Published online Aug 15, 2025. doi: 10.4251/wjgo.v17.i8.106663
Revised: April 8, 2025
Accepted: July 4, 2025
Published online: August 15, 2025
Processing time: 163 Days and 14.9 Hours
Detection and treatment of colorectal cancer (CRC) at an early stage is vital for long-term survival. Liquid biopsy has emerged as a promising new avenue for non-invasive screening of CRC as well as prognostication and surveillance of minimal residual disease. Cell free DNA (cfDNA) is a promising liquid biopsy analyte and has been approved for use in clinical practice. Here, we explore the current challenges of utilizing cfDNA in the screening and prognostication of CRC but also for detecting driver mutations in healthy, presymptomatic patients with normal colonic crypts. CfDNA for the detection of cancerous or premalig
Core Tip: Cell free DNA (cfDNA) carries information about colorectal cancer-specific genetic and epigenetic alterations which can aid in screening, detection and prognostication. Detection of genetic alterations is made difficult by low signal-to-noise ratio owing to an abundance of background non-tumorigenic mutations. Low amounts of cfDNA in healthy individuals negatively affects the sequencing performance and limit of detection of assays making screening non-feasible. One solution is to harvest cfDNA from peritoneal fluid or stool as this is more representative of the primary tumour compared to plasma-derived cfDNA. Alternatively, increasing the sensitivity of sequencing technologies would allow for the detection of low frequency mutations.
- Citation: Chua MWE, Chan DKH. Challenges and proposed solutions to the adoption of cell free DNA in screening, detecting and prognosticating colorectal cancer. World J Gastrointest Oncol 2025; 17(8): 106663
- URL: https://www.wjgnet.com/1948-5204/full/v17/i8/106663.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i8.106663
Colorectal cancer (CRC) is the second leading cause of cancer death worldwide, with a lifetime risk in average individuals of approximately 4% to 5%[1]. Novel approaches in screening and detection of CRC are needed to reduce overall mortality. Current approaches in the detection of CRC include modalities such as endoscopy and faecal occult blood tests. Liquid biopsy-based approaches are a promising avenue for non-invasive cancer detection, prognostication and surveillance. Liquid biopsy is a molecular-biological diagnostic approach for detecting significant tumour-derived markers in bodily fluids without the need for invasive tissue biopsy. This includes circulating tumour cells (CTCs), cell-free DNA (cfDNA), mRNA, microRNA, exosomes, nucleosomes, and various glycoproteins and antigens[2]. Although each liquid biopsy analyte has unique advantages and disadvantages with regards to detection, this present review will focus on cfDNA, which are DNA fragments released into circulation during cellular apoptosis and necrosis[3].
This review focuses on cfDNA because it is an attractive candidate for non-invasive cancer detection. CfDNA carries information about cancer-specific genetic and epigenetic mutations[4]. CfDNA levels during treatment have been shown to correlate with oncologic outcome and provide direct evidence of minimal residual disease (MRD) in patients post-surgical resection[5]. Some studies report cfDNA to outperform imaging modalities like computer tomography in the detection of recurrent tumour[6]. Moreover, cancer patients’ blood has been shown to contain increased levels of cfDNA compared to that of healthy individuals[7]. The analysis of cfDNA methylation patterns has also shown to successfully detect a wide range of cancers with specificity and sensitivity performance approaching the standard for population-level screening[8]. However, few studies have explored the use of cfDNA analysis in healthy, presymptomatic individuals. This is a novel concept that is difficult to implement for a few reasons: Lack of knowledge regarding the molecular basis of tumour initiation, significantly lower cfDNA concentration in the plasma of healthy individuals compared to cancer patients[9], and lack of mutant ctDNA molecules present in the plasma of healthy individuals with zero tumour burden[10].
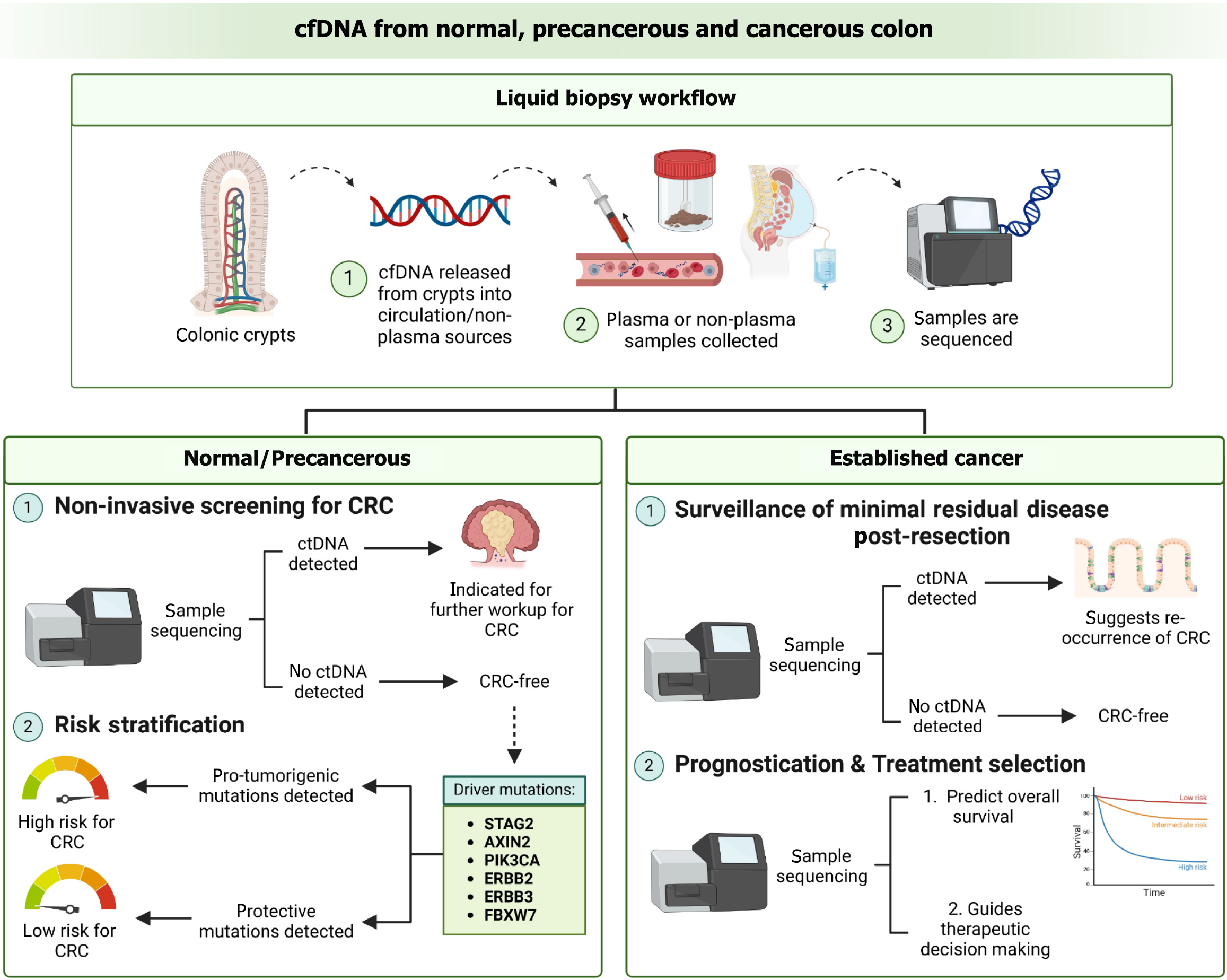
In this article, we will explore the role of cfDNA analysis across the oncogenic spectrum of CRC, beginning during tumour initiation at a point when the tissue is phenotypically normal, then proceeding into a precancerous polypoidal lesion, and finally at the point when cancer has been established. The clinical roles of cfDNA with regards to normal, precancerous and cancerous colonic epithelium are summarized in Figure 1.
CfDNA refers to extracellular DNA fragments of around 140-170 base pairs in length found in the plasma or non-plasma bodily fluids. CfDNA was first detected in the blood of individuals in 1948 by Mandel and Metais[11], but only in the last few decades have researchers started to explore the potential of cfDNA as a minimally invasive modality of obtaining biological information. CfDNA mainly originates from the apoptosis of hematopoietic cells[12], however, numerous other forms of cell release mechanisms have been hypothesised. A specific component of cfDNA is circulating tumour DNA (ctDNA), which represents DNA shed from tumour cells[13]. The tumour burden and its degree of metastasis have been positively correlated to ctDNA fraction (ctDNA%) in the plasma[14]. CtDNA constitutes 0.01%-90% of cfDNA[15] and has a half-life of 1-2.4 hours[16]. CtDNA in the plasma or bodily fluids can be detected through cfDNA-based assays that search for cancer-specific mutations with high sensitivity and specificity.
Existing literature suggests that cfDNA is released into the circulation via two different routes: Passive release mechanisms and active release mechanisms. Passive release mechanisms include processes like apoptosis, necrosis, and breakage of CTCs. Active release mechanisms involve cfDNA release from extracellular vesicles (EVs).
Apoptosis is a type of programmed cell death carried out by caspases occurring in both physiological and pathological conditions. This process is triggered by a complex signalling cascade and involving morphological changes like cell shrinkage, pyknosis, plasma and chromatin condensation. Membrane blebbing leads to the release of apoptotic bodies from cells. These apoptotic bodies are subsequently engulfed by phagocytic cells and their components recycled[17,18]. It is believed that the large majority of cfDNA originates from apoptotic processes.
Due to the multiple cancer hallmarks and cell-death mechanisms involved in tumorigenesis, an origin of cfDNA from apoptosis alone is unlikely[19]. Besides apoptosis, necrosis has been mentioned as a potential source of cfDNA in cancer patients. Cells undergoing necrosis exhibit organelle dysfunction and degradation of the plasma membrane, exposing its intracellular DNA to degradative agents such as nucleases and leading to the release of DNA into the extracellular space. CfDNA molecules are then fragmented by the various nucleases. Other passive mechanisms include cfDNA release from CTCs and chromosome fragments due to chromosomal instability. Other cell death mechanisms that have also been hypothesised to contribute to cfDNA load include necroptosis, oncosis, pyroptosis and ferroptosis[20-22].
Despite the abundant literature suggesting that cfDNA is mainly associated with apoptotic and necrotic processes, one recent study showed that cfDNA concentration had no correlation with apoptosis and necrosis. Active release mechanisms via exosomes constitute an alternative mechanism in which cfDNA may be found in the blood. The study found that breast cancer cells in the G1 phase released cfDNA via exosomes, and that the majority of cfDNA from breast cancer cells was released via active cellular secretion processes[23]. Current literature mentions active release mechanisms via exosomes, apoptotic blebs, shedding vesicles, and microparticles[24,25], however, the majority of active secretion of cfDNA occurs via EVs, which are spherical lipid-bound particles acting as mediators of physiological and biological processes[26] such as homeostasis[27]. Tumour-derived EVs are known to promote tumour invasion, metastasis and tumour migration as they can transfer tumour traits by entering other cells[18].
Genetic mutations such as single-nucleotide variations (SNVs) and copy number variations (CNVs) have been used as diagnostic biomarkers for cfDNA-based modalities[28]. Modalities which detect genetic mutations (i.e., SNVs and CNVs) include quantitative polymerase chain reaction (PCR), targeted sequencing, whole-genome sequencing (WGS), and whole-exome sequencing. However, these mutation-based diagnostic modalities might not be adequately sensitive for patients with precancerous lesions or early-stage cancer given the lower number of recurrent mutations. A possibly superior approach may be through the detection of large-scale epigenetic alterations instead as they are tissue and cancer-type specific, and therefore are not constrained by low cell numbers[29].
Though initially discovered in the blood, cfDNA fragments have now been found in all human body fluid types, such as pleural and peritoneal effusions, cerebrospinal fluids, urine, saliva, stool and seminal fluid. The advantages of using plasma as a source of cfDNA are as follows: It is easily obtainable hence allowing for longitudinal sampling at multiple timepoints, and tumour heterogeneity might be better captured than with tissue biopsy sampling. However, the low ctDNA to cfDNA ratio due to the predominance of clonal haematopoiesis makes using plasma cfDNA as a diagnostic modality difficult[30].
In contrast, the lower proportion of cfDNA originating from haematopoietic cells in non-plasma bodily fluids suggests a higher ctDNA fraction (ctDNA%) and higher variant allele frequencies (VAFs) in non-plasma sources compared to plasma[31]. This reduction in cfDNA levels can be beneficial when searching for low-frequency genetic alterations due to a reduction of the background noise created by clonal haematopoiesis. Furthermore, ctDNA from non-plasma sources might be more representative of the primary tumour, as shown in the pleural fluid of patients with advanced stage lung cancer[31] or in CSF from patients with leptomeningeal metastases[32]. Specifically in the context of CRC, stool-derived cfDNA can be particularly advantageous due to physical proximity to the colorectal neoplastic tissue. A multitarget stool DNA test (Cologuard®) showed a sensitivity of 92.3% and specificity of 86.6% for the detection of CRC[33]. Hence, depending on the specific tumour type, non-plasma cfDNA can be a more viable option for cancer detection.
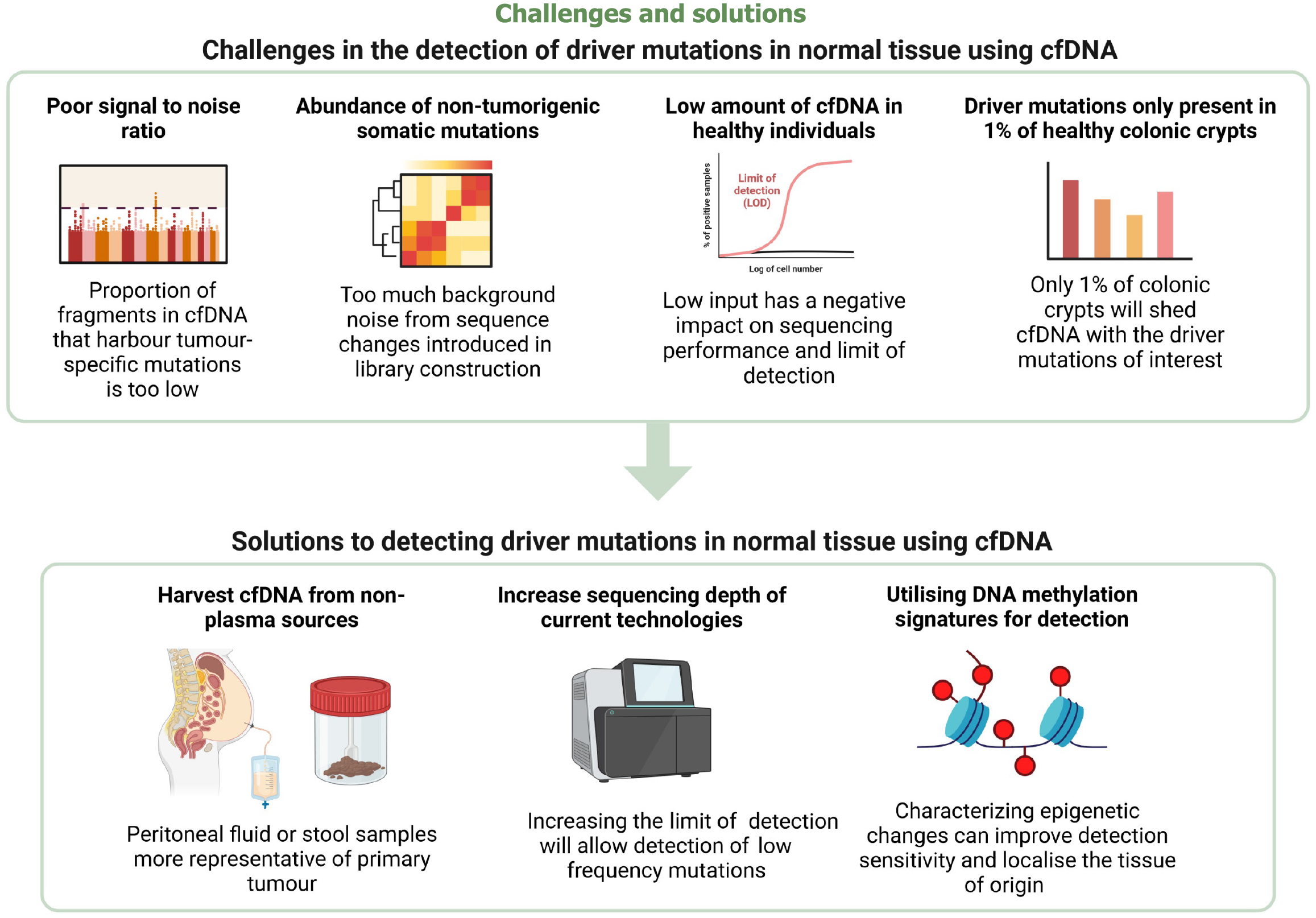
Mutation-based diagnostic modalities involve utilizing CNVs[34,35] and SNVs[36-38] as discriminative molecular features to reliably assess tumour-derived cfDNA. Current tumour fraction prediction methods based on CNVs rely on WGS with higher-coverage of more than 100-fold sequence coverage. Cutting edge algorithms, ichorCNA14[33] and ACE23[39], were initially developed from low-coverage WGS to generate an estimate of tumour fraction in cfDNA. However, both fail to provide an accurate estimate of tumour fractions due to high levels of aneuploidy and chromosomal instability[40,41]. Moreover, CNVs and SNVs are challenging to detect given the low number of mutated ctDNA fragments in early-stage cancer or certain tumour types[19]. While next-generation sequencing (NGS) technology enables high degrees of target multiplexing, the current depth of NGS sequencing is not deep enough to reliably search for mutations in a background of non-tumour-derived cfDNA[12,42]. The proportion of cfDNA fragments which harbour tumorigenic mutations is too low[43] which makes it difficult to search for bona fide variations amidst background signal from sequence changes introduced in library preparation. Extensive efforts have been made to improve the signal-to-noise ratio for more sensitive mutation detections, however these new methods rely on high-throughput sequencing and only analyze specific parts of the genome[37,42]. Such methods have limited efficacy for detecting cancer, especially at early stages, due to the low number of tumour genome equivalents in cfDNA[44,45]. The specificity of mutation detection is also hindered by the presence of somatic mutations in normal, non-malignant tissues[46,47]. Additionally, mutation-based screening modalities are largely incapable of localising the tissue of origin (TOO) of the tumour as the same driver mutation can be shared by many different cancers[37].
In contrast, DNA methylation signatures exhibit significant differences between healthy individuals and those with malignant tumours[19]. Compared to searching for point mutations, characterizing plasma epigenetic changes has shown to improve detection sensitivity by studies exploring the utility of cfDNA methylation for cancer detection[48]. Moreover, cfDNA methylation has shown utility in locating the cancer’s TOO[8], otherwise known as tissue deconvolution. This is possible as different tissues have different DNA methylation signatures[49,50] and even between different cell types within the same tissue[51]. Analyzing differentially methylated positions or regions can even allow detection of patients with malignant tumours[52-54]. Since cfDNA has various release sources, the methylation level measured at each cytosine-phosphate-guanine dinucleotide site is essentially a mixed signal originating from multiple tissues, including those harbouring tumorigenic driver mutations[55]. Hence, pinpointing the TOO is possible by deconvolution of blended methylation signatures.
Carcinogenesis is classically considered to occur as a result of the gradual accumulation of multiple oncogenic cancer gene mutations, acquired from an early stage[56]. A study by Lee-Six et al[57] found that driver mutations were present in about 1% of normal colorectal crypts. The driver mutations found in the normal colonic epithelium included truncating mutations in the cancer genes STAG2 and AXIN2, and hotspot mutations in ERBB2 (R678Q, T862A, V842I), ERBB3 (R667 L, R475W), PIK3CA (E542K, R38H), and FBXW7 (R658Q, R505C)[57]. This list is unexpected as these genes rarely harbour mutations in cancerous colonic epithelium. Mutations in ERBB2 and ERBB3 were common in normal crypts with driver mutations (5 in 14), but rare in CRC (7 in 631). There is also a surprising lack of established CRC driver mutations such as APC, KRAS and TP53 which are common in colorectal neoplasms and account for 56% of base-substitution and indel driver mutations but were comparatively rare in normal epithelium (1 in 14).
Pre-symptomatic screening of such mutations in cancer-free individuals, however, is still largely unfeasible due to low input volume and poor signal-to-noise ratio. To date, only a few studies have reported the analysis of cfDNA in healthy, presymptomatic controls. One such study by Alborelli et al[10] detected genetic mutations in 7 out of 55 clinically healthy subjects from plasma-derived cfDNA using NGS, of which 6 were germline variants (APC, PDGFRA, TP53) and 4 were cancer hotspot driver mutations (GNAS, IHD2, PIK3CA, TP53)[10]. The 4 cancer hotspot driver mutations detected were PIK3CA, TP53, IDH2, and GNAS, which are clinically classified as pathogenic or likely pathogenic. This study proved that it was possible for cancer-associated driver mutations to be detected in healthy individuals by analysing the cfDNA. The development of error-corrected NGS methods in recent years has also allowed for the identification of low-frequency mutations in normal tissue by reducing sequencing errors or bias[58-62]. For instance, Duplex sequencing[59,63] was able to detect mutations clustered in cancer-associated TP53 hotspots at low frequencies of < 0.01% in peritoneal fluid of women without cancer[64]. Another similar study found considerable levels of driver mutations including KRAS, TP53, PTEN, PIK3CA and several others in women with endometriosis, a largely benign gynecological disease. Other studies have identified RAS and P53 mutations in the cfDNA from saliva of patients up to 2 years before lung cancer insurgence[65], or the presence of TP53 or KRAS2 mutations in the plasma of healthy individuals who subsequently developed bladder cancer[66]. However, there are still no studies which have evaluated mutations from the cfDNA of common mutations found in normal colonic epithelium, such as STAG2, AXIN2, PIK3CA, ERBB2, ERBB3 and FBXW7.
The detection of driver mutations in the normal colon of presymptomatic individuals is hindered by several challenges, as summarized in Figure 2. To achieve sufficient sensitivity and specificity, a cfDNA-based modality must be able to distinguish between the high volume of background noise due to non-tumorigenic processes and the driver mutations of clinical interest. However, our current knowledge of how driver mutations impact on oncogenesis is unknown. Previous studies have found mutations in normal skin tissue which do indeed contribute to clonal hematopoiesis[37] or clonal expansion but not tumorigenesis[67,68]. However, the role of different mutations in healthy colonic crypts is still widely unknown, which hampers the identification of pro-tumorigenic driver mutations which are clinically significant amidst a background of non-tumorigenic somatic mutations.
As first reported in 1977 by Leon et al[9], the total plasma cfDNA concentration is significantly lower in healthy subjects compared to cancer patients. This makes the recovery and characterization of driver mutations from cfDNA in healthy individuals challenging due to the negative effect on NGS library concentration. In a study by Alborelli et al[10], NGS library concentration was found to be significantly affected by the lower plasma cfDNA concentration in healthy individuals as limited DNA input was used for library preparation, often below the minimal manufacturers’ recommended volume. The low level of input was shown to have a negative impact on sequencing performance and limit of detection (LOD) of certain assays, whereas generally higher molecular coverage was found in plasma cfDNA samples with higher amount of input cfDNA[10]. Hence, the low level of signal as well as the heterogeneity of mutations present in normal tissue still needs to be overcome to achieve efficacy. Specific to the colon, driver mutations are present in only 1% of colorectal crypts in healthy middle-aged individuals[57], making the cfDNA concentration shed by this small proportion of crypts incredibly low. This further exacerbates the poor signal-to-noise ratio. Despite the development of cutting-edge biotechnology, such as quantitative PCR or fluorescent dyes, whether the sequencing is deep enough to detect the ultralow cfDNA concentration is still unknown.
One possible solution is to analyze the cfDNA derived from non-plasma sources. CfDNA from non-blood sources has been previously shown to be more representative of the primary tumour[31,32]. Detection of stool-derived cfDNA for CRC detection is one of the areas where non-plasma cfDNA testing is already being utilized clinically - the United States Food and Drug Association-approved screening test, Cologuard®, is able to analyze stool DNA samples using sequencing panels comprising mutations in KRAS, TP53, APC and BAT-26[33]. Similarly, stool cfDNA might be more sensitive in detecting mutations in the normal colonic epithelium compared to plasma cfDNA. This might be due to stool’s physical proximity to the colonic epithelium, as human DNA is hypothesised to directly enter the stool from the colonic crypts via a combination of cellular shedding and colonocyte apoptosis[69]. However, human DNA accounts for only 0.01% of the total DNA content in stool, with the majority of DNA derived from the diverse consortium of microorganisms which inhabit the gastrointestinal tract[69,70]. The low cfDNA component poses a challenge to the detection of mutations from the normal colon. Another possible non-plasma source would be peritoneal fluid, which has previously been shown to have high detectability in CRC patients with peritoneal metastasis[71].
Another solution would be to improve the depth of current sequencing technologies. However, even with the recent advent of various NGS technologies and methods where the LOD has decreased to < 0.01%, the LODs are unlikely to be low enough to detect low-frequency driver mutations amidst background noise from the abundance of somatic mutations in normal tissues. Another possible avenue would be utilizing DNA methylation signatures of normal colonic cfDNA instead as epigenetic changes have been shown to improve detection sensitivity[48]. If this is possible, cfDNA methylation might even allow location of the driver mutation’s TOO[8].
Despite the efficacy of colonoscopy as a screening test for CRC, its invasive nature, interobserver variability leading to interpretive error, and need for bowel preparation, significantly limit its potential for population-wide screening. In contrast, liquid biopsy is a potential non-invasive modality for screening CRC through the detection of ctDNA which possess CRC-related genetic and epigenetic mutations. To date, many liquid biopsy kits have already been commercialised and approved for clinical use. Table 1 summarizes the current commercial liquid biopsy kits which analyze cfDNA, while Table 2 summarizes the liquid biopsy kits which analyze epigenetic changes.
| Name | Company | CfDNA source | Function | Detection of CRC sensitivity | Main limitation | Price per kit (dollar) |
| CancerSEEK | Thrive Earlier Detection Corp (Cambridge, Massachusetts, United States) | Plasma | Detection of 8 different cancers | 64.0% | Low sensitivity for CRC detection | 500 |
| ColoAlert® | Pharm Genomics (Mainz, Germany) | Stool | Detection of CRC | 84.6% | Low to very low certainty of reliable evidence available | 151 |
| Guardant360® CDx | Guardant Health (Palo Alto, California, United States) | Plasma | Genome profiling | NIL | Only some tumours shed detectable ctDNA into circulation | 5000 |
| FoundationOne® Liquid CDx | Foundation Medicine (Ontario, Canada) | Plasma | Genome profiling | NIL | Majority of the mutations detected are non-actionable | 5800 |
| Oncomine™ Colon cfDNA Assay | Thermo Fisher (Yokohama, Japan) | Plasma | Prognostication | 78.6% | Low feasibility in clinical practise | 12000 |
| Signatera™ | Natera (Austin, Texas, United States) | Plasma | Surveillance of MRD | 99.9% | Not as sensitive as standard-of-care imaging surveillance | 1750 |
| Name | Company | cfDNA source | Function | Detection of CRC sensitivity | Main limitation | Price per kit (dollar) |
| Cologuard® | EXACT Sciences Corporation (Madison, Wisconsin, United States) | Stool | Detection of CRC | 92.3% | Poor compliance rate due to the inconvenience of collecting stool sample | 649 |
| Epi proColon® | Epigenomics AG (Berlin, Germany) | Plasma | Detection of CRC | 74.0% | Large amount of blood plasma (> 3.5 mL) is required | 192 |
| ColoProbe | NIL | Plasma | Detection of CRC | 82.7% | Performance could exhibit variability since age was shown to be a confounding factor | NIL |
| ColonSecure | NIL | Plasma | Detection of CRC | 85.3% | Performance could exhibit variability since age was shown to be a confounding factor | NIL |
| Guardant Reveal™ | Guardant Health (Palo Alto, California, United States) | Plasma | Surveillance of MRD | 91.0% | High false-negative and high false-positive rates | 5000 |
CancerSEEK evaluates the presence of mutations in 16 cancer genes and 8 tumour-associated protein biomarkers in the plasma. CancerSEEK can detect the presence of multiple cancers through assessment of cfDNA, and its specificity for detection of CRC was over 99% but the sensitivity was only around 60%[37]. However, multi-analyte tests like CancerSEEK are not meant to replace stool-based cfDNA assays for CRC detection, but to provide additional information that could help identify patients most likely to harbour a malignancy.
Cologuard® on the other hand is a stool-based cfDNA assay specifically designed to detect CRC, involving NGS of the methylation of NDRG4, bone morphogenic protein, 7 mutation sites of KRAS and an immunohistochemical assay for haemoglobin. It was previously evaluated in a large prospective study by Imperiale et al[72] including 9989 participants with an average risk for CRC. Its evaluated sensitivity is higher than fecal immunohistochemistry test (FIT) (92.3% vs 73.8%) however with lower specificity (89.8% vs 96.4%)[72]. However, the stool-based tests usually have suboptimal compliance rates due to inconvenience[73], hence, blood-based detection assays still need to be explored.
To date, Epi proColon® is the only FDA-approved blood-based detection assay for CRC. However, its usage is currently limited for individuals aged 50 and above who are noncompliant to traditional screening modalities like colonoscopy or FIT tests[74]. It utilizes a PCR with a hydrolysis probe for the detection of methylated Septin-9 DNA (mSEPT9), which has been associated with the occurrence of CRC[75,76]. Multiple studies have demonstrated Epi proColon®’s high sensitivity and specificity of mSEPT9 for the detection of CRC[77-79]. A recent study evaluating the performance of mSEPT9 showed an aggregate sensitivity and specificity of 74% and 84% respectively when comparing CRC patients to healthy individuals[80]. Another recent study by Loomans-Kropp et al[81] evaluated the accuracy of Epi proColon® V2.0 as a screening tool for early-onset CRC, which are CRC cases diagnosed at an age of 50 years or younger[81]. They showed that the mSEPT9 assay’s detection of early-onset CRC had a sensitivity of 90.8% and specificity of 88.9%.
Other upcoming cfDNA assays for CRC detection include ColoAlert®, ColonSecure and ColoProbe. For example, ColoProbe is a multitarget plasma-based assay which detects three methylation markers, mSEPT9, SDC2, and ALX4. ColoProbe exhibited sensitivity of 82.7% for detecting CRC and 55.0% for detecting precancer, along with a specificity of 90.1%. Hence, compared to Epi proColon®, ColoProbe has a significant advantage in detecting precancerous lesions. Another upcoming blood-based assay known as ColonSecure demonstrated excellent sensitivity (85.3% and 87.0% respectively) in discriminating CRC patients from healthy controls in control and test groups[82], surpassing even conventional biomarkers such as carcinoembryonic antigen (CEA), C-reactive protein and carbohydrate antigen 19-9. Compared to the mSEPT9 assay used in Epi proColon® and ColoProbe, ColonSecure exhibited increased sensitivity and comparable specificity[83]. ColonSecure has shown similar detection sensitivity compared to the stool-based Cologuard® test and clear superiority over the FIT test, in addition to its likely advantage of having a significantly improved compliance rate[72,73].
Comprehensive genome profiling cfDNA tests for CRC - Guardant360® CDx and FoundationOne® Liquid CDx - were recently approved by the FDA for the detection of genomic changes in cancer-associated genes. Both assays are recommended as companion diagnostics for guidance of therapeutic decision making in multiple cancers. In particular, Guardant360® CDx has been shown to accurately identify 28 of 29 (96%) of pre-treatment plasma of CRC patients as bearing an amplification of ERBB2, which as previously stated in this review, is a common driver mutation in normal colonic epithelium. This might potentially allow clinicians to identify patients at a higher risk of malignant colonic transformation. This would also allow the prediction of the patients’ response to HER2-targeted therapy[84].
Another test that could have prognostication utility in CRC patients is the commercial Oncomine™ cfDNA assay which constructs 48 amplicons covering key hotspot mutations of 14 genes. In a pre-planned analysis of the VALENTINO trial using Oncomine™, Manca et al[85] studied the ctDNA VAF as a prognostic marker in patients with wild-type RAS metastatic CRC treated with an anti-EGFR-based treatment (folinic acid, fluorouracil, and oxaliplatin). They noted that higher VAF was found in patients with liver metastases, and that patients with high VAF had shorter overall survival (OS) compared to those with low VAF (21.8 months vs 36.5 months). The prognostication value of VAF hence exceeded that of baseline CEA by being significantly correlated with OS (P = 0.003), hence confirming its reliability for this purpose.
CfDNA based assays are also emerging as promising noninvasive approaches for detection of CRC recurrence post-resection and evaluating treatment response. The DYNAMIC trial highlighted that the ctDNA-based detection approach significantly reduced the use of adjuvant chemotherapy without increasing the risk of CRC recurrence in patients with stage II CRC[5]. Various NGS assays for surveillance of MRD post CRC resection have been developed over the past decade. One such example is Signatera™ (Natera), a personalized, tumour-informed, multiplex PCR-based NGS assay for ctDNA detection currently commercially available in the United States. A large observational surveillance trial by Reinert et al[86] evaluated CEA levels, computed tomography imaging, and ctDNA in patients with stage I to III CRC. Patients underwent post-resection surveillance by Signatera™ after adjuvant chemotherapy as well as by radiographic imaging. They found that Signatera™ identified disease recurrence at a median of 8.7 months before radiographic imaging. More notably, while patients were awaiting radiologic detection, their ctDNA levels increased 5-fold, indicating that tumour burden increased markedly during the 8.7 months of lead time.
Guardant Reveal™ is another NGS assay that utilizes ctDNA detection as a surveillance strategy. Unlike Signatera™, it is a tumour-uninformed assay which analyses epigenetic signatures related to anomalous DNA methylation in addition to the detection of CRC-specific somatic alterations employed by most MRD assays. A prospective cohort study by Parikh et al[87] found that the augmentation of MRD detection by the integration of epigenomic signatures increased sensitivity by 36% compared to somatic alterations alone. In most CRC patients, ctDNA was detected from both genetic and epigenetic alterations, however a significant proportion were detected as positive by either genetic or epigenetic alterations, demonstrating that combining these two modalities may improve sensitivity for MRD detection. Other than its utility as a postoperative surveillance tool, the Guardant Reveal™ assay is also being tested in prospective clinical trials to assess its efficacy as a guide for adjuvant therapy.
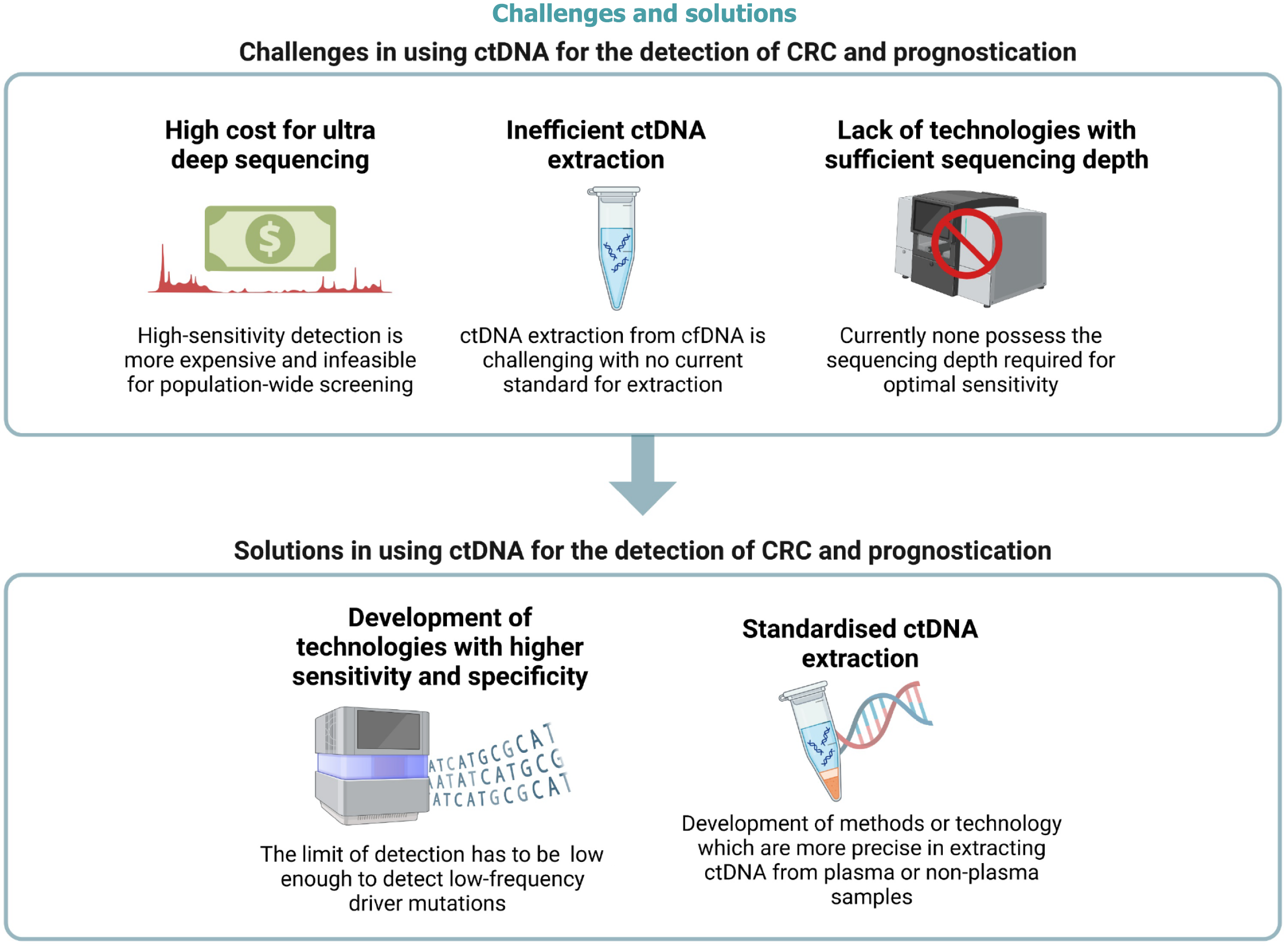
Despite the major strides of progress in the last decade regarding cfDNA assay for CRC detection, surveillance and prognostication, only a handful of tests have been approved for widespread clinical use due to multiple limitations, as summarized in Figure 3.
One of the main limitations is that ctDNA is not readily available in patients with early-stage tumours due to the low input volume and poor signal-to-noise ratio. Since ctDNA is not very different from normal cfDNA, specific extraction is challenging with no current standard for extraction. Extraction efficiency is pivotol for a successful ctDNA analysis, especially in the early stages of CRC when ctDNA load is low to begin with. However, there is currently no standardized protocol for ctDNA isolation. Most methods require centrifugation of plasma which is time-consuming, inefficient and drains resources. Most methods also lack extraction efficiency as they are unable to detect for low molecular weight DNA which is typical of most cfDNA fragments[88]. Hence, the entire process of ctDNA isolation is not only labour-intensive but also costly, calling for the need of a standardized and efficient purification protocol.
Additionally, somatic mosaicism in blood plasma remains an immense challenge for accurate cfDNA analysis[89]. Clonal hematopoiesis is a common age-related process involving the expansion of a clonal population of hematopoietic stem cells[90]. However, the detection of these non-tumorigenic clonal hematopoietic mutations is a common source of background signal for cfDNA-based assays[89]. Hence, the detection of CRC-specific mutations from plasma-derived cfDNA remains largely infeasible.
In order to address the issue of poor signal-to-noise ratio, we need detection technologies with higher sensitivity and specificity to detect the < 1.0% of ctDNA in total cfDNA to allow for earlier intervention. However, the cost of high-sensitivity sequencing is generally more costly and is hence infeasible for population-wide screening. It might be advantageous to explore the use of new cutting edge sequencing technologies such as Beads, Emulsion, Amplification, and Magnetics, clustered regularly interspaced short palindromic repeats-mediated, Ultrasensitive detection of Target DNA-PCR-PCR, or CAncer Personalised Profiling by Deep Sequencing for the augmentation of cfDNA analysis to increase sensitivity and specificity[91].
As the consistency of mutation profiles between paired plasma and tumour tissue samples increases, it is becoming clear that cfDNA-based liquid biopsy for CRC detection, surveillance and prognostication has immense potential for clinical and precision medicine. However, two main challenges still stand in the way. The first challenge is the poor signal-to-noise ratio amidst a background of somatic non-tumorigenic mutations, which is further compounded by a lack of technologies with sufficient sequencing depth. The only way to circumvent this issue would be to develop technologies capable of deep sequencing to pick up mutations more sensitively. However, even if such technologies are developed, the cost of high-sensitivity detection is likely to be expensive and infeasible for population-wide screening. The second challenge is the low quantity of plasma ctDNA found in healthy, precancerous and even early-stage CRC patients. This significantly affects the sequencing performance of some, if not all, ctDNA assays. It might be possible to circumvent this issue by harvesting ctDNA from non-plasma sources like the stool or peritoneal fluid, which might be more representative of the primary tumour. However, obtaining peritoneal fluid is unrealistic for population-wide screening due to the invasive nature of a paracentesis. On the other hand, obtaining stool as a non-plasma source of ctDNA is more feasible and is already being used clinically. However, due to the gastrointestinal microbiome, only 0.01% of the total DNA content of stool is human-derived. This leaves us to face the first challenge again: The issue of poor signal-to-noise ratio and the lack of technologies with sufficiently deep sequencing. Hence, there is no conceivable way to utilize ctDNA assay as a mainstream modality of CRC detection and prognostication if the above two challenges are not addressed. If they are circumvented, however, the potential of liquid biopsy for the detection, surveillance and prognostication of CRC would be limitless.
The potential for cfDNA to revolutionize the screening, detection and prognostication of patients with CRC rests on overcoming the challenges detailed in this manuscript. Given the potential for improved screening, as well as improved OS in patients with CRC, efforts need to be focused on overcoming these challenges with expediency.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64702] [Article Influence: 16175.5] [Reference Citation Analysis (177)] |
| 2. | Kustanovich A, Schwartz R, Peretz T, Grinshpun A. Life and death of circulating cell-free DNA. Cancer Biol Ther. 2019;20:1057-1067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 339] [Cited by in RCA: 395] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 3. | Wang S, Meng F, Li M, Bao H, Chen X, Zhu M, Liu R, Xu X, Yang S, Wu X, Shao Y, Xu L, Yin R. Multidimensional Cell-Free DNA Fragmentomic Assay for Detection of Early-Stage Lung Cancer. Am J Respir Crit Care Med. 2023;207:1203-1213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 4. | Lo YM, Lam WK. Tracing the tissue of origin of plasma DNA-feasibility and implications. Ann N Y Acad Sci. 2016;1376:14-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Tie J, Wang Y, Tomasetti C, Li L, Springer S, Kinde I, Silliman N, Tacey M, Wong HL, Christie M, Kosmider S, Skinner I, Wong R, Steel M, Tran B, Desai J, Jones I, Haydon A, Hayes T, Price TJ, Strausberg RL, Diaz LA Jr, Papadopoulos N, Kinzler KW, Vogelstein B, Gibbs P. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. 2016;8:346ra92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 1041] [Article Influence: 130.1] [Reference Citation Analysis (0)] |
| 6. | Muhanna N, Di Grappa MA, Chan HHL, Khan T, Jin CS, Zheng Y, Irish JC, Bratman SV. Cell-Free DNA Kinetics in a Pre-Clinical Model of Head and Neck Cancer. Sci Rep. 2017;7:16723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Angeles AK, Janke F, Bauer S, Christopoulos P, Riediger AL, Sültmann H. Liquid Biopsies beyond Mutation Calling: Genomic and Epigenomic Features of Cell-Free DNA in Cancer. Cancers (Basel). 2021;13:5615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Liu MC, Oxnard GR, Klein EA, Swanton C, Seiden MV; CCGA Consortium. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol. 2020;31:745-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 707] [Cited by in RCA: 873] [Article Influence: 174.6] [Reference Citation Analysis (0)] |
| 9. | Leon SA, Shapiro B, Sklaroff DM, Yaros MJ. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977;37:646-650. [PubMed] |
| 10. | Alborelli I, Generali D, Jermann P, Cappelletti MR, Ferrero G, Scaggiante B, Bortul M, Zanconati F, Nicolet S, Haegele J, Bubendorf L, Aceto N, Scaltriti M, Mucci G, Quagliata L, Novelli G. Cell-free DNA analysis in healthy individuals by next-generation sequencing: a proof of concept and technical validation study. Cell Death Dis. 2019;10:534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 11. | Mandel P, Metais P. [Nuclear Acids In Human Blood Plasma]. C R Seances Soc Biol Fil. 1948;142:241-243. [PubMed] |
| 12. | Wan JCM, Massie C, Garcia-Corbacho J, Mouliere F, Brenton JD, Caldas C, Pacey S, Baird R, Rosenfeld N. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1634] [Cited by in RCA: 1773] [Article Influence: 221.6] [Reference Citation Analysis (0)] |
| 13. | Fece de la Cruz F, Corcoran RB. Methylation in cell-free DNA for early cancer detection. Ann Oncol. 2018;29:1351-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Abbosh C, Birkbak NJ, Wilson GA, Jamal-Hanjani M, Constantin T, Salari R, Le Quesne J, Moore DA, Veeriah S, Rosenthal R, Marafioti T, Kirkizlar E, Watkins TBK, McGranahan N, Ward S, Martinson L, Riley J, Fraioli F, Al Bakir M, Grönroos E, Zambrana F, Endozo R, Bi WL, Fennessy FM, Sponer N, Johnson D, Laycock J, Shafi S, Czyzewska-Khan J, Rowan A, Chambers T, Matthews N, Turajlic S, Hiley C, Lee SM, Forster MD, Ahmad T, Falzon M, Borg E, Lawrence D, Hayward M, Kolvekar S, Panagiotopoulos N, Janes SM, Thakrar R, Ahmed A, Blackhall F, Summers Y, Hafez D, Naik A, Ganguly A, Kareht S, Shah R, Joseph L, Quinn AM, Crosbie PA, Naidu B, Middleton G, Langman G, Trotter S, Nicolson M, Remmen H, Kerr K, Chetty M, Gomersall L, Fennell DA, Nakas A, Rathinam S, Anand G, Khan S, Russell P, Ezhil V, Ismail B, Irvin-Sellers M, Prakash V, Lester JF, Kornaszewska M, Attanoos R, Adams H, Davies H, Oukrif D, Akarca AU, Hartley JA, Lowe HL, Lock S, Iles N, Bell H, Ngai Y, Elgar G, Szallasi Z, Schwarz RF, Herrero J, Stewart A, Quezada SA, Peggs KS, Van Loo P, Dive C, Lin CJ, Rabinowitz M, Aerts HJWL, Hackshaw A, Shaw JA, Zimmermann BG, Swanton C. Corrigendum: Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. 2018;554:264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Corcoran RB, Chabner BA. Application of Cell-free DNA Analysis to Cancer Treatment. N Engl J Med. 2018;379:1754-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 674] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 16. | Mohme M, Riethdorf S, Pantel K. Circulating and disseminated tumour cells - mechanisms of immune surveillance and escape. Nat Rev Clin Oncol. 2017;14:155-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 444] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 17. | Chistiakov DA, Chekhonin VP. Extracellular vesicles shed by glioma cells: pathogenic role and clinical value. Tumour Biol. 2014;35:8425-8438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Thierry AR, El Messaoudi S, Gahan PB, Anker P, Stroun M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016;35:347-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 418] [Cited by in RCA: 591] [Article Influence: 73.9] [Reference Citation Analysis (0)] |
| 19. | van der Pol Y, Mouliere F. Toward the Early Detection of Cancer by Decoding the Epigenetic and Environmental Fingerprints of Cell-Free DNA. Cancer Cell. 2019;36:350-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 204] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 20. | Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146:3-15. [PubMed] |
| 21. | Weerasinghe P, Buja LM. Oncosis: an important non-apoptotic mode of cell death. Exp Mol Pathol. 2012;93:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 22. | Heitzer E, Auinger L, Speicher MR. Cell-Free DNA and Apoptosis: How Dead Cells Inform About the Living. Trends Mol Med. 2020;26:519-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 164] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 23. | Wang W, Kong P, Ma G, Li L, Zhu J, Xia T, Xie H, Zhou W, Wang S. Characterization of the release and biological significance of cell-free DNA from breast cancer cell lines. Oncotarget. 2017;8:43180-43191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 24. | Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1478] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 25. | Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, Zheng Y, Hoshino A, Brazier H, Xiang J, Williams C, Rodriguez-Barrueco R, Silva JM, Zhang W, Hearn S, Elemento O, Paknejad N, Manova-Todorova K, Welte K, Bromberg J, Peinado H, Lyden D. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24:766-769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 997] [Cited by in RCA: 1258] [Article Influence: 114.4] [Reference Citation Analysis (0)] |
| 26. | Lázaro-Ibáñez E, Sanz-Garcia A, Visakorpi T, Escobedo-Lucea C, Siljander P, Ayuso-Sacido A, Yliperttula M. Different gDNA content in the subpopulations of prostate cancer extracellular vesicles: apoptotic bodies, microvesicles, and exosomes. Prostate. 2014;74:1379-1390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 222] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 27. | Zou W, Lai M, Zhang Y, Zheng L, Xing Z, Li T, Zou Z, Song Q, Zhao X, Xia L, Yang J, Liu A, Zhang H, Cui ZK, Jiang Y, Bai X. Exosome Release Is Regulated by mTORC1. Adv Sci (Weinh). 2019;6:1801313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 28. | Piñero F, Dirchwolf M, Pessôa MG. Biomarkers in Hepatocellular Carcinoma: Diagnosis, Prognosis and Treatment Response Assessment. Cells. 2020;9:1370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 330] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 29. | Hoadley KA, Yau C, Wolf DM, Cherniack AD, Tamborero D, Ng S, Leiserson MDM, Niu B, McLellan MD, Uzunangelov V, Zhang J, Kandoth C, Akbani R, Shen H, Omberg L, Chu A, Margolin AA, Van't Veer LJ, Lopez-Bigas N, Laird PW, Raphael BJ, Ding L, Robertson AG, Byers LA, Mills GB, Weinstein JN, Van Waes C, Chen Z, Collisson EA; Cancer Genome Atlas Research Network, Benz CC, Perou CM, Stuart JM. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell. 2014;158:929-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1229] [Cited by in RCA: 1056] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 30. | De Mattos-Arruda L, Weigelt B, Cortes J, Won HH, Ng CKY, Nuciforo P, Bidard FC, Aura C, Saura C, Peg V, Piscuoglio S, Oliveira M, Smolders Y, Patel P, Norton L, Tabernero J, Berger MF, Seoane J, Reis-Filho JS. Capturing intra-tumor genetic heterogeneity by de novo mutation profiling of circulating cell-free tumor DNA: a proof-of-principle. Ann Oncol. 2014;25:1729-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 298] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 31. | Tong L, Ding N, Tong X, Li J, Zhang Y, Wang X, Xu X, Ye M, Li C, Wu X, Bao H, Zhang X, Hong Q, Song Y, Shao YW, Bai C, Zhou J, Hu J. Tumor-derived DNA from pleural effusion supernatant as a promising alternative to tumor tissue in genomic profiling of advanced lung cancer. Theranostics. 2019;9:5532-5541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 32. | Li YS, Jiang BY, Yang JJ, Zhang XC, Zhang Z, Ye JY, Zhong WZ, Tu HY, Chen HJ, Wang Z, Xu CR, Wang BC, Du HJ, Chuai S, Han-Zhang H, Su J, Zhou Q, Yang XN, Guo WB, Yan HH, Liu YH, Yan LX, Huang B, Zheng MM, Wu YL. Unique genetic profiles from cerebrospinal fluid cell-free DNA in leptomeningeal metastases of EGFR-mutant non-small-cell lung cancer: a new medium of liquid biopsy. Ann Oncol. 2018;29:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 214] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 33. | van Lanschot MC, Carvalho B, Coupé VM, van Engeland M, Dekker E, Meijer GA. Molecular stool testing as an alternative for surveillance colonoscopy: a cross-sectional cohort study. BMC Cancer. 2017;17:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Adalsteinsson VA, Ha G, Freeman SS, Choudhury AD, Stover DG, Parsons HA, Gydush G, Reed SC, Rotem D, Rhoades J, Loginov D, Livitz D, Rosebrock D, Leshchiner I, Kim J, Stewart C, Rosenberg M, Francis JM, Zhang CZ, Cohen O, Oh C, Ding H, Polak P, Lloyd M, Mahmud S, Helvie K, Merrill MS, Santiago RA, O'Connor EP, Jeong SH, Leeson R, Barry RM, Kramkowski JF, Zhang Z, Polacek L, Lohr JG, Schleicher M, Lipscomb E, Saltzman A, Oliver NM, Marini L, Waks AG, Harshman LC, Tolaney SM, Van Allen EM, Winer EP, Lin NU, Nakabayashi M, Taplin ME, Johannessen CM, Garraway LA, Golub TR, Boehm JS, Wagle N, Getz G, Love JC, Meyerson M. Scalable whole-exome sequencing of cell-free DNA reveals high concordance with metastatic tumors. Nat Commun. 2017;8:1324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 385] [Cited by in RCA: 652] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 35. | Carter SL, Cibulskis K, Helman E, McKenna A, Shen H, Zack T, Laird PW, Onofrio RC, Winckler W, Weir BA, Beroukhim R, Pellman D, Levine DA, Lander ES, Meyerson M, Getz G. Absolute quantification of somatic DNA alterations in human cancer. Nat Biotechnol. 2012;30:413-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1637] [Cited by in RCA: 1533] [Article Influence: 117.9] [Reference Citation Analysis (0)] |
| 36. | Cohen JD, Javed AA, Thoburn C, Wong F, Tie J, Gibbs P, Schmidt CM, Yip-Schneider MT, Allen PJ, Schattner M, Brand RE, Singhi AD, Petersen GM, Hong SM, Kim SC, Falconi M, Doglioni C, Weiss MJ, Ahuja N, He J, Makary MA, Maitra A, Hanash SM, Dal Molin M, Wang Y, Li L, Ptak J, Dobbyn L, Schaefer J, Silliman N, Popoli M, Goggins MG, Hruban RH, Wolfgang CL, Klein AP, Tomasetti C, Papadopoulos N, Kinzler KW, Vogelstein B, Lennon AM. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc Natl Acad Sci U S A. 2017;114:10202-10207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 435] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 37. | Cohen JD, Li L, Wang Y, Thoburn C, Afsari B, Danilova L, Douville C, Javed AA, Wong F, Mattox A, Hruban RH, Wolfgang CL, Goggins MG, Dal Molin M, Wang TL, Roden R, Klein AP, Ptak J, Dobbyn L, Schaefer J, Silliman N, Popoli M, Vogelstein JT, Browne JD, Schoen RE, Brand RE, Tie J, Gibbs P, Wong HL, Mansfield AS, Jen J, Hanash SM, Falconi M, Allen PJ, Zhou S, Bettegowda C, Diaz LA Jr, Tomasetti C, Kinzler KW, Vogelstein B, Lennon AM, Papadopoulos N. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. 2018;359:926-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1752] [Cited by in RCA: 1885] [Article Influence: 269.3] [Reference Citation Analysis (0)] |
| 38. | Lennon AM, Buchanan AH, Kinde I, Warren A, Honushefsky A, Cohain AT, Ledbetter DH, Sanfilippo F, Sheridan K, Rosica D, Adonizio CS, Hwang HJ, Lahouel K, Cohen JD, Douville C, Patel AA, Hagmann LN, Rolston DD, Malani N, Zhou S, Bettegowda C, Diehl DL, Urban B, Still CD, Kann L, Woods JI, Salvati ZM, Vadakara J, Leeming R, Bhattacharya P, Walter C, Parker A, Lengauer C, Klein A, Tomasetti C, Fishman EK, Hruban RH, Kinzler KW, Vogelstein B, Papadopoulos N. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science. 2020;369:eabb9601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 416] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 39. | Poell JB, Mendeville M, Sie D, Brink A, Brakenhoff RH, Ylstra B. ACE: absolute copy number estimation from low-coverage whole-genome sequencing data. Bioinformatics. 2019;35:2847-2849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Taylor AM, Shih J, Ha G, Gao GF, Zhang X, Berger AC, Schumacher SE, Wang C, Hu H, Liu J, Lazar AJ; Cancer Genome Atlas Research Network, Cherniack AD, Beroukhim R, Meyerson M. Genomic and Functional Approaches to Understanding Cancer Aneuploidy. Cancer Cell. 2018;33:676-689.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 716] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 41. | Albertson DG, Collins C, McCormick F, Gray JW. Chromosome aberrations in solid tumors. Nat Genet. 2003;34:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 529] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 42. | Newman AM, Lovejoy AF, Klass DM, Kurtz DM, Chabon JJ, Scherer F, Stehr H, Liu CL, Bratman SV, Say C, Zhou L, Carter JN, West RB, Sledge GW, Shrager JB, Loo BW Jr, Neal JW, Wakelee HA, Diehn M, Alizadeh AA. Integrated digital error suppression for improved detection of circulating tumor DNA. Nat Biotechnol. 2016;34:547-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 641] [Cited by in RCA: 800] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 43. | Razavi P, Li BT, Brown DN, Jung B, Hubbell E, Shen R, Abida W, Juluru K, De Bruijn I, Hou C, Venn O, Lim R, Anand A, Maddala T, Gnerre S, Vijaya Satya R, Liu Q, Shen L, Eattock N, Yue J, Blocker AW, Lee M, Sehnert A, Xu H, Hall MP, Santiago-Zayas A, Novotny WF, Isbell JM, Rusch VW, Plitas G, Heerdt AS, Ladanyi M, Hyman DM, Jones DR, Morrow M, Riely GJ, Scher HI, Rudin CM, Robson ME, Diaz LA Jr, Solit DB, Aravanis AM, Reis-Filho JS. High-intensity sequencing reveals the sources of plasma circulating cell-free DNA variants. Nat Med. 2019;25:1928-1937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 321] [Cited by in RCA: 510] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 44. | Cristiano S, Leal A, Phallen J, Fiksel J, Adleff V, Bruhm DC, Jensen SØ, Medina JE, Hruban C, White JR, Palsgrove DN, Niknafs N, Anagnostou V, Forde P, Naidoo J, Marrone K, Brahmer J, Woodward BD, Husain H, van Rooijen KL, Ørntoft MW, Madsen AH, van de Velde CJH, Verheij M, Cats A, Punt CJA, Vink GR, van Grieken NCT, Koopman M, Fijneman RJA, Johansen JS, Nielsen HJ, Meijer GA, Andersen CL, Scharpf RB, Velculescu VE. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature. 2019;570:385-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 797] [Cited by in RCA: 861] [Article Influence: 143.5] [Reference Citation Analysis (0)] |
| 45. | Chabon JJ, Hamilton EG, Kurtz DM, Esfahani MS, Moding EJ, Stehr H, Schroers-Martin J, Nabet BY, Chen B, Chaudhuri AA, Liu CL, Hui AB, Jin MC, Azad TD, Almanza D, Jeon YJ, Nesselbush MC, Co Ting Keh L, Bonilla RF, Yoo CH, Ko RB, Chen EL, Merriott DJ, Massion PP, Mansfield AS, Jen J, Ren HZ, Lin SH, Costantino CL, Burr R, Tibshirani R, Gambhir SS, Berry GJ, Jensen KC, West RB, Neal JW, Wakelee HA, Loo BW Jr, Kunder CA, Leung AN, Lui NS, Berry MF, Shrager JB, Nair VS, Haber DA, Sequist LV, Alizadeh AA, Diehn M. Integrating genomic features for non-invasive early lung cancer detection. Nature. 2020;580:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 444] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 46. | Yizhak K, Aguet F, Kim J, Hess JM, Kübler K, Grimsby J, Frazer R, Zhang H, Haradhvala NJ, Rosebrock D, Livitz D, Li X, Arich-Landkof E, Shoresh N, Stewart C, Segrè AV, Branton PA, Polak P, Ardlie KG, Getz G. RNA sequence analysis reveals macroscopic somatic clonal expansion across normal tissues. Science. 2019;364:eaaw0726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 356] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 47. | Li R, Di L, Li J, Fan W, Liu Y, Guo W, Liu W, Liu L, Li Q, Chen L, Chen Y, Miao C, Liu H, Wang Y, Ma Y, Xu D, Lin D, Huang Y, Wang J, Bai F, Wu C. A body map of somatic mutagenesis in morphologically normal human tissues. Nature. 2021;597:398-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 127] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 48. | Shen SY, Singhania R, Fehringer G, Chakravarthy A, Roehrl MHA, Chadwick D, Zuzarte PC, Borgida A, Wang TT, Li T, Kis O, Zhao Z, Spreafico A, Medina TDS, Wang Y, Roulois D, Ettayebi I, Chen Z, Chow S, Murphy T, Arruda A, O'Kane GM, Liu J, Mansour M, McPherson JD, O'Brien C, Leighl N, Bedard PL, Fleshner N, Liu G, Minden MD, Gallinger S, Goldenberg A, Pugh TJ, Hoffman MM, Bratman SV, Hung RJ, De Carvalho DD. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 617] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 49. | Fernandez AF, Assenov Y, Martin-Subero JI, Balint B, Siebert R, Taniguchi H, Yamamoto H, Hidalgo M, Tan AC, Galm O, Ferrer I, Sanchez-Cespedes M, Villanueva A, Carmona J, Sanchez-Mut JV, Berdasco M, Moreno V, Capella G, Monk D, Ballestar E, Ropero S, Martinez R, Sanchez-Carbayo M, Prosper F, Agirre X, Fraga MF, Graña O, Perez-Jurado L, Mora J, Puig S, Prat J, Badimon L, Puca AA, Meltzer SJ, Lengauer T, Bridgewater J, Bock C, Esteller M. A DNA methylation fingerprint of 1628 human samples. Genome Res. 2012;22:407-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 296] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 50. | Roadmap Epigenomics Consortium; Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, Heravi-Moussavi A, Kheradpour P, Zhang Z, Wang J, Ziller MJ, Amin V, Whitaker JW, Schultz MD, Ward LD, Sarkar A, Quon G, Sandstrom RS, Eaton ML, Wu YC, Pfenning AR, Wang X, Claussnitzer M, Liu Y, Coarfa C, Harris RA, Shoresh N, Epstein CB, Gjoneska E, Leung D, Xie W, Hawkins RD, Lister R, Hong C, Gascard P, Mungall AJ, Moore R, Chuah E, Tam A, Canfield TK, Hansen RS, Kaul R, Sabo PJ, Bansal MS, Carles A, Dixon JR, Farh KH, Feizi S, Karlic R, Kim AR, Kulkarni A, Li D, Lowdon R, Elliott G, Mercer TR, Neph SJ, Onuchic V, Polak P, Rajagopal N, Ray P, Sallari RC, Siebenthall KT, Sinnott-Armstrong NA, Stevens M, Thurman RE, Wu J, Zhang B, Zhou X, Beaudet AE, Boyer LA, De Jager PL, Farnham PJ, Fisher SJ, Haussler D, Jones SJ, Li W, Marra MA, McManus MT, Sunyaev S, Thomson JA, Tlsty TD, Tsai LH, Wang W, Waterland RA, Zhang MQ, Chadwick LH, Bernstein BE, Costello JF, Ecker JR, Hirst M, Meissner A, Milosavljevic A, Ren B, Stamatoyannopoulos JA, Wang T, Kellis M. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518:317-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5261] [Cited by in RCA: 4463] [Article Influence: 446.3] [Reference Citation Analysis (0)] |
| 51. | Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, Wiencke JK, Kelsey KT. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics. 2012;13:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2024] [Cited by in RCA: 2399] [Article Influence: 184.5] [Reference Citation Analysis (0)] |
| 52. | Xu RH, Wei W, Krawczyk M, Wang W, Luo H, Flagg K, Yi S, Shi W, Quan Q, Li K, Zheng L, Zhang H, Caughey BA, Zhao Q, Hou J, Zhang R, Xu Y, Cai H, Li G, Hou R, Zhong Z, Lin D, Fu X, Zhu J, Duan Y, Yu M, Ying B, Zhang W, Wang J, Zhang E, Zhang C, Li O, Guo R, Carter H, Zhu JK, Hao X, Zhang K. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat Mater. 2017;16:1155-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 635] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 53. | Luo H, Zhao Q, Wei W, Zheng L, Yi S, Li G, Wang W, Sheng H, Pu H, Mo H, Zuo Z, Liu Z, Li C, Xie C, Zeng Z, Li W, Hao X, Liu Y, Cao S, Liu W, Gibson S, Zhang K, Xu G, Xu RH. Circulating tumor DNA methylation profiles enable early diagnosis, prognosis prediction, and screening for colorectal cancer. Sci Transl Med. 2020;12:eaax7533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 282] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 54. | Chen X, Gole J, Gore A, He Q, Lu M, Min J, Yuan Z, Yang X, Jiang Y, Zhang T, Suo C, Li X, Cheng L, Zhang Z, Niu H, Li Z, Xie Z, Shi H, Zhang X, Fan M, Wang X, Yang Y, Dang J, McConnell C, Zhang J, Wang J, Yu S, Ye W, Gao Y, Zhang K, Liu R, Jin L. Non-invasive early detection of cancer four years before conventional diagnosis using a blood test. Nat Commun. 2020;11:3475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 374] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 55. | Sun K, Jiang P, Chan KC, Wong J, Cheng YK, Liang RH, Chan WK, Ma ES, Chan SL, Cheng SH, Chan RW, Tong YK, Ng SS, Wong RS, Hui DS, Leung TN, Leung TY, Lai PB, Chiu RW, Lo YM. Plasma DNA tissue mapping by genome-wide methylation sequencing for noninvasive prenatal, cancer, and transplantation assessments. Proc Natl Acad Sci U S A. 2015;112:E5503-E5512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 540] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 56. | Calabrese P, Tavaré S, Shibata D. Pretumor progression: clonal evolution of human stem cell populations. Am J Pathol. 2004;164:1337-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 57. | Lee-Six H, Olafsson S, Ellis P, Osborne RJ, Sanders MA, Moore L, Georgakopoulos N, Torrente F, Noorani A, Goddard M, Robinson P, Coorens THH, O'Neill L, Alder C, Wang J, Fitzgerald RC, Zilbauer M, Coleman N, Saeb-Parsy K, Martincorena I, Campbell PJ, Stratton MR. The landscape of somatic mutation in normal colorectal epithelial cells. Nature. 2019;574:532-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 460] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 58. | Kinde I, Wu J, Papadopoulos N, Kinzler KW, Vogelstein B. Detection and quantification of rare mutations with massively parallel sequencing. Proc Natl Acad Sci U S A. 2011;108:9530-9535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1040] [Cited by in RCA: 920] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 59. | Schmitt MW, Kennedy SR, Salk JJ, Fox EJ, Hiatt JB, Loeb LA. Detection of ultra-rare mutations by next-generation sequencing. Proc Natl Acad Sci U S A. 2012;109:14508-14513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 762] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 60. | Mattox AK, Wang Y, Springer S, Cohen JD, Yegnasubramanian S, Nelson WG, Kinzler KW, Vogelstein B, Papadopoulos N. Bisulfite-converted duplexes for the strand-specific detection and quantification of rare mutations. Proc Natl Acad Sci U S A. 2017;114:4733-4738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 61. | Hoang ML, Kinde I, Tomasetti C, McMahon KW, Rosenquist TA, Grollman AP, Kinzler KW, Vogelstein B, Papadopoulos N. Genome-wide quantification of rare somatic mutations in normal human tissues using massively parallel sequencing. Proc Natl Acad Sci U S A. 2016;113:9846-9851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 62. | Dong X, Zhang L, Milholland B, Lee M, Maslov AY, Wang T, Vijg J. Accurate identification of single-nucleotide variants in whole-genome-amplified single cells. Nat Methods. 2017;14:491-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 63. | Kennedy SR, Schmitt MW, Fox EJ, Kohrn BF, Salk JJ, Ahn EH, Prindle MJ, Kuong KJ, Shen JC, Risques RA, Loeb LA. Detecting ultralow-frequency mutations by Duplex Sequencing. Nat Protoc. 2014;9:2586-2606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 352] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 64. | Krimmel JD, Schmitt MW, Harrell MI, Agnew KJ, Kennedy SR, Emond MJ, Loeb LA, Swisher EM, Risques RA. Ultra-deep sequencing detects ovarian cancer cells in peritoneal fluid and reveals somatic TP53 mutations in noncancerous tissues. Proc Natl Acad Sci U S A. 2016;113:6005-6010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 65. | Gormally E, Vineis P, Matullo G, Veglia F, Caboux E, Le Roux E, Peluso M, Garte S, Guarrera S, Munnia A, Airoldi L, Autrup H, Malaveille C, Dunning A, Overvad K, Tjønneland A, Lund E, Clavel-Chapelon F, Boeing H, Trichopoulou A, Palli D, Krogh V, Tumino R, Panico S, Bueno-de-Mesquita HB, Peeters PH, Pera G, Martinez C, Dorronsoro M, Barricarte A, Navarro C, Quirós JR, Hallmans G, Day NE, Key TJ, Saracci R, Kaaks R, Riboli E, Hainaut P. TP53 and KRAS2 mutations in plasma DNA of healthy subjects and subsequent cancer occurrence: a prospective study. Cancer Res. 2006;66:6871-6876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 66. | Ding RF, Zhang Y, Wu LY, You P, Fang ZX, Li ZY, Zhang ZY, Ji ZL. Discovering Innate Driver Variants for Risk Assessment of Early Colorectal Cancer Metastasis. Front Oncol. 2022;12:898117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 67. | Aghili L, Foo J, DeGregori J, De S. Patterns of somatically acquired amplifications and deletions in apparently normal tissues of ovarian cancer patients. Cell Rep. 2014;7:1310-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Martincorena I, Roshan A, Gerstung M, Ellis P, Van Loo P, McLaren S, Wedge DC, Fullam A, Alexandrov LB, Tubio JM, Stebbings L, Menzies A, Widaa S, Stratton MR, Jones PH, Campbell PJ. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science. 2015;348:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1271] [Article Influence: 127.1] [Reference Citation Analysis (0)] |
| 69. | Aghagolzadeh P, Radpour R. New trends in molecular and cellular biomarker discovery for colorectal cancer. World J Gastroenterol. 2016;22:5678-5693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (2)] |
| 70. | Klaassen CH, Jeunink MA, Prinsen CF, Ruers TJ, Tan AC, Strobbe LJ, Thunnissen FB. Quantification of human DNA in feces as a diagnostic test for the presence of colorectal cancer. Clin Chem. 2003;49:1185-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 71. | Van't Erve I, Rovers KP, Constantinides A, Bolhuis K, Wassenaar EC, Lurvink RJ, Huysentruyt CJ, Snaebjornsson P, Boerma D, van den Broek D, Buffart TE, Lahaye MJ, Aalbers AG, Kok NF, Meijer GA, Punt CJ, Kranenburg O, de Hingh IH, Fijneman RJ. Detection of tumor-derived cell-free DNA from colorectal cancer peritoneal metastases in plasma and peritoneal fluid. J Pathol Clin Res. 2021;7:203-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 72. | Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, Ahlquist DA, Berger BM. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1241] [Article Influence: 112.8] [Reference Citation Analysis (1)] |
| 73. | Piscitello A, Saoud L, Fendrick AM, Borah BJ, Hassmiller Lich K, Matney M, Ozbay AB, Parton M, Limburg PJ. Estimating the impact of differential adherence on the comparative effectiveness of stool-based colorectal cancer screening using the CRC-AIM microsimulation model. PLoS One. 2020;15:e0244431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 74. | US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr, García FAR, Gillman MW, Harper DM, Kemper AR, Krist AH, Kurth AE, Landefeld CS, Mangione CM, Owens DK, Phillips WR, Phipps MG, Pignone MP, Siu AL. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315:2564-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1249] [Cited by in RCA: 1388] [Article Influence: 154.2] [Reference Citation Analysis (1)] |
| 75. | deVos T, Tetzner R, Model F, Weiss G, Schuster M, Distler J, Steiger KV, Grützmann R, Pilarsky C, Habermann JK, Fleshner PR, Oubre BM, Day R, Sledziewski AZ, Lofton-Day C. Circulating methylated SEPT9 DNA in plasma is a biomarker for colorectal cancer. Clin Chem. 2009;55:1337-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 404] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 76. | Lofton-Day C, Model F, Devos T, Tetzner R, Distler J, Schuster M, Song X, Lesche R, Liebenberg V, Ebert M, Molnar B, Grützmann R, Pilarsky C, Sledziewski A. DNA methylation biomarkers for blood-based colorectal cancer screening. Clin Chem. 2008;54:414-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 375] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 77. | Fu B, Yan P, Zhang S, Lu Y, Pan L, Tang W, Chen S, Chen S, Zhang A, Liu W. Cell-Free Circulating Methylated SEPT9 for Noninvasive Diagnosis and Monitoring of Colorectal Cancer. Dis Markers. 2018;2018:6437104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 78. | Sun J, Fei F, Zhang M, Li Y, Zhang X, Zhu S, Zhang S. The role of (m)SEPT9 in screening, diagnosis, and recurrence monitoring of colorectal cancer. BMC Cancer. 2019;19:450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 79. | Church TR, Wandell M, Lofton-Day C, Mongin SJ, Burger M, Payne SR, Castaños-Vélez E, Blumenstein BA, Rösch T, Osborn N, Snover D, Day RW, Ransohoff DF; PRESEPT Clinical Study Steering Committee, Investigators and Study Team. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut. 2014;63:317-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 486] [Cited by in RCA: 592] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 80. | Sun G, Meng J, Duan H, Zhang D, Tang Y. Diagnostic Assessment of septin9 DNA Methylation for Colorectal Cancer Using Blood Detection: A Meta-Analysis. Pathol Oncol Res. 2019;25:1525-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 81. | Loomans-Kropp HA, Song Y, Gala M, Parikh AR, Van Seventer EE, Alvarez R, Hitchins MP, Shoemaker RH, Umar A. Methylated Septin9 (mSEPT9): A promising blood-based biomarker for the detection and screening of early-onset colorectal cancer. Cancer Res Commun. 2022;2:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 82. | Zhao F, Bai P, Xu J, Li Z, Muhammad S, Li D, Zhang Z, Gao Y, Liu Q. Efficacy of cell-free DNA methylation-based blood test for colorectal cancer screening in high-risk population: a prospective cohort study. Mol Cancer. 2023;22:157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 83. | Zygulska AL, Pierzchalski P. Novel Diagnostic Biomarkers in Colorectal Cancer. Int J Mol Sci. 2022;23:852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 135] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 84. | Chan HT, Chin YM, Low SK. Circulating Tumor DNA-Based Genomic Profiling Assays in Adult Solid Tumors for Precision Oncology: Recent Advancements and Future Challenges. Cancers (Basel). 2022;14:3275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 85. | Manca P, Corallo S, Lonardi S, Fucà G, Busico A, Leone AG, Corti F, Antoniotti C, Procaccio L, Smiroldo V, Ratti M, Murialdo R, Racca P, Pagani F, Randon G, Martinetti A, Sottotetti E, Prisciandaro M, Ambrosini M, Raimondi A, Morano F, Pietrantonio F. Variant allele frequency in baseline circulating tumour DNA to measure tumour burden and to stratify outcomes in patients with RAS wild-type metastatic colorectal cancer: a translational objective of the Valentino study. Br J Cancer. 2022;126:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 86. | Reinert T, Henriksen TV, Christensen E, Sharma S, Salari R, Sethi H, Knudsen M, Nordentoft I, Wu HT, Tin AS, Heilskov Rasmussen M, Vang S, Shchegrova S, Frydendahl Boll Johansen A, Srinivasan R, Assaf Z, Balcioglu M, Olson A, Dashner S, Hafez D, Navarro S, Goel S, Rabinowitz M, Billings P, Sigurjonsson S, Dyrskjøt L, Swenerton R, Aleshin A, Laurberg S, Husted Madsen A, Kannerup AS, Stribolt K, Palmelund Krag S, Iversen LH, Gotschalck Sunesen K, Lin CJ, Zimmermann BG, Lindbjerg Andersen C. Analysis of Plasma Cell-Free DNA by Ultradeep Sequencing in Patients With Stages I to III Colorectal Cancer. JAMA Oncol. 2019;5:1124-1131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 645] [Article Influence: 107.5] [Reference Citation Analysis (0)] |
| 87. | Parikh AR, Van Seventer EE, Siravegna G, Hartwig AV, Jaimovich A, He Y, Kanter K, Fish MG, Fosbenner KD, Miao B, Phillips S, Carmichael JH, Sharma N, Jarnagin J, Baiev I, Shah YS, Fetter IJ, Shahzade HA, Allen JN, Blaszkowsky LS, Clark JW, Dubois JS, Franses JW, Giantonio BJ, Goyal L, Klempner SJ, Nipp RD, Roeland EJ, Ryan DP, Weekes CD, Wo JY, Hong TS, Bordeianou L, Ferrone CR, Qadan M, Kunitake H, Berger D, Ricciardi R, Cusack JC, Raymond VM, Talasaz A, Boland GM, Corcoran RB. Minimal Residual Disease Detection using a Plasma-only Circulating Tumor DNA Assay in Patients with Colorectal Cancer. Clin Cancer Res. 2021;27:5586-5594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 242] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 88. | Diefenbach RJ, Lee JH, Kefford RF, Rizos H. Evaluation of commercial kits for purification of circulating free DNA. Cancer Genet. 2018;228-229:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 89. | Chan HT, Chin YM, Nakamura Y, Low SK. Clonal Hematopoiesis in Liquid Biopsy: From Biological Noise to Valuable Clinical Implications. Cancers (Basel). 2020;12:2277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 90. | Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, Ebert BL. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488-2498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3384] [Cited by in RCA: 3508] [Article Influence: 318.9] [Reference Citation Analysis (0)] |
| 91. | Myint NNM, Verma AM, Fernandez-Garcia D, Sarmah P, Tarpey PS, Al-Aqbi SS, Cai H, Trigg R, West K, Howells LM, Thomas A, Brown K, Guttery DS, Singh B, Pringle HJ, McDermott U, Shaw JA, Rufini A. Circulating tumor DNA in patients with colorectal adenomas: assessment of detectability and genetic heterogeneity. Cell Death Dis. 2018;9:894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |