Published online Dec 15, 2021. doi: 10.4251/wjgo.v13.i12.1939
Peer-review started: March 16, 2021
First decision: May 3, 2021
Revised: May 14, 2021
Accepted: October 14, 2021
Article in press: October 14, 2021
Published online: December 15, 2021
Processing time: 273 Days and 9.9 Hours
Despite being the second most frequent primary liver tumor in humans, early diagnosis and treatment of cholangiocarcinoma (CCA) are still unsatisfactory. In fact, survival after 5 years is expected in less than one fourth of patients diagnosed with this disease. Rare incidence, late appearance of symptoms and heterogeneous biology are all factors contributing to our limited knowledge of this cancer and determining its poor prognosis in the clinical setting. Several efforts have been made in the last decades in order to achieve an improved classification/understanding with regard to the diverse CCA forms. Location within the biliary tree has helped to distinguish between intrahepatic, perihilar and distal CCA types. Sequence analysis contributed to identifying several characteristic genetic aberrations in CCA that may also serve as possible targets for therapy. Novel findings are expected to significantly improve the management of this malignancy in the near future. In this changing scenario our review focuses on the current and future strategies for CCA treatment. Both systemic and surgical treatments are discussed in detail. The results of the main studies in this field are reported, together with the ongoing trials. The current findings suggest that an integrated multidisciplinary approach to this malignancy would be helpful to improve its outcome.
Core Tip: Cholangiocarcinoma is a lethal malignancy characterized by a poor survival. In this review we discuss in detail the actual treatment and the future therapeutic perspectives for this cancer. Systemic and surgical strategies are reported with the corresponding results. Improved knowledge of this malignancy and a multidisciplinary therapeutic approach are likely to improve the cholangiocarcinoma outcome in the future.
- Citation: Manzia TM, Parente A, Lenci I, Sensi B, Milana M, Gazia C, Signorello A, Angelico R, Grassi G, Tisone G, Baiocchi L. Moving forward in the treatment of cholangiocarcinoma. World J Gastrointest Oncol 2021; 13(12): 1939-1955
- URL: https://www.wjgnet.com/1948-5204/full/v13/i12/1939.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i12.1939
Cholangiocarcinoma (CCA) is a primary malignancy of the biliary system and represents the second most common primary hepatic malignancy after hepatocellular carcinoma (HCC), constituting around 15% of primary liver tumors and 3% of gastrointestinal malignancies[1,2]. It is a rare tumor with a global incidence of 0.3-6 per 100000 inhabitants per year, displaying an increasing trend in the last decades[1]. However, in some Asian countries, such as Thailand, Cambodia and Laos, rates can be as high as 85 per 100000 due to infection with liver flukes[2].
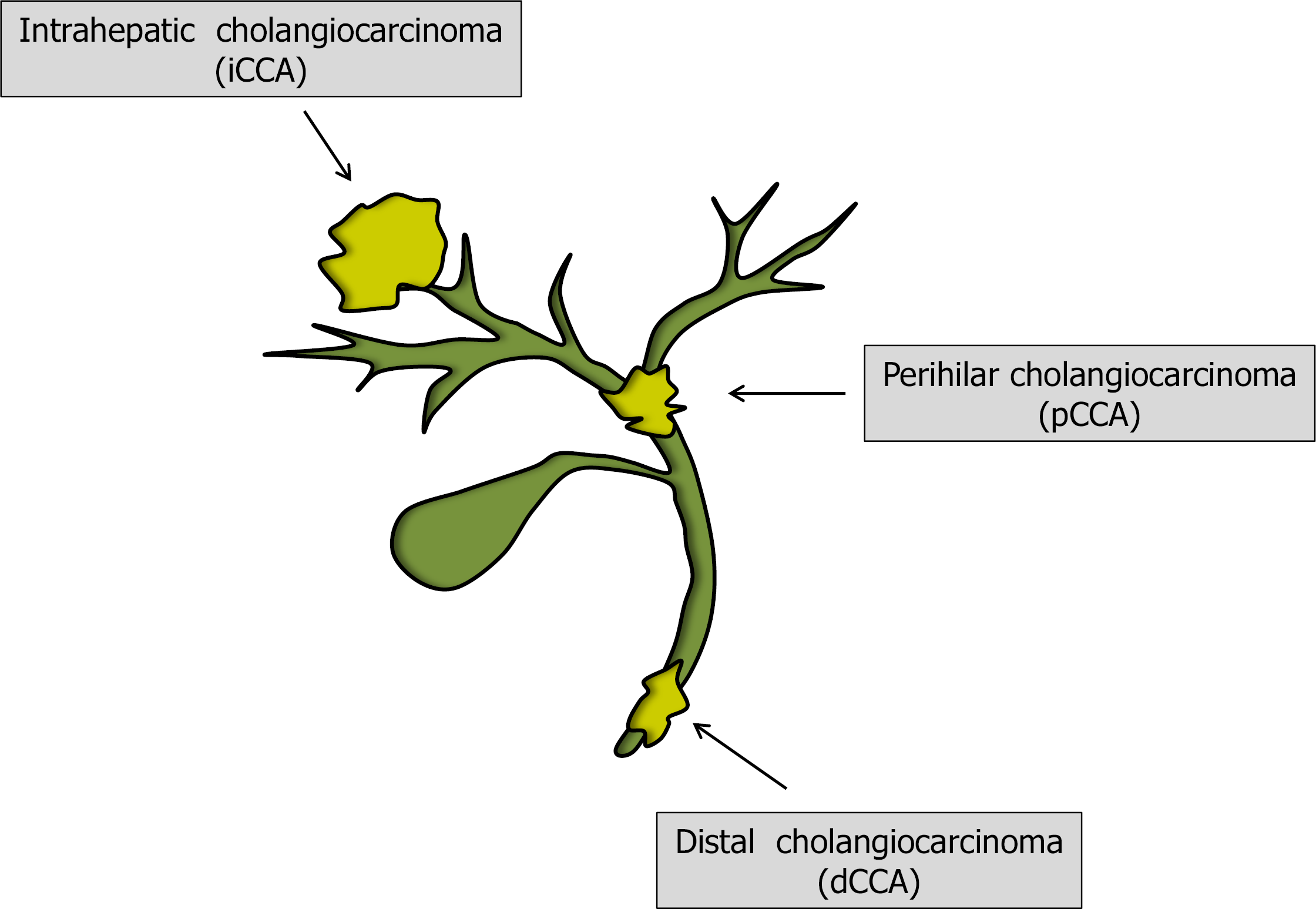
Distinction into subgroups of CCA is anatomical: intrahepatic CCA (iCCA) arises in the liver above the second order bile ducts; perihilar CCA (pCCA), also known as Klatskin tumor, arises in the first order or main bile duct above the junction with the cystic duct; and distal CCA (dCCA) originates distally to the cystic duct (Figure 1). This classification is crucial as each subtype has distinct clinical characteristics and therapeutic strategies. pCCA accounts for the majority of diagnoses (50%-60%), with dCCA (20%-30%) and iCCA (10-20%) being less frequent[3]. iCCA can be further classified on the basis of the cells of origin as large and small duct types, with chronic biliary inflammation and chronic hepatis as risk factors, respectively[4]. On top of this, a recent interesting study involved the epigenomic and transcriptomic analysis of CCAs from 10 different countries in order to further understand and classify the genetic basis of CCA. The authors performed the analysis on CCA samples associated with liver flukes (mainly Opisthorchis viverrine and Clonorchis sinensis) and non-fluke cases. Four CCA clusters were likely driven by distinct etiologies, with separate genetic, epigenetic and clinical features found, highlighting how distinct cancer subtypes in the same organ may arise through different carcinogenic pathways[5].
Unfortunately, symptoms often appear when the disease is already advanced, resulting in a poor prognosis. In fact, this malignancy has an overall survival rate at 5 years of 5%-20%[1,3]. Nonetheless, many promising new approaches are currently under investigation.
Several issues have been encountered in the pursuit of a curative treatment for CCA in humans. Despite the evidence of different biological and epidemiological risk factors and genetic aberrations between diverse types of CCAs, these tumors are still frequently pooled together (also with gallbladder cancer) or misclassified in studies focusing on natural history or treatment[6,7]. On the other hand, histological classification (in particular for iCCA forms) remains suboptimal and also relies on heterogeneous genetic aberrations identified in this cancer[2]. The difficulties in CCA classification and in the comprehension of its biology therefore affect both clinical and basic research in this field. For instance, despite next generation models now attempting the construction of complex 3D CCA systems in culture (such as organoids or spheroids), an adequate reproduction of this tumor remains difficult in the preclinical experimental setting[8].
From the clinical side, CCA symptoms are generally not specific and share similarities with inflammatory diseases of the biliary tract. Moreover, general biomarkers used in medical practice, such as carbohydrate antigen 19-9 exhibit a sensitivity and specificity lower than 70%, underscoring the importance of the identification of possible novel genomic or proteomic biomarkers[9]. Also the appropriate surveillance of CCA-predisposing conditions, such as primary sclerosing cholangitis, remains undefined, leading to disappointing late-stage tumor identification in the majority of patients[10].
Furthermore, CCA remains an infrequent cancer in the majority of countries, several cases arise in the absence of recognized risk factors, and when some intraductal papillary or tubular forms are excluded[11], there is usually a short-term poor prog
As described in detail in the dedicated paragraphs, the opportunity for a complete CCA cure should be offered in rare cases just employing surgical techniques. On the other hand, despite the fact that current drug therapy for this cancer is unsatisfactory, the pharmacological approach may present a larger margin of improvement in the future in comparison with operative methods.
At present, in subjects with unresectable, advanced disease, the best option is represented by cisplatin/gemcitabine first-line treatment. Confirmation of the utility of this treatment was obtained by a large study comparing this association with gem
With regard to adjuvant therapy in subjects amenable to surgical resection, the major indication came from the BILCAP trial[14]. In this study, patients undergoing surgical treatment of biliary cancer (n = 447) were allocated to receive capecitabine or just observation after a macroscopically complete tumor resection. Capecitabine increased survival by almost one third. This difference was statistically significant in the per-protocol (53 mo vs 36 mo, P = 0.02) but not in the intention-to-treat analysis. Serious adverse events occurred in the two groups at a similar rate. A randomized Phase 3 clinical trial conducted with adjuvant gemcitabine chemotherapy did not show significant improvement in overall survival or relapse-free survival in comparison with untreated control[15]. An attempt was also conducted with adjuvant gemci
In conclusion, excluding the modest, above-described, therapeutic options, phy
The possible evolution of systemic therapy for CCA is largely dependent on the resolution of some issues with regard to this cancer[19]. First, scientists are still searching for an appropriate preclinical model of CCA[20]. CCA cell culture and tumor xenotransplantation in nude mice are the most commonly used strategies, but they do not adequately reproduce the neoplastic microenvironment[21]. From the clinical experimental side, the rarity of this neoplasm and competition between new molecules do not facilitate the performance of trials with an adequate number and homogeneous type of CCAs. While exploring this undefined horizon, research efforts are oriented in some main fronts, as reported in the following subparagraphs.
One of the main issues greatly limiting chemotherapy effectiveness in CCA is represented by chemoresistance[22]. Chemoresistance describes the capacity of cancer cells to escape or attenuate therapeutic drug effects[23]. Several mechanisms have been identified as the basis of chemoresistance, some opposing drug uptake or increasing its extracellular export and others reducing cellular necrosis/apoptosis or stimulating tumoral phenotypic changes. For instance, the reduced expression of organic cation transporter 1, as observed in both CCA and HCC, has been related to a poor response to tyrosine kinase inhibitors such as sorafenib[24]. On the other hand, the phenotypic CCA evolution from an epithelial to a mesenchymal trait (so-called epithelial-mesenchymal-transition) not only counteracts chemotherapy effects but also seems to favor metastatic progression[25]. Several strategies have been attempted in preclinical experimental studies to improve therapeutic response to chemotherapy, such as drug transporter induction or export pump inhibition in CCA cells or targeting cells with specific organic molecules such as bile acids or vesicles. With regard to human trials, a gemcitabine analogue (NUC-1031)[26] not requiring nucleoside cellular transport or intracellular kinase activation is currently being tested in a Phase 3 trial (NCT 04163900).
Several genetic aberrations have been identified in CCA, with a different distribution among intrahepatic, perihilar or distal CCA[27]. Kirsten rat sarcoma gene mutations are frequently encountered, ranging from 9%-40% of cases according to CCA location within the biliary tract[28]. A specific molecule (AMG 510) targeting the Kirsten rat sarcoma/G12C mutation is currently being tested in a Phase1/2 trial (NCT03600883); however, downstream pathway suppression, obtained by kinase inhibition (such as those of the Raf or MEK family) also may be attempted. In this perspective, the dual suppression of BRAF and MEK, obtained with dabrafenib and trametinib, gave excellent results in anecdotal cases[29], thus stimulating the Phase 2 ROAR study in patients with the BRAFV600E solid tumor mutation (NCT02034110). In an interim analysis of this trial of 43 patients with biliary tract cancer, the overall response rate (after external data review) accounted for 20% of cases[30].
The fibroblast growth factor (FGF) family comprises a group of proteins that react with their specific receptors (FGFRs) to stimulate several developmental and proliferative processes, also involving stem cell differentiation[31]. FGFR (subtype 2) genetic alterations, characterized by fusion with other genes, have been observed in nearly 15% of iCCAs, so FGF/FGFR signaling has emerged as a possible target to cure this cancer[32]. Among the FGFR inhibitors, infigratinib and pemigatinib have been eva
Since isocitrate dehydrogenase 1 mutations have been identified in approximately 13% of iCCA and 0.8% of other CCAs and the impairment of this enzyme may lead to the accumulation of the pro-oncogenic metabolite D-2-hydroxyglutarate, isocitrate dehydrogenase 1 inhibitors have been suggested for treatment of this cancer. The ClarIDHy phase 3 trial tested the isocitrate dehydrogenase 1 inhibitor ivosidenib in CCAs with a mutation of this enzyme and refractory to previous systemic therapy[35]. Six-month progression-free survival was 32% in the ivosidenib group in comparison with 0% in the placebo group. Other inhibitors are currently being examined in different trials, as summarized in a recent review on this issue[36]. Other genetic aberrations, such as those involving the ERRB family and proto-oncogene tyrosine-protein kinase 1, represent possible targets for CCA therapy; some drugs are under evaluation[37].
The activation of immune checkpoint (IC) pathways seems to be involved, under normal conditions, in tolerance and the prevention of autoimmune diseases[38]; however, tumor-mediated stimulation, hindering immune surveillance, may favor cancer proliferation and spread[39]. In this perspective, IC inhibitors have recently gained major importance with regard to cancer therapy, achieving a complete response in 20% of melanoma patients[40]. Among diverse IC pathways, the cytotoxic T-lymphocyte antigen 4 and programmed cell death protein 1/programmed cell death protein ligand 1 are those that are mainly recognized and targeted in oncology. In another study, 22 patients harboring CCA characterized by microsatellite instability and mismatch repair reduced protein (findings related to IC upregulation) were treated with the programmed cell death protein ligand 1 inhibitor pembrolizumab, obtaining a median progression free survival of 4.2 mo and a median overall survival of 24.3 mo[41]. However, these results might be improved with careful patient selection since an increased response has been observed as a function of programmed cell death protein ligand 1 expression[42]. Several trials with IC inhibitors alone or in combination and including CCA patients are ongoing.
The neuroendocrine regulation of CCA expansion (as shown by preclinical experimental studies) might be an important factor to consider while searching for a therapy for this cancer[43]. Secretin, somatostatin and melatonin have all been demonstrated to decrease CCA growth, as observed in cancer cell lines or in animal models such as tumor xenotransplantation in nude mice[44-46]. At present, however, no clinical data are available with either secretin or melatonin for CCA treatment, while a trial with somatostatin gave negative results[47]. Also, angiogenic factors such as vascular endothelial growth factor are considered possible targets for CCA therapy. Vascular endothelial growth factor in fact seems to be increased in half of human biliary tract cancers[48]. A trial using the anti- vascular endothelial growth factor antibody bevacizumab, in association with standard chemotherapy (gemcitabine, oxaliplatin), however, gave modest results[49].
Surgery remains the best treatment option for long-term patient survival in CCA, and it is recommended to undertake surgical treatments in highly specialized centers to minimize morbidity and mortality[50].
Preoperative workup and biliary drainage have been widely discussed in recent decades. The current consensus is that preoperative biliary drainage is required in cases of concomitant cholangitis, need for neoadjuvant therapy, malnutrition, hepatic or renal failure and need for portal vein embolization (PVE)[1]. When jaundice is the only indication, need for decompression is still a matter of debate. Asian guidelines recommend preoperative drainage because of the higher risk of patients with cholangitis[51,52]. Furthermore, drainage may help restore liver function, decreasing the chance of postoperative liver failure[52]. On the other hand some studies have shown that, while biliary drainage is beneficial by reducing morbidity and mortality in patients with small future liver remnant (FLR), it is equally detrimental when FLR is large enough[53,54]. In Western countries, many centers prefer to use selective biliary drainage when FLR is less than 30%-40%[55]. When stenting is required, both endoscopic and percutaneous methods are used. Percutaneous transhepatic biliary drainage has some advantages, such as reducing the need for re-intervention, reducing the time to achieving a therapeutic effect and fewer procedural risks. However, a recent randomized trial of percutaneous vs endoscopic stenting was terminated early due to excess mortality in the percutaneous group (41% vs 11%), mandating further prospective studies and a reconsideration of drainage strategies[55]. Alternatively, nasobiliary drainage may be a valid option, showing good success rates and low morbidity despite greater patient discomfort[56,57]. The optimal timing of surgery in drained patients is currently unknown. A recent study identified a preoperative bilirubin level of < 75 µmol/L (2.9 mg/dL) to be correlated with fewer complications, less mortality and longer 5-year overall survival[58].
Patients are considered eligible for surgery whenever complete resection of the tumor with negative margins (R0) can be achieved, providing sufficient FLR. Bilateral multifocal or multicentric disease is associated in many studies to a significantly shorter overall survival (OS)[59,60]. In practice, only 32% of iCCAs satisfy resectability criteria at presentation. On top of this, around 30% of iCCAs will be deemed inoperable on the operating table. Staging laparoscopy can detect unresectable disease in around 36% of patients with minimal costs[61] and is advocated by current guidelines[62].
Principles: The established principles of surgery for iCCA are to achieve R0 resections and to provide adequate staging with hilar lymphadenectomy, sparing at the same time as much parenchyma as possible to avoid post-hepatectomy liver failure. Margin status is the primary objective in iCCA surgery. Evidence mainly derives from large single center and multicenter studies, which have demonstrated a significant survival impact of R0 resection. Overall survival at 5 years for R0, R1 and R2 resections are reported to be 28.7%, 13.9% and 0%, respectively[63], with an increased survival benefit for > 5 mm margins[64].
Lymphadenectomy and nodal disease: Nodal disease is recognized as the most important prognostic factor in most studies[59,63-66]. In fact, some authors have reported that margin status may have limited impact in the presence of nodal metastases[64]. Most guidelines suggest routine consideration of regional lymphadenectomy and a minimum of six lymph nodes are needed for accurate staging[2,62,67]. Nonetheless, the role of lymphadenectomy remains controversial in Western coun
Extended procedures: Given the poor prognosis (0% 5-year OS) of unresectable disease or R2 resection[63,65], in recent years, some groups have explored the benefits of major vascular resections to obtain R0 resection, resulting in up to 84% of patients[66] with morbidity and mortality rates comparable to standard resection[69]. Overall survival of these patients is also comparable to patients who did not undergo vascular resection[66,69,70]. In general, all patients with localized iCCA should be considered for resection even if this implies major hepatectomy or vascular resection[62]. In recent decades, based on the principles of liver regeneration, some authors have pushed the boundaries for resectability in liver surgery by introducing the concept of two-stage hepatectomies, namely portal vein ligation and PVE. The latter can enhance the resectability rates of liver tumors, allowing extensive resection with adequate FLR and are usually well-tolerated by the patient. However, a major drawback is the long waiting time for the second stage procedure, which can take up to several weeks, carrying the risk of tumor progression. To solve these problems, a German group of authors developed a new technique, known as associating liver partition and portal vein ligation for staged hepatectomy (ALPPS), which was found to allow rapid growth of the FLR, with a median period of 9 d[71]. Another study investigated benchmark outcomes in ALPPS, demonstrating that it has a comparable standard outcome as other types of major liver surgery[72]. ALPPS for iCCA has been evaluated in an international multicenter study in which 102 patients underwent first-stage ALPPS; 99 completed the second procedure, and R0 resection was obtained in 85% of cases with 29% major morbidity and 7% mortality[60]. When disease is considered unresectable, neoadjuvant chemotherapy can convert as many as 53% of cases to secondary resectable disease[73].
Recurrent disease: Recurrence of iCCA is frequent. Most recurrences are intrahepatic and therefore potentially amenable to re-resection[74], with satisfactory outcomes when repeated resections are undertaken. These results lead to the recommendation that the same principles for resectability should be applied in consideration of primary and secondary resection[75].
pCCA represents a surgical challenge due to its intrinsic anatomical location. None
In pCCA, the main criteria that define surgical unresectability are inadequate FLR, absence of a suitable field for biliary reconstruction (i.e. bilateral segmental ductal extension) and major vascular infiltration[51]. Growth of FLR may be induced with two-stage hepatectomy techniques, broadening indications for resection. Nonetheless, 20%-50% of patients are deemed to be unresectable upon surgical exploration, making explorative laparoscopy a useful tool to avoid unnecessary laparotomies.
Principles: Surgery for pCCA routinely involves en bloc hemi-hepatectomy and bile duct resection to achieve negative biliary and parenchymal margins, with additional resection of the caudate lobe, regional lymphadenectomy[51,76] and biliary recon
Lymphadenectomy and nodal disease: European guidelines affirm that lymphadenectomy should be considered the standard of care, but there is no consensus on the extent of lymphadenectomy for pCCA[81]. A recent systematic review identified a minimum of seven lymph nodes to convey sufficient information avoiding under-staging, with no benefit coming from higher lymph node counts (≥ 15) which could only be achieved with extended lymphadenectomy[82]. The regional nodes for pCCA are cystic, biliary, hepatic artery, portal vein and retropancreatic. The impact of extended lymphadenectomy of N2 nodes (dissection of celiac, superior mesenteric and paraaortic nodes) on survival has not been established, but trials are ongoing[83,84]. For known N2 positive disease, current expert consensus suggests no benefit of resection[2].
Extended procedures: Extended resections have been explored for pCCA, including two-stage hepatectomies such as portal vein ligation/PVE and ALPPS with acceptable outcomes. The first reports of 29 ALPPS procedures for this indication featured a strikingly high mortality rate, although statistically comparable to results of 29 matched patients who underwent non-ALPPS resection[85]. These poor initial results have dramatically improved for most ALPPS indications with better patient selection and inter-stage management and will hopefully improve for hCCA as well[86]. As of 2020, ALPPS should only be considered in highly experienced institutions. Hepatopancreaticoduodenectomy entails resection of the entire extrahepatic biliary tree, thus necessitating resection of the pancreatic head and duodenum. It is used for tumors with concomitant distal bile duct spread. This procedure is associated with high major morbidity rates of up to 37%. Nonetheless, the latest reports from highly specialized centers have been encouraging and suggest that hepatopancreaticoduodenectomy could be considered in young, fit patients when it represents the only chance of a cure[87]. Vascular resection can be adopted to increase R0 rates. Long-term oncological results are in the range of 25%-45%[88,89].
dCCA affects the third portion of the extrahepatic biliary duct, which lies in a retro/intra pancreatic position. This particular anatomical configuration translates into a completely different surgical approach compared to iCCA and pCCA. In particular, resection involves pancreaticoduodenectomy, as for cancer of the pancreatic head. Negative margin status is imperative, as positive margins increase anastomotic recurrence rates and herald poor survival. An aggressive approach is justified in cases with vascular infiltration. Resection of the superior mesenteric or portal vein and reconstruction to obtain R0 obtains survival comparable to patients without vascular resection with no additional morbidity and mortality[90]. Data on arterial resection is more limited[91]. Specific for dCCA is the need to resect the bile duct high in the liver hilum as well as a lymphadenectomy of the porta hepatis and gastroduodenal ligament[76]. Unfortunately, dCCA diagnosis is not always defined preoperatively, and these steps may be omitted, increasing the chance of R1 if the tumor has prominent intraductal spread.
Progress in the field of surgery for CCA has been limited for many years, yet the coming decade harbors great promise, with numerous innovations on the horizon. The main ongoing surgical trials are reported in Table 1.
| CCA Type | Domain | Trial name | Summary |
| iCCA/pCCA | Hepatic venous deprivation | NCT03841305 | Randomized trial of portal vein embolization vs hepatic venous deprivation. Primary endpoint: future liver remnant at 3 wk |
| iCCA | Liver transplantation | NCT02878473 | Liver transplantation for early (< 3 cm) iCCA. Single group assignment |
| iCCA | Liver transplantation | NCT04556214 | Liver transplantation for stable (> 6 mo), advanced (unresectable) iCCA. Single group assignment |
| iCCA | Liver transplantation | NCT04195503 | Liver transplantation for stable (> 6 mo), advanced (unresectable) iCCA. Single group assignment |
| pCCA | Lymphadenectomy | ChiCTR1800015688 | Randomized trial of extended vs regional lymphadenectomy for resectable pCCA. Primary endpoint: overall survival |
| pCCA | Liver transplantation | NCT02232932 | Randomized trial of liver transplantation vs resection for resectable pCCA (< 3 cm). Primary endpoint: overall survival at 5 yr |
Resectability for CCA is limited mainly by inadequate FLR, especially when extensive resections are required. Portal vein ligation, PVE and ALPPS are compelling procedures for enhancing resectability with adequate FLR. On top of this, recently Guiu et al[92] described an interesting new technique, named liver venous deprivation, which involves PVE with simultaneous embolization of one or two hepatic veins. In a subsequent study, the same group demonstrated that liver venous deprivation permits a significantly greater increase in both FLR volume and function compared to PVE[93]. A randomized trial is ongoing with the aim of establishing the superiority of this technique (NCT03841305). Liver venous deprivation could represent an important advancement in liver surgery, combining the low morbidity of PVE with the greater efficacy and rapidity of ALPPS.
Minimally invasive approaches have developed slowly in liver surgery. Few studies specifically address the use of minimally invasive surgery for CCA with comparable outcomes, although no benefit has been clearly demonstrated so far[94]. For pCCA, the literature is discordant, but nevertheless it is possible that it could develop further in the near future[94].
Liver transplantation (LT) for hCCA has been investigated for many years, but the practice was abandoned due to very poor results compared to other indications, in the setting of the ongoing organ shortage. Initial experiences featured 5-year OS survival rates of 23%-38%, mainly due to early recurrence[95].
In the early 2000s, the idea of LT for unresectable iCCA changed thanks to the work of Vreede et al[96] at the Mayo Clinic. They developed a very rigorous protocol to optimize the selection of patients who were most likely to benefit from LT. In particular, patients with a diagnosis of unresectable, non-metastatic hCCA were treated with external beam radiotherapy (4500 cGy in 30 fractions) with concomitant intravenous 5 fluorouracil followed 3 wk later by transcatheter brachytherapy with an iridium-193 wire and finally maintenance oral capecitabine (as tolerated) until transplantation. Before LT, patients underwent staging laparotomy to exclude any intra-abdominal disease, including distant lymph node sampling. With this protocol, they reported a 5-year survival of 82% for patients undergoing LT[97]. Of note, almost half of the patients who enrolled in the protocol were not transplanted due to death or disease progression. Surgical exploration resulted in findings that precluded trans
Sahai et al[98] reported similar efficacy with a different neoadjuvant protocol consisting of higher brachytherapy doses and the omission of external beam ra
Notably, patients with hCCA developing in the setting of primary sclerosing cholangitis had a significantly better outlook than sporadic hCCA[104]. Given the technical and management complexity of this surgery, outcomes are influenced by center experience, with centers having performed at least six procedures providing the best results[104,105]. These experiences have led neoadjuvant therapy followed by LT to become the current standard of care for locally advanced non-metastatic unre
To date, iCCA is generally considered a contraindication to LT due to poor results in initial experiences[106]. Vilchez analyzed 440 patients with iCCA from the UNOS database and reported a significantly reduced OS with respect to HCC patients undergoing LT[107]. Yet, it may not be correct to generalize these poor results as analysis of the National Cancer Data Base revealed that only 2.2% of patients with iCCA underwent LT[107]. Furthermore, none of the studies cited so far have investigated the benefits of preoperative neoadjuvant therapy. The success and implemen
Whether mixed HCC-iCCA should be considered for LT is also debated. The literature is conflicting, with some studies reporting outcomes similar to HCC and others to iCCA[107,109].
Successful results of LT after neoadjuvant therapy have induced investigators to compare them with conventional resection for resectable hCCA. Rea et al[97] reported a significantly improved OS at 5 years for patients undergoing LT compared to those undergoing resection. Ethun et al[110] showed similar results in an intention-to-treat analysis as well. They also went further and analyzed results for a subgroup of patients that were selected to be more comparable to patients in the resection group (i.e. hCCA not associated with primary sclerosing cholangitis, < 3 cm and lymph node negative). Even in this case, results were significantly better in the LT group. The authors suggest that the available data should prompt consideration of LT for hCCA patients with resectable disease. Indeed, this may be the new frontier in hCCA surgery. Nonetheless, some obstacles remain before the implementation of this strategy becomes widespread, the main one being the scarcity of allograft availability. In fact, critics of this approach argue that the benefit of LT (14% 5-year survival increase) is too little compared to the minimum benefit commonly applied to LT (50% at 5 years) and does not justify use of a deceased or living donor allograft. Better identification of patients who would benefit most from LT (e.g., patients who are less likely to undergo an R0 resection) could maximize the benefit and justify an LT program. In any case, a randomized trial is currently ongoing (NCT02232932).
Regarding resectable iCCA, Facciuto et al[111] recently published a small series of patients transplanted for HCC or iCCA. Their analysis showed that when iCCA features were within the Milan Criteria survival was comparable to that achieved for HCC. Further insights have come in recent years. Sapisochin et al[109] reported that the subgroup of patients transplanted for small iCCA (< 2 cm) had similar survival to HCC. In two subsequent studies, these results were confirmed with OS being significantly different between small (< 2 cm) and large tumors (> 2 cm)[71,112], 65%-73% vs 40%-45% respectively. Trials of LT for small iCCA are currently ongoing (NCT02878473).
Experience with neoadjuvant therapy followed by LT has shown that disease can be stabilized in more than 50% of patients and that 57% of patients who ultimately undergo LT benefit from a complete response[97,103]. While LT seems to offer superior survival compared to resection, it is unknown to what extent neoadjuvant therapy or strict selection criteria contribute to the effect[113]. Neoadjuvant therapy may therefore prove useful in cases of resectable disease as well to increase chances of R0 resection. Consideration should be given to the risk of disease progression and loss of chance of resection. To date, there is little data available on this possible approach[114].
Poor CCA prognosis requires important therapeutic improvements in the next few years. Table 2 summarizes the new approaches in CCA therapy. Several attempts are being made or hypothesized at present, as described above in this review, with regard to systemic and/or surgical treatment for this cancer. The heterogeneity and rare occurrence of this tumor, however, impede the design of large trials with homo
| Approaches | |
| Systemic therapy | (1) Overcoming chemoresistance; (2) Genetic aberration targeted therapy; (3) Immune checkpoint inhibitors; and (4) Neuroendocrine modulation of cancer growth |
| Surgical therapy | (1) Liver venous deprivation; (2) Minimally invasive surgery; and (3) Liver transplantation |
| Combined therapy | Liver transplantation or surgical resection after radiotherapy and/or neoadjuvant treatment |
Provenance and peer review: Invited article; Externally peer reviewed.
Corresponding Author’s Membership in Professional Societies: Società Italiana Di Gastroenterologia Ed Endoscopia Digestiva.
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Titapun A S-Editor: Chang KL L-Editor: Filipodia P-Editor: Chang KL
| 1. | Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, La Vecchia C, Negri E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 425] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 2. | Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, Calvisi DF, Perugorria MJ, Fabris L, Boulter L, Macias RIR, Gaudio E, Alvaro D, Gradilone SA, Strazzabosco M, Marzioni M, Coulouarn C, Fouassier L, Raggi C, Invernizzi P, Mertens JC, Moncsek A, Rizvi S, Heimbach J, Koerkamp BG, Bruix J, Forner A, Bridgewater J, Valle JW, Gores GJ. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1555] [Cited by in RCA: 1554] [Article Influence: 310.8] [Reference Citation Analysis (0)] |
| 3. | Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, Hruban RH, Lillemoe KD, Yeo CJ, Cameron JL. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463-473; discussion 473-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 865] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 4. | Aishima S, Oda Y. Pathogenesis and classification of intrahepatic cholangiocarcinoma: different characters of perihilar large duct type vs peripheral small duct type. J Hepatobiliary Pancreat Sci. 2015;22:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 167] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 5. | Jusakul A, Cutcutache I, Yong CH, Lim JQ, Huang MN, Padmanabhan N, Nellore V, Kongpetch S, Ng AWT, Ng LM, Choo SP, Myint SS, Thanan R, Nagarajan S, Lim WK, Ng CCY, Boot A, Liu M, Ong CK, Rajasegaran V, Lie S, Lim AST, Lim TH, Tan J, Loh JL, McPherson JR, Khuntikeo N, Bhudhisawasdi V, Yongvanit P, Wongkham S, Totoki Y, Nakamura H, Arai Y, Yamasaki S, Chow PK, Chung AYF, Ooi LLPJ, Lim KH, Dima S, Duda DG, Popescu I, Broet P, Hsieh SY, Yu MC, Scarpa A, Lai J, Luo DX, Carvalho AL, Vettore AL, Rhee H, Park YN, Alexandrov LB, Gordân R, Rozen SG, Shibata T, Pairojkul C, Teh BT, Tan P. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017;7:1116-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 678] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 6. | Cholangiocarcinoma Working Group. Italian Clinical Practice Guidelines on Cholangiocarcinoma - Part I: Classification, diagnosis and staging. Dig Liver Dis. 2020;52:1282-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Cardinale V. Classifications and misclassification in cholangiocarcinoma. Liver Int. 2019;39:260-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Sato K, Zhang W, Safarikia S, Isidan A, Chen AM, Li P, Francis H, Kennedy L, Baiocchi L, Alvaro D, Glaser S, Ekser B, Alpini G. Organoids and spheroids as novel models for studying cholestatic liver injury and cholangiocarcinoma. Hepatology. 2020;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 9. | Macias RIR, Banales JM, Sangro B, Muntané J, Avila MA, Lozano E, Perugorria MJ, Padillo FJ, Bujanda L, Marin JJG. The search for novel diagnostic and prognostic biomarkers in cholangiocarcinoma. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1468-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 10. | Song J, Li Y, Bowlus CL, Yang G, Leung PSC, Gershwin ME. Cholangiocarcinoma in Patients with Primary Sclerosing Cholangitis (PSC): a Comprehensive Review. Clin Rev Allergy Immunol. 2020;58:134-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 11. | Tsukahara T, Shimoyama Y, Ebata T, Yokoyama Y, Igami T, Sugawara G, Mizuno T, Yamaguchi J, Nakamura S, Nagino M. Cholangiocarcinoma with intraductal tubular growth pattern vs intraductal papillary growth pattern. Mod Pathol. 2016;29:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine vs gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3170] [Article Influence: 211.3] [Reference Citation Analysis (1)] |
| 13. | Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, Falk S, Gillmore R, Wadsley J, Patel K, Anthoney A, Maraveyas A, Iveson T, Waters JS, Hobbs C, Barber S, Ryder WD, Ramage J, Davies LM, Bridgewater JA, Valle JW; Advanced Biliary Cancer Working Group. Second-line FOLFOX chemotherapy vs active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22:690-701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 390] [Cited by in RCA: 494] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 14. | Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, Anthony A, Corrie P, Falk S, Finch-Jones M, Wasan H, Ross P, Wall L, Wadsley J, Evans JTR, Stocken D, Praseedom R, Ma YT, Davidson B, Neoptolemos JP, Iveson T, Raftery J, Zhu S, Cunningham D, Garden OJ, Stubbs C, Valle JW, Bridgewater J; BILCAP study group. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 824] [Article Influence: 137.3] [Reference Citation Analysis (0)] |
| 15. | Ebata T, Hirano S, Konishi M, Uesaka K, Tsuchiya Y, Ohtsuka M, Kaneoka Y, Yamamoto M, Ambo Y, Shimizu Y, Ozawa F, Fukutomi A, Ando M, Nimura Y, Nagino M; Bile Duct Cancer Adjuvant Trial (BCAT) Study Group. Randomized clinical trial of adjuvant gemcitabine chemotherapy vs observation in resected bile duct cancer. Br J Surg. 2018;105:192-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 287] [Article Influence: 41.0] [Reference Citation Analysis (1)] |
| 16. | Edeline J, Benabdelghani M, Bertaut A, Watelet J, Hammel P, Joly JP, Boudjema K, Fartoux L, Bouhier-Leporrier K, Jouve JL, Faroux R, Guerin-Meyer V, Kurtz JE, Assénat E, Seitz JF, Baumgaertner I, Tougeron D, de la Fouchardière C, Lombard-Bohas C, Boucher E, Stanbury T, Louvet C, Malka D, Phelip JM. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J Clin Oncol. 2019;37:658-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 367] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 17. | Lischalk JW, Repka MC, Unger K. Radiation therapy for hepatobiliary malignancies. J Gastrointest Oncol. 2017;8:279-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Cholangiocarcinoma Working Group. Italian Clinical Practice Guidelines on Cholangiocarcinoma - Part II: Treatment. Dig Liver Dis. 2020;52:1430-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Baiocchi L, Sato K, Ekser B, Kennedy L, Francis H, Ceci L, Lenci I, Alvaro D, Franchitto A, Onori P, Gaudio E, Wu C, Chakraborty S, Glaser S, Alpini G. Cholangiocarcinoma: bridging the translational gap from preclinical to clinical development and implications for future therapy. Expert Opin Investig Drugs. 2021;30:365-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Vicent S, Lieshout R, Saborowski A, Verstegen MMA, Raggi C, Recalcati S, Invernizzi P, van der Laan LJW, Alvaro D, Calvisi DF, Cardinale V. Experimental models to unravel the molecular pathogenesis, cell of origin and stem cell properties of cholangiocarcinoma. Liver Int. 2019;39 Suppl 1:79-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Fabris L, Sato K, Alpini G, Strazzabosco M. The Tumor Microenvironment in Cholangiocarcinoma Progression. Hepatology. 2021;73 Suppl 1:75-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 144] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 22. | Marin JJG, Lozano E, Herraez E, Asensio M, Di Giacomo S, Romero MR, Briz O, Serrano MA, Efferth T, Macias RIR. Chemoresistance and chemosensitization in cholangiocarcinoma. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1444-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 23. | Yeldag G, Rice A, Del Río Hernández A. Chemoresistance and the Self-Maintaining Tumor Microenvironment. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 155] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 24. | Herraez E, Lozano E, Macias RI, Vaquero J, Bujanda L, Banales JM, Marin JJ, Briz O. Expression of SLC22A1 variants may affect the response of hepatocellular carcinoma and cholangiocarcinoma to sorafenib. Hepatology. 2013;58:1065-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 25. | Vaquero J, Guedj N, Clapéron A, Nguyen Ho-Bouldoires TH, Paradis V, Fouassier L. Epithelial-mesenchymal transition in cholangiocarcinoma: From clinical evidence to regulatory networks. J Hepatol. 2017;66:424-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 26. | Kapacee ZA, Knox JJ, Palmer D, Blagden SP, Lamarca A, Valle JW, McNamara MG. NUC-1031, use of ProTide technology to circumvent gemcitabine resistance: current status in clinical trials. Med Oncol. 2020;37:61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Kayhanian H, Smyth EC, Braconi C. Emerging molecular targets and therapy for cholangiocarcinoma. World J Gastrointest Oncol. 2017;9:268-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Massironi S, Pilla L, Elvevi A, Longarini R, Rossi RE, Bidoli P, Invernizzi P. New and Emerging Systemic Therapeutic Options for Advanced Cholangiocarcinoma. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 29. | Lavingia V, Fakih M. Impressive response to dual BRAF and MEK inhibition in patients with BRAF mutant intrahepatic cholangiocarcinoma-2 case reports and a brief review. J Gastrointest Oncol. 2016;7:E98-E102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Subbiah V, Lassen U, Élez E, Italiano A, Curigliano G, Javle M, de Braud F, Prager GW, Greil R, Stein A, Fasolo A, Schellens JHM, Wen PY, Viele K, Boran AD, Gasal E, Burgess P, Ilankumaran P, Wainberg ZA. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020;21:1234-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 331] [Article Influence: 66.2] [Reference Citation Analysis (1)] |
| 31. | Mossahebi-Mohammadi M, Quan M, Zhang JS, Li X. FGF Signaling Pathway: A Key Regulator of Stem Cell Pluripotency. Front Cell Dev Biol. 2020;8:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 32. | Mahipal A, Tella SH, Kommalapati A, Anaya D, Kim R. FGFR2 genomic aberrations: Achilles heel in the management of advanced cholangiocarcinoma. Cancer Treat Rev. 2019;78:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 33. | Javle M, Lowery M, Shroff RT, Weiss KH, Springfeld C, Borad MJ, Ramanathan RK, Goyal L, Sadeghi S, Macarulla T, El-Khoueiry A, Kelley RK, Borbath I, Choo SP, Oh DY, Philip PA, Chen LT, Reungwetwattana T, Van Cutsem E, Yeh KH, Ciombor K, Finn RS, Patel A, Sen S, Porter D, Isaacs R, Zhu AX, Abou-Alfa GK, Bekaii-Saab T. Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. J Clin Oncol. 2018;36:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 518] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 34. | Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, Paulson AS, Borad MJ, Gallinson D, Murphy AG, Oh DY, Dotan E, Catenacci DV, Van Cutsem E, Ji T, Lihou CF, Zhen H, Féliz L, Vogel A. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 1074] [Article Influence: 214.8] [Reference Citation Analysis (0)] |
| 35. | Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, Cleary JM, Catenacci DV, Borad MJ, Bridgewater J, Harris WP, Murphy AG, Oh DY, Whisenant J, Lowery MA, Goyal L, Shroff RT, El-Khoueiry AB, Fan B, Wu B, Chamberlain CX, Jiang L, Gliser C, Pandya SS, Valle JW, Zhu AX. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:796-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 717] [Article Influence: 143.4] [Reference Citation Analysis (0)] |
| 36. | Crispo F, Pietrafesa M, Condelli V, Maddalena F, Bruno G, Piscazzi A, Sgambato A, Esposito F, Landriscina M. IDH1 Targeting as a New Potential Option for Intrahepatic Cholangiocarcinoma Treatment-Current State and Future Perspectives. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Jin W. ErBb Family Proteins in Cholangiocarcinoma and Clinical Implications. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Paluch C, Santos AM, Anzilotti C, Cornall RJ, Davis SJ. Immune Checkpoints as Therapeutic Targets in Autoimmunity. Front Immunol. 2018;9:2306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 39. | Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1293] [Cited by in RCA: 1482] [Article Influence: 211.7] [Reference Citation Analysis (0)] |
| 40. | Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11:3801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 421] [Cited by in RCA: 1098] [Article Influence: 219.6] [Reference Citation Analysis (0)] |
| 41. | Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, Piha-Paul SA, Doi T, Gao B, Chung HC, Lopez-Martin J, Bang YJ, Frommer RS, Shah M, Ghori R, Joe AK, Pruitt SK, Diaz LA Jr. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol. 2020;38:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2120] [Cited by in RCA: 2023] [Article Influence: 404.6] [Reference Citation Analysis (0)] |
| 42. | Ahn S, Lee JC, Shin DW, Kim J, Hwang JH. High PD-L1 expression is associated with therapeutic response to pembrolizumab in patients with advanced biliary tract cancer. Sci Rep. 2020;10:12348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 43. | Sato K, Francis H, Zhou T, Meng F, Kennedy L, Ekser B, Baiocchi L, Onori P, Mancinelli R, Gaudio E, Franchitto A, Glaser S, Alpini G. Neuroendocrine Changes in Cholangiocarcinoma Growth. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Onori P, Wise C, Gaudio E, Franchitto A, Francis H, Carpino G, Lee V, Lam I, Miller T, Dostal DE, Glaser SS. Secretin inhibits cholangiocarcinoma growth via dysregulation of the cAMP-dependent signaling mechanisms of secretin receptor. Int J Cancer. 2010;127:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Tan CK, Podila PV, Taylor JE, Nagorney DM, Wiseman GA, Gores GJ, LaRusso NF. Human cholangiocarcinomas express somatostatin receptors and respond to somatostatin with growth inhibition. Gastroenterology. 1995;108:1908-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Han Y, Demorrow S, Invernizzi P, Jing Q, Glaser S, Renzi A, Meng F, Venter J, Bernuzzi F, White M, Francis H, Lleo A, Marzioni M, Onori P, Alvaro D, Torzilli G, Gaudio E, Alpini G. Melatonin exerts by an autocrine loop antiproliferative effects in cholangiocarcinoma: its synthesis is reduced favoring cholangiocarcinoma growth. Am J Physiol Gastrointest Liver Physiol. 2011;301:G623-G633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Fiebiger WC, Scheithauer W, Traub T, Kurtaran A, Gedlicka C, Kornek GV, Virgolini I, Raderer M. Absence of therapeutic efficacy of the somatostatin analogue lanreotide in advanced primary hepatic cholangiocellular cancer and adenocarcinoma of the gallbladder despite in vivo somatostatin-receptor expression. Scand J Gastroenterol. 2002;37:222-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Yoshikawa D, Ojima H, Iwasaki M, Hiraoka N, Kosuge T, Kasai S, Hirohashi S, Shibata T. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer. 2008;98:418-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 318] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 49. | Zhu AX, Meyerhardt JA, Blaszkowsky LS, Kambadakone AR, Muzikansky A, Zheng H, Clark JW, Abrams TA, Chan JA, Enzinger PC, Bhargava P, Kwak EL, Allen JN, Jain SR, Stuart K, Horgan K, Sheehan S, Fuchs CS, Ryan DP, Sahani DV. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol. 2010;11:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 50. | Idrees JJ, Merath K, Gani F, Bagante F, Mehta R, Beal E, Cloyd JM, Pawlik TM. Trends in centralization of surgical care and compliance with National Cancer Center Network guidelines for resected cholangiocarcinoma. HPB (Oxford). 2019;21:981-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 51. | Mansour JC, Aloia TA, Crane CH, Heimbach JK, Nagino M, Vauthey JN. Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17:691-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 280] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 52. | Miyazaki M, Yoshitomi H, Miyakawa S, Uesaka K, Unno M, Endo I, Ota T, Ohtsuka M, Kinoshita H, Shimada K, Shimizu H, Tabata M, Chijiiwa K, Nagino M, Hirano S, Wakai T, Wada K, Isayama H, Okusaka T, Tsuyuguchi T, Fujita N, Furuse J, Yamao K, Murakami K, Yamazaki H, Kijima H, Nakanuma Y, Yoshida M, Takayashiki T, Takada T. Clinical practice guidelines for the management of biliary tract cancers 2015: the 2nd English edition. J Hepatobiliary Pancreat Sci. 2015;22:249-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 166] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 53. | Wiggers JK, Groot Koerkamp B, Cieslak KP, Doussot A, van Klaveren D, Allen PJ, Besselink MG, Busch OR, D'Angelica MI, DeMatteo RP, Gouma DJ, Kingham TP, van Gulik TM, Jarnagin WR. Postoperative Mortality after Liver Resection for Perihilar Cholangiocarcinoma: Development of a Risk Score and Importance of Biliary Drainage of the Future Liver Remnant. J Am Coll Surg. 2016;223:321-331.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 54. | Kennedy TJ, Yopp A, Qin Y, Zhao B, Guo P, Liu F, Schwartz LH, Allen P, D'Angelica M, Fong Y, DeMatteo RP, Blumgart LH, Jarnagin WR. Role of preoperative biliary drainage of liver remnant prior to extended liver resection for hilar cholangiocarcinoma. HPB (Oxford). 2009;11:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 55. | Lidsky ME, Jarnagin WR. Surgical management of hilar cholangiocarcinoma at Memorial Sloan Kettering Cancer Center. Ann Gastroenterol Surg. 2018;2:304-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 56. | Kawakami H, Kuwatani M, Onodera M, Haba S, Eto K, Ehira N, Yamato H, Kudo T, Tanaka E, Hirano S, Kondo S, Asaka M. Endoscopic nasobiliary drainage is the most suitable preoperative biliary drainage method in the management of patients with hilar cholangiocarcinoma. J Gastroenterol. 2011;46:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 57. | Kawashima H, Itoh A, Ohno E, Itoh Y, Ebata T, Nagino M, Goto H, Hirooka Y. Preoperative endoscopic nasobiliary drainage in 164 consecutive patients with suspected perihilar cholangiocarcinoma: a retrospective study of efficacy and risk factors related to complications. Ann Surg. 2013;257:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 58. | She WH, Cheung TT, Ma KW, Tsang SHY, Dai WC, Chan ACY, Lo CM. Defining the optimal bilirubin level before hepatectomy for hilar cholangiocarcinoma. BMC Cancer. 2020;20:914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 59. | Li J, Moustafa M, Linecker M, Lurje G, Capobianco I, Baumgart J, Ratti F, Rauchfuss F, Balci D, Fernandes E, Montalti R, Robles-Campos R, Bjornsson B, Topp SA, Fronek J, Liu C, Wahba R, Bruns C, Brunner SM, Schlitt HJ, Heumann A, Stüben BO, Izbicki JR, Bednarsch J, Gringeri E, Fasolo E, Rolinger J, Kristek J, Hernandez-Alejandro R, Schnitzbauer A, Nuessler N, Schön MR, Voskanyan S, Petrou AS, Hahn O, Soejima Y, Vicente E, Castro-Benitez C, Adam R, Tomassini F, Troisi RI, Kantas A, Oldhafer KJ, Ardiles V, de Santibanes E, Malago M, Clavien PA, Vivarelli M, Settmacher U, Aldrighetti L, Neumann U, Petrowsky H, Cillo U, Lang H, Nadalin S. ALPPS for Locally Advanced Intrahepatic Cholangiocarcinoma: Did Aggressive Surgery Lead to the Oncological Benefit? Ann Surg Oncol. 2020;27:1372-1384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 60. | Goere D, Wagholikar GD, Pessaux P, Carrère N, Sibert A, Vilgrain V, Sauvanet A, Belghiti J. Utility of staging laparoscopy in subsets of biliary cancers : laparoscopy is a powerful diagnostic tool in patients with intrahepatic and gallbladder carcinoma. Surg Endosc. 2006;20:721-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 61. | Weber SM, Ribero D, O'Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17:669-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 341] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 62. | Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, D'Angelica M, DeMatteo RP, Fong Y, Schwartz L, Kemeny N, O'Reilly E, Abou-Alfa GK, Shimada H, Blumgart LH, Jarnagin WR. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 653] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 63. | Luo X, Yuan L, Wang Y, Ge R, Sun Y, Wei G. Survival outcomes and prognostic factors of surgical therapy for all potentially resectable intrahepatic cholangiocarcinoma: a large single-center cohort study. J Gastrointest Surg. 2014;18:562-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 64. | Farges O, Fuks D, Boleslawski E, Le Treut YP, Castaing D, Laurent A, Ducerf C, Rivoire M, Bachellier P, Chiche L, Nuzzo G, Regimbeau JM. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg. 2011;254:824-829; discussion 830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 187] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 65. | Nakagawa T, Kamiyama T, Kurauchi N, Matsushita M, Nakanishi K, Kamachi H, Kudo T, Todo S. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg. 2005;29:728-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 66. | Ribero D, Pinna AD, Guglielmi A, Ponti A, Nuzzo G, Giulini SM, Aldrighetti L, Calise F, Gerunda GE, Tomatis M, Amisano M, Berloco P, Torzilli G, Capussotti L; Italian Intrahepatic Cholangiocarcinoma Study Group. Surgical Approach for Long-term Survival of Patients With Intrahepatic Cholangiocarcinoma: A Multi-institutional Analysis of 434 Patients. Arch Surg. 2012;147:1107-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 67. | Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 862] [Cited by in RCA: 1078] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 68. | Nathan H, Aloia TA, Vauthey JN, Abdalla EK, Zhu AX, Schulick RD, Choti MA, Pawlik TM. A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009;16:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 224] [Article Influence: 14.0] [Reference Citation Analysis (2)] |
| 69. | Reames BN, Ejaz A, Koerkamp BG, Alexandrescu S, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Martel G, Marsh JW, Pawlik TM. Impact of major vascular resection on outcomes and survival in patients with intrahepatic cholangiocarcinoma: A multi-institutional analysis. J Surg Oncol. 2017;116:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 70. | Lang H, Sotiropoulos GC, Sgourakis G, Schmitz KJ, Paul A, Hilgard P, Zöpf T, Trarbach T, Malagó M, Baba HA, Broelsch CE. Operations for intrahepatic cholangiocarcinoma: single-institution experience of 158 patients. J Am Coll Surg. 2009;208:218-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 71. | Sapisochin G, Rodríguez de Lope C, Gastaca M, Ortiz de Urbina J, Suarez MA, Santoyo J, Castroagudín JF, Varo E, López-Andujar R, Palacios F, Sanchez Antolín G, Perez B, Guiberteau A, Blanco G, González-Diéguez ML, Rodriguez M, Varona MA, Barrera MA, Fundora Y, Ferron JA, Ramos E, Fabregat J, Ciria R, Rufian S, Otero A, Vazquez MA, Pons JA, Parrilla P, Zozaya G, Herrero JI, Charco R, Bruix J. "Very early" intrahepatic cholangiocarcinoma in cirrhotic patients: should liver transplantation be reconsidered in these patients? Am J Transplant. 2014;14:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 72. | Raptis DA, Linecker M, Kambakamba P, Tschuor C, Müller PC, Hadjittofi C, Stavrou GA, Fard-Aghaie MH, Tun-Abraham M, Ardiles V, Malagó M, Campos RR, Oldhafer KJ, Hernandez-Alejandro R, de Santibañes E, Machado MA, Petrowsky H, Clavien PA. Defining Benchmark Outcomes for ALPPS. Ann Surg. 2019;270:835-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 73. | Le Roy B, Gelli M, Pittau G, Allard MA, Pereira B, Serji B, Vibert E, Castaing D, Adam R, Cherqui D, Sa Cunha A. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg. 2018;105:839-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 74. | Spolverato G, Kim Y, Alexandrescu S, Marques HP, Lamelas J, Aldrighetti L, Clark Gamblin T, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Tran TB, Wallis Marsh J, Pawlik TM. Management and Outcomes of Patients with Recurrent Intrahepatic Cholangiocarcinoma Following Previous Curative-Intent Surgical Resection. Ann Surg Oncol. 2016;23:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 201] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 75. | Yoh T, Hatano E, Seo S, Okuda Y, Fuji H, Ikeno Y, Taura K, Yasuchika K, Okajima H, Kaido T, Uemoto S. Long-Term Survival of Recurrent Intrahepatic Cholangiocarcinoma: The Impact and Selection of Repeat Surgery. World J Surg. 2018;42:1848-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 76. | Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D; ESMO Guidelines Committee. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v28-v37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 484] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 77. | Watson MD, Baimas-George MR, Passeri MJ, Sulzer JK, Baker EH, Ocuin LM, Martinie JB, Iannitti DA, Vrochides D. Effect of Margin Status on Survival After Resection of Hilar Cholangiocarcinoma in the Modern Era of Adjuvant Therapies. Am Surg. 2020;3134820973401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 78. | Otsuka S, Ebata T, Yokoyama Y, Mizuno T, Tsukahara T, Shimoyama Y, Ando M, Nagino M. Clinical value of additional resection of a margin-positive distal bile duct in perihilar cholangiocarcinoma. Br J Surg. 2019;106:774-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 79. | Zhang XF, Squires MH 3rd, Bagante F, Ethun CG, Salem A, Weber SM, Tran T, Poultsides G, Son AY, Hatzaras I, Jin L, Fields RC, Weiss M, Scoggins C, Martin RCG, Isom CA, Idrees K, Mogal HD, Shen P, Maithel SK, Schmidt CR, Pawlik TM. The Impact of Intraoperative Re-Resection of a Positive Bile Duct Margin on Clinical Outcomes for Hilar Cholangiocarcinoma. Ann Surg Oncol. 2018;25:1140-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 80. | Stremitzer S, Stift J, Laengle J, Schwarz C, Kaczirek K, Jones RP, Quinn LM, Fenwick SW, Diaz-Nieto R, Poston GJ, Malik HZ. Prognosis and Circumferential Margin in Patients with Resected Hilar Cholangiocarcinoma. Ann Surg Oncol. 2021;28:1493-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 81. | Cai Y, Cheng N, Ye H, Li F, Song P, Tang W. The current management of cholangiocarcinoma: A comparison of current guidelines. Biosci Trends. 2016;10:92-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 82. | Kambakamba P, Linecker M, Slankamenac K, DeOliveira ML. Lymph node dissection in resectable perihilar cholangiocarcinoma: a systematic review. Am J Surg. 2015;210:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 83. | He M, Xu X, Feng H, Chen W, Liu H, Zhang Y, Wang J, Geng Z, Qiu Y, Duan W, Li X, Zhi X, Zhu W, Li F, Li J, Li S, He Y, Quan Z. Regional lymphadenectomy vs. extended lymphadenectomy for hilar cholangiocarcinoma (Relay-HC trial): study protocol for a prospective, multicenter, randomized controlled trial. Trials. 2019;20:528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 84. | Ma WJ, Wu ZR, Hu HJ, Wang JK, Yin CH, Shi YJ, Li FY, Cheng NS. Extended Lymphadenectomy Versus Regional Lymphadenectomy in Resectable Hilar Cholangiocarcinoma. J Gastrointest Surg. 2020;24:1619-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 85. | Olthof PB, Coelen RJS, Wiggers JK, Groot Koerkamp B, Malago M, Hernandez-Alejandro R, Topp SA, Vivarelli M, Aldrighetti LA, Robles Campos R, Oldhafer KJ, Jarnagin WR, van Gulik TM. High mortality after ALPPS for perihilar cholangiocarcinoma: case-control analysis including the first series from the international ALPPS registry. HPB (Oxford). 2017;19:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 86. | Balci D, Sakamoto Y, Li J, Di Benedetto F, Kirimker EO, Petrowsky H. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) procedure for cholangiocarcinoma. Int J Surg. 2020;82S:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 87. | Mizuno T, Ebata T, Nagino M. Advanced hilar cholangiocarcinoma: An aggressive surgical approach for the treatment of advanced hilar cholangiocarcinoma: Perioperative management, extended procedures, and multidisciplinary approaches. Surg Oncol. 2020;33:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 88. | Aoki T, Sakamoto Y, Kohno Y, Akamatsu N, Kaneko J, Sugawara Y, Hasegawa K, Makuuchi M, Kokudo N. Hepatopancreaticoduodenectomy for Biliary Cancer: Strategies for Near-zero Operative Mortality and Acceptable Long-term Outcome. Ann Surg. 2018;267:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 89. | Sakamoto Y, Nara S, Kishi Y, Esaki M, Shimada K, Kokudo N, Kosuge T. Is extended hemihepatectomy plus pancreaticoduodenectomy justified for advanced bile duct cancer and gallbladder cancer? Surgery. 2013;153:794-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 90. | Riediger H, Makowiec F, Fischer E, Adam U, Hopt UT. Postoperative morbidity and long-term survival after pancreaticoduodenectomy with superior mesenterico-portal vein resection. J Gastrointest Surg. 2006;10:1106-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 91. | Stitzenberg KB, Watson JC, Roberts A, Kagan SA, Cohen SJ, Konski AA, Hoffman JP. Survival after pancreatectomy with major arterial resection and reconstruction. Ann Surg Oncol. 2008;15:1399-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 92. | Guiu B, Chevallier P, Denys A, Delhom E, Pierredon-Foulongne MA, Rouanet P, Fabre JM, Quenet F, Herrero A, Panaro F, Baudin G, Ramos J. Simultaneous trans-hepatic portal and hepatic vein embolization before major hepatectomy: the liver venous deprivation technique. Eur Radiol. 2016;26:4259-4267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 93. | Guiu B, Quenet F, Panaro F, Piron L, Cassinotto C, Herrerro A, Souche FR, Hermida M, Pierredon-Foulongne MA, Belgour A, Aho-Glele S, Deshayes E. Liver venous deprivation vs portal vein embolization before major hepatectomy: future liver remnant volumetric and functional changes. Hepatobiliary Surg Nutr. 2020;9:564-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 94. | Shiraiwa DK, Carvalho PFDC, Maeda CT, Silva LC, Forones NM, Lopes-Filho GJ, Linhares MM, Araujo RLC. The role of minimally invasive hepatectomy for hilar and intrahepatic cholangiocarcinoma: A systematic review of the literature. J Surg Oncol. 2020;121:863-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 95. | Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69:1633-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 348] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 96. | De Vreede I, Steers JL, Burch PA, Rosen CB, Gunderson LL, Haddock MG, Burgart L, Gores GJ. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transpl. 2000;6:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 239] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 97. | Rea DJ, Heimbach JK, Rosen CB, Haddock MG, Alberts SR, Kremers WK, Gores GJ, Nagorney DM. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451-458; discussion 458-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 428] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 98. | Sahai P, Kumar S. External radiotherapy and brachytherapy in the management of extrahepatic and intrahepatic cholangiocarcinoma: available evidence. Br J Radiol. 2017;90:20170061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 99. | Duignan S, Maguire D, Ravichand CS, Geoghegan J, Hoti E, Fennelly D, Armstrong J, Rock K, Mohan H, Traynor O. Neoadjuvant chemoradiotherapy followed by liver transplantation for unresectable cholangiocarcinoma: a single-centre national experience. HPB (Oxford). 2014;16:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 100. | Sio TT, Martenson JA Jr, Haddock MG, Novotny PJ, Gores GJ, Alberts SR, Miller RC, Heimbach JK, Rosen CB. Outcome of Transplant-fallout Patients With Unresectable Cholangiocarcinoma. Am J Clin Oncol. 2016;39:271-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 101. | Darwish Murad S, Kim WR, Harnois DM, Douglas DD, Burton J, Kulik LM, Botha JF, Mezrich JD, Chapman WC, Schwartz JJ, Hong JC, Emond JC, Jeon H, Rosen CB, Gores GJ, Heimbach JK. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143:88-98.e3; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 395] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 102. | Darwish Murad S, Kim WR, Therneau T, Gores GJ, Rosen CB, Martenson JA, Alberts SR, Heimbach JK. Predictors of pretransplant dropout and posttransplant recurrence in patients with perihilar cholangiocarcinoma. Hepatology. 2012;56:972-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 103. | Lehrke HD, Heimbach JK, Wu TT, Jenkins SM, Gores GJ, Rosen CB, Mounajjed T. Prognostic Significance of the Histologic Response of Perihilar Cholangiocarcinoma to Preoperative Neoadjuvant Chemoradiation in Liver Explants. Am J Surg Pathol. 2016;40:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 104. | Tan EK, Taner T, Heimbach JK, Gores GJ, Rosen CB. Liver Transplantation for Peri-hilar Cholangiocarcinoma. J Gastrointest Surg. 2020;24:2679-2685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 105. | Kitajima T, Hibi T, Moonka D, Sapisochin G, Abouljoud MS, Nagai S. Center Experience Affects Liver Transplant Outcomes in Patients with Hilar Cholangiocarcinoma. Ann Surg Oncol. 2020;27:5209-5221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 106. | O'Grady JG, Polson RJ, Rolles K, Calne RY, Williams R. Liver transplantation for malignant disease. Results in 93 consecutive patients. Ann Surg. 1988;207:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 329] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 107. | Vilchez V, Shah MB, Daily MF, Pena L, Tzeng CW, Davenport D, Hosein PJ, Gedaly R, Maynard E. Long-term outcome of patients undergoing liver transplantation for mixed hepatocellular carcinoma and cholangiocarcinoma: an analysis of the UNOS database. HPB (Oxford). 2016;18:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 108. | Lunsford KE, Javle M, Heyne K, Shroff RT, Abdel-Wahab R, Gupta N, Mobley CM, Saharia A, Victor DW, Nguyen DT, Graviss EA, Kaseb AO, McFadden RS, Aloia TA, Conrad C, Li XC, Monsour HP, Gaber AO, Vauthey JN, Ghobrial RM; Methodist–MD Anderson Joint Cholangiocarcinoma Collaborative Committee (MMAJCCC). Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol. 2018;3:337-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 200] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 109. | Sapisochin G, de Lope CR, Gastaca M, de Urbina JO, López-Andujar R, Palacios F, Ramos E, Fabregat J, Castroagudín JF, Varo E, Pons JA, Parrilla P, González-Diéguez ML, Rodriguez M, Otero A, Vazquez MA, Zozaya G, Herrero JI, Antolin GS, Perez B, Ciria R, Rufian S, Fundora Y, Ferron JA, Guiberteau A, Blanco G, Varona MA, Barrera MA, Suarez MA, Santoyo J, Bruix J, Charco R. Intrahepatic cholangiocarcinoma or mixed hepatocellular-cholangiocarcinoma in patients undergoing liver transplantation: a Spanish matched cohort multicenter study. Ann Surg. 2014;259:944-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 110. | Ethun CG, Lopez-Aguiar AG, Anderson DJ, Adams AB, Fields RC, Doyle MB, Chapman WC, Krasnick BA, Weber SM, Mezrich JD, Salem A, Pawlik TM, Poultsides G, Tran TB, Idrees K, Isom CA, Martin RCG, Scoggins CR, Shen P, Mogal HD, Schmidt C, Beal E, Hatzaras I, Shenoy R, Cardona K, Maithel SK. Transplantation Versus Resection for Hilar Cholangiocarcinoma: An Argument for Shifting Treatment Paradigms for Resectable Disease. Ann Surg. 2018;267:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 111. | Facciuto ME, Singh MK, Lubezky N, Selim MA, Robinson D, Kim-Schluger L, Florman S, Ward SC, Thung SN, Fiel M, Schiano TD. Tumors with intrahepatic bile duct differentiation in cirrhosis: implications on outcomes after liver transplantation. Transplantation. 2015;99:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 112. | Sapisochin G, Facciuto M, Rubbia-Brandt L, Marti J, Mehta N, Yao FY, Vibert E, Cherqui D, Grant DR, Hernandez-Alejandro R, Dale CH, Cucchetti A, Pinna A, Hwang S, Lee SG, Agopian VG, Busuttil RW, Rizvi S, Heimbach JK, Montenovo M, Reyes J, Cesaretti M, Soubrane O, Reichman T, Seal J, Kim PT, Klintmalm G, Sposito C, Mazzaferro V, Dutkowski P, Clavien PA, Toso C, Majno P, Kneteman N, Saunders C, Bruix J; iCCA International Consortium. Liver transplantation for "very early" intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology. 2016;64:1178-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 252] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 113. | Moris D, Kostakis ID, Machairas N, Prodromidou A, Tsilimigras DI, Ravindra KV, Sudan DL, Knechtle SJ, Barbas AS. Comparison between liver transplantation and resection for hilar cholangiocarcinoma: A systematic review and meta-analysis. PLoS One. 2019;14:e0220527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 114. | Baltatzis M, Jegatheeswaran S, Siriwardena AK. Neoadjuvant chemoradiotherapy before resection of perihilar cholangiocarcinoma: A systematic review. Hepatobiliary Pancreat Dis Int. 2020;19:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |