Published online Jul 15, 2022. doi: 10.4239/wjd.v13.i7.498
Peer-review started: February 1, 2022
First decision: April 18, 2022
Revised: April 19, 2022
Accepted: June 24, 2022
Article in press: June 24, 2022
Published online: July 15, 2022
Processing time: 159 Days and 14.7 Hours
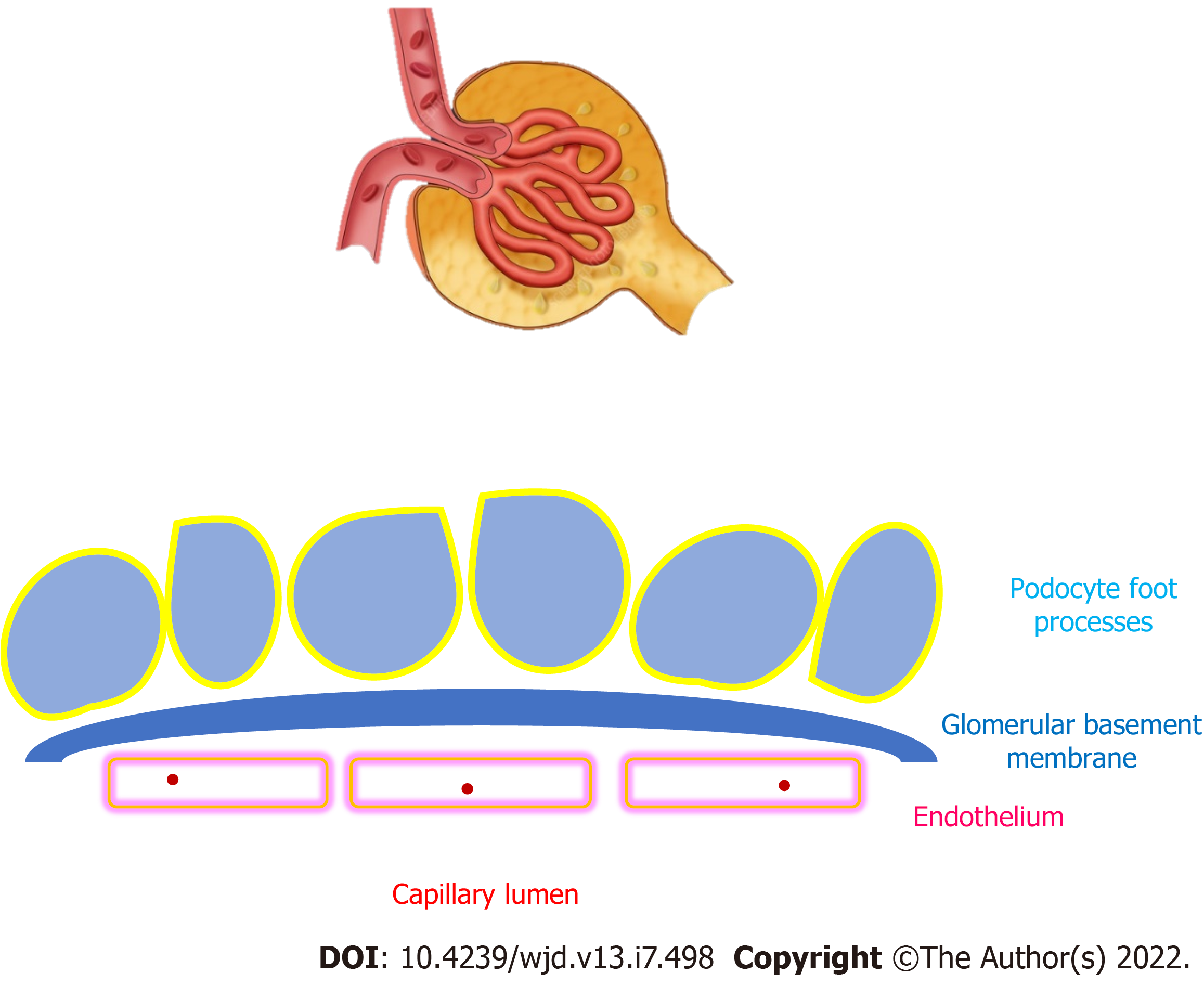
In the glomeruli, mesangial cells produce mesangial matrix while podocytes wrap glomerular capillaries with cellular extensions named foot processes and tether the glomerular basement membrane (GBM). The turnover of the mature GBM and the ability of adult podocytes to repair injured GBM are unclear. The actin cytoskeleton is a major cytoplasmic component of podocyte foot processes and links the cell to the GBM. Predominant components of the normal glomerular extracellular matrix (ECM) include glycosaminoglycans, proteoglycans, laminins, fibronectin-1, and several types of collagen. In patients with diabetes, multiorgan composition of extracellular tissues is anomalous, including the kidney, so that the constitution and arrangement of glomerular ECM is profoundly altered. In patients with diabetic kidney disease (DKD), the global quantity of glomerular ECM is increased. The level of sulfated proteoglycans is reduced while hyaluronic acid is augmented, compared to control subjects. The concentration of mesangial fibronectin-1 varies depending on the stage of DKD. Mesangial type III collagen is abundant in patients with DKD, unlike normal kidneys. The amount of type V and type VI collagens is higher in DKD and increases with the progression of the disease. The GBM contains lower amount of type IV collagen in DKD compared to normal tissue. Further, genetic variants in the α3 chain of type IV collagen may modulate susceptibility to DKD and end-stage kidney disease. Human cellular models of glomerular cells, analyses of human glomerular proteome, and improved microscopy procedures have been developed to investigate the molecular composition and organization of the human glomerular ECM.
Core Tip: Diabetic kidney disease is associated with profound disturbance in glomerular extracellular matrix (ECM). Understanding the mechanisms that regulate glomerular ECM synthesis and repair may contribute to design therapeutic strategies that improve clinical outcomes. The cytoskeleton inside the foot processes of podocytes is connected to the glomerular basement membrane (GBM) via associated proteins. There is a reciprocal interaction between the cellular cytoskeleton and the extracellular tissue that contribute to regulate ECM composition. Loss of anchor points in the GBM may lead to podocyte detachment. Likewise, alterations in the podocyte cytoskeleton may unfasten the cell and impair the filtration barrier.
- Citation: Adeva-Andany MM, Carneiro-Freire N. Biochemical composition of the glomerular extracellular matrix in patients with diabetic kidney disease. World J Diabetes 2022; 13(7): 498-520
- URL: https://www.wjgnet.com/1948-9358/full/v13/i7/498.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i7.498
In human glomeruli, mesangial cells (which are thought to be akin to vascular smooth muscle cells) produce mesangial matrix while podocytes wrap glomerular capillaries with cellular extensions named foot processes and tether underneath glomerular basement membrane (GBM) (Figure 1). The turnover of the GBM present at birth and the ability of adult podocytes to restore damaged GBM are unclear. These cells have limited proliferation capacity, but they can undergo hypertrophy to compensate for the detachment and loss of contiguous podocytes, thus avoiding uncovered GBM areas to preserve the filtration barrier. Podocyte detachment may be caused by an altered composition of the GBM with deficiency of anchor points or by anomalies in the connection apparatus that links the foot processes to the GBM. The actin cytoskeleton is a major cytoplasmic component of the podocyte foot processes and connects the cell to the GBM. Actin-associated proteins such as α-actinin-4 and inverted formin-2 attach the actin cytoskeleton to plasma membrane components (such as integrins, syndecans and dystroglycans), which in turn bind to their ligands in the GBM, including laminin and fibronectin-1[1-4]. The integrity of the GBM is crucial to maintain the filtration barrier, as highlighted by the clinical consequences of disorders that alter GBM components, such as laminin or collagen. Diabetes and other conditions associated with insulin resistance (such as Alström syndrome) are associated with a systemic and pronounced alteration in the composition of extracellular matrix (ECM), including the kidney and the blood vessels, that leads to multi-organ interstitial fibrosis[5]. Pathogenic mechanisms underlying this disturbance are unclear. Understanding the pathways of ECM assembly and remodeling and the cell-ECM interactions is crucial for designing therapeutic strategies and tissue engineering. A growing number of procedures have been improved and developed to investigate the biochemical composition and architecture of the ECM and its mutual interaction with the contiguous cells. Among them are biochemical assays to identify and quantify ECM components, genetic methods to investigate gene expression, imaging procedures, human cell cultures, and in vitro pharmacological evaluations to assess metabolic pathways.
Nuclear magnetic resonance spectroscopy and soft-ionization mass spectrometry represent complimentary techniques for ECM research. Mass spectrometry techniques (such as matrix-assisted laser desorption and ionization) are useful for compositional analysis whereas nuclear magnetic resonance spectroscopy evaluates the molecular architecture of the ECM and its dynamics[6]. Raman spectroscopy is a label-free vibrational technique that contributes to characterize the molecular ECM structure and composition[7,8].
Histological methods for ECM analysis with conventional microscopy include immunohistochemistry and zymography. The former can be utilized to determine the localization of various ECM proteins while the latter may be used to evaluate proteinase activity in the ECM. In addition, imaging methods have been designed to characterize the human ECM and the adjacent cells at the molecular and cellular level. Scanning electron microscopy and multi-harmonic generation microscopy can be used to visualize ECM components and assess their structural properties[9]. Multiphoton imaging has been described to analyze the human structural organization of elastin and collagen during mechanical loading[10].
The construction of flat and tubular collagen gel-based scaffolds cellularized with vascular smooth muscle cells have enriched vascular tissue engineering[11]. Microgel assembly, a macroscopic aggregate formed by assembly of microgels, can be applied to tissue engineering and cell cultures[12].
A variety of genetic techniques are useful on ECM research. MicroRNAs are noncoding RNAs that regulate gene expression and participate in ECM pathophysiology. Microarrays can be used to determine microRNA profiles[13]. Weighted gene co-expression network analysis enables the identification of clusters of related genes that can be associated with specific clinical phenotypes. This technique has been used to assess differentially expressed ECM genes in patients with diabetic kidney disease (DKD) and other glomerular diseases[14,15]. The Matrisome Project has been developed to characterize genes encoding structural and associated ECM proteins (http://matrisomeproject.mit.edu).
Human cell culture technology and pharmacological investigations on cultured cells are instrumental tools on ECM research. Human pluripotent stem cells in culture have been used to generate models of various tissues. The presence of ECM in the cultures provides cues to the cells that modify their behavior and improves similarity to the native human tissue, including the kidney[16]. Stem cells reside in “niches” within the ECM and the composition of the ECM contributes to define the degree of quiescence and turnover of these cells[17]. Manufacture of a suitable ECM is crucial to alter growth, differentiation, and proliferation of stem cells and use them for tissue engineering[18]. Three-dimensional decellularized ECM derived from mesenchymal stem cell cultures has been attained by application of macromolecular crowding[19]. Three-dimensional tissue constructs that recapitulate human fibrous connective tissue have been achieved by using cultures of primary human fibroblasts, enabling the quantification of cell-derived changes in ECM synthesis in response to several stimuli, such as nutrient composition or pharmacological compounds[20]. Treatment of human bone marrow-derived mesenchymal cells with high molecular weight hyaluronic acid increases fibronectin production and ECM deposition, suggesting that hyaluronic acid-based biomaterials may be useful to promote ECM formation[21]. A pulsatile perfusion culture of progenitor cells has been developed as an in vitro system to construct vascular tissue[22]. Cell-matrix interactions may be investigated by micro-electro-mechanical systems and Organ-on-a-Chip technology[23].
In the kidney, human cellular models of glomerular epithelial cells have been developed that can be used to evaluate podocyte pathophysiology and investigate therapeutic strategies[24]. Investigations of the glomerular proteome have provided information on the proteins expressed in the glomerular ECM of adult normal human kidney. A database has been created that may be used for clinical research on the pathophysiology of kidney diseases[25-27]. Proteomic analysis of human glomerular ECM may be conducted from sections retrieved by kidney biopsy samples[28]. The sub-diffraction resolution stochastic optical reconstruction microscopy (STORM) facilitates the investigation of the molecular organization within the human GBM[29].
The analysis of the specific composition of the GBM and mesangial matrix is hindered by the technical obstacle of adequately separate these two compartments of glomerular ECM, as procedures that isolate glomerular ECM achieve samples that contain both GBM and mesangial matrix. However, immunohistochemical analyses contribute to determine the differential constitution of the two structures (Table 1)[27,30-32]. Major components of the normal glomerular ECM are laminin and collagen. In addition, glycosaminoglycans, proteoglycans, sialic acid, and fibronectin-1 are important constituents of the kidney ECM in humans.
| Glomerular basement membrane | Mesangial matrix | |
| Heparan sulfate proteoglycan | Abundant | Abundant |
| Laminin | Major component | Minor component |
| Fibronectin | Minor component | Major component |
| Type I collagen | Absent in most studies | Absent in most studies |
| Type III collagen | Absent in most studies | Absent in most studies |
| Type IV collagen | Major component | Present (inconsistent amounts) |
| Type V collagen | Present | Present |
| Type VI collagen | Present | Present |
| Type XVII collagen | Present | Unknown |
| Type XVIII collagen | Present | Present |
| Tubulointerstitial nephritis antigen-like-1 | Low abundance | High abundance |
| Nidogen / Entactin | Present | Low abundance |
| Fibulin-1 | Present | Present |
| Fibrillin-1 | Present | Present |
| Nephronectin | Present | Present |
| Vitronectin | Absent | Present |
| Microfibril-associated proteins | Absent | Present |
Proteoglycans consist of a core protein attached to one or more glycosaminoglycan chains, which are formed by linear polysaccharides. Sulfate groups are usually bound to the unbranched polysaccharide chains, creating a high negative charge[33,34].
In kidney specimens from healthy humans, heparan sulfate is the predominant glycosaminoglycan present in the glomerular ECM, followed by hyaluronate, dermatan sulfate, and chondroitin sulfate isomers 4-sulfate and 6-sulfate[35-37].
Immunohistochemical studies show that both GBM and mesangial matrix contain heparan sulfate proteoglycans[33,38-40]. Among them, mass spectrometry-based analyses of normal human kidney samples reveal that agrin and perlecan are present in the glomerular proteome[27]. Agrin is a major heparan sulfate proteoglycan present in human GBM. Immunoelectron microscopy shows a linear distribution of agrin throughout the width of the normal GBM. In addition to the GBM, agrin mRNA and protein are detected in normal lungs[34,41]. Localization of agrin to the human GBM has been confirmed by STORM. Using this procedure, agrin is predominantly detected at the epithelial surface compared to the endothelial aspect of the GBM[29]. The precise function of agrin in the human kidney has not been defined, but it may contribute to the adhesion of the GBM to the podocyte by tethering laminins to cell surface receptors such as integrins or α-dystroglycan[34,41]. In normal human kidney specimens, perlecan stained the GBM only slightly, in contrast to the strong staining of the mesangium, the Bowman’s capsule, and the tubular basement membrane. The function of this heparan sulfate proteoglycan in the normal kidney remains to be clarified[34,42,43]. Unlike agrin, immunoelectron microscopy shows that perlecan is distributed only on the endothelial side of the GBM[34].
Sialic acid (neuraminic acid) is a nine-carbon carbohydrate that may exist as several derivatives. In humans, the most common sialic acid byproduct is the acetylated compound N-acetyl-neuraminic acid. Sialic acid typically occupies the terminal domain of oligosaccharide chains of some glycolipids and glycoproteins and usually protrudes from the cell surface. Sialidases (neuraminidases) are enzymes that remove sialic acid residues from glycosaminoglycans attached to proteins or lipids on the cell surface (desialylation). Sialyltransferases catalyze the addition of sialic acid residues to glycosaminoglycans (sialylation). Sialylated conjugates are identified by specific binding to lectins or by cationic dyes such as alcian blue[44,45]. In normal human kidney specimens, sialic acid stains strongly the podocytes, unlike glomerular capillaries and Bowman’s capsule[44].
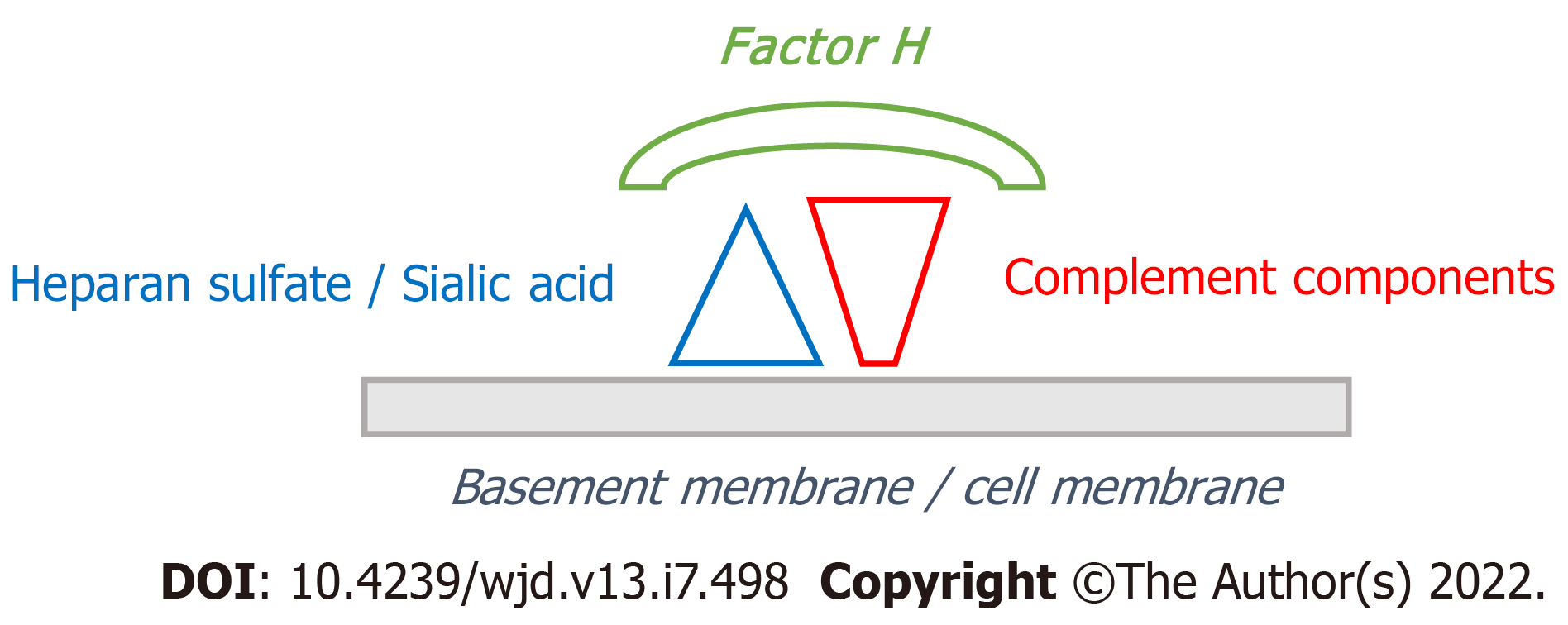
Both sulfated glycosaminoglycans and sialic acid are polyanions that have an essential role in the identification of “self” structures to avoid complement activation and subsequent complement-mediated injury in host tissues[46-49]. Sulfation can occur at various positions within the glycosaminoglycan structure creating the potential for high molecular variability. The unique position of sulfate groups in the glycosaminoglycan molecule is named sulfation code and defines functional characteristics of the sulfated glycosaminoglycan, such as its interaction with proteins. Hyaluronic acid is a glycosaminoglycan that lacks sulfate groups and is not attached to a protein core to form proteoglycans[50]. Factor H is a glycoprotein that inhibits the alternative pathway of complement in “self” structures (as opposed to foreign elements such as pathogens), by recognizing sialic acid or sulfated glycosaminoglycans present on “self” biological surfaces. The interaction between factor H and sulfated glycosaminoglycans is highly specific and depends upon the sulfation code. Little or no binding occurs with hyaluronic acid. The binding of factor H to sialic acid or sulfated glycosaminoglycans on biological surfaces protects the host from autolytic complement attack (Figure 2). Deficit of binding sites for factor H due to loss of sulfated glycosaminoglycans or sialic acid (or alteration of the sulfation code or the sialylation pattern) impairs factor H binding to “self” structures and may result in complement-mediated damage due to unrestrained activation of the alternative pathway of complement[46,48,49,51-54].
Protein quantification of the glomerular ECM proteome by mass spectrometry reveals that laminin isoforms and type IV collagen are the most abundant proteins in the glomerular ECM[27]. Laminins are heterotrimeric proteins composed of α, β, and γ glycoprotein chains. Different α, β, and γ chains create diverse isoforms of laminin heterotrimers, such as laminin α5/β2/γ1 (laminin 521). Laminin heterotrimers polymerize in the extracellular space to form a network. Laminin polymerization is required for initiation of basement membrane formation. The actin cytoskeleton plays an important role in extracellular laminin polymerization. In vitro studies using myotubes reveal that the organization of extracellular laminin into networks is abnormal when the actin cytoskeleton is disrupted with cytochalasin (an agent that prevents actin polymerization) compared to control myotubes free of this compound. Cytochalasin-treated myotubes show no arrangement of surface laminin into complex networks, unlike control myotubes that show normal laminin array. However, no detrimental effect on laminin network formation was observed with wortmannin, an inhibitor of phosphatidylinositol 3-kinase[55].
Laminin isoforms has been identified in the mesangial matrix of normal human kidneys, although this protein is predominantly detected in the GBM. Immunohistochemical studies show a continuous staining for laminin in the normal GBM. Immunogold electron microscopy reveals that laminin is distributed throughout the entire thickness of the GBM[37-40,56]. The organization of laminin 521 in normal human GBM has been investigated with STORM. Laminin 521 (and agrin) have their N-terminal domains facing the interior of the GBM while their C-terminal domains are oriented towards the surface of endothelial cells and podocytes[29].
The important functional role of laminin in the glomeruli is underlined by the clinical consequences of genetic mutations that alter the protein. Mutations in the gene that codes the β2 chain of laminin cause an autosomal recessive clinical spectrum of disorders that ranges from isolated congenital nephrotic syndrome (type 5) to Pierson syndrome, which consists of a combination of ocular abnormalities, neurological manifestations due to defects of the neuromuscular junction, and congenital nephrotic syndrome with diffuse mesangial sclerosis progressing rapidly to end-stage kidney disease (ESKD)[57,58].
Fibronectin-1 is a dimeric glycoprotein circulating in normal plasma and present in the healthy human kidney. Immunohistochemical studies show that fibronectin-1 is mainly present in the mesangium and to a far less degree in the GBM. Staining for fibronectin-1 also occurs in the Bowman’s capsule and the peritubular interstitium[27,37,39,40,59,60]. The function of fibronectin-1 in the kidney is largely unknown. In vitro studies using cultured fibronectin-null cell lines find that fibronectin-1 polymerization in the ECM is involved in the deposition of other ECM components, such fibulin, type III collagen, and type I collagen[61]. Fibronectin-1 possesses several domains that may function as binding sites for other molecules, including collagen and cell surface proteins such as integrins. Fibronectin-1 may connect to plasma membrane proteins which in turn are linked to the intracellular actin cytoskeleton. There is a reciprocal relationship between fibronectin-1 and the actin cytoskeleton. Agents that disrupt actin polymerization block the extracellular organization of fibronectin-1 into a network. In turn, inhibition of fibronectin-1 polymerization in the ECM induces changes in the actin cytoskeleton[62]. In vitro studies using cultured human podocytes show that fibronectin-1 is essential for the attachment of podocytes to the GBM during mechanical stress. Mechanical stretch induces a marked upregulation of fibronectin-1 in normal podocytes. Accordingly, in podocyte cell lines lacking fibronectin-1, a loss of podocytes greater than 80% is observed following mechanical stress[4].
An abnormal glomerular accumulation of fibronectin-1 may occur in acquired disorders, such as DKD and other diseases that feature mesangial expansion, such as lupus nephritis, IgA nephropathy, and membranoproliferative glomerulonephritis[62-65]. In addition, glomerulopathy with fibronectin-1 deposits (fibronectin nephropathy) is an autosomal dominant disease characterized by deposits of fibronectin-1 in the mesangial matrix and subendothelial space. Mutations in the FN1 locus (that encodes fibronectin-1) at 2q32 have been identified as the genetic cause of the disease[63]. Clinical manifestations include proteinuria, hematuria, hypertension, and kidney failure that may progress to ESKD. Asymptomatic patients harboring FN1 mutations have been documented. Light microscopy reveals enlarged glomeruli with deposits of eosinophilic material in the mesangium and subendothelial space that shows reactivity with periodic acid Schiff (PAS) and trichrome stains while methenamine silver and Congo red stain negative. No immunoglobulin or complement factors are detectable by immunofluorescence studies. Electron microscopy reveals a normal GBM and large electron-dense deposits in the mesangium extending to the subendothelial space. Diagnosis can be established by specific immunohistochemical analysis, as the glomerular deposits stain intensely with anti-fibronectin-1 antibodies (Table 2)[62-65].
| Periodic acid Schiff | Methenamine silver | Congo red | Specific analysis | |
| Diabetic kidney disease | Positive | Positive | Negative | Unknown material |
| Fibronectin-1 nephropathy | Positive | Negative | Negative | Fibronectin-1 |
| Type III collagen nephropathy | Negative | Negative | Negative | Type III collagen |
Initial studies suggested the presence of collagen in normal human glomerular ECM by the high amount of glycine, hydroxyproline, and hydroxylysine in glomerular extracts[30,66-68]. Relative protein quantification confirms an abundant amount of type I, type IV, and type VI collagen in human glomerular ECM. As mentioned, type IV collagen and laminin are the most abundant proteins in the normal glomerular ECM[27].
Type I collagen in the normal glomerulus: Some studies fail to find type I collagen in normal human glomerular ECM, either the GBM or the mesangium[39,40,69]. However, mass spectrometry performed in adult kidney samples identifies abundant type I collagen in the glomerular ECM, although its localization to a specific glomerular ECM sector (GBM, mesangial matrix, or other) is undefined[27].
Type III collagen in the normal glomerulus: Type III collagen mRNA or protein have not been detected in healthy human glomeruli. Neither the mesangial matrix nor the GBM normally possess type III collagen[33,38-40,69-71]. However, type III collagen has been observed in sclerotic glomeruli, suggesting that production of this collagen type is linked to the progression of glomerular sclerosis[69]. In addition, glomerular type III collagen has been demonstrated in human kidney diseases, such as DKD, LIM homeodomain transcription factor-1β (LMX1β)-associated nephropathy (LAN) and type III collagen glomerulopathy.
Heterozygous loss of function mutations in the LMXIβ gene (located on chromosome 9q34) cause LAN. Patients with LAN may present with isolated nephropathy or may exhibit additional extrarenal clinical manifestations composing the nail-patella syndrome[72,73]. The LMXIβ protein is a transcription factor that possesses two LIM domains (cysteine rich sequences that usually mediate protein-protein interactions) and a homeodomain that regulates target gene transcription. The precise role of the LIM-homeodomain protein LMXIβ in humans remains unknown[74,75]. Nail-patella syndrome or onycho-osteodysplasia is characterized by the association of nail hypoplasia or dysplasia, bone abnormalities that affect the knees, elbows, and pelvis, glaucoma, sensorineural hearing impairment, and nephropathy. Renal manifestations include hematuria, proteinuria, and kidney failure that may evolve to ESKD. LMX1β mutations may also cause isolated autosomal dominant kidney involvement with no extrarenal manifestations[72,73,75-78]. LAN is characterized by deposition of type III collagen within the GBM on electron microscopy examination. Fibrillar type III collagen bundles may be seen occasionally in the mesangial matrix as well. The GBM may demonstrate focal irregular thickening, thinning, splitting, or wrinkling and may contain patchy electron-lucent (“moth-eaten”) areas. Hyperplasia and effacement of podocyte foot processes is usually observed. The basement membrane of kidney tubules appears markedly thickened and demonstrates type III collagen deposition[78,79]. Light microscopy examination may reveal focal segmental glomerulosclerosis (FSGS) or unremarkable findings, such as mild interstitial fibrosis or mesangial proliferation. Immunofluorescence microscopy yields negative or non-specific findings, such as slight granular deposits of C3 in the mesangium. Specific immunohistochemical analyses show that the fibrillar material present within the GBM (and occasionally the mesangial matrix) is type III collagen[78-82]. The histological phenotype of LAN is expanding, as heterozygous mutations in the LMXIβ gene have been reported in patients with autosomal dominant FSGS without ultrastructural abnormalities of the GBM and in families with FSGS and myelin figures and zebra bodies (electron-dense multilamellar inclusions) in podocytes, mesangial cells, and tubular epithelium. Patients affected with LAN and myelin figures and zebra bodies are free of Fabry’s disease, which is the typical cause of these inclusions. Therefore, LMX1β pathogenic variants should be ruled out as a potential cause of autosomal dominant kidney disease, sporadic and hereditary forms of FSGS, and steroid-resistant nephrotic syndrome, regardless of extrarenal manifestations. In addition, the presence of myelin figures or zebra bodies may hint toward LAN diagnosis in patients free of lysosomal storage disorders or drug-induced phospholipidosis, although the mechanism underlying the appearance of these structures in LAN is unclear[80,82,83].
Type III collagen glomerulopathy (collagenofibrotic glomerulopathy) is characterized by deposition of type III collagen fibrils within the mesangial matrix and along the subendothelial aspect of a normal GBM. The cause of the excessive production and deposition of type III collagen in the glomeruli is unknown. The diagnosis of the disease is confirmed by electron microscopy and specific immunohistochemistry demonstrating the presence of mesangial type III collagen. Light microscopy reveals diffuse mesangial expansion that cause glomerular enlargement. In the advanced stage, the expanded mesangium shows a lobular appearance reminiscent of Kimmelstiel-Wilson nodules of DKD. In addition, the subendothelial accumulation of this material causes double contour or “reduplication” of the GBM. Unlike DKD, the amorphous material present in the mesangium stains negative with PAS. Masson’s trichrome stain identifies the blue-colored collagen within the mesangium. Immunofluorescence microscopy studies are negative for immunoglobulins and complement components. Immunohistochemistry reveals strong staining for antibodies to type III collagen in the widened mesangial and subendothelial areas. Electron microscopy reveals a normal GBM and confirms the accumulation of electron-dense fibrillar material consistent with dense collagen bundles in mesangial and subendothelial zones. The fibrils exhibit a transverse band structure with distinctive periodicity suggesting type III collagen fibers. The mesangium and subendothelium acquire a mottled appearance due to the presence of collagen fibrils[84-86]. Accumulation of mesangial type III collagen has been reported in one patient with inherited factor H deficiency[87].
Type IV collagen in the normal glomerulus: Type IV collagen is an abundant protein of the glomerular ECM and may be observed in the GBM and the mesangium. In the normal GBM, a distinct continuous staining for type IV collagen indicates that this collagen type is a predominant component[27,31,37,39,71,88].
The molecule of type IV collagen consists of three α chains. Six genes (COL4A1-6) encode six different α chains that create several isoforms of type IV collagen. The α3, α4, and α5 chains of type IV collagen contain more cysteine than the chains α1 and α2. Therefore, α1α1α2 (α112) trimers possess fewer disulfide bonds than α3α4α5 (α345) heterotrimers. The relative protein abundance of the α1 and α2 chains in normal adult glomerular ECM has been reported higher compared to the richness of the α3, α4, and α5 chains[27].
Ultrastructural examination with immunogold technique reveals that type IV collagen is concentrated in the endothelial zone and decreases towards the epithelial third of the GBM in normal human glomeruli. In addition, the α1 chain is distributed primarily along the endothelial side of GBM whereas the α3 and α4 chains are seen throughout the thickness of the GBM[56,89]. Kidney assessment with STORM reveals that type IV collagen α345 trimers are localized at the center of the GBM while type IV collagen α112 trimers are located to the endothelial side. Co-labeling for both trimers of type IV collagen (α345 and α112) suggest the α112 network occupies the space between the central α345 Layer and the endothelial surface of the GBM[29].
In addition to the GBM, type IV collagen is detectable in the mesangial matrix of normal human glomeruli[38-40,43,71]. Investigations using quantitative immunogold electron microscopy show that mesangial type IV collagen labeling appears uniform throughout the mesangial matrix and extends to the subendothelial side of the GBM[56,89]. Electron microscopy examination with immunogold technique shows that the α1 chain of type IV collagen is distributed primarily along the mesangial matrix and the endothelial side of GBM whereas the α3 chain of type IV collagen is not detected in normal human mesangial matrix[32].
The relevance of type IV collagen to kidney structure and function is highlighted by the clinical consequences of mutations in genes that code the α chains of this collagen type. Mutations in the COL4A3-5 genes cause type IV collagen-related kidney disease (Table 3). The COL4A5 gene encodes the α5 chain and maps to the X chromosome. Mutations in this gene account for X-linked Alport syndrome. Males harbor hemizygous mutations whereas females carry heterozygous mutations. The COL4A4 and COL4A3 genes encode respectively the α4 and α3 chains of type IV collagen and are located on chromosome locus 2q36-37. Mutations in these genes cause autosomal Alport syndrome. Biallelic (homozygous or compound heterozygous) mutations in either one of them result in autosomal recessive Alport syndrome whereas autosomal dominant Alport syndrome is due to heterozygous mutations in either the COL4A4 or COL4A3 genes. Mutations in two of the COL4A5, COL4A4, or COL4A3 genes cause digenic Alport syndrome[90-94].
| Gene/location | Protein | Mutation | Risk of progression to end-stage kidney disease | |
| X-linked Alport syndrome | COL4A5/X chromosome | α5 chain of type IV collagen | Hemizygous (males) or heterozygous (females) mutations | Hemizygous: 100%; Heterozygous: 25% |
| Autosomal recessive Alport syndrome | COL4A4 or COL4A3/2q36-37 | α4 and α3 chains of type IV collagen | Biallelic (homozygous or compound heterozygous) mutations | 100% |
| Autosomal dominant Alport syndrome | COL4A4 or/COL4A32q36-37 | α4 and α3 chains of type IV collagen | Heterozygous mutations in the α4 or α3 chains | 20% in patients with risk factors for progression |
| Digenic Alport syndrome | Two of the COL4A3-5 genes | Two of the α3-5 chains |
Mutations in type IV collagen are highly prevalent. Genome-wide association studies show that 1 in 600 subjects from the Icelandic population carry a variant in the COL4A3 gene associated with hematuria and albuminuria. In the UK population, the COL4A4 variant rs35138315 (Ser969X) has a carrier frequency of 0.13% and is also associated with hematuria and albuminuria[95]. Among 24 Greek families with familial microscopic hematuria, next generation sequencing identifies pathogenic mutations in the COL4A3-5 genes in 17 (71%) of them[96]. Mutations in the COL4A3-5 genes are also frequently found in patients with sporadic and familial FSGS[94,95,97-99]. Pathogenic variants in any of the COL4A3-5 genes are found in up to 10% of patients with renal failure of unknown cause and in some families with IgA nephropathy[94]. Therefore, indications for screening for pathogenic variants in the COL4A5, COL4A4, or COL4A3 genes have been extended beyond the classical Alport syndrome phenotype (hematuria, renal failure, family history of hematuria or renal failure) to include FSGS, persistent proteinuria, steroid-resistant nephrotic syndrome, familial IgA nephropathy, and ESKD without an obvious cause[94].
The phenotypical expression of mutations in type IV collagen (COL4A3-5 genes) is heterogeneous. Patients with type IV collagen-related nephropathy may exhibit isolated microscopic hematuria, proteinuria, or kidney failure that evolves to ESKD. In addition, patients with type IV collagen mutations may experience extrarenal manifestations such as sensorineural hearing loss, lenticonus, and retinopathy[90-93,97]. In patients with mutations in the COL4A5 gene (X-linked Alport syndrome), hemizygous males have a 100% risk of progression to ESKD while heterozygous females (formerly called carriers) have substantial risk associated with proteinuria, progressive renal disease, and sensorineural hearing loss. Their lifetime risk of progression to ESKD is approximately 25%. Patients with autosomal recessive Alport syndrome (due to biallelic mutations in COL4A4 or COL4A3 genes) have a 100% risk of ESKD. Patients with heterozygous mutations in COL4A4 or COL4A3 genes (autosomal dominant Alport syndrome) may be asymptomatic or may exhibit hematuria or proteinuria and include patients previously diagnosed with thin basement membrane nephropathy. Risk factors for progression to ESKD in these subjects include proteinuria, sensorineural deafness, family history of progression to ESKD and renal biopsy findings of FSGS or GBM thickening and disarray. The risk of ESKD is up to 20% among those with risk factors. Patients with heterozygous mutations in COL4A4 or COL4A3 genes without kidney manifestations (hematuria or proteinuria) generally have a good prognosis but should be screened in a yearly basis[93].
The kidney histological phenotype of mutations in type IV collagen is characterized by GBM alterations, effacement of podocyte foot processes, and FSGS. Light microscopy examination of kidney samples from patients with Alport syndrome may reveal normal glomeruli or only minor mesangial widening. Immunofluorescent staining generally renders negative or nonspecific results. Electron microscopy usually provides the diagnosis, revealing changes in the GBM that may include areas of thinning, thickening, lamellation, and splitting. Initially, the GBM exhibits segmental thinning followed by progressive thickening and disorganization. In addition, diffuse podocyte foot process effacement occurs very frequently[74,97,99-101]. Patients with pathogenic variants affecting the α chains of type IV collagen may display FSGS with or without GBM changes[91,97-99,102,103].
Type V collagen in the normal glomerulus: In normal human glomeruli, type V collagen shows a distribution similar to type IV, being detectable in the GBM and the mesangium[38-40].
Type VI collagen in the normal glomerulus: In human adult glomerular tissue, mass spectrometry quantitative analyses show that type VI collagen is highly abundant[27]. In normal kidney samples, immunogold electron microscopy and immunohistochemical analyses show that the glomerular distribution of type VI collagen is comparable to that of the α1 chain of type IV collagen, namely along the mesangial matrix and the endothelial aspect of the GBM mainly[27,31,32,38,40].
Other types of collagen (type XV, type XVII and type XVIII collagen) in the normal glomerulus: Type XV collagen (α1 chain) has been found among ECM proteins in the glomerular proteome although its biological significance is uncertain[27].
Type XVII collagen is a transmembrane molecule involved in epithelial adhesion that has been identified as an autoantigen in bullous pemphigoid, a blistering skin disease of autoimmune origin. The association of bullous pemphigoid and a glomerular disease with characteristics of anti-GBM disease and membranous nephropathy has been reported in a 75-year-old man that also had circulating IgG against BP180, the 180-kDa bullous pemphigoid antigen (type XVII collagen). The kidney biopsy exhibited endocapillary inflammation without crescents. Direct immunofluorescence showed strong IgG and C3 staining in a combined granular and linear pattern along the GBM. Electron microscopy revealed subepithelial deposits[104]. In a kidney biopsy sample collected from a 4-year-old girl with hematuria, immunoelectron microscopy reveals that type XVII collagen is expressed in the foot processes of podocytes. In addition, type XVII collagen can be seen in the adjacent lamina rara externa of the GBM[105].
Type XVIII collagen has been identified among the ECM proteins in the glomerular proteome, being present in the GBM and the mesangium. Its expression pattern is similar to that of the α1 and α2 chains of type IV collagen[27,106].
The tubulointerstitial nephritis antigen-like-1 (TINAGL1) is highly abundant in normal glomerular ECM, being predominantly localized to the mesangial matrix. TINAGL1 is a glycoprotein structurally related to the tubulointerstitial nephritis antigen, a protein of the tubular basement membrane that is the antigenic target in autoimmune anti-tubular basement membrane disease. Nephronectin, vitronectin, fibulin-1, and fibrillin-1 have been identified as components of glomerular proteome using mass spectrometry. Nephronectin is present both in the GBM and mesangial matrix while vitronectin is localized in the mesangial matrix alone[27].
Matrix metalloproteinases and their inhibitors: Matrix metalloproteinases (MMPs) and their inhibitors are present in the ECM, but the particular isoforms distributed to the human kidney and their specific pathophysiologic role remain largely unknown. Disruption of the balance between MMPs and their inhibitors in the extracellular space has been implicated in the development of kidney fibrosis. Plasma concentration of MMPs and their inhibitors have been correlated with insulin resistance and kidney disease in clinical studies, suggesting that the composition of the ECM is altered in these conditions[107-109]. In the Renal Iohexol Clearance Survey, higher MMP-7 Levels were independently associated with increased risk of accelerated glomerular filtration rate (GFR) decline and incident chronic kidney disease among 1324 adults from the general population free of baseline diabetes, kidney disease or cardiovascular disease, over a median observation period of 5.6 years. In contrast, MMP2 and tissue inhibitor of metalloproteinase-1 (TIMP-1) showed no association with kidney disease[107]. Patients with insulin resistance display increased plasma TIMP-1 Level compared with healthy subjects. Accordingly, elevated plasma TIMP-1 concentration may be a marker of interstitial fibrosis due to excessive collagen deposition[108,109].
In vitro studies show that the expression of MMPs in the kidney ECM may be regulated at least in part by growth factors and ECM components[110,111]. In human kidney tubular cells, transforming growth factor-β1 (TGF-β1) induces MMP-2 expression via up-regulation of integrin-linked kinase[110], while elevated glucose concentration decreases MMP-9 and MMP-2 expression and increases TIMP-1 expression[112]. In cultured human glomerular epithelial cells, the expression of MMP-2 and MMP-9 is down-regulated by the presence of the α3 chain of type IV collagen[111], while high glucose concentration reduces MMP-2 expression and up-regulates TIMP-2[113].
Growth factors: The ECM composition is modulated by growth factors such as TGF-β1, platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF). TGF-β1 and PDGFs may promote ECM fibrosis in the kidney at least in part via integrins and integrin-associated proteins[114,115]. In vitro investigations using human mesangial cells show that TGF-β1 induces mesangial matrix expansion[116]. Accordingly, up-regulation of TGF-β1 is observed in the areas of interstitial and fibrosis in human fibrotic kidneys, compared with control kidneys[117]. Likewise, the expression of TGF-β1 and type IV collagen is increased in kidney allografts with interstitial fibrosis compared to normal kidney tissue[118]. VEGF and its receptors are expressed in normal human kidney, particularly in podocytes and mesangial cells. In normal podocytes, transmission electron microscopy examination reveals that VEGF may be detected in the intracellular compartment (36%) and associated with the cell membrane (63%)[119]. In vitro studies show that VEGF induces a proliferative effect on human mesangial cells[120,121]. The role of VEGF in glomerular pathophysiology is largely unknown, but neutralizing VEGF activity may increase the risk of kidney disease, as bevacizumab (a monoclonal antibody against human VEGF-A) therapy has been associated with elevated risk of proteinuria and hypertension among cancer patients in a systematic review and meta-analysis of clinical trials[122]. Rapamycin therapy has been associated with reduced VEGF expression in the human kidney that might contribute to explain the renal side-effects of this drug[123].
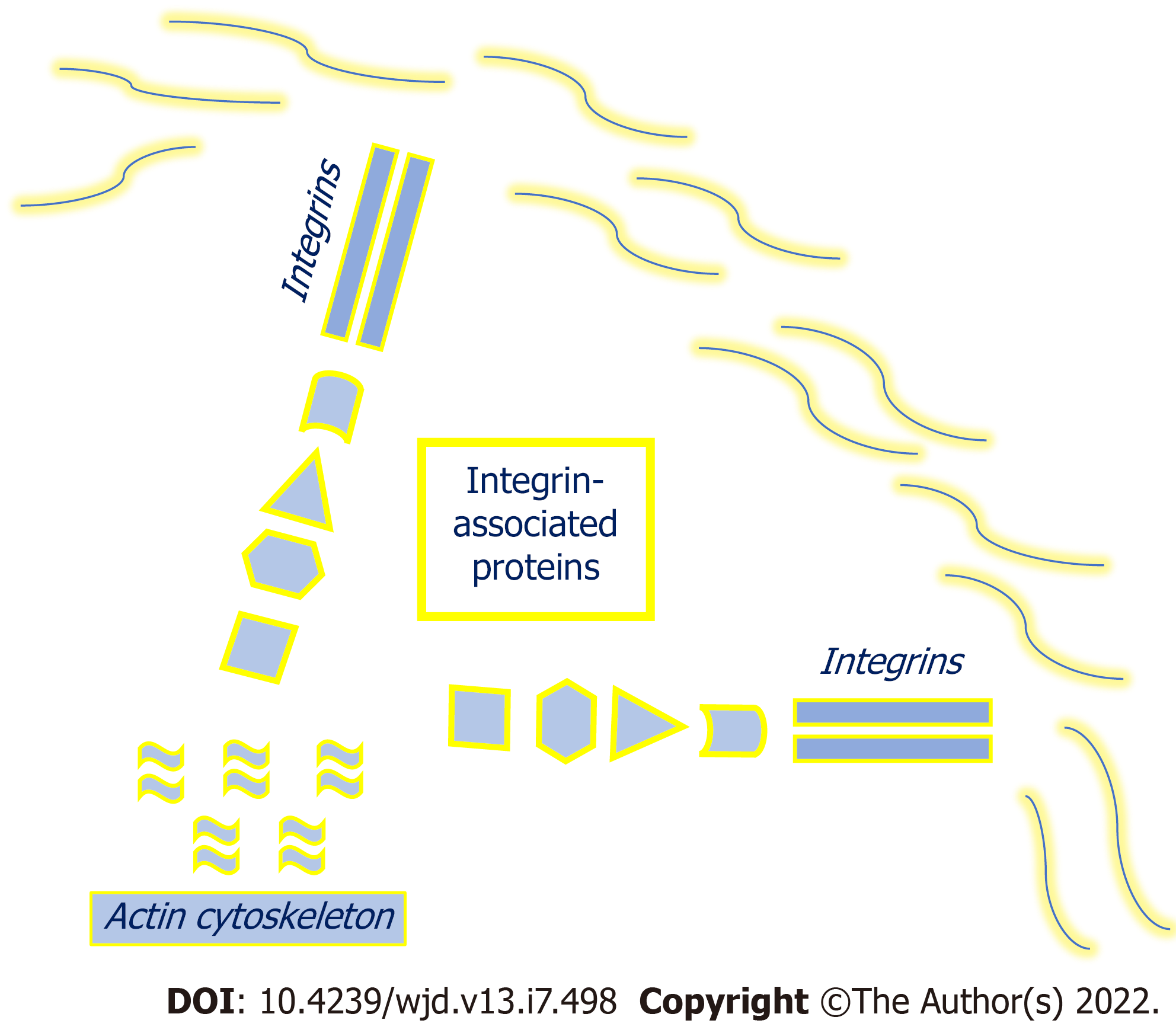
Integrins and integrin-associated proteins: Growth factors may interact with integrins to initiate signaling cascades. Integrins are plasma membrane proteins that link structurally and functionally the cell cytoskeleton with the extracellular space (Figure 3). Inside the cell, the cytoplasmic domain of integrins connects with the cytoskeleton via integrin-associated proteins, including integrin-linked kinase, particularly interesting new cysteine-histidine-rich protein (PINCH1), parvin proteins, and calponin homology domain-containing integrin-linked kinase-binding protein (CH-ILKBP)[114,124-128]. PINCH1 is an adaptor protein that comprises five LIM domains and interacts with integrin-linked kinase[129]. The parvins are partner proteins to integrin-linked kinase and PINCH1[130]. CH-ILKBP interacts with integrin-linked kinase, PINCH1, and the cytoskeleton. The interaction with integrin-linked kinase mediates the plasma membrane localization of CH-ILKBP. Northern blot analyses show widespread CH-ILKBP expression in human tissues, particularly in the heart, skeletal muscle, and kidney[131]. In vitro studies using human cell lines (HeLa cells) show that depletion of CH-ILKBP prevents the membrane translocation and the phosphorylation of protein kinase B (AKT), suggesting that CH-ILKBP facilitates the activation of this kinase in response to extracellular signals[132]. Integrins and integrin-associated proteins convey cues from growth factors and ECM components to intracellular pathways, although specific signaling cascades are not fully elucidated in humans[110,117,118]. Integrin signaling via integrin-associated proteins has been implicated in the regulation of ECM deposition and may be involved in the development of kidney fibrosis, both in native kidneys and kidney allografts, although underlying mechanisms remain largely unsolved[118]. An up-regulation of β1 integrin and integrin-linked kinase has been observed in areas of interstitial fibrosis in human fibrotic kidneys, compared with control kidneys[117]. In vitro experiments using cultured human proximal tubular cells reveal that overexpression of integrin-linked kinase and PINCH1 increases fibronectin-1 expression and its extracellular assembly, whereas PINCH1 knockdown reduces TGFβ1-mediated fibronectin-1 expression[110,124]. In vitro studies show that α3β1 integrin largely mediates the adhesion of human glomerular epithelial cells to type IV collagen[133]. Glucose concentration in the medium may alter integrin expression and the binding to type IV collagen in human glomerular epithelial cells[113], and human proximal tubular epithelial cells[112].
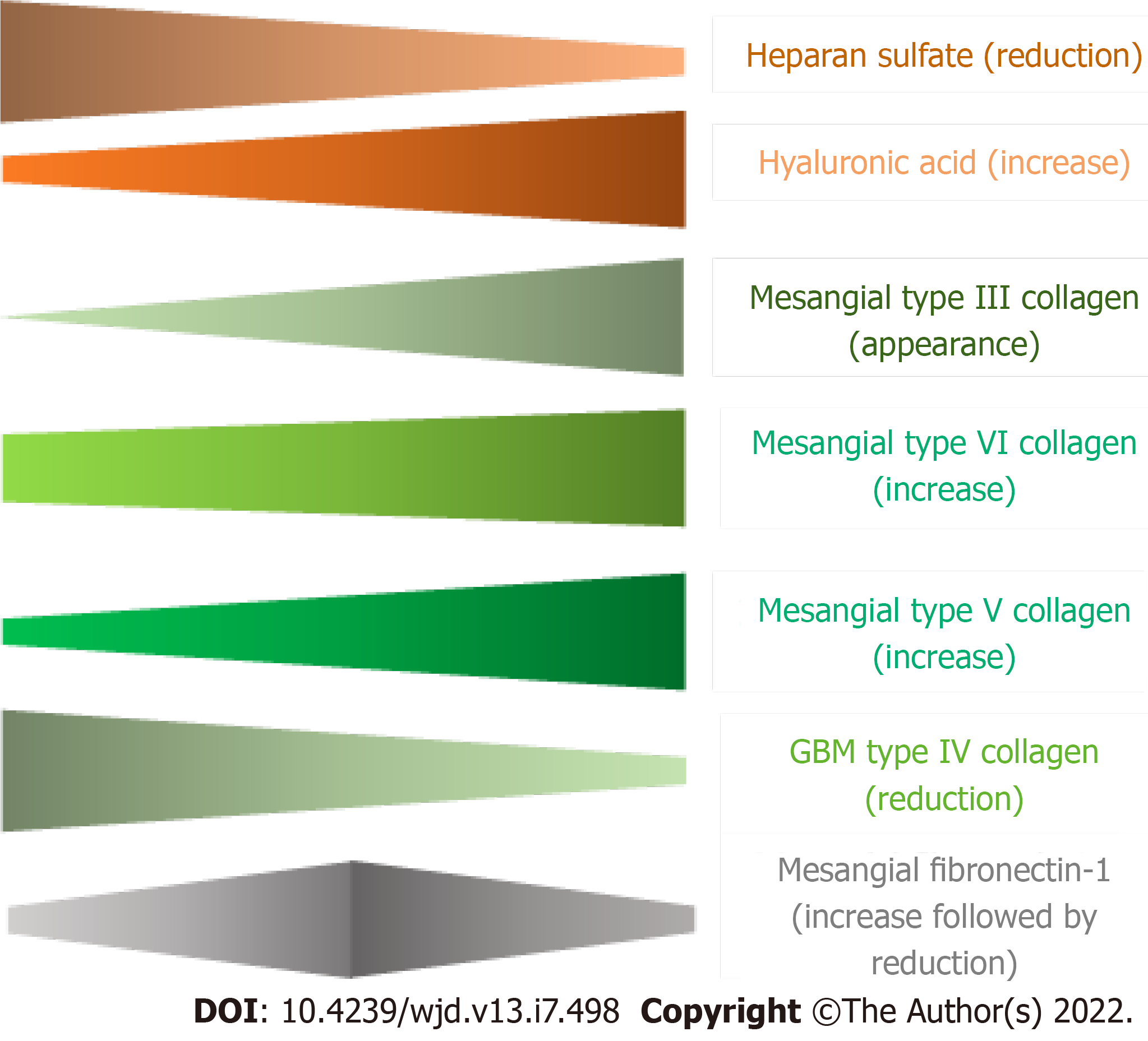
Diabetes is associated with a profound alteration in the composition of extracellular tissues throughout the body, including the kidney and the blood vessels. Patients with diabetes demonstrate increased interstitial collagen production and deposition that leads to fibrosis. Alström syndrome is an autosomal recessive disease due to mutations in the ALMS1 protein, characterized by the presence of early childhood insulin resistance. Like diabetes, patients with Alström syndrome typically show systemic fibrosis of extracellular tissues[5,36,134-136]. In patients with DKD, the amount and biochemical composition of the GBM and mesangial matrix are markedly anomalous. The global amount of glomerular ECM is increased, the level of heparan sulfate proteoglycans is reduced, and the collagen content is augmented compared to normal kidneys (Table 4)[36,134]. Furthermore, the abnormal composition of the glomerular ECM becomes more pronounced with the progression of DKD. Advanced sclerotic lesions show increased type III collagen and reduced amount of heparan sulfate proteoglycan and fibronectin-1 compared to earlier stages of DKD (Figure 4)[39,137,138].
| Normal glomeruli | Diabetic kidney disease | |
| Heparan sulfate proteoglycans | GBM and mesangial matrix | Decreased amount |
| Laminin | Predominantly in the GBM | Inconsistent |
| Fibronectin-1 | Mainly in the mesangial matrix | It varies according to DKD stage |
| Type I collagen | Inconsistent | No detectable |
| Type III collagen | Absent | Abundant |
| Type IV collagen | Abundant in the GBM | Reduced GBM amount |
| Type V collagen | Similar to type IV collagen | Increased mesangial amount |
| Type VI collagen | GBM and mesangial matrix | Increased mesangial amount |
In patients with diabetes, the content of heparan sulfate in the glomerular ECM is prominently reduced while the global amount of extracellular tissue is increased. A quantitative assessment conducted by immunochemical procedures reveals that the abundance of heparan sulfate proteoglycan in the GBM of patients with diabetes is 30% lower than that of control subjects. The decrease in glomerular heparan sulfate has been also observed in other diseases, such as C3 glomerulopathy, membranous nephropathy, minimal change disease, and lupus nephritis[33,36,37,139].
The reduction in glomerular heparan sulfate proteoglycan associated with DKD starts to occur early and becomes more severe with the advance of the disorder. In patients with mild diffuse glomerulosclerosis, the staining pattern of heparan sulfate proteoglycan is reduced in the thickened mesangial matrix while in more pronounced diffuse glomerulosclerosis and mesangial nodules the enlarged matrix lacks heparan sulfate proteoglycan completely[33,39]. In contrast, the amount of hyaluronic acid is increased in the glomerular ECM of patients with DKD compared to control subjects[140]. As mentioned, heparan sulfate is a major ligand for factor H, an inhibitor of the alternative pathway of complement on “self” biological surfaces. Loss of heparan sulfate (or altered sulfation pattern) may result in reduced factor H attachment to “self” structures, subsequent activation of the alternative pathway and complement-mediated injury, like occurs in the presence of mutated factor H (C3 glomerulopathy). Complement activation via the alternative pathway may contribute to the progression of renal and vascular complications in human diabetes. In patients with biopsy-proven DKD, a higher level of factor H in the urine has been independently associated with worse kidney outcomes, including onset of ESKD and faster kidney function decline, compared to control subjects[141]. Further, clinical studies have shown an association between single nucleotide polymorphisms in factor H and adverse clinical outcomes in different population groups of non-diabetic and diabetic patients[142,143]. In African American patients, genetic changes in factor H gene, such as the intronic variant rs379489, have been associated with ESKD in both non-diabetic and type 2 diabetes (T2D), compared to controls[142]. In 1158 T2D patients prospectively followed in the randomized controlled trial Bergamo Nephrologic Complications of T2D (BENEDICT), the single nucleotide polymorphism in the factor H gene c.2808G>T (p.Glu936Asp) is independently associated with increased risk of microalbuminuria and cardiovascular complications (Asp/Asp homozygotes, recessive model). T2D patients Asp/Asp homozygotes are at increased risk of microalbuminuria and cardiovascular events compared to carriers of one or two wild type Glu alleles[143].
Among patients with diabetes, the reported amount of glomerular sialic acid has been inconsistent. A decline in the content of sialic acid has been detected in the glomerular ECM of patients with diabetes, compared to normal kidney samples[68,144]. However, an increased expression of sialic acid on podocytes has been observed in patients with DKD and other kidney diseases without differences among them[44].
A study that applied weighted gene co-expression network analysis to 179 human glomeruli reveals that two small leucine-rich proteoglycans (lumican and fibromodulin) are more abundant in the ECM of patients with DKD compared to controls and other glomerular diseases, such as IgA nephropathy or membranous nephropathy. Further, the expression level of lumican and fibromodulin is negatively correlated with kidney function. The specificity of lumican and fibromodulin in kidney samples from patients with DKD in comparison to normal specimens and patients with other glomerular diseases suggests that these ECM components may become potential diagnostic biomarkers for DKD[14].
The laminin content of the glomerular ECM in patients with diabetes has been barely reported and the values are variable[37,39]. In human kidneys obtained at autopsy, there is a marked reduction in laminin content in the diabetic GBM compared to non-diabetic control subjects. Radioimmunoassays indicated that GBM from patients with diabetes contains average values of laminin that were 60% of control subjects[37]. However, immunohistochemical studies show an increased glomerular deposition of laminin in kidney biopsy samples from type 1 diabetes (T1D) and T2D patients with diffuse and nodular glomerulosclerosis[39].
Immunohistochemical studies reveal that the amount of mesangial fibronectin-1 is abnormal in patients with diabetes and varies with the advance of DKD. Antibodies to fibronectin-1 normally stain the mesangium and the subendothelial aspect of the GBM. Early lesions of mesangial expansion are associated with increased staining for fibronectin-1. However, a marked diminution in fluorescent intensity for fibronectin-1 is documented in more advanced mesangial enlargement (nodular lesions). Compared with normal tissues and early lesions of DKD, the progression of the disease is associated with a noticeable reduction in the amount of glomerular fibronectin-1[39,40,137]. The increase in mesangial fibronectin-1 that occurs in the early stage of DKD also takes place in other glomerular diseases characterized by mesangial expansion, such as mesangiocapillary glomerulonephritis[59,60]. An up-regulation of fibronectin-1 expression in the glomeruli has been also observed in patients with hypertension compared to normal kidneys[4]. Likewise, the amount of fibronectin-1 in vascular tissue is increased in patients with diabetes before the development of atherosclerosis lesions[59,145]. The content of fibronectin-1 in the intima-media of normal aorta specimens is more elevated in patients with diabetes (T1D and T2D) compared to control subjects, suggesting that diabetic patients develop structural alterations in the connective tissue of their arteries before the appearance of vascular disease[145]. In patients with DKD, the thickened capillary walls also contain a markedly elevated amount of fibronectin-1[59].
Earlier studies found elevated glomerular ECM levels of glycine, hydroxyproline, hydroxylysine, and hexoses in patients with diabetes compared to normal kidney samples, suggesting an increase in the amount of collagen in the glomerular ECM from diabetic patients[30,36,66-68]. Radioimmunoassays confirmed collagen enrichment in the glomerular ECM of patients with diabetes[37]. Accordingly, electron microscopy examination shows accumulation of collagen fibrils in the mesangium of patients with DKD[146].
Glomerular type I collagen in DKD: No glomerular type I collagen has been detected in DKD at any stage of the disorder[33,39,40,137,147].
Glomerular type III collagen in DKD: Unlike normal glomeruli, type III collagen is identified in the mesangium of patients with DKD and its amount increases gradually with the progression of the disease. Early DKD lesions (diffuse glomerulosclerosis) show positive staining for type III collagen that increases in more advanced mesangial nodular lesions. In the late stage of global sclerosis, type III collagen is diffusely present in the sclerotic mesangial matrix. Therefore, de novo synthesis of type III collagen in glomeruli occurs in patients with DKD[33,39,40,70,71]. A patient with T1D and collagenofibrotic glomerulopathy has been reported[148].
Glomerular type IV collagen in DKD: In patients with diabetes, immunohistochemical estimates of type IV collagen in the GBM reveal reduced staining compared to normal tissue. Accordingly, the density of gold particles for type IV collagen is decreased in the GBM of T1D patients on quantitative immunogold electron microscopy examination. Like in normal subjects, the labeling of antibody against type IV collagen in the GBM is concentrated in the endothelial zone and decreases towards the epithelial aspect of the GMB in diabetic patients[33,89].
In the mesangial matrix, immunohistochemical studies show that the amount of type IV collagen changes according to the stage of DKD. In earlier lesions of diffuse glomerulosclerosis, mesangial staining for type IV collagen is increased while more advanced nodular glomerulosclerosis showed marked reduction in the mesangial staining for type IV collagen, suggesting that type IV collagen is progressively substituted for other collagen types such as type VI and type III during the transition from diffuse to nodular glomerulosclerosis[39,40,89]. However, an elevated mesangial staining for type IV collagen has been observed in specific nodular lesions, called non-mesangiolytic nodules, compared to normal kidney[33,71]. The amount of type IV collagen in nodular lesions may depend on the type of the lesion, mesangiolytic or non-mesangiolytic. In a study aimed to investigate collagen staining of mesangial nodules from 67 patients with DKD, type IV collagen staining was only robust in nodular lesions with strong PAS/periodic acid methenamine silver (PAMS) staining (non-mesangiolytic nodular lesions). In contrast, nodular lesions with faint PAS/PAMS staining (mesangiolytic nodular lesions) did not show type IV collagen. The amount of type IV collagen correlates with the PAS and PAMS staining pattern. Non-mesangiolytic nodules (with prominent PAS/PAMS staining) are strongly positive for type IV collagen whereas mesangiolytic nodules (with weak or negative PAS/PAMS staining) show weak or negative staining for type IV collagen[147]. Immunofluorescence studies performed in 918 kidney biopsy samples from patients with diabetes (T1D and T2D) show accumulation of α3 and α5 chains of type IV collagen in diffuse mesangial sclerosis while minimal amounts of these α3 and α5 chains were seen within the mesangium of control subjects[43].
In patients with diabetes, two large clinical investigations with different population groups (African American and European descent subjects) have shown that genetic variants in the gene that codes the α3 chain of type IV collagen (COL4A3) may modulate susceptibility to DKD and ESKD[149]. In 4885 African American patients with T2D, an association between the genetic variant R408H (rs34505188) in COL4A3 and ESKD has been observed, suggesting that genetic changes in the COL4A3 locus may contribute to ESKD susceptibility in patients with diabetes[149]. In 19406 T1D patients of European descent from 17 cohorts, a genome-wide association meta-analysis reveals that a single nucleotide polymorphism in the COL4A3 gene is associated with protection from DKD (proteinuria and ESKD)[150].
Glomerular type V collagen in DKD: Immunohistochemical studies have documented an enrichment in mesangial type V collagen in diffuse glomerulosclerosis and nodular lesions in patients with DKD compared to control subjects. Increased staining for type V collagen is observed in advanced mesangial disease, compared to normal tissues and early mesangial disease.
Staining for type V collagen was strongly positive in all nodular lesions, mesangiolytic and non-mesangiolytic[39,40,137,147].
Glomerular type VI collagen in DKD: In patients with DKD, the amount of mesangial type VI collagen is elevated. Quantitation by radioimmunoassay reveals that the level of type VI collagen is 2.8-fold higher in the diabetic preparations compared to control subjects. Furthermore, the amount of mesangial type VI collagen increases with the progression of DKD. In earlier lesions of diffuse glomerulosclerosis, the contribution of type VI collagen deposition to the overall matrix expansion is minor. However, type VI collagen is a major component in the expanded mesangial matrix of nodular glomerulosclerosis. A marked increase in type VI collagen deposition is observed in nodular lesions where the strong positivity for type VI collagen is evenly distributed throughout the entire nodules[31,39,40,147].
As kidney ECM remodeling is profoundly altered in patients with DKD, the expression of MMPs, TIMPs, integrins, integrin-associated proteins, and signaling pathways from growth factors have been reported abnormal among these patients. In addition, the nuclear factor-kappa-B (NF-κB) family of transcription factors and advanced glycation end-products (AGEs) have been proposed as potential contributors to the ECM disturbance present in patients with DKD.
MMPs and their inhibitors in patients with DKD: The expression of MMPs and their inhibitors is altered in patients with DKD. Clinical studies have suggested that these ECM components might be useful to evaluate the risk for cardiovascular disease, kidney disease, and all-cause mortality among patients with diabetes. In a cross-sectional study, T1D patients with cardiovascular disease showed higher levels of TIMP-1 compared to T1D patients without cardiovascular disease[151]. In a prospective study that followed 337 T1D patients for a median period of 12.3 years, elevated MMP-2 plasma levels were associated with higher incidence of cardiovascular events, but this relationship was attenuated after adjustment for estimated GFR, suggesting that kidney function may mediate the association[152]. In a cross-sectional pooled analysis of three groups of T1D patients, circulating MMP-1, MMP-2, and MMP-3 Levels were associated with arterial stiffening independent of confounding factors while no association with TIMPs was observed[153]. In a case-control study that evaluated 120 control women and 120 women with a history of gestational diabetes 3.7 years after delivery, both serum TIMP-1 Levels and arterial stiffness were higher in subjects with previous gestational diabetes compared to control individuals[154]. In T1D patients, MMPs and their inhibitors have been associated with albuminuria in a cross-sectional study[151], and with kidney function decline in a prospective study[152]. In a prospective observational cohort study, urinary excretion of MMP-7 was independently associated with higher mortality rate over a median follow-up period of 3.0 years, in T2D patients with DKD. In contrast, no association between serum MMP-7 Level and mortality was observed[155]. In T1D patients, the association of MMP-1, MMP-2 and MMP-3 with all-cause mortality was attenuated after adjustment for estimated GFR, suggesting that the known association between kidney function and mortality may mediate the relation between MMPs and death[152].
However, MMPs expression in glomeruli may be altered in other glomerular diseases, such as IgA nephropathy, which is associated with extensive changes of the glomerular ECM proteome, including higher abundance of MMP-9, MMP-2, α1 chain of type IV collagen, fibronectin, and β1-laminin[156].
Growth factors (TGF-β, PDGF, and VEGF) in patients with DKD: In T2D patients with albuminuria, serum TGF-β1 Level is higher compared to healthy controls and T2D patients with normal urinary albumin excretion rate, suggesting that serum TGF-β1 might be used to evaluate progression of DKD[157,158]. Several meta-analyses indicate a potential value of serum TGF-β1 Levels to evaluate the risk of DKD and the advance of the disease[159-161]. However, the administration of a neutralizing monoclonal antibody against TGF-β1 to T1D and T2D patients with DKD failed to slow progression of the disease compared to placebo in a randomized controlled clinical trial, suggesting that TGF-β1 is not a major determinant of kidney function decline in patients with diabetes[162].
The glomerular expression of PDGF-B and its receptor (PDGFR-β) is higher in T2D patients with DKD compared to normal kidneys, particularly in samples with mild mesangial expansion. In contrast, the expression of PDGF-A and its receptor (PDGFR-α) is comparable in normal kidneys and patients with DKD[163].
In patients with DKD, VEGF-A expression is lower compared to normal kidney tissue[164]. VEGF signaling has been reported to be differentially regulated in patients with DKD in a study that examined gene-expression changes in human DKD[165]. However, in a prospective study that recruited 155 T1D patients with proteinuria, plasma VEGF failed to predict kidney function decline over 3-year follow-up[166].
Integrins and integrin-associated proteins in patients with DKD: Glomerular integrin and integrin-linked kinase signaling pathways have been found differentially regulated in patients with DKD[165]. In addition, the expression of integrin-linked kinase in the mesangium is increased in kidney specimens from patients with diabetes and diffuse mesangial expansion, compared to control samples. In contrast, integrin-linked kinase level is reduced in glomeruli with advanced nodular sclerosis and global sclerosis, suggesting that integrin-linked kinase expression increases during early stages of DKD[114].
NF-κB in patients with DKD: NF-κB is a transcription factor that regulates the expression of several genes. NF-κB-inducing kinase activates the NF-κB signaling pathway. Dysregulation of NF-κB signaling has been implicated in DKD, but its role remains uncertain. In vitro studies using human proximal tubular epithelial cells (HK-2 cells) suggest a role for NF-κB pathway in modulating diabetes-induced disease in renal tubular epithelium[167,168].
Advanced glycation products in patients with DKD: AGEs are molecules that result from nonenzymatic glycation of proteins and lipids. They may bind to cell surface receptors (RAGEs). AGEs have been hypothesized to be involved in the development of human DKD, but their participation remain undefined. In kidney biopsies from patients with DKD, AGEs are detected in the expanded mesangial matrix while they are not identified in control samples[169,170]. In addition, AGEs are identified in areas of glomerulosclerosis and arteriosclerosis in other diseases, such as FSGS, hypertensive nephrosclerosis, and lupus nephritis[169]. RAGE expression was detected in mesangial cells and glomerular epithelial cells, in both patients with DKD and control subjects[170]. In a cross-sectional study, the level of AGEs was positively associated with serum concentration of MMP-2, MMP3, and TIMP-1 while an inverse association with MMP-9 was observed in T1D patients[171].
Understanding mechanisms that regulate glomerular ECM injury and repair may contribute to develop therapeutic strategies for DKD and other kidney diseases. During adult life, mesangial cells produce mesangial matrix. The turnover of the GBM present at birth is unknown. Podocyte foot processes surround and attach entirely the GBM. Adult podocytes may sustain hypertrophy following the loss of adjacent cells to prevent bared GBM areas that compromise the filtration barrier. Glycosaminoglycans, such as heparan sulfate and hyaluronic acid, are major constituents of the glomerular ECM. The specific pattern of sulfation of glycosaminoglycans allows the identification of these molecules as “self” by complement components and avoid complement-mediated self-damage. Sialic acid is also present in glomerular ECM and may serve to a similar function. Fibronectin-1 is important for the normal deposition of other ECM components, such as collagen. Type IV, V, and VI collagens are predominant types of collagen normally present in the glomerular ECM while type III collagen appears in diseased states, such as diabetes and glomerulosclerosis. The composition and arrangement of the glomerular ECM is profoundly altered in patients with diabetes. The global quantity of glomerular ECM is increased while the amount of sulfated proteoglycans is reduced and hyaluronic acid is augmented, compared to control tissue. Fibronectin-1 is increased in early lesions of mesangial expansion. Likewise, the amount of fibronectin-1 in capillary walls and aorta is increased before the development of vascular disease in patients with diabetes. Mesangial type III, type V, and type VI collagen amount is elevated in patients with DKD and increases progressively with the advance of the disease. Genetic variants in the gene that codes the α3 chain of type IV collagen (COL4A3) may modulate susceptibility to DKD and ESKD.
We are very grateful to Ms. Gema Souto, MHS for her help and support during the writing of this manuscript.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: Suriname
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bayoumi RAL, United Arab Emirates; Javor E, Croatia S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Saleem MA, O'Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13:630-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 909] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 2. | Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007;17:428-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 429] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 3. | Pollak MR. Familial FSGS. Adv Chronic Kidney Dis. 2014;21:422-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Kliewe F, Kaling S, Lötzsch H, Artelt N, Schindler M, Rogge H, Schröder S, Scharf C, Amann K, Daniel C, Lindenmeyer MT, Cohen CD, Endlich K, Endlich N. Fibronectin is up-regulated in podocytes by mechanical stress. FASEB J. 2019;33:14450-14460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Baig S, Paisey R, Dawson C, Barrett T, Maffei P, Hodson J, Rambhatla SB, Chauhan P, Bolton S, Dassie F, Francomano C, Marshall RP, Belal M, Skordilis K, Hayer M, Price AM, Cramb R, Edwards N, Steeds RP, Geberhiwot T. Defining renal phenotype in Alström syndrome. Nephrol Dial Transplant. 2020;35:994-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Riemer T, Nimptsch A, Nimptsch K, Schiller J. Determination of the glycosaminoglycan and collagen contents in tissue samples by high-resolution 1H NMR spectroscopy after DCl-induced hydrolysis. Biomacromolecules. 2012;13:2110-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Bergholt MS, Serio A, Albro MB. Raman Spectroscopy: Guiding Light for the Extracellular Matrix. Front Bioeng Biotechnol. 2019;7:303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 8. | Sherlock BE, Chen J, Mansfield JC, Green E, Winlove CP. Biophotonic tools for probing extracellular matrix mechanics. Matrix Biol Plus. 2021;12:100093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Reimann C, Brangsch J, Colletini F, Walter T, Hamm B, Botnar RM, Makowski MR. Molecular imaging of the extracellular matrix in the context of atherosclerosis. Adv Drug Deliv Rev. 2017;113:49-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Chow MJ, Turcotte R, Lin CP, Zhang Y. Arterial extracellular matrix: a mechanobiological study of the contributions and interactions of elastin and collagen. Biophys J. 2014;106:2684-2692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 148] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 11. | Pezzoli D, Di Paolo J, Kumra H, Fois G, Candiani G, Reinhardt DP, Mantovani D. Fibronectin promotes elastin deposition, elasticity and mechanical strength in cellularised collagen-based scaffolds. Biomaterials. 2018;180:130-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Feng Q, Li D, Li Q, Cao X, Dong H. Microgel assembly: Fabrication, characteristics and application in tissue engineering and regenerative medicine. Bioact Mater. 2022;9:105-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 13. | Suh EJ, Remillard MY, Legesse-Miller A, Johnson EL, Lemons JM, Chapman TR, Forman JJ, Kojima M, Silberman ES, Coller HA. A microRNA network regulates proliferative timing and extracellular matrix synthesis during cellular quiescence in fibroblasts. Genome Biol. 2012;13:R121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Feng S, Gao Y, Yin D, Lv L, Wen Y, Li Z, Wang B, Wu M, Liu B. Identification of Lumican and Fibromodulin as Hub Genes Associated with Accumulation of Extracellular Matrix in Diabetic Nephropathy. Kidney Blood Press Res. 2021;46:275-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Wang Z, Chen X, Li C, Tang W. Application of weighted gene co-expression network analysis to identify novel key genes in diabetic nephropathy. J Diabetes Investig. 2022;13:112-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Chen FM, Liu X. Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci. 2016;53:86-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 655] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 17. | Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121:255-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 688] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 18. | Karamanos NK, Theocharis AD, Piperigkou Z, Manou D, Passi A, Skandalis SS, Vynios DH, Orian-Rousseau V, Ricard-Blum S, Schmelzer CEH, Duca L, Durbeej M, Afratis NA, Troeberg L, Franchi M, Masola V, Onisto M. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021;288:6850-6912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 542] [Article Influence: 135.5] [Reference Citation Analysis (1)] |
| 19. | Chiang CE, Fang YQ, Ho CT, Assunção M, Lin SJ, Wang YC, Blocki A, Huang CC. Bioactive Decellularized Extracellular Matrix Derived from 3D Stem Cell Spheroids under Macromolecular Crowding Serves as a Scaffold for Tissue Engineering. Adv Healthc Mater. 2021;10:e2100024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 20. | Wilks BT, Evans EB, Howes A, Hopkins CM, Nakhla MN, Williams G, Morgan JR. Quantifying Cell-Derived Changes in Collagen Synthesis, Alignment, and Mechanics in a 3D Connective Tissue Model. Adv Sci (Weinh). 2022;9:e2103939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Assunção M, Yiu CHK, Wan HY, Wang D, Ker DFE, Tuan RS, Blocki A. Hyaluronic acid drives mesenchymal stromal cell-derived extracellular matrix assembly by promoting fibronectin fibrillogenesis. J Mater Chem B. 2021;9:7205-7215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Lin S, Mequanint K. Bioreactor-induced mesenchymal progenitor cell differentiation and elastic fiber assembly in engineered vascular tissues. Acta Biomater. 2017;59:200-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Urbanczyk M, Layland SL, Schenke-Layland K. The role of extracellular matrix in biomechanics and its impact on bioengineering of cells and 3D tissues. Matrix Biol. 2020;85-86:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 24. | Abraham VC, Miller LN, Pratt SD, Putman B, Kim L, Gopalakrishnan SM, King A. Implementation of a human podocyte injury model of chronic kidney disease for profiling of renoprotective compounds. Eur J Pharmacol. 2017;815:219-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Yoshida Y, Miyazaki K, Kamiie J, Sato M, Okuizumi S, Kenmochi A, Kamijo K, Nabetani T, Tsugita A, Xu B, Zhang Y, Yaoita E, Osawa T, Yamamoto T. Two-dimensional electrophoretic profiling of normal human kidney glomerulus proteome and construction of an extensible markup language (XML)-based database. Proteomics. 2005;5:1083-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Miyamoto M, Yoshida Y, Taguchi I, Nagasaka Y, Tasaki M, Zhang Y, Xu B, Nameta M, Sezaki H, Cuellar LM, Osawa T, Morishita H, Sekiyama S, Yaoita E, Kimura K, Yamamoto T. In-depth proteomic profiling of the normal human kidney glomerulus using two-dimensional protein prefractionation in combination with liquid chromatography-tandem mass spectrometry. J Proteome Res. 2007;6:3680-3690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Lennon R, Byron A, Humphries JD, Randles MJ, Carisey A, Murphy S, Knight D, Brenchley PE, Zent R, Humphries MJ. Global analysis reveals the complexity of the human glomerular extracellular matrix. J Am Soc Nephrol. 2014;25:939-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 28. | Hobeika L, Barati MT, Caster DJ, McLeish KR, Merchant ML. Characterization of glomerular extracellular matrix by proteomic analysis of laser-captured microdissected glomeruli. Kidney Int. 2017;91:501-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Suleiman H, Zhang L, Roth R, Heuser JE, Miner JH, Shaw AS, Dani A. Nanoscale protein architecture of the kidney glomerular basement membrane. Elife. 2013;2:e01149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 30. | Beisswenger PJ, Spiro RG. Studies on the human glomerular basement membrane. Composition, nature of the carbohydrate units and chemical changes in diabetes mellitus. Diabetes. 1973;22:180-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 103] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Mohan PS, Carter WG, Spiro RG. Occurrence of type VI collagen in extracellular matrix of renal glomeruli and its increase in diabetes. Diabetes. 1990;39:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Zhu D, Kim Y, Steffes MW, Groppoli TJ, Butkowski RJ, Mauer SM. Application of electron microscopic immunocytochemistry to the human kidney: distribution of type IV and type VI collagen in normal human kidney. J Histochem Cytochem. 1994;42:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Tamsma JT, van den Born J, Bruijn JA, Assmann KJ, Weening JJ, Berden JH, Wieslander J, Schrama E, Hermans J, Veerkamp JH. Expression of glomerular extracellular matrix components in human diabetic nephropathy: decrease of heparan sulphate in the glomerular basement membrane. Diabetologia. 1994;37:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 125] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Groffen AJ, Ruegg MA, Dijkman H, van de Velden TJ, Buskens CA, van den Born J, Assmann KJ, Monnens LA, Veerkamp JH, van den Heuvel LP. Agrin is a major heparan sulfate proteoglycan in the human glomerular basement membrane. J Histochem Cytochem. 1998;46:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 131] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Murata K. Acidic glycosaminoglycans in human kidney tissue. Clin Chim Acta. 1975;63:157-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Parthasarathy N, Spiro RG. Effect of diabetes on the glycosaminoglycan component of the human glomerular basement membrane. Diabetes. 1982;31:738-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 115] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Shimomura H, Spiro RG. Studies on macromolecular components of human glomerular basement membrane and alterations in diabetes. Decreased levels of heparan sulfate proteoglycan and laminin. Diabetes. 1987;36:374-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Oomura A, Nakamura T, Arakawa M, Ooshima A, Isemura M. Alterations in the extracellular matrix components in human glomerular diseases. Virchows Arch A Pathol Anat Histopathol. 1989;415:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Nerlich A, Schleicher E. Immunohistochemical localization of extracellular matrix components in human diabetic glomerular lesions. Am J Pathol. 1991;139:889-899. [PubMed] |
| 40. | Nerlich AG, Schleicher ED, Wiest I, Specks U, Timpl R. Immunohistochemical localization of collagen VI in diabetic glomeruli. Kidney Int. 1994;45:1648-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Groffen AJ, Buskens CA, van Kuppevelt TH, Veerkamp JH, Monnens LA, van den Heuvel LP. Primary structure and high expression of human agrin in basement membranes of adult lung and kidney. Eur J Biochem. 1998;254:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Groffen AJ, Hop FW, Tryggvason K, Dijkman H, Assmann KJ, Veerkamp JH, Monnens LA, Van den Heuvel LP. Evidence for the existence of multiple heparan sulfate proteoglycans in the human glomerular basement membrane and mesangial matrix. Eur J Biochem. 1997;247:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Kriz W, Löwen J, Federico G, van den Born J, Gröne E, Gröne HJ. Accumulation of worn-out GBM material substantially contributes to mesangial matrix expansion in diabetic nephropathy. Am J Physiol Renal Physiol. 2017;312:F1101-F1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Babal P, Slugen I, Danis D, Zaviacic M, Gardner WA Jr. Sialic acid expression in normal and diseased human kidney. Acta Histochem. 1996;98:71-77. [PubMed] |
| 45. | Bowles WHD, Gloster TM. Sialidase and Sialyltransferase Inhibitors: Targeting Pathogenicity and Disease. Front Mol Biosci. 2021;8:705133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 46. | Fearon DT. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci U S A. 1978;75:1971-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 292] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 47. | Blackmore TK, Sadlon TA, Ward HM, Lublin DM, Gordon DL. Identification of a heparin binding domain in the seventh short consensus repeat of complement factor H. J Immunol. 1996;157:5422-5427. [PubMed] |
| 48. | Ram S, Sharma AK, Simpson SD, Gulati S, McQuillen DP, Pangburn MK, Rice PA. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J Exp Med. 1998;187:743-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 310] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 49. | Hellwage J, Jokiranta TS, Friese MA, Wolk TU, Kampen E, Zipfel PF, Meri S. Complement C3b/C3d and cell surface polyanions are recognized by overlapping binding sites on the most carboxyl-terminal domain of complement factor H. J Immunol. 2002;169:6935-6944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 50. | Feyzi E, Saldeen T, Larsson E, Lindahl U, Salmivirta M. Age-dependent modulation of heparan sulfate structure and function. J Biol Chem. 1998;273:13395-13398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 121] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 51. | Pangburn MK, Müller-Eberhard HJ. Complement C3 convertase: cell surface restriction of beta1H control and generation of restriction on neuraminidase-treated cells. Proc Natl Acad Sci U S A. 1978;75:2416-2420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 271] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 52. | Meri S, Pangburn MK. Discrimination between activators and nonactivators of the alternative pathway of complement: regulation via a sialic acid/polyanion binding site on factor H. Proc Natl Acad Sci U S A. 1990;87:3982-3986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 301] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 53. | Blackmore TK, Hellwage J, Sadlon TA, Higgs N, Zipfel PF, Ward HM, Gordon DL. Identification of the second heparin-binding domain in human complement factor H. J Immunol. 1998;160:3342-3348. [PubMed] |
| 54. | Loeven MA, Rops AL, Berden JH, Daha MR, Rabelink TJ, van der Vlag J. The role of heparan sulfate as determining pathogenic factor in complement factor H-associated diseases. Mol Immunol. 2015;63:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 55. | Colognato H, Winkelmann DA, Yurchenco PD. Laminin polymerization induces a receptor-cytoskeleton network. J Cell Biol. 1999;145:619-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 243] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 56. | Smith PS, Fanning JC, Aarons I. The structure of the normal human glomerular basement membrane. Ultrastructural localization of type IV collagen and laminin. Pathology. 1989;21:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Zenker M, Aigner T, Wendler O, Tralau T, Müntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wühl E, Cochat P, Bouvier R, Kraus C, Mark K, Madlon H, Dötsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A. Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet. 2004;13:2625-2632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 338] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 58. | Hasselbacher K, Wiggins RC, Matejas V, Hinkes BG, Mucha B, Hoskins BE, Ozaltin F, Nürnberg G, Becker C, Hangan D, Pohl M, Kuwertz-Bröking E, Griebel M, Schumacher V, Royer-Pokora B, Bakkaloglu A, Nürnberg P, Zenker M, Hildebrandt F. Recessive missense mutations in LAMB2 expand the clinical spectrum of LAMB2-associated disorders. Kidney Int. 2006;70:1008-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 59. | Weiss MA, Ooi BS, Ooi YM, Engvall E, Ruoslahti E. Immunofluorescent localization of fibronectin in the human kidney. Lab Invest. 1979;41:340-347. [PubMed] |
| 60. | Dixon AJ, Burns J, Dunnill MS, McGee JO. Distribution of fibronectin in normal and diseased human kidneys. J Clin Pathol. 1980;33:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 61. | Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol Biol Cell. 2002;13:3546-3559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 475] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 62. | Assmann KJ, Koene RA, Wetzels JF. Familial glomerulonephritis characterized by massive deposits of fibronectin. Am J Kidney Dis. 1995;25:781-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 63. | Castelletti F, Donadelli R, Banterla F, Hildebrandt F, Zipfel PF, Bresin E, Otto E, Skerka C, Renieri A, Todeschini M, Caprioli J, Caruso RM, Artuso R, Remuzzi G, Noris M. Mutations in FN1 cause glomerulopathy with fibronectin deposits. Proc Natl Acad Sci U S A. 2008;105:2538-2543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 64. | Ertoy Baydar D, Kutlugun AA, Bresin E, Piras R. A case of familial glomerulopathy with fibronectin deposits caused by the Y973C mutation in fibronectin. Am J Kidney Dis. 2013;61:514-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 65. | Ohtsubo H, Okada T, Nozu K, Takaoka Y, Shono A, Asanuma K, Zhang L, Nakanishi K, Taniguchi-Ikeda M, Kaito H, Iijima K, Nakamura S. Identification of mutations in FN1 leading to glomerulopathy with fibronectin deposits. Pediatr Nephrol. 2016;31:1459-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 66. | Bonting SL, De Bruin H, Pollak VE. QUANTITATIVE HISTOCHEMISTRY OF THE NEPHRON. VI. HYDROXYPROLINE IN THE HUMAN GLOMERULUS. J Clin Invest. 1961;40:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 67. | Beisswenger PG, Spiro RG. Human glomerular basement membrane: chemical alteration in diabetes mellitus. Science. 1970;168:596-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 122] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 68. | Kefalides NA. Biochemical properties of human glomerular basement membrane in normal and diabetic kidneys. J Clin Invest. 1974;53:403-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 69. | Funabiki K, Horikoshi S, Tomino Y, Nagai Y, Koide H. Immunohistochemical analysis of extracellular components in the glomerular sclerosis of patients with glomerulonephritis. Clin Nephrol. 1990;34:239-246. [PubMed] |
| 70. | Yoshioka K, Takemura T, Tohda M, Akano N, Miyamoto H, Ooshima A, Maki S. Glomerular localization of type III collagen in human kidney disease. Kidney Int. 1989;35:1203-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Razzaque MS, Koji T, Taguchi T, Harada T, Nakane PK. In situ localization of type III and type IV collagen-expressing cells in human diabetic nephropathy. J Pathol. 1994;174:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 72. | Bongers EM, Huysmans FT, Levtchenko E, de Rooy JW, Blickman JG, Admiraal RJ, Huygen PL, Cruysberg JR, Toolens PA, Prins JB, Krabbe PF, Borm GF, Schoots J, van Bokhoven H, van Remortele AM, Hoefsloot LH, van Kampen A, Knoers NV. Genotype-phenotype studies in nail-patella syndrome show that LMX1B mutation location is involved in the risk of developing nephropathy. Eur J Hum Genet. 2005;13:935-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 73. | Oe Y, Mishima E, Mori T, Okamoto K, Honkura Y, Nagasawa T, Yoshida M, Sato H, Suzuki J, Ikeda R, Sohara E, Uchida S, Katori Y, Miyazaki M. A Novel Mutation in LMX1B (p.Pro219Ala) Causes Focal Segmental Glomerulosclerosis with Alport Syndrome-like Phenotype. Intern Med. 2021;60:2991-2996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 74. | Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG. Alport's syndrome, Goodpasture's syndrome, and type IV collagen. N Engl J Med. 2003;348:2543-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 638] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 75. | Edwards N, Rice SJ, Raman S, Hynes AM, Srivastava S, Moore I, Al-Hamed M, Xu Y, Santibanez-Koref M, Thwaites DT, Gale DP, Sayer JA. A novel LMX1B mutation in a family with end-stage renal disease of 'unknown cause'. Clin Kidney J. 2015;8:113-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 76. | Isojima T, Harita Y, Furuyama M, Sugawara N, Ishizuka K, Horita S, Kajiho Y, Miura K, Igarashi T, Hattori M, Kitanaka S. LMX1B mutation with residual transcriptional activity as a cause of isolated glomerulopathy. Nephrol Dial Transplant. 2014;29:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 77. | Konomoto T, Imamura H, Orita M, Tanaka E, Moritake H, Sato Y, Fujimoto S, Harita Y, Hisano S, Yoshiura K, Nunoi H. Clinical and histological findings of autosomal dominant renal-limited disease with LMX1B mutation. Nephrology (Carlton). 2016;21:765-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 78. | Andeen NK, Schleit J, Blosser CD, Dorschner MO, Hisama FM, Smith KD. LMX1B-Associated Nephropathy With Type III Collagen Deposition in the Glomerular and Tubular Basement Membranes. Am J Kidney Dis. 2018;72:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | Silverman ME, Goodman RM, Cuppage FE. The Nail-Patella syndrome. Clinical findings and ultrastructural observations in the kidney. Arch Intern Med. 1967;120:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 80. | Boyer O, Woerner S, Yang F, Oakeley EJ, Linghu B, Gribouval O, Tête MJ, Duca JS, Klickstein L, Damask AJ, Szustakowski JD, Heibel F, Matignon M, Baudouin V, Chantrel F, Champigneulle J, Martin L, Nitschké P, Gubler MC, Johnson KJ, Chibout SD, Antignac C. LMX1B mutations cause hereditary FSGS without extrarenal involvement. J Am Soc Nephrol. 2013;24:1216-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 81. | Ghoumid J, Petit F, Holder-Espinasse M, Jourdain AS, Guerra J, Dieux-Coeslier A, Figeac M, Porchet N, Manouvrier-Hanu S, Escande F. Nail-Patella Syndrome: clinical and molecular data in 55 families raising the hypothesis of a genetic heterogeneity. Eur J Hum Genet. 2016;24:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 82. | Shimohata H, Miyake Y, Yoshida Y, Usui J, Mori T, Sohara E, Uchida S, Hirayama K, Kobayashi M. LMX1B-associated nephropathy that showed myelin figures on electron microscopy. CEN Case Rep. 2021;10:588-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 83. | Lei L, Oh G, Sutherland S, Abra G, Higgins J, Sibley R, Troxell M, Kambham N. Myelin bodies in LMX1B-associated nephropathy: potential for misdiagnosis. Pediatr Nephrol. 2020;35:1647-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 84. | Duggal R, Nada R, Rayat CS, Rane SU, Sakhuja V, Joshi K. Collagenofibrotic glomerulopathy-a review. Clin Kidney J. 2012;5:7-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 85. | Kurien AA, Larsen CP, Cossey LN. Collagenofibrotic glomerulopathy. Clin Kidney J. 2015;8:543-547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 86. | Aoki T, Hayashi K, Morinaga T, Tomida H, Hishida M, Yamamoto S, Kajiwara N, Tamai H. Two brothers with collagenofibrotic glomerulopathy. CEN Case Rep. 2015;4:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 87. | Vogt BA, Wyatt RJ, Burke BA, Simonton SC, Kashtan CE. Inherited factor H deficiency and collagen type III glomerulopathy. Pediatr Nephrol. 1995;9:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 88. | Kleppel MM, Santi PA, Cameron JD, Wieslander J, Michael AF. Human tissue distribution of novel basement membrane collagen. Am J Pathol. 1989;134:813-825. [PubMed] |
| 89. | Zhu D, Kim Y, Steffes MW, Groppoli TJ, Butkowski RJ, Mauer SM. Glomerular distribution of type IV collagen in diabetes by high resolution quantitative immunochemistry. Kidney Int. 1994;45:425-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 90. | Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, Gregory MC, Skolnick MH, Atkin CL, Tryggvason K. Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science. 1990;248:1224-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 540] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 91. | Voskarides K, Damianou L, Neocleous V, Zouvani I, Christodoulidou S, Hadjiconstantinou V, Ioannou K, Athanasiou Y, Patsias C, Alexopoulos E, Pierides A, Kyriacou K, Deltas C. COL4A3/COL4A4 mutations producing focal segmental glomerulosclerosis and renal failure in thin basement membrane nephropathy. J Am Soc Nephrol. 2007;18:3004-3016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 92. | Parkin JD, San Antonio JD, Pedchenko V, Hudson B, Jensen ST, Savige J. Mapping structural landmarks, ligand binding sites, and missense mutations to the collagen IV heterotrimers predicts major functional domains, novel interactions, and variation in phenotypes in inherited diseases affecting basement membranes. Hum Mutat. 2011;32:127-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 93. | Kashtan CE, Ding J, Garosi G, Heidet L, Massella L, Nakanishi K, Nozu K, Renieri A, Rheault M, Wang F, Gross O. Alport syndrome: a unified classification of genetic disorders of collagen IV α345: a position paper of the Alport Syndrome Classification Working Group. Kidney Int. 2018;93:1045-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 208] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 94. | Savige J, Storey H, Watson E, Hertz JM, Deltas C, Renieri A, Mari F, Hilbert P, Plevova P, Byers P, Cerkauskaite A, Gregory M, Cerkauskiene R, Ljubanovic DG, Becherucci F, Errichiello C, Massella L, Aiello V, Lennon R, Hopkinson L, Koziell A, Lungu A, Rothe HM, Hoefele J, Zacchia M, Martic TN, Gupta A, van Eerde A, Gear S, Landini S, Palazzo V, Al-Rabadi L, Claes K, Corveleyn A, Van Hoof E, van Geel M, Williams M, Ashton E, Belge H, Ars E, Bierzynska A, Gangemi C, Lipska-Ziętkiewicz BS. Consensus statement on standards and guidelines for the molecular diagnostics of Alport syndrome: refining the ACMG criteria. Eur J Hum Genet. 2021;29:1186-1197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 95. | Barua M, Paterson AD. Population-based studies reveal an additive role of type IV collagen variants in hematuria and albuminuria. Pediatr Nephrol. 2022;37:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 96. | Papazachariou L, Papagregoriou G, Hadjipanagi D, Demosthenous P, Voskarides K, Koutsofti C, Stylianou K, Ioannou P, Xydakis D, Tzanakis I, Papadaki A, Kallivretakis N, Nikolakakis N, Perysinaki G, Gale DP, Diamantopoulos A, Goudas P, Goumenos D, Soloukides A, Boletis I, Melexopoulou C, Georgaki E, Frysira E, Komianou F, Grekas D, Paliouras C, Alivanis P, Vergoulas G, Pierides A, Daphnis E, Deltas C. Frequent COL4 mutations in familial microhematuria accompanied by later-onset Alport nephropathy due to focal segmental glomerulosclerosis. Clin Genet. 2017;92:517-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 97. | Malone AF, Phelan PJ, Hall G, Cetincelik U, Homstad A, Alonso AS, Jiang R, Lindsey TB, Wu G, Sparks MA, Smith SR, Webb NJ, Kalra PA, Adeyemo AA, Shaw AS, Conlon PJ, Jennette JC, Howell DN, Winn MP, Gbadegesin RA. Rare hereditary COL4A3/COL4A4 variants may be mistaken for familial focal segmental glomerulosclerosis. Kidney Int. 2014;86:1253-1259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 98. | Xie J, Wu X, Ren H, Wang W, Wang Z, Pan X, Hao X, Tong J, Ma J, Ye Z, Meng G, Zhu Y, Kiryluk K, Kong X, Hu L, Chen N. COL4A3 mutations cause focal segmental glomerulosclerosis. J Mol Cell Biol. 2014;6:498-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 99. | Yao T, Udwan K, John R, Rana A, Haghighi A, Xu L, Hack S, Reich HN, Hladunewich MA, Cattran DC, Paterson AD, Pei Y, Barua M. Integration of Genetic Testing and Pathology for the Diagnosis of Adults with FSGS. Clin J Am Soc Nephrol. 2019;14:213-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 100. | Dische FE. Measurement of glomerular basement membrane thickness and its application to the diagnosis of thin-membrane nephropathy. Arch Pathol Lab Med. 1992;116:43-49. [PubMed] |
| 101. | Kashtan CE, Segal Y. Genetic disorders of glomerular basement membranes. Nephron Clin Pract. 2011;118:c9-c18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 102. | Nogueira M, Cartwright J Jr, Horn K, Doe N, Shappell S, Barrios R, Coroneos E, Truong LD. Thin basement membrane disease with heavy proteinuria or nephrotic syndrome at presentation. Am J Kidney Dis. 2000;35:E15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 103. | Quinlan C, Rheault MN. Genetic Basis of Type IV Collagen Disorders of the Kidney. Clin J Am Soc Nephrol. 2021;16:1101-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 104. | Hoorn EJ, Taams NE, Hurskainen T, Salih M, Weening JJ, Jonkman MF, Pas HH, Schreurs MW. Bullous Pemphigoid With a Dual Pattern of Glomerular Immune Complex Disease. Am J Kidney Dis. 2016;67:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 105. | Hurskainen T, Moilanen J, Sormunen R, Franzke CW, Soininen R, Loeffek S, Huilaja L, Nuutinen M, Bruckner-Tuderman L, Autio-Harmainen H, Tasanen K. Transmembrane collagen XVII is a novel component of the glomerular filtration barrier. Cell Tissue Res. 2012;348:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 106. | Saarela J, Rehn M, Oikarinen A, Autio-Harmainen H, Pihlajaniemi T. The short and long forms of type XVIII collagen show clear tissue specificities in their expression and location in basement membrane zones in humans. Am J Pathol. 1998;153:611-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 184] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 107. | Enoksen ITT, Svistounov D, Norvik JV, Stefansson VTN, Solbu MD, Eriksen BO, Melsom T. Serum Matrix Metalloproteinase 7 and accelerated GFR decline in a general non-diabetic population. Nephrol Dial Transplant. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 108. | Głowińska-Olszewska B, Urban M. Elevated matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 in obese children and adolescents. Metabolism. 2007;56:799-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 109. | Ishikawa J, Hoshide S, Eguchi K, Ishikawa S, Pickering TG, Shimada K, Kario K. Plasma tissue inhibitor of matrix metalloproteinase-1 level is increased in normotensive non-dippers in association with impaired glucose metabolism. Hypertens Res. 2008;31:2045-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 110. | Li Y, Yang J, Dai C, Wu C, Liu Y. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest. 2003;112:503-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 111. | Aggeli AS, Kitsiou PV, Tzinia AK, Boutaud A, Hudson BG, Tsilibary EC. Selective binding of integrins from different renal cell types to the NC1 domain of alpha3 and alpha1 chains of type IV collagen. J Nephrol. 2009;22:130-136. [PubMed] |
| 112. | Karamessinis PM, Tzinia AK, Kitsiou PV, Stetler-Stevenson WG, Michael AF, Fan WW, Zhou B, Margaritis LH, Tsilibary EC. Proximal tubular epithelial cell integrins respond to high glucose by altered cell-matrix interactions and differentially regulate matrixin expression. Lab Invest. 2002;82:1081-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 113. | Kitsiou PV, Tzinia AK, Stetler-Stevenson WG, Michael AF, Fan WW, Zhou B, Tsilibary EC. Glucose-induced changes in integrins and matrix-related functions in cultured human glomerular epithelial cells. Am J Physiol Renal Physiol. 2003;284:F671-F679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 114. | Guo L, Sanders PW, Woods A, Wu C. The distribution and regulation of integrin-linked kinase in normal and diabetic kidneys. Am J Pathol. 2001;159:1735-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 115. | van Roeyen CR, Ostendorf T, Denecke B, Bokemeyer D, Behrmann I, Strutz F, Lichenstein HS, LaRochelle WJ, Pena CE, Chaudhuri A, Floege J. Biological responses to PDGF-BB vs PDGF-DD in human mesangial cells. Kidney Int. 2006;69:1393-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 116. | Dey N, Ghosh-Choudhury N, Kasinath BS, Choudhury GG. TGFβ-stimulated microRNA-21 utilizes PTEN to orchestrate AKT/mTORC1 signaling for mesangial cell hypertrophy and matrix expansion. PLoS One. 2012;7:e42316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 117. | Kim MK, Maeng YI, Sung WJ, Oh HK, Park JB, Yoon GS, Cho CH, Park KK. The differential expression of TGF-β1, ILK and wnt signaling inducing epithelial to mesenchymal transition in human renal fibrogenesis: an immunohistochemical study. Int J Clin Exp Pathol. 2013;6:1747-1758. [PubMed] |
| 118. | Yan Q, Sui W, Xie S, Chen H, Zou G, Guo J, Zou H. Expression and role of integrin-linked kinase and collagen IV in human renal allografts with interstitial fibrosis and tubular atrophy. Transpl Immunol. 2010;23:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 119. | Foster RR, Hole R, Anderson K, Satchell SC, Coward RJ, Mathieson PW, Gillatt DA, Saleem MA, Bates DO, Harper SJ. Functional evidence that vascular endothelial growth factor may act as an autocrine factor on human podocytes. Am J Physiol Renal Physiol. 2003;284:F1263-F1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 139] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 120. | Simon M, Gröne HJ, Jöhren O, Kullmer J, Plate KH, Risau W, Fuchs E. Expression of vascular endothelial growth factor and its receptors in human renal ontogenesis and in adult kidney. Am J Physiol. 1995;268:F240-F250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 87] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 121. | Thomas S, Vanuystel J, Gruden G, Rodríguez V, Burt D, Gnudi L, Hartley B, Viberti G. Vascular endothelial growth factor receptors in human mesangium in vitro and in glomerular disease. J Am Soc Nephrol. 2000;11:1236-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 122. | Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007;49:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 460] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 123. | Sartelet H, Toupance O, Lorenzato M, Fadel F, Noel LH, Lagonotte E, Birembaut P, Chanard J, Rieu P. Sirolimus-induced thrombotic microangiopathy is associated with decreased expression of vascular endothelial growth factor in kidneys. Am J Transplant. 2005;5:2441-2447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 124. | Kovalevich J, Tracy B, Langford D. PINCH: More than just an adaptor protein in cellular response. J Cell Physiol. 2011;226:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 125. | Ghatak S, Morgner J, Wickström SA. ILK: a pseudokinase with a unique function in the integrin-actin linkage. Biochem Soc Trans. 2013;41:995-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 126. | Kim YC, Gonzalez-Nieves R, Cutler ML. Rsu1 contributes to cell adhesion and spreading in MCF10A cells via effects on P38 map kinase signaling. Cell Adh Migr. 2015;9:227-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 127. | Yang H, Lin L, Sun K, Zhang T, Chen W, Li L, Xie Y, Wu C, Wei Z, Yu C. Complex structures of Rsu1 and PINCH1 reveal a regulatory mechanism of the ILK/PINCH/Parvin complex for F-actin dynamics. Elife. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 128. | Górska A, Mazur AJ. Integrin-linked kinase (ILK): the known vs. the unknown and perspectives. Cell Mol Life Sci. 2022;79:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 129. | Velyvis A, Yang Y, Wu C, Qin J. Solution structure of the focal adhesion adaptor PINCH LIM1 domain and characterization of its interaction with the integrin-linked kinase ankyrin repeat domain. J Biol Chem. 2001;276:4932-4939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 130. | Sepulveda JL, Wu C. The parvins. Cell Mol Life Sci. 2006;63:25-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 131. | Tu Y, Huang Y, Zhang Y, Hua Y, Wu C. A new focal adhesion protein that interacts with integrin-linked kinase and regulates cell adhesion and spreading. J Cell Biol. 2001;153:585-598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 188] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 132. | Fukuda T, Guo L, Shi X, Wu C. CH-ILKBP regulates cell survival by facilitating the membrane translocation of protein kinase B/Akt. J Cell Biol. 2003;160:1001-1008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 133. | Krishnamurti U, Chen Y, Michael A, Kim Y, Fan WW, Wieslander J, Brunmark C, Rondeau E, Sraer JD, Delarue F, Tsilibary EC. Integrin-mediated interactions between primary/T-sv40 immortalized human glomerular epithelial cells and type IV collagen. Lab Invest. 1996;74:650-657. [PubMed] |
| 134. | Spiro RG. Biochemistry of the renal glomerular basement membrane and its alterations in diabetes mellitus. N Engl J Med. 1973;288:1337-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 135. | Goldstein JL, Fialkow PJ. The Alström syndrome. Report of three cases with further delineation of the clinical, pathophysiological, and genetic aspects of the disorder. Medicine (Baltimore). 1973;52:53-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 62] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 136. | Marshall JD, Bronson RT, Collin GB, Nordstrom AD, Maffei P, Paisey RB, Carey C, Macdermott S, Russell-Eggitt I, Shea SE, Davis J, Beck S, Shatirishvili G, Mihai CM, Hoeltzenbein M, Pozzan GB, Hopkinson I, Sicolo N, Naggert JK, Nishina PM. New Alström syndrome phenotypes based on the evaluation of 182 cases. Arch Intern Med. 2005;165:675-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 224] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 137. | Falk RJ, Scheinman JI, Mauer SM, Michael AF. Polyantigenic expansion of basement membrane constituents in diabetic nephropathy. Diabetes. 1983;32 Suppl 2:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 135] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 138. | Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer E, Joh K, Noël LH, Radhakrishnan J, Seshan SV, Bajema IM, Bruijn JA; Renal Pathology Society. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21:556-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 842] [Cited by in RCA: 1119] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 139. | Schleicher E, Wieland OH. Changes of human glomerular basement membrane in diabetes mellitus. J Clin Chem Clin Biochem. 1984;22:223-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 140. | Berenson GS, Ruiz H, Dalferes ER Jr, Dugan FA, Radhakrishnamurthy B. Acid mucopolysaccharide changes in diabetic kidneys. Diabetes. 1970;19:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 141. | Zhao L, Zhang Y, Liu F, Yang H, Zhong Y, Wang Y, Li S, Su Q, Tang L, Bai L, Ren H, Zou Y, Wang S, Zheng S, Xu H, Li L, Zhang J, Chai Z, Cooper ME, Tong N. Urinary complement proteins and risk of end-stage renal disease: quantitative urinary proteomics in patients with type 2 diabetes and biopsy-proven diabetic nephropathy. J Endocrinol Invest. 2021;44:2709-2723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 142. | Bonomo JA, Palmer ND, Hicks PJ, Lea JP, Okusa MD, Langefeld CD, Bowden DW, Freedman BI. Complement factor H gene associations with end-stage kidney disease in African Americans. Nephrol Dial Transplant. 2014;29:1409-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 143. | Valoti E, Noris M, Perna A, Rurali E, Gherardi G, Breno M, Parvanova Ilieva A, Petrov Iliev I, Bossi A, Trevisan R, Dodesini AR, Ferrari S, Stucchi N, Benigni A, Remuzzi G, Ruggenenti P. Impact of a Complement Factor H Gene Variant on Renal Dysfunction, Cardiovascular Events, and Response to ACE Inhibitor Therapy in Type 2 Diabetes. Front Genet. 2019;10:681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 144. | Westberg NG, Michael AF. Human glomerular basement membrane: chemical composition in diabetes mellitus. Acta Med Scand. 1973;1-2:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 145. | Rasmussen LM, Heickendorff L. Accumulation of fibronectin in aortas from diabetic patients. A quantitative immunohistochemical and biochemical study. Lab Invest. 1989;61:440-446. [PubMed] |
| 146. | Kimmelstiel P, Kim OJ, Beres J. Studies on renal biopsy specimens, with the aid of the electron microscope. I. Glomeruli in diabetes. Am J Clin Pathol. 1962;38:270-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 147. | Mise K, Ueno T, Hoshino J, Hazue R, Sumida K, Yamanouchi M, Hayami N, Suwabe T, Hiramatsu R, Hasegawa E, Sawa N, Fujii T, Hara S, Wada J, Makino H, Takaichi K, Ohashi K, Ubara Y. Nodular lesions in diabetic nephropathy: Collagen staining and renal prognosis. Diabetes Res Clin Pract. 2017;127:187-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 148. | Alsaad KO, Edrees B, Rahim KA, Alanazi A, Ahmad M, Aloudah N. Collagenofibrotic (Collagen Type III) glomerulopathy in association with diabetic nephropathy. Saudi J Kidney Dis Transpl. 2017;28:898-905. [PubMed] |
| 149. | Guan M, Ma J, Keaton JM, Dimitrov L, Mudgal P, Stromberg M, Bonomo JA, Hicks PJ, Freedman BI, Bowden DW, Ng MC. Association of kidney structure-related gene variants with type 2 diabetes-attributed end-stage kidney disease in African Americans. Hum Genet. 2016;135:1251-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 150. | Salem RM, Todd JN, Sandholm N, Cole JB, Chen WM, Andrews D, Pezzolesi MG, McKeigue PM, Hiraki LT, Qiu C, Nair V, Di Liao C, Cao JJ, Valo E, Onengut-Gumuscu S, Smiles AM, McGurnaghan SJ, Haukka JK, Harjutsalo V, Brennan EP, van Zuydam N, Ahlqvist E, Doyle R, Ahluwalia TS, Lajer M, Hughes MF, Park J, Skupien J, Spiliopoulou A, Liu A, Menon R, Boustany-Kari CM, Kang HM, Nelson RG, Klein R, Klein BE, Lee KE, Gao X, Mauer M, Maestroni S, Caramori ML, de Boer IH, Miller RG, Guo J, Boright AP, Tregouet D, Gyorgy B, Snell-Bergeon JK, Maahs DM, Bull SB, Canty AJ, Palmer CNA, Stechemesser L, Paulweber B, Weitgasser R, Sokolovska J, Rovīte V, Pīrāgs V, Prakapiene E, Radzeviciene L, Verkauskiene R, Panduru NM, Groop LC, McCarthy MI, Gu HF, Möllsten A, Falhammar H, Brismar K, Martin F, Rossing P, Costacou T, Zerbini G, Marre M, Hadjadj S, McKnight AJ, Forsblom C, McKay G, Godson C, Maxwell AP, Kretzler M, Susztak K, Colhoun HM, Krolewski A, Paterson AD, Groop PH, Rich SS, Hirschhorn JN, Florez JC; SUMMIT Consortium, DCCT/EDIC Research Group, GENIE Consortium. Genome-Wide Association Study of Diabetic Kidney Disease Highlights Biology Involved in Glomerular Basement Membrane Collagen. J Am Soc Nephrol. 2019;30:2000-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 144] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 151. | Peeters SA, Engelen L, Buijs J, Chaturvedi N, Fuller JH, Schalkwijk CG, Stehouwer CD; EURODIAB Prospective Complications Study Group. Plasma levels of matrix metalloproteinase-2, -3, -10, and tissue inhibitor of metalloproteinase-1 are associated with vascular complications in patients with type 1 diabetes: the EURODIAB Prospective Complications Study. Cardiovasc Diabetol. 2015;14:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 152. | Peeters SA, Engelen L, Buijs J, Jorsal A, Parving HH, Tarnow L, Rossing P, Schalkwijk CG, Stehouwer CDA. Plasma matrix metalloproteinases are associated with incident cardiovascular disease and all-cause mortality in patients with type 1 diabetes: a 12-year follow-up study. Cardiovasc Diabetol. 2017;16:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 153. | Peeters SA, Engelen L, Buijs J, Chaturvedi N, Fuller JH, Jorsal A, Parving HH, Tarnow L, Theilade S, Rossing P, Schalkwijk CG, Stehouwer CDA. Circulating matrix metalloproteinases are associated with arterial stiffness in patients with type 1 diabetes: pooled analysis of three cohort studies. Cardiovasc Diabetol. 2017;16:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 154. | Vilmi-Kerälä T, Lauhio A, Tervahartiala T, Palomäki O, Uotila J, Sorsa T, Palomäki A. Subclinical inflammation associated with prolonged TIMP-1 upregulation and arterial stiffness after gestational diabetes mellitus: a hospital-based cohort study. Cardiovasc Diabetol. 2017;16:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 155. | Afkarian M, Zelnick LR, Ruzinski J, Kestenbaum B, Himmelfarb J, de Boer IH, Mehrotra R. Urine matrix metalloproteinase-7 and risk of kidney disease progression and mortality in type 2 diabetes. J Diabetes Complications. 2015;29:1024-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 156. | Paunas FTI, Finne K, Leh S, Osman TA, Marti HP, Berven F, Vikse BE. Characterization of glomerular extracellular matrix in IgA nephropathy by proteomic analysis of laser-captured microdissected glomeruli. BMC Nephrol. 2019;20:410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 157. | Ibrahim S, Rashed L. Estimation of transforming growth factor-beta 1 as a marker of renal injury in type II diabetes mellitus. Saudi Med J. 2007;28:519-523. [PubMed] |
| 158. | Shaker OG, Sadik NA. Transforming growth factor beta 1 and monocyte chemoattractant protein-1 as prognostic markers of diabetic nephropathy. Hum Exp Toxicol. 2013;32:1089-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 159. | Mou X, Zhou DY, Ma JR, Liu YH, Chen HP, Hu YB, Shou CM, Chen JW, Liu WH, Ma GL. Serum TGF-β1 as a Biomarker for Type 2 Diabetic Nephropathy: A Meta-Analysis of Randomized Controlled Trials. PLoS One. 2016;11:e0149513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 160. | Qiao YC, Chen YL, Pan YH, Ling W, Tian F, Zhang XX, Zhao HL. Changes of transforming growth factor beta 1 in patients with type 2 diabetes and diabetic nephropathy: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). 2017;96:e6583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 161. | Zhou T, Li HY, Zhong H, Zhong Z. Relationship between transforming growth factor-β1 and type 2 diabetic nephropathy risk in Chinese population. BMC Med Genet. 2018;19:201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 162. | Voelker J, Berg PH, Sheetz M, Duffin K, Shen T, Moser B, Greene T, Blumenthal SS, Rychlik I, Yagil Y, Zaoui P, Lewis JB. Anti-TGF-β1 Antibody Therapy in Patients with Diabetic Nephropathy. J Am Soc Nephrol. 2017;28:953-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 211] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 163. | Uehara G, Suzuki D, Toyoda M, Umezono T, Sakai H. Glomerular expression of platelet-derived growth factor (PDGF)-A, -B chain and PDGF receptor-alpha, -beta in human diabetic nephropathy. Clin Exp Nephrol. 2004;8:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 164. | Baelde HJ, Eikmans M, Lappin DW, Doran PP, Hohenadel D, Brinkkoetter PT, van der Woude FJ, Waldherr R, Rabelink TJ, de Heer E, Bruijn JA. Reduction of VEGF-A and CTGF expression in diabetic nephropathy is associated with podocyte loss. Kidney Int. 2007;71:637-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 165. | Woroniecka KI, Park AS, Mohtat D, Thomas DB, Pullman JM, Susztak K. Transcriptome analysis of human diabetic kidney disease. Diabetes. 2011;60:2354-2369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 502] [Cited by in RCA: 497] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 166. | Hovind P, Tarnow L, Oestergaard PB, Parving HH. Elevated vascular endothelial growth factor in type 1 diabetic patients with diabetic nephropathy. Kidney Int Suppl. 2000;75:S56-S61. [PubMed] |
| 167. | Zhao Y, Banerjee S, LeJeune WS, Choudhary S, Tilton RG. NF-κB-inducing kinase increases renal tubule epithelial inflammation associated with diabetes. Exp Diabetes Res. 2011;2011:192564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 168. | Bae EH, Cho S, Joo SY, Ma SK, Kim SH, Lee J, Kim SW. 4-Hydroxy-2-hexenal-induced apoptosis in human renal proximal tubular epithelial cells. Nephrol Dial Transplant. 2011;26:3866-3873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 169. | Tanji N, Markowitz GS, Fu C, Kislinger T, Taguchi A, Pischetsrieder M, Stern D, Schmidt AM, D'Agati VD. Expression of advanced glycation end products and their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal disease. J Am Soc Nephrol. 2000;11:1656-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 359] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 170. | Suzuki D, Toyoda M, Yamamoto N, Miyauchi M, Katoh M, Kimura M, Maruyama M, Honma M, Umezono T, Yagame M. Relationship between the expression of advanced glycation end-products (AGE) and the receptor for AGE (RAGE) mRNA in diabetic nephropathy. Intern Med. 2006;45:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 171. | Peeters SA, Engelen L, Buijs J, Theilade S, Rossing P, Schalkwijk CG, Stehouwer CDA. Associations between advanced glycation endproducts and matrix metalloproteinases and its inhibitor in individuals with type 1 diabetes. J Diabetes Complications. 2018;32:325-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |