Published online Oct 15, 2020. doi: 10.4239/wjd.v11.i10.459
Peer-review started: April 24, 2020
First decision: June 15, 2020
Revised: July 8, 2020
Accepted: August 31, 2020
Article in press: August 31, 2020
Published online: October 15, 2020
Processing time: 172 Days and 10.8 Hours
Diabetes distress is an important factor in treatment outcomes and results in poor behavioral and biological consequences. Technology has been used in management programs of diabetes to improve communication between patients and health care providers and to promote education about the disease and its psychological aspects, which can impact the self-efficacy of the programs. However, the true impact of technological approaches on the management of type 2 diabetes distress remains controversial.
To investigate the effectiveness of technology interventions on the management of type 2 diabetes distress.
Studies published from 2014 to 2019 were searched in five databases: MEDLINE, PubMed, Library and Information Science Source, Academic Search Ultimate and PsycINFO. The Boolean logic search terms were: (1) T2Diabetes; (2) diabetes distress; and (3) technology OR mobile OR phone OR application OR web. We also systematically searched the reference lists of the included studies and relevant reviews. Randomized controlled trials with technology interventions, type 2 diabetes patients and diabetes distress as the outcome were selected. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement was followed.
Of the 88 studies selected, nine full articles met the inclusion criteria and were subjected to final careful review. On the JADAD scale, one article was classified as having poor quality and eight as having good quality. Six out of nine articles showed that technology interventions had a positive impact on diabetes distress scale scores when compared with the initial data. Among the six articles, five showed a greater reduction in the diabetes distress scores from control interventions. Web-based interventions had good results when users received personalized feedback and routine caregiver support and attention.
Technology interventions can contribute positively to the management of type 2 diabetes distress, especially with a tailored approach in conjunction with caregiver interaction with patients.
Core Tip: Technology interventions can impact the reduction of diabetes distress and improve the outcome and quality of life of patients with type 2 diabetes mellitus.
- Citation: Vieira P, Kobayasi R, Pereira F, Zaia I, Sasaki SU. Impact of technology use in type 2 diabetes distress: A systematic review. World J Diabetes 2020; 11(10): 459-467
- URL: https://www.wjgnet.com/1948-9358/full/v11/i10/459.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i10.459
Diabetes mellitus is one of the most prevalent diseases in the world. Today, the worldwide incidence of diabetes is estimated to be over 450 million[1]. Type 2 diabetes (T2D) comprises approximately 90% of cases and is associated with modifiable factors, genetics and aging. Target organ lesions such as nephropathy, neuropathy, retinopathy and cardiopathy are long-term results of hyperglycemia. Organ complications and attempts at diabetes control may affect physical and emotional health and patient quality of life, leading to negative psychological conditions. Patients with depressive symptoms present with more hospitalization days, poorer self-management behavior, more absenteeism, and increased morbidity and mortality. However, it is important to highlight that most diabetic patients with high levels of depressive symptoms are not clinically depressed, rather, they could be suffering from diabetic-specific distress consequences[2].
Diabetes distress (DD) is defined as the fears, worries and concerns of individuals with type 1 or type 2 diabetes related to the emotional responses to diagnosis, risk of complications, self-management demands, unresponsive providers and quality of interpersonal relationships. Identifying patients with DD and addressing the social, personal and health-related causes of distress might have a greater impact than prescribing treatments for clinical depression[2]. Initially, to serve as a screening measure for DD, the Problem Areas in Diabetes scale (PAID), a 20-item questionnaire with no subscales, was developed and has been linked to diabetes self-care behaviors and glycemic controls[3,4]. In 2009, McGuire et al[5] validated the PAID-5, a short version of PAID with items 3, 6, 12, 16 and 19 of the original scale, with 94% sensitivity and 80% specificity.
In 2005, Polonsky et al[3] validated a specific diabetes distress scale (DDS) with a 17-item self-reported questionnaire that captures four critical dimensions of distress: Emotional burden, regimen distress, interpersonal distress and physician distress. Higher DDS scores are associated with poorer diabetes outcomes, such as high HbA1c, low self-efficacy, choosing unhealthy foods[3] and even an increase in coronary artery disease incidence[6].
In 2008, Fisher et al[4] presented a two-item screening version of the DDS, with items “feeling overwhelmed by the demands of living with diabetes” and “feeling that I am often failing with my diabetes regimen”, which showed good sensitivity (95%) and specificity (85%).
Simultaneously, in an attempt to measure DD, data management technologies, web-based interventions, telemedicine, mobile phones, applications and others have been used as modern tools of communication to improve healthcare. Some authors have already demonstrated that technological devices could enhance engagement, adherence, cost effectiveness and access to health interventions[7,8], having an impact on blood glucose control and T2D self-management[9-12]. However, review authors have are in disagreement about the benefits of this technology in T2D distress[13,14]. The primary aim of this review is to determine the impact of programs with technological interventions regarding disease management, not just as a communication alternative, on T2D distress through a DDS measurement study.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and checklist were followed in this study. The review protocol is registered on PROSPERO (https://www.crd.york.ac.uk/PROSPERO/), an international prospective register of systematic reviews with 160386 registered numbers.
The electronic databases used were MEDLINE, PubMed and EBSCO (which includes three databases with duplicates removed automatically: Library and Information Science Source, Academic Search Ultimate and PsycINFO) for studies published in English from January 2014 through December 2019. The Boolean logic search terms were: (1) T2Diabetes; (2) Diabetes distress; and (3) Technology OR mobile OR phone OR application OR web. We also systematically searched the reference lists of the included studies and relevant reviews.
Following the removal of duplicates, titles and/or abstracts were screened by the first reviewer and then a second reviewer. If a disagreement occurred, a third reviewer was consulted.
Inclusion criteria were studies with: (1) Only subjects over 18-years-old; (2) Subjects with T2D; (3) Randomized controlled trials (RCTs); (4) Any intervention with technology; use and (5) DDS present in main or secondary outcomes.
Exclusion criteria were studies with: (1) Non-English language text; (2) absence of a control group without technological intervention; and (3) the inclusion of only pregnant women.
The investigators collected the following from each eligible study in a full article screening: (1) Number of subjects recruited for randomization, including the presence of sample size calculations; (2) Main demographic descriptions, including age with standard deviation, gender and study design including duration; (3) Description of intervention and control groups; (4) distress outcome measures; and (5) Statistical significance results.
The JADAD scale was used to measure the likelihood of bias and was applied to each selected study by the two reviewers independently.
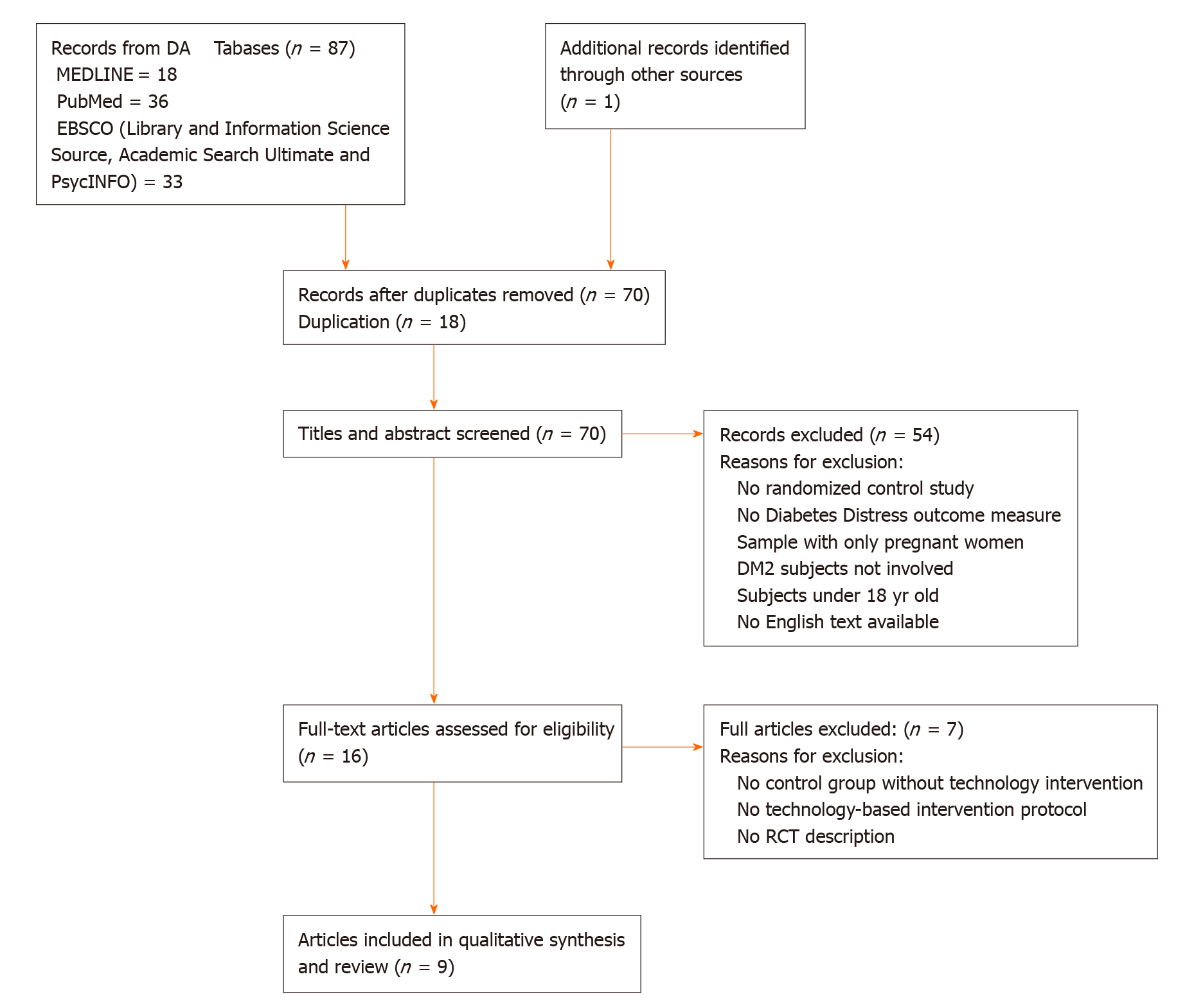
A PRISMA flowchart (Figure 1) summarizes the results of the search, screening process and reasons for exclusion. From the database sources, we collected 87 studies: (1) MEDLINE: 18; (2) PubMed: 36; and (3) EBSCO (Library and Information Science Source, Academic Search Ultimate and PsycINFO): 33. One article from the reference lists was included. After 18 duplicates were excluded (EBSCO system automatically excluded duplicated references from its three databases) by the two reviewers’ analysis, 70 references with titles and abstracts remained for screening. Studies with no randomized control design, no DD outcome measure, no involvement of T2D subjects, no English text available, samples with only pregnant women or individuals less than 18-years-old were excluded. Next, out of the 16 full text articles selected, 7 were excluded due to the absence of a control group without technology-based intervention or for only containing an RCT protocol description; the remaining nine full articles were analyzed for data extraction[15-23] (Figure 1).
Data extraction items are shown in Table 1. Of the nine articles analyzed, two did not present sample size calculations[15,22] including technique description. Three RCTs[17,19,23] used the DDS-17 items for the DD measure, four used the PAID-5[16,18,21,23], one used two subscales of the DDS, the five-item Regimen Distress subscale and the five-item Emotional Burden subscale version[15], and one used the PAID with 20 items[15]. In terms of demographics, female gender was the majority in six articles[15,16,19,20,22,23], and in one, no gender reporting was found[17]. Follow-up varied from 8 to 48 wk. One study had an 8-wk[16] period, one had 10 wk[20], two studies had 12 wk[17,23], three studies had 24 wk[18,21,22] and two had 48 wk[15,19] (Table 1).
| Ref. | Sample | SSC | Age average | Gender | Duration | IEWT | CGITE | DDS version used and data results | Statistical significance between groups |
| Fisher et al[15], 2014 | 392 | No | 56 | 53.8% Female, 46.2% Male | 48 wk | My path to a healthy life computer-assisted self-management plus problem-solving therapyTechnology: Phone calls and web-based diabetes self-management and diabetes distress change program | Leap ahead program delivers diabetes information only, and participants were not directed to use the information to engage in a specific or structured program of self-management or diabetes distress change | DDS (5- item Regimen Distress Subscale and 5-item Emotional Burden Subscale from DDS) | P = 0.50, No significant |
| Nobis et al[16], 2015 | 260 | Yes | 51 | 63% Female, 37% Male | 8 wk | GET.ON Mood Enhancer personalized, guided, Internet-based diabetes self-help intervention with personalized feedback from psychologist | Control Group: Unguided psychoeducation program | DDS (PAID-5) | P < 0.001, Significant |
| Bajaj et al[17], 2016 | 139 | Yes | 56.4 | NR | 12 wk | Long-acting insulin glargine Titration Web Tool (LTHome), instructions on insulin administration and dosing, as well as the use of the web-based LTHome tool (containing a rules engine-based algorithm for titration), provided by a delegated nonhealthcare professional Technology: Web-based insulin titration algorithm embedded in a range of platforms, including glucometer, personal computer and mobile phones | EUT of Glargine Titration: Insulin dosing and titration instructions were provided by CDEs according to a standard protocol | 17-item DDS | P = 0.04; Significant |
| Rondags et al[18], 2016 | 137 | Yes | 52 | 46% Female, 54% Male | 24 wk | HypoAware consists of three group sessions and is combined with two online modules. Group sessions are highly interactive and aimed at patient empowerment to improve symptom recognition, risk awareness, preventive and problem-solving strategies and coping with (the risk of) hypoglycemia Technology: two online modules | Care as usual had access to comprehensive diabetes care as normally provided by their diabetes team | DDS (PAID-5) | P = 0.365, No significant |
| Holland-Carter et al[19], 2017 | 563 | Yes | 55.1 | 71% Female, 29% Male | 48 wk | WW approach, supplemented with phone and email counseling with a CDE Technology: WW online tools, unlimited phone calls and email diabetes educator consultation | SC, one session of face-to-face T2DM nutritional counseling by a registered dietitian as well as follow-up written information | 17-item DDS | P < 0.001, Significant |
| Newby et al[20], 2017 | 106 | Yes | 47 | 71% Female, 29% Male | 10 wk | ICBT not tailored to diabetes | TAU control group | DDS (PAID 20 items) | P < 0.001, Significant |
| Ebert et al[21], 2017 | 260 | Yes | 50.8 | 43.8% Female, 56.2% Male | 24 wk | GET.ON Mood Enhancer personalized, guided, Internet-based diabetes self-help intervention with personalized feedback from psychologist Technology: active online training on diabetes and depression, personalized approach | Control: Usual treatment | DDS (PAID-5) | P < 0.001, Significant |
| Schlicker et al[22], 2019 | 253 | No | 50.7 | 62.8% Female, 37.2% Male | 24 wk | GET.ON Mood Enhancer personalized, guided, Internet-based diabetes self-help intervention with personalized feedback from psychologist | Placebo online, online psychoeducation control condition | DDS (PAID-5) | P = 0.75, No significant |
| Clarke et al[23], 2019 | 780 | Yes | 58 | 68.8% Female, 31.2% Male | 12 wk | My compass program is a fully automated, web- based cognitive behavioral, self-guided public health treatment program for common mental health problems with a personalized treatment plan based on an assessment of user symptoms. Technology: Web-based, fully automated program with self-guided cognitive behavioral treatment through personal computer or mobile phone | Healthy lifestyles: Placebo without therapeutic, only informative, no feedback content | 17-item DDS | P = 0.36, No significant |
The quality assessment of the studies with the JADAD scale is presented in Table 2. Eight RCTs were scored with good quality (total score ≥ 3) and low bias risk. One study[22] was scored as poor quality with high bias risk. The main topic with the fewest points was blindness, and three articles had a double-blind design[19,20,23] (Table 2).
| Ref. | Randomization, method | Double blind | Descriptions of withdrawals and dropouts | Total |
| Bajaj et al[17] | 1 + 1 | 0 | 1 | 3 |
| Clarke et al[23] | 1 + 1 | 1 + 1 | 1 | 5 |
| Ebert et al[21] | 1 + 1 | 1 | 1 | 4 |
| Fisher et al[15] | 1 + 1 | 0 | 1 | 3 |
| Holland-Carter et al[19] | 1 + 1 | 1 + 1 | 1 | 5 |
| Nobis et al[16] | 1 + 1 | 0 | 1 | 3 |
| Rondags et al[18] | 1 + 1 | 0 | 1 | 3 |
| Schlicker et al[22] | 1 | 0 | 0 | 1 |
| Newby et al[20] | 1 + 1 | 1 + 1 | 1 | 5 |
This review suggests that technology could have a positive impact on DD in T2D patients. The majority of articles selected for qualitative synthesis (six[16,17,19,20,23] out of nine[15,18,22]) showed significant DD scale improvement in the technology intervention groups over the initial data. Five articles[16,17,19-21] showed significant differences between groups. Studies with technological interventions had significantly lower DDS scores at the end than at baseline. Although a study did not find significant differences between groups, all participants showed symptom improvement in DDS scores, including the control group.
Rondags et al[18] did not find a significant difference in the DDS scores between the groups but described a 30% drop in the HypoAware Group (technology-based intervention) in distress concerning hypoglycemia.
Regarding quality, perhaps as a result of the RCT inclusion criteria, only one article had a high bias risk on the JADAD scale, and two had no sample size calculation, reflecting good scientific quality of the articles reviewed.
Newby et al[20] and Clarke et al[23] showed different findings for generic web-based interventions with psychological content and highlighted the necessity of a diabetes-specific web-based approach. In contrast, Nobis et al[16] and Ebert et al[21] found similar results concerning diabetic distress improvement with the same web-based mood enhancer intervention (GET.ON MED).
Web-based interventions had better results when users received program feedback personalized in its content[24]. According to this affirmative, all publications with DDS improvement in our review presented tailored technology interventions with personal adjustments in their programs[16,17,19,20,23]. Thus, it seems that more than a diabetes-tailored approach, patient-tailored and-guided technological programs were more successful in type 2 DDS improvement.
However, these findings are in contrast to the results of Mathiesen et al[13] in their trial about the influence of technology interventions on T2D distress. They attributed their findings to the vulnerability of T2D patients facing tailored digital interventions, resulting in an increase in distress, such as “suffering informational confusion, experiencing digital alienation, and missing the human touch”, mainly because “navigating a complex digital portal on diabetes might be more challenging than simply accessing the site” and the digital caregivers’ approach. Despite the limitations of this study, such as the small sample size (12 subjects) and less scientific evidence compared to the nine articles analyzed in the present review, we considered some of the authors’ conclusions to improve our considerations. For example, the success of a digital T2D-tailored program depends on the quality of the caregiver-patient relationship, and topics such as digital buddy rights choice and training for vulnerable T2D patient care or a wider social network must be included in the technology T2D intervention framework.
Possible biases arising from the different scales of DD in the included studies (17-item DDS, PAID and PAID-5) became null due to the use of the same distress scale in the intervention and control groups. This is the main reason to consider only DDS scales in the review and not any of the isolated distress symptoms or other measures in the outcome.
First, our review did not discriminate psychological level in article subject recruitment, and some authors attributed differences in the impact of DD approaches to baseline depression levels[20,23], and RCT studies may not be comparable in their demographic composition. However, we selected only RCTs, and the outcome was DDS improvement between groups with the same inclusion and exclusion criteria. That reduces possible biases such as better intervention results for populations with worse baseline levels of depression. Second, we did not exclude studies that also included type 1 diabetes patients, but we limited the participants’ ages to over 18-years-old; thus, the late depression symptoms and increased somatic burden associated with patients in the age range of T2D[25] were minimized with the exclusion of younger subjects.
These review findings could contribute to future new approaches on the elaboration of technological strategies to cope with T2D distress and consequently improve organ complications, patient well-being and cost effectiveness in the management of T2D.
The findings of the present study show that management of T2D distress can have positive outcomes with technology-based interventions and highlight that the best results come from programs that offer not only a personalized digital/technological experience for patients but also routine caregiver support and attention.
High diabetes distress is associated with poorer diabetes outcomes. Technological interventions have been used as modern tools of communication to improve communication and can impact diabetes self-management, engagement and adherence. Understanding the impact of programs with technological interventions regarding disease management on type 2 diabetes distress bears clinical significance.
Review authors disagree about the benefits of this technology in type 2 diabetes distress. We systematically reviewed randomized controlled trials that studied the impact of technology interventions on type 2 diabetes distress.
The goal of this study is to provide comprehensive overview of the impact of technology interventions on type 2 diabetes distress.
We systematically searched MEDLINE, PubMed and EBSCO with the Boolean logic search terms were: (1) T2Diabetes; (2) Diabetes distress and (3) Technology OR mobile OR phone OR application OR web. We also systematically searched the reference lists of the included studies and relevant reviews.
We found nine full articles that met the inclusion criteria. Six out of nine articles showed that technology interventions had a positive impact on diabetes distress scale scores when compared with the initial data. Among these six articles, five showed a greater reduction in the diabetes distress scores from control interventions. Web-based interventions had good results when users received personalized feedback and routine caregiver support and attention.
Technology-based interventions have a positive impact on type 2 diabetes distress management, and programs that include routine caregiver support and attention show the best results.
These review findings could contribute to the development of new approaches on the elaboration of technological strategies to cope with type 2 diabetes distress and consequently improve treatment outcomes, resulting in patient well-being and better biological consequences in the management of type 2 diabetes.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wan TT S-Editor: Zhang L L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271-281. |
| 2. | Fisher L, Skaff MM, Mullan JT, Arean P, Mohr D, Masharani U, Glasgow R, Laurencin G. Clinical depression vs distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30:542-548. |
| 3. | Polonsky WH, Fisher L, Earles J, Dudl RJ, Lees J, Mullan J, Jackson RA. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28:626-631. |
| 4. | Fisher L, Glasgow RE, Mullan JT, Skaff MM, Polonsky WH. Development of a brief diabetes distress screening instrument. Ann Fam Med. 2008;6:246-252. |
| 5. | McGuire BE, Morrison TG, Hermanns N, Skovlund S, Eldrup E, Gagliardino J, Kokoszka A, Matthews D, Pibernik-Okanović M, Rodríguez-Saldaña J, de Wit M, Snoek FJ. Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia. 2010;53:66-69. |
| 6. | Kubzansky LD, Davidson KW, Rozanski A. The clinical impact of negative psychological states: expanding the spectrum of risk for coronary artery disease. Psychosom Med. 2005;67 Suppl 1:S10-S14. |
| 7. | Martínez-Pérez B, de la Torre-Díez I, López-Coronado M, Herreros-González J. Mobile apps in cardiology: review. JMIR Mhealth Uhealth. 2013;1:e15. |
| 8. | Maddison R, Rawstorn JC, Shariful Islam SM, Ball K, Tighe S, Gant N, Whittaker RM, Chow CK. mHealth Interventions for Exercise and Risk Factor Modification in Cardiovascular Disease. Exerc Sport Sci Rev. 2019;47:86-90. |
| 9. | Quinn CC, Shardell MD, Terrin ML, Barr EA, Ballew SH, Gruber-Baldini AL. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care. 2011;34:1934-1942. |
| 10. | Or CK, Tao D. Does the use of consumer health information technology improve outcomes in the patient self-management of diabetes? Int J Med Inform. 2014;83:320-329. |
| 11. | Hou C, Carter B, Hewitt J, Francisa T, Mayor S. Do Mobile Phone Applications Improve Glycemic Control (HbA1c) in the Self-management of Diabetes? Diabetes Care. 2016;39:2089-2095. |
| 12. | Hunt CW. Technology and diabetes self-management: An integrative review. World J Diabetes. 2015;6:225-233. |
| 13. | Mathiesen AS, Thomsen T, Jensen T, Schiøtz C, Langberg H, Egerod I. The influence of diabetes distress on digital interventions for diabetes management in vulnerable people with type 2 diabetes: A qualitative study of patient perspectives. J Clin Transl Endocrinol. 2017;9:41-47. |
| 14. | Alcántara-Aragón V. Improving patient self-care using diabetes technologies. Ther Adv Endocrinol Metab. 2019;10:2042018818824215. |
| 15. | Fisher L, Hessler D, Masharani U, Strycker L. Impact of baseline patient characteristics on interventions to reduce diabetes distress: the role of personal conscientiousness and diabetes self-efficacy. Diabet Med. 2014;31:739-746. |
| 16. | Nobis S, Lehr D, Ebert DD, Baumeister H, Snoek F, Riper H, Berking M. Efficacy of a web-based intervention with mobile phone support in treating depressive symptoms in adults with type 1 and type 2 diabetes: a randomized controlled trial. Diabetes Care. 2015;38:776-783. |
| 17. | Bajaj HS, Venn K, Ye C, Aronson R. Randomized Trial of Long-Acting Insulin Glargine Titration Web Tool (LTHome) Versus Enhanced Usual Therapy of Glargine Titration (INNOVATE Trial). Diabetes Technol Ther. 2016;18:610-615. |
| 18. | Rondags SM, de Wit M, Twisk JW, Snoek FJ. Effectiveness of HypoAware, a Brief Partly Web-Based Psychoeducational Intervention for Adults With Type 1 and Insulin-Treated Type 2 Diabetes and Problematic Hypoglycemia: A Cluster Randomized Controlled Trial. Diabetes Care. 2016;39:2190-2196. |
| 19. | Holland-Carter L, Tuerk PW, Wadden TA, Fujioka KN, Becker LE, Miller-Kovach K, Hollander PL, Garvey WT, Weiss D, Rubino DM, Kushner RF, Malcolm RJ, Raum WJ, Hermayer KL, Veliko JL, Rost SL, Sora ND, Salyer JL, O'Neil PM. Impact on psychosocial outcomes of a nationally available weight management program tailored for individuals with type 2 diabetes: Results of a randomized controlled trial. J Diabetes Complications. 2017;31:891-897. |
| 20. | Newby J, Robins L, Wilhelm K, Smith J, Fletcher T, Gillis I, Ma T, Finch A, Campbell L, Andrews G. Web-Based Cognitive Behavior Therapy for Depression in People With Diabetes Mellitus: A Randomized Controlled Trial. J Med Internet Res. 2017;19:e157. |
| 21. | Ebert DD, Nobis S, Lehr D, Baumeister H, Riper H, Auerbach RP, Snoek F, Cuijpers P, Berking M. The 6-month effectiveness of Internet-based guided self-help for depression in adults with Type 1 and 2 diabetes mellitus. Diabet Med. 2017;34:99-107. |
| 22. | Schlicker S, Weisel KK, Buntrock C, Berking M, Nobis S, Lehr D, Baumeister H, Snoek FJ, Riper H, Ebert DD. Do Nonsuicidal Severely Depressed Individuals with Diabetes Profit from Internet-Based Guided Self-Help? J Diabetes Res. 2019;2019:2634094. |
| 23. | Clarke J, Sanatkar S, Baldwin PA, Fletcher S, Gunn J, Wilhelm K, Campbell L, Zwar N, Harris M, Lapsley H, Hadzi-Pavlovic D, Christensen H, Proudfoot J. A Web-Based Cognitive Behavior Therapy Intervention to Improve Social and Occupational Functioning in Adults With Type 2 Diabetes (The SpringboarD Trial): Randomized Controlled Trial. J Med Internet Res. 2019;21:e12246. |
| 24. | Clarke G, Eubanks D, Reid E, Kelleher C, O'Connor E, DeBar LL, Lynch F, Nunley S, Gullion C. Overcoming Depression on the Internet (ODIN) (2): a randomized trial of a self-help depression skills program with reminders. J Med Internet Res. 2005;7:e16. |