Published online Aug 15, 2020. doi: 10.4239/wjd.v11.i8.322
Peer-review started: March 20, 2020
First decision: April 26, 2020
Revised: May 27, 2020
Accepted: June 14, 2020
Article in press: June 14, 2020
Published online: August 15, 2020
Obesity and obesity-related co-morbidities, diabetes mellitus, and hypertension are among the fastest-growing risk factors of heart failure and kidney disease worldwide. Obesity, which is not a unitary concept, or a static process, ranges from alterations in distribution to the amount of adiposity. Visceral adiposity, which includes intraabdominal visceral fat mass and ectopic fat deposition such as hepatic, cardiac, or renal, was robustly associated with a greater risk for cardiorenal morbidity than subcutaneous adiposity. In addition, morbid obesity has also demonstrated a negative effect on cardiac and renal functioning. The mechanisms by which adipose tissue is linked with the cardiorenal syndrome (CRS) are hemodynamic and mechanical changes, as well neurohumoral pathways such as insulin resistance, endothelial dysfunction, nitric oxide bioavailability, renin-angiotensin-aldosterone, oxidative stress, sympathetic nervous systems, natriuretic peptides, adipokines and inflammation. Adiposity and other associated co-morbidities induce adverse cardiac remodeling and interstitial fibrosis. Heart failure with preserved ejection fraction has been associated with obesity-related functional and structural abnormalities. Obesity might also impair kidney function through hyperfiltration, increased glomerular capillary wall tension, and podocyte dysfunction, which leads to tubulointerstitial fibrosis and loss of nephrons and, finally, chronic kidney disease. The development of new treatments with renal and cardiac effects in the context of type 2 diabetes, which improves mortality outcome, has highlighted the importance of CRS and its prevalence. Increased body fat triggers cellular, neuro-humoral and metabolic pathways, which create a phenotype of the CRS with specific cellular and biochemical biomarkers. Obesity has become a single cardiorenal umbrella or type of cardiorenal metabolic syndrome. This review article provides a clinical overview of the available data on the relationship between a range of adiposity and CRS, the support for obesity as a single cardiorenal umbrella, and the most relevant studies on the recent therapeutic approaches.
Core tip: Visceral adiposity and morbid obesity are risk factors for heart and kidney disease, configuring a cardiorenal syndrome. Adipose tissue results in hemodynamic and mechanical derangements in addition to activating neuro-humoral systems such as endothelial dysfunction, adipokines, renin-angiotensin-aldosterone, sympathetic nervous system, natriuretic peptides, inflammation, and oxidative stress. Obesity induces cardiac remodeling and fibrosis, leading to heart failure (HF). HF with preserved ejection fraction is characteristically linked to obesity. Hyperfiltration, increased glomerular capillary wall tension, podocyte dysfunction, and, finally, chronic kidney disease has been linked to obesity. Most of the new treatments for diabetes mellitus type 2, which have favorable cardiovascular outcomes, improve the cardiometabolic renal syndrome associated with obesity.
- Citation: Pazos F. Range of adiposity and cardiorenal syndrome. World J Diabetes 2020; 11(8): 322-350
- URL: https://www.wjgnet.com/1948-9358/full/v11/i8/322.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i8.322
The prevalence of obesity has increased dramatically in recent years, and the increase has been more significant in the high ranges of body mass index (BMI) that represents almost 5% of the population. The rise in obesity is associated with an increase in cardiometabolic disease. Both the distribution and the amount of adiposity have been related to the pathophysiology of arterial hypertension, atherosclerotic vascular disease, heart failure (HF), chronic kidney disease (CKD), global mortality and cardiovascular disease[1].
The global prevalence of obesity is 5% among children and 12% among adults, and is higher in women. The peak of obesity is observed between the ages of 50 and 64 years. High BMI represented 7.1% of all deaths, and nearly 70% of these deaths related to high BMI were due to cardiovascular disease. CKD was the cause of 18% of disability-adjusted life-years that occurred at a BMI greater than 30 but represented less than 10% of all BMI-related deaths in 2015[2]. Obesity, diabetes mellitus, the second leading cause of BMI-related deaths, and CKD are among the fastest-growing causes of deaths worldwide[3]. Furthermore, waist circumference and visceral obesity might be increasing beyond what is expected according to BMI. Janssen et al[4] demonstrated that waist circumference increased in a sample of the Canadian population with a one-unit increase in BMI from 1981 to 2007-2009. This data suggests that the trend in obesity is related to a risker profile in which greater visceral obesity and the burden of adiposity is taking place.
The risk factors associated with increased cardiovascular disease (CVD) are similar across countries. In a standardized case-control study of myocardial infarction in 52 countries, abdominal obesity was found to be related to myocardial infarction (OR of 1.12 for top vs lowest tertile and 1.62 for middle vs lowest tertile), and a population attributable risk (PAR) of 20.1% for the top two tertiles vs the lowest tertiles (from 5 in China to 63.5 in western Europe)[5]. These associations were noted in men and women, old and young, and in all regions of the world. Collectively, these nine risk factors, such as smoking, history of hypertension or diabetes, waist-hip-ratio, dietary patterns, physical activity, consumption of alcohol, blood apolipoproteins, and psychosocial factors; all of them accounted for 90% of the PAR in men and 94% in women. South Asians have been found to have a higher proportional incidence and mortality rate from ischemic heart disease and CKD compared with other ethnic groups such as East Asian (Chinese, Japanese, and Korean) populations and non-Hispanic whites[6].
This increase in the prevalence of obesity and visceral adiposity (VA) and its related diseases is paralleled with the fastest growth in severe/morbid obesity. Population-based studies have reported that 2.3% (2.0-2.7) of the world's men and 5.0% (4.4-5.6) of women were severely obese (BMI ≥ 35 kg/m2). Furthermore, the prevalence of morbid obesity was 0.64% (0.46-0.86) in men and 1.6% (1.3-1.9) in women (BMI ≥ 40 kg/m2)[7]. The association of cardiometabolic-renal risk factors that are related to cardiovascular events is likely to be bigger with greater BMI. According to this, severe/morbid obesity (BMI ≥ 35 kg/m2) was associated with higher triglycerides, hs-C-reactive protein (CRP), insulin and insulin resistance, diastolic blood pressure and higher odds of hypertension than grade I obesity both in women and men and in those who were physically inactive[8]. In addition, recently, Santos et al[9] reported that hypertension, CRP, systolic blood pressure, waist circumference, body fat percentage, and visceral area were significantly higher among individuals with the highest BMI. It appears that most of the cardiometabolic risk factors are related to cardiorenal syndrome (CRS).
Once the individual threshold is exceeded, severe visceral, parenchymal and generalized adiposity is accompanied by an inflammatory, neurohormonal, vascular, and metabolic response that converges in cardiac and renal damage. In addition, the presence of hypertension and diabetes mellitus, where obesity plays a central role, along with the increasing number of aggregated cardiovascular risk factors amplify and perpetuate the CRS[10].
The prevalence of HF is 1% to 2% in the general population and, in the United States, at the age of 45 years, the lifetime risk of HF ranges from 20% to 46% depending on the black or the white population, and is higher in women[11]. At least, more than half of HF is in the form of heart failure with preserved ejection fraction (HFpEF), which is increasing in the developed world associated with the increasing risk factors for vascular disease and markers of renal dysfunction[12]. HFpEF is characterized by relaxation abnormalities during diastole and increased venous congestion. Obesity and insulin resistance, along with endothelial dysfunction, impaired insulin signaling, and nitric oxide (NO) bioavailability. In addition, VA and the metabolic syndrome induce oxidative stress and the inflammatory response, which exert their effects on the heart and kidney[13]. The increased ventricular mass, biventricular dependency, and interstitial fibrosis compromise diastolic relaxation and predispose the failing heart to acute decompensated HF (ADHF) and hospitalization[14].
The association between obesity and CKD has been reported. With a BMI > 30, the disability-adjusted life years were 18% due to CKD compared to 7.2% with a BMI < 30[2]. Epidemiological studies have found that VA has raised the incidence of CKD and predisposition to acute kidney injury (AKI)[15]. In addition, there is a strong relationship between increases in body weight measured by BMI and the progression of kidney disease. In a global meta-analysis of over five million adults followed over an average of 8 years, Chang et al[16] found that a BMI of 30, 35, and 40 were associated with an 18%, 69%, and 102% increased risk of glomerular filtration rate (GFR) decline, respectively, compared with a BMI of 25. In this regard, glomerular hyperfiltration was reported to have been an early functional alteration shared with diabetes mellitus, adiposity, insulin resistance, and hypertension[17]. Several obesity-related mediators have been implicated, such as renin-angiotensin-aldosterone system (RAAS) activation, NO production, pro-inflammatory adipokines, and adipose-related mediators[18]. To which extent adiposity might influence the progression from glomerular to tubulointerstitial fibrosis and loss of nephrons depends on the combination of the rest of the kidney risk factors and the degree of obesity.
The development of a new therapy for diabetes mellitus with cardiorenal improvements and benefits in mortality outcomes, which has been used in HF as well, has opened up a new horizon in the management of this syndrome. We should use the term cardiorenal metabolic syndrome previously reported by Sowers et al[19], which may be applied to an adiposity-related cardiorenal syndrome. This term considers obesity and diabetes mellitus or metabolic syndrome as a pivotal factor in the development of kidney and heart disease.
It is necessary to move toward a model in which the heart and the kidney are affected by the same risk factors such as obesity and adiposity, but in different degrees and time frames. We have to consider obesity as a single CRS umbrella, differentiating patients according to the predominant pathophysiological systems, which may be identified with a biomarker phenotyping or a biomarker profile. The aim of this article is to propose new concepts for the basis of adiposity in the pathophysiology of CRS, and furthermore, to highlight the importance of obesity in response to new treatments of CRS and new trials. We have tried to develop a phenotype of CRS associated with obesity, which is considered as being a heterogeneous entity within a range of adiposity that determines the specific profile of biomarkers for VA and morbid obesity.
This is a narrative review; however, to ensure all relevant literature is considered, systematic searches were carried out on Medline, Ovid and EBSCO using the terms “cardiorenal”, “obesity”, “adiposity”, “HF, and “CKD” limited to English language papers with human subjects. This was supplemented by manual reference searches; many papers reporting effects on the heart and the kidney in humans were identified. This review is limited to describing studies of obesity, adiposity, HF, and acute and CKD. No exclusions were made based on participant characteristics, co-morbidity and study design. To assist with the direct interpretation of data, the study design, participants, and results from studies, which met the inclusion criteria and assessed current key areas of interest, are described in tables. Specifically, these deal with the concept of the range of adiposity, which refers to adipose tissue and ectopic fat distribution and the BMI classification, the classification of CRS, and tables in relation to key studies of the new therapeutic alternatives for adiposity-related CRS.
Cardiorenal syndromes are defined as alterations of the heart and the kidneys where the dysfunction of one can induce acute or chronic dysfunction of the other, as defined in the international consensus[20]. At the 2008 conference, the Acute Dialysis Quality Initiative reported a classification for CRS, categorized into five types, based on which organ or process is the causation and which are the consequence as well as the time frame of development, as summarized in Table 1.
| Type | Primary and secondary organs and processes affected in the syndromes | |
| Primary | Secondary | |
| Type 1 | Cardiac impairment, acute | Renal impairment |
| Type 2 | Cardiac impairment, chronic | Renal impairment |
| Type 3 | Renal impairment, acute | Cardiac impairment |
| Type 4 | Renal impairment, chronic | Cardiac impairment |
| Type 5 | Systemic condition | Cardiac and renal impairment |
Type 1 CRS, acute cardiorenal is characterized by the acute worsening of cardiac function leading to AKI, and it occurs in about 25% of patients with ADHF[21]. Furthermore, in the HFpEF individuals from the ADHERE database, urea nitrogen or creatinine levels were the most powerful predictors of in-hospital mortality. CRS type 2 is characterized by chronic abnormalities in cardiac function, leading to chronic kidney injury or dysfunction. In a prospective sequence of selected individuals with systolic cardiac dysfunction [heart failure with reduced ejection fraction (HFrEF)], CKD was found in 26% with a lower survival prognosis compared with patients without CKD.
CRS type 3 or acute kidney-heart syndrome is characterized by AKI, leading to heart diseases (acute) such as ADHF, arrhythmias, and acute coronary syndrome (ACS)[22]. Many pathological situations such as infections lead to AKI and then ADHF, where a vicious cycle is established, worsening the prognosis and mortality rates[23].
CRS type 4 or chronic kidney-heart syndrome is characterized by cardiac dysfunction in patients with CKD at any stage but predominantly from stages 4 and 5. Go et al[24] reported that in a large-scale community-based population with CKD, reduced estimated GFR (eGFR) had an independent and graded risk of death, cardiovascular events, and hospitalization.
CRS type 5 or secondary is characterized when cardiac and renal injury coincides, resulting from a common underlying pathological process such as connective tissue disorders or sepsis[25,26]. Despite the existence of the underlying disease, the hemodynamic and pro-inflammatory mediators are similar to those in the other types[27].
In a retrospective cohort study of 30681 patients who underwent at least one transthoracic echocardiography, 8% of patients developed at least one of the CRSs, of which 19% subsequently developed an acute syndrome. The development of an acute syndrome was associated with the highest risk of death (HR = 3.13, 95%CI: 0.37 to 0.61, P < 0.001)[28]. Furthermore, in a meta-analysis of studies on HF, the prevalence of CKD was 32% and worsening renal function was present in 23%, both of which are associated and independent predictors of all-cause mortality[29]. In addition, a retrospective analysis of studies on left ventricular (LV) dysfunction showed that moderate renal insufficiency was associated with an increased risk of all-cause mortality (RR = 1.41; P = 0.001)[30].
Obesity is defined by the BMI, calculated according to WHO recommendations, which allows not only the establishment of criteria for clinical studies but also classification into different degrees of obesity. Classifications and thresholds of adiposity are summarized in Table 2. However, obesity in terms of BMI is remarkably heterogeneous with various co-morbidities and levels of health risk[31]. Most studies have shown that the increase in visceral adipose tissue is the primary determinant of the metabolic risk associated with obesity. Thus, VA is the result of the inability of subcutaneous adipose tissue to expand in response to increased demand for triglycerides, that among individuals determines the concept of the personal fat threshold[32]. Numerous factors influence visceral or subcutaneous adiposity such as age, sex, race or ethnicity, genetic and epigenetic traits, even the type of diet[32,33]. Excess VA is composed of visceral and ectopic adiposity, which has been related to adipocyte dysfunction, inflammatory response, adipokine and neurohormonal dysregulation, insulin resistance, and endothelial dysfunction[34].
| General adiposity (SAT and VAT) BMI, kg/m2 | Thresholds and classification |
| < 24.9 | Normal |
| 25-29.9 | Overweight |
| 30-34.9 | Class I |
| 35-39.9 | Class II |
| ≥ 40 | Class III |
| Central adiposity (VA) | Thresholds |
| WC | M: ≥ 94 cm, W: ≥ 80 cm |
| Thresholds depend on BMI and ethnicity | |
| Waist-to-height ratio (index of central obesity) | > 50 yr: ≥ 0.6, < 40 yr: ≥ 0.5 |
| Waist-to-hip ratio | M: ≥ 0.9, W: ≥ 0.85 |
| Neck circumference | M: ≥ 40.5 cm W: ≥ 34.2 cm |
| Sagittal abdominal diameter | > 30 cm correlates with CV risk |
| Visceral adiposity index[189] | The formula for M and W depends on WC,BMI, TG and HDL-cholesterol |
| Ectopic and parenchymal adiposity | |
| Liver, epicardial and renal fat tissue | Continuous variable, MRI or TC |
The development of CT and MRI has allowed the analysis and measurement of fat content in different compartments using tomographic slices and computerized analysis[35]. This has allowed quantitative and qualitative analysis of the different areas and organs such as the pancreas, liver, kidney, and heart of the so-called ectopic fat and the risk for cardiovascular disease[36]. Recently, the use of dual-energy X-ray absorptiometry, which gives an estimate of the distribution and amount of body fat, has become popular. In fact, it is an acceptable alternative to the use of MRI or CT along with waist circumference and BMI[37]. The International Study of Prediction of Intra-abdominal Adiposity and Its Relationship with Cardiometabolic Risk/Intra-abdominal Adiposity, based on data from CT, has demonstrated that within each BMI, the measurement of abdominal circumference correlates with abdominal, visceral fat and cardiometabolic risk[38]. Furthermore, these data and those from prospective studies have shown that abdominal circumference is associated with an increase in cardiovascular risk within each category of BMI; thus, the risk of CVD should be established after having been adjusted for the BMI[39]. Finally, the presence of an increase in abdominal circumference associated with an increase in triglyceride levels has been strongly correlated with increased visceral fat (VAT) levels giving rise to an index called the hypertriglyceridemic waist phenotype[40].
The general increase in adiposity that characterizes severe or morbid obesity has special characteristics that combine a massive increase in visceral and subcutaneous adiposity, which adds additional morbidity to the traditional concept of obesity[41]. Despite the increased fat mass, severe/morbid obesity was associated with higher triglycerides, hs-CRP, insulin and insulin resistance, diastolic blood pressure and higher odds of hypertension than grade I obesity in both women and men and among them[42], physically inactive individuals presented the least favorable cardiometabolic profile (P < 0.05)[8]. Moreover, Santos et al[9] reported that hypertension, CRP, systolic, and diastolic blood pressure were more prevalent in a patient with BMI > 45. Also, the waist circumference, body fat percentage, visceral fat area and systolic blood pressure were significantly higher in patients with a BMI > 45 compared with a BMI 35-44.9.
Taken together, there is a gradual increase in dangerous adiposity, from VA and ectopic adiposity to generalized morbidly obese patients, where not only VA but also SA plays a key role in the higher prevalence of hypertension, HF and CKD.
Adiposity triggers a metabolic, neurohormonal, inflammatory, endothelial, immunological and fibrotic response that configures a characteristic pathophysiological profile for each type of obesity. These pathophysiological systems may have variable clinical expressions in the heart and in the kidney.
The renin-angiotensin system (RAS) is likely to be one of the main factors that links the kidney and the heart in the CRS. The augmented renin-induced angiotensin II (AngII) has many renal, cardiac, and systemic effects[43]. In addition, Ang II stimulates the synthesis and release of aldosterone, which stimulates sodium and water retention, and it has other harmful effects on the cardiovascular system[44]. At the glomerular level, the activation of RAS and AngII cause sodium retention and efferent artery vasoconstriction, augmenting the intraglomerular pressure. Also, intrarenal Ang II is likely to play a role in kidney injury by acting directly at the cellular level. It has been shown that Ang II stimulates the proliferation of mesangial cells and the production of collagen in vitro[45]. Ang II has also been implicated in the inflammatory and oxidative responses not only in mesangial cells but also in endothelial cells[46].
Ang II also exerts its effect on the heart by inducing cardiac hypertrophy. AngII stimulates protein synthesis and cell growth in cardiomyocytes in vitro and in vivo. It has also been shown that AngII exerts its effects via AT1 receptors independently of blood pressure[47]. Within the signaling pathways of the action of AngII, it has been suggested that, at least in part, they act through the generation of reactive oxygen species (ROS) and that this effect can be modified by the administration of antioxidants[48]. On the other hand, RAS has been implicated in cardiac fibrosis by stimulating cardiac fibroblasts proliferation and the synthesis and secretion of collagen[49]. However, the exact signaling pathways involved in the effect of AngII-induced cardiac fibrosis are still not well known. It is currently considered that most of the impact on renal and heart fibrosis seems to depend on the augmented mineralocorticoid activity.
The activation of RAS and increased AngII cause an increase in the release and production of aldosterone, which in turn, induces sodium and water retention contributing to fluid overload. Experimental mineralocorticoid receptor (MR) blockade markedly attenuated sodium retention, glomerular hyperfiltration, and blood pressure[50]. However, the clinical benefits of aldosterone receptor blockade occur despite the concomitant blockade with angiotensin blockade or ACE inhibitors, that support MR activation independently of AngII-mediated stimulation of aldosterone secretion[51].
Additionally, activation of the MR may lead to fibrosis in the vessels, heart and kidneys. In an experimental model of hypertensive aldosterone-salt-treated rats, galectin-3 is involved in mediating aldosterone-induced cardiac and renal fibrosis[52]. Furthermore, acute and chronic kidney injury induced by ischemia/reperfusion in rats displayed kidney dysfunction, increased proteinuria, extensive tubule-interstitial fibrosis, TGFβ, and collagen-I mRNA. All of these chronic and acute alterations have been prevented by the administration of the novel nonsteroidal MR antagonist finerenone[53].
The augmented activity of the sympathetic nervous system (SNS) characterizes HF and worsening of renal function (WRF) in the CRS. Thus, an activated SNS has harmful effects on the heart and the kidney. This raised renal SNS activity leads to an increase in both tubular sodium reabsorption and arteriolar vascular contraction, which, results in increased renin release[54,55]. RAS activation and sympathetic activity are processes that self-amplify each other. On the other hand, an increase in norepinephrine is observed in HF as well as an activated SNS, all of which correlate with a higher degree of congestion and a worse prognosis[56].
The ROS, which are usually generated as by-products in the mitochondrial respiratory chain activity, inactivate the endothelium-derived relaxing factor, NO, by forming ONOO-[57]. This inactivation of NO results in protein damage, loss of NO bioavailability, and impaired vasodilatation, which further diminishes renal and cardiac function[58]. In addition, the imbalance between ROS and NOS is augmented by the activation of RAS and SNS, perpetuating the damage in the CRS[59]. Oxidative stress has been studied as a reversible process, and it should be emphasized that there are therapeutic attempts with promising results, but to date, there is not enough evidence to recommend these types of treatments[60].
The inflammatory response may cause tissue injury and organ dysfunction. The HF and WRF in CRS may deteriorate due to local and systemic inflammation. Moreover, pathological processes such as diabetes, hypertension, obesity, and dyslipidemia are linked to CRS, which involves systemic inflammation[61]. Furthermore, the activated RAAS and SAS, as well as the hemodynamic derangements observed in CRS, may be additional sources of inflammatory mediators[61]. In the kidneys, tumor necrosis factor alpha and other pro-inflammatory cytokines have been shown to induce mesangial apoptosis, renal hypertrophy, ROS production, and fibrosis[62]. In the same way, these pro-inflammatory mediators are involved in LV remodeling, LV hypertrophy, and are detrimental to ventricular function[63].
A reduction in effective circulation fluid volume in HF is associated with reduced renal blood flow (RBF), which stimulates renin release from the juxtaglomerular apparatus in the renal afferent artery. Activation of the RAS generates sodium and volume retention, arterial vasoconstriction, reduced glomerular perfusion and the release of aldosterone. These hemodynamic changes may worsen HF and renal function, which directs therapy towards the improvement of cardiac indices. However, this association between cardiac output and renal function has not always been demonstrated in the clinical setting. In fact, there are two HF clinical pictures, HFrEF and HFpEF which accounts for more than 50% of all HF[64].
Furthermore, when the risk factors for acute WRF (AWRF) in patients with acute decompensated HF were studied, a history of hypertension but not elevated blood pressure at admission was an independent risk factor for HFpEF. In contrast, diastolic blood pressure at admission was the only risk factor observed for AWRF in the HFrEF group[65]. In addition, analyses of the ESCAPE database revealed that baseline hemodynamics or the change in hemodynamics parameters was not correlated with WRF and clinical outcomes[66]. From the above, we can conclude that the mechanisms linking HF and WRF are multiple and not fully known.
It has been suggested that venous congestion may be a critical factor in CRS that impairs renal function. In patients with advanced decompensated HF, venous congestion was the most important hemodynamic factor associated with WRF measured as central venous pressure (CVP)[67]. Also, during the ten-year follow-up of a broad spectrum of cardiovascular patients, increased CVP was associated with impaired renal function all-cause mortality. In summary, fluid overload and increased CVP resulted in decreased renal perfusion and reduced GFR that results from the activation of deleterious neurohormonal pathways[68].
Atrial natriuretic peptide (ANP), which is a peptide produced by cardiomyocytes in response to myocardial stretch, activates the guanylyl cyclase A receptor (GC-A), whereas ANP is degraded by enzymes like neprilysin (NEP) and insulin-degrading enzyme[69]. ANP regulates the volume and pressures by inducing natriuresis and vasodilatation. ANP also antagonizes the RAAS system by inhibiting renin secretion, aldosterone production and SNS activation[70]. Wang et al[71] reported that obese individuals had low plasma N-ANP compared with lean individuals. Furthermore, in a group of ADHF patients, serum levels and the effect of ANP were inversely related to BMI[72]. Hypertension, HF, obesity and metabolic syndrome are processes characterized by ANP deficiency or insufficiency, which makes ANP a key target for the treatment of cardiorenal metabolic disease.
The increase in insulin resistance with obesity and adiposity is well established. However, there is no linear relationship between insulin resistance levels and BMI. It has been published that resistance levels in severe obesity with BMI above 45 are similar to the values found in individuals with moderate BMI[9]. These results suggest that the main determinants of insulin resistance are trunk obesity or adiposity in organs and tissues rather than the global and subcutaneous fat tissue.
Insulin resistance is considered pivotal in the pathogenesis of cardiorenal metabolic syndrome, being at the center of obesity, diabetes mellitus, hypertension, hyperinsulinemia, dyslipidemia and albuminuria[73]. The specific clinical condition associated with insulin resistance obesity is insulin resistance, and the physiopathology picture is RAAS activation, oxidative stress, and endothelial dysfunction[74]. The latter evidenced as lower NO production and augmented endothelial-derived inflammatory markers. Decreased NO production by impaired insulin signaling and the destruction of NO by oxidative stress lead to a decrease in available NO, the mechanism by which vasodilator response is altered[42]. The pathophysiological picture of impaired NO-mediated vasodilatation, endothelial-damaged lost, microvascular remodeling, pro-inflammatory and prothrombotic states lead to fibrosis in the heart and the kidneys, which has been suggested to be the underlying causes of cardiorenal metabolic syndrome[75]. One of the pathways that disturb insulin signaling is mTOR/S6K1 signaling, which with excessive activation, can lead to impaired insulin metabolic signaling by phosphorylation of IRS-1 and IRS-2. This hyperactive mTOR/S6K1 signaling was also associated with dysfunction of endothelial cells, vascular smooth muscle cells and cardiomyocytes[76].
Activation of vascular MRs was associated with impairment in IRS-1/PI3K/AKT signaling, lowering endothelial NO. This signaling pathway is activated by insulin metabolic action and flow-mediated shear stress. Endothelium Na+ channel (EnNaC) activation by endothelium MR stimulation increases Na+ entry, promotes actin polymerization, and stiffness of the cellular membrane. EnNaC and MR activation results in a further decrease in NO production and arterial stiffness[77]. Also, endothelial MR and low NO promote translocation of transglutaminase 2 to the extracellular matrix where it crosslinks several substrates, including collagen, which promotes CV fibrosis and maladaptive remodeling[78].
It should be mentioned that the metabolic syndrome is to be a new target in the management of CRS due to new drugs with cardiovascular, renal, and metabolic effects[79].
Adiponectin is a hormone produced by adipose tissue with anti-inflammatory, insulin-sensitizing, and vascular effects. Adiponectin has been inversely correlated with body weight, visceral fat, cardiac disease, endothelial dysfunction, renal dysfunction, and proteinuria[80]. By contrast, CKD and end-stage CKD were associated with high serum levels of adiponectin. The vascular protective effect of adiponectin in the setting of renal dysfunction remains controversial[81].
Leptin is a hormone produced by fat cells and enterocytes, implicated in the regulation of energy balance. The leptin receptor is located in the CNS and other body organs such as the kidneys, inducing natriuresis, insulin sensitization, vascular dilatation, NO production, and increased heart rate and blood pressure[82]. Therefore, the kidneys are not only leptin-clearance organs but also a target of leptin action. In addition, experimental and human obesity are associated with increased serum levels of leptin, adrenergic activation rises in arterial pressure, and relative renal resistance being a risk for WRF[83]. Although leptin has been suggested as being a link between obesity and CRS, the underlying pathways and mechanisms remain to be elucidated[84].
The metabolic activity of more extensive adipose tissue in obesity-related insulin resistance disorder, generates a flux of free fatty acids (FFA)[85]. This FFA-induced lipotoxicity inhibits insulin-stimulated glucose uptake, and insulin-mediated vasodilatation[86]. The insulin-resistance of the glucose-lowering effect impairs NO production by endothelial cells which might contribute, at least partly, to vascular stiffening[87].
Many studies have linked vascular risk with BMI, giving results that may be contradictory. It has been seen that BMI is very heterogeneous and that similar values of BMI are related to very different cardiovascular co-morbidity. For many years, it has been well established that abdominal, central, or visceral obesity is an independent cardiometabolic risk factor. In addition, the meta-analysis carried out by Mottillo et al[88] showed that metabolic syndrome, including abdominal circumference, was associated with an increased risk of CVD and cardiovascular mortality (RR = 2.40; 95%CI: 1.87 to 3.08). Variations in VAT have shown to be more relevant than BMI measures in the cardiometabolic effect of intervention measures, and this relationship was independent after adjustment for BMI. This heterogeneity of the BMI, especially in patients with severe obesity, when quantifying risk and cardiovascular disease, has prompted the study of methods that allow quantification of the different compartments of fatty tissue in the body. Recently, the relationship between VA measured by standard techniques such as NMR and CT and the different cardiometabolic parameters has been reported. Also, it has been observed that 25% of variations in abdominal or visceral fat, which are seen when losing 5%-10% of body weight, are associated with a significant decrease in cardiovascular risk factors.
VA has been shown to be associated with cardiac and vascular remodeling, fibrosis, and diastolic dysfunction, and these structural and functional changes were associated with blood fibrosis biomarkers[89]. In addition, Lee et al[90] demonstrated that LV mass and geometry worsened over the period of observation in obesity and that the waist-to-hip ratio and waist-to-height ratio remained significantly associated with ventricular mass even after adjusting for BMI. However, VA also includes VAT and ectopic fat or adipose tissue inside or surrounding the organs such as the pancreas, kidney and heart. In this regard, most studies have found an association between non-alcoholic fatty liver disease (NAFLD) and CVD or mortality, but the association disappears when it is adjusted for abdominal circumference and other metabolic variables. However, the severity of NAFLD has been associated with aortic stiffness, a surrogate CV risk factor, and independently of the abdominal circumference. This data suggested the independent role of liver fat in the pathogenesis of cardiovascular disease[91].
Furthermore, in 2529 participants from the Framingham Heart Study[92], the fat depots were evaluated by TC and RMN and were associated with cardiometabolic risk factors. The VA and intrahepatic fat, but not SA, were more robust and continuously correlated with most of the risk factors after further adjustment for BMI and waist circumference. The authors suggested that VA and intrahepatic fat were correlates of cardiometabolic risk factors, above and beyond standard anthropometric indices such as BMI and SA[92].
Obesity is also considered a major risk factor for HFpEF, which is being associated with morbi-mortality[93]. HFpEF is the most common phenotype in the community, mostly associated with adiposity and aging. Data from more than 100000 patients hospitalized for acute decompensated HF have also shown that HFpEF represents 50.4% of patients with an in-hospital assessment of LV function[94]. The study of vascular structures and function among HFpEF patients revealed an obesity-related HFpEF phenotype, which has increased plasma volume, increased concentric LV remodeling, greater right ventricular dilatation, and augmented epicardial fat thickness[95]. This increased heart volumes and greater pericardial restrain augmented ventricular interdependence. The RV to LV filling pressure relative to transmural pressure has led to higher biventricular filling and pulmonary venous pressures[95]. In addition, in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial, similar echocardiographic findings with plasma volume expansion and enlarged left atrial size have been reported[96]. In addition, obesity-associated high NEP levels and low natriuretic peptides might also contribute to the volume expansion observed in patients with HFpEF[97].
The increased prevalence of CKD is around 10%. It has been demonstrated that CKD increases CVD and death[98]. Also, a recent meta-analysis from nine studies showed that VA measured by waist-to-height-ratio was a better predictor of CKD relative to other physical measurements[99]. Adiposity measured by BMI and waist circumference impair kidney function by inducing hyperfiltration, increasing glomerular capillary wall tension, and podocyte stress acting both directly and through obesity-related diabetes and hypertension, the two most common risk factors for end-stage kidney disease[100].
The mechanisms that link excess of adiposity, systemic and visceral, and renal damage have implicated neurohumoral pathways such as inflammation, oxidative stress, impaired lipid metabolism, endothelial dysfunction, and activation of the RAS and production of aldosterone and fibrosis. Early in the development of obesity, there is a decline in the renal plasma flow that deteriorates renal hemodynamics[101]. In this setting, the GFR is maintained by either efferent vasoconstriction or afferent vasodilatation[102]. The rise in glomerular pressure in turn affects hyperfiltration and albuminuria, expansion of mesangial matrix, podocyte disorder, and interstitial fibrosis. Finally, the damaged glomerular basement membrane and dysfunctional podocytes deteriorate renal function, declining GFR which is associated with nephron loss that predisposes to AKI or CKD. In this regard, there are many clinical studies that have reported the correlation between visceral and retroperitoneal fat and WRF[103].
Ectopic adiposity with systemic repercussions observed in non-alcoholic steatohepatitis with or without advanced fibrosis was associated with a higher prevalence and incidence of CKD. The magnitude of this damage was positively associated with each CKD stage, but unaffected by diabetes status[104]. Perinephric fat acting locally contributed to renal dysfunction and fibrosis[105]. In addition, in the Framingham Heart Study, the perinephric fat size was an independent risk factor for hypertension and CKD[106]. In addition, renal sinus fat was correlated with the number of prescribed antihypertensive medications, stage II hypertension, and renal size[107].
Furthermore, the perinephric and visceral fat may be a source of local lipid-induced damage and a source of adipokines such as leptin, adiponectin and FGF21. Overall, vascular dysfunction and tubule-interstitial fibrosis have been proposed as the key mechanism implicated in the progression of obesity-related kidney disease. Accordingly, in vitro and in vivo studies showed that chronic stimulation of G protein-coupled receptors (GPCRs) as well as endothelin G-protein beta-gamma-subunit (Gbetagamma) signaling were associated with experimental CRS and pathological fibroblast activation. These data suggested GPCR-Gbetagamma inhibition as a novel therapeutic approach for treating CRS and AKI[108].
The concept of metabolically healthy obesity (MHO) implies a subgroup of obese individuals with few metabolic risk factors, in contrast to metabolically unhealthy obesity (MUO) which is equivalent to metabolic syndrome[109]. In the Framingham Heart Study offspring cohort participants who were followed up for more than 15 years, there was a modest, positive association of obesity with CVD and CKD. The association of metabolically unhealthy with CVD was more robust than being obese, but it was comparable with incident CKD[110]. In a population-based prospective study, during 12 years of follow-up, the risk of HF was similarly increased in MH and metabolically unhealthy in moderate obesity (BMI < 35)[111]. Although the risk of incident CKD in MHO was slightly higher than that in non-obese MH, the evolution to a metabolically unhealthy status increases the risk for incident CKD[112]. Individuals characterized as MHO constitute a dynamic and heterogeneous group with a low-risk cardiorenal phenotype but not an absence of risk for HF and CKD.
The relationships between obesity and CRS are not always linear. A recent meta-analysis has shown that the relationship between BMI and HF risk has a J-shape, where there was a non-significant slightly greater risk of incident HF between underweight and overweight individuals[113]. Similarly, it has been observed that there are some clinical situations such as HF or CKD in which obesity improves survival, which has been called the obesity paradox. A sub-analysis of the MAGGIC meta-analysis has shown that BMI was paradoxically associated with survival rates in both HFpEF and HFrEF patients, and demonstrated that mortality risk had a U-shaped curve with a nadir at 30.0-34.9 kg/m2[114]. The paradoxical decline in mortality rate with increasing BMI and the U-shape curve for CV mortality was also confirmed recently[113]. Although obesity and increased adiposity are a risk factor for the incidence and progression of CKD, the relationship of BMI with survival in both CKD and end-stage renal disease has also been reported as a U-shaped curve, where higher BMI values have a potential protective effect[115].
Obesity is associated with type 2 diabetes, CVD, diminished life expectancy, and impairment in quality of life. The analysis of cardiometabolic risk factors in morbid obesity revealed that hypertension, CRP, and family history were more prevalent in a patient with BMI greater than 45[9]. In the same study, there was also an increase in waist circumference, body fat, visceral fat area, and systolic blood pressure in the highest BMI and suggested an enhanced risk for the occurrence of CVDs[9]. In fact, the mortality rate in morbidly obese patients without effective treatment was remarkably higher than that observed in surgically treated patients[116].
Several specific markers of inflammation and fibrosis have been specifically associated with morbid obesity. Growth differentiation factor 15, which is a marker of CVD and diabetes, has been seen to increase in severe obesity and correlates with abdominal circumference and glucose metabolism parameters[117]. GlyA, a marker of systemic inflammation and associated with cardiovascular risk and mortality, has been shown to increase in severe obesity. The values of GlyA normalized in 77 severe obese patients (BMI: 44.1 ± 6.4 kg/m2) one year after surgery, and more than 41% of patients still had a BMI > 30[118]. In addition, in a cohort of 36258 type 1 patients followed-up over 9.7 years, severe obesity (BMI > 35 kg/m2), but not moderated obesity, was associated with the development of atrial fibrillation[119]. Figliuzzi et al[120] studied 24 h, day-time and night-time systolic/diastolic BP levels in a large cohort of adult outpatients with different classes of BMI and controls. BMI was significantly and independently related to the clinic (r = 0.053; P < 0.001), 24 h (r = 0.098; P < 0.001) and night-time systolic BP (r = 0.126; P < 0.001), and LV mass indexed by height (r = 0.311; P < 0.001).
The cardiorenal obesity syndrome with HFpEF in morbid obese patients could predispose to AKI, right-side volume overload, and right-side volume overload in the setting of complications such as sepsis or infection. This type of CRS carried a significant mortality rate of 50%[121]. In fact, echocardiographic and hemodynamic assessment of severe obesity with HFpEF showed increased plasma volume, more concentric LV remodeling, more right ventricular dysfunction, increased epicardial fat thickness, than that in non-obese HFpEF, supporting the previously mentioned concept of obese-specific HFpEF[95].
Obesity is a risk factor for incident HF, and weight loss is an established approach for preventing or managing HF, with a role for bariatric surgery in patients with severe obesity. However, weight loss for patients with existing HF and obesity is a more controversial topic owing to an obesity survival paradox[122]. Dietary interventions and pharmacologic weight-loss therapies are understudied in HF populations and with modest weight reduction success[123]. However, given the challenges in morbid obese patients with CVD or HF syndromes in particular, the optimal treatment, which includes bariatric surgery should be considered in a multidisciplinary team approach in order to indicate surgery for these patients. Cohort studies, and head-to-head clinical trials, randomized and non-randomized, comparing medical vs surgical weight loss in severe/morbid obesity have demonstrated the superiority of surgical procedures in controlling diabetes mellitus, cardiovascular events and mortality rate[124]. Meta-analyses involving 29208 patients who underwent bariatric surgery and 166200 controls showed that bariatric surgery reduced total mortality by 50%, the risk of composite cardiovascular adverse events (OR = 0.45), myocardial infarction (OR = 0.46), and stroke (OR = 0.49)[125]. These data indicate that patients undergoing bariatric surgery have a reduced risk of cardiovascular events, but HF and hospitalization for HF have not been extensively studied in RCTs or meta-analyses.
Thus, we will focus on the main studies on the effect of bariatric surgery on HF outcomes in morbidly obese patients. The studies regarding bariatric surgery in severely obese patients with HF are summarized in Table 3. In patients with HF, obesity surgery has been shown to improve LV remodeling[126], reduce LV mass in HFrEF[127] and in normotensive asymptomatic morbid obesity at risk of HFpEF[128]. Thus, bariatric surgery was able to reduce the need for hospitalization and emergency department visits, improving functional capacity and quality of life along with the control of blood pressure, diabetes mellitus, and the metabolic syndrome components[129,130]. In addition, bariatric surgery in advanced HF may avoid the need for immediate transplants or LV assist devices as well as cardiac transplantation[131].
| Year, country | ParticipantsSurgical/Control | Follow-up Surgical procedures | HF type | HF and LV outcomes | |
| Alpert et al[190] | 1985, United States | 62 vs none | 4.3 ± 0.3 mo | NA | A decrease in LV dimensions |
| Surgical gastric restriction. | (↑ LVS, ↑ LVpW) | Lower mean blood pressure | |||
| Ramani et al[131] | 2008, United States | 12 vs 10 | 1 yr | HFrEF (treated) | Lower hospital readmission |
| Mostly LRYGB | LVEF improved | ||||
| NYHA improved | |||||
| Miranda et al[130] | 2013, United States | 13 vs 6 | 4.3 yr | HFrEF 77% | Better Quality of life |
| Mostly RYGB | HFpEF 23% | Better functional capacity | |||
| Less leg edema | |||||
| Vest et al[127] | 2016, United States | 38 vs 2588 non surgical obese | 2.6 yr | HFrEF | Improvement in LVEF; 28% improved; LVEF > 10% vs < 1% control |
| RYGB, AGB, SG | |||||
| Shimada et al[126] | 2016, United States | 524 vs none | 2 yr | NA | Lower rate of HF exacerbations (ED visits), 1 to 2 yr after surgery |
| Lower rate of hospitalizations | |||||
| Berger et al[191] | 2018, Switzerland | 676 (meta-analysis of surgery vs conventional treatment) | NA | NA | HR for the incidence of HF in MO without pre-existing HF 0.44 (0.36, 0.55) vs conventional treatment |
| Reduced ED visits and readmission | |||||
| Increase left ventricular ejection | |||||
| Improve the quality of life and symptoms |
The obesity-related cardiometabolic and renal pathways have been previously mentioned. Several specific effects have been reported in morbid or severe obesity with WRF and hypertension. Despite early reports of the renal effect of obesity, most of our data are from bariatric surgery clinical trials, with few data from experimental studies. Morbid obesity might exert specific alterations based on the amount of adiposity. Increasing abdominal and retroperitoneal fat mass can physically compress the kidneys[132]. The high intraabdominal pressure compresses the veins, lymph vessels and ureters; it increases intrarenal pressure and elevates interstitial hydrostatic pressure[133]. This increase in pressure can compromise or reduce tubular blood flow and increase sodium tubular reabsorption reducing tubular sodium delivery. Finally, activation of the RAS, vasodilation of the afferent arterioles, increased intraglomerular pressure and glomerular hyperfiltration result in arterial hypertension and WRF[133]. In addition, the equation for eGFR based on creatinine may overestimate renal function in patients with significant weight reductions which occurs after bariatric surgery, likely due to changes in muscle mass. Therefore, measured GFR (mGFR) adjusted for body surface area, which appears to be unchanged, along with cystatin C based equations have been recommended[134].
With regard to cardiovascular complications in severe obesity, the only proven strategy to reduce visceral, retroperitoneal fat mass and renal fat and their adverse effects on renal function in severe/morbid obesity is bariatric surgery. Thus, we will focus on the main studies on the effect of bariatric surgery in morbidly obese patients on renal outcomes. Studies on the effects of bariatric surgery on kidney function are summarized in Table 4. Bariatric surgery in patients with previous preserved kidney function reduces the risk of worsening eGFR, end-stage renal disease[135], and reduces albuminuria by lowering systemic inflammation[136]. Also, metabolic parameters have been associated with improvement in renal function after surgery[137]. In addition, improvements in vascular indices such as a renal resistive index or carotid intima-media thickness were found to increase GFR following surgery[138].
| Authors | Year, countryFollow up | PatientsSurgical/control | Surgical procedure | Diabetes, CVD, RD | Outcomes |
| Serra et al[192] | 2015, Spain (76 ± 42 mo) | 92 vs none | GB | D2: 14% | No WRF |
| Renal biopsy | Glomerulopathy 75% | A decrease in creatinine and albuminuria | |||
| No progression (not related to glomerular lesions) | |||||
| Neff et al[142] | 201, France (1 and 5 yr) | 190 vs 271 | RYGB vs | D2: 39%. CVD: | Improvement in eGFR in both procedures |
| LAGB | 28%. CKD: 4% | ||||
| RYGB better in remission of hypertension | |||||
| RYGB better in diabetes | |||||
| Nehus et al[143] | 2017, United States | 242 vs none | 3 yr | D2: 12.6% | eGFR increased by 3.9 mL/min per 1.73 m2 for each 10-unit loss of BMI. |
| RYGB 66.5% | Albuminuria: 17% | ||||
| SG: 27.7% | A decrease in ACR | ||||
| AGB: 5.8% | |||||
| Wakamatsu[141] | 2018, Japan | 254 | LSG 24 | D2: 51% | Improvement of eGFRcys in mild CKD (eGFRcys ≥ 60 mL/min per 1.73 m2) |
| LSG-DJB 94 | |||||
| LRYGB 26 | |||||
| LAGB 10 | |||||
| NS: eGFRcys in moderate CKD (< 60 mL/min per 1.73 m2) | |||||
| Solini et al[138] | 2019, Italy | 25 vs none | 1 yr | No D2. No HTA | Improvement in mGFR |
| RYGB | Improvement in a renal resistive index and correlates with mGFR | ||||
| Lowers carotid intima-media thickness | |||||
| Inge et al[144] | 2019, United States | Adoles vs adults | 5 yr | D2: 14% vs 31% | HTA and D2 remissions are higher in adolescents than in adults. Rate of death (NS) |
| 161 vs 396 | RYGB | HTA: 30% vs 61% |
In patients with established or advanced renal disease following bariatric surgery, although there may be an increase in early postoperative renal complications[139], the long term effects have been demonstrated to improve albuminuria, hypertension and further renal stabilization in renal disease[140]. However, in a Japanese cohort who underwent bariatric surgery, no benefit due to surgery was found in those with more advanced renal disease[141]. Indeed, there were observed differences among surgery procedures regarding renal response, with Roux-en-Y gastric bypass surgery having better results than adjustable gastric banding[142].
Also, there were differences in the age of morbidly obese patients regarding bariatric surgery outcomes. First, Nehus et al[143] reported a significant improvement in terms of eGFR and proteinuria in morbidly obese adolescents undergoing bariatric surgery with preoperative renal disease. Moreover, Inge et al[144] showed that 5 years after bariatric surgery, the remission rate of hypertension and diabetes was greater in adolescents than in adults. These data suggested that excess of adiposity seen in morbid obesity predispose to renal disease and early intervention appears to improve the outcome.
There are no specific treatments for the CRS, therefore they are usually based on the underlying pathophysiological alterations in each type and the timeframe of development. Diuretics, ultrafiltration (UF), RAS inhibitors, vasodilators, and inotropes are mainly used in CRS.
Diuretics play a primary role in the management of patients with CRS, both acute and chronic. The effectiveness of volume overload management with diuretics decreases with the progression of HF along with CKD leading to complex and challenging scenarios. Firstly, diuretic resistance is thought to result due to neurohormonal activation along with vascular, tubular, and glomerular effects[145]. Diuretic resistance is associated with the worst prognosis and an increase in hospitalization and cardiovascular mortality[146]. Despite the optimal dose of diuretic therapy, the Diuretic Optimization Strategies Evaluation (DOSE) trial found that 42% of patients died, were rehospitalized, or had an emergency department visit within the 60-d follow-up period[147]. Diuretic therapy has been shown to be insufficient in the acute management of HF, which has led to additional treatments such as dopamine, vasopressin, and adenosine A1 antagonists, and extracorporeal UF. Although these strategies improve liquid overflow in the short-term, they have shown conflicting long-term outcomes. Secondly, greater volume expansion and renal perfusion pressure along with right ventricular dysfunction and ventricular interdependence are characteristic of HFpEF associated with obesity where decongestion might undermine cardiorenal function. In acute decompensated HFpEF obese patients from several trials, Reddy et al[148] showed that decongestive therapies increased the incidence of WRF by 2-fold and the incidence of severe WRF (9% vs 0%, P = 0.002) compared to non-obese patients and demonstrated that there were no differences in survival and rehospitalization in obese as compared to non-obese patients with HFpEF. According to these data, in a post hoc analysis of the DOSE study[149], the composite outcome of death, hospitalization or an emergency room visit was strongly related to improvement in renal function, whereas a linear increase in creatinine was paradoxically associated with improved outcomes. The authors suggested that some patients with improved creatinine levels were suboptimally decongested, and changes in serum creatinine did not reflect poor outcomes and should be evaluated in the context of the clinical picture. Thus, in the short-term effects of medication omission, reductions in serum creatinine by 8% were associated with increases in NT-proBNP and left atrial volume[150].
Another class of new drugs that could be effective in patients with HF are the NEP inhibitors, which increase the natriuretic peptides and vasodilators. In a prospective randomized controlled trial of 8442 patients with HFrEF, the angiotensin-receptor NEP inhibitor (ARNi) sacubitril/valsartan was superior to enalapril in reducing the risk of death due to cardiovascular causes (HR = 0.8, CI: 0.71 to 0.89) and reduced the risk of hospitalization for HF by 21%, compared with enalapril[151]. By contrast, in the PARAGON-HF trial, sacubitril-valsartan did not result in a significantly lower rate of total hospitalization for HF and death from cardiovascular causes among patients with HF and an ejection fraction of 45% or higher, although, the difference was of borderline statistical significance[152]. However, in a post hoc analysis of the PARAGON-HF trial, when compared with valsartan, sacubitril-valsartan seemed to reduce the risk of HF hospitalization and death more in women (HR = 0.73 95%CI: 0.59-0.90) than in men (HR = 1.03 95%CI: 0.84-1.25). This study also reported that the women in this study were older, more were obese (BMI > 30, women, 51.3% vs male, 46.7%) more had VA (women, 82.7% vs male, 61.6%) and had lower eGFR than men[153].
RAS inhibitors also play a key role in the management of not only hypertension, CKD and HF but also in the framework of metabolic and functional processes such as obesity, proteinuria or albuminuria and diabetes mellitus. However, despite the guidelines, clinicians are reluctant to use this class of drugs in patients with CRS and CKD in which WRF defined by increasing serum creatinine is usually seen after the initiation of RAS inhibitors. It has been previously suggested that early WRF in the setting of angiotensin converting enzyme inhibitor (ACE-I) introduction might not only result in the absence of adverse prognostic significance but also a survival advantage in those who remained on enalapril therapy[154]. However, despite RAS inhibition-induced WRF in patients with HFrEF, this was associated with a lower increased relative risk of mortality. By contrast, in patients with HFpEF, RAS inhibitor-related WRF was strongly associated with worse outcomes compared with placebo[155]. Overall, an early decrease in GFR after initiation of RAS inhibitors might represent impaired renal perfusion and not real kidney damage, maintaining the long-term cardiac and renal function stabilizing effect in the CRS[156]. Finally, the demonstrated effects of RAS inhibitor on survival and morbidity in HFrEF have seen its use recommended in numerous guidelines[157], but it contrasts with a lack of definitive benefit for patients with HFpEF.
The aldosterone-related effects in promoting the development of interstitial cardiac fibrosis, platelet aggregation, endothelial dysfunction, concentric LV hypertrophy, hypertension and CKD indicated the possibility of MR as a key target in the CRS. Clinical trials on the MA antagonist (MRA) on cardiorenal endpoints in obesity are summarized in Table 5. Obesity-related activation of RAAS and augmented aldosterone production along with an increase in MR signaling increase the risk of hypertension and CRS[158]. MRA therapy improves mortality in patients with chronic HFrEF and mild symptoms (EMPHASIS-HF trial)[159], in acute symptomatic post-myocardial infarction HFrEF (EPHESUS trial)[160] and in severe NYHA class III–IV systolic HF (RALES trial)[161]. Also, in a recent post hoc analysis of the EMPHASIS-HF trial, the benefit of eplerenone was greater in patients with abdominal obesity and HFrEF[162]. However, MRA failed to decrease mortality and the primary composite outcome in HFpEF patients in the TOPCAT trial[96]. However, in a recent meta-analysis, Kapelios et al[163] reported that MRA treatment improved indices of cardiac structure and function and suggested that MRA decreased LV filling pressures and improved cardiac remodeling. In a post hoc analysis of the TOPCAT trial based on plasma biomarkers, echocardiographic measurements and clinical features, Cohen et al[164] identified phenogroup 3 with the highest significant reduction in the risk of the primary endpoint (HR = 0.75; 95%CI: 0.59 to 0.95). This phenogroup 3 exhibited more functional impairment, obesity, diabetes, CKD, LV hypertrophy, high renin, and biomarkers of inflammation and fibrosis. Furthermore, MRA added to ACEI/ARB reduced urinary protein/albumin excretion in diabetic nephropathy[165]. The side effects of hyperkalemia and WRF explain the underuse and discontinuation of these drugs during the treatment of CRS[166]. Therefore, a new potassium binder patiromer was associated with less hyperkalemia in patients with CKD treated with higher spironolactone doses[167].
| Trial | n (follow-up) | BMI > 30 % | eGFR % < 60 mL/min | CVD(%) vs HF(%) | DM2 | CV and RO (HR, significant) |
| EMPHASIS-HF[159] (eplerenone vs PBO) | 2737 (21 mo) | 27% | 33% | 70% (IHD) | 31% | CVO1,2,3,4,5 |
| HFrEF (NYHAII) | RO: NS | |||||
| High WC: Greater benefit of eplerenone[163] | ||||||
| TOPCAT[96] (spironolactone vs PBO) | 3445 (3.3 yr) | 50% | 39% | 59%(IHD) | 32% | CVO4 |
| HFpEF (NYHAII-IV) | ||||||
| TOPCAT post hoc[193] (BMI&NP categories) | 997 (3.3 yr) | NR | NR | NR | NR | High BMI/high NP1,4,5 |
| High NP5 | ||||||
| TOPCAT post hoc[166] (eGFR categories) | 1767 (3.3 yr) | 70% | 53.4% | MI (20.3%) | 44.5% | AE increased with declining eGFR eGFR ≥ 60 vs eGFR ≤ 451,2,4,5 |
| FIDELIO-DKD[170] (finerenone vs PBO) | 5734 (< 48 mo) | 58% | 87% | 45.9% & 7.5 (HFpEF) | 100% | Outcomes expected in 2020 (composite RO and secondary endpoints CV ) |
| FIGARO-DKD[171] (finerenone vs PBO) | 7437 (< 53 mo) | 60% | 38% | 44.3% & 7.6% (HFpEF) | 100% | Outcomes expected in 2021 (composite RO and secondary endpoints CV ) |
| AMBER[167] (patiromer vs PBO) | 295 (3 mo) | NR | 100% | 19.3% (MI) & 45% (HF) | 49.1% | Les hyperkaliemia |
| Less Spironolactone withdrawal |
Recently the new non-steroidal MRA finerenone has been evaluated in the CRS. In the Mineralocorticoid Receptor antagonist Tolerability Study-Heart Failure (ARTS-HF) trial with the primary outcome of NT-proBNP decrease and exploratory endpoint of death and cardiovascular events, finerenone achieved the primary and composite outcomes slightly better compared with eplerenone group[168]. Furthermore, in the ARTS-DN trial[169], finerenone improved the urinary albumin-creatinine ratio in diabetic nephropathy patients receiving ACE inhibitors or AIIR blockers without worsening renal function. In both studies, hyperkalemia was reported in the highest doses of finerenone. Two relevant clinical trials are ongoing in patients with CKD, the FIDELIO-DKD[170], and the FIGARO-DKD[171], which were designed to assess renal and CV outcomes in patients with type 2 diabetes and CKD.
The set of cardiac and renal pathophysiological alterations that characterize obesity-related cardiometabolic and renal syndrome, which includes diabetes mellitus and hypertension, are important for establishing therapeutic targets. The hypervolemic state, cardiomegaly and worsening intracardiac filling pressure in the obese patient might be treated with diuretics and with the new antidiabetic volume-reducing therapies, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors. In this regard, the new diabetic therapies glucagon-like peptide-1 (GLP-1) receptor agonists, which were shown to reduce both global and ectopic fat mass such as epicardial and renal fat, were effective in treating CRS. In addition, bariatric surgery has previously been reported to improve functional capacity in the heart and the kidneys. Studies regarding the effects of SGLT2 inhibitors on the cardiorenal endpoints are summarized in Table 6.
| Trial | n (follow-up) | BMI > 30 | eGFR < 60 mL/min per 1.73 m2 | CVD and HF | Diabetes | CVO and RO (HR; significant) |
| EMPA-REG[175] (empagliflozin vs PBO) | 7020 (3.1 yr) | 51% | 25.9% | 99.2% and 10.1% | About 100% | CVO1,2,3,4 RO6,7,8,9,10 |
| CANVAS[172] (canagliflozin vs PBO) | 10142 (2.4 yr) | 59% | 20,1% | 65.6% and 14.4% | About 100% | CVO1,3 RO6,7,8,9 |
| DECLARE-TIMI[174] (dapagliflozin vs PBO) | 17160 (4.2 yr) | 60% | 7.4% | 40.6% and 10% | About 100% | CVO1,3 RO6,7 |
| CREDENCE[173] (canaglifozin vs PBO) | 4401 (2.6 yr) | 54.4% | 60% | 50.4% and 15% | 52% | RO6,7,8,9 CVO1,2,3,4 |
| DAPA-HF[176] (dapagliflozin vs PBO) | 2373 (18.2 mo) | 35% | 26.1% | 55.5% (IHD) and 100% (HFrEF) | 41% | CVO1,2,3 RO: NS HFrEF: Better dapagliflozin |
| DAPA-CKD[194] (dapagliflozina vs PBO) | 4304 (NA) | NA | About 90% | NA | Non-DM: ≥ 30% | Outcomes expected in 2020 (composite renal and secondary CV endpoints) |
| EMPEROR-Preserved[195] (empagliflozin vs PBO) | 5988 (NA) | NA | NA (eGFR ≥ 20) | HFpEF (100%) | NA | Outcomes expected late in 2020 (composite CV, HF and secondary R endpoints) |
| EMPEROR-Reduced[196] (empaglifozin vs PBO) | 3730 (NA) | NA | NA (eGFR ≥ 20) | HFrEF (100%) | NA | Outcomes expected late in 2020 (composite CV, HF and secondary RO) |
SGLT2 inhibitors, canagliflozin[172,173], dapagliflozin[174], and empagliflozin[175] have demonstrated cardiovascular and renal benefits in several large international cardiovascular outcome trials (CVOT) in both diabetic and non-diabetic patients[176]. A recent meta-analysis of the first three trials showed that SGLT2 inhibitors reduced the risk of myocardial infarction by 11%, cardiovascular death by 16% and all-cause death by 15%[177]. This study further demonstrated that SGLT2 inhibitors significantly reduced hospitalization for HF by 31% (HR: 0.61–0.79), in both patients with atherosclerotic disease and patients with multiple risk factors. Also, SGLT2 inhibitors were renoprotective and reduced the composite of WRF, ESRD, or renal death by 45% (HR = 0.55, 0.48-0.64) across all baseline eGFR levels, although this was greater in those with preserved renal function[177]. The CREDENCE trial specifically evaluated the renal endpoint of canagliflozin in patients with pre-existing renal disease, albuminuria and eGFR 30-89 mL/min[173]. In this study, canagliflozin reduced renal composite outcome by 30% (HR = 0.7; 0.59-0.82) and the secondary composite CV outcome (CV death, nonfatal MI, or nonfatal stroke) by 20% (HR = 0.8; 0.67–0.95). The DAPA HF trial was designed to evaluate the efficacy and safety of the SGLT2 inhibitor dapagliflozin in patients with HF and reduced ejection fraction[176]. This study showed that dapagliflozin reduced the primary composite outcome (worsening HF or cardiovascular death) vs placebo (HR = 0.74, CI: 0.65–0.85)[176].
Different mechanisms have been proposed by which SGLT2 inhibitors exert their cardiac and nephroprotective effects. Firstly, the augmented sodium delivery to the macula densa, by affecting the tubular-glomerular feedback loop, released vasoconstrictors which reduced the intraglomerular pressure and hyperfiltration without altering serum NO or activating RAAS[178]. Furthermore, in the RED study, a randomized controlled parallel study comparing dapagliflozin with gliclazide in patients with diabetes mellitus type 2, dapagliflozin reduced mGFR and filtration fraction without increasing renal vascular resistance or increasing urinary adenosine and prostaglandin concentrations[179]. Secondly, in streptozotocin-induced diabetic rats, Ojima et al[180] demonstrated that empagliflozin may inhibit oxidative, inflammatory, and fibrotic pathways in the kidneys partly via the suppression of oxidative stress and RAGE expression. Recently, it has been reported that SGLT2 inhibitors activated SIRT1/AMPK and suppressed Akt/mTOR signaling, the mechanisms by which these drugs might, at least partly, reduce oxidative stress, normalize mitochondrial structure and function, suppress inflammation, reduce coronary microvascular injury, enhance contractile performance, and ameliorate the progression of cardiomyopathy and nephropathy[181].
GLP-1 receptor agonists are a new class of drugs that lower HbA1c, reduce glucagon secretion, and lower blood pressure and body weight. Cardiovascular and renal outcomes have been studied in different trials and the results have been evaluated in a recent meta-analysis. The studies on the effects of GLP-1 antagonists on cardiorenal outcomes are summarized in Table 7. The combination of seven trials in 56006 participants showed that GLP-1 reduced major adverse cardiovascular events by 12% (HR = 0.88, 95%CI: 0.82-0.94; P < 0.0001), death from cardiovascular causes by 12% (HR = 0.88; 95%CI: 0.81-0.96; P = 0.003) and fatal or non-fatal stroke by 16% (HR = 0.84; 95%CI: 0.76-0.93; P < 0.0001)[182]. Interestingly, this study has shown that GPL-1 antagonists reduced hospital admission for HF by 9% (HR = 0.91; 95%CI: 0.83-0.99; P = 0.028), and a broad composite kidney outcome (development of new-onset macroalbuminuria, a decline in eGFR or increase in creatinine, progression to end-stage kidney disease, or death attributable to kidney causes) by 17% (0.83, 0.78-0.89; P < 0.0001), mainly due to a reduction in urinary albumin excretion[182]. Previously, GLP-1 receptor antagonists have been reported not to affect cardiovascular or mortality outcomes in patients with HFrEF after hospitalization[183]. Therefore, HF benefits were likely attributable to a reduction in myocardial infarction, weight loss, and blood-pressure-lowering effects. However, GLP-1 receptors are expressed in the kidneys and support the idea of a direct renal effect. Muskiet et al[184] first reported that intravenous exenatide administration in healthy overweight men acutely increased glomerular pressure in a NO-dependent manner and absolute and fractional sodium excretion in a NO-independent manner. In an in vitro study, Carraro-Lacroix et al[185] demonstrated that exedin-4 regulated sodium-hydrogen exchanger (NHE) 3 activity and suggested that GLP-1 receptor agonists modulate sodium homeostasis in the kidney by modifying NHE activity. Furthermore, it has been suggested that activation of the NHE in the heart and vasculature (NHE1 isoform) and the kidneys (NHE3 isoform) by neurohormonal derangements may serve as a common mechanism that links diabetes mellitus, obesity and CRS[186]. Taken together these data indicate that GLP-1 receptor activation might increase distal sodium transport to the macula densa, resulting in a reduction in intraglomerular pressure, and RAAS activation[187]. Finally, although the cardiorenal effects of SGLT2 inhibitors are much greater than those of GLP-1 receptor agonists, they might be an alternative in the case of intolerance or contraindication to SGLT2 inhibitors[188].
| Trial | n(follow-up) | BMI >30 % | eGFR %< 60 mL/min | CVD% vsHF% | DM2 | CVO & RO (HR, significant) |
| LEADER[197] (liraglutide vs PBO) | 9340 (3.8 yr) | 61% | 23.1% | 81% vs 14% (NYHAII-III) | ALL | CVO12 |
| RO6: Reduction in progression to Macroalbuminuria | ||||||
| FIGHT[183] (liraglutide vs PBO) | 300 (180 d) | 50% | 40% | 100% vs 100%HFrEF (NYHAIII-IV) | 59% | CVO:NS |
| RO: Increase in cystatin C in the liraglutide group | ||||||
| SUSTAIN-6[198] (Semaglutide vs PBO) | 3297 (2.1 yr) | 64% | 28.5% | 83% vs 24% | ALL | CVO1245 |
| RO6: Reduction in progression to macroalbuminuria | ||||||
| EXSCEL[199] (exenatide-ER vs PBO) | 14752 (3.2 yr) | 63% | 21.6% | 73% vs 16% | ALL | CVO: NS; RO: NA |
| HARMONY OUTCOMES[200] (Albiglutide vs PBO) | 9463 (1.6 yr) | 62% | NA | 100% vs 20% | ALL | CVO13; RO: NA |
| REWIND[201] (dulaglutide vs PBO) | 9901 (5.4 yr) | 46% | 22% | 31.5% vs 9% (NYHAII-III) | ALL | CVO14 |
| RO6: Reduction in Macroalbuminuria and eGFR (dulaglutide group) | ||||||
| PIONEER 6[202] | 3183 (1.33 yr) | 60% | 26.9%% | 85% vs 12% | ALL | CVO12 for noninferiority |
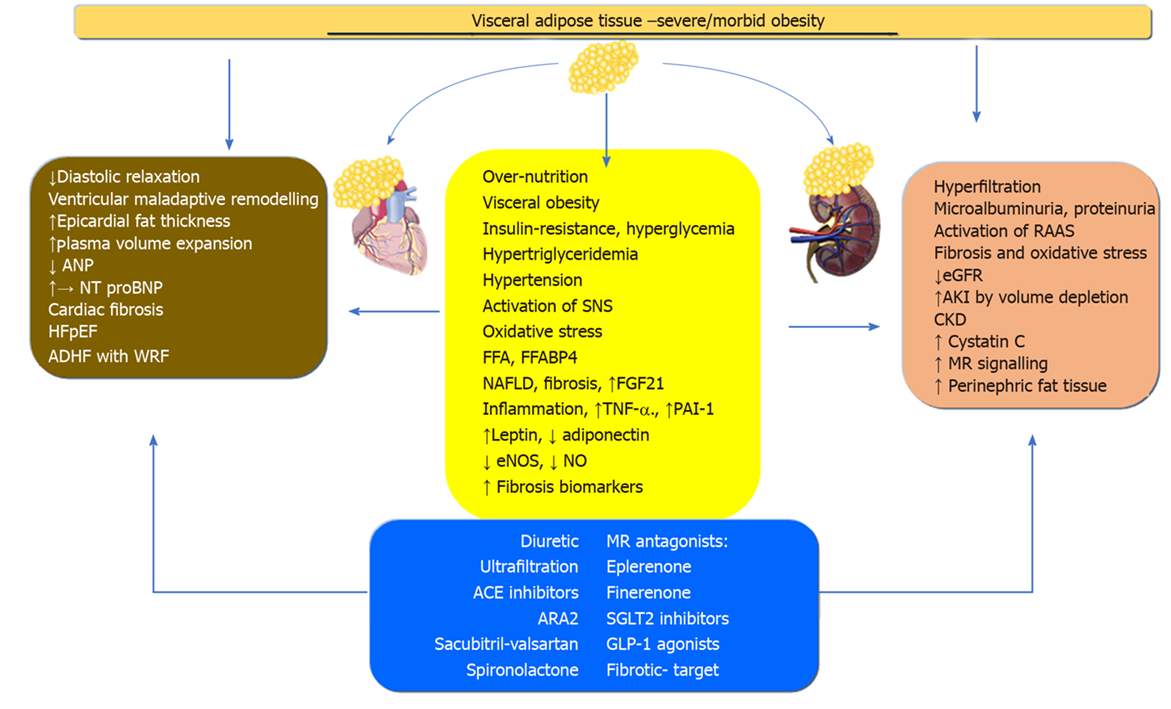
Obesity and obesity-related diabetes mellitus and hypertension contribute to the onset of CRS. Obesity is a heterogeneous and complex process in which a wide range of adiposity such as VAT, ectopic, subcutaneous or BMI level confers different associated morbi–mortality settings. It appears that most studies have linked VA to the onset and progression of HFpEF and CKD. In the initial stages, the activation of RAAS, inflammation, oxidative stress, and other neurohormonal mediators of fatty tissue acting systemically and locally, induce ventricular remodeling and glomerular hyperfiltration in the kidney. These pathophysiological pathways cause fibrosis and failure of the heart and kidney. Acute and chronic cardiovascular events increase the risk for WRF and conversely, acute and chronic renal events increase the risk of cardiovascular events, at least in diabetes. We propose obesity as a single cardiorenal umbrella, which includes the clinical spectrum of cardiac and renal disease along with obesity-related neurohormonal inflammatory, endothelial and fibrotic consequences (Figure 1). This concept is further supported by the development of new treatments for CRS in the context of metabolic disease, obesity, and type 2 diabetes mellitus. The new MRA finerenone, SGLT-2 inhibitors and GLP-1 agonists, with cardiovascular and renal outcomes, along with bariatric surgery in very severe obesity, have been shown to be a new and promising therapeutic strategy in patients with obesity or obesity-related CRS.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Majanovic SK, Stavroulopoulos A S-Editor: Ma RY L-Editor: Webster JR E-Editor: Ma YJ
| 1. | Hiuge-Shimizu A, Kishida K, Funahashi T, Ishizaka Y, Oka R, Okada M, Suzuki S, Takaya N, Nakagawa T, Fukui T, Fukuda H, Watanabe N, Yoshizumi T, Nakamura T, Matsuzawa Y, Yamakado M, Shimomura I. Absolute value of visceral fat area measured on computed tomography scans and obesity-related cardiovascular risk factors in large-scale Japanese general population (the VACATION-J study). Ann Med. 2012;44:82-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 2. | GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377:13-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3869] [Cited by in F6Publishing: 4184] [Article Influence: 597.7] [Reference Citation Analysis (2)] |
| 3. | Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, Pletcher MA, Smith AE, Tang K, Yuan CW, Brown JC, Friedman J, He J, Heuton KR, Holmberg M, Patel DJ, Reidy P, Carter A, Cercy K, Chapin A, Douwes-Schultz D, Frank T, Goettsch F, Liu PY, Nandakumar V, Reitsma MB, Reuter V, Sadat N, Sorensen RJD, Srinivasan V, Updike RL, York H, Lopez AD, Lozano R, Lim SS, Mokdad AH, Vollset SE, Murray CJL. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. 2018;392:2052-2090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1036] [Cited by in F6Publishing: 1062] [Article Influence: 177.0] [Reference Citation Analysis (0)] |
| 4. | Janssen I, Shields M, Craig CL, Tremblay MS. Changes in the obesity phenotype within Canadian children and adults, 1981 to 2007-2009. Obesity (Silver Spring). 2012;20:916-919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L; INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7301] [Cited by in F6Publishing: 7008] [Article Influence: 350.4] [Reference Citation Analysis (1)] |
| 6. | Vijayaraghavan K, McCullough PA, Singh B, Gupta M, Enas E, Mohan V, Misra A, Deedwania P, Brinton EA; for the Consensus Panel Steering Committee. Cardiometabolic-Renal Disease in South Asians: Consensus Recommendations from the Cardio Renal Society of America. Cardiorenal Med. 2019;9:240-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387:1377-1396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3351] [Cited by in F6Publishing: 3152] [Article Influence: 394.0] [Reference Citation Analysis (0)] |
| 8. | Soriano-Maldonado A, Aparicio VA, Félix-Redondo FJ, Fernández-Bergés D. Severity of obesity and cardiometabolic risk factors in adults: Sex differences and role of physical activity. The HERMEX study. Int J Cardiol. 2016;223:352-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Santos ASAC, Rodrigues APS, Rosa LPS, Sarrafzadegan N, Silveira EA. Cardiometabolic risk factors and Framingham Risk Score in severely obese patients: Baseline data from DieTBra trial. Nutr Metab Cardiovasc Dis. 2020;30:474-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Caleyachetty R, Thomas GN, Toulis KA, Mohammed N, Gokhale KM, Balachandran K, Nirantharakumar K. Metabolically Healthy Obese and Incident Cardiovascular Disease Events Among 3.5 Million Men and Women. J Am Coll Cardiol. 2017;70:1429-1437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 313] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 11. | Christiansen MN, Køber L, Weeke P, Vasan RS, Jeppesen JL, Smith JG, Gislason GH, Torp-Pedersen C, Andersson C. Age-Specific Trends in Incidence, Mortality, and Comorbidities of Heart Failure in Denmark, 1995 to 2012. Circulation. 2017;135:1214-1223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 12. | Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14:591-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 559] [Cited by in F6Publishing: 765] [Article Influence: 109.3] [Reference Citation Analysis (0)] |
| 13. | Costanzo MR. The Cardiorenal Syndrome in Heart Failure. Heart Fail Clin. 2020;16:81-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 36] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 14. | Van Aelst LNL, Arrigo M, Placido R, Akiyama E, Girerd N, Zannad F, Manivet P, Rossignol P, Badoz M, Sadoune M, Launay JM, Gayat E, Lam CSP, Cohen-Solal A, Mebazaa A, Seronde MF. Acutely decompensated heart failure with preserved and reduced ejection fraction present with comparable haemodynamic congestion. Eur J Heart Fail. 2018;20:738-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 15. | Aglae C, Muller L, Reboul P, Cariou S, Saber Davide B, Trusson R, Messikh Z, De Brauwere DP, Lefrant JY, Moranne O. Heterogeneity of Cause, Care, and Prognosis in Severe Acute Kidney Injury in Hospitalized Patients: A Prospective Observational Study. Can J Kidney Health Dis. 2019;6:2054358119892174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Chang AR, Grams ME, Ballew SH, Bilo H, Correa A, Evans M, Gutierrez OM, Hosseinpanah F, Iseki K, Kenealy T, Klein B, Kronenberg F, Lee BJ, Li Y, Miura K, Navaneethan SD, Roderick PJ, Valdivielso JM, Visseren FLJ, Zhang L, Gansevoort RT, Hallan SI, Levey AS, Matsushita K, Shalev V, Woodward M; CKD Prognosis Consortium (CKD-PC). Adiposity and risk of decline in glomerular filtration rate: meta-analysis of individual participant data in a global consortium. BMJ. 2019;364:k5301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 17. | Turer CB, Baum M, Dubourg L, Selistre LS, Skinner AC. Prevalence of hyperfiltration among US youth/young adults with overweight and obesity: A population-based association study. Obes Sci Pract. 2019;5:570-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116:991-1006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 605] [Cited by in F6Publishing: 670] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 19. | Whaley-Connell A, Sowers JR. Basic science: Pathophysiology: the cardiorenal metabolic syndrome. J Am Soc Hypertens. 2014;8:604-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Ronco C, McCullough PA, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House A, Katz NM, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P. Cardiorenal syndromes: an executive summary from the consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol. 2010;165:54-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Dar O, Cowie MR. Acute heart failure in the intensive care unit: epidemiology. Crit Care Med. 2008;36:S3-S8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Bellomo R, Kellum JA, Ronco C. Defining and classifying acute renal failure: from advocacy to consensus and validation of the RIFLE criteria. Intensive Care Med. 2007;33:409-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 267] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 23. | Siirilä-Waris K, Lassus J, Melin J, Peuhkurinen K, Nieminen MS, Harjola VP; FINN-AKVA Study Group. Characteristics, outcomes, and predictors of 1-year mortality in patients hospitalized for acute heart failure. Eur Heart J. 2006;27:3011-3017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 24. | Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296-1305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7995] [Cited by in F6Publishing: 8096] [Article Influence: 404.8] [Reference Citation Analysis (0)] |
| 25. | Sharma A, Sartori M, Zaragoza JJ, Villa G, Lu R, Faggiana E, Brocca A, Di Lullo L, Feriozzi S, Ronco C. Fabry's disease: an example of cardiorenal syndrome type 5. Heart Fail Rev. 2015;20:689-708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Clementi A, Virzì GM, Brocca A, Ronco C. The Role of Endotoxin in the Setting of Cardiorenal Syndrome Type 5. Cardiorenal Med. 2017;7:276-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Reinhart K, Bauer M, Riedemann NC, Hartog CS. New approaches to sepsis: molecular diagnostics and biomarkers. Clin Microbiol Rev. 2012;25:609-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 324] [Cited by in F6Publishing: 316] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 28. | Mavrakanas TA, Khattak A, Singh K, Charytan DM. Epidemiology and Natural History of the Cardiorenal Syndromes in a Cohort with Echocardiography. Clin J Am Soc Nephrol. 2017;12:1624-1633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35:455-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 554] [Cited by in F6Publishing: 667] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 30. | Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 635] [Cited by in F6Publishing: 621] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 31. | González-Muniesa P, Mártinez-González MA, Hu FB, Després JP, Matsuzawa Y, Loos RJF, Moreno LA, Bray GA, Martinez JA. Obesity. Nat Rev Dis Primers. 2017;3:17034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 593] [Cited by in F6Publishing: 659] [Article Influence: 94.1] [Reference Citation Analysis (0)] |
| 32. | Cuthbertson DJ, Steele T, Wilding JP, Halford JC, Harrold JA, Hamer M, Karpe F. What have human experimental overfeeding studies taught us about adipose tissue expansion and susceptibility to obesity and metabolic complications? Int J Obes (Lond). 2017;41:853-865. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 33. | Bouchard C, Tremblay A, Després JP, Nadeau A, Lupien PJ, Thériault G, Dussault J, Moorjani S, Pinault S, Fournier G. The response to long-term overfeeding in identical twins. N Engl J Med. 1990;322:1477-1482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 886] [Cited by in F6Publishing: 889] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 34. | Neeland IJ, Poirier P, Després JP. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation. 2018;137:1391-1406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 479] [Cited by in F6Publishing: 431] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 35. | Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr. 1982;36:172-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 357] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 36. | Mathieu P, Boulanger MC, Després JP. Ectopic visceral fat: a clinical and molecular perspective on the cardiometabolic risk. Rev Endocr Metab Disord. 2014;15:289-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Gradmark AM, Rydh A, Renström F, De Lucia-Rolfe E, Sleigh A, Nordström P, Brage S, Franks PW. Computed tomography-based validation of abdominal adiposity measurements from ultrasonography, dual-energy X-ray absorptiometry and anthropometry. Br J Nutr. 2010;104:582-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 38. | Nazare JA, Smith J, Borel AL, Aschner P, Barter P, Van Gaal L, Tan CE, Wittchen HU, Matsuzawa Y, Kadowaki T, Ross R, Brulle-Wohlhueter C, Alméras N, Haffner SM, Balkau B, Després JP; INSPIRE ME IAA Investigators. Usefulness of measuring both body mass index and waist circumference for the estimation of visceral adiposity and related cardiometabolic risk profile (from the INSPIRE ME IAA study). Am J Cardiol. 2015;115:307-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 39. | Lassale C, Tzoulaki I, Moons KGM, Sweeting M, Boer J, Johnson L, Huerta JM, Agnoli C, Freisling H, Weiderpass E, Wennberg P, van der A DL, Arriola L, Benetou V, Boeing H, Bonnet F, Colorado-Yohar SM, Engström G, Eriksen AK, Ferrari P, Grioni S, Johansson M, Kaaks R, Katsoulis M, Katzke V, Key TJ, Matullo G, Melander O, Molina-Portillo E, Moreno-Iribas C, Norberg M, Overvad K, Panico S, Quirós JR, Saieva C, Skeie G, Steffen A, Stepien M, Tjønneland A, Trichopoulou A, Tumino R, van der Schouw YT, Verschuren WMM, Langenberg C, Di Angelantonio E, Riboli E, Wareham NJ, Danesh J, Butterworth AS. Separate and combined associations of obesity and metabolic health with coronary heart disease: a pan-European case-cohort analysis. Eur Heart J. 2018;39:397-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 173] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 40. | Lemieux I, Pascot A, Couillard C, Lamarche B, Tchernof A, Alméras N, Bergeron J, Gaudet D, Tremblay G, Prud'homme D, Nadeau A, Després JP. Hypertriglyceridemic waist: A marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation. 2000;102:179-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 647] [Cited by in F6Publishing: 625] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 41. | Murphy NF, MacIntyre K, Stewart S, Hart CL, Hole D, McMurray JJ. Long-term cardiovascular consequences of obesity: 20-year follow-up of more than 15 000 middle-aged men and women (the Renfrew-Paisley study). Eur Heart J. 2006;27:96-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 201] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 42. | Vázquez LA, Pazos F, Berrazueta JR, Fernández-Escalante C, García-Unzueta MT, Freijanes J, Amado JA. Effects of changes in body weight and insulin resistance on inflammation and endothelial function in morbid obesity after bariatric surgery. J Clin Endocrinol Metab. 2005;90:316-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 43. | Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341:577-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 876] [Cited by in F6Publishing: 780] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 44. | Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345:1689-1697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 537] [Cited by in F6Publishing: 543] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 45. | Wolf G, Haberstroh U, Neilson EG. Angiotensin II stimulates the proliferation and biosynthesis of type I collagen in cultured murine mesangial cells. Am J Pathol. 1992;140:95-107. [PubMed] [Cited in This Article: ] |
| 46. | Border WA, Noble NA. Interactions of transforming growth factor-beta and angiotensin II in renal fibrosis. Hypertension. 1998;31:181-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 331] [Cited by in F6Publishing: 350] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 47. | Dostal DE, Booz GW, Baker KM. Angiotensin II signalling pathways in cardiac fibroblasts: conventional versus novel mechanisms in mediating cardiac growth and function. Mol Cell Biochem. 1996;157:15-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Nakamura K, Fushimi K, Kouchi H, Mihara K, Miyazaki M, Ohe T, Namba M. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-alpha and angiotensin II. Circulation. 1998;98:794-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 345] [Cited by in F6Publishing: 362] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 49. | Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82-C97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1315] [Cited by in F6Publishing: 1397] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 50. | de Paula RB, da Silva AA, Hall JE. Aldosterone antagonism attenuates obesity-induced hypertension and glomerular hyperfiltration. Hypertension. 2004;43:41-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 132] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 51. | de Souza F, Muxfeldt E, Fiszman R, Salles G. Efficacy of spironolactone therapy in patients with true resistant hypertension. Hypertension. 2010;55:147-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 52. | Calvier L, Martinez-Martinez E, Miana M, Cachofeiro V, Rousseau E, Sádaba JR, Zannad F, Rossignol P, López-Andrés N. The impact of galectin-3 inhibition on aldosterone-induced cardiac and renal injuries. JACC Heart Fail. 2015;3:59-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 53. | Lattenist L, Lechner SM, Messaoudi S, Le Mercier A, El Moghrabi S, Prince S, Bobadilla NA, Kolkhof P, Jaisser F, Barrera-Chimal J. Nonsteroidal Mineralocorticoid Receptor Antagonist Finerenone Protects Against Acute Kidney Injury-Mediated Chronic Kidney Disease: Role of Oxidative Stress. Hypertension. 2017;69:870-878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 54. | Joles JA, Koomans HA. Causes and consequences of increased sympathetic activity in renal disease. Hypertension. 2004;43:699-706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 55. | Schlaich MP, Socratous F, Hennebry S, Eikelis N, Lambert EA, Straznicky N, Esler MD, Lambert GW. Sympathetic activation in chronic renal failure. J Am Soc Nephrol. 2009;20:933-939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 302] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 56. | Barretto AC, Santos AC, Munhoz R, Rondon MU, Franco FG, Trombetta IC, Roveda F, de Matos LN, Braga AM, Middlekauff HR, Negrão CE. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol. 2009;135:302-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 211] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 57. | Rubattu S, Mennuni S, Testa M, Mennuni M, Pierelli G, Pagliaro B, Gabriele E, Coluccia R, Autore C, Volpe M. Pathogenesis of chronic cardiorenal syndrome: is there a role for oxidative stress? Int J Mol Sci. 2013;14:23011-23032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 58. | Modlinger PS, Wilcox CS, Aslam S. Nitric oxide, oxidative stress, and progression of chronic renal failure. Semin Nephrol. 2004;24:354-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 194] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 59. | Braam B. Renal endothelial and macula densa NOS: integrated response to changes in extracellular fluid volume. Am J Physiol. 1999;276:R1551-R1561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Treuer AV, Gonzalez DR. Nitric oxide synthases, S-nitrosylation and cardiovascular health: from molecular mechanisms to therapeutic opportunities (review). Mol Med Rep. 2015;11:1555-1565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 61. | Colombo PC, Ganda A, Lin J, Onat D, Harxhi A, Iyasere JE, Uriel N, Cotter G. Inflammatory activation: cardiac, renal, and cardio-renal interactions in patients with the cardiorenal syndrome. Heart Fail Rev. 2012;17:177-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 62. | DiPetrillo K, Coutermarsh B, Gesek FA. Urinary tumor necrosis factor contributes to sodium retention and renal hypertrophy during diabetes. Am J Physiol Renal Physiol. 2003;284:F113-F121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 63. | Hedayat M, Mahmoudi MJ, Rose NR, Rezaei N. Proinflammatory cytokines in heart failure: double-edged swords. Heart Fail Rev. 2010;15:543-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 64. | Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3022] [Cited by in F6Publishing: 2972] [Article Influence: 165.1] [Reference Citation Analysis (1)] |
| 65. | Yamagishi T, Matsushita K, Minamishima T, Goda A, Sakata K, Satoh T, Yoshino H. Comparison of risk factors for acute worsening renal function in heart failure patients with and without preserved ejection fraction. Eur J Intern Med. 2015;26:599-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 66. | Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW, Califf RM, Stevenson LW, Hill JA. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51:1268-1274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 421] [Cited by in F6Publishing: 383] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 67. | Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WHW. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1048] [Cited by in F6Publishing: 1058] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 68. | Dupont M, Mullens W, Tang WH. Impact of systemic venous congestion in heart failure. Curr Heart Fail Rep. 2011;8:233-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 69. | Volpe M, Carnovali M, Mastromarino V. The natriuretic peptides system in the pathophysiology of heart failure: from molecular basis to treatment. Clin Sci (Lond). 2016;130:57-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 70. | Rubattu S, Volpe M. Natriuretic Peptides in the Cardiovascular System: Multifaceted Roles in Physiology, Pathology and Therapeutics. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 71. | Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, Vasan RS. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 701] [Cited by in F6Publishing: 719] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 72. | Reginauld SH, Cannone V, Iyer S, Scott C, Bailey K, Schaefer J, Chen Y, Sangaralingham SJ, Burnett JC. Differential Regulation of ANP and BNP in Acute Decompensated Heart Failure: Deficiency of ANP. JACC Heart Fail. 2019;7:891-898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 73. | Reaven GM. Role of insulin resistance in human disease (syndrome X): an expanded definition. Annu Rev Med. 1993;44:121-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 573] [Cited by in F6Publishing: 540] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 74. | Templeman NM, Skovsø S, Page MM, Lim GE, Johnson JD. A causal role for hyperinsulinemia in obesity. J Endocrinol. 2017;232:R173-R183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 75. | Cabandugama PK, Gardner MJ, Sowers JR. The Renin Angiotensin Aldosterone System in Obesity and Hypertension: Roles in the Cardiorenal Metabolic Syndrome. Med Clin North Am. 2017;101:129-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 76. | Jia G, Aroor AR, Martinez-Lemus LA, Sowers JR. Overnutrition, mTOR signaling, and cardiovascular diseases. Am J Physiol Regul Integr Comp Physiol. 2014;307:R1198-R1206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 77. | Aroor AR, Jia G, Sowers JR. Cellular mechanisms underlying obesity-induced arterial stiffness. Am J Physiol Regul Integr Comp Physiol. 2018;314:R387-R398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 78. | Jia G, Jia Y, Sowers JR. Role of mineralocorticoid receptor activation in cardiac diastolic dysfunction. Biochim Biophys Acta Mol Basis Dis. 2017;1863:2012-2018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 79. | Rask Larsen J, Dima L, Correll CU, Manu P. The pharmacological management of metabolic syndrome. Expert Rev Clin Pharmacol. 2018;11:397-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 80. | Liberale L, Carbone F, Bertolotto M, Bonaventura A, Vecchié A, Mach F, Burger F, Pende A, Spinella G, Pane B, Palombo D, Dallegri F, Montecucco F. Serum adiponectin levels predict acute coronary syndrome (ACS) in patients with severe carotid stenosis. Vascul Pharmacol. 2018;102:37-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 81. | Cui J, Panse S, Falkner B. The role of adiponectin in metabolic and vascular disease: a review. Clin Nephrol. 2011;75:26-33. [PubMed] [Cited in This Article: ] |
| 82. | Bełtowski J. Leptin and the Regulation of Renal Sodium Handling and Renal Na-Transporting ATPases: Role in the Pathogenesis of Arterial Hypertension. Curr Cardiol Rev. 2010;6:31-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 83. | D'Elia L, Manfredi M, Perna L, Iacone R, Russo O, Strazzullo P, Galletti F. Circulating leptin levels predict the decline in renal function with age in a sample of adult men (The Olivetti Heart Study). Intern Emerg Med. 2019;14:507-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 84. | Katsiki N, Mikhailidis DP, Banach M. Leptin, cardiovascular diseases and type 2 diabetes mellitus. Acta Pharmacol Sin. 2018;39:1176-1188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 148] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 85. | Tumova J, Andel M, Trnka J. Excess of free fatty acids as a cause of metabolic dysfunction in skeletal muscle. Physiol Res. 2016;65:193-207. [PubMed] [Cited in This Article: ] |
| 86. | Steinberg HO, Paradisi G, Hook G, Crowder K, Cronin J, Baron AD. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes. 2000;49:1231-1238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 267] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 87. | Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016;12:144-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 450] [Cited by in F6Publishing: 524] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 88. | Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113-1132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1623] [Cited by in F6Publishing: 1753] [Article Influence: 125.2] [Reference Citation Analysis (0)] |
| 89. | Eschalier R, Rossignol P, Kearney-Schwartz A, Adamopoulos C, Karatzidou K, Fay R, Mandry D, Marie PY, Zannad F. Features of cardiac remodeling, associated with blood pressure and fibrosis biomarkers, are frequent in subjects with abdominal obesity. Hypertension. 2014;63:740-746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 90. | Lee TC, Jin Z, Homma S, Nakanishi K, Elkind MSV, Rundek T, Tugcu A, Matsumoto K, Sacco RL, Di Tullio MR. Changes in Left Ventricular Mass and Geometry in the Older Adults: Role of Body Mass and Central Obesity. J Am Soc Echocardiogr. 2019;32:1318-1325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 91. | Harada PH, Bensenõr IJM, Drager LF, Goulart AC, Mill JG, Lotufo PA. Non-alcoholic fatty liver disease presence and severity are associated with aortic stiffness beyond abdominal obesity: The ELSA-Brasil. Atherosclerosis. 2019;284:59-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 92. | Lee JJ, Pedley A, Hoffmann U, Massaro JM, Levy D, Long MT. Visceral and Intrahepatic Fat Are Associated with Cardiometabolic Risk Factors Above Other Ectopic Fat Depots: The Framingham Heart Study. Am J Med. 2018;131:684-692.e12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 93. | Tsujimoto T, Kajio H. Abdominal Obesity Is Associated With an Increased Risk of All-Cause Mortality in Patients With HFpEF. J Am Coll Cardiol. 2017;70:2739-2749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 94. | Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC; ADHERE Scientific Advisory Committee and Investigators. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 688] [Cited by in F6Publishing: 726] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 95. | Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure With Preserved Ejection Fraction. Circulation. 2017;136:6-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 502] [Cited by in F6Publishing: 621] [Article Influence: 88.7] [Reference Citation Analysis (0)] |
| 96. | Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383-1392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1656] [Cited by in F6Publishing: 1690] [Article Influence: 169.0] [Reference Citation Analysis (0)] |
| 97. | Standeven KF, Hess K, Carter AM, Rice GI, Cordell PA, Balmforth AJ, Lu B, Scott DJ, Turner AJ, Hooper NM, Grant PJ. Neprilysin, obesity and the metabolic syndrome. Int J Obes (Lond). 2011;35:1031-1040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 98. | Grams ME, Juraschek SP, Selvin E, Foster MC, Inker LA, Eckfeldt JH, Levey AS, Coresh J. Trends in the prevalence of reduced GFR in the United States: a comparison of creatinine- and cystatin C-based estimates. Am J Kidney Dis. 2013;62:253-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 99. | Upadhya B, Amjad A, Stacey RB. Optimizing The Management of Obese HFpEF Phenotype: Can We Mind Both The Heart and The Kidney? J Card Fail. 2020;26:108-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 100. | Wickman C, Kramer H. Obesity and kidney disease: potential mechanisms. Semin Nephrol. 2013;33:14-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 122] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 101. | Engeli S. Role of the renin-angiotensin- aldosterone system in the metabolic syndrome. Contrib Nephrol. 2006;151:122-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 102. | Bosma RJ, Kwakernaak AJ, van der Heide JJ, de Jong PE, Navis GJ. Body mass index and glomerular hyperfiltration in renal transplant recipients: cross-sectional analysis and long-term impact. Am J Transplant. 2007;7:645-652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 103. | Chandra A, Neeland IJ, Berry JD, Ayers CR, Rohatgi A, Das SR, Khera A, McGuire DK, de Lemos JA, Turer AT. The relationship of body mass and fat distribution with incident hypertension: observations from the Dallas Heart Study. J Am Coll Cardiol. 2014;64:997-1002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 170] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 104. | Musso G, Gambino R, Tabibian JH, Ekstedt M, Kechagias S, Hamaguchi M, Hultcrantz R, Hagström H, Yoon SK, Charatcharoenwitthaya P, George J, Barrera F, Hafliðadóttir S, Björnsson ES, Armstrong MJ, Hopkins LJ, Gao X, Francque S, Verrijken A, Yilmaz Y, Lindor KD, Charlton M, Haring R, Lerch MM, Rettig R, Völzke H, Ryu S, Li G, Wong LL, Machado M, Cortez-Pinto H, Yasui K, Cassader M. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 408] [Cited by in F6Publishing: 442] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 105. | Engeli S, Böhnke J, Gorzelniak K, Janke J, Schling P, Bader M, Luft FC, Sharma AM. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45:356-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 446] [Cited by in F6Publishing: 442] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 106. | Foster MC, Hwang SJ, Porter SA, Massaro JM, Hoffmann U, Fox CS. Fatty kidney, hypertension, and chronic kidney disease: the Framingham Heart Study. Hypertension. 2011;58:784-790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 228] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 107. | Chughtai HL, Morgan TM, Rocco M, Stacey B, Brinkley TE, Ding J, Nicklas B, Hamilton C, Hundley WG. Renal sinus fat and poor blood pressure control in middle-aged and elderly individuals at risk for cardiovascular events. Hypertension. 2010;56:901-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 108. | Kamal FA, Travers JG, Schafer AE, Ma Q, Devarajan P, Blaxall BC. G Protein-Coupled Receptor-G-Protein βγ-Subunit Signaling Mediates Renal Dysfunction and Fibrosis in Heart Failure. J Am Soc Nephrol. 2017;28:197-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 109. | Blüher M. Metabolically Healthy Obesity. Endocr Rev. 2020;41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 356] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 110. | Sponholtz TR, van den Heuvel ER, Xanthakis V, Vasan RS. Association of Variability in Body Mass Index and Metabolic Health With Cardiometabolic Disease Risk. J Am Heart Assoc. 2019;8:e010793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 111. | Mørkedal B, Vatten LJ, Romundstad PR, Laugsand LE, Janszky I. Risk of myocardial infarction and heart failure among metabolically healthy but obese individuals: HUNT (Nord-Trøndelag Health Study), Norway. J Am Coll Cardiol. 2014;63:1071-1078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 112. | Cho YK, Lee J, Kim HS, Park JY, Lee WJ, Kim YJ, Jung CH. Impact of Transition in Metabolic Health and Obesity on the Incident Chronic Kidney Disease: A Nationwide Cohort Study. J Clin Endocrinol Metab. 2020;105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 113. | Mahajan R, Stokes M, Elliott A, Munawar DA, Khokhar KB, Thiyagarajah A, Hendriks J, Linz D, Gallagher C, Kaye D, Lau D, Sanders P. Complex interaction of obesity, intentional weight loss and heart failure: a systematic review and meta-analysis. Heart. 2020;106:58-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 114. | Padwal R, McAlister FA, McMurray JJ, Cowie MR, Rich M, Pocock S, Swedberg K, Maggioni A, Gamble G, Ariti C, Earle N, Whalley G, Poppe KK, Doughty RN, Bayes-Genis A; Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). The obesity paradox in heart failure patients with preserved versus reduced ejection fraction: a meta-analysis of individual patient data. Int J Obes (Lond). 2014;38:1110-1114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 115. | Naderi N, Kleine CE, Park C, Hsiung JT, Soohoo M, Tantisattamo E, Streja E, Kalantar-Zadeh K, Moradi H. Obesity Paradox in Advanced Kidney Disease: From Bedside to the Bench. Prog Cardiovasc Dis. 2018;61:168-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 116. | Sowemimo OA, Yood SM, Courtney J, Moore J, Huang M, Ross R, McMillian U, Ojo P, Reinhold RB. Natural history of morbid obesity without surgical intervention. Surg Obes Relat Dis. 2007;3:73-7; discussion 77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 117. | Schernthaner-Reiter MH, Itariu BK, Krebs M, Promintzer-Schifferl M, Stulnig TM, Tura A, Anderwald CH, Clodi M, Ludvik B, Pacini G, Luger A, Vila G. GDF15 reflects beta cell function in obese patients independently of the grade of impairment of glucose metabolism. Nutr Metab Cardiovasc Dis. 2019;29:334-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 118. | Manmadhan A, Lin BX, Zhong J, Parikh M, Berger JS, Fisher EA, Heffron SP. Elevated GlycA in severe obesity is normalized by bariatric surgery. Diabetes Obes Metab. 2019;21:178-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 119. | Hallström S, Pivodic A, Rosengren A, Ólafsdóttir AF, Svensson AM, Lind M. Risk Factors for Atrial Fibrillation in People With Type 1 Diabetes: An Observational Cohort Study of 36,258 Patients From the Swedish National Diabetes Registry. Diabetes Care. 2019;42:1530-1538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 120. | Figliuzzi I, Presta V, Miceli F, Citoni B, Coluccia R, Ceccarini G, Salvetti G, Santini F, Musumeci MB, Ferrucci A, Volpe M, Tocci G. 24-Hour ambulatory blood pressure levels and control in a large cohort of adult outpatients with different classes of obesity. J Hum Hypertens. 2019;33:298-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 121. | Nelson R, Antonetti I, Bisognano JD, Sloand J. Obesity-related cardiorenal syndrome. J Clin Hypertens (Greenwich). 2010;12:59-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 122. | Tadic M, Cuspidi C. Obesity and heart failure with preserved ejection fraction: a paradox or something else? Heart Fail Rev. 2019;24:379-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 123. | Appel LJ, Clark JM, Yeh HC, Wang NY, Coughlin JW, Daumit G, Miller ER, Dalcin A, Jerome GJ, Geller S, Noronha G, Pozefsky T, Charleston J, Reynolds JB, Durkin N, Rubin RR, Louis TA, Brancati FL. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365:1959-1968. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 579] [Cited by in F6Publishing: 528] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 124. | Hofsø D, Nordstrand N, Johnson LK, Karlsen TI, Hager H, Jenssen T, Bollerslev J, Godang K, Sandbu R, Røislien J, Hjelmesaeth J. Obesity-related cardiovascular risk factors after weight loss: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. Eur J Endocrinol. 2010;163:735-745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 125. | Kwok CS, Pradhan A, Khan MA, Anderson SG, Keavney BD, Myint PK, Mamas MA, Loke YK. Bariatric surgery and its impact on cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Cardiol. 2014;173:20-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 126. | Shimada YJ, Tsugawa Y, Brown DF, Hasegawa K. Bariatric Surgery and Emergency Department Visits and Hospitalizations for Heart Failure Exacerbation: Population-Based, Self-Controlled Series. J Am Coll Cardiol. 2016;67:895-903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 127. | Vest AR, Patel P, Schauer PR, Satava ME, Cavalcante JL, Brethauer S, Young JB. Clinical and Echocardiographic Outcomes After Bariatric Surgery in Obese Patients With Left Ventricular Systolic Dysfunction. Circ Heart Fail. 2016;9:e002260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 128. | Mukerji R, Petruc M, Fresen JL, Terry BE, Govindarajan G, Alpert MA. Effect of weight loss after bariatric surgery on left ventricular mass and ventricular repolarization in normotensive morbidly obese patients. Am J Cardiol. 2012;110:415-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 129. | Vest AR, Schauer PR, Young JB. Failure and Fatness: Could Surgical Management of Obesity Reduce Heart Failure Hospitalizations? J Am Coll Cardiol. 2016;67:904-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 130. | Miranda WR, Batsis JA, Sarr MG, Collazo-Clavell ML, Clark MM, Somers VK, Lopez-Jimenez F. Impact of bariatric surgery on quality of life, functional capacity, and symptoms in patients with heart failure. Obes Surg. 2013;23:1011-1015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 131. | Ramani GV, McCloskey C, Ramanathan RC, Mathier MA. Safety and efficacy of bariatric surgery in morbidly obese patients with severe systolic heart failure. Clin Cardiol. 2008;31:516-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 132. | Sugerman H, Windsor A, Bessos M, Wolfe L. Intra-abdominal pressure, sagittal abdominal diameter and obesity comorbidity. J Intern Med. 1997;241:71-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 346] [Cited by in F6Publishing: 315] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 133. | Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat Rev Nephrol. 2019;15:367-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 278] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 134. | von Scholten BJ, Persson F, Svane MS, Hansen TW, Madsbad S, Rossing P. Effect of large weight reductions on measured and estimated kidney function. BMC Nephrol. 2017;18:52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 135. | Chang AR, Chen Y, Still C, Wood GC, Kirchner HL, Lewis M, Kramer H, Hartle JE, Carey D, Appel LJ, Grams ME. Bariatric surgery is associated with improvement in kidney outcomes. Kidney Int. 2016;90:164-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 136. | Park S, Kim YJ, Choi CY, Cho NJ, Gil HW, Lee EY. Bariatric Surgery can Reduce Albuminuria in Patients with Severe Obesity and Normal Kidney Function by Reducing Systemic Inflammation. Obes Surg. 2018;28:831-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 137. | Seghieri M, Vitolo E, Giannini L, Santini E, Rossi C, Salvati A, Solini A. Determinants of glomerular filtration rate following bariatric surgery in individuals with severe, otherwise uncomplicated, obesity: an observational, prospective study. Acta Diabetol. 2017;54:593-598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 138. | Solini A, Seghieri M, Santini E, Giannini L, Biancalana E, Taddei S, Volterrani D, Bruno RM. Renal Resistive Index Predicts Post-Bariatric Surgery Renal Outcome in Nondiabetic Individuals with Severe Obesity. Obesity (Silver Spring). 2019;27:68-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 139. | Saleh F, Kim SJ, Okrainec A, Jackson TD. Bariatric surgery in patients with reduced kidney function: an analysis of short-term outcomes. Surg Obes Relat Dis. 2015;11:828-835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 140. | Navarro-Díaz M, Serra A, Romero R, Bonet J, Bayés B, Homs M, Pérez N, Bonal J. Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. J Am Soc Nephrol. 2006;17:S213-S217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 141. | Wakamatsu K, Seki Y, Kasama K, Uno K, Hashimoto K, Seto Y, Kurokawa Y. Prevalence of Chronic Kidney Disease in Morbidly Obese Japanese and the Impact of Bariatric Surgery on Disease Progression. Obes Surg. 2018;28:489-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 142. | Neff KJ, Baud G, Raverdy V, Caiazzo R, Verkindt H, Noel C, le Roux CW, Pattou F. Renal Function and Remission of Hypertension After Bariatric Surgery: a 5-Year Prospective Cohort Study. Obes Surg. 2017;27:613-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 143. | Nehus EJ, Khoury JC, Inge TH, Xiao N, Jenkins TM, Moxey-Mims MM, Mitsnefes MM. Kidney outcomes three years after bariatric surgery in severely obese adolescents. Kidney Int. 2017;91:451-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 144. | Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Brandt ML, Xanthakos SA, Dixon JB, Harmon CM, Chen MK, Xie C, Evans ME, Helmrath MA; Teen–LABS Consortium. Five-Year Outcomes of Gastric Bypass in Adolescents as Compared with Adults. N Engl J Med. 2019;380:2136-2145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 186] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 145. | ter Maaten JM, Valente MA, Damman K, Hillege HL, Navis G, Voors AA. Diuretic response in acute heart failure-pathophysiology, evaluation, and therapy. Nat Rev Cardiol. 2015;12:184-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 166] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 146. | Voors AA, Davison BA, Teerlink JR, Felker GM, Cotter G, Filippatos G, Greenberg BH, Pang PS, Levin B, Hua TA, Severin T, Ponikowski P, Metra M; RELAX-AHF Investigators. Diuretic response in patients with acute decompensated heart failure: characteristics and clinical outcome--an analysis from RELAX-AHF. Eur J Heart Fail. 2014;16:1230-1240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 147. | Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O'Connor CM; NHLBI Heart Failure Clinical Research Network. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797-805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1222] [Cited by in F6Publishing: 1105] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 148. | Reddy YNV, Obokata M, Testani JM, Felker GM, Tang WHW, Abou-Ezzeddine OF, Sun JL, Chakrabothy H, McNulty S, Shah SJ, Lewis GD, Stevenson LW, Redfield MM, Borlaug BA. Adverse Renal Response to Decongestion in the Obese Phenotype of Heart Failure With Preserved Ejection Fraction. J Card Fail. 2020;26:101-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 149. | Brisco MA, Zile MR, Hanberg JS, Wilson FP, Parikh CR, Coca SG, Tang WH, Testani JM. Relevance of Changes in Serum Creatinine During a Heart Failure Trial of Decongestive Strategies: Insights From the DOSE Trial. J Card Fail. 2016;22:753-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 150. | Dovancescu S, Pellicori P, Mabote T, Torabi A, Clark AL, Cleland JGF. The effects of short-term omission of daily medication on the pathophysiology of heart failure. Eur J Heart Fail. 2017;19:643-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 151. | McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4078] [Cited by in F6Publishing: 4160] [Article Influence: 416.0] [Reference Citation Analysis (0)] |
| 152. | Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin-Colet J, Cleland J, Düngen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP; PARAGON-HF Investigators and Committees. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2019;381:1609-1620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1097] [Cited by in F6Publishing: 1272] [Article Influence: 254.4] [Reference Citation Analysis (0)] |
| 153. | McMurray JJV, Jackson AM, Lam CSP, Redfield MM, Anand IS, Ge J, Lefkowitz MP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Rizkala AR, Sabarwal SV, Shah AM, Shah SJ, Shi VC, van Veldhuisen DJ, Zannad F, Zile MR, Cikes M, Goncalvesova E, Katova T, Kosztin A, Lelonek M, Sweitzer N, Vardeny O, Claggett B, Jhund PS, Solomon SD. Effects of Sacubitril-Valsartan Versus Valsartan in Women Compared With Men With Heart Failure and Preserved Ejection Fraction: Insights From PARAGON-HF. Circulation. 2020;141:338-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 201] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 154. | Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail. 2011;4:685-691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 155. | Beldhuis IE, Streng KW, Ter Maaten JM, Voors AA, van der Meer P, Rossignol P, McMurray JJ, Damman K. Renin-Angiotensin System Inhibition, Worsening Renal Function, and Outcome in Heart Failure Patients With Reduced and Preserved Ejection Fraction: A Meta-Analysis of Published Study Data. Circ Heart Fail. 2017;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 156. | Valika AA, Gheorghiade M. Ace inhibitor therapy for heart failure in patients with impaired renal function: a review of the literature. Heart Fail Rev. 2013;18:135-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 157. | Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. [2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure]. Kardiol Pol. 2016;74:1037-1147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 158. | De Pergola G, Nardecchia A, Giagulli VA, Triggiani V, Guastamacchia E, Minischetti MC, Silvestris F. Obesity and heart failure. Endocr Metab Immune Disord Drug Targets. 2013;13:51-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 159. | Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2034] [Cited by in F6Publishing: 1972] [Article Influence: 151.7] [Reference Citation Analysis (0)] |
| 160. | Pitt B, Williams G, Remme W, Martinez F, Lopez-Sendon J, Zannad F, Neaton J, Roniker B, Hurley S, Burns D, Bittman R, Kleiman J. The EPHESUS trial: eplerenone in patients with heart failure due to systolic dysfunction complicating acute myocardial infarction. Eplerenone Post-AMI Heart Failure Efficacy and Survival Study. Cardiovasc Drugs Ther. 2001;15:79-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 231] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 161. | Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6409] [Cited by in F6Publishing: 5883] [Article Influence: 235.3] [Reference Citation Analysis (0)] |
| 162. | Olivier A, Pitt B, Girerd N, Lamiral Z, Machu JL, McMurray JJV, Swedberg K, van Veldhuisen DJ, Collier TJ, Pocock SJ, Rossignol P, Zannad F, Pizard A. Effect of eplerenone in patients with heart failure and reduced ejection fraction: potential effect modification by abdominal obesity. Insight from the EMPHASIS-HF trial. Eur J Heart Fail. 2017;19:1186-1197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 163. | Kapelios CJ, Murrow JR, Nührenberg TG, Montoro Lopez MN. Effect of mineralocorticoid receptor antagonists on cardiac function in patients with heart failure and preserved ejection fraction: a systematic review and meta-analysis of randomized controlled trials. Heart Fail Rev. 2019;24:367-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 164. | Cohen JB, Schrauben SJ, Zhao L, Basso MD, Cvijic ME, Li Z, Yarde M, Wang Z, Bhattacharya PT, Chirinos DA, Prenner S, Zamani P, Seiffert DA, Car BD, Gordon DA, Margulies K, Cappola T, Chirinos JA. Clinical Phenogroups in Heart Failure With Preserved Ejection Fraction: Detailed Phenotypes, Prognosis, and Response to Spironolactone. JACC Heart Fail. 2020;8:172-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 171] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 165. | Zuo C, Xu G. Efficacy and safety of mineralocorticoid receptor antagonists with ACEI/ARB treatment for diabetic nephropathy: A meta-analysis. Int J Clin Pract. 2019;e13413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 166. | Beldhuis IE, Myhre PL, Claggett B, Damman K, Fang JC, Lewis EF, O'Meara E, Pitt B, Shah SJ, Voors AA, Pfeffer MA, Solomon SD, Desai AS. Efficacy and Safety of Spironolactone in Patients With HFpEF and Chronic Kidney Disease. JACC Heart Fail. 2019;7:25-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 167. | Agarwal R, Rossignol P, Romero A, Garza D, Mayo MR, Warren S, Ma J, White WB, Williams B. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2019;394:1540-1550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 205] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 168. | Filippatos G, Anker SD, Böhm M, Gheorghiade M, Køber L, Krum H, Maggioni AP, Ponikowski P, Voors AA, Zannad F, Kim SY, Nowack C, Palombo G, Kolkhof P, Kimmeskamp-Kirschbaum N, Pieper A, Pitt B. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J. 2016;37:2105-2114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 231] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 169. | Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, Remuzzi G, Rossing P, Schmieder RE, Nowack C, Kolkhof P, Joseph A, Pieper A, Kimmeskamp-Kirschbaum N, Ruilope LM; Mineralocorticoid Receptor Antagonist Tolerability Study–Diabetic Nephropathy (ARTS-DN) Study Group. Effect of Finerenone on Albuminuria in Patients With Diabetic Nephropathy: A Randomized Clinical Trial. JAMA. 2015;314:884-894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 427] [Cited by in F6Publishing: 432] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 170. | Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Nowack C, Kolkhof P, Ferreira AC, Schloemer P, Filippatos G; on behalf of the FIDELIO-DKD study investigators; FIDELIO-DKD study investigators. Design and Baseline Characteristics of the Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease Trial. Am J Nephrol. 2019;50:333-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 171. | Ruilope LM, Agarwal R, Anker SD, Bakris GL, Filippatos G, Nowack C, Kolkhof P, Joseph A, Mentenich N, Pitt B; FIGARO-DKD study investigators. Design and Baseline Characteristics of the Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease Trial. Am J Nephrol. 2019;50:345-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 172. | Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4498] [Cited by in F6Publishing: 4696] [Article Influence: 670.9] [Reference Citation Analysis (0)] |
| 173. | Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380:2295-2306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2826] [Cited by in F6Publishing: 3206] [Article Influence: 641.2] [Reference Citation Analysis (0)] |
| 174. | Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380:347-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3164] [Cited by in F6Publishing: 3518] [Article Influence: 703.6] [Reference Citation Analysis (0)] |
| 175. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7124] [Cited by in F6Publishing: 7288] [Article Influence: 809.8] [Reference Citation Analysis (0)] |
| 176. | McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995-2008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2953] [Cited by in F6Publishing: 3502] [Article Influence: 700.4] [Reference Citation Analysis (0)] |
| 177. | Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1634] [Cited by in F6Publishing: 1690] [Article Influence: 338.0] [Reference Citation Analysis (0)] |
| 178. | Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC, von Eynatten M. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 847] [Cited by in F6Publishing: 898] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 179. | van Bommel EJM, Muskiet MHA, van Baar MJB, Tonneijck L, Smits MM, Emanuel AL, Bozovic A, Danser AHJ, Geurts F, Hoorn EJ, Touw DJ, Larsen EL, Poulsen HE, Kramer MHH, Nieuwdorp M, Joles JA, van Raalte DH. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int. 2020;97:202-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 180. | Ojima A, Matsui T, Nishino Y, Nakamura N, Yamagishi S. Empagliflozin, an Inhibitor of Sodium-Glucose Cotransporter 2 Exerts Anti-Inflammatory and Antifibrotic Effects on Experimental Diabetic Nephropathy Partly by Suppressing AGEs-Receptor Axis. Horm Metab Res. 2015;47:686-692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 159] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 181. | Packer M. SGLT2 Inhibitors Produce Cardiorenal Benefits by Promoting Adaptive Cellular Reprogramming to Induce a State of Fasting Mimicry: A Paradigm Shift in Understanding Their Mechanism of Action. Diabetes Care. 2020;43:508-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 182. | Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Køber L, Petrie MC, McMurray JJV. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776-785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 729] [Cited by in F6Publishing: 827] [Article Influence: 165.4] [Reference Citation Analysis (0)] |
| 183. | Margulies KB, Hernandez AF, Redfield MM, Givertz MM, Oliveira GH, Cole R, Mann DL, Whellan DJ, Kiernan MS, Felker GM, McNulty SE, Anstrom KJ, Shah MR, Braunwald E, Cappola TP; NHLBI Heart Failure Clinical Research Network. Effects of Liraglutide on Clinical Stability Among Patients With Advanced Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA. 2016;316:500-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 408] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 184. | Muskiet MH, Tonneijck L, Smits MM, Kramer MH, Diamant M, Joles JA, van Raalte DH. Acute renal haemodynamic effects of glucagon-like peptide-1 receptor agonist exenatide in healthy overweight men. Diabetes Obes Metab. 2016;18:178-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 185. | Carraro-Lacroix LR, Malnic G, Girardi AC. Regulation of Na+/H+ exchanger NHE3 by glucagon-like peptide 1 receptor agonist exendin-4 in renal proximal tubule cells. Am J Physiol Renal Physiol. 2009;297:F1647-F1655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 186. | Packer M. Activation and Inhibition of Sodium-Hydrogen Exchanger Is a Mechanism That Links the Pathophysiology and Treatment of Diabetes Mellitus With That of Heart Failure. Circulation. 2017;136:1548-1559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 187. | Tonneijck L, Smits MM, Muskiet MHA, Hoekstra T, Kramer MHH, Danser AHJ, Diamant M, Joles JA, van Raalte DH. Acute renal effects of the GLP-1 receptor agonist exenatide in overweight type 2 diabetes patients: a randomised, double-blind, placebo-controlled trial. Diabetologia. 2016;59:1412-1421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 188. | Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. Comparison of the Effects of Glucagon-Like Peptide Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors for Prevention of Major Adverse Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus. Circulation. 2019;139:2022-2031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 399] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 189. | Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, Galluzzo A; AlkaMeSy Study Group. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33:920-922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 778] [Cited by in F6Publishing: 903] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 190. | Alpert MA, Terry BE, Kelly DL. Effect of weight loss on cardiac chamber size, wall thickness and left ventricular function in morbid obesity. Am J Cardiol. 1985;55:783-786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 150] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 191. | Berger S, Meyre P, Blum S, Aeschbacher S, Ruegg M, Briel M, Conen D. Bariatric surgery among patients with heart failure: a systematic review and meta-analysis. Open Heart. 2018;5:e000910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 192. | Serra A, Esteve A, Navarro-Díaz M, López D, Bancu I, Romero R. Long-Term Normal Renal Function after Drastic Weight Reduction in Patients with Obesity-Related Glomerulopathy. Obes Facts. 2015;8:188-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 193. | Pandey A, Berry JD, Drazner MH, Fang JC, Tang WHW, Grodin JL. Body Mass Index, Natriuretic Peptides, and Risk of Adverse Outcomes in Patients With Heart Failure and Preserved Ejection Fraction: Analysis From the TOPCAT Trial. J Am Heart Assoc. 2018;7:e009664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 194. | Heerspink HJL, Stefansson BV, Chertow GM, Correa-Rotter R, Greene T, Hou FF, Lindberg M, McMurray J, Rossing P, Toto R, Langkilde AM, Wheeler DC; DAPA-CKD Investigators. Rationale and protocol of the Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant. 2020;35:274-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 195. | Ingelheim B. EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Preserved Ejection Fraction (EMPEROR-Preserved). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 1 May 2020]. https://clinicaltrials.gov/ct2/show/NCT03057951 NLM Identifier: NCT03057951. [Cited in This Article: ] |
| 196. | Ingelheim B. EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Reduced Ejection Fraction (EMPEROR-Reduced). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 1 May 2020]. https://clinicaltrials.gov/ct2/show/NCT03057977 NLM Identifier: NCT03057977. [Cited in This Article: ] |
| 197. | Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4164] [Cited by in F6Publishing: 4200] [Article Influence: 525.0] [Reference Citation Analysis (0)] |
| 198. | Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T; SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375:1834-1844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3025] [Cited by in F6Publishing: 3222] [Article Influence: 402.8] [Reference Citation Analysis (0)] |
| 199. | Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, Maggioni AP, Marso SP, Öhman P, Pagidipati NJ, Poulter N, Ramachandran A, Zinman B, Hernandez AF; EXSCEL Study Group. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2017;377:1228-1239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1210] [Cited by in F6Publishing: 1249] [Article Influence: 178.4] [Reference Citation Analysis (0)] |
| 200. | Hernandez AF, Green JB, Janmohamed S, D'Agostino RB Sr, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC, Thorpe KM, McMurray JJV, Del Prato S; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519-1529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 957] [Cited by in F6Publishing: 1005] [Article Influence: 167.5] [Reference Citation Analysis (0)] |
| 201. | Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, Xavier D, Atisso CM, Dyal L, Hall S, Rao-Melacini P, Wong G, Avezum A, Basile J, Chung N, Conget I, Cushman WC, Franek E, Hancu N, Hanefeld M, Holt S, Jansky P, Keltai M, Lanas F, Leiter LA, Lopez-Jaramillo P, Cardona Munoz EG, Pirags V, Pogosova N, Raubenheimer PJ, Shaw JE, Sheu WH, Temelkova-Kurktschiev T; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1213] [Cited by in F6Publishing: 1356] [Article Influence: 271.2] [Reference Citation Analysis (0)] |
| 202. | Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, Tack CJ, Thomsen M, Vilsbøll T, Warren ML, Bain SC; PIONEER 6 Investigators. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2019;381:841-851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 764] [Cited by in F6Publishing: 856] [Article Influence: 171.2] [Reference Citation Analysis (0)] |