoparticles, and biodegradable polymer nanoparticles[131]. Polymer-based delivery approaches commonly incorporate polyethylene glycol (PEG), wherein peptide or protein medications such as insulin are conjugated with PEG to enhance solubility, permeability, and stability during oral administration. Likewise, there have been notable advancements in the utilization of insulin via the oral route with the application of micellar formulations[132] (Figure 4)[133].
Liposomes are considered to be more appropriate and enduring structures compared to micelles. Consequently, certain variations of liposomes have been created and examined in animal models to assess their efficacy in delivering insulin. The oral administration of liposomal insulin has demonstrated enhanced bioavailability compared to the free version[134]. Nanoparticles loaded with insulin have been created utilizing a range of polymers such as CS, polylactide-co-glycolic acid, and dextran. The utilization of solid lipid nanoparticles has been explored to deliver insulin[135].
The investigation of genetic aspects of childhood monogenic diabetes not only provides valuable insights into the existing body of knowledge but also establishes a foundation for promising future avenues of research. With the progression of genetic analysis, there is an increasing potential to discover new gene mutations and comprehend their complex involvement in the development of monogenic diabetes. This can potentially reveal previously unknown disease subtypes and enhance our comprehension of the underlying mechanisms[136]. Furthermore, incorporating genomic data in conjunction with other 'omics' fields, like transcriptomics and metabolomics, can offer a comprehensive understanding of the molecular landscape of the disease. The construction of comprehensive databases through collaborative efforts in data sharing and multinational consortia can significantly assist clinicians in accurately diagnosing patients and selecting appropriate treatment options[137]. In addition, the prospect of gene treatments and precision medicine strategies presents a promising perspective, wherein customized interventions aimed at specific genetic abnormalities have the potential to profoundly transform the treatment of pediatric monogenic diabetes[138]. In essence, comprehending and effectively handling monogenic diabetes is closely linked to the ever-evolving field of genetics. It offers the potential for groundbreaking progress that will significantly impact the provision of diabetes care for children.
As a result of the challenges associated with identifying monogenic forms of diabetes in pediatrics, there is an increasing tendency for these conditions to be underdiagnosed, thereby overlooking potential opportunities for treatment strategies based on genetic factors. The misdiagnosis of diabetes can be attributed to several factors, including the clinical and hereditary variability of its subtypes, the complex relationship between clinical and polygenic types, the high cost of genetic screening, lack of healthcare insurance coverage, and limited knowledge of the condition among medical professionals. Integrating biomarkers with phenotype is a promising approach that can potentially speed up and improve the accuracy of genetic diagnoses. The clinical implications of this discovery for both the patient and their family, notwithstanding the relatively low prevalence of monogenic forms of diabetes, support the appropriate utilization of genetic testing. Assessing an inherited genetic form of diabetes necessitates specific consideration of several factors, including the absence of typical symptoms associated with type 1 or type 2 diabetes, early onset of the condition, familial predisposition, and extrapancreatic abnormalities. The prognosis and management of monogenic diabetes in pediatric and adolescent populations can be improved by expanding knowledge regarding the condition and facilitating a more approachable assessment process.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dabla PK, India; Liao Z, Singapore S-Editor: Qu XL L-Editor: A P-Editor: Chen YX
| 1. | Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1068] [Cited by in RCA: 977] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 2. | Yoshiji S, Horikawa Y, Kubota S, Enya M, Iwasaki Y, Keidai Y, Aizawa-Abe M, Iwasaki K, Honjo S, Hosomichi K, Yabe D, Hamasaki A. First Japanese Family With PDX1-MODY (MODY4): A Novel PDX1 Frameshift Mutation, Clinical Characteristics, and Implications. J Endocr Soc. 2022;6:bvab159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Aydogan HY, Gul N, Demirci DK, Mutlu U, Gulfidan G, Arga KY, Ozder A, Camli AA, Tutuncu Y, Ozturk O, Cacina C, Darendeliler F, Poyrazoglu S, Satman I. Precision Diagnosis of Maturity-Onset Diabetes of the Young with Next-Generation Sequencing: Findings from the MODY-IST Study in Adult Patients. OMICS. 2022;26:218-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Murphy R, Colclough K, Pollin TI, Ikle JM, Svalastoga P, Maloney KA, Saint-Martin C, Molnes J; ADA/EASD Precision Medicine Diabetes Initiative, Misra S, Aukrust I, de Franco A, Flanagan SE, Njølstad PR, Billings LK, Owen KR, Gloyn AL. A Systematic Review of the use of Precision Diagnostics in Monogenic Diabetes. medRxiv. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Rodrigues KF, Yong WTL, Bhuiyan MSA, Siddiquee S, Shah MD, Venmathi Maran BA. Current Understanding on the Genetic Basis of Key Metabolic Disorders: A Review. Biology (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 6. | Thomas CC, Philipson LH. Update on diabetes classification. Med Clin North Am. 2015;99:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 7. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S14-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 2130] [Article Influence: 426.0] [Reference Citation Analysis (0)] |
| 8. | Riddle MC, Philipson LH, Rich SS, Carlsson A, Franks PW, Greeley SAW, Nolan JJ, Pearson ER, Zeitler PS, Hattersley AT. Monogenic Diabetes: From Genetic Insights to Population-Based Precision in Care. Reflections From a Diabetes Care Editors' Expert Forum. Diabetes Care. 2020;43:3117-3128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 9. | Shepherd M, Shields B, Hammersley S, Hudson M, McDonald TJ, Colclough K, Oram RA, Knight B, Hyde C, Cox J, Mallam K, Moudiotis C, Smith R, Fraser B, Robertson S, Greene S, Ellard S, Pearson ER, Hattersley AT; UNITED Team. Systematic Population Screening, Using Biomarkers and Genetic Testing, Identifies 2.5% of the U.K. Pediatric Diabetes Population With Monogenic Diabetes. Diabetes Care. 2016;39:1879-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 10. | Hattersley AT, Patel KA. Precision diabetes: learning from monogenic diabetes. Diabetologia. 2017;60:769-777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 199] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 11. | Sanyoura M, Philipson LH, Naylor R. Monogenic Diabetes in Children and Adolescents: Recognition and Treatment Options. Curr Diab Rep. 2018;18:58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Nasykhova YA, Barbitoff YA, Serebryakova EA, Katserov DS, Glotov AS. Recent advances and perspectives in next generation sequencing application to the genetic research of type 2 diabetes. World J Diabetes. 2019;10:376-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Vora LK, Gholap AD, Jetha K, Thakur RRS, Solanki HK, Chavda VP. Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design. Pharmaceutics. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 211] [Reference Citation Analysis (0)] |
| 14. | Yang Y, Chen S, Liu Y, Huang Y, Cheong KL, Teng B, Liu W. Long-term treatment of polysaccharides-based hydrogel microparticles as oral insulin delivery in streptozotocin-induced type 2 diabetic mice. Biomed Pharmacother. 2021;133:110941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Pillon NJ, Loos RJF, Marshall SM, Zierath JR. Metabolic consequences of obesity and type 2 diabetes: Balancing genes and environment for personalized care. Cell. 2021;184:1530-1544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 16. | Sweeting A, Wong J, Murphy HR, Ross GP. A Clinical Update on Gestational Diabetes Mellitus. Endocr Rev. 2022;43:763-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 396] [Article Influence: 132.0] [Reference Citation Analysis (1)] |
| 17. | Kotagama OW, Jayasinghe CD, Abeysinghe T. Era of Genomic Medicine: A Narrative Review on CRISPR Technology as a Potential Therapeutic Tool for Human Diseases. Biomed Res Int. 2019;2019:1369682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Akil AA, Yassin E, Al-Maraghi A, Aliyev E, Al-Malki K, Fakhro KA. Diagnosis and treatment of type 1 diabetes at the dawn of the personalized medicine era. J Transl Med. 2021;19:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 19. | Shields BM, Hicks S, Shepherd MH, Colclough K, Hattersley AT, Ellard S. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia. 2010;53:2504-2508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 466] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 20. | Kim SH. Maturity-Onset Diabetes of the Young: What Do Clinicians Need to Know? Diabetes Metab J. 2015;39:468-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Urakami T. Maturity-onset diabetes of the young (MODY): current perspectives on diagnosis and treatment. Diabetes Metab Syndr Obes. 2019;12:1047-1056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 22. | Yang YS, Kwak SH, Park KS. Update on Monogenic Diabetes in Korea. Diabetes Metab J. 2020;44:627-639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S19-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1310] [Article Influence: 655.0] [Reference Citation Analysis (70)] |
| 24. | Marble A. Glibenclamide, a new sulphonylurea: whither oral hypoglycaemic agents? Drugs. 1971;1:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Johansson BB, Irgens HU, Molnes J, Sztromwasser P, Aukrust I, Juliusson PB, Søvik O, Levy S, Skrivarhaug T, Joner G, Molven A, Johansson S, Njølstad PR. Targeted next-generation sequencing reveals MODY in up to 6.5% of antibody-negative diabetes cases listed in the Norwegian Childhood Diabetes Registry. Diabetologia. 2017;60:625-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 26. | Owen KR. Monogenic diabetes: old and new approaches to diagnosis. Clin Med (Lond). 2013;13:278-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Fajans SS, Bell GI. MODY: history, genetics, pathophysiology, and clinical decision making. Diabetes Care. 2011;34:1878-1884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 28. | Yamagata K, Oda N, Kaisaki PJ, Menzel S, Furuta H, Vaxillaire M, Southam L, Cox RD, Lathrop GM, Boriraj VV, Chen X, Cox NJ, Oda Y, Yano H, Le Beau MM, Yamada S, Nishigori H, Takeda J, Fajans SS, Hattersley AT, Iwasaki N, Hansen T, Pedersen O, Polonsky KS, Bell GI. Mutations in the hepatocyte nuclear factor-1alpha gene in maturity-onset diabetes of the young (MODY3). Nature. 1996;384:455-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 785] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 29. | Flanagan SE, Kapoor RR, Mali G, Cody D, Murphy N, Schwahn B, Siahanidou T, Banerjee I, Akcay T, Rubio-Cabezas O, Shield JP, Hussain K, Ellard S. Diazoxide-responsive hyperinsulinemic hypoglycemia caused by HNF4A gene mutations. Eur J Endocrinol. 2010;162:987-992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 30. | Chakera AJ, Spyer G, Vincent N, Ellard S, Hattersley AT, Dunne FP. The 0.1% of the population with glucokinase monogenic diabetes can be recognized by clinical characteristics in pregnancy: the Atlantic Diabetes in Pregnancy cohort. Diabetes Care. 2014;37:1230-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 31. | Matschinsky FM. Glucokinase as glucose sensor and metabolic signal generator in pancreatic beta-cells and hepatocytes. Diabetes. 1990;39:647-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 360] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 32. | Froguel P, Zouali H, Vionnet N, Velho G, Vaxillaire M, Sun F, Lesage S, Stoffel M, Takeda J, Passa P. Familial hyperglycemia due to mutations in glucokinase. Definition of a subtype of diabetes mellitus. N Engl J Med. 1993;328:697-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 446] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 33. | Gidh-Jain M, Takeda J, Xu LZ, Lange AJ, Vionnet N, Stoffel M, Froguel P, Velho G, Sun F, Cohen D. Glucokinase mutations associated with non-insulin-dependent (type 2) diabetes mellitus have decreased enzymatic activity: implications for structure/function relationships. Proc Natl Acad Sci U S A. 1993;90:1932-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 133] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Byrne MM, Sturis J, Clément K, Vionnet N, Pueyo ME, Stoffel M, Takeda J, Passa P, Cohen D, Bell GI. Insulin secretory abnormalities in subjects with hyperglycemia due to glucokinase mutations. J Clin Invest. 1994;93:1120-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 182] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 35. | Steele AM, Shields BM, Wensley KJ, Colclough K, Ellard S, Hattersley AT. Prevalence of vascular complications among patients with glucokinase mutations and prolonged, mild hyperglycemia. JAMA. 2014;311:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 223] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 36. | Steele AM, Wensley KJ, Ellard S, Murphy R, Shepherd M, Colclough K, Hattersley AT, Shields BM. Use of HbA1c in the identification of patients with hyperglycaemia caused by a glucokinase mutation: observational case control studies. PLoS One. 2013;8:e65326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 37. | Lorini R, Klersy C, d'Annunzio G, Massa O, Minuto N, Iafusco D, Bellannè-Chantelot C, Frongia AP, Toni S, Meschi F, Cerutti F, Barbetti F; Italian Society of Pediatric Endocrinology and Diabetology (ISPED) Study Group. Maturity-onset diabetes of the young in children with incidental hyperglycemia: a multicenter Italian study of 172 families. Diabetes Care. 2009;32:1864-1866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Codner E, Rocha A, Deng L, Martínez-Aguayo A, Godoy C, Mericq V, Chung WK. Mild fasting hyperglycemia in children: high rate of glucokinase mutations and some risk of developing type 1 diabetes mellitus. Pediatr Diabetes. 2009;10:382-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Ellard S, Beards F, Allen LI, Shepherd M, Ballantyne E, Harvey R, Hattersley AT. A high prevalence of glucokinase mutations in gestational diabetic subjects selected by clinical criteria. Diabetologia. 2000;43:250-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 125] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 40. | Dickens LT, Naylor RN. Clinical Management of Women with Monogenic Diabetes During Pregnancy. Curr Diab Rep. 2018;18:12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 41. | Clissold RL, Hamilton AJ, Hattersley AT, Ellard S, Bingham C. HNF1B-associated renal and extra-renal disease-an expanding clinical spectrum. Nat Rev Nephrol. 2015;11:102-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 215] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 42. | Harsunen M, Kettunen JLT, Härkönen T, Dwivedi O, Lehtovirta M, Vähäsalo P, Veijola R, Ilonen J, Miettinen PJ, Knip M, Tuomi T. Identification of monogenic variants in more than ten per cent of children without type 1 diabetes-related autoantibodies at diagnosis in the Finnish Pediatric Diabetes Register. Diabetologia. 2023;66:438-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 43. | De Franco E, Flanagan SE, Houghton JA, Lango Allen H, Mackay DJ, Temple IK, Ellard S, Hattersley AT. The effect of early, comprehensive genomic testing on clinical care in neonatal diabetes: an international cohort study. Lancet. 2015;386:957-963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 231] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 44. | Gloyn AL, Reimann F, Girard C, Edghill EL, Proks P, Pearson ER, Temple IK, Mackay DJ, Shield JP, Freedenberg D, Noyes K, Ellard S, Ashcroft FM, Gribble FM, Hattersley AT. Relapsing diabetes can result from moderately activating mutations in KCNJ11. Hum Mol Genet. 2005;14:925-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 45. | Bowman P, McDonald TJ, Knight BA, Flanagan SE, Leveridge M, Spaull SR, Shields BM, Hammersley S, Shepherd MH, Andrews RC, Patel KA, Hattersley AT. Patterns of postmeal insulin secretion in individuals with sulfonylurea-treated KCNJ11 neonatal diabetes show predominance of non-K(ATP)-channel pathways. BMJ Open Diabetes Res Care. 2019;7:e000721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Pearson ER, Flechtner I, Njølstad PR, Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E, Iotova V, Slingerland AS, Shield J, Robert JJ, Holst JJ, Clark PM, Ellard S, Søvik O, Polak M, Hattersley AT; Neonatal Diabetes International Collaborative Group. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355:467-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 670] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 47. | Chung WK, Erion K, Florez JC, Hattersley AT, Hivert MF, Lee CG, McCarthy MI, Nolan JJ, Norris JM, Pearson ER, Philipson L, McElvaine AT, Cefalu WT, Rich SS, Franks PW. Precision medicine in diabetes: a Consensus Report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2020;63:1671-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 48. | Proks P, Antcliff JF, Lippiat J, Gloyn AL, Hattersley AT, Ashcroft FM. Molecular basis of Kir6.2 mutations associated with neonatal diabetes or neonatal diabetes plus neurological features. Proc Natl Acad Sci U S A. 2004;101:17539-17544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 179] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 49. | Pipatpolkai T, Usher S, Stansfeld PJ, Ashcroft FM. New insights into K(ATP) channel gene mutations and neonatal diabetes mellitus. Nat Rev Endocrinol. 2020;16:378-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 50. | Flanagan SE, Patch AM, Mackay DJ, Edghill EL, Gloyn AL, Robinson D, Shield JP, Temple K, Ellard S, Hattersley AT. Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes. 2007;56:1930-1937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 245] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 51. | Bowman P, Flanagan SE, Edghill EL, Damhuis A, Shepherd MH, Paisey R, Hattersley AT, Ellard S. Heterozygous ABCC8 mutations are a cause of MODY. Diabetologia. 2012;55:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 52. | Ellard S, Flanagan SE, Girard CA, Patch AM, Harries LW, Parrish A, Edghill EL, Mackay DJ, Proks P, Shimomura K, Haberland H, Carson DJ, Shield JP, Hattersley AT, Ashcroft FM. Permanent neonatal diabetes caused by dominant, recessive, or compound heterozygous SUR1 mutations with opposite functional effects. Am J Hum Genet. 2007;81:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 53. | De Franco E, Saint-Martin C, Brusgaard K, Knight Johnson AE, Aguilar-Bryan L, Bowman P, Arnoux JB, Larsen AR, Sanyoura M, Greeley SAW, Calzada-León R, Harman B, Houghton JAL, Nishimura-Meguro E, Laver TW, Ellard S, Del Gaudio D, Christesen HT, Bellanné-Chantelot C, Flanagan SE. Update of variants identified in the pancreatic β-cell K(ATP) channel genes KCNJ11 and ABCC8 in individuals with congenital hyperinsulinism and diabetes. Hum Mutat. 2020;41:884-905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 54. | Støy J, Edghill EL, Flanagan SE, Ye H, Paz VP, Pluzhnikov A, Below JE, Hayes MG, Cox NJ, Lipkind GM, Lipton RB, Greeley SA, Patch AM, Ellard S, Steiner DF, Hattersley AT, Philipson LH, Bell GI; Neonatal Diabetes International Collaborative Group. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci U S A. 2007;104:15040-15044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 412] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 55. | Letourneau LR, Carmody D, Philipson LH, Greeley SAW. Early Intensive Insulin Use May Preserve β-Cell Function in Neonatal Diabetes Due to Mutations in the Proinsulin Gene. J Endocr Soc. 2018;2:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 56. | Carmody D, Park SY, Ye H, Perrone ME, Alkorta-Aranburu G, Highland HM, Hanis CL, Philipson LH, Bell GI, Greeley SA. Continued lessons from the INS gene: an intronic mutation causing diabetes through a novel mechanism. J Med Genet. 2015;52:612-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 57. | Křížková K, Veverka V, Maletínská L, Hexnerová R, Brzozowski AM, Jiráček J, Žáková L. Structural and functional study of the GlnB22-insulin mutant responsible for maturity-onset diabetes of the young. PLoS One. 2014;9:e112883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 58. | Støy J, Olsen J, Park SY, Gregersen S, Hjørringgaard CU, Bell GI. In vivo measurement and biological characterisation of the diabetes-associated mutant insulin p.R46Q (GlnB22-insulin). Diabetologia. 2017;60:1423-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | Lemelman MB, Letourneau L, Greeley SAW. Neonatal Diabetes Mellitus: An Update on Diagnosis and Management. Clin Perinatol. 2018;45:41-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 60. | Temple IK, Gardner RJ, Mackay DJ, Barber JC, Robinson DO, Shield JP. Transient neonatal diabetes: widening the understanding of the etiopathogenesis of diabetes. Diabetes. 2000;49:1359-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 173] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 61. | Gardner RJ, Mackay DJ, Mungall AJ, Polychronakos C, Siebert R, Shield JP, Temple IK, Robinson DO. An imprinted locus associated with transient neonatal diabetes mellitus. Hum Mol Genet. 2000;9:589-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 151] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 62. | Polak M, Shield J. Neonatal and very-early-onset diabetes mellitus. Semin Neonatol. 2004;9:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 63. | Slingerland AS, Hattersley AT. Mutations in the Kir6.2 subunit of the KATP channel and permanent neonatal diabetes: new insights and new treatment. Ann Med. 2005;37:186-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 64. | Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JM, Molnes J, Edghill EL, Frayling TM, Temple IK, Mackay D, Shield JP, Sumnik Z, van Rhijn A, Wales JK, Clark P, Gorman S, Aisenberg J, Ellard S, Njølstad PR, Ashcroft FM, Hattersley AT. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350:1838-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 858] [Cited by in RCA: 817] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 65. | Ehtisham S, Hattersley AT, Dunger DB, Barrett TG; British Society for Paediatric Endocrinology and Diabetes Clinical Trials Group. First UK survey of paediatric type 2 diabetes and MODY. Arch Dis Child. 2004;89:526-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 66. | Pihoker C, Gilliam LK, Ellard S, Dabelea D, Davis C, Dolan LM, Greenbaum CJ, Imperatore G, Lawrence JM, Marcovina SM, Mayer-Davis E, Rodriguez BL, Steck AK, Williams DE, Hattersley AT; SEARCH for Diabetes in Youth Study Group. Prevalence, characteristics and clinical diagnosis of maturity onset diabetes of the young due to mutations in HNF1A, HNF4A, and glucokinase: results from the SEARCH for Diabetes in Youth. J Clin Endocrinol Metab. 2013;98:4055-4062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 266] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 67. | Kanakatti Shankar R, Pihoker C, Dolan LM, Standiford D, Badaru A, Dabelea D, Rodriguez B, Black MH, Imperatore G, Hattersley A, Ellard S, Gilliam LK; SEARCH for Diabetes in Youth Study Group. Permanent neonatal diabetes mellitus: prevalence and genetic diagnosis in the SEARCH for Diabetes in Youth Study. Pediatr Diabetes. 2013;14:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 68. | Bacon S, Kyithar MP, Rizvi SR, Donnelly E, McCarthy A, Burke M, Colclough K, Ellard S, Byrne MM. Successful maintenance on sulphonylurea therapy and low diabetes complication rates in a HNF1A-MODY cohort. Diabet Med. 2016;33:976-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 69. | Shepherd M, Shields B, Ellard S, Rubio-Cabezas O, Hattersley AT. A genetic diagnosis of HNF1A diabetes alters treatment and improves glycaemic control in the majority of insulin-treated patients. Diabet Med. 2009;26:437-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 70. | Greeley SA, John PM, Winn AN, Ornelas J, Lipton RB, Philipson LH, Bell GI, Huang ES. The cost-effectiveness of personalized genetic medicine: the case of genetic testing in neonatal diabetes. Diabetes Care. 2011;34:622-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 71. | Naylor RN, John PM, Winn AN, Carmody D, Greeley SA, Philipson LH, Bell GI, Huang ES. Cost-effectiveness of MODY genetic testing: translating genomic advances into practical health applications. Diabetes Care. 2014;37:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 72. | Naylor R, Philipson LH. Who should have genetic testing for maturity-onset diabetes of the young? Clin Endocrinol (Oxf). 2011;75:422-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 73. | Clement A, Guo S, Jansen-Olesen I, Christensen SL. ATP-Sensitive Potassium Channels in Migraine: Translational Findings and Therapeutic Potential. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 74. | Kleinberger JW, Pollin TI. Undiagnosed MODY: Time for Action. Curr Diab Rep. 2015;15:110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 75. | Carroll RW, Murphy R. Monogenic diabetes: a diagnostic algorithm for clinicians. Genes (Basel). 2013;4:522-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 76. | Alkorta-Aranburu G, Carmody D, Cheng YW, Nelakuditi V, Ma L, Dickens JT, Das S, Greeley SAW, Del Gaudio D. Phenotypic heterogeneity in monogenic diabetes: the clinical and diagnostic utility of a gene panel-based next-generation sequencing approach. Mol Genet Metab. 2014;113:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 77. | Miki T, Seino S. Roles of KATP channels as metabolic sensors in acute metabolic changes. J Mol Cell Cardiol. 2005;38:917-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 78. | Flanagan SE, Edghill EL, Gloyn AL, Ellard S, Hattersley AT. Mutations in KCNJ11, which encodes Kir6.2, are a common cause of diabetes diagnosed in the first 6 months of life, with the phenotype determined by genotype. Diabetologia. 2006;49:1190-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 79. | Shimomura K, Girard CA, Proks P, Nazim J, Lippiat JD, Cerutti F, Lorini R, Ellard S, Hattersley AT, Barbetti F, Ashcroft FM. Mutations at the same residue (R50) of Kir6.2 (KCNJ11) that cause neonatal diabetes produce different functional effects. Diabetes. 2006;55:1705-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 80. | Hattersley AT, Ashcroft FM. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes. 2005;54:2503-2513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 301] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 81. | Gloyn AL, Diatloff-Zito C, Edghill EL, Bellanné-Chantelot C, Nivot S, Coutant R, Ellard S, Hattersley AT, Robert JJ. KCNJ11 activating mutations are associated with developmental delay, epilepsy and neonatal diabetes syndrome and other neurological features. Eur J Hum Genet. 2006;14:824-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 82. | Babenko AP, Polak M, Cavé H, Busiah K, Czernichow P, Scharfmann R, Bryan J, Aguilar-Bryan L, Vaxillaire M, Froguel P. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med. 2006;355:456-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 447] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 83. | Rafiq M, Flanagan SE, Patch AM, Shields BM, Ellard S, Hattersley AT; Neonatal Diabetes International Collaborative Group. Effective treatment with oral sulfonylureas in patients with diabetes due to sulfonylurea receptor 1 (SUR1) mutations. Diabetes Care. 2008;31:204-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 84. | Rubio-Cabezas O, Argente J. Current insights into the genetic basis of diabetes mellitus in children and adolescents. J Pediatr Endocrinol Metab. 2008;21:917-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 85. | Molven A, Ringdal M, Nordbø AM, Raeder H, Støy J, Lipkind GM, Steiner DF, Philipson LH, Bergmann I, Aarskog D, Undlien DE, Joner G, Søvik O; Norwegian Childhood Diabetes Study Group, Bell GI, Njølstad PR. Mutations in the insulin gene can cause MODY and autoantibody-negative type 1 diabetes. Diabetes. 2008;57:1131-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 86. | Edghill EL, Flanagan SE, Patch AM, Boustred C, Parrish A, Shields B, Shepherd MH, Hussain K, Kapoor RR, Malecki M, MacDonald MJ, Støy J, Steiner DF, Philipson LH, Bell GI; Neonatal Diabetes International Collaborative Group, Hattersley AT, Ellard S. Insulin mutation screening in 1,044 patients with diabetes: mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood. Diabetes. 2008;57:1034-1042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 279] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 87. | Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 891] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 88. | Matschinsky FM. Glucokinase, glucose homeostasis, and diabetes mellitus. Curr Diab Rep. 2005;5:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 89. | Njølstad PR, Søvik O, Cuesta-Muñoz A, Bjørkhaug L, Massa O, Barbetti F, Undlien DE, Shiota C, Magnuson MA, Molven A, Matschinsky FM, Bell GI. Neonatal diabetes mellitus due to complete glucokinase deficiency. N Engl J Med. 2001;344:1588-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 278] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 90. | Njølstad PR, Sagen JV, Bjørkhaug L, Odili S, Shehadeh N, Bakry D, Sarici SU, Alpay F, Molnes J, Molven A, Søvik O, Matschinsky FM. Permanent neonatal diabetes caused by glucokinase deficiency: inborn error of the glucose-insulin signaling pathway. Diabetes. 2003;52:2854-2860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 91. | Osbak KK, Colclough K, Saint-Martin C, Beer NL, Bellanné-Chantelot C, Ellard S, Gloyn AL. Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum Mutat. 2009;30:1512-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 368] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 92. | Kharroubi AT, Darwish HM. Diabetes mellitus: The epidemic of the century. World J Diabetes. 2015;6:850-867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 584] [Cited by in RCA: 575] [Article Influence: 57.5] [Reference Citation Analysis (42)] |
| 93. | Vaxillaire M, Froguel P. Genetic basis of maturity-onset diabetes of the young. Endocrinol Metab Clin North Am. 2006;35:371-384, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 94. | Musso C, Cochran E, Moran SA, Skarulis MC, Oral EA, Taylor S, Gorden P. Clinical course of genetic diseases of the insulin receptor (type A and Rabson-Mendenhall syndromes): a 30-year prospective. Medicine (Baltimore). 2004;83:209-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 176] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 95. | Longo N, Wang Y, Smith SA, Langley SD, DiMeglio LA, Giannella-Neto D. Genotype-phenotype correlation in inherited severe insulin resistance. Hum Mol Genet. 2002;11:1465-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 96. | Oral EA. Lipoatrophic diabetes and other related syndromes. Rev Endocr Metab Disord. 2003;4:61-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 97. | Peters JM, Barnes R, Bennett L, Gitomer WM, Bowcock AM, Garg A. Localization of the gene for familial partial lipodystrophy (Dunnigan variety) to chromosome 1q21-22. Nat Genet. 1998;18:292-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 102] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 98. | Schwitzgebel VM, Mamin A, Brun T, Ritz-Laser B, Zaiko M, Maret A, Jornayvaz FR, Theintz GE, Michielin O, Melloul D, Philippe J. Agenesis of human pancreas due to decreased half-life of insulin promoter factor 1. J Clin Endocrinol Metab. 2003;88:4398-4406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 99. | Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet. 1997;15:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 745] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 100. | Gittes GK. Developmental biology of the pancreas: a comprehensive review. Dev Biol. 2009;326:4-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 317] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 101. | Stoffers DA, Ferrer J, Clarke WL, Habener JF. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet. 1997;17:138-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 598] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 102. | Hoveyda N, Shield JP, Garrett C, Chong WK, Beardsall K, Bentsi-Enchill E, Mallya H, Thompson MH. Neonatal diabetes mellitus and cerebellar hypoplasia/agenesis: report of a new recessive syndrome. J Med Genet. 1999;36:700-704. [PubMed] |
| 103. | Sellick GS, Barker KT, Stolte-Dijkstra I, Fleischmann C, Coleman RJ, Garrett C, Gloyn AL, Edghill EL, Hattersley AT, Wellauer PK, Goodwin G, Houlston RS. Mutations in PTF1A cause pancreatic and cerebellar agenesis. Nat Genet. 2004;36:1301-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 292] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 104. | Taha D, Barbar M, Kanaan H, Williamson Balfe J. Neonatal diabetes mellitus, congenital hypothyroidism, hepatic fibrosis, polycystic kidneys, and congenital glaucoma: a new autosomal recessive syndrome? Am J Med Genet A. 2003;122A:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 105. | Senée V, Chelala C, Duchatelet S, Feng D, Blanc H, Cossec JC, Charon C, Nicolino M, Boileau P, Cavener DR, Bougnères P, Taha D, Julier C. Mutations in GLIS3 are responsible for a rare syndrome with neonatal diabetes mellitus and congenital hypothyroidism. Nat Genet. 2006;38:682-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 271] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 106. | Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;39:537-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 519] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 107. | Ochs HD, Gambineri E, Torgerson TR. IPEX, FOXP3 and regulatory T-cells: a model for autoimmunity. Immunol Res. 2007;38:112-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 108. | Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked: forkhead box protein 3 mutations and lack of regulatory T cells. J Allergy Clin Immunol. 2007;120:744-50; quiz 751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 179] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 109. | Barrett TG. Differential diagnosis of type 1 diabetes: which genetic syndromes need to be considered? Pediatr Diabetes. 2007;8 Suppl 6:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 110. | Goto Y, Nonaka I, Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990;348:651-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1439] [Cited by in RCA: 1434] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 111. | Murphy R, Turnbull DM, Walker M, Hattersley AT. Clinical features, diagnosis and management of maternally inherited diabetes and deafness (MIDD) associated with the 3243A>G mitochondrial point mutation. Diabet Med. 2008;25:383-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 112. | van ven Ouweland JM, Cryns K, Pennings RJ, Walraven I, Janssen GM, Maassen JA, Veldhuijzen BF, Arntzenius AB, Lindhout D, Cremers CW, Van Camp G, Dikkeschei LD. Molecular characterization of WFS1 in patients with Wolfram syndrome. J Mol Diagn. 2003;5:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 113. | Urano F. Wolfram syndrome iPS cells: the first human cell model of endoplasmic reticulum disease. Diabetes. 2014;63:844-846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 114. | Bonnycastle LL, Chines PS, Hara T, Huyghe JR, Swift AJ, Heikinheimo P, Mahadevan J, Peltonen S, Huopio H, Nuutila P, Narisu N, Goldfeder RL, Stitzel ML, Lu S, Boehnke M, Urano F, Collins FS, Laakso M. Autosomal dominant diabetes arising from a Wolfram syndrome 1 mutation. Diabetes. 2013;62:3943-3950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 115. | Jurgens SJ, Choi SH, Morrill VN, Chaffin M, Pirruccello JP, Halford JL, Weng LC, Nauffal V, Roselli C, Hall AW, Oetjens MT, Lagerman B, vanMaanen DP; Regeneron Genetics Center, Aragam KG, Lunetta KL, Haggerty CM, Lubitz SA, Ellinor PT. Analysis of rare genetic variation underlying cardiometabolic diseases and traits among 200,000 individuals in the UK Biobank. Nat Genet. 2022;54:240-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 101] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 116. | Mirshahi UL, Colclough K, Wright CF, Wood AR, Beaumont RN, Tyrrell J, Laver TW, Stahl R, Golden A, Goehringer JM; Geisinger-Regeneron DiscovEHR Collaboration, Frayling TF, Hattersley AT, Carey DJ, Weedon MN, Patel KA. Reduced penetrance of MODY-associated HNF1A/HNF4A variants but not GCK variants in clinically unselected cohorts. Am J Hum Genet. 2022;109:2018-2028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 117. | Shepherd M, Pearson ER, Houghton J, Salt G, Ellard S, Hattersley AT. No deterioration in glycemic control in HNF-1alpha maturity-onset diabetes of the young following transfer from long-term insulin to sulphonylureas. Diabetes Care. 2003;26:3191-3192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 118. | Tuomi T, Honkanen EH, Isomaa B, Sarelin L, Groop LC. Improved prandial glucose control with lower risk of hypoglycemia with nateglinide than with glibenclamide in patients with maturity-onset diabetes of the young type 3. Diabetes Care. 2006;29:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 119. | Becker M, Galler A, Raile K. Meglitinide analogues in adolescent patients with HNF1A-MODY (MODY 3). Pediatrics. 2014;133:e775-e779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 120. | Østoft SH, Bagger JI, Hansen T, Pedersen O, Faber J, Holst JJ, Knop FK, Vilsbøll T. Glucose-lowering effects and low risk of hypoglycemia in patients with maturity-onset diabetes of the young when treated with a GLP-1 receptor agonist: a double-blind, randomized, crossover trial. Diabetes Care. 2014;37:1797-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 121. | Hohendorff J, Szopa M, Skupien J, Kapusta M, Zapala B, Platek T, Mrozinska S, Parpan T, Glodzik W, Ludwig-Galezowska A, Kiec-Wilk B, Klupa T, Malecki MT. A single dose of dapagliflozin, an SGLT-2 inhibitor, induces higher glycosuria in GCK- and HNF1A-MODY than in type 2 diabetes mellitus. Endocrine. 2017;57:272-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 122. | Ogurtsova K, Guariguata L, Barengo NC, Ruiz PL, Sacre JW, Karuranga S, Sun H, Boyko EJ, Magliano DJ. IDF diabetes Atlas: Global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res Clin Pract. 2022;183:109118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 441] [Article Influence: 147.0] [Reference Citation Analysis (0)] |
| 123. | Wang HC, Lee AR. Recent developments in blood glucose sensors. J Food Drug Anal. 2015;23:191-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 124. | Rodbard D. Continuous Glucose Monitoring: A Review of Successes, Challenges, and Opportunities. Diabetes Technol Ther. 2016;18 Suppl 2:S3-S13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 378] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 125. | Kim W, Bang A, Kim S, Lee GJ, Kim YH, Choi S. Adiponectin-targeted SERS immunoassay biosensing platform for early detection of gestational diabetes mellitus. Biosens Bioelectron. 2022;213:114488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 126. | Gharravi AM, Jafar A, Ebrahimi M, Mahmodi A, Pourhashemi E, Haseli N, Talaie N, Hajiasgarli P. Current status of stem cell therapy, scaffolds for the treatment of diabetes mellitus. Diabetes Metab Syndr. 2018;12:1133-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 127. | Makaram P, Owens D, Aceros J. Trends in Nanomaterial-Based Non-Invasive Diabetes Sensing Technologies. Diagnostics (Basel). 2014;4:27-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 128. | Zhang W, Zhang L, Gao H, Yang W, Wang S, Xing L, Xue X. Self-Powered Implantable Skin-Like Glucometer for Real-Time Detection of Blood Glucose Level In Vivo. Nanomicro Lett. 2018;10:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 129. | Shoaib A, Darraj A, Khan ME, Azmi L, Alalwan A, Alamri O, Tabish M, Khan AU. A Nanotechnology-Based Approach to Biosensor Application in Current Diabetes Management Practices. Nanomaterials (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 130. | Sabbagh F, Muhamad II, Niazmand R, Dikshit PK, Kim BS. Recent progress in polymeric non-invasive insulin delivery. Int J Biol Macromol. 2022;203:222-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 131. | Nie X, Chen Z, Pang L, Wang L, Jiang H, Chen Y, Zhang Z, Fu C, Ren B, Zhang J. Oral Nano Drug Delivery Systems for the Treatment of Type 2 Diabetes Mellitus: An Available Administration Strategy for Antidiabetic Phytocompounds. Int J Nanomedicine. 2020;15:10215-10240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 132. | Iyer G, Dyawanapelly S, Jain R, Dandekar P. An overview of oral insulin delivery strategies (OIDS). Int J Biol Macromol. 2022;208:565-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 133. | Zhang T, Tang JZ, Fei X, Li Y, Song Y, Qian Z, Peng Q. Can nanoparticles and nano-protein interactions bring a bright future for insulin delivery? Acta Pharm Sin B. 2021;11:651-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 134. | Cui M, Wu W, Hovgaard L, Lu Y, Chen D, Qi J. Liposomes containing cholesterol analogues of botanical origin as drug delivery systems to enhance the oral absorption of insulin. Int J Pharm. 2015;489:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 135. | Allawadhi P, Singh V, Govindaraj K, Khurana I, Sarode LP, Navik U, Banothu AK, Weiskirchen R, Bharani KK, Khurana A. Biomedical applications of polysaccharide nanoparticles for chronic inflammatory disorders: Focus on rheumatoid arthritis, diabetes and organ fibrosis. Carbohydr Polym. 2022;281:118923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 136. | Raguraman R, Srivastava A, Munshi A, Ramesh R. Therapeutic approaches targeting molecular signaling pathways common to diabetes, lung diseases and cancer. Adv Drug Deliv Rev. 2021;178:113918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 137. | Goo YT, Lee S, Choi JY, Kim MS, Sin GH, Hong SH, Kim CH, Song SH, Choi YW. Enhanced oral absorption of insulin: hydrophobic ion pairing and a self-microemulsifying drug delivery system using a D-optimal mixture design. Drug Deliv. 2022;29:2831-2845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 138. | Qiu A, Wang Y, Zhang G, Wang H. Natural Polysaccharide-Based Nanodrug Delivery Systems for Treatment of Diabetes. Polymers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |