Published online Jun 15, 2025. doi: 10.4251/wjgo.v17.i6.106608
Revised: April 19, 2025
Accepted: May 6, 2025
Published online: June 15, 2025
Processing time: 103 Days and 9.8 Hours
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy. Ablation therapy is one of the first-line treatments for early HCC. Accurately predicting early recurrence (ER) is crucial for making precise treatment plans and improving patient prognosis.
To establish an intratumoral and peritumoral model for predicting ER in HCC patients following curative ablation.
This study included a total of 288 patients from three Centers. The patients were divided into a primary cohort (n = 222) and an external cohort (n = 66). Radiomics and deep learning methods were combined for feature extraction, and models were constructed following a three-step feature selection process. Model performance was evaluated using the area under the receiver operating characteristic curve (AUC), while calibration curves and decision curve analysis (DCA) were used to assess calibration and clinical utility. Finally, Kaplan-Meier (K-M) analysis was used to stratify patients according to progression-free survival (PFS) and overall survival (OS).
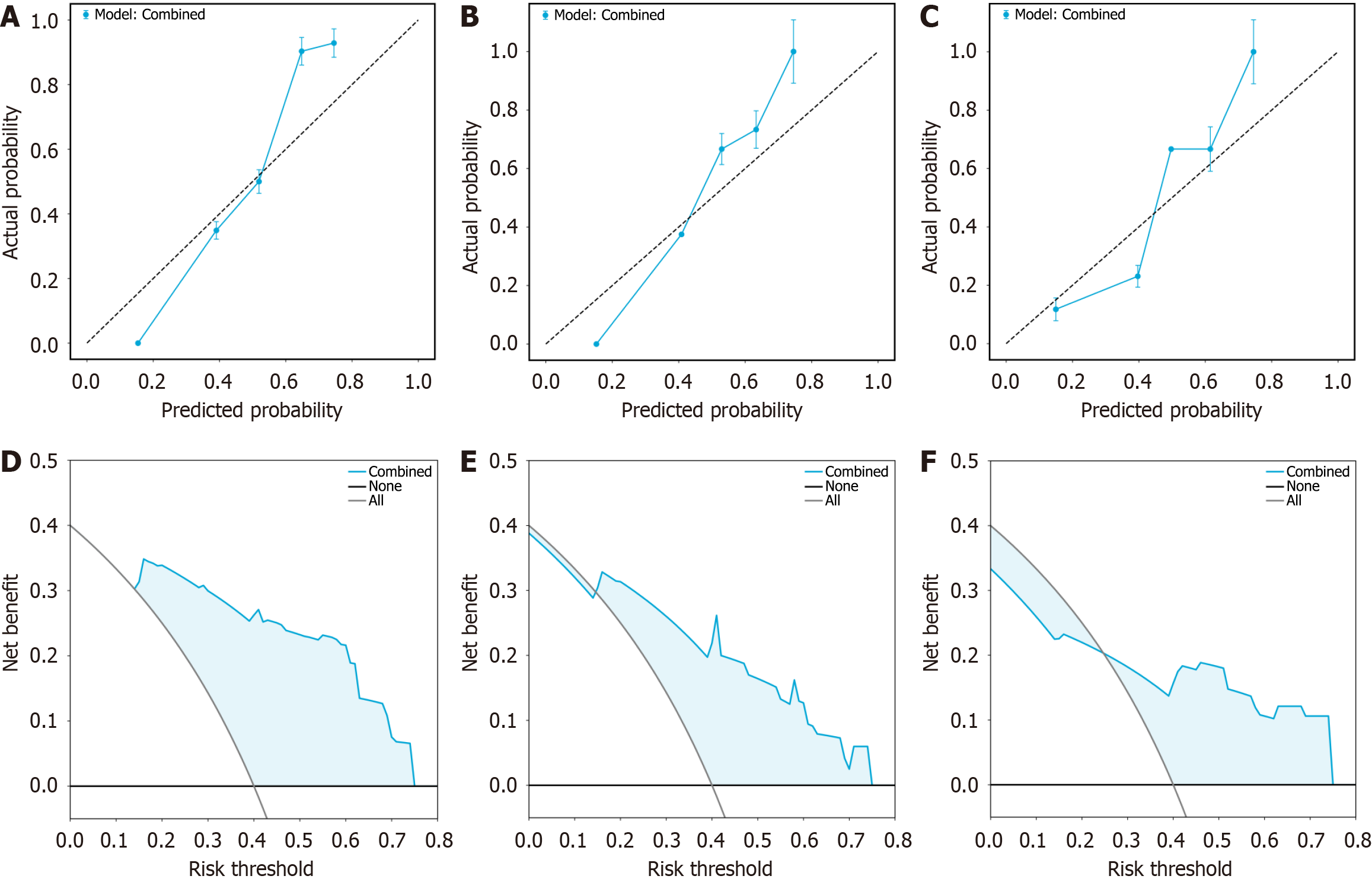
The combined model, which utilizes the light gradient boosting machine learning algorithm and incorporates both intratumoral and peritumoral regions (5 mm and 10 mm), demonstrated the best predictive performance for ER following HCC ablation, achieving AUCs of 0.924 in the training set, 0.899 in the internal validation set, and 0.839 in the external validation set. Calibration and DCA curves confirmed strong calibration and clinical utility, whereas K-M curves provided risk stratification for PFS and OS in HCC patients.
The most efficient model integrated the tumor region with the peritumoral 5 mm and 10 mm regions. This model provides a noninvasive, effective, and reliable method for predicting ER after curative ablation of HCC.
Core Tip: This study developed a predictive model for early recurrence (ER) in hepatocellular carcinoma (HCC) patients postablation by combining radiomics and deep learning. The model, which integrates intratumoral and peritumoral regions, demonstrated strong predictive performance, with area under the receiver operating characteristic curve of 0.924, 0.899, and 0.839 in the training, internal, and external validation sets, respectively. It offers a noninvasive and reliable method for ER prediction, providing valuable insights for treatment planning and prognosis in HCC patients.
- Citation: Li YH, Qian GX, Yao L, Lei XD, Zhu Y, Tang L, Xu ZL, Bu XY, Wei MT, Lu JL, Jia WD. Preoperative model for predicting early recurrence in hepatocellular carcinoma patients using radiomics and deep learning: A multicenter study. World J Gastrointest Oncol 2025; 17(6): 106608
- URL: https://www.wjgnet.com/1948-5204/full/v17/i6/106608.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i6.106608
Primary liver cancer is the sixth most prevalent malignant tumor and the third leading cause of cancer-related death globally, with hepatocellular carcinoma (HCC) accounting for approximately 90% of cases[1]. Ablation is a minimally invasive and effective treatment for early-stage HCC. However, its higher early recurrence (ER) rate than surgical resection remains a significant challenge[2,3]. ER in HCC is defined as recurrence occurring within two years and is often associated with a poorer prognosis than late recurrence[4,5]. Xing et al[6] reported that patients with ER had a median recurrence-free survival (RFS) of only 8.4 months, which was markedly shorter than the 21.3 months observed in patients with late recurrence. This finding underscores the importance of identifying patients at high risk of ER before treatment to optimize therapeutic strategies, strengthen postoperative surveillance, and ultimately improve long-term survival. Pathological grade and microvascular invasion (MVI) have been recognized as independent risk factors for postoperative ER. However, surgical treatment allows for the collection of pathological tissue, whereas ablation treatment does not. Therefore, predicting ER after radiofrequency ablation (RFA) in clinical practice remains a significant challenge.
Radiomics, in which imaging features are quantitatively extracted to delve into tumor phenotypes, has shown great potential in revealing tumor heterogeneity and predicting tumor prognosis[7,8]. Our team previously developed a comprehensive model integrating radiomic and clinical imaging features, which achieved an area under the receiver operating characteristic (ROC) curve (AUC) of 0.790 in predicting ER in liver resection patients in the validation set[9]. Deep learning has demonstrated significant promise in the field of medical image analysis. In deep learning-based radiomics (DLR), features are extracted through deep learning networks and combined with those obtained via traditional radiomic methods, effectively reducing overfitting and improving the accuracy of associated deep learning models. Li et al[10] tested and validated the accuracy of a DLR model on multimodal imaging and reported that the DLR model had a greater AUC than did the radiomic model. Hu et al[11] explored the combination of deep learning and radiomic models to predict Ki-67 expression in HCC, providing more detailed information on tumor characteristics and serving as a critical parameter for assessing HCC tumor proliferation status. The model that used both deep learning and radiomic features achieved better performance, possibly because more imaging biomarkers were introduced into the predictive model and provided more information on tumor characteristics[12].
The peritumoral region refers to the boundary between the tumor mass and healthy tissue, where the macroscopic appearance of peritumoral tissue is similar to that of normal tissue but implies microscopic heterogeneity[13]. Gu et al[14] first proposed the concept of the peritumor microenvironment (PME) and confirmed that the PME in HCC manifests in terms of protein composition and function fundamentally different from that of normal tissue. Radiomic models constructed using peritumoral features have shown good diagnostic efficacy and predictive effects for tumors, with some studies even finding that peritumoral models have better predictive value than do intertumoral models[15-17]. However, the optimal peritumoral range for predicting HCC recurrence remains unknown[18,19].
Therefore, this study aimed to develop a combined model that integrates traditional radiomics and deep learning to extract multiphase and multiregional contrast-enhanced computed tomography (CECT) features from tumors and peritumoral regions. By employing six machine learning algorithms, the model predicts ER in HCC patients following ablation and is designed to assist clinicians in treatment planning and prognosis management, promoting precision therapy in HCC.
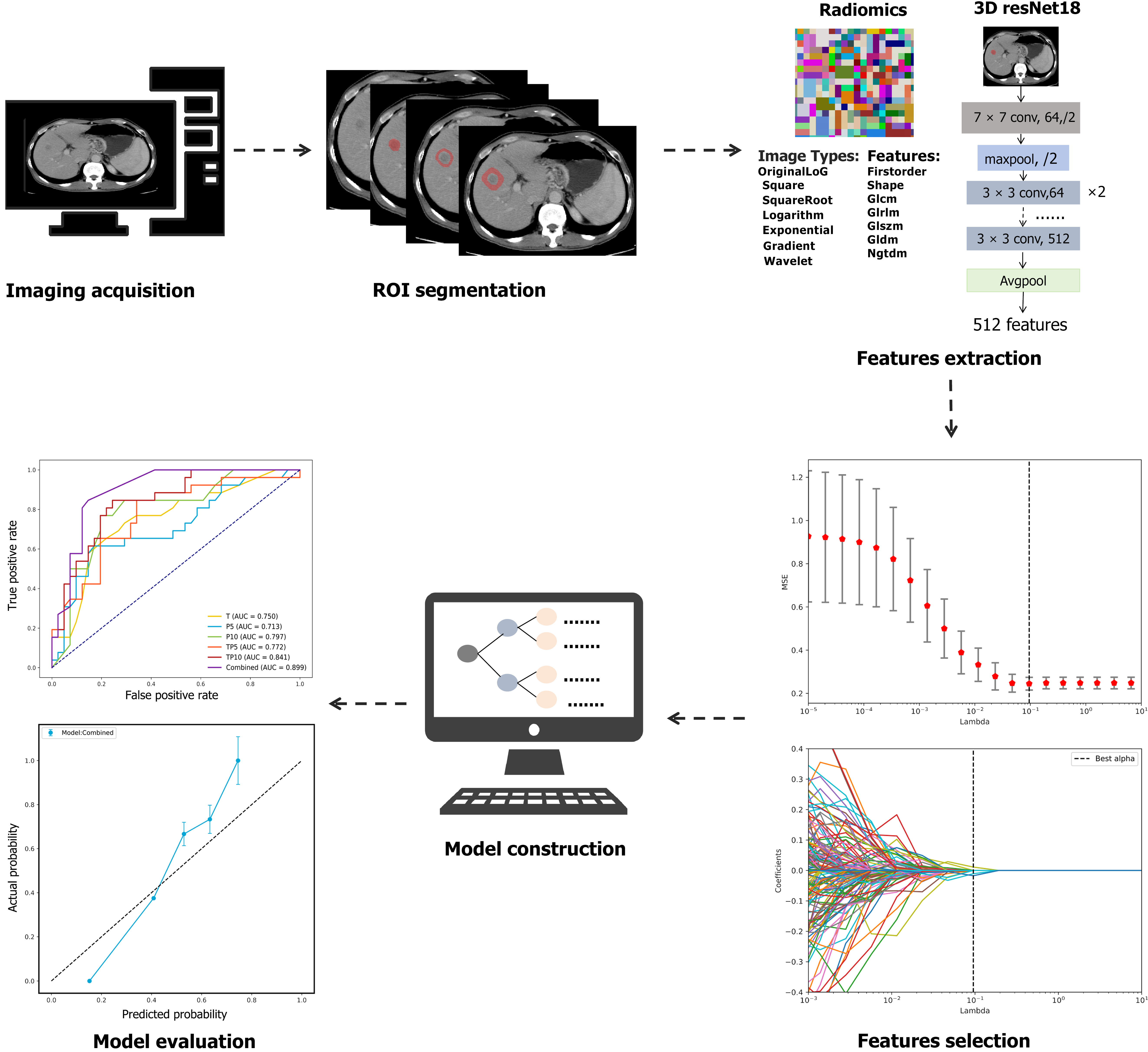
This study was conducted in accordance with the Declaration of Helsinki and received ethical approval from the Ethics Committee of The First Affiliated Hospital of University of Science and Technology of China (No. 2021-RE-043). The committee waived the requirement for patient consent to access medical records, as all the data were anonymized and deidentified prior to analysis. The research process is shown in Figure 1.
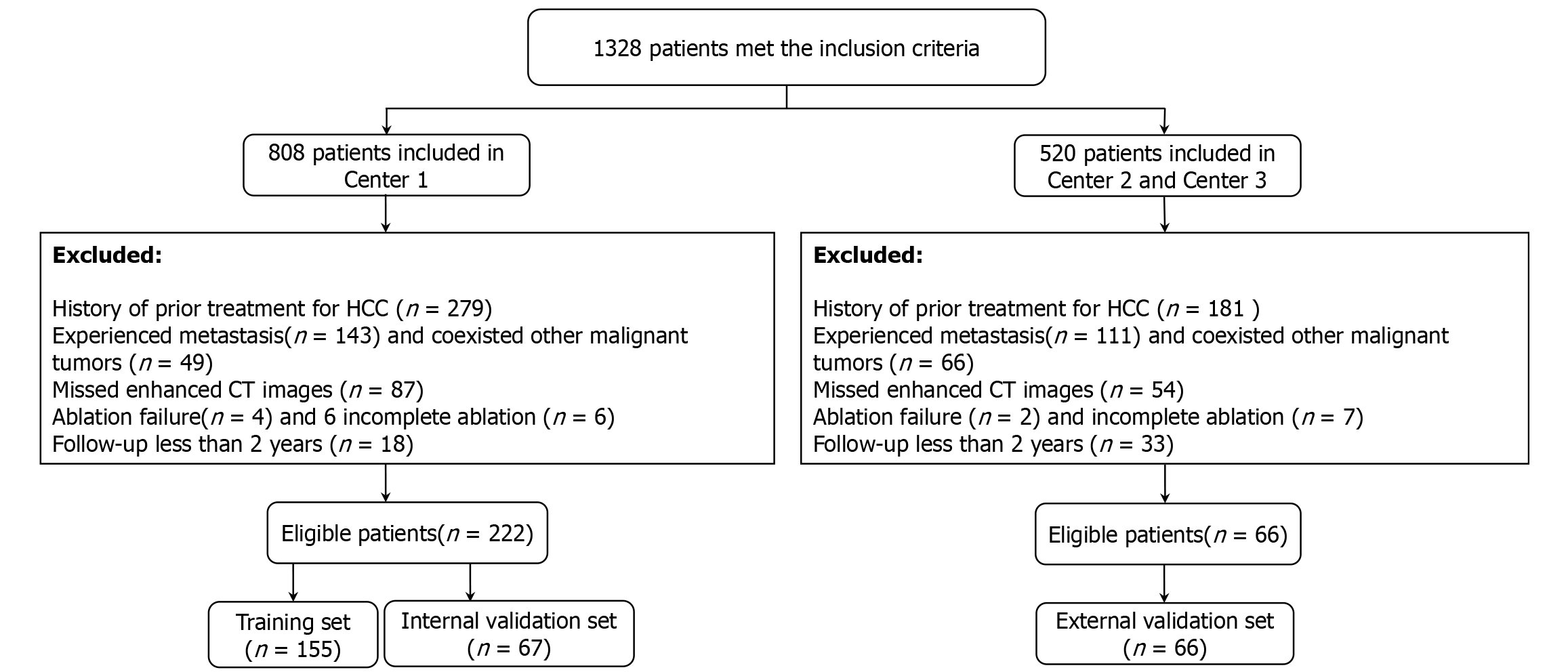
This study included patients with HCC at The First Affiliated Hospital of University of Science and Technology of China (Center 1), The Second Affiliated Hospital of Anhui Medical University (Center 2), and Taizhou Hospital in Zhejiang Province (Center 3) from April 2008 to March 2022. The inclusion criteria were as follows: (1) Diagnosis of HCC based on noninvasive criteria defined by the American Association for the Study of Liver Diseases-specific imaging features[20]; (2) Single tumor with a diameter of ≤ 5 cm or ≤ 3 tumors with a diameter of ≤ 3 cm; (3) Initial treatment with ablation therapy [RFA or microwave ablation (MWA)]; (4) Age between 18 years and 75 years; and (5) Child-Pugh classification grade A. The exclusion criteria were as follows: (1) Vascular, biliary, or adjacent organ invasion or distant metastasis; (2) Discovery of hepatic artery tumor thrombus; (3) Coexistence of other malignant tumors; (4) Prior out-of-hospital treatment history for HCC; (5) No CECT examination within one month of ablation; (6) Postablation assessment as failure or incomplete ablation; and (7) Follow-up of less than 2 years. The specific inclusion and exclusion process is shown in Figure 2. The ablation procedure was carried out by skilled physicians. Prior to treatment, each patient underwent a thorough evaluation to determine the most suitable ablation method (RFA or MWA), one antenna or two, based on tumor characteristics such as shape, size, location, and its proximity to surrounding organs. Patients were positioned supine or oblique, and ultrasound (US) was used to guide positioning before puncture to ensure a precise and safe approach. Local anesthesia with 1% lidocaine was administered along the predetermined path from skin to peritoneum, and intravenous anesthesia was given during the ablation. Ablation needles were accurately placed into the target lesion under US guidance. During the procedure, the surgeon monitored the ablation zone (hyperechoic area) to ensure it fully covered the target lesion. After the ablation, a US examination was conducted to confirm complete ablation, defined as an ablation margin encompassing at least 5 mm of peritumor liver tissue. The ablation was performed using either the ECO-100C microwave treatment system (Nanjing Yigao, China) or the HCCF-3000 cold pole radiofrequency tumor system (Zhuhai Hejia, China).
Before data collection, each Center's researchers received training in data extraction. To ensure a high correlation between the extracted data and the patient's condition before curative ablation treatment, data were extracted from the patient's last clinical record and the enhanced computed tomography (CT) image before curative ablation treatment. The researchers reviewed the medical records of eligible patients in detail and standardized the clinical and imaging data extracted from the patients. Extreme outliers were marked and re-evaluated by the designated physician at each Center to confirm the validity of the data.
CECT was performed using a GE Lightspeed 4-row, GE gem CT 128-layer spiral CT scanner, or GE Lightspeed voluntary counseling and testing 64-row. Patients first underwent a plain abdominal CT scan to determine the lesion extent. Abdominal CT parameters were: (1) Tube voltage 120 kVp; (2) Tube current 250-350 mA; (3) Layer thickness 5 mm; (4) Layer spacing 5 mm; (5) Visual field 35-50 cm; (6) Matrix range 512 × 512; (7) Rotation time 0.7 second; and (8) Pitch 1.375:1.
After scanning, non-ionic iodine contrast was injected at 3.0 mL/second with a dose of 1.5 mL/kg. The arterial phase (A) scan was monitored 35 seconds after the descending aorta density reached 95 HU. The portal vein phase (P) and delayed phase (D) scans began 35 seconds and 3 minutes after the AP scan, respectively. The images were downloaded from the Picture Archiving and Communication System at each Center in Digital Imaging and Communications in Medicine format. A radiologist with a decade of experience in abdominal CT imaging at Center 1 delineated the three-dimensional volume of interest (VOI) for the tumor during the arterial phase, portal venous phase, and delayed phase via ITK-SNAP software (version 3.6.0; www.itksnap.org). The VOI was defined as the area of the tumor without blood vessels or bile ducts. If a patient had multiple tumors, the largest tumor was selected for segmentation. The tumor-adjacent VOI was processed via morphological erosion and dilation algorithms in Python, which automatically expanded the boundaries of each lesion by 5 mm and 10 mm. The radiologist lacked access to the clinical information of all included patients.
To ensure the reproducibility of image feature extraction, two weeks after the initial extraction, physician A and another radiologist randomly selected 50 patients for a second VOI delineation. They then compared the extracted imaging features with those obtained from the first extraction. The intratumoral and intergroup correlation coefficients (ICCs) were calculated to assess the degree of consistency between the data derived from both extractions.
We employed PyRadiomics (version 3.1.0) software within the Python environment (version 3.8.4) for the extraction of radiomic features, with detailed parameter settings. Before extraction, we applied the following settings: (1) First, based on the original image layer thickness of 5 mm, we resampled the coronal and sagittal positions to 1 mm × 1 mm × 5 mm. The image types included the original image and those processed with square, square root, log, exponential, gradient operation, Gauss-Laplacian filter, and wavelet filter. These settings enabled further processing and analysis to extract more relevant feature information. The extracted feature categories included 19 first-order features, 17 three-dimensional (3D) shape features, 10 two-dimensional shape features, 24 gray-level co-occurrence matrix features, 16 gray-level size zone matrix features, 16 gray-level run length matrix features, 5 neighboring gray-tone difference matrix (NGTDM) features, and 14 gray-level dependence matrix features. For deep learning feature extraction, we utilized a 3D ResNet-18 architecture, whose corresponding parameter configurations are outlined; (2) We utilized a pre-trained 3D ResNet-18 model in Pytorch 2.1.2 for depth feature extraction. As this model is designed for three-channel 3D input images, but CT images are single-channel 3D grayscale images, we adapted the input layer of 3D ResNet-18 to accept single-channel images; and (3) Additionally, we modified the network structure by removing the final fully connected layer, allowing the model to output deep learning features with 512 feature vectors. The temporal range for feature extraction encompassed the arterial, venous, and delayed phases, whereas the spatial scope included three tumor regions: (1) The tumor itself; (2) Areas extending 5 mm; and (3) Areas extending 10 mm from its periphery. The results from both the radiomics and deep learning extractions are presented in Supplementary Tables 1 and 2.
In this study, a three-step feature selection strategy was implemented to reduce dimensionality and enhance the robustness of the model. First, features with an ICC greater than 0.8 were retained to ensure reproducibility and interreader consistency. Second, the retained features were standardized using Z score normalization, followed by least absolute shrinkage and selection operator (LASSO) regression. LASSO regression, by introducing an L1 regularization term, effectively shrinks the coefficients of redundant variables to zero, selecting features with significant impact while reducing model complexity and avoiding overfitting. Third, for models where multiple features remained after LASSO, recursive feature elimination (RFE) using a decision tree as the base classifier was employed to determine the optimal feature subset. RFE refines the feature set by iteratively training the model and removing the least influential features, ultimately selecting those that contribute the most to prediction and further optimizing the model's performance.
The final radiomic and deep learning scores were calculated as linear combinations of the selected features weighted by their respective regression coefficients from the LASSO model. All features underwent this uniform selection process to ensure consistency and comparability across models. A detailed breakdown of the feature selection results, including the number and types of features retained at each stage, is provided in Supplementary Table 3.
We built our model via six machine learning algorithms, including support vector machine, logistic regression, random forest, K-nearest neighbor, light gradient boosting machine (LightGBM), and extreme gradient boosting. For each region, we identified the machine learning algorithm with the highest AUC. To enhance the model’s generalization ability, we applied five-fold cross-validation to the model hyperparameter selection and training process on the training set. Finally, we selected the best model by evaluating its AUC on the internal validation set.
One month following the ablation procedure, the efficacy of the intervention was evaluated. Patients exhibiting complete ablation were subjected to regular follow-up evaluations every 3 months to 6 months, which included serum alpha-fetoprotein levels, liver function tests, abdominal US, and CT imaging. Magnetic resonance imaging (MRI) or contrast-enhanced US (CEUS) examinations were conducted as necessary to ascertain the presence of recurrence. The study commenced on the date of ablation and concluded upon the identification of ER. Complete ablation was characterized by an absence of enhancement on dynamic CT, MRI, or CEUS during the arterial phase, signifying total tumor necrosis. ER was defined as the emergence of new intrahepatic lesions or metastases within two years after ablation that exhibit typical imaging or pathological features consistent with HCC. The final follow-up date for this study was March 31, 2024.
The sample size calculation in this study adhered to the 10 events per variable principle to ensure model stability and prevent overfitting[21]. This study excluded parameters with missing values exceeding 20%, whereas those with missing values below this threshold were imputed via multiple imputation techniques. We employed the ‘Tableone’ package in R software (version 4.3.0) to analyze the baseline characteristics of the patients. Categorical variables were assessed via χ2 tests or Fisher’s exact tests, whereas continuous variables were analyzed via paired t tests or Mann-Whitney U tests. The predictive performance of the model was evaluated through the AUC, and we also calculated the accuracy, positive predictive value, and negative predictive value for each model. Model comparisons were performed via the net reclassification improvement (NRI) and integrated discrimination improvement (IDI), whereas calibration and clinical applicability were assessed via calibration curves and decision curve analysis (DCA). Survival curves were generated via the Kaplan-Meier (K-M) method, with comparisons conducted via log-rank tests. A two-sided P < 0.05 was considered statistically significant.
This study included a total of 288 HCC patients, with patients from Center 1 being randomly allocated to the training set (155 patients) and the internal validation set (67 patients) at a ratio of 7:3 and patients from Centers 2 and 3 serving as the external validation set (66 patients). The average age of patients in the training set, internal validation set, and external validation set was 57.9 years ± 11.7 years, 58.9 years ± 11.3 years, and 61.8 years ± 9.1 years, respectively, and the ER incidence rate was 40.0%, 38.8%, and 33.3%, respectively, as shown in Table 1.
| Training set (n = 155) | Internal validation set (n = 67) | External validation set (n = 66) | P value | |
| Sex | 0.386 | |||
| Female | 30 (19.4) | 13 (19.4) | 18 (27.3) | |
| Male | 125 (80.6) | 54 (80.6) | 48 (72.7) | |
| Age (years) | 57.9 ± 11.7 | 58.9 ± 11.3 | 61.8 ± 9.07 | 0.06 |
| ≤ 50 | 38 (24.5) | 20 (29.9) | 12 (18.2) | 0.290 |
| > 50 | 117 (75.5) | 47 (70.1) | 54 (81.8) | |
| History of hepatitis | 0.110 | |||
| Negative | 57 (36.8) | 30 (44.8) | 18 (27.3) | |
| Positive | 98 (63.2) | 37 (55.2) | 48 (72.7) | |
| Cirrhosis | 0.424 | |||
| Negative | 28 (18.1) | 17 (25.4) | 12 (18.2) | |
| Positive | 127 (81.9) | 50 (74.6) | 54 (81.8) | |
| Platelets (× 109/L) | 0.236 | |||
| > 100 | 62 (40.0) | 35 (52.2) | 28 (42.4) | |
| ≤ 100 | 93 (60.0) | 32 (47.8) | 38 (57.6) | |
| Alanine transferase (U/L) | 0.806 | |||
| ≤ 50 | 115 (74.2) | 47 (70.1) | 49 (74.2) | |
| > 50 | 40 (25.8) | 20 (29.9) | 17 (25.8) | |
| Aspartate transferase (U/L) | 0.784 | |||
| > 40 | 68 (43.9) | 27 (40.3) | 26 (39.4) | |
| ≤ 40 | 87 (56.1) | 40 (59.7) | 40 (60.6) | |
| Gammaglutamyl transferase (U/L) | 0.606 | |||
| ≤ 60 | 85 (54.8) | 38 (56.7) | 41 (62.1) | |
| > 60 | 70 (45.2) | 29 (43.3) | 25 (37.9) | |
| Total bilirubin (umol/L) | 0.07 | |||
| ≤ 21 | 88 (56.8) | 47 (70.1) | 46 (69.7) | |
| > 21 | 67 (43.2) | 20 (29.9) | 20 (30.3) | |
| Activated partial thromboplastin time (second) | 0.207 | |||
| < 42 | 130 (83.9) | 62 (92.5) | 58 (87.9) | |
| ≥ 42 | 25 (16.1) | 5 (7.46) | 8 (12.1) | |
| Fibrinogen (g/L) | 0.075 | |||
| ≥ 2 | 90 (58.1) | 42 (62.7) | 49 (74.2) | |
| < 2 | 65 (41.9) | 25 (37.3) | 17 (25.8) | |
| International normalized ratio | 0.265 | |||
| ≤ 1.2 | 101 (65.2) | 51 (76.1) | 44 (66.7) | |
| > 1.2 | 54 (34.8) | 16 (23.9) | 22 (33.3) | |
| Hepatitis B surface antigen | 0.313 | |||
| Negative | 28 (18.1) | 13 (19.4) | 7 (10.6) | |
| Positive | 127 (81.9) | 54 (80.6) | 59 (89.4) | |
| Alpha fetoprotein (ng/mL) | 0.374 | |||
| ≤ 400 | 131 (84.5) | 53 (79.1) | 58 (87.9) | |
| > 400 | 24 (15.5) | 14 (20.9) | 8 (12.1) | |
| Size (cm) | 2.7 ± 1.05 | 2.70 ± 1.10 | 2.57 ± 0.90 | 0.586 |
| Early recurrence | 0.642 | |||
| No | 93 (60.0) | 41 (61.2) | 44 (66.7) | |
| Yes | 62 (40.0) | 26 (38.8) | 22 (33.3) |
We extracted 13587 features from the imaging data of each patient via radiomics, resulting in 15 relevant features after feature selection. Among these 15 features, 13 features were located in the peritumoral region, and 10 features were from the 10 mm peritumoral region. In addition, 4608 features were obtained from the imaging data of each patient via deep learning methods, resulting in 18 relevant features after feature selection. Among these 18 features, 11 features were located in the peritumoral region, and 8 features were from the 10 mm peritumoral region. The specific features selected are shown in Supplementary Table 3.
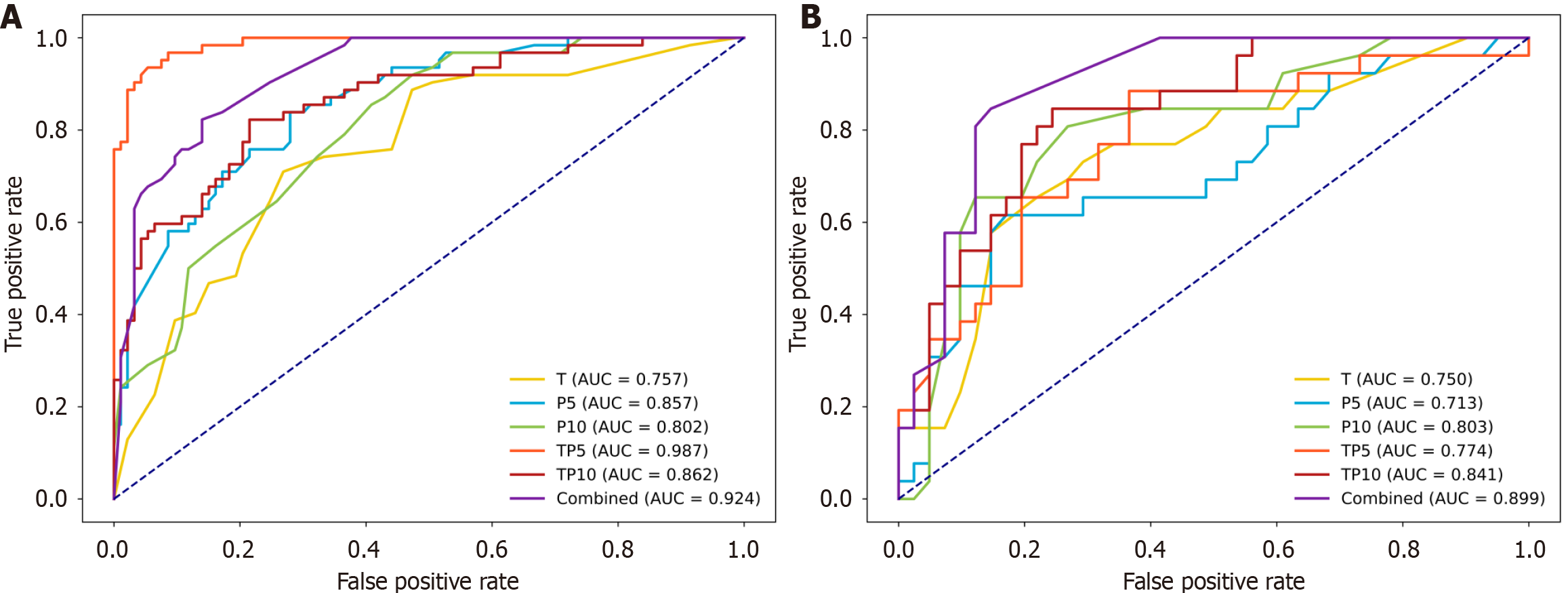
We integrated radiomics with deep learning features to develop both single-region and multiregional models. The three single-region models were the tumor-only model (T), the 5 mm peritumoral model (P5), and the 10 mm peritumoral model (P10). The three multiregional models were the tumor-peritumor 5 mm model (TP5), the tumor-peritumor 10 mm model (TP10), and a comprehensive combined model incorporating features from all regions: (1) The tumor; (2) Peritumoral 5 mm; and (3) 10 mm regions. Owing to space limitations, Table 2 presents only the optimal machine learning approach for each model. The results of all the machine learning methods corresponding to each model can be found in Supplementary Table 4, and their training and validation ROC curves are illustrated in Figure 3.
| Model | Training set | Internal-Validation set | ||||||
| Accuracy | AUC (95%CI) | NPV | PPV | Accuracy | AUC (95%CI) | NPV | PPV | |
| The tumor-only model (KNN) | 0.690 | 0.757 (0.724-0.789) | 0.532 | 0.796 | 0.731 | 0.750 (0.700-0.801) | 0.654 | 0.780 |
| The 5 mm peri-tumor model (LightGBM) | 0.768 | 0.857 (0.824-0.891) | 0.629 | 0.860 | 0.746 | 0.713 (0.665-0.761) | 0.577 | 0.854 |
| The 10 mm peri-tumor model (KNN) | 0.723 | 0.802 (0.776-0.829) | 0.548 | 0.839 | 0.776 | 0.803 (0.763-0.842) | 0.577 | 0.902 |
| The tumor-peri-tumor 5 mm model (extreme gradient boosting) | 0.935 | 0.987 (0.934-1.000) | 0.887 | 0.968 | 0.701 | 0.774 (0.696-0.853) | 0.654 | 0.732 |
| The tumor-peri-tumor 10 mm model (logistic regression) | 0.781 | 0.862 (0.821-0.904) | 0.774 | 0.785 | 0.761 | 0.841 (0.777-0.904) | 0.692 | 0.805 |
| The model that incorporates features from all regions—tumor, 5 mm, and 10 mm (LightGBM) | 0.832 | 0.924 (0.890-0.958) | 0.726 | 0.903 | 0.776 | 0.899 (0.850-0.948) | 0.615 | 0.878 |
As shown in Figure 3, the combined model utilizing LightGBM demonstrated the best overall performance, with AUCs of 0.924 in the training set and 0.899 in the internal validation set. These values were notably higher than those of the single-region models (T: 0.750; P5: 0.713; P10: 0.803) and other multiregional models (TP5: 0.774; TP10: 0.841). Additionally, the combined model outperformed the other models in the NRI and IDI analyses, further supporting its selection as the final predictive model (Table 3).
| Model 1 | Model 2 | Net reclassification improvement | P value | Integrated discrimination improvement | P value |
| The tumor-only model | Combined | 0.261 | 0.021 | 0.493 | < 0.0001 |
| The 5 mm peri-tumor model | Combined | 0.321 | 0.002 | 0.353 | < 0.0001 |
| The 10 mm peri-tumor model | Combined | 0.292 | 0 | 0.166 | 0.00033 |
| The tumor-peri-tumor 5 mm model | Combined | 0.189 | 0.049 | 0.166 | 0.00052 |
| The tumor-peri-tumor 10 mm model | Combined | 0.220 | 0.02 | 0.231 | 0.00311 |
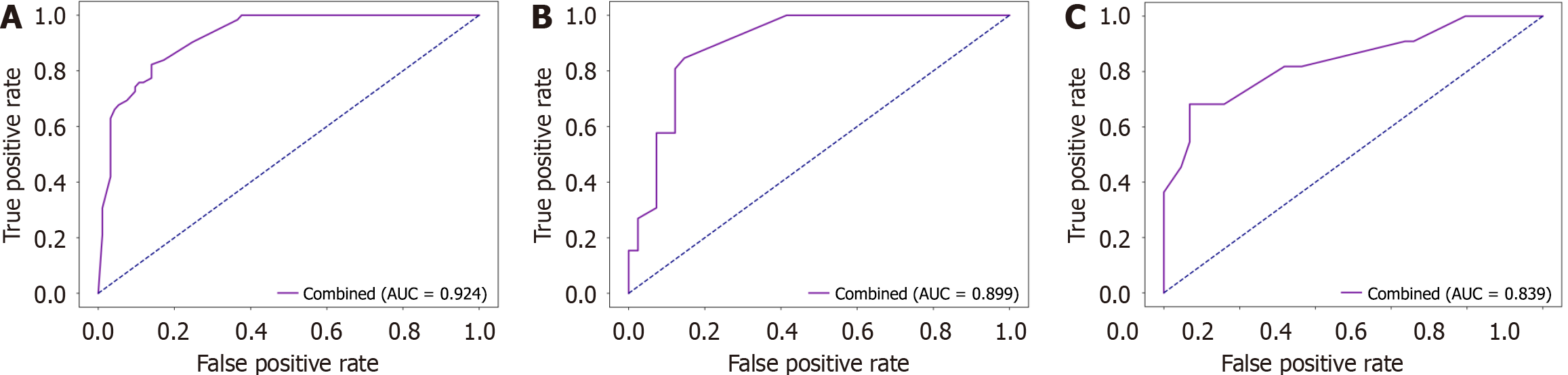
To further assess the generalizability of the combined model, we evaluated its performance on the external validation set, achieving an AUC of 0.839 (Figure 4). To evaluate the model’s calibration ability, calibration curves were plotted across the training, internal validation, and external validation sets (Figure 5A-C), which demonstrated strong agreement between the predicted and actual outcomes.
To assess the clinical utility of the model, DCA was performed separately for each dataset (Figure 5D-F). The DCA curves indicated that the combined model provides a favorable net benefit across a range of threshold probabilities in all cohorts.
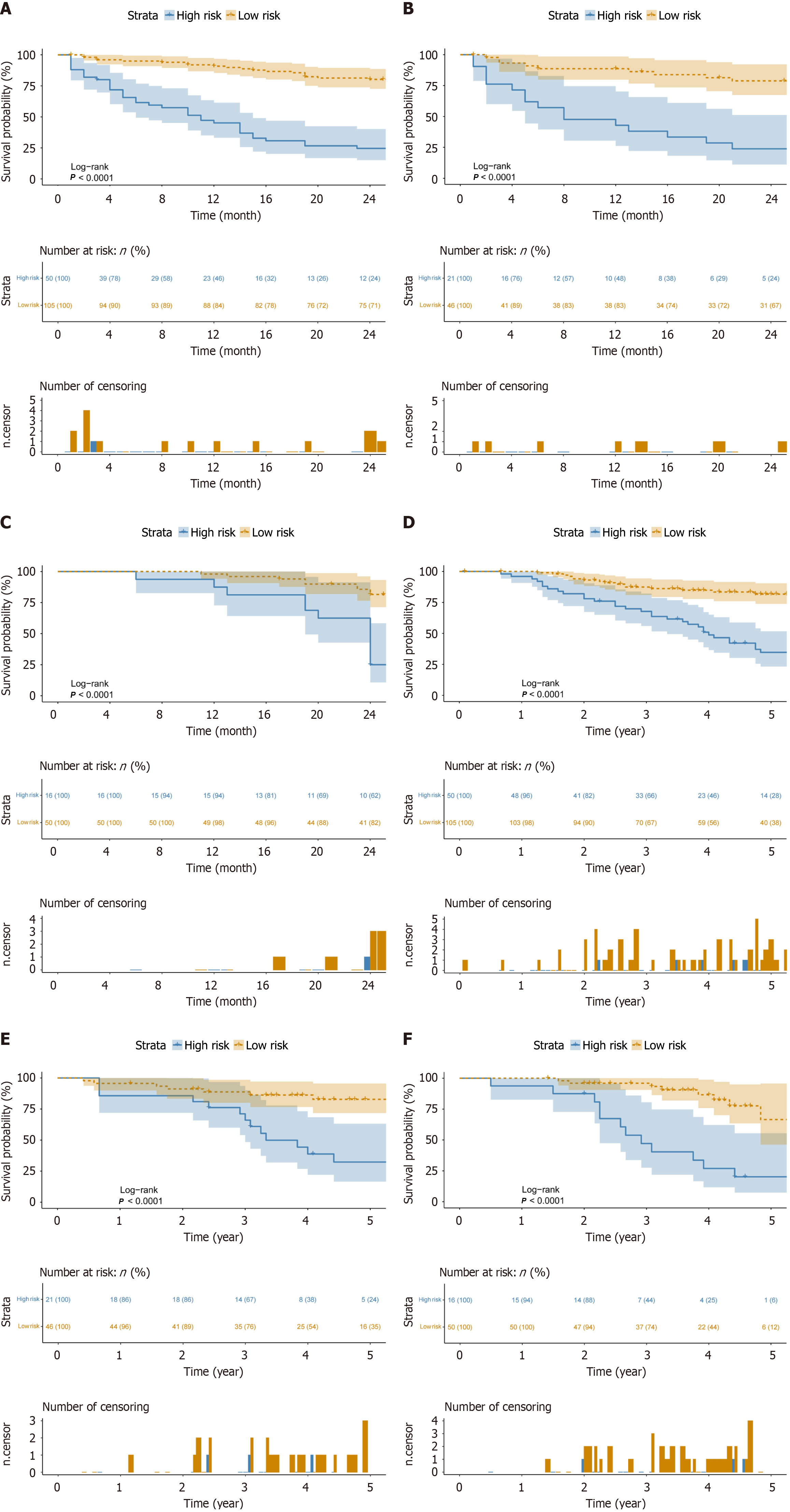
Furthermore, patients were stratified into high-risk and low-risk groups using the predicted recurrence probabilities from the combined model. K–M survival analysis revealed significant differences in 2-year progression-free survival (PFS) (Figure 6A-C) and 5-year overall survival (OS) (Figure 6D-F) between risk groups in all three cohorts (P < 0.001), highlighting the model’s effectiveness in risk stratification and its potential clinical value.
This study developed a preoperative prediction model for ER after curative ablation of HCC by combining radiomics with deep learning. The main findings of this study are as follows: (1) Models that combined intertumoral and peritumoral features outperformed models that used either feature alone; and (2) The combined model, which integrated tumor, 5 mm, and 10 mm peritumoral features, achieved the best predictive performance. Calibration and DCA curves confirmed the strong calibration and clinical utility of the model. K-M curves indicated that the combined model could provide accurate risk stratification for 2-year PFS and 5-year OS in patients with HCC.
Compared with previously published ER prediction models for HCC, which have typically exhibited validation AUCs between 0.70 and 0.80, our model demonstrated superior performance. For example, Yuan et al[22] developed a CECT-based radiomic nomogram with a validation C-index of 0.755 without incorporating deep learning. Wang et al[23] reported AUCs of 0.777 and 0.787 using an MRI-based model combining radiomics, deep learning, and clinical data but without peritumoral analysis. In contrast, our CECT-based model integrated radiomic and deep learning features from both intratumoral and peritumoral regions (5 mm and 10 mm), achieving AUCs of 0.899 and 0.839 in the internal and external validation sets, respectively.
Deep learning methods, including ResNet, visual geometry group (VGG), and DenseNet, are widely used in medical image analysis. While VGG is known for its simplicity and high accuracy, its deep layers and large number of parameters limit its practical flexibility. DenseNet, a network architecture that enhances feature reuse through dense connectivity, imposes higher computational costs because of its complex design. In contrast, ResNet-18 offers a lightweight design and efficient feature extraction, significantly reducing computational complexity and resource consumption while maintaining high predictive performance, making it particularly suitable for clinical medical imaging. For example, Tian et al[24] demonstrated that ResNet-18 outperformed ResNet-34 and DenseNet121 in predicting lymph node metastasis in lung adenocarcinoma, with an AUC of 0.754. Wu et al[25] also developed and validated a combined model based on ResNet-18 for predicting HCC recurrence and differentiation, which showed strong predictive ability and potential for noninvasive acquisition of pathological information. These advantages form a solid foundation for integrating deep learning with radiomics in our study.
While previous radiomic studies have focused mainly on tumor regions, interest in peritumoral regions has increased[26,27]. Shan et al[18] developed a model using peritumoral radiomic features based on CT images to predict ER after curative resection or ablation of HCC and reported that the accuracy of the peritumoral radiomic model improved by 0.22 compared with that of the intertumoral model (P < 0.01). Kang et al[28] studied preoperative CECT images of 160 HCC patients and extracted radiomic features from peritumoral regions of 1–5 mm to predict ER after surgical resection. They identified the 3 mm peritumoral model as the best, with an AUC of 0.807 in the validation set. However, their study focused predominantly on patients undergoing liver resection, with over 60% of tumors exceeding 5 cm, which may involve recurrence mechanisms distinct from those in our study. In contrast, our research targeted early-stage HCC patients undergoing curative ablation, where recurrence is more strongly influenced by the peritumoral microenvironment. Thus, we prioritized the 5 mm and 10 mm regions to capture microenvironmental heterogeneity and enhance the model’s robustness and clinical applicability. Park et al[29] extracted radiomic features from 3-phase CECT scans to predict clinical outcomes in HCC patients undergoing liver-directed radiotherapy. Their study revealed that the peritumoral 10 mm radiomics model demonstrated better predictive performance, which is consistent with our findings and suggests that the peritumoral 10 mm region might be one of the optimal choices. This finding aligns with the pathological extent of MVI, an independent risk factor for postoperative recurrence of HCC.
This study also revealed that models combining intertumoral and peritumoral features demonstrated improved predictive performance. Several studies also support that integrating intertumoral and peritumoral features can increase the predictive accuracy. For example, Xu et al[30] integrated tumor and peritumoral radiomic features to predict recurrence in intrahepatic cholangiocarcinoma and reported that the best models for predicting both early and late recurrence included features from both tumor and peritumoral regions. Li et al[31] included 329 Barcelona Clinic Liver Cancer stage 0-B HCC patients and developed a radiomic model to predict early and late recurrence after liver resection. Their results indicated that including peritumoral features improved model discrimination accuracy, with the combined model outperforming the tumor-only model (C-index: 0.727 vs 0.690)[31]. Liu et al[32] extracted radiomic features from MRI to predict pathological differentiation in HCC patients. They reported that the peritumoral 10 mm model outperformed the 5 mm and 20 mm models, with AUCs of 0.80, 0.77, and 0.68, respectively. The combined model of the tumor and peritumoral 10 mm regions significantly improved the predictive performance, with an AUC of 0.86 in the validation set. Despite differences in HCC types, treatment methods, and imaging modalities, all three studies suggest that integrating tumor and peritumoral features enhances predictive model performance. However, these models had lower predictive performance than our model did, likely because we used radiomics combined with deep learning for more comprehensive feature extraction and included both 5 mm and 10 mm peritumoral regions, providing richer predictive information.
The reason why peritumoral features can enhance model performance remains unclear. In 1889, Paget[33] proposed the "seed and soil" hypothesis, where "soil" refers to the microenvironment that allows tumor cells to transfer and establish themselves in the body. Research on the tumor microenvironment (TME) typically focuses on the tumor itself and its relationship with tumor progression. The protein composition and function of the PME differ significantly from those of the TME, showing minimal overlap; this highlights a strong association between the PME and the biological behavior of HCC[14]. Therefore, preoperative assessment of PME could provide insights into HCC occurrence, recurrence or metastasis. Radiomics, as a noninvasive method, has the potential to decode the PME. Research by Prior et al[34] demonstrated that 3D radiomic features derived from CT images, analyzed via unsupervised learning algorithms, can reliably model the TME and assess cancer heterogeneity. It is widely recognized that tumors with greater structural heterogeneity tend to be more aggressive. Thus, leveraging radiomics to extract key peritumoral features and quantify the TME has become an important and promising area of research. This study revealed that wavelet features in the peritumoral area were most abundant across all filtering types, similar to the findings reported by Zhou et al[35]. These wavelet features serve as quantitative indicators of intratumor heterogeneity closely related to morphology, pathophysiology, and prognosis[36,37]. Additionally, this study revealed that NGTDM_Coarseness was prevalent in 33.33% of the cases, indicating textural roughness in the images. Lower coarseness values are associated with more pronounced texture variations within tumors, reflecting increased heterogeneity and suggesting a greater risk of recurrence[38]. We believe that extracting features such as wavelets and NGTDM_Coarseness from the peritumoral area via radiomics can indicate changes in the TME and provide valuable insights for predicting the ER of HCC. Additionally, these features could be integrated with genomic, proteomic, and other multiomic approaches to increase prediction accuracy.
This study introduces an innovative intratumoral and peritumoral CECT-based model that integrates deep learning and radiomics to predict ER following curative ablation of HCC. However, this study has certain limitations. First, as a retrospective multicenter study, it may introduce selection bias. Second, the extended study period across the three Centers could have resulted in imaging-related variability. Third, the sample size was relatively small because of the limited number of early-stage HCC patients who underwent ablation. Differences in cohort sizes may introduce variability in AUC estimation, which should be considered when interpreting model performance. Finally, manual delineation of the VOI by radiologists introduces subjectivity, increases workload, and may lead to potential errors.
Our model may face challenges due to variations in imaging protocols and patient populations across different institutions, making the standardization of feature extraction and model recalibration crucial. Future research should focus on validating the model in larger, multicenter cohorts and exploring the underlying biological mechanisms of recurrence by integrating genomic, transcriptomic, and proteomic data. Additionally, implementing automated or semiautomated segmentation methods could increase efficiency and reduce interobserver variability in clinical practice.
In conclusion, the integration of intertumoral and peritumoral features significantly improved the predictive performance of the model. The optimal model, which incorporated intertumoral, peritumoral 5 mm, and 10 mm features, demonstrated superior efficacy in accurately stratifying patients with ER by recurrence risk. Furthermore, this model supports the development of individualized treatment plans and postoperative follow-up strategies. As a noninvasive predictive tool, it holds considerable promise for clinical application.
The authors would like to thank Professor Jing-Wei Wei from the Institute of Automation, Chinese Academy of Sciences, for his support in writing this article. The authors also extend their gratitude to Professor Song Wu from Anhui University of Chinese Medicine for his guidance on statistical methodology in this manuscript.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 8187] [Article Influence: 8187.0] [Reference Citation Analysis (2)] |
| 2. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6064] [Article Influence: 866.3] [Reference Citation Analysis (3)] |
| 3. | Doyle A, Gorgen A, Muaddi H, Aravinthan AD, Issachar A, Mironov O, Zhang W, Kachura J, Beecroft R, Cleary SP, Ghanekar A, Greig PD, McGilvray ID, Selzner M, Cattral MS, Grant DR, Lilly LB, Selzner N, Renner EL, Sherman M, Sapisochin G. Outcomes of radiofrequency ablation as first-line therapy for hepatocellular carcinoma less than 3 cm in potentially transplantable patients. J Hepatol. 2019;70:866-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 4. | Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye QH, Wang L, Zhou J, Qiu SJ, Li Y, Ji XN, Liu H, Xia JL, Wu ZQ, Fan J, Ma ZC, Zhou XD, Lin ZY, Liu KD. A decade's studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:187-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 352] [Cited by in RCA: 362] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 5. | Yang Y, Nagano H, Ota H, Morimoto O, Nakamura M, Wada H, Noda T, Damdinsuren B, Marubashi S, Miyamoto A, Takeda Y, Dono K, Umeshita K, Nakamori S, Wakasa K, Sakon M, Monden M. Patterns and clinicopathologic features of extrahepatic recurrence of hepatocellular carcinoma after curative resection. Surgery. 2007;141:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 6. | Xing H, Zhang WG, Cescon M, Liang L, Li C, Wang MD, Wu H, Lau WY, Zhou YH, Gu WM, Wang H, Chen TH, Zeng YY, Schwartz M, Pawlik TM, Serenari M, Shen F, Wu MC, Yang T. Defining and predicting early recurrence after liver resection of hepatocellular carcinoma: a multi-institutional study. HPB (Oxford). 2020;22:677-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 7. | Bera K, Braman N, Gupta A, Velcheti V, Madabhushi A. Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nat Rev Clin Oncol. 2022;19:132-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 501] [Cited by in RCA: 421] [Article Influence: 140.3] [Reference Citation Analysis (1)] |
| 8. | Pinker K, Chin J, Melsaether AN, Morris EA, Moy L. Precision Medicine and Radiogenomics in Breast Cancer: New Approaches toward Diagnosis and Treatment. Radiology. 2018;287:732-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 198] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 9. | Qian GX, Xu ZL, Li YH, Lu JL, Bu XY, Wei MT, Jia WD. Computed tomography-based radiomics to predict early recurrence of hepatocellular carcinoma post-hepatectomy in patients background on cirrhosis. World J Gastroenterol. 2024;30:2128-2142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (2)] |
| 10. | Li Z, Wang Y, Yu J, Guo Y, Cao W. Deep Learning based Radiomics (DLR) and its usage in noninvasive IDH1 prediction for low grade glioma. Sci Rep. 2017;7:5467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 194] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 11. | Hu X, Zhou J, Li Y, Wang Y, Guo J, Sack I, Chen W, Yan F, Li R, Wang C. Added Value of Viscoelasticity for MRI-Based Prediction of Ki-67 Expression of Hepatocellular Carcinoma Using a Deep Learning Combined Radiomics (DLCR) Model. Cancers (Basel). 2022;14:2575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Zhang J, Zhao Y, Lu Y, Li P, Dang S, Li X, Yin B, Zhao L. Meningioma consistency assessment based on the fusion of deep learning features and radiomics features. Eur J Radiol. 2024;170:111250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 13. | Silva M, Vivancos C, Duffau H. The Concept of «Peritumoral Zone» in Diffuse Low-Grade Gliomas: Oncological and Functional Implications for a Connectome-Guided Therapeutic Attitude. Brain Sci. 2022;12:504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Gu Y, Guo Y, Gao N, Fang Y, Xu C, Hu G, Guo M, Ma Y, Zhang Y, Zhou J, Luo Y, Zhang H, Wen Q, Qiao H. The proteomic characterization of the peritumor microenvironment in human hepatocellular carcinoma. Oncogene. 2022;41:2480-2491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Algohary A, Shiradkar R, Pahwa S, Purysko A, Verma S, Moses D, Shnier R, Haynes AM, Delprado W, Thompson J, Tirumani S, Mahran A, Rastinehad AR, Ponsky L, Stricker PD, Madabhushi A. Combination of Peri-Tumoral and Intra-Tumoral Radiomic Features on Bi-Parametric MRI Accurately Stratifies Prostate Cancer Risk: A Multi-Site Study. Cancers (Basel). 2020;12:2200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 16. | Hu Y, Xie C, Yang H, Ho JWK, Wen J, Han L, Chiu KWH, Fu J, Vardhanabhuti V. Assessment of Intratumoral and Peritumoral Computed Tomography Radiomics for Predicting Pathological Complete Response to Neoadjuvant Chemoradiation in Patients With Esophageal Squamous Cell Carcinoma. JAMA Netw Open. 2020;3:e2015927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 17. | Duan Y, Chen X, Li W, Li S, Zhang C. Multimodal radiomics and nomogram-based prediction of axillary lymph node metastasis in breast cancer: An analysis considering optimal peritumoral region. J Clin Ultrasound. 2023;51:1231-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Shan QY, Hu HT, Feng ST, Peng ZP, Chen SL, Zhou Q, Li X, Xie XY, Lu MD, Wang W, Kuang M. CT-based peritumoral radiomics signatures to predict early recurrence in hepatocellular carcinoma after curative tumor resection or ablation. Cancer Imaging. 2019;19:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (1)] |
| 19. | Wang F, Cheng M, Du B, Li LM, Huang WP, Gao JB. Use of radiomics containing an effective peritumoral area to predict early recurrence of solitary hepatocellular carcinoma ≤5 cm in diameter. Front Oncol. 2022;12:1032115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 20. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3031] [Article Influence: 433.0] [Reference Citation Analysis (3)] |
| 21. | Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. Berlin: Springer, 2001; 608. |
| 22. | Yuan C, Wang Z, Gu D, Tian J, Zhao P, Wei J, Yang X, Hao X, Dong D, He N, Sun Y, Gao W, Feng J. Prediction early recurrence of hepatocellular carcinoma eligible for curative ablation using a Radiomics nomogram. Cancer Imaging. 2019;19:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 23. | Wang Y, Zhang Y, Xiao J, Geng X, Han L, Luo J. Multicenter Integration of MR Radiomics, Deep Learning, and Clinical Indicators for Predicting Hepatocellular Carcinoma Recurrence After Thermal Ablation. J Hepatocell Carcinoma. 2024;11:1861-1874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 24. | Tian W, Yan Q, Huang X, Feng R, Shan F, Geng D, Zhang Z. Predicting occult lymph node metastasis in solid-predominantly invasive lung adenocarcinoma across multiple centers using radiomics-deep learning fusion model. Cancer Imaging. 2024;24:8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 25. | Wu JP, Ding WZ, Wang YL, Liu S, Zhang XQ, Yang Q, Cai WJ, Yu XL, Liu FY, Kong D, Zhong H, Yu J, Liang P. Radiomics analysis of ultrasound to predict recurrence of hepatocellular carcinoma after microwave ablation. Int J Hyperthermia. 2022;39:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 26. | Yusa T, Yamashita YI, Okabe H, Nakao Y, Itoyama R, Kitano Y, Kaida T, Miyata T, Mima K, Imai K, Hayashi H, Baba H. Survival impact of immune cells infiltrating peritumoral area of hepatocellular carcinoma. Cancer Sci. 2022;113:4048-4058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Qian H, Huang Y, Xu L, Fu H, Lu B. Role of peritumoral tissue analysis in predicting characteristics of hepatocellular carcinoma using ultrasound-based radiomics. Sci Rep. 2024;14:11538. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Kang W, Cao X, Luo J. Effect of multiple peritumoral regions of interest ranges based on computed tomography radiomics for the prediction of early recurrence of hepatocellular carcinoma after resection. Quant Imaging Med Surg. 2023;13:6668-6682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 29. | Park JW, Lee H, Hong H, Seong J. Efficacy of Radiomics in Predicting Oncologic Outcome of Liver-Directed Combined Radiotherapy in Locally Advanced Hepatocellular Carcinoma. Cancers (Basel). 2023;15:5405. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Xu L, Wan Y, Luo C, Yang J, Yang P, Chen F, Wang J, Niu T. Integrating intratumoral and peritumoral features to predict tumor recurrence in intrahepatic cholangiocarcinoma. Phys Med Biol. 2021;66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Li N, Wan X, Zhang H, Zhang Z, Guo Y, Hong D. Tumor and peritumor radiomics analysis based on contrast-enhanced CT for predicting early and late recurrence of hepatocellular carcinoma after liver resection. BMC Cancer. 2022;22:664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 32. | Liu HF, Wang M, Wang Q, Lu Y, Lu YJ, Sheng Y, Xing F, Zhang JL, Yu SN, Xing W. Multiparametric MRI-based intratumoral and peritumoral radiomics for predicting the pathological differentiation of hepatocellular carcinoma. Insights Imaging. 2024;15:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 33. | Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98-101. [PubMed] |
| 34. | Prior O, Macarro C, Navarro V, Monreal C, Ligero M, Garcia-Ruiz A, Serna G, Simonetti S, Braña I, Vieito M, Escobar M, Capdevila J, Byrne AT, Dienstmann R, Toledo R, Nuciforo P, Garralda E, Grussu F, Bernatowicz K, Perez-Lopez R. Identification of Precise 3D CT Radiomics for Habitat Computation by Machine Learning in Cancer. Radiol Artif Intell. 2024;6:e230118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (5)] |
| 35. | Zhou Y, Zhou G, Zhang J, Xu C, Wang X, Xu P. Radiomics signature on dynamic contrast-enhanced MR images: a potential imaging biomarker for prediction of microvascular invasion in mass-forming intrahepatic cholangiocarcinoma. Eur Radiol. 2021;31:6846-6855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 36. | Chen S, Zhu Y, Liu Z, Liang C. Texture analysis of baseline multiphasic hepatic computed tomography images for the prognosis of single hepatocellular carcinoma after hepatectomy: A retrospective pilot study. Eur J Radiol. 2017;90:198-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 37. | Liang W, Xu L, Yang P, Zhang L, Wan D, Huang Q, Niu T, Chen F. Novel Nomogram for Preoperative Prediction of Early Recurrence in Intrahepatic Cholangiocarcinoma. Front Oncol. 2018;8:360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 38. | Li W, Shen H, Han L, Liu J, Xiao B, Li X, Ye Z. A Multiparametric Fusion Radiomics Signature Based on Contrast-Enhanced MRI for Predicting Early Recurrence of Hepatocellular Carcinoma. J Oncol. 2022;2022:3704987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |