Published online Jan 15, 2023. doi: 10.4251/wjgo.v15.i1.90
Peer-review started: September 30, 2022
First decision: October 21, 2022
Revised: October 28, 2022
Accepted: December 21, 2022
Article in press: December 21, 2022
Published online: January 15, 2023
Processing time: 101 Days and 23 Hours
Heat-clearing and detoxifying drugs has protective effect on colorectal cancer (CRC). Given the complicated features of Traditional Chinese medicine formulas, network pharmacology is an effective approach for studying the multiple interactions between drugs and diseases.
To systematically explore the anticancer mechanism of heat-clearing and detoxifying drug JC724.
This study obtained the active compounds and their targets in JC724 from Traditional Chinese Medicine System Pharmacology Database. In addition, the CRC targets were obtained from Drugbank, TTD, DisGeNET and GeneCards databases. We performed transcriptome analysis of differentially expressed genes in CRC treated with JC724. Venn diagram was used to screen the JC724-CRC intersection targets as candidate targets. Core targets were selected by protein-protein interaction network and herb ingredient-target-disease network analysis. The functional and pathway of core targets were analysed by enrichment analysis.
We found 174 active ingredients and 283 compound targets from JC724. 940 CRC-related targets were reserved from the four databases and 304 CRC differentially expressed genes were obtained by transcriptome analysis. We constructed the network and found that the five core ingredients were quercetin, β Beta sitosterol, wogonin, kaempferol and baicalein. The core JC724-CRC targets were CYP1A1, HMOX1, CXCL8, NQO1 and FOSL1. JC724 acts on multiple signaling pathways associated with CRC, including the Nrf2 signaling pathway, oxidative stress, and the IL-17 signaling pathway.
In this study, we systematically analyzed the active ingredients, core targets and main mechanisms of JC724 in the treatment of CRC. This study could bring a new perspective to the heat-clearing and detoxifying therapy of CRC.
Core Tip: We analyzed the core drug ingredients, genes and pathways of heat-clearing and detoxifying drug JC724 on the treatment of colorectal cancer (CRC), and illustrated the potential mechanisms comprehensively. We revealed that heat-clearing and detoxifying treatment can inhibit oxidative damage and inflammatory damage in CRC.
- Citation: Tang HZ, Yang ZP, Lu S, Wang B, Wang YY, Sun XB, Qu JX, Rao BQ. Network pharmacology-based analysis of heat clearing and detoxifying drug JC724 on the treatment of colorectal cancer. World J Gastrointest Oncol 2023; 15(1): 90-101
- URL: https://www.wjgnet.com/1948-5204/full/v15/i1/90.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i1.90
Colorectal cancer (CRC) has a high incidence and mortality, which seriously threatens human health. Traditional Chinese medicine (TCM) believes that the pathogenesis of colorectal cancer is the imbalance of Yin and Yang, which makes the heat toxins accumulate in the intestinal and eventually leads to tumour occurrence. Heat-clearing and detoxifying therapy has a history of more than 2000 years since it was first recorded in Huangdi Neijing. It can eliminate heat toxins and is the most frequently used TCM method (39.59%) for the treatment of colorectal cancer[1]. Huangqin decoction (HQD), originally recorded in Shang Han Lun, is a classic heat-clearing and detoxifying formula of four herbs, which has been used for the treatment of gastrointestinal diseases for nearly 1800 years[2]. HQD is composed of Huangqin (Scutellaria baicalensis Georgi), Gancao (Licorice), Baishao (Radix Paeoniae Alba), Dazao (Jujube), which exert multiple anticancer effects. Based on our previous research, we added Baihuasheshecao (Hedyotis diffusa) and Banzhilian (Scutellaria barbata) on the basis of HQD to improve the anticancer effect[3]. The new heat-clearing and detoxifying drug is called JC724. Pre-clinical trials have shown that JC724 combined with 5-fluorouracil in the treatment of advanced cancer patients could obtain better curative effect and reduce the toxicity of 5- fluorouracil[3,4]. However, the potential mechanism of the anticancer effect of heat-clearing and detoxifying drugs remains unknown. Therefore, further research is needed to determine the targets interacting with heat-clearing and detoxifying drug JC724 and elucidate its underlying mechanism in CRC treatment.
The function features of TCM are multi-ingredients, multi-targets and multi-pathways. Given the complicated features of TCM formulas, network pharmacology is an effective approach for studying the multiple interactions between drugs and diseases. It offers computational techniques to explore the treatment targets of drugs, which is more effective than experimental approaches. The interaction of drug targets and disease targets collected from the public databases may reveal many targets that are not the most critical. However, combined with transcriptional analysis, the core key targets of drug-disease can be found more comprehensively and accurately[5,6]. In this study, we used network pharmacology and transcriptional analysis to analyze the effectively active ingredients, potential targets, and core mechanisms of JC724 anti-cancer therapy. This can more accurately guide the next research direction and bring new perspective to heat-clearing and detoxifying drug in anti-cancer treatment.
Pharmacology platform database named Traditional Chinese Medicine System Pharmacology Database (TCMSP) (https://tcmspw.com/tcmsp.php) was used to collect the components of JC724[7]. The ADME screening criteria of active compounds were drug-likeness (DL) ≥ 0.18, oral bioavailability (OB) ≥ 30%, and half-life (HL) ≥ 4 h. The TCMSP was also used to identify targets of each active ingredient. We eliminated the duplicate data and retained the human targets. Subsequently, the Universal Protein Resource (Uniport, https://www.uniprot.org) was utilized to standardize all the targets into consistent terms.
We collected CRC-related targets from the following databases: DisGeNET (http://www.disgenet.org), Drugbank (https://www.drugbank.ca), TTD (http://db.idrblab.net/ttd/), and GeneCards (https://www.genecards.org). The retrieval results were merged, and the duplicate data had been eliminated.
Human colorectal cancer cell line HCT116 cells are widely used in the study of heat-clearing and detoxifying drugs against colorectal cancer and have better effect than other cell lines[8-12]. In this study, HCT116 cells were obtained from the Central Laboratory of Beijing Shijitan Hospital (Beijing, China), and the cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco, Grand Island, United States) added with 10% fetal bovine serum (Gibco, Australia Origin) and 1% penicillin/streptomycin (Gibco, United States). HCT116 cells were cultured in a 5% CO2 humidified incubator at 37 ℃.
HCT116 cells were seeded at a density of 1 × 106 cells/well in 6-well plates. After growing to 80% confluence, cells were treated with JC724 for 24h, while the untreated cells were considered as the control group. The drug extraction and concentration of JC724 in this study were determined according to our preliminary experimental results[3].
RNA-seq difference analysis Gene sequencing was conducted by Wuhan Huada Genoin Biotechnology Co., Ltd. After removing adapter or poly-N and low-quality reads, clean reads were used for the downstream analysis. Mean FPKM was utilized to screen the differential gene. The significant difference genes with the absolute value of log2foldchange (log2FC) > 1 were selected as CRC targets.
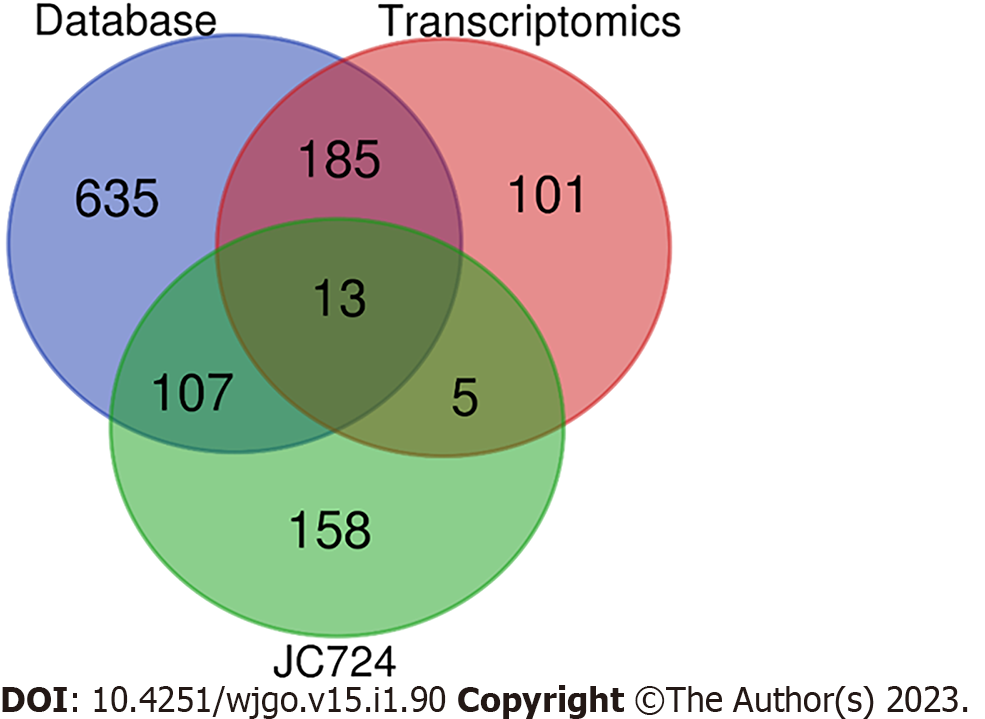
We plotted and visualized a Venn diagram of the JC724-CRC targets by using an online resource (https://bioinfogp.cnb.csic.es/tools/venny/index.html). The overlapped targets were regarded as correlative targets between JC724 and CRC.
The STRING database was used to integrate biomolecular interactions between various proteins, including physical and functional interactions[13]. Candidate targets were put into the STRING database Version 11.0 (https://string-db.org/) to acquire the interactions information between the candidate targets of JC724 and CRC. Protein-protein interaction (PPI) network analysis with interaction score > 0.4 as medium confidence and ‘Homo sapiens’ were selected.
Cytoscape 3.8.0 software can visualize the interaction networks of candidate targets[14]. We analyzed core genes of PPI network by MCODE and CytoHubba plugins of Cytoscape 3.8.0. The parameters of CytoHubba were core genes = top 10 nodes ranked by degree, maximal clique centrality (MCC) and maximum neighborhood component (MNC) . And we set the parameters of MCODE as node score cut off = 0.2, degree cut off = 2, K-score = 2 and Max depth = 100. The results were analyzed to get overlapping genes(core genes).
The Database for Enrichr (https://maayanlab.cloud/Enrichr/) uniquely integrates the comprehensive information of gene sets of many well-known projects. The platform can compute gene set enrichment and visualize in a variety of interactive ways[15]. We used the Enrichr Database for GO enrichment analysis, KEGG pathway analysis and Wiki pathway analysis. The results of Enriched GO terms are divided into molecular function (MF), cellular component (CC), and biological process (BP). The analysed pathway with P < 0.05 is statistically significant.
A total of 232 active herbal ingredients and 7812 compound targets of JC724 were identified from the TCMSP. Supplementary Table 1 showed 174 active herbal ingredients and 283 compound targets retained after removing duplicate data.
85, 112, 422, and 451 CRC targets were obtained from Drugbank, DisGeNET, TTD, and GeneCards, respectively. After deduplication, a total of 940 CRC related targets were retained. Transcriptomic results showed that there were 5289 differentially expressed genes. According to |log2FC| ≥ 1, a total of 304 genes were significantly differentially expressed. Combined with database and transcriptomics, there were 1046 CRC related targets.
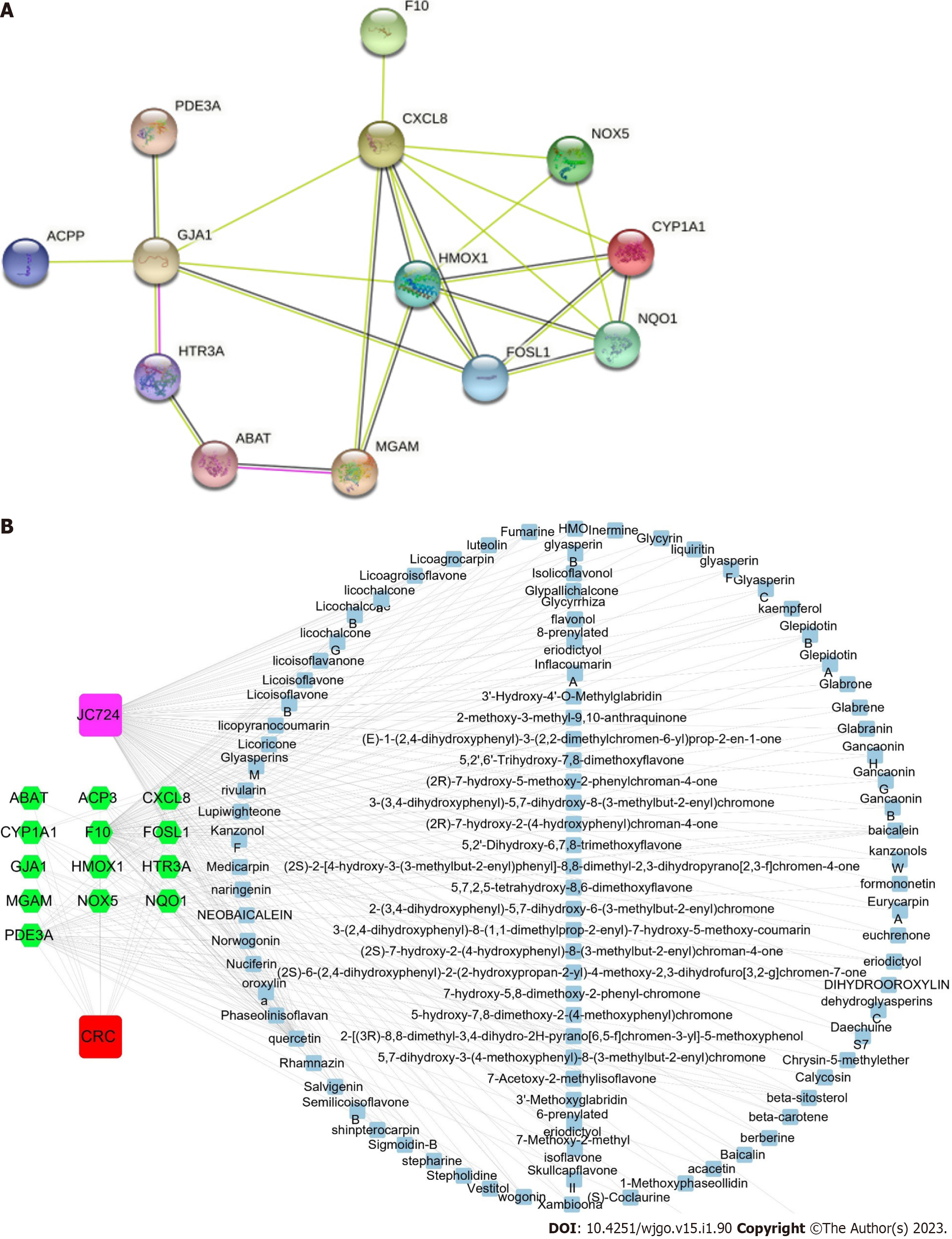
We intersected the potential targets of ingredients and CRC through Venn diagram. A total of 120 intersecting targets were obtained from JC724 targets and databases, and 18 intersecting targets were obtained from JC724 targets and transcriptomics. After screening and verification, 13 overlapped targets were selected as the candidate targets (Figure 1). They were MGAM, CYP1A1, HMOX1, CXCL8, NQO1, FOSL1, ABAT, NOX5, F10, PDE3A, GJA1, ACP3 and HTR3A. The results suggest that JC724 may exert anticancer effects on CRC through these 13 genes.
The interactions of targets in PPI network is represented by nodes and edges. The result showed that PPI network had 13 nodes and 24 edges, and the characteristics of the specific network topology were calculated (Figure 2A). The active ingredients and potential targets were imported into Cytoscape software for network construction (Figure 2B). Combining the results of CytoHubba and MCODE, we found that CYP1A1, HMOX1, CXCL8, NQO1, FOSL1 were core genes (Supplementary Figure 1). The active ingredients were screened according to the degree value greater than 2 times the median, we found that quercetin, β Beta sitosterol, wogonin, kaempferol and baicalein can be connected with more than 5 targets. The nodes of these active ingredients had more edges connected with other nodes, and their degree values were higher. Therefore, these 5 active ingredients may be the core ingredients of JC724.
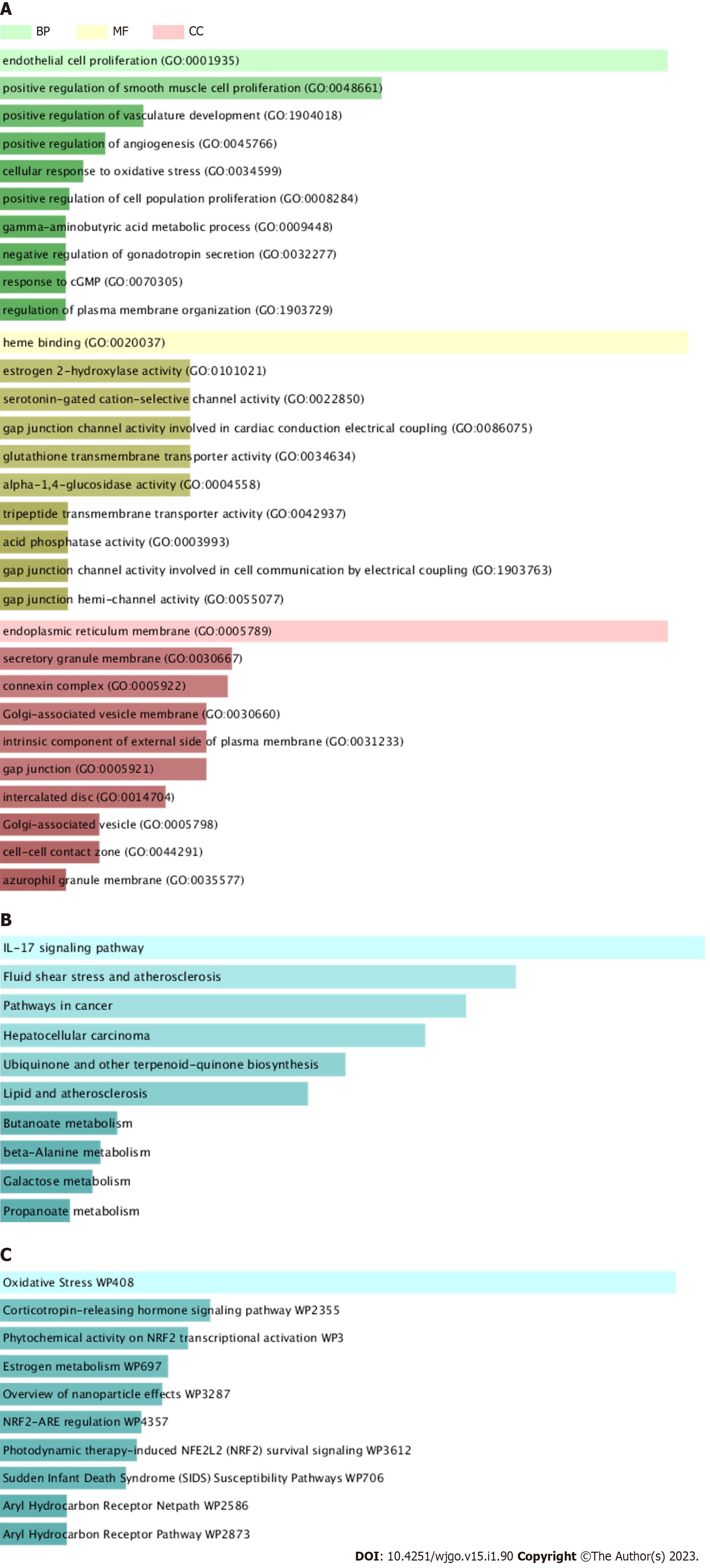
BP analysis indicated that the core targets were significantly enriched in cellular response to oxidative stress, cell proliferation, vasculature development, gamma-aminobutyric acid metabolic process and so forth (Figure 3A). MF analysis showed that the core targets were enriched in heme binding, serotonin-gated cation-selective channel activity, gap junction channel activity, glutathione transmembrane transporter activity and the like (Figure 3A). CC analysis revealed that the core targets were remarkably enriched in endoplasmic reticulum membrane, secretory granule membrane, connexin complex and so forth (Figure 3A). The analysis of KEGG pathway showed that the core targets were significantly enriched in the regulation of IL-17 signaling pathway, pathways in cancer, lipid and atherosclerosis and so on (Figure 3B). Wiki pathway analysis pointed out that the core targets were significantly enriched in oxidative stress, phytochemical activity on Nrf2 transcriptional activation, Nrf2-ARE regulation, Aryl Hydrocarbon Receptor Pathway, IL-10 Anti-inflammatory Signaling Pathway and so on (Figure 3C). The results showed that JC724 can treat CRC mainly through NRF signal pathway and IL-17 signal pathway, which can regulate inflammation and oxidative stress. In addition, JC724 can regulate cell proliferation, angiogenesis and metabolic reprogramming.
TCM compound has many components, which are characterized by wide coverage, low side effects, and can regulate the body's metabolism and microenvironment through multiple channels and targets, so it has natural therapeutic advantages. Most of the Chinese medicines with direct anti-cancer effects recorded in the Pharmacopoeia of the People's Republic of China are heat-clearing and detoxifying drugs[16]. Heat-clearing and detoxifying drugs have the functions of clearing ‘heat evil’ and ‘cancer toxin’. In TCM theory, heat evil is an invisible pathogenic qi that accumulates in the viscera, blocking the meridians, leading to fever and other manifestations. Cancer toxins can be divided into exogenous poisons such as environment and virus, and endogenous poisons produced by the body such as inflammatory factors and ROS. Cancer toxins have the characteristics of invisibility, destructiveness, intractability, etc. They damage organs, consumes nutrients, and drive the occurrence and development of cancer. The JC724 used in this study is a modified Huangqing decoction. Huangqin Decoction (HQD) is a traditional Chinese medicine formula recorded in Shanghan Lun, which is often used to treat gastrointestinal diseases. HQD can prevent CRC possibly by preventing oxidative stress induced cellular damage and inhibiting inflammation[17]. Furthermore, HQD can enhance the antitumor effect of chemotherapy agents and reduce the GI toxicity[18]. The mechanisms of HQD include increasing the nuclear expression of Nrf2 to protect antioxidant[19,20] and inhibiting inflammation through a variety of a variety of inflammation related signaling pathways[21,22]. We added heat-clearing and detoxifying drug Hedyotis diffusa and Scutellaria barbata to HQD to enhance the anticancer effect. They were the core couplet drugs of anti-inflammation and anticancer treatments in China[23]. The anti-colorectal cancer mechanism of Hedyotis diffusa is mainly achieved by attenuating the migration and tube formation abilities, inhibiting angiogenesis, enhancing immune function, reducing ROS to protect antioxidant and inducing tumor cell apoptosis[10,24,25]. Scutellaria barbata can treat colorectal cancer by inducing tumor cell apoptosis, inhibiting tumor cell growth, inhibiting angiogenesis, inhibiting migration and invasion and regulating immunity[26-28]. In a word, they have the same functions of inducing apoptosis, enhancing cellular immunity and protecting antioxidants. They also have their own unique functions, making them play a comprehensive role in TCM.
In our study, the important active ingredients of JC724 are quercetin, beta-sitosterol, wogonin, kaempferol and baicalein. Quercetin and kaempferol are flavonoids, which have the functions of reducing resistance of antibiotics and chemotherapeutics, detoxification of xenobiotics, anti-inflammatory and regulating tumour proliferation signaling pathways, etc. In addition, they can be used as antioxidants to prevent DNA damage and enhance DNA repair by scavenging reactive oxygen radicals[29]. Quercetin is responsible for increasing in Ca2+ production, cytochrome C, reducing mitochondrial membrane, and promoting the apoptosis of cancer cells[30]. Kaempferol can also activate caspase-3 and other apoptosis-inducing factors to promote apoptosis[31]. Furthermore, it was demonstrated that quercetin and kaempferol can reduce the activity of matrix metalloproteinase (MMP) by inhibiting the ERK signaling pathway, thereby reducing the invasion and metastasis of cancer cells[32,33]. Baicalein and wogonin are compounds extracted from Scutellaria baicalensis. They are flavonoids and have anti-cancer effects[18]. They can effectively relieve inflammation via activate PPARγ and downregulate the NF-κB/MAPK signaling pathway[34]. In addition, baicalein and wogonin can regulate the expression of Bax, Bad and other apoptotic factors through PI3K/Akt and STAT3 signaling pathways, therefore they induce apoptosis and autophagy to inhibit cancer cell proliferation[35,36]. Moreover, they act as antioxidants to clear superoxide radical by inhibiting xanthine oxidase[37]. β-Sitosterol has anti-cancer effects, which can induce mitochondrial mediated apoptotic cell death, inhibit the inflammatory reaction and protect DNA from oxidative damage[38,39]. In summary, the mechanism of these important active ingredients is mainly through anti-inflammatory, anti-oxidative stress and promoting apoptosis.
In our study, the CRC-related targets were not only from the transcriptome of HCT116 cells, but also from databases, so we can more comprehensively elucidated the anti-cancer mechanism. After screening and verification, we found that CYP1A1, HMOX1, CXCL8, NQO1 and FOSL1 were the core genes. NQO1 is a flavin adenine dinucleotide-dependent flavoprotein, which promotes the two-electron reduction of quinone-like compounds[40]. NQO1 has an antioxidative stress effect in cellular defense by activating the Nrf2/NQO1 signaling pathway[41]. In addition, NQO1 can play an important role in mediating the anti-inflammatory effect by regulating AMPK signaling pathways[42]. HMOX1 is a stress-inducing enzyme, which can catalyze the degradation of heme to iron, carbon monoxide and biliverdin[43]. It can reduce oxidative stress damage by ERK/Nrf2 pathway and eliminate toxic intracellular heme[44]. It can also generate carbon monoxide and activate the PPP3-TFEB signal axis through IFNG to induce autophagy[45]. CYP1A1 is a subtype of the cytochrome P450 family. It can catalyze the activation of polycyclic aromatic hydrocarbons such as benzo[a]pyrene, which is transcriptionally regulated by the AhR/ARNT complex[46]. These reactive metabolites can induce mutagenic DNA adducts and lead to cancer[47]. CXCL8 is a pivotal chemokine secreted by tumor-associated macrophages, which can regulate epithelial mesenchymal transition, cancer migration and invasion ability through PI3K/Akt/NF-κB pathway[48,49]. CXCL8 can inhibits CD8T cells function through inducing the expression of PD-L1 on macrophages[50]. Furthermore, CXCL8 can also participate in the activation and transport of inflammatory mediators through the CXCL8-CXCR1/2 signaling axis[51]. FOSL1 serves as the dominant activating protein 1 family member, can promote tumorigenesis in CRC through the FBXL2/Wnt/β-catenin axis [52]. Furthermore, FOSL1 can regulate inflammation through TCF7/FOL1/MMP9 axis [53]. Therefore, the main functions of these five core genes are antioxidant stress, anti-inflammatory and detoxification. The Nrf2 signaling pathway can regulate the oxidative stress function by downstream NQO1 and HMOX1 in CRC[54]. Nrf2 signaling pathway can also regulate CYP1A1[55], CYP1A1 can form a stable complex with HMOX1 to play physiological function[56]. Wiki enrichment analysis showed that the Nrf2 pathway can regulate oxidative stress, which is a key pathway for JC724 to treat CRC. In addition, CXCL8 and FOSL1 regulate inflammation through the IL-17 signaling pathway[57,58]. The IL-17 signaling pathway is the most significant pathway in our KEGG enrichment analysis, and it is also the key pathway of JC724 in the treatment of CRC. Therefore, the heat-clearing and detoxifying drug JC724 plays an anti-cancer effect mainly through the Nrf2 signaling pathway, IL-17 signaling pathway and the key regulatory genes.
In conclusion, the analysis results of the core drug components, genes and pathways provide a reference for the further research on specific mechanisms of TCM therapy on CRC. The results showed that the anticancer mechanism of heat-clearing and detoxifying drugs is anti-oxidative stress, anti-inflammatory and detoxification. Based on the theory of traditional Chinese medicine, we speculated that the heat toxin in Chinese medicine refers to reactive oxygen species, inflammatory factors and toxins, etc. Heat-clearing and detoxifying treatment can inhibit oxidative damage and inflammatory damage by clearing heat toxin, thus inhibiting the development of CRC. The complicated features of TCM and active ingredients are multi-targets and multi-pathways. Therefore, the mechanism of JC724 against colorectal cancer may not be specific, which needs further experiments to prove. However, network pharmacology research has its own limitations. First, network pharmacology ignores drug dosage, and different doses of different components in TCM may have different comprehensive therapeutic effects. In addition, network pharmacology cannot elucidate the possible new compounds in the process of drug decoction and the metabolism in the human body. Furthermore, another limitation is the high false positive rate of network pharmacology. However, network pharmacology saves the scientific research resources, helps researchers effectively screen drug components and core targets, and provides direction for subsequent mechanism validation. In the future, it is necessary to optimize the technology of drug screening and verify the effect of JC724 on CRC treatment at the molecular level. We hope that our study can provide a new perspective to the anti-cancer mechanism of heat-clearing and detoxifying therapy, and bring new drugs for the treatment of CRC.
Heat clearing and detoxification drugs are commonly used in traditional Chinese medicine to treat colorectal cancer, but the mechanism is not clear.
We hope that our study can provide a new perspective to the anti-cancer mechanism of heat-clearing and detoxifying therapy, and bring new drugs for the treatment of colorectal cancer (CRC).
This study aims to systematically explore the anticancer mechanism of heat clearing and detoxifying drug JC724.
We identified JC724 active components and targets by database, and then determined CRC related targets through the database and transcriptome analysis. We used network pharmacology to analyze the active herbal ingredients, core targets and main mechanisms of JC724 in the treatment of CRC.
We found that the five ingredients with the most targets were quercetin, β Beta sitosterol, wogonin, kaempferol and baicalein, which were the core ingredients. The core JC724-CRC targets were CYP1A1, HMOX1, CXCL8, NQO1 and FOSL1. JC724 mainly acts on CRC through Nrf2 signaling pathway, oxidative stress and IL-17 signaling pathway.
This study systematically analyzed the mechanism of heat clearing and detoxification drug JC724 in the treatment of CRC, providing direction for further experiments.
This study provides a new perspective on the anti-cancer mechanism of heat clearing and detoxifying drug drugs. Combined with the theory of traditional Chinese medicine, this study can provide direction for research and clinical treatment in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pharmacology and pharmacy
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Zaatari M, United States; Ozturk S, Turkey S-Editor: Gong ZM L-Editor: A P-Editor: Chen YX
| 1. | Wu LN, Xie YM, Liu H, Zhang Y, Lu Q, Zhuang Y. [Real-world study on syndrome distribution and medication characteristics of colonic malignant tumors]. Zhongguo Zhong Yao Za Zhi. 2020;45:1174-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Li MY, Li MX, Xu N, Li ZH, Zhang YM, Gan YX, Luo HJ, Zhou CL, Liu YH, Su ZR, Huang XQ, Zheng XB. Effects of Huangqin Decoction on ulcerative colitis by targeting estrogen receptor alpha and ameliorating endothelial dysfunction based on system pharmacology. J Ethnopharmacol. 2021;271:113886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Deng L, Ding Y, Rao BQ, Yang J. JC724 extraction and its effect on colorectal cancer. Zhongliu Yu Daixie Dianzi Zazhi. 2016;3:234-238. [DOI] [Full Text] |
| 4. | Rao BQ, Ding Y, Deng L. Clinical trial of the JC724 combined capecitabine on advanced cancer. Zhongliu Yu Daixie Dianzi Zazhi. 2017;4:78-82. [DOI] [Full Text] |
| 5. | Liu Z, Ma H, Lai Z. Revealing the potential mechanism of Astragalus membranaceus improving prognosis of hepatocellular carcinoma by combining transcriptomics and network pharmacology. BMC Complement Med Ther. 2021;21:263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Sun L, Yang Z, Zhao W, Chen Q, Bai H, Wang S, Yang L, Bi C, Shi Y, Liu Y. Integrated lipidomics, transcriptomics and network pharmacology analysis to reveal the mechanisms of Danggui Buxue Decoction in the treatment of diabetic nephropathy in type 2 diabetes mellitus. J Ethnopharmacol. 2022;283:114699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 7. | Ru J, Li P, Wang J, Zhou W, Li B, Huang C, Guo Z, Tao W, Yang Y, Xu X, Li Y, Wang Y, Yang L. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1623] [Cited by in RCA: 3120] [Article Influence: 283.6] [Reference Citation Analysis (0)] |
| 8. | Havermann S, Chovolou Y, Humpf HU, Wätjen W. Modulation of the Nrf2 signalling pathway in Hct116 colon carcinoma cells by baicalein and its methylated derivative negletein. Pharm Biol. 2016;54:1491-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Phan T, Nguyen VH, A'lincourt Salazar M, Wong P, Diamond DJ, Yim JH, Melstrom LG. Inhibition of Autophagy Amplifies Baicalein-Induced Apoptosis in Human Colorectal Cancer. Mol Ther Oncolytics. 2020;19:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Feng J, Jin Y, Peng J, Wei L, Cai Q, Yan Z, Lai Z, Lin J. Hedyotis diffusa willd extract suppresses colorectal cancer growth through multiple cellular pathways. Oncol Lett. 2017;14:8197-8205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Zhao Y, Zhang L, Wu Y, Dai Q, Zhou Y, Li Z, Yang L, Guo Q, Lu N. Selective anti-tumor activity of wogonin targeting the Warburg effect through stablizing p53. Pharmacol Res. 2018;135:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Pan D, Zeng C, Zhang W, Li T, Qin Z, Yao X, Dai Y, Yao Z, Yu Y. Non-volatile pungent compounds isolated from Zingiber officinale and their mechanisms of action. Food Funct. 2019;10:1203-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, Jensen LJ, von Mering C. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362-D368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4345] [Cited by in RCA: 5062] [Article Influence: 562.4] [Reference Citation Analysis (0)] |
| 14. | Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498-2504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24663] [Cited by in RCA: 33302] [Article Influence: 1585.8] [Reference Citation Analysis (0)] |
| 15. | Xie Z, Bailey A, Kuleshov MV, Clarke DJB, Evangelista JE, Jenkins SL, Lachmann A, Wojciechowicz ML, Kropiwnicki E, Jagodnik KM, Jeon M, Ma'ayan A. Gene Set Knowledge Discovery with Enrichr. Curr Protoc. 2021;1:e90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 1899] [Article Influence: 474.8] [Reference Citation Analysis (0)] |
| 16. | Xu X, Xu H, Shang Y, Zhu R, Hong X, Song Z, Yang Z. Development of the general chapters of the Chinese Pharmacopoeia 2020 edition: A review. J Pharm Anal. 2021;11:398-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 17. | Chen G, Yang Y, Hu C, Cheng X, Xu Y, Cai X, Wang M, Yang CS, Cao P. Protective effects of Huangqin Decoction against ulcerative colitis and associated cancer in mice. Oncotarget. 2016;7:61643-61655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Xu DD, Hou XY, Wang O, Wang D, Li DT, Qin SY, Lv B, Dai XM, Zhang ZJ, Wan JB, Xu FG. A four-component combination derived from Huang-Qin Decoction significantly enhances anticancer activity of irinotecan. Chin J Nat Med. 2021;19:364-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Li WK, Wang GF, Wang TM, Li YY, Li YF, Lu XY, Wang YH, Zhang H, Liu P, Wu JS, Ma YM. Protective effect of herbal medicine Huangqi decoction against chronic cholestatic liver injury by inhibiting bile acid-stimulated inflammation in DDC-induced mice. Phytomedicine. 2019;62:152948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Han H, Cao A, Wang L, Guo H, Zang Y, Li Z, Zhang X, Peng W. Huangqi Decoction Ameliorates Streptozotocin-Induced Rat Diabetic Nephropathy through Antioxidant and Regulation of the TGF-β/MAPK/PPAR-γ Signaling. Cell Physiol Biochem. 2017;42:1934-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Li MY, Luo HJ, Wu X, Liu YH, Gan YX, Xu N, Zhang YM, Zhang SH, Zhou CL, Su ZR, Huang XQ, Zheng XB. Anti-Inflammatory Effects of Huangqin Decoction on Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice Through Regulation of the Gut Microbiota and Suppression of the Ras-PI3K-Akt-HIF-1α and NF-κB Pathways. Front Pharmacol. 2019;10:1552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 22. | Gu LM, Li H, Xia JQ, Pan CY, Gu C, Tian YZ. Huangqin Decoction Attenuates DSS-Induced Mucosal Damage and Promotes Epithelial Repair via Inhibiting TNF-α-Induced NF-κB Activation. Chin J Integr Med. 2022;28:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 23. | Xu Y, Chen XX, Jiang YX, Zhang DD. Ethyl Acetate Fraction from Hedyotis diffusa plus Scutellaria barbata Exerts Anti-Inflammatory Effects by Regulating miR-155 Expression and JNK Signaling Pathway. Evid Based Complement Alternat Med. 2018;2018:3593408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Li H, Lai Z, Yang H, Peng J, Chen Y, Lin J. Hedyotis diffusa Willd. inhibits VEGFCmediated lymphangiogenesis in colorectal cancer via multiple signaling pathways. Oncol Rep. 2019;42:1225-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Li YL, Chen X, Niu SQ, Zhou HY, Li QS. Protective Antioxidant Effects of Amentoflavone and Total Flavonoids from Hedyotis diffusa on H2 O2 -Induced HL-O2 Cells through ASK1/p38 MAPK Pathway. Chem Biodivers. 2020;17:e2000251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Wei L, Lin J, Xu W, Cai Q, Shen A, Hong Z, Peng J. Scutellaria barbata D. Don inhibits tumor angiogenesis via suppression of Hedgehog pathway in a mouse model of colorectal cancer. Int J Mol Sci. 2012;13:9419-9430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 27. | Jin Y, Chen W, Yang H, Yan Z, Lai Z, Feng J, Peng J, Lin J. Scutellaria barbata D. Don inhibits migration and invasion of colorectal cancer cells via suppression of PI3K/AKT and TGF-β/Smad signaling pathways. Exp Ther Med. 2017;14:5527-5534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Zeng S, Chen L, Sun Q, Zhao H, Yang H, Ren S, Liu M, Meng X, Xu H. Scutellarin ameliorates colitis-associated colorectal cancer by suppressing Wnt/β-catenin signaling cascade. Eur J Pharmacol. 2021;906:174253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 29. | Kubina R, Iriti M, Kabała-Dzik A. Anticancer Potential of Selected Flavonols: Fisetin, Kaempferol, and Quercetin on Head and Neck Cancers. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 30. | Ma YS, Yao CN, Liu HC, Yu FS, Lin JJ, Lu KW, Liao CL, Chueh FS, Chung JG. Quercetin induced apoptosis of human oral cancer SAS cells through mitochondria and endoplasmic reticulum mediated signaling pathways. Oncol Lett. 2018;15:9663-9672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Kang JW, Kim JH, Song K, Kim SH, Yoon JH, Kim KS. Kaempferol and quercetin, components of Ginkgo biloba extract (EGb 761), induce caspase-3-dependent apoptosis in oral cavity cancer cells. Phytother Res. 2010;24 Suppl 1:S77-S82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Lin CW, Chen PN, Chen MK, Yang WE, Tang CH, Yang SF, Hsieh YS. Kaempferol reduces matrix metalloproteinase-2 expression by down-regulating ERK1/2 and the activator protein-1 signaling pathways in oral cancer cells. PLoS One. 2013;8:e80883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 33. | Lai WW, Hsu SC, Chueh FS, Chen YY, Yang JS, Lin JP, Lien JC, Tsai CH, Chung JG. Quercetin inhibits migration and invasion of SAS human oral cancer cells through inhibition of NF-κB and matrix metalloproteinase-2/-9 signaling pathways. Anticancer Res. 2013;33:1941-1950. [PubMed] |
| 34. | Ji Y, Han J, Lee N, Yoon JH, Youn K, Ha HJ, Yoon E, Kim DH, Jun M. Neuroprotective Effects of Baicalein, Wogonin, and Oroxylin A on Amyloid Beta-Induced Toxicity via NF-κB/MAPK Pathway Modulation. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 35. | Kim SJ, Kim HJ, Kim HR, Lee SH, Cho SD, Choi CS, Nam JS, Jung JY. Antitumor actions of baicalein and wogonin in HT-29 human colorectal cancer cells. Mol Med Rep. 2012;6:1443-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | Tan H, Li X, Yang WH, Kang Y. A flavone, Wogonin from Scutellaria baicalensis inhibits the proliferation of human colorectal cancer cells by inducing of autophagy, apoptosis and G2/M cell cycle arrest via modulating the PI3K/AKT and STAT3 signalling pathways. J BUON. 2019;24:1143-1149. [PubMed] |
| 37. | Shieh DE, Liu LT, Lin CC. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res. 2000;20:2861-2865. [PubMed] |
| 38. | Rajavel T, Packiyaraj P, Suryanarayanan V, Singh SK, Ruckmani K, Pandima Devi K. β-Sitosterol targets Trx/Trx1 reductase to induce apoptosis in A549 cells via ROS mediated mitochondrial dysregulation and p53 activation. Sci Rep. 2018;8:2071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 39. | Sharmila R, Sindhu G. Evaluate the Antigenotoxicity and Anticancer Role of β-Sitosterol by Determining Oxidative DNA Damage and the Expression of Phosphorylated Mitogen-activated Protein Kinases', C-fos, C-jun, and Endothelial Growth Factor Receptor. Pharmacogn Mag. 2017;13:95-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 40. | Rashid MH, Babu D, Siraki AG. Interactions of the antioxidant enzymes NAD(P)H: Quinone oxidoreductase 1 (NQO1) and NRH: Quinone oxidoreductase 2 (NQO2) with pharmacological agents, endogenous biochemicals and environmental contaminants. Chem Biol Interact. 2021;345:109574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 41. | Shu M, Lei W, Su S, Wen Y, Luo F, Zhao L, Chen L, Lu C, Zhou Z, Li Z. Chlamydia trachomatis Pgp3 protein regulates oxidative stress via activation of the Nrf2/NQO1 signal pathway. Life Sci. 2021;277:119502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Park JE, Park JS, Leem YH, Kim DY, Kim HS. NQO1 mediates the anti-inflammatory effects of nootkatone in lipopolysaccharide-induced neuroinflammation by modulating the AMPK signaling pathway. Free Radic Biol Med. 2021;164:354-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 43. | Dunn LL, Kong SMY, Tumanov S, Chen W, Cantley J, Ayer A, Maghzal GJ, Midwinter RG, Chan KH, Ng MKC, Stocker R. Hmox1 (Heme Oxygenase-1) Protects Against Ischemia-Mediated Injury via Stabilization of HIF-1α (Hypoxia-Inducible Factor-1α). Arterioscler Thromb Vasc Biol. 2021;41:317-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 44. | Tian R, Yang Z, Lu N, Peng YY. Quercetin, but not rutin, attenuated hydrogen peroxide-induced cell damage via heme oxygenase-1 induction in endothelial cells. Arch Biochem Biophys. 2019;676:108157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Singh N, Kansal P, Ahmad Z, Baid N, Kushwaha H, Khatri N, Kumar A. Antimycobacterial effect of IFNG (interferon gamma)-induced autophagy depends on HMOX1 (heme oxygenase 1)-mediated increase in intracellular calcium levels and modulation of PPP3/calcineurin-TFEB (transcription factor EB) axis. Autophagy. 2018;14:972-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 46. | Nakano N, Sakata N, Katsu Y, Nochise D, Sato E, Takahashi Y, Yamaguchi S, Haga Y, Ikeno S, Motizuki M, Sano K, Yamasaki K, Miyazawa K, Itoh S. Dissociation of the AhR/ARNT complex by TGF-β/Smad signaling represses CYP1A1 gene expression and inhibits benze[a]pyrene-mediated cytotoxicity. J Biol Chem. 2020;295:9033-9051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 47. | Gastelum G, Jiang W, Wang L, Zhou G, Borkar R, Putluri N, Moorthy B. Polycyclic Aromatic Hydrocarbon-induced Pulmonary Carcinogenesis in Cytochrome P450 (CYP)1A1- and 1A2-Null Mice: Roles of CYP1A1 and CYP1A2. Toxicol Sci. 2020;177:347-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 48. | Nie G, Cao X, Mao Y, Lv Z, Lv M, Wang Y, Wang H, Liu C. Tumor-associated macrophages-mediated CXCL8 infiltration enhances breast cancer metastasis: Suppression by Danirixin. Int Immunopharmacol. 2021;95:107153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 49. | Shen T, Yang Z, Cheng X, Xiao Y, Yu K, Cai X, Xia C, Li Y. CXCL8 induces epithelial-mesenchymal transition in colon cancer cells via the PI3K/Akt/NF-κB signaling pathway. Oncol Rep. 2017;37:2095-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 50. | Lin C, He H, Liu H, Li R, Chen Y, Qi Y, Jiang Q, Chen L, Zhang P, Zhang H, Li H, Zhang W, Sun Y, Xu J. Tumour-associated macrophages-derived CXCL8 determines immune evasion through autonomous PD-L1 expression in gastric cancer. Gut. 2019;68:1764-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 265] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 51. | Ha H, Debnath B, Neamati N. Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases. Theranostics. 2017;7:1543-1588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 562] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 52. | Liu Y, Yue M, Li Z. FOSL1 promotes tumorigenesis in colorectal carcinoma by mediating the FBXL2/Wnt/β-catenin axis via Smurf1. Pharmacol Res. 2021;165:105405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 53. | Yao T, Zhang L, Fu Y, Yao L, Zhou C, Chen G. Saikosaponin-d Alleviates Renal Inflammation and Cell Apoptosis in a Mouse Model of Sepsis via TCF7/FOSL1/Matrix Metalloproteinase 9 Inhibition. Mol Cell Biol. 2021;41:e0033221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 54. | Jang HJ, Hong EM, Kim M, Kim JH, Jang J, Park SW, Byun HW, Koh DH, Choi MH, Kae SH, Lee J. Simvastatin induces heme oxygenase-1 via NF-E2-related factor 2 (Nrf2) activation through ERK and PI3K/Akt pathway in colon cancer. Oncotarget. 2016;7:46219-46229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 55. | Ye W, Lin R, Chen X, Chen J, Chen R, Xie X, Deng Y, Wen J. T-2 toxin upregulates the expression of human cytochrome P450 1A1 (CYP1A1) by enhancing NRF1 and Sp1 interaction. Toxicol Lett. 2019;315:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Connick JP, Reed JR, Cawley GF, Backes WL. Heteromeric complex formation between human cytochrome P450 CYP1A1 and heme oxygenase-1. Biochem J. 2021;478:377-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Qi L, Bao W, Li W, Ding X, Yan A. IL-17 signaling pathway plays a key role in laryngeal squamous cell carcinoma with ethnic specificity. Am J Cancer Res. 2021;11:2684-2695. [PubMed] |
| 58. | Moon YM, Lee SY, Kwok SK, Lee SH, Kim D, Kim WK, Her YM, Son HJ, Kim EK, Ryu JG, Seo HB, Kwon JE, Hwang SY, Youn J, Seong RH, Jue DM, Park SH, Kim HY, Ahn SM, Cho ML. The Fos-Related Antigen 1-JUNB/Activator Protein 1 Transcription Complex, a Downstream Target of Signal Transducer and Activator of Transcription 3, Induces T Helper 17 Differentiation and Promotes Experimental Autoimmune Arthritis. Front Immunol. 2017;8:1793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |