Published online Jan 15, 2024. doi: 10.4251/wjgo.v16.i1.13
Peer-review started: November 11, 2023
First decision: December 6, 2023
Revised: December 6, 2023
Accepted: December 19, 2023
Article in press: December 19, 2023
Published online: January 15, 2024
Processing time: 61 Days and 1.1 Hours
Gallbladder (GB) carcinoma, although relatively rare, is the most common biliary tree cholangiocarcinoma with aggressiveness and poor prognosis. It is closely associated with cholelithiasis and long-standing large (> 3 cm) gallstones in up to 90% of cases. The other main predisposing factors for GB carcinoma include molecular factors such as mutated genes, GB wall calcification (porcelain) or mainly mucosal microcalcifications, and GB polyps ≥ 1 cm in size. Diagnosis is made by ultrasound, computed tomography (CT), and, more precisely, magnetic resonance imaging (MRI). Preoperative staging is of great importance in decision-making regarding therapeutic management. Preoperative staging is based on MRI findings, the leading technique for liver metastasis imaging, enhanced three-phase CT angiography, or magnetic resonance angiography for major vessel assessment. It is also necessary to use positron emission tomography (PET)-CT or 18F-FDG PET-MRI to more accurately detect metastases and any other occult deposits with active metabolic uptake. Staging laparoscopy may detect dissemination not otherwise found in 20%-28.6% of cases. Multimodality treatment is needed, including surgical resection, targeted therapy by biological agents according to molecular testing gene mapping, chemotherapy, radiation therapy, and immunotherapy. It is of great importance to understand the updated guidelines and current treatment options. The extent of surgical intervention depends on the disease stage, ranging from simple cholecystectomy (T1a) to extended resections and including extended cholecystectomy (T1b), with wide lymph node resection in every case or IV-V segmentectomy (T2), hepatic trisegmentectomy or major hepatectomy accompanied by hepaticojejunostomy Roux-Y, and adjacent organ resection if necessary (T3). Laparoscopic or robotic surgery shows fewer posto
Core Tip: Gallbladder (GB) carcinoma is a rare but aggressive malignancy with a poor prognosis. Therapeutic surgical resection constitutes the only chance of cure, but only in earlier stages. For advanced-stage patients, multimodality treatment is necessary. Early and accurate preoperative evaluation is essential for the best choice of surgical management, determining the correct extent of resection and the type of novel adjuvant or neoadjuvant treatment. Application of targeted treatment and immunotherapy has broadened the management armamentarium for GB carcinomas, but future work must focus on increasing efficiency. The most extended operative procedures remain under debate. An individualized approach may be more suitable.
- Citation: Pavlidis ET, Galanis IN, Pavlidis TE. New trends in diagnosis and management of gallbladder carcinoma. World J Gastrointest Oncol 2024; 16(1): 13-29
- URL: https://www.wjgnet.com/1948-5204/full/v16/i1/13.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i1.13
Gallbladder (GB) carcinoma, although relatively uncommon, is the most common biliary tree cholangiocarcinoma and constitutes an aggressive, lethal malignancy with a dismal prognosis[1-5]. It is the 6th most common gastrointestinal tract carcinoma and is more common in women. Although 5-year survival has progressively increased to between 7% and 20%, patients with advanced disease still have a lower than 5% 5-year survival[1,4,6-8].
Gene mutations, socioeconomic status and environmental factors may explain the differences in geographical incidence distribution[9-11]. The global incidence is 1.2 cases per 100000 inhabitants, with the highest rates in Asia, mainly China and India (1.4 cases per 100000 inhabitants), and the lowest rates in Europe (0.66 cases per 100000 inhabitants) and North America (0.67 cases per 100000 inhabitants). However, with an increased aging population and obesity, a substantial increase in GB carcinoma rates is estimated to occur by 2040[1].
GB carcinoma is closely associated with cholelithiasis and long-standing (> 20 years) large (> 3 cm) gallstones[12] in up to 90% of cases[9,13-15]. In approximately half of all cases, GB carcinoma is revealed in cholecystectomy specimens, and its incidence among specimens fluctuates between 0.19% and 1.6%[9,16,17]. In addition, because of its cooccurrence with cholelithiasis, it is associated with female sex, obesity, and increased age[9,18]. Gallstones are the main cause of GB mucosa inflammation and secondary infection by Helicobacter pylori or Salmonella. This chronic inflammation induces mediator release, which in the long term may cause gene alterations, ultimately leading to carcinogenesis[15] through previous mucosal damage, metaplasia and dysplasia[12]. Among other notable predisposing factors for GB carcinogenesis are mutated genes, mainly TP53 (up to 91%) and KRAS (29.0%)[15,19,20], GB wall calcification (porcelain GB) or mainly mucosal microcalcifications, GB neoplastic true polyps ≥ 1 cm in size[12,16] and aflatoxin[21] or the older contrast medium Thorotrast[10].
GB carcinoma arising from the epithelium is typically adenocarcinoma (95.7%) but can also infrequently be squamous carcinoma (2.4%) or adenosquamous carcinoma (1.9%), both with more aggressiveness and worse prognoses than adenocarcinoma[12,22-24].
There are two precancerous lesions of GB adenocarcinoma: (1) Intracholecystic papillary-tubular neoplasm; and (2) biliary intraepithelial neoplasia. The malignant transformation of these precursors has a better prognosis than the original carcinoma. Multivariate analysis showed that the absence of coexistence with such lesions in adenocarcinoma was associated with symptomatic cases and advanced stage at presentation, poor cancer cell differentiation, and poor prognosis[25].
Mucinous adenocarcinoma is a rare subtype of GB adenocarcinoma with more aggressiveness, but it has the same management[26].
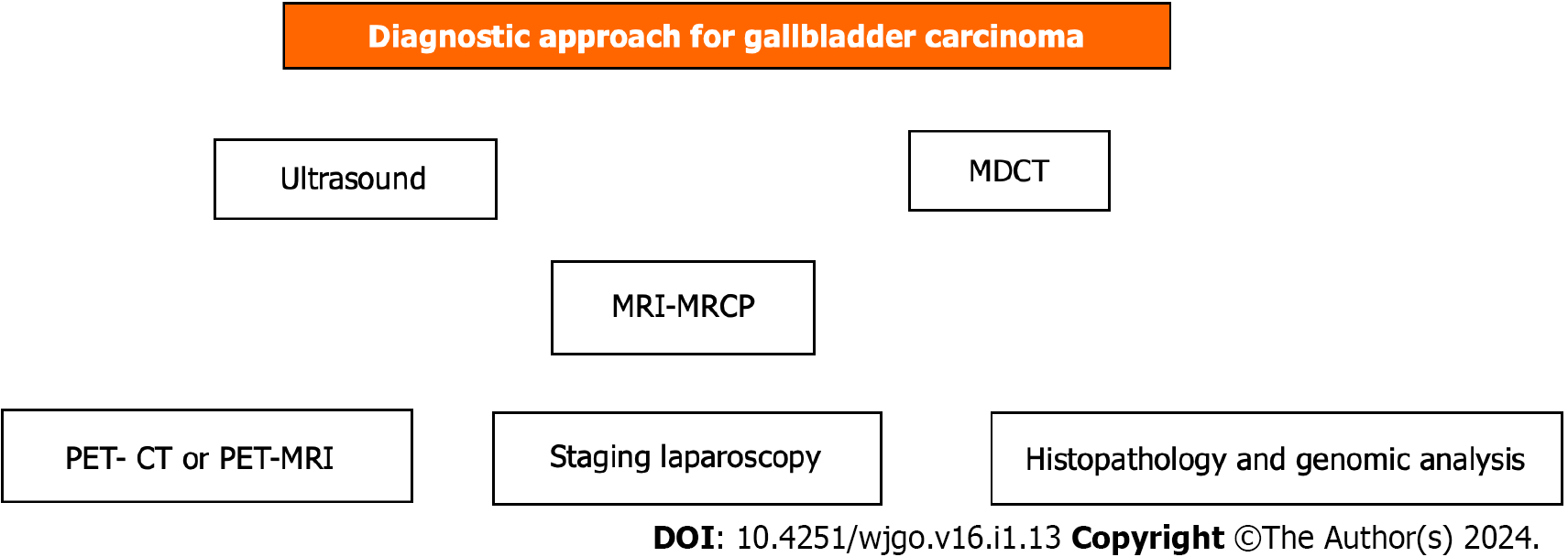
Diagnosis is made by ultrasound (US), computed tomography (CT)[27], and, more precisely, magnetic resonance imaging (MRI) by detecting a filling defect in the GB or wall thickening and assessing the depth of invasion[7,12]. Preoperative staging is of great importance in the decision-making plan of therapeutic management. Preoperative staging is based on MRI contrast-enhanced findings, the leading technique for liver metastasis imaging[28], enhanced three-phase CT angiography or magnetic resonance angiography for major vessel assessment. It is also necessary to use 18F-FDG positron emission tomography (PET)-CT[29,30] or 18F-FDG PET-MRI[28] to more accurately detect metastases and any other occult deposits with active metabolic uptake. PET is complementary to traditional imaging for primary lesions and imperative for doubtful metastases with high specificity, including follow-up[31]. Staging laparoscopy may detect dissemination that cannot otherwise be found preoperatively in 20%-28.6% of cases; thus, it must be included in evaluation planning[32,33].
A prediction model of prognosis that is based on multiple clinical indications, in combination with artificial intelligence algorithms, has been recently developed[34].
Surgical curative resection is the cornerstone of treatment but is often impossible in advanced metastatic disease. Its extent depends on staging ranging from cholecystectomy to major resections with wide lymphadenectomy[3,29,35-37]. However, a debate exists about extended operations, which increase morbidity and mortality with unclear long-term outcomes[3].
Laparoscopic or robotic surgery has the advantages of minimally invasive surgery and equivalent long-term outcomes to open surgery but requires much attention and expertise to avoid major complications[38-40].
Other management options include chemotherapy and radiotherapy, immunotherapy, and targeted therapy by monoclonal antibody biological agents[41-45]. The current rapidly evolving, multimodal therapeutic approach[46] and Medicaid expansion[47] have improved the results, opening new perspectives even in inoperable cases involving palliative treatment[4,13,48-50].
Nanotechnology using photodynamic nanoparticles to target the tumor has been reported recently as a novel treatment[51].
In this narrative review, we evaluate the updated knowledge on GB carcinoma, emphasizing current diagnosis and surgical management. This study was based on an extensive literature review from PubMed until October 2023, focusing particularly on full-text papers published only in the English language over the last six years.
The diagnostic steps are shown schematically in Figure 1.
Unfortunately, GB carcinoma is usually asymptomatic in the early stages, or the symptoms of coexisting cholelithiasis predominate, and GB carcinomas may be detected incidentally by histopathological examination of the cholecystectomy specimen or by imaging performed for other reasons. The appearance of pain, mainly obstructive jaundice, anorexia, weakness, and weight loss, indicate a more advanced disease[12].
The presence of preoperative obstructive jaundice may be considered a contraindication for curative resection because it is accompanied by increased morbidity and mortality and worse overall survival; obviously, a careful high selection of jaundiced patients is mandatory[35,52].
GB carcinomas are located in the GB fundus (60%), body (30%) and/or neck (10%). On imaging, it can be found as an intraluminal mass or occupying the GB along with focal or diffuse wall thickening[12].
US plain, contrast-enhanced US, high-frequency US, high-resolution US, and endoscopic US in combination with three-phase contrast-enhanced multidetector CT[53] and contrast-enhanced MRI constitute the basic steps for diagnosis and preoperative staging[7,12,27,28].
18F-FDG PET-CT[54,55] or 18F-FDG PET-MRI[28] must be performed before every intended major curative resection to exclude any occult metastatic lesion, which is not visible by the other imaging techniques, particularly in port-site metastases after laparoscopic cholecystectomy[29] and in ambiguous cases or at follow-up[12,31,56].
Artificial intelligence may analyze computerized imaging. Radiomics is a sophisticated method of imaging analysis machine learning that ensures, on the one hand, precise diagnosis and staging, including lymph node involvement and depth of wall invasion, particularly serosal infiltration, and, on the other hand, reliable prognosis predicting oncological outcomes, including survival and recurrence[27].
In addition to magnetic resonance cholangiopancreatography and percutaneous transhepatic cholangiography, endoscopic retrograde cholangiopancreatography (ERCP) and cholangioscopy may be used to evaluate the common bile duct in the presence of obstructive jaundice. ERCP may also provide bile samples or brushings for cytological exami
Endoscopic ultrasound (EUS) provides high-quality images. In addition, EUS-guided fine needle aspiration biopsy may be used to safely diagnose GB malignancy in suspected cases but has the drawback of bile leakage and potent intraperitoneal dissemination of cancer cells[58]. Additionally, imaging-guided fine needle aspiration cytology of GB bile is sometimes used[22].
Transpapillary GB biopsy by insertion of a novel cholangioscope via the cystic duct may overcome these disadvantages and has been recently introduced[57,59].
The tumor markers CA 19-9, CA 125, CA 242, CA 15-3, and CEA have been found to be elevated in GB carcinoma and could contribute to its early diagnosis and in the follow-up for prompt detection of recurrence[12,16].
The preoperative value of CA 125 was found to be an independent risk factor for early recurrence[60]. CA 19-9 is valuable for detecting recurrence, with a sensitivity of 79.1%, specificity of 97.2%, positive predictive value of 95%, and negative predictive value of 87.5%[61].
Circulating tumor cell assessment is feasible and constitutes a determinant factor of the management plan and preoperative prognostic marker[62].
To date, there is no indication for including gut microbiota assessment in the diagnosis and management of GB carcinoma[63].
Knowledge of molecular mechanisms is valuable for aiding in the development of novel therapeutic targets. He et al[64] recently demonstrated that zinc finger protein 64 (ZFP64) plays an important role in GB carcinogenesis and progression through the ZFP64-Notch1-HDAC1 pathway. It promotes in vitro and in vivo cell proliferation, anti-apoptosis, migration, and invasion[64]. Thus, targeting this protein may be the basis for a prospective efficient treatment.
Topological alterations in genomic structure lead to genomic dysfunction that promotes malignant transformation[65]. Genomic analysis may be useful as a diagnostic biomarker or, most importantly, for potent targeted therapy. Genes of interest include VEGF, VEGFR, EGFR, MEK1, MEK2, MET, mTOR, HER2, N-cadherin, PI3K, PDL-1 and PD-1[15].
Zhou et al[23] recently reported the genetic characteristics of GB carcinoma in elderly individuals over 65 years. They found increased expression of the cell cycle-related genes AURKA, AURKB, CCNA2, CCNB1, CDK1, DLGAP5, KIF11, MELK, NCAPG, and TPX2 and decreased expression of the mitochondrial function-associated genes ND1, ND2, ND3, ND4, ND4L, CYTB, COX1, COX2, ATP6, and ATP8. These genes promote cancer cell overgrowth and metastasis. In addition, Zhou et al[23] found that elderly patients were less responsive to gemcitabine and cisplatin chemotherapy, similar to immunotherapy, with high PD-L1 and CTLA-4 expression[23].
Mishra et al[19] studied 37 formalin-fixed GB carcinoma paraffin-embedded cubes of specimens and found that the most common mutations occur in combination in TP53 (90.9%), SMAD4 (60.6%), NOTCH1 (45.45), ERBB2 (45.45%), PIK3CA (33.33%), MET (30.30%), PTEN (30.3%), EGFR (24.24%), KRAS (21.21%) and BRAF (9%). Mutations that could be targeted constituted 89.91% of cases and included all the above genes, except the TP53 gene. CTNNB1, KRAS and NRAS gene mutations were more highly related to metastatic disease[19].
Chae et al[20] conducted a study of 124 patients in Korea and found that 83.8% of patient samples contained genetic alterations, and the most common mutations were in TP53 (44.4%), KRAS (29.0%), ERBB2-3 (20.0%), ARID1A (12.1%), and IDH1 (4%)[20].
Expression of the Her2/neu gene, a receptor of tyrosine kinase 2, is found, apart from breast carcinoma, in GB carcinoma and is associated with the degree of cell differentiation and advanced stage (III, IV). It could be used for prognosis and in follow-up[66]. HER2/ERBB2 gene overexpression has been found in 20% of advanced GB carcinoma stages and may be a target of relevant treatment[67].
A study of 157 GB carcinoma patients in China found that mutated ERBB2/ERBB3 genes (7%-8%) promoted proliferation and migration, upregulated PD-L1 expression, and were associated with a worse prognosis. These mutations may be useful biomarkers for targeted therapy[68].
The expression of the FASN gene (fatty acid synthase), a key enzyme of lipid metabolism that has great importance in cancer progression through the PI3K/AKT pathway, was found to promote GB carcinoma growth and is related to a decreased response to gemcitabine chemotherapy[69].
The expression of the EMP3 gene was decreased in GB carcinoma and was related to poor prognosis. Its therapeutic targeting may inhibit carcinoma progression through the miR-663a/EMP3/MAPK/ERK pathway[70].
The expression of the YTHDF2 gene promoted GB carcinoma cell proliferation, growth, migration, and invasion and inhibited apoptosis in vitro and in vivo. Additionally, it was related to a decreased response to gemcitabine treatment and may be a therapeutic target[71].
The expression of the FOXA2 suppressive gene is poor in GB carcinoma[72].
The expression of the m6A gene, which modifies CLDN4 gene stability, induces immunosuppression and poor prognosis in GB carcinoma due to an aggressive phenotype; thus, it may be a therapeutic target[73].
The CEP55 gene was overexpressed in GB carcinoma and associated with a decrease in apoptosis, advanced stage, lower cell differentiation, and dismal prognosis. Thus, it constitutes not only a diagnostic and prognostic biomarker but also a potent prospective therapeutic target[74].
It has been found that DLGAP5 gene expression was increased in GB cancer and associated with poor prognosis by promoting proliferation and migration; it could be a therapeutic target[75].
The mutated PBRM1 gene has been found in a small proportion of GB carcinomas related to DNA damage repair. It could be amenable to targeted therapy[76].
It has been found that the mutated TRIM37 gene was increased in GB carcinoma, promoting progression by increased proliferation and decreased apoptosis. It was related to lower cell differentiation, advanced stage, and reduced survival; it could be a therapeutic target[77].
A recent study of 148 GB carcinoma patients in Korea performed multivariate analysis and found that PAK4 gene expression and the associated PHF8 gene were independent predictive factors of reduced survival and dismal prognosis[78].
miRNAs may be valuable biomarkers and therapeutic targets[6,15]. The most dysregulated miRNAs in GB carcinoma were the upregulated miRNAs miR-4430 and miR-642α-3p and the downregulated miRNAs miR-451α and miR-145-5p. miR-642α-3p and miR-145-5p were associated with invasion and metastasis and could constitute a prospective therapeutic target[79]. miR-214-3p, which is expressed in mesenchymal stem cells of the umbilical cord, may decrease proliferation and increase apoptosis of GB carcinoma by inhibiting ACLY/GLUT1 gene expression[80].
Histopathological staging is based on the TNM system (primary tumor invasion, lymph node involvement, and distant metastases). The applicable 8th Edition of TNM classification and staging by the American Joint Committee on Cancer (AJCC), which entered into force on January 1, 2018, replacing the 7th Edition, are shown in Tables 1 and 2[81]. Jiang et al[81] proposed a small modification of this staging system in 2020 to improve its diagnostic accuracy, but the AJCC has not made any change thus far[81].
| T | Invasion |
| Tis | In situ |
| T1a | Only lamina propria |
| T1b | Lamina propria and muscle layer |
| T2a | Perimuscular fibrous tissue on the side of the peritoneum |
| T2b | Perimuscular fibrous tissue on the side of the liver |
| T3 | Serosa, and/or liver, and/or adjacent organs (stomach, duodenum, colon, pancreas, omentum, common bile duct) |
| T4 | Hepatic artery or main portal vein or ≥ 2 adjacent organs |
| Stage | Parameters |
| 0 | Tis, N0, M0 |
| I | T1, N0, M0 |
| IIa | T2a, N0, M0 |
| IIb | T2b, N0, M0 |
| IIIa | T3, N0, M0 |
| IIIb | T1-T3, N1, M0 |
| IVa | T4, N0-N1, M0 |
| IVb | Any T, N2, M0 or any T, any N, M1 |
Surgery remains the first-choice basic treatment for long-sustained oncological outcomes even in elderly patients (70-84 years)[82], but unfortunately, the disease is usually inoperable at presentation. Curative resection may be performed in only 15%-35% of cases and is associated with high recurrence. Thus, multimodal management is imperative, and the current efforts have been focused in this direction[46,47,83].
Precise and evidence-based decisions must be made to determine the best management strategy. The optimal extent of surgical resection and the appropriate adjuvant treatment should be individualized based on the patient’s physical status, comorbidity, imaging staging and molecular genetic factors[41,83,84].
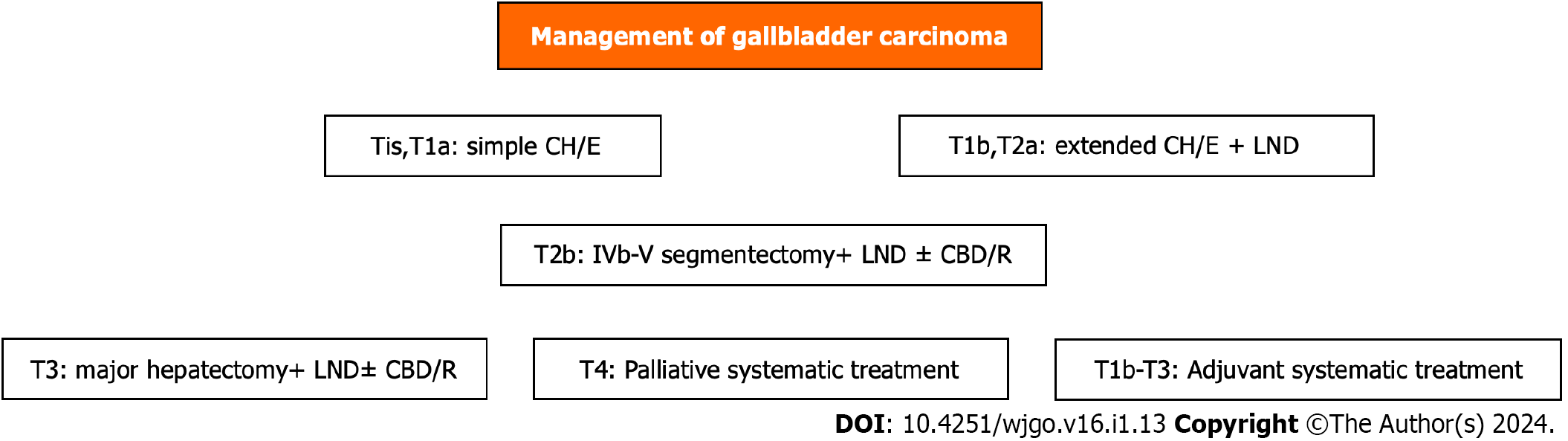
Therapeutic radical operative resection is the cornerstone of management. The extent of resection depends on the T classification stage of tumor invasion[85-88]. For Tis and T1a, simple cholecystectomy is an adequate treatment. For T1b and T2a, extended cholecystectomy with removal of the GB bed or segment IVb and V resection (T2b) and regional lymphadenectomy have gained wide acceptance[37,85-87,89]. For T3, major hepatectomy (trisegmentectomy or right hepatectomy), extended lymphadenectomy, and common bile duct resection with hepaticojejunostomy or hepato-pancreato-duodenectomy in more advanced cases can be performed[85,86,90]. For inoperable stage T4, palliative systematic treatment (chemoradiation, immunotherapy, targeted therapy) is recommended[46]. The management policy according to T stage is shown schematically in Figure 2[46,85,86]. Extended cholecystectomy in the T1b stage was related to better survival than simple cholecystectomy, particularly in elderly patients (≥ 67 years)[91].
A recent study in China of 144 patients with T2-T4 stage advanced GB carcinoma determined that the total number of removed lymph nodes must be at least 6, and the optimal lymph node ratio (positive to total) is 0.28. Lymphadenectomy may be D1 (lymph nodes of hepatoduodenal ligament and common hepatic artery) or D2 (lymph nodes of posterior duodenum and celiac artery)[85]. Adequate lymphadenectomy has an increasing trend in the United States and Japan, affecting survival[92-94]. Extrahepatic bile duct resection was performed only in jaundiced patients and cystic duct invasion[85,95]. Resection of adjacent organs and portal vein or hepatic artery reconstruction was possible if there was related infiltration. Multivariate analysis identified T stage, R (residual) resection and G (grade) of cell differentiation as independent prognostic factors[85].
The method of lymphadenectomy influences the outcome. Another study in China of 133 patients found that fusion lymphadenectomy, which uses 3D-SPECT (photon emission CT) and CT or MRI data, may increase the number of both overall retrieved lymph nodes and positive lymph nodes. It improved both the diagnostic accuracy and survival[96]. A study of 227 GB carcinoma patients assessed the effect of three-dimensional visualization preoperative evaluation and enhanced recovery after surgery and found that precise surgery improved oncological outcomes. The researchers found that this effect, compared to the control group, showed a higher R0 resection rate (67.4% vs 20.9%) and number of retrieved lymph nodes (26.6 ± 12.6 vs 16.3 ± 7.6%) and better median overall survival (27.4 mo vs 12.7%), 1-year survival (84.4% vs 53.5%) and 3-year survival (29.8% vs 15.1%)[97].
A recent large national cohort study in Japan of 4917 GB carcinoma patients, including 1609 T2 stage patients, evaluated the efficacy of extrahepatic bile duct resection in this stage compared to nonresection. They found that resection was associated with higher lymph node involvement (38.2% vs 20.7%), postoperative complications (32.4% vs 11.7%), and 5-year survival (64% vs 54%) but comparable disease-specific survival (76% vs 79%); they recommended much caution in decision-making for such resection in the T2 stage[98]. A recent systematic review and meta-analysis including 2086 patients and 9 studies with T2 stage GB carcinoma who underwent wedge hepatectomy (extended cholecystectomy) or hepatic segment IV and V resection found that segment resection provided better disease-free survival than wedge resection but more postoperative complications[99]. Another recent meta-analysis including 7 studies and 1795 GB carcinoma patients with stage T2 and T3 disease found that extended cholecystectomy had better short-term outcomes than hepatic segment IV and V resection and equivalent oncological outcomes[100]. Wu et al[90] demonstrated that extended lymphadenectomy provided a significant survival benefit in patients with preoperative N0 stage disease[90]. A recent study of 197 stage T2 GB carcinoma patients found that liver resection added to lymphadenectomy did not improve survival compared to lymphadenectomy alone, while it involved more blood loss and hospitalization. The 5-year disease-free survival was equivalent (82.7% vs 77.9%) in both T2a and T2b stages. A multivariate analysis showed lymph node involvement and perineural invasion, but not the absence of liver resection, as risk factors for worse survival outcomes. The researchers recommended T2 stage extended cholecystectomy with lymphadenectomy, without hepatic segment IV and V resection in selected cases[101]. Perineural invasion has been considered an important indicator of early recurrence and worse prognosis. In such cases, adjuvant chemotherapy has been associated with improvement in survival[102].
The importance of GB carcinoma location has been evaluated. Proximal tumors (neck and cystic duct) are less common, more frequently associated with obstructive jaundice, and had more aggressiveness with worse prognosis compared to distal tumors (body and fundus)[103]. Cystic duct location was an independent prognostic risk factor that did not have any survival benefit by common bile duct resection. Likewise, the latter also applies to distal locations, where extrahepatic bile resection may even be harmful[104]. A systematic review and meta-analysis confirmed increased morbidity, particularly in patients without jaundice[95].
For incidentally discovered GB carcinoma (stage ≥ Ib) after cholecystectomy, there have been different conflicting aspects about the optimal extent following surgical resection of the liver GB bed (≥ 2 cm wedge excision). A recent study of 111 patients found that there were no significant differences in overall survival after R0 resection independent of precise excised hepatic volume. However, a volume ≥ 105 cm3 was associated with increased morbidity. Thus, for ≥ T3 stage, the authors recommended a volume of 77.5 cm3-105 cm3, but some concerns have been raised regarding overall survival[105].
A recent study conducted in the United States including 791 GB carcinoma patients incidentally discovered after cholecystectomy stage T1b-T3 evaluated the optimal reresection time. The researchers found that the optimal reoperation time for improved overall survival was over 4 wk after initial surgery, but there were no significant differences when surgery was performed at 5-8 wk, 8-12 wk or more[106].
Hepatectomy for countable, treatable metastatic liver disease with increased CA 19-9 values after initial curative surgery for GB carcinoma has been described, but with high recurrence, mainly within the following 6 mo[107].
A recent large multicenter, retrospective, international study (Omega) including 3676 patients showed that hepatectomy did not improve survival, and extended operations increased morbidity and mortality without oncological benefit[3]. Likewise, Creasy et al[108] reported that there was a trend in the United States to perform fewer biliary and major liver resections[108]. Cho et al[109] reported that liver resection is not essential for curative treatment in the T2b stage[109].
Therapeutic excision has been recently proposed in selected cases for stage IV GB carcinoma, but with limited metastases. A study conducted in India of 1040 GB carcinoma patients, 234 of which were stage IV with low volume metastases (among them, 62 patients underwent R0 resection and adjuvant systemic therapy) found that patients in the operation group had better median overall survival (19 mo vs 12 mo) compared to those receiving only palliative chemotherapy[8].
The repeated surgical resection for GB carcinoma, as for intrahepatic cholangiocarcinoma, has recently gained attention, and Laurenzi et al[4] reported good outcomes[4]. Xie et al[110] found that the excision of primary tumors in metastatic advanced GB carcinoma may improve survival[110]. Chan et al[111] proposed frozen sections in suspected cases of GB carcinoma to confirm the diagnosis[111].
Textbook outcomes in liver surgery (TOLS) is a new assessment method designed by experts to evaluate the optimal course after liver excision. A multicenter study conducted in China including 11 tertiary hospitals and 242 patients found that TOLS was achieved in almost half of GB carcinoma patients who were treated by pressurized therapeutic excision. A multivariate analysis determined the following as independent prognostic factors for optimal outcome: Age ≤ 70 years; preoperative bilirubin ≤ 3 mg/dL; T1 stage; N0 stage; extended cholecystectomy; and no need for neoadjuvant therapy. Subsequently, a relevant accurate prediction nomogram by logistic regression analysis has been proposed[112].
High-quality surgery is essential in the management of GB carcinoma. An international multicenter study including 13 high-volume centers and 906 GB carcinoma patients with indented therapeutic surgery assessed the outcomes. Among them, 245 operations (27%) fulfilled the evaluation criteria, which included the following benchmark values: (1) Retrieved lymph nodes ≥ 4; (2) blood loss ≤ 350 mL; (3) blood transfusion ≤ 13%; (4) duration of operation ≤ 322 min; (5) hospitalization ≤ 8 d; (6) R1 resection (margin macroscopic infiltration) ≤ 7%; (7) overall complications ≤ 22%; and (8) complications of grade ≥ 3 at a rate of ≤ 11%[113].
Laparoscopic hepatic bisegmentectomy (segment IVb and V) and lymph node dissection for T1b or T2a are feasible, safe and effective with better short-term outcomes than open procedures and equivalent long-term outcomes[114]. Equivalent short-term and long-term oncological outcomes were exhibited by laparoscopic and open surgery for T3 stage incidentally discovered GB carcinoma[40].
However, a debate still exists between laparoscopic and open procedures. According to applicable guidelines of the National Comprehensive Cancer Network, the European Society of Medical Oncology, the Japanese Society of Hepato-Biliary-Pancreatic Surgery, and the Chinese Surgical Society and Chinese Committee of Biliary Surgeons, open surgery is recommended as a rule and laparoscopic surgery only in selected cases and for research purposes[115-118]. This reco
Chemotherapy has seen increased acceptance in the management of GB carcinoma in combination with systemic therapy, either as neoadjuvant therapy for downstaging in borderline resectable cases and adjuvant therapy in operable cases or as palliative treatment in inoperable cases[128-130]. Perioperative chemotherapy is increasingly being used[131]. Neoadjuvant chemotherapy may increase survival[132].
There are several schemes, but gemcitabine with cisplatin (the standard chemotherapy) in combination with immunotherapy with pembrolizumad or durvalumab and targeted treatment as first-line therapy is widely preferable[128,133]. For second-line therapy, folonic acid (leucovorin), 5-fluorouracil, oxaliplatin (FOLFOX) or irinotecan with 5-fluorouracil is indicated[128]. FOLFOX chemotherapy has been proven effective after gemcitabine-cisplatin treatment in increasing overall survival at 6 and 12 mo[134].
Adjuvant chemotherapy alone or combined with radiotherapy is recommended in every case of ≥ T2 disease stage. However, it has absolute indications in high-risk patients, which are those with positive lymph node involvement, microscopic infiltration of the resection margin (R1 resection), perineural invasion or vascular invasion[135-137].
Neoadjuvant chemotherapy with gemcitabine-cisplatin followed by curative surgery increased survival compared to surgery alone[138]. For advanced inoperable GB carcinoma, the combination of gemcitabine and either cisplatin or S-1 (modified regimens of 5-fluorouracil) or gemcitabine, cisplatin, and S-1 has been used as first-line chemotherapy[116].
Chemoresistance, particularly in gemcitabine of GB carcinoma or even cisplatin, occurs frequently and constitutes an important problem that affects treatment efficacy[139]. This is attributed to the effects of the produced cytokines against apoptosis. Expression of some miRNAs (miR-125b-5p, miR-205-5p, miR-31) are implicated in this drug resistance and could be used as diagnostic biomarkers. In addition, recent research efforts have focused on the innovation of novel drugs that could overcome chemoresistance[140].
In the case of high microsatellite instability and chemoresistance, the immunotherapeutic pembrolizumad may be efficient[116]. In cases of locally advanced or inoperable metastatic stage IV GB carcinoma, the addition of nab-paclitaxel to the standard gemcitabine-cisplatin treatment (GCNP scheme) as first-line therapy exhibited an increased response with potential resection after downstaging and prolonged survival[141]. Patients with resistance to gemcitabine-cisplatin as first-line therapy may benefit from second-line therapy with trifluridine-tipiracil and irinotecan[48].
It has been recently proposed that adjuvant combined chemoradiation therapy instead of chemotherapy alone provides improved survival after curative surgical resection, particularly for those with advanced stage (III, IV) disease and lymph node-positive cases[142-144]. Chemoradiotherapy had a beneficial effect independent of lymph node status[145].
Despite the increasing application of immunotherapy in many carcinomas, its use in GB carcinoma is still limited[146-148]. Immunotherapy includes targeting checkpoint inhibitors such as monotherapy, vaccines, oncolytic viruses, adoptive cells, and cytokines (interleukin-2, interferon-α, granulocyte-macrophage colony-stimulating factor). Monoclonal antibodies against programmed cell death 1 (PD-1) (pembrolizumab, nivolumab and camrelizumab) and PD-L1 (durvalumab and atezolizumab) have been applied during the last decade in various solid tumors[146,147]. However, there are still no reliable markers predicting immunotherapy effectiveness[147].
A heterogeneous immune microenvironment and increased expression of the immune checkpoint inhibitors PD-1 and TIM3 have been correlated to worse prognosis. Thus, their simultaneous blockage as potential targets could be an effective novel treatment[145].
In inoperable advanced cases, the combination of treatment (KEYNOT-966 trial) by pembrolizumab with gemcitabine and cisplatin improved survival compared to treatment with gemcitabine and cisplatin alone[133]. Additionally, the TOPAZ 1 trial used durvalumab instead of pembrolizumab as immunotherapy in the same scheme for such cases, with similar results[148]. Nevertheless, immunotherapy in such advanced cases improved unbearable pain relief, reducing opioid use[149].
Molecular profiles and a better understanding of the mechanisms involved in carcinogenesis, growth, invasion and metastasis have led to the search for certain targetable mutated genes by monoclonal antibody biological targeted treatment in solid tumors. This endeavor may improve survival and constitute a short future research direction. However, for GB carcinoma, more work is needed in the field[42,44,150].
Several targeted genomics, including potent sensitive mutations, have been assessed against the genes PD-1, PDL-1, HER2, VEGF, N-cadherin, VEGFR, EGFR, mTOR, MET, PI3K, MEK1, and MEK2[15]. For targeted therapy, the following factors are used: (1) NTRK gene alterations (larotrectinib or entrectinib); (2) BRAF V600E (B-Raf proto-oncogene serine/threonine kinase) mutated gene (combination of dabrafenib with trametinib)[151]; (3) HER2 (human epidermal growth factor-2) gene reinforcement (trastuzumab with pertuzumab or trastuzumab with deruxtecan)[49]; (4) RET gene alterations (selpercatinib); (5) FGFR-2 gene (fibroblast growth factor receptor-2) alterations (multityrosine kinase inhibitors, i.e. pemigatinib, infigratinib, futibatinib, derazantinib, erdafitinib, ponatinib, debio-1347); (6) EGFR (cetuximab, panitumumab); and (7) IDH inhibitors (ivosidenib)[20,42,46,128,152].
The role of radiation therapy in GB carcinoma is limited. It is usually combined with chemotherapy as adjuvant or neoadjuvant treatment[142-144]. The progress made by precise stereotactic body radiotherapy has limited side effects by providing accurate targeting and increased effectiveness[128,153]. Patients with lung and lymph node metastases had better response to radiotherapy[154]. It has also been recommended in combination with chemotherapy after R1 resection[155].
Seventy percent of GB carcinomas are not amenable to radical surgery. However, for those receiving curative resection, recurrence is common. Early recurrence predicts worse prognosis[60]. A recent study of 1601 GB carcinoma patients who underwent surgical resection were followed for survival. The 5-year survival rate for stage I was 82.7%, 73.4% for stage II, 31.9% for stage IIIA, 24.1% for stage IIIB, and 10% for stage IV. They found that adjuvant treatment had a beneficial effect[156]. Likewise, a study in Japan of 200 GB carcinoma patients who underwent surgical resection reported the following 5-year overall survival rates: Stage I, 90.8%; stage IIA, 94.4%; stage IIB, 73.6%; stage IIIA, 33.7%; stage IIIB, 57.7%; stage IVA, 14.3%; and stage IVB, 11.8%[88]. A study conducted in Australia including 104 patients with GB carcinoma and a median follow-up of 60 mo found a median overall survival of 35 mo in those with intended curative resection and 4 mo in inoperable cases with palliative treatment[36]. For a T1b follow-up of 69.9 mo, the disease-free survival was 92%[50]. For advanced stage III-IV GB carcinoma patients, the overall survival was as follows: 1-year at 47.6%; 2-year at 29.1%; and 3-year at 19.9%[157].
The site of metastases (liver, distant lymph nodes, lung, bone, brain) may have an effect on survival. The most common sites of liver and lymph nodes can be affected by primary tumor resection[158]. More removed lymph nodes (above 6) are associated with better survival when they are negative[159].
Revealing residual disease in the specimen of the second operation after incidental discovery of GB carcinoma with cholecystectomy predicts worse prognosis, despite the achievement of R0 resection[160]. The ratio of γ-glutamyl transferase to platelet count may predict GB carcinoma patient survival and was included in a relevant nomogram[161]. There are several prognostic scoring systems and nomograms predicting survival[162-166] or lymph node metastases[167]. Three immunological markers (TRAIL, TIE2, CSF1) in plasma were associated with survival after surgery[168]. Infiltrated lymph nodes and the degree of cell differentiation in stage T1b-T2 GB carcinoma restrict survival[169]. The presence of acute cholecystitis in GB carcinoma patients had a negative influence on prognosis[170].
A recent multicenter study conducted in Japan of 290 patients found that postoperative infectious complications negatively affect prognosis. They reported a median overall survival in the infection group of 38 mo vs 62 mo in the noninfection group[171].
Food insecurity may affect long-term postoperative outcomes, reducing survival[172].
It was found that immunohistochemical markers MRP2, CXCR4, and PD-L1 were associated with increased survival and could be used as prognostic biomarkers[173]. Additionally, PD-L1 and NY-ESO1 are indicative markers for immunotherapy[150,174]. The predictive factors for favorable prognosis are shown in Table 3[34,112,156,169,170].
| No. | Factor |
| 1 | Age ≤ 70 yr |
| 2 | Stage Tis, T1-T2 |
| 3 | R0 resection |
| 4 | G1 cell differentiation |
| 5 | Absence of lymph node infiltration |
| 6 | Absence of perineural invasion |
| 7 | Distant tumor location (body, fundus) |
| 8 | Preoperative bilirubin ≤ 3 mg/dL |
| 9 | Absence of concurrent acute cholecystitis |
| 10 | Absence of postoperative infectious complications |
| 11 | Absence of early recurrence |
| 12 | Multimodality treatment |
In summary, surgery is the main therapeutic first step; nevertheless, it is impracticable in most cases, and when it is achieved, it is often accompanied by recurrence. Neoadjuvant treatment may ensure better local control in a manner of downstaging the disease. Moreover, it allows potential R0 resection, contributing to limited recurrence[175]. The use of 6 mo of capecitabine treatment after therapeutic excision represents a standard cure. However, novel adjuvant systemic treatment opens new perspectives[176]. First-line treatment with cisplatin and gemcitabine combined with immune checkpoint inhibitors and targeted therapy has broadened the management horizons of advanced biliary tract carcinoma[177,178].
GB carcinoma is relatively uncommon but has a dismal prognosis. It is usually diagnosed in an advanced, inoperable stage, where palliative systematic treatment is the only option. However, progress in diagnostic modalities may allow for earlier detection, and its management is rapidly evolving. Radical surgery followed by adjuvant therapy with capecitabine and cisplatin as the first-line therapy, followed by 5-fluorouracil and oxaliplatin-irinotecan as the second-line therapy, offers the best chance for a cure. Management through surgery is dependent on T stage classifications. However, the most extended operative procedures remain in recent debate. Multimodality treatment is needed. Targeted therapies and immunotherapy constitute novel treatments but do not have the same efficacy as in other solid tumors. Research efforts are ongoing with promising prospects. An individualized approach could be more appropriate.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ricci AD, Italia S-Editor: Fan JR L-Editor: Filipodia P-Editor: Zhang XD
| 1. | Vuthaluru S, Sharma P, Chowdhury S, Are C. Global epidemiological trends and variations in the burden of gallbladder cancer. J Surg Oncol. 2023;128:980-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (1)] |
| 2. | de Reuver PR, van der Post RS. Clinicopathological and Molecular Insights into Gallbladder Cancer. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 3. | Balakrishnan A, Barmpounakis P, Demiris N, Jah A, Spiers HVM, Talukder S, Martin JL, Gibbs P, Harper SJF, Huguet EL, Kosmoliaptsis V, Liau SS, Praseedom RK, Basu B, de Aretxabala X, Lendoire J, Maithel S, Branes A, Andersson B, Serrablo A, Adsay V; OMEGA Study Investigators. Surgical outcomes of gallbladder cancer: the OMEGA retrospective, multicentre, international cohort study. EClinicalMedicine. 2023;59:101951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (2)] |
| 4. | Laurenzi A, Brandi G, Greco F, Prosperi E, Palloni A, Serenari M, Frega G, Ravaioli M, Rizzo A, Cescon M. Can repeated surgical resection offer a chance of cure for recurrent cholangiocarcinoma? Langenbecks Arch Surg. 2023;408:102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 5. | Kim D, Konyn P, Cholankeril G, Bonham CA, Ahmed A. Trends in the Mortality of Biliary Tract Cancers Based on Their Anatomical Site in the United States From 2009 to 2018. Am J Gastroenterol. 2021;116:1053-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (2)] |
| 6. | Shahin RK, Elkady MA, Abulsoud AI, Abdelmaksoud NM, Abdel Mageed SS, El-Dakroury WA, Zewail MB, Elazazy M, Sobhy MH, Nomier Y, Elazazy O, Elballal MS, Mohammed OA, Midan HM, Elrebehy MA, Ziada BO, Doghish AS. miRNAs orchestration of gallbladder cancer - Particular emphasis on diagnosis, progression and drug resistance. Pathol Res Pract. 2023;248:154684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 7. | Cassese G, Han HS, Yoon YS, Lee JS, Cho JY, Lee HW, Lee B, Troisi RI. Preoperative Assessment and Perioperative Management of Resectable Gallbladder Cancer in the Era of Precision Medicine and Novel Technologies: State of the Art and Future Perspectives. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Reference Citation Analysis (1)] |
| 8. | Patkar S, Patel S, Kazi M, Goel M. Radical surgery for stage IV gallbladder cancers: Treatment strategies in patients with limited metastatic burden. Ann Hepatobiliary Pancreat Surg. 2023;27:180-188. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Malik H, Izwan S, Ng J, Teng R, Chan E, Damodaran Prabha R, Puhalla H. Incidence and management of gallbladder cancer in cholecystectomy specimens: a 5-year tertiary centre experience. ANZ J Surg. 2023;93:2481-2486. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Hemminki K, Försti A, Hemminki O, Liska V, Hemminki A. Long-term incidence and survival trends in cancer of the gallbladder and extrahepatic bile ducts in Denmark, Finland, Norway and Sweden with etiological implications related to Thorotrast. Int J Cancer. 2022;151:200-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Van Dyke AL, Shiels MS, Jones GS, Pfeiffer RM, Petrick JL, Beebe-Dimmer JL, Koshiol J. Biliary tract cancer incidence and trends in the United States by demographic group, 1999-2013. Cancer. 2019;125:1489-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 12. | Ganeshan D, Kambadakone A, Nikolaidis P, Subbiah V, Subbiah IM, Devine C. Current update on gallbladder carcinoma. Abdom Radiol (NY). 2021;46:2474-2489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Akbar N, Yaseen T, Muhammad A, Danish M, Adeel M, Khan SA, Ismail H, Bajaj K, Ali I, Panezai MQ, Tareen M, Tasneem AA, Laeeq SM, Hanif F, Luck NH. A Tertiary Care Center's Experience with Clinicopathological Characteristics of Gallbladder Carcinoma in Our Population. Euroasian J Hepatogastroenterol. 2022;12:35-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Singh N, Zaidi A, Kaur R, Kaur J, Nijhawan VS. Incidental Gallbladder Neoplasms: A Growing Global Burden. Cureus. 2022;14:e25805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Mishra SK, Kumari N, Krishnani N. Molecular pathogenesis of gallbladder cancer: An update. Mutat Res. 2019;816-818:111674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Kellil T, Chaouch MA, Aloui E, Tormane MA, Taieb SK, Noomen F, Zouari K. Incidence and Preoperative Predictor Factors of Gallbladder Cancer Before Laparoscopic Cholecystectomy: a Systematic Review. J Gastrointest Cancer. 2021;52:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Corten BJGA, van Kuijk SMJ, Leclercq WKG, Janssen L, Roumen RMH, Dejong CHC, Slooter GD; incidental Gallbladder Cancer Collaborative Group. A Dutch prediction tool to assess the risk of incidental gallbladder cancers after cholecystectomies for benign gallstone disease. HPB (Oxford). 2023;25:409-416. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Jackson SS, Marks MA, Katki HA, Cook MB, Hyun N, Freedman ND, Kahle LL, Castle PE, Graubard BI, Chaturvedi AK. Sex disparities in the incidence of 21 cancer types: Quantification of the contribution of risk factors. Cancer. 2022;128:3531-3540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 91] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 19. | Mishra S, Kumari S, Srivastava P, Pandey A, Shukla S, Husain N. Genomic profiling of gallbladder carcinoma: Targetable mutations and pathways involved. Pathol Res Pract. 2022;232:153806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (1)] |
| 20. | Chae H, Kim D, Yoo C, Kim KP, Jeong JH, Chang HM, Lee SS, Park DH, Song TJ, Hwang S, Kim KH, Song GW, Ahn CS, Lee JH, Hwang DW, Kim SC, Jang SJ, Hong SM, Kim TW, Ryoo BY. Therapeutic relevance of targeted sequencing in management of patients with advanced biliary tract cancer: DNA damage repair gene mutations as a predictive biomarker. Eur J Cancer. 2019;120:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 21. | Koshiol J, Zhu B, Wang R, Hildesheim A, Gao YT, Egner PA, Yuan JM, Groopman JD. Association of aflatoxin with gallbladder cancer in a case-control study nested within a Chinese cohort. Int J Cancer. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 22. | Goyal S, Prasad G, Chaudhary D, Sakhuja P, Srivastava S, Aggarwal AK. Role of Guided FNA in Gallbladder Cancer: A Retrospective 3-Year Study. J Cytol. 2023;40:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Zhou H, Chen J, Jin H, Liu K. Genetic characteristics and clinical-specific survival prediction in elderly patients with gallbladder cancer: a genetic and population-based study. Front Endocrinol (Lausanne). 2023;14:1159235. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Samuel S, Mukherjee S, Ammannagari N, Pokuri VK, Kuvshinoff B, Groman A, LeVea CM, Iyer R. Clinicopathological characteristics and outcomes of rare histologic subtypes of gallbladder cancer over two decades: A population-based study. PLoS One. 2018;13:e0198809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Mochidome N, Koga Y, Ohishi Y, Miyazaki T, Matsuda R, Yamada Y, Aishima S, Nakamura M, Oda Y. Prognostic implications of the coexisting precursor lesion types in invasive gallbladder cancer. Hum Pathol. 2021;114:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Yang WW, Fang YT, Niu YR, Sun YK. Comparison of clinicopathological characteristics and survival outcomes between gallbladder mucinous adenocarcinoma and gallbladder adenocarcinoma: A propensity score-matched study. World J Gastrointest Oncol. 2023;15:1436-1450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 27. | Zhou S, Han S, Chen W, Bai X, Pan W, Han X, He X. Radiomics-based machine learning and deep learning to predict serosal involvement in gallbladder cancer. Abdom Radiol (NY). 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Obmann VC, Grosse-Hokamp N, Alberts I, Fulton N, Rassouli N, Siegel C, Avril N, Herrmann KA. Diagnosis and staging of hepatobiliary malignancies: Potential incremental value of (18)F-FDG-PET/MRI compared to MRI of the liver. Nuklearmedizin. 2021;60:355-367. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Saini VK, Hassan A, Singh AK, Ora M, Gambhir S. Port-Site Metastases in Incidentally Detected Carcinoma Gall Bladder: Role of 18F-FDG PET/CT and Patient Outcome. J Gastrointest Cancer. 2022;53:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Ma KW, Cheung TT, She WH, Chok KSH, Chan ACY, Dai WC, Chiu WH, Lo CM. Diagnostic and Prognostic Role of 18-FDG PET/CT in the Management of Resectable Biliary Tract Cancer. World J Surg. 2018;42:823-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Moradi F, Iagaru A. The Role of Positron Emission Tomography in Pancreatic Cancer and Gallbladder Cancer. Semin Nucl Med. 2020;50:434-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | van Dooren M, de Savornin Lohman EAJ, Brekelmans E, Vissers PAJ, Erdmann JI, Braat AE, Hagendoorn J, Daams F, van Dam RM, de Boer MT, van den Boezem PB, Koerkamp BG, de Reuver PR. The diagnostic value of staging laparoscopy in gallbladder cancer: a nationwide cohort study. World J Surg Oncol. 2023;21:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 33. | Rahul, Haldeniya K, Singh A, Bhatt N, Mishra P, Singh RK, Saxena R. Determinants of curative resection in incidental gallbladder carcinoma with special reference to timing of referral. Ann Hepatobiliary Pancreat Surg. 2021;25:492-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 34. | Zhou Y, Chen S, Wu Y, Li L, Lou Q, Chen Y, Xu S. Multi-clinical index classifier combined with AI algorithm model to predict the prognosis of gallbladder cancer. Front Oncol. 2023;13:1171837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 35. | Goel M, Gupta AM, Patkar S, Parray AM, Shetty N, Ramaswamy A, Patil P, Chopra S, Ostwal V, Kulkarni S, Engineer R, Mehta S. Towards standardization of management of gallbladder carcinoma with obstructive jaundice: Analysis of 113 cases over 10 years at a single institution. J Surg Oncol. 2021;124:572-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Wietsma MFT, Molloy C, Bhimani N, de Savornin Lohman EAJ, Gill AJ, Andrici J, Samra J, de Reuver PR, Hugh TJ. Gallbladder carcinoma outcomes in an Australian tertiary referral hospital. ANZ J Surg. 2021;91:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Kwon W, Kim H, Han Y, Hwang YJ, Kim SG, Kwon HJ, Vinuela E, Járufe N, Roa JC, Han IW, Heo JS, Choi SH, Choi DW, Ahn KS, Kang KJ, Lee W, Jeong CY, Hong SC, Troncoso AT, Losada HM, Han SS, Park SJ, Kim SW, Yanagimoto H, Endo I, Kubota K, Wakai T, Ajiki T, Adsay NV, Jang JY. Role of tumour location and surgical extent on prognosis in T2 gallbladder cancer: an international multicentre study. Br J Surg. 2020;107:1334-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 38. | Kim KH, Thrastardottir TO, Choi SH. The technique of laparoscopic and robotic extended cholecystectomy for gallbladder cancer. J Minim Invasive Surg. 2023;26:43-45. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Ahmed SH, Usmani SUR, Mushtaq R, Samad S, Abid M, Moeed A, Atif AR, Farhan SA, Saif A. Role of laparoscopic surgery in the management of gallbladder cancer: Systematic review & meta-analysis. Am J Surg. 2023;225:975-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Jin YW, Ma WJ, Gao W, Li FY, Cheng NS. Laparoscopic versus open oncological extended re-resection for incidental gallbladder adenocarcinoma: we can do more than T1/2. Surg Endosc. 2023;37:3642-3656. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 41. | Ruff SM, Cloyd JM, Pawlik TM. Annals of Surgical Oncology Practice Guidelines Series: Management of Primary Liver and Biliary Tract Cancers. Ann Surg Oncol. 2023;30:7935-7949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | LaPelusa M, Heumann T, Goff L, Agarwal R. Targeted therapies in advanced biliary tract cancers-a narrative review. Chin Clin Oncol. 2023;12:14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | White MJ, Marmor S, Brauer D, Ankeny J, Gupta A, Prakash A, Jensen EH. Physician decision-making in the use of adjuvant chemotherapy for lymph node-positive gallbladder cancer. HPB (Oxford). 2023;25:1603-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Queiroz MM, Lima NF Jr, Biachi de Castria T. Immunotherapy and Targeted Therapy for Advanced Biliary Tract Cancer: Adding New Flavors to the Pizza. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 45. | Khan TM, Verbus EA, Hong H, Ethun CG, Maithel SK, Hernandez JM. Perioperative Versus Adjuvant Chemotherapy in the Management of Incidentally Found Gallbladder Cancer (OPT-IN). Ann Surg Oncol. 2022;29:37-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Lamarca A, Edeline J, Goyal L. How I treat biliary tract cancer. ESMO Open. 2022;7:100378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 47. | Ortiz Rueda B, Endo Y, Tsilimigras DI, Araujo Lima H, Munir MM, Woldesenbet S, Dillhoff M, Ejaz A, Cloyd J, Pawlik TM. Impact of Medicaid expansion on the multimodal treatment of biliary tract cancer. J Surg Oncol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 48. | Kehmann L, Berres ML, Gonzalez-Carmona M, Modest DP, Mohr R, Wree A, Venerito M, Strassburg C, Keitel V, Trautwein C, Luedde T, Roderburg C. Study protocol of an open-label, single arm phase II trial investigating the efficacy and safety of Trifluridine/Tipiracil combined with irinotecan as a second line therapy in patients with cholangiocarcinoma (TRITICC). BMC Cancer. 2023;23:470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 49. | Neuzillet C, Artru P, Assenat E, Edeline J, Adhoute X, Sabourin JC, Turpin A, Coriat R, Malka D. Optimizing Patient Pathways in Advanced Biliary Tract Cancers: Recent Advances and a French Perspective. Target Oncol. 2023;18:51-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 50. | Pehlivanoglu B, Akkas G, Memis B, Basturk O, Reid MD, Saka B, Dursun N, Bagci P, Balci S, Sarmiento J, Maithel SK, Bandyopadhyay S, Escalona OT, Araya JC, Losada H, Goodman M, Knight JH, Roa JC, Adsay V. Reappraisal of T1b gallbladder cancer (GBC): clinicopathologic analysis of 473 in situ and invasive GBCs and critical review of the literature highlights its rarity, and that it has a very good prognosis. Virchows Arch. 2023;482:311-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 51. | Juengpanich S, Li S, Yang T, Xie T, Chen J, Shan Y, Lee J, Lu Z, Chen T, Zhang B, Cao J, Hu J, Yu J, Wang Y, Topatana W, Gu Z, Cai X, Chen M. Pre-activated nanoparticles with persistent luminescence for deep tumor photodynamic therapy in gallbladder cancer. Nat Commun. 2023;14:5699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 52. | Lv TR, Hu HJ, Regmi P, Liu F, Li FY. The effect of preoperative jaundice in the surgical management of gallbladder carcinoma: An updated meta-analysis. ANZ J Surg. 2021;91:E455-E464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 53. | Mohakud S, Sidhu S, Deep N, Naik S. Panorama of multidetector-row computed tomography findings of carcinoma gall bladder - A retrospective observational study. J Cancer Res Ther. 2022;18:661-667. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 54. | Parida GK, Panda RA, Agrawal K. Impact of fluorine-18-fluorodeoxyglucose PET/computed tomography in staging of patients with gallbladder cancer: a systematic review and meta-analysis. Nucl Med Commun. 2021;42:846-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Goel S, Aggarwal A, Iqbal A, Gupta M, Rao A, Singh S. 18-FDG PET-CT should be included in preoperative staging of gall bladder cancer. Eur J Surg Oncol. 2020;46:1711-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 56. | Sabaté-Llobera A, Reynés-Llompart G, Mestres-Martí J, Gràcia-Sánchez L, Lladó L, Serrano T, Ramos E, Cortés-Romera M. Imaging Gallbladder Lesions: What Can Positron Emission Tomography/Computed Tomography Add to the Conventional Imaging Approach? J Comput Assist Tomogr. 2023;47:343-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 57. | Ogura T, Bessho K, Hattori N, Tomita M, Nishikawa H. Technical aspects of transpapillary biopsy for gallbladder cancer using a novel cholangioscope. Endoscopy. 2023;55:E1085-E1086. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 58. | Crino SF, Rimbaș M, Gabbrielli A, Larghi A. Endoscopic Ultrasound Guided Gallbladder Interventions: a Review of the Current Literature. J Gastrointestin Liver Dis. 2019;28:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Yane K, Tomita Y, Yoshida M, Sasaki K, Imagawa T, Morita K, Ihara H, Minagawa T, Okagawa Y, Hirayama M, Sumiyoshi T, Kondo H. Initial experience of transpapillary gallbladder biopsy using newly designed device delivery system. Endosc Int Open. 2023;11:E613-E617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 60. | Li Q, Li N, Gao Q, Liu H, Xue F, Cheng Y, Li W, Chen C, Zhang D, Geng Z. The clinical impact of early recurrence and its recurrence patterns in patients with gallbladder carcinoma after radical resection. Eur J Surg Oncol. 2023;49:106959. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 61. | Agrawal S, Saxena R. Raised carbohydrate antigen 19-9 levels detect recurrences and impacts overall and disease-free survival in radically resected gallbladder cancer: A simple surveillance marker? J Cancer Res Ther. 2023;19:273-277. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 62. | Yan C, Xiao Y, Zhang W, Sun Y, Lin Y, Cai W. Circulating Tumor Cells are an Independent Risk Factor for Poor Prognosis in Patients with Gallbladder Adenocarcinoma. Ann Surg Oncol. 2023;30:7966-7975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | Lederer AK, Rasel H, Kohnert E, Kreutz C, Huber R, Badr MT, Dellweg PKE, Bartsch F, Lang H. Gut Microbiota in Diagnosis, Therapy and Prognosis of Cholangiocarcinoma and Gallbladder Carcinoma-A Scoping Review. Microorganisms. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 64. | He Z, Zhong Y, Hu H, Li F. ZFP64 Promotes Gallbladder Cancer Progression through Recruiting HDAC1 to Activate NOTCH1 Signaling Pathway. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 65. | Li G, Pu P, Pan M, Weng X, Qiu S, Li Y, Abbas SJ, Zou L, Liu K, Wang Z, Shao Z, Jiang L, Wu W, Liu Y, Shao R, Liu F. Topological reorganization and functional alteration of distinct genomic components in gallbladder cancer. Front Med. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 66. | Mani R, Gupta A, Gupta S, Goyal B, Mishra R, Tandon A, Sharma O, Rohilla KK, Kishore S, Dhar P. Expression of ER, PR, and HER-2 Neu and correlation with tumor markers in gall bladder carcinoma. J Cancer Res Ther. 2023;19:1279-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 67. | Verma P, Gupta P, Gupta N, Srinivasan R, Dutta U, Sharma S, Uppal R, Nada R, Lal A. HER2/ERBB2 overexpression in advanced gallbladder carcinoma: comprehensive evaluation by immunocytochemistry and fluorescence in situ hybridisation on fine-needle aspiration cytology samples. J Clin Pathol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 68. | Li M, Liu F, Zhang F, Zhou W, Jiang X, Yang Y, Qu K, Wang Y, Ma Q, Wang T, Bai L, Wang Z, Song X, Zhu Y, Yuan R, Gao Y, Liu Y, Jin Y, Li H, Xiang S, Ye Y, Zhang Y, Jiang L, Hu Y, Hao Y, Lu W, Chen S, Gu J, Zhou J, Gong W, Wang X, Liu X, Liu C, Liu H. Genomic ERBB2/ERBB3 mutations promote PD-L1-mediated immune escape in gallbladder cancer: a whole-exome sequencing analysis. Gut. 2019;68:1024-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (1)] |
| 69. | Cheng H, Sun Y, Yu X, Zhou D, Ding J, Wang S, Ma F. FASN promotes gallbladder cancer progression and reduces cancer cell sensitivity to gemcitabine through PI3K/AKT signaling. Drug Discov Ther. 2023;17:328-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 70. | Ma Q, Zhang Y, Liang H, Zhang F, Liu F, Chen S, Hu Y, Jiang L, Hao Y, Li M, Liu Y. EMP3 as a key downstream target of miR-663a regulation interferes with MAPK/ERK signaling pathway to inhibit gallbladder cancer progression. Cancer Lett. 2023;575:216398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 71. | Bai X, Chen J, Zhang W, Zhou S, Dong L, Huang J, He X. YTHDF2 promotes gallbladder cancer progression and gemcitabine resistance via m6A-dependent DAPK3 degradation. Cancer Sci. 2023;114:4299-4313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 72. | Hong L, Chen M, Huang M, Chen W, Abudukeremu X, She F, Chen Y. FOXA2 suppresses gallbladder carcinoma cell migration, invasion, and epithelial-mesenchymal transition by targeting SERPINB5. Environ Toxicol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 73. | Qin J, Cui Z, Zhou J, Zhang B, Lu R, Ding Y, Hu H, Cai J. IGF2BP3 drives gallbladder cancer progression by m6A-modified CLDN4 and inducing macrophage immunosuppressive polarization. Transl Oncol. 2023;37:101764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 74. | Huang M, Zhong F, Chen M, Hong L, Chen W, Abudukeremu X, She F, Chen Y. CEP55 as a promising biomarker and therapeutic target on gallbladder cancer. Front Oncol. 2023;13:1156177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 75. | Huang J, Zheng M, Li Y, Xu D, Tian D. DLGAP5 promotes gallbladder cancer migration and tumor-associated macrophage M2 polarization by activating cAMP. Cancer Immunol Immunother. 2023;72:3203-3216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 76. | Zimmer K, Kocher F, Untergasser G, Kircher B, Amann A, Baca Y, Xiu J, Korn WM, Berger MD, Lenz HJ, Puccini A, Fontana E, Shields AF, Marshall JL, Hall M, El-Deiry WS, Hsiehchen D, Macarulla T, Tabernero J, Pichler R, Khushman M, Manne U, Lou E, Wolf D, Sokolova V, Schnaiter S, Zeimet AG, Gulhati P, Widmann G, Seeber A. PBRM1 mutations might render a subtype of biliary tract cancers sensitive to drugs targeting the DNA damage repair system. NPJ Precis Oncol. 2023;7:64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 77. | Xu M, Jiang B, Man Z, Zhu H. TRIM37 promotes gallbladder cancer proliferation by activating the Wnt/β-catenin pathway via ubiquitination of Axin1. Transl Oncol. 2023;35:101732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 78. | Ahn AR, Karamikheirabad M, Park MS, Zhang J, Kim HS, Jeong JS, Kim KM, Park HS, Jang KY. Expression Patterns of PAK4 and PHF8 Are Associated with the Survival of Gallbladder Carcinoma Patients. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 79. | Cao J, Shao H, Hu J, Jin R, Feng A, Zhang B, Li S, Chen T, Jeungpanich S, Topatana W, Tian Y, Lu Z, Cai X, Chen M. Identification of invasion-metastasis associated MiRNAs in gallbladder cancer by bioinformatics and experimental validation. J Transl Med. 2022;20:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 80. | Liu L, Xiao W, Yang Z, Wang Q, Yi J. Human umbilical cord mesenchymal stem cell-derived exosomal miR-214-3p regulates the progression of gallbladder cancer by regulating ACLY/GLUT1. Adv Clin Exp Med. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 81. | Jiang W, Zhao B, Li Y, Qi D, Wang D. Modification of the 8th American Joint Committee on Cancer staging system for gallbladder carcinoma to improve prognostic precision. BMC Cancer. 2020;20:1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 82. | Xu X, Wang J, Duan Q. Effects of surgery on survival of elderly patients with gallbladder cancer: A propensity score matching analysis of the SEER database. Front Oncol. 2023;13:1083618. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 83. | Zhou Y, Yuan K, Yang Y, Ji Z, Zhou D, Ouyang J, Wang Z, Wang F, Liu C, Li Q, Zhang Q, Shan X, Zhou J. Gallbladder cancer: current and future treatment options. Front Pharmacol. 2023;14:1183619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 84. | Mellado S, Chirban AM, Rivera B, Panettieri E, Vega EA. ASO Author Reflections: Precision in Gallbladder Cancer Care: Present Challenges and Future Directions. Ann Surg Oncol. 2023;30:6601-6602. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 85. | Wang J, Liu F, Ma W, Hu H, Li F. Metastatic lymph node ratio as an important prognostic factor in advanced gallbladder carcinoma with at least 6 lymph nodes retrieved. Langenbecks Arch Surg. 2023;408:382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 86. | Sun J, Xie TG, Ma ZY, Wu X, Li BL. Current status and progress in laparoscopic surgery for gallbladder carcinoma. World J Gastroenterol. 2023;29:2369-2379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (1)] |
| 87. | Washington K, Rocha F. Approach to Resectable Biliary Cancers. Curr Treat Options Oncol. 2021;22:97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 88. | Yuza K, Sakata J, Hirose Y, Miura K, Ando T, Katada T, Takizawa K, Kobayashi T, Ichikawa H, Shimada Y, Nagahashi M, Wakai T. Outcome of radical surgery for gallbladder carcinoma according to TNM stage: implications for adjuvant therapeutic strategies. Langenbecks Arch Surg. 2021;406:801-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 89. | Kasumova GG, Tabatabaie O, Najarian RM, Callery MP, Ng SC, Bullock AJ, Fisher RA, Tseng JF. Surgical Management of Gallbladder Cancer: Simple Versus Extended Cholecystectomy and the Role of Adjuvant Therapy. Ann Surg. 2017;266:625-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 90. | Wu B, Shen Y, Chen X, Wang X, Zhong Z. Effect of lymphadenectomy on the prognosis for N0 gallbladder carcinoma patients: A study based on SEER database. Cancer Med. 2021;10:7136-7143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 91. | Ji Z, Ren L, Liu F, Liu L, Song J, Zhu J, Ji G, Huang G. Effect of different surgical options on the long-term survival of stage I gallbladder cancer: a retrospective study based on SEER database and Chinese Multi-institutional Registry. J Cancer Res Clin Oncol. 2023;149:12297-12313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 92. | Maegawa FB, Ashouri Y, Hamidi M, Hsu CH, Riall TS. Gallbladder Cancer Surgery in the United States: Lymphadenectomy Trends and Impact on Survival. J Surg Res. 2021;258:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 93. | Toge K, Sakata J, Hirose Y, Yuza K, Ando T, Soma D, Katada T, Miura K, Takizawa K, Kobayashi T, Wakai T. Lymphatic spread of T2 gallbladder carcinoma: Regional lymphadenectomy is required independent of tumor location. Eur J Surg Oncol. 2019;45:1446-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 94. | Tran Cao HS, Zhang Q, Sada YH, Chai C, Curley SA, Massarweh NN. The role of surgery and adjuvant therapy in lymph node-positive cancers of the gallbladder and intrahepatic bile ducts. Cancer. 2018;124:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 95. | Lv TR, Liu F, Hu HJ, Regmi P, Ma WJ, Yang Q, Jin YW, Li FY. The role of extra-hepatic bile duct resection in the surgical management of gallbladder carcinoma. A first meta-analysis. Eur J Surg Oncol. 2022;48:482-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 96. | Tang X, Zheng K, Huang J, Hu W, Xu L, Xu Q, Fan Y, Liu J, Li B, Ran L, Liu T, Liang B, Xiong H, Li W, Fu X, Fang L. Effect of different lymph node dissection methods on the number of lymph nodes detected and prognosis in gallbladder cancer. Medicine (Baltimore). 2023;102:e34163. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 97. | Jia ZY, Zhu YD, Wu XS, Yang JX, Wu WG, Wang XA, He M, Wang H, Yang LH, Zhang J, Li XC, Zou L, Li HF, Zhang F, Bao RF, Cui XY, Song XL, Chen W, Gong W, Li ML, Liu YB. Improved long-term outcomes after innovative preoperative evaluation and conception of precise surgery for gallbladder cancer. Cancer Med. 2023;12:18861-18871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 98. | Kato H, Horiguchi A, Ishihara S, Nakamura M, Endo I. Clinical significance of extrahepatic bile duct resection for T2 gallbladder cancer using data from the Japanese Biliary Tract Cancer Registry between 2014 and 2018. J Hepatobiliary Pancreat Sci. 2023;30:1316-1323. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (5)] |
| 99. | Chen Z, Yu J, Cao J, Lin C, Hu J, Zhang B, Shen J, Feng X, Topatana W, Chen M, Fang H. Wedge resection versus segment IVb and V resection of the liver for T2 gallbladder cancer: a systematic review and meta-analysis. Front Oncol. 2023;13:1186378. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (2)] |
| 100. | Matsui S, Tanioka T, Nakajima K, Saito T, Kato S, Tomii C, Hasegawa F, Muramatsu S, Kaito A, Ito K. Surgical and Oncological Outcomes of Wedge Resection Versus Segment 4b + 5 Resection for T2 and T3 Gallbladder Cancer: a Meta-Analysis. J Gastrointest Surg. 2023;27:1954-1962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 101. | Park Y, Lee JS, Lee B, Jo Y, Lee E, Kang M, Kwon W, Lim CS, Jang JY, Han HS, Yoon YS. Prognostic Effect of Liver Resection in Extended Cholecystectomy for T2 Gallbladder Cancer Revisited: A Retrospective Cohort Study With Propensity Score-matched Analysis. Ann Surg. 2023;278:985-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 102. | Lv TR, Hu HJ, Liu F, Ma WJ, Jin YW, Li FY. The significance of peri-neural invasion in patients with gallbladder carcinoma after curative surgery: a 10 year experience in China. Updates Surg. 2023;75:1123-1133. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 103. | Leigh N, Pletcher E, Solomon D, Sarpel U, Labow DM, Magge DR, Golas BJ. The significance of anatomic tumor location in gallbladder cancer. J Surg Oncol. 2021;123:932-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (3)] |
| 104. | Lv TR, Wang JK, Hu HJ, Ma WJ, Li FY. The Significance of Tumor Locations in Patients with Gallbladder Carcinoma After Curative-Intent Resection. J Gastrointest Surg. 2023;27:1387-1399. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 105. | Vega EA, Mellado S, Chirban AM, Panettieri E, Sanhueza M, Mege R, Diaz C, Brañes A, Briceño E, Viñuela E. Analysis of the Extent of Liver Oncological Extended Resection for Incidental Gallbladder Cancer: How Much Is Too Much? Ann Surg Oncol. 2023;30:6594-6600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 106. | Shah S, Sweeney R, Wegner RE. Survival Benefit with Re-resection and Optimal Time to Re-resection in Gallbladder Cancer: a National Cancer Database Study. J Gastrointest Cancer. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 107. | Lv TR, Liu F, Ma WJ, Hu HJ, Jin YW, Li FY. The significance of countable and treatable metastatic liver disease in patients with gallbladder carcinoma after curative-intent surgery: A 10-year experience in China. Cancer Med. 2023;12:18503-18515. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 108. | Creasy JM, Goldman DA, Gonen M, Dudeja V, O'Reilly EM, Abou-Alfa GK, Cercek A, Harding JJ, Balachandran VP, Drebin JA, Allen PJ, Kingham TP, D'Angelica MI, Jarnagin WR. Evolution of surgical management of gallbladder carcinoma and impact on outcome: results from two decades at a single-institution. HPB (Oxford). 2019;21:1541-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 109. | Cho JK, Lee W, Jang JY, Kim HG, Kim JM, Kwag SJ, Park JH, Kim JY, Park T, Jeong SH, Ju YT, Jung EJ, Lee YJ, Hong SC, Jeong CY. Validation of the oncologic effect of hepatic resection for T2 gallbladder cancer: a retrospective study. World J Surg Oncol. 2019;17:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 110. | Xie J, Liao Z, Sun C, Chen Z. Impact of Primary Tumor Resection on Survival of Patients with Metastatic Gallbladder Carcinoma: A Population-Based, PropensityMatched Study. Med Sci Monit. 2022;28:e934447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 111. | Chan BKY, Carrion-Alvarez L, Telfer R, Rehman AH, Bird N, Mann K, Jones RP, Malik HZ, Fenwick SW, Diaz-Nieto R. Surgical management of suspected gallbladder cancer: The role of intraoperative frozen section for diagnostic confirmation. J Surg Oncol. 2022;125:399-404. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 112. | Liu ZP, Guo W, Yin DL, Chen WY, Wang JY, Li XL, Yue P, Yu C, Wu ZP, Ding R, Zhu Y, Huang F, Zhou JX, Zhang D, Chen W, Jiang Y, Bai J, Wang JJ, Zhang YQ, Dai HS, Lau WY, Chen ZY. Textbook outcomes in liver surgery for gallbladder cancer patients treated with curative-intent resection: a multicenter observational study. Int J Surg. 2023;109:2751-2761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 113. | Vega EA, Newhook TE, Mellado S, Ruzzenente A, Okuno M, De Bellis M, Panettieri E, Ahmad MU, Merlo I, Rojas J, De Rose AM, Nishino H, Sinnamon AJ, Donadon M, Hauger MS, Guevara OA, Munoz C, Denbo JW, Chun YS, Tran Cao HS, Sanchez Claria R, Tzeng CD, De Aretxabala X, Vivanco M, Brudvik KW, Seo S, Pekolj J, Poultsides GA, Torzilli G, Giuliante F, Anaya DA, Guglielmi A, Vinuela E, Vauthey JN. Benchmarks and Geographic Differences in Gallbladder Cancer Surgery: An International Multicenter Study. Ann Surg Oncol. 2023;30:4904-4911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 114. | Fontana AP, Russolillo N, Di Menno Stavron J, Langella S, Tesoriere RL, Ricotti A, Ferrero A. Inverse probability of treatment weighting analysis of laparoscopic versus open Sg4b-5 bi-segmentectomy in patients with gallbladder cancer. Updates Surg. 2023;75:1471-1480. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 115. | Benson AB, D'Angelica MI, Abrams T, Abbott DE, Ahmed A, Anaya DA, Anders R, Are C, Bachini M, Binder D, Borad M, Bowlus C, Brown D, Burgoyne A, Castellanos J, Chahal P, Cloyd J, Covey AM, Glazer ES, Hawkins WG, Iyer R, Jacob R, Jennings L, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Ronnekleiv-Kelly S, Sahai V, Singh G, Stein S, Turk A, Vauthey JN, Venook AP, Yopp A, McMillian N, Schonfeld R, Hochstetler C. NCCN Guidelines® Insights: Biliary Tract Cancers, Version 2.2023. J Natl Compr Canc Netw. 2023;21:694-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 92] [Reference Citation Analysis (0)] |
| 116. | Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D; ESMO Guidelines Committee. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v28-v37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 483] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 117. | Nagino M, Hirano S, Yoshitomi H, Aoki T, Uesaka K, Unno M, Ebata T, Konishi M, Sano K, Shimada K, Shimizu H, Higuchi R, Wakai T, Isayama H, Okusaka T, Tsuyuguchi T, Hirooka Y, Furuse J, Maguchi H, Suzuki K, Yamazaki H, Kijima H, Yanagisawa A, Yoshida M, Yokoyama Y, Mizuno T, Endo I. Clinical practice guidelines for the management of biliary tract cancers 2019: The 3rd English edition. J Hepatobiliary Pancreat Sci. 2021;28:26-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 142] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 118. | Branch of Biliary Surgery, Chinese Surgical Society; Chinese Committee of Biliary Surgeons. [Guideline for the diagnosis and treatment of gallbladder carcinoma (2019 edition)]. Zhonghua Wai Ke Za Zhi. 2020;58:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 119. | Wu X, Li BL, Zheng CJ. Application of laparoscopic surgery in gallbladder carcinoma. World J Clin Cases. 2023;11:3694-3705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 120. | Nakanishi H, Miangul S, Oluwaremi TT, Sim BL, Hong SS, Than CA. Open versus laparoscopic surgery in the management of patients with gallbladder cancer: A systematic review and meta-analysis. Am J Surg. 2022;224:348-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 121. | Lv TR, Yang C, Regmi P, Ma WJ, Hu HJ, Liu F, Yin CH, Jin YW, Li FY. The role of laparoscopic surgery in the surgical management of gallbladder carcinoma: A systematic review and meta-analysis. Asian J Surg. 2021;44:1493-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 122. | Takala S, Lassen K, Søreide K, Sparrelid E, Angelsen JH, Bringeland EA, Eilard MS, Hemmingsson O, Isaksson B, Karjula H, Lammi JP, Larsen PN, Lavonius M, Lindell G, Mortensen FV, Mortensen K, Nordin A, Pless T, Sandström P, Sandvik O, Vaalavuo Y, Villard C, Sallinen V. Practice patterns in diagnostics, staging, and management strategies of gallbladder cancer among Nordic tertiary centers. Scand J Surg. 2023;112:147-156. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 123. | Minagawa T, Itano O, Hasegawa S, Wada H, Abe Y, Kitago M, Katsura Y, Takeda Y, Adachi T, Eguchi S, Oshima G, Aiko S, Ome Y, Kobayashi T, Hashida K, Nara S, Esaki M, Watanabe J, Ohtani H, Endo Y, Shirobe T, Tokumitsu Y, Nagano H. Short- and long-term outcomes of laparoscopic radical gallbladder resection for gallbladder carcinoma: A multi-institutional retrospective study in Japan. J Hepatobiliary Pancreat Sci. 2023;30:1046-1054. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 124. | Cheng J, Liu J, Dou CW, Xie ZC, Fan BF, Jin LM, Liang L, Zhang CW. Standardized lymph node dissection for gallbladder cancer under laparoscopy: en-bloc resection technique. Langenbecks Arch Surg. 2023;408:183. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 125. | Navarro JG, Kang I, Hwang HK, Yoon DS, Lee WJ, Kang CM. Oncologic safety of laparoscopic radical cholecystectomy in pT2 gallbladder cancer: A propensity score matching analysis compared to open approach. Medicine (Baltimore). 2020;99:e20039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 126. | Chin KM, Chua DWQ, Lee SY, Chan CY, Goh BKP. Outcome of minimally invasive liver resection for extrapancreatic biliary malignancies: A single-institutional experience. J Minim Access Surg. 2021;17:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 127. | Cho YJ, Yun WG, Jung HS, Lee M, Han Y, Kwon W, Jang JY. Oncologic safety of robotic extended cholecystectomy for gallbladder cancer. Surg Endosc. 2023;37:9089-9097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 128. | Ramjeesingh R, Chaudhury P, Tam VC, Roberge D, Lim HJ, Knox JJ, Asselah J, Doucette S, Chhiber N, Goodwin R. A Practical Guide for the Systemic Treatment of Biliary Tract Cancer in Canada. Curr Oncol. 2023;30:7132-7150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 129. | Nara S, Esaki M, Ban D, Takamoto T, Shimada K, Ioka T, Okusaka T, Ishii H, Furuse J. Adjuvant and neoadjuvant therapy for biliary tract cancer: a review of clinical trials. Jpn J Clin Oncol. 2020;50:1353-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 130. | Wang J, Bo X, Nan L, Wang CC, Gao Z, Suo T, Ni X, Liu H, Lu P, Wang Y. Landscape of distant metastasis mode and current chemotherapy efficacy of the advanced biliary tract cancer in the United States, 2010-2016. Cancer Med. 2020;9:1335-1348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 131. | Patkar S, Patel S, Gupta A, Ostwal V, Ramaswamy A, Shetty N, Goel M. Lessons learnt from 1300 consecutive gallbladder cancer surgeries: Evolving role of peri-operative chemotherapy in the treatment paradigm. Eur J Surg Oncol. 2023;49:107035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 132. | Toyoda J, Sahara K, Takahashi T, Miyake K, Yabushita Y, Sawada Y, Homma Y, Matsuyama R, Endo I, Pawlik TM. Neoadjuvant Therapy for Extrahepatic Biliary Tract Cancer: A Propensity Score-Matched Survival Analysis. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 133. | Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, Yau T, Klümpen HJ, Chan SL, Ozaka M, Verslype C, Bouattour M, Park JO, Barajas O, Pelzer U, Valle JW, Yu L, Malhotra U, Siegel AB, Edeline J, Vogel A; KEYNOTE-966 Investigators. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401:1853-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 455] [Article Influence: 227.5] [Reference Citation Analysis (0)] |
| 134. | Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, Falk S, Gillmore R, Wadsley J, Patel K, Anthoney A, Maraveyas A, Iveson T, Waters JS, Hobbs C, Barber S, Ryder WD, Ramage J, Davies LM, Bridgewater JA, Valle JW; Advanced Biliary Cancer Working Group. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22:690-701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 390] [Cited by in RCA: 490] [Article Influence: 122.5] [Reference Citation Analysis (0)] |
| 135. | Song S, Yang W, Tian H, Gong S, Lei C, Lv K, Lu T, Cheng Q, Yang K, Guo T. The efficacy and safety of 5-fluorouracil based adjuvant therapy in resected biliary tract cancer: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2022;46:101788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 136. | Geng ZM, Cai ZQ, Zhang Z, Tang ZH, Xue F, Chen C, Zhang D, Li Q, Zhang R, Li WZ, Wang L, Si SB. Estimating survival benefit of adjuvant therapy based on a Bayesian network prediction model in curatively resected advanced gallbladder adenocarcinoma. World J Gastroenterol. 2019;25:5655-5666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 137. | Bergquist JR, Shah HN, Habermann EB, Hernandez MC, Ivanics T, Kendrick ML, Smoot RL, Nagorney DM, Borad MJ, McWilliams RR, Truty MJ. Adjuvant systemic therapy after resection of node positive gallbladder cancer: Time for a well-designed trial? (Results of a US-national retrospective cohort study). Int J Surg. 2018;52:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 138. | Goetze TO, Bechstein WO, Bankstahl US, Keck T, Königsrainer A, Lang SA, Pauligk C, Piso P, Vogel A, Al-Batran SE. Neoadjuvant chemotherapy with gemcitabine plus cisplatin followed by radical liver resection versus immediate radical liver resection alone with or without adjuvant chemotherapy in incidentally detected gallbladder carcinoma after simple cholecystectomy or in front of radical resection of BTC (ICC/ECC) - a phase III study of the German registry of incidental gallbladder carcinoma platform (GR)- the AIO/ CALGP/ ACO- GAIN-trial. BMC Cancer. 2020;20:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 139. | Mayr C, Kiesslich T, Modest DP, Stintzing S, Ocker M, Neureiter D. Chemoresistance and resistance to targeted therapies in biliary tract cancer: what have we learned? Expert Opin Investig Drugs. 2022;31:221-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 140. | Sun Y, Li X, Cheng H, Wang S, Zhou D, Ding J, Ma F. Drug resistance and new therapies in gallbladder cancer. Drug Discov Ther. 2023;17:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 141. | Gedela S, Munot P, Vaidyanathan A, Joarder R, Chaugule D, Parulekar M, Nashikkar C, Ghadi A, Vadodaria D, Goel M, Patkar S, Mandavkar S, Ramaswamy A, Bhargava P, Srinivas S, Ostwal V. Gemcitabine, Cisplatin, and Nab-Paclitaxel as a First-Line Therapy for Advanced Biliary Tract Cancers. J Gastrointest Cancer. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 142. | Zhu Y, Liu X, Lin Y, Tang L, Yi X, Xu H, Yuan Y, Chen Y. Adjuvant chemoradiotherapy vs chemotherapy for resectable biliary tract cancer: a propensity score matching analysis based on the SEER database. Eur J Med Res. 2023;28:310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 143. | Jiang Y, Jiang L, Li H, Yuan S, Huang S, Fu Y, Li S, Li F, Li Q, Yan X, Chen J, Liu J. Adjuvant chemoradiotherapy in resected gallbladder cancer: A SEER-based study. Heliyon. 2023;9:e14574. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 144. | Gholami S, Colby S, Horowitz DP, Guthrie KA, Ben-Josef E, El-Khoueiry AB, Blanke CD, Philip PA, Kachnic LA, Ahmad SA, Rocha FG. Adjuvant Chemoradiation in Patients with Lymph Node-Positive Biliary Tract Cancers: Secondary Analysis of a Single-Arm Clinical Trial (SWOG 0809). Ann Surg Oncol. 2023;30:1354-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 145. | He X, Peng Y, He G, Ye H, Liu L, Zhou Q, Shi J, Fu S, Wang J, Zhou Z, Li W. Increased co-expression of PD1 and TIM3 is associated with poor prognosis and immune microenvironment heterogeneity in gallbladder cancer. J Transl Med. 2023;21:717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 146. | Zhao Y, Yang M, Feng J, Wang X, Liu Y. Advances in immunotherapy for biliary tract cancers. Chin Med J (Engl). 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 147. | Frega G, Cossio FP, Banales JM, Cardinale V, Macias RIR, Braconi C, Lamarca A. Lacking Immunotherapy Biomarkers for Biliary Tract Cancer: A Comprehensive Systematic Literature Review and Meta-Analysis. Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 148. | Lo JH, Agarwal R, Goff LW, Heumann TR. Immunotherapy in Biliary Tract Cancers: Current Standard-of-Care and Emerging Strategies. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 149. | Wu X, Qin F, Zhang Q, Qiao J, Qi Y, Liu B. Immunotherapy improved cancer related pain management in patients with advanced Hepato-Pancreatic Biliary Cancers: A propensity score-matched (PSM) analysis. Front Oncol. 2022;12:914591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 150. | Wu T, Pu C, Wu X, Wang Q, Zhang K. Chemo-Free Treatment Using Anti-PD-1 Antibodies with Lenvatinib in Unresectable Gallbladder Cancer: PD-L1 May Be a Potential Biomarker for a Better Outcome. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 151. | Rizzo A, Federico AD, Ricci AD, Frega G, Palloni A, Pagani R, Tavolari S, Marco MD, Brandi G. Targeting BRAF-Mutant Biliary Tract Cancer: Recent Advances and Future Challenges. Cancer Control. 2020;27:1073274820983013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 152. | Zugman M, Botrus G, Pestana RC, Uson Junior PLS. Precision Medicine Targeting FGFR2 Genomic Alterations in Advanced Cholangiocarcinoma: Current State and Future Perspectives. Front Oncol. 2022;12:860453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 153. | Franzese C, Bonu ML, Comito T, Clerici E, Loi M, Navarria P, Franceschini D, Pressiani T, Rimassa L, Scorsetti M. Stereotactic body radiotherapy in the management of oligometastatic and recurrent biliary tract cancer: single-institution analysis of outcome and toxicity. J Cancer Res Clin Oncol. 2020;146:2289-2297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 154. | Cai YL, Lin YX, Xiong XZ, Ye H, Li FY, Cheng NS. Postsurgical radiotherapy in stage IIIB gallbladder cancer patients with one to three lymph nodes metastases: A propensity score matching analysis. Am J Surg. 2021;221:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 155. | Gamboa AC, Maithel SK. The Landmark Series: Gallbladder Cancer. Ann Surg Oncol. 2020;27:2846-2858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 156. | Zhu J, Wu Y, Xiao W, Li Y. Survival Predictors of Resectable Gallbladder Carcinoma: An Analysis of the Surveillance, Epidemiology, and End Results Database. Am Surg. 2023;89:1629-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 157. | Wang J, Yang Y, Pan J, Qiu Y, Shen S, Wang W. Competing-risk nomogram for predicting survival in patients with advanced (stage III/IV) gallbladder cancer: A SEER population-based study. Jpn J Clin Oncol. 2022;52:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 158. | Yang Y, Tu Z, Ye C, Cai H, Yang S, Chen X, Tu J. Site-specific metastases of gallbladder adenocarcinoma and their prognostic value for survival: a SEER-based study. BMC Surg. 2021;21:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 159. | Fan DX, Xu RW, Li YC, Zhao BQ, Sun MY. Impact of the number of examined lymph nodes on outcomes in patients with lymph node-negative gallbladder carcinoma. World J Gastroenterol. 2018;24:2886-2892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 160. | Ramos E, Lluis N, Llado L, Torras J, Busquets J, Rafecas A, Serrano T, Mils K, Leiva D, Fabregat J. Prognostic value and risk stratification of residual disease in patients with incidental gallbladder cancer. World J Surg Oncol. 2020;18:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 161. | Sun LJ, Guan A, Xu WY, Liu MX, Yin HH, Jin B, Xu G, Xie FH, Xu HF, Du SD, Xu YY, Zhao HT, Lu X, Sang XT, Yang HY, Mao YL. γ-glutamyl transferase-to-platelet ratio based nomogram predicting overall survival of gallbladder carcinoma. World J Gastrointest Oncol. 2020;12:1014-1030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 162. | Yang J, Lv L, Zhao F, Mei X, Zhou H, Yu F. The value of the preoperative Naples prognostic score in predicting prognosis in gallbladder cancer surgery patients. World J Surg Oncol. 2023;21:303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 163. | Jiang S, Zhang J, Zhang L, Xu Y, Zhao H, Sang X, Lu X. A novel nomogram based on log odds of positive lymph nodes to predict survival for non-metastatic gallbladder adenocarcinoma after surgery. Sci Rep. 2022;12:16466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 164. | Han D, Yang J, Xu F, Huang Q, Bai L, Wei YL, Kaaya RE, Wang S, Lyu J. Prognostic factors in patients with gallbladder adenocarcinoma identified using competing-risks analysis: A study of cases in the SEER database. Medicine (Baltimore). 2020;99:e21322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 165. | Li P, Song L. Prognostic Evaluation for Patients over 45 Years Old with Gallbladder Adenocarcinoma Resection: A SEER-Based Nomogram Analysis. Biomed Res Int. 2020;2020:6370946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 166. | Mochizuki T, Abe T, Amano H, Hanada K, Hattori M, Kobayashi T, Nakahara M, Ohdan H, Noriyuki T. Efficacy of the Gallbladder Cancer Predictive Risk Score Based on Pathological Findings: A Propensity Score-Matched Analysis. Ann Surg Oncol. 2018;25:1699-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 167. | Cai YL, Lin YX, Jiang LS, Ye H, Li FY, Cheng NS. A Novel Nomogram Predicting Distant Metastasis in T1 and T2 Gallbladder Cancer: A SEER-based Study. Int J Med Sci. 2020;17:1704-1712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 168. | Jansson H, Cornillet M, Sun D, Filipovic I, Sturesson C, O'Rourke CJ, Andersen JB, Björkström NK, Sparrelid E. Preoperative immunological plasma markers TRAIL, CSF1 and TIE2 predict survival after resection for biliary tract cancer. Front Oncol. 2023;13:1169537. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 169. | Cao J, Yan J, Hu J, Zhang B, Topatana W, Li S, Chen T, Jeungpanich S, Lu Z, Peng S, Cai X, Chen M. Estimating the influencing factors for T1b/T2 gallbladder cancer on survival and surgical approaches selection. Cancer Med. 2023;12:16744-16755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 170. | Kihara Y, Yokomizo H, Murotani K. Impact of acute cholecystitis comorbidity on prognosis after surgery for gallbladder cancer: a propensity score analysis. World J Surg Oncol. 2023;21:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 171. | Iseki M, Mizuma M, Unno M, Maruyama H, Akagi S, Shimoda M, Uemura K, Inoue T, Shiomi H, Watanabe M, Kobayashi M, Matsuda A, Mizuuchi Y, Aoki T, Shinkawa H, Takahata R, Makino K, Arai H, Yokoyama Y, Takeda S, Yaguchi Y, Kitagawa Y. Prognostic impact of postoperative infection after resection of biliary malignancy: A multicenter retrospective cohort study. Surgery. 2023;174:1145-1152. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 172. | Chinaemelum A, Munir MM, Azap L, Woldesenbet S, Dillhoff M, Cloyd J, Ejaz A, Pawlik TM. Impact of Food Insecurity on Outcomes Following Resection of Hepatopancreaticobiliary Cancer. Ann Surg Oncol. 2023;30:5365-5373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 173. | Tittarelli A, Barría O, Sanders E, Bergqvist A, Brange DU, Vidal M, Gleisner MA, Vergara JR, Niechi I, Flores I, Pereda C, Carrasco C, Quezada-Monrás C, Salazar-Onfray F. Co-Expression of Immunohistochemical Markers MRP2, CXCR4, and PD-L1 in Gallbladder Tumors Is Associated with Prolonged Patient Survival. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 174. | Ibukić A, Ramić S, Zovak M, Bilić Z, Tomas D, Demirović A. Expression and Prognostic Significance of PD-L1 and NY-ESO1 in Gallbladder Carcinoma. In Vivo. 2023;37:1828-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 175. | Rizzo A, Brandi G. Neoadjuvant therapy for cholangiocarcinoma: A comprehensive literature review. Cancer Treat Res Commun. 2021;27:100354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 176. | Rizzo A, Brandi G. Pitfalls, challenges, and updates in adjuvant systemic treatment for resected biliary tract cancer. Expert Rev Gastroenterol Hepatol. 2021;15:547-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 177. | Rizzo A, Brandi G. First-line Chemotherapy in Advanced Biliary Tract Cancer Ten Years After the ABC-02 Trial: "And Yet It Moves!". Cancer Treat Res Commun. 2021;27:100335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 178. | Santoni M, Rizzo A, Mollica V, Matrana MR, Rosellini M, Faloppi L, Marchetti A, Battelli N, Massari F. The impact of gender on The efficacy of immune checkpoint inhibitors in cancer patients: The MOUSEION-01 study. Crit Rev Oncol Hematol. 2022;170:103596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 118] [Article Influence: 39.3] [Reference Citation Analysis (0)] |