Published online Aug 15, 2025. doi: 10.4251/wjgo.v17.i8.108870
Revised: June 8, 2025
Accepted: July 15, 2025
Published online: August 15, 2025
Processing time: 91 Days and 21.4 Hours
Colorectal cancer (CRC) remains one of the leading causes of cancer-related morbidity and mortality worldwide. Growing evidence suggests that gut microbial dysbiosis plays a crucial role in tumorigenesis and can influence therapeutic responses.
To explore the associations between serum S100A12 and soluble CD14 (sCD14) levels and gut microbiota alterations in patients with CRC, and to assess the predictive utility of these biomarkers in forecasting chemotherapy response.
A retrospective analysis was conducted on 104 patients diagnosed with advanced CRC (CRC group) and 104 age-matched and sex-matched healthy controls. Serum concentrations of S100A12 and sCD14 were measured using enzyme-linked immunosorbent assay. Fecal samples collected before chemotherapy were subjected to 16S rRNA sequencing to profile gut microbial composition. Pearson correlation analysis was used to evaluate the relationship between biomarker levels and microbial abundance. Receiver operating characteristic (ROC) curves were used to assess the predictive performance of S100A12 and sCD14 for chemotherapy response.
CRC patients exhibited significantly higher serum levels of S100A12 and sCD14 compared to healthy individuals (P < 0.05). Patients with moderate to severe gut dysbiosis showed the highest elevations of these biomarkers (P < 0.05). Elevated levels of S100A12 and sCD14 were positively correlated with Fusobacterium nucleatum and Prevotella abundance, and negatively correlated with Faecalibacterium prausnitzii and Akkermansia muciniphila (P < 0.05). Both biomarkers significantly decreased following chemotherapy (P < 0.05). Non-responders to chemotherapy had higher pre-treatment levels of S100A12 and sCD14 compared to responders (P < 0.05). Combined ROC analysis showed improved diagnostic accuracy compared to either marker alone.
Serum S100A12 and sCD14 levels are closely associated with gut microbiota imbalance and chemotherapy response in CRC patients. These markers may serve as promising predictive indicators for treatment efficacy and offer potential value in individualized treatment strategies.
Core Tip: This study highlights the potential of S100A12 and soluble CD14 as non-invasive biomarkers for assessing gut microbial imbalance and predicting chemotherapy responsiveness in colorectal cancer. Their integration into clinical decision-making could support more personalized and effective treatment planning.
- Citation: Ling MZ, Wan Z, Hu B, Zhao MJ, Gong HS, Li G. Associations between serum biomarkers and gut microbial imbalance in predicting chemotherapy response in colorectal cancer: A retrospective analysis. World J Gastrointest Oncol 2025; 17(8): 108870
- URL: https://www.wjgnet.com/1948-5204/full/v17/i8/108870.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i8.108870
Colorectal cancer (CRC) is among the most common malignancies worldwide and remains a major contributor to global cancer-related mortality. The incidence of CRC has shown a steady increase in recent years, influenced by a combination of dietary patterns, environmental exposures, and host biological factors[1]. Notably, accumulating evidence underscores the significant role of gut microbiota in CRC pathogenesis, with specific bacterial populations implicated in promoting tumorigenesis, modulating immune responses, and affecting the efficacy of cancer therapies[2,3]. Gut microbial dysbiosis—a disruption in the normal composition and function of intestinal microbiota—has been associated with inflammatory microenvironments that support colorectal carcinogenesis[4]. In particular, enrichment of pathogenic species such as Fusobacterium nucleatum (F. nucleatum) and Prevotella abundance and depletion of beneficial bacteria like Faecalibacterium prausnitzii (F. prausnitzii) and Akkermansia muciniphila (A. muciniphila) have been observed in CRC patients[5,6]. These microbial alterations can influence epithelial integrity, immune regulation, and even resistance to chemotherapy by affecting drug metabolism or tumor immune evasion[7].
Systemic inflammatory mediators provide a crucial link between microbial dysbiosis and cancer progression. S100A12, a calcium-binding protein secreted by activated neutrophils, functions as a pro-inflammatory alarmin capable of amplifying immune responses through pattern recognition receptors[8]. Meanwhile, soluble CD14 (sCD14), a co-receptor for lipopolysaccharide (LPS), reflects monocyte activation and microbial translocation from the gut lumen, and has recently garnered attention for its prognostic relevance in various cancers[9]. Despite their individual significance, few studies have evaluated the relationship between these circulating biomarkers, gut microbiota composition, and therapeutic response in CRC. Given that chemotherapy remains a cornerstone of treatment for advanced CRC, the identification of novel predictive biomarkers is essential for optimizing outcomes. As both systemic inflammation and microbial ecology are increasingly recognized as determinants of treatment success, exploring the combined utility of inflammatory markers and microbial profiling holds promise for guiding personalized interventions. Therefore, this study aims to investigate the association between serum levels of S100A12 and sCD14 and gut microbiota composition in CRC patients, and to determine their predictive value in chemotherapy responsiveness. By integrating microbial and immune biomarkers, this research seeks to contribute to precision oncology approaches that tailor treatment strategies based on host-microbiome interactions.
This retrospective study included 104 patients diagnosed with advanced CRC who were treated at Henan Provincial People's Hospital between August 2022 and May 2024. All participants provided written informed consent prior to enrollment, and the study was approved by the hospital’s ethics committee, in accordance with the Declaration of Helsinki. The CRC group comprised 62 males and 42 females, aged between 42 years and 78 years, with a mean age of 63.84 ± 7.95 years. According to the American Joint Committee on Cancer TNM staging system[10], 58 patients were classified as stage IIIb and 46 patients as stage IV. All patients were deemed unsuitable for surgical resection and were scheduled to receive first-line chemotherapy. None had previously undergone chemotherapy, radiotherapy, immunotherapy, or surgical interventions for cancer. In parallel, 104 age-matched and sex-matched healthy individuals were recruited as the control group. These individuals underwent routine physical examinations at the same hospital during the same period and had no history of malignancy, major inflammatory or infectious disease, or chronic medication use. The control group included 66 males and 38 females, aged between 40 years and 76 years, with a mean age of 62.91 ± 7.28 years. There were no statistically significant differences in age or gender between the two groups (P > 0.05), confirming baseline comparability.
Inclusion criteria: (1) Age ≥ 40 years, irrespective of sex; (2) Histologically and radiologically confirmed diagnosis of colorectal adenocarcinoma; (3) Stage IIIb or IV disease based on TNM classification, with documented lymph node involvement and/or distant metastases; (4) No prior oncologic treatments including chemotherapy, radiation, immunotherapy, or surgery; (5) Eastern Cooperative Oncology Group performance status of 0–2[11], and an expected survival duration of at least three months; (6) Availability of complete clinical and laboratory data, including serum and stool samples collected prior to chemotherapy initiation; and (7) Informed consent obtained and ability to participate in clinical follow-up.
Exclusion criteria: (1) Diagnosis of other primary malignant tumors; (2) Severe organ dysfunction (e.g., cardiac insufficiency, renal failure, liver cirrhosis) or decompensated chronic comorbidities; (3) Acute infections (bacterial, viral, or fungal) within the preceding three months; (4) Known immunodeficiency disorders (e.g., human immunodeficiency virus, post-transplant immunosuppression); (5) Use of antibiotics, probiotics, prebiotics, or corticosteroids within three months prior to sample collection; (6) Known hypersensitivity to chemotherapeutic agents planned for use; (7) Mental illness, neurocognitive impairment, or other conditions impairing the ability to provide consent or adhere to treatment protocols; (8) Missing or incomplete clinical documentation; and (9) Loss to follow-up or death before evaluation of chemotherapy response.
Peripheral venous blood samples were collected from all study participants. For healthy controls, blood was drawn during routine physical examinations. In the CRC group, blood samples were obtained at two time points: Prior to initiation of chemotherapy and after the completion of the planned chemotherapy cycles. All blood samples were collected in the early morning after an overnight fast (≥ 8 hours). Using standard aseptic technique, approximately 5 mL of whole blood was drawn from the antecubital vein and centrifuged at 3000 rpm for 15 minutes at 4 °C to isolate serum. The supernatant was aliquoted into sterile cryotubes and stored at -70 °C until analysis. Serum levels of S100A12 and sCD14 were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits following the manufacturers' protocols. The S100A12 detection kit (Catalog No. ZY-S100A12-Hu) was supplied by Shanghai Zeye Biotechnology Co., Ltd., and the sCD14 kit (Catalog No. SEA953Hu) was obtained from Wuhan USCN Life Science Inc. All samples were tested in duplicate, and absorbance values were read using a microplate reader set to 450 nm.
Fresh fecal samples were collected from CRC patients before chemotherapy initiation. Each sample (approximately 0.2 g) was placed in a sterile container and transported on ice for immediate analysis. Total DNA was extracted using the QIAamp DNA Stool Mini Kit (Qiagen, Germany) according to manufacturer instructions. The V3–V4 region of the 16S rRNA gene was amplified using specific primers, and high-throughput sequencing was performed on the Illumina MiSeq platform. Operational taxonomic units were assigned using the SILVA 138 database.
Particular attention was given to quantifying the relative abundance of the following bacteria, previously associated with CRC and gut homeostasis: (1) F. nucleatum; (2) Prevotella abundance; (3) F. prausnitzii; and (4) A. muciniphila. Microbial dysbiosis was graded based on alpha diversity indices (Shannon and Simpson) and changes in the abundance of key bacterial taxa. Two severity grades were defined: (1) Grade I dysbiosis: Moderate reduction in microbial diversity, with relative overrepresentation of F. nucleatum or Prevotella abundance, and moderate depletion of F. prausnitzii and A. muciniphila; and (2) Grade II dysbiosis: Marked decline in diversity and profound dominance of pro-inflammatory taxa, with severe depletion or near-absence of beneficial species.
All CRC patients received first-line chemotherapy with the CapeOX regimen. Oxaliplatin was administered intravenously at 130 mg/m² on day 1 of each cycle. Concurrently, capecitabine was administered orally at 1000 mg/m² twice daily for 14 days. Each chemotherapy cycle lasted 21 days and was repeated for four cycles in total. No patients received concurrent immunotherapy or targeted therapy during the study period.
Therapeutic efficacy was evaluated using the Response Evaluation Criteria in Solid Tumors version 1.1[12]. Based on radiological assessments and clinical follow-up, patients were categorized as: (1) Complete response (CR): Disappearance of all target lesions; (2) Partial response (PR): ≥ 30% reduction in total lesion diameter; (3) Stable disease (SD): No sufficient shrinkage or progression to meet PR or progressive disease (PD) criteria; (4) PD: ≥ 20% increase in lesion size or emergence of new lesions; and (5) Patients achieving CR or PR were classified as responders, while those with SD or PD were classified as non-responders for statistical analysis.
Statistical analyses were performed using Statistical Package for the Social Sciences version 25.0 (IBM Corp., Armonk, NY, United States) and GraphPad Prism 8.0 for visualizations. Continuous variables conforming to normal distribution were presented as means ± SD. Inter-group comparisons were made using independent-samples t-tests, while within-group comparisons (e.g., pre-treatment vs post-treatment biomarker levels) employed paired-samples t-tests. Multi-group comparisons (e.g., different dysbiosis grades) were conducted using one-way analysis of variance followed by Student–Newman–Keuls post hoc tests. Pearson correlation coefficients were calculated to assess associations between serum biomarker levels and the relative abundance of specific gut microbes. Receiver operating characteristic (ROC) curve analysis was used to evaluate the predictive performance of S100A12, sCD14, and their combination in identifying chemotherapy non-responders. An area under the curve (AUC) value > 0.7 was considered indicative of good discriminative ability. A two-tailed P value < 0.05 was regarded as statistically significant.
Pre-treatment serum levels of both S100A12 and sCD14 were significantly elevated in the CRC group compared to healthy controls (P < 0.01). This suggests heightened systemic inflammation and immune activation in CRC patients prior to therapy. Detailed quantitative data are provided in Table 1.
| Group | S100A12 (ng/mL) | Soluble CD14 (ng/mL) |
| Colorectal cancer patients (n = 104) | 158.64 ± 21.37 | 3345.29 ± 412.84 |
| Healthy controls (n = 104) | 89.72 ± 18.95 | 2384.61 ± 376.21 |
| t value | 78.611 | 17.540 |
| P value | < 0.001 | < 0.001 |
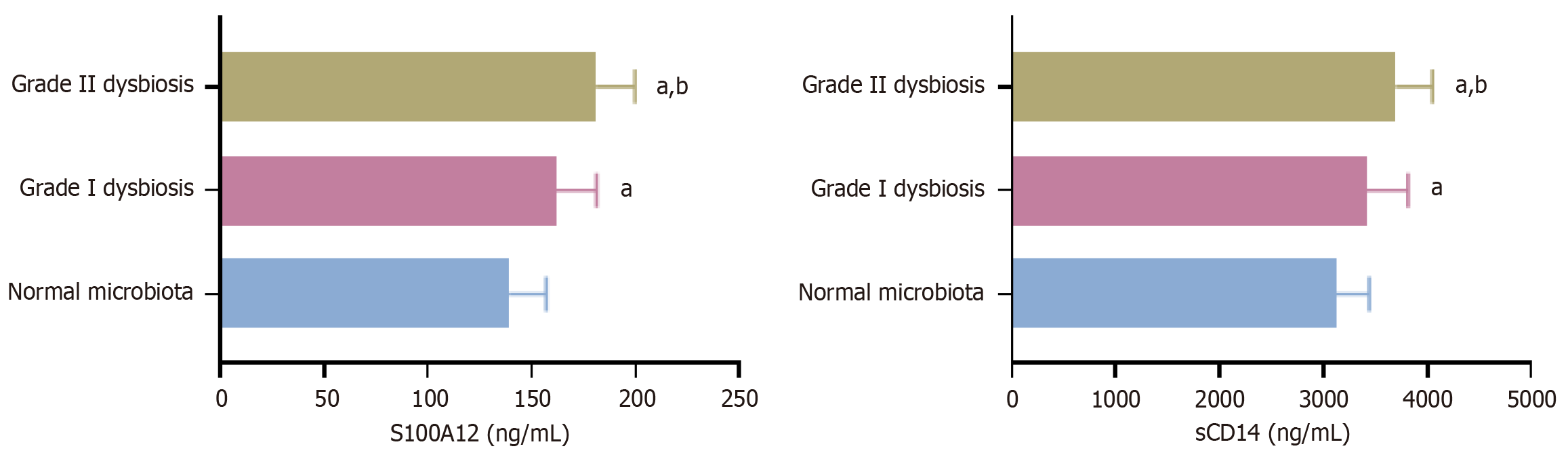
Based on 16S rRNA sequencing of fecal samples, 104 CRC patients were stratified into three groups: (1) Normal microbiota composition (n = 46); (2) Grade I dysbiosis (n = 37); and (3) Grade II dysbiosis (n = 21). Serum S100A12 and sCD14 Levels were significantly higher in patients with dysbiosis than in those with normal microbiota (P < 0.05). Furthermore, grade II dysbiosis was associated with the highest biomarker concentrations, exceeding those in grade I dysbiosis (P < 0.05). This stepwise increase highlights a potential relationship between microbiome disruption severity and systemic inflammatory markers. These results are visualized in Figure 1.
Pearson correlation analysis revealed that both serum S100A12 and sCD14 Levels were positively correlated with the relative abundance of F. nucleatum and Prevotella abundance (P < 0.01), while showing negative correlations with F. prausnitzii and A. muciniphila (P < 0.01). These findings support the hypothesis that pro-inflammatory and immunomodulatory microbial taxa are closely associated with elevated systemic inflammatory status in CRC. Full correlation coefficients and P values are summarized in Table 2.
| Microbial indicator | S100A12 | Soluble CD14 | ||
| r value | P value | r value | P value | |
| Fusobacterium nucleatum | 0.482 | < 0.01 | 0.446 | < 0.01 |
| Prevotella abundance | 0.457 | < 0.01 | 0.429 | < 0.01 |
| Faecalibacterium prausnitzii | -0.505 | < 0.01 | -0.489 | < 0.01 |
| Akkermansia muciniphila | -0.462 | < 0.01 | -0.438 | < 0.01 |
Following four cycles of CapeOX chemotherapy, a significant reduction in both S100A12 and sCD14 Levels was observed among CRC patients (P < 0.001), indicating a decline in systemic inflammation potentially linked to therapeutic response. These dynamic changes are presented in Table 3.
| Time point | S100A12 (ng/mL) | Soluble CD14 (ng/mL) |
| Pre-chemotherapy | 158.64 ± 21.37 | 3345.29 ± 412.84 |
| Post-chemotherapy | 112.89 ± 19.84 | 2771.03 ± 389.67 |
| t value | 16.000 | 10.315 |
| P value | < 0.001 | < 0.001 |
Treatment response was assessed in all 104 patients, with outcomes classified as CR (n = 15), PR (n = 57), SD (n = 21), and PD (n = 11). Based on these, 72 patients were grouped as responders (CR + PR) and 32 patients as non-responders (SD + PD). Notably, pre-treatment serum S100A12 and sCD14 Levels were significantly higher in the non-responder group (P < 0.01), suggesting that elevated baseline inflammation may be predictive of suboptimal chemotherapy response. Data comparisons are shown in Table 4.
| Group | S100A12 (μg/L) | Soluble receptor for advanced glycation end-products (ng/L) |
| Responders (complete response + partial response) | 146.27 ± 18.93 | 3172.84 ± 401.22 |
| Non-responders (stable disease + progressive disease) | 182.96 ± 19.74 | 3682.31 ± 375.45 |
| t value | 13.680 | 9.455 |
| P value | < 0.001 | < 0.001 |
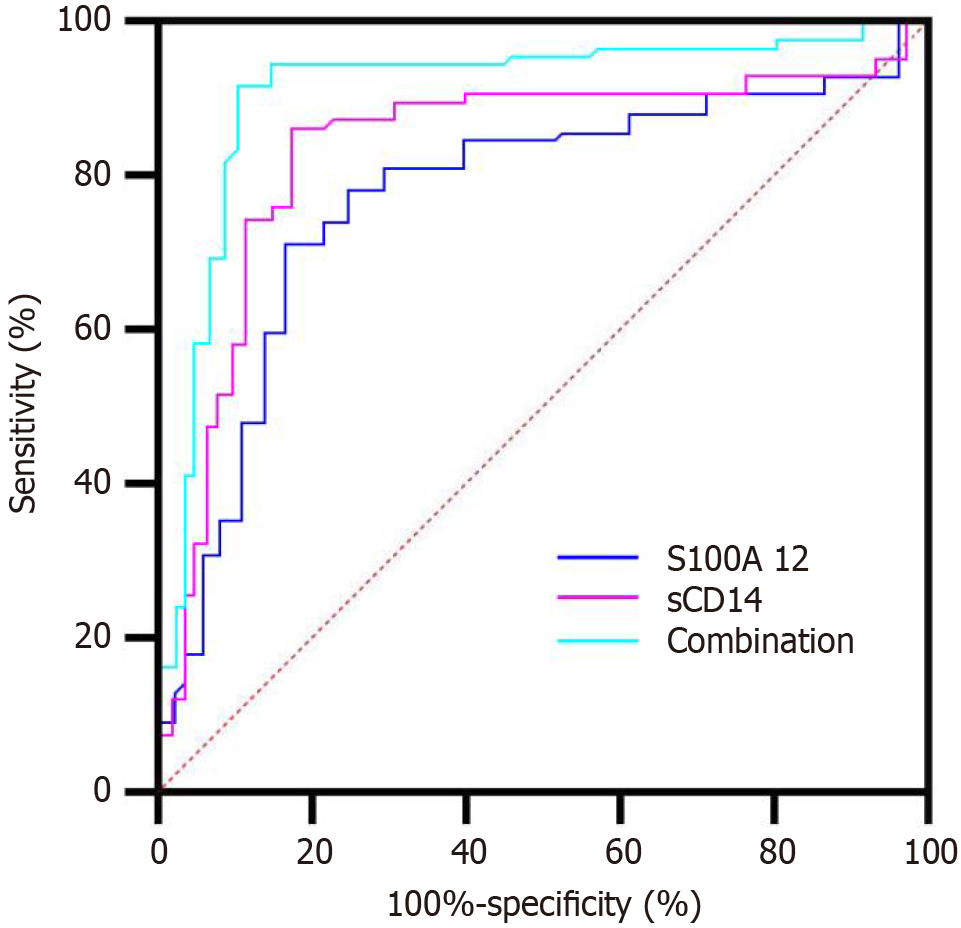
ROC curve analysis demonstrated that both S100A12 and sCD14 had good individual predictive capacity for chemotherapy non-response, with AUC values of 0.781 and 0.766, respectively. When combined, the diagnostic performance improved further (AUC = 0.842), indicating that the joint assessment of these markers enhances chemoresistance prediction. Detailed results are provided in Table 5, and the corresponding ROC curves are illustrated in Figure 2.
| Indicator | An area under the curve | 95%CI | P value | Sensitivity (%) | Specificity (%) |
| S100A12 | 0.776 | 0.692–0.854 | < 0.001 | 78.1 | 73.6 |
| Soluble CD14 | 0.759 | 0.678–0.843 | < 0.001 | 75.0 | 72.2 |
| Combined | 0.838 | 0.764–0.902 | < 0.001 | 84.4 | 76.4 |
CRC remains a significant global health burden, with therapeutic outcomes still hindered by interindividual variability in chemotherapy response. Accumulating evidence suggests that the gut microbiota—a dynamic microbial ecosystem intimately linked with host immunity and systemic inflammation—plays a key role in shaping the tumor microenvironment and modulating treatment efficacy[13-15]. Microbial dysbiosis has been implicated in tumorigenesis and immune evasion in CRC and is increasingly recognized as a potential contributor to chemoresistance. In this study, we explored the relationship between gut microbiota composition and circulating levels of the inflammatory markers S100A12 and sCD14, and assessed their predictive value for chemotherapy response in CRC patients.
Our results revealed that both serum S100A12 and sCD14 Levels were significantly elevated in CRC patients compared to healthy individuals, consistent with previous findings that associate chronic inflammation with colorectal tumor progression[16-19]. Importantly, these elevations were correlated with the degree of microbial imbalance. Specifically, patients with more severe dysbiosis—characterized by higher relative abundances of F. nucleatum and Prevotella abundance, and decreased levels of F. prausnitzii and A. muciniphila—exhibited significantly higher biomarker levels. These microbial shifts have previously been linked to pro-inflammatory states and epithelial barrier dysfunction, both of which contribute to colorectal carcinogenesis and poor treatment response[20-22].
The biomarker sCD14 serves as a co-receptor for LPS and reflects monocyte activation in response to microbial components. Elevated sCD14 Levels have been observed in inflammatory conditions and are thought to represent a systemic response to endotoxemia or microbiota-driven immune activation[23]. Meanwhile, S100A12, secreted by activated neutrophils, binds to pattern recognition receptors such as soluble receptor for advanced glycation end-products and toll-like receptor 4 (TLR4), triggering downstream pro-inflammatory signaling pathways, including nuclear factor kappa B and mitogen-activated protein kinase cascades[24,25]. These pathways contribute to immune evasion, cytokine release, and tumor-supportive inflammation—hallmarks of the chemoresistant phenotype in CRC.
Our findings further demonstrated that baseline levels of S100A12 and sCD14 were significantly higher in patients who later failed to respond to chemotherapy (SD/PD), compared to those with favorable clinical outcomes (CR/PR). ROC analysis supported their predictive utility, with the combined biomarker model achieving superior sensitivity and specificity than either marker alone. This suggests that systemic inflammation, as reflected by these two indicators, may serve as a barrier to effective chemotherapeutic cytotoxicity. These results align with prior studies indicating that a heightened inflammatory state impairs treatment response by reducing tumor immunogenicity and promoting a suppressive tumor microenvironment[26,27].
Mechanistically, gut microbial dysbiosis may act upstream in this axis by enhancing mucosal permeability and driving chronic immune activation. F. nucleatum, for instance, has been implicated in chemoresistance via TLR4/MyD88 signaling, increased expression of BIRC3 (an anti-apoptotic factor), and recruitment of myeloid-derived suppressor cells[28]. The observed correlations between high F. nucleatum abundance and elevated sCD14/S100A12 Levels in our cohort support this interplay. Conversely, protective commensals such as A. muciniphila and F. prausnitzii are known to maintain epithelial integrity and produce anti-inflammatory metabolites (e.g., butyrate), which may enhance drug sensitivity and immune surveillance[29,30].
These insights underscore the potential clinical utility of monitoring inflammation-related serum biomarkers in conjunction with microbiota profiling to predict chemotherapy outcomes. Notably, both S100A12 and sCD14 are measurable via routine ELISA assays, providing a feasible and cost-effective tool for patient stratification. With advances in metagenomic sequencing and integrative bioinformatics, these markers could eventually be incorporated into composite indices alongside microbial and genetic data for precision therapy planning.
However, this study has certain limitations. First, the retrospective design and single-center sample restrict external validity. Second, while we characterized microbiota composition, we did not assess microbial function (e.g., metabolite production or virulence factors), which may more directly influence host responses. Third, the mechanistic links between S100A12/sCD14 expression and chemotherapy resistance remain speculative and warrant validation through in vitro and in vivo experiments. Lastly, we did not control for potential confounders such as diet, body mass index, and baseline immune status, which could affect both microbiota and biomarker levels.
Future studies should address these gaps by incorporating functional metagenomics, mechanistic immunology, and multicenter prospective cohorts. Moreover, interventional trials that modulate gut microbiota—through prebiotics, probiotics, or fecal microbiota transplantation—may provide additional evidence supporting the causal role of microbiota–inflammation interactions in CRC therapy response.
This study highlights that elevated serum levels of S100A12 and sCD14 in CRC patients are closely associated with gut microbial dysbiosis and reduced chemotherapy responsiveness. These biomarkers reflect systemic inflammatory status driven by microbial alterations and may serve as valuable predictors for treatment stratification. Combined detection of S100A12 and sCD14 improves the accuracy of chemotherapy response prediction and offers a potential tool for guiding personalized therapeutic strategies. Future research integrating microbiota-host biomarkers with multi-omics data will be crucial for advancing precision oncology in CRC.
| 1. | National Cancer Center, China; Expert Group of the Development of China Guideline for the Screening; Early Detection and Early Treatment of Colorectal Cancer. [China guideline for the screening, early detection and early treatment of colorectal cancer (2020, Beijing)]. Zhonghua Zhong Liu Za Zhi. 2021;43:16-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 2. | Liu X, Cui S, Zhang L, Wu S, Feng C, Liu B, Yang H. Gut microbiota affects the activation of STING pathway and thus participates in the progression of colorectal cancer. World J Surg Oncol. 2024;22:192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Novoa Díaz MB, Carriere P, Gentili C. How the interplay among the tumor microenvironment and the gut microbiota influences the stemness of colorectal cancer cells. World J Stem Cells. 2023;15:281-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Wang X, Sun X, Chu J, Sun W, Yan S, Wang Y. Gut microbiota and microbiota-derived metabolites in colorectal cancer: enemy or friend. World J Microbiol Biotechnol. 2023;39:291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 5. | Lo CH, Wu DC, Jao SW, Wu CC, Lin CY, Chuang CH, Lin YB, Chen CH, Chen YT, Chen JH, Hsiao KH, Chen YJ, Chen YT, Wang JY, Li LH. Enrichment of Prevotella intermedia in human colorectal cancer and its additive effects with Fusobacterium nucleatum on the malignant transformation of colorectal adenomas. J Biomed Sci. 2022;29:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 6. | Lopez-Siles M, Enrich-Capó N, Aldeguer X, Sabat-Mir M, Duncan SH, Garcia-Gil LJ, Martinez-Medina M. Alterations in the Abundance and Co-occurrence of Akkermansia muciniphila and Faecalibacterium prausnitzii in the Colonic Mucosa of Inflammatory Bowel Disease Subjects. Front Cell Infect Microbiol. 2018;8:281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 7. | Li HM, Liu Y, Hao MD, Liang XQ, Yuan DJ, Huang WB, Li WJ, Ding L. Research status and hotspots of tight junctions and colorectal cancer: A bibliometric and visualization analysis. World J Gastrointest Oncol. 2024;16:3705-3715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 8. | Bobek D, Sestan M, Mijacika L, Kovacic N, Lukic IK, Grcevic D, Jelusic M. Serum S100A12 levels in children with childhood-onset systemic lupus erythematosus, systemic juvenile arthritis, and systemic undefined recurrent fevers. Z Rheumatol. 2023;82:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Wang W, Liu Q, Xu W, Liu T, Zhu B, Qi H, Xiao Q, Wang P, Li Y. [Effects and significance of continuous hemoperfusion on patients with diquat poisoning]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2022;34:1320-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Liu SX, Zhang L, Shi YK, Han XH. [Analysis of related factors of prognosis after surgical treatment of patients with non-metastatic colorectal cancer and construction of a normagram prediction model]. Zhonghua Zhong Liu Za Zhi. 2022;44:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Hanaoka M, Hino H, Shiomi A, Kagawa H, Manabe S, Yamaoka Y, Kato S, Yamauchi S, Kinugasa Y, Sugihara K. The Eastern Cooperative Oncology Group Performance Status as a prognostic factor of stage I-III colorectal cancer surgery for elderly patients: a multi-institutional retrospective analysis. Surg Today. 2022;52:1081-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Qiu MZ, Zhang Y, Guo Y, Guo W, Nian W, Liao W, Xu Z, Zhang W, Zhao HY, Wei X, Xue L, Tang W, Wu Y, Ren G, Wang L, Xi J, Jin Y, Li H, Hu C, Xu RH. Evaluation of Safety of Treatment With Anti-Epidermal Growth Factor Receptor Antibody Drug Conjugate MRG003 in Patients With Advanced Solid Tumors: A Phase 1 Nonrandomized Clinical Trial. JAMA Oncol. 2022;8:1042-1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 13. | Tinazzi M, Sacilotto A, Cocetta V, Giacomini I, Raso F, Bulferi G, De Togni H, Lanza R, Consolo P, Berretta M, Montopoli M. Bowel Inflammation and Nutrient Supplementation: Effects of a Fixed Combination of Probiotics, Vitamins, and Herbal Extracts in an In Vitro Model of Intestinal Epithelial Barrier Dysfunction. Yale J Biol Med. 2024;97:297-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Zhan ZS, Zheng ZS, Shi J, Chen J, Wu SY, Zhang SY. Unraveling colorectal cancer prevention: The vitamin D - gut flora - immune system nexus. World J Gastrointest Oncol. 2024;16:2394-2403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Koustas E, Trifylli EM, Sarantis P, Papadopoulos N, Aloizos G, Tsagarakis A, Damaskos C, Garmpis N, Garmpi A, Papavassiliou AG, Karamouzis MV. Implication of gut microbiome in immunotherapy for colorectal cancer. World J Gastrointest Oncol. 2022;14:1665-1674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (2)] |
| 16. | Qiao SB, Niu ML, Liang WT, Zhang LJ, Chen X, Zhu YK. Analysis of serum S100A12, soluble advanced glycation end products receptor, and gut microbiome in elderly patients with colorectal cancer. World J Gastrointest Oncol. 2025;17:106393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (4)] |
| 17. | Shi M, Zong X, Hur J, Birmann BM, Martinez-Maza O, Epeldegui M, Chan AT, Giovannucci EL, Cao Y. Circulating markers of microbial translocation and host response to bacteria with risk of colorectal cancer: a prospective, nested case-control study in men. EBioMedicine. 2023;91:104566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Turgunov Y, Ogizbayeva A, Avdiyenko O, Mugazov M, Shakeyev K, Komarov T, Asamidanova S. The sCD14-ST predictive value in the development of adverse outcomes in operated colorectal cancer patients (diagnostic study). Ann Med Surg (Lond). 2023;85:796-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Ren JX, Chen L, Guo W, Feng KY, Cai YD, Huang T. Patterns of Gene Expression Profiles Associated with Colorectal Cancer in Colorectal Mucosa by Using Machine Learning Methods. Comb Chem High Throughput Screen. 2024;27:2921-2934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 20. | Wang T, Yu H, Yu J, Shao J, Zheng R. [Research progress in the mechanism of intestinal environmental disturbance on the occurrence and development of sepsis-associated liver injury]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2024;36:660-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Tian X, Song W, Xia G, Tan C, Yin J. [Research progress of the effect of enteral nutrition on intestinal microecology in neurocritical ill patients]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021;33:1393-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Wang ZY, Gong JF. [Gut microbiota and immune-related diseases]. Zhonghua Wei Chang Wai Ke Za Zhi. 2022;25:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Chen Y, He Y, Qin H, Qin S. [Annexin A1 activates the G protein-coupled formyl peptide receptor type 2-dependent endothelial nitric oxide synthase pathway to alleviate sepsis associated acute lung injury]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2024;36:924-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Lin CP, Huang PH, Chen CY, Wu MY, Chen JS, Chen JW, Lin SJ. Sitagliptin attenuates arterial calcification by downregulating oxidative stress-induced receptor for advanced glycation end products in LDLR knockout mice. Sci Rep. 2021;11:17851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Singh H, Rai V, Agrawal DK. LPS and oxLDL-induced S100A12 and RAGE expression in carotid arteries of atherosclerotic Yucatan microswine. Mol Biol Rep. 2022;49:8663-8672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 26. | Bao YF, Hu DY, Shao XX, Dai CX, Lu JH, Wu JH, Jiang Y. [Clinical efficacy and its influencing factors of ustekinumab in the treatment of patients with Crohn's disease]. Zhonghua Yi Xue Za Zhi. 2024;104:3840-3843. [PubMed] [DOI] [Full Text] |
| 27. | Xu HJ, Qi YJ, Wu DR, Liu QW, Chen P, Li MX, Jiao YL, Ruan HJ, Li ZT, Gao SG. [Porphyromonas gingivalis promotes the occurrence of esophageal squamous cell carcinoma via an inflammatory microenvironment]. Zhonghua Zhong Liu Za Zhi. 2024;46:746-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Hsueh CY, Lau HC, Huang Q, Gong H, Sun J, Cao P, Hu C, Zhang M, Tao L, Zhou L. Fusobacterium nucleatum impairs DNA mismatch repair and stability in patients with squamous cell carcinoma of the head and neck. Cancer. 2022;128:3170-3184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 29. | Lee Y, Byeon HR, Jang SY, Hong MG, Kim D, Lee D, Shin JH, Kim Y, Kang SG, Seo JG. Oral administration of Faecalibacterium prausnitzii and Akkermansia muciniphila strains from humans improves atopic dermatitis symptoms in DNCB induced NC/Nga mice. Sci Rep. 2022;12:7324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Yaghoubfar R, Behrouzi A, Zare Banadkoki E, Ashrafian F, Lari A, Vaziri F, Nojoumi SA, Fateh A, Khatami S, Siadat SD. Effect of Akkermansia muciniphila, Faecalibacterium prausnitzii, and Their Extracellular Vesicles on the Serotonin System in Intestinal Epithelial Cells. Probiotics Antimicrob Proteins. 2021;13:1546-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |