Published online Jul 15, 2022. doi: 10.4251/wjgo.v14.i7.1281
Peer-review started: February 1, 2022
First decision: April 17, 2022
Revised: April 29, 2022
Accepted: June 4, 2022
Article in press: June 4, 2022
Published online: July 15, 2022
Processing time: 161 Days and 20.5 Hours
The liver is the most common metastatic site of colorectal cancer. Hepatectomy is the mainstay of treatment for patients with colorectal liver metastases (CRLMs). However, there are cases of early recurrence after upfront hepatectomy alone. In selected high-risk patients, neoadjuvant chemotherapy (NAC) may improve long-term survival.
To determine the efficacy of NAC for initially resectable CRLMs.
Among 644 patients who underwent their first hepatectomy for CRLMs at our institution, 297 resectable cases were stratified into an upfront hepatectomy group (238 patients) and a NAC group (59 patients). Poor prognostic factors for upfront hepatectomy were identified using multivariate logistic regression analysis. Propensity score matching was used to compare clinical outcomes between the upfront hepatectomy and NAC groups, according to the number of poor prognostic factors. Survival curves were estimated using the Kaplan–Meier method and compared using the log-rank test.
Preoperative carcinoembryonic antigen levels (≥ 10 ng/mL) (P = 0.003), primary histological type (other than well/moderately differentiated) (P = 0.04), and primary lymph node metastases (≥ 1) (P = 0.04) were identified as independent poor prognostic factors for overall survival (OS) in the upfront hepatectomy group. High-risk status was defined as the presence of two or more risk factors. After propensity score matching, 50 patients were matched in each group. Among high-risk patients, the 5-year OS rate was significantly higher in the NAC group (13 patients) than in the upfront hepatectomy group (18 patients) (100% vs 34%; P = 0.02).
NAC may improve the prognosis of high-risk patients with resectable CRLMs who have two or more risk factors.
Core Tip: Hepatectomy is the mainstay of treatment for patients with colorectal liver metastases (CRLMs). However, there are cases of early recurrence after upfront hepatectomy alone. In selected high-risk patients, neoadjuvant chemotherapy (NAC) may improve long-term survival. Although several studies have identified risk factors for recurrence and prognosis after hepatectomy for CRLMs, they could not show a benefit of NAC for resectable CRLMs. This article demonstrated the effectiveness of NAC for initially resectable CRLMs, based on risk stratification according to prognostic factors.
- Citation: Takeda K, Sawada Y, Yabushita Y, Honma Y, Kumamoto T, Watanabe J, Matsuyama R, Kunisaki C, Misumi T, Endo I. Efficacy of neoadjuvant chemotherapy for initially resectable colorectal liver metastases: A retrospective cohort study. World J Gastrointest Oncol 2022; 14(7): 1281-1294
- URL: https://www.wjgnet.com/1948-5204/full/v14/i7/1281.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i7.1281
Colorectal cancer (CRC) is a leading cause of cancer-related deaths worldwide. Approximately 20% of patients with CRC present with synchronous distant metastases, and another 20% develop metachronous metastases[1].
The liver is the most common metastatic site of CRC[2]. Hepatectomy is the mainstay of treatment for patients with colorectal liver metastases (CRLMs). The 5-year overall survival (OS) rate after curative hepatectomy has been reported to range from 45 to 61%. However, the postoperative recurrence rate is high (approximately 75%), especially in the remnant liver[3]. To improve surgical outcomes, neo
Several studies[6-9] have identified risk factors for recurrence and prognosis after hepatectomy for CRLMs, including positive lymph node status of the primary colorectal lesion, appearance time, largest tumor diameter, number and distribution of CRLMs, and preoperative carcinoembryonic antigen (CEA)/carbohydrate antigen 19-9 Levels. A greater number of risk factors were associated with early recurrence or poor prognosis. Hence, there are cases of early recurrence after upfront hepatectomy alone in the resectable CRLMs, and in selected high-risk patients, NAC may improve long-term survival. We investigated the effectiveness of NAC for initially resectable CRLMs, based on risk stratification according to prognostic factors.
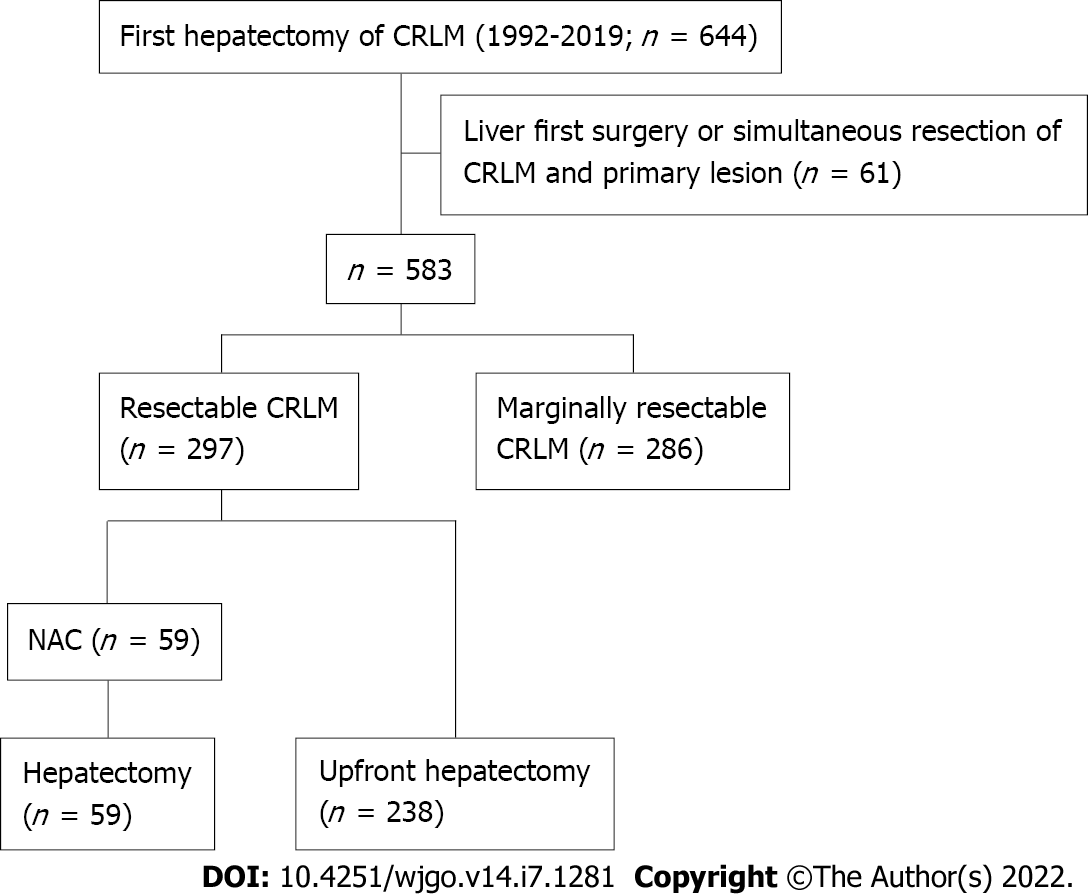
A total of 644 patients underwent their first hepatectomy for CRLMs at our institution between January 1992 and December 2019. Among them, 297 resectable cases were included in this study. Among these cases, patients with synchronous liver metastases who received liver-first surgery or simultaneous resection of CRLM and the primary lesion were excluded. Patients were stratified into an upfront hepatectomy group (238 patients) and a NAC group (59 patients) (Figure 1). No patient received preoperative chemotherapy before resection of the primary lesion. Poor prognostic factors for upfront hepatectomy were identified using multivariate logistic regression analysis. Propensity score matching was performed using baseline characteristics, and clinical outcomes were compared between the groups, according to the number of poor prognostic factors.
The following clinicopathological variables were analyzed: Patient-related: Age (< 60 vs ≥ 60 years), sex (male vs female), and initial CEA level (< 10 vs ≥ 10 ng/mL); primary tumor-related: Site of the primary lesion (right vs left), primary histological type (well/moderately differentiated vs others), lymph node metastases (0 vs ≥ 1), depth of tumor invasion [adjacent organ invasion (T4b) vs others], lymphatic invasion (0 vs ≥ 1), and venous invasion (0 vs ≥ 1); liver metastasis-related: Number (1–3 vs ≥ 4), maximum diameter (< 40 vs ≥ 40 mm), appearance time (synchronous vs metachronous), and tumor distribution (unilobar vs bilobar); and treatment-related: Staged hepatectomy (performed vs not performed), surgical margins (exposed vs not exposed), and adjuvant chemotherapy after primary resection and after hepatectomy (administered vs not administered). In addition, left-sided tumors included carcinomas in the descending colon, sigmoid colon, and rectum; and right-sided tumors included carcinomas in the cecum, ascending colon, and transverse colon.
Propensity score matching was performed to minimize the differences in baseline characteristics between the upfront hepatectomy and NAC groups. The propensity score for each patient was estimated by logistic regression analysis using the primary tumor- and liver metastasis-related variables.
The criteria for resectable CRLMs were: (1) No extrahepatic metastases; (2) Liver tumor in one lobe only, or no more than three tumors in both lobes; (3) Favorable tumor location, without invasion of major vascular structures; (4) Maximum tumor diameter ≤ 80 mm; and (5) Sufficient planned residual liver volume[10]. The criteria for unresectable CRLMs were uncontrollable extrahepatic metastases and insufficient residual liver capacity. Originally, NAC was administered to those with marginally resectable CRLMs who did not satisfy either of these criteria[10]. However, there were patients who underwent upfront hepatectomy (at their own request) although they met the criteria for NAC initially. Conversely, there were patients who received NAC although they met the criteria for resectable CRLM initially. Therefore, patients who met the criteria for resectable CRLM included those who underwent upfront hepatectomy or received NAC.
Patients received NAC according to the abovementioned criteria. Some patients in the NAC group were treated with chemotherapy by another physician, who considered the CRLMs to be unresectable. However, when the patients were referred to our hospital, the CRLMs were judged to have met the criteria for resection prior to the start of chemotherapy. Regarding NAC regimens, fluoracyl and folinic acid had been used. After oxaliplatin- and irinotecan-based regimens became available, these were widely used as NAC. The combined use of molecularly-targeted agents was also considered, based on RAS status. Hepatic arterial infusion was considered for elderly patients, or those who could not continue systemic chemotherapy due to side effects. The response to NAC was evaluated by contrast-enhanced computed tomography (CT)/magnetic resonance imaging, according to the Response Evaluation Criteria in Solid Tumors (version 1.1)[11]. The number of treatment cycles varied because of the retrospective nature of the study. Hepatectomy was performed ≥ 4 wk after the last administration of chemotherapy. When bevacizumab was used, an interval of ≥ 6 wk was maintained.
Adjuvant chemotherapy (hepatic arterial or intravenous infusion or systemic or oral administration of fluoracyl and folinic acid, oxaliplatin, or irinotecan) was considered for all patients who underwent hepatectomy[10]. However, it has not been administered actively since 2019, as few studies have shown a survival benefit[12,13].
Hepatectomy with negative surgical margins was performed in principle with non-anatomical procedures. Anatomical hepatectomy was performed, if it was advantageous, in terms of complete resection (R0), operative time, blood loss, or invasiveness. Portal vein embolization or two-stage hepatectomy was planned when the remnant prognostic score was low, based on volumetry, the indocyanine green retention rate, and patients’ age[14]. Intraoperative ultrasonography was performed in all cases to detect occult tumors undetected by preoperative imaging, and to confirm the anatomical relationships between tumors and vasculobiliary structures, and the absence of residual tumors in the remnant liver. Parenchymal dissection was performed mainly using ultrasonic dissectors[14]. R0 resection was considered complete when the pathologist assessed free resection margins.
OS was defined as the time from hepatectomy until death from any cause. Disease-free survival (DFS) was defined as the time from hepatectomy until the first recurrence. Tumor response was evaluated according to the Response Evaluation Criteria in Solid Tumors (version 1.1)[11]. Synchronous CRLMs were defined as metastases to the liver at the time of resection of the primary CRC.
Patients were examined for recurrence after hepatectomy using contrast-enhanced CT (every 4–6 mo), blood tests, and tumor markers (every 2–3 mo). When recurrence in the remnant liver was suspected, magnetic resonance imaging was performed, and the appearance of new lesions was investigated. Extrahepatic recurrence in the chest and pelvis was detected on CT. Fluorodeoxyglucose positron emission tomography was sometimes performed to detect other distant metastases. Recurrence was diagnosed when imaging studies confirmed new lesions showing typical features of CRC/CRLMs, compared with previous images. Recurrent CRLMs were treated with repeat resection, if applicable. When there was no indication for resection, chemotherapy, radiotherapy, or palliative care was chosen.
Quantitative variables were expressed as medians (interquartile ranges), and categorical variables as numbers and percentages. Continuous data were compared using the Mann–Whitney U test, and categorical data using the chi-square test. Survival curves were estimated using the Kaplan–Meier method and compared using the log-rank test. Multivariate analysis was performed using stepwise logistic regression. Statistically significant variables in the univariate analysis were included in the multivariate analysis. All statistical analyses were conducted using SPSS Base 11.0 J (Chicago, IL, United States). A P value < 0.05 was considered statistically significant.
Before propensity score matching, there were 238 patients in the upfront hepatectomy group and 59 patients in the NAC group (Table 1). Variables that were significantly different between the upfront hepatectomy and NAC groups included age (≥ 60 years) (P < 0.001), primary tumor location (right) (P = 0.03), lymph node metastases (≥ 1) (P < 0.001), depth of tumor invasion [adjacent organ invasion (T4b)] (P = 0.01), number of liver metastases (≥ 4) (P < 0.001), appearance time (synchronous) (P < 0.001), tumor distribution (bilobar) (P < 0.001), and staged hepatectomy (performed) (P = 0.04). The NAC regimens were as follows: Oxaliplatin-based chemotherapy (35 patients), with molecularly-targeted agents [bevacizumab (14 patients), cetuximab (three patients), and panitumumab (seven patients)]; irinotecan-based chemotherapy (four patients), with molecularly-targeted agents [bevacizumab (two patients) and panitumumab (two patients)]; oxaliplatin- and irinotecan-based chemotherapy (nine patients), with molecularly-targeted agents [bevacizumab (six patients) and cetuximab (one patient)]; fluorouracil and folinic acid (nine patients), with cisplatin (seven patients); and chemotherapy, including hepatic arterial infusion (two patients). Responses to NAC were defined as follows: Complete response (no patient), partial response (34 patients), stable disease (22 patients), or progressive disease (three patients). The median number of treatment cycles was 6 (range from 2 to 25).
| Variables | Upfront hepatectomy (n = 238) | NAC (n = 59) | P value | |
| Patient-related | ||||
| Age | < 60 | 123 | 14 | < 0.001 |
| ≥ 60 | 115 | 45 | ||
| Gender | Male | 184 | 39 | 0.07 |
| Female | 54 | 20 | ||
| CEA level (ng/mL) | < 10 | 73 | 19 | 0.82 |
| ≥ 10 | 165 | 40 | ||
| Primary tumor-related | ||||
| Site | Right | 34 | 15 | 0.03 |
| Left | 204 | 44 | ||
| Histology | Well/moderately differentiated | 236 | 57 | 0.128 |
| Others | 2 | 2 | ||
| Lymph node metastases | 0 | 144 | 17 | < 0.001 |
| ≥ 1 | 94 | 42 | ||
| Depth of invasion | Adjacent organ invasion (T4b) | 14 | 9 | 0.01 |
| Others | 224 | 50 | ||
| Lymphatic invasion | 0 | 146 | 29 | 0.08 |
| ≥ 1 | 92 | 30 | ||
| Venous invasion | 0 | 91 | 18 | 0.27 |
| ≥ 1 | 147 | 41 | ||
| Liver metastasis-related | ||||
| Number | 1–3 | 233 | 48 | < 0.001 |
| ≥ 4 | 5 | 11 | ||
| Size (max) | < 40 | 180 | 18 | 0.06 |
| ≥ 40 | 58 | 41 | ||
| Timing of the appearance | Synchronous | 40 | 37 | < 0.001 |
| Metachronous | 198 | 22 | ||
| Distribution | Unilobar | 211 | 38 | < 0.001 |
| Bilobar | 27 | 21 | ||
| Treatment-related | ||||
| Staged hepatectomy | Performed | 0 | 1 | 0.04 |
| Not performed | 238 | 58 | ||
| Surgical margin | Exposed | 13 | 6 | 0.186 |
| Not exposed | 225 | 53 | ||
| Adjuvant chemotherapy after primary resection | Administered | 69 | 32 | < 0.001 |
| Not administered | 169 | 27 | ||
| Adjuvant chemotherapy after hepatectomy | Administered | 86 | 23 | 0.684 |
| Not administered | 152 | 36 |
In univariate analysis, preoperative CEA levels (≥ 10 ng/mL) (P = 0.01), primary histological type (other than well/moderately differentiated) (P = 0.01), primary lymph node metastases (≥ 1) (P = 0.001), lymphatic invasion (≥ 1) (P = 0.02), and adjuvant chemotherapy (performed) (P = 0.02) were associated with poor OS in the upfront hepatectomy group (238 patients). Preoperative CEA levels [hazard ratio (HR), 1.948; 95% confidence interval (CI): 1.252–3.031; P = 0.003], primary histological type (HR, 2.971; 95%CI: 1.038–8.503; P = 0.04), and primary lymph node metastases (HR, 1.623; 95%CI: 1.020–2.583; P = 0.04) were independent prognostic factors in multivariate analysis (Table 2).
| Variables | n | 5-yr OS rate (%) | P value | Hazard ratio (95%CI) | P value | |
| Patient-related | ||||||
| Age (yr) | < 60 | 60 | 65.7 | 0.24 | ||
| ≥ 60 | 178 | 66.7 | ||||
| Sex | Male | 167 | 66.5 | 0.28 | ||
| Female | 71 | 65.8 | ||||
| CEA level (ng/mL) | < 10 | 122 | 75 | 0.01 | 1.948 (1.252–3.031) | 0.003 |
| ≥ 10 | 116 | 58.4 | ||||
| Primary tumor-related | ||||||
| Site | Left | 190 | 56.2 | 0.14 | ||
| Right | 48 | 68.5 | ||||
| Histology | Well/moderately differentiated | 234 | 67.1 | 0.01 | 2.971 (1.038–8.503) | 0.04 |
| Others | 4 | 25 | ||||
| Lymph node metastases | 0 | 108 | 79 | 0.001 | 1.623 (1.020–2.583) | 0.04 |
| ≥ 1 | 130 | 56.6 | ||||
| Depth of invasion | Adjacent organ invasion (T4b) | 19 | 64.8 | 0.64 | ||
| Others | 219 | 66.4 | ||||
| Lymphatic invasion | 0 | 116 | 73.1 | 0.02 | 1.418 (0.897–2.242) | 0.135 |
| ≥ 1 | 122 | 60.8 | ||||
| Venous invasion | 0 | 81 | 69.7 | 0.73 | ||
| ≥ 1 | 157 | 63.9 | ||||
| Number | 1–3 | 228 | 67.5 | 0.07 | ||
| ≥ 4 | 10 | 38.1 | ||||
| Maximum diameter (mm) | < 40 | 180 | 70.9 | 0.05 | ||
| ≥ 40 | 58 | 52.9 | ||||
| Timing of the appearance | Synchronous | 54 | 61.2 | 0.94 | ||
| Metachronous | 184 | 67.8 | ||||
| Distribution | Unilobar | 198 | 67.1 | 0.12 | ||
| Bilobar | 40 | 63 | ||||
| Treatment-related | ||||||
| Staged hepatectomy | Performed | 0 | – | – | ||
| Not performed | 238 | 66.3 | ||||
| Surgical margins | Exposed | 19 | 40.1 | 0.09 | ||
| Not exposed | 219 | 69 | ||||
| Adjuvant chemotherapy after primary resection | Administered | 69 | 64.9 | 0.16 | ||
| Not administered | 169 | 66.7 | ||||
| Adjuvant chemotherapy after hepatectomy | Administered | 126 | 56.2 | 0.02 | 0.646 (0.414–1.009) | 0.05 |
| Not administered | 112 | 71.7 |
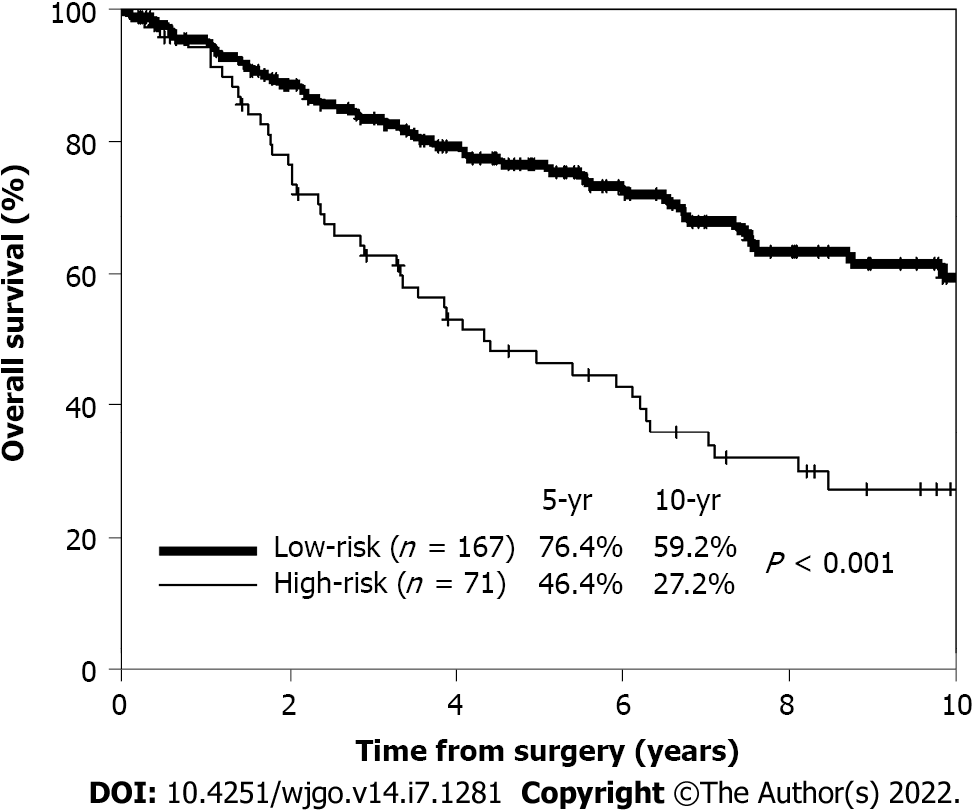
The 5-year OS rates of patients with zero (59 patients), one (108 patients), and two (71 patients) risk factors were 83%, 73%, and 46%, respectively. No patient had three risk factors. High-risk patients were defined as those with two or more risk factors, while low-risk patients were defined as those with zero or one risk factor. The 5-year OS rate of high-risk patients (71 patients) was significantly worse than that of low-risk patients (167 patients) (46.4% vs 76.4%; P < 0.001) (Figure 2).
Fifty patients in the upfront hepatectomy group were matched with 50 patients in the NAC group. Patients with insufficient preoperative data or without a suitable match were excluded. After matching preoperative baseline characteristics, treatment-related factors (staged hepatectomy, surgical margins, and adjuvant chemotherapy) were comparable between the two groups (Table 3). The NAC regimens after propensity score matching were as follows: Oxaliplatin-based chemotherapy (30 patients), with molecularly-targeted agents [bevacizumab (11 patients), cetuximab (three patients), and panitumumab (seven patients)]; irinotecan-based chemotherapy (four patients), with molecularly-targeted agents [bevacizumab (two patients) and panitumumab (two patients)]; oxaliplatin- and irinotecan-based chemotherapy (eight patients), with molecularly-targeted agents [bevacizumab (five patients) and cetuximab (one patient)]; fluorouracil and folinic acid (six patients), with cisplatin (four patients); and chemotherapy, including hepatic arterial infusion (two patients). Responses to NAC were defined as follows: Partial response (30 patients), stable disease (17 patients), or progressive disease (three patients). In total, there were 30 responders and 20 non-responders. The median number of treatment cycles was 6 (range from 2 to 25). The upfront hepatectomy group comprised 18 high-risk patients and 32 low-risk patients. The NAC group comprised 13 high-risk patients and 37 low-risk patients (Table 3). The background characteristics were comparable when stratified by high- and low-risk, respectively.
| Variables | Upfront hepatectomy group (n = 50) | NAC group (n = 50) | P value | |
| Patient-related | ||||
| Age (yr) | < 60 | 14 | 10 | 0.349 |
| ≥ 60 | 36 | 40 | ||
| Sex | Male | 33 | 34 | 0.832 |
| Female | 17 | 16 | ||
| CEA level (ng/mL) | < 10 | 28 | 34 | 0.216 |
| ≥ 10 | 22 | 16 | ||
| Primary tumor-related | ||||
| Site | Right | 10 | 14 | 0.349 |
| Left | 40 | 36 | ||
| Histology | Well/moderately differentiated | 49 | 50 | 0.315 |
| Others | 1 | 0 | ||
| Lymph node metastases | 0 | 15 | 15 | 1.0 |
| ≥ 1 | 35 | 35 | ||
| Depth of invasion | Adjacent organ invasion (T4b) | 5 | 3 | 0.461 |
| Others | 45 | 47 | ||
| Lymphatic invasion | 0 | 19 | 23 | 0.418 |
| ≥ 1 | 31 | 27 | ||
| Venous invasion | 0 | 16 | 15 | 0.829 |
| ≥ 1 | 34 | 35 | ||
| Liver metastasis-related | ||||
| Number | 1–3 | 44 | 42 | 0.564 |
| ≥ 4 | 6 | 8 | ||
| Maximum diameter (mm) | < 40 | 15 | 15 | 1.0 |
| ≥ 40 | 35 | 35 | ||
| Timing of the appearance | Synchronous | 28 | 28 | 1.0 |
| Metachronous | 22 | 22 | ||
| Distribution | Unilobar | 33 | 33 | 1.0 |
| Bilobar | 17 | 17 | ||
| Treatment-related | ||||
| Staged hepatectomy | Performed | 0 | 1 | 0.315 |
| Not performed | 50 | 49 | ||
| Surgical margins | Exposed | 4 | 4 | 1.0 |
| Not exposed | 46 | 46 | ||
| Adjuvant chemotherapy after primary resection | Administered | 17 | 29 | 0.144 |
| Not administered | 23 | 21 | ||
| Adjuvant chemotherapy after hepatectomy | Administered | 23 | 17 | 0.221 |
| Not administered | 27 | 33 | ||
| Risk stratification | High-risk | 18 | 13 | 0.515 |
| Low-risk | 32 | 37 | ||
Short-term outcomes, including the amount of intraoperative bleeding, frequency of red blood cell transfusions, postoperative complications, and length of postoperative hospital stay, were not significantly different between the two groups. In the NAC group, there was one complication of Clavien–Dindo grade IV. In this patient, five cycles of irinotecan-based chemotherapy were ad
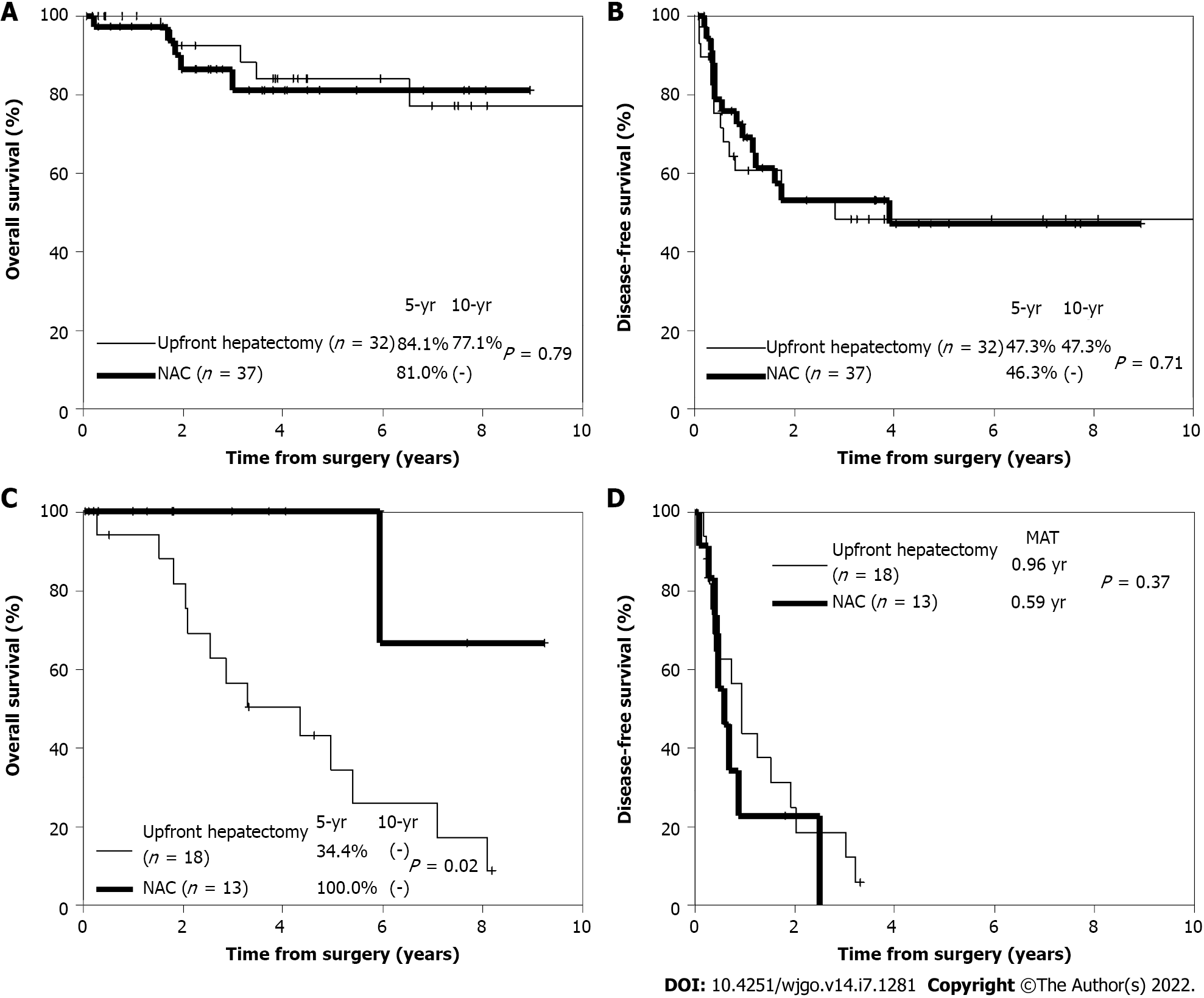
Regarding long-term outcomes, there was no significant difference in the 5-year OS rate between the upfront hepatectomy and NAC groups (63% vs 83%; P = 0.13) after propensity score matching. Among low-risk patients, there was also no significant difference in the 5-year OS rate (84.1% vs 81.0%; P = 0.79) (Figure 3A) or 5-year DFS rate (47.3% vs 46.3%; P = 0.71) (Figure 3B) between the two groups. Conversely, among high-risk patients, the 5-year OS rate was significantly higher in the NAC group than in the upfront hepatectomy group (100% vs 34.4%; P = 0.02) (Figure 3C). However, there was no significant difference in the 5-year DFS rate between the two groups (P = 0.37) (Figure 3D).
Recurrence after hepatectomy was observed in 30 (60%) patients in the upfront hepatectomy group and 24 (48%) patients in the NAC group. The difference between them was not statistically significant. The lung and remnant liver were the most frequent sites of recurrence in the upfront hepatectomy and NAC groups, respectively, and there was no significant difference in the distribution of initial recurrence sites. Regarding the initial treatment strategy for recurrence, resection and chemotherapy were adopted in 26.7% and 57.7% of patients in the upfront hepatectomy group and 25.0% and 66.7% of patients in the NAC group, respectively. The differences between them were not statistically significant (Table 4). Especially among high-risk patients, recurrence was observed in 15 (83%) of the 18 patients in the upfront hepatectomy group. Resection was adopted as the initial treatment strategy for recurrence in four patients, chemotherapy in six patients, and other therapies in five patients. None of the patients who received chemotherapy were converted to resection, and resection could only be performed in 27% of patients with recurrence. Conversely, recurrence was observed in nine (69%) of the 13 high-risk patients in the NAC group. Resection was adopted as the initial treatment strategy for recurrence in two patients. Chemotherapy was adopted as the initial treatment strategy for recurrence in seven patients (the same regimen was used in all responders; a different regimen was used in non-responders), three of whom were converted to resection (Table 5). Consequently, resection was performed in 56% of patients with recurrence in the NAC group, which was higher than the proportion of high-risk patients in the upfront hepatectomy group (27%). The 5-year OS rate after the first recurrence in the NAC group was significantly higher than that in the upfront hepatectomy group (66.7% vs 17.9%; P = 0.04).
| Case | NAC regimen | Course | Efficacy | First recurrence site | Initial treatment for recurrence | Conversion |
| Therapy | ||||||
| 1 | FU, FOL + CDDP | 2 | SD | Peritoneum | LV/5-FU + CPT-11 (IFL) | Resection |
| 2 | FU, FOL + CDDP | 2 | SD | Liver | LV/5-FU + CPT-11 (IFL) | Resection |
| 3 | FU, FOL | 2 | PD | Other | FOLFOX + Bmab | |
| 4 | FU, FOL + CDDP | 4 | SD | Lung | Resection | |
| 5 | FOLFOX + Bmab | 8 | PD | Liver | FOLFIRI + Bmab | Resection |
| 6 | XELOX + Bmab | 14 | SD | Lung | IRIS + Bmab | |
| 7 | FOLFOX + Cmab | 6 | PR | Liver | Resection | |
| 8 | IRI + Pmab | 6 | PR | Peritoneum | IRI + Pmab | |
| 9 | FOLFIRI + Bmab | 6 | PR | Liver | FOLFIRI + Bmab |
This study revealed a significantly worse OS rate of patients in resectable CRLMs with two or more risk factors [primary histological type (other than well/moderately differentiated), lymph node metastases (≥ 1), and preoperative CEA levels (≥ 10 g/mL)] who met the high-risk criteria compared to those who met the low-risk criteria. Among high-risk patients, the OS rate of those who received NAC was significantly higher than that of those who underwent upfront hepatectomy after propensity score matching. It is a novel finding that the efficacy of NAC for resectable CRLMs was demonstrated after risk stratification and propensity score matching.
The definition of resectable CRLM varies in the literature[3,4,6,15]. In studies that examined the effectiveness of NAC for resectable CRLMs, resectable CRLM was defined as a maximum of four tumors[4]; four or fewer tumors with a maximum diameter of < 5 cm[3]; or (1) A ≥ 30% residual liver volume (regardless of tumor number and size); (2) Resectable or already resected primary tumor; and (3) No unresectable extrahepatic metastases[16]. Some studies did not show a benefit of NAC for resectable CRLMs[3,4]. This may be because the criteria for resectable CRLMs were not specific enough to restrict the patient group to those for whom NAC is truly effective. Even when NAC was shown to be effective, it was considered without propensity score matching[16]. The definition of resectable CRLM in our database is more detailed and the efficacy of NAC was assessed by risk stratification.
We demonstrated that the OS rate, but not the DFS rate, of high-risk patients was significantly higher in the NAC group than in the upfront hepatectomy group. The post-recurrence clinical course after the first hepatectomy differed between the two groups. The treatment strategy for recurrence showed that chemotherapy was initially selected most frequently in both the upfront hepatectomy and NAC groups, although resection of not only the intrahepatic, but also the extrahepatic, recurrence site is crucial for prolonging the survival of patients with CRLMs[17]. However, in the NAC group, there were conversion cases from chemotherapy to resection, and consequently, there were more resection cases in the NAC group than in the upfront hepatectomy group (56% vs 27%), although this was not significant. Based on these results, the reason for a better OS rate among high-risk patients in the NAC group may be that the most effective and tolerable chemotherapy regimen has already been established in patients receiving NAC before their first hepatectomy, and appropriate regimens may be available from the start of treatment for recurrence. In fact, the OS rate of the NAC group after recurrence was significantly higher than that of the upfront hepatectomy group (P = 0.04).
Conversely, disadvantages of NAC include the risk that hepatectomy may not be performed in patients who do not respond to NAC. We showed that the effect of chemotherapy was progressive in 6% of NAC cases. To avoid missing the timing of hepatectomy, it is important to evaluate the efficacy of chemotherapy every 2–3 cycles. Other disadvantages of NAC include liver damage and perioperative complications induced by the NAC drugs. Sinusoidal dilation caused by oxaliplatin and steatohepatitis caused by irinotecan have been reported[18]. Furthermore, prolonged systemic NAC alters the liver parenchyma and increases morbidity after major resection[19]. Although many centers specializing in hepatobiliary procedures have reported mortality rates of < 5% after major liver surgery, the morbidity of hepatectomy may have increased with the advent of NAC, due to the hepatic parenchymal damage caused by chemotherapy[5]. The short-term outcomes of the NAC group in this study were comparable to those of a previous study[5]. However, one case of postoperative bleeding was observed after irinotecan-based chemotherapy. As hepatectomy was performed after a sufficient drug interval, no sinusoidal dilation or steatohepatitis was observed in the resected specimen. Postoperative bleeding in this case may have resulted from a complicated hepatic dissection surface. Therefore, careful surgical procedures are required, even after a sufficient drug interval.
This study has several limitations. The first is its single-center design with limited sample size. Second, its retrospective nature introduces the inevitable risk of selection bias, which could not be completely eradicated, despite using propensity score matching to reduce confounding by indication. Lastly, it has been reported that molecular biological factors, such as RAS status and microsatellite instability, are prognostic[20,21]. However, this information was unavailable in the present study.
Our findings suggest that NAC may improve the prognosis of patients with resectable CRLMs who have at least two of the following risk factors: Preoperative CEA levels (≥ 10 ng/mL), primary histological type (other than well/moderately differentiated), and lymph node metastases (≥ 1). Future prospective, multicenter studies with larger sample sizes are needed to validate these findings
Colorectal cancer (CRC) is a leading cause of cancer-related deaths worldwide. The liver is the most common metastatic site of CRC, and hepatectomy is the mainstay of treatment for patients with colorectal liver metastases (CRLMs). Upfront hepatectomy is recommended for patients with resectable CRLMs. However, there are cases of early recurrence after upfront hepatectomy alone in the resectable CRLMs. In selected patients, neoadjuvant chemotherapy (NAC) may improve long-term survival.
Identifying the poor prognostic factors for upfront hepatectomy in resectable CRLMs and investigating the effectiveness of NAC are urgently needed to improve long-term survival of patients with resectable CRLMs.
To determine the efficacy of NAC for initially resectable CRLMs.
Among 644 patients who underwent their first hepatectomy for CRLMs at our institution, 297 resectable cases were stratified into an upfront hepatectomy group (238 patients) and NAC group (59 patients). Poor prognostic factors for upfront hepatectomy were identified using multivariate logistic regression analysis. Propensity score matching was used, and clinical outcomes between the upfront hepatectomy and NAC groups were compared according to the number of poor prognostic factors. Survival curves were estimated using the Kaplan–Meier method and compared using the log-rank test.
As independent poor prognostic factors for overall survival (OS) in the upfront hepatectomy group, preoperative carcinoembryonic antigen (CEA) levels (≥ 10 ng/mL) (P = 0.003), primary histological type (other than well/moderately differentiated) (P = 0.04), and primary lymph node metastases (≥ 1) (P = 0.04) were identified. High-risk status was defined as the presence of two or more risk factors. Fifty patients were matched in upfront hepatectomy and NAC groups respectively, after propensity score matching. Among high-risk patients, the 5-year OS rate was significantly higher in the NAC group (13 patients) than in the upfront hepatectomy group (18 patients) (100% vs 34%; P = 0.02).
NAC was effective in patients with resectable CRLMs who had at least two of the following risk factors: Preoperative CEA levels (≥ 10 ng/mL), primary histological type (other than well/moderately differentiated), and lymph node metastases (≥ 1).
NAC therapy may improve the prognosis of high-risk patients with resectable CRLMs.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barisani D, Italy; de Melo FF, Brazil; Habashy HO, Egypt; Planellas P, Spain A-Editor: Zhou S, United States S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | de Ridder JAM, van der Stok EP, Mekenkamp LJ, Wiering B, Koopman M, Punt CJA, Verhoef C, de Wilt JH. Management of liver metastases in colorectal cancer patients: A retrospective case-control study of systemic therapy vs liver resection. Eur J Cancer. 2016;59:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Ishida K, Uesugi N, Hasegawa Y, Sugimoto R, Takahara T, Otsuka K, Nitta H, Kawasaki T, Wakabayashi G, Sugai T. Proposal for novel histological findings of colorectal liver metastases with preoperative chemotherapy. Pathol Int. 2015;65:367-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Hirokawa F, Asakuma M, Komeda K, Shimizu T, Inoue Y, Kagota S, Tomioka A, Uchiyama K. Is neoadjuvant chemotherapy appropriate for patients with resectable liver metastases from colorectal cancer? Surg Today. 2019;49:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Mauer M, Tanis E, Van Cutsem E, Scheithauer W, Gruenberger T; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und–tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative FOLFOX4 chemotherapy and surgery vs surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 921] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 5. | Boostrom SY, Nagorney DM, Donohue JH, Harmsen S, Thomsen K, Que F, Kendrick M, Reid-Lombardo KM. Impact of neoadjuvant chemotherapy with FOLFOX/FOLFIRI on disease-free and overall survival of patients with colorectal metastases. J Gastrointest Surg. 2009;13:2003-9; discussion 2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309-18; discussion 318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2800] [Article Influence: 107.7] [Reference Citation Analysis (1)] |
| 7. | Kanemitsu Y, Kato T. Prognostic models for predicting death after hepatectomy in individuals with hepatic metastases from colorectal cancer. World J Surg. 2008;32:1097-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC, Geller DA, Gayowski TJ, Fung JJ, Starzl TE. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999;189:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 328] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 9. | Mann CD, Metcalfe MS, Leopardi LN, Maddern GJ. The clinical risk score: emerging as a reliable preoperative prognostic index in hepatectomy for colorectal metastases. Arch Surg. 2004;139:1168-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Tanaka K, Nojiri K, Kumamoto T, Takeda K, Endo I. R1 resection for aggressive or advanced colorectal liver metastases is justified in combination with effective prehepatectomy chemotherapy. Eur J Surg Oncol. 2011;37:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21630] [Article Influence: 1351.9] [Reference Citation Analysis (1)] |
| 12. | Portier G, Elias D, Bouche O, Rougier P, Bosset JF, Saric J, Belghiti J, Piedbois P, Guimbaud R, Nordlinger B, Bugat R. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24:4976-4982. [RCA] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 414] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 13. | Hasegawa K, Saiura A, Takayama T, Miyagawa S, Yamamoto J, Ijichi M, Teruya M, Yoshimi F, Kawasaki S, Koyama H, Oba M, Takahashi M, Mizunuma N, Matsuyama Y, Watanabe T, Makuuchi M, Kokudo N. Adjuvant Oral Uracil-Tegafur with Leucovorin for Colorectal Cancer Liver Metastases: A Randomized Controlled Trial. PLoS One. 2016;11:e0162400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 14. | Tanaka K, Shimada H, Kubota K, Ueda M, Endo I, Sekido H, Togo S. Effectiveness of prehepatectomy intra-arterial chemotherapy for multiple bilobar colorectal cancer metastases to the liver: a clinicopathologic study of peritumoral vasculobiliary invasion. Surgery. 2005;137:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Gayowski TJ, Iwatsuki S, Madariaga JR, Selby R, Todo S, Irish W, Starzl TE. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery. 1994;116:703-10; discussion 710. [PubMed] |
| 16. | Ninomiya M, Emi Y, Motomura T, Tomino T, Iguchi T, Kayashima H, Harada N, Uchiyama H, Nishizaki T, Higashi H, Kuwano H. Efficacy of neoadjuvant chemotherapy in patients with high-risk resectable colorectal liver metastases. Int J Clin Oncol. 2021;26:2255-2264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Imai K, Yamashita YI, Miyamoto Y, Nakagawa S, Okabe H, Hashimoto D, Chikamoto A, Baba H. The predictors and oncological outcomes of repeat surgery for recurrence after hepatectomy for colorectal liver metastases. Int J Clin Oncol. 2018;23:908-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, Xiong HQ, Eng C, Lauwers GY, Mino-Kenudson M, Risio M, Muratore A, Capussotti L, Curley SA, Abdalla EK. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 954] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 19. | Karoui M, Penna C, Amin-Hashem M, Mitry E, Benoist S, Franc B, Rougier P, Nordlinger B. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 519] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 20. | Jácome AA, Vreeland TJ, Johnson B, Kawaguchi Y, Wei SH, Nancy You Y, Vilar E, Vauthey JN, Eng C. The prognostic impact of RAS on overall survival following liver resection in early vs late-onset colorectal cancer patients. Br J Cancer. 2021;124:797-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Fujiyoshi K, Yamamoto G, Takahashi A, Arai Y, Yamada M, Kakuta M, Yamaguchi K, Akagi Y, Nishimura Y, Sakamoto H, Akagi K. High concordance rate of KRAS/BRAF mutations and MSI-H between primary colorectal cancer and corresponding metastases. Oncol Rep. 2017;37:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |