Published online Dec 15, 2013. doi: 10.4251/wjgo.v5.i12.222
Revised: September 22, 2013
Accepted: October 11, 2013
Published online: December 15, 2013
Processing time: 116 Days and 11.6 Hours
Pneumo-computed tomography (PnCT) is a technique primarily developed and used to study stenotic lesions of the esophagus, gastroesophageal junction and stomach for pre-surgical planning. It helps to define both upper and lower borders of neoplasms located in the aforementioned areas. It achieves maximum lumen distension with CO2 highlighting thickened areas of the esophageal wall, thus allowing an accurate quantification of their extents. Although there are other alternatives for distension (oral contrast agents, water and effervescent granules), they may be suboptimal. Patients with locally advanced esophageal cancer have a dismal prognosis despite surgical resection. Therefore, neoadjuvant treatment strategies using radiation therapy and chemotherapy were developed to improve survival. Neoadjuvant therapy improves esophageal tumor prognosis in a substantial proportion of patients, and the use of imaging techniques is mandatory to detect their response. PnCT combined with virtual endoscopy and multiplanar reconstruction enhances morphologic details in esophageal cancer, and thus would allow an improved assessment of response to neoadjuvant treatment. Therefore, more information could be provided to assess the efficacy of pre-surgical treatment. We describe the potential use of PnCT to assess the response to neoadjuvant therapy in esophageal cancer with an imaging pathologic correlation.
Core tip: Pneumo-computed tomography may be a useful technique to monitor neoadjuvant therapy response as it enhances morphologic details. Besides, it provides key information for surgical planning as it helps to define both upper and lower borders of esophageal or gastro-esophageal neoplasms in a single examination.
- Citation: Ulla M, Gentile E, Yeyati EL, Diez ML, Cavadas D, Garcia-Monaco RD, Ros PR. Pneumo-CT assessing response to neoadjuvant therapy in esophageal cancer: Imaging-pathological correlation. World J Gastrointest Oncol 2013; 5(12): 222-229
- URL: https://www.wjgnet.com/1948-5204/full/v5/i12/222.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v5.i12.222
Esophageal carcinoma is the sixth most common cause of cancer deaths worldwide[1]. There are approximately 14000 new cases of esophageal cancer per year in the United States, half of which are adenocarcinomas[2]. It is currently the most rapidly increasing cancer in the United States and Western Europe[3]. Prognosis is poor, with an overall survival of less than 10% within 5 years[4,5]. Therefore, there is need to improve the survival rate for patients with this disease, by earlier diagnosis when prognosis is more favorable, and/or by improving its therapy[6].
Neoadjuvant treatment strategies have been developed to improve survival[7]. Under current standards, chemoradiation is administered preoperatively in the majority of patients with advanced locoregional disease[8]. Thence, the ability to predict response to this combined therapy is clearly desirable.
Despite the importance of accurate pre-operative staging of esophageal neoplasm, there isn’t a well accepted method to do so by imaging[9]. The spectrum of diagnostic modalities most commonly used used besides computed tomography (CT), includes endoscopic ultrasound, positron emission computed tomography (PET)/CT and magnetic resonance imaging (MRI). The strength of endoscopic ultrasound is in its role in the initial staging of esophageal cancer. One weakness is its inaccuracy for staging after neoadjuvant therapy because of its inability to distinguish inflammation and fibrosis from residual cancer[10].
Even though PET/CT is primarily indicated to look for distant metastases, it has also been used as a noninvasive test to evaluate response after neoadjuvant therapy before surgical resection[11,12]. One of its drawbacks lies in the fact that it does not provide accurate information for surgical planning because of lack of distension of the esophageal lumen.
MRI studies using routine clinical protocols have demonstrated a limited ability to evaluate esophageal anatomy[6]. Dynamic contrast-enhanced MRI is an emerging approach that needs further validation to be used in daily practice.
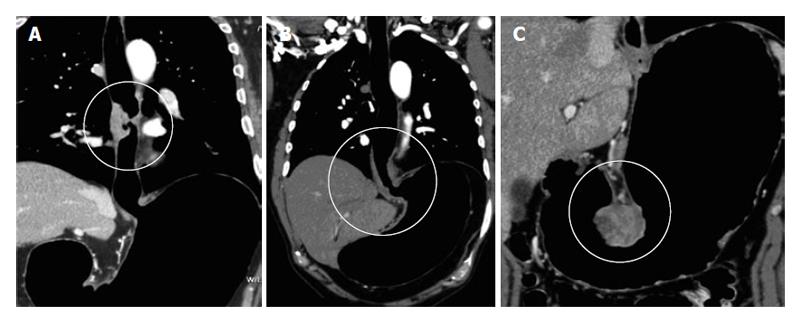
PneumoCT (PnCT) is a recently described technique that optimizes tumor visualization in the esophageal wall, gastroesophageal junction (GEJ) and stomach[13] (Figure 1). It achieves maximum lumen distension, highlighting thickened wall areas[13,14].
The purpose of this review is to present a correlation between PnCT findings and post surgical pathology in order to evaluate the response of esophageal cancer to neoadjuvant therapy.
First, we discuss the basic technique of how to perform PnCT. Second, we describe the reconstruction steps followed by the classification and CT parameters used to assess response to neoadjuvant therapy. Finally, we present clinical examples of pre- and post-neoadjuvant cases with imaging-pathological correlation.
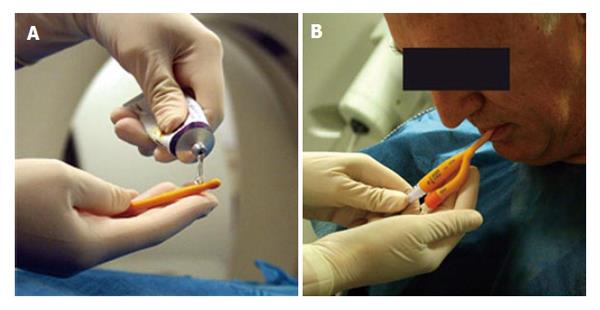
Patients are given before their imaging appointment an information sheet describing the procedure in detail including mention of slight discomfort that may be experienced by the esophageal distension. Patients with an 8-h pre-procedural fasting are received in the radiology department by a nurse, and a peripheral venous line is placed. Once in the CT suite, after administration via spray of a local anesthetic a lubricated Foley catheter is introduced transorally or transnasally, placing its distal tip below the cricopharyngeal muscles (Figure 2).
Continuous and sustained supply of CO2 is maintained during the CT acquisition, with a pressure between 10 and 20 mmHg. We use the the same CO2 pump as in CT-Colonography (Protocol pump, PROTOC02L, E-Z-EM, Inc., Lake Success, NY, United States). Patients are instructed to hold the air and avoid burping during the procedure.
Pn6CTs are performed at our institution with Aquilion 64-row multidetector computed tomography (MDCT) (Toshiba Inc, Tokyo, Japan) with the following technical parameters: 0.5 mm slices, 0.25 mm table feed, 50 mAs, 120 kV, 0.75 s rotation time and 0.875 pitch. Anterior and lateral scout views are obtained to protocol the scans. We perform two cervico-thoraco-abdominal phases, the first non-enhanced and the second enhanced. The time required for each acquisition is approximately of 8 seconds. Nonionic iodinated contrast (Iobitridol, Xenetix® 350; Guerbet, France) at a dose of 1 mL/kg is infused using an automatic injection pump at a flow of 2.5 mL/s. No oral contrast is used. We haven’t experienced a major adverse events specific to the CO2 insufflation. The typical effective radiation dose is 18 mSv.
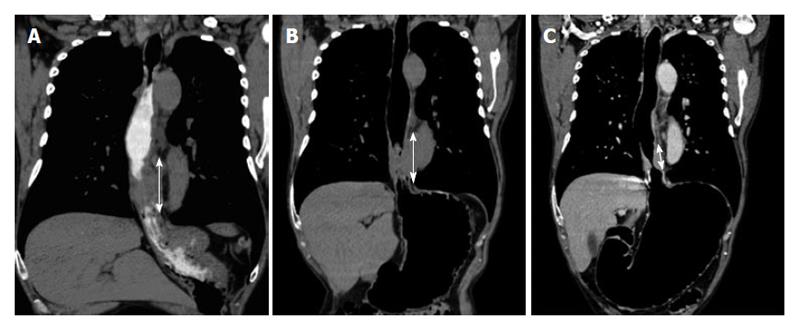
Other well-known alternatives for esophageal and gastric lumen distension (i.e., oral contrast agents or effervescent granules) are used in daily practice[15]. However, distention may be suboptimal due to rapid transit of contrast and the required esophageal distension cannot always be achieved[16]. Oral contrast enhancement may generate confusing images, with the same density as the tumor[17,18]. Moreover, a suboptimal distension can cause distortion both in the quantification of the extension and in the degree of wall thickening (Figure 3).
Once acquired, images are sent to a Vitrea 2 working station (Vital Images, Inc, Minnesota, United States) for evaluation. Multiplanar reconstructions (MPR) and curved MPRs are performed with different window settings in order to characterize the lesion. In addition, we performed 3D reconstructions with different window settings (surface-shaded and transparent modes similar to the images obtained in single- and double-contrast barium studies). These images are easy to understand and allow visualization of the tumor. Finally, we obtain fly through views of the esophageal lumen and generate endoluminal views akin to esophagoscopy, to further asses lesion morphology.
A description of shape and location of the lesion as well as measurements of size and wall thickening are performed. Also, we evaluate for presence of periesophageal fat stranding, adenopathy, and extraesophageal disease Information regarding panoramic and longitudinal extent of the esophageal lesion is obtained.
This technique was originally developed for GEJ stenosis, since the GEJ is a known difficult area to distend with traditional double contrast esophagograms[13,14]. The obtained gastric distension led to an adequate definition of both the upper and lower borders of GEJ lesions and in turn proved to be helpful for surgical planning[13,14].
We then extended its use to all cases of esophageal stenosis impeding the passage an endoscope and also to cases were endoscopy was contraindicated. The latter include cases in which a noninvasive method for endoluminal lesion characterization stenosis grading, and beyond stricture visualization of the esophagus and/or the stomach is needed.
Palliation therapy with self-expandable stenting is the method of choice[19] both in unresectable esophageal tumors due to distant metastasis or local invasion, and in high-risk patients. In these patients, the definition of both upper and lower limits of the lesion in the longitudinal axis provided by PnCT allowed determining the stent graft length and the need for a valved stent. Thus no further barium studies are required.
Although the stomach can be well distended with other contrasts, such as water, milk, effervescent granules, the pyloric area is also a known difficult area to distend. Due to the optimal distension obtained with PnCT, we began to use this technique for distal stomach pathology with suspected pyloric involvement (Figure 4).
Esophageal tumors exhibit increased expression of proangiogenic factors such as basic fibroblast growth factor (bFGF) and vascular endothelial growth factor[20,21]. This form of malignancy is associated with greater vascularization and has a higher microvascular density compared with normal esophageal tissue and precancerous lesions[20-23]. These properties suggest a valid pathologic basis upon which quantifying the density with PnCT could not only detect esophageal tumors and but also evaluate their response to neoadjuvant therapy.
The following CT parameters are measured to evaluate (or restage) response to neoadjuvant therapy: wall thickening, density, and presence of adenopathy.
We compare the maximum diameters of pre- and post-neoadjuvant therapy for wall thickening evaluation. To analyze its density, we make the same pre- and post- neoadjuvant therapy comparison by placing a region of interests (ROI) at the same site of greatest wall thickening, excluding areas of low density representing wall necrosis. Finally, we compare adenopathy sizes as well before and after neoadjuvant therapy.
The use of neoadjuvant therapy began to be practiced for rectal cancer[24]. Its use was extended to esophageal and other gastrointestinal cancers[24]. Since neoadjuvant therapy changes the internal structure of tumor, pathologists have been faced to reconsider the staging of these neoplasms resected after receiving chemo-radiation. The appearance of fibrosis and necrosis confirms the action of therapy and constitutes the basis for several new staging classifications[24].
In 1997, Dworak et al[24] presented a pathological classification for rectal cancer based mainly on the difficulty of highlighting viable tumor cells in a fibronecrotic stroma. Thus, five categories were identified (Table 1). Due to its usefulness, this classification, has also been extrapolated to other gastrointestinal tumors[25].
| Dworak classification | |
| Dworak grade 0 | Is define as no regression |
| Dworak grade 1 | Is define as prevalence of active cells and fibrosis/necrosis |
| Dworak grade 2 | Is define as many of fibrosis or necrosis with active cells easier to find |
| Dworak grade 3 | Is define as scarce neoplastic cells, hard to detect |
| Dworak grade 4 | Is define as absence of tumor cells |
We use clinical examples to show the change observed in the CT findings comparing pre- and post-therapy PnCT scans with correlation with the Dworak classification obtained after surgery.
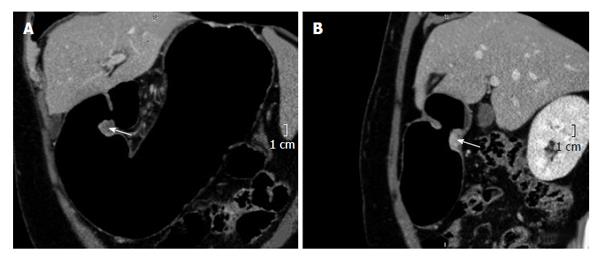
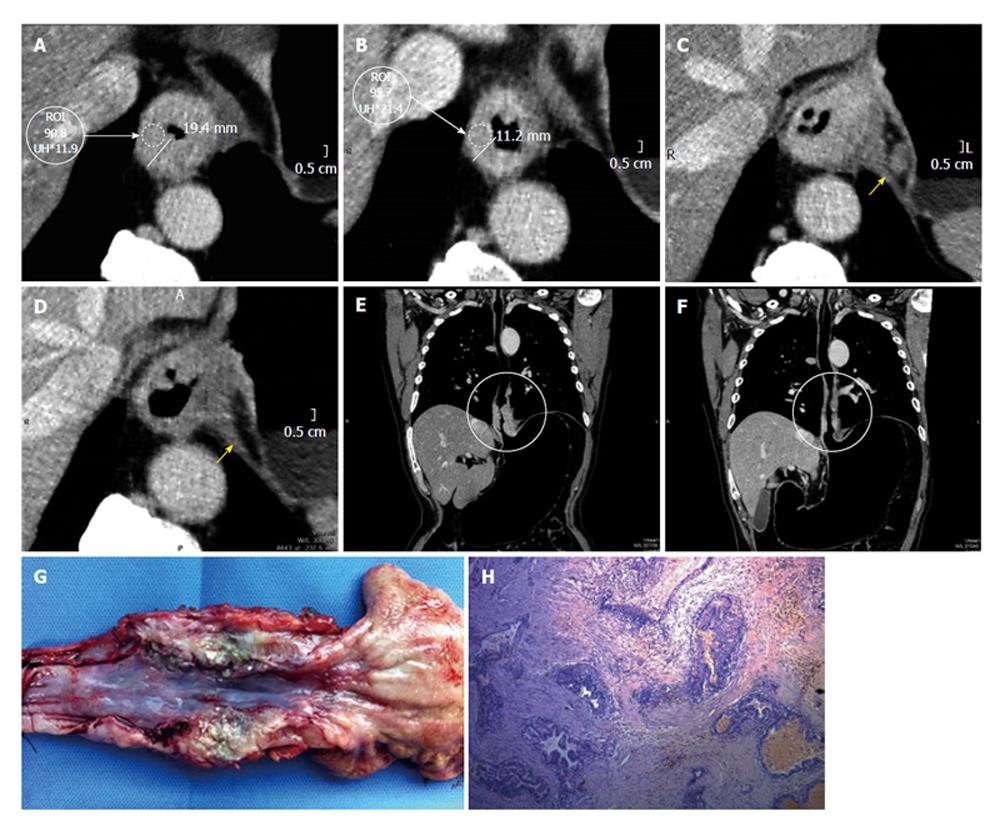
Wall thickening persistence is evident in the pre- and post-neoadjuvant therapy measure comparison at the same level (Figure 5). Placing the ROI on the site of maximum wall thickening, the density in Hounsfield Units (HU) increases from (90.8 HU) before to 95.7 HU after therapy. The increase in the density may be given by the persistence of active cells containing proangiogenic factors (Figure 5). Additionally, an adenopathy monitoring can be performed, determining it’s evolution.
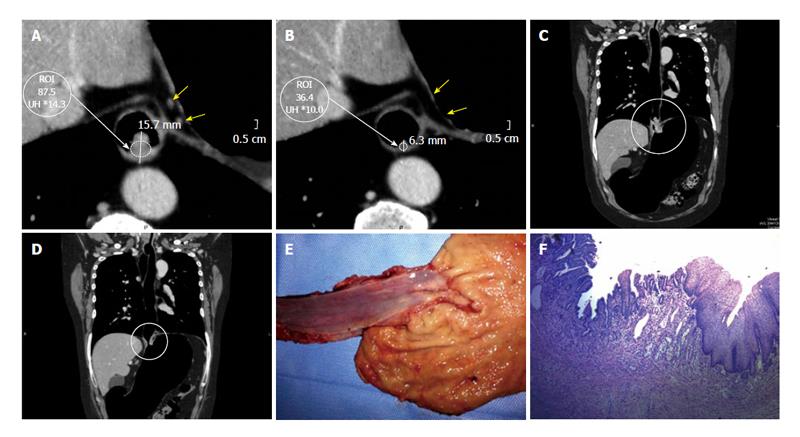
On the other hand, the pre- and post- comparison of the wall thickness in Figures 6 and 7 at the same level demonstrate a decrease of over 50% in the measured density, for instance before 85.7 HU and after 36.4 HU chemotherapy and radiotherapy (Figures 6-8). This decrease in density could be explained by the presence of extensive areas lacking proangiogenic factors. The disappearance of the lymphadenopathy can also be observed.
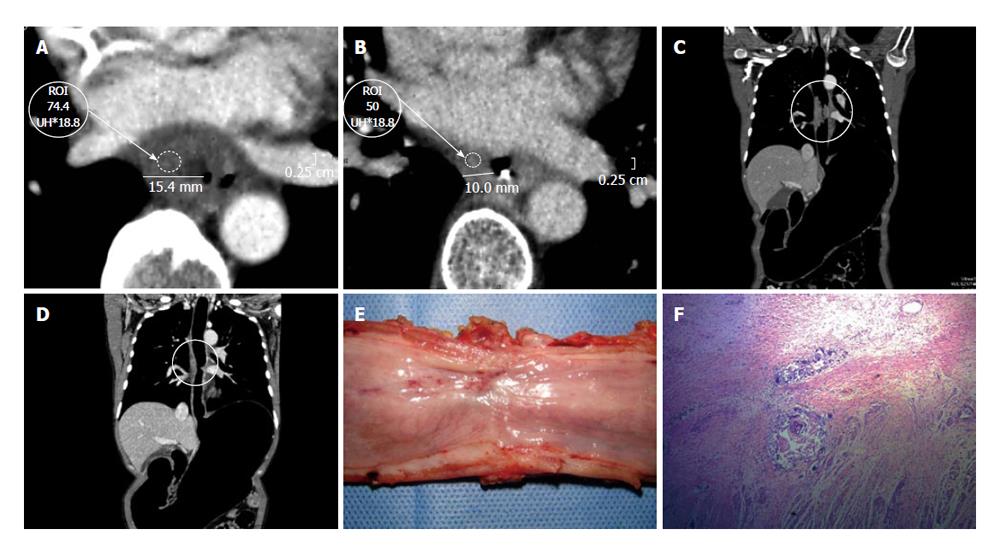
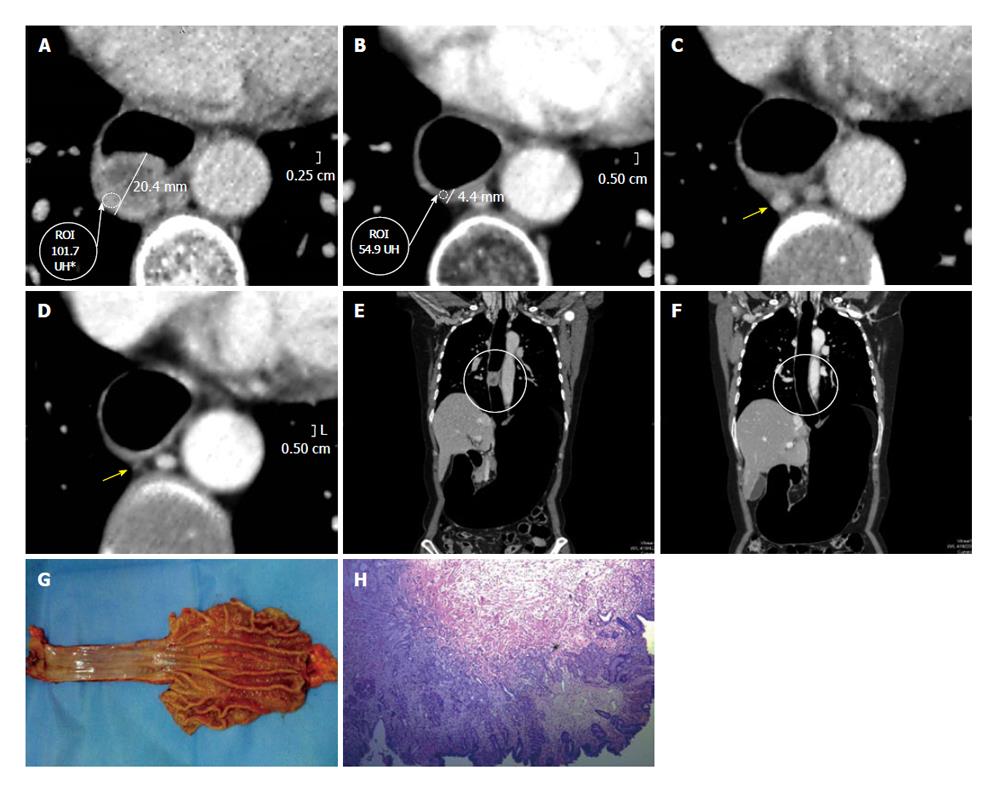
In patients without response to neoadjuvant therapy (Dworak I), there was no change in the density of the tumor when we compared the PnCT pre- and post- therapy. This finding could be explained by the presence in the active cells of bFGF and vascular endothelial growth factor. Conversely, there is reduction in the thickness and in the Hounsfield Units density (between 33% and 46% less) of the tumor in those patients with a good response to therapy, correlating well with a significant regression in the post-surgical pathological staging of Dworak III or IV neoplasms.
PnCT is a useful non-invasive imaging technique for evaluating esophageal and gastroesophageal tumors, allowing a precise evaluation of their size, location, local extension and regional adenopathy a single examination[13,14].
Further, PnCT provides key pre-surgical planning information, since it defines both upper and lower borders of neoplasms located in the GE junction[13,14] .
We also demonstrate the PnCT findings correlate with the Dworak pathologic classification and could allow assessing the response of esophageal neoplasms to neoadjuvant therapy. This is crucial regarding the overall prognosis and therapeutic strategy.
Our next goal is to prove in a prospective, blinded and randomized study the accuracy of PnCT to asses response to neoadjuvant therapy in esophageal neoplasms and its correlation with post resection pathological staging.
Authors wish to thank Osvaldo Castro for his imaging assistance.
P- Reviewer: Vieth M S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
| 1. | Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6112] [Cited by in RCA: 5989] [Article Influence: 332.7] [Reference Citation Analysis (0)] |
| 2. | Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3094] [Cited by in RCA: 2973] [Article Influence: 141.6] [Reference Citation Analysis (0)] |
| 3. | Bollschweiler E, Wolfgarten E, Gutschow C, Hölscher AH. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer. 2001;92:549-555. [PubMed] |
| 4. | Ide H, Nakamura T, Hayashi K, Endo T, Kobayashi A, Eguchi R, Hanyu F. Esophageal squamous cell carcinoma: pathology and prognosis. World J Surg. 1994;18:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Torres C, Turner JR, Wang HH, Richards W, Sugarbaker D, Shahsafaei A, Odze RD. Pathologic prognostic factors in Barrett’s associated adenocarcinoma: a follow-up study of 96 patients. Cancer. 1999;85:520-528. [PubMed] |
| 6. | Chang EY, Li X, Jerosch-Herold M, Priest RA, Enestvedt CK, Xu J, Springer CS, Jobe BA. The evaluation of esophageal adenocarcinoma using dynamic contrast-enhanced magnetic resonance imaging. J Gastrointest Surg. 2008;12:166-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Sherman CA, Turrisi AT, Wallace MB, Reed CE. Locally advanced esophageal cancer. Curr Treat Options Oncol. 2002;3:475-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Stahl M, Wilke H, Stuschke M, Walz MK, Fink U, Molls M, Siewert JR, Schroeder M, Makoski HB, Schmidt U. Clinical response to induction chemotherapy predicts local control and long-term survival in multimodal treatment of patients with locally advanced esophageal cancer. J Cancer Res Clin Oncol. 2005;131:67-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Chandawarkar RY, Kakegawa T, Fujita H, Yamana H, Hayabuthi N. Comparative analysis of imaging modalities in the preoperative assessment of nodal metastasis in esophageal cancer. J Surg Oncol. 1996;61:214-217. [PubMed] |
| 10. | Lightdale CJ, Kulkarni KG. Role of endoscopic ultrasonography in the staging and follow-up of esophageal cancer. J Clin Oncol. 2005;23:4483-4489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Swisher SG, Maish M, Erasmus JJ, Correa AM, Ajani JA, Bresalier R, Komaki R, Macapinlac H, Munden RF, Putnam JB. Utility of PET, CT, and EUS to identify pathologic responders in esophageal cancer. Ann Thorac Surg. 2004;78:1152-1160; discussion 1152-1160;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 215] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Levine EA, Farmer MR, Clark P, Mishra G, Ho C, Geisinger KR, Melin SA, Lovato J, Oaks T, Blackstock AW. Predictive value of 18-fluoro-deoxy-glucose-positron emission tomography (18F-FDG-PET) in the identification of responders to chemoradiation therapy for the treatment of locally advanced esophageal cancer. Ann Surg. 2006;243:472-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Ulla M, Cavadas D, Muñoz I, Beskow A, Seehaus A, García-Mónaco R. Esophageal cancer: pneumo-64-MDCT. Abdom Imaging. 2010;35:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Ulla M, Gentile EM, Cavadas D, Yeyati EL, Frank L, Argerich JI, Garcia Mónaco R. Esophageal cancer characterization with pneumo-64-MDCT. Abdom Imaging. 2012;37:501-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Carrascosa P, Capuñay C, Martín López E, Salis G, Mazzadi S, Carrascosa J. Esophageal stenosis: three-dimensional multidetector CT and virtual endoscopy. Abdom Imaging. 2009;34:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Thompson WM. Esophageal carcinoma. Abdom Imaging. 1997;22:138-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Mazzeo S, Caramella D, Gennai A, Giusti P, Neri E, Melai L, Cappelli C, Bertini R, Capria A, Rossi M. Multidetector CT and virtual endoscopy in the evaluation of the esophagus. Abdom Imaging. 2004;29:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Kim AY, Kim HJ, Ha HK. Gastric cancer by multidetector row CT: preoperative staging. Abdom Imaging. 2005;30:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Siddiqui AA, Sarkar A, Beltz S, Lewis J, Loren D, Kowalski T, Fang J, Hilden K, Adler DG. Placement of fully covered self-expandable metal stents in patients with locally advanced esophageal cancer before neoadjuvant therapy. Gastrointest Endosc. 2012;76:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Couvelard A, Paraf F, Gratio V, Scoazec JY, Hénin D, Degott C, Fléjou JF. Angiogenesis in the neoplastic sequence of Barrett’s oesophagus. Correlation with VEGF expression. J Pathol. 2000;192:14-18. [PubMed] |
| 21. | Lord RV, Park JM, Wickramasinghe K, DeMeester SR, Oberg S, Salonga D, Singer J, Peters JH, Danenberg KD, Demeester TR. Vascular endothelial growth factor and basic fibroblast growth factor expression in esophageal adenocarcinoma and Barrett esophagus. J Thorac Cardiovasc Surg. 2003;125:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Torres C, Wang H, Turner J, Shahsafaei A, Odze RD. Prognostic significance and effect of chemoradiotherapy on microvessel density (angiogenesis) in esophageal Barrett’s esophagus-associated adenocarcinoma and squamous cell carcinoma. Hum Pathol. 1999;30:753-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Auvinen MI, Sihvo EI, Ruohtula T, Salminen JT, Koivistoinen A, Siivola P, Rönnholm R, Rämö JO, Bergman M, Salo JA. Incipient angiogenesis in Barrett’s epithelium and lymphangiogenesis in Barrett’s adenocarcinoma. J Clin Oncol. 2002;20:2971-2979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 995] [Cited by in RCA: 1061] [Article Influence: 37.9] [Reference Citation Analysis (1)] |
| 25. | Turrini O, Viret F, Moureau L, Guiramand J, Lelong B, Bège T, Giovannini M, Delpero JR. A modified Dworak classification applied to pancreatic adenocarcinoma: a useful prognostic factor. Bull Cancer. 2007;94:897-901. [PubMed] |