Published online Dec 15, 2022. doi: 10.4251/wjgo.v14.i12.2415
Peer-review started: August 30, 2022
First decision: September 23, 2022
Revised: October 2, 2022
Accepted: November 6, 2022
Article in press: November 6, 2022
Published online: December 15, 2022
Processing time: 103 Days and 14.4 Hours
Hepatic hemangioblastoma is an extremely rare disease; only three cases have been reported in the literature, and its magnetic resonance imaging (MRI) findings are unreported.
We report a case of incidental hepatic hemangioblastoma. The patient had no history of von Hippel-Lindau disease or associated clinical signs. Computed tomography and MRI showed a large tumor occupying almost half of the right side of the liver with expansive growth, well-defined borders, heterogeneous mildly progressive enhancement, and visibly enlarged blood supply vessels. Flow voids were observed on T2-weighted imaging. Both diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) map findings of the mass were predominantly inhomogeneous. Postoperative pathology indicated a diagnosis of hemangioblastoma.
Enlarged peripheral blood-supplying vessels and progressive enhancement seem to be typical imaging features of hepatic hemangioblastoma. However, a solid significantly enhanced mass with a low signal on DWI and a high signal on ADC may also be helpful for the diagnosis of hepatic hemangioblastoma.
Core Tip: Hepatic hemangioblastoma is mostly huge in size, and images of flow void vessels within the tumor can be seen on T2-weighted imaging, and enlarged peripheral blood-supplying vessels and progressive enhancement seem to be typical imaging features of hepatic hemangioblastoma. However, a solid significantly enhanced mass with a low signal on diffusion-weighted imaging and a high signal on apparent diffusion coefficient may also be helpful for the diagnosis of hepatic hemangioblastoma.
- Citation: Li DF, Guo XJ, Song SP, Li HB. Rare massive hepatic hemangioblastoma: A case report. World J Gastrointest Oncol 2022; 14(12): 2415-2421
- URL: https://www.wjgnet.com/1948-5204/full/v14/i12/2415.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i12.2415
Hemangioblastomas are rare benign tumors, accounting for 1%-2% of all central nervous system tumors[1,2]. The cerebellum is the most common location, followed by the spinal cord and brainstem[3]; however, hemangioblastomas can also occur in the peripheral nervous system, adrenal gland, and liver[4]. Hemangioblastomas are present in 25%-30% of patients with von Hippel-Lindau disease (VHLD), and in these cases, retinal hemangioblastomas and endolymphatic sac tumors may also be detected[5].
Only three cases of VHLD with hepatic hemangioblastoma have been reported in the literature. Here, we report a case of hepatic hemangioblastoma that was not clearly related to VHLD, and we combined computed tomography (CT) and magnetic resonance imaging (MRI) findings with postoperative pathology findings and reports in the literature to further elucidate the imaging findings of hepatic hemangioblastoma.
A 42-year-old Chinese man complained of left lumbar abdominal pain for 3 d and decreased vision in his right eye for 3 mo.
Three days earlier, there was no obvious cause of the left-sided lumbar abdominal pain and discomfort with persistent distension, which radiated to the left lower abdomen and was accompanied by urine frequency, urgency, and dysuria. Ultrasonography performed at the local community health center showed a calculus in the distal portion of the left ureter with mild dilated effusion in the ureter and kidney.
The patient had a history of hyperlipidemia for 3 years and denied any history of infectious diseases, such as “hepatitis or tuberculosis”.
The patient’s family members were fit and healthy with no genetic diseases or history of hepatitis B.
On physical examination, the abdomen was flat, and a hard mass was palpable under the hepatic rib cage. No enlargement was palpable under the splenic rib cage. There was no percussion pain in the liver or left kidney areas.
The laboratory examinations were as follows: Aspartate aminotransferase 86 U/L, alanine aminotransferase 95 U/L, total bilirubin 25.2 μmol/L, direct bilirubin 11.6 μmol/L, hepatitis B virus surface antibody (luminescence method) 106.90 mIU/mL, hepatitis B virus core antibody (luminescence method) 3.90 cut off index, erythrocyte count 4.07 × 1012/L, and hemoglobin concentration 114 g/L.
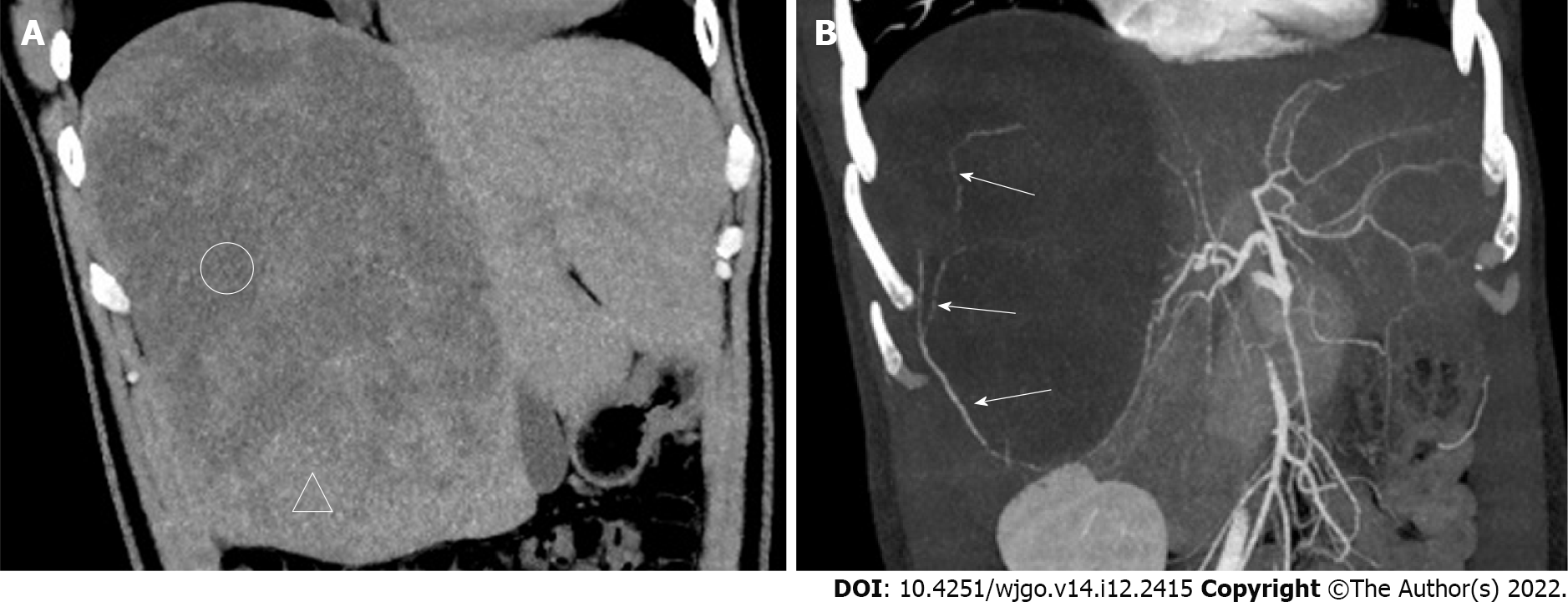
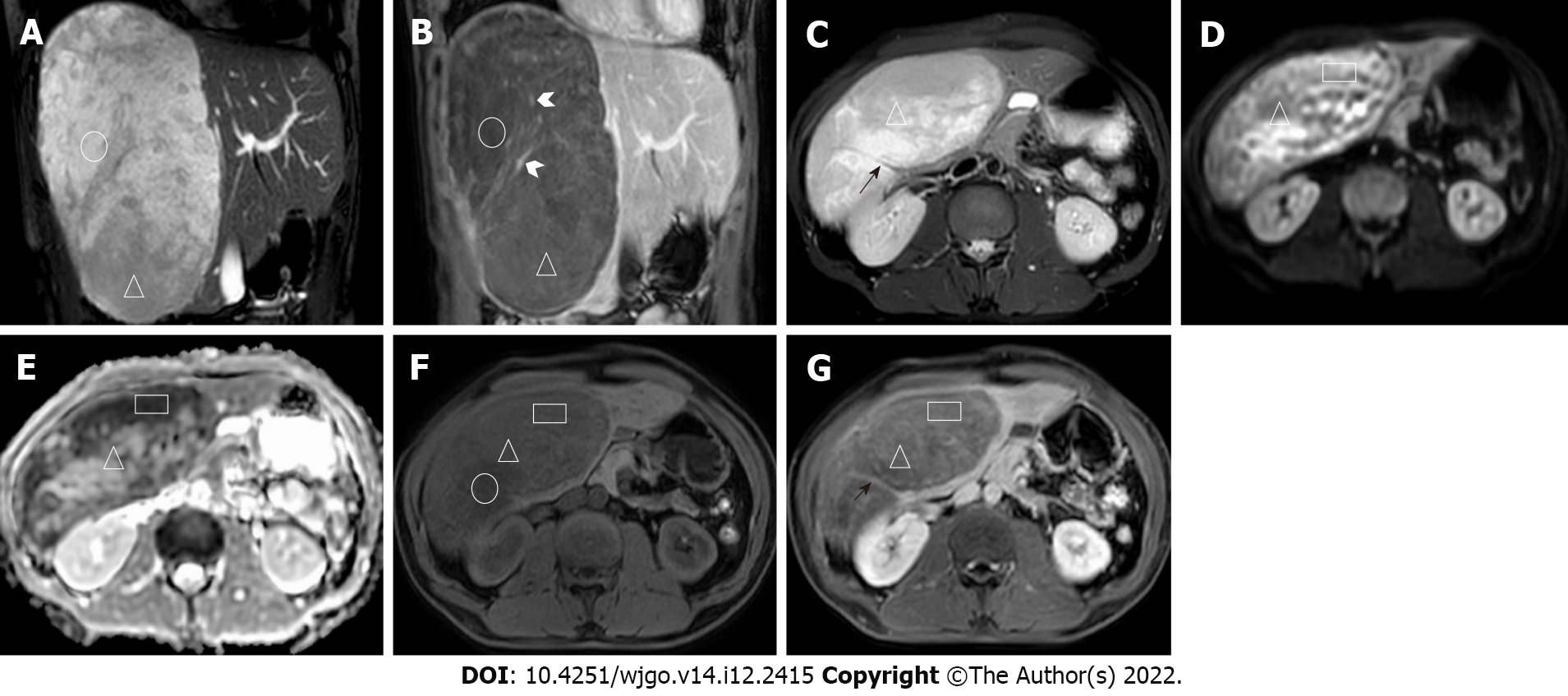
Ultrasonography revealed a left distal ureteral calculus with dilatation. A mixed-density mass (approximately 160 mm × 184 mm × 122 mm in size) in the right lobe of the liver was accidentally found on a plain CT scan of the urinary system after admission. Subsequently, CT and MRI images of the liver revealed an oval-shaped mass with heterogeneous density and signal (Figures 1 and 2), which resembled different types of meteorites in different series images. A multidisciplinary team consu
Hepatic hemangioblastoma.
After all examinations and a full assessment were made, the intrahepatic mass was surgically resected with the patient’s signed consent. Intraoperatively, the total volume of the liver was significantly enlarged, and there was a huge mass (200 mm in diameter) in the right lobe of the liver, occupying half of the right liver, with the left lower margin closing to the left edge of the gallbladder bed and the upper margin closing to the right edge of the inferior vena cava. The right liver was partially lifted out of the abdominal cavity. The right hemihepatic mass was completely resected. The mass had an intact envelope during the resection. The surgical procedure was uneventful, with satisfactory intraoperative anesthesia, no adverse effects, stable vital signs, and bleeding of about 400 mL. Postoperative radiotherapy and chemotherapy were not performed. The patient was advised to undergo genetic examination for VHLD, but the patient refused.
The patient recovered well without complications after 8 mo of follow-up.
The current patient had no clinical features related to VHLD. MRI did not reveal hemangioblastoma in the cerebellum, the red blood cell count was normal, and the hemoglobin concentration was close to normal, indicating a sporadic hemangioblastoma. However, three previous reports indicated that hepatic hemangioblastoma can be related to VHLD[6-8]. The three previous cases were also found to have hemangioblastoma in the cerebellum, spinal cord, retina, lung, or mesentery, with a history of surgery for cerebellar and spinal cord hemangioblastoma. The features of the three cases of hepatic hemangioblastoma are shown in Table 1.
| Case | Sex | Age (yr) | Location | Size (cm2) | US | CT | DSA | VHLD | First or preoperative diagnosis | Other parts |
| 1 | Female | 42 | Left and right liver | 1.5 × 1.5 (largest) | A central hypoechoic solid mass | Enlarged liver with multiple nodules, some of which have necrosis or bleeding within them | No abnormalities in the kidneys and other abdominal organs | Yes | / | Cerebellum, cervical spine |
| 2 | Female | 39 | The right lobe of the liver | 9 × 11 | Solid hyperechoic mass with a hypoechoic central portion | Hypodense lesion, significant peripheral enhancement, and incomplete filling inward after enhancement | Early stage with obvious vascularization and extensive tumor redness involving almost the whole liver | Yes | A giant cavernous hemangioma | Cerebellum, cervical medulla, lung |
| 3 | Male | 30 | The right lobe of the liver | / | Two hypoechoic solid masses with simple cysts in the kidneys and pancreas | Progressively enhanced liver and mesenteric mass with collateral veins, and pancreatic cysts | Richly vascularized and supplied by the right hepatic and right renal perineural arteries and collateral veins | Yes | Metastases from hepatic and mesenteric hemangioblastoma or renal cell carcinoma | Mesentery, retina, cerebellum, and spinal cord |
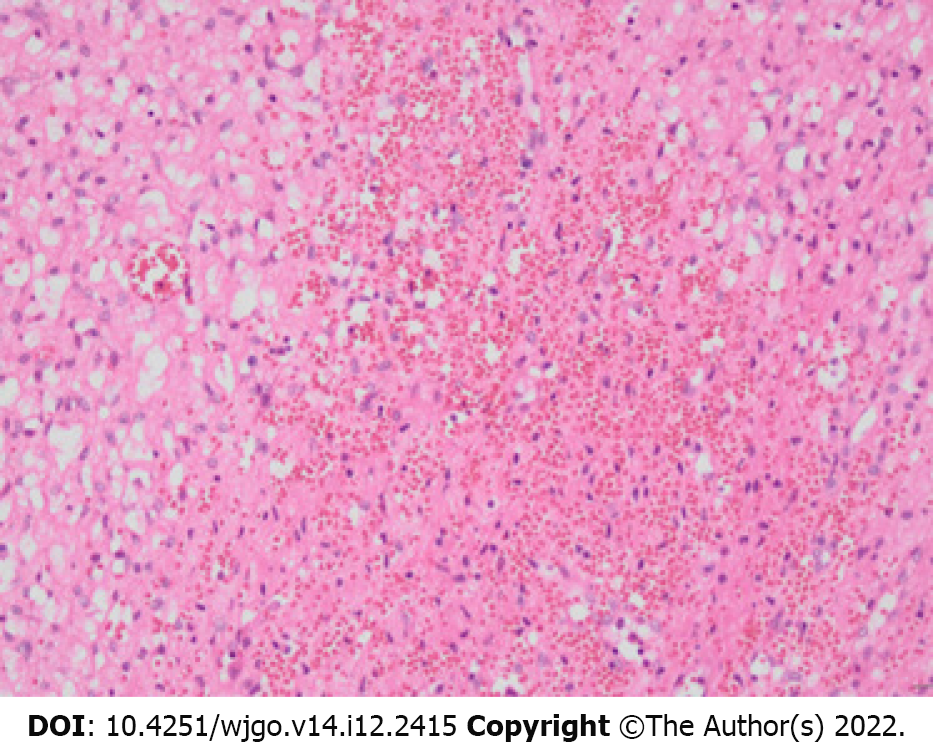
The imaging features of the lesion in this case are as follows: The tumor was massive, with expansive growth and well-defined boundaries, the real capsule was not observed on pathology, and there was no invasion of adjacent hepatic vessels or breakthrough of the hepatic capsule. There were no emboli in the portal vein or enlarged lymph nodes in the hepatic portal or retroperitoneum. Contrast-enhanced scans showed heterogeneous mild progressive enhancement. There was no definitive liquefaction or necrosis in the tumor, which may be because the tumor itself was composed of many capillary components and numerous vacuolar stromal cells, and it had a low tumor proliferation index and low oxygen demand. The density or signal of the tumor parenchyma was heterogeneous, with low density on unenhanced CT and obvious high signal on T2-weighted imaging (T2WI) (Figures 1 and 2). There was no enhancement in those areas of the lesion on the enhanced scan, and it is considered that the cytoplasm of tumor cells contained more liquid components and some lipid-like bubbles. Patchy areas with high diffusion-weighted imaging (DWI) and low apparent diffusion coefficient (ADC) signals appeared in some parts of the lesion, and the CT findings were relatively high-density (rectangular areas in Figures 2D-G), which may be related to two aspects. First, intratumoral hemorrhage was confirmed by pathology. The incidence of hemorrhage in hemangioblastomas is low[9]. The intratumoral hemorrhage occurred possibly because the tumor contained more immature thin-walled vessels and the tumor volume was large. Second, the tumor-feeding artery originated from the blood vessels below the tumor and the blood supply in the lower part of the tumor was relatively abundant; as such, there were many closely arranged spindle stromal cells. A vascular flow void signal can be seen on the T2WI sequence (black arrow in Figure 2B), which may indicate a hemangioblastoma. In other tumors, a vascular flow void signal occurs only when the blood supply is very abundant[1]. The lower part of the tumor and the scattered areas showed a slightly high signal on T2WI and a slightly low signal on T1WI, and the low signal on DWI and high signal on ADC were mildly enhanced (triangles in Figures 2D, 2E and 2G). These findings are suggestive of hemangioblastoma. The solid area of cerebellar hemangioblastoma often exhibits a low signal on DWI with ADC corresponding to a high signal, which is significantly enhanced, and it may be related to the tumor having abundant vascular interstitial spaces[10]. The signal in the other areas on DWI and ADC was equally highly inhomogeneous (Figures 2D and 2E), and this may be related to the tumor cell composition, arrangement, and vascular network structure. The lesions had heterogeneous and progressive enhancement (Figures 1 and 2), but the degree of enhancement was significantly lower than reported previously[7,8]. After reviewing the pathology (Figure 3), we speculated that this was due to the following reasons: (1) There were many interstitial cells with vacuolar structures in the tumor; (2) Although there were many capillaries in the tumor, most were immature vessels lacking a normal vascular structure (a large amount of blood remained in the capillaries, resulting in increased vascular resistance, which made it difficult for contrast agents to penetrate the tumor parenchyma); and (3) The tumor was too large and the feeding arteries were relatively small, resulting in insufficient blood supply. Multiple irregular strip-like obvious enhan
We carefully compared the features of this case with those of the lesions of the previous three cases and found some similar features. The patients were aged 30-45 years when the lesion was found, the tumors were large, all were predominantly solid with heterogeneous density and were mostly found in the right lobe of the liver, with obvious blood supply vessels visible on either angiography or MRI, and all were progressively enhanced. However, clinical symptoms were variable and most could have been related to VHLD. MRI or CT can replace angiography for detecting vascular supply, thus reducing unnecessary invasive examinations.
The present report has some limitations. The first limitation is the lack of pathological sections consistent with CT or MRI, thereby preventing the appropriate analysis of the imaging features corresponding to the pathology. Further, our report only included a single case, which was too few to allow a summary of lesion characteristics.
The main advantage of CT and MRI is the ability to optimally demonstrate the internal composition of the tumor, blood supply, and relationship with surrounding structures, which provide the necessary basis for surgical planning. Because of the abundance of capillaries in hemangioblastoma related to the increased expression of vascular endothelial growth factor, this tumor demonstrates elevated relative cerebral blood volume seen in perfusion sequences. Thus, DWI imaging combined with perfusion cerebral blood volume can provide more diagnostic information[11].
Because of the rarity of hepatic hemangioblastoma, the understanding of its imaging features is in the preliminary stages. The patient had no clinical features of VHLD, indicating a sporadic hemangioblastoma. Enlarged peripheral supplying arteries and progressive enhancement may be typical imaging features of hepatic hemangioblastoma. Low signal on DWI and high signal on ADC with significant enhancement may be indicative of a diagnosis of hepatic hemangioblastoma.
The authors thank Dr. Wang Rui-An (Department of Pathology, Shenzhen Hospital, Southern Medical University, Guangdong Province, China) for providing pathological pictures, consultation, and annotations.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mahmoud MZ, Saudi Arabia; Reis F, Brazil S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | Duan M, Yang L, Kang J, Wang R, You H, Feng M. Neuroimaging Features of Optic Nerve Hemangioblastoma Identified by Conventional and Advanced Magnetic Resonance Techniques: A Case Report and Literature Review. Front Oncol. 2021;11:763696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 2. | Kano H, Shuto T, Iwai Y, Sheehan J, Yamamoto M, McBride HL, Sato M, Serizawa T, Yomo S, Moriki A, Kohda Y, Young B, Suzuki S, Kenai H, Duma C, Kikuchi Y, Mathieu D, Akabane A, Nagano O, Kondziolka D, Lunsford LD. Stereotactic radiosurgery for intracranial hemangioblastomas: a retrospective international outcome study. J Neurosurg. 2015;122:1469-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Hussein MR. Central nervous system capillary haemangioblastoma: the pathologist's viewpoint. Int J Exp Pathol. 2007;88:311-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Bisceglia M, Muscarella LA, Galliani CA, Zidar N, Ben-Dor D, Pasquinelli G, la Torre A, Sparaneo A, Fanburg-Smith JC, Lamovec J, Michal M, Bacchi CE. Extraneuraxial Hemangioblastoma: Clinicopathologic Features and Review of the Literature. Adv Anat Pathol. 2018;25:197-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Mourão JLV, Borella LFM, Duarte JÁ, Dalaqua M, Fernandes DA, Reis F. Imaging manifestations of von Hippel-Lindau disease: an illustrated guide focusing on the central nervous system. Radiol Bras. 2022;55:188-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Rojiani AM, Owen DA, Berry K, Woodhurst B, Anderson FH, Scudamore CH, Erb S. Hepatic hemangioblastoma. An unusual presentation in a patient with von Hippel-Lindau disease. Am J Surg Pathol. 1991;15:81-86. [PubMed] |
| 7. | McGrath FP, Gibney RG, Morris DC, Owen DA, Erb SR. Case report: multiple hepatic and pulmonary haemangioblastomas--a new manifestation of von Hippel-Lindau disease. Clin Radiol. 1992;45:37-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Hayasaka K, Tanaka Y, Satoh T, Mutoh H. Hepatic hemangioblastoma: an unusual presentation of von Hippel-Lindau disease. J Comput Assist Tomogr. 1999;23:565-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Ene CI, Morton RP, Ferreira M Jr, Sekhar LN, Kim LJ. Spontaneous Hemorrhage from Central Nervous System Hemangioblastomas. World Neurosurg. 2015;83:1180.e13-1180.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | She D, Yang X, Xing Z, Cao D. Differentiating Hemangioblastomas from Brain Metastases Using Diffusion-Weighted Imaging and Dynamic Susceptibility Contrast-Enhanced Perfusion-Weighted MR Imaging. AJNR Am J Neuroradiol. 2016;37:1844-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Neska-Matuszewska M, Zimny A, Bladowska J, Czarnecka A, Sąsiadek M. The role of diffusion and perfusion magnetic resonance imaging in differentiation of haemangioblastomas and pilocytic astrocytomas. Pol J Radiol. 2018;83:e197-e203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |