Published online May 15, 2025. doi: 10.4251/wjgo.v17.i5.105448
Revised: February 28, 2025
Accepted: March 26, 2025
Published online: May 15, 2025
Processing time: 113 Days and 4.7 Hours
In addition to nodal lesions, over 30% of mantle cell lymphomas (MCLs) also have gastrointestinal involvement, characteristically presenting as multiple lymphomatous polyposis (MLP), which rarely involve the esophagus. Most related papers have been case reports, and no comprehensive studies have been con
To elucidate the actual clinical situation of esophageal involvement of MCL presenting with MLP, including its prognosis.
From January 2001 to December 2021, among MCL patients whose gastro
In all patients, multiple lesions were present in the gastrointestinal tract other than the esophagus and in the lymph nodes throughout the body, and most patients also had lesions involving the bone marrow or spleen. Most of the treatments include chemotherapy, with a 50% survival period of less than 2 years and a 5-year survival rate of approximately 30%, indicating a poor prognosis.
Patients with esophageal involvement of the MCL who presented with MLP had a large tumor burden and poor survival.
Core Tip: Multiple lymphomatous polyposis, a main characteristic form of gastrointestinal involvement of mantle cell lymphoma, rarely affects the esophagus; the clinical status of this condition, including survival prognosis, has long remained unknown. In this study, we selected 6 patients and retrospectively examined their clinical features. In all patients, multiple lesions were present in the gastrointestinal tract other than the esophagus and in lymph nodes throughout the body, and most patients had lesions involving the bone marrow or spleen. The median survival time was less than 2 years, and the 5-year survival rate was approximately 30%, indicating a poor prognosis.
- Citation: Saito M, Oda Y, Sugino H, Suzuki T, Yokoyama E, Kanaya M, Izumiyama K, Mori A, Morioka M, Kondo T. Esophageal involvement of mantle cell lymphoma presenting with multiple lymphomatous polyposis: A single-center study. World J Gastrointest Oncol 2025; 17(5): 105448
- URL: https://www.wjgnet.com/1948-5204/full/v17/i5/105448.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i5.105448
Mantle cell lymphoma (MCL) is an independent disease unit that, at the molecular level, is genetically more than 95% characterized by the BCL-1 (CCND1) gene rearrangement associated with chromosomal translocation t (11; 14) (q13; q32)[1]. The incidence of this disease is estimated to be 5%-7% of all malignant lymphomas in Western countries[2] and 3%-5% in Japan[3], and its incidence is gradually increasing annually[4]. The median age of onset is in the middle-60s, and it is more common in men[1,5]. Approximately 90% of cases are diagnosed in advanced stages (III or IV) at the time of initial onset[5]. In addition to nodal lesions, many patients also have extranodal lesions, with bone marrow involvement in more than half of patients, splenomegaly in more than 30% of patients, and gastrointestinal involvement in more than 30% of patients[5]. The prognosis of this disease is reportedly worse than that of other lymphomas[6].
The main characteristic form of gastrointestinal involvement in MCL is called multiple lymphomatous polyposis (MLP)[7], which rarely spreads to the esophagus. Excluding a few case reports[8-10], there are currently no comprehensive studies about the esophageal involvement of MCL patients presenting with MLP. To elucidate the actual clinical condition of MCL with MLP in the esophagus, including survival prognosis, we selected 6 patients who were treated at our center and conducted a retrospective study.
This was a retrospective study at our center.
For 21 years, from January 2001 to December 2021, we selected 6 patients with MLP in the esophagus by esophagogastroduodenoscopy (EGD; 5 patients were confirmed by esophageal biopsy) from among MCL patients whose gastrointestinal lesions were histopathologically confirmed by endoscopic biopsy at our hospital, and we retrospectively examined their clinical features. To investigate the spread of the disease, in addition to EGD, patients were examined by colonoscopy, contrast computed tomography (CT), bone marrow aspiration/biopsy, and, when possible, positron emission tomography-CT. Extraesophageal gastrointestinal lesions are classified as MLP or non-MLP according to their form, and the latter are further subdivided into the following types: Protruded, ulcerative, fold thickening, and superficial[7]. Patient status was evaluated via the MCL international prognostic index (MIPI)[11]. In addition, we investigated the main treatment methods, history of recurrence, and survival time.
Because this study was conducted as part of a standard clinical treatment under the Japanese health insurance system, no special treatments were administered, and bendamustine was not used to treat patients prior to 2010. In addition, no peripheral blood stem cell transplants were performed in any of the patients.
The empirical survival probability was calculated via the Kaplan-Meier method.
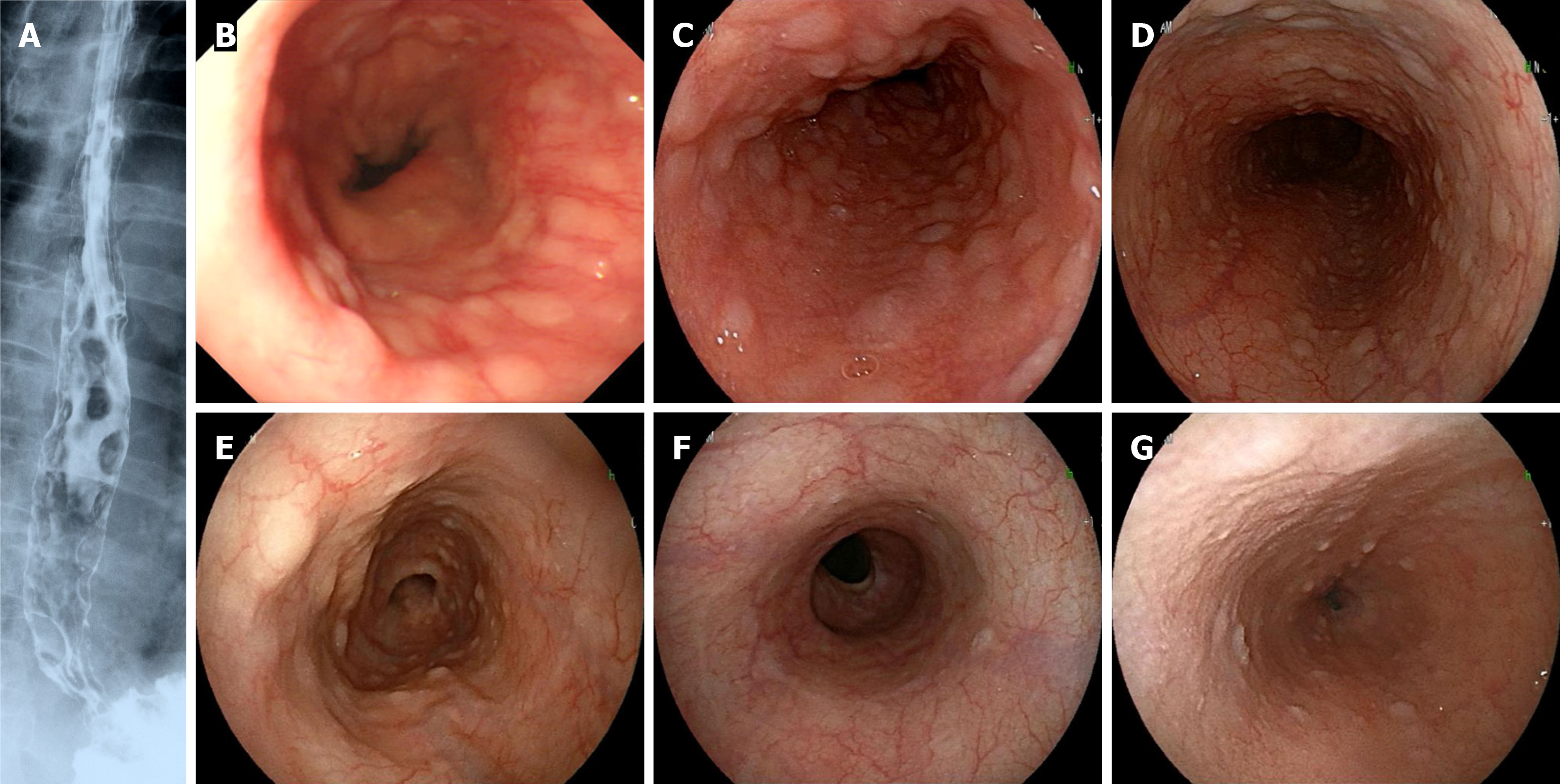
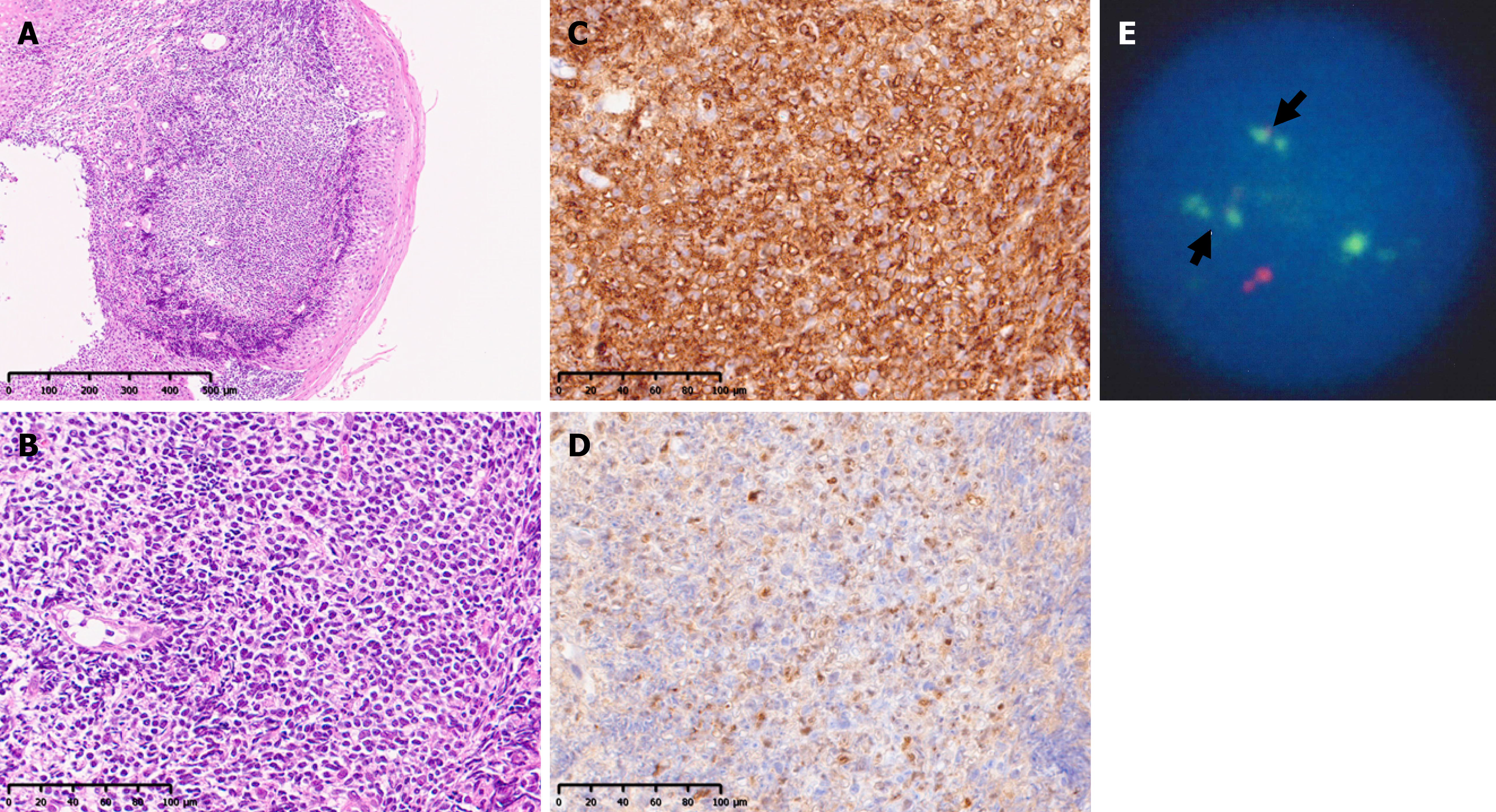
With respect to esophageal lesions (Figure 1), MLPs are often recognized as numerous small, flat protrusions connected from the middle to lower esophagus. The lesions ranged from clear lesions that could be observed even with contrast X-rays (case 1) to inconspicuous lesions where a biopsy was required to confirm the diagnosis (case 6). Although a biopsy of the esophageal lesion was not performed for case 5, the lesion disappeared after anticancer drug treatment, and the initial findings were considered indicative of MCL involvement; this patient was included in the current study. The histopathological findings of the esophageal biopsy of case 6 are shown in Figure 2. The monotonous proliferation of small- to medium-sized lymphoid cells with slightly atypical nuclei was observed under the mucosa (Figure 2A and B). The atypical lymphocytes were positive for CD20, CD5, and cyclin D1 expression (Figure 2C and D), and the pathological diagnosis was MCL. In addition, IgH-BCL-1 (CCND1) expression was strongly positive (98.8%) according to fluorescence in situ hybridization (Figure 2E).
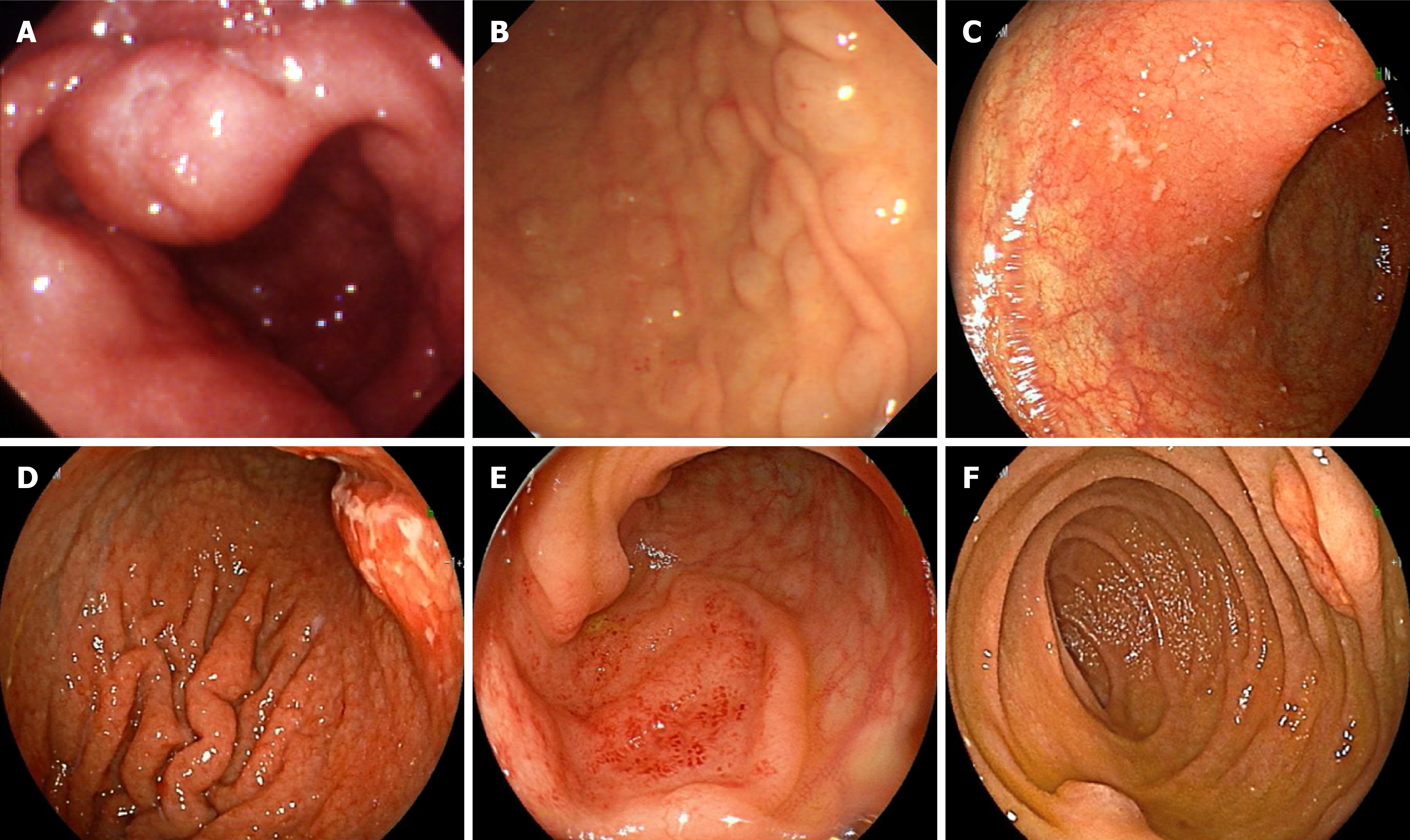
The characteristics of the gastrointestinal lesions in the 6 patients are shown in Table 1. There were no patients with only one resion in the esophagus alone; furthermore, all the patients had multiple lesions in the gastrointestinal tract other than the esophagus (Figure 3). There were lesions with not only MLP but also non-MLP (mostly protruded types, and no ulcer types). MLP was observed in 2 of the 5 patients with gastric lesions, in all 5 patients with duodenal lesions, and in 4 of the 6 patients with colonic lesions. In case 2, the MLP was intermittently observed from the esophagus to the rectum.
| Case | Esophagus | Stomach | Duodenum | Terminal ileum-colon |
| 1 | MLP | Non-MLP (fold thickening + protruded) | MLP | MLP |
| 2 | MLP | MLP | MLP | MLP |
| 3 | MLP | Non-MLP (superficial) | MLP | Non-MLP (protruded) |
| 4 | MLP | Non-MLP (fold thickening + protruded) | MLP | MLP |
| 5 | MLP | MLP | (-) | Non-MLP (protruded) |
| 6 | MLP | (-) | MLP | MLP |
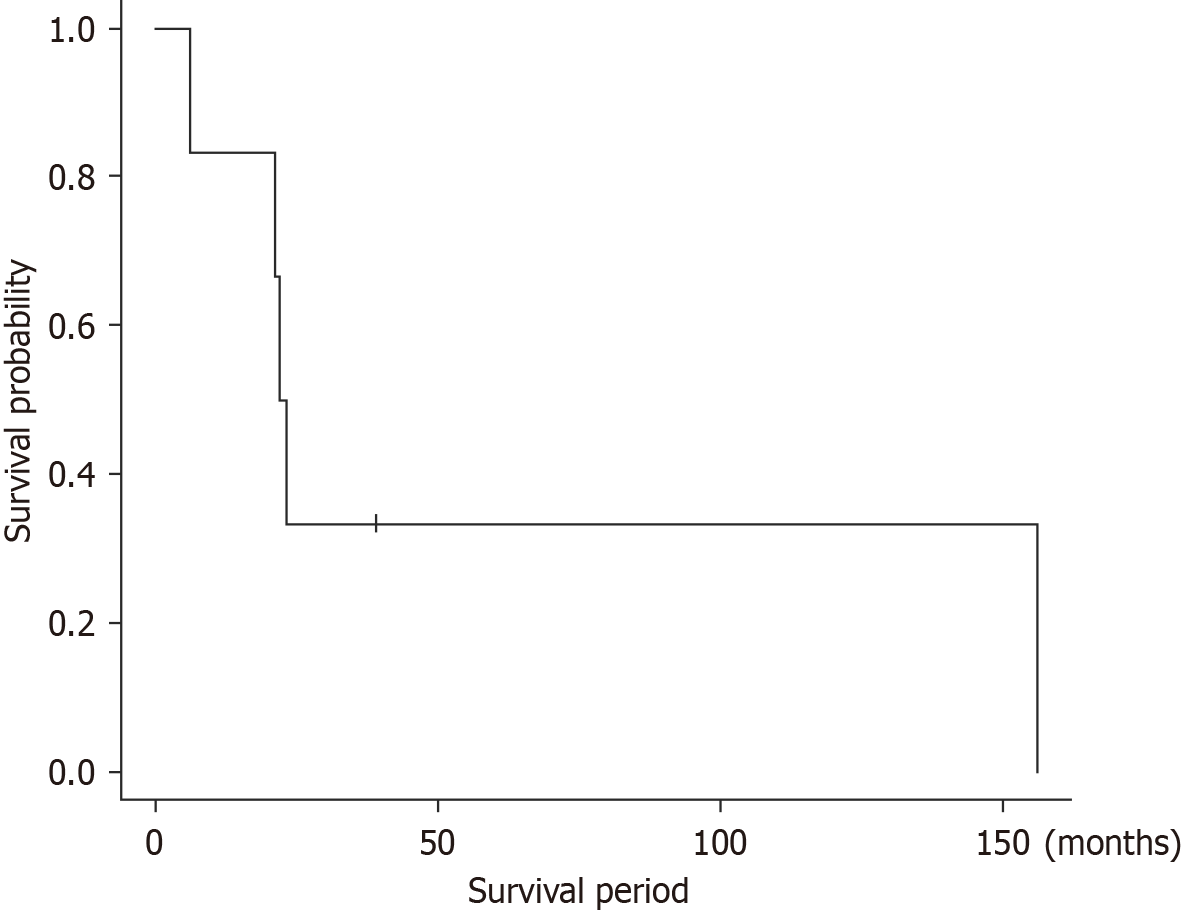
The clinical characteristics of the 6 patients are shown in Table 2. The median age of the patients was 71.5 years (ranging between 52-84 years), and 5 patients were male. All the patients had multiple nodal lesions, many of which also involved the bone marrow (5 patients, 4 of whom were leukemic) or the spleen (4 patients). In the MIPI, the low-risk, intermediate-risk, and high-risk groups each included 2 patients, and most of the treatments were chemotherapy. Various drugs were used to treat the patients, with the exception of rituximab (RTX), which was used to treat all 6 patients. Four patients achieved complete remission (CR); however, two subsequently relapsed, and one patient who nearly achieved CR died from treatment-related causes. Two patients who did not achieve CR died of MCL, and most lived for less than 2 years. In the survival curves for the 6 patients (Figure 4), the 50% survival period was less than 2 years, and the 5-year survival rate was approximately 30%, indicating a poor prognosis.
| Case | Age (year) | Sex | Lesion site (other than GI tract) | MIPI (score) | Treatment | Recurrence | Survival period |
| 1 | 70 | M | Cervical LN, inguinal LN, intra-abdominal LN, BM | Low (5.5) | THP•COP, Radiation ProMACE/CytaBOM, RTX (/+chemo) | (+) | 13 years (death from MCL) |
| 2 | 66 | F | Submandibular LN, axillary LN, thyroid gland | Low (5.2) | R-CHOP, RTX + Cladribine, ProMACE/CytaBOM | (+) | 1year 9 months (death from MCL) |
| 3 | 75 | M | Cervical LN, supraclavicular LN, axillary LN, hilar/mediastinal LN, intra-abdominal LN, inguinal LN, BM, PB, spleen, nasopharynx/palate | High (7.2) | THP•COP, R-BAC | Nearly CR | 6 months (treatment-related deaths) |
| 4 | 84 | M | Intra-abdominal LN, BM, PB, spleen | Intermediate (6.1) | RTX (monotherapy) | Non-CR | 1 year 10 months (death from MCL) |
| 5 | 73 | M | Submandibular-cervical LN, supraclavicular LN, axillary LN, intra-abdominal LN, inguinal LN, BM, PB, spleen, tonsil | High (6.7) | BR | Non-CR | 1 year 11 months (death from MCL) |
| 6 | 52 | M | Submandibular-cervical LN, axillary LN, mediastinal LN, abdominal-pelvic LN, Inguinal LN, BM, PB, spleen, liver, tonsil | Intermediate (6.1) | CHOP, RTX + Ara-C/ETP R-BAC | (-) | 3 year 3 months (alive with CR maintained) |
The normal esophageal mucosa lacks structured lymphatic tissue and lymphatic follicles[12]. Therefore, the esophagus is considered to be the digestive organ that is least commonly affected by malignant lymphoma[13], and less than 1% of lymphoma patients experience esophageal involvement[14]. On the other hand, even in MCL patients, esophageal lesions are rare; in previous reviews of gastrointestinal MCL published by Iwamuro et al[15], the incidence rates were reported to be 2 of 35 (5.7%) patients. Because the number of patients with MCL in whom the MLP involved the esophagus was small, the actual clinical conditions were unknown for a long period of time. Therefore, this clinical study is the first to conduct such long-term observations.
In all 6 patients, MCL involvement was observed in multiple gastrointestinal regions other than the esophagus, regardless of whether MLP or non-MLP was present. There were no patients whose pathological diagnosis was difficult, except for case 5, for whom no biopsy was performed.
When MCL patients with gastric, duodenum, or both lesions undergo EGD examination, careful observation and appropriate biopsy of the esophagus are needed. If a biopsy is omitted, reexamination via EGD is recommended after therapeutic intervention with anticancer drugs. In certain cases, such as case 5, the initial diagnosis can be confirmed based on clear changes in the endoscopic findings.
Although the prognosis of MCL has improved since 2000 with the advent of RTX[16], the survival curve has never plateaued; furthermore, MCL has a poorer prognosis than other lymphomas according to previous reports[6]. As a prognostic prediction model for MCL, MIPI has been proposed[11], which assigns points to 4 factors, namely, age, performance status, serum lactate dehydrogenase, and white blood cell count in peripheral blood, and stratifies prognosis into three groups (low-risk, intermediate-risk, or high-risk) base on the total score. As a result, the 6 patients in this study were equally divided into 3 groups. On the other hand, Ambinder et al[17] reviewed 4477 patients with MCL (349 of whom had gastrointestinal MCL) from a United States database and reported that the median survival period and 5-year survival rate were significantly better in patients with gastrointestinal lesions (66 months and 55%, respectively) than in those with nodal MCL (48 months and 43%, respectively). There is still no established standard treatment for MCL. In patients aged 66 years or older or for whom intensive chemotherapy is difficult, bendamustine, RTX (BR) therapy[18], RTX maintenance therapy following R-CHOP (RTX, cyclophosphamide, doxorubicin, vincristine and prednisolone)[19], and VR-CAP therapy (bortezomib, RTX, cyclophosphamide, doxorubicin and prednisolone)[20] are recommended[16,21]. In particular, as in this study, BR therapy is often selected in clinical practice because it is simple and highly effective in treating other low-grade B-cell lymphomas, such as follicular lymphoma[22]. RTX was administered to all 6 patients; many patients have been actively using bendamustine since 2010. Regardless, the survival curve of the patients in this study was similar to that from 1990 to 1999[14], before the advent of RTX. The prognosis was clearly poor, and this may be due to the large tumor burden; as all patients had multiple gastrointestinal lesions other than those in the esophagus, all patients had multiple nodal lesions, and many patients had extranodal lesions involving the bone marrow or spleen. Alternatively, this may also be because the current study included several elderly patients who had poor activities of daily living before the intervention.
This study was retrospective in design and included only 6 patients, which limited the ability to analyze details such as the causes of poor prognosis. Another limitation of this study was that only patients presenting with MLP could be examined. The possibility of detecting non-MLP esophageal involvement of the MCL is a subject for future study.
In this study, patients with esophageal involvement of MCL with MLP had multiple lesions presenting in gastrointestinal regions other than the esophagus, and in lymph nodes throughout the body, a high tumor burden including bone marrow or spleen involvement, and a poor prognosis. In MCL patients, when lesions are identified in the stomach, duodenum, or both via EGD, careful observation of the esophagus and appropriate biopsy are needed.
We would like to thank Ikuo Sato (Sapporo Clinical Laboratory Inc.) for the technical support provided in the laboratory aspects of this study.
| 1. | International Agency for Research on Cancer. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. [cited 20 January 2025]. Available from: https://www.iarc.who.int/news-events/who-classification-of-tumours-of-haematopoietic-and-lymphoid-tissues-2/. |
| 2. | Wu H, Wang J, Zhang X, Yang H, Wang Y, Sun P, Cai Q, Xia Y, Liu P. Survival Trends in Patients Under Age 65 Years With Mantle Cell Lymphoma, 1995-2016: A SEER-Based Analysis. Front Oncol. 2020;10:588314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | The world health organization classification of malignant lymphomas in japan: incidence of recently recognized entities. Lymphoma Study Group of Japanese Pathologists. Pathol Int. 2000;50:696-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 343] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 4. | Chihara D, Ito H, Matsuda T, Shibata A, Katsumi A, Nakamura S, Tomotaka S, Morton LM, Weisenburger DD, Matsuo K. Differences in incidence and trends of haematological malignancies in Japan and the United States. Br J Haematol. 2014;164:536-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 260] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 5. | Chihara D, Asano N, Ohmachi K, Kinoshita T, Okamoto M, Maeda Y, Mizuno I, Matsue K, Uchida T, Nagai H, Nishikori M, Nakamura S, Ogura M, Suzuki R. Prognostic model for mantle cell lymphoma in the rituximab era: a nationwide study in Japan. Br J Haematol. 2015;170:657-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Smith A, Crouch S, Lax S, Li J, Painter D, Howell D, Patmore R, Jack A, Roman E. Lymphoma incidence, survival and prevalence 2004-2014: sub-type analyses from the UK's Haematological Malignancy Research Network. Br J Cancer. 2015;112:1575-1584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 309] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 7. | Okada H, Yoshino T, Shinagawa K, Yamamoto K. Gastrointestinal mantle cell lymphoma. Gastroenterol Endosc. 2013;55:3067-30. |
| 8. | Remes-Troche JM, De-Anda J, Ochoa V, Barreto-Zuñiga R, Arista-Nasr J, Valdovinos MA. A rare case of multiple lymphomatous polyposis with widespread involvement of the gastrointestinal tract. Arch Pathol Lab Med. 2003;127:1028-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Shen KH, Chen CJ, Yen HH. Multiple polyposis of the esophagus: mantle cell lymphoma. Clin Gastroenterol Hepatol. 2012;10:e65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Zullo A, Cerro P, Chios A, Andriani A, Balsamo G, Francesco VD, Bruzzese V. A very unusual cause of dysphagia: mantle cell lymphoma. Ann Gastroenterol. 2016;29:383-385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Hoster E, Dreyling M, Klapper W, Gisselbrecht C, van Hoof A, Kluin-Nelemans HC, Pfreundschuh M, Reiser M, Metzner B, Einsele H, Peter N, Jung W, Wörmann B, Ludwig WD, Dührsen U, Eimermacher H, Wandt H, Hasford J, Hiddemann W, Unterhalt M; German Low Grade Lymphoma Study Group (GLSG); European Mantle Cell Lymphoma Network. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 701] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 12. | Ma Q, Zhang C, Fang S, Zhong P, Zhu X, Lin L, Xiao H. Primary esophageal mucosa-associated lymphoid tissue lymphoma: A case report and review of literature. Medicine (Baltimore). 2017;96:e6478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Hosaka S, Nakamura N, Akamatsu T, Fujisawa T, Ogiwara Y, Kiyosawa K, Hidaka E, Ota H, Katsuyama T, Inagaki H. A case of primary low grade mucosa associated lymphoid tissue (MALT) lymphoma of the oesophagus. Gut. 2002;51:281-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Kalogeropoulos IV, Chalazonitis AN, Tsolaki S, Laspas F, Ptohis N, Neofytou I, Rontogianni D. A case of primary isolated non-Hodgkin's lymphoma of the esophagus in an immunocompetent patient. World J Gastroenterol. 2009;15:1901-1903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Iwamuro M, Okada H, Kawahara Y, Shinagawa K, Morito T, Yoshino T, Yamamoto K. Endoscopic features and prognoses of mantle cell lymphoma with gastrointestinal involvement. World J Gastroenterol. 2010;16:4661-4669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Armitage JO, Longo DL. Mantle-Cell Lymphoma. N Engl J Med. 2022;386:2495-2506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 17. | Ambinder AJ, Shenoy PJ, Nastoupil LJ, Flowers CR. Using primary site as a predictor of survival in mantle cell lymphoma. Cancer. 2013;119:1570-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Flinn IW, van der Jagt R, Kahl B, Wood P, Hawkins T, MacDonald D, Simpson D, Kolibaba K, Issa S, Chang J, Trotman J, Hallman D, Chen L, Burke JM. First-Line Treatment of Patients With Indolent Non-Hodgkin Lymphoma or Mantle-Cell Lymphoma With Bendamustine Plus Rituximab Versus R-CHOP or R-CVP: Results of the BRIGHT 5-Year Follow-Up Study. J Clin Oncol. 2019;37:984-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 213] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 19. | Kluin-Nelemans HC, Hoster E, Hermine O, Walewski J, Geisler CH, Trneny M, Stilgenbauer S, Kaiser F, Doorduijn JK, Salles G, Szymczyk M, Tilly H, Kanz L, Schmidt C, Feugier P, Thieblemont C, Zijlstra JM, Ribrag V, Klapper W, Pott C, Unterhalt M, Dreyling MH. Treatment of Older Patients With Mantle Cell Lymphoma (MCL): Long-Term Follow-Up of the Randomized European MCL Elderly Trial. J Clin Oncol. 2020;38:248-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 20. | Robak T, Jin J, Pylypenko H, Verhoef G, Siritanaratkul N, Drach J, Raderer M, Mayer J, Pereira J, Tumyan G, Okamoto R, Nakahara S, Hu P, Appiani C, Nemat S, Cavalli F; LYM-3002 investigators. Frontline bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in transplantation-ineligible patients with newly diagnosed mantle cell lymphoma: final overall survival results of a randomised, open-label, phase 3 study. Lancet Oncol. 2018;19:1449-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 21. | Maddocks K. Update on mantle cell lymphoma. Blood. 2018;132:1647-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 22. | Martin P, Cohen JB, Wang M, Kumar A, Hill B, Villa D, Switchenko JM, Kahl B, Maddocks K, Grover NS, Qi K, Parisi L, Daly K, Zhu A, Salles G. Treatment Outcomes and Roles of Transplantation and Maintenance Rituximab in Patients With Previously Untreated Mantle Cell Lymphoma: Results From Large Real-World Cohorts. J Clin Oncol. 2023;41:541-554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 42] [Article Influence: 21.0] [Reference Citation Analysis (0)] |