Published online Oct 15, 2022. doi: 10.4251/wjgo.v14.i10.2048
Peer-review started: March 1, 2022
First decision: April 19, 2022
Revised: May 17, 2022
Accepted: August 24, 2022
Article in press: August 24, 2022
Published online: October 15, 2022
Processing time: 227 Days and 2.6 Hours
The role of HER2 overexpression in rectal cancer is controversial.
To assess the role of HER2 overexpression in the long-term prognosis of rectal cancer.
Data from patients with locally advanced rectal cancer who underwent total mesorectal excision after short-course radiotherapy at Beijing Cancer Hospital between May 2002 and October 2005 were collected. A total of 151 tissue samples of rectal cancer were obtained using rigid proctoscopy before neoadjuvant radiotherapy, followed by immunohistochemistry and fluorescence in situ hybridisation to determine the patients’ HER2 expression status. Univariate and multivariate analyses of the associations between the clinicopathological factors and HER2 status were performed. Survival was estimated and compared using the Kaplan-Meier method based on HER2 expression status, and the differences between groups were verified using the log-rank test.
A total of 151 patients were enrolled in this study. A total of 27 (17.9%) patients were ultimately confirmed to be HER2-positive. The follow-up duration ranged from 9 mo to 210 mo, with a median of 134 mo. Distant metastasis and local recurrence occurred in 60 (39.7%) and 24 (15.9%) patients, respectively. HER2 positivity was significantly associated with the pre-treatment lymph node stage (pre-N) (P = 0.040), while there were no differences between HER2 status and age, sex, preoperative CEA levels (pre-CEA), T stage, and lympho-vascular invasion. In terms of prognosis, HER2 overexpression was correlated with distant meta
HER2 overexpression is a potential biomarker for predicting lymph node metastasis and distant metastasis, which are associated with worse long-term DFS and OS in rectal cancer patients with locally advanced disease.
Core Tip: Long-term follow-up of rectal cancer patients treated with neoadjuvant radiotherapy demonstrated that pre-treatment HER2 overexpression was significantly correlated with lymph node metastasis and long-term distant metastasis. Furthermore, HER2 overexpression, elevated CEA and lymph node positivity were independent risk factors, predictive for poorer survival.
- Citation: Chen N, Li CL, Peng YF, Yao YF. Long-term follow-up of HER2 overexpression in patients with rectal cancer after preoperative radiotherapy: A prospective cohort study. World J Gastrointest Oncol 2022; 14(10): 2048-2060
- URL: https://www.wjgnet.com/1948-5204/full/v14/i10/2048.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i10.2048
Colorectal cancer (CRC) is a great challenge for people worldwide and is the 3rd most commonly diagnosed cancer and the 2nd leading cause of cancer-related deaths. In China, the incidence and death rates of CRC are the 3rd and 5th highest, respectively[1,2]. In 2020, there were about 43340 new cases of rectal cancer in the United States each year, while this number was 376000 in China[2,3]. Due to its insidious onset, most patients are diagnosed with locally advanced disease, and approximately 20% of CRC patients have distant metastasis at the time of diagnosis[4]. A series of studies, including the SWEDISH RECTAL CANCER TRIAL in the 1990s[5], the CAO/ARO/AIO-94 trial performed in Germany in the early 21st century[6], and the subsequent EORTC22921 study[7], have shown the benefits of tumour regression, sphincter preservation, and decreased local recurrence, establishing the status of neoadjuvant radiotherapy. In recent years, the treatment mode of rectal cancer guided by TNM staging has been evolving; notably, the long-term survival rates have appeared to remain stable[8-10]. In addition, even patients with the same TNM stage may have different prognoses, prompting us to search for new therapeutic biomarkers to improve patient outcomes. In recent years, the molecular mechanisms of CRC have been further studied, and biological markers such as RAS and BRAF mutations have been identified as prognostic targets. In contrast, HER2, a member of the epidermal growth factor receptor family of receptor tyrosine kinases, is highly expressed in a variety of tumour cells. HER2 is currently considered a potential target for CRC therapy, as reported by previous findings[11]. Although there have been several studies on the role of HER2 in CRC, the results are controversial and lack long-term outcomes. The present study aimed to report the long-term outcomes of patients with HER2 overexpression in locally advanced rectal cancer with preoperative radiotherapy.
Data from patients with locally advanced rectal cancer who underwent total mesorectal excision (TME) after short-course radiotherapy at the Beijing Cancer Hospital between May 2002 and October 2005 were collected. All patients underwent preoperative staging using magnetic resonance imaging, computed tomography, endorectal ultrasonography, and/or endoscopy. Each patient enrolled in our study satisfied the following criteria: (1) Age > 18 years; (2) Primary rectal adenocarcinoma below the peritoneal folds; (3) Clinically staged as cT3-4 and/or N+ rectal tumours, with a tumour that could be resected radically; and (4) Willingness to participate in long-term follow-up. Patients were excluded if they: (1) Had synchronous tumours or a history of other malignant tumours within the previous 5 years; (2) Had a previous history of cytotoxic chemotherapy, previous pelvic radiation therapy, or a known hypersensitivity to any drug included in the treatment protocol; (3) Had a diagnosis of hereditary nonpolyposis colorectal carcinoma; (4) Were being treated with other experimental drugs or had previously participated in a clinical trial of other experimental agents for rectal carcinoma; and (5) Had clinical evidence of distant metastasis. There were no restrictions based on sex, race, or disability.
All patients received 30 Gy/10 F/2 W neoadjuvant radiotherapy (SIMENS PRIMUS 2916 Linear Accelerator), and radical surgeries (LAR or APR ) were performed 2 wk after the end of radiotherapy, according to the TME principle. All patients received adjuvant chemotherapy (standard regimen, 5-FU or capecitabine) within 6 mo after surgery, according to the pathologic stage. Patient epidemiological information and primary tumour features, including distance from the anal verge and TNM stage before and after neoadjuvant radiotherapy, were collected prospectively. Regular follow-up visits were performed every 3 mo for the first 2 years, and then every 6 mo for a total of 5 years. After 5 years, follow-up was performed once per year. Follow-up examinations included blood tests for CEA and CA199, thoracic and abdominal/pelvic computed tomography or magnetic resonance imaging, and enteroscopy for timely detection of recurrence or metastasis. The study was approved by the medical ethics committee of the Peking University Cancer Hospital, and the requirement for informed consent was waived.
All patients underwent rigid proctoscopy before neoadjuvant therapy, and a sufficient amount of tumour tissue was obtained. HER2 expression was evaluated using immunohistochemistry. Immunostained samples were examined and scored independently and in a blinded manner by two experienced pathologists. The scoring criteria were as follows[12]: 0 (no staining), 1+ (1%-25% positive cells), 2+ (26%-75% positive cells), or 3+ (76%-100% positive cells). A score of 0 or 1+ was considered negative (low expression) and a score of 3+ was considered positive (overexpression). In samples when the score was 2+ (moderate expression), fluorescence in situ hybridization (FISH) was performed to confirm the HER2 expression.
Data were analysed using R4.1.0 software. The ‘survival’ and ‘survminer’ package were used for statistical analysis, and the ‘ggplot2’ package was used for plotting. Categorical variables were assessed using the chi-square (2 × 2) or Fisher's exact test (2 × C) when applicable. Multivariate analysis was performed using a binary logistic regression model (forward: LR). Survival analysis was performed using the Kaplan-Meier method, and the differences between groups were verified using the log-rank test. P values < 0.05 were considered significant statistically.
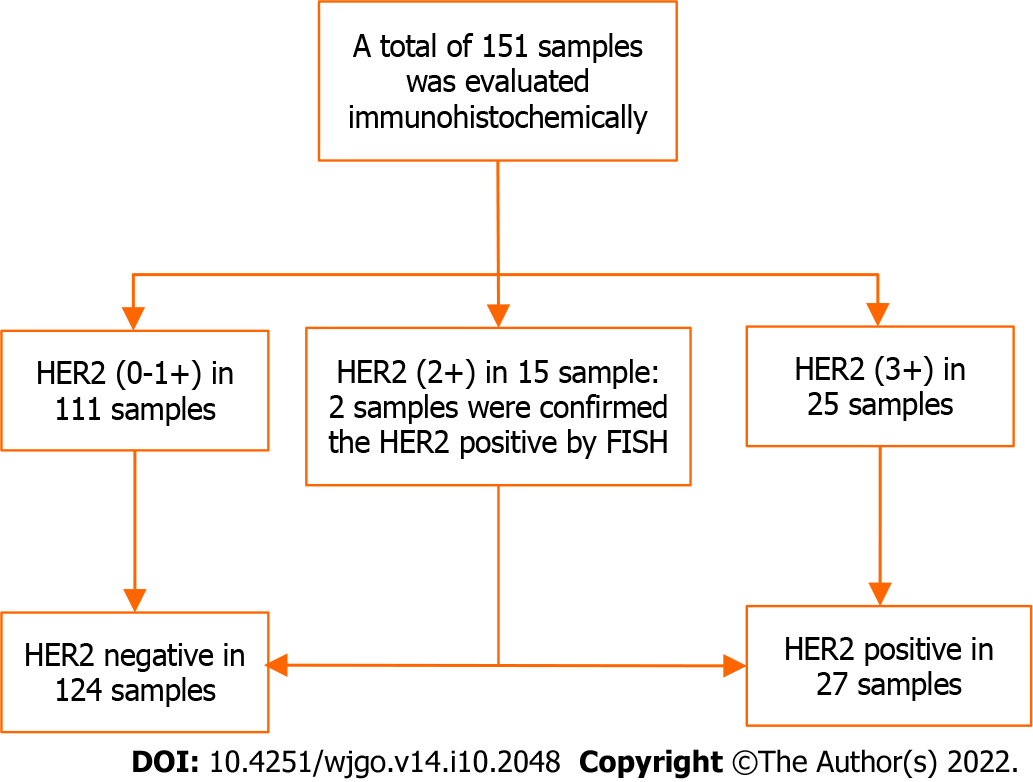
In our previous reports, a total of 142 rectal cancer patients with locally advanced diseases were enrolled, followed by nine more patients meeting the inclusion criteria; finally, 151 patients were included in the final analysis (Figure 1). The mean age was 55.85 ± 13.26 years, with 93 (61.6%) men and 58 (38.4%) women. The patient characteristics are listed in Table 1.
| Characteristic | Result |
| Age diagnosis (yr) | 55.85 ± 13.26 |
| Gender (%) | |
| Male | 93 (61.6) |
| Female | 58 (38.4) |
| Distance to anal verge (cm) (%) | 4.91 ± 2.04 |
| > 6 | 45 (29.8) |
| ≤ 6 | 106 (70.2) |
| Pre-CEA (%) | |
| Normal range | 98 (64.9) |
| Elevated | 53 (35.1) |
| Pre-T (%) | |
| T1 | 2 (1.3) |
| T2 | 10 (6.6) |
| T3 | 133 (88.1) |
| T4 | 6 (4.0) |
| Pre-N (%) | |
| N0 | 38 (25.2) |
| N+ | 113 (74.8) |
| Post-T (%) | |
| pT0 | 7 (4.6) |
| pT1 | 6 (4.0) |
| pT2 | 48 (31.8) |
| pT3 | 84 (55.6) |
| pT4 | 4 (4.0) |
| Post-N (%) | |
| pN0 | 89 (58.9) |
| pN+ | 62 (41.1) |
| Tumor regression grade (%) | |
| Grade 0 | 9 (6.3) |
| Grade 1 | 34 (23.9) |
| Grade 2 | 42 (29.6) |
| Grade 3 | 57 (40.1) |
| HER2 (%) | |
| - | 124 (82.1) |
| + | 27 (17.9) |
| LVI (%) | |
| + | 20 (13.2) |
| - | 131 (86.8) |
| Median follow-up period | 134 |
| Distant metastasis, number (%) | |
| Yes | 60 (39.7) |
| No | 91 (60.3) |
| Local recurrence, number (%) | |
| Yes | 24 (15.9) |
| No | 127 (84.1) |
The immunohistochemical results showed that HER2 over-expression was detected in 16.6% (25/151) of the tissue samples, with low expression (0-1+) in 73.51% (111/151) and moderate expression in 9.93% (15/151) of samples. In samples (n = 2) that scored 2+, we confirmed the positive expression of HER2 using FISH. A total of 27/151 (17.9%) samples were ultimately confirmed to have HER2 positivity. The median follow-up period for all patients was 134 mo. Distant metastasis and local recurrence occurred in 60 (39.7%) and 24 (15.9%) patients, respectively.
There were no significant differences between HER2 status and age, sex, preoperative CEA levels (pre-CEA), T stage, lymph-vascular invasion (LVI), and local recurrence. HER2 positivity was associated with pre-treatment N(+) stage (pre-N; P = 0.040) and distant metastasis (P = 0.002). Distant metastasis occurred in 66.7% (18/27) of HER2-positive patients compared to in 33.9% (42/124) of HER2-negative patients (Table 2).
| Variable | HER2- (%) | HER2+ (%) | χ2 | P value |
| Age (yr) | 0.558 | 0.455 | ||
| < 60 | 74 (59.7) | 14 (51.9) | ||
| ≥ 60 | 50 (40.3) | 13 (48.1) | ||
| Sex | 2.166 | 0.141 | ||
| Male | 73 (58.9) | 20 (74.1) | ||
| Female | 51 (41.1) | 7 (25.9) | ||
| Pre-CEA | 0.045 | 0.832 | ||
| Normal range | 80 (64.5) | 18 (66.7) | ||
| Elevated | 44 (35.5) | 9 (33.3) | ||
| Distance to anal verge (cm) | 0.236 | 0.627 | ||
| > 6 | 38 (30.6) | 7 (25.9) | ||
| ≤ 6 | 86 (69.4) | 20 (74.1) | ||
| Pre-T | 0.6941 | |||
| T1-2 | 11 | 1 | ||
| T3-4 | 113 | 26 | ||
| Pre-N | 4.235 | 0.040 | ||
| N0 | 27 (21.8) | 11 (40.7) | ||
| N+ | 97 (78.2) | 16 (59.3) | ||
| Post-T | 1.583 | 0.208 | ||
| T1-2 | 53 | 8 | ||
| T3-4 | 71 | 19 | ||
| Post-N | 1.582 | 0.208 | ||
| N0 | 76 (61.3) | 13 (48.1) | ||
| N+ | 48 (38.7) | 14 (51.9) | ||
| LVI | ||||
| + | 17 (13.7) | 3 (11.1) | 0.002 | 0.9622 |
| - | 107 (86.3) | 24 (88.9) | ||
| Distant metastasis | 9.959 | 0.002 | ||
| + | 42 (33.9) | 18 (66.7) | ||
| - | 82 (66.1) | 9 (33.3) | ||
| Local recurrence | 1.083 | 0.2982 | ||
| + | 22 (17.7) | 2 (7.4) | ||
| - | 102 (82.3) | 25 (92.6) |
Univariate analysis showed that pre-CEA, pre-treatment T stage (pre-T), post-treatment N status (Post-N), and HER2 status were correlated with distant metastasis (Table 3). These variables were further included in the binary logistic regression analysis, and the P value (0.052) of post-T was close to 0.05; therefore, it was also included in the multivariate analysis. The final analysis showed that elevated pre-CEA [P = 0.002, odds ratio (OR) = 3.277, 97.5% confidence interval (CI): 1.543-7.163], post-N(+) (P = 0.022, OR = 2.437, 97.5%CI: 1.143-5.308), and HER2(+) (P = 0.003, OR = 4.222, 97.5%CI: 1.667-11.409) were risk factors for distant metastasis, as demonstrated in Table 4.
| Variable | Distant metastasis | χ2 | P value | |
| - (%) | + (%) | |||
| Age (yr) | 0.121 | 0.728 | ||
| < 60 | 52 (59.1) | 36 (40.9) | ||
| ≥ 60 | 39 (61.9) | 24 (38.1) | ||
| Sex | 0.446 | 0.504 | ||
| Male | 58 (62.4) | 35 (37.6) | ||
| Female | 33 (56.9) | 25 (43.1) | ||
| Pre-CEA | 9.704 | 0.002 | ||
| Normal range | 68 (69.4) | 30 (30.6) | ||
| Elevated | 23 (43.4) | 30 (56.6) | ||
| Distance to anal verge (cm) | 0.103 | 0.749 | ||
| > 6 | 28 (62.2) | 17 (37.8) | ||
| ≤ 6 | 63 (59.4) | 43 (40.6) | ||
| Pre-T | 0.2211 | |||
| T1-2 | 5 | 7 | ||
| T3-4 | 86 | 53 | ||
| Pre-N | 0.001 | 1.000 | ||
| N0 | 23 (60.5) | 15 (39.5) | ||
| N+ | 68 (60.2) | 45 (39.8) | ||
| Post-T | 3.782 | 0.052 | ||
| T1-2 | 43 | 18 | ||
| T3-4 | 48 | 42 | ||
| Post-N | 7.995 | 0.005 | ||
| N0 | 62 (69.7) | 27 (30.3) | ||
| N+ | 29 (46.8) | 33 (53.2) | ||
| LVI | 1.014 | 0.314 | ||
| + | 10 (50.0) | 10 (50.0) | ||
| - | 81 (61.8) | 50 (38.2) | ||
| HER2, number | 9.959 | 0.002 | ||
| - | 82 (66.1) | 42 (33.9) | ||
| + | 9 (33.3) | 18 (66.7) | ||
| Variable | Odds ratio | 97.5%CI | P value |
| CEA level before radiotherapy | |||
| Normal range | 1 | ||
| Elevated | 3.277 | 1.543-7.163 | 0.002 |
| Post-treatment N | |||
| N0 | 1 | ||
| N+ | 2.437 | 1.143-5.308 | 0.022 |
| HER2 | |||
| - | 1 | ||
| + | 4.222 | 1.667-11.409 | 0.003 |
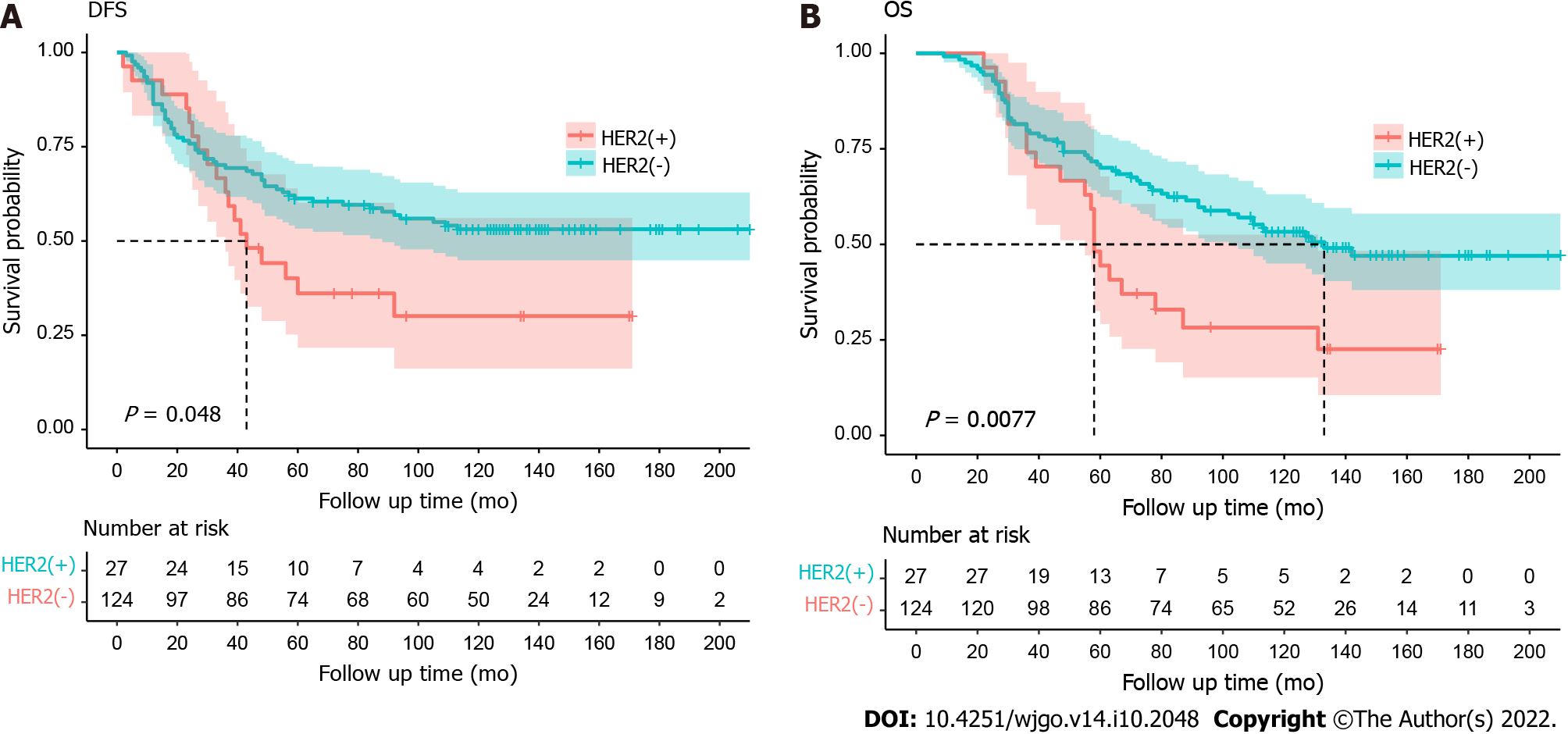
The follow-up time ranged from 9 mo to 210 mo, with a median follow-up of 134 mo. During the follow-up period, 18 (66.7%) of the 27 HER2-positive patients experienced distant metastasis or recurrence, and 2 of these patients suffered from both metastasis and recurrence; thus, in terms of disease progression, there were a total of 18 (66.7%) distant metastases and 2 (7.4%) local recurrence events. The median disease-free survival (DFS) of HER2-positive patients was 43 mo. For HER2-negative patients, 57 (46.0%) out of 124 developed distant metastasis or recurrence. Death occurred in 20 (74.1%) of 27 HER2(+) patients, with a median overall survival (OS) of 58 mo, and in 60 (48.4%) of 124 HER2(-) patients, with a median OS of 133 mo. There were significant differences between the HER2-positive group and HER2-negative group with respect to both DFS [hazard ratio (HR): 1.69 (95%CI: 0.91-3.14); P = 0.048] and OS [1.95 (1.05-3.63); P = 0.0077], as shown in Figure 2A and B.
HER2, also known as C-erbB-2, neu, or p185, is a member of the EGFR/ErbB family. It is a transmembrane protein encoded by the HER2 proto-oncogene, which participates in the signalling transduction pathway leading to cell growth and differentiation, potentially affecting the invasion and migration of tumour cells in the network of tumorigenesis. Previous studies have confirmed that HER2 is one of a predictors for poor prognosis in breast cancer, gastric cancer, endometrial cancer, and other tumours, in which HER2 overexpression is associated with poorer tumour biological properties[13-17]. In recent years, HER2 overexpression has also been found in CRC tumours; however, the data reported in the literature vary greatly, with positivity rates of approximately 2.6%-17%[18-22]. The effects of HER2 on the prognosis of CRC are also controversial, and the relationship between HER2 and the prognosis of patients with CRC remains under discussion. Our study is a 10-year long-term follow-up report on the prognosis of HER2 overexpression in locally advanced rectal cancer, which is of great significance in exploring the role of HER2 in rectal cancer.
In our study, the results from the correlation analysis using the chi-square test revealed that there were no significant differences between HER2 status and age, sex, pre-CEA, T stage, LVI, and local recurrence, which was consistent with the majority of previous reported results. Furthermore, HER2 positivity was associated with pre-N(+) (P = 0.040) and distant metastasis (P = 0.002), which is consistent with our previous short-term results[12]. Similar to our results, a study involving 1645 cases of primary colorectal adenocarcinoma showed that HER2 overexpression was associated with lymph node metastasis[23]. In addition, logistic regression was used to verify the predictive effect of HER2 overexpression on distant metastasis in rectal cancer. Univariate and multivariate analyses demonstrated that HER2 overexpression was associated with distant metastasis. The risk of distant metastasis in HER2-positive patients was 4.222 times higher than that in HER2-negative patients (P = 0.003, OR = 4.222, 97.5%CI: 1.667-11.409). We propose that these results suggest that overexpression of HER2 may promote the aggressiveness of rectal cancer. As in breast and gastric cancers, HER2 might play an important role in local failure and distant metastasis in patients with rectal cancer, featuring a higher possibility of lymph node metastasis.
Our results also showed that two other risk factors predictive of distant metastasis of rectal cancer were elevated pre-CEA (P = 0.002, OR = 3.277, 97.5%CI: 1.543-7.163) and post-N(+) (P = 0.022, OR = 2.437, 97.5%CI: 1.143-5.308). Studies have shown that pre-CEA is associated with neoadjuvant treatment response and clinical outcomes in patients with rectal cancer as a predictor of poorer prognoses[24-27]. Similarly, LN(+) status after neoadjuvant therapy was related to higher tumour stages and poorer treatment responses, which are obviously related to the prognoses of patients[28]. Therefore, we assumed that these might explain the increased rate of distant metastasis in elevated pre-CEA and post-N(+) patients.
A few studies have suggested that HER2 overexpression is a poor prognostic factor for CRC, playing an important role in its progression[29-31]. In the PETACC-8 trial, 1689 patients with stage III colon cancer received postoperative adjuvant chemotherapy. The results showed that HER2 overexpression was associated with a shorter time to recurrence [HR: 1.55 (95%CI: 1.02-2.36) P = 0.04] and shorter OS [HR: 1.57 (0.99-2.5) P = 0.05][32]. A meta-analysis of 1761 CRC patients from 11 studies by Li et al[33] showed that HER2 overexpression was negatively correlated with OS, and similar results were obtained in different subgroups, although the authors admitted that this effect might not be significant. However, some studies have shown that HER2 overexpression is not associated with the prognosis of CRC. In a large-pool study, three randomised controlled trials (the QUASAR, FOCUS, and PICCOLO trials) were analysed. Within a total of 3256 CRC patients enrolled, the results showed that there was no correlation between HER2 overexpression and survival (either progression-free survival or OS)[34].
Some studies have shown that cytoplasmic HER2-positive patients are associated with longer survival as an independent risk factor in Duke C stage patients[35]. In our short-term results report (median follow-up: 38 mo), the association between HER2 positivity and survival (DFS or OS) did not reach statistical significance. These results may be related to the short follow-up period. In this long-term study, with a median follow-up time of 134 mo, more events of distant metastasis or local recurrence occurred: 18 of 27 HER2-positive patients (66.7%) experienced disease progression, including 18 distant metastases and 2 Local recurrence events. There were significant differences between the HER2-positive and HER2-negative groups with respect to both DFS [HR: 1.69 (95%CI: 0.91-3.14); P = 0.048] and OS [HR: 1.95 (95%CI: 1.05-3.63); P = 0.0077] (Figures 1 and 2). The median DFS of HER2-positive patients was 43 mo. Of the 124 HER2-negative patients, 57 (46.0%) had distant metastases or recurrence events. Death occurred in 20 of 27 (74.1%) HER2-positive patients and 60 of 124 (48.4%) HER2-negative patients. The median OS was significantly shorter in HER2-positive patients than in HER2-negative patients (58 mo vs 133 mo). We hypothesis that HER2 overexpression, as a proto-oncogene, may be associated with increased tumour recurrence and poor prognosis. One explanation might be related to HER2’s inhibition of tumour cell apoptosis, resulting in tumour cell proliferation and acceleration of tumour aggressiveness. This suggests that, in order to pay special attention to local recurrence or distant metastasis, regular postoperative follow-up might be particularly important for patients with HER2-positive diseases, thereby improving their long-term survival. It can also provide possible suggestions for the enhanced treatment of these patients (combined with targeted therapy).
Recurrence and distant metastasis have been the main causes of treatment failure for locally advanced rectal cancer, despite many efforts having been made. The effect of anti-HER2 targeted drugs, such as trastuzumab and lapatinib, on breast cancer with high HER2 expression has been confirmed by several international multicentre open randomised controlled studies[36,37]. Studies have shown that metastatic CRC with wild-type RAS and HER2 overexpression might benefit from HER2 dual-targeted therapy[38,39]. Other studies have shown that trastuzumab, a HER2 inhibitor, can inhibit colony formation in colon cancer cells and reduce the viability of CRC cells in vitro[40]. Although this study did not involve targeted therapy, the results demonstrated that HER2-positive patients were associated with a higher rate of distant metastasis, which was related to poorer DFS and OS. These findings imply that more active treatments for patients with HER2-positive rectal cancer with distant metastasis or locally recurrent diseases are warranted, such as chemotherapy combined with single-targeted or even dual-targeted therapy. Moreover, HER2 overexpression in CRC may not only be an important prognostic determinant but also a potential therapeutic factor; however, this needs to be investigated further. Given that HER2-positive tumour cells are more likely to be aggressive in nature, anti-HER2 treatment may be beneficial for improving patient outcomes.
This study had a few limitations. The results showed that 27 (17.9%) patients were confirmed to be HER2-positive, a rate higher than that reported in most previous studies. However, this result is still considerably consistent with the results of previous studies. Most studies demonstrated that the variability of HER2 positivity might be caused by non-uniform judgment criteria, cohort heterogeneity, small study populations, different regimes of preoperative chemoradiotherapy, antibody selection methods, staining platforms, and so on[41]. In this study, all patients underwent rigid proctoscopy before neoadjuvant therapy, and a sufficient amount of tumour tissue was obtained. All samples were evaluated using immunohistochemistry, and the evaluation criteria were similar to those used when evaluating HER2 expression in gastric cancer. Two experienced pathologists independently performed the blind examinations and scoring. For controversial results, the FISH method was used to verify the accuracy of the results and exclude the possibility of false positives. A possible reason for the high rate of positivity is the potential selection bias resulting from an inadequate number of patients from a relatively larger HER2-negative population. However, we believe that due to the higher aggressiveness and poor prognoses of HER2-positive patients, our results may be more meaningful when compared with the results of these negative patients.
In conclusion, a considerable proportion of patients with rectal cancer showed HER2 overexpression. HER2 overexpression plays an important role in rectal cancer, which may promote the aggressiveness of rectal cancer and may be a potential prognostic biological predictor. For rectal cancer patients receiving preoperative neoadjuvant radiotherapy, HER2 overexpression predicts lymph node metastasis and distant metastasis and is associated with worse long-term DFS and long-term OS.
Predictive factors for long-term survival in locally advanced rectal cancer remained controversial. The roles of HER2 over-expression was still under discussion.
The effects of HER2 over-expression on the long-term survival was investigated in this prospective cohort study.
The associations between clinico-pathological factors and long-term survival were evaluated.
Categorical variables were assessed using the Chi square (2 × 2) or Fisher's exact test (2 × C), when applicable. Multivariate analysis was performed using a binary logistic regression model (forward: LR). Survival analysis was performed by the Kaplan-Meier method, and the differences between groups were verified by log-rank test.
The immunohistochemical results showed that HER2 over-expression was detected in 16.6% (25/151) of the tissue samples. HER2 positivity was associated with the pre-treatment N(+) stage (Pre-N) (P = 0.040) and the distant metastasis (P = 0.002). There were significant differences between HER2 positive group and HER2 negative group with respect to both disease-free survival (DFS) [hazard ratio: 1.69 (95% confidence interval: 0.91-3.14); P = 0.048] and overall survival (OS) [1.95 (1.05-3.63); P = 0.0077].
A considerable part of rectal cancer patients showed HER2 overexpression. HER2 overexpression plays an important role in rectal cancer, which may promote the aggressiveness of rectal cancer, and it may be a potential prognostic biological predictor. For those rectal cancer patients receiving preoperative neoadjuvant radiotherapy, HER2 overexpression predicts lymph node metastasis and distant metastasis, and it is associated with worse long-term DFS and long-term OS.
For rectal cancer patients, with HER2 over-expression, conventional treatment combined with targeted therapy might be of help.
We gave special thanks to faculty members (Professor Gu J, Professor Wu AW, etc.) of Gastro-intestinal center Ward III, Beijing Cancer Hospital.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Society of Colon and Rectal Surgeons, No. 24353.
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bartholomeyczik S, Germany; Shinozaki E, Japan; Yano M, Japan S-Editor: Gao CC L-Editor: A P-Editor: Yuan YY
| 1. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Gunn A, Hoffe S, Hubbard J, Hunt S, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Johnson-Chilla A, Gurski LA. NCCN Guidelines Insights: Rectal Cancer, Version 6. 2020. J Natl Compr Canc Netw. 2020;18:806-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 332] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 2. | National Health Commission of the People's Republic of China. Chinese Protocol of Diagnosis and Treatment of Colorectal Cancer (2020 edition). Zhonghua Waike Zazhi. 2020;58:561-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 3. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15320] [Article Influence: 3064.0] [Reference Citation Analysis (4)] |
| 4. | Leufkens AM, van den Bosch MA, van Leeuwen MS, Siersema PD. Diagnostic accuracy of computed tomography for colon cancer staging: a systematic review. Scand J Gastroenterol. 2011;46:887-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Swedish Rectal Cancer Trial, Cedermark B, Dahlberg M, Glimelius B, Påhlman L, Rutqvist LE, Wilking N. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1818] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 6. | Sauer R, Fietkau R, Wittekind C, Rödel C, Martus P, Hohenberger W, Tschmelitsch J, Sabitzer H, Karstens JH, Becker H, Hess C, Raab R; German Rectal Cancer Group. Adjuvant vs. neoadjuvant radiochemotherapy for locally advanced rectal cancer: the German trial CAO/ARO/AIO-94. Colorectal Dis. 2003;5:406-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 154] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC; EORTC Radiotherapy Group Trial 22921. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1993] [Cited by in RCA: 2041] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 8. | van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ; Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1348] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 9. | Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, Leer JW, van de Velde CJ; Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3104] [Cited by in RCA: 3120] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 10. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R; German Rectal Cancer Study Group. Preoperative vs postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4342] [Cited by in RCA: 4466] [Article Influence: 212.7] [Reference Citation Analysis (1)] |
| 11. | Hsieh AC, Moasser MM. Targeting HER proteins in cancer therapy and the role of the non-target HER3. Br J Cancer. 2007;97:453-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 211] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 12. | Yao YF, Du CZ, Chen N, Chen P, Gu J. Expression of HER-2 in rectal cancers treated with preoperative radiotherapy: a potential biomarker predictive of metastasis. Dis Colon Rectum. 2014;57:602-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Barros-Silva JD, Leitão D, Afonso L, Vieira J, Dinis-Ribeiro M, Fragoso M, Bento MJ, Santos L, Ferreira P, Rêgo S, Brandão C, Carneiro F, Lopes C, Schmitt F, Teixeira MR. Association of ERBB2 gene status with histopathological parameters and disease-specific survival in gastric carcinoma patients. Br J Cancer. 2009;100:487-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Kalogiannidis I, Petousis S, Bobos M, Margioula-Siarkou C, Topalidou M, Papanikolaou A, Vergote I, Agorastos T. HER-2/neu is an independent prognostic factor in type I endometrial adenocarcinoma. Arch Gynecol Obstet. 2014;290:1231-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 872] [Article Influence: 51.3] [Reference Citation Analysis (2)] |
| 16. | Kaptain S, Tan LK, Chen B. Her-2/neu and breast cancer. Diagn Mol Pathol. 2001;10:139-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 129] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, Blair SL, Burstein HJ, Dang C, Elias AD, Giordano SH, Goetz MP, Goldstein LJ, Hurvitz SA, Isakoff SJ, Jankowitz RC, Javid SH, Krishnamurthy J, Leitch M, Lyons J, Matro J, Mayer IA, Mortimer J, O'Regan RM, Patel SA, Pierce LJ, Rugo HS, Sitapati A, Smith KL, Smith ML, Soliman H, Stringer-Reasor EM, Telli ML, Ward JH, Wisinski KB, Young JS, Burns JL, Kumar R. NCCN Guidelines® Insights: Breast Cancer, Version 4.2021. J Natl Compr Canc Netw. 2021;19:484-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 209] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 18. | Koeppen HK, Wright BD, Burt AD, Quirke P, McNicol AM, Dybdal NO, Sliwkowski MX, Hillan KJ. Overexpression of HER2/neu in solid tumours: an immunohistochemical survey. Histopathology. 2001;38:96-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Kruszewski WJ, Rzepko R, Ciesielski M, Szefel J, Zieliński J, Szajewski M, Jasiński W, Kawecki K, Wojtacki J. Expression of HER2 in colorectal cancer does not correlate with prognosis. Dis Markers. 2010;29:207-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 20. | Liu F, Ren C, Jin Y, Xi S, He C, Wang F, Wang Z, Xu RH. Assessment of two different HER2 scoring systems and clinical relevance for colorectal cancer. Virchows Arch. 2020;476:391-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Meng X, Huang Z, Di J, Mu D, Wang Y, Zhao X, Zhao H, Zhu W, Li X, Kong L, Xing L. Expression of Human Epidermal Growth Factor Receptor-2 in Resected Rectal Cancer. Medicine (Baltimore). 2015;94:e2106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Kountourakis P, Pavlakis K, Psyrri A, Rontogianni D, Xiros N, Patsouris E, Pectasides D, Economopoulos T. Clinicopathologic significance of EGFR and Her-2/neu in colorectal adenocarcinomas. Cancer J. 2006;12:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Ingold Heppner B, Behrens HM, Balschun K, Haag J, Krüger S, Becker T, Röcken C. HER2/neu testing in primary colorectal carcinoma. Br J Cancer. 2014;111:1977-1984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 24. | Tarantino I, Warschkow R, Worni M, Merati-Kashani K, Köberle D, Schmied BM, Müller SA, Steffen T, Cerny T, Güller U. Elevated preoperative CEA is associated with worse survival in stage I-III rectal cancer patients. Br J Cancer. 2012;107:266-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Park YA, Lee KY, Kim NK, Baik SH, Sohn SK, Cho CW. Prognostic effect of perioperative change of serum carcinoembryonic antigen level: a useful tool for detection of systemic recurrence in rectal cancer. Ann Surg Oncol. 2006;13:645-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Moureau-Zabotto L, Farnault B, de Chaisemartin C, Esterni B, Lelong B, Viret F, Giovannini M, Monges G, Delpero JR, Bories E, Turrini O, Viens P, Salem N. Predictive factors of tumor response after neoadjuvant chemoradiation for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2011;80:483-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Armstrong D, Raissouni S, Price Hiller J, Mercer J, Powell E, MacLean A, Jiang M, Doll C, Goodwin R, Batuyong E, Zhou K, Monzon JG, Tang PA, Heng DY, Cheung WY, Vickers MM. Predictors of Pathologic Complete Response After Neoadjuvant Treatment for Rectal Cancer: A Multicenter Study. Clin Colorectal Cancer. 2015;14:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Mirbagheri N, Kumar B, Deb S, Poh BR, Dark JG, Leow CC, Teoh WM. Lymph node status as a prognostic indicator after preoperative neoadjuvant chemoradiotherapy of rectal cancer. Colorectal Dis. 2014;16:O339-O346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (2)] |
| 29. | Osako T, Miyahara M, Uchino S, Inomata M, Kitano S, Kobayashi M. Immunohistochemical study of c-erbB-2 protein in colorectal cancer and the correlation with patient survival. Oncology. 1998;55:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Kapitanović S, Radosević S, Kapitanović M, Andelinović S, Ferencić Z, Tavassoli M, Primorać D, Sonicki Z, Spaventi S, Pavelic K, Spaventi R. The expression of p185(HER-2/neu) correlates with the stage of disease and survival in colorectal cancer. Gastroenterology. 1997;112:1103-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 152] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Knösel T, Yu Y, Stein U, Schwabe H, Schlüns K, Schlag PM, Dietel M, Petersen I. Overexpression of c-erbB-2 protein correlates with chromosomal gain at the c-erbB-2 Locus and patient survival in advanced colorectal carcinomas. Clin Exp Metastasis. 2002;19:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Laurent-Puig P, Balogoun R, Cayre A, Le Malicot K, Tabernero J, Mini E, Folprecht G, Van Laethem JL, Thaler J, Nørgård Petersen L, Sanchez E, Bridgewater J, Ellis S, Locher C, Lagorce C, Ramé JF, Lepage C, Penault-Llorca F, Taieb J. ERBB2 alterations a new prognostic biomarker in stage III colon cancer from a FOLFOX based adjuvant trial (PETACC8). Ann Oncol. 2016;27:VI151. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Li C, Liu DR, Ye LY, Huang LN, Jaiswal S, Li XW, Wang HH, Chen L. HER-2 overexpression and survival in colorectal cancer: a meta-analysis. J Zhejiang Univ Sci B. 2014;15:582-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Richman SD, Southward K, Chambers P, Cross D, Barrett J, Hemmings G, Taylor M, Wood H, Hutchins G, Foster JM, Oumie A, Spink KG, Brown SR, Jones M, Kerr D, Handley K, Gray R, Seymour M, Quirke P. HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials. J Pathol. 2016;238:562-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 190] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 35. | Essapen S, Thomas H, Green M, De Vries C, Cook MG, Marks C, Topham C, Modjtahedi H. The expression and prognostic significance of HER-2 in colorectal cancer and its relationship with clinicopathological parameters. Int J Oncol. 2004;24:241-248. [PubMed] |
| 36. | Gianni L, Dafni U, Gelber RD, Azambuja E, Muehlbauer S, Goldhirsch A, Untch M, Smith I, Baselga J, Jackisch C, Cameron D, Mano M, Pedrini JL, Veronesi A, Mendiola C, Pluzanska A, Semiglazov V, Vrdoljak E, Eckart MJ, Shen Z, Skiadopoulos G, Procter M, Pritchard KI, Piccart-Gebhart MJ, Bell R; Herceptin Adjuvant (HERA) Trial Study Team. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12:236-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 480] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 37. | Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, Gómez H, Dinh P, Fauria K, Van Dooren V, Aktan G, Goldhirsch A, Chang TW, Horváth Z, Coccia-Portugal M, Domont J, Tseng LM, Kunz G, Sohn JH, Semiglazov V, Lerzo G, Palacova M, Probachai V, Pusztai L, Untch M, Gelber RD, Piccart-Gebhart M; NeoALTTO Study Team. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 996] [Cited by in RCA: 1027] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 38. | Martinelli E, Troiani T, Sforza V, Martini G, Cardone C, Vitiello PP, Ciardiello D, Rachiglio AM, Normanno N, Sartore-Bianchi A, Marsoni S, Bardelli A, Siena S, Ciardiello F. Sequential HER2 blockade as effective therapy in chemorefractory, HER2 gene-amplified, RAS wild-type, metastatic colorectal cancer: learning from a clinical case. ESMO Open. 2018;3:e000299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 39. | Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, Zagonel V, Leone F, Depetris I, Martinelli E, Troiani T, Ciardiello F, Racca P, Bertotti A, Siravegna G, Torri V, Amatu A, Ghezzi S, Marrapese G, Palmeri L, Valtorta E, Cassingena A, Lauricella C, Vanzulli A, Regge D, Veronese S, Comoglio PM, Bardelli A, Marsoni S, Siena S. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 742] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 40. | Conradi LC, Spitzner M, Metzger AL, Kisly M, Middel P, Bohnenberger H, Gaedcke J, Ghadimi MB, Liersch T, Rüschoff J, Beißbarth T, König A, Grade M. Combined targeting of HER-2 and HER-3 represents a promising therapeutic strategy in colorectal cancer. BMC Cancer. 2019;19:880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Guarini C, Grassi T, Pezzicoli G, Porta C. Beyond RAS and BRAF: HER2, a New Actionable Oncotarget in Advanced Colorectal Cancer. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |