Published online May 15, 2025. doi: 10.4251/wjgo.v17.i5.105604
Revised: March 11, 2025
Accepted: April 18, 2025
Published online: May 15, 2025
Processing time: 106 Days and 6.7 Hours
Differential diagnosis among atypical hyperplasia (AH) (including reparative hyperplasia and intestinal metaplasia), low-grade dysplasia (LGD), high-grade dysplasia (HGD), and adenocarcinoma (AC) in gastric mucosal biopsies is cha
To evaluate the diagnostic utility of P53, Ki67, P504S, and IMP3 in gastric cancer and its precancerous lesions, focusing on their effectiveness in distinguishing AH, LGD, HGD, and AC.
From January 2018 to September 2020, a total of 185 gastric mucosal biopsy spe
The expression rate of P504S was highest in the LGD group (53.3%, 16/30), while IMP3 expression was highest in the AC group (41.9%, 26/62), followed by the HGD group (33.3%). Significant differences in P504S and IMP3 expression levels were observed among the four lesion groups (P < 0.001). Pairwise comparisons revealed statistically significant differences in P504S expression between the AH group and the LGD, HGD, and AC groups (P < 0.001), as well as significant variations in IMP3 expression between the AH group and the HGD and AC groups, and between the LGD group and the HGD and AC groups (P < 0.001). Additionally, significant correlations were found between P504S and the polarity expression pattern of Ki67, and between IMP3 and the mutation expression pattern of P53 (P < 0.001). The combined detection of P504S with Ki67 and IMP3 with P53 increased the diagnostic sensitivity for LGD and HGD/AC, respectively.
P504S is highly expressed in LGD and is associated with the Ki67 “polarity” expression pattern. IMP3 is highly expressed in HGD/AC and is correlated with the P53 mutation expression pattern. The combined detection of P504S with Ki67 and IMP3 with P53 increased the diagnostic sensitivity for LGD and HGD/AC, respectively. The rational use of P504S, Ki67, IMP3, and p53 can help distinguish gastric cancer and precancerous lesions, improving the early cancer diagnosis rate.
Core Tip: The combined detection of P504S and Ki67 for low-grade dysplasia (LGD), and IMP3 and P53 for high-grade dysplasia (HGD) and adenocarcinoma (AC), enhances diagnostic sensitivity, aiding in the differentiation of gastric cancer and its precancerous lesions, thereby improving early cancer diagnosis. The immunohistochemical markers P504S, Ki67, IMP3, and P53 are valuable in distinguishing gastric lesions, including atypical hyperplasia, LGD, HGD, and AC. P504S is predominantly expressed in LGD and shows a correlation with the Ki67 “polarity” expression pattern, while IMP3 is highly expressed in HGD/AC and correlates with the P53 mutation pattern. The combined detection of P504S with Ki67 and IMP3 with P53 significantly enhances the diagnostic sensitivity for LGD and HGD/AC, respectively, aiding in the early detection of gastric cancer and its precancerous lesions. This approach can improve the accuracy of pathological diagnoses, thereby facilitating timely intervention.
- Citation: Miao LF, Sun YY, Du XJ, Xu N, Shen JW, Hua H, Guo M, Yang HJ, Li JK, Zhu L. Combined detection of P53, Ki67, P504S, and IMP3: Diagnostic implications for gastric cancer and precursor lesions. World J Gastrointest Oncol 2025; 17(5): 105604
- URL: https://www.wjgnet.com/1948-5204/full/v17/i5/105604.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i5.105604
The World Health Organization (WHO) Classification of Tumors of the Digestive System, 5th edition (2019), categorizes gastric mucosal epithelial lesions into five classifications: No dysplasia, indeterminate dysplasia, low-grade dysplasia (LGD), high-grade dysplasia (HGD), and intramucosal carcinoma[1]. While the WHO classification offers validated crite
P504S is expressed at a higher rate in LGD[4], while IMP3 shows higher expression in HGD and AC[5]. Our previous research has demonstrated that the distinct expression patterns of P53 and Ki67 are critically significant in differentiating various lesions in gastric mucosal biopsies. With the progression of lesions, the mutation rate of P53 increases, while the polarity and diffuse expression patterns of Ki67 suggest LGD and HGD, respectively[6]. However, there is a paucity of literature regarding the concurrent detection of P504S and IMP3 in gastric mucosal biopsies, and the correlation between these two biomarkers and Ki67 and P53 has not been documented.
This study investigated the relationship between the expression of P504S and IMP3 and the expression patterns of P53 and Ki67, thereby providing robust evidence for the combined use of these markers in the diagnosis of gastric mucosal biopsies, aiming to establish a more objective diagnostic framework to enhance the detection rates of early gastric cancer and precancerous lesions, ultimately contributing to improved five-year survival rates for patients with gastric tumors.
From January 2018 to September 2020, a total of 185 gastroscopic biopsy specimens were examined in the Department of Pathology at Anyang Tumor Hospital. The diagnoses were established in accordance with the WHO Classification of Tumors of the Digestive System, 5th edition (2019)[1]. Two deputy chief physicians experienced in digestive system patho
All specimens were fixed in 10% neutral buffered formalin, followed by standard histopathological processing that included dehydration, paraffin embedding, and sectioning at a thickness of 3 μm. The slices were then stained with he
P504S and IMP3 showed positive expression in the cytoplasm, whereas P53 and Ki67 showed positive expression in the nucleus. P504S expression was classified as positive when ≥ 5% of the tumor cells were positive[4], while IMP3 expression was considered positive when ≥ 10% of the tumor cells showed positivity[7]. The interpretation criteria for P53 and Ki67 were established based on our prior research findings[6].
Statistical analyses were conducted using SPSS23.0 and R4.3.3. Counting data are presented as n (%). Intergroup comparisons were performed using the χ2 test or Fisher's exact probability test, with the level of significance set at α = 0.05. Pairwise comparisons between groups were performed using the χ2 test (adjusted α' = α/comparisons). Sensitivity, specificity, and area under the receiver operating characteristic (ROC) curve (AUC) were used to assess the diagnostic value of P53, Ki67, P504S, and IMP3. Sensitivity and specificity were calculated as follows: Sensitivity = (true positives)/(true positives + false negatives); specificity = (true negatives)/(true negatives + false positives).
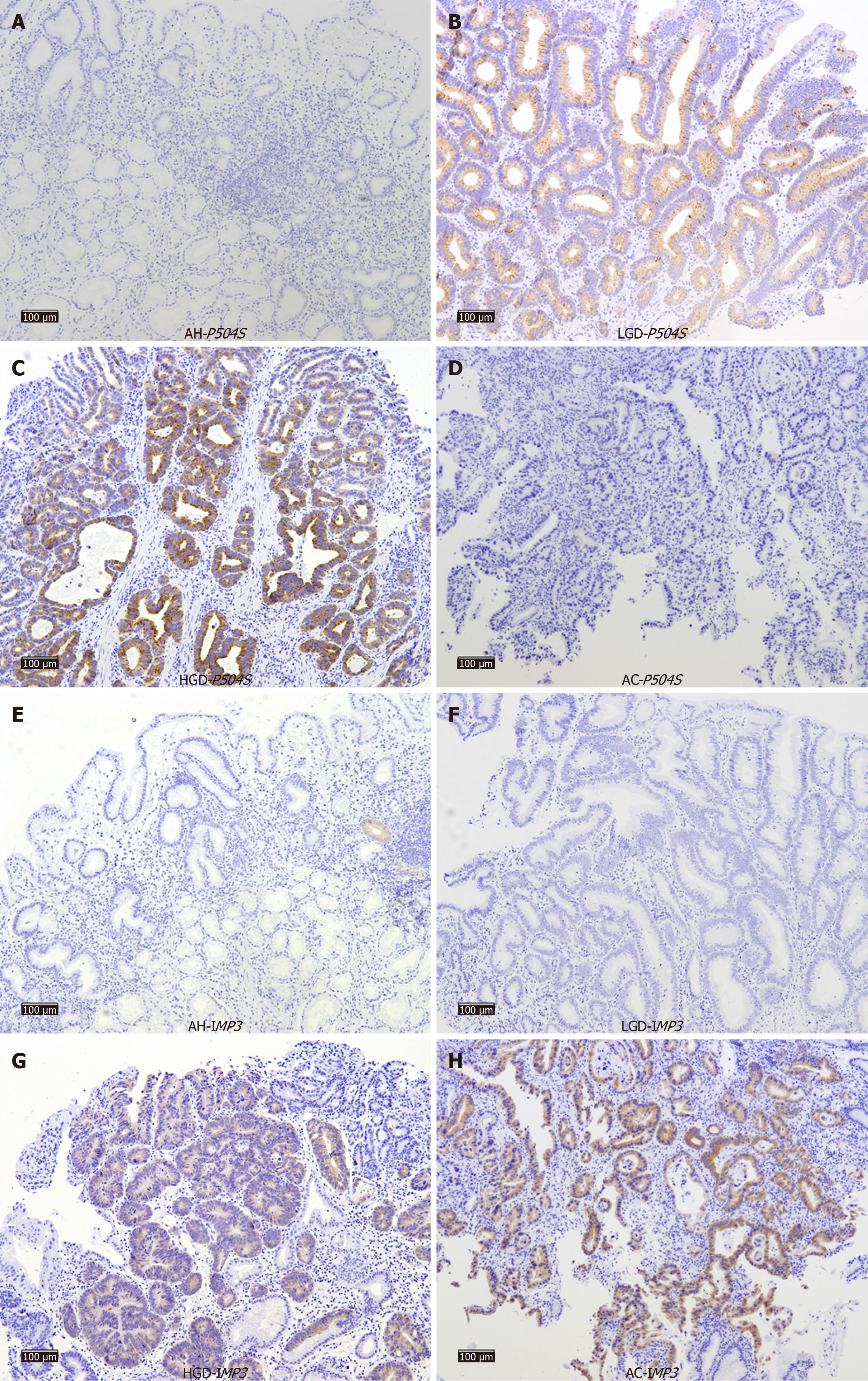
P504S was not detected in the AH group (Figure 1A), while its expression rate was highest in the LGD group with a positive rate of 53.3% (16/30) (Figure 1B). The expression rates in the HGD and AC groups were comparable (35.6% and 35.5%, respectively), yet slightly lower than that observed in the LGD group (Figure 1C and D). The differences in expression rates between the groups were statistically significant (P < 0.001; Table 1).
| AH | LGD | HGD | AC | P value | |
| P504S | < 0.001 | ||||
| - | 48 (100) | 14 (46.7) | 29 (64.4) | 40 (64.5) | |
| + | 0 | 16 (53.3) | 16 (35.6) | 22 (35.5) | |
| IMP3 | < 0.001 | ||||
| - | 47 (97.9) | 29 (96.7) | 30 (66.7) | 36 (58.1) | |
| + | 1 (2.1) | 1 (3.3) | 15 (33.3) | 26 (41.9) |
The highest positive expression rate for IMP3 was observed in the AC group at 41.9% (26/62), whereas the expression rates in the AH and LGD groups were significantly lower at 2.1% and 3.3%, respectively (Figure 1E-H). The differences in IMP3 expression rate between the four groups were also statistically significant (P < 0.001).
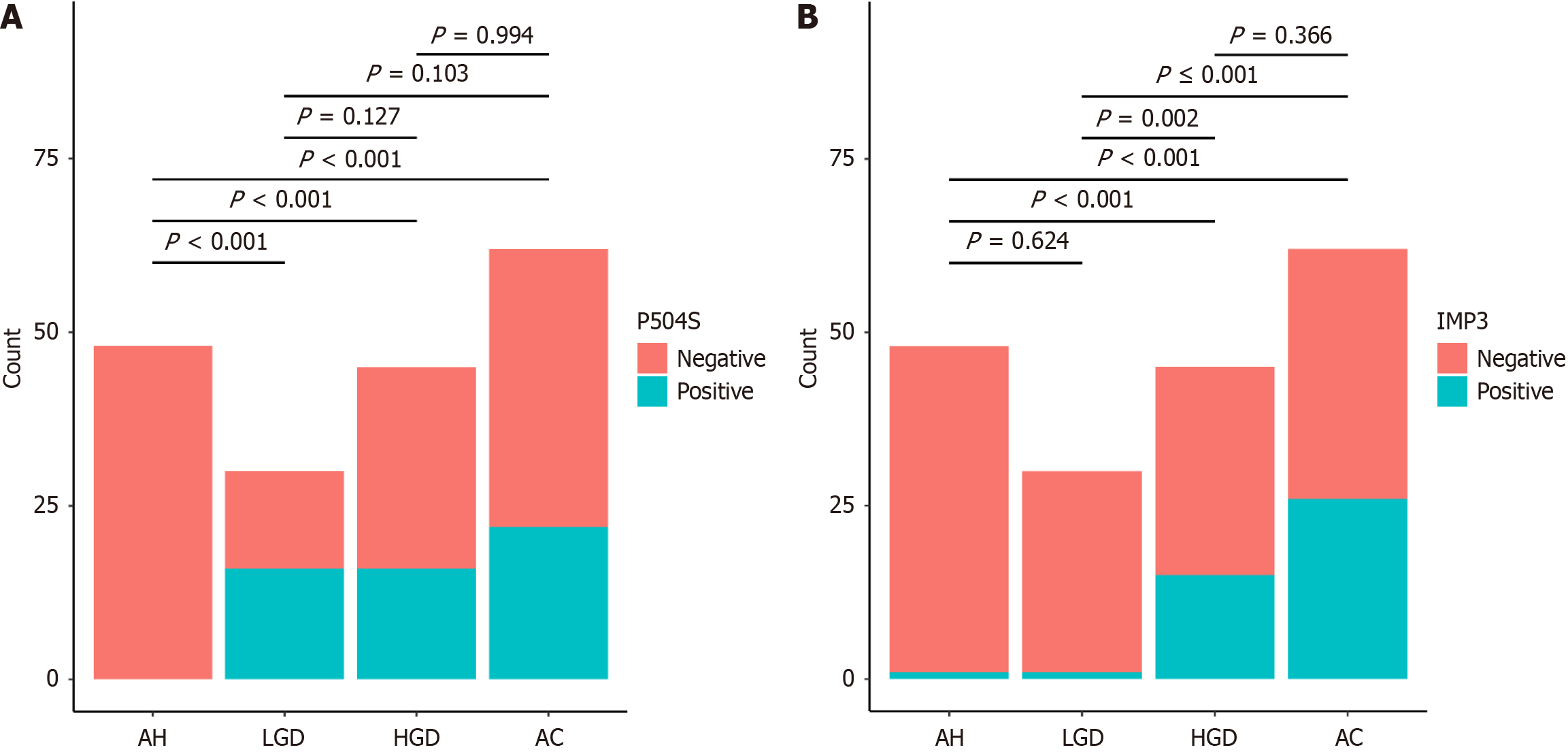
The expression status of P504S and IMP3 was compared across the four groups. Notably, significant differences in P504S expression were observed between the AH group and the LGD, HGD, and AC groups (P < 0.001). However, no significant differences were observed between the remaining groups (P > 0.05; Figure 2).
The expression rate of IMP3 was highest in the AC group, but very similar to that in the HGD group, with no statistically significant difference between the two groups (P > 0.05). Conversely, the expression rates of IMP3 in the AH and LGD groups were lower and were not significantly different (P > 0.05). Significant differences were observed between the AH group and the HGD and AC groups, as well as between the LGD group and the HGD and AC groups (P < 0.001).
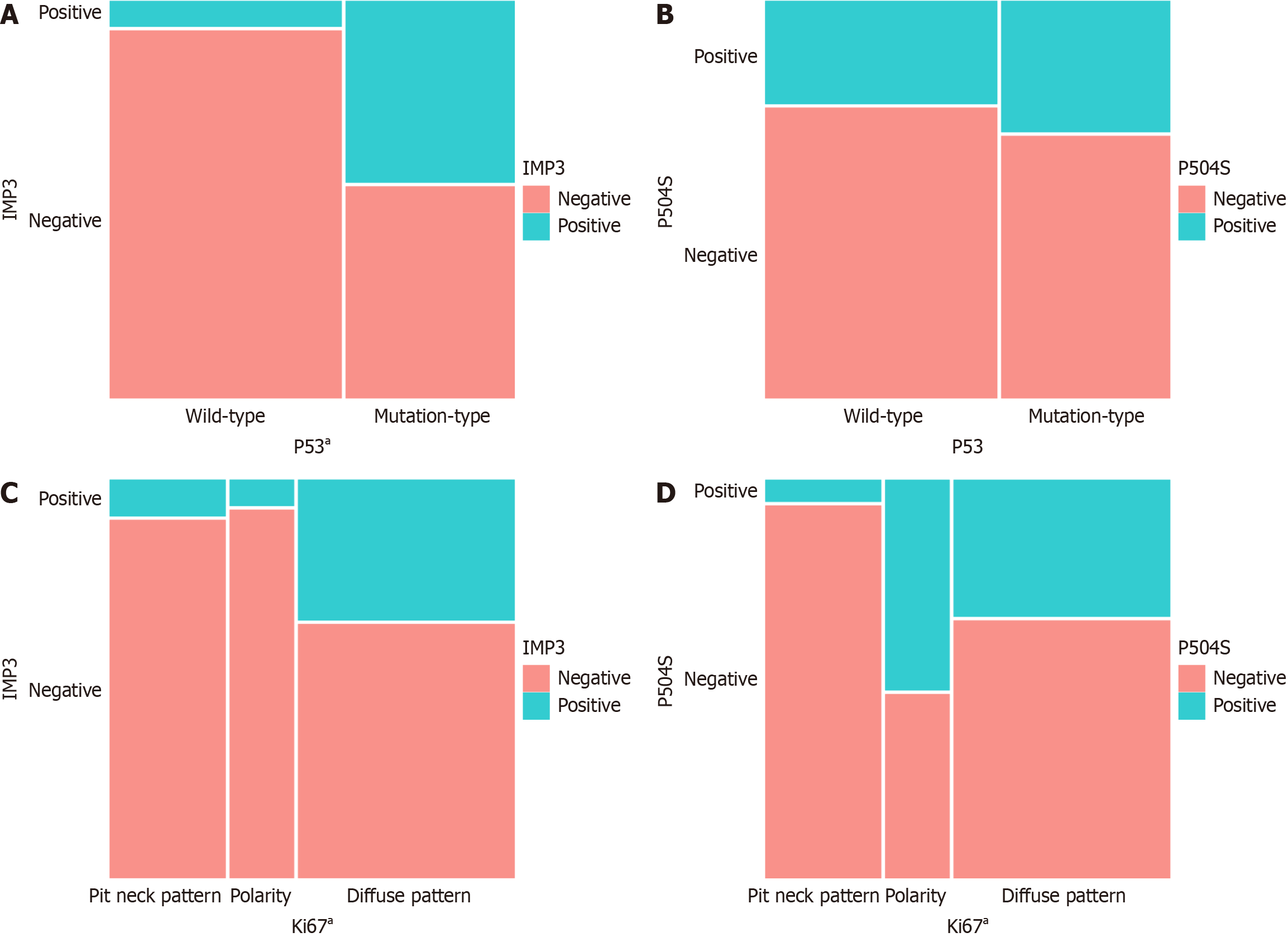
Comparative analysis of the expression status of IMP3 and P504S relative to the expression patterns of P53 and Ki67 revealed that the P53 mutation rate was significantly elevated in patients with positive IMP3 expression (P < 0.001). Additionally, the "polarity" expression pattern of Ki67 was predominantly higher in patients exhibiting positive P504S expression (P < 0.001; Figure 3).
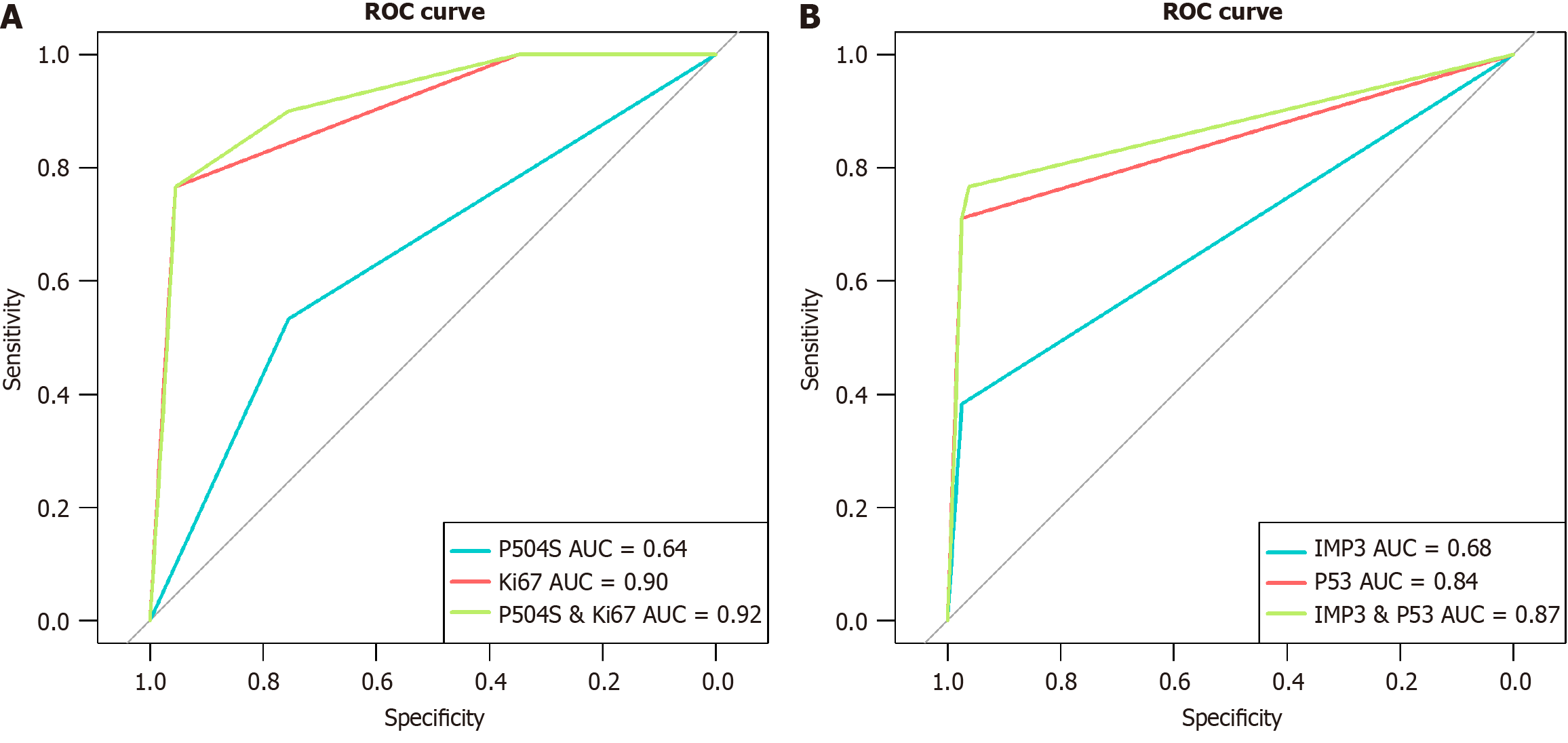
The sensitivity of P504S and Ki67 in diagnosing LGD was 53.33% and 76.67%, respectively, while the specificity was 75.48% and 95.48%, respectively. The combination of P504S and Ki67 demonstrated a sensitivity of 90% and specificity of 73.55% in the diagnosis of LGD (Table 2). The AUC of P504S, Ki67, and their combination in predicting LGD was 0.64, 0.90 and 0.92, respectively (Figure 4A). For HGD/AC, the sensitivity of IMP3 and P53 was 38.32% and 71.03%, respectively, both exhibiting a specificity of 97.44%. The combined evaluation of IMP3 and P53 resulted in a sensitivity of 76.64% and specificity of 96.15% for diagnosing HGD/AC (Table 3). The AUC of IMP3, P53, and their combination in predicting HGD/AC was 0.68, 0.84 and 0.87, respectively (Figure 4B).
The objective of this study was to identify more reliable IHC markers to enhance the diagnosis of gastric mucosal biopsy specimens. A panel of IHC assays was employed to evaluate gastric mucosal lesions, including AH, LGD, HGD, and AC. The expression of P504S is associated with the polar expression pattern of Ki67, while IMP3 is linked to the mutation expression pattern of P53. Furthermore, the combined detection of P504S and Ki67 enhances the diagnostic sensitivity for LGD, while the concurrent detection of IMP3 and P53 improves the diagnostic sensitivity for HGD/AC.
Previous investigations demonstrated the absence of P53 mutation in AH cases, with mutation expression rates of 6.7% in LGD and substantially elevated rates of 66.7% and 74.2% in HGD and AC, respectively[6]. These findings suggest an increasing mutation frequency in P53 that correlates with lesion progression. Mutant P53 can disrupt intracellular growth factor signaling pathways, thereby promoting cellular proliferation, inhibiting apoptosis, and ultimately driving cellular transformation and tumorigenesis[8]. Mutations in P53 have been shown to be associated with an increased risk of malignant transformation[9,10]. Multiple studies using exome sequencing have shown that LGD has APC mutations, while HGD and AC have mainly TP53 mutations[11,12].
The expression of IMP3 has been frequently observed in esophageal AC, as well as in bladder, lung, gastric, and pancreatic cancers. Its expression is often correlated with the invasive properties of tumors, thereby classifying it as a prognostic biomarker for various malignancies. Research suggests that IMP3 expression correlates with advanced gastric cancer stages, with multivariate analysis showing that IMP3 is a predictor of independent survival[13]. Notably, IMP3 was found to be negative in 96% of non-neoplastic gastric mucosa and LGD samples, while positive expression rates rose significantly in HGD (83%) and AC (65%)[5]. Consistent with our research findings, the expression of IMP3 is instrumental in facilitating the pathological diagnosis of HGD and AC. Furthermore, IMP3 expression plays a critical role in differentiating AH from HGD, as well as LGD from AC, and is correlated with the mutational expression pattern of P53.
P504S has been recognized as an IHC marker for prostate cancer. One potential mechanism by which P504S contributes to gastric cancer pathogenesis is through its function as an activator of PPAR-γ, a nuclear receptor primarily expressed in adipose tissue that plays a crucial role in initiating adipocyte differentiation. Consequently, P504S may facilitate gastric cancer cell proliferation via the activation of PPAR-γ signaling[14]. Recent studies suggest that its application extends beyond prostate cancer as it has been used in the diagnosis of breast cancer, urogenital system tumors, gastrointestinal tract cancers, and their precursor lesions[15]. Research[4] indicates that P504S is not expressed in the normal gastric mucosal epithelium, whereas sporadic granular staining may occur in intestinal metaplasia and gastric mucosal glands without reaching diagnostic positivity. The expression of P504S is upregulated significantly in neoplastic lesions and is observed in 100% of adenomas, compared to 54.5% in AC[4]. Other studies[16] have reported positive rates for P504S expression in gastric mucosal intestinal metaplasia, adenoma, and AC tissues of 7.7%, 79.3%, and 62.9%, respectively. Our study demonstrated that only focal mucosal glandular expression was observed in the AH group, while the LGD group exhibited the highest expression rate (53.3%). Positive expression rates in the HGD (35.6%) and AC (35.5%) groups were comparable and lower than those in the LGD group, and this finding aligns with those of previously reported trends. The pairwise comparisons among the four groups indicated a significant difference in P504S expression between the AH group and the LGD, HGD, and AC groups (P < 0.001). Hence, P504S may function as a reliable biomarker for distinguishing neoplastic from non-neoplastic gastric mucosa, with particular relevance in the diagnosis of LGD.
There is evidence that Ki67 serves as an established marker for cellular proliferation activity[17]. A traditional Chinese medicine recipe called Modified Chaishao Liujunzi Decoction improved bile acid-induced gastric intestinal metaplasia by reducing the expression of pro-inflammatory cytokines (pro-ICs) and Ki-67, thereby inhibiting the occurrence of precancerous lesions in the stomach. This suggests that Ki-67 plays a promoting role in the development of gastric cancer and precancerous lesions[18]. In the normal gastric mucosa, Ki67 is expressed predominantly in the neck region of gastric mucosal glands, constituting the proliferation zone of the epithelium. Ki67-positive cells appear sporadically throughout this region[19]. Ki67 expression is markedly upregulated in gastric mucosal reparative hyperplasia and intestinal meta
The results of this study demonstrate that the expression of IMP3 is significantly associated with the mutation pattern of P53, while the expression of P504S is significantly associated with the polar expression pattern of Ki67. IGF2BP3/IMP3 directly interacts with the deubiquitinase ubiquitin-specific peptidase 10 (USP10) and diminishes its role in stabilizing P53 protein. Knockdown of IGF2BP3 expression in lung cancer cells consistently prolongs the half life and elevates the protein levels of P53, resulting in G0/G1 phase cell cycle arrest. These results indicate that IGF2BP3 facilitates lung carcinogenesis by compromising P53 protein stability[22]. IMP3 inhibits the normal function of the P53 gene through USP10, providing a theoretical basis for our findings that IMP3 is associated with the P53 mutation pattern, and offering a better explanation for how the combined detection of IMP3 and P53 can enhance the diagnostic sensitivity for HGD/AC. In the cases exhibiting a Ki67 "polarity" pattern, the positive expression rate of P504S was significantly elevated, with statistically significant results. Moreover, the combined detection of P504S and Ki67 enhanced the diagnostic sensitivity for LGD. Consequently, we hypothesize that P504S is implicated in the development of LGD, and its association with the APC pathway warrants further investigation. The integrated assessment of P504S and Ki67 in differentiating LGD from AH and HGD/AC, along with the concurrent analysis of IMP3 and P53 in distinguishing HGD/AC from AH and LGD, while not enhancing diagnostic specificity, significantly improves diagnostic sensitivity, thereby minimizing the risk of false-negative diagnoses. When coupled with morphological characteristics, it facilitates a more accurate diagnosis. The improvement of specificity requires further research to identify better biological markers or more specific molecular diagnostics.
The collaborative analysis of P504S, Ki67, IMP3, and P53 expression by immunohistochemistry presents a robust framework for the accurate diagnosis of gastric cancer and its precursors. By addressing the morphological and IHC challenges inherent in diagnosing gastric lesions, this approach has the potential to significantly enhance diagnostic sensitivity, reduce missed diagnoses, and ultimately improve patient management strategies. This study has several limitations, including the use of a single-center sample, the need to establish a stable IHC operating system and stan
P504S positive expression and/or Ki67 exhibit a "polarity" expression pattern suggests the possibility of LGD, while positive expression of IMP3 and/or mutation pattern in P53 may indicate the potential for HGD/AC. The rational use of P504S, Ki67, IMP3, and P53 can help distinguish gastric cancer and precancerous lesions, improving the early cancer diagnosis rate.
We sincerely appreciate Professor Meng-Fei Liu and his graduate student Xiang-Wen Cheng from the Department of Genetics at Peking University Cancer Hospital/Beijing Cancer Research Institute for their valuable help and support in the statistical analysis of this manuscript.
| 1. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2442] [Article Influence: 488.4] [Reference Citation Analysis (3)] |
| 2. | Kato M. Diagnosis and therapies for gastric non-invasive neoplasia. World J Gastroenterol. 2015;21:12513-12518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Ushiku T, Lauwers GY. Pathology and Clinical Relevance of Gastric Epithelial Dysplasia. Gastroenterol Clin North Am. 2024;53:39-55. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Nozawa Y, Nishikura K, Ajioka Y, Aoyagi Y. Relationship between alpha-methylacyl-coenzyme A racemase expression and mucin phenotype in gastric cancer. Hum Pathol. 2012;43:878-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Strehl JD, Hoegel J, Hornicek I, Hartmann A, Riener MO. Immunohistochemical expression of IMP3 and p53 in inflammatory lesions and neoplastic lesions of the gastric mucosa. Int J Clin Exp Pathol. 2014;7:2091-2101. [PubMed] |
| 6. | Miao L, Sun Y, Guo M, Yang H, Du X, Li J, Shen J, Wang X, Lei R. Unique immunohistochemical profiles of MUC5AC, MUC6, P53, and Ki67 in gastric atypical hyperplasia and dysplasia. Int J Clin Exp Pathol. 2024;17:63-71. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Lee D, Yu EJ, Ham IH, Hur H. Clinicopathological Implication of Insulin-like Growth Factor-II mRNA-Binding Protein 3 (IMP3) Expression in Gastric Cancer. Anticancer Res. 2017;37:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Aas T, Børresen AL, Geisler S, Smith-Sørensen B, Johnsen H, Varhaug JE, Akslen LA, Lønning PE. Specific P53 mutations are associated with de novo resistance to doxorubicin in breast cancer patients. Nat Med. 1996;2:811-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 534] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 9. | Song B, Yang P, Zhang S. Cell fate regulation governed by p53: Friends or reversible foes in cancer therapy. Cancer Commun (Lond). 2024;44:297-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 10. | Leroy B, Anderson M, Soussi T. TP53 mutations in human cancer: database reassessment and prospects for the next decade. Hum Mutat. 2014;35:672-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 283] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 11. | Lee JH, Abraham SC, Kim HS, Nam JH, Choi C, Lee MC, Park CS, Juhng SW, Rashid A, Hamilton SR, Wu TT. Inverse relationship between APC gene mutation in gastric adenomas and development of adenocarcinoma. Am J Pathol. 2002;161:611-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Rokutan H, Abe H, Nakamura H, Ushiku T, Arakawa E, Hosoda F, Yachida S, Tsuji Y, Fujishiro M, Koike K, Totoki Y, Fukayama M, Shibata T. Initial and crucial genetic events in intestinal-type gastric intramucosal neoplasia. J Pathol. 2019;247:494-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Chen L, Xie Y, Li X, Gu L, Gao Y, Tang L, Chen J, Zhang X. Prognostic value of high IMP3 expression in solid tumors: a meta-analysis. Onco Targets Ther. 2017;10:2849-2863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Sato H, Ishihara S, Kawashima K, Moriyama N, Suetsugu H, Kazumori H, Okuyama T, Rumi MA, Fukuda R, Nagasue N, Kinoshita Y. Expression of peroxisome proliferator-activated receptor (PPAR)gamma in gastric cancer and inhibitory effects of PPARgamma agonists. Br J Cancer. 2000;83:1394-1400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 181] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Jiang Z, Fanger GR, Woda BA, Banner BF, Algate P, Dresser K, Xu J, Chu PG. Expression of alpha-methylacyl-CoA racemase (P504s) in various malignant neoplasms and normal tissues: astudy of 761 cases. Hum Pathol. 2003;34:792-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Cho EY, Kim KM, Park CK, Kim JJ, Sohn TS, Kim DW. AMACR is highly expressed in gastric adenomas and intestinal-type carcinomas. APMIS. 2007;115:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol. 2005;23:7212-7220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 602] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 18. | Sun Z, Liu Y, Deng H, Wang S, Zhang J, Xing C, Xu C. Modified Chaishao Liujunzi Decoction inhibits bile acid-induced gastric intestinal metaplasia: from network prediction to experimental verification. Aging (Albany NY). 2023;15:13998-14018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Wang YK, Ran DM, Li YY, Zhu CY, Zhang RB, Jiang B, Wang SN. Histopathological features of glandular atrophy of the lamina propria of the gastric mucosa during its occurrence and development. BMC Gastroenterol. 2023;23:395. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Park JH, Seo AN, Kim M. Diagnostic Usefulness of p53 Immunostaining in Gastric Cancer and Dysplasia: A Real-world Clinical Experience. In Vivo. 2024;38:1865-1874. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Ma C, Pai RK. Predictive value of immunohistochemistry in pre-malignant lesions of the gastrointestinal tract. Semin Diagn Pathol. 2015;32:334-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Zhao W, Lu D, Liu L, Cai J, Zhou Y, Yang Y, Zhang Y, Zhang J. Insulin-like growth factor 2 mRNA binding protein 3 (IGF2BP3) promotes lung tumorigenesis via attenuating p53 stability. Oncotarget. 2017;8:93672-93687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |