Published online Mar 15, 2023. doi: 10.4251/wjgo.v15.i3.490
Peer-review started: December 4, 2022
First decision: December 24, 2022
Revised: January 6, 2023
Accepted: February 14, 2023
Article in press: February 14, 2023
Published online: March 15, 2023
Processing time: 100 Days and 12.4 Hours
F-box and leucine-rich repeat 6 (FBXL6) have reportedly been associated with several cancer types. However, the role and mechanisms of FBXL6 in gastric cancer (GC) require further elucidation.
To investigate the effect of FBXL6 in GC tissues and cells and the underlying mechanisms.
TCGA and GEO database analysis was performed to evaluate the expression of FBXL6 in GC tissues and adjacent normal tissues. Reverse transcription-quan
FBXL6 expression was upregulated more in tumor tissues than in adjacent normal tissues and positively associated with clinicopathological characteristics. The outcomes of CCK-8, clone formation, and Edu assays demonstrated that FBXL6 knockdown inhibited cell proliferation, whereas upregulation of FBXL6 promoted proliferation in GC cells. Additionally, the transwell migration assay revealed that FBXL6 knockdown suppressed migration and invasion, whereas the overexpression of FBXL6 showed the opposite results. Through the subcutaneous tumor implantation assay, it was evident that the knockdown of FBXL6 inhibited GC graft tumor growth in vivo. Western blotting showed that the effects of FBXL6 on the expression of the proteins associated with the epithelial-mesenchymal transition-associated proteins in GC cells.
Silencing of FBXL6 inactivated the EMT pathway to suppress GC malignancy in vitro. FBXL6 can potentially be used for the diagnosis and targeted therapy of patients with GC.
Core Tip: F-box and leucine-rich repeat 6 (FBXL6) is up-regulated in gastric cancer (GC) cell lines and tissues, which is correlated with tumor size, grade of differentiation, and TNM stage. Knockdown of TRIM55 in GC cells suppressed proliferation, migration and invasion of cells and affected the expression of cell epithelial-mesenchymal transition-related proteins. Our study provides novel evidence that FBXL6 contributes the growth and metastasis of GC.
- Citation: Meng L, Hu YT, Xu AM. F-box and leucine-rich repeat 6 promotes gastric cancer progression via the promotion of epithelial-mesenchymal transition. World J Gastrointest Oncol 2023; 15(3): 490-503
- URL: https://www.wjgnet.com/1948-5204/full/v15/i3/490.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i3.490
Globally, gastric cancer (GC) is the second most common cause of cancer-related deaths and the fourth most common cancer[1,2]. Approximately 950000 new cases of patients with GC are diagnosed worldwide each year; however, a decline in incidence and mortality rates has been observed in recent years[3]. As traditional treatment strategies for gastric cancer, surgical resection, chemotherapy, and radiotherapy continue to show shortcomings; this is the main reason for the < 30% 5-year overall survival (OS) for patients with GC[4,5]. Therefore, further research is warranted to help researchers elucidate the underlying molecular mechanisms and identify effective therapeutic avenues to enhance survival in GC.
F-box and leucine-rich repeat 6 (FBXL6) is an FBXL protein that is closely associated with the degradation of ETV6, which is involved in nucleoplasm formation in the intercellular phase through the ubiquitin-proteasome system[6]. Recent studies have reported that FBXL6 activates the estrogen receptor by promoting its transcription and mediating its protein hydrolysis[7]. Furthermore, FBXL6 expression is reportedly associated with the occurrence of tumors in humans. To illustrate, Li et al[8] reported that FBXL6 is a unique prognostic marker, and it demonstrates the occurrence of the malignant progression of renal cell carcinoma. Other studies found that FBXL6 is upregulated and connected with poor prognosis in CRC, in which FBXL6 targets phosphorylated p53 to regulate its polyubiquitination and degradation in cases of colorectal cancer. However, it is unclear whether FBXL6 is closely related to the progression and function in GC.
Epithelial-mesenchymal transition (EMT) has been identified as a vital factor in promoting metastasis in multiple tumors[9,10]. Several EMT-related factors are abnormal, including E-cadherin, vimentin and N-cadherin, which is a biological phenomenon in the EMT progression[11]. The findings of previous studies indicated that the AKT signaling pathway suppresses GSK3β-mediated phosphorylation of β-catenin, thereby causing the β-catenin-mediated transcription of EMT[12]. Furthermore, Song et al[13] reported that HOXA10 mediates EMT to promote gastric cancer metastasis through TGF
Here, we described the relationship between FBXL6 and clinicopathological characteristics and the potential role of FBXL6 in GC cell proliferation and invasion in vitro and in vivo. Our results showed that FBXL6 is significantly upregulated in GC tissues and that high expression of FBXL6 in patients is commonly associated with poor OS. Therefore, FBXL6 can evidently be considered a novel prognostic biomarker and direction of treatment in GC.
At the first afflation hospital of Anhui Medical University, 68 pairs of GC tissues and precancerous tissue samples of patients were gathered between January 2020 and December 2020. The clinical samples were stored at -80 °C for reverse transcription-quantitative polymerase chain reaction (RT-qPCR), extraction of proteins, or embedded in paraffin for immunohistochemistry. This study had been approved by the Ethics review committee of the First Affiliated Hospital of Anhui Medical University (approval: Quick-PJ 2019-10-11) and written informed consent was obtained from all cancer patients.
The RNA-seq expression files for GC were downloaded from The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/) and GEO database was used to compare FBXL6 expression between tumors and normal tissue in GC. The correlation relation between FBXL6 expression and (OS) in GC was analyzed using the Kaplan-Meier plots (http://kmplot.com/), and their statistical significance was obtained by the log-rank test.
Three gastric cancer cell lines (MKN-45, HGC-27, and MGC-803) were purchased from Fenghui Biotechnology Co., Ltd (Hunan Province, China), and gastric mucosal epithelial cell line (GES-1) was maintained in laboratory. Cell cultured was performed using standard media supplemented 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37 °C. Transfections were performed using Fugene® HD (Promega) as per manufacturer instructions. Two short hairpin RNAs (sh-FBXL6#1 and sh-FBXL6#2) and sh-NC negative control and overexpressed FBXL6 plasmid were obtained from GeneChem company (Shanghai, China). the sequences of shRNA were as follows: sh-FBXL6#1: 5’-CACCGGCATCAACCGTAATAG-3’; sh-FBXL6#2: 5’-TGGAGTGGCTTATGCCCAATC-3’; infection of HGC-27 and MKN45 cell lines with shFBXL6#1 and shFBXL6#2 and MGC-803 with OE-FBXL6, and screening with puromycin (10 µg/mL) was performed for 1 wk to establish stable FBXL6 knockdown and overexpression cell lines.
Tissues and cells were processed by performing lysis in the RIPA reagent containing 1 mmol/L PMSF, and the protein was then prepared and quantified through bicinchoninic acid (BCA) analysis. The 20-µg protein sample was separated via SDS-PAGE (8%-10%); the isolated proteins were then placed onto polyvinylidene fluoride membranes (Millipore). Blocking was performed with 3% bovine serum albumin (BSA) for 2 h, and the membrane was subsequently incubated at 4 °C for 12 h with the following primary antibody: FBXL6 (1:1000; Abcam, United Kingdom) or anti-GAPDH (ZSGB, China), Vimentin, E-cadherin, and N-cadherin (1:1000, Proteintech, China), matrix metalloproteinase-2 (MMP-2; 1:500, Abcam, United Kingdom), and MMP9 (1:500, Abcam, United Kingdom). The membranes were then incubated with goat anti-rabbit and goat anti-mouse HRP (1:10000, Proteintech, China) for 2 h at 25 °C. Finally, the protein membranes were visualized using the ECL system (Tanon, Shanghai, China).
RT-qPCR was performed as described previously[16]. Total RNA was extracted from cells and sample tissues using Trizol (Life Technologies, United States). The reverse transcriptase enzyme was used to conduct reverse transcription of 2 μg of purified (Yeasen, Shanghai, China). RT-qPCR was conducted with the appropriate primers using 2 × SYBR Green PCR Master Mix (Yeasen, Shanghai, China) and using the light cycler 96 qPCR System (Roche, United States), and GAPDH as the control. The primer sequences are as follows: FBXL6 forward, 5’-GGAGACCGCATTCCCTTGG-3’; reverse, 5’-AAA
Tissue and cellular immunofluorescence were performed as described in protocol. In this study, GC cells were inoculated in 24-well plates (containing crawlers) and reached the appropriate density. The cells on crawl sheets were then washed with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde for 15 min. FBXL6-specific antibodies (1:100, Abcam, United Kingdom) were used overnight and FBXL6-stained samples were incubated with secondary antibodies (Proteintech, China). Following this, DAPI staining of cell nuclei was performed.
IHC experimental methods are identical to those reported in the literature[16]. The Intensity scores were analyzed as follows: 0: No staining; 1: Low staining; 2: Medium staining; and 3: High staining. Proportional scores were further classified as 0: 0%; 1: 1%-25%; 2: 26%-50%; 3: 51%-75%; and 4: > 75%. Immunoreactivity scores were calculated by multiplying the intensity and percentage scores. Staining results were compared and scored by two independent pathologists.
Stably transfected GC cells were incubated in 12-well plates. Then 50 μM 5-ethynyl-2’-deoxyuridine (EdU, Beyotime, Shanghai, China) was added to each well and incubated at 37 °C; however, the incubation time varied on a cell-to-cell basis. Then the cells were fixated, permeabilized, and processed with 200 μL of Hoechst 33342 to achieve nuclear staining. A fluorescence microscope was used to capture images to determine the proportion of EdU-positive cells.
Transfected GC cells were digested when their content reached 80%; these cells were then inoculated into 96-well culture plates at 2 × 103 cells per well, cultured in 96-well contain standard medium, and cell viability was assessed at 0 h, 24 h, 48 h, and 72 h applying the CCK-8 kit (Beyotime, Shanghai, China). The absorbance value was then detected at 450 nm. For the cell clone formation assay, the stably transfected cells were seeded into 6-well plates. After 2 wk, the cell debris was washed, clone cells were fixed and stained with crystal violet (Beyotime, Shanghai China), and the number of clone cells was calculated by the camera.
Transfected GC cells were prepared into a 6-well plate, and when their density reached 80% and confluence and were sectioned with a 200 mL pipette. The surface of the cells was washed once with a serum-free medium, and the cell debris was removed. The cells were then recorded and captured under a microscope with a 100-fold magnification, and their position in the photograph was recorded. Subsequently, each group of cells was then continued to incubate at 37 °C for 24 h. Finally, photographs were captured and recorded, and the migration area of each group was calculated.
The Transwell cell assay was used to evaluate the migration and invasion abilities of tumor cells. Typically, invasion assays were constructed using BD Matrigel (Corning, United States) and covered the upper chamber and GC cells (1 × 105) were starved for 24 h in a medium without FBS, and the bottom plate supplemented standard medium. After 24 h, the upper cells were removed and adhered to the membrane’s lower surface, fixed, and stained with crystal violet. The cells visible in the field of view were recorded and counted.
BALB/c female mice (age, 4 wk) were obtained from SLAC Laboratory Animal Company (Shanghai, China). All nude mice were randomly raised and grouped in an SPF environment. Subcutaneous injection of stable knockout sh-FBXL6#1 and sh-NC HGC-27 cells in nude mice. Furthermore, the length and width of the tumor was recorded and calculated every 4 days using vernier calipers. Finally, the weight of the tumor (mg) was recorded after the mice were euthanized. All animal experiments were approved by the Experimental Animal Ethics Committee of Anhui Medical University (Approval: LLSC2020513).
The Student’s two-tailed t-test was used to perform comparisons between the two groups, and comparisons between multiple groups were performed using a one-way ANOVA. Associations between FBXL6 levels and GC clinical data were analyzed using logistic analysis. All statistical analyses were carried out using GraphPad Prism 8.0 software (GraphPad Software, United States) and SPSS 22.0 (IBM Corp). All data are mean ± SD. aP < 0.05, and bP < 0.01 indicated statistical significance.
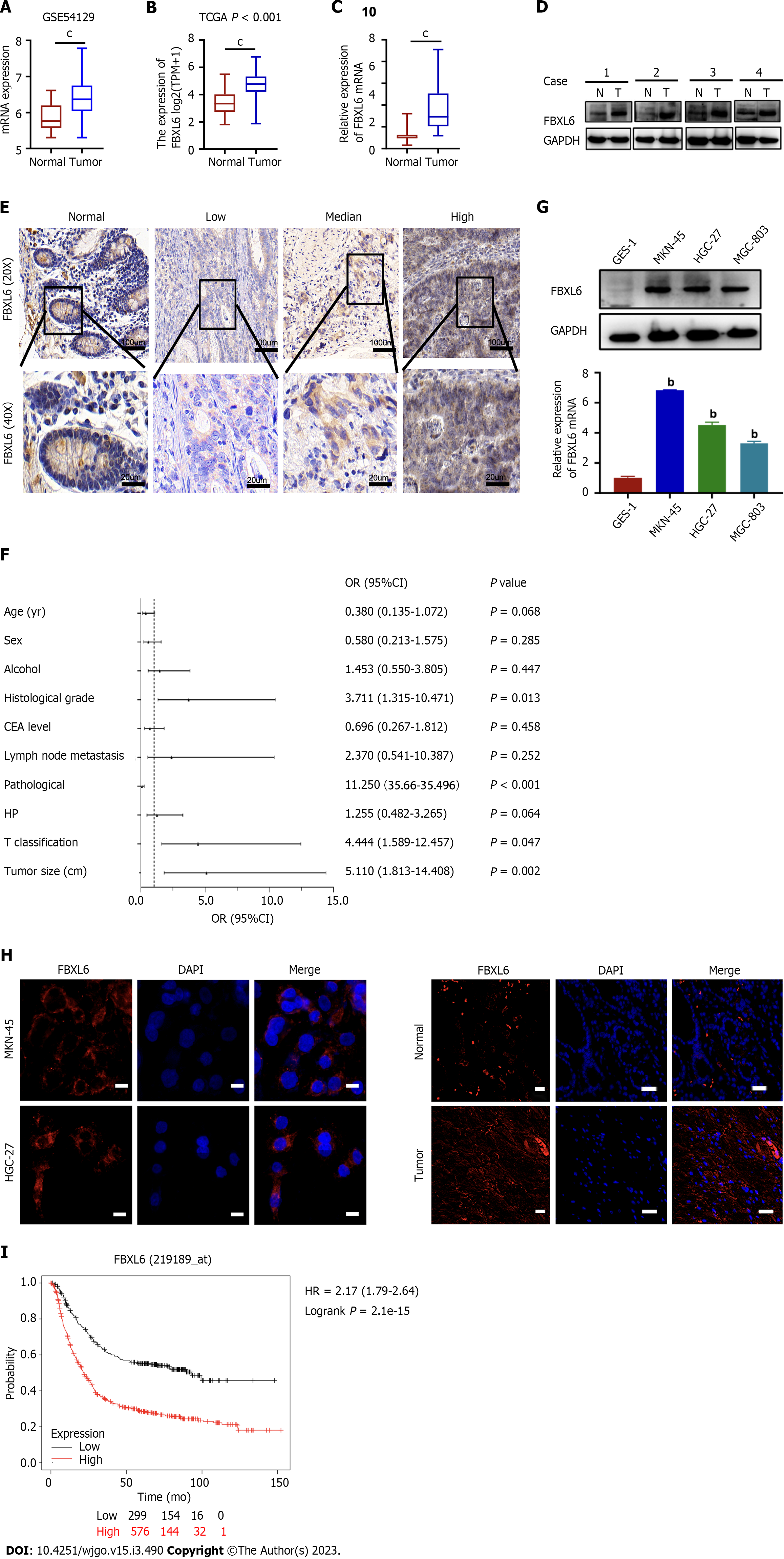
To identify potential FBXL6 associations with GC, our analysis of the TCGA and GSE54129 datasets revealed that FBXL6 expression was upregulated considerably more in GC sample than in adjacent normal samples (Figure 1A and B, P < 0.001). Additionally, RT-qPCR and western blotting were used to measure the expression of FBXL6 in primary GC samples. FBXL6 mRNA levels were significantly elevated in GC tumors (Figure 1C, P < 0.001) and FBXL6 protein was significantly more enriched in GC than in the normal tissue (Figure 1D, n = 4). IHC analysis showed that FBXL6 expression was dramatically more abundant in GC tissues than in normal adjacent tissues (Figure 1E). To analyze the relationship between FBXL6 and the clinical data of GC, As shown in Figure 1F, FBXL6 expression was strongly associated with histological grade [3.711 (1.315-10.471), P = 0.013], pathological stage [11.250 (3.566-35.496), P < 0.001], T grade [4.444 (1.589-12.457), P = 0.047], and tumor size [5.111 (1.813-14.408), P = 0.002]. However, this correlation was not observed in terms of age [0.380 (0.135-1.072), P = 0.068], sex [0.580 (0.213-1.575), P = 0.285], alcohol [1.453 (0.550-3.805), P = 0.447], CEA levels [0.696 (0.267-1.812), P = 0.458), lymph node metastasis [2.370 (0.541-10.387), P = 0.252], and Helicobacter pylori (H. pylori) infection [1.255 (0.482-3.265), P = 0.642]. We subsequently investigated the FBXL6 protein and mRNA expression levels in three GC cell lines. FBXL6 mRNA and protein (Figure 1G) were higher in the HGC-27, MGC-803, and MKN-45 cell lines than in the GES-1 cell line. We consequently performed IF staining of the GC tissues and cells. In MKN-45 and HGC-27 cell lines, FBXL6 was predominantly located in the cytoplasm and nucleus (Figure 1H); FBXL6 protein staining in normal tissues was relatively lower than that in tumor tissues (Figure 1I). Notably, according to the Kaplan-Meier online database, patients with higher FBXL6 Levels had shorter OS (Figure 1J, HR = 2.7, P = 2.1e-15). Furthermore, these results strongly suggest high expression and clinical relevance in GC patents.
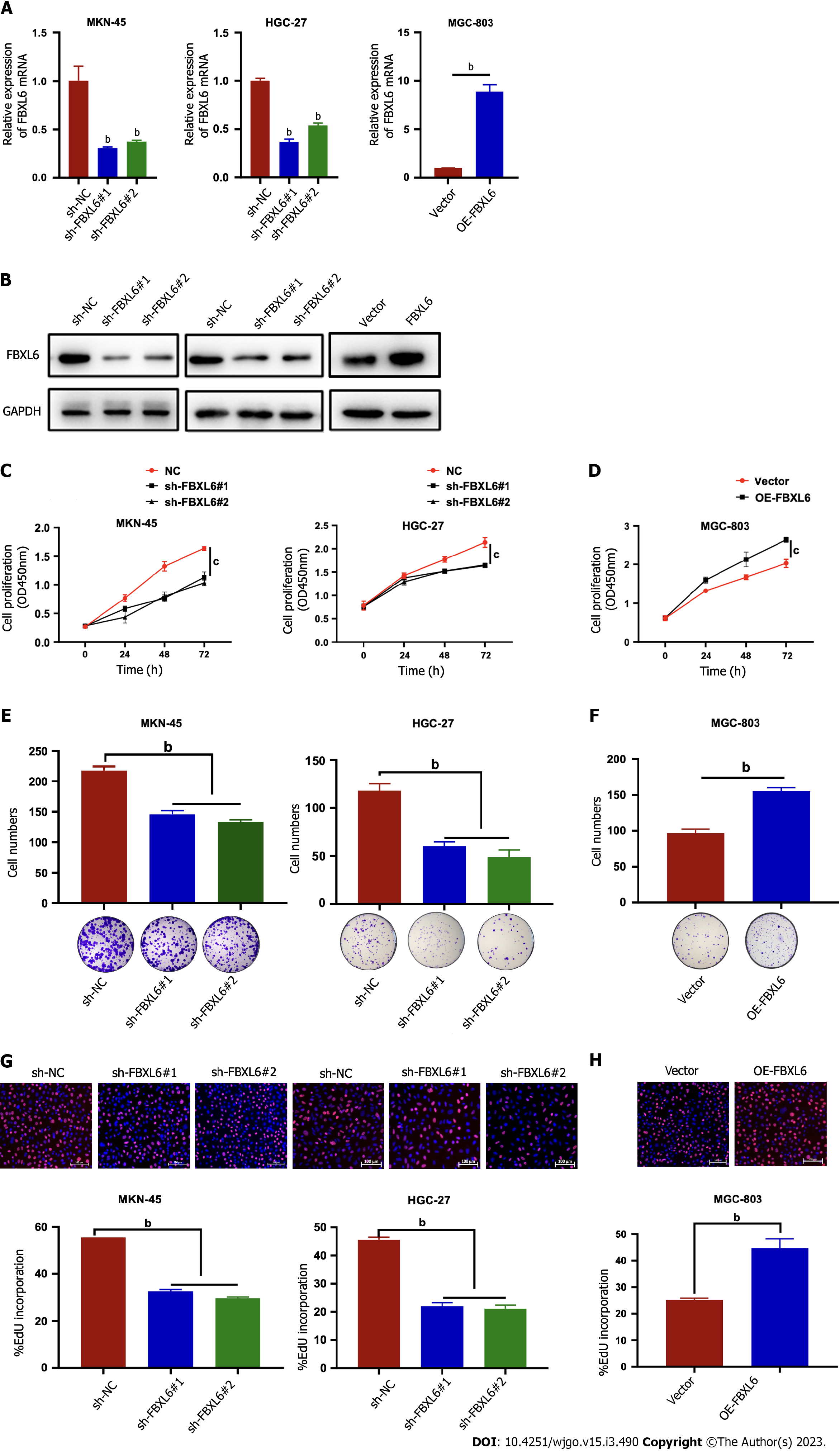
In order to investigate the underlying role of FBXL6 on gastric cancer cells, a stable knockdown FBXL6 was constructed in MKN-45 and HGC-27 cells and overexpression in MGC-803 cells. The knockdown could be effectively detected via RT-qPCR and western blot (Figure 2A and B, P < 0.01). Knockdown of FBXL6 could suppress the proliferation ability of both HGC-27 and MKN-45 cells; notably, similar results were observed in colony formation assays (Figure 2C-F, P < 0.01). In particular, this was noted when knocking down FBXL6 resulted in a decrease in the number of clones. Furthermore, outcomes of the EdU experiment indicated that FBXL6 knockdown suppressed the viability of GC cells (Figure 2G, P < 0.01), whereas its overexpression promoted GC cell viability (Figure 2H, P < 0.01). These results demonstrated that FBXL6 accelerated the proliferation of GC cells.
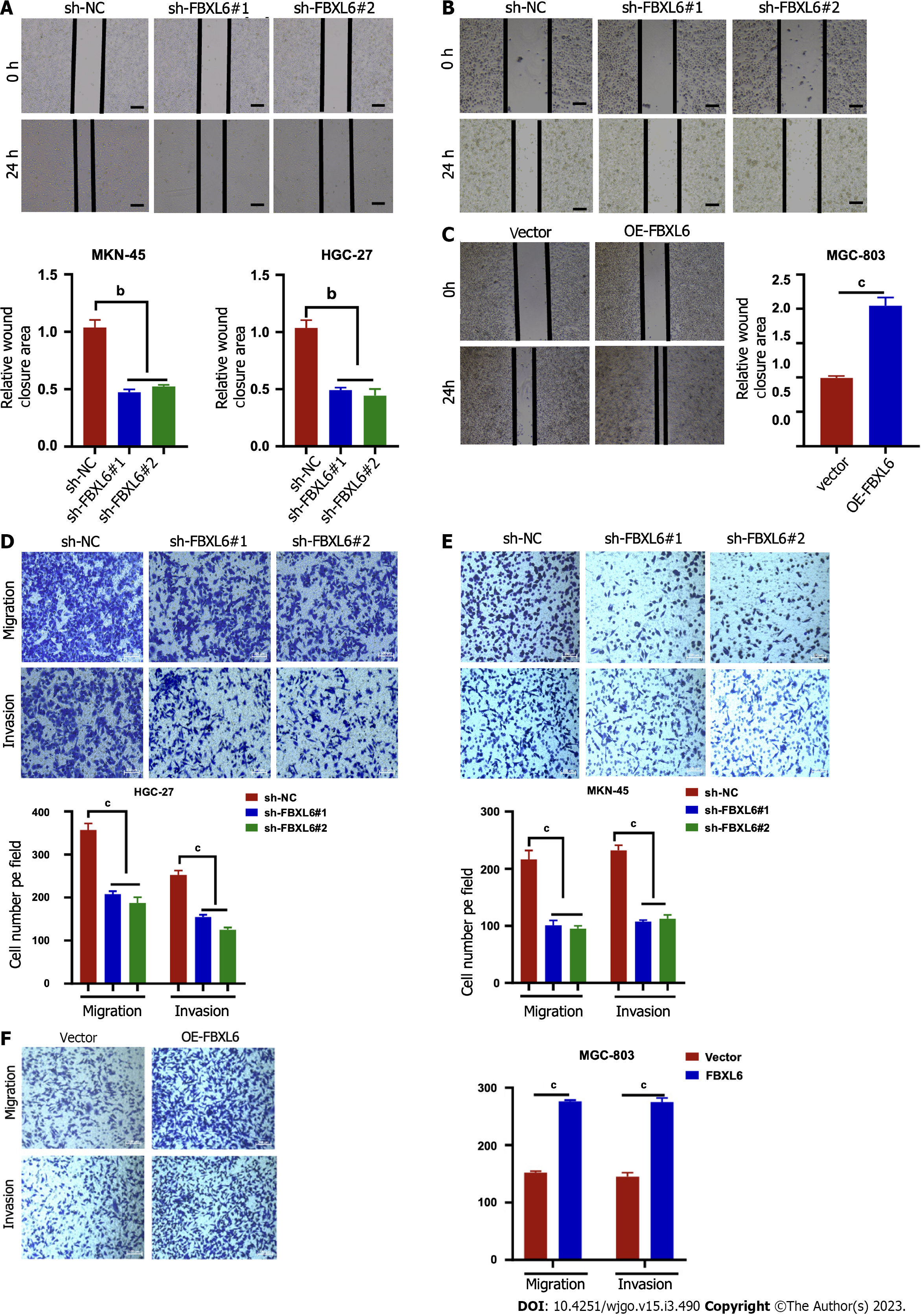
To accurately determine whether FBXL6 has an effect on the development of migratory and invasive phenotypes cells, The findings of the wound healing assays demonstrated that the migration rate was slower in the stable knockdown MKN-45 and HGC-27 cells (Figure 3A and B, P < 0.01); however, we noted that FBXL6 overexpression promoted cell migration in MGC-803 (Figure 3C, P < 0.001). To evaluate the impact of FBXL6 on the transwell assays were performed using both MGC-803 and MKN45, and HGC-27 cells. The experimental results revealed that the knockdown of FBXL6 significantly reduced migration and invasion of HGC-27 and MKN-45 cells (Figures 3D and E, P < 0.001), whereas the overexpression of FBXL6 increased migration in MGC-803 (Figure 3F, P < 0.001). Therefore, the outcomes of the present study suggested that FBXL6 could promote metastasis ability in GC cells.
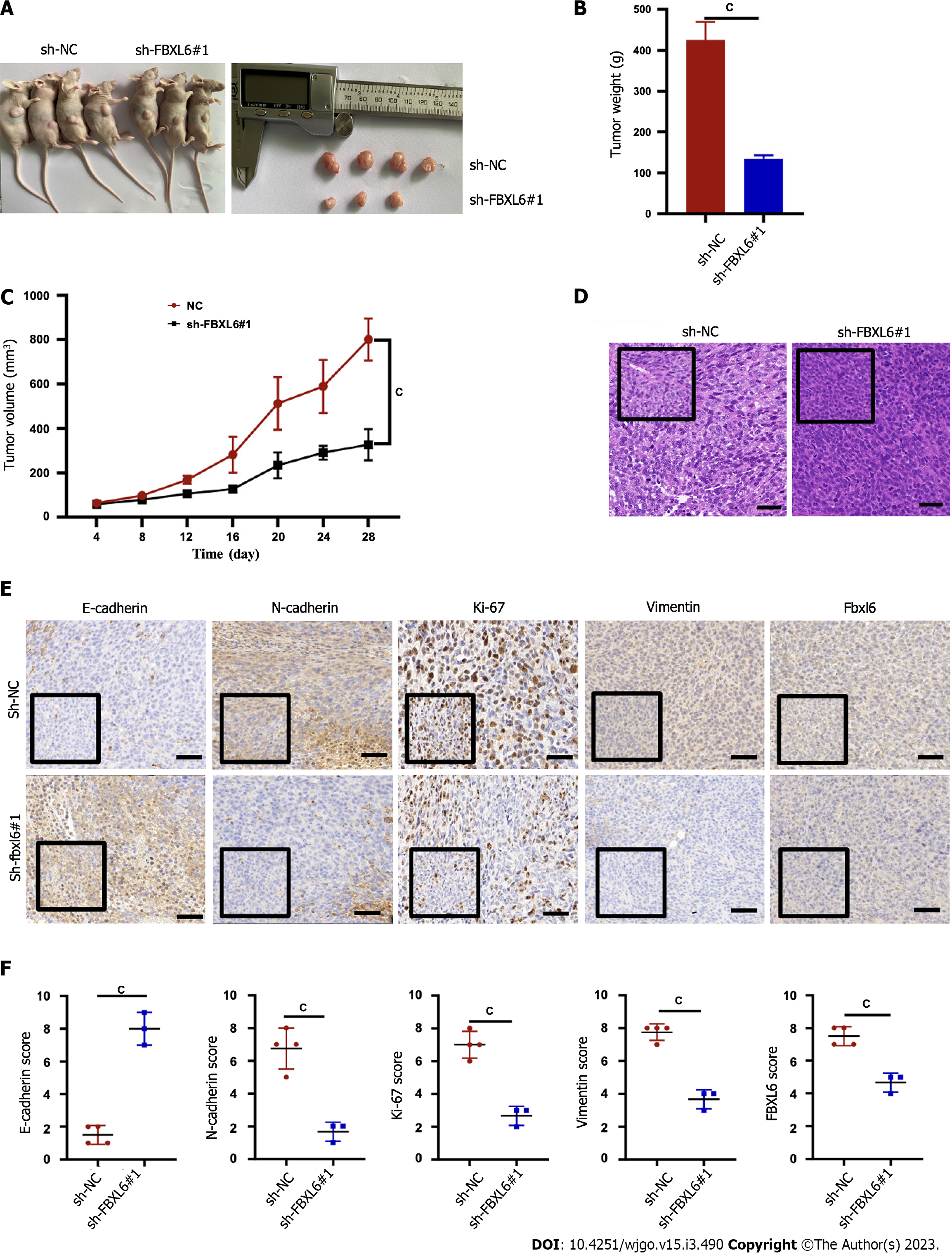
The potential effects of FBXL6 on tumor growth in nude mice were evaluated that the HGC-27 cells were stably transfected with sh-NC, and sh-FBXL6#1 was injected subcutaneously into each BALB/c nude mouse. Figure 4A-C shows that the subcutaneous tumors in the sh-FBXL6#1 knockout group are significantly lower in terms of volume and mean weight than the sh-NC group. Additionally, H&E staining indicated that the sh-FBXL6#1 group had a lower nuclear malignancy than the sh-NC group (Figure 4D, P < 0.001). Notably, IHC staining results revealed that FBXL6, N-cadherin, vimentin, and Ki-67 expression were reduced in the sh-FBXL6#1 group. However, the expression levels of E-cadherin appeared to increase (Figure 4E and F, P < 0.001). Therefore, our result proposed that the knockdown of FBXL6 could reduce tumorigenesis and proliferation in vivo.
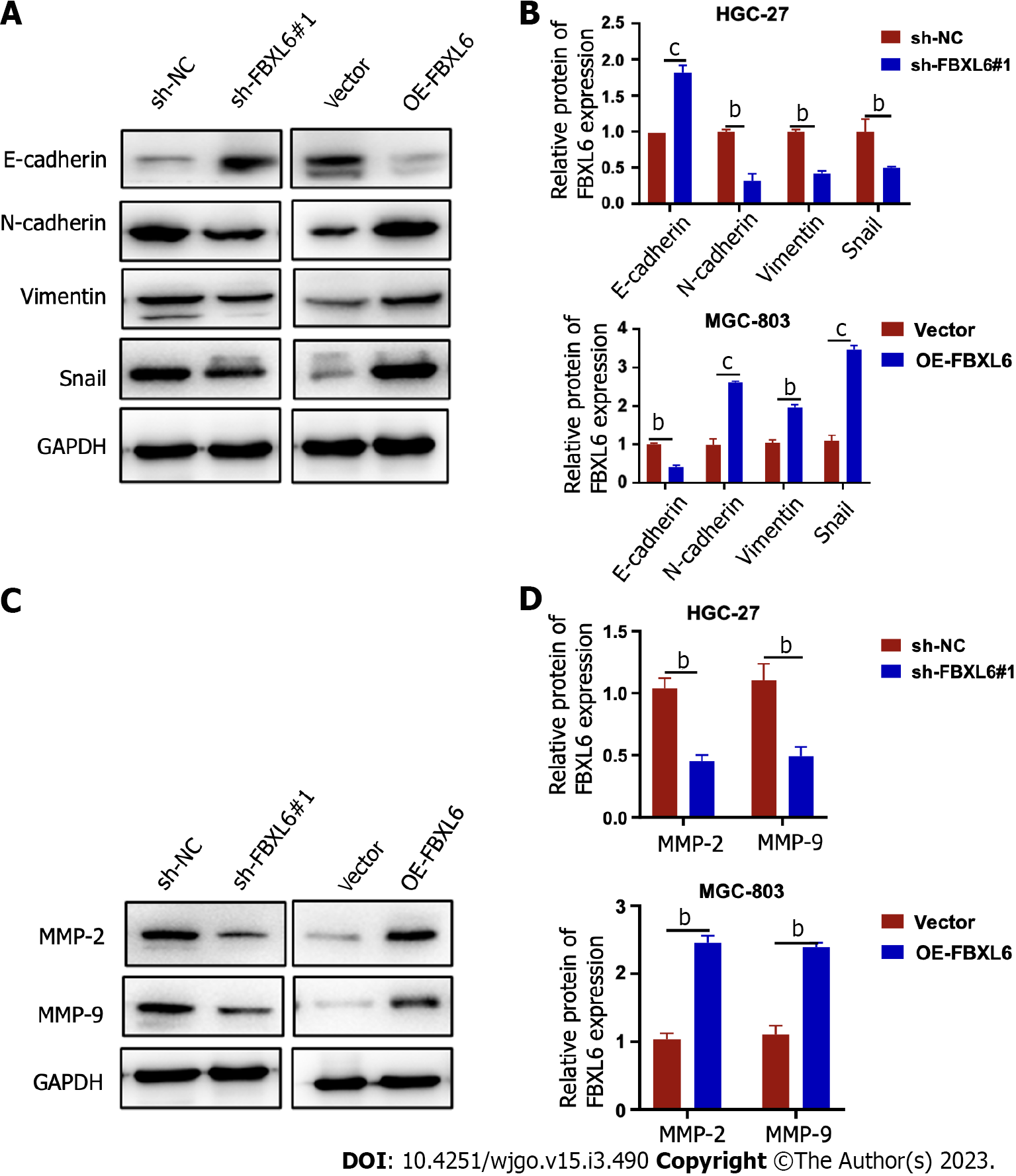
EMT has been recognized as a key factor present in all types of tumor metastases. These results showed that the EMT pathway was dramatically inhibited, which led to an increase in the expression of E-cadherin protein and a decrease in that of vimentin and N-cadherin protein in FBXL6-silenced HGC-27 cells. However, overexpression of FBXL6 showed the opposite effect in MGC-803 cells (Figure 5A and B, P < 0.001). MMP-9 and MMP2 are essential MMPs associated with EMT and cell metastasis. Our results demonstrated that MMP2 and MMP-9 expression was reduced in HGC-27 cells following the knockdown of FBXL6, and were increased following the overexpression of FBXL6 in MGC-803 cells (Figure 5C and D, P < 0.001). These studies demonstrated that FBXL6 promotes GC cell invasion and metastasis by inducing EMT.
Identifying genes that are imperative to ensure GC development and progression or its behavioral processes is of paramount importance to successfully explore potentially effective treatments. FBXL6 is a member of the F-box protein (FBP) family and is reported to promote colon cancer progression and play an anti-metastatic role in colon cancer[8]. Additionally, FBXL6 facilitates the stabilization and activation of c-Myc protein through the prevention of HSP90AA1 degradation, which combines directly with the FBXL6 promoter region to enhance mRNA expression in patients with hepatocellular carcinoma[17]. However, the effects of FBXL6 on GC remain unclear and require further elucidation. A new finding from our results indicated that FBXL6 protein and mRNA levels were higher in GC tissues than those normal tissues. Furthermore, the interaction between FBXL6 expression and clinicopathological features was assessed in the current study and a positive correlation was identified between FBXL6 and tumor size, histological grade, and TNM stage. Noteworthy, The Kaplan-Meier Plotter database showed that FBXL6 expression was associated with a potentially poor prognosis of GC. Our results suggested that FBXL6 is a prognostic factor and oncogenic gene in GC patients.
The FBPs are substrate receptors for the SCF E3 ubiquitin ligase and play a critical role in recognizing and recruiting polyubiquitinated substrate proteins[18]. Several studies have reported that FBPs are strongly associated with human cancers and activity of the pertinent oncogenes[19,20]. Reportedly, FBXL16 mechanistically promotes cell growth and migration through the antagonization of the activity of FBW7 and enhancement of the stability of c-Myc[21]. Furthermore, Yang et al[22] also noted that FBXO39 was highly expressed in invasive cervical squamous cell carcinoma and that patients with high FBXO39 expression presented with poorer disease prognosis than low expression patients.
In the past, studies have reported that the loss of FBXL6 reduces the growth and induces apoptosis in ccRCC cells[23]. Consistent with our results related to GC cells, silencing of FBXL6 may inhibit the proliferation and colony-forming ability, whereas FBXL6 overexpression demonstrated the opposite effect. These findings were coherent with the effect of FBXL6 in vivo assays. In addition, FBXL6 expression knockout decreased the migration viability of GC cells, whereas the overexpression of FBXL6 enhanced cell migration ability. Therefore, our results suggested that FBXL6 acts as an oncogene in gastric cancer by enhancing cell proliferation and invasion ability in vitro and in vivo. Nonetheless, the molecular mechanisms through which FBXL6 regulates proliferation, migration, and invasion require elucidation. Evidently, EMT is believed to play a critical influence in cancer invasion and metastasis, with the main effects on the expression of E-and N-cadherin proteins[24]. Furthermore, EMT also has been widely recognized as an essential factor in GC metastasis[25,26]. In this research, we determined that silencing FBXL6 downregulated the N-cadherin and vimentin proteins, and increase E-cadherin protein expression. Conversely, FBXL6 overexpression demonstrated the opposite results in GC cells. Recent studies suggest that FBPs play a vital part in tumorigenesis and metastasis, and the research on F-box proteins and EMT factors in cancers is increasing annually. To illustrate, FBXO22 protein significantly reduced RCC cell metastasis ability by reversing EMT and inhibiting MMP-9 expression in vitro[27]. Li et al[8] identified the fact that FBXW7 regulated MMP-2 and MMP-9 expression and suppresses RCC metastasis through the EMT single pathway. The activation of MMP9 has been implicated in the invasion and metastasis of GC[28]. Furthermore, MMP2 and MMP-9 are dramatically associated with cell invasion and metastasis in particular; this progression may occur through the digestion of the extra
The fact that we did not further investigate the mechanism of action of FBXL6 in GC is a limitation of this study. Owing to the limitations related to follow-up time, no clinical data were recorded that did not allow for the use of statistics to elucidate the survival of the patients. Additionally, Whether or not FBXL6 can bind other genes to induce the ubiquitination process in GC remain unknown. Future studies warranted to investigate the potential mechanisms of FBXL6-mediated regulation of the expression of EMT.
In conclusion, this study reported that FBXL6 expression was significantly enhanced in GC tissues and GC cells, and the positive of FBXL6 expression was significantly associated with the prognostic significance of GC patients and correlation between its expression and clinical features. Notably, FBXL6 promoted growth, migration, and invasion through the EMT signaling pathway of GC cells. Therefore, future research needs concern that FBXL6 may have potential as an important prognostic indicator and therapeutic destination for GC.
F-box and leucine-rich repeat 6 (FBXL6) have reportedly been associated with several cancer types. However, the role of FBXL6 in the proliferation and epithelial-mesenchymal transition (EMT) of gastric cancer (GC) remains to be investigated.
To investigate the effect of FBXL6 on the proliferation of GC cells and to find new therapeutic targets for the treatment of GC.
The present study to clarify the effect of FBXL6 on the prognosis of GC patients and the proliferation and EMT of GC cells.
The expression of FBXL6 expression in GC tissues and cells was detected using RT-qPCR and Western blotting. In vitro, stable FBXL6 knockdown and overexpressed GC cell lines were cultured, and the proliferation, clone formation, migration and invasion ability of GC cells were examined using cholecystokinin-8 assay, clone formation assay, wound healing assay and transwell assay, respectively. In vivo tumor assays were performed to prove whether FBXL6 promoted cell proliferation in vivo. Western blotting was used to detect the association of FBXL6 protein with EMT-related protein expression levels.
FBXL6 expression is elevated in GC cells and tissues, and FBXL6 expression levels correlated with histological grade, pathological stage, T grade, and tumor size. In vitro, endogenous silenced of FBXL6 suppressed GC cell proliferation, migration, invasion and EMT. In vivo, knockdown of FBXL6 inhibited subcutaneous graft tumor growth in nude mice.
FBXL6 expression is increased in GC tissues and cell lines. FBXL6 promotes the proliferation migration, invasion, and epithelial-mesenchymal transition of GC cells.
FBXL6 may have potential as an important prognostic indicator and therapeutic destination for GC. Further search for potential cancer-promoting mechanisms of FBXL6 is needed in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Biochemistry and molecular biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang HL, Japan; Wu CN, China S-Editor: Chen YL L-Editor: A P-Editor: Zhang XD
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11439] [Article Influence: 3813.0] [Reference Citation Analysis (4)] |
| 2. | Lin Y, Totsuka Y, Shan B, Wang C, Wei W, Qiao Y, Kikuchi S, Inoue M, Tanaka H, He Y. Esophageal cancer in high-risk areas of China: research progress and challenges. Ann Epidemiol. 2017;27:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 157] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 3. | Virgilio E, Proietti A, D'Urso R, Cardelli P, Giarnieri E, Montagnini M, Giovagnoli MR, Mercantini P, Balducci G, Cavallini M. Measuring Intragastric Tumor Markers in Gastric Cancer Patients: a Systematic Literature Review on Significance and Reliability. Anticancer Res. 2017;37:2817-2821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Kim JH, Zang DY, Jang HJ, Kim HS. A Bayesian network meta-analysis on systemic therapy for previously treated gastric cancer. Crit Rev Oncol Hematol. 2021;167:103505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Zhao Y, Bai Y, Shen M, Li Y. Therapeutic strategies for gastric cancer targeting immune cells: Future directions. Front Immunol. 2022;13:992762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 6. | Roukens MG, Alloul-Ramdhani M, Moghadasi S, Op den Brouw M, Baker DA. Downregulation of vertebrate Tel (ETV6) and Drosophila Yan is facilitated by an evolutionarily conserved mechanism of F-box-mediated ubiquitination. Mol Cell Biol. 2008;28:4394-4406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Chen D, Liu X, Xia T, Tekcham DS, Wang W, Chen H, Li T, Lu C, Ning Z, Liu J, Qi H, He H, Piao HL. A Multidimensional Characterization of E3 Ubiquitin Ligase and Substrate Interaction Network. iScience. 2019;16:177-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Li Y, Cui K, Zhang Q, Li X, Lin X, Tang Y, Prochownik EV, Li Y. FBXL6 degrades phosphorylated p53 to promote tumor growth. Cell Death Differ. 2021;28:2112-2125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Creighton CJ, Chang JC, Rosen JM. Epithelial-mesenchymal transition (EMT) in tumor-initiating cells and its clinical implications in breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 10. | Franco-Chuaire ML, Magda Carolina SC, Chuaire-Noack L. Epithelial-mesenchymal transition (EMT): principles and clinical impact in cancer therapy. Invest Clin. 2013;54:186-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 370] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 11. | Sánchez-Tilló E, Liu Y, de Barrios O, Siles L, Fanlo L, Cuatrecasas M, Darling DS, Dean DC, Castells A, Postigo A. EMT-activating transcription factors in cancer: beyond EMT and tumor invasiveness. Cell Mol Life Sci. 2012;69:3429-3456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 408] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 12. | Ozman Z, Ozbek Iptec B, Sahin E, Guney Eskiler G, Deveci Ozkan A, Kaleli S. Regulation of valproic acid induced EMT by AKT/GSK3β/β-catenin signaling pathway in triple negative breast cancer. Mol Biol Rep. 2021;48:1335-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Song C, Zhou C. HOXA10 mediates epithelial-mesenchymal transition to promote gastric cancer metastasis partly via modulation of TGFB2/Smad/METTL3 signaling axis. J Exp Clin Cancer Res. 2021;40:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 14. | Kang X, Xu E, Wang X, Qian L, Yang Z, Yu H, Wang C, Ren C, Wang Y, Lu X, Xia X, Guan W, Qiao T. Tenascin-c knockdown suppresses vasculogenic mimicry of gastric cancer by inhibiting ERK- triggered EMT. Cell Death Dis. 2021;12:890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Li R, Zhuang C, Jiang S, Du N, Zhao W, Tu L, Cao H, Zhang Z, Chen X. ITGBL1 Predicts a Poor Prognosis and Correlates EMT Phenotype in Gastric Cancer. J Cancer. 2017;8:3764-3773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Meng L, Chen Z, Jiang Z, Huang T, Hu J, Luo P, Zhang H, Huang M, Huang L, Chen Y, Lu M, Xu AM, Ying S. MiR-122-5p suppresses the proliferation, migration, and invasion of gastric cancer cells by targeting LYN. Acta Biochim Biophys Sin (Shanghai). 2020;52:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Shi W, Feng L, Dong S, Ning Z, Hua Y, Liu L, Chen Z, Meng Z. FBXL6 governs c-MYC to promote hepatocellular carcinoma through ubiquitination and stabilization of HSP90AA1. Cell Commun Signal. 2020;18:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 18. | Randle SJ, Laman H. F-box protein interactions with the hallmark pathways in cancer. Semin Cancer Biol. 2016;36:3-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Ji J, Shen J, Xu Y, Xie M, Qian Q, Qiu T, Shi W, Ren D, Ma J, Liu W, Liu B. FBXO2 targets glycosylated SUN2 for ubiquitination and degradation to promote ovarian cancer development. Cell Death Dis. 2022;13:442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Uddin S, Bhat AA, Krishnankutty R, Mir F, Kulinski M, Mohammad RM. Involvement of F-BOX proteins in progression and development of human malignancies. Semin Cancer Biol. 2016;36:18-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Morel M, Shah KN, Long W. The F-box protein FBXL16 up-regulates the stability of C-MYC oncoprotein by antagonizing the activity of the F-box protein FBW7. J Biol Chem. 2020;295:7970-7980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Yang Y, Zhao Y, Sun G, Zuo S, Chai J, Xu T, Liu J, Li L, Song J, Qian S, Kang Y, Sui F, Li M, Jia Q. FBXO39 predicts poor prognosis and correlates with tumor progression in cervical squamous cell carcinoma. Pathol Res Pract. 2022;238:154090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Yu Y, Yao W, Wang T, Xue W, Meng Y, Cai L, Jian W, Yu Y, Zhang C. FBXL6 depletion restrains clear cell renal cell carcinoma progression. Transl Oncol. 2022;26:101550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Pastushenko I, Blanpain C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019;29:212-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 920] [Cited by in RCA: 1892] [Article Influence: 270.3] [Reference Citation Analysis (0)] |
| 25. | Li S, Cong X, Gao H, Lan X, Li Z, Wang W, Song S, Wang Y, Li C, Zhang H, Zhao Y, Xue Y. Tumor-associated neutrophils induce EMT by IL-17a to promote migration and invasion in gastric cancer cells. J Exp Clin Cancer Res. 2019;38:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 201] [Article Influence: 33.5] [Reference Citation Analysis (1)] |
| 26. | Zhang Y, Yuan Y, Zhang Y, Cheng L, Zhou X, Chen K. SNHG7 accelerates cell migration and invasion through regulating miR-34a-Snail-EMT axis in gastric cancer. Cell Cycle. 2020;19:142-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 27. | Guo F, Liu J, Han X, Zhang X, Lin T, Wang Y, Bai J, Han J. FBXO22 Suppresses Metastasis in Human Renal Cell Carcinoma via Inhibiting MMP-9-Mediated Migration and Invasion and VEGF-Mediated Angiogenesis. Int J Biol Sci. 2019;15:647-656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Zhao L, Niu H, Liu Y, Wang L, Zhang N, Zhang G, Liu R, Han M. LOX inhibition downregulates MMP-2 and MMP-9 in gastric cancer tissues and cells. J Cancer. 2019;10:6481-6490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Kumar P, Sebastian A, Verma K, Dixit R, Kumari S, Singh J, Tiwary SK, Narayan G. mRNA Expression Analysis of E-Cadherin, VEGF, and MMPs in Gastric Cancer: a Pilot Study. Indian J Surg Oncol. 2021;12:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |