Published online Jul 15, 2025. doi: 10.4251/wjgo.v17.i7.103337
Revised: March 19, 2025
Accepted: March 20, 2025
Published online: July 15, 2025
Processing time: 241 Days and 5 Hours
Pancreatic ductal adenocarcinoma (PDAC) is a global health challenge and remains one of the most lethal malignancies; there are only a few therapeutic options. However, significant efforts have led to the identification of major genetic factors that drive the progression and pathogenesis of PDAC. Notably, the research and application of molecular targeted therapies and immunotherapies have rapidly increased and facilitated great progress in the treatment of many malignant tumors, additional targeted therapies and immunotherapy based on next-generation sequencing results provide new opportunities for the diagnosis and treatment of pancreatic tumors. Immune checkpoint inhibitors are also now being incorporated as PDAC therapies, and combinations of molecularly targeted therapies with immunotherapies are emerging as strategies for boosting the immune response. The investigation of biomarkers of a response or primary resistance to immunotherapies is also an emerging research area. Herein, we further discuss the recent technological advances that continue to expand our understanding of PDAC complexity. We discuss the advancements expected in the near future, including biomarker-driven treatments and immunotherapies. We presume that the clinical translation of these research efforts will improve the survival outcomes of this challenging disease, which are currently dismal.
Core Tip: Pancreatic ductal adenocarcinoma is the fourth leading cause of cancer death globally and is projected to be the second leading cause later. Kirsten rat sarcoma oncogene is a critical target for treatment regime evaluation in pancreatic ductal adenocarcinoma and proof of principle approaches have validated targeting in Kirsten rat sarcoma oncogene G12C. FOLFIRINOX and nab-paclitaxel gemcitabine are gold standard therapeutic in patients. The combinations were shown a survival benefit over previously standard gemcitabine monotherapy. Therapies targeting as well as immuno-therapies hold promise for the future but are currently not standard of care.
- Citation: Hendi M, Zhang B, Mou YP, Cai XJ. Importance of landscape exploration and progress in molecular therapies and precision medicine for pancreatic ductal adenocarcinoma. World J Gastrointest Oncol 2025; 17(7): 103337
- URL: https://www.wjgnet.com/1948-5204/full/v17/i7/103337.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i7.103337
Pancreatic ductal adenocarcinoma (PDAC) is a deadly disease; it remains one of the most lethal cancers and aggressive diseases and is predicted to be the second leading cause of cancer-related deaths globally within a short time frame. Only small improvements in patient outcomes have occurred over the past decade[1]. PDAC is a devastating disease with a five-year overall survival (OS) rate were less than 11%. Following more than 50% of individuals are diagnosed with metastatic disease, which is associated only 3% with a 5-year survival rate[1,2]. Recent progress in chemotherapy regimens has modestly improved the outcomes of the majority of patients treated with chemotherapy at the cost of marked toxicity. Recently, adjuvant therapy with fluorouarcil, oxaliplatin, irinotecan, and leucovorin (modified FOLFIRINOX) led to a median disease-free survival time of 21.6 months (compared with 12.8 months with gemcitabine treatment)[3]. However, approximately 80% of individuals were diagnosed at an advanced stage, precluding curative intent surgery[4]. Studies over the past decade has cemented gemcitabine and fluoropyrimidine based regimens as the standard for chemotherapy care for patients with metastatic pancreatic cancer (MPC)[5]. Moreover, gemcitabine & fluoropyrimidine-based regimens have become the standard of care for suitable patients with removable tumor. Regrettably, the median OS time remains approximately one year[6]. Additionally, the Italian Association of Medical Oncology recommends additional treatment after first-line therapy on the basis of primer testing for actionable genomic changes[7].
To date, there are no predictive molecular markers that identify which patients are likely to benefit from FOLFIRINOX vs gemcitabine/nab-paclitaxel. Thus, there is an urgent and important need for insight into the biology and genetics of PDAC. Immunotherapeutic strategies are capable of inducing strong immune responses against tumors. Immunomodulators, immune checkpoint inhibitors, adoptive cell therapies and vaccine therapy could be promising treatment options[8]. Novel therapies for PDAC remain unused medical tools for improving the survival rate and quality of life. This review summarizes the current therapeutics paradigms for PDAC and then expanded upon the biological underpinnings driving its pathogenesis and progression. Additionally, we discuss how the clinical trial landscape of PDAC has evolved over the past decade and provide a prospective outlook on precision therapy for PDAC in the future years.
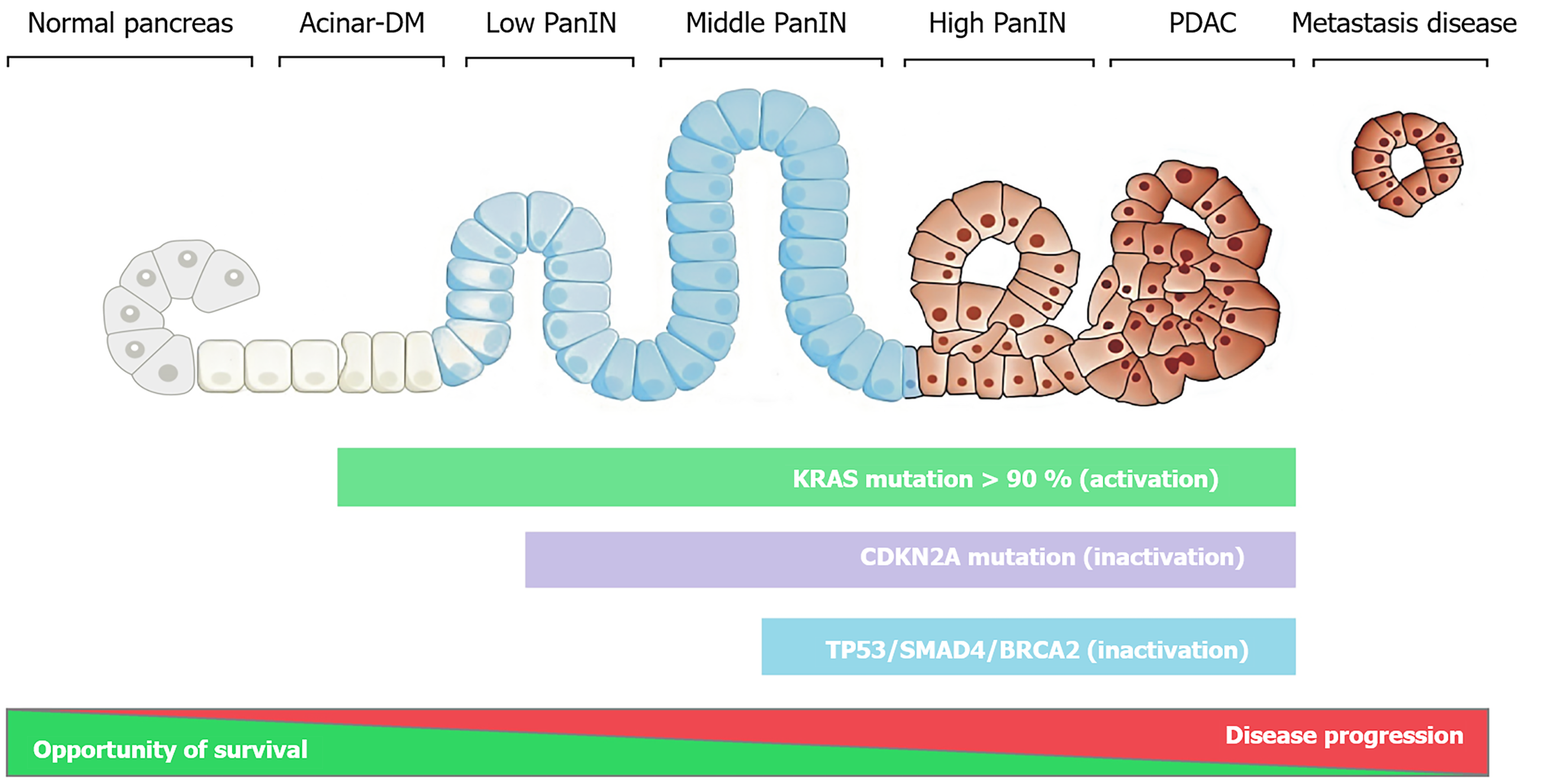
Next-generation sequencing (NGS) has revolutionized the care of patients with various types of cancer. NGS has enabled molecular profiling to be increasingly accessible and integrated into standard therapeutic paradigms because of its lower cost and wider availability and the availability of advanced hardware and software[9]. In the first NGS study by Jones et al[10], the authors analyzed a cohort of 24 pancreatic cancer patients and found an average of 63 genetic alterations per case. The Know Your Tumour program revealed that 25%-35% of the PDAC profiles harbored actionable molecular alterations. Precise molecular matched therapy for individuals with PDAC substantially increased the median OS times of patients with PDAC (approximately 30 months in patients with actionable alterations on matched therapy compared with 18 months in patients with unmatched treatments). This indicates that potentially actionable genes should be evaluated for most individuals who have advanced-stage pancreatic cancer[11]. Currently, the next generation is used globally for PDAC patients. Recently, the American Society of Clinical Oncology guidelines and European Society for Medical Oncology Guideline were recommended germline genetic testing for most PDAC patients[12,13]. Genetic profiling is used to identify some actionable somatic mutations and fusions [such as Kirsten rat sarcoma oncogene (KRAS), B-raf proto oncogene, neuregulin-1, neurotrophic receptor tyrosine kinase (NTRK), anaplastic lymphoma kinase, breast cancer susceptibility gene 1 (BRCA1), BRCA2, HER2, partner and localizer of breast cancer 2 (PALB2), rearranged during transfection, fibroblast growth factor receptor 2, and mismatch repair deficient (MMR-D)][13]. Additionally, from a genetic point of view, pancreatic cancer is a complex disease; a number of genes are altered through different point mutations, chromosomal aberrations, and epigenetic mechanisms, resulting in intermediate tumor mutational burden[14,15]. The predominant gene mutations appear to occur sequentially as pancreatic intraepithelial neoplasms progresses (Figure 1). Furthermore, most of the studies have shown that more than 90% of patients have oncogenic activating KRAS mutations. The KRAS mutation was detected in most stages of pancreatic cancer. Pancreatic intraepithelial neoplasms transform into an invasive adenocarcinoma in a typically slow process involving some primary driver mutations, including mutations in KRAS, CDKN2A, TP53, SMAD4, and BRCA2. These common mutations in major genes have been used to characterize PDAC and provide a pleiotropic path for identifying ideal targets that may benefit most patients[15-17]. The Cancer Genome Atlas Research Network reported that some patients with PDAC present with multiple KRAS mutations and that alternative driver events occur in KRAS wild-type tumors[18]. Another work by Witkiewicz et al[19] used exome sequencing to reveal that the B-raf proto oncogene V600E mutation is present in only 3% of patients and exclusively in KRAS wild-type PDAC. On the other hand, the proton oncogene KRAS is mutated and found in approximately 88%-95% of patients with PDAC. Nevertheless, no therapies successfully target mutated KRAS. This is a hot topic for many researchers. A variety of oncogenes, including KRAS and RAS isoforms, represent the most prevalent oncogene in human cancers.
In recent decades, considerable efforts toward the discovery of therapies targeting proteins downstream of activated RAS have been largely unsuccessful owing to the activation of adjacent pathways to obtain clinically approved drugs. Additionally, promising progress in the exploration of treatment opportunities in RAS inhibition has been made in the last few years[20,21]. Notably, platinum-based chemotherapy improved survival outcomes in patients with BRCA1 mutations and BRCA2 mutations[22]. Two studies revealed that patients with BRCA1 and BRCA2 mutations displayed better response rates and improved survival with platinum-based chemotherapy[23,24]. Seeber et al[25] studied 2818 PDAC samples and analyzed BRCA1, BRCA2 and PALB2 via NGS. Mutations in these genes were observed in 1.3%, 3.1%, and 0.6% of patients, respectively. The findings revealed both BRCA, PALB2 mutations in a significant subgroup of PDAC individuals. These mutations were associated with a distinct molecular profile potentially predictive of the response to immune checkpoint inhibitor treatment[25].
BRCA1/2 mutation is positively correlated with increased programmed-death ligand 1 (PD-L1) staining in human PDAC cancer cells[25]. Targeting poly(ADP-ribose) (PAR) polymerase (PARP), a critical enzyme for single-stranded DNA repair, in BRCA1 and BRCA2 deficient pre-clinical cancer models is an effective treatment strategy[26]. The Pancreatic Cancer Olaparib Ongoing (POLO) study demonstrated that the PARP inhibitor olaparib exhibits greater efficacy in patients with platinum-sensitive metastasis PDAC with germline BRCA1 or BRCA2 mutations[27,28]. Kindler et al[29] reported that phase III POLO demonstrated significant progression-free survival (PFS) benefit and preserved health-related quality of life for active maintenance treatment with olaparib vs placebo in patients with MPC and a germline BRCA mutation. Additionally, 5% to 9% of PDAC patients have a germline BRCA1/2 or PALB2 mutation[30]. Moreover, the molecular characterization offers a unique opportunity to identify new prognostic and predictive biomarkers to improve patient selection for treatments PDAC patients.
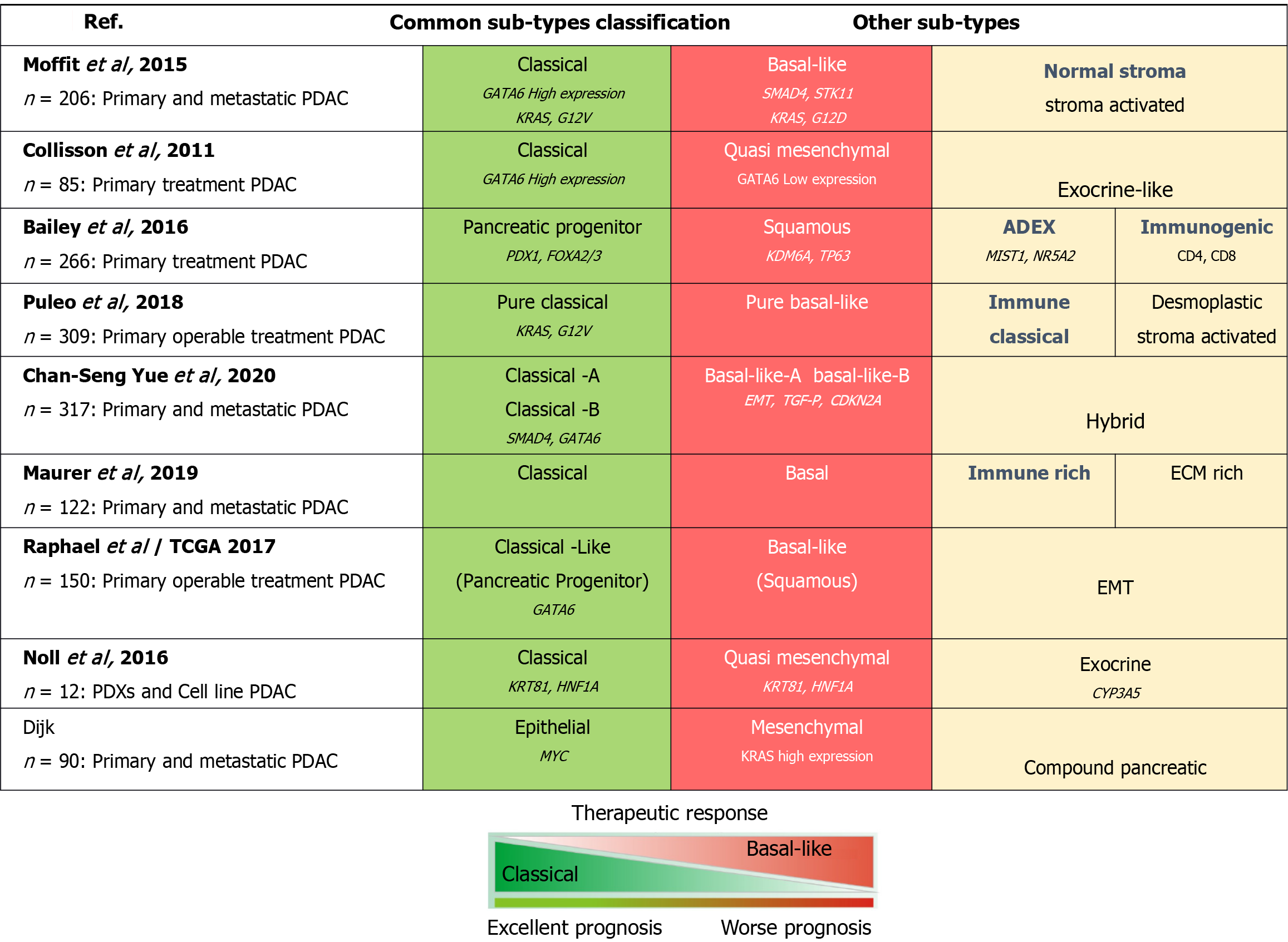
Over the years, several studies have been performed to analyze pancreatic tumors via hybridization arrays based on mRNA expression data from primary untreated resected PDAC to suggest broad classifications; these studies revealed specific genes that characterize different subtypes of PDAC (Figure 2). Each of those studies involved the use of different approaches to address the low cellularity and stromal contribution, leading to some debate regarding the value of those subtype classification systems. Collisson et al[31] originally identified three molecular subtypes of PDAC tumors; classical, exocrine-like and quasimesenchymal.
Moffitt et al[32] analyzed bulk tumor tissues from treated primary resected PDAC to identify two tumor-specific subtypes. The normal and activated subtypes were independent prognostic factors. Another two tumor subtypes are the classical and basal-like subtypes[32]. In both studies on these subtypes, basal subtype tumors were associated with a poorer OS than classical tumors, which overlapped significantly with the Collisson classical subtype[31,32]. Bailey et al[33] described several stable subtype classifications using samples with > 40% cellularity from resectable primary pancreatic cancer; these classifications were maintained in an extended set of mRNA hybridization data for 232 PDACs covering the full range of tumor cellularity values (from 12%-100%). On the basis of the expression profiles, the molecular subtypes are as follows: Progenitor, immunogenic, aberrantly differentiated endocrine exocrine, and squamous; each subtype is related to the differential expression of transcription factors and their targets involved in lineage specification and differentiation during pancreatic development[33]. Maurer et al[34] were suggested that the Collisson exocrine and Bailey aberrantly differentiated endocrine exocrine sub-types might be a function of the degree of tumor cellularity rather than distinct types, as most of the sub-type defining genes are largely derived from bulk tumor tissue. Puleo et al[35] reported the sub-types of PDAC by using paraffin-embedded samples and then divided them into five sub-types (pure classical, pure basal-like, immune classical, desmoplastic, and stroma activated) according to the features of both cells and the microenvironment. The pure basal-like subtype had the worst prognostic outcome, with a median OS of 10.3 months, whereas the pure classical sub-type had a good prognostic outcome with a median OS of 43.1 months[35].
Currently, basal-like tumors are associated with a poor prognosis, worse survival, and resistance to therapy, whereas classical tumors are more differentiated, have good survival, and increased response to therapy. Chan-Seng-Yue et al[36] reported that, as single-cell resolution studies have indicated, most PDAC tumors harbor both classical and basal-like subtypes, whereas the hybrid subtype was inconsistently classified by previous systems due to heterogeneous expression profiles. This stratification system still needs validation, but it does demonstrate the importance of including metastatic sites in PDAC molecular studies[36]. Another report by Noll et al[37] revealed different molecular subtypes via immunohistochemistry of the hepatocyte nuclear factor 1 alpha and keratin 81. The two-marker combination identified the quasi-mesenchymal subtype, which was associated with shorter survival; the exocrine-like subtype, which is associated with longer survival; and the classical subtype, which was associated with intermediate survival. Clear subtype-specific treatment vulnerabilities have not yet been demonstrated for these classifications, despite their established relevance for prognosis[38,39].
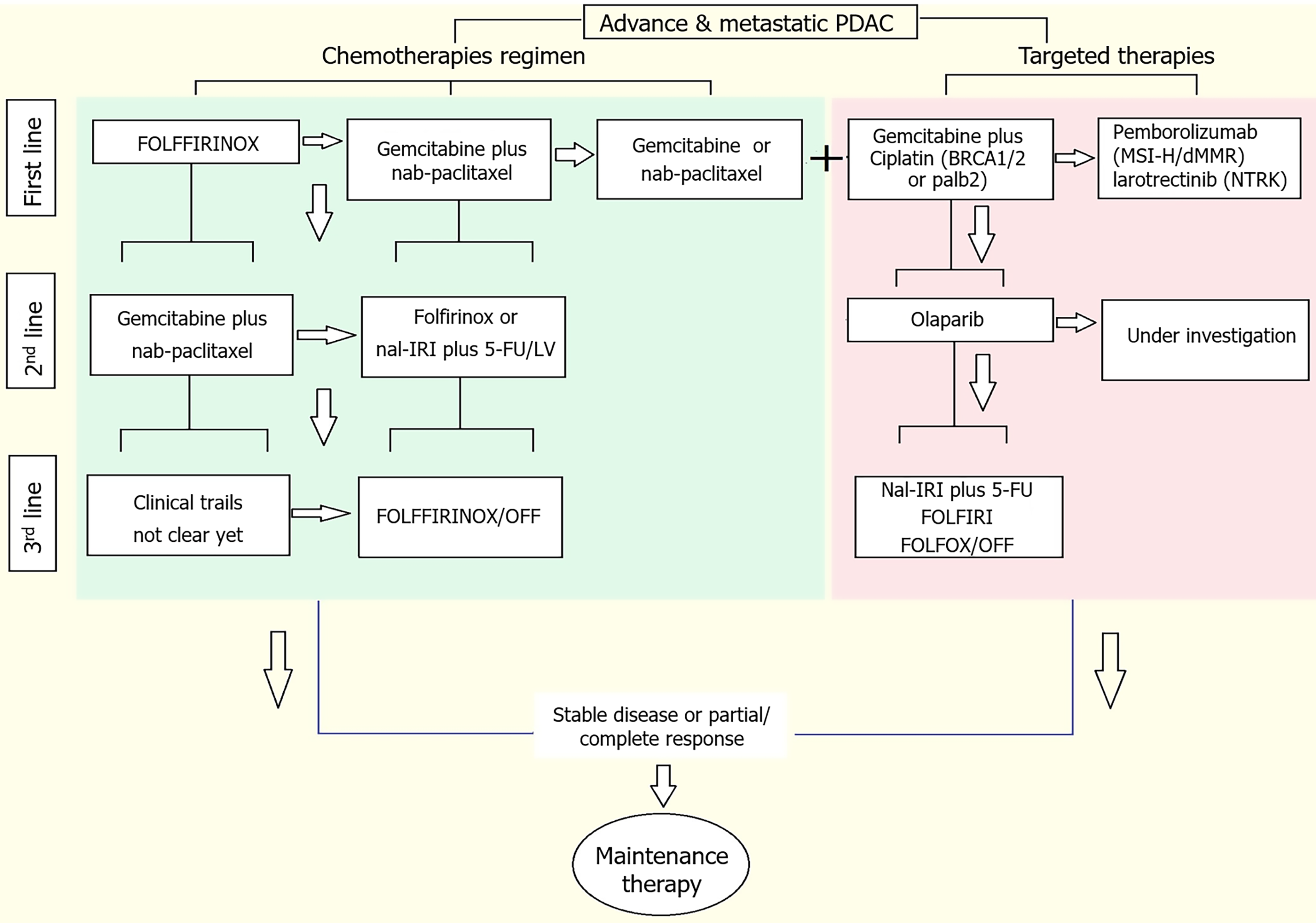
Chemotherapy and surgery are the initial therapeutic options for patients with PDAC. Furthermore, only a few patients are eligible for resection at diagnosis (15%-20%)[40]. Most of the PDAC patients present with metastasis at first diagnosis, where removal of the primary lesion through a major surgical procedure is not suitable. The clinical care for most patients, regardless of removal eligibility, includes systemic chemotherapy. In the past decade, new combination regimens have emerged as first-line therapy in patients with advanced PDAC. The first is a combination of 5-fluorouracil (5-FU), leucovorin, irinotecan, and oxaliplatin, which is named FOLFIRINOX. The second line is the combination of gemcitabine and an albumin nanoparticle conjugate of paclitaxel (nab-paclitaxel). The third-line regimen includes a liposomal formulation of irinotecan in combination with 5-FU[41-43]. Patients progressing on the first-line and second regimens can be offered other regimens, such as targeted therapy options (Figure 3). Although chemotherapy and surgery are encouraged for the clinical management of PDAC, there have been several compelling developments in the application of targeted therapies in PDAC, which will be discussed in the next section.
The ASCO Clinical Practice Guideline recommended two main chemotherapy regimens for first-line treatment of MPC, FOLFIRINOX and gemcitabine plus nab-paclitaxe is recommended for patients who meet an Eastern Cooperative Oncology Group PS of 0 to 1[44]. Moreover, a randomized phase II trial was performed to select a regimen from mFOLFIRINOX or gemcitabine plus nab-paclitaxel as a promising candidate for a subsequent phase III study[45]. Another important aspect of the PROPANC trial, the PREOPANC trial revealed that neoadjuvant gemcitabine-based chemoradiotherapy followed by surgery and adjuvant gemcitabine improved OS with upfront surgery and adjuvant gemcitabine in patients with resectable and borderline resectable PDAC. Neoadjuvant FOLFIRINOX is currently being tested in a few randomized trials for pancreatic cancer[46,47]. The 2023 NAPOLI 3 study were compared the efficacy and safety of NALIRIFOX vs nab-paclitaxel and gemcitabine as first-line treatment for metastatic PDAC. A total of 770 patients were randomly assigned to the NALIRI FOX group (n = 383) or the nab-paclitaxel gemcitabine (n = 387) group. The median follow-up was 16.1 months; the median survival time was 11.1 months with NALIRI-FOX vs 9.2 months with nab-paclitaxel-gemcitabine, and the median PFS was 7.4 months with NALIRIFOX and 5.6 months with nab-paclitaxel and gemcitabine. In the NAPOLI 3 trial, the NALIRIFOX regimen demonstrated statistical significant and clinical better improvements in OS and PFS compared with nab-paclitaxel and gemcitabine in patients with metastatic PDAC[48]. Another study showed the comparable effectiveness of FOLFIRINOX and gemcitabine plus nab-paclitaxel, comprising 274 selected individuals. No differences were reported regarding median OS. Following FOLFIRINOX and gemcitabine plus nab-paclitaxel are regimens of choice for neoadjuvant chemotherapy in treating PDAC when patient conditions are acceptable[49] (Table 1). A study from the United States enrolled approximately 654 selected patients; the median OS was significantly longer in the FOLFIRINOX group (13.8 months) than in the G/nab-P group (12.1 months)[50]. A German study revealed the benefit of the first-line treatment in combination therapy in a phase III trial[51]. The MPACT trial revealed a survival benefit of nab-paclitaxel gemcitabine over gemcitabine monotherapy. The median OS was 8.7 months vs 6.6 months[42].
| Clinical trial identifier | Year | Patients | Clinical phase | Tumor stage | Treatment regimen | Median OS (months) | Median PFS (months) | Ref. |
| JCOG1407 UMIN000023143 | 2022 | 126 | Phase II | LAPC | mFOLFIRINOX/gemcitabine + nab-paclitaxel | 23.0 vs 21.3 | 11.2 vs 9.4 | [48] |
| PREOPANC Eudra CT 2012-003181-40 | 2022 | 246 | Phase III | rPC | Gemcitabine + surgery | 15.7 vs 14.3 | NA | [49] |
| NAPOLI 3 NCT04083235 | 2023 | 770 | Phase III | mPDAC | NALIRIFOX vs nab-paclitaxel and gemcitabine | 11.1 vs 9.2 | 7.4 vs 5.6 | [53] |
| PRODIG-4 ACCO TD-11 | 2022 | 406 | Phase III | PDAC | Gemcitabine plus nab-paclitaxel vs FOLFIRINOX | 9.4 vs 7.5 | Failure | [61] |
| MPACT NCT00844649 | 2013 | 861 | Phase III | mPDAC | Nab-paclitaxel plus gemcitabine + gemcitabine | 8.5 vs 6.7 | 5.5 vs 3.7 | [57] |
| NEONAX NCT02047513 | 2023 | 127 | Phase II | rPADC | Gemcitabine plus nab-paclitaxel | 15.7 vs 14.3 | 11.5 vs 5.9 | [62] |
| NAPOLI 3 | 2024 | 2581 | Phase III | mPC | NALIRIFOX, gemcitabine plus nab-paclitaxel + FOLFIRINOX | 11.1 vs 10.4 vs 11.7 | 7.4 vs 5.7 vs 7.3 | [63] |
| PRODIGE 65 NCT03943667 | 2024 | 211 | Phase III | mPDAC | Gemcitabine and paclitaxel vs gemcitabine (GEMPAX) | 6.4 vs 5.9 | 3.1 vs 2.0 | [64] |
Furthermore, FOLFIRINOX and nab-paclitaxel combined with gemcitabine as a combination therapeutic has been evaluated in randomized trials for patients only in large randomized clinical trials evaluating both protocols for local advanced disease with or without the addition of sequential chemoradiation (for example, the NEOLAP study NCT02125136)[52,53]. Finally, the PDAC Signature Stratification for Treatment (PASS-01) trial was a phase II, multicenter clinical trial in which patients with metastatic PDAC were randomized to either FOLFIRINOX or gemcitabine plus nab-paclitaxel treatment groups (NCT04469556)[54]. Other groups performed studies on fist-line therapies with better outcomes[55-58].
Since the approval of the FOLFIRINOX regimen in 2010, it has been the second-line treatment after disease progression (Table 2). Conroy[59] reported second-line therapies after progression on gemcitabine alone in PDAC. In the CONKO-003 trial, with disease progression after gemcitabine treatment were randomized into two arms: L-OHP plus 5-FU and leucovorin. A significant improvement in PFS (13 weeks vs 9 weeks, P = 0.012) and OS (26 weeks vs 13 weeks, P = 0.014) was achieved for patients in the oxaliplatin arm[59].
| Clinical trial identifier | Year | Patients | Study phase | Tumor stage | Treatment regimen | Median OS (months) | Median PFS (months) | Ref. |
| NAPOLI-1 NCT01494506 | 2016 | 417 | Phase III | mPDAC | Nanoliposomal irinotecan with fluorouracil and folinic acid | 6.1 vs 4.2 | NA | [66] |
| MPACT | 2021 | 378 | Phase III | mPDAC | Liposomal irinotecan + fluorouracil/leucovorin vs FOLFIRINOX | 9.8 vs 6.6 | 3.7 vs 4.6 | [68] |
| PANCREOX NCT01121848 | 2016 | 108 | Phase III | LAPC | Nanoliposomal irinotecan + fluorouracil and folinic acid | 6.1 vs 9.9 | 3.1 vs 2.9 | [69] |
| MPACT | 2015 | 57 | mPDAC | Nab-paclitaxel plus gemcitabine | 8.8 | 5.1 | [71] | |
| NCT05074589 | 2022 | 298 | Phase III | mPDAC | Liposomal formulation of irinotecan plus 5-fluorouracil and folinic acid, or placebo plus 5-fluorouracil and folinic acid | 7.39 vs 4.99 | 4.21 vs 1.48 | [73] |
| NCT01834235 | 2023 | 78 | Phase III | APDAC | Gemcitabine and nab-paclitaxel | 6.6 vs 5.0 | 2.7 vs 3.4 | [74] |
| NAPOLI-1 | 2024 | 115 | Phase III | APDAC | Nanoliposomal irinotecan and 5-fluorouracil/leucovorin | 8.4 vs 7.9 | 3.6 vs 3.4 | [75] |
| NAPOLI-1 | 2023 | 296 | Phase III | APDAC | Nanoliposomal irinotecan plus 5-fluorouracil/leucovorin | 6 vs 12 | 12.4 vs NA | [76] |
Moreover, a phase III NAPOLI-1 trial demonstrated that liposomal-irinotecan nanoliposomal irinotecan (nal-IRI) plus 5-FU were more effective in patients was treated with gemcitabine previously than in those treated with 5-FU monotherapy (median OS of 6.2 months vs 4.2 months; P = 0.012)[43]. Another choice in the second-line setting is the OFF regimen consisting of 5-FU/leucovorin and oxaliplatin. This regimen can be employed in patients who progress under gemcitabine[60].
Korean Cancer Group studies were analyzed to compare second-line nal-IRI + 5-FU + folinic acid for second-line treatment after FOLFIRINOX failure. Interestingly, patients treated with FOLFIRINOX had better OS if they were more than 70 years, whereas patients treated with nal-IRI + 5-FU + folinic acid had better PFS and OS if they were less than 70 years[61]. Another study recommended that nal-IRI + 5-FU plus folinic acid might be a better treatment option for elderly patients with PDAC[21]. Gill et al[62] reported that the PANCREAS trial did not indicate a benefit from the addition of oxaliplatin (FOLFOX6) to 5-FU-FA. The median survival was 6.1 months vs 9.9 months (P = 0.02)[62]. Also retrospective NAPA study, individuals who received Gem-NaP as the first-line therapy were treated with FOLFOX or FOLFIRI as second-line treatment after progression; the median PFS was slightly but no significant better in the FOLFIRI group than in the FOLFOX group, and the median OS was significantly better in the FOLFIRI group compared to the FOLFOX[63].
Data regarding second-line treatment following this regimen are sparse[42]. 57 patients were treated with nab-paclitaxel and gemcitabine following FOLFIRINOX, with a median survival of 8.8 months and an acceptable toxicity profile[64]. A couple studies reported better evidence in phase II and III trials, second-line treatment was effective and well tolerated in patients, who have good statuses despite progression on first-line therapy[53,65]. However, phase III trials in which 298 patients with PDAC failed gemcitabine based treatment were presented at the European Society for Medical Oncology 2022 meeting. Patients were randomized to receive a liposomal formulation of irinotecan plus 5-FU, folinic acid or placebo plus 5-FU and folinic acid, this followed the second line treatment of PDAC[66]. Other studies provided evidence supporting second-line therapeutic options[67-69]. Insights from this review into the use and efficacy of treatments in a real-world setting may influence the development of effective therapeutics plans, potentially improving patient outcomes in the future.
Unfortunately, most patients with advanced pancreatic cancer experience challenges such as tumor metastatic, chemotherapy resistance and treatment related toxicity after first or second-line chemotherapy. Chemotherapy remains the standard therapies for advanced and metastatic disease. First-line therapy (FOLFIRINOX or gemcitabine plus nab-paclitaxel) improved the OS rate of patients with advanced pancreatic cancer, and 5-FU/leucovorin plus nal-IRI is proven to be a solid second-line therapy (Table 3).
| Clinical trial identifier | Year | Patients | Study phase | Tumor stage | Treatment regimes | Median OS (months) | Median FPS (months) | Ref. |
| NAPOLI-1 | 2019 | 86 | Retrospective | mPDAC | Nanoliposomal irinotecan + 5-fluorouracil and leucovorin | 9.4 | 3.5 | [77] |
| NAPOLI-1NCT01494506 | 2020 | 417 | Phase III | mPDAC | Nanoliposomal irinotecan + 5-fluorouracil/leucovorin | 5.8 vs 4.3 | 2.8 vs 1.4 | [78] |
| NR | 2023 | 29 | Phase II/III | LA-mPC | Nanoliposomal irinotecan plus 5-fluorouracil/leucovorin | 10.27 vs 9.33 | 2.90 vs 3.60 | [80] |
Third-line treatment by using a nal-IRI + 5-FU/leucovorin regimen is routine in the clinic. Furthermore, two reports analyzed the clinical efficacy and safety of the use of nal-IRI in combination with 5-FU/leucovorin[70-72]. Möhring et al[73] reported that 19 patients were treated with nal-IRI plus 5-FU/leucovorin as a third-line therapy after the FOLFIRINOX regimen, and only one patient did not have complete therapy data concerning FOLFIRINOX therapy; among 18 patients receiving nal-IRI plus 5-FU/leucovorin as a third-line therapy, the OS was 9.33 months, and the median PFS was 2.53 months; the final response to nal-IRI plus 5-FU/leucovorin was not statistically significant. Furthermore, the therapeutic benefits and safety of nal-IRI + 5-FU/leucovorin continue improving for patients with advanced and MPC. Kim et al[74] reported the efficacy of third-line chemotherapy for patients with advanced pancreatic cancer; the outcomes of 125 patients who received a third-line chemotherapy regimen with nal-IRI plus 5-FU/leucovorin were as follows: The median OS from diagnosis was 18.0 months in all patients, and the median OS after progression on second-line therapy was 15.1 months. This study suggests that proceeding with third-line chemotherapy could be recommended in some selected patients; notably, females benefitted significantly more than males did[74]. The efficacy and safety of various third-line treatments in individuals with PDAC are still awaiting confirm.
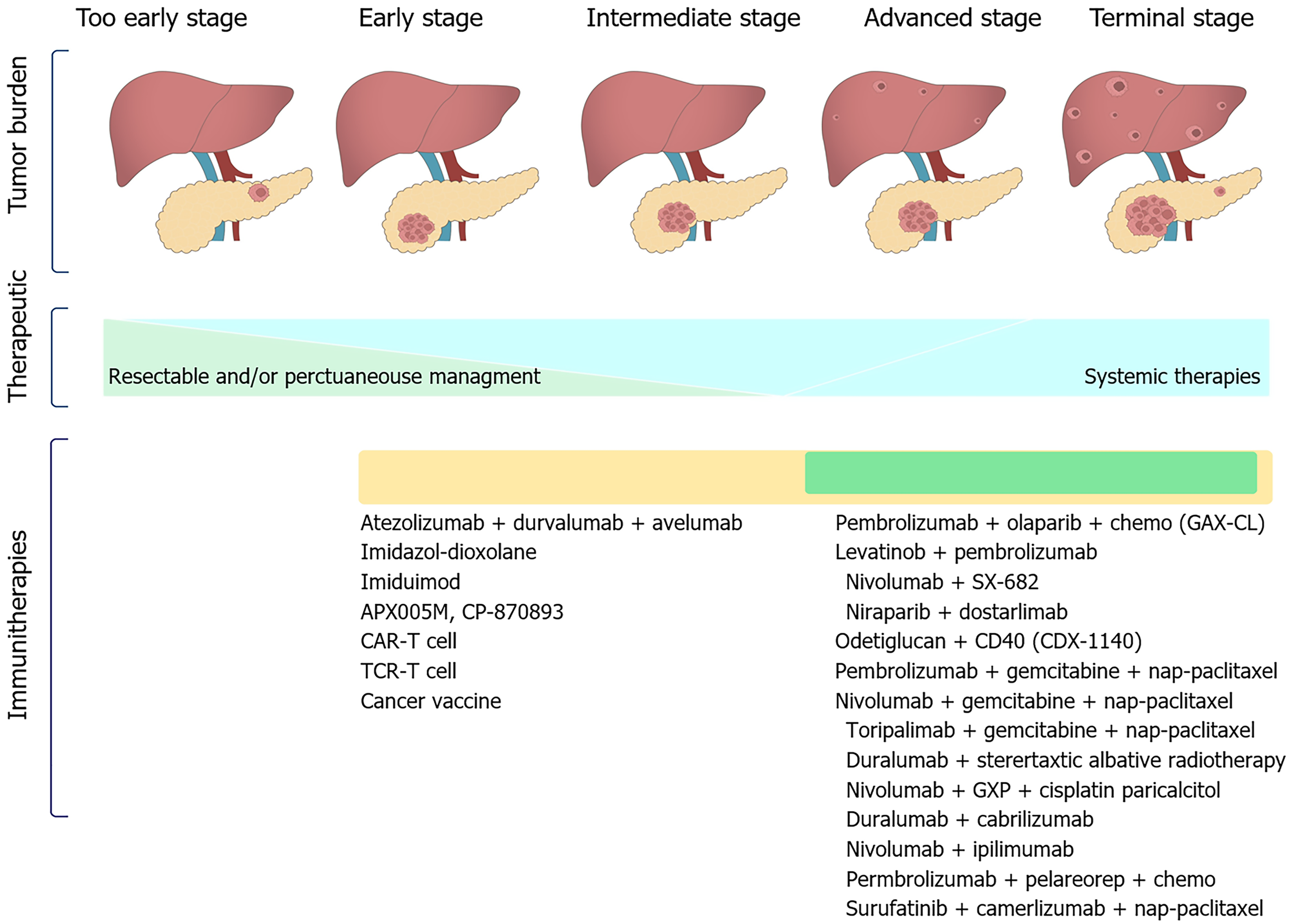
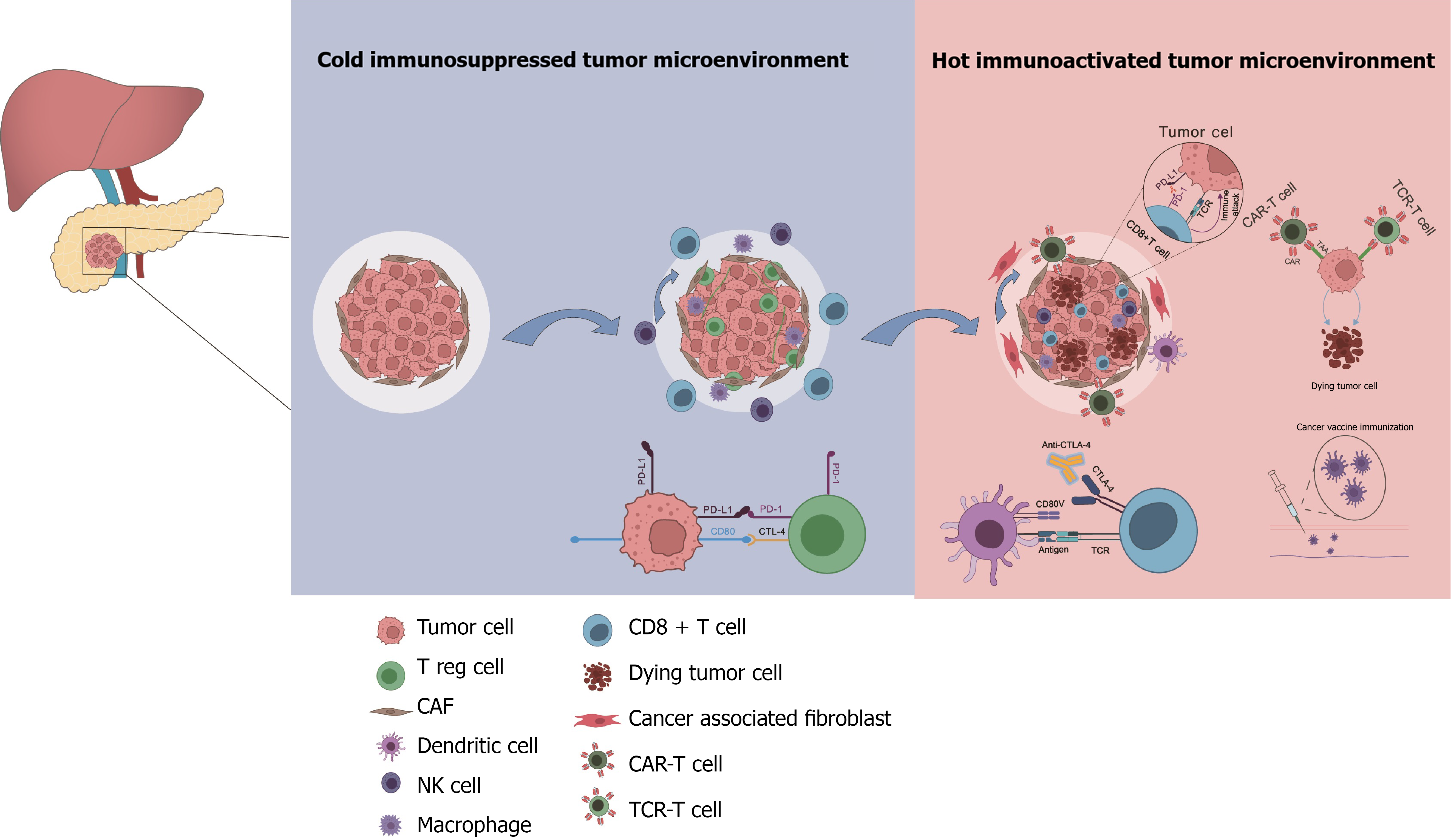
In the past decade, immunotherapy, especially immune checkpoint inhibitors, has revolutionized the management of a broad spectrum of malignancies and immunotherapy agents have provided novel and great opportunities for expanding the treatment of PDAC[75]. Immunotherapy has been shown to be effective and safe for treating many tumors. PDAC is a typical tumor that is known to occur in the context of immunosuppression, making immunotherapy a potentially attractive therapeutic option (Figure 4). Farren et al[76] suggested that the strength of the immune response predicts the survival outcomes of pancreatic cancer patients, and manipulation of this feature might be of clinical benefit.
Tumor vaccination is an option for tumor immunotherapy whose use has increased rapidly recently and can improve outcomes. There are several kinds of tumor vaccines, including whole-cell, peptide-based vaccines, dendritic cell vaccines, DNA vaccines (plasmid vaccines, virus-based vaccines, bacterial vector vaccines, and yeast-based recombinant vaccines), and mRNA vaccines[77]. Hopkins et al[78] revealed via T cell receptor Vβ sequencing (HTTPS) analysis that increased responses mediated by CD4+ and CD8+ T cells were observed in PDAC patients who received immune checkpoint therapy [anti-programmed cell death protein 1 (PD-1) and anti-cytotoxic T lymphocyte associated antigen 4 (CTLA-4)] combined with GVAX, as evidenced by more significant T cell receptor library diversity, and a certain degree of clinical response. In another study on cancer vaccines, targeting of KRAS caused a KRAS-specific immune response, indicating that this approach is seemingly promising and safe with clinical benefit[79].
The use of immunotherapy targeting CTLA-4 and PD-1 is rapidly increasing for solid tumors. However, pancreatic cancer is considered a cold tumor. A number of clinical trials have researched immune checkpoint blockade of CTLA4 and PD-1/PD-L1 pathways to directly increase effective T-cell activity in PDAC. Blocking CTLA4 modulates T-cell activity and enhances T-cell priming[80]. As an important part of precision therapy strategies, immune checkpoint inhibitors represented by PD-1 inhibitors are an important direction for immunotherapy. MMR-D and microsatellite instability high status can lead to an increase in the tumor mutational burden and has important indications in predicting the efficacy of immune checkpoint inhibitors. However, MMR-D/microsatellite instability high tumors account for < 1% of all cases of pancreatic cancer cases treated with pembrolizumab. The objective response rate (ORR) was 18.2%; 22 patients with pancreatic cancer had a complete response, and 3 patients achieved a partial response. The median survival was 4 months[81]. An AGEO European cohort study in 2023 revealed that it was effective and well tolerated in patients with advanced PDAC, supporting the usage of immune checkpoint inhibitors for MSI/MMR-D PDAC cases in advanced stages and also providing a good foundation for future explorations[82].
Owing to the limited efficacy of immunotherapy, monotherapy combined with chemotherapy and other regimens, a phase II clinical study in which 27 patients with advanced pancreatic cancer was reported at ASCO 2023. The treatment groups were as follows: The surufatinib + camrelizumab + nab-paclitaxel + S-1 group (NASCA group, n = 14), nab-paclitaxel + gemcitabine group (AG group, n = 13). The ORR was 55.0% for the NASCA group and 23.1% in the AG group; the median PFS was 8.8 months in the NASCA and 5.8 months in the AG group, with a median follow-up of 8.9 months. Overall, the results showed that the NASCA regimen presented greater clinical benefit than did the standard AG therapy and had a manageable safety profile. This trial is ongoing, and the NASCA regimen deserves further exploration in metastatic PDAC [clinical trial (NCT05218889)][83].
In this context, chimeric antigen receptor-engineered T (CAR-T) cells constitute a hotspot of immunotherapy. Therefore, novel therapeutic strategies are rapidly needed to improve the outcomes of pancreatic cancer. Another promising therapeutic could be immunotherapy with CAR-T cells. However, it has emphasized that CAR-T cell treatment achieved in pancreatic cancer therapy is hindered by the tumor microenvironment (TME)[84]. CAR-T cell-based therapy has revolutionized therapies whether they are used for benign or malignant tumors[84,85]. Primarily, six CAR-T cell therapies are available and were approved by the Food and Drug Administration and the European Medicines Agency[86]. CAR-T cell therapy trials in pancreatic cancer illuminate the path of both potential therapies and challenges in treating these diseases. Safety outlines were generally acceptable, with manageable and reversible adverse events[87].
Despite the recent significant success of targeted therapies in the management of more cancers, PDAC has been associated with a poor prognosis. This lack of progress can be partly explained by the scarcity of PDAC samples, especially for advanced stages; thus, sample availability represents a major barrier to understanding the relationship between tumor biology and therapeutic response (Table 4). In this context, the development of relevant preclinical models is particularly important. KRAS(G12C) mutation has emerged as a promising therapy for a series of solid tumors, and AMG-510 was the first-line treatment for KRAS(G12C) tumors. AMG-510 has shown great promise in treating tumors in both preclinical and clinical studies[88]. Hong et al[89] reported a study on 22 pancreatic cancer patients treated with sotorasib (AMG510). Nine patients had stable disease after treatment, and one patient exhibited a favorable response to treatment[89]. Strickler et al[90] showed that sotorasib therapy had clinically meaningful efficacy and an acceptable safety profile in patients with advanced and metastatic PDAC. The outcomes of a phase I-II clinical trial of sotorasib were follows: The median PFS of patients was 4.0 months; 36.1% of patients had PFS > 6 months; the median OS was 6.9 months; and 19.6% of patients had OS > 12 months. Sotorasib has shown good efficacy as a second-line treatment for advanced pancreatic cancer[90]. Ou et al[91] reported phase I/IB adagrasib dose finding components of the first in the human KRYSTAL-1 trial. This trial evaluated the safety of sustained exposure to adagrasib above the target threshold. A dosage of 600 mg twice a day was predicted to enable the inhibition of newly synthesized KRAS(G12C)[91]. However, KRAS(G12C) mutation accounts for only 1% of the overall population affected by pancreatic cancer.
| Targeted pathway | Frequency of mutation | Clinical phase | Targeted therapies approved by the FDA |
| KRAS [G12D, G12V, G12R, G12C, G13 (D, C, S, R)] | 95% | Phase I-II | Sotorasib (AMG 510), adagrasib (MRTX849) |
| BRAF (V600E) | 13.7% | Phase I-III | Dabrafenib, trametinib |
| NTRK fusion | 0.3% | Phase II | Larotrectinib, entrectinib |
| NRG-1 fusion | 0.5% | Phase I-II | Afatinib, zenocutuzumab |
| BRCA1/2/PALB2 | 3% | Phase I-III | Olaparib, cisplatin, niraparib |
| MSI-H/dMMR | 4.7% | Phase I-II | Pembrolizumab |
| FGFR2 | 43%-69% | Phase II | Pemigatinib |
| MEK/ERK | 1% | Phase II | Trametinib |
| RET fusion | 1% | Phase II | Selpercatinib, pralsetinib (BLU-667) |
Benefit is lower, which cannot significantly improve the prognosis of pancreatic cancer. In 2023, a drug targeting small interfering RNA with KRAS(G12D) mutation (SiG12D-LODER) entered phase II clinical trials (NCT01676259). At the same time, T cell receptor cell therapy targeting KRAS(G12D) mutants, and tumor antigens has exhibited efficacy in patients with MPC who have received multiple prior treatments[92]. The KRAS(G12D) mutation was found in nearly 43% of pancreatic cancer cases. In 2021, KRAS(G12C) mutation was approved by the Food and Drug Administration as a therapeutic target. Although the targeted therapies for pancreatic cancer have been very slow to progress, the POLO trial reported the efficacy and safety of maintenance olaparib compared with placebo in patients with MPC with a germline BRCA mutation[93]. The results for maintenance olaparib were as follows: Median PFS with placebo (7.4 months vs 3.8 months), where progression was defined as disease progression or death (P = 0.004); however, no difference in OS between the olaparib and placebo groups was observed. Grade ≥ 3 adverse drug events were more frequently reported in the olaparib group than in the placebo group (40% vs 23%). Thus, the maintenance olaparib regimen seems to be an attractive option for the treatment of MPC patients with a germline BRCA mutation[93]. In 2023 study trials, the phase III POLO trial was conducted to investigate the efficacy of olaparib as a switch maintenance therapy in PDAC patients with germline BRCA mutations[29].
Again, international guidelines recommended olaparib in a heavily pretreated patient with BRCA2 germline mutation for maintenance treatment of patients with PDAC. In 2022, the NOTABLE, phase III clinical study confirming the efficacy and safety of nimotuzumab plus gemcitabine combination regimen with wild-type KRAS for patients with advanced or metastatic pancreatic cancer (ClinicalTrials.gov identifier: NCT02395016). The outcome of the median OS and the median PFS was 4.2 months vs 3.6 months, and both OS and PFS were longer in the nimotuzumab group than in the placebo group. The ORRs and disease control rates were 7% vs 10% and 68% vs 63%, respectively. investigational and control groups, respectively[94]. Thus, several studies reported NTRK gene fusion-positive cancer occurs in very rare patients with pancreatic cancer. The counterparts larotrectinib and entrectinib are multitarget tyrosine receptor kinase inhibitors[95,96]. Solomon et al[97] reported that NTRK gene fusions in 1492 pancreatic adenocarcinomas were very rare (0.34%). In 2023, Demols et al[98] reported overall results in patients after screening via immunohistochemistry and confirmation via NGS, the percentage of NTRK fusions was 0.7% for pancreatic cancers.
PDAC has a complex immune microenvironment that plays a crucial role in disease progression of pancreatic cancer; the therapeutic purpose of the immune microenvironment of pancreatic cancer has been a research topic. Thus, the cellular components of the TME, combined with the extracellular environment and signaling pathways in surrounding tissues. As the field evolves to better understand the dynamic interactions within the TME, treatment strategies for drug delivery and unique immune targets will provide new hope for curing disease[99]. However, T lymphocytes are very limited in the immune microenvironment, intrinsic immune cells are significantly enriched as a major component of the immune microenvironment regulating pancreatic cancer[100].
In 2023, cancer cellular heterogeneity, circulating tumor cells, and cancer-associated fibroblasts, the immune microenvironment, and pancreatic precancerous lesions were explored by single-cell sequencing. This research is expected to increase our knowledge about pancreatic neoplasms and improve their diagnosis and prognosis[101]. Additionally, macrophages are crucial innate immune effector cells that perform many functions in response to pathogens and tissue inflammatory signals, including promotion of tumor development. High levels of tumor-associated macrophages (TAMs) are associated with a poor prognosis and correlate with chemotherapy resistance in most cancers[102,103]. Chen et al[103] reported that deletion of immune response gene 1 in vivo combined with and PD-1/PD-L1 blockade enhanced antitumor immunity; that is, while aconitate decarboxylase 1 inhibitors target monocytes/macrophages to reprogram TAM polarization and promotes antigen presentation and T cell trafficking, PD-L1 blockades target and reinvigorate CD8+ T cells to restore their antitumor effector functions. Another study aimed to clear the PDAC microenvironment to drive local macrophage proliferation; the results suggested that cancer-associated fibroblast-induced macrophage proliferation is important for sustaining TAM number[104]. The induction of p21 in TAMs by stromal colony stimulating factor 1 results in immunosuppression and tumor progression, and the expression of p21 in TAMs might sensitize tumors to CD40 agonist treatment[104]. Additionally, Caronni et al[105] reported that interleukin-1β+ TAMs represent a subset of monocyte-derived macrophages endowed with inflammatory activity, but not cytotoxic activity; these cells are programmed in pancreatic cancer, and the prostaglandin E2-L-interleukin axis as a driver of the spatial and transcriptional heterogeneity of immune and tumor cells in PDAC. The study revealed that the cysteine-rich intestinal protein 1/nuclear factor kappa B/C-X-C motif ligand axis is critical for triggering immune evasion and tumour immune microenvironment development in PDAC[106]. One group aimed to explore neutrophil heterogeneity in PDAC TME at the single-cell level, and mechanistic studies revealed the targets if both hypoxia and endoplasmic reticulum stress, two potent stimulators in the TME driving neutrophils toward the protumor TAN-1 phenotype. Importantly, this demonstrated that BHLHE40 is a key regulator of protumor neutrophils[107]. In the microenvironment, T cells and B cells, as adaptive immune cells, follow tertiary lymphoid structures associated with a good prognosis which are known to play an active role in a variety of tumors, including PDAC[108,109]. Although immunotherapy has not been very successful in PDAC, the development of new immunotherapeutic regimens is continually unceasing. Given the difficulty in infiltrating acquired immune cells but the relative abundance of intrinsic immune cells in the microenvironment of PDACs, intrinsic immune cells are considered to be very promising as targets or vectors for immunotherapies for patients with pancreatic cancer[110].
An increasing understanding of the mechanisms of cancers and the map of the tumor host interactions have accelerated improvements in novel immunotherapies[111]. Although immunotherapy for patients with pancreatic cancer has made progress in some patient subpopulations, the overall therapeutic response to current treatments remains very limited.
In the near future, we expect to improve the immunotherapeutic response to individuals with pancreatic cancer, by performing in-depth research, exploring new therapeutic targets, improving technology; this will facilitate a clear understanding of its parthenogenesis. Notably, immunotherapy has made breakthroughs in the fight against some cancers and has become a better path for PDAC treatment. PDAC is defined as a “cold tumor”. Thus, its response to most immunotherapies is not very satisfactory. Therefore, one of the current significant endeavors in PDAC immunotherapy is to transform the “cold tumor” into a targetable “hot tumor”[112,113] (Figure 5).
Currently, the recent increase in therapeutic targets has led to an improvement in the immunotherapy response and OS rate of pancreatic cancer patients, suggesting that manipulation of this feature might lead to new and promising therapeutic strategies[114,115]. Therefore, immunotherapy, including immune checkpoint inhibitors that target PD-1 immunotherapy, CTLA4 and CAR-T cell therapy have been shown to be promising strategies for hematological malignancies.
Previous works to reactivate immune responses by antagonizing the inhibitory signals utilized by tumors have shown promise, with antibody blockade of T-cell inhibitory receptors such as PD-1, PD-L1, or CTLA-4 achieving response rates of up to 50% in bladder, lung, and renal cancers and melanoma[116,117]. According to Michelakos et al[118], the B7-H3 receptor, where the tumor antigens that target monoclonal antibodies is not only a promising technically feasible target; further examination of B7-H3 characteristics and development of B7-H3-targeting strategies is timely and warranted. Additionally, two studies by Tate et al[119] and Balachandran et al[120] showed that immunotherapies targeting shared neoantigens constitute good approaches for cancer treatment.
However, with improvements in both molecular biology and immunology, new technologies bring hope for the development of new biomaterials based on approaches as new anticancer immunotherapies for patients with pancreatic cancer[113]. Zemek et al[121] showed that high-throughput sequencing technology in the pretreatment TME predicts responses to immune checkpoint blockade. A report by Falcomatà et al[122] revealed that the multikinase inhibitor nintedanib, inducing cell death and widespread reprogramming of the immunosuppressive microenvironment can activate an anti-tumor immune response, resulting in the recruitment of cytotoxic T cells. Another study by Stadtmauer et al[123] revealed that CRISPR-Cas9 is a revolutionary gene-editing technology that offers the potential to treat diseases such as cancer, and a phase I clinical trial to assess the safety and feasibility of CRISPR-Cas9 gene editing in patients with advanced cancer. Therefore, the immunogenicity demonstrated the feasibility of CRISPR gene editing for cancer immunotherapy[123]. Diagnosis with ultrasound therapy combined with microbubbles has achieved immense success in cancer treatment, and terahertz therapy; its combination with traditional chemotherapy and targeted therapy and immunotherapy have slowly moved from basic research to clinical trials showing benefits. However, many unanswered questions remain regarding the pathogenesis mechanism of pancreatic cancer, and understanding the tumor immune escape therapy mechanism should facilitate the improvement of clinical therapeutic strategies. Therefore, the best option is to perform further basic research on pancreatic cancer that might lead to new therapeutic targets and choices.
While tumor tissue is still the gold standard for identifying cancer-specific biomarkers, the use of liquid biopsies in new screening methods for the early detection of pancreatic cancer is promising[124]. Currently, the potential targets of liquid biopsies are circulating tumor cells, circulating tumor DNA, exosomes, noncoding RNAs, mRNAs, and extracellular vesicles; assessment of these markers may provide a clearer picture and more information about tumor genomics, transcriptomics, and proteomics[125,126]. Hence, liquid biopsy has become an appealing standard source of biomarkers for some applications in most cancers including pancreatic cancer. The applications were involved in early diagnosis, treatment response, prognosis evaluation, prognosis and prediction of treatment response[125,127]. However, the liquid biopsy had a large positive effect of allowing longitudinal monitoring of tumors and may capture the spatial and temporal Intratumor heterogeneity is not easy with traditional procedures[125,128]. Currently, carbohydrate antigen carbohydrate antigen 19-9 is the most commonly used serum protein biomarker for the diagnosis and monitoring of patients with PDAC[129]. Finally, liquid biopsy is likely to revolutionize clinical cancer management, but there is limited research on this subject.
Artificial intelligence (AI) has gained considerable interest in oncology because it has potential to be useful for the analysis of big data can be used to produce individualized recommendations based on patient’s clinical profile[130]. With the development of digital technology, AI has become a great tool in the health care field. There is a great deal of repetitive work in clinics, and AI can reduce the workload for physicians[131]. Despite these promising early outcomes, the identification of primary lesions and secondary anatomic signs has not been well studied in AI-based detection research. Most of the reports do not assess the performance of AI-trained models on the basis of tumor size and stage[132]. Schuurmans et al[132] have shown great potential for AI-driven PDAC diagnosis via computed tomography (CT).
Diagnostic images are the classic application of AI in health care; however, Jiang et al’s proof-of-concept studies have provided promising evidence that AI can be useful for predicting responses to various treatments[133]. Another study revealed that an AI model was able to accurately diagnose pancreatic cancer via CT images with a mean accuracy of approximately 92%. The sensitivity was 88%, and the specificity was approximately 95%. Therefore, AI models can be highly accurate and be applied to automate the detection of PDAC on diagnostic CT as well as the detection of visually occult PDAC on prediagnostic CT[134].
A study from China revealed that an AI model (area under the receiver operating characteristic curve: 0.92) performed better than radiologist examination, a clinical model and a radiomics model (area under the receiver operating characteristic curves of 0.65, 0.77, and 0.68, respectively) in the prediction of lymph node metastasis (P < 0.001). An AI model predicted that lymph node metastasis was associated with worse survival. This AI model was a fully automated noninvasive tool considering both lymph node and tumor imaging characteristics and showed favorable accuracy for the prediction of lymph node metastatic via CT in patients with PDAC[135]. At present, in the medical field, AI models can utilize relatively objective data to assist in the screening and diagnosis of diseases. In the near future, medical AI models should be developed to analyze patients’ medical history, symptoms, medical testing reports, medical images, and even sociological characteristics. These models may facilitate prevention and risk control. Overall, AI may bring new hope by facilitating disease diagnosis in the clinical setting and facilitating the development of novel therapeutic strategies in the near future[136].
An improved understanding of the biology of PDAC has led to many clinical trials to improve treatment efficacy and outcomes. Outcomes have consistently improved over the past few decades. Unfortunately, these findings have not translated into a breakthrough in clinical care for the most of patients. Furthermore, pancreatic cancer remains a disease with a poor prognosis; it has an OS rate of approximately 5%, irrespective of the patient’s socioeconomic status.
Primary and secondary prevention policies and increased implementation of surveillance programs will be essential in reducing the mortality and morbidity associated with pancreatic cancer. The implementation of effective precision medicine is still hampered by several barriers. Thus, there is still a lack of reliable biomarkers to predict responses to current treatments, and novel effective therapies are needed. A clear understanding of the epistatic gene interactions between mutated driver genes may help to increase the efficacy of combination therapies. Ongoing improvements in PDAC treatment with KRAS-specific inhibitors are encouraging. Additionally, we have observed the recent success of PARP inhibitors and PD-1 blockade for molecularly defined subclasses of PDAC. In the past decade, there were many improvements in preclinical and clinical work demonstrating the importance of the adaptive and innate immune systems in PDAC immunosurveillance. Since the outcomes of immunotherapy clinical trials on patients with PDAC have thus far been modest, more hard work has to be undertaken to increase new and relevant preclinical models; these studies are designed to consider the TME in particular. On the other hand, molecular targeted therapies and immunotherapies are emerging as tools to promote immune system response against PDAC-derived neoantigens. In this scenario, systematic tumor biopsies and blood samples should be collected from individuals with pancreatic cancer, and more biomarker-based clinical trials should be implemented in the near future. More importantly, the concept of multidisciplinary team is involved in the entire process of treating pancreatic cancer, and the determination and implementation of the major decisions should be carried out with more options and ideas from the multidisciplinary team, with dynamic evaluation and follow-up. In the management of the whole course of disease, clinicians should pay attention to systematic supportive treatment and nutritional status. Finally, we are optimistic that these collective efforts will soon transform PDAC from a recalcitrant disease to a manageable disease.
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15472] [Article Influence: 2578.7] [Reference Citation Analysis (2)] |
| 2. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11431] [Article Influence: 3810.3] [Reference Citation Analysis (4)] |
| 3. | Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O'Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379:2395-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 1945] [Article Influence: 277.9] [Reference Citation Analysis (0)] |
| 4. | Gobbi PG, Bergonzi M, Comelli M, Villano L, Pozzoli D, Vanoli A, Dionigi P. The prognostic role of time to diagnosis and presenting symptoms in patients with pancreatic cancer. Cancer Epidemiol. 2013;37:186-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Sohal DPS, Kennedy EB, Khorana A, Copur MS, Crane CH, Garrido-Laguna I, Krishnamurthi S, Moravek C, O'Reilly EM, Philip PA, Ramanathan RK, Ruggiero JT, Shah MA, Urba S, Uronis HE, Lau MW, Laheru D. Metastatic Pancreatic Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2018;36:2545-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 191] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 6. | Di Costanzo F, Di Costanzo F, Antonuzzo L, Mazza E, Giommoni E. Optimizing First-Line Chemotherapy in Metastatic Pancreatic Cancer: Efficacy of FOLFIRINOX versus Nab-Paclitaxel Plus Gemcitabine. Cancers (Basel). 2023;15:416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 7. | Reni M, Giommoni E, Bergamo F, Milella M, Cavanna L, Di Marco MC, Spada M, Cordio S, Aprile G, Cardellino GG, Maiello E, Bernardini I, Ghidini M, Bozzarelli S, Macchini M, Orsi G, De Simone I, Rulli E, Porcu L, Torri V, Pinto C; GARIBALDI Study Group. Guideline Application in Real world: multi-Institutional Based survey of Adjuvant and first-Line pancreatic Ductal adenocarcinoma treatment in Italy. Primary analysis of the GARIBALDI survey. ESMO Open. 2023;8:100777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Zhao Z, Zheng L, Chen W, Weng W, Song J, Ji J. Delivery strategies of cancer immunotherapy: recent advances and future perspectives. J Hematol Oncol. 2019;12:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 9. | Muir P, Li S, Lou S, Wang D, Spakowicz DJ, Salichos L, Zhang J, Weinstock GM, Isaacs F, Rozowsky J, Gerstein M. The real cost of sequencing: scaling computation to keep pace with data generation. Genome Biol. 2016;17:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 182] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 10. | Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801-1806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3216] [Cited by in RCA: 3027] [Article Influence: 178.1] [Reference Citation Analysis (0)] |
| 11. | Pishvaian MJ, Blais EM, Brody JR, Lyons E, DeArbeloa P, Hendifar A, Mikhail S, Chung V, Sahai V, Sohal DPS, Bellakbira S, Thach D, Rahib L, Madhavan S, Matrisian LM, Petricoin EF 3rd. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol. 2020;21:508-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 366] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 12. | Conroy T, Ducreux M; ESMO Guidelines Committee. ESMO Clinical Practice Guideline Express Update on the management of metastatic pancreatic cancer. ESMO Open. 2025;10:104528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, Dillhoff M, Donahue TR, Dotan E, Ferrone CR, Fountzilas C, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Moravek C, Nakakura EK, Narang AK, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Vollmer C, Wolff RA, Wolpin BM, Lynn B, George GV. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:439-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 694] [Article Influence: 173.5] [Reference Citation Analysis (0)] |
| 14. | Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, Kiezun A, Hammerman PS, McKenna A, Drier Y, Zou L, Ramos AH, Pugh TJ, Stransky N, Helman E, Kim J, Sougnez C, Ambrogio L, Nickerson E, Shefler E, Cortés ML, Auclair D, Saksena G, Voet D, Noble M, DiCara D, Lin P, Lichtenstein L, Heiman DI, Fennell T, Imielinski M, Hernandez B, Hodis E, Baca S, Dulak AM, Lohr J, Landau DA, Wu CJ, Melendez-Zajgla J, Hidalgo-Miranda A, Koren A, McCarroll SA, Mora J, Crompton B, Onofrio R, Parkin M, Winckler W, Ardlie K, Gabriel SB, Roberts CWM, Biegel JA, Stegmaier K, Bass AJ, Garraway LA, Meyerson M, Golub TR, Gordenin DA, Sunyaev S, Lander ES, Getz G. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3848] [Cited by in RCA: 4127] [Article Influence: 343.9] [Reference Citation Analysis (0)] |
| 15. | Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J, Chang DK, Cowley MJ, Gardiner BB, Song S, Harliwong I, Idrisoglu S, Nourse C, Nourbakhsh E, Manning S, Wani S, Gongora M, Pajic M, Scarlett CJ, Gill AJ, Pinho AV, Rooman I, Anderson M, Holmes O, Leonard C, Taylor D, Wood S, Xu Q, Nones K, Fink JL, Christ A, Bruxner T, Cloonan N, Kolle G, Newell F, Pinese M, Mead RS, Humphris JL, Kaplan W, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chou A, Chin VT, Chantrill LA, Mawson A, Samra JS, Kench JG, Lovell JA, Daly RJ, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N; Australian Pancreatic Cancer Genome Initiative, Kakkar N, Zhao F, Wu YQ, Wang M, Muzny DM, Fisher WE, Brunicardi FC, Hodges SE, Reid JG, Drummond J, Chang K, Han Y, Lewis LR, Dinh H, Buhay CJ, Beck T, Timms L, Sam M, Begley K, Brown A, Pai D, Panchal A, Buchner N, De Borja R, Denroche RE, Yung CK, Serra S, Onetto N, Mukhopadhyay D, Tsao MS, Shaw PA, Petersen GM, Gallinger S, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Schulick RD, Wolfgang CL, Morgan RA, Lawlor RT, Capelli P, Corbo V, Scardoni M, Tortora G, Tempero MA, Mann KM, Jenkins NA, Perez-Mancera PA, Adams DJ, Largaespada DA, Wessels LF, Rust AG, Stein LD, Tuveson DA, Copeland NG, Musgrove EA, Scarpa A, Eshleman JR, Hudson TJ, Sutherland RL, Wheeler DA, Pearson JV, McPherson JD, Gibbs RA, Grimmond SM. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1690] [Cited by in RCA: 1647] [Article Influence: 126.7] [Reference Citation Analysis (0)] |
| 16. | Philip PA, Azar I, Xiu J, Hall MJ, Hendifar AE, Lou E, Hwang JJ, Gong J, Feldman R, Ellis M, Stafford P, Spetzler D, Khushman MM, Sohal D, Lockhart AC, Weinberg BA, El-Deiry WS, Marshall J, Shields AF, Korn WM. Molecular Characterization of KRAS Wild-type Tumors in Patients with Pancreatic Adenocarcinoma. Clin Cancer Res. 2022;28:2704-2714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 110] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 17. | Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K, Quinn MC, Robertson AJ, Fadlullah MZ, Bruxner TJ, Christ AN, Harliwong I, Idrisoglu S, Manning S, Nourse C, Nourbakhsh E, Wani S, Wilson PJ, Markham E, Cloonan N, Anderson MJ, Fink JL, Holmes O, Kazakoff SH, Leonard C, Newell F, Poudel B, Song S, Taylor D, Waddell N, Wood S, Xu Q, Wu J, Pinese M, Cowley MJ, Lee HC, Jones MD, Nagrial AM, Humphris J, Chantrill LA, Chin V, Steinmann AM, Mawson A, Humphrey ES, Colvin EK, Chou A, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Pettitt JA, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N, Jamieson NB, Graham JS, Niclou SP, Bjerkvig R, Grützmann R, Aust D, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Falconi M, Zamboni G, Tortora G, Tempero MA; Australian Pancreatic Cancer Genome Initiative, Gill AJ, Eshleman JR, Pilarsky C, Scarpa A, Musgrove EA, Pearson JV, Biankin AV, Grimmond SM. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2130] [Cited by in RCA: 1993] [Article Influence: 199.3] [Reference Citation Analysis (1)] |
| 18. | Cancer Genome Atlas Research Network. Cancer Genome Atlas Research Network. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2017;32:185-203.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1247] [Cited by in RCA: 1389] [Article Influence: 173.6] [Reference Citation Analysis (0)] |
| 19. | Witkiewicz AK, McMillan EA, Balaji U, Baek G, Lin WC, Mansour J, Mollaee M, Wagner KU, Koduru P, Yopp A, Choti MA, Yeo CJ, McCue P, White MA, Knudsen ES. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun. 2015;6:6744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 704] [Cited by in RCA: 856] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 20. | Chatani PD, Yang JC. Mutated RAS: Targeting the "Untargetable" with T Cells. Clin Cancer Res. 2020;26:537-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Nissley DV, McCormick F. RAS at 40: Update from the RAS Initiative. Cancer Discov. 2022;12:895-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Blair AB, Groot VP, Gemenetzis G, Wei J, Cameron JL, Weiss MJ, Goggins M, Wolfgang CL, Yu J, He J. BRCA1/BRCA2 Germline Mutation Carriers and Sporadic Pancreatic Ductal Adenocarcinoma. J Am Coll Surg. 2018;226:630-637.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Golan T, Sella T, O'Reilly EM, Katz MH, Epelbaum R, Kelsen DP, Borgida A, Maynard H, Kindler H, Friedmen E, Javle M, Gallinger S. Overall survival and clinical characteristics of BRCA mutation carriers with stage I/II pancreatic cancer. Br J Cancer. 2017;116:697-702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 24. | Golan T, Kanji ZS, Epelbaum R, Devaud N, Dagan E, Holter S, Aderka D, Paluch-Shimon S, Kaufman B, Gershoni-Baruch R, Hedley D, Moore MJ, Friedman E, Gallinger S. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer. 2014;111:1132-1138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 335] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 25. | Seeber A, Zimmer K, Kocher F, Puccini A, Xiu J, Nabhan C, Elliott A, Goldberg RM, Grothey A, Shields AF, Battaglin F, El-Deiry WS, Philip PA, Marshall JL, Hall M, Korn WM, Lenz HJ, Wolf D, Feistritzer C, Spizzo G. Molecular characteristics of BRCA1/2 and PALB2 mutations in pancreatic ductal adenocarcinoma. ESMO Open. 2020;5:e000942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, Smith GC, Ashworth A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4316] [Cited by in RCA: 4886] [Article Influence: 244.3] [Reference Citation Analysis (0)] |
| 27. | Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick AC, Tortora G, Algül H, O'Reilly EM, Mcguinness D, Cui K, Schlienger K, Locker GY, Kindler HL. Overall survival from the phase 3 POLO trial: Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. J Clin Oncol. 2021;39:378-378. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 28. | Wu B, Shi L. Cost-Effectiveness of Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. J Natl Compr Canc Netw. 2020;18:1528-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Kindler HL, Yoo HK, Hettle R, Cui KY, Joo S, Locker GY, Golan T. Patient-centered outcomes in the POLO study of active maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. Cancer. 2023;129:1411-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 30. | O'Reilly EM, Lee JW, Zalupski M, Capanu M, Park J, Golan T, Tahover E, Lowery MA, Chou JF, Sahai V, Brenner R, Kindler HL, Yu KH, Zervoudakis A, Vemuri S, Stadler ZK, Do RKG, Dhani N, Chen AP, Kelsen DP. Randomized, Multicenter, Phase II Trial of Gemcitabine and Cisplatin With or Without Veliparib in Patients With Pancreas Adenocarcinoma and a Germline BRCA/PALB2 Mutation. J Clin Oncol. 2020;38:1378-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 31. | Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, Cooc J, Weinkle J, Kim GE, Jakkula L, Feiler HS, Ko AH, Olshen AB, Danenberg KL, Tempero MA, Spellman PT, Hanahan D, Gray JW. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17:500-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1536] [Cited by in RCA: 1364] [Article Influence: 97.4] [Reference Citation Analysis (0)] |
| 32. | Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA, Rashid NU, Williams LA, Eaton SC, Chung AH, Smyla JK, Anderson JM, Kim HJ, Bentrem DJ, Talamonti MS, Iacobuzio-Donahue CA, Hollingsworth MA, Yeh JJ. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47:1168-1178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1041] [Cited by in RCA: 1479] [Article Influence: 147.9] [Reference Citation Analysis (0)] |
| 33. | Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, Nourse C, Murtaugh LC, Harliwong I, Idrisoglu S, Manning S, Nourbakhsh E, Wani S, Fink L, Holmes O, Chin V, Anderson MJ, Kazakoff S, Leonard C, Newell F, Waddell N, Wood S, Xu Q, Wilson PJ, Cloonan N, Kassahn KS, Taylor D, Quek K, Robertson A, Pantano L, Mincarelli L, Sanchez LN, Evers L, Wu J, Pinese M, Cowley MJ, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chantrill LA, Mawson A, Humphris J, Chou A, Pajic M, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Lovell JA, Merrett ND, Toon CW, Epari K, Nguyen NQ, Barbour A, Zeps N, Moran-Jones K, Jamieson NB, Graham JS, Duthie F, Oien K, Hair J, Grützmann R, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Rusev B, Capelli P, Salvia R, Tortora G, Mukhopadhyay D, Petersen GM; Australian Pancreatic Cancer Genome Initiative, Munzy DM, Fisher WE, Karim SA, Eshleman JR, Hruban RH, Pilarsky C, Morton JP, Sansom OJ, Scarpa A, Musgrove EA, Bailey UM, Hofmann O, Sutherland RL, Wheeler DA, Gill AJ, Gibbs RA, Pearson JV, Waddell N, Biankin AV, Grimmond SM. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2848] [Cited by in RCA: 2556] [Article Influence: 284.0] [Reference Citation Analysis (0)] |
| 34. | Maurer C, Holmstrom SR, He J, Laise P, Su T, Ahmed A, Hibshoosh H, Chabot JA, Oberstein PE, Sepulveda AR, Genkinger JM, Zhang J, Iuga AC, Bansal M, Califano A, Olive KP. Experimental microdissection enables functional harmonisation of pancreatic cancer subtypes. Gut. 2019;68:1034-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 35. | Puleo F, Nicolle R, Blum Y, Cros J, Marisa L, Demetter P, Quertinmont E, Svrcek M, Elarouci N, Iovanna J, Franchimont D, Verset L, Galdon MG, Devière J, de Reyniès A, Laurent-Puig P, Van Laethem JL, Bachet JB, Maréchal R. Stratification of Pancreatic Ductal Adenocarcinomas Based on Tumor and Microenvironment Features. Gastroenterology. 2018;155:1999-2013.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 361] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 36. | Chan-Seng-Yue M, Kim JC, Wilson GW, Ng K, Figueroa EF, O'Kane GM, Connor AA, Denroche RE, Grant RC, McLeod J, Wilson JM, Jang GH, Zhang A, Dodd A, Liang SB, Borgida A, Chadwick D, Kalimuthu S, Lungu I, Bartlett JMS, Krzyzanowski PM, Sandhu V, Tiriac H, Froeling FEM, Karasinska JM, Topham JT, Renouf DJ, Schaeffer DF, Jones SJM, Marra MA, Laskin J, Chetty R, Stein LD, Zogopoulos G, Haibe-Kains B, Campbell PJ, Tuveson DA, Knox JJ, Fischer SE, Gallinger S, Notta F. Transcription phenotypes of pancreatic cancer are driven by genomic events during tumor evolution. Nat Genet. 2020;52:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 415] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 37. | Noll EM, Eisen C, Stenzinger A, Espinet E, Muckenhuber A, Klein C, Vogel V, Klaus B, Nadler W, Rösli C, Lutz C, Kulke M, Engelhardt J, Zickgraf FM, Espinosa O, Schlesner M, Jiang X, Kopp-Schneider A, Neuhaus P, Bahra M, Sinn BV, Eils R, Giese NA, Hackert T, Strobel O, Werner J, Büchler MW, Weichert W, Trumpp A, Sprick MR. CYP3A5 mediates basal and acquired therapy resistance in different subtypes of pancreatic ductal adenocarcinoma. Nat Med. 2016;22:278-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 38. | Sivakumar S, de Santiago I, Chlon L, Markowetz F. Master Regulators of Oncogenic KRAS Response in Pancreatic Cancer: An Integrative Network Biology Analysis. PLoS Med. 2017;14:e1002223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 39. | de Santiago I, Yau C, Heij L, Middleton MR, Markowetz F, Grabsch HI, Dustin ML, Sivakumar S. Immunophenotypes of pancreatic ductal adenocarcinoma: Meta-analysis of transcriptional subtypes. Int J Cancer. 2019;145:1125-1137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 40. | Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH, Neoptolemos JP. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1442] [Cited by in RCA: 1344] [Article Influence: 149.3] [Reference Citation Analysis (2)] |
| 41. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5639] [Article Influence: 402.8] [Reference Citation Analysis (1)] |
| 42. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4889] [Article Influence: 407.4] [Reference Citation Analysis (0)] |
| 43. | Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, Macarulla T, Lee KH, Cunningham D, Blanc JF, Hubner RA, Chiu CF, Schwartsmann G, Siveke JT, Braiteh F, Moyo V, Belanger B, Dhindsa N, Bayever E, Von Hoff DD, Chen LT; NAPOLI-1 Study Group. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 830] [Article Influence: 92.2] [Reference Citation Analysis (0)] |
| 44. | Sohal DPS, Kennedy EB, Cinar P, Conroy T, Copur MS, Crane CH, Garrido-Laguna I, Lau MW, Johnson T, Krishnamurthi S, Moravek C, O'Reilly EM, Philip PA, Pant S, Shah MA, Sahai V, Uronis HE, Zaidi N, Laheru D. Metastatic Pancreatic Cancer: ASCO Guideline Update. J Clin Oncol. 2020;38:3217-3230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 45. | Ozaka M, Nakachi K, Kobayashi S, Ohba A, Imaoka H, Terashima T, Ishii H, Mizusawa J, Katayama H, Kataoka T, Okusaka T, Ikeda M, Sasahira N, Miwa H, Mizukoshi E, Okano N, Mizuno N, Yamamoto T, Komatsu Y, Todaka A, Kamata K, Furukawa M, Fujimori N, Katanuma A, Takayama Y, Tsumura H, Fukuda H, Ueno M, Furuse J; Hepatobiliary and Pancreatic Oncology Group of Japan Clinical Oncology Group (JCOG). A randomised phase II study of modified FOLFIRINOX versus gemcitabine plus nab-paclitaxel for locally advanced pancreatic cancer (JCOG1407). Eur J Cancer. 2023;181:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 46. | Versteijne E, van Dam JL, Suker M, Janssen QP, Groothuis K, Akkermans-Vogelaar JM, Besselink MG, Bonsing BA, Buijsen J, Busch OR, Creemers GM, van Dam RM, Eskens FALM, Festen S, de Groot JWB, Groot Koerkamp B, de Hingh IH, Homs MYV, van Hooft JE, Kerver ED, Luelmo SAC, Neelis KJ, Nuyttens J, Paardekooper GMRM, Patijn GA, van der Sangen MJC, de Vos-Geelen J, Wilmink JW, Zwinderman AH, Punt CJ, van Tienhoven G, van Eijck CHJ; Dutch Pancreatic Cancer Group. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J Clin Oncol. 2022;40:1220-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 415] [Article Influence: 138.3] [Reference Citation Analysis (0)] |
| 47. | Schwarz L, Vernerey D, Bachet JB, Tuech JJ, Portales F, Michel P, Cunha AS. Resectable pancreatic adenocarcinoma neo-adjuvant FOLF(IRIN)OX-based chemotherapy - a multicenter, non-comparative, randomized, phase II trial (PANACHE01-PRODIGE48 study). BMC Cancer. 2018;18:762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 48. | Wainberg ZA, Melisi D, Macarulla T, Pazo Cid R, Chandana SR, De La Fouchardière C, Dean A, Kiss I, Lee WJ, Goetze TO, Van Cutsem E, Paulson AS, Bekaii-Saab T, Pant S, Hubner RA, Xiao Z, Chen H, Benzaghou F, O'Reilly EM. NALIRIFOX versus nab-paclitaxel and gemcitabine in treatment-naive patients with metastatic pancreatic ductal adenocarcinoma (NAPOLI 3): a randomised, open-label, phase 3 trial. Lancet. 2023;402:1272-1281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 174] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 49. | Macedo FI, Ryon E, Maithel SK, Lee RM, Kooby DA, Fields RC, Hawkins WG, Williams G, Maduekwe U, Kim HJ, Ahmad SA, Patel SH, Abbott DE, Schwartz P, Weber SM, Scoggins CR, Martin RCG, Dudeja V, Franceschi D, Livingstone AS, Merchant NB. Survival Outcomes Associated With Clinical and Pathological Response Following Neoadjuvant FOLFIRINOX or Gemcitabine/Nab-Paclitaxel Chemotherapy in Resected Pancreatic Cancer. Ann Surg. 2019;270:400-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 50. | Kim S, Signorovitch JE, Yang H, Patterson-Lomba O, Xiang CQ, Ung B, Parisi M, Marshall JL. Comparative Effectiveness of nab-Paclitaxel Plus Gemcitabine vs FOLFIRINOX in Metastatic Pancreatic Cancer: A Retrospective Nationwide Chart Review in the United States. Adv Ther. 2018;35:1564-1577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 51. | Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias JL, Zhang H, Soon-Shiong P, Shi T, Rajeshkumar NV, Maitra A, Hidalgo M. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548-4554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 870] [Article Influence: 62.1] [Reference Citation Analysis (2)] |
| 52. | Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, Scheithauer W, Siena S, Tabernero J, Teixeira L, Tortora G, Van Laethem JL, Young R, Penenberg DN, Lu B, Romano A, Von Hoff DD. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. 2015;107:dju413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 452] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 53. | Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15:333-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 805] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 54. | Thomas AM, Fidelle M, Routy B, Kroemer G, Wargo JA, Segata N, Zitvogel L. Gut OncoMicrobiome Signatures (GOMS) as next-generation biomarkers for cancer immunotherapy. Nat Rev Clin Oncol. 2023;20:583-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 74] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 55. | Hatashima A, Arango MJ, Reardon J, Freeman T, Williams T, McLaughlin EM, Abushahin L. First-line gemcitabine plus nab-paclitaxel versus FOLFIRINOX for metastatic pancreatic cancer in a real-world population. Future Oncol. 2022;18:2521-2532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 56. | Seufferlein T, Uhl W, Kornmann M, Algül H, Friess H, König A, Ghadimi M, Gallmeier E, Bartsch DK, Lutz MP, Metzger R, Wille K, Gerdes B, Schimanski CC, Graupe F, Kunzmann V, Klein I, Geissler M, Staib L, Waldschmidt D, Bruns C, Wittel U, Fichtner-Feigl S, Daum S, Hinke A, Blome L, Tannapfel A, Kleger A, Berger AW, Kestler AMR, Schuhbaur JS, Perkhofer L, Tempero M, Reinacher-Schick AC, Ettrich TJ. Perioperative or only adjuvant gemcitabine plus nab-paclitaxel for resectable pancreatic cancer (NEONAX)-a randomized phase II trial of the AIO pancreatic cancer group. Ann Oncol. 2023;34:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 89] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 57. | Nichetti F, Rota S, Ambrosini P, Pircher C, Gusmaroli E, Droz Dit Busset M, Pusceddu S, Sposito C, Coppa J, Morano F, Pietrantonio F, Di Bartolomeo M, Mariani L, Mazzaferro V, de Braud F, Niger M. NALIRIFOX, FOLFIRINOX, and Gemcitabine With Nab-Paclitaxel as First-Line Chemotherapy for Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis. JAMA Netw Open. 2024;7:e2350756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 58. | De La Fouchardière C, Malka D, Cropet C, Chabaud S, Raimbourg J, Botsen D, Launay S, Evesque L, Vienot A, Perrier H, Jary M, Rinaldi Y, Coutzac C, Bachet JB, Neuzillet C, Williet N, Desgrippes R, Grainville T, Aparicio T, Peytier A, Lecomte T, Roth GS, Thirot-Bidault A, Lachaux N, Bouché O, Ghiringhelli F. Gemcitabine and Paclitaxel Versus Gemcitabine Alone After 5-Fluorouracil, Oxaliplatin, and Irinotecan in Metastatic Pancreatic Adenocarcinoma: A Randomized Phase III PRODIGE 65-UCGI 36-GEMPAX UNICANCER Study. J Clin Oncol. 2024;42:1055-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 59. | Conroy T. FOLFIRINOX versus gemcitabine in patients with metastatic pancreatic cancer. Cancéro Digest. 2011;. [DOI] [Full Text] |
| 60. | Pelzer U, Schwaner I, Stieler J, Adler M, Seraphin J, Dörken B, Riess H, Oettle H. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer. 2011;47:1676-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 250] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 61. | Park HS, Kang B, Chon HJ, Im HS, Lee CK, Kim I, Kang MJ, Hwang JE, Bae WK, Cheon J, Park JO, Hong JY, Kang JH, Kim JH, Lim SH, Kim JW, Kim JW, Yoo C, Choi HJ. Liposomal irinotecan plus fluorouracil/leucovorin versus FOLFIRINOX as the second-line chemotherapy for patients with metastatic pancreatic cancer: a multicenter retrospective study of the Korean Cancer Study Group (KCSG). ESMO Open. 2021;6:100049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 62. | Gill S, Ko YJ, Cripps C, Beaudoin A, Dhesy-Thind S, Zulfiqar M, Zalewski P, Do T, Cano P, Lam WYH, Dowden S, Grassin H, Stewart J, Moore M. PANCREOX: A Randomized Phase III Study of Fluorouracil/Leucovorin With or Without Oxaliplatin for Second-Line Advanced Pancreatic Cancer in Patients Who Have Received Gemcitabine-Based Chemotherapy. J Clin Oncol. 2016;34:3914-3920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 182] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 63. | Catalano M, Conca R, Petrioli R, Ramello M, Roviello G. FOLFOX vs FOLFIRI as Second-line of Therapy After Progression to Gemcitabine/Nab-paclitaxel in Patients with Metastatic Pancreatic Cancer. Cancer Manag Res. 2020;12:10271-10278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 64. | Portal A, Pernot S, Tougeron D, Arbaud C, Bidault AT, de la Fouchardière C, Hammel P, Lecomte T, Dréanic J, Coriat R, Bachet JB, Dubreuil O, Marthey L, Dahan L, Tchoundjeu B, Locher C, Lepère C, Bonnetain F, Taieb J. Nab-paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: an AGEO prospective multicentre cohort. Br J Cancer. 2015;113:989-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 65. | Nagrial AM, Chin VT, Sjoquist KM, Pajic M, Horvath LG, Biankin AV, Yip D. Second-line treatment in inoperable pancreatic adenocarcinoma: A systematic review and synthesis of all clinical trials. Crit Rev Oncol Hematol. 2015;96:483-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 66. | Lu W, Wang L, Li X, Tang K. Efficacy and safety of FOLFIRINOX as second-line chemotherapy for advanced pancreatic cancer after gemcitabine-based therapy: A systematic review and meta-analysis. J Int Med Res. 2022;50:3000605221093225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 67. | Huffman BM, Basu Mallick A, Horick NK, Wang-Gillam A, Hosein PJ, Morse MA, Beg MS, Murphy JE, Mavroukakis S, Zaki A, Schlechter BL, Sanoff H, Manz C, Wolpin BM, Arlen P, Lacy J, Cleary JM. Effect of a MUC5AC Antibody (NPC-1C) Administered With Second-Line Gemcitabine and Nab-Paclitaxel on the Survival of Patients With Advanced Pancreatic Ductal Adenocarcinoma: A Randomized Clinical Trial. JAMA Netw Open. 2023;6:e2249720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 68. | Nagashima S, Kobayashi S, Tsunoda S, Yamachika Y, Tozuka Y, Fukushima T, Morimoto M, Ueno M, Furuse J, Maeda S. Liposomal irinotecan plus fluorouracil/leucovorin in older patients with advanced pancreatic cancer: a single-center retrospective study. Int J Clin Oncol. 2024;29:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 69. | Procaccio L, Merz V, Fasano M, Vaccaro V, Giommoni E, Pretta A, Noventa S, Satolli MA, Giordano G, Zichi C, Pinto C, Zecchetto C, Barsotti G, De Vita F, Milella M, Antonuzzo L, Scartozzi M, Zaniboni A, Spadi R, Casalino S, Bergamo F, De Toni C, Melisi D, Lonardi S. The role of nanoliposomal irinotecan plus fluorouracil/leucovorin in the continuum of care of patients with metastatic pancreatic ductal adenocarcinoma. Cancer Med. 2023;12:14337-14345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 70. | Yoo C, Im HS, Kim KP, Oh DY, Lee KH, Chon HJ, Kim JH, Kang M, Kim I, Lee GJ, Oh SY, Choi Y, Choi HJ, Kim ST, Park JO, Ryoo BY. Real-world efficacy and safety of liposomal irinotecan plus fluorouracil/leucovorin in patients with metastatic pancreatic adenocarcinoma: a study by the Korean Cancer Study Group. Ther Adv Med Oncol. 2019;11:1758835919871126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 71. | Bang YJ, Li CP, Lee KH, Chiu CF, Park JO, Shan YS, Kim JS, Chen JS, Shim HJ, Rau KM, Choi HJ, Oh DY, Belanger B, Chen LT. Liposomal irinotecan in metastatic pancreatic adenocarcinoma in Asian patients: Subgroup analysis of the NAPOLI-1 study. Cancer Sci. 2020;111:513-527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 72. | Su YY, Chiang NJ, Tsai HJ, Yen CJ, Shan YS, Chen LT. The Impact of Liposomal Irinotecan on the Treatment of Advanced Pancreatic Adenocarcinoma: Real-World Experience in a Taiwanese Cohort. Sci Rep. 2020;10:7420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 73. | Möhring C, Frontado Graffe FJ, Bartels A, Sadeghlar F, Zhou T, Mahn R, Marinova M, Feldmann G, Brossart P, Glowka TR, Kalff JC, Strassburg CP, Gonzalez-Carmona MA. Second-line and third-line therapy with nanoliposomal irinotecan (nal-IRI) in pancreatic cancer: a single-center experience and review of literature. J Gastrointest Oncol. 2023;14:352-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 74. | Kim B, Ahn J, Jung JH, Jung K, Lee J, Hwang J, Kim J. Efficacy of the third-line chemotherapy in patients with advanced pancreatic cancer. J Clin Oncol. 2023;41:711-711. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 75. | Hosein AN, Brekken RA, Maitra A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat Rev Gastroenterol Hepatol. 2020;17:487-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 616] [Article Influence: 123.2] [Reference Citation Analysis (0)] |
| 76. | Farren MR, Mace TA, Geyer S, Mikhail S, Wu C, Ciombor K, Tahiri S, Ahn D, Noonan AM, Villalona-Calero M, Bekaii-Saab T, Lesinski GB. Systemic Immune Activity Predicts Overall Survival in Treatment-Naïve Patients with Metastatic Pancreatic Cancer. Clin Cancer Res. 2016;22:2565-2574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 77. | Luo W, Yang G, Luo W, Cao Z, Liu Y, Qiu J, Chen G, You L, Zhao F, Zheng L, Zhang T. Novel therapeutic strategies and perspectives for metastatic pancreatic cancer: vaccine therapy is more than just a theory. Cancer Cell Int. 2020;20:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 78. | Hopkins AC, Yarchoan M, Durham JN, Yusko EC, Rytlewski JA, Robins HS, Laheru DA, Le DT, Lutz ER, Jaffee EM. T cell receptor repertoire features associated with survival in immunotherapy-treated pancreatic ductal adenocarcinoma. JCI Insight. 2018;3:e122092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 185] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 79. | Zhang Y, Ma JA, Zhang HX, Jiang YN, Luo WH. Cancer vaccines: Targeting KRAS-driven cancers. Expert Rev Vaccines. 2020;19:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 80. | Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8:1069-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1430] [Cited by in RCA: 2197] [Article Influence: 313.9] [Reference Citation Analysis (0)] |
| 81. | Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, Piha-Paul SA, Doi T, Gao B, Chung HC, Lopez-Martin J, Bang YJ, Frommer RS, Shah M, Ghori R, Joe AK, Pruitt SK, Diaz LA Jr. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol. 2020;38:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2120] [Cited by in RCA: 2019] [Article Influence: 403.8] [Reference Citation Analysis (0)] |
| 82. | Taïeb J, Sayah L, Heinrich K, Kunzmann V, Boileve A, Cirkel G, Lonardi S, Chibaudel B, Turpin A, Beller T, Hautefeuille V, Vivaldi C, Mazard T, Bauguion L, Niger M, Prager GW, Coutzac C, Benedikt Westphalen C, Auclin E, Pilla L. Efficacy of immune checkpoint inhibitors in microsatellite unstable/mismatch repair-deficient advanced pancreatic adenocarcinoma: an AGEO European Cohort. Eur J Cancer. 2023;188:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 40] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 83. | Dai G, Jia R, Si H, Wang Z, Deng G, Zhang N, Liu F, Shi Y, Zhang Y, Jia Y, Zhang B, Tong S. A phase 1b/2 study of surufatinib plus camrelizumab, nab-paclitaxel, and S-1 (NASCA) as first-line therapy for metastatic pancreatic adenocarcinoma (mPDAC). J Clin Oncol. 2023;41:4142-4142. [DOI] [Full Text] |
| 84. | Tang HKC, Wang B, Tan HX, Sarwar MA, Baraka B, Shafiq T, Rao AR. CAR T-Cell Therapy for Cancer: Latest Updates and Challenges, with a Focus on B-Lymphoid Malignancies and Selected Solid Tumours. Cells. 2023;12:1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 85. | Yan T, Zhu L, Chen J. Current advances and challenges in CAR T-Cell therapy for solid tumors: tumor-associated antigens and the tumor microenvironment. Exp Hematol Oncol. 2023;12:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 101] [Reference Citation Analysis (0)] |
| 86. | Śledź M, Wojciechowska A, Zagożdżon R, Kaleta B. In Situ Programming of CAR-T Cells: A Pressing Need in Modern Immunotherapy. Arch Immunol Ther Exp (Warsz). 2023;71:18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 87. | Czaplicka A, Lachota M, Pączek L, Zagożdżon R, Kaleta B. Chimeric Antigen Receptor T Cell Therapy for Pancreatic Cancer: A Review of Current Evidence. Cells. 2024;13:101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 88. | Lanman BA, Allen JR, Allen JG, Amegadzie AK, Ashton KS, Booker SK, Chen JJ, Chen N, Frohn MJ, Goodman G, Kopecky DJ, Liu L, Lopez P, Low JD, Ma V, Minatti AE, Nguyen TT, Nishimura N, Pickrell AJ, Reed AB, Shin Y, Siegmund AC, Tamayo NA, Tegley CM, Walton MC, Wang HL, Wurz RP, Xue M, Yang KC, Achanta P, Bartberger MD, Canon J, Hollis LS, McCarter JD, Mohr C, Rex K, Saiki AY, San Miguel T, Volak LP, Wang KH, Whittington DA, Zech SG, Lipford JR, Cee VJ. Discovery of a Covalent Inhibitor of KRAS(G12C) (AMG 510) for the Treatment of Solid Tumors. J Med Chem. 2020;63:52-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 488] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 89. | Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, Falchook GS, Price TJ, Sacher A, Denlinger CS, Bang YJ, Dy GK, Krauss JC, Kuboki Y, Kuo JC, Coveler AL, Park K, Kim TW, Barlesi F, Munster PN, Ramalingam SS, Burns TF, Meric-Bernstam F, Henary H, Ngang J, Ngarmchamnanrith G, Kim J, Houk BE, Canon J, Lipford JR, Friberg G, Lito P, Govindan R, Li BT. KRAS(G12C) Inhibition with Sotorasib in Advanced Solid Tumors. N Engl J Med. 2020;383:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1183] [Article Influence: 236.6] [Reference Citation Analysis (0)] |
| 90. | Strickler JH, Satake H, George TJ, Yaeger R, Hollebecque A, Garrido-Laguna I, Schuler M, Burns TF, Coveler AL, Falchook GS, Vincent M, Sunakawa Y, Dahan L, Bajor D, Rha SY, Lemech C, Juric D, Rehn M, Ngarmchamnanrith G, Jafarinasabian P, Tran Q, Hong DS. Sotorasib in KRAS p.G12C-Mutated Advanced Pancreatic Cancer. N Engl J Med. 2023;388:33-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 258] [Article Influence: 129.0] [Reference Citation Analysis (0)] |
| 91. | Ou SI, Jänne PA, Leal TA, Rybkin II, Sabari JK, Barve MA, Bazhenova L, Johnson ML, Velastegui KL, Cilliers C, Christensen JG, Yan X, Chao RC, Papadopoulos KP. First-in-Human Phase I/IB Dose-Finding Study of Adagrasib (MRTX849) in Patients With Advanced KRAS(G12C) Solid Tumors (KRYSTAL-1). J Clin Oncol. 2022;40:2530-2538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 194] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 92. | Leidner R, Sanjuan Silva N, Huang H, Sprott D, Zheng C, Shih YP, Leung A, Payne R, Sutcliffe K, Cramer J, Rosenberg SA, Fox BA, Urba WJ, Tran E. Neoantigen T-Cell Receptor Gene Therapy in Pancreatic Cancer. N Engl J Med. 2022;386:2112-2119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 331] [Article Influence: 110.3] [Reference Citation Analysis (0)] |
| 93. | Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick A, Tortora G, Algül H, O'Reilly EM, McGuinness D, Cui KY, Schlienger K, Locker GY, Kindler HL. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med. 2019;381:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1628] [Article Influence: 271.3] [Reference Citation Analysis (0)] |
| 94. | Qin S, Li J, Bai Y, Wang Z, Chen Z, Xu R, Xu J, Zhang H, Chen J, Yuan Y, Liu T, Yang L, Zhong H, Chen D, Shen L, Hao C, Fu D, Cheng Y, Yang J, Wang Q, Qin B, Pan H, Zhang J, Bai X, Zheng Q. Nimotuzumab Plus Gemcitabine for K-Ras Wild-Type Locally Advanced or Metastatic Pancreatic Cancer. J Clin Oncol. 2023;41:5163-5173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 95. | Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, Blakely CM, Seto T, Cho BC, Tosi D, Besse B, Chawla SP, Bazhenova L, Krauss JC, Chae YK, Barve M, Garrido-Laguna I, Liu SV, Conkling P, John T, Fakih M, Sigal D, Loong HH, Buchschacher GL Jr, Garrido P, Nieva J, Steuer C, Overbeck TR, Bowles DW, Fox E, Riehl T, Chow-Maneval E, Simmons B, Cui N, Johnson A, Eng S, Wilson TR, Demetri GD; trial investigators. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 1136] [Article Influence: 189.3] [Reference Citation Analysis (0)] |
| 96. | Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo AS, Turpin B, Dowlati A, Brose MS, Mascarenhas L, Federman N, Berlin J, El-Deiry WS, Baik C, Deeken J, Boni V, Nagasubramanian R, Taylor M, Rudzinski ER, Meric-Bernstam F, Sohal DPS, Ma PC, Raez LE, Hechtman JF, Benayed R, Ladanyi M, Tuch BB, Ebata K, Cruickshank S, Ku NC, Cox MC, Hawkins DS, Hong DS, Hyman DM. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med. 2018;378:731-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1577] [Cited by in RCA: 1940] [Article Influence: 277.1] [Reference Citation Analysis (0)] |
| 97. | Solomon JP, Linkov I, Rosado A, Mullaney K, Rosen EY, Frosina D, Jungbluth AA, Zehir A, Benayed R, Drilon A, Hyman DM, Ladanyi M, Sireci AN, Hechtman JF. NTRK fusion detection across multiple assays and 33,997 cases: diagnostic implications and pitfalls. Mod Pathol. 2020;33:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 379] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 98. | Demols A, Rocq L, Perez-Casanova L, Charry M, De Nève N, Ramadhan A, Van Campenhout C, De Clercq S, Maris C, Closset J, Lucidi V, Salmon I, D'Haene N. A Two-Step Diagnostic Approach for NTRK Gene Fusion Detection in Biliary Tract and Pancreatic Adenocarcinomas. Oncologist. 2023;28:e520-e525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 99. | Hartupee C, Nagalo BM, Chabu CY, Tesfay MZ, Coleman-Barnett J, West JT, Moaven O. Pancreatic cancer tumor microenvironment is a major therapeutic barrier and target. Front Immunol. 2024;15:1287459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 100. | Ye L, Shi S, Chen W. Innate immunity in pancreatic cancer: Lineage tracing and function. Front Immunol. 2022;13:1081919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 101. | Lv G, Zhang L, Gao L, Cui J, Liu Z, Sun B, Wang G, Tang Q. The application of single-cell sequencing in pancreatic neoplasm: analysis, diagnosis and treatment. Br J Cancer. 2023;128:206-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 102. | Roy P, Orecchioni M, Ley K. How the immune system shapes atherosclerosis: roles of innate and adaptive immunity. Nat Rev Immunol. 2022;22:251-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 297] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 103. | Chen YJ, Li GN, Li XJ, Wei LX, Fu MJ, Cheng ZL, Yang Z, Zhu GQ, Wang XD, Zhang C, Zhang JY, Sun YP, Saiyin H, Zhang J, Liu WR, Zhu WW, Guan KL, Xiong Y, Yang Y, Ye D, Chen LL. Targeting IRG1 reverses the immunosuppressive function of tumor-associated macrophages and enhances cancer immunotherapy. Sci Adv. 2023;9:eadg0654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
| 104. | Zuo C, Baer JM, Knolhoff BL, Belle JI, Liu X, Alarcon De La Lastra A, Fu C, Hogg GD, Kingston NL, Breden MA, Dodhiawala PB, Zhou DC, Lander VE, James CA, Ding L, Lim KH, Fields RC, Hawkins WG, Weber JD, Zhao G, DeNardo DG. Stromal and therapy-induced macrophage proliferation promotes PDAC progression and susceptibility to innate immunotherapy. J Exp Med. 2023;220:e20212062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 105. | Caronni N, La Terza F, Vittoria FM, Barbiera G, Mezzanzanica L, Cuzzola V, Barresi S, Pellegatta M, Canevazzi P, Dunsmore G, Leonardi C, Montaldo E, Lusito E, Dugnani E, Citro A, Ng MSF, Schiavo Lena M, Drago D, Andolfo A, Brugiapaglia S, Scagliotti A, Mortellaro A, Corbo V, Liu Z, Mondino A, Dellabona P, Piemonti L, Taveggia C, Doglioni C, Cappello P, Novelli F, Iannacone M, Ng LG, Ginhoux F, Crippa S, Falconi M, Bonini C, Naldini L, Genua M, Ostuni R. IL-1β(+) macrophages fuel pathogenic inflammation in pancreatic cancer. Nature. 2023;623:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 158] [Reference Citation Analysis (0)] |
| 106. | Liu X, Tang R, Xu J, Tan Z, Liang C, Meng Q, Lei Y, Hua J, Zhang Y, Liu J, Zhang B, Wang W, Yu X, Shi S. CRIP1 fosters MDSC trafficking and resets tumour microenvironment via facilitating NF-κB/p65 nuclear translocation in pancreatic ductal adenocarcinoma. Gut. 2023;72:2329-2343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 107. | Wang L, Liu Y, Dai Y, Tang X, Yin T, Wang C, Wang T, Dong L, Shi M, Qin J, Xue M, Cao Y, Liu J, Liu P, Huang J, Wen C, Zhang J, Xu Z, Bai F, Deng X, Peng C, Chen H, Jiang L, Chen S, Shen B. Single-cell RNA-seq analysis reveals BHLHE40-driven pro-tumour neutrophils with hyperactivated glycolysis in pancreatic tumour microenvironment. Gut. 2023;72:958-971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 158] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 108. | Zou X, Guan C, Gao J, Shi W, Cui Y, Zhong X. Tertiary lymphoid structures in pancreatic cancer: a new target for immunotherapy. Front Immunol. 2023;14:1222719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 109. | Kinker GS, Vitiello GAF, Diniz AB, Cabral-Piccin MP, Pereira PHB, Carvalho MLR, Ferreira WAS, Chaves AS, Rondinelli A, Gusmão AF, Defelicibus A, Dos Santos GO, Nunes WA, Claro LCL, Bernardo TM, Nishio RT, Pacheco AM, Laus AC, Arantes LMRB, Fleck JL, de Jesus VHF, de Moricz A, Weinlich R, Coimbra FJF, de Lima VCC, Medina TDS. Mature tertiary lymphoid structures are key niches of tumour-specific immune responses in pancreatic ductal adenocarcinomas. Gut. 2023;72:1927-1941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 60] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 110. | Rohila D, Park IH, Pham TV, Weitz J, Hurtado de Mendoza T, Madheswaran S, Ishfaq M, Beaman C, Tapia E, Sun S, Patel J, Tamayo P, Lowy AM, Joshi S. Syk Inhibition Reprograms Tumor-Associated Macrophages and Overcomes Gemcitabine-Induced Immunosuppression in Pancreatic Ductal Adenocarcinoma. Cancer Res. 2023;83:2675-2689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 111. | O'Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16:151-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 1202] [Article Influence: 200.3] [Reference Citation Analysis (0)] |
| 112. | Luo W, Wen T, Qu X. Tumor immune microenvironment-based therapies in pancreatic ductal adenocarcinoma: time to update the concept. J Exp Clin Cancer Res. 2024;43:8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 113. | Luo W, Wen T, Qu X. Correction: Tumor immune microenvironment-based therapies in pancreatic ductal adenocarcinoma: time to update the concept. J Exp Clin Cancer Res. 2024;43:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 114. | Lai E, Puzzoni M, Ziranu P, Pretta A, Impera V, Mariani S, Liscia N, Soro P, Musio F, Persano M, Donisi C, Tolu S, Balconi F, Pireddu A, Demurtas L, Pusceddu V, Camera S, Sclafani F, Scartozzi M. New therapeutic targets in pancreatic cancer. Cancer Treat Rev. 2019;81:101926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 115. | Jin S, Zhang Y, Zhou F, Chen X, Sheng J, Zhang J. TIGIT: A promising target to overcome the barrier of immunotherapy in hematological malignancies. Front Oncol. 2022;12:1091782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 116. | Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3297] [Cited by in RCA: 4772] [Article Influence: 397.7] [Reference Citation Analysis (1)] |
| 117. | Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, Eaton DL, Grogan JL. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26:923-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 882] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 118. | Michelakos T, Kontos F, Barakat O, Maggs L, Schwab JH, Ferrone CR, Ferrone S. B7-H3 targeted antibody-based immunotherapy of malignant diseases. Expert Opin Biol Ther. 2021;21:587-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 119. | Tate T, Matsumoto S, Nemoto K, Leisegang M, Nagayama S, Obama K, Nakamura Y, Kiyotani K. Identification of T Cell Receptors Targeting a Neoantigen Derived from Recurrently Mutated FGFR3. Cancers (Basel). 2023;15:1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 120. | Balachandran VP, Łuksza M, Zhao JN, Makarov V, Moral JA, Remark R, Herbst B, Askan G, Bhanot U, Senbabaoglu Y, Wells DK, Cary CIO, Grbovic-Huezo O, Attiyeh M, Medina B, Zhang J, Loo J, Saglimbeni J, Abu-Akeel M, Zappasodi R, Riaz N, Smoragiewicz M, Kelley ZL, Basturk O; Australian Pancreatic Cancer Genome Initiative; Garvan Institute of Medical Research; Prince of Wales Hospital; Royal North Shore Hospital; University of Glasgow; St Vincent’s Hospital; QIMR Berghofer Medical Research Institute; University of Melbourne, Centre for Cancer Research; University of Queensland, Institute for Molecular Bioscience; Bankstown Hospital; Liverpool Hospital; Royal Prince Alfred Hospital, Chris O’Brien Lifehouse; Westmead Hospital; Fremantle Hospital; St John of God Healthcare; Royal Adelaide Hospital; Flinders Medical Centre; Envoi Pathology; Princess Alexandria Hospital; Austin Hospital; Johns Hopkins Medical Institutes; ARC-Net Centre for Applied Research on Cancer, Gönen M, Levine AJ, Allen PJ, Fearon DT, Merad M, Gnjatic S, Iacobuzio-Donahue CA, Wolchok JD, DeMatteo RP, Chan TA, Greenbaum BD, Merghoub T, Leach SD. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature. 2017;551:512-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 920] [Cited by in RCA: 879] [Article Influence: 109.9] [Reference Citation Analysis (0)] |
| 121. | Zemek RM, De Jong E, Chin WL, Schuster IS, Fear VS, Casey TH, Forbes C, Dart SJ, Leslie C, Zaitouny A, Small M, Boon L, Forrest ARR, Muiri DO, Degli-Esposti MA, Millward MJ, Nowak AK, Lassmann T, Bosco A, Lake RA, Lesterhuis WJ. Sensitization to immune checkpoint blockade through activation of a STAT1/NK axis in the tumor microenvironment. Sci Transl Med. 2019;11:eaav7816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 122. | Falcomatà C, Bärthel S, Widholz SA, Schneeweis C, Montero JJ, Toska A, Mir J, Kaltenbacher T, Heetmeyer J, Swietlik JJ, Cheng JY, Teodorescu B, Reichert O, Schmitt C, Grabichler K, Coluccio A, Boniolo F, Veltkamp C, Zukowska M, Vargas AA, Paik WH, Jesinghaus M, Steiger K, Maresch R, Öllinger R, Ammon T, Baranov O, Robles MS, Rechenberger J, Kuster B, Meissner F, Reichert M, Flossdorf M, Rad R, Schmidt-Supprian M, Schneider G, Saur D. Selective multi-kinase inhibition sensitizes mesenchymal pancreatic cancer to immune checkpoint blockade by remodeling the tumor microenvironment. Nat Cancer. 2022;3:318-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 123. | Stadtmauer EA, Fraietta JA, Davis MM, Cohen AD, Weber KL, Lancaster E, Mangan PA, Kulikovskaya I, Gupta M, Chen F, Tian L, Gonzalez VE, Xu J, Jung IY, Melenhorst JJ, Plesa G, Shea J, Matlawski T, Cervini A, Gaymon AL, Desjardins S, Lamontagne A, Salas-Mckee J, Fesnak A, Siegel DL, Levine BL, Jadlowsky JK, Young RM, Chew A, Hwang WT, Hexner EO, Carreno BM, Nobles CL, Bushman FD, Parker KR, Qi Y, Satpathy AT, Chang HY, Zhao Y, Lacey SF, June CH. CRISPR-engineered T cells in patients with refractory cancer. Science. 2020;367:eaba7365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 943] [Cited by in RCA: 961] [Article Influence: 192.2] [Reference Citation Analysis (0)] |
| 124. | Zhao Y, Tang J, Jiang K, Liu SY, Aicher A, Heeschen C. Liquid biopsy in pancreatic cancer - Current perspective and future outlook. Biochim Biophys Acta Rev Cancer. 2023;1878:188868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 125. | Wang K, Wang X, Pan Q, Zhao B. Liquid biopsy techniques and pancreatic cancer: diagnosis, monitoring, and evaluation. Mol Cancer. 2023;22:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 60] [Reference Citation Analysis (0)] |
| 126. | Raufi AG, May MS, Hadfield MJ, Seyhan AA, El-Deiry WS. Advances in Liquid Biopsy Technology and Implications for Pancreatic Cancer. Int J Mol Sci. 2023;24:4238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 127. | Kaczor-Urbanowicz KE, Cheng J, King JC, Sedarat A, Pandol SJ, Farrell JJ, Wong DTW, Kim Y. Reviews on Current Liquid Biopsy for Detection and Management of Pancreatic Cancers. Pancreas. 2020;49:1141-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 128. | Anta JA, Martínez-Ballestero I, Eiroa D, García J, Rodríguez-Comas J. Artificial intelligence for the detection of pancreatic lesions. Int J Comput Assist Radiol Surg. 2022;17:1855-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 129. | Sok CP, Polireddy K, Kooby DA. Molecular pathology and protein markers for pancreatic cancer: relevance in staging, in adjuvant therapy, in determination of minimal residual disease, and follow-up. Hepatobiliary Surg Nutr. 2024;13:56-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 130. | Kann BH, Hosny A, Aerts HJWL. Artificial intelligence for clinical oncology. Cancer Cell. 2021;39:916-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 174] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 131. | Schuurmans M, Alves N, Vendittelli P, Huisman H, Hermans J; PANCAIM Consortium. Artificial Intelligence in Pancreatic Ductal Adenocarcinoma Imaging: A Commentary on Potential Future Applications. Gastroenterology. 2023;165:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 132. | Schuurmans M, Alves N, Vendittelli P, Huisman H, Hermans J. Setting the Research Agenda for Clinical Artificial Intelligence in Pancreatic Adenocarcinoma Imaging. Cancers (Basel). 2022;14:3498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 133. | Jiang J, Chao WL, Culp S, Krishna SG. Artificial Intelligence in the Diagnosis and Treatment of Pancreatic Cystic Lesions and Adenocarcinoma. Cancers (Basel). 2023;15:2410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 134. | Korfiatis P, Suman G, Patnam NG, Trivedi KH, Karbhari A, Mukherjee S, Cook C, Klug JR, Patra A, Khasawneh H, Rajamohan N, Fletcher JG, Truty MJ, Majumder S, Bolan CW, Sandrasegaran K, Chari ST, Goenka AH. Automated Artificial Intelligence Model Trained on a Large Data Set Can Detect Pancreas Cancer on Diagnostic Computed Tomography Scans As Well As Visually Occult Preinvasive Cancer on Prediagnostic Computed Tomography Scans. Gastroenterology. 2023;165:1533-1546.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 135. | Bian Y, Zheng Z, Fang X, Jiang H, Zhu M, Yu J, Zhao H, Zhang L, Yao J, Lu L, Lu J, Shao C. Artificial Intelligence to Predict Lymph Node Metastasis at CT in Pancreatic Ductal Adenocarcinoma. Radiology. 2023;306:160-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 60] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 136. | Zhang C, Xu J, Tang R, Yang J, Wang W, Yu X, Shi S. Novel research and future prospects of artificial intelligence in cancer diagnosis and treatment. J Hematol Oncol. 2023;16:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 22.0] [Reference Citation Analysis (0)] |