Copyright
©The Author(s) 2022.
World J Diabetes. Nov 15, 2022; 13(11): 926-939
Published online Nov 15, 2022. doi: 10.4239/wjd.v13.i11.926
Published online Nov 15, 2022. doi: 10.4239/wjd.v13.i11.926
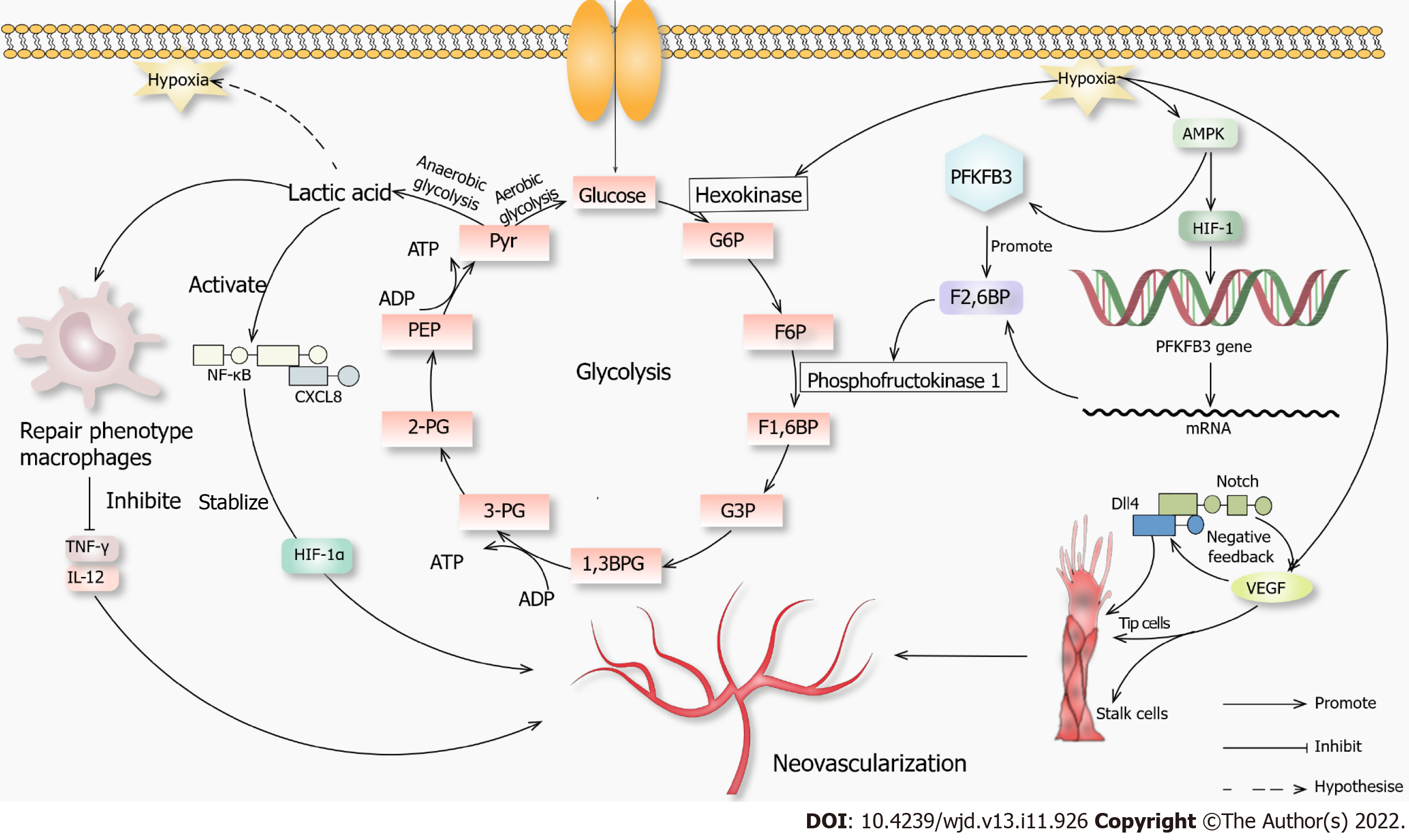
Figure 1 Novel mechanisms of neovascularization.
When glucose enters the cell through channels that initiate glycolysis in an aerobic environment, when the anaerobic glycolysis, lactic acid can cause macrophages to convert to a repair phenotype. These macrophages will not produce inflammatory factors such as tumor necrosis factor γ and interleukin 12, which promotes angiogenesis. Lactic acid can activate the nuclear factor kappa-B-C-X-C motif chemokine 8 pathway, promote vascular endothelial growth factor (VEGF) secretion, stabilize hypoxia-inducible factor-1α (HIF-1α), and promote angiogenesis. When hypoxia occurs in the environment, it promotes hexokinase, which promotes glycolysis, and AMPK, which promotes hypoxia-inducible factor-1. It then acts on 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3. AMPK also acts directly on 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3, and they jointly promote fructose-2,6-biphosphatase, which acts on phosphofructokinase 1 and promotes glycolysis. Hypoxia also promotes VEGF secretion, and a high concentration of VEGF activates the Notch-DLL4 pathway, directly or indirectly promoting the proliferation of tip cells. VEGF also promotes the growth of stalk cells, and both promote angiogenesis. 1,3BPG: 1,3 diphosphoglycerate; 2-PG: 2-phosphoglycerate; 3-PG: 3-phosphoglycerate; F1,6BP: Fructose 1,6-diphosphate; F2,6BP: Fructose 2,6-diphosphate; F6P: Fructose 6 phosphate; G3P: Glyceraldehyde-3-phosphate; G6P: Glucose 6-phosphate; PEP: Phosphoenolpyruvate; Pyr: Pyruvic acid.
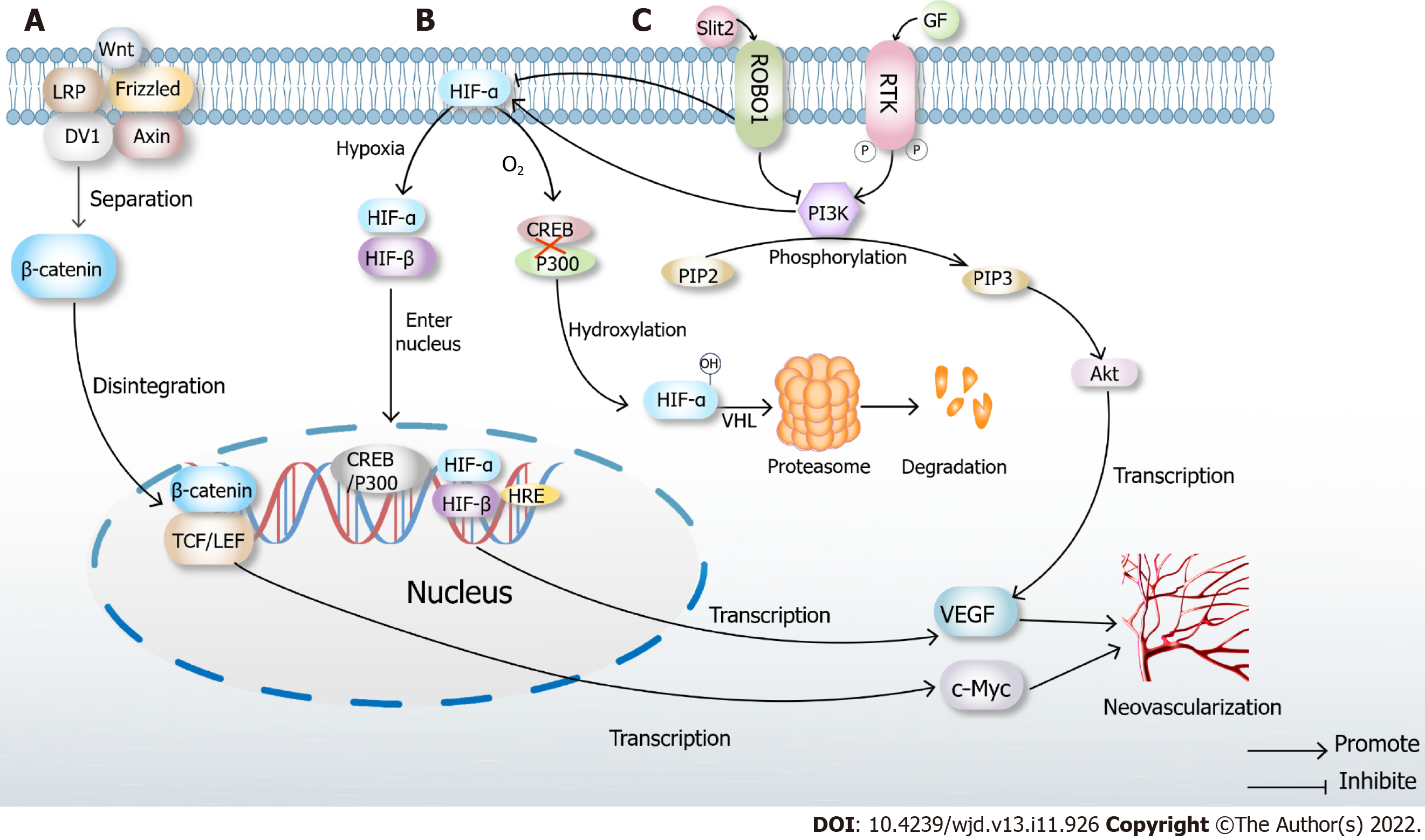
Figure 2 Classical mechanisms of neovascularization.
A: Classical Wnt pathway. When there is no Wnt signal, LRP, Frizzled, DV1 and Axin are closely combined with β-catenin. In the presence of Wnt signaling, the complex disintegrates, and β-catenin enters the nucleus, binds to TCF/LEF, transcribes multiple downstream signals such as c-Myc and ultimately promotes neovascularization; B: Under normoxic conditions, hypoxia-inducible factor (HIF)-α inhibits the binding of cAMP-response element binding protein (CREB) and P300, which causes hydroxylation of HIF, then leads to proteasome degradation and inactivation after VHL ubiquitination. When hypoxia occurs, prolyl hydroxylase domains become inactive, allowing HIF-α to migrate to the nucleus, where it dimerizes with HIF-β. The dimer binds to the hypoxia response elements of specific genes in DNA, transcribes thousands of genes such as vascular endothelial growth factor (VEGF) and promotes neovascularization; C: High glucose induces Slit2/Roundabout 1 (Robo1) binding, and Robo1 inhibits the activation of phosphatidylinositol 3kinase (PI3K) /protein kinase B (Akt) and HIF-1α/VEGF signaling pathway and inhibits angiogenesis. PI3K inhibitors also inhibit the HIF-1α/VEGF signaling pathway. After activation of the PI3K/Akt signaling pathway, a variety of cytokines including VEGF will be transcribed to promote angiogenesis.
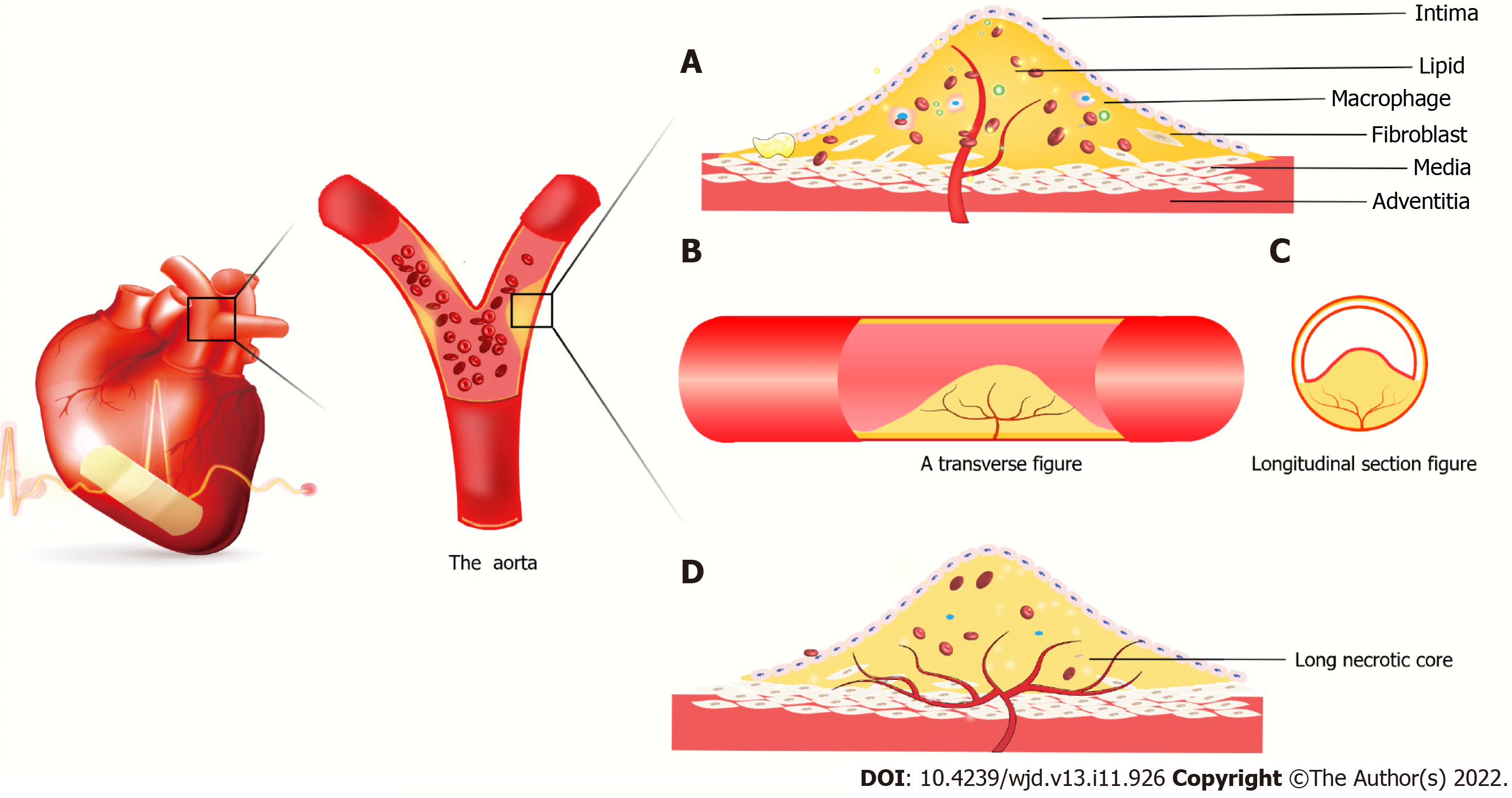
Figure 3 Atherosclerotic plaque forms in coronary vessels.
A: New blood vessels from the outer membrane to the media and intima, macrophages and smooth muscle cells gobble up from the film in lipid foam cell formation. The red blood cells, cytokines, etc extend the blood vessels into the plaque. It is because the spin-off of new blood vessels leads to unstable plaques, plaque hemorrhage and rupture resulting in acute coronary syndrome; B: Axial slice of intravascular plaque and neovascularization grows axially in the plaque; C: Transverse view of intravascular plaque; D: It is believed that neovascularization grows in the axial direction in plaques. Due to insufficient growth of neovascularization, long necrotic plaques are ruptured due to ischemia and hypoxia in the plaque, resulting in acute coronary syndrome.
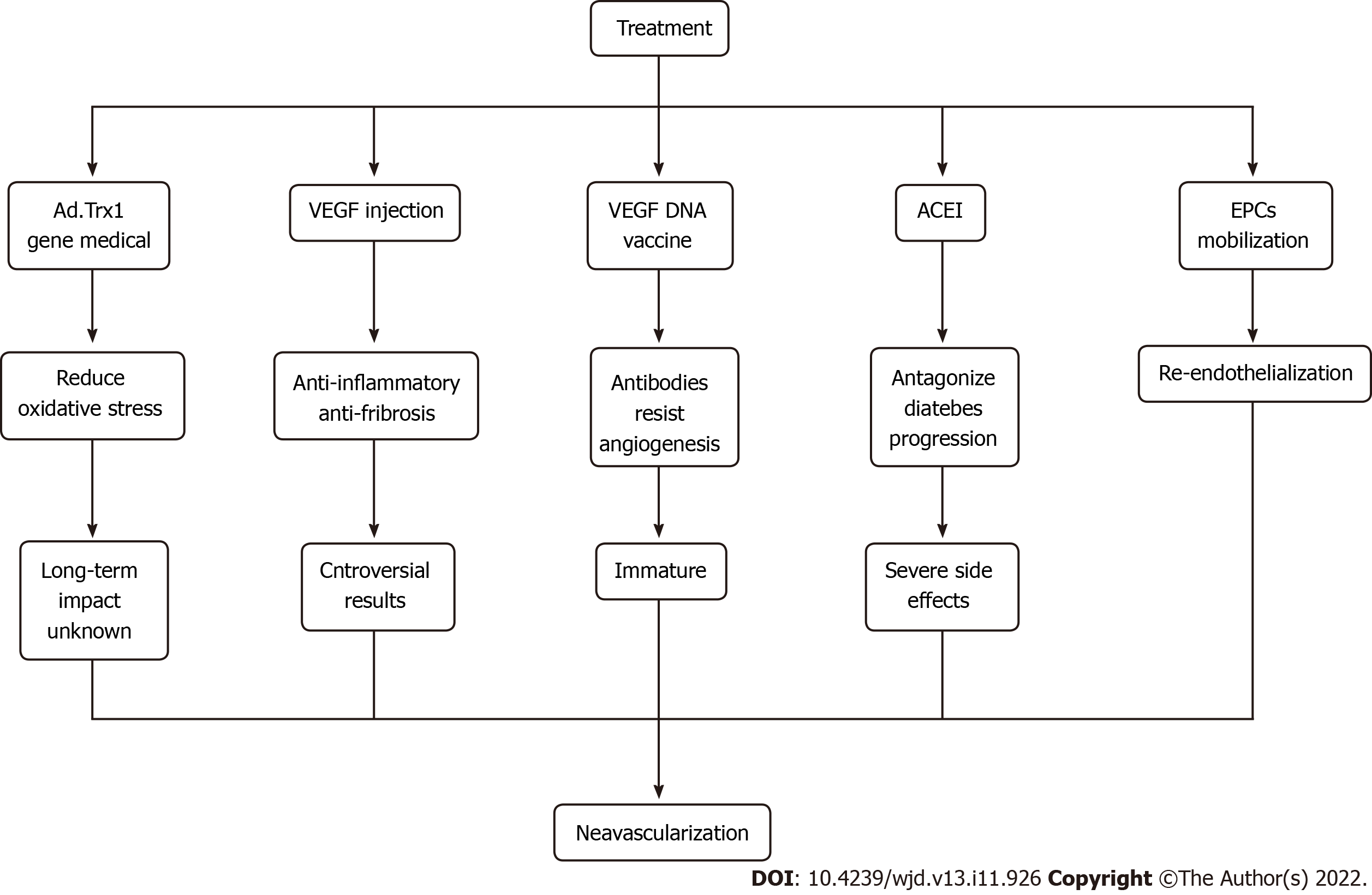
Figure 4 Cutting-edge approaches to therapeutic angiogenesis.
ACEI: Angiotensin converting enzyme inhibitors; EPCs: Endothelial progenitor cells; VEGF: Vascular endothelial growth factor.
- Citation: Cai Y, Zang GY, Huang Y, Sun Z, Zhang LL, Qian YJ, Yuan W, Wang ZQ. Advances in neovascularization after diabetic ischemia. World J Diabetes 2022; 13(11): 926-939
- URL: https://www.wjgnet.com/1948-9358/full/v13/i11/926.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i11.926